Abstract
Background
Hepatocellular carcinoma (HCC) has variable etiological risk factors. Radiofrequency ablation (RFA) and surgical resection (SR) are frequently used as curative treatment options. In the present study, we assessed the etiological factors and efficacy of RFA and SR in patients with unifocal HCC in a real-life setting.
Methods
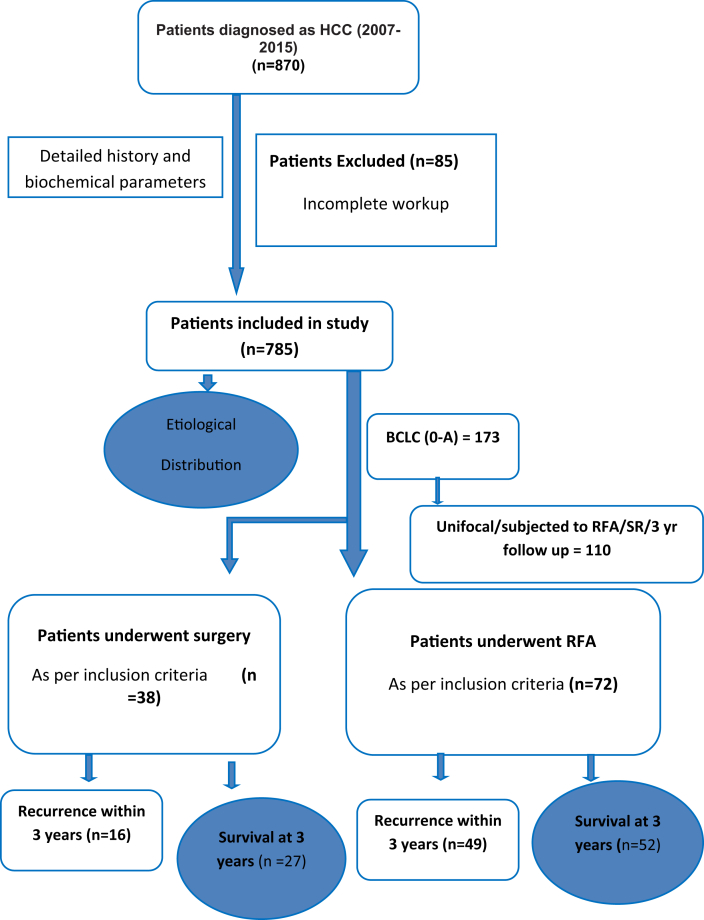
Of 870 patients with HCC seen over a period of nine years, 785 patients were assessed for stage and etiological risk factors. Of these, 110 (14%) patients with single HCC who were either treated with RFA (n = 72) or SR (n = 38) were evaluated for their outcomes in terms of overall survival (OS) and disease-free survival (DFS) over 3 years.
Results
Of 785 patients [median age 60 (range 51–65) years, males (n = 685, 87.3%)] with HCC, viral hepatitis [HBV and HCV with or without alcohol = 502 (63.9%)] was the most common etiology; nonalcoholic steatohepatitis (NASH) and alcohol as an etiology showed increase over the years. About 677 (86.2%) patients had evidence of cirrhosis; NASH and HBV were predominant causes in noncirrhotic patients. Even though the groups were not matched, in 110 patients subjected to either RFA [mean tumor size, 2.2 (1.9–2.8) cm] or SR [mean tumor size, 7.1 (4.8–9.7) cm], tumor progression was observed in 49 (68%) and 16 (42%) patients in RFA and SR groups, respectively, with superior DFS in the SR group (P < 0.01). Of total 31 deaths, 20 (27.8%) deaths were in the RFA group and 11 (28.9%) in the SR group with no difference in OS at 3 years.
Conclusion
Viral hepatitis with or without alcohol is the commonest etiological factor for HCC in Northern India; NASH and alcohol are increasing over the years. In a real-life setting, in patients with unifocal HCC, there is no difference in overall 3-year survival subjected to SR or RFA with better DFS in the SR group.
Keywords: hepatitis B virus, hepatitis C virus, alcohol, nonalcoholic fatty liver disease, NASH
Abbreviations: AFP, Alpha Fetoprotein; BCLC, Barcelona Clinic Liver Cancer; CEMR, Contrast-Enhanced Magnetic Resonance Imaging; CLD, Chronic Liver Disease; DFS, Disease-free Survival; HBV, Hepatitis B Virus; HCV, Hepatitis C Virus; HCC, Hepatocellular Carcinoma; LT, Liver Transplantation; NAFLD, Non -Alcoholic Fatty Liver Disease; NASH, Non-alcoholic Steatohepatitis; OS, Overall Survival; RFA, Radiofrequency Ablation; SR, Surgical Resection; TPCT, Triple-Phase Contrast-enhanced computerized Tomography
Hepatocellular carcinoma (HCC) is a lethal tumor of the liver and is a common cause of cancer-related deaths the world over.1,2 However, the incidence of HCC shows considerable heterogeneity in various countries, reflecting the regional differences in etiological factors as well as in ethnicity.3,4 In Asia, HBV- and HCV-related chronic liver disease is among the top causes of HCC.5 Alcohol abuse and nonalcoholic fatty liver disease (NAFLD) are other foremost reasons for HCC.5,6 India is reported as low incidence zone for HCC. Estimates suggest that crude incidence rate of HCC in India in the year 2015 was 2.8 cases per 100,000 population per year (males: 3.9, females: 1.6); however, the incidence of HCC has been rising in India over last few decades.6, 7, 8
Despite surveillance strategies for HCC in high-risk patients, HCC cases are usually diagnosed late in intermediate or advanced stage of HCC which is not suitable for potential curative therapies.8 Around 30% patients diagnosed of HCC at an early stage have the possibility of curative therapies such as liver transplantation and surgical resection (SR) that are expected to yield good long-term outcome.9 However, shortage of donor and poor hepatic reserve with liver cirrhosis restrict these options in patients with HCC.10 Recently, the Barcelona Clinic Liver Cancer (BCLC) group suggested radiofrequency ablation (RFA) as an alternate to SR in patients with early HCC.11 RFA is an in situ ablative therapy used to selectively destroy tumor tissue; considered as safe for cirrhotic patients, cost-effective, and achieves outcomes similar to SR in patients with single small HCC (2–3 cm).12 RFA has been widely performed in patients with HCC who are unwilling to be treated with SR or may not be candidate for surgery.13 However, RFA has limitations in performance for lesions located close to the gall bladder, diaphragm, etc., because of the risk of collateral damage and for those situated close to blood vessels because of the ‘heat sink phenomenon’.14
Even though both RFA and SR are the mainstay curative treatment modalities for HCC in clinical practice, both modalities may be limited by the intrahepatic and extrahepatic recurrence of HCC.13, 14, 15, 16, 17, 18, 19 Globally, comparative data for smaller lesions showed equal efficacy for both the modalities with SR scoring over RFA for lesions more than 2 cm in size.13,15
The aim of the present study was to assess the clinical profile and etiological risk factors of a large cohort of patients with HCC and to assess the efficacy of SR and RFA as the curative treatment modalities for HCC in a real-life setting.
Methods
Study population
In a retrospective data of nine years (January 2007–December 2015), medical records of 870 patients diagnosed to have HCC were retrieved for the analysis. Of these, 85 patients with HCC were excluded because of the incomplete work up/record. Finally, 785 patients were analyzed for the clinical profile and etiological risk factors (Figure 1). Of 785 patients, 173 (22%) patients were in early stage (BCLC 0-A) and were offered ablation or SR [percutaneous ethanol injection (PEI) or RFA or SR]. Because follow-up data in patients subjected to PEI were incomplete, one hundred and ten of these patients who were subjected to either RFA [n = 72 (65%)] or SR [n = 38 (35%)] in a real-life setting, who had unifocal nodule and available three-year follow-up data (up to December 2018) were analyzed in this study (Figure 1). Liver transplantation could not be offered to any patient because of either nonavailability (liver transplant programme started in our institute in 2011) or nonaffordability. The diagnosis of HCC was based on triple-phase contrast-enhanced computerized tomography (TPCT) or contrast-enhanced magnetic resonance imaging (CEMR) showing characteristic findings (hypervascularity on arterial phase with washout on portal venous or delayed phase) with or without elevated alpha fetoprotein (AFP) and tissue diagnosis. Patients who underwent SR had confirmed diagnosis of HCC on the resected specimen. Fine-needle aspiration cytology was performed only in patients with inconclusive radiology or in the presence of a noncirrhotic liver. Inclusion criteria for patients who underwent RFA were those with a single nodule of HCC ≤ 3 cm on size with or without underlying cirrhosis and/or portal hypertension whereas patients were included for SR only if they had single nodule with normal platelet count and no evidence of gastroesophageal varices and had at least 30% remnant liver volume in a noncirrhotic liver and 60% remnant liver volume in a cirrhotic liver as per the CT volumetric study.20 Exclusion criteria for both RFA and SR groups were age<18 years, macroscopic vascular invasion, and distant metastasis before initial therapy. Because patients were managed in a real-life setting, patients subjected to RFA and SR were not matched but were assessed for the disease-free survival (DFS), overall survival (OS), and deaths up to 3 years after therapy.
Figure 1.
Consort diagram showing the inclusion of patients and their outcome.
Patient evaluation
A detailed clinical history and laboratory investigations were recorded for all 785 included patients. Laboratory tests such as hemogram, liver function test, renal function test, coagulogram, viral markers (HBsAg and/or anti-HBc, anti-HCV, HIV serology), other etiological workup if clinically indicated, serum AFP, and radiological (USG/CT/MRI) findings were recorded in a standard performa. Diagnosis of cirrhosis was based on combination of the presence of thrombocytopenia, radiological findings of a heterogenous liver, irregular liver margin with or without features of portal hypertension, transient elastography (where available), liver histology and gross findings during surgery in those subjected to SR. Patients were diagnosed as HBV-related HCC based on the positive HBsAg status with or without positivity for HBeAg and HBV DNA. Similarly, diagnosis of HCV-related HCC was made on positive anti-HCV status with or without HCV RNA positivity. Diagnosis of alcohol-related HCC was made based on the history of significant alcohol intake (≥40-60 gm per day for ≥ 10 years). Patients without history of alcohol intake, negative for viral markers and other etiologies, with the presence or history of two of the metabolic risk factors (obesity, type 2 diabetes mellitus, hypertension, high triglycerides and low high-density lipoprotein) were diagnosed as having nonalcoholic steatohepatitis (NASH)-related cirrhosis. Patients were diagnosed as having cirrhosis related to Budd-Chiari syndrome, autoimmune hepatitis, and cholestatic liver disease in appropriate clinical setting, in the presence of autoimmune markers, radiological findings, liver histology, and exclusion of other causes. Patients were divided into different stages as per the BCLC staging.21
Treatment and follow-up
A multidisciplinary team that included hepatologists, hepatobiliary surgeons, and interventional radiologists decided the treatment modality of each patient with HCC. SR was performed under general anesthesia using standard hepatectomy technique by experienced team of hepatobiliary surgeons. Type of surgery, anatomical or nonanatomical resection, was decided in accordance with tumor location and underlying liver status. RFA was carried out by experienced radiologists on an in-patient basis in the department of Radiodiagnosis and Imaging, using a Radionics, Cool-Tip System (USA) as per manufacturer instructions. The RFA procedure was performed under conscious sedation using USG/CT guidance. The tumors were considered as ablated completely if no viability was found on TPCT or CEMR scan at one month after the intervention. Thereafter, patients were followed up in the liver clinic with biochemical tests/TPCT or CEMR every 3 months for the first year and every 4–6 months thereafter for a period of three years or death whichever was earlier. The median (interquartile range [IQR]) duration of follow-up of the RFA and SR groups were 36 (34–36 months) and 36 (31.5–36 months), respectively.
Tumor recurrence was defined as one or more emerging lesion within the liver (local/distant recurrence—at the site/away from target nodule) and/or extrahepatic metastases on follow-up imaging (TPCT/CEMR [abdomen or chest], bone scan or PET-CT). Recurrence-free survival was defined as interval between date of first treatment and first recurrence or last follow-up. When recurrence was detected, the patient was aggressively treated with appropriate treatment modalities. OS was defined as time interval from first treatment to date of death from any cause or censoring.
Statistical analysis
All the statistical analysis was carried out by using IBM SPSS version 22 (IBM, Armonk, NY). Categorical data variables were expressed as number (percentage) and continuous data were expressed as mean ± standard deviations or median and IQR. For categorical variables, the Pearson Chi-square test or Fisher's exact test was applied. Quantitative data distribution was checked by means of skewness and Kolmogorov-Smirnov test. Student's t test or Wilcoxon signed rank test was used for comparison of continues variables between the two groups. OS of patients and cumulative probability of HCC recurrence were estimated using Kaplan–Meier Method. A two tailed P value<0.05 was considered for statistical significance.
Results
Etiological and demographic profile of the patients with HCC
A total of 870 patients with HCC were prospectively screened over nine years (2007–2015), of which 785 patients with complete workup for HCC were analyzed in this study (Figure 1). The median age of the patients at presentation was 60 (range 51–65) years and majority of them were male (n = 685, 87.3%). Detailed demographic and clinical parameters of patients with HCC are shown in Table 1. Viral hepatitis (HBV and HCV with or without alcohol) was the most common etiology present in 502 (63.9%) patients whereas nonviral etiology was observed in 283 (36.1%) patients (Table 2). Overall, 293 (37.3%) patients were related only to viral hepatitis [HBV—158 (20%) and HCV—133 (17%)] whereas 209 (26.6%) patients had virus plus alcohol [HBV+ alcohol—97 (12.3%), HCV+ alcohol—110 (14%), HBV+ HCV+ alcohol—2 (0.002%)] as the etiology. Alcohol alone accounted for 141 (18%) patients and NASH for 138 (17.6%) patients with HCC (Table 2).
Table 1.
Demographic and Clinical Characteristics of 785 Patients With HCC patients.
| Variables | Number (%) or Median (IQR) |
|---|---|
| Age (years) | 60 (51–65) |
| Gender (male, %) | 685 (87.2%) |
| Diabetes mellitus | 206 (26.2%) |
| Smokers | 159 (20.2%) |
| Clinicalcharacteristics | |
| Abdomen pain | 387 (49.3%) |
| Loss of appetite | 362 (46.1%) |
| Loss of weight | 327 (41.6%) |
| Ascites | 194 (24.7%) |
| Jaundice | 150 (19.1%) |
| Cirrhosis liver, n (%) | 677 (86.2%) |
| Noncirrhotic liver, n (%) | 108 (14%) |
| HBV | 57 (53%) |
| NASH | 46 (42%) |
| HCV | 5 (5%) |
| AFP, ng/mL [mean (range)] | 100 (10–640) |
| >10 ng/mL | 580 (73.9%) |
| Cirrhosis—CTP (n = 677) | |
| Class A | 302 (38.4%) |
| Class B | 300 (38.2%) |
| Class C | 75 (9.5%) |
| BCLC | |
| Stage A | 173 (22%) |
| Stage B | 204 (26%) |
| Stage C | 276 (35.2%) |
| Stage D | 132 (16.8%) |
| Tumor size (cm) | 6 (3.5–9.5) |
| Single tumor Multiple tumors |
343 (43.7%) 442 (56.3%) |
AFP, alpha fetoprotein; CTP, Child-Turcotte-Pugh; BCLC, Barcelona clinic liver cancer.
Table 2.
Etiological Factors Associated With 785 Patients With HCC.
| Etiology | Etiological agents | Number of HCC patients (%) |
|---|---|---|
| Viral etiology (n = 502; 63.9%) | HBV | 158 (20.1%) |
| HCV | 133 (16.9%) | |
| HBV+HCV | 2 (0.002%) | |
| HBV+ALD | 97 (12.3%) | |
| HCV+ALD | 110 (14.%) | |
| HBV+HCV+ALD | 2 (0.002%) | |
| Nonviral etiology (n = 283, 36.1%) | ALD | 141 (18.0%) |
| NASH | 138 (17.6%) | |
| Miscellaneous (BCS, AIH, PBC) | 4 (0.5%) |
HBV, hepatitis B virus; HCV, hepatitis C virus; ALD, alcohol-associated liver disease; NASH, nonalcoholic steatohepatitis; BCS, Budd-Chiari syndrome; AIH, autoimmune hepatitis; PBC, primary biliary cholangitis.
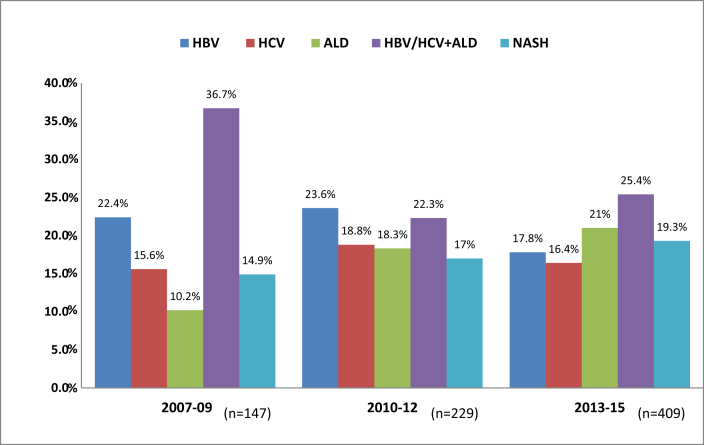
Presence of type 2 diabetes mellitus was frequently reported both in viral and nonviral etiology (Table 1). Patients with viral etiology were comparatively younger than patients with nonviral etiology. Analysis of patients with HCC over the years revealed not only an increase in the number of patients but also a change in the etiology of HCC (Figure 2). As per the trends shown in Figure 2, there was an increase in patients with HCC due to alcohol and NASH; HBV as the etiology of HCC decreased over the years; HCV was static after the initial increase and combination of HBV and/or HCV with alcohol continued to remain a common cause.
Figure 2.
Increasing number and changing etiology of HCC over nine years. HBV, hepatitis B virus; HCV, hepatitis C virus; ALD, alcoholic liver disease; NASH, nonalcoholic steatohepatitis.
Clinical or radiological evidence of cirrhosis was found in 677 (86.2%) patients with HCC; among 108 patients with noncirrhotic liver, HBV and NASH constituted the most (Table 1). HCC lesions were predominantly located in the right lobe of the liver (n = 451, 57.4%), followed by bilobar involvement (n = 224, 28.5%), and left lobe involvement (n = 110, 14%). Vascular invasion of portal vein was seen in 361 (46%) patients. Median tumor size was 6 cm (IQR 3.5–9.5 cm) and multifocal tumors were present in 442 (56.3%) of the patients with HCC. As per the BCLC staging, 173 (22%) patients were in early stage (BCLC 0-A), 204 (26%) patients in intermediate stage (BCLC-B) and 408 (52%) patients were in BCLC stage C/D. AFP was raised above upper limit normal (ULN=10 ng/mL) in 580 (73.9%) patients with HCC.
Therapy
The study is the retrospective analysis of the real-life data of the two curative nontransplant modalities (RFA and SR) for patients with HCC. Since the treatment decisions were taken in a real-life setting; two groups of patients (RFA and SR) were not matched and the results described for treatment response are only descriptive.
There was no statistically significant difference between the RFA group [n = 72 (65%)] and SR group [n = 38 (35%)] with respect to age and gender (Table 3). However, the RFA group demonstrated higher frequency of patients with cirrhosis (71/72, 98.6% Vs 23/38, 60.5%, P < 0.01) and worse liver synthetic functions with low albumin, low platelets, and poorer Child-Pugh class than the SR group. Of 23 patients with cirrhosis, all but one patient (95.6%) in the SR group were in CTP A in contrast to 48 of 71 cirrhosis (67.6%) in the RFA group (Table 3). Patients in the SR group had significantly higher median AFP value (90 ng/mL) and larger tumor size of 7.1 cm (IQR 4.1–9.7 cm) than the RFA group (≤3 cm) (Table 3).
Table 3.
Characteristics of the Treated Patients Along With Treatment Groups.
| Parameters No. (%) or Median (IQR) |
Overall patients (n = 110) | RFAa (n = 72) | Surgical resectionb (n = 38) | P-Valueavs.b |
|---|---|---|---|---|
| Age, years | 60 (53–65) | 60 (52–65) | 60 (55.5–65) | NS |
| Gender, male | 100 (91%) | 62 (86.1%) | 36 (94.7%) | NS |
| Diabetes | 31 (28.2%) | 24 (33.3%) | 7 (21.8%) | 0.043 |
| Underlying liver disease | 0.032 | |||
| Hepatitis B | 32 (29.1%) | 15 (20.8%) | 18 (47.4%) | |
| Hepatitis C | 42 (38.2%) | 34 (47.2%) | 8 (21%) | |
| ALD | 17 (15.5%) | 12 (16.7%) | 5 (13.1%) | |
| NASH | 19 (17.3%) | 11 (15.3%) | 7 (18.4%) | |
| Smokers | 13 (11.8%) | 8 (11.1%) | 5 (13.2%) | NS |
| Bilirubin (total), mg/Dl | 1.05 (0.7–1.4) | 1.1 (0.8–1.4) | 0.8 (0.6–1.3) | NS |
| AST, IU/L | 60.5 (45–89) | 61.5 (45.7–89) | 55.8 (42.7–89.5) | NS |
| ALT, IU/L | 55.5 (37–78.7) | 55 (35.7–76.2) | 55.5 (39–84.7) | NS |
| Serum albumin, g/dL | 3.5 (3.18–4) | 3.4 (3.1–3.9) | 3.8 (3.4–4.1) | <001 |
| Platelet, nx103/mm3 | 129.5 (95.2–185) | 108 (88.5–147) | 187 (145–239) | <0.01 |
| AFP, ng/ml | 20.4 (7.3–147.7) | 11.5 (6.8–48.2) | 90 (10–307.7) | <0.01 |
| Cirrhosis liver | 94 (85.4%) | 71 (98.6%) | 23 (60.5%) | <0.01 |
| CTP A | 70 (74.5%) | 48 (67.6%) | 22 (95.6%) | 0.019 |
| Tumor size (cm) | 2.85 (2–4.9) | 2.2 (1.9–2.8) | 7.1 (4.8–9.7) | <0.01 |
AFP, alpha fetoprotein; CTP, Child-Turcotte-Pugh; ALD, alcohol-associated liver disease; NASH, non-alcoholic steatohepatitis.
Outcomes
The median duration of hospital stay in the RFA group was shorter (2 days, range 2–4 days) than the SR group (8 days, range 6–15 days) (P < 0.01). There was no immediate postprocedure hepatic decompensation and in-hospital deaths in the either group but one patient in the surgical group expired due to hepatic decompensation within 6 months of surgery. The number of patients who required hospital admission within 6 months of the initial procedure was higher in the SR group than in the RFA group (n = 4, 10.5% Vs 1, 1.3%, P < 0.01).
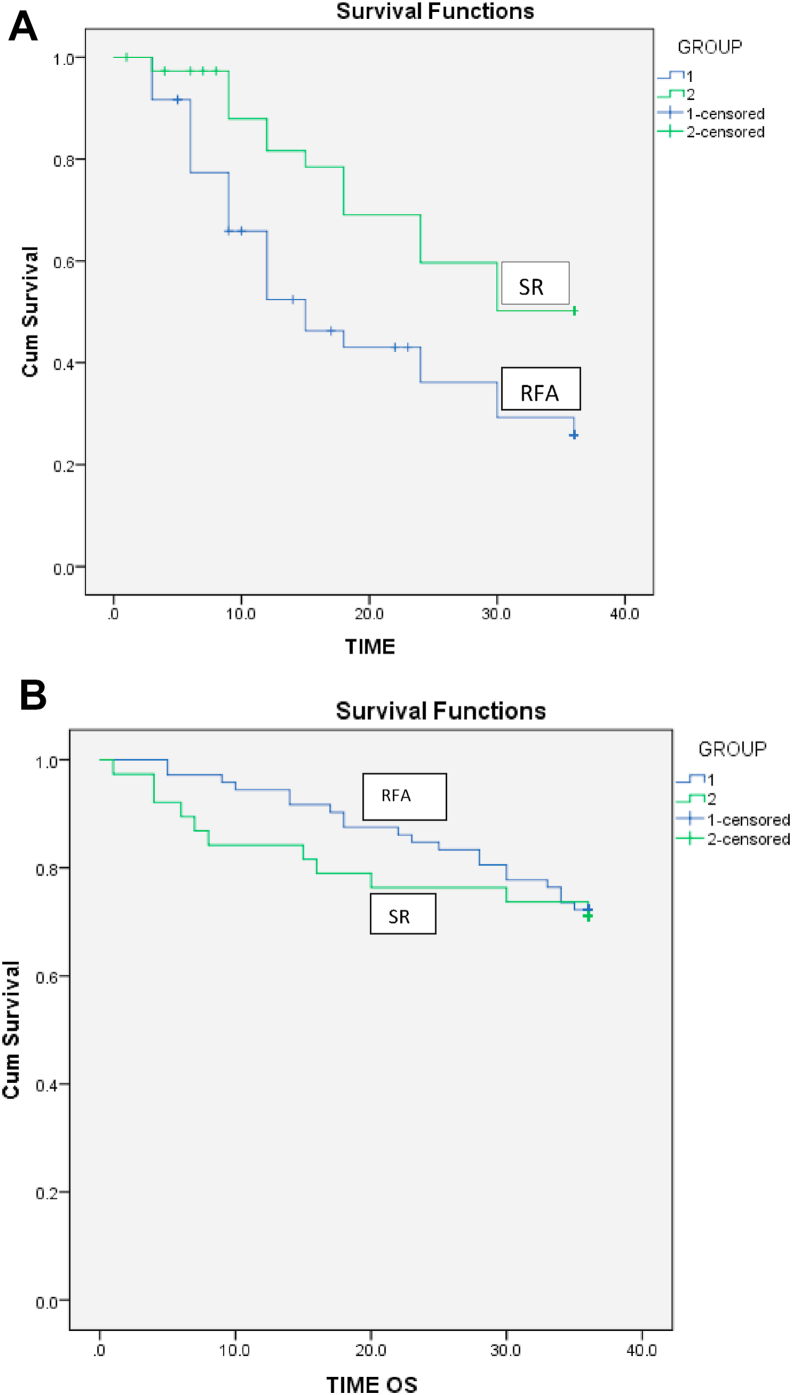
During follow-up, tumor recurrence occurred in 49 (68%) patients in the RFA group in a median time of 12 (IQR 6–15) months compared with 16 (42%) patients in the SR group at a median time of 18 (IQR 11.2–24) months. Cumulative recurrence rates at 1, 2, and 3 years were higher in the RFA group than in the SR group [33 (45.8%), 43 (59.7%), 49 (68%) Vs6 (15.8%), 13 (34.2%), 16(42.1%)] (Figure 3A) (P < 0.01). Intrahepatic tumor recurrence was more common in both RFA and SR groups [44 (89.8%) and 13 (81.2%) patients]; and distant intrahepatic recurrence was more common than the local recurrence in the both RFA and SR groups [29 (66%) and 10 (76.9%)]. Extrahepatic recurrence in the lungs and bones was reported in 5 (10.2%) and 3 (18.8%) patients in the RFA and SR groups, respectively.
Figure 3.
A: Kaplan-Meier curves for disease-free survival (DFS) in patients treated with surgical resection vs. radiofrequency ablation. Three-year DFS rates were significantly higher for the SR group than for the RFA group. (P < 0.001). Figure 3B: Comparison of overall survival rates in patients treated with surgical resection (SR) and radiofrequency ablation (RFA). There was no significant difference in OS rates between the SR group and RFA group. (P = 0.775).
There were 31 deaths (28.2%) during the follow-up, 20 (27.8%) deaths in the RFA group at a median time of 22.5 (IQR 14–30) months and 11 (28.9%) in the SR group at a median time of 8 (IQR 5–18) months. There was no statistically significant difference in the 1-, 2-, and 3-year survival rates between the RFA and SR groups [ 68 (94.4%), 61 (84.7%), 52(72.2%) Vs 32(84.2%), 29 (76.3%), and 27 (71%)] (Figure 3B) (P = 0.75).
Discussion
The present study is the largest study from India providing the clinical profile and etiological details of patients with HCC. Although, our data confirms that viral hepatitis (HBV+HCV) when combined with alcohol is still the commonest etiology of HCC in India; ours is the first study from India showing a trend in the change of etiology of HCC.6, 7, 8,22, 23, 24, 25 The decrease in HBV as the etiology over the years could be related to the awareness, vaccination, and effective treatment. Similarly, HCV becoming static after the initial increase could be related to the effective treatment for HCV. Alcohol-associated liver disease, NAFLD, and their combination are very common in this part of the country26, 27, 28, 29 and could be responsible for the rising incidence of alcohol and NASH as the etiology for HCC. Previous Indian and global experience also indicate NASH as the emerging cause of HCC.6,7,30 Higher prevalence of type 2 diabetes mellitus in our cohort of patients could have also contributed toward overall rise in HCC in patients with both viral and nonviral etiology. In addition, improvement in the radiological modalities and techniques for the diagnosis of HCC over the years could have contributed to the rising number of patients with HCC over last nine years. Consistent with the available literature, majority [677 (86%)] of our patients had evidence of cirrhosis and occurrence of HCC without cirrhosis was mostly related to HBV and NASH.31
Of all the patients in our study, only 173 (22%) patients were in early stage (BCLC 0-A) that could be offered potential curative therapies, whereas other 78% patients were offered either potentially curative or palliative treatment modalities. This calls for an urgent need of new strategies and biomarkers for early diagnosis of HCC in India.
Various treatment modalities are available for patients with HCC depending on the stage of the tumor and status of underlying liver disease.18 Surgical tumor resection and RFA are considered as the curative treatment modalities for patients with HCC in an early stage.32 SR has an advantage of complete excision of tumor tissue and hepatic parenchyma around the tumor. It also allows the analysis of the surgical specimen for pathological aggressiveness. Conversely, RFA is less invasive, safe with low risk of hepatic decompensation and short-term mortality compared with SR.33 For a small tumor with a diameter of less than 3 cm, RFA usually requires only one ablation session to achieve satisfactory results but for HCC of 3–5 cm, complete ablation may require more than one session.34
The 5-year survival rates after RFA have been reported to range from 55 to 77.8%. Various studies suggested that RFA for small HCC ≤3 cm achieved survival rates similar to that of SR.35,36 On the other hand, for lesions of size 3–5 cm, SR has been shown to be is superior to RFA.34 Our results however have shown no difference in OS and better DFS in patients subjected to SR in comparison with those treated with RFA in spite of larger tumor size in the SR group compared with the RFA group. All but one patient (98.6%) in the RFA group had cirrhosis. Since, most of the intrahepatic recurrences were distant recurrences (away from the ablated lesion), underlying cirrhosis could be the main reason for higher intrahepatic recurrence in the RFA group.
Previous studies have reported 5-year postoperative recurrence rate of 50–81% and correlated it with poor survival.37 In our study, most of the recurrences occurred within first two years in both RFA and surgical groups. We observed tumor recurrence in 42% patients within the SR group at 3 years, which is comparable with previous studies.37 The DFS at 3 years was comparatively better in the SR group than in the RFA group (68% vs 37.2%, P < 0.01) of our study. However, as mentioned earlier, the patients in our study were managed in real-life setting and because the two groups were not matched, the results of recurrence and survival need to interpreted in that context.
In spite of the availability of comparative data between RFA and SR, higher number of patients being subjected to RFA than SR in our real-life data could be related to multiple factors including the patient preference and preference by the treating team for an ablative procedure over surgery because of the presence of cirrhosis in large number of patients.
Management of patients with larger tumors (>5 cm) is still debated and aggressive curative therapy is often denied in these patients. Earlier studies looking at outcomes after resection in HCC>5 cm reported 5-year survival ranging from 16.7 to 33%. Although the advent of modern liver surgical techniques and other contemporary methods in past 10 years has led to a favorable outcome in recent studies with a 5-year survival of >50–70% after resection of solitary large HCC (>5 cm).38 In our SR group, 26 (68%) patients had large unifocal tumors (>5 cm) and 23 (60%) patients had cirrhosis liver and had similar 3-year OS and better DFS in comparison with those treated with RFA.
Our study has the strengths of being the largest study on HCC from India; showing for the first time the changing etiology of HCC in India and provides the real-life experience of RFA and SR. The study, however, has inherent limitations of its retrospective design and analysis of real-life data. Patients were included till 2015 to assess the outcome over next three years; hence has missed capturing of the recent clinical profile and etiology of patients. A trend was noticed regarding changing etiology of HCC but the data were limited only to nine years (2007–2015). Even though most patients in the SR group had larger tumor size (>5 cm), surgical techniques used in SR patients were not available for analysis. In addition, separate data on DFS and recurrence was not available as per the status of underlying cirrhosis in both RFA and SR groups. Because the decision for RFA and SR was taken in a real-life setting depending on the tumor stage, underlying liver disease, and patients’ general condition and preference; two modalities cannot be compared based on this noncomparative descriptive data. However, the study does give an insight into the outcome of RFA and SR in a real-life setting.
In conclusion, results of our real-life study show that viral hepatitis (HBV/HCV) with or without alcohol is the commonest risk factor for HCC in Northern India; nonviral causes of NASH and alcohol are increasing over the years. Most patients with HCC have underlying cirrhosis; NASH and HBV are predominant causes in noncirrhotic patients. In a real-life setting, in patients with unifocal HCC, there is no difference in overall 3-year survival subjected to SR or RFA with better DFS in the SR group.
CRediT authorship contribution statement
Suneel Tohra: Formal analysis, Writing - original draft, Writing - review & editing. Ajay Duseja: Conceptualization, Patient management, critical revision of manuscript, Writing - review & editing. Sunil Taneja: Patient management, critical revision of manuscript. Naveen Kalra: Patient management, critical revision of manuscript. Ujjwal Gorsi: Patient management, critical revision of manuscript. Arunanshu Behera: Patient management, critical revision of manuscript. Lileswar Kaman: Patient management, critical revision of manuscript. Divya Dahiya: Patient management, critical revision of manuscript. Srimanta Sahu: Formal analysis, Patient management. Balkrishan Sharma: Formal analysis, Writing - review & editing. Virendra Singh: Patient management, critical revision of manuscript. Radha K. Dhiman: Patient management, critical revision of manuscript. Yogesh Chawla: Patient management, critical revision of manuscript.
Conflicts of interest
The authors have none to declare.
Funding
None.
References
- 1.Balogh J., Victor D., 3rd, Asham E.H., et al. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016 5;3:41–53. doi: 10.2147/JHC.S61146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang Y.Q., Wang Z.X., Deng Y.N., Yang Y., Wang G.Y., Chen G.H. Efficacy of hepatic resection vs. Radiofrequency ablation for patients with very-early-stage or early-stage hepatocellular carcinoma: a population-based study with stratification by age and tumor size. Front Oncol. 2019;9:113. doi: 10.3389/fonc.2019.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGlynn K.A., London W.T. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis. 2011;15:223–243. doi: 10.1016/j.cld.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Serag H.B. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raihan R., Azzeri A., H Shabaruddin F., Mohamed R. Hepatocellular carcinoma in Malaysia and its changing trend. Euroasian J Hepato-Gastroenterol. 2018;8:54–56. doi: 10.5005/jp-journals-10018-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duseja A., Sharma B., Kumar A., et al. Nonalcoholic fatty liver in a developing country is responsible for significant liver disease. Hepatology. 2010;52:2248–2249. doi: 10.1002/hep.23838. [DOI] [PubMed] [Google Scholar]
- 7.Kumar A., Acharya S.K., Singh S.P., et al. INASL task-force on hepatocellular carcinoma. 2019 update of Indian national association for study of the liver consensus on prevention, diagnosis, and management of hepatocellular carcinoma in India: the puri II recommendations. J Clin Exp Hepatol. 2020;10:43–80. doi: 10.1016/j.jceh.2019.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sood A., Midha V., Goyal O., Goyal P., Sood N., Sharma S.K. Profile of hepatocellular carcinoma in a tertiary care hospital in Punjab in northern India. Indian J Gastroenterol. 2014;33:35–40. doi: 10.1007/s12664-013-0373-7. [DOI] [PubMed] [Google Scholar]
- 9.Xie H., Yu H., Tian S., et al. MEIS-1 level in unresectable hepatocellular carcinoma can predict the post-treatment outcomes of radiofrequency ablation. Oncotarget. 2018;9:15252–15265. doi: 10.18632/oncotarget.24165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rekik S., Allaire M., Mumana A., et al. Transient elastography predicts survival after radiofrequency ablation of hepatocellular carcinoma developing on cirrhosis. J Gastroenterol Hepatol. 2020;35:142–150. doi: 10.1111/jgh.14763. [DOI] [PubMed] [Google Scholar]
- 11.Pan T., Mu L.W., Wu C., et al. Comparison of combined transcatheter arterial chemoembolization and CT-guided radiofrequency ablation with surgical resection in patients with hepatocellular carcinoma within the up-to-seven criteria: a multicenter case-matched study. J Cancer. 2017;8:3506–3513. doi: 10.7150/jca.19964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chong C.C., Chan A.W., Wong J., et al. Albumin-bilirubin grade predicts the outcomes of liver resection versus radiofrequency ablation for very early/early stage of hepatocellular carcinoma. Surgeon. 2018;16:163–170. doi: 10.1016/j.surge.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Uhlig J., Sellers C.M., Stein S.M., Kim H.S. Radiofrequency ablation versus surgical resection of hepatocellular carcinoma: contemporary treatment trends and outcomes from the United States National Cancer Database. Eur Radiol. 2019;29:2679–2689. doi: 10.1007/s00330-018-5902-4. [DOI] [PubMed] [Google Scholar]
- 14.Kim J.M., Kang T.W., Kwon C.H., et al. Single hepatocellular carcinoma ≤ 3 cm in left lateral segment: liver resection or radiofrequency ablation? World J Gastroenterol. 2014;20:4059–4065. doi: 10.3748/wjg.v20.i14.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S., Kang T.W., Cha D.I., et al. Radiofrequency ablation vs. surgery for perivascular hepatocellular carcinoma: propensity score analyses of long-term outcomes. J Hepatol. 2018;69:70–78. doi: 10.1016/j.jhep.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Shimagaki T., Yoshizumi T., Harimoto N., et al. MicroRNA-125b expression and intrahepatic metastasis are predictors for early recurrence after hepatocellular carcinoma resection. Hepatol Res. 2018;48:313–321. doi: 10.1111/hepr.12990. [DOI] [PubMed] [Google Scholar]
- 17.Yamashita Y.I., Imai K., Yusa T., et al. Microvascular invasion of single small hepatocellular carcinoma ≤3 cm: predictors and optimal treatments. Ann Gastroenterol Surg. 2018;2:197–203. doi: 10.1002/ags3.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sucandy I., Cheek S., Golas B.J., Tsung A., Geller D.A., Marsh J.W. Long term survival outcomes of patients undergoing treatment with radiofrequency ablation for hepatocellular carcinoma and metastatic colorectal cancer liver tumors. HPB. 2016 Sep;18:756–763. doi: 10.1016/j.hpb.2016.06.010. (Oxford) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shindoh J., Kawamura Y., Kobayashi Y., et al. Time-to-Interventional failure as a new surrogate measure for survival outcomes after resection of hepatocellular carcinoma. J Gastrointest Surg. 2019 Jun 12 doi: 10.1007/s11605-019-04277-y. [Epub ahead of print] PubMed PMID: 31190124. [DOI] [PubMed] [Google Scholar]
- 20.Guglielmi A., Ruzzenente A., Conci S., Valdegamberi A., Iacono C. How much remnant is enough in liver resection? Dig Surg. 2012;29:6–17. doi: 10.1159/000335713. [DOI] [PubMed] [Google Scholar]
- 21.Llovet J.M., Brú C., Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 22.Kumar R., Saraswat M.K., Sharma B.C., Sakhuja P., Sarin S.K. Characteristics of hepatocellular carcinoma in India: a retrospective analysis of 191 cases. QJM. 2008;101:479–485. doi: 10.1093/qjmed/hcn033. [DOI] [PubMed] [Google Scholar]
- 23.Saini N., Bhagat A., Sharma S., Duseja A., Chawla Y. Evaluation of clinical and biochemical parameters in hepatocellular carcinoma: experience from an Indian center. Clin Chim Acta. 2006;371:183–186. doi: 10.1016/j.cca.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 24.Bhattacharyya G.S., Babu K.G., Malhotra H., Ranade A.A., Murshed S., Datta D. Hepatocellular carcinoma in India. Chin Clin Oncol. 2013;2:41. doi: 10.3978/j.issn.2304-3865.2013.09.05. [DOI] [PubMed] [Google Scholar]
- 25.Paul S.B., Chalamalasetty S.B., Vishnubhatla S., et al. Clinical profile, etiology and therapeutic outcome in 324 hepatocellular carcinoma patients at a tertiary care center in India. Oncology. 2009;77:162–171. doi: 10.1159/000231886. [DOI] [PubMed] [Google Scholar]
- 26.Duseja A., Najmy S., Sachdev S., et al. High prevalence of non-alcoholic fatty liver disease among healthy male blood donors of urban India. JGH Open. 2019;3:133–139. doi: 10.1002/jgh3.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duseja A., Singh S.P., Saraswat V.A., et al. Non-alcoholic fatty liver disease and metabolic syndrome-position paper of the Indian national association for the study of the liver, endocrine society of India, Indian college of cardiology and Indian society of gastroenterology. J Clin Exp Hepatol. 2015;5:51–68. doi: 10.1016/j.jceh.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehta M., Satsangi S., Duseja A., Taneja S., Dhiman R.K., Chawla Y. Can alcoholic liver disease and nonalcoholic fatty liver disease Co-exist? J Clin Exp Hepatol. 2017;7:121–126. doi: 10.1016/j.jceh.2017.01.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jain D., Nayak N.C., Saigal S. Hepatocellular carcinoma in nonalcoholic fatty liver cirrhosis and alcoholic cirrhosis: risk factor analysis in liver transplant recipients. Eur J Gastroenterol Hepatol. 2012;24:840–848. doi: 10.1097/MEG.0b013e3283534b40. [DOI] [PubMed] [Google Scholar]
- 30.Younossi Z., Stepanova M., Ong J.P., et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol. 2019;17:748–755. doi: 10.1016/j.cgh.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 31.Gawrieh S., Dakhoul L., Miller E., et al. Characteristics, aetiologies and trends of hepatocellular carcinoma in patients without cirrhosis: a United States multicentre study. Aliment Pharmacol Ther. 2019 Oct;50:809–821. doi: 10.1111/apt.15464. [DOI] [PubMed] [Google Scholar]
- 32.Hemming A.W., Cattral M.S., Reed A.I., Van Der Werf W.J., Greig P.D., Howard R.J. Liver transplantation for hepatocellular carcinoma. Ann Surg. 2001;233:652–659. doi: 10.1097/00000658-200105000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohkam K., Dumont P.N., Manichon A.F., et al. No-touch multibipolar radiofrequency ablation vs. surgical resection for solitary hepatocellular carcinoma ranging from 2 to 5 cm. J Hepatol. 2018;68:1172–1180. doi: 10.1016/j.jhep.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 34.Kalra N., Kang M., Bhatia A., et al. Role of radiofrequency ablation in unresectable hepatocellular carcinoma: an Indian experience. Indian J Radiol Imaging. 2013;23:139–144. doi: 10.4103/0971-3026.116569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng Z.W., Liu F.R., Ye S., et al. Radiofrequency ablation versus open hepatic resection for elderly patients (> 65 years) with very early or early hepatocellular carcinoma. Cancer. 2013;119:3812–3820. doi: 10.1002/cncr.28293. [DOI] [PubMed] [Google Scholar]
- 36.Lei J.Y., Wang W.T., Yan L.N., Wen T.F., Li B. Radiofrequency ablation versus surgical resection for small unifocal hepatocellular carcinomas. Medicine. 2014;93:e271. doi: 10.1097/MD.0000000000000271. (Baltimore) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Truty M.J., Vauthey J.N. Surgical resection of high-risk hepatocellular carcinoma: patient selection, preoperative considerations, and operative technique. Ann Surg Oncol. 2010;17:1219–1225. doi: 10.1245/s10434-010-0976-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crissien A.M., Frenette C. Current management of hepatocellular carcinoma. Gastroenterol Hepatol. 2014;10:153–161. (N Y) [PMC free article] [PubMed] [Google Scholar]