Significance
Cannabis use is becoming more prevalent, including during developmentally sensitive periods such as pregnancy. Here we find that maternal cannabis use is associated with increased cortisol, anxiety, aggression, and hyperactivity in young children. This corresponded with widespread reductions in immune-related gene expression in the placenta which correlated with anxiety and hyperactivity. Future studies are needed to examine the effects of cannabis on immune function during pregnancy as a potential regulatory mechanism shaping neurobehavioral development.
Keywords: anxiety, cortisol, immune system, cannabinoid receptor, transcriptome
Abstract
While cannabis is among the most used recreational drugs during pregnancy, the impact of maternal cannabis use (mCB) on fetal and child development remains unclear. Here, we assessed the effects of mCB on psychosocial and physiological measures in young children along with the potential relevance of the in utero environment reflected in the placental transcriptome. Children (∼3 to 6 y) were assessed for hair hormone levels, neurobehavioral traits on the Behavioral Assessment System for Children (BASC-2) survey, and heart rate variability (HRV) at rest and during auditory startle. For a subset of children with behavioral assessments, placental specimens collected at birth were processed for RNA sequencing. Hair hormone analysis revealed increased cortisol levels in mCB children. In addition, mCB was associated with greater anxiety, aggression, and hyperactivity. Children with mCB also showed a reduction in the high-frequency component of HRV at baseline, reflecting reduced vagal tone. In the placenta, there was reduced expression of many genes involved in immune system function including type I interferon, neutrophil, and cytokine-signaling pathways. Finally, several of these mCB-linked immune genes organized into coexpression networks that correlated with child anxiety and hyperactivity. Overall, our findings reveal a relationship between mCB and immune response gene networks in the placenta as a potential mediator of risk for anxiety-related problems in early childhood.
Along with the progressive legalization of recreational cannabis across the world, there is a prevalent misconception that cannabis use is without significant health risks. In line with this softening public perception, cannabis has emerged as one of the most consumed recreational drugs of abuse during pregnancy, with past-month cannabis use ranging from 3 to 16% among pregnant women (1, 2). In addition, the concentration of the psychoactive component in cannabis, tetrahydrocannabinol (THC), has increased consistently over the past decade, further increasing its potential harm (3). Gestational development is highly vulnerable to environmental factors which are transduced by the mother through the placenta to impact fetal health (4). Indeed, THC freely passes from the maternal blood to the fetus via the placenta (5). Thus, although cannabis is considered to provide relief from symptoms such as nausea during pregnancy (6), it may also present a significant xenobiotic challenge to the developing fetus (7).
THC targets the endocannabinoid system in both the placental tissue and developing brain (8, 9), highlighting the vulnerability of the developing fetus to prenatal cannabis exposure. Maternal cannabis use (mCB) during pregnancy is associated with fetal growth restriction (7, 10), low birth weight (7, 11), and preterm birth (12). Ex vivo treatment of placenta with THC (40 μM) shows increasing levels of the endocannabinoid anandamide (13). Moreover, rodent studies have demonstrated that THC exposure during gestation induces growth restriction and alters placental vasculature (14). Neurobiological consequences are also evident. Cannabis use during pregnancy is associated with reduced dopaminergic receptor expression in the fetal brain (15, 16), altered cognitive development (17), increased incidence of autism (18), and depression and psychosis symptomology (19, 20). Preclinical studies directly examining the effects of THC and cannabinoid (CB) receptor agonists during pregnancy also report adverse effects on adult offspring such as anxiogenic phenotypes (21), altered learning behavior (22), and increased drug-seeking behavior (23). Collectively, this evidence suggests that cannabis use or THC during pregnancy may exert long-lasting effects on neurobehavioral development.
Despite the abundance of evidence associating mCB with fetal health and child development independently (24), few studies have concurrently investigated the role of the in utero environment. This is noteworthy, as many clinical and preclinical studies have elucidated an important role for the placenta in the neurodevelopmental effects associated with prenatal exposures such as chronic stress (25). Therefore, in the current study, we examined the effect of mCB on the placental transcriptome in relation to offspring development, leveraging a longitudinal cohort of mother–child dyads from the New York metropolitan area. The results provide a unique multimodal interrogation of the impact of mCB.
Results
Demographics.
The present study utilized longitudinal assessments from a total cohort of 322 mother–child dyads (see Table 1 for demographics and SI Appendix, Fig. S1 for a consort flowchart). mCB was associated with reduced maternal and paternal age and more single-mother pregnancies, state anxiety, trait anxiety, depression, and cigarette smoking. There was also a significant difference in the race demographic including slightly more African American (25.4 vs. 19.1%) and fewer Caucasian (12.7 vs. 21.5%) and Asian (4.2 vs. 10.4%) mCB cases vs. non–cannabis users (non-CB). As a result, each of these presented confounds was adjusted for in all subsequent analyses. Notably, there was no difference across all measures of perinatal health or frequency of pregnancy complications for mCB vs. non-CB cases.
Table 1.
Demographics and reproductive characteristics of the participants in the total sample grouped by mCB status
| Demographics | Non-CB (n = 251) | mCB (n = 71) | F (df) | P value | ||
| Mean | SD | Mean | SD | |||
| Maternal age, y | 28.46 | 6.09 | 25.91 | 5.2 | F(1,320) = 10.28 | 0.001 |
| Paternal age, y | 31.34 | 7.59 | 28.08 | 6.35 | F(1,291) = 9.74 | 0.002 |
| Parity | 2.57 | 1.88 | 2.89 | 2.57 | F(1,320) = 1.30 | 0.26 |
| Birthweight, kg | 3.25 | 0.55 | 3.16 | 0.65 | F(1,266) = 1.21 | 0.27 |
| Gestational age, wk | 38.74 | 2.71 | 39.41 | 1.83 | F(1,255) = 3.32 | 0.07 |
| Placenta weight, g* | 658.04 | 169.85 | 599.64 | 113.52 | F(1,97) = 2.56 | 0.001 |
| Prenatal trait anxiety | 38.10 | 10.42 | 42.94 | 10.29 | F(1,285) = 10.88 | <0.001 |
| Prenatal state anxiety | 36.76 | 11.53 | 42.76 | 11.28 | F(1,284) = 13.76 | 0.001 |
| Depression | 9.08 | 4.67 | 11.31 | 5.51 | F(1,301) = 10.97 | 0.01 |
| N | % total | N | % total | Chi-square(df) | P value | |
| Child sex (male) | 137 | 54.6 | 33 | 46.5 | X2(1) = 1.46 | 0.23 |
| Marital status | X2(4) = 20.93 | <0.001 | ||||
| Married | 128 | 51 | 18 | 25.4 | ||
| Common law | 14 | 5.6 | 2 | 2.8 | ||
| Single | 106 | 42.3 | 49 | 69 | ||
| Widowed | 0 | 0 | 1 | 1.4 | ||
| Divorced/separated | 3 | 1.2 | 1 | 1.4 | ||
| Education | X2(6) = 6.5 | 0.36 | ||||
| Primary school | 6 | 2.4 | 3 | 4.2 | ||
| Some high school | 25 | 10 | 12 | 16.9 | ||
| High school/GED | 51 | 20.3 | 13 | 16.3 | ||
| Some college | 58 | 23.1 | 19 | 26.8 | ||
| Associate degree | 24 | 9.6 | 8 | 11.3 | ||
| Bachelor’s degree | 45 | 17.9 | 10 | 14.1 | ||
| Graduate degree | 42 | 16.7 | 6 | 8.5 | ||
| Child race | X2(4) = 10.03 | 0.04 | ||||
| Asian | 26 | 10.4 | 3 | 4.2 | ||
| Black | 48 | 19.1 | 18 | 25.4 | ||
| Hispanic | 114 | 45.4 | 34 | 47.9 | ||
| White | 54 | 21.5 | 9 | 12.7 | ||
| Other | 9 | 3.6 | 7 | 9.9 | ||
| Prenatal alcohol use | 15 | 6 | 7 | 9.9 | X2(1) = 1.30 | 0.25 |
| Prenatal cigarette smoke | 14 | 5.6 | 19 | 26.8 | X2(1) = 27.13 | <0.001 |
| Preeclampsia | 15 | 6 | 8 | 11.3 | X2(1) = 2.28 | 0.13 |
| Gestational diabetes | 17 | 6.8 | 4 | 5.6 | X2(1) = 0.13 | 0.99† |
| Vaginal infection | 13 | 5.2 | 3 | 4.2 | X2(1) = 0.11 | 0.99† |
| C-section | 59 | 23.5 | 23 | 32.4 | X2(1) = 2.30 | 0.13 |
Bold indicates significance. DF, degrees of freedom; GED, General Educational Development test.
*Based on 96 available placenta weights (72 for non-CB and 24 for mCB).
†Fisher’s exact test was used due to a small count (<5) in one of the cells.
Steroid Hormone Analysis.
Hair samples (taken close to the scalp from the posterior vertex of the head) collected from young children were used to evaluate stable steroid hormone concentrations accumulated over recent months (26) (Table 2, Upper). The results revealed increased cortisol levels in mCB children [F(1,220) = 15.67, adjusted (adj.) P < 0.0001]. There were no significant group differences for cortisone, dehydroepiandrosterone (DHEA), or cortisol:DHEA ratio.
Table 2.
mCB in relation to hair steroid hormones and clinical behavior scores in early childhood
| Hair hormone levels |
Non-CB (n = 180) | mCB (n = 49) | F test (1,220) | Adj. P value | ||
| Mean | SD | Mean | SD | |||
| Cortisol, pg/mg | 63.57 | 73.65 | 124.45 | 101.15 | 15.67 | <0.0001 |
| Cortisone, pg/mg | 65.86 | 55.81 | 62.94 | 41.23 | 0.36 | 0.55 |
| DHEA, pg/mg | 17.96 | 24.28 | 16.3 | 12.81 | 1.69 | 0.20 |
| Cortisol:DHEA ratio | 8.29 | 14.89 | 13.01 | 22.05 | 2.06 | 0.74 |
| BASC-2 assessments | Non-CB (n = 236) | mCB (n = 68) | F test (1,298) | Adj. P value | ||
| Mean | SD | Mean | SD | |||
| Aggression | 46.14 | 8.02 | 51.1 | 10.19 | 16.73 | 0.00006 |
| Anxiety | 50.23 | 8.02 | 54.77 | 12.83 | 10.07 | 0.002 |
| Attention problems | 50.32 | 8.73 | 51.63 | 10.21 | 1.94 | 0.17 |
| Atypical behavior | 50.28 | 9.73 | 54.52 | 11.82 | 3.04 | 0.08 |
| Depression | 48.24 | 10.56 | 51.29 | 12.7 | 3.87 | 0.05 |
| Hyperactivity | 48.52 | 8.99 | 52.19 | 9.92 | 8.5 | 0.004 |
| Somatization | 49.19 | 8.94 | 48.71 | 9.45 | 0.05 | 0.83 |
| Withdrawn behavior | 49.43 | 8.5 | 48.81 | 9.81 | 0.26 | 0.61 |
Bold indicates significance.
Neurobehavioral Profile: Clinical and Adaptive Behaviors.
To assess neurobehavioral traits in early childhood, we utilized the Behavior Assessment System for Children, Second Edition (BASC-2) (27) as reported in Table 2, Lower. The BASC-2 is a well-standardized, multidimensional evaluation of the behavior of young children which measures clinical dimensions of behaviors. The instrument produces standardized T scores, with 50 being the mean and 10 being the SD, which are normalized for the sex and age for the category. Here, we found that mCB was associated with increased anxiety [F(1,298) = 16.73, adj. P = 0.002], aggression [F(1,298) = 10.07, adj. P < 0.001], and hyperactivity [F(1,298) = 8.50, adj. P < 0.001]. In addition, when we evaluated neurobehavioral problems meeting a clinically significant threshold (>60 on BASC-2), mCB was associated with significantly increased risk for clinical-level problems with aggression, anxiety, and hyperactivity (SI Appendix, Table S1). Next, we evaluated BASC-2 measures for mCB × child sex interaction effects (SI Appendix, Table S2). There was a significant mCB × sex interaction for aggression [F(1,296) = 4.96, adj. P = 0.027] and univariate post hoc tests revealed a significant increase in aggression specifically in mCB females [F(1,135) = 20.85, adj. P < 0.001] and not males [F(1,163) =1.87, adj. P > 0.05].
Heart Rate Variability.
Heart rate variability (HRV) describes the change in time interval between heart beats. HRV measured in the high-frequency (HF) domain is associated with active engagement of the parasympathetic nervous system (28). Reductions in HF-HRV are linked with various anxiety-related disorders in both adults and children (29). Here we examined HRV during three sequential 1-min testing phases (prestartle, intermittent auditory startle, and poststartle). As reported in Table 3, when we evaluated HF-HRV normalized to low-frequency (LF) HRV (reflecting parasympathetic–sympathetic balance), there was a significant main effect of mCB [F(1,144) = 5.67, P = 0.02] and significantly reduced HF-HRV both prestartle [F(1,146) = 4.30, P = 0.04] and during startle [F(1,146) = 6.37, P = 0.01] for mCB children. There were no differences in heart rate or startle response magnitude/amplitude between mCB and non-CB groups (data not shown). For the subset of subjects with both BASC-2 and HRV measures (n = 126 subjects), there was no relationship between behavioral traits such as anxiety and HF-HRV or normalized HF-HRV (SI Appendix, Table S3).
Table 3.
Relationship between mCB and HRV in early childhood
| Testing phase | Non-CB (n = 116) | mCB (n = 31) | Main effect | Univariate tests | |||||
| Mean | SD | Mean | SD | F (1,144) | P | F (1,146) | P | ||
| HF-HRV* | Resting (prestartle) | 4.12 | 1.48 | 3.51 | 1.43 | 2.77 | 0.10 | 3.28 | 0.07 |
| Auditory startle | 4.13 | 1.45 | 3.72 | 1.52 | 1.5 | 0.22 | |||
| Resting (poststartle) | 3.99 | 1.62 | 3.39 | 1.29 | 2.96 | 0.09 | |||
| HF-HRV/LF-HRV* | Resting (prestartle) | 0.59 | 0.54 | 0.30 | 0.65 | 5.67 | 0.02 | 4.3 | 0.04 |
| Auditory startle | 0.51 | 0.62 | 0.19 | 0.68 | 6.37 | 0.01 | |||
| Resting (poststartle) | 0.54 | 0.53 | 0.28 | 0.78 | 1.11 | 0.3 | |||
*Log10-transformed values are represented.
mCB and the Placental Transcriptome.
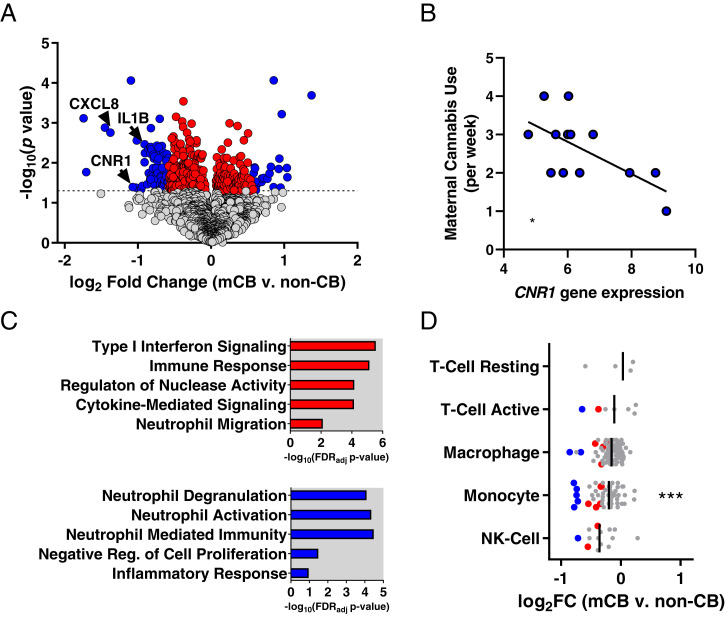
Placental biopsies were collected at the time of birth (gestational age 39.13 ± 2.63 wk, μ ± SD) and processed for RNA sequencing (RNA-seq) to carry out gene expression analysis (see SI Appendix, Table S4 for demographics and Dataset S1 and SI Appendix, Fig. S2 for RNA-seq quality metrics). Differential expression analysis revealed 480 significant genes (359 decreased, 121 increased) in mCB placentas at P < 0.05 (Fig. 1A, SI Appendix, Fig. S3, and Dataset S2) with no genes passing false discovery rate (FDR) adjustment (q < 0.05). While the high stringency of accounting for numerous covariates limited our statistical power to pass FDR adjustment, we evaluated the function of significant unadjusted genes (P < 0.05) which allows the possibility to identify potential transcriptome pathways to inform future validation studies. Among the most robustly differentially expressed genes (DEGs) were many proinflammatory cytokines and chemokines (e.g., IL1B, CXCL8) which were also reduced in mCB placentas (Fig. 1A). CB receptor 1 (CNR1) was among the significantly reduced genes and was inversely correlated with weekly cannabis use (R = −0.64, P < 0.05; Fig. 1B). Gene ontology analysis revealed that DEGs were significantly enriched for immune response functions including type I interferon pathway, cytokine-mediated signaling, and neutrophil migration (Fig. 1C and Dataset S3). Using a more stringent significance criterion (>1.5-fold change), 86 genes remained differentially expressed with gene ontology enrichment for neutrophil-related functions (Fig. 1C). Examining immune cell–type marker genes previously identified with single-cell RNA-seq in term placenta (30) (Dataset S4), Fisher’s exact tests revealed significant enrichment of mCB DEGs among monocyte-marker genes (odds ratio [OR] = 6.30, P < 0.001; Fig. 1D).
Fig. 1.
mCB is associated with reduced immune-related gene expression in placenta. (A) Volcano plot showing DEGs for mCB placentas (DEGs < 1.5-fold change [FC], red points; DEGs ≥ 1.5 FC, blue points). Note: 1.5 FC ∼0.58 log2 FC on x axis. (B) Significant inverse correlation between self-reported prenatal cannabis use and placental CNR1 gene expression (R = 0.64, P < 0.05). (C) Gene ontology analysis revealed that all DEGs (Top) and DEGs >1.5 FC (Bottom) were enriched for immune-related gene sets. (D) Log FCs (mCB vs. non-CB) for immune cell–type marker genes in placenta (mCB DEGs in red [<1.5 FC] or blue [≥1.5 FC] with bars depicting the median). NK-cell, natural killer cell. ***P < 0.001.
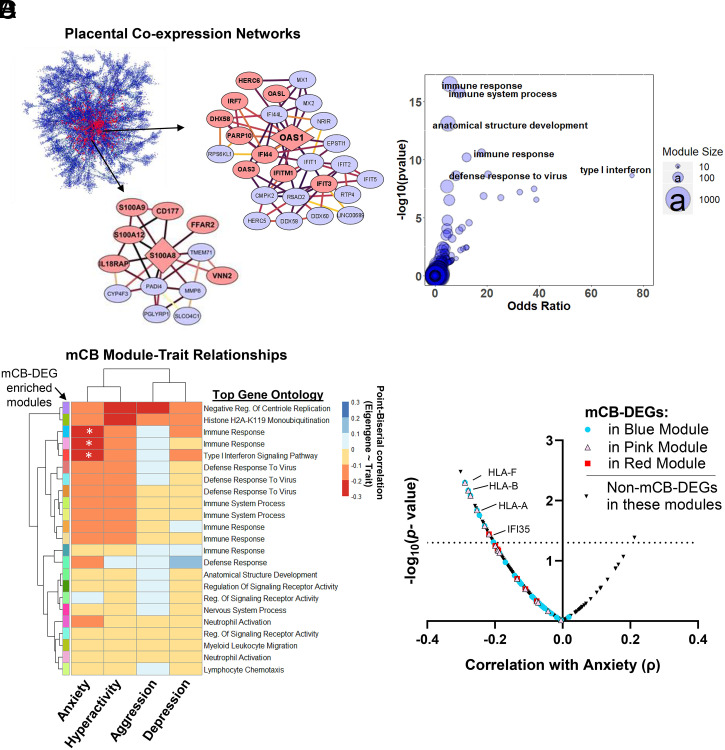
To further examine the functional organization of immune genes associated with mCB, we constructed gene coexpression modules using multiscale embedded gene coexpression network analysis (MEGENA) (31). This identified 600 interconnected gene modules (i.e., one gene can be present in multiple modules) ranging from 10 to 1,000 genes per module (Fig. 2A and Dataset S5). Among these networks, many of the key driver genes (i.e., those with the strongest intranetwork correlations) were significantly reduced in mCB placentas including monocyte-marker genes (e.g., S100A8) and interferon-induced genes (e.g., OAS1) (Fig. 2A and Dataset S6). Furthermore, Fisher’s exact tests revealed 23 modules enriched for mCB DEGs with most containing top gene ontology categories involved in immune-signaling pathways (Fig. 2B and Dataset S6).
Fig. 2.
mCB is associated with placental gene coexpression networks related to immune function and heightened anxiety. (A) Multiscale embedded gene coexpression network composed of highly correlated gene modules. Arrows zoom in on two modules significantly enriched for mCB DEGs. Red nodes, DEGs; diamond shape, network hub genes. Darker edges between nodes represent stronger gene–gene correlations. (B) Bubble plot showing modules (blue circles) that are most significantly enriched for mCB DEGs are also enriched for immune-related gene ontology terms. (C) Heatmap of correlation coefficients between module eigengenes and child behavioral problems. Rows feature all modules enriched for mCB DEGs annotated with a unique color and top gene ontology term. *P < 0.05. (D) For each mCB DEG in the teal, pink, and red (C) modules correlated with anxiety, correlation coefficients with anxiety (x axis) and the −log10-transformed P values (y axis) are depicted. The dotted line represents the significance threshold for gene–anxiety correlations.
Placental Immune Gene Networks Are Associated with Behavioral Traits in Early Childhood.
We next examined the relationship between the 23 mCB-associated placental gene networks and neurobehavioral traits. We found that module eigengene values for three mCB-linked networks were significantly associated with heightened anxiety (Fig. 2C, SI Appendix, Fig. S4, and Dataset S6). Directly examining the mCB-linked DEGs within these networks, major histocompatibility complex (MHC) genes (HLA-A/B/F) involved in adaptive immunity, as well as interferon-induced genes (IFI-35) supporting innate immunity, were among DEGs most associated with elevated anxiety (Fig. 2D and Dataset S7). In addition, we found two mCB-linked networks with eigengenes significantly correlated with hyperactivity levels (SI Appendix, Fig. S5A and Dataset S6). Each network featured the differentially expressed proinflammatory cytokines IL1B and CXCL8 which were significantly associated with hyperactivity levels (SI Appendix, Fig. S5B and Dataset S7).
Discussion
The present study identified significant relationships between cannabis use during pregnancy and many facets of early-childhood development. Collectively, we report increased hair cortisol levels, elevated anxiety, aggression, and hyperactivity and a reduction in normalized HRV associated with mCB. In placenta, mCB was associated with reduced immune-related gene expression including proinflammatory cytokines and immune cell–type markers. Using gene coexpression analysis, we revealed immune-related gene networks in placenta significantly correlated with anxiety problems and hyperactivity. Overall, the results indicate an association between the placental transcriptome and developmental trajectories related to in utero cannabis exposure.
In line with previous investigations reporting greater risk for psychiatric illness in mCB children with prenatal cannabis exposure (18, 32), the current study showed that mCB is associated with increased anxiety, aggression, and hyperactivity in young children. Moreover, along with several altered psychobehavioral traits, mCB children exhibited endocrine and physiological phenotypes consistent with aberrant stress and anxiety regulation. First, mCB children had increased hair cortisol, a biomarker for long-term hypothalamic–pituitary–adrenal (HPA) axis activity. Early-life HPA dysregulation is a risk for mental health disorders over the lifetime (33). Second, mCB decreased HRV, a measure of cardiac modulation of the autonomic nervous system, suggesting attenuated parasympathetic tone in mCB children. Low resting HRV is associated with multiple anxiety-related disorders in both young children and adults and is a risk factor for cardiovascular disease (29). Moreover, low anticipatory HRV is associated with increased stress-induced cortisol (34). Thus, while we did not observe significant relationships across behavioral assessments for the subset of children evaluated across multiple assessments in the current study, more robust intersubject assessments will be important for future studies. Altogether, the current multimodal assessment revealed several novel phenotypes associated with stress and emotional dysregulation in mCB children that will be important measures for continued longitudinal and mechanistic evaluation.
The finding that mCB is correlated with reduced CNR1 expression in the placenta may reflect an adaptive response of the fetal endocannabinoid system as the CNR1 and brain CB receptor expression is normally reduced in human cannabis users (35). In rodents, prenatal THC reduces CNR1 protein levels in the gestational cortex (36). As CNR1 is highly expressed in the brain, particularly in limbic regions like the hippocampus and amygdala (37), which contribute to stress regulation and anxiety, fetal endocannabinoid disturbances have implications for neurodevelopment and emotional regulation. Thus, monitoring endocannabinoid signaling in the placenta may be a valuable noninvasive indicator of in utero cannabis exposure severity.
The current finding of significant gene expression alterations centered on the placental immune-related transcriptome of mCB cases is intriguing. Pregnancy requires well-coordinated immunological activity, promoting maternal tolerance, while maintaining innate immune activity to protect against pathogens and viruses (38). For example, this delicate balance is reflected by type I interferon-mediated signaling in placenta which is essential for preventing viral replication, yet can also lead to significant pathology and fetal mortality (39). The reduced immune-related gene expression observed in the placenta of cannabis users mirrors other studies and is consistent with an immunosuppressive role for CBs. For instance, in preclinical studies, THC during pregnancy suppresses the cytokine-sensitive STAT3-signaling pathway in the placenta (40). Moreover, experienced cannabis users have reduced monocyte chemotaxis in response to CB1 receptor agonists (41) and CB2 agonists modulate monocyte migration via reduced interferon-γ activation (42). Both interferon-signaling genes and several monocyte markers were prominently reduced in mCB placentas in the current study.
Within gene coexpression networks enriched for DEGs, those most linked with increased child anxiety were HLA-A/B-F, which encode MHC proteins. MHC proteins are cell-surface antigen-presenting proteins recognized by T cells to promote adaptive immune activation. Induction of histocompatibility leukocyte antigen (HLA) gene expression can be triggered by the interferon-signaling pathway (43), which was among the gene ontologies associated with mCB use. Reduced basal expression of immune activating genes could potentially increase vulnerability to pathogens or viruses during pregnancy. For instance, the infection efficacy of Zika virus is mediated by suppression of the interferon-signaling pathway (44). Importantly, the immune system (and thereby immune-related gene expression) is intertwined with stress, metabolic, and microbial environments (45). In addition, the complete impact of mCB on immune function (i.e., activation, suppression) cannot be evaluated from placental gene expression alone. It will be critical for future human studies to examine cytokines over the course of pregnancy and their associations with other signaling factors in other tissues such as maternal and fetal cord blood in addition to placentas. Overall, our findings suggest that the immune-modulating effects previously associated with cannabis and THC may be pertinent to gestational development.
The potential risks of immune system dysregulation during pregnancy to offspring development have been studied with both activating and suppressing exogenous immunomodulators. For example, abuse of nonsteroidal antiinflammatory drugs has been linked to miscarriage and altered fetal maturation (46). In addition, maternal immune activation in rodents increases anxiety and depression phenotypes in offspring (47). Here we found that mCB-linked suppression of immune-related gene networks in placenta correlated with increased anxiety and hyperactivity phenotypes. Previously, socioeconomically disadvantaged pregnant mothers were found to have reduced interleukin-8 levels in serum during the third trimester that associated with increased neurological deficits (48). In rodents, prenatal stress induces placental inflammation linked to hyperactivity in males (49). While we observed a reduction in proinflammatory cytokine-signaling genes like IL1B and CXCL8 in association with increased hyperactivity, this finding may reflect the importance of balanced immune regulation during pregnancy. More mechanistic preclinical studies are needed to directly and concurrently evaluate the impact of CBs on the placental immunotranscriptome and offspring neurobehavioral development.
The current report has important limitations. First, in order to reduce stigma, the accuracy of our self-reported cannabis use was not verified. Furthermore, there was not sufficient power to examine prenatal and postnatal cannabis use separately and thus the findings can only be interpreted broadly as mCB. However, it is likely that some mothers reporting postnatal use were also prenatal users and the correlation evident between maternal cannabis use and placental CNR1 expression shows that the self-report information is robust to detect expected biological changes. Another limitation relates to the fact that DEGs identified in mCB+ placentas did not pass FDR adjustment for multiple comparisons. Thus, further studies aimed at validating key findings, such as reduced CNR1 gene expression, will be necessary. Also, while we controlled for various confounds, maternal cannabis use had significant differences in demographic profile and it is difficult to rule out important interaction effects (e.g., cannabis × stress or cannabis × nicotine) or other potential unmeasured confounds influencing child outcomes (e.g., parental investment) contributing to the main effects. Finally, while many assessments were made in the current study, most mother–child dyads did not undergo all evaluations. This may have limited our ability to detect robust correlations across different measures such as between HRV and behavioral traits.
In summary, mCB is associated with anxiety-related traits during early childhood in addition to altered stress hormone levels and psychophysiological activity. The heightened anxiety associated with reduced placental gene expression within immune response coexpression networks suggests that child development may be linked to in utero immunomodulatory effects of cannabis. These results have significant implications for defining mental health risks attributable to cannabis use during pregnancy.
Materials and Methods
Procedures and Participants.
The study cohort was composed of mother–child dyads from an ongoing Stress in Pregnancy project (50). Expectant mothers were recruited from obstetrics clinics at Mount Sinai Hospital and NewYork-Presbyterian/Queens in New York City. Women were excluded based on HIV infection, maternal psychosis, maternal age <15 y, life-threatening maternal medical complications, and congenital or chromosomal abnormalities in the fetus. Written informed consent was obtained from eligible women for all procedures. The institutional review boards at the City University of New York (CUNY), Icahn School of Medicine at Mount Sinai, and NewYork-Presbyterian/Queens approved the study. Demographic and clinical information was obtained at an initial face-to-face evaluation with a social worker as previously described (50). Annual assessments continued at Queens College, CUNY. At each annual visit, children’s neurobehavioral development and mother’s psychological functioning were ascertained. (As child behavioral, hormonal, and psychophysiological assays were each carried out during separate evaluations with uneven participation, group sizes varied across analyses; Tables 2 and 3.)
Hair Hormone Levels.
Cortisol, cortisone, and DHEA were measured in hair samples collected in children at ∼36 mo. Briefly, a bundle of ∼100 hairs was cut from the posterior vertex of the child’s head, close to the scalp. Samples of 3-cm sections were processed using methanol extraction and assayed (C. Kirschbaum’s laboratory, Technical University of Dresden, Dresden, Germany) (51).
Behavioral and Emotional Functioning during Childhood.
Clinical dimensions of child behavior and emotions were ascertained by administering the BASC-2 to parents (27). Dimensions included hyperactivity, aggression, anxiety, depression, somatization, atypicality, withdrawal, and attention problems. The standardized t score assessment was generated based on the child’s biological age and sex. The mean age (SD) at the time of the assessments was 44.9 (10.9) mo. Furthermore, we tested the effect of mCB on clinically significant and at-risk conditions; in accordance with the BASC-2 manual, scores ≥60 on the standardized dimensions reflect clinical diagnosis with a behavioral or emotional problem.
Electrocardiogram Analysis of HRV.
Electrocardiogram (ECG) recordings were made using the BIOPAC MP150 system and ECG100C BioNomadix amplifier with three prejelled Ag-AgCl disposable vinyl electrodes placed at the upper left collarbone, upper right collarbone, and lower left ribcage with a band-pass filter of 60 Hz. ECG signals were visually inspected for artifacts and corrected for false or undetected R waves in AcqKnowledge 4.4. HRV was quantified by spectral analysis of the interbeat interval series analyzed at both HF (0.15 to 0.40 Hz) and LF (0.04 to 0.24 Hz) power. Log10 transformation was applied to correct the skewed distribution of HF and LF measures. The normalized HRV was computed by calculating log10(HF-HRV/LF-HRV).
The psychophysiological assessment was carried out in children between 48 and 72 mo with mean (SD) age of 54.39 (1.95) mo. The testing session began with a 1-min rest period during which children were instructed to sit still and be quiet, followed by a 1-min startle paradigm, presented on the computer monitor. The startle paradigm involved a calm video suddenly interrupted by six startle probes of tone burst at 90 dB. After the startle paradigm, there was another 1-min resting period with continued ECG monitoring. Throughout testing, the mother sat next to the child and could talk to the child to maintain the child’s attention and stillness. Notably, during the trial, measurements of electrodermal activity were also recorded. However, since usable data were recovered for only a small percentage (<25%) of the experimental cohort, we were not able to perform statistical analysis for startle magnitude and amplitude.
Statistical Analysis.
Self-report of either prenatal or postnatal cannabis use was collectively considered “maternal cannabis use” throughout the present study. Parental age, education, marital status, prenatal substance use, child’s sex, child’s age, and race were a priori defined as confounders. Additionally, aspects of maternal stress including pregnancy during Hurricane Sandy in 2012 and measures of maternal state/trait anxiety (derived from the State-Trait Anxiety Inventory) were controlled for in all statistical models.
For the statistical analysis, descriptive statistics were first conducted to evaluate mean and SD of outcome variables. The general linear model (GLM) was used as the primary analytical method. A univariate GLM was used for hair hormones. For neurobehavioral (BASC-2) outcomes, a multivariable GLM was used which adjusts for correlations among dependent variables. The effect of mCB on values of neurobehavioral outcomes (i.e., classifying children with at-risk and clinically significant problems) was tested with the ordinal logistic link function in the GLM. The exponentiation of the β-coefficient was obtained to calculate the OR and associated 95% CI. Prior to the analysis, each subscale was evaluated for normality. If the assumption of univariate normal distribution was violated, log transformation was applied to achieve normality. Finally, the ECG assessments of HRV used a linear mixed-effects model with trial as a repeated measure and subject as a random effect along with univariate GLM tests for each trial as presented in Table 2. Age at the time of testing was also included as a covariate for this assessment.
Placental Tissue Collection.
Placental samples were acquired and processed as previously described (50). Briefly, biopsies from the outer layer of the blastocyst, free of maternal decidua, were collected from each placental quadrant midway between the cord insertion and the placental rim within an hour of delivery. Specimens were frozen in liquid nitrogen and stored at −80 °C.
RNA-Seq Data Analysis.
Transcriptome analysis (RNA-seq) was carried out on a representative subset (n = 131) of the total cohort. RNA was extracted from pulverized placental tissue and processed to remove ribosomal RNA and prepare sequencing libraries (Ribo-Zero kit and TruSeq commercial kits; Illumina). Libraries were then paired end–sequenced (2 × 75 bp) at a depth of 28.0 ± 0.3 million reads (conducted by Novogene). Fastq sequencing output was then mapped to the hg38 genomic assembly using the STAR read aligner with standard mapping parameters. Uniquely mapped reads (91.6 ± 1.73% [μ ± SD] of total reads) were annotated to genomic features from the Ensembl genome database and quantified using the –quantMode argument in STAR. Uniquely mapped reads with minimal average expression (<0.3 fragments per kilobase million) were removed prior to subsequent analyses. All samples were evaluated with principal-component analysis to confirm the absence of technical outliers (SI Appendix, Fig. S2).
For differential gene expression analysis, all group comparisons were made using the R package DESeq2 for moderated estimation of fold change and statistical analysis. All analyses were performed examining main effects of mCB on each gene with a general linearized model weighted for library sizes and gene-level variance. The model accounted for many covariates with maternal age, paternal age, fetal weight, and maternal trait anxiety evaluated as continuous variables and pregnancy during Hurricane Sandy, cigarette smoking, alcohol use, fetal sex, marital status, race, and educational attainment as discrete variables. While FDR adjustment was applied, significant genes with uncorrected P values were also considered in order to elucidate gene sets and networks that can be directly tested and evaluated in future studies without false discovery burden. Identified DEGs were used for gene set enrichment analyses using the curated transcriptional, pathway, and ontology libraries managed on the Enrichr web server. All gene ontology analysis utilized the databases from the Gene Ontology Consortium and statistical significance was determined with Fisher’s exact test in the Enrichr R package (52). Placental immune subtype–specific genes were defined using marker genes previously identified with single-cell RNA-seq in human term placenta (30).
MEGENA was performed on all protein-coding genes using the R package MEGENA. Briefly, Pearson correlation coefficients were computed for all gene pairs to identify all significant gene pair correlations (FDR adj. P < 0.05). Next, planar filtered network and multiscale clustering analyses were carried out to identify coexpression modules with 10/1,000 sets as min/max module sizes. Significance testing for overlap between DEGs and network module gene sets was performed using one-tailed Fisher’s exact test with Benjamini and Hochberg’s FDR adjustment (q < 0.05). Module eigengene values were calculated with the moduleEigengenes function in the WGCNA R package (53). In calculating eigengenes, all gene members of each module were considered. Point-biserial or Spearman correlations were used to associate eigengenes/genes with dichotomous and continuous neurobehavioral traits, respectively.
Supplementary Material
Acknowledgments
This work was supported by Grants MH102729 from the National Institute of Mental Health (to Y.N.) and DA030359 from the National Institute on Drug Abuse (to Y.L.H.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. T.B. is a guest editor invited by the Editorial Board.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2106115118/-/DCSupplemental.
Data Availability
The RNA-seq datasets generated and analyzed in the current study were submitted to the National Center for Biotechnology Information Sequence Read Archive (accession no. PRJNA719417) and Gene Expression Omnibus (accession no. GSE179930). All software used for transcriptomic analyses are publicly available.
Anonymized RNA sequence data have been deposited in the NIH database of Genotypes and Phenotypes (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE179930; https://www.ncbi.nlm.nih.gov/bioproject/PRJNA719417).
References
- 1.Volkow N. D., Han B., Compton W. M., McCance-Katz E. F., Self-reported medical and nonmedical cannabis use among pregnant women in the United States. JAMA 322, 167–169 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown Q. L., et al. , Trends in marijuana use among pregnant and nonpregnant reproductive-aged women, 2002–2014. JAMA 317, 207–209 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chandra S., et al. , New trends in cannabis potency in USA and Europe during the last decade (2008–2017). Eur. Arch. Psychiatry Clin. Neurosci. 269, 5–15 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Ross E. J., Graham D. L., Money K. M., Stanwood G. D., Developmental consequences of fetal exposure to drugs: What we know and what we still must learn. Neuropsychopharmacology 40, 61–87 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costa M. A., The endocannabinoid system: A novel player in human placentation. Reprod. Toxicol. 61, 58–67 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Pertwee R. G., Targeting the endocannabinoid system with cannabinoid receptor agonists: Pharmacological strategies and therapeutic possibilities. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367, 3353–3363 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurd Y. L., et al. , Marijuana impairs growth in mid-gestation fetuses. Neurotoxicol. Teratol. 27, 221–229 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Mato S., Del Olmo E., Pazos A., Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. Eur. J. Neurosci. 17, 1747–1754 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Harkany T., et al. , The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol. Sci. 28, 83–92 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Fergusson D. M., Horwood L. J., Northstone K., ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood, Maternal use of cannabis and pregnancy outcome. BJOG 109, 21–27 (2002). [DOI] [PubMed] [Google Scholar]
- 11.Fried P. A., Watkinson B., Gray R., Growth from birth to early adolescence in offspring prenatally exposed to cigarettes and marijuana. Neurotoxicol. Teratol. 21, 513–525 (1999). [DOI] [PubMed] [Google Scholar]
- 12.Corsi D. J., et al. , Association between self-reported prenatal cannabis use and maternal, perinatal, and neonatal outcomes. JAMA 322, 145–152 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maia J., et al. , Effects of cannabis tetrahydrocannabinol on endocannabinoid homeostasis in human placenta. Arch. Toxicol. 93, 649–658 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Natale B. V., et al. , Δ9-Tetrahydrocannabinol exposure during rat pregnancy leads to symmetrical fetal growth restriction and labyrinth-specific vascular defects in the placenta. Sci. Rep. 10, 544 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X., Dow-Edwards D., Anderson V., Minkoff H., Hurd Y. L., In utero marijuana exposure associated with abnormal amygdala dopamine D2 gene expression in the human fetus. Biol. Psychiatry 56, 909–915 (2004). [DOI] [PubMed] [Google Scholar]
- 16.DiNieri J. A., et al. , Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biol. Psychiatry 70, 763–769 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bourque J., Afzali M. H., Conrod P. J., Association of cannabis use with adolescent psychotic symptoms. JAMA Psychiatry 75, 864–866 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corsi D. J., et al. , Maternal cannabis use in pregnancy and child neurodevelopmental outcomes. Nat. Med. 26, 1536–1540 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Gray K. A., Day N. L., Leech S., Richardson G. A., Prenatal marijuana exposure: Effect on child depressive symptoms at ten years of age. Neurotoxicol. Teratol. 27, 439–448 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Fine J. D., et al. , Association of prenatal cannabis exposure with psychosis proneness among children in the Adolescent Brain Cognitive Development (ABCD) study. JAMA Psychiatry 76, 762–764 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trezza V., et al. , Effects of perinatal exposure to delta-9-tetrahydrocannabinol on the emotional reactivity of the offspring: A longitudinal behavioral study in Wistar rats. Psychopharmacology (Berl.) 198, 529–537 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Mereu G., et al. , Prenatal exposure to a cannabinoid agonist produces memory deficits linked to dysfunction in hippocampal long-term potentiation and glutamate release. Proc. Natl. Acad. Sci. U.S.A. 100, 4915–4920 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spano M. S., Ellgren M., Wang X., Hurd Y. L., Prenatal cannabis exposure increases heroin seeking with allostatic changes in limbic enkephalin systems in adulthood. Biol. Psychiatry 61, 554–563 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Roncero C., et al. , Cannabis use during pregnancy and its relationship with fetal developmental outcomes and psychiatric disorders. A systematic review. Reprod. Health 17, 25 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bronson S. L., Bale T. L., The placenta as a mediator of stress effects on neurodevelopmental reprogramming. Neuropsychopharmacology 41, 207–218 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sauvé B., Koren G., Walsh G., Tokmakejian S., Van Uum S. H., Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin. Invest. Med. 30, E183–E191 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Musuva R., et al. , Change in children’s school behavior after mass administration of praziquantel for Schistosoma mansoni infection in endemic areas of western Kenya: A pilot study using the Behavioral Assessment System for Children (BASC-2). PLoS One 12, e0181975 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berntson G. G., et al. , Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology 34, 623–648 (1997). [DOI] [PubMed] [Google Scholar]
- 29.Chalmers J. A., Quintana D. S., Abbott M. J., Kemp A. H., Anxiety disorders are associated with reduced heart rate variability: A meta-analysis. Front. Psychiatry 5, 80 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pique-Regi R., et al. , Single cell transcriptional signatures of the human placenta in term and preterm parturition. eLife 8, e52004 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song W. M., Zhang B., Multiscale embedded gene co-expression network analysis. PLoS Comput. Biol. 11, e1004574 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paul S. E., et al. , Associations between prenatal cannabis exposure and childhood outcomes: Results from the ABCD study. JAMA Psychiatry 78, 64–76 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Staufenbiel S. M., Penninx B. W., Spijker A. T., Elzinga B. M., van Rossum E. F., Hair cortisol, stress exposure, and mental health in humans: A systematic review. Psychoneuroendocrinology 38, 1220–1235 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Pulopulos M. M., Vanderhasselt M. A., De Raedt R., Association between changes in heart rate variability during the anticipation of a stressful situation and the stress-induced cortisol response. Psychoneuroendocrinology 94, 63–71 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferland J. N., Hurd Y. L., Deconstructing the neurobiology of cannabis use disorder. Nat. Neurosci. 23, 600–610 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Salas-Quiroga A., et al. , Prenatal exposure to cannabinoids evokes long-lasting functional alterations by targeting CB1 receptors on developing cortical neurons. Proc. Natl. Acad. Sci. U.S.A. 112, 13693–13698 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X., Dow-Edwards D., Keller E., Hurd Y. L., Preferential limbic expression of the cannabinoid receptor mRNA in the human fetal brain. Neuroscience 118, 681–694 (2003). [DOI] [PubMed] [Google Scholar]
- 38.Ander S. E., Diamond M. S., Coyne C. B., Immune responses at the maternal-fetal interface. Sci. Immunol. 4, eaat6114 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yockey L. J., et al. , Type I interferons instigate fetal demise after Zika virus infection. Sci. Immunol. 3, eaao1680 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang X., et al. , Suppression of STAT3 signaling by Δ9-tetrahydrocannabinol (THC) induces trophoblast dysfunction. Cell. Physiol. Biochem. 42, 537–550 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Sexton M., Silvestroni A., Möller T., Stella N., Differential migratory properties of monocytes isolated from human subjects naïve and non-naïve to cannabis. Inflammopharmacology 21, 253–259 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montecucco F., Burger F., Mach F., Steffens S., CB2 cannabinoid receptor agonist JWH-015 modulates human monocyte migration through defined intracellular signaling pathways. Am. J. Physiol. Heart Circ. Physiol. 294, H1145–H1155 (2008). [DOI] [PubMed] [Google Scholar]
- 43.Keskinen P., Ronni T., Matikainen S., Lehtonen A., Julkunen I., Regulation of HLA class I and II expression by interferons and influenza A virus in human peripheral blood mononuclear cells. Immunology 91, 421–429 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu Y., et al. , Zika virus antagonizes interferon response in patients and disrupts RIG-I-MAVS interaction through its CARD-TM domains. Cell Biosci. 9, 46 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romano-Keeler J., Weitkamp J. H., Maternal influences on fetal microbial colonization and immune development. Pediatr. Res. 77, 189–195 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Antonucci R., et al. , Use of non-steroidal anti-inflammatory drugs in pregnancy: Impact on the fetus and newborn. Curr. Drug Metab. 13, 474–490 (2012). [DOI] [PubMed] [Google Scholar]
- 47.Minakova E., Warner B. B., Maternal immune activation, central nervous system development and behavioral phenotypes. Birth Defects Res. 110, 1539–1550 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Gilman S. E., et al. , Socioeconomic disadvantage, gestational immune activity, and neurodevelopment in early childhood. Proc. Natl. Acad. Sci. U.S.A. 114, 6728–6733 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bronson S. L., Bale T. L., Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology 155, 2635–2646 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Finik J., Nomura Y., Cohort profile: Stress in Pregnancy (SIP) study. Int. J. Epidemiol. 46, 1388–1388k (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stalder T., Kirschbaum C., Analysis of cortisol in hair—State of the art and future directions. Brain Behav. Immun. 26, 1019–1029 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Kuleshov M. V., et al. , Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 44, W90–W97 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Langfelder P., Horvath S., WGCNA: An R package for weighted correlation network analysis. BMC Bioinformatics 9, 559 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-seq datasets generated and analyzed in the current study were submitted to the National Center for Biotechnology Information Sequence Read Archive (accession no. PRJNA719417) and Gene Expression Omnibus (accession no. GSE179930). All software used for transcriptomic analyses are publicly available.
Anonymized RNA sequence data have been deposited in the NIH database of Genotypes and Phenotypes (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE179930; https://www.ncbi.nlm.nih.gov/bioproject/PRJNA719417).