Significance
The resting brain consumes enormous energy and shows highly organized spontaneous activity as often measured by functional MRI (fMRI). Using large-scale recordings from thousands of neurons, we showed a highly structured brain activity that involves the majority (∼70%) of surveyed neurons from various brain regions. It takes the form of sequential activations between two distinct neuronal ensembles and relates to low-frequency (∼0.1 Hz) modulations of arousal and hippocampal ripple activity. The finding provides a cellular-level understanding of the resting-state global brain activity often observed with fMRI and further suggests that this global activity may represent an “offline” process that links cholinergic function, memory consolidation, and perivascular clearance of brain waste.
Keywords: low-frequency resting-state activity, global signal, neuronal population dynamics, sequential activations, hippocampal ripples
Abstract
The resting brain consumes enormous energy and shows highly organized spontaneous activity. To investigate how this activity is manifest among single neurons, we analyzed spiking discharges of ∼10,000 isolated cells recorded from multiple cortical and subcortical regions of the mouse brain during immobile rest. We found that firing of a significant proportion (∼70%) of neurons conformed to a ubiquitous, temporally sequenced cascade of spiking that was synchronized with global events and elapsed over timescales of 5 to 10 s. Across the brain, two intermixed populations of neurons supported orthogonal cascades. The relative phases of these cascades determined, at each moment, the response magnitude evoked by an external visual stimulus. Furthermore, the spiking of individual neurons embedded in these cascades was time locked to physiological indicators of arousal, including local field potential power, pupil diameter, and hippocampal ripples. These findings demonstrate that the large-scale coordination of low-frequency spontaneous activity, which is commonly observed in brain imaging and linked to arousal, sensory processing, and memory, is underpinned by sequential, large-scale temporal cascades of neuronal spiking across the brain.
The brain at rest exhibits slow (<0.1 Hz) but highly organized spontaneous activity as measured by functional MRI (fMRI) (1, 2). Much research in this area has utilized the temporal coordination of these signals to assess the functional organization a large number of brain networks. In recent years, however, new attention has been directed to a less-studied aspect of this signal, namely the conspicuous and discrete spontaneous events that occur simultaneously across the brain (3–5). These global resting-state fMRI events appear to reflect transient arousal modulations at a timescale of ∼10 s (4, 6) and also to be closely related to activity among clusters of cholinergic projection neurons in the basal forebrain (4, 5).
The nature of global brain events is of great interest, as is their spatiotemporal dynamics. Some evidence suggests they take the form of traveling waves, propagating coherently according to the principles of the cortical hierarchy (7, 8), and shaping functional connectivity measures important for assessing the healthy and diseased brain (7, 9, 10). Other work has linked such global activity to phenomena as varied as modulation of the autonomic nervous system (11–14), cleansing circulation of cerebrospinal fluid in the glymphatic system (15–19), and memory consolidation mediated by hippocampal sharp-wave ripples (20, 21). In general, the global activity measured through brain imaging appears coordinated over timescales of seconds with a range of other neural and physiological events (11, 12, 14, 21–23). In a few cases, the relationship between local and global neural events has been studied using simultaneous measurements. For example, brain-wide fMRI fluctuations and local field potential (LFP) power changes are locked to the issuance of hippocampal ripples (21, 24). However, very little is understood about the extent to which single neurons participate in the expression and coordination of global spontaneous events of the seconds timescale. To approach this topic, recent technological advances have made it possible to track and compare the spiking activity of a large number of isolated neurons recorded simultaneously across multiple brain areas.
A few recent studies utilizing high-density neuronal recording (25) have accumulated initial evidence suggesting a close relationship between the brain state and neuronal population dynamics (26–28). A large proportion of neurons, regardless of their location, showed strong modulations in their discharging rate that were coordinated in time with physiological arousal measures (28), across thirsty and sated states (26), and during exploratory and nonexploratory behaviors (27). Nevertheless, these studies leave open the question of how neuronal population dynamics are organized at a finer timescale of seconds surrounding spontaneous global events during immobile rest, and whether and how such dynamics are coincident with arousal modulations, hippocampal ripples, and sensory excitability. To investigate this topic, we examine the spiking activity recorded from large neuronal populations of neurons in immobilized mice, focusing on their seconds-scale coordination with global events and with one another. We further studied the impact of this spontaneous spiking on the magnitude of visually evoked responses and its time locking with other physiological signals related to arousal, such as LFP changes, hippocampal ripples, and changes in pupil diameter.
Results
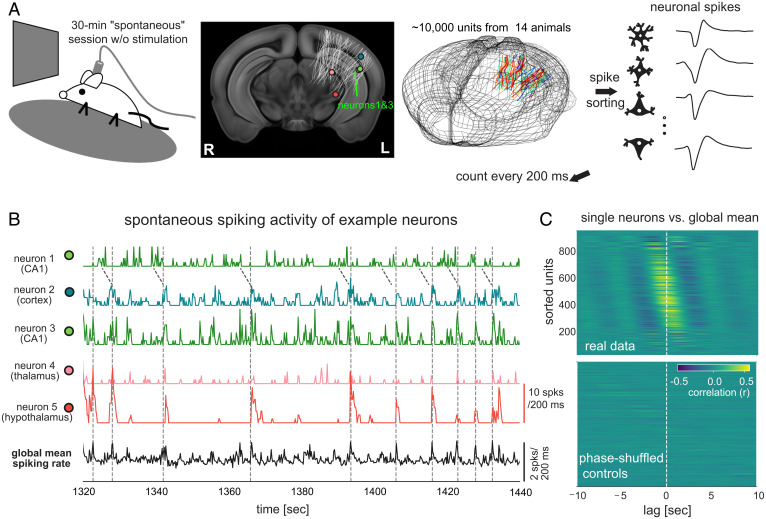
We analyzed neural activity, pupillary changes, and locomotor activity obtained from the Visual Coding – Neuropixels project of the Allen Institute (29). We focused on the spontaneous spiking activity of ∼10,000 neurons from 44 brain regions of 14 mice (730 ± 178 neurons per mouse, mean ± SD) sustained during periods of immobile rest (Fig. 1A and SI Appendix, Table S1).
Fig. 1.
Neuronal population is engaged in global activity of the seconds timescale during immobile rest. (A) Illustration of the experimental setup of the Visual Coding – Neuropixels project that included a 30-min “spontaneous” session without any stimulation (Top Left). The locations of 6,171 channels on 79 probes from 14 mice were collapsed along the anterior-posterior direction and mapped onto a middle slice of a mouse brain template and also shown in a three-dimensional representation of the mouse brain (the second and third columns). (B) Spontaneous spiking rate of five example neurons from the hippocampus (CA1), visual cortex (VISal), thalamus (LP), and hypothalamus (ZI) of a representative mouse. Their locations were marked in (A). The neurons 1 and 3 were recorded by two channels with a distance of 14 μm. The Bottom trace is the global mean spiking rate of all 930 surveyed neurons. (C) Cross-correlation functions between individual neurons’ spiking rate and the global mean spiking rate (reference) during the stationary period from the representative mouse (Top). The same cross-correlation functions were computed after phase-shuffling the real data (Bottom).
Global Coordination of Spontaneous Spiking Activity during Rest.
The 30-min “spontaneous” sessions were characterized by two distinct behavioral states. The rest state was defined as extended (≥50 s) periods of immobility in which there was no activity registered on the running wheel, whereas the running state was defined as periods dominated by running, with brief (<50 s) periods of immobility. The two behavioral states were marked by distinct patterns of neural and ocular signals (SI Appendix, Fig. S1A), with the immobile rest associated with reduced pupil diameter (P = 2.2 × 10−7, n = 14; paired Student’s t test), pupil motion (P = 8.2 × 10−7), eye-area height (P = 0.0008) (SI Appendix, Fig. S1B), and mean spiking rate (P = 1.1 × 10−5), all of which suggested a less-aroused state.
Close inspection of neural activity during immobile rest revealed bouts of elevated spiking in a majority of neurons across distant brain areas that included the visual cortex, hippocampus, thalamus, and hypothalamus (Fig. 1B). These ubiquitous and coarsely synchronous events occurred aperiodically, with typical intervals in the range of 5 to 20 s, although the spiking bursts observed between a given pair of neurons were frequently shifted in time (see neurons 1 and 3 in Fig. 1B). These quasisynchronized firing events led to large peaks in the global mean spiking rate (here and throughout, “global” refers to the surveyed population) of all survey neurons (dashed lines, Fig. 1B). As a result, the stationary period was characterized by a large fluctuation in the global mean spiking rate and a high level of global synchronization as measured by the Fano factor (SI Appendix, Fig. S1C) (30, 31).
To understand the temporal relationship between the firing of individual neurons and the average population dynamics during immobile rest, we correlated each neuron’s spiking activity with the global mean spiking rate. Cross-correlation functions were computed from lowpass-filtered (<0.5 Hz) spiking rate time courses in order to focus on slow modulations. This analysis revealed two main findings. First, the spontaneous firing of the majority of surveyed neurons (71.4 ± 9.3% across the 14 mice; SI Appendix, Fig. S1D), regardless of the location (SI Appendix, Fig. S2), were significantly correlated with the global mean spiking rate (P < 0.05; permutation test as compared to spectral-profile–preserved but phase-shuffled controls, Fig. 1C; the percentage dropped to 48.4 ± 16.7% at the significance level of P < 0.001). Second, the peak correlations for individual neurons spanned time lags ranging between ±5 s (Fig. 1C and SI Appendix, Fig. S1E), demonstrating a unique and consistent time delay between a single neuron’s firing and the global spiking activity. We also estimated the coherence between the spiking activity of individual neurons and their mean, which was strongest in the frequency range of ∼0.1 to 0.4 Hz and exhibited systematic phase shifts across neurons (SI Appendix, Fig. S3).
Sequenced Activity Cascades Underlie Spontaneous Resting Activity.
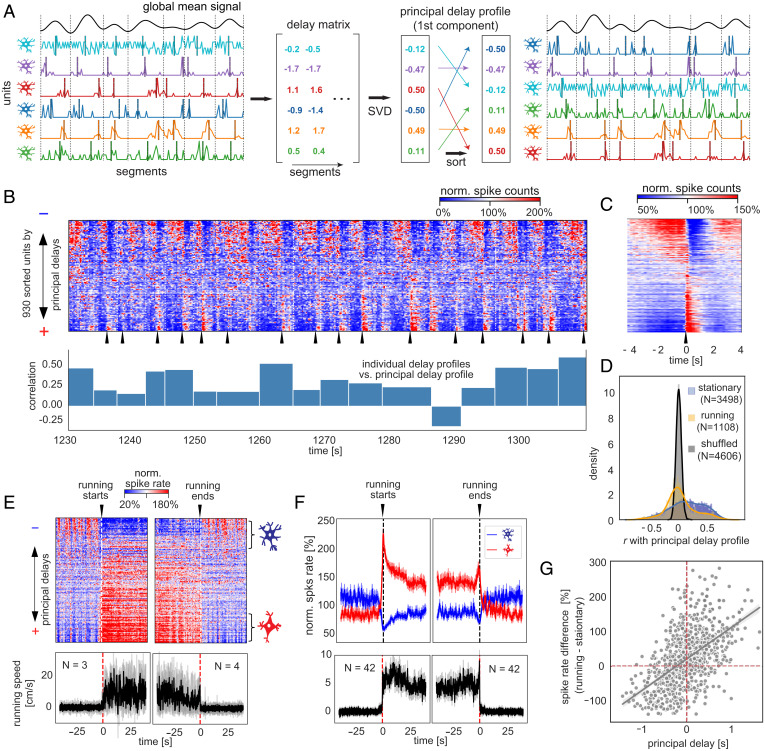
Given that individual neurons appeared to be entrained into the global activity with distinct time delays, we thus examined if the structured patterns of sequential activations, which have been observed in resting-state fMRI signals (7, 8), exist in populational spiking data. We adapted a delay-profile decomposition method, which has been validated with simulation and successfully extracted propagating patterns of resting-state fMRI signals (8), to achieve this goal. Briefly, the spiking data were cut into time segments based on troughs of the global mean signal, and a delay profile was computed for each segment to describe relative delays of each neuron’s spiking activity based on the centroid of spiking time. A singular value decomposition was then applied to all delay profiles to extract their first principal component (i.e., the principal delay profile) that would represent the major direction of sequential activations if any exists (Fig. 2A; see Materials and Methods for details). Applying this method to the spiking data extracted a principal delay profile that accounts for a significant portion of delay profile variance (SI Appendix, Fig. S4).
Fig. 2.
Sequential activations between two distinct neuronal ensembles define the resting-state neuronal dynamics. (A) Illustration of the delay-profile decomposition method to identify and extract sequential activation patterns in neuronal spiking data. The spiking data were divided into segments according to troughs of the filtered (<0.5 Hz) global mean signal. A delay profile was computed for each segment based on the centroid of spiking activity and then assembled to a delay matrix. The singular value decomposition (SVD) was applied to the delay matrix to extract the principal delay profile (i.e., the first principal component) that represents the major direction of sequential activations across neurons. Sorting neurons according to this principal delay profile allows us to visualize sequential activations of neurons. (B) An 80-s example of spontaneous spiking activity of the representative mouse with all 930 neurons sorted according to the principal delay profile (Top). The spiking counts of each neuron were normalized to percentage changes with respect to their temporal mean. The sharp activations of the positive-delay neurons were marked by black arrows. The bar plot (Bottom) shows the correlations between the principal delay profile and the delay profile of individual time segments, whose boundaries were marked by white dashed lines. (C) The mean pattern of sequential activation of the representative mouse was obtained by aligning and averaging the time segments of sequential activation according to the sharp activations of the positive-delay neurons. (D) Distributions of the correlations of individual delay profiles with the principal delay profile. They were summarized for the stationary (blue) and running (orange) periods, as well as the randomly shuffled (black) individual delay profiles. (E) Spiking activity (Top) and running speed (Bottom) at the transitions of running and stationary states for the representative mouse. The black and gray lines represent the mean and individual traces, respectively. (F) Spiking activity of the negative- and positive-delay neurons (Top) and running speed (Bottom) at the transitions of running and stationary states were summarized across all 14 mice. The thick lines represent the mean, whereas shadow regions denote areas within 1 SEM. (G) The principal delay value of individual neurons of the representative mouse is significantly correlated (r = 0.54, P = 2.5 × 10−71) with their spiking rate change across the running and stationary states.
Sorting the neuronal spiking time courses according to the derived principal delay profile revealed sequenced spiking cascades, with individual neurons exhibiting a gradation in their temporal shifts relative to shared global events (Fig. 2 B, Top). Each cascade was repeated with each cycle of the global mean signal, with individual neurons having fixed temporal lags. These cascades were most prominent during periods of immobile rest. Across time, 51.1 ± 13.7% of stationary time segments exhibited a significant (P < 0.001, permutation test) positive correlation with the principal delay profile. The running time segments showed much lower correlations (P = 2.6 × 10−35, Mann–Whitney U test) with the principal delay profile (Fig. 2D), with relatively strong correlations occurring mostly at short epochs of immobility between running, which were classified to the running state (see Materials and Methods for details).
To better visualize the average temporal structure of each neuron in the population, we aligned to sharp rate increases evident in positive-delay neurons (black arrows in Fig. 2B; see Materials and Methods for the definition of the positive- and negative-delay neurons, which account for 23.6 ± 4.7 and 21.0 ± 4.9% of the surveyed neurons, respectively). This analysis revealed the sequence of events in the neural population (Fig. 2C), beginning with the increased spiking of some negative-delay neurons, with gradual recruitment of additional neurons, followed by an abrupt transition in which the negative-delay cells ceased firing, and the positive-delay cells showed a sudden firing increase, which decayed within ∼1 s. This pattern was highly reproducible across animals (SI Appendix, Fig. S5).
Analysis of the same neurons during periods of active running revealed that the negative- and positive-delay groups constituted different functional subpopulations. Specifically, the spiking rate of negative-delay neurons was suppressed during running, whereas that of positive-delay neurons was substantially increased (Fig. 2 E and F). Across the population, the correlation between a neuron’s principal delay value at rest and its spike-rate changes during running was robust (r = 0.55 ± 0.08, P = 2.9 × 10−12), with negative delays largely associated with spiking reduction during running (Fig. 2G).
To summarize, activity during rest was composed of highly structured, repeating patterns of sequenced spiking activation that fell into two distinct neuronal groups with ostensibly different functional roles during behavior.
Spatial Organization of the Negative- and Positive-Delay Neurons.
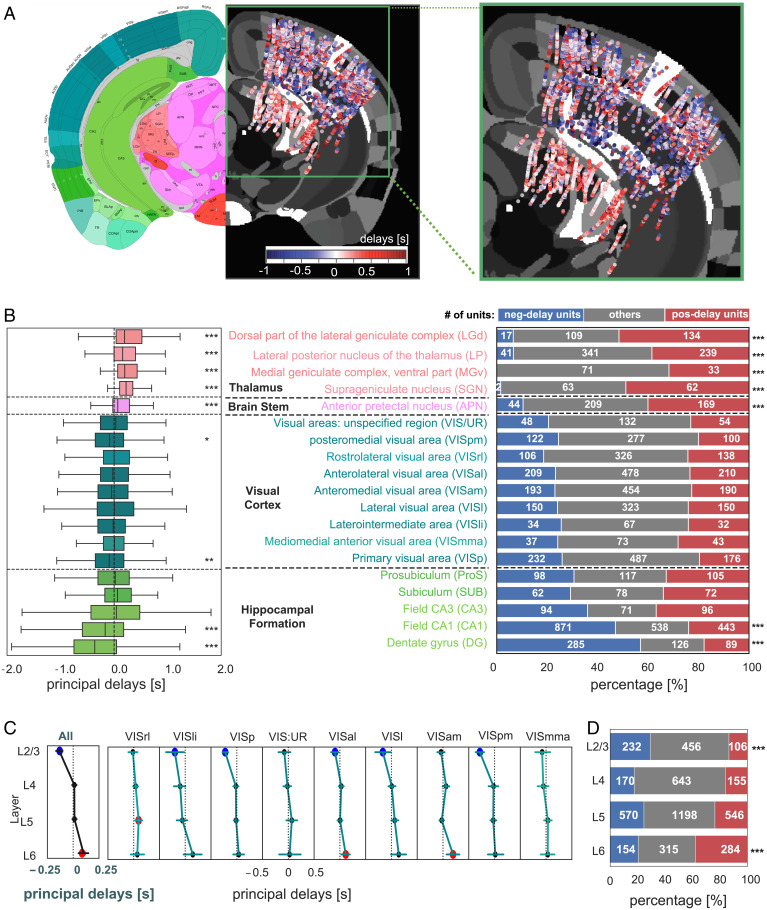
Negative- and positive-delay neurons were often spatially intermingled within the same brain areas, suggesting that the expression of spontaneous events at the single neuron level was not that of a coherent spatiotemporal wave (Fig. 3A). While cortical areas had a roughly even balance of leading versus lagging neuronal activity, CA1 and dentate gyrus (DG) of the hippocampus were dominated by negative delays (P < 2.8 × 10−29, Bonferroni corrected) whereas the thalamus was dominated by positive delays (P < 3.0 × 10−12, Bonferroni corrected) (Fig. 3 B, Left). These systematic biases stemmed from unbalanced proportions of negative- and positive-delay neurons: the CA1 and DG regions contain significantly more (P < 6.5 × 10−29, proportion z-test, Bonferroni corrected) negative-delay neurons, whereas the thalamic structures have significant less (P < 9.8 × 10−53), as compared with the overall distribution (Fig. 3 B, Right). Repeating the same analysis with controlling the equal numbers of neurons at the visual cortex, hippocampal formation, and thalamus resulted in a very similar result (SI Appendix, Fig. S6).
Fig. 3.
Spatial organization of the negative- and positive-delay neurons. (A) A map of the principal delay values. The principal delay values were averaged across units detected by the same channel, combined across all 14 animals, collapsed along the anterior-posterior direction, presented on an axial brain slice in the middle of recording sites, and shown along with a corresponding mouse brain atlas (Left; image credit: Allen Institute). The recording areas were amplified and shown on the Right. (B) A boxplot showing the region-specific distributions of the principal delay values (Left) is displayed together with a bar plot showing the percentages of three groups of neurons with distinct delays (Right). (C) The distributions of the principal delays across different cortical layers at various visual regions (pooled results in the first column). Error bars indicate 95% CIs of the mean delay. Brain regions with a mean delay significantly (P < 0.05) different from 0 are marked by colored circles (blue: negative; red: positive). (D) The percentages of the three types of neurons at different cortical layers. Asterisks represent the significance level (Bonferroni corrected: *0.01 < P < 0.05; **0.001 < P < 0.01; ***P < 0.001).
Similar biased distributions of the two types of neurons were also observed across cortical layers (Fig. 3A). Despite an overall balanced distribution of the principal delay values in the cortex, the mean delay value of layers 2/3 (L2/3) neurons was significantly (P = 7.5 × 10−17) lower than 0, whereas that of layer 6 (L6) neurons was significantly positive (P = 1.2 × 10−5) (Fig. 3C). Accordingly, relative proportions of the leading and lagging neurons was significantly different in the L2/3 (P = 6.1 × 10−12) and L6 (P = 2.9 × 10−11) layers as compared with the overall distribution (Fig. 3D). We further examined the subsecond time delay among the leading/lagging neurons of different visual cortical layers with those in the hippocampus and thalamus. According to the peak correlation offset, the CA1 and DG leading neurons preceded the visual L2/3 leading neurons by 138 ± 126 ms (P = 0.0019) in their spontaneous firing, whereas the visual L6 lagging neurons led the thalamic lagging neurons by 169 ± 160 ms (P = 0.0024) (SI Appendix, Fig. S7).
Together, these findings suggest that the observed spontaneous activation sequences are expressed earliest in the hippocampus and then propagate to the cortex, first affecting the supragranular layers, followed by the infragranular layers and then the thalamic projection targets.
Sequential Activation, Arousal, and Hippocampal Ripples.
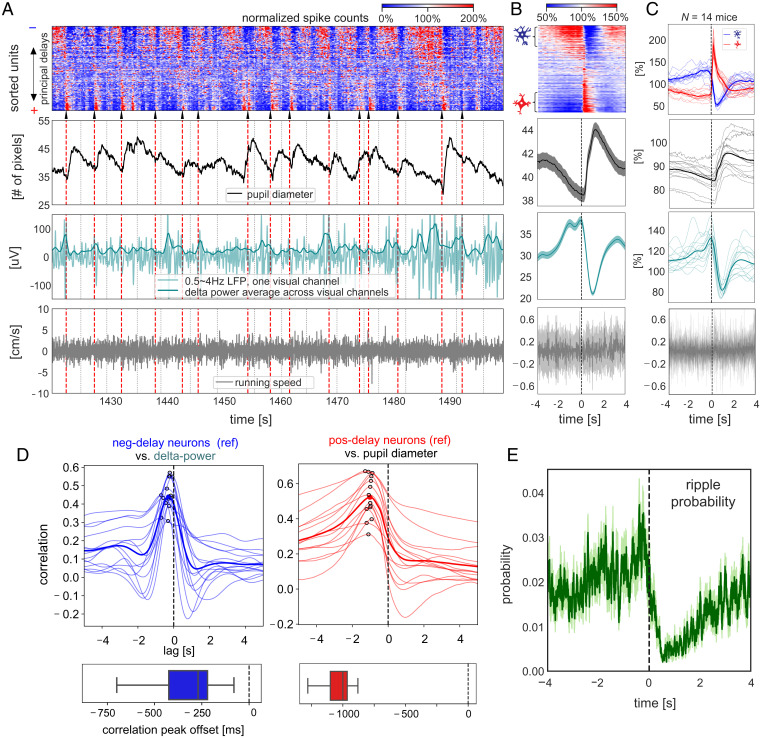
We further examined changes of arousal-related measures across the cycle of global sequential activation events, since the global resting-state fMRI activity has been linked to transient arousal modulations (4). First, we found that pupil diameter fluctuations were phase locked to the sharp activation of lagging positive-delay neurons (Fig. 4A, the second row). Second, we found that the cortical delta-band (0.5 to 4 Hz) activity demonstrated a concurrent decrease in its power, resulting in slow modulations of seconds timescale (Fig. 4A, the third row). For both of these arousal indicators, the average patterns within (Fig. 4B) and across animals (Fig. 4C) showed strong and distinct modulations across the cycle of sequential activation. The delta-power changes closely followed the temporal dynamics of the negative-delay neurons with a mean delay of 339 ± 199 ms (P = 2.5 × 10−5), as estimated by the peak correlation offset (Fig. 4 D, Left), whereas the pupil diameter changes followed the spontaneous firing of the positive-delay neurons by 1,046 ± 118 ms (P = 6.2 × 10−15) (Fig. 4 D, Right). These changes were spontaneous, with the animal at rest, and thus could not be explained by potential residual motion of animals (Fig. 4 A–C, Bottom).
Fig. 4.
Sequential activation pattern is accompanied by seconds-scale modulations of arousal measures and hippocampal ripples activity. (A) An 80-s section of various neuronal and behavioral signals from the representative mouse. Sequential activation patterns of the spiking activity (Top) are associated with changes in pupil diameter (the second row) and delta (0.5 to 4 Hz) activity (the third row) but not running speed (the Bottom row). White/black dashed lines denote time segment boundaries. Black arrows and red dashed lines mark the sharp activation of the positive-delay neurons, according to which the time segments were aligned and averaged. The time segments with delay profile positively correlated with the principal delay profile were aligned and averaged according to the sharp activation of the positive-delay neurons within the representative mouse (B) and across all 14 mice (C). For the group results (C), the spiking dynamics of the negative- and positive-delay neurons were shown, and the thicker, darker lines represent the mean curves, whereas the thinner, lighter lines denote the results from individual animals. For the results of the representative mouse (B), the thicker, darker lines represent the mean, whereas the shadowed regions denote the regions within 1 SEM across the 14 animals. (D) Cross-correlation functions were computed between the spiking activity of negative-delay neurons (reference) and the cortical delta-power (Left), as well as between the positive-delay neurons’ spiking dynamics (reference) and the pupil diameter (Right), during the stationary period. Thin and thick lines present the results of individual animals and their average respectively, and the peak correlations are marked by hollow circles. The boxplots (Bottom) show the distribution of the time offset of peak cross-correlations. (E) The hippocampal ripple events were aligned and averaged across the time segment of sequential activations during the stationary period, similar to the other neural and behavioral signals in (C). The shadow regions represent areas within 1 SEM across the 14 animals.
We also examined how this global sequential activation might be related to hippocampal ripples, which have been shown to comodulate with cortical delta-power (24), pupil microdilations (32), and brain-wide fMRI changes (21) on a similar seconds timescale. Adapting an existing algorithm (33) (see Materials and Methods for details), we detected 606 ± 184 hippocampal ripple events (average life: 46.2 ± 17.8 ms) per mouse/session from LFPs recordings in the CA1 region, and they were predominantly during the stationary period as expected (SI Appendix, Fig. S8). The occurrence probability of these ripple events was strongly modulated across the cycle of sequential activation (Fig. 4E). Specifically, it closely followed spiking dynamics of the negative-delay neurons and occurred with a probability close to 0 during the sharp activation of the lagging positive-delay neurons. Altogether, the sequential activation of two distinct neuronal populations was orchestrated with the seconds-scale modulation of both arousal measures and hippocampal ripple activity.
Ongoing Activity of the Two Neuronal Ensembles Affects Stimulus-Evoked Responses.
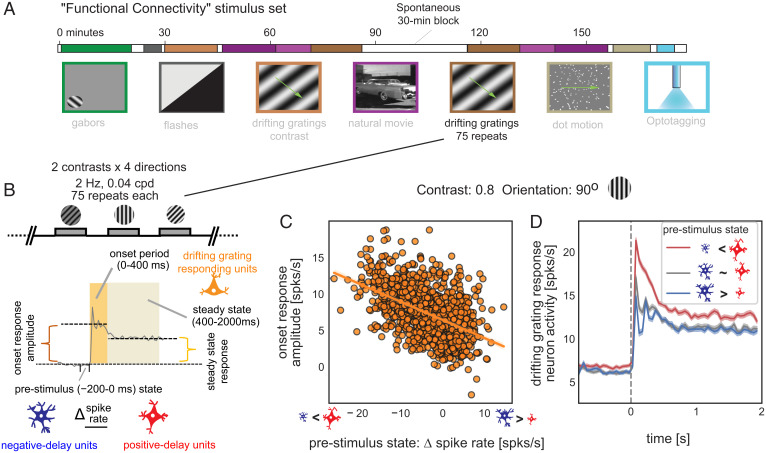
Given the prominent role of the negative- and positive-delay neurons in resting-state brain dynamics, we investigated whether their activity state might affect responses to external sensory stimuli (34–36). To achieve this goal, we used spiking data in another 30-min session with drifting grating stimulation (Fig. 5A). We quantified the prestimulus state (−0.2 to 0 s to the stimulus onset) by the firing rate difference between the negative- and positive-delay neurons (those responding to the drifting grating were excluded). The response amplitude of the third group of neurons, which were significantly activated by the drifting gratings (those with significant negative or positive principal delays were excluded), was quantified for both an early onset phase (0 to 0.4 s) and a later steady-state period (0.4 to 2 s) during stimulation (Fig. 5B).
Fig. 5.
Ongoing activity of the two neuronal ensembles predicts the amplitude of visual responses. (A) A diagram showing the “Functional Connectivity” stimulus set of the “Visual Coding – Neuropixels” project, which included a 30-min session with drifting gratings stimuli. (Image credit: Allen Institute.) (B) Illustration of the definition of the prestimulus state and the response amplitude to drifting gratings stimuli. The prestimulus state was defined as the spiking rate difference between the negative- and positive-delay neurons right before the stimulus onset (−200 to 0 ms), whereas the response amplitude was defined as the spiking rate change of the drifting-grating-responding neurons during the onset (0 to 400 ms) and steady-state (400 to 2,000 ms) stimulation periods as compared with the baseline period (−800 to 0 ms) without stimulation. (C) The prestimulus state of the negative- and positive-delay neurons is significantly correlated with the onset response amplitude of the drifting-grating-responding neurons to a drifting gratings stimulus (orientation: 90 degree; contrast: 0.8) across 1,050 trials pooled from all 14 mice (r = −0.50, P = 3.9 × 10−66). (D) Spiking rate of the drifting-grating-responding neurons were averaged within three groups of trials divided based on the prestimulus state: the bottom 350 trials (red) with the positive-delay neurons more active, the middle 350 trials (gray) with similar spiking rate between the negative- and positive-delay neurons, and the top 350 trials (blue) showing more active negative-delay neurons. Shadows represent regions within ±1 SEM across trials.
As shown by the example for a specific stimulus (90° and 0.8 contrast), the onset response amplitude of the drifting-grating-responding neurons to the visual stimulus was strongly dependent on the prestimulus state of the negative- and positive-delay neurons (r = −0.50, P = 3.9 × 10−66) (Fig. 5C). The neurons’ response to identical drifting grating patterns was often several fold higher when positive-delay neurons were more active right before simulation (Fig. 5C). The association was reproducible for all eight stimulus types (r = −0.52 ± 0.03, P = 3.9 × 10−10) and became weaker (P = 8.7 × 10−5) but still significant (r = −0.32 ± 0.05, P = 5.6 × 10−7) for the steady-state response (SI Appendix, Fig. S9). Consistent with these correlative analyses, the drifting-grating-responding neurons showed distinct dynamics, particularly during the onset phase, in trials with distinct prestimulus states (Fig. 5D). Overall, the prestimulus activity of the negative- and positive-delay neurons accounted for a significant portion of trial-to-trial variability in neuronal response to identical visual stimuli.
Discussion
Here we showed, using large-scale single-unit recordings in mice, that a surprisingly large proportion (∼70%) of surveyed neuronal population, regardless of their location, were entrained to slow (∼5 to 10 s) but highly structured spontaneous brain activity at immobile rest. Activity relative to global events was characterized by sequential activations between two distinct neuronal ensembles that showed orthogonal modulations across running/stationary states. The cortical delta-band power and pupil diameter showed similar but slightly delayed dynamics following the spontaneous firing of the leading, rest-active neurons and the lagging, running-active neurons, respectively, leading to their strong modulations across the sequential activation cycle. In addition, the occurrence of the hippocampal ripples was tightly linked to this global sequential activation and closely followed the dynamics of leading rest-active neurons. Lastly, the prestimulus activity of the two neuronal ensembles accounts for the trial-to-trial variability in neuronal responses to subsequent visual stimuli. Overall, these findings provide a single-cell perspective on resting-state seconds-scale brain dynamics at the cellular level and suggest a specific entity that may link arousal and memory functions of the brain, which are often concurrently impaired in neurodegenerative diseases.
While our recordings were extensive, they only covered a subregion of the brain, thus for cortical and subcortical structures that we did not analyze, we cannot know for certain whether the same cascades are present. However, there is some reason to believe that these signals are, to a first approximation, “global.” First, all 44 surveyed brain regions from various cortical and subcortical structures (e.g., the visual cortex, thalamus, brainstem, and hippocampus) in our study were replete with neurons that were active during these widespread events (SI Appendix, Fig. S2). Second, the recent studies reporting ensembles of neurons showing opposite modulation across distinct brain states (26–28), including the running and stationary state (28), were similarly found at all recording sites across an even-wider span of brain regions. Those neuronal ensembles are likely overlapped with the negative- and positive-delay neurons found during rest in the present study. Third, this global neuronal spiking activity shares important features with the global resting-state fMRI signal, which has been shown to involve brain-wide changes (4). For example, the global resting-state fMRI is coupled to cortical delta-power modulation (4, 5) and pupil diameter change (37) of a similar timescale (∼10 to 20 s in humans). It takes the form of sequential activations in the cortex mostly between the task-negative (i.e., the default mode network) and task-positive regions that often show opposite responses to task performance (7, 8, 38, 39), similar to those observed here between the rest-active (negative-delay) and running-active (positive-delay) neurons. It is interesting to speculate that the apparently coherent propagations at the macroscale fMRI signals arose from the biased distributions of the two types of neurons across brain regions, particularly along the cortical hierarchy gradient. The test of this hypothesis would remain a challenge for future studies capable of recording the whole-brain dynamics at both micro and macro levels. The structured spiking cascades are unlikely to be a straightforward consequence of particular spontaneous behaviors, such as pupil dilation, whisking, or fidgeting (40), since they are observed broadly across the forebrain, in areas involved in very different cognitive functions. At the same time, the specific mechanisms linking the observed spiking cascades and associated behaviors (i.e., the pupil dilations) remain unclear. Changes in the large-scale dynamics neuronal activity is often linked to brain state changes, as are changes in behavior (26–28, 40). Future studies will be needed to establish the causal relationships underlying the initiation of neuromodulation, motivated behaviors, brain state changes, and the broad patterns of organized spiking dynamics described here.
The functional relevance of this resting-state global brain activity remains unclear, but speculations can be made based on existing literature. First, this global activity is likely related to memory function due to its comodulation with the hippocampal ripples. The hippocampal ripples have been found to be comodulated, on a slow timescale of seconds, with cortical delta power (24, 41), sleep spindles (42), cortical membrane potentials, and pupil microdilations (32). There was also evidence for slow covariation of the pupillometry and cortical neuronal activity during quiet wakefulness (43, 44). Our findings here extend these previous observations by showing that the slow modulations of the hippocampal ripples and other neural and physiological changes actually are embedded in the highly organized global activity involving the majority of the neuronal population. This highly structured activity may provide local network dynamics critical for generating hippocampal ripples (41) and also a time window for their interaction with a large population of distant neurons, particularly those in the cortex.
This resting-state global activity may also represent a specific entity enabling an offline interaction between the memory function and cholinergic arousal system. A close relationship between the cholinergic system and memory function, particularly their concurrent impairments in Alzheimer’s disease (AD) (45, 46), has long been evident (47, 48), and various theories have been proposed to explain this relationship (49). The global resting-state fMRI activity has been linked to the cholinergic region at the basal forebrain (4, 5), since pharmaceutically deactivating the region greatly suppressed this global fMRI component (5). Spontaneous low-frequency pupil diameter change during immobile rest, similar to what we observed here, has also been linked to the activity of corticopetal cholinergic neurons from the basal forebrain (44). Together with its potential relationship with memory function as discussed in the preceding paragraph, the global resting-state brain activity may represent an “offline” process that links the memory consolidation and cholinergic system, which is distinct from the “online” influence of the cholinergic system on memory encoding through the attention regulation (49). This “offline” interaction is presumably mediated by extensive neuronal connections through the septohippocampal pathway linking the basal forebrain and hippocampus (50, 51).
Lastly, the resting-state global activity may orchestrate low-frequency (∼0.1 Hz) physiological modulations that are important for peri- and paravascular drainage of brain waste. The available data only allowed us to see spontaneous low-frequency pupil microdilations in the present study, but other sympathetic responses may concur. The global resting-state fMRI activity has been associated with slow (∼10 s) modulation of various physiological signals, including respiration (12, 23), cardiac pulsation (11, 13, 22), heart rate variability (52), and arterial tone (14), presumably mediated through sympathetic innervation (13). These low-frequency physiological modulations could be important for the clearance of toxic brain proteins by driving cerebrospinal fluid flow (53–56) through the perivascular and interstitial spaces through a newly discovered glymphatic pathway (16, 57). Consistent with this notion, the low-frequency spontaneous vasomotion was recently linked to paravascular clearance in mice (17). In humans, the global resting-state fMRI activity indeed was found to be coupled by cerebrospinal fluid movements (15). Moreover, the reduction of this coupling has been found to correlate with various AD pathology, particularly the increased accumulation of amyloid-beta protein (18). Altogether, the resting-state global activity and associated physiological modulations may represent a highly coordinated neuronal and physiological process that is potentially related to the cholinergic deficiency, memory dysfunction, and toxic protein accumulation, the three major pathological features of AD. It remains a challenge for future studies to further test this hypothesis and understand the role of this resting-state process in neurodegenerative diseases and dementia.
Materials and Methods
Our analysis was performed on the Neuropixels dataset, which contains neural and behavioral signals recorded from 14 mice for both spontaneous state and drifting grating stimulation (29). The spontaneous state was further divided into running and stationary state based on the mouse running speed. We focused on the neural spike data sorted by Kilosort2 (28). The neural spikes were binned with intervals of 200 ms to obtain the spiking time course for each neuron, which was normalized by its temporal mean and then averaged across population to obtain the global mean spiking activity (global signal).
Cross-correlation functions were computed between the spiking activity of individual neurons and the global mean spiking activity that was calculated with excluding the neuron to be correlated. The Fano factor was defined as the ratio between the variance and the mean of population spike count.
To extract the major direction of sequential activation across the neuronal population, we adapted a delay profile decomposition method to derive the principal delay profile of the neuronal spiking activity (8). The principal delay profile was rescaled to the seconds unit based on the average pattern of spiking cascade. We define the negative- and positive-delay neurons as those whose mean delay value, averaged across the stationary time segments of spiking cascade, is significantly (P < 0.001) lower or higher than 0, respectively.
The delta-band power was computed as the average of the band-pass–filtered (0.5 to 4 Hz), rectified, and low-pass–filtered (<0.72 Hz) LFPs over all channels in visual cortex. To detect hippocampal ripples, we adapted an existing offline ripple detection algorithm on LFP recorded from the CA1 region (33). The delta-band LFP power and the occurrence of the hippocampal ripples, along with other neural and behavioral measures, were aligned and averaged at the time segments of spiking cascades.
We correlated the prestimulus state of the negative- and positive-delay neurons and the response amplitude of the drifting-grating-responding neurons. The prestimulus state was quantified by the spiking rate difference between the negative- and positive-delay neurons within a 200-ms time window before the stimulus onset. The drifting-grating-responding neurons were defined as those showing a significantly (P < 0.001, paired Student’s t test) higher spiking rate during the simulation period than the baseline period. Their response amplitude to drifting grating stimuli was quantified by their spiking rate difference between the stimulation and baseline periods.
More details are available in SI Appendix, SI Materials and Methods .
Supplementary Material
Acknowledgments
This work was supported by NIH Pathway to Independence Award K99/R00 5R00NS092996-03, Brain Initiative Award 1RF1MH123247-01, NIH R01 Award 1R01NS113889-01A1, and the Intramural Research Program of the National Institute of Mental Health (Grant ZIAMH002896).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2105395118/-/DCSupplemental.
Data Availability
Python and Matlab code data have been deposited in GitHub (https://github.com/psu-mcnl/Neural-Cascade). Previously published data were used for this work (29). All the multimodal data are available at https://allensdk.readthedocs.io/en/latest/visual_coding_neuropixels.html.
References
- 1.Biswal B., Yetkin F. Z., Haughton V. M., Hyde J. S., Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 34, 537–541 (1995). [DOI] [PubMed] [Google Scholar]
- 2.Fox M. D., Raichle M. E., Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 8, 700–711 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Liu T. T., Nalci A., Falahpour M., The global signal in fMRI: Nuisance or Information? Neuroimage 150, 213–229 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu X., et al. , Subcortical evidence for a contribution of arousal to fMRI studies of brain activity. Nat. Commun. 9, 395 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turchi J., et al. , The basal forebrain regulates global resting-state fMRI fluctuations. Neuron 97, 940–952.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong C. W., Olafsson V., Tal O., Liu T. T., The amplitude of the resting-state fMRI global signal is related to EEG vigilance measures. Neuroimage 83, 983–990 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitra A., Snyder A. Z., Blazey T., Raichle M. E., Lag threads organize the brain’s intrinsic activity. Proc. Natl. Acad. Sci. U.S.A. 112, E2235–E2244 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu Y., et al. , Brain activity fluctuations propagate as waves traversing the cortical hierarchy. Cereb. Cortex 31, 3986–4005 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang D., Raichle M. E., Disease and the brain’s dark energy. Nat. Rev. Neurol. 6, 15–28 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Abbas A., et al. , Quasi-periodic patterns contribute to functional connectivity in the brain. Neuroimage 191, 193–204 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang C., Cunningham J. P., Glover G. H., Influence of heart rate on the BOLD signal: The cardiac response function. Neuroimage 44, 857–869 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birn R. M., Diamond J. B., Smith M. A., Bandettini P. A., Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage 31, 1536–1548 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Özbay P. S., et al. , Contribution of systemic vascular effects to fMRI activity in white matter. Neuroimage 176, 541–549 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tong Y., Yao J. (Fiona), Chen J. J., de B. Frederick B., The resting-state fMRI arterial signal predicts differential blood transit time through the brain. J. Cereb. Blood Flow Metab. 39, 1148–1160 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fultz N. E., et al. , Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science 366, 628–631 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jessen N. A., Munk A. S. F., Lundgaard I., Nedergaard M., The glymphatic system: A beginner’s guide. Neurochem. Res. 40, 2583–2599 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Veluw S. J., et al. , Vasomotion as a driving force for paravascular clearance in the awake mouse brain. Neuron 105, 549–561.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han F., et al. , Alzheimer’s Disease Neuroimaging Initiative, Reduced coupling between cerebrospinal fluid flow and global brain activity is linked to Alzheimer disease-related pathology. PLoS Biol. 19, e3001233 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han F., et al. , Decoupling of global brain activity and cerebrospinal fluid flow in Parkinson’s disease cognitive decline. Mov. Disord. 36, 2066–2076 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buzsáki G., Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus 25, 1073–1188 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Logothetis N. K., et al. , Hippocampal-cortical interaction during periods of subcortical silence. Nature 491, 547–553 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Özbay P. S., et al. , Sympathetic activity contributes to the fMRI signal. Commun. Biol. 2, 421 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu Y., Han F., Sainburg L. E., Liu X., Transient Arousal Modulations Contribute to Resting-State Functional Connectivity Changes Associated with Head Motion Parameters. Cereb. Cortex 30, 5242–5256 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sirota A., Csicsvari J., Buhl D., Buzsáki G., Communication between neocortex and hippocampus during sleep in rodents. Proc. Natl. Acad. Sci. U.S.A. 100, 2065–2069 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jun J. J., et al. , Fully integrated silicon probes for high-density recording of neural activity. Nature 551, 232–236 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen W. E., et al. , Thirst regulates motivated behavior through modulation of brainwide neural population dynamics. Science 364, 253 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gründemann J., et al. , Amygdala ensembles encode behavioral states. Science 364, eaav8736 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Stringer C., et al. , Spontaneous behaviors drive multidimensional, brainwide activity. Science 364, 255 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Vries S. E. J., et al. , A large-scale standardized physiological survey reveals functional organization of the mouse visual cortex. Nat. Neurosci. 23, 138–151 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brunel N., Hakim V., Fast global oscillations in networks of integrate-and-fire neurons with low firing rates. Neural Comput. 11, 1621–1671 (1999). [DOI] [PubMed] [Google Scholar]
- 31.Hahn G., et al. , Spontaneous cortical activity is transiently poised close to criticality. PLOS Comput. Biol. 13, e1005543 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGinley M. J., David S. V., McCormick D. A., Cortical membrane potential signature of optimal states for sensory signal detection. Neuron 87, 179–192 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stark E., et al. , Pyramidal cell-interneuron interactions underlie hippocampal ripple oscillations. Neuron 83, 467–480 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fox M. D., Snyder A. Z., Zacks J. M., Raichle M. E., Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat. Neurosci. 9, 23–25 (2006). [DOI] [PubMed] [Google Scholar]
- 35.He B. J., Spontaneous and task-evoked brain activity negatively interact. J. Neurosci. 33, 4672–4682 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arieli A., Sterkin A., Grinvald A., Aertsen A., Dynamics of ongoing activity: Explanation of the large variability in evoked cortical responses. Science 273, 1868–1871 (1996). [DOI] [PubMed] [Google Scholar]
- 37.Pais-Roldán P., et al. , Indexing brain state-dependent pupil dynamics with simultaneous fMRI and optical fiber calcium recording. Proc. Natl. Acad. Sci. U.S.A. 117, 6875–6882 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox M. D., et al. , The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U.S.A. 102, 9673–9678 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yousefi B., Shin J., Schumacher E. H., Keilholz S. D., Quasi-periodic patterns of intrinsic brain activity in individuals and their relationship to global signal. Neuroimage 167, 297–308 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drew P. J., Winder A. T., Zhang Q., Twitches, blinks, and fidgets: Important generators of ongoing neural activity. Neuroscientist 25, 298–313 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanda P., et al. , Bidirectional interaction of hippocampal ripples and cortical slow waves leads to coordinated spiking activity during NREM sleep. Cereb. Cortex 31, 324–340 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siapas A. G., Wilson M. A., Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron 21, 1123–1128 (1998). [DOI] [PubMed] [Google Scholar]
- 43.Reimer J., et al. , Pupil fluctuations track fast switching of cortical states during quiet wakefulness. Neuron 84, 355–362 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reimer J., et al. , Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nat. Commun. 7, 13289 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitehouse P. J., et al. , Alzheimer’s disease and senile dementia: Loss of neurons in the basal forebrain. Science 215, 1237–1239 (1982). [DOI] [PubMed] [Google Scholar]
- 46.Bartus R. T., On neurodegenerative diseases, models, and treatment strategies: Lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp. Neurol. 163, 495–529 (2000). [DOI] [PubMed] [Google Scholar]
- 47.Drachman D. A., Leavitt J., Human memory and the cholinergic system. A relationship to aging? Arch. Neurol. 30, 113–121 (1974). [DOI] [PubMed] [Google Scholar]
- 48.Haam J., Yakel J. L., Cholinergic modulation of the hippocampal region and memory function. J. Neurochem. 142 (suppl. 2), 111–121 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hasselmo M. E., The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 16, 710–715 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dragoi G., Carpi D., Recce M., Csicsvari J., Buzsáki G., Interactions between hippocampus and medial septum during sharp waves and theta oscillation in the behaving rat. J. Neurosci. 19, 6191–6199 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petsche H., Gogolak G., Vanzwieten P. A., Rhythmicity of septal cell discharges at various levels of reticular excitation. Electroencephalogr. Clin. Neurophysiol. 19, 25–33 (1965). [DOI] [PubMed] [Google Scholar]
- 52.Chang C., et al. , Association between heart rate variability and fluctuations in resting-state functional connectivity. Neuroimage 68, 93–104 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klose U., Strik C., Kiefer C., Grodd W., Detection of a relation between respiration and CSF pulsation with an echoplanar technique. J. Magn. Reson. Imaging 11, 438–444 (2000). [DOI] [PubMed] [Google Scholar]
- 54.Schley D., Carare-Nnadi R., Please C. P., Perry V. H., Weller R. O., Mechanisms to explain the reverse perivascular transport of solutes out of the brain. J. Theor. Biol. 238, 962–974 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Iliff J. J., et al. , Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J. Neurosci. 33, 18190–18199 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamada S., et al. , Influence of respiration on cerebrospinal fluid movement using magnetic resonance spin labeling. Fluids Barriers CNS 10, 36 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie L., et al. , Sleep drives metabolite clearance from the adult brain. Science 342, 373–377 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Python and Matlab code data have been deposited in GitHub (https://github.com/psu-mcnl/Neural-Cascade). Previously published data were used for this work (29). All the multimodal data are available at https://allensdk.readthedocs.io/en/latest/visual_coding_neuropixels.html.