Abstract
The synthetic cathinones are derived from the naturally occurring drug cathinone found in the khat plant (Catha edulis) and have chemical structures and neurochemical consequences similar to other psychostimulants. This class of new psychoactive substances (NPS) also has potential for use and abuse coupled with a range of possible adverse effects including neurotoxicity and lethality. This review provides a general background of the synthetic cathinones in terms of the motivation for and patterns and demographics of their use as well as the behavioral and physiological effects that led to their spread as abused substances and consequent regulatory control. This background is followed by a review focusing on their rewarding and aversive effects as assessed in various preclinical animal models and the contribution of these effects to their self-administration (implicating their use and abuse potential). The review closes with an overview of the consequences of synthetic cathinone use and abuse in terms of their potential to produce neurotoxicity and lethality. These characterizations are discussed in the context of other classical psychostimulants.
Keywords: New psychoactive substances (NPS), Synthetic cathinones, “Bath salts”, Drug abuse, Neurotoxicity, Lethality
1. Introduction
“New psychoactive substances” (NPS) is a relatively new term used to describe analogues of traditionally abused drugs such as cannabinoids, hallucinogens and psychostimulants. Many of these new drugs are both rewarding and aversive, have abuse liability, are potentially toxic and lethal and pose an imminent threat to public health. What places a drug in this grouping is not uniformly accepted or defined, as such there are multiple ways in which this assignment has been made. Some classify new psychoactive substances as “substances of abuse, either in a pure form or a preparation, that are not controlled by the 1961 Single Convention on Narcotic Drugs or the 1971 Convention on Psychotropic Substances” (UNODC, 2014; see also Pirona et al., 2017). The extent to which drugs fit this definition changes over time as new substances come into more widespread use and under regulatory scrutiny. Yet others classify NPS as drugs with abuse potential that have recently emerged in popularity in the illicit market (the compounds themselves may not actually be novel; the word ‘new’ refers to their recent introduction and not necessarily their recent creation) (Perrone, 2016). Finally, others refer to these compounds as those that (while not necessarily new) are deliberately designed to mimic existing scheduled drugs of abuse with the intention of bypassing legal regulations (Baumann et al., 2014; Brunt et al., 2017; Karila et al., 2015). These assignments are not necessarily mutually exclusive, but independent of the basis for their classification, there is a general consensus as to the various drugs that fit within this grouping. For example, such drugs include many synthetic cannabinoids, phenethylamines, tryptamines, aminonindanes, arylcyclohexylamines, novel benzodiazepines, novel opioids, plant-based compounds such as kratom, piperazines and synthetic cathinones (Assi et al., 2017; Brunt et al., 2017; UNODC, 2014). In 2015, the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) reported the emergence of 560 new psychoactive substances, a number that increased to 620 by 2016 (EMCDDA, 2016). In 2016, the rate that new NPS were being introduced was on the order of one new compound per week (EMCDDA, 2017). Although the number of new drugs being introduced had slowed by 2017, there is no evidence that overall NPS availability has changed (EMCDDA, 2017).
1.1. Background: the synthetic cathinones
The present review focuses on one class of NPS, the synthetic cathinones. Cathinone itself is a naturally-occurring psychoactive compound found in the khat plant (Catha edulis), a small leafy green shrub native to East Africa and the Arabian Peninsula (Capriola, 2013; Coppola and Mondola, 2012; DeRuiter et al., 1994). Cathinone is at its highest concentrations in young and fresh khat leaves, and it rapidly degrades as the leaves begin to wilt (Glennon et al., 1995; Kalix, 1992). Khat leaves are either chewed or brewed in teas for their stimulant effects, and its use was initially reported in areas in which the small Catha edulis tree naturally grows, e.g., Kenya and Ethiopia (Coppola and Mondola, 2012; DeRuiter et al., 1994; Kalix, 1992).
Cathinone, the β-ketone analogue of amphetamine, is often referred to as a “natural amphetamine” due to its similar chemical structure and behavioral effects (see Fig. 1). Synthetic cathinones were originally marketed using other labels to avoid regulatory controls and were initially sold as “bath salts”, although the terms “plant food”, “carpet cleaner” or “stain remover” have also been used to hide their actual intended use as intoxicants (Fratantonio et al., 2015; Schneir et al., 2014). In the early 21st century, these products could easily be purchased over the internet or in small physical retail locations such as “head shops” or “smart shops”, smoke shops, convenience stores, gas stations and adult book stores (Benschop et al., 2017; DEA, 2014; Ross et al., 2011; Spiller et al., 2011). These items are often labeled “not for human consumption”, “research chemicals” or “for external use only” as a way also to circumvent legal restrictions (DEA, 2014; Fratantonio et al., 2015; Ross et al., 2011; Schneir et al., 2014). A number of synthetic cathinones were made illegal in the United States beginning in 2011 (DEA, 2017, 2013, 2011), as well as in Europe and many other nations during this period, which eliminated the “legal” sale of these drugs at “brick and mortar” stores, but internet sales persist. Because of their initial legal status, they were also known as “legal highs” and have often been presumed by users to be safe alternatives to other popularly abused stimulants, e.g., cocaine and amphetamine (DEA, 2014; Prosser and Nelson, 2012). Further, each product packet could contain differing amounts of drug, or even types of drugs, than specified on their labels, meaning users may not always know what they are consuming or how much (Brandt et al., 2010; Schneir et al., 2014; Spiller et al., 2011). This problem continues, of course, now that these drugs are generally illegal, but this is certainly not limited to the synthetic cathinones. Indeed, an analysis of hair samples from self-reported “ecstasy” (3,4-methylenedioxymethamphetamine; MDMA) users found the presence of a wide variety of cathinones and other amphetamine derivatives (Kalasinsky et al., 2004; Palamar et al., 2016). Many of these individuals did not test positive for MDMA at all, but did test positive for one or more cathinones, especially methylone and butylone.
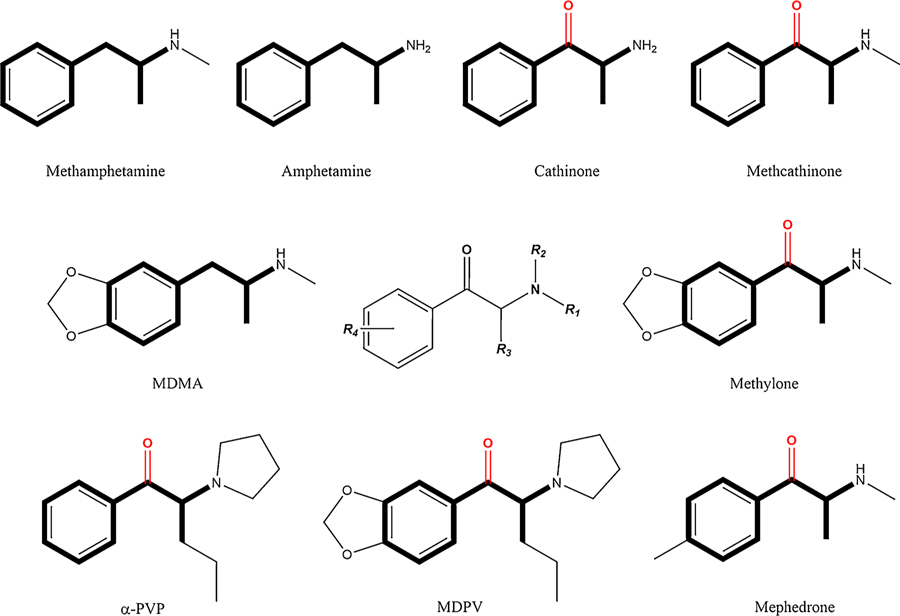
Fig. 1.
Chemical structures of amphetamines and synthetic cathinones (and their common pharmacophore) generated by lengthening of the alkyl chain, expansion of the nitrogen terminus and/or ring substitution. Synthetic cathinones have an oxygen addition at the β-carbon position (β-ketone).
One of the issues with increased synthetic cathinone use, either knowingly or unknowingly, is the reported potential of these compounds for adverse effects that can include agitation, combative violent behavior, tachycardia, palpitations, chest pain, hallucinations, paranoia, confusion, myoclonus, hypertension, mydriasis, vomiting, hyperthermia, seizures and death (DEA, 2014; James et al., 2011; Marinetti and Antonides, 2013; Spiller et al., 2011). These drugs initially began to appear in reports from the Centers for Disease Control (CDC) and emergency rooms across the United States in 2010, at which time the three primary cathinone products detected were 3,4-methylenedioxypyrovalerone (MDPV), 4-methylmethcathinone (mephedrone; 4-MMC), and 3,4-methylenedioxymethcathinone (methylone; MDMC) (Karch, 2015). MDPV was initially the most prevalent cathinone found in “bath salt” products in the United States, while in Europe these compounds tended to contain mephedrone (German et al., 2014). The rapidity with which these drugs appeared in the wider drug-using population and the magnitude of their adverse effects resulted in emergency Drug Enforcement Administration (DEA) scheduling for these three synthetic cathinones in 2011, and just a year later, they were classified as Schedule 1 (Baumann et al., 2013a; DEA, 2014, 2013, 2011; German et al., 2014). Since this classification, reports from poison control centers involving these specific compounds have decreased significantly, although given the abovementioned discussion of the relative purity of “bath salts” products, it may be difficult to make such conclusions. This reduced availability has resulted in a wide array of “replacement” compounds in which slight chemical modifications have been made in order to side-step law enforcement, resulting in a variety of synthetic cathinones including 4-methyl-α-pyrrolidinopropiophenone (4′-MePPP), 4-methylethcathinone (4-MEC), 4- methylenedioxybutiophenone (MDPBP), butylone, pentylone, pentedrone, 3-fluoromethcathinone (3-FMC), 4-fluoromethcathinone (4-FMC, flephedrone), α-pyrrolidinopropiobutiophenone (α-PBP), α-pyrrolidinoenanthophenone (α-PEP), α-pyrrolidinohexiophenone (α-PHP), α-pyrrolidinovalerophenone (α-PVP) and α-pyrrolidinopropiophenone (α-PPP) (for more complete lists of synthetic cathinones, see Baumann, 2014; Brunt et al., 2017; Gatch et al., 2017, 2015a; Karila et al., 2017; Naylor et al., 2015; Paillet-Loilier et al., 2014; Watterson and Olive, 2014; Zawilska and Wojcieszak, 2017). In 2014, the DEA temporarily placed an additional 10 synthetic cathinones into Schedule I, including many of the most popular second-generation “bath salts” (see also, DEA, 2016). In 2017, the DEA filed a ruling to permanently place these in Schedule I. As of 2017, the number of synthetic cathinones that had been reported in the European Union was 118, with new ones still being introduced, although at a slower rate than in prior years (EMCDDA, 2017).
1.2. Motivations for use
Currently, there are a diverse set of reasons for why and how the synthetic cathinones are used. For example, they are taken to enhance social and sexual experiences often in lieu of more expensive drugs of abuse such as cocaine and amphetamines (German et al., 2014). Doses vary from 1 to 200 mg, depending on the specific cathinone and its route of administration, but they have been reported to be most commonly used orally (either sublingually or swallowing) or nasally (insufflation, ‘snorting’) (Zawilska et al., 2017). A recent report from Hungary found a range of cathinones including pentedrone, mephedrone, α-PVP, α-PHP and α-PEP (as well as opiates) in discarded intravenous drug injection paraphernalia sampled from 2015 to 2016 (Gyarmathy et al., 2017). From these data, it would appear that individuals are not only intravenously injecting the cathinones, but that they are in at least some places combining opiates with these compounds, as they have previously done with opiates and other stimulants. Several reports have recently warned about the spread of intravenous synthetic cathinone use (EMCDDA, 2014; Hope et al., 2016; Kelly et al., 2013; Kirby and Thornber-Dunwell, 2013; Péterfi et al., 2014; Peyrière et al., 2013). Interestingly, “slamming” (the intravenous injection of drugs of abuse), has recently been applied to the synthetic cathinones. For example, mephedrone, sometimes mixed with other drugs, such as gamma-hydroxybutyric acid (GHB) or methamphetamine (METH), has been reported to be used during so-called “chemsex” parties, which is a new trend in sexual relations focused on enhancing sexual experiences through the use of psychoactive substances (Daskalopoulou et al., 2014; Dolengevich-Segal et al., 2016; McCall et al., 2015). Other routes of administration include vaping or smoking, rectal insertion, intramuscular injection and less commonly “eyeballing” (dropping a synthetic cathinone solution directly onto the surface of the eye) (Assi et al., 2017; Johnson and Johnson, 2014; Paillet-Loilier et al., 2014; Zawilska and Wojcieszak, 2017). The way in which these compounds are consumed is also worth noting. For example, mephedrone consumption patterns have been reported as binge-like with multiple doses within a few hours and usually in social settings (e.g., friends’ homes, house parties, night clubs) (German et al., 2014). Notable factors of specific use motivation were found when 419 regular psychostimulant users in Australia were interviewed to assess their basis for synthetic cathinone use. While there were differences in the fundamental motivations for NPS use between different classes of NPS [see Table 2 in Sutherland et al. (2017) for complete results], the top two synthetic cathinone use factors were availability and affordability.
1.3. User demographics
The demographics of synthetic cathinone use are critical to understanding the dynamics of their diffusion into the drug marketplace and their abuse potential among vulnerable populations. A common finding of reports assessing the demographics of the synthetic cathinone user base is that the participants surveyed tend to be young and male (Carhart-Harris et al., 2011; Johnson and Johnson, 2014; Matthews et al., 2017; Orsolini et al., 2015; Winstock et al., 2011). An analysis of NPS online threads was recently conducted and resulted in the confirmation of an active online community of users sharing knowledge on synthetic cathinone use, effects, drug-drug interactions and psychological experiences (Assi et al., 2017). In another review of data compiled from six EU countries, the online community was found to be made up of young adults between the ages of 18–25; however, the user profile of the nightlife scene was most likely to be older with an age range of 18–35 years of age (Benschop et al., 2017). A synthetic cathinone user base was also found in the electronic dance music (EDM) attendee population in which these substances (similar to MDMA) were used to enhance the musical experience (Mohr et al., 2018). Although a national study found a very low prevalence rate of cathinone use among high school seniors, the extremely small population of students that self-reported “bath salt” use were also users of other drugs (Palamar, 2015). Such co-use of recreational drugs was reported in a survey paired with biological specimen sampling that was conducted at a large EDM festival in Miami, Florida, in which a prevalence of both α-PVP and ethylone was confirmed along with a variety of other illicit substances (Mohr et al., 2018). Supporting the initial concern for the abuse potential of the synthetic cathinones were data from the National Drug Intelligence Center which indicated that while in 2009 there were no recorded “bath salts” incidents, in 2010 there were 302 reports, and in the first five months of 2011 alone there were 2237 (US Department of Justice, 2011). Importantly, a vulnerable population may be illicit drug users that do not have a direct intention to use synthetic cathinones but instead receive tablets that contain these compounds, e.g., α-PVP sold as ecstasy (Zawilska and Andrzejczak, 2015; for a description of the analysis of samples purchased and examined from top online shops based throughout five EU countries, see Brunt et al., 2017). A recent investigation by Johnson and Johnson (2014) that surveyed 113 individuals revealed that 50% of those using “bath salts” met DSM-V criteria for substance use disorder.
1.4. Structural and functional relationships
The use and abuse of synthetic cathinones are not surprising in light of their chemical structure and pharmacological activity. As noted above, cathinone (and its synthetic derivatives) share a common phenethylamine pharmacophore with the psychostimulant amphetamine (Banks et al., 2014), and like amphetamine they increase levels of brain monoamines such as norepinephrine (NE), serotonin (5-HT) and dopamine (DA), thought to be involved in the stimulant and rewarding effects of a number of drugs of abuse (Baumann et al., 2013a; Koob and Volkow, 2016; López-Arnau et al., 2012; Watterson and Olive, 2014). Although all synthetic cathinones have a carbonyl group (=O) at the β-carbon (β-ketone addition; see Fig. 1), other variations in the basic chemical structure of the phenethylamine pharmacophore generate a wide array of compounds that differ in a number of ways, including the expansion/modification on the nitrogen terminus (R1 and/or R2), the length of the alkyl chain at the α-carbon (R3) and a number of different ring substitutions (R4) (Banks et al., 2014). These variations (along with the β-ketone addition itself) have been reported to impact the mechanism of action of specific synthetic cathinones (monoamine release and/or monoamine reuptake inhibition) as well as their relative selectivity for the dopamine, norepinephrine and serotonin transporters (DAT, NET and SERT, respectively), e.g., shortening the alkyl chain and abbreviation or expansion of the pyrrolidine moiety reduce DAT activity (Kolanos et al., 2015b; for a review, see Glennon and Young, 2016; for a demonstration of the effects of such variations on hyperthermia, see Grecco and Sprague, 2016; for pharmacological characterization of synthetic cathinones, see Eshleman et al., 2017; Simmler et al., 2014, 2013).
It has also been reported that small structural changes of the phenethylamine pharmacophore are capable of modifying the affinity of synthetic cathinones for the trace amine associated receptor 1 (TAAR1), a receptor involved in the regulation of dopaminergic activity. Unlike amphetamines, most of the synthetic cathinones do not appear to have affinity for TAAR1 (Simmler et al., 2016); however, a sub-micromolar affinity was observed for 2,3- and 2,4-dimethylmethcathinone (Luethi et al., 2017a).
The fact that synthetic cathinones are self-administered by humans and act on neurotransmitter substrates associated with reward, i.e., the monoamines and particularly DA, suggests that they have some measure of abuse potential (Baumann et al., 2016, 2013a; Glennon, 2014; Glennon and Young, 2016; Hataoka et al., 2017; Negus and Banks, 2016; Simmler et al., 2014; Watterson and Olive, 2016, 2014). Although such data implicate these compounds as drugs of abuse, to more thoroughly assess this potential, particularly given the wide variety of these compounds presently on the illegal drug market and the obvious limitations on such experimentation in humans, it is important to turn to preclinical animal models of drug use and abuse.
2. Self-administration and implications for abuse liability
The model with the most face validity for human drug taking is intravenous self-administration (IVSA; O’Connor et al., 2011; see also Bozarth, 1985). In such models, animals surgically prepared with indwelling catheters for drug delivery are trained to respond, e.g., press a lever, for the infusion of a specific drug. The rate or level of responding is generally concluded to be a function of the rewarding effects of that drug (Sanchis-Segura and Spanagel, 2006; Zernig et al., 2007). Such a procedure allows for assessments of its rewarding effects, the dose-specificity of these effects (via dose-response analyses), the similarity and differences between various other drugs (via drug substitution assessments), the motivation to use the drug through response requirements that assess effort [e.g., progressive ratio (PR) schedules; see below] and the role of drug, stress and various drug-associated cues in reinstatement of drug-taking behavior (i.e., relapse) (see Koob et al., 2014). The basic IVSA design has been widely used since its initial demonstration (see Weeks, 1962), and the high correspondence between IVSA of drugs in animals and the use and abuse of these same compounds in human support the validity of this model for abuse liability (Ator and Griffiths, 2003, 1987; Balster and Lukas, 1985; Fischman, 1988).
Available data on cathinones have shown that they are also reliably self-administered in animal models of drug intake. For example, Johanson and Schuster (1981) reported that l- and dl-cathinone (as well as d-amphetamine) supported IVSA in Rhesus monkeys. Lower doses of l-cathinone than d-amphetamine maintained responding, whereas the response function for dl-cathinone was shifted to the right of that for d-amphetamine, suggestive of a greater potency for the l isomer than d-amphetamine or dl-cathinone. Given that both l- and dl-cathinone generated greater overall responding than d-amphetamine (i.e., displayed greater efficacy), the authors cautioned against general conclusions about relative potency (for a discussion, see Levine, 1990). Follow-up work by Woolverton and Johanson (1984) reported that both dl-cathinone and cocaine were self-administered by Rhesus monkeys when either was given in a choice with saline. When cocaine and dl-cathinone were made available and animals had to choose between these two alternatives, the reinforcing efficacy of the two appeared equivalent, e.g., the dose of cocaine had to be increased to support cocaine IVSA when the dose of dl-cathinone was increased (see also Gosnell et al., 1996 for cathinone self-administration in rats). Subsequent to these demonstrations of IVSA with cathinone, Kaminski and Griffiths (1994) demonstrated that the cathinone derivative, methcathinone (0.01–1 mg/kg/infusion; the β-ketone analogue of METH) supported IVSA in the baboon and at the higher doses (0.1–1 mg/kg/infusion) did so at rates comparable to those supported by cocaine.
Additional work with the more recent derivatives of cathinone has also shown consistent IVSA for most of these drugs (for reviews, see Glennon and Young, 2016; Watterson and Olive, 2016). In one of the first such assessments, Watterson et al. (2012) trained male Sprague-Dawley rats to self-administer methylone at either 0.05, 0.1, 0.2 or 0.5 mg/kg (per infusion) in daily short-access (ShA; 2-h) sessions. A series of long-access sessions (LgA; 6 h/day) followed the ShA exposure to assess escalation of intake that were in turn followed by PR schedules to assess any changes in reinforcer efficacy subsequent to the extended access conditions (for a review of general drug self-administration under extended access and choice conditions, see Ahmed, 2012). As reported, all doses of methylone supported self-administration. Similar to the patterns reported elsewhere with cocaine and amphetamine, methylone self-administration was dose-dependent as rats took a greater number of infusions at the 0.5 mg/kg dose compared to 0.05 and 0.1 mg/kg and more animals met the criterion for drug self-administration as the dose increased. Unlike the patterns seen with other psychostimulants (Kitamura et al., 2006; Roth and Carroll, 2004), however, intake did not escalate during the extended access condition and PR responding did not increase (compared to PR responding prior to extended access). Methylone has also been shown to support self-administration in female Wistar rats at a dose of 0.5 mg/kg/infusion (Creehan et al., 2015). In this work, direct comparisons were made between methylone, mephedrone and MDMA. The levels of methylone self-administered were less than those supported by mephedrone (at 0.5 mg/kg; MDMA did not support IVSA at this dose). To date, no direct comparisons have been made between males and females (see Vandewater et al., 2015 for self-administration of methylone in male Wistar rats). Together, these data support the position that methylone is self-administered, but that it possesses weaker reinforcing efficacy than other psychostimulants but greater than that of MDMA (see also Javadi-Paydar et al., 2017 for comparisons of methylone with pentedrone and pentylone in which methylone was less efficacious; see also Nguyen et al., 2017; Schindler et al., 2016; Vandewater et al., 2015). These relative effects of methylone are possibly related to the significant release of serotonin (and the resulting lower DAT/SERT affinity ratio) that may impact its overall reinforcing effects; see Baumann et al., 2016, 2012; Glennon and Young, 2016; Negus and Banks, 2016; Schindler et al., 2016).
The initial work with methylone has now been extended to a variety of other synthetic cathinones, including mephedrone and MDPV. For example, in one of the initial assessments of mephedrone, Hadlock et al. (2011) noted that male Sprague-Dawley rats infused with mephedrone (at 0.24 mg/kg/infusion) acquired IVSA (4-h sessions for 7–8 days) and responded at higher rates for mephedrone than the same dose of METH. Subsequent to this demonstration, a number of investigations with mephedrone have demonstrated its IVSA under a variety of conditions. For example, Aarde et al. (2013a) reported mephedrone self-administration at 0.5 and 1 mg/kg/infusion in both Sprague-Dawley and Wistar rats. In subsequent dose-response substitution and progressive ratio assessments, the level of responding was dose-dependent. Further, mephedrone dose-dependently substituted for METH in animals trained to self-administer this drug (at 0.1 mg/kg/infusion). These two drugs appeared comparable in the level of self-administration at this dose. Interestingly, Motbey et al. (2013) reported a greater number of drug infusions for mephedrone than METH in adolescent male Sprague-Dawley rats – both at the training dose and in dose-substitution comparisons. It is important to note that a different training dose was used for mephedrone (0.3 mg/kg/infusion) than METH (0.03 mg/kg/infusion), making it somewhat difficult to directly compare reinforcing efficacy (there were also no differences under subsequent PR assessments). As noted above, in direct comparisons between mephedrone and methylone (and at comparable doses; 0.5 mg/kg/infusion) in female Sprague-Dawley rats (Creehan et al., 2015), acquisition was greater for mephedrone than methylone (with no evidence of self-administration for MDMA; see also Vandewater et al., 2015). In assessments of short and long access IVSA (both at 0.5 mg/kg/infusion) in male Wistar rats, mephedrone had a greater reinforcing efficacy than methylone, producing overall greater responding (see Nguyen et al., 2017). No differences were evident in short-access sessions. In this report, mephedrone had a comparable reinforcing efficacy to METH in PR dose-response drug substitution tests (animals tested for METH in mephedrone-trained animals); however, METH was reported to be approximately 10 times more potent than mephedrone.
The synthetic cathinone that has received the most attention in relation to its self-administration has been MDPV. In one of the first assessments of IVSA of MDPV, Aarde et al. (2013b) reported that MDPV supported self-administration (at 0.05 mg/kg/infusion; there was no significant difference between MDPV and METH in the acquisition of IVSA at the same dose). However, in subsequent dose-response and progressive ratio assessments, MDPV generated greater responding than METH, indicating greater reinforcing efficacy. Similar findings have been reported by Watterson et al. (2014a) who demonstrated that male Sprague-Dawley rats dose-dependently acquired MDPV IVSA at 0.05, 0.1 and 0.2 mg/kg/infusion. In subsequent progressive ratio assessments, MDPV and METH generated comparable levels of responding at 0.05 mg/kg/infusion (only a single dose of METH was tested making assessments of potency and efficacy limited). Responding escalated for both MDPV and METH under long-access conditions (6 h). Recently, Schindler and colleagues (2016) assessed MDPV self-administration in male Sprague-Dawley rats and directly compared levels of self-administration generated by MDPV (0.03 mg/kg/infusion) to those of cocaine (0.5) and methylone (0.3). From analyses in acquisition and dose-response assessments, MDPV and cocaine appeared to have greater efficacy than methylone and MDPV was more potent than cocaine (peak levels were similar for MDPV and cocaine at 0.01 and 0.1 mg/kg/infusion, respectively; see also Simmons et al., 2016). Assessments of efficacy and potency between MDPV, cocaine and METH (see Gannon et al., 2017b) parallel those of Schindler et al. and reveal that MDPV is more efficacious than both cocaine and METH (as assessed via PR responding). Further, the authors concluded that MDPV was approximately 10 times more potent than cocaine and approximately 2 times more potent than METH, although, as noted, there were differences in overall efficacy that may preclude such conclusions (Levine, 1990). Additional work with MDPV has supported its clear reinforcing effects in IVSA (see Gannon et al., 2018, 2017c; Sewalia et al., 2017).
These initial descriptions have focused on cathinone itself and some of the first generation synthetic derivatives. The data, in general, confirm their abuse liability as reflected in the fact that they all support self-administration. Subsequent to these initial investigations, other synthetic cathinones have been examined, including α-PVP, α-piperidinopropiophenone (PIPP), α-piperidinopentiothiophenone (PIVT), α-PHP, α-pyrrolidinopentiothiophenone (α-PVT), 2-cyclohexyl-2-(methylamino)-1-phenylethanone (MACHP), 2-(methylamino)-1-phenyloctan-1-one (MAOP), 4-MePPP, 4-MEC, pentedrone and pentylone. The most studied of these compounds is α-PVP, and like the earlier assessments it is also reinforcing and supports IVSA. For example, Aarde and his colleagues (2015a) have recently demonstrated that in male Wistar rats α-PVP and MDPV IVSA (both at 0.05 mg/kg/infusion) were acquired at comparable rates (with the same percentage of animals acquiring the response) and the two compounds generated functionally identical dose-response substitution patterns with no statistical differences in potency or efficacy (see also Gannon et al., 2017c for similar findings identifying the S isomer of each as the active constituent).
Subsequently, Huskinson et al. (2017) examined α-PVP substitution in male Sprague-Dawley rats trained to self-administer 0.05 mg/kg/infusion METH (and compared α-PVP responding to those of two other synthetic cathinones, 4-MEC and 4-MePPP). In these comparisons, α-PVP generated levels of responding comparable to 4-MePPP (although at lower doses, which is suggestive of greater potency). In turn, α-PVP produced greater responding than 4-MEC and METH (suggestive of greater reinforcing efficacy). These conclusions were supported by demand functions in which animals had to respond at higher levels to obtain infusions. These differences were discussed in terms of the differential effects on 5-HT release by METH and 4-MEC that may have reduced the overall reinforcing effects of these compounds relative to α-PVP and 4-MEPPP that appear to have limited effects on 5-HT reuptake or release. Work with other second generation cathinones substantiate their reinforcing effects. For example, Cheong et al. (2017) reported dose-dependent IVSA of α-PVT (an analog of α-PVP) in male Sprague-Dawley rats (maximal rates at 0.3 mg/kg/infusion; weaker, though significant self-administration, was evident at 0.1 and 1 mg/kg/infusion, an inverted dose-response function characteristic of other psychostimulant IVSA). Dose-dependent progressive ratio responding was also evident (highest at 1 mg/kg/infusion). Interestingly, α-PVT IVSA was greater than that reported for the positive control METH (trained at 0.05 mg/kg/infusion); however, direct comparisons were not made and the METH assessment was made at only a single dose.
Javadi-Paydar et al. (2017) have recently focused on how modification of the methylenedioxy substitution on the aromatic ring (generating pentylone and methylone) as well as extension of the α-alkyl chain (associated with pentedrone and pentylone) affect the relative reinforcing effects of the synthetic cathinones. They report that in female Wistar rats the reinforcing efficacy in IVSA was pentedrone = pentylone > methylone, suggestive of the importance of α-alkyl extension. Pentedrone, pentylone and methylone IVSA was less than that for α-PVP and α-PHP, suggestive of the importance of the pyrrolidine motif associated with these latter compounds. Botanas et al. (2017a) has recently examined the effects of changes in the alpha-carbon position by examining different IVSA rates with MACHP and MAOP in male Sprague-Dawley rats. In this assessment, MAOP was without effect (from 0.1–3 mg/kg/infusion). Although MACHP supported self-administration (at 1 mg/kg/infusion), these levels were substantially lower than those supported by METH (at 0.1 mg/kg/infusion). Interestingly, Botanas et al. (2017c) failed to find any IVSA of two synthetic cathinones with piperidine ring substitutions on nitrogen, i.e., PIPP or PIVT (0.1–3 mg/kg/infusion), in male Sprague-Dawley rats (METH was consistently self-administered at high levels at 0.1 mg/kg/infusion). While MAOP (Botanas et al., 2017a) and PIPP and PIVT (Botanas et al., 2017c) did not support IVSA, they did condition place preferences (in male ICR mice), suggestive of rewarding effects (see below). Finally, Botanas et al. (2017b) recently reported that the novel synthetic cathinone, 2-(methylamino)-1-(naphthalen-2-yl) propan-1-one (BMAPN), with a naphthalene substitution on the aromatic ring, induced modest IVSA in male Sprague-Dawley rats (at 0.3 mg/kg/infusion). For related work assessing modification of the α-alkyl side chain and methylenedioxy moiety on the reinforcing effects of the synthetic cathinones, see Gannon et al. (2017a).
The focus of the foregoing review of the synthetic cathinones has been on their ability to support IVSA and their relative effects (and potency) in this preparation. Under various procedures, e.g., acquisition, dose-response determinations, drug substitutions and progressive ratio assessments and with various doses during acquisition and substitution testing, these compounds are generally reinforcing as indexed by their self-administration. With that said, it is nonetheless difficult to make direct comparisons among the various synthetic cathinones given that many of these assessments were made in different studies, with different species and strains, under different behavioral preparations, with different training and testing doses and with varying specificities of statistical analyses. More direct work needs to be done to establish relationships among the various compounds (and at comparable doses or with extended dose-response functions) that will allow statistical evaluations of relative potencies (ED50) and efficacies (Emax). Further, although work examining the effects of various manipulations on the relative abuse potentials of these compounds has begun (e.g., see Creehan et al., 2015; Gannon et al., 2018, 2017d; Hicks et., al., 2018; Nguyen et al., 2017; Simmons et al., 2017; Vandewater et al., 2015), such assessments are relatively few and additional work is needed to determine the role of a host of factors, e.g., sex, age, strain, structure, drug history and drug interactions, on the reinforcing effects of the synthetic cathinones (see below).
2.1. Reward and aversion in abuse vulnerability
Traditionally, drug taking in animals (as assessed in self-administration models) has been assumed to reflect the drug’s positive (rewarding) effects, an assumption supported by the fact that in a host of other models of drug reward, e.g., intracranial self-stimulation (ICSS), drug discrimination learning (DDL), conditioned place preferences (CPP) (see below; see also Bozarth, 1987), these same drugs appear to be rewarding (Koob et al., 2014). Although certainly important to the initiation and maintenance of drug-taking behavior, the rewarding effects of a drug are not the only stimulus effects produced. In fact, many drugs of abuse are not only rewarding, but are also aversive, as indexed by their ability to induce a conditioned taste avoidance (CTA; see Freeman and Riley, 2009).
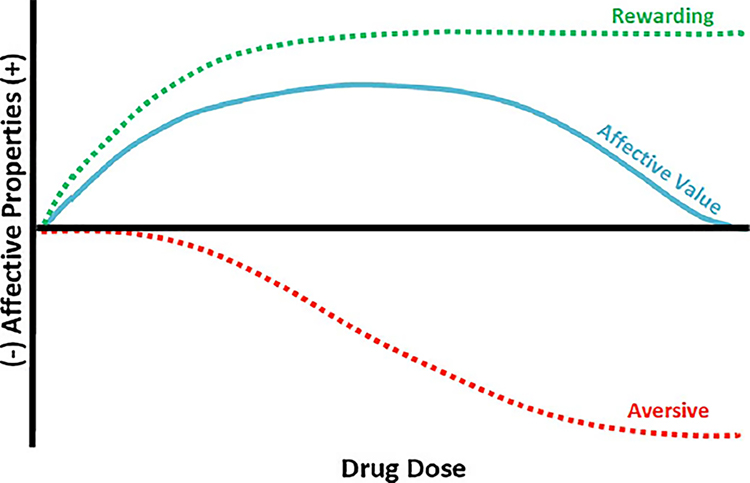
Thus, somewhat paradoxically (Goudie, 1979; Hunt and Amit, 1987), most drugs of abuse appear to have both rewarding and aversive effects which immediately raises the issue of whether these two effects are both important in affecting abuse liability (Riley, 2011; Stolerman and D’Mello, 1981; Verendeev and Riley, 2013). As described, traditionally it is assumed that the major factor in drug self-administration (or human drug intake) is the drug’s rewarding (and positive) effects. The fact that drugs also have aversive effects suggests that this factor, too, may be important in drug-taking behavior, in this case a limiting factor in that intake. This position can be best illustrated as drug intake being a function of the balance between these two affective states (see Fig. 2).
Fig. 2.
A model of the manner by which the balance between the rewarding and aversive effects of a drug might influence the affective value of the drug and its intake. As illustrated the drug produces both dose-dependent rewarding (green-dotted line) and aversive (red-dotted line) effects. The overall affective balance of these effects determines its affective balance (blue-solid line) and the amount of drug self-administered. Changes in the rewarding and aversive effects (resulting from experience with the drug or specific characteristics of the individual) impact a user's likelihood of subsequent use or abuse of the drug. The onset, asymptotic level and offset of these affective properties are likely to be drug specific, all of which will impact the nature of their interaction and its likelihood of use and/or abuse. Reprinted from Riley et al. (2018) with permission from Elsevier (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Understanding and predicting abuse vulnerability, accordingly, necessitates an awareness of each of these properties and the various factors that may influence them and their balance. One immediate response to such demonstrations of a drug’s aversive and rewarding effects, and conclusions about their relative contributions, is that these effects are dependent upon the specific parameters under which they have been reported. Although various parametric factors undoubtedly impact the observation of rewarding and aversive drug effects, direct tests reveal that many drugs of abuse are both rewarding and aversive in the same animal under identical parametric conditions, suggesting that a simple difference in these parametric conditions does not explain these two affective properties (for assessments with amphetamine, see Sherman et al., 1980; Verendeev and Riley, 2011; for caffeine, see Brockwell et al., 1991; for morphine, see Simpson and Riley, 2005; White et al., 1977; for nicotine, see Pomfrey et al., 2015).
If drug use and abuse are a function of the balance of its rewarding and aversive effects, determining these effects for specific drugs and the various factors known to affect them is critical in predicting abuse vulnerability. Factors relevant to use and abuse include concurrent drug intake, age, drug history, species, strain, drug dose, drug duration, route of administration and sex (Cunningham et al., 2009; Riley et al., 2018, 2009). These factors can also interact to alter the subjective effects of drugs (see Schramm-Sapyta et al., 2014), making it clear that individual susceptibility to a particular drug can depend on a variety of overlapping and interacting factors. It is, therefore, important, particularly as we begin to investigate and characterize new synthetic cathinones (and NPS in general), to examine both of these effects and the range of factors that may influence them, in order to determine overall abuse liability.
3. Assessments of the rewarding effects of synthetic cathinones
3.1. Intracranial self stimulation
In this procedure, electrodes are implanted into an animal’s brain (usually into areas such as the medial forebrain bundle that are highly implicated and associated with reward or reinforcement) and the animal is trained to press a lever for pulses of electrical stimulation to those areas (see Fouriezos and Nawiesniak, 1987; Reid, 1987). Threshold determinations are commonly made for the level of stimulation sufficient to maintain responding. Once this threshold is determined, drugs of abuse are injected and changes in the threshold needed to maintain responding for the electrical stimulation is re-determined. Under these conditions, most drugs of abuse lower the threshold for responding, indicative of a summation of the drug’s and electrical brain stimulation’s rewarding effects (it is interesting to note that drugs classified as being aversive in other procedures raise this threshold; see Todtenkopf et al., 2004; Vlachou et al., 2005). The ability of a number of synthetic cathinones to lower the threshold for ICSS (or increase the rate of responding for the stimulation) has been well-established, and we will provide a brief description of these data.
In one of the first such examinations of the synthetic cathinones with this procedure, Robinson et al. (2012) assessed the impact of mephedrone administration on ICSS reward thresholds in male C57BL/6 J mice. In this work, mice were implanted with electrodes in the lateral hypothalamus and brain stimulation reward (BSR) thresholds, response frequencies and maximum response rates were measured before and after a range of doses of mephedrone or cocaine (1, 3 or 10 mg/kg for both drugs). Mephedrone dose-dependently lowered BSR thresholds (and decreased overall response frequency and the maximum response rates – an effect likely due to increased stereotypies). The same doses of cocaine dose-dependently lowered BSR thresholds, but did not change the maximum response rate. These results suggest that like cocaine, mephedrone may possess a high abuse potential. The major difference between the two was in the time-course impacting ICSS thresholds in which the effects with cocaine were greater than mephedrone immediately post-injection, whereas mephedrone’s effects were greater than cocaine 15–30 minutes after drug administration. Interestingly, Gregg et al. (2015) reported that in male Sprague-Dawley rats the R(+) isomer of mephedrone produced greater facilitation of ICSS than the S(−) isomer, but was less potent as a 5-HT substrate (the two isomers displayed similar potencies as releasers for DA; for a similar structural analysis with the mephedrone metabolite 4-methylcathinone in which the R(+) isomer had greater ICSS facilitation and greater relative potency for DAT over SERT as a substrate, see Hutsell et al., 2015; for a related discussion, see Kolanos et al., 2015a).
In subsequent examinations, Watterson et al. (2014b, 2012) examined the effects of both MDPV and methylone on ICSS thresholds in male Sprague-Dawley rats. While MDPV produced reductions in ICSS thresholds across all doses tested (0.1, 0.5, 1 and 2 mg/kg), methylone only produced trends towards this effect (non-significant) at 0.1, 0.5, 1, 3, 5 and 10 mg/kg. In a related study, Bonano et al. (2014a) compared the abilities of methcathinone (0.1–1 mg/kg), MDPV (0.32–3.2 mg/kg), methylone (1–10 mg/kg) and mephedrone (1–10 mg/kg) to lower ICSS thresholds in male Sprague-Dawley rats. Although all drugs produced dose- and time-dependent facilitation of ICSS, these effects differed between compounds (methcathinone ≥ MDPV ≥ methylone > mephedrone). Further, while methcathinone was the most potent of the compounds in reducing ICSS thresholds, MDPV was the longest acting. It is important to note that the initial examination reporting significant effects of mephedrone on ICSS (see Robinson et al., 2012) used C57BL/6 J mice, whereas the work by Bonano et al. (2014a) reported data in male Sprague-Dawley rats, suggesting that the reinforcing effects of these drugs as assessed in this procedure may differ not only based on dose, but also on the particular species used. MDPV (3.2 mg/kg) also facilitated ICSS in male Sprague-Dawley rats [and to a level comparable to that produced by cocaine (10 mg/kg; Bonano et al. (2014b)].
3.2. Drug discrimination learning
In the DDL procedure, the interoceptive effects of the compound can be established and compared to other drugs with known abuse potential (see Ator and Griffiths, 2003; Colpaert, 1987; Järbe, 1986; Palmatier et al., 2005; Solinas et al., 2006). In this work, responding on one lever for a reinforcer (typically food) is preceded by an injection of a training drug, whereas responding on another lever for the same reinforcer is preceded by injection of the drug vehicle. Under such conditions, animals acquire discriminative control and distribute lever choice based on the subjective properties of the administered drug (or its vehicle) (for a review of drug discrimination learning, see Colpaert, 1987; Overton, 1991; for alternative DDL procedures, see Mastropaolo et al., 1989; Riley et al., 2016). Subsequent drug tests can be run to determine whether a novel drug substitutes for the training drug (and if it has similar interoceptive effects) and whether known pharmacological antagonists can attenuate or block discriminative control (providing some insight into possible receptor mediation of the drug’s discriminative stimulus effects).
In one such examination, Gatch et al. (2013) trained male Swiss-Webster mice to respond on one lever for a food pellet following an injection of METH or cocaine, and a second lever following an injection of the drug vehicle. Once the discriminations were acquired, methylone (0.5–5 mg/kg), mephedrone (0.5–5 mg/kg) and MDPV (0.05–2.5 mg/kg) were assessed for their ability to substitute for METH and cocaine. As reported, each of the synthetic cathinones fully substituted for the discriminative stimulus effects of both METH and cocaine, suggesting common interoceptive effects (and presumably similar abuse potential; see also BBerquist and Baker, 2017; Dolan et al., 2018; Harvey et al., 2017; though see Varner et al., 2013 for a failure of a range of stimulants to substitute for mephedrone). Gatch et al. (2015a) extended their work to other synthetic cathinones, i.e., α-PVP, 4′-MePPP and α-PBP, using male Sprague-Dawley rats trained to discriminate cocaine or METH from saline. Both α-PBP and α-PVP fully substituted for the discriminative stimulus effects of cocaine and METH, where 4′-MePPP only fully substituted for the discriminative stimulus effects of METH. Under similar training conditions, Gatch et al. (2017) reported that α-PPP, α-PHP, α-PVT, MDPBP and ethylone all dose-dependently (and fully) substituted for the discriminative stimulus effects of METH, whereas only α-PPP, α-PHP and ethylone displayed complete substitution for the discriminative stimulus effects of cocaine. The authors suggested that the failure of MDPBP and α-PVT to substitute completely for cocaine may indicate lesser abuse vulnerability compared to the other compounds that were tested. The findings of these latter two studies together add to their previous work suggesting that common interoceptive effects among these stimulants may indicate similar abuse potential. (For other work involving second generation cathinones in rodent models, see Cheong et al., 2017; Javadi-Paydar et al., 2017; Naylor et al., 2015; for work in monkeys, see Smith et al., 2017.)
Consistent with the work by Gatch et al. (2017, 2015a, 2013) that synthetic cathinones can substitute for METH and cocaine in the DDL design, Fantegrossi et al. (2013) demonstrated the reverse. Specifically, in their work male NIH Swiss mice were trained to discriminate MDPV (0.3 mg/kg) from its vehicle and were then given various doses of MDMA and METH to assess their ability to substitute for MDPV’s stimulus effects. During test sessions, a multiple-component cumulative dosing procedure was utilized in the absence of reinforcement which allowed for testing of four doses of each drug in a single session. MDMA dose-dependently and fully substituted for the MDPV training dose, with near exclusive choice of the MDPV-associated lever at a cumulative dose of 0.3 mg/kg. The interpolated ED50 for cumulative MDMA was identical to that of MDPV (0.03 ± 0.01 mg/kg). Substitution doses of MDMA dose-dependently suppressed response rates. Similarly, cumulative injections of METH dose-dependently and fully substituted for MDPV, with > 80% of the total responses on the drug-associated lever at a cumulative dose of 0.3 mg/kg. The interpolated ED50 for cumulative METH was 0.08 ± 0.03 mg/kg. As with MDMA, METH dose-dependently suppressed response rates. Substitution assessments with negative controls, JWH-018 and morphine, resulted primarily in responding on the saline-associated lever. These results again suggest abuse potential and rewarding efficacy of MDPV that are similar to those reported for other known psychostimulants (see also, Gannon and Fantegrossi, 2016).
Assessments of drug mixtures have begun to be examined for discriminative stimulus effects using the DDL design as “bath salt” products are often either intentionally mixed, or otherwise adulterated, with other compounds prior to sale. For instance, Collins et al. (2016) used DDL to assess the effects of cocaine, MDPV and caffeine, alone and as binary combinations, in male Sprague-Dawley rats. A primary goal of the study was to determine the effect caffeine may have when used in tandem with another psychostimulant, as well as to determine if the effect these combinations of drugs that have either similar or dissimilar mechanisms of action are simply additive. Rats were trained to discriminate cocaine (10 mg/kg) from saline in a two-lever drug discrimination procedure, and binary drug combinations (cocaine:caffeine, MDPV:caffeine and cocaine:MDPV) were each prepared at three separate dose ratios (3:1, 1:1 and 1:3 with respect to their ED50 values). Single-drug assessments of their ability to induce dose-dependent cocaine-appropriate responding revealed that METH = MDPV > cocaine > caffeine, an indication of at least partially intersecting stimulus effects. Binary mixtures of cocaine:caffeine, MDPV:caffeine and cocaine:MDPV showed mostly additive effects, although there were some supra-additive effects seen in some combinations as well as some individual differences across subjects. The significance of these results comes from evidence of cocaine-like discriminative effects among these drug combinations and a demonstration of supra-additive interactions occurring between drugs that possess different mechanisms of action and different binding affinities for DAT and SERT (for another study assessing stimulant combinations with synthetic cathinones, see Harvey and Baker, 2016; for a study assessing the discriminative stimulus effects of MDPV, see Gannon et al., 2016; for a list of other DDL studies involving the synthetic cathinones, see Glennon, 2014).
3.3. Conditioned place preference
Place preference conditioning is an often-used method for assessing the potentially rewarding effects of drugs of abuse (for reviews, see Tzschentke, 2007, 1998). CPP typically utilizes a two-chambered apparatus, with distinct environmental and tactile cues associated with each chamber (or sometimes a three-chambered apparatus, in which a smaller “neutral” chamber connects the two conditioning chambers). Following a baseline test assessing each subject’s unconditioned (initial) chamber preference, animals are given an injection of a drug paired with one chamber of the place conditioning apparatus. On other days (or at other times), injections of the drug vehicle are paired with the opposite chamber (the drug vehicle is generally paired with both chambers for control subjects). Any change in the conditioned animal’s preference for the chamber paired with the drug (usually measured by change in time spent on the drug-paired side) is taken to be an index of the rewarding value of the drug. If a drug is rewarding, animals generally increase time spent in the drug-paired chamber (from their own preconditioning baseline or from controls). Place preference conditioning has been widely used to evaluate the rewarding effects of a host of drugs of abuse (for reviews, see Sanchis-Segura and Spanagel, 2006; Tzschentke, 2007, 1998), including psychostimulant drugs (see Bardo et al., 1999; Bedingfield et al., 1998; Bilsky et al., 1990; Yates et al., 2013).
In one of the first assessments of conditioned place preferences with the synthetic cathinones, Lisek et al. (2012) reported that male Sprague-Dawley rats conditioned with 30 mg/kg mephedrone (but not 3 or 10) displayed a significant shift in their preference for the drug-paired side compared to that of vehicle-treated controls (see also Ramoz et al., 2012 for similar conditioning in an invertebrate planarian assay). CD-1 mice injected with 30 mg/kg mephedrone also displayed a significant place preference [see Gregg et al., 2015, for a structural analysis reporting place preference conditioning with the R(+) isomer of mephedrone at 30 mg/kg but not the S(−) isomer in Sprague-Dawley rats; see Vouga et al., 2015 for similar stereospecificity with mephedrone in planaria]. MDPV has also been reported to condition place preferences when administered either orally (0.30 mg/ml) or intraperitoneally (0.30 mg/kg) in male Swiss mice (Gannon et al., 2017d) or intraperitoneally (1.8 and 3.2 mg/kg) in male and female Sprague-Dawley rats (King et al., 2015ab; see also Gregg et al., 2016). In a comparison of mephedrone, methylone and MDPV (dose range of 0.5–20 mg/kg) in C57BL/6 mice, Karlsson et al. (2014) reported that in male C57BL/6 mice all compounds induced dose-dependent place preferences with MDPV inducing preferences at lower doses than those induced by either mephedrone or methylone (as well as those induced by amphetamine; see Miyazawa et al., 2011 for place preference conditioning with methylone at 2.5 and 5 mg/kg in male ddY mice).
More recently, a number of other synthetic cathinones have been reported to support place preference conditioning, including α-PVP (Gatch et al., 2015a; Hataoka et al., 2017; Marusich et al., 2016; Nelson et al., 2017), α-PVT (Cheong et al., 2017), α-PHP (Gatch et al., 2017), 4-MEPPP (Gatch et al., 2017) and pentedrone (Hwang et al., 2017). Several other synthetic cathinones have been examined in this context as well, with interesting dissociations from data examining their ability to support self-administration. For example, Botanas et al. (2017a) has reported that MACHP and MAOP both conditioned place preferences (at 10 and 30 mg/kg) in male ICR mice, yet only MACHP was self-administered (at 1 mg/kg/infusion; see above). Similarly, while neither the newly synthesized cathinones PIPP nor PIVT (analogs of methcathinone and a-PVP, respectively) support self-administration, PIPP (at 10 and 30 mg/kg) induces place preferences, but PIVT at these same doses is ineffective (see Botanas et al., 2017c). BMAPN (at 30 mg/kg) induces place preferences comparable to those induced by METH at 1 mg/kg (Botanas et al., 2017b).
Given the available data, the synthetic cathinones clearly possess rewarding effects in a variety of preparations (including ICSS, DDL and CPP). Nonetheless, more work needs to be done extending such findings to other species, assessing structure-activity relationships (Botanas et al., 2017a,b,c), including females in more comparisons, addressing effects in adolescent and adult animals (given the greater vulnerability to drugs of abuse in adolescents; see Schramm-Sapyta et al., 2014), examining drug history (or concurrent drug access; see Ciudad-Roberts et al., 2015; Gannon et al., 2018; Listos et al., 2017; López-Arnau et al., 2018, 2017a, 2017b) and pharmacological antagonism (Bonano et al., 2014b; Ciudad-Roberts et al., 2015; Gannon et al., 2017d; Gregg et al., 2016).
4. Assessments of the aversive effects of synthetic cathinones
Although the rewarding effects of the synthetic cathinones are becoming well characterized, as noted overall drug intake (i.e., self-administration) is a function of the balance of the drug’s rewarding and aversive effects. As such, it is important also to assess the aversive effects of these drugs before making conclusions regarding their abuse potential. In that context, the following discussion focuses on the aversive effects of the synthetic cathinones (see King and Riley, 2016).
4.1. Conditioned taste avoidance
In relation to the aversive effects of drugs, one procedure has been primarily used, i.e., CTA, in which animals tend to avoid consuming solutions associated with the drug (see Freeman and Riley, 2009; Parker, 2003; Riley, 2011; for parallel work on place avoidance conditioning, see Prus et al., 2009; though see Gore-Langton et al., 2015). Although initially reported with radiation and classical emetics like LiCl, emetine, and apomorphine (see Garcia et al., 1955; Garcia and Koelling, 1967), such acquired avoidance has now been reported with a wide variety of compounds, including most drugs of abuse, e.g., ethanol (Lester et al., 1970), amphetamine/mescaline (Cappell and LeBlanc, 1971), Δ9-tetrahydrocannabinol (Elsmore and Fletcher, 1972), METH (Martin and Ellinwood, 1973) and cocaine (Goudie et al., 1978) (for reviews, see Davis and Riley, 2010; Hunt and Amit, 1987; Riley et al., 2009; Verendeev and Riley, 2012). In this preparation, a novel solution (e.g., saccharin) is paired with injections of a drug, usually over multiple conditioning trials. The aversive effects of the drug are indexed via any reduction in consumption of the drug-paired saccharin solution. This avoidance is referred to as a conditioned taste avoidance given that it is acquired via the association of the taste with the aversive effects of the drug, possibly reflecting an evolutionary mechanism that prevents repeated consumption of toxic or poisonous substances (see Garcia and Ervin, 1968; Lin et al., 2015; Revusky and Garcia, 1970; Rozin and Kalat, 1971; for a history of conditioned taste avoidance, see Freeman and Riley, 2009; for a database, see www.CTAlearning.com).
Although CTAs are readily induced by a wide variety of drugs of abuse, assessments of the synthetic cathinones in this preparation are quite limited. In the initial study examining the ability of MDPV to induce a conditioned taste avoidance, Merluzzi et al. (2014) gave experimentally naïve, adult Sprague-Dawley rats limited access to saccharin (45 min) followed immediately by MDPV (0, 1, 1.8 or 3.2 mg/kg). This pairing was given every other day for a total of five conditioning trials each of which was separated by an intervening water-recovery day in which animals were given access to water alone. In addition to this conditioning procedure, the animals’ temperatures were taken via scans of implanted telemetry probes immediately prior to drug administration, as well as at 30-, 60-, 90- and 120-minutes post-injection, in order to determine whether body temperature was affected by MDPV at these doses and if any reported effects might be associated with MDPV-induced taste avoidance (for comparison, see Fantegrossi et al., 2013). Under these conditions, MDPV induced dose-dependent taste avoidance such that drug-injected subjects drank significantly less saccharin than controls. Further, MDPV produced a significant hyperthermic effect (for examples of drug-induced changes in temperature for psychostimulants, see Cappon et al., 1998; Dafters and Lynch, 1998; Fukumura et al., 1998; for MDPV, see Fantegrossi et al., 2013), although there was no dose-dependent relationship between hyperthermia and taste avoidance, suggesting that the aversive effects of the drug were not likely a function of the drug-induced changes in temperature (for a discussion of the role of hyperthermia in ethanol-induced taste avoidance, see Cunningham et al., 1992).
In a follow-up assessment, Merluzzi et al. (2014) extended this analysis of MDPV-induced taste avoidance to adolescents, given that for a variety of other abused compounds, including nicotine (Shram et al., 2006), amphetamine (Infurna and Spear, 1979), MDMA (Cobuzzi et al., 2014), cocaine (Schramm-Sapyta et al., 2006) and morphine (Hurwitz et al., 2013), taste avoidance is age-related with adolescent animals generally showing weaker avoidance than adults (for reviews, see Doremus-Fitzwater et al., 2010; Spear, 2011). Under procedures similar to that described for adults (see above), adolescent animals also acquired significant MDPV-induced taste avoidance, although no differences between drug-treated groups were observed. Although both adults and adolescents acquired avoidance of the MDPV-paired saccharin solution, this avoidance was significantly weaker and acquired slower in adolescent rats, a difference suggestive that adolescents may be relatively insensitive to MDPV’s aversive effects and more vulnerable to MDPV abuse. Little is known about possible age differences in the rewarding effects of MDPV (age differences have yet to be explored for ICSS, CPP, DDL or self-administration), and the inclusion of adolescent and adult animals in these assessments of reward will be important in assessing its overall abuse vulnerability. Interestingly, MDPV produced a hypothermic effect in adolescents (as opposed to the hyperthermic effect reported above in adults). Similar to adults, these changes in the thermic response were unrelated to the strength of the MDPV-induced taste avoidance.
Subsequently, King et al. (2014) extended this analysis of MDPV’s aversive effects by examining potential differences in MDPV-induced taste avoidance in the F344 and LEW rat strains. Although primarily characterized for their differences in the rewarding effects of drugs, where the LEW strain displays greater self-administration of, and stronger conditioned place preferences with, a host of compounds (Horan et al., 1997; Kosten et al., 1994; Picetti et al., 2012, 2010; Stöhr et al., 1998; although see Davis et al., 2007), the F344 and LEW strains also display differential sensitivity to the aversive effects of a number of drugs of abuse, effects which might limit drug intake (for reviews, see Davis and Riley, 2010; Riley, 2011). For example, Lancellotti et al. (2001) reported that LEW rats displayed attenuated morphine-induced taste avoidance compared to F344 rats (Davis et al., 2007), an effect also seen with nicotine and ethanol (see Pescatore et al., 2005; Roma et al., 2006). Interestingly, LEW rats acquire stronger cocaine-induced taste avoidance compared to F344 rats (Glowa et al., 1994; Grigson and Freet, 2000; for other drug comparisons, see Davis and Riley, 2010). Such differences have been interpreted as being a function of differential sensitivity to the aversive effects of these compounds, with the direction of the difference being drug-dependent. Given the pharmacological similarities between cocaine and MDPV and the fact that LEW rats appear more sensitive to cocaine’s aversive effects (see above), stronger MDPV-induced taste avoidance might be expected in this strain. To test this, King et al. (2014) gave male F344 and LEW rats access to a novel saccharin solution and then injected them with either vehicle or one of three doses of MDPV (1.0, 1.8 or 3.2 mg/kg) in a taste avoidance procedure similar to that used by Merluzzi et al. (2014). Each conditioning trial was followed by a water-recovery day. This procedure was repeated every other day for a total of five conditioning cycles (with water access on intervening days). Again, the animals’ temperatures were taken immediately prior to drug administration, as well as at 30-, 60-, 90- and 120-minutes post-injection. Similar to the work in outbred adult rats (Merluzzi et al., 2014), MDPV induced dose-dependent taste avoidance, but no differences between strains. The lack of strain difference in MDPV-induced avoidance is somewhat surprising given prior data showing stronger cocaine-induced avoidance in LEW animals (see Glowa et al., 1994). The degree of avoidance was strong for both groups at the two highest doses (between 50–75% reductions in consumption) with some animals in both groups displaying complete suppression on the final conditioning trial, a floor effect that may have precluded seeing differences among groups. MDPV produced non dose-dependent hyperthermia, again unrelated to taste avoidance.
4.2. Combined CTA/CPP
Recently, King et al. (2015b) assessed the aversive and rewarding effects of MDPV in male and female Sprague-Dawley rats using the combined CTA/CPP design which allows for the assessment of both affective properties of a drug in the same animal and under identical parametric conditions (see above). Using this procedure, animals were given access to saccharin, injected with MDPV (1, 1.8 or 3.2 mg/kg) and then placed in a distinct chamber of a place preference apparatus. Given reported sex differences in taste and place conditioning (with females displaying both stronger taste avoidance and place preferences induced by a variety of psychostimulants; Roma et al., 2008; Russo et al., 2003; Torres et al., 2009; for a review, see Riley et al., 2018), both males and females were included. Under these conditions, males and females differed in the acquisition and degree of MDPV-induced taste avoidance (males > females; see Chambers and Sengstake, 1976; Roma et al., 2008; Torres et al., 2009 for similar effects with other compounds). In regard to place preference conditioning, all groups (independent of dose) significantly increased time on the drug-paired side, but these preferences did not vary as a function of sex. Given the neurochemical similarities to cocaine, it might have been predicted that MDPV-induced preferences would have been stronger in females than males (Russo et al., 2003; Zakharova et al., 2009). However, prior work assessing place preference conditioning with cocaine in males and females used different strains (Russo et al., 2003) and conditioned at different ages and with a different procedure (Zakharova et al., 2009), indicating the potential role of a host of other factors in detection or production of sex differences (for a discussion, see Riley et al., 2018). Although there were no sex differences in MDPV-induced place preference conditioning, the fact that females showed a weaker taste avoidance compared to males suggests that sex may nonetheless be important in impacting susceptibility to MDPV use and abuse and that combined assessments of reward and aversion are important in evaluating abuse vulnerability to new synthetic compounds. The data further suggest a dissociation between these two affective properties, i.e., these effects likely function independently (for a review of such a dissociation, see Cunningham et al., 2002; King and Riley, 2013; Turenne et al., 1996; Verendeev and Riley, 2011).
To date, only one other synthetic cathinone, α-PVP, has been investigated in relation to its aversive effects. In this assessment, Nelson et al. (2017) used a combined CTA/CPP procedure similar to that described above to examine concurrently the aversive and rewarding effects of this second generation synthetic cathinone. Adult male Sprague-Dawley rats were given access to a novel saccharin solution, injected with α-PVP (0, 0.3, 1 or 3 mg/kg) and then placed in their non-preferred compartment of a conditioned place preference apparatus (a total of four such trials). Under these conditions, the highest dose of α-PVP induced significant avoidance of the drug-associated saccharin (different from controls and their own baseline). Interestingly, place preferences were conditioned at all three doses (a dissociation of reward and aversion similar to that reported by King and her colleagues with MDPV (2015b; see above). Collateral temperature assessments revealed that α-PVP induced dose- and time-dependent hyperthermia (see Aarde et al., 2015a for hypothermic effects of α-PVP in mice). The failure to see any association between hyperthermia and taste avoidance again argues against its mediation of α-PVP’s aversive effects. Comparisons with work on MDPV and cocaine under identical procedures in which comparable avoidance was induced by differing doses of these compounds (see Freeman and Riley, 2005; Freeman et al., 2005; Serafine et al., 2012a, 2012b) indicate that MDPV is more potent than α-PVP which in turn is more potent than cocaine, a relative potency consistent with their binding affinities to the brain amine transporters (see Aarde et al., 2015b; Baumann et al., 2013b; Marusich et al., 2014; Simmler et al., 2014). Other synthetic cathinones have not been investigated in the taste avoidance design. This is a gap in the literature that needs to be addressed in order to assess the abuse potential of these drugs.
5. Implications for abuse vulnerability
From the above discussion, a variety of animal models clearly indicate that the synthetic cathinones have both aversive and rewarding effects and are self-administered. The logic underlying the exploration of the affective properties of these drugs was to assess their relationship to self-administration, primarily by focusing on factors that might impact aversion and reward, their relative balance and their contribution to eventual drug taking (i.e., self-administration) that may have implications for their abuse potential. At this point, it is important to note that while the aversive and rewarding effects of the cathinones are becoming well-characterized, their relationship to self-administration has not been established.
In fact, few such studies exist for any drug. In one such assessment, Ettenberg et al. (2015) exposed male Sprague-Dawley rats to cocaine (1 mg/kg, iv) in a place conditioning procedure, followed by runway training using the same dose. During place conditioning, animals received alternating daily 5-min sessions of either cocaine paired with one chamber or saline paired with the opposite chamber for 16 days (or a total of eight sessions for both cocaine and saline). Half of the animals were placed in the conditioning chamber immediately post-injection, and half were given a 15-min delay post-injection, modifications known to produce CPP and CPA, respectively. Following the 16 conditioning trials, a final test was administered to determine any change in side preference from baseline assessments. Forty-eight hours following place conditioning, animals were trained to traverse a runway to enter a goal box in which they received an injection of cocaine. Animals were given once-daily runway trials for 14 days; start latencies, run times and retreat behaviors were recorded. Forty-eight hours following runway conditioning, animals were trained to self-administer cocaine. Once stable responding was established, animals underwent 10 days of 1- or 6-h/day access to cocaine IVSA (0.4 mg/injection). Under these conditions, the magnitude of the conditioned place preference was significantly and positively correlated with the ranked magnitude of response escalation during self-administration (suggesting that a stronger positive initial response to place conditioning predicted more self-administration); conversely, the magnitude of the conditioned place avoidance was unrelated to response rates during self-administration. Further, runway start latencies were strongly and positively correlated with the latency to initiate self-administration responding each day (suggesting that high cocaine seeking in the runway test was predictive of cocaine seeking in subsequent self-administration). Run time was significantly and negatively correlated with the average number of injections that animals earned during each self-administration trial, the mean number of injections earned during the first hour of testing and the degree of response escalation observed over self-administration (i.e., the difference score between the first and last day of cocaine self-administration). In general, these results suggest that animals that have a greater positive than negative initial response to cocaine exhibit greater subsequent escalation of self-administration (see Cunningham et al., 2009 who found an inverse relationship between the aversive effects of ethanol (as indexed by taste avoidance conditioning) and ethanol self-administration in mice; see also Cason and Grigson, 2013; Grigson and Twining, 2002; Imperio and Grigson, 2015; Twining et al., 2009 for an assessment of the relationship between cocaine-induced taste avoidance and cocaine self-administration).
Although suggestive of specific relationships between drug reward/aversion and self-administration, one major issue in such demonstrations concerns the fact that the procedures needed to assess this relationship are generally serial in nature. That is, one first assesses reward and aversion (using such designs as place preference conditioning and taste avoidance; see above) and then examines the ability of the drug to support self-administration. The difficulty with this procedure is that exposure to the drug during the assessments of reward and aversion could impact the drug’s self-administration, precluding an unconfounded assessment of drug taking. For example, if the initial exposures to the drug during taste avoidance and/or place preference conditioning adapted (or sensitized) the animal to the drug effect this could impact the likelihood or degree of self-administration and affect any relationships being examined (Horger et al., 1992, 1990; Schenk and Partridge, 1997).
Establishing associations and relationships between the drug’s rewarding and aversive effects and its self-administration may necessitate assessments in which both affective properties of the drug as well as its self-administration are established in independent groups of animals and the effects of various manipulations on these behavioral models are independently evaluated and correlated. In so doing, one can determine to what degree these models are affected and if changes in reward and aversion (as well as self-administration) are similar or different. This approach requires the use of many of the specific models described above for reward (e.g., ICSS, CPP and DDL), aversion (CTA) and drug intake (IVSA) as well as systematic evaluation of many of the issues noted in the present review, i.e., sex, age, dose, route, drug history.
In relation to drug history, for example, synthetic cathinone exposure has been reported to sensitize its own motoric effects (for cross-sensitization between the synthetic cathinones and other drugs of abuse, see Berquist et al., 2016; Buenrostro-Jàuregui et al., 2016; Gregg et al., 2013b; López-Arnau et al., 2017b). Behavioral sensitization refers to the progressive increase in the locomotor response to repeated exposures to drugs of abuse (Robinson and Berridge, 1993; Steketee and Kalivas, 2011). It is often used as a surrogate for psychostimulants’ rewarding effects and abuse potential (Kalivas and Weber, 1988; Pierce and Kalivas, 1997), given the relationship of behavioral sensitization to striatal activity and elevated brain amines (primarily DA) in this area and the relationship of activity in this area to the incentive and motivational properties of drugs of abuse (see Robinson and Berridge, 2008). Interestingly, several studies suggest that enhanced DA transmission in the striatum and nucleus accumbens is associated with locomotor sensitization (Johnson and Glick, 1993; Kalivas and Duffy, 1990; Parsons and Justice, 1993; Robinson et al., 1988; Shoaib et al., 1994) and direct evidence for DA’s role in behavioral sensitization action comes from the fact that mice lacking DAT or D1 receptors fail to develop cocaine sensitization (Kelly et al., 2008; Mead et al., 2002; Morice et al., 2010; Xu et al., 1994; Yao et al., 2004). The relationship between the neurochemical consequences and the motor stimulating effects of synthetic cathinones has been investigated widely (see Table 1), but only a few studies have focused on behavioral sensitization with these compounds. Although only a handful have been examined in this context, it is clear that repeated exposure to the cathinones can induce behavioral sensitization and that this sensitization is consistent with relative increases in DA levels subsequent to DA release or reuptake inhibition via DAT binding (see Simmler and Liechti, 2016 for a summary).
Table 1.
Inhibition (IC50 values) of dopamine and serotonin uptake by synthetic cathinones; doses of cathinones eliciting motor activation and motor sensitization in rodents.
| Synthetic Cathinones | IC50 DA uptake (μM) | IC50 5-HT uptake (μM) | Motor activation (mg/kg) | Motor sensitization (mg/kg) | Reference |
|---|---|---|---|---|---|
|
| |||||
| α-PBP | 0.06331; 0.0782 | > 101,2 | 3–101 or 2.5–2517 (mice) | N/A | 1 Marusich et al., 2014 |
| α-PPP | 0.19671; 0.5402 | > 101,2 | 10–30 (mice)1 | N/A | 2 Eshleman et al., 2017 |
| α-PVP | 0.01281; 0.01972; 0.01753 | > 101,2 | 3–101 or 2.5–2517 (mice) | N/A | 3 Kolanos et al., 2015a |
| 1–1020 (rats) | 4 Simmler et al., 2013 | ||||
| Butylone | 2.904; 0.2117; 1.716 | 6.224; 1.977; 0.686 | 5–256 or 10–305 (mice) | N/A | 5 Gatch et al., 2013 |
| Cathinone | 14.04; 0.8569 | > 104,9 | 1–48 (rats) | 1–48 (rats) | 6 López-Arnau et al., 2012 |
| 4-FMC | 6.354; 0.2737 | > 104,7 | 105 or 17–3010 (mice) | N/A | 7 Eshleman et al., 2013 |
| MDPV | 0.00411,11; 0.01267; 0.0048512 | 3.3051; 3.34911; 1.387 | 1–3, 17 or 301,5,10,20 (mice) | 0.325 (mice) | 8 Shortall et al., 2013 |
| 0.524 or 123,27 (rats) | 9 Fleckenstein et al., 1999 | ||||
| 0.3–3 29 or 1–1019 (rats) | 10 Marusich et al., 2012 | ||||
| Mephedrone | 3.314; 0.0987; 0.9713; 0.76211 | 4.644; 0.5107; 0.3113; 0.42211 | 3–105, 5–256 or 3–3010 (mice) | 3 28 (mice) | 11 Baumann et al., 2014 |
| 0.521, 224, 108 or 1526 (rats) | 12 Kolanos et al., 2015b | ||||
| 108 or 3–3021 (rats) | 13 Martínez-Clemente et al., 2012 | ||||
| Methcathinone | 1.124; 0.1447; 0.3449; 0.35615 | > 104,7,9,15 | 1–3018 (mice) | N/A | |
| Methedrone | 3514 | 4.7314 | 3010 (mice) | N/A | 14 Simmler et al., 2014 |
| Methylone | 4.824; 0.3437; 0.566; 2.916; 0.81915; 1.23211 | 15.54; 1.927; 0.236; 2.316; 5.7515; 1.01711 | 3, 10–305,10 or 5–256 (mice) | N/A | 15 Cozzi et al., 1999 |
| 16 Nagai et al., 2007 | |||||
| Naphyrone | 0.474; 0.01267 | 0.964; 0.05937 | 10–1005 (mice) | N/A | 17 Gatch et al., 2015a |
| Pentedrone | 2.5014; 0.17622 | > 1014; 92 | 2.5–2518 (mice) | N/A | 18 Gatch et al., 2015b |
| Pentylone | 1.3414; 0.1672 | 8.3714; 0.812 | 10–10018 (mice) | N/A | 19 Aarde et al., 2015a |
| 3-FMC | 1.714; 0.2142 | > 102,14 | 1, 3–3010,18 (mice) | N/A | 20 Fantegrossi et al., 2013 |
| 4-MEC | 4.2814; 0.962 | 7.9314; 0.2182 | 30–10019 (mice) | 3022 (rats) | 21 Lisek et al., 2012 |
| 3022 (rats) | 22 Xu et al., 2016 | ||||
| 23 Gregg et al., 2016 | |||||
| 24 Berquist et al., 2016 | |||||
| 25 Buenrostro-Jàuregui et al., 2016 | |||||
| 26 Gregg et al., 2013a | |||||
| 27 Watterson et al., 2016 | |||||
| 28 Berquist et al., 2015 | |||||
| 29 Novellas et al., 2015 | |||||
| N/A: no available data | |||||
Consistent with this work on behavioral sensitization and its relation to abuse potential, recent studies have shown that a history of synthetic cathinone exposure also affects place preference conditioning by, and self-administration of, itself and other drugs. In relation to place preference conditioning, Listos et al. (2017) demonstrated that adolescent mephedrone pre-exposure in male Wistar rats increased the rewarding effects of morphine in adulthood (see also Listos et al., 2017 for the facilitating effects of adolescent mephedrone exposure on morphine-induced conditioned place preferences in adult male Wistar rats). López-Arnau et al. (2017b) also recently reported that adult male Swiss CD-1 mice with exposure to MDPV during adolescence displayed a higher reactivity to cocaine in terms of locomotion, increased cocaine-induced CPP, greater PR responding for IVSA of cocaine and greater reinstatement of cocaine IVSA following extinction. Further, Creehan et al. (2015) demonstrated that female Wistar rats pre-exposed to mephedrone IVSA procedures subsequently self-administered more methylone, mephedrone and MDMA than animals without such a history. Interestingly, exposure to MDPV has also been reported to attenuate the aversive effects of both MDPV and cocaine (Woloshchuk et al., 2016). It remains to be determined how general such a drug history is on drug intake (and the relation of these changes to reward sensitization and aversion reduction).
The ability of a variety of synthetic cathinones to establish and support drug intake is well established and their rewarding and aversive effects are being characterized in various models. More work is now required to assess how these affective properties and their relationship to cathinone abuse are impacted by the host of experiential and subject factors known to affect drug use in general (see Cunningham et al., 2009; Riley, 2011; Riley et al., 2018,). Understanding these relationships and how they are affected by these variables will likely provide critical insight into the vulnerability of the abuse of the synthetic cathinones and into the development of behavioral and pharmacological strategies for their prevention and treatment.
6. Neurotoxicology
As described above, the synthetic cathinones share many of the structural, pharmacological and behavioral effects of the amphetamine-like drugs, differing in chemical structure from their cathinone analogues only by their lack of a β-keto substitution. Some examples of amphetamines and their associated β-keto containing cathinones are amphetamine-cathinone, METH-methcathinone and MDMA-methylone (Kelly, 2011). These β-keto containing cathinones and amphetamines share the ability to interact with monoamine transporters to cause the release of DA, 5-HT or NE (see Baumann et al., 2012; Cameron et al., 2013a,b; Eshleman et al., 2017, 2013; López-Arnau et al., 2012; Simmler et al., 2013). Further, they alter thermoregulation (Aarde et al., 2015a; Baumann et al., 2012; Fantegrossi et al., 2013; Kiyatkin et al., 2015; López-Arnau et al., 2014a, 2014b; Martinez-Clemente et al., 2014; Shortall et al., 2016), increase locomotor activity (Aarde et al., 2013a; Baumann et al., 2012; Fantegrossi et al., 2013; Gatch et al., 2013; López-Arnau et al., 2012; Marusich et al., 2012; Motbey et al., 2012a; Wright et al., 2012) and have high abuse potential as affirmed in a variety of animal models of addiction (see above). Based on the extensive similarities in the effects of these drug classes, it might also be predicted that the cathinone-like NPS would cause neurotoxicity to DA and 5-HT nerve endings like their non-β-ketone congeners METH and MDMA (Cadet et al., 2007; Colado et al., 2001; Halpin et al., 2014a; Jablonski et al., 2016). However, investigations into the neurotoxic potential of the synthetic cathinones have revealed some very surprising and unexpected results, as reviewed below.
6.1. Effects of Mephedrone on DA nerve endings of the striatum
The initial studies into the neurotoxic potential of the synthetic cathinones involved a comparison of mephedrone to METH. Mice were treated with mephedrone (4X - 20 or 40 mg/kg, intraperitoneal), using the standard binge dosing regimen which is traditionally used in studies of METH-induced neurotoxicity. This regimen involves four injections with a 2-h interval between injections. Mice were sacrificed 2 or 7 days after treatment to determine the effects of mephedrone at those times when METH toxicity is maximal and sustained, respectively (Thomas et al., 2004b). The status of DA nerve terminals in the striatum was assessed through measures of tyrosine hydroxylase (TH), DA and DAT, each of which is a specific marker for DA nerve terminals. The results in Fig. 3 indicate that large doses of mephedrone did not change TH, DA or DAT levels by comparison to vehicle controls at 2 days. Given that neither dose of mephedrone caused changes in DA terminals at that time point, only the highest dose of mephedrone (4X - 40 mg/kg) was selected to evaluate its possible sustained effects at 7 days. As observed at 2 days, no significant differences were induced by mephedrone at 7 days. By comparison, METH significantly lowers striatal levels of TH, DA and DAT by 70–80%, depending on the dose used and these signs of nerve ending damage show no evidence of recovery (Thomas et al., 2004b). Because binge dosing with METH increases reactive gliosis in mice (Thomas et al., 2004a) and humans (Sekine et al., 2008), the effects of mephedrone on microglia and astrocyte status in striatum were determined at the 4X-20 and 4X-40 mg/kg doses. Neither dose of mephedrone changed the number of activated microglia or astrocytes in striatum 2 or 7 days after treatment, and as a result of these initial findings it was concluded that mephedrone did not cause damage to DA nerve endings of the striatum (Angoa-Perez et al., 2012). These results are in agreement with those reported by Martinez-Clemente et al. (2014) and López-Arnau et al. (2015) for which no signs of microglial or astrocytic activation or even persistent (7 days) changes in TH and DAT levels in rat or mouse striatum were observed after mephedrone exposure (3 × 25 mg/kg, 2 h intervals, for 2 consecutive days). Moreover, mephedrone does not appear to cause any overt depletion of striatal brain monoamine levels in rodents 2 (Baumann et al., 2012; den Hollander et al., 2013) or 7 weeks (Motbey et al., 2012b) after treatment.
Fig. 3.
Effect of MEPH on DA nerve endings of the striatum 2 or 7 days after treatment. Mice (n = 6 per group) were treated with MEPH and the levels of DA, TH and DAT were determined at 2 days (doses of 4X- 20 or 4X- 40 mg/kg) and 7 days (dose of 4X- 40 mg/kg) after the last injection of MEPH. Immunoblots show only three representative samples for each treatment group. All immunoblots were scanned and presented as means ± SEM relative to controls. The effects of either dose of MEPH on DA, TH and DAT were not significantly different from control or from each other with the exception that DAT levels after the higher dose of MEPH were significantly increased over those of the lower dose (*p < 0.05, one-way ANOVA followed by Tukey’s test). Reprinted from Angoa-Perez et al. (2012) with permission from Wiley.
The synthetic cathinones are often taken with other drugs of abuse, including METH, to either heighten or diminish their psychoactive effects (Miller and Stogner, 2014; Winstock et al., 2011). In a test of this, Angoa-Perez et al. (2013) examined whether mephedrone would modify the damaging effects of METH on DA nerve terminals. Amphetamine and MDMA were also tested in combination with mephedrone. Fig. 4 shows that mephedrone significantly enhanced the reductions in TH, DA and DAT caused by METH (4X - 2.5 or 5 mg/kg). This effect of mephedrone was also seen when administered along with amphetamine or MDMA, using doses of each drug that were sub-maximally neurotoxic. It is clear from these results that the enhancing effect of mephedrone on drug-induced damage to the DA nerve ending generalizes to other psychostimulants of the amphetamine class (Angoa-Perez et al., 2013).
Fig. 4.
Effects of mephedrone on methamphetamine-induced reductions in striatal DA, DAT and TH (upper panels). Mice were treated with the indicated doses of mephedrone (MEPH) 30 min prior to each injection of 2.5 (●) or 5.0 mg/kg (■) methamphetamine (METH) and killed 2d later for determination of striatal levels of DA by HPLC or DAT and TH by immunoblotting. Effects of mephedrone (4 × 20 mg/kg) in combination with other amphetamines on DA, DAT and TH levels (bottom panels). Immunoblots for DAT and TH (lower panels) are numbered 1–8 to represent the following treatments: 1,5: control; 2,6: MEPH; 3: amphetamine (AMPH); 4: AMPH + MEPH; 7: MDMA; and 8: MDMA + MEPH. Data are mean ± SEM for 5–7 mice per group. Some error bars were too small to exceed the size of the symbols and are not visible. ***p < 0.001 versus controls and #p < 0.01, ##p < 0.001 or ###p < 0.0001 versus the respective dose of methamphetamine (Tukey’s multiple comparison test). Reprinted from Angoa-Perez et al. (2013) with permission from Wiley.
Previous studies have shown that the damaging effects of METH on the DA nerve terminal depend on the newly synthesized pool of DA (Thomas et al., 2008). Treatments that increase the amount of DA in this pool, including the DA precursor L-DOPA and the monoamine oxidase inhibitor pargyline, significantly increase the toxicity of METH, whereas α-methyl-para-tyrosine, which decreases this presynaptic pool of DA, significantly reduces METH-induced nerve ending damage (Thomas et al., 2008). In light of results indicating that mephedrone does not cause damage to DA nerve endings, Anneken et al. (2017a) treated mice with either L-DOPA or pargyline to determine if increases in the newly synthesized pool of DA would provoke damage by mephedrone. Neither L-DOPA nor pargyline interacted with mephedrone to cause damage to DA terminals. In agreement with previous findings, the only drug combination that showed enhanced reductions in TH, DA or DAT was mephedrone + METH (Anneken et al., 2017a). Taken together, these results indicate that mephedrone does not cause damage to DA nerve terminals, even under conditions that are known to increase METH-induced damage (i.e., L-DOPA, pargyline). These findings are surprising in light of the extensive overlap in the chemical structures of METH and mephedrone and in their pharmacological and behavioral effects (see above).
6.2. Effects of MDPV and methylone on DA nerve endings of the striatum
MDPV (like other pyrovalerone analogues) is somewhat unique among the synthetic cathinones in that it is not a substrate for transport by DAT but instead serves as an extremely potent inhibitor of DAT, much like cocaine (Baumann et al., 2016, 2013b; Cameron et al., 2013a,b; Eshleman et al., 2013; Kolanos et al., 2015a; Simmler et al., 2013). Methylone, on the other hand, is the β-keto analogue of MDMA. Methylone functions as a substrate for DAT, as well as for SERT and NET, which underlies its ability to cause the release of the monoamine neurotransmitters (Eshleman et al., 2013). Because MDPV and methylone are often found in “bath salt” formulations, but differ considerably in the mechanism by which they interact with monoamine transporters, Anneken et al. (2015) tested each for its effects on the status of the DA nerve terminal. Like mephedrone, MDPV does not lower the levels of TH, DA or DAT over a wide dosage range (i.e., 4X - 10, 20 or 30 mg/kg, intraperitoneal). However, if MDPV is given prior to a neurotoxic regimen of METH, the reductions in TH, DA or DAT caused by the latter drug are completely prevented. The non-toxic standing of MDPV is reinforced by its failure to cause increases in astrocytosis (assessed via measures of GFAP) and by its ability to prevent the increases in GFAP caused by METH. Methylone, over the same dosage range, is devoid of damaging effects on the DA nerve terminal (Anneken et al., 2015). Like mephedrone, methylone has the very interesting property of enhancing the neurotoxicity of METH, evidenced by greater reductions in TH, DA and DAT, as well as greater increases in striatal GFAP levels (Anneken et al., 2015). These findings with MDPV and methylone provide added insight into the mechanisms by which these drugs interact with the DA nerve terminal to influence the effects of METH on DA neurochemistry. MDPV, like other blockers of DAT, is non-toxic and provides protection against METH-induced damage (Anneken et al., 2015; Pu et al., 1994; Schmidt and Gibb, 1985). Methylone is slightly less potent than mephedrone in causing the release of DA (Eshleman et al., 2013) and is likewise non-toxic to DA nerve terminals and somewhat less potent than mephedrone in enhancing the neurotoxic properties of METH (Anneken et al., 2015). It is becoming clearer that the manner by which the synthetic cathinones interact with DAT (i.e., blocker or substrate) influences their pharmacological effects and abuse potential, but it does not explain why these cathinone derivatives lack neurotoxic effects on the DA nerve terminal.
6.3. Effects of synthetic cathinones combinations on DA nerve endings of the striatum
It is quite clear that at least mephedrone, MDPV and methylone, when administered alone, do not cause damage to striatal DA nerve endings like the non-β-keto-amphetamines. However, these compounds are often taken as complex mixtures by human abusers of these drugs, so it is important to test these drugs in combination to determine if alterations in the status of the striatal DA nerve ending would emerge. In this regard, Anneken et al. (2015) addressed the effects of mephedrone, MDPV and methylone in all possible two-drug combinations on striatal DA, DAT and TH. The possibility that MDPV would combine with either mephedrone or methylone to cause DA nerve ending damage was predicted to be very low because of the potency of MDPV in preventing the neurotoxicity caused by METH, amphetamine and MDMA (Anneken et al., 2015). This prediction was verified by results showing that neither MDPV + mephedrone nor MDPV + methylone caused a change in striatal DA, DAT or TH. Likewise, these drug combinations did not increase GFAP levels. Although neither mephedrone nor methylone alone is neurotoxic to striatal DA nerve endings, the possibility that their combination could be neurotoxic was important to examine because of each drug’s ability to enhance METH-induced neurotoxicity. Consistent with prior results, the combined treatment with mephedrone + methylone was non-toxic, leaving striatal levels of TH, DA, DAT and GFAP unchanged from vehicle controls (Anneken et al., 2015). Despite numerous attempts to provoke METH-like damage to DA nerve endings by the “bath salts”, the data suggest that mephedrone, MDPV and methylone are non-neurotoxic. This lack of neurotoxicity by the cathinone derivatives stands in stark contrast to the numerous other similarities shared by these drugs and the amphetamine class of psychostimulants.
6.4. Defining the structural elements that differentiate non-neurotoxic synthetic cathinones from neurotoxic amphetamines
Mephedrone and METH share remarkable overlap in terms of their chemical structures, pharmacological properties, behavioral effects and abuse potential, but they fall at opposite ends of the neurotoxicity continuum with mephedrone non-neurotoxic and METH highly neurotoxic. From a structural perspective, mephedrone differs from METH by the presence of a β-ketone substitution and the presence of a 4-methyl group on the phenyl ring. Removal of the β-ketone from mephedrone or the addition of a 4-methyl group to METH would yield 4-methyl-methamphetamine (4-MM). Correspondingly, removal of the 4-methyl group from mephedrone or addition of a β-ketone to METH yields methcathinone. These four compounds, therefore, offer an interesting possibility for a structure-activity comparison with regard to DA nerve ending toxicity. The neural and behavioral pharmacological properties of methcathinone have been studied extensively, and it has been shown to possess moderate neurotoxicity in the DA neuronal system in three older publications (Gygi et al., 1997, 1996; Sparago et al., 1996). 4-MM has not been studied in this regard heretofore. Comparisons of methcathinone and 4-MM offer an excellent opportunity to assess the relative contributions of the β-keto and 4-methyl substituents in determining neurotoxic potential.
4-MM was synthesized as described (Davis et al., 2012), and its structure was confirmed by NMR and mass spectrometry. This drug was then tested for its effects on DA nerve endings along with methcathinone (Anneken et al., 2017b). The results are presented in Fig. 5. Several interesting findings emerge from these experiments. First, 4-MM, over the dose range 5 to 40 mg/kg (administered 4X in a standard binge procedure), did not change the levels of TH, DA or DAT with the exception of a ~20% reduction in DA content at the highest dose. Second, 4-MM did not increase the levels of GFAP at any dose. Third, methcathinone over a dose range 10–80 mg/kg (administered 4X in a standard binge procedure) showed signs of DA nerve ending damage as evidenced by significant reductions in TH, DA and DAT, as well as significant increases in striatal GFAP. The effects of METH and MEPH on striatal TH, DA, DAT and GFAP are included in Fig. 5 for reference (see Annekin et al., 2015) and show that METH (but not MEPH) appear more potent than methcathinone in causing damage to DA nerve endings. Based on the results in Fig. 5, 4-MM does not appear to cause damage to DA nerve endings. Because 4-MM has not been tested, heretofore, in animal models of drug-induced neurotoxicity, it was important to include analyses of body temperature and locomotor activity to ensure that it was pharmacologically active. These studies documented significant increases in body temperature and locomotor activity, suggesting that 4-MM was indeed reaching the brain and exerting psychostimulant-like effects (Anneken et al., 2017b). Methcathinone showed signs of causing significant damage to DA nerve endings, but its effects were not entirely dose-related because the highest dose used (4X - 80 mg/kg) was not associated with the greatest degree of damage.
Fig. 5.
Effects of 4 MM and MeCa on markers of dopaminergic toxicity. Mice were treated with 4 MM (4X- 2.5, 5, 10, 20, or 40 mg/kg), MeCa (4X- 10, 20, 40, or 80 mg/kg) or saline vehicle every 2 h for a total of four injections at the given dose. Levels of DA (A), DAT (B), TH (C) and GFAP (D) were determined 48 h after drug exposure. Data are means S.E.M. (for 4 MM: control, n = 18; 2.5 mg/kg, n = 8; 5 mg/kg, n = 8; 10 mg/kg, n = 9; 20 mg/kg, n = 12; 40 mg/kg, n = 12; for methcathinone (MeCa): control, n = 15; 10 mg/kg, n = 6; 20 mg/kg, n = 6; 40 mg/kg, n = 11; 80 mg/kg, n = 10). Each experiment was performed three times, with differing doses, and results were then pooled for analysis. One-way ANOVA, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with controls, with Bonferroni’s post-hoc test. Reprinted from Anneken et al. (2017b) with permission from the American Society for Pharmacology and Experimental Therapeutics. METH and MEPH data were reprinted for comparison from Anneken et al. (2015) with permission from John Wiley and Sons, Inc. These data are not statistically compared to 4-MM and MeCa in the present figure, but METH elicited highly significant toxic effects on each measure in the original experiments, whereas MEPH had no significant effects.
The structure-activity studies using methcathinone and 4-MM suggest that the β-ketone substitution (METH to methcathinone) significantly reduces the neurotoxic potential of METH. The addition of a methyl group (i.e., METH to 4-MM) renders METH completely non-neurotoxic. Although mephedrone differs from METH by its content of both substituents, the results from studies with 4-MM suggest that the 4-methyl group is playing the most influential role in rendering mephedrone non-neurotoxic.
In light of results showing that elevations in DA significantly increase the damaging effects of METH on DA nerve endings (Thomas et al., 2008), Anneken et al. (2017a) recently tested 4-MM and methcathinone for their responses to L-DOPA and pargyline, recalling that mephedrone is not rendered neurotoxic by either treatment. The results in Fig. 6 show that combined treatment of mice with 4-MM plus L-DOPA or pargyline leads to significant reductions in striatal DA, DAT and TH. Fig. 6 also shows that METH significantly increases DA nerve ending damage when paired with 4-MM (Panels A, B and C). Parallel experiments with methcathinone reveal another influence of the β-keto substitution on neurotoxicity. It can be seen in Fig. 6 (Panels D, E and F) that the effects of methcathinone on striatal TH, DA and DAT are enhanced slightly after combined treatment with L-DOPA, but not after combined treatment with pargyline. Fig. 6 also shows that METH increases the effects of methcathinone to a very small extent. Taken together, these experiments suggest that the 4-methyl group slightly reduces the DA-induced enhancement of damage to DA nerve endings (i.e., 4-MM compared to METH), whereas the β-ketone substituent has a much greater effect in reducing the DA-induced enhancement (i.e., methcathinone compared to METH). Likewise, the 4-methyl group is permissive of greater additivity with METH in damaging DA nerve endings, whereas the β-ketone substituent almost completely prevents it.
Fig. 6.
Effects of 4 MM and MeCa ± L-DOPA, PARG or METH on markers of striatal dopaminergic toxicity. Levels of DA (A, D), DAT (B, E), and TH (C, F) were determined in the striatum following treatment with 4- MM and MeCa ± L-DOPA (LeD), pargyline (PARG), or METH. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared to controls. ##p < 0.01, ###p < 0.001, ####p < 0.0001 compared to 4 MM. @@@p < 0.001, @@@@p < 0.0001 compared to METH. Data are presented as mean ± SEM. N = 5–16 mice per group. Reprinted from Anneken et al. (2017b) with permission from Elsevier.
6.5. Why are the “bath salts” compounds not neurotoxic to DA nerve endings?
The remarkable overlap between the synthetic cathinones and the amphetamine psychostimulants in terms of their pharmacological, neurochemical, behavioral and addictive effects can be attributed largely to their structural similarities. However, the synthetic cathinones are enigmatic with regard to neurotoxicity because the structural differences between them and their corresponding amphetamines, albeit small, are extremely important in determining their neurotoxic potential. The relative lack of neurotoxic potential to the striatal DA nerve endings of the cathinone family of compounds may be determined by the presence of the β-ketone substituent. Methcathinone does cause damage to DA nerve endings, but far less than that caused by METH. We are not aware of studies of cathinone neurotoxicity, but based on the lower neurotoxic potential of its non-β-keto-analog, compared to METH, we would predict that cathinone possesses low neurotoxic potential. The addition of a 4-methyl group to the β-keto-containing methcathinone yields mephedrone and further reduces the neurotoxic potential of this cathinone derivative. The substitution that has the greatest influence on the neurotoxic potential of mephedrone is the 4-methyl group. The addition of a 4-methyl group to METH, to yield 4-MM, results in a drug with significant psychoactive effects, but devoid of neurotoxic potential (Anneken et al., 2017b). Therefore, this modification probably outweighs the influence of the β-keto substituent in reducing the neurotoxic potential of the cathinones.
The rhetorical question at the beginning of this section could be cast in another way, i.e., why is METH (and amphetamine and MDMA) neurotoxic? A complete review of the possible mechanisms by which METH damage the brain is well beyond the scope of this section of this review, but the predominant theory underlying its neurotoxicity holds that METH enters the DA nerve ending via DAT. Once inside the presynaptic terminal, METH causes a modification in DAT so that DA is transported out of the presynaptic process via “reverse transport”. At the same time, the weak base properties of METH cause a collapse of the pH gradient across the vesicular monoamine transporter (VMAT), allowing its stored DA to leak into the cytoplasm. The gradient of DA leaking from storage vesicles into the cytoplasm is continuously released into the synapse via reverse DAT uptake under the influence of METH (Sitte and Freissmuth, 2015; Sulzer, 2011; Sulzer et al., 1993). DA can be oxidatively labile when dislocated from its protective neuronal compartments and initiate numerous downstream reactions that ultimately lead to damage (Angoa-Perez et al., 2017). The interactions of the synthetic cathinones with monoamine transporters has been very well delineated, establishing that these drugs are either substrates for transport or non-substrate blockers of uptake (Baumann et al., 2016, 2013b, 2012; Cameron et al., 2013a,b; Eshleman et al., 2014, 2013; López-Arnau et al., 2012; Simmler et al., 2013). Those drugs that are transported by monoamine transporters, therefore, cause release of the neurotransmitters much like METH. However, it is not yet clear if these drugs interact with VMAT in a manner that resembles the actions of METH. A limited number of studies have indicated that the cathinone-derivatives have much lower affinities for VMAT than the amphetamines and they result in lower levels of monoamine release via VMAT (Eshleman et al., 2013; Pifl et al., 2015). We hypothesize, therefore, that the far less potent actions of the cathinone-derivatives at VMAT to cause leakage of DA into the cytoplasm, by comparison to METH, is the mechanism that differentiates the non-neurotoxic cathinones from the neurotoxic amphetamines. Further support for this hypothesis must await additional studies on cathinone-VMAT interactions.
We must also point out that transient reductions of DA or DAT (or 5-HT and SERT) content in neuronal terminals may not always reflect neurotoxic effects, but instead homeostatic adaptions of these terminals which can be reflective of a neuroadaptive response to the increase in DA or 5-HT release/levels induced by psychostimulants (see reviews Baumann et al., 2007; Biezonski and Meyer, 2011; Siciliano et al., 2015). Moreover, although synthetic cathinone compounds are not neurotoxic to striatal DA nerve endings (unlike amphetamines), they can affect other neuronal systems and brain regions.
Among the synthetic cathinones, mephedrone is one of the most extensively studied in terms of its neurotoxic effects. It was initially hypothesized that mephedrone would cause damage to striatal DA nerve endings, much like METH. Although the literature regarding the neurotoxicity of mephedrone in 5-HT and DA systems remains controversial, the data clearly suggest that mephedrone, as with other synthetic cathinones, does not damage DA nerve endings, at least in the striatum. This finding does not indicate that mephedrone is safe for human ingestion, as it still retains substantial abuse potential (Karila et al., 2016; Papaseit et al., 2017a). Further, mephedrone when administered with sub-optimally neurotoxic doses of METH significantly enhances damage to DA nerve endings. L-DOPA and pargyline, treatments that enhance METH-induced neurotoxicity, do not combine with mephedrone to damage the DA system. On the other hand, some report that mephedrone alone does not produce damage to 5-HT nerve endings in the hippocampus (Angoa-Perez et al., 2014). This is consistent with the suggestion that mephedrone has little neurotoxic potential, while others (Naseri et al., 2018) have demonstrated that repeated use of mephedrone during gestation impairs learning and memory processes by inducing hippocampal damage in the offspring of mice. Other laboratories have also noted that mephedrone can cause CNS damage at high doses (Martinez-Clemente et al., 2014; Hadlock et al., 2011) and that mephedrone or methylone can damage 5-HT neuronal systems (den Hollander et al., 2013; López-Arnau et al., 2015, 2014a,b; for a review, see Pantano et al., 2017). The results of these last studies may have been influenced by the elevated ambient temperatures utilized, as such conditions exacerbate the toxicity of comparable drugs (Escubedo et al., 1998; Malberg and Seiden, 1998; Parrott, 2004; Pubill et al., 2003). Other authors have also found oxidative damage of DNA induced by mephedrone in cortical cells (Kamińska et al., 2018) which is in accordance with the findings published by López-Arnau et al. (2015) demonstrating that the frontal cortex was one of the brain areas most affected. Other synthetic cathinones, e.g., MDPV, do not damage DA nerve endings in the striatum, leaving methcathinone as the only major member of the cathinone-derivatives that produces robust dopaminergic neurotoxicity (Anneken et al., 2017a; Gygi et al., 1997, 1996; Miner et al., 2017; Sparago et al., 1996). However, Sewalia and colleagues (2017) have recently reported that prolonged MDPV intake induced cognitive deficits that were paralleled by neurodegeneration in perirhinal and entorhinal cortical regions, while other areas, such as the striatum, were unaffected (Sewalia et al., 2017) as mentioned in previous sections.
7. Clinical and preclinical toxicity and lethality
7.1. Clinical findings
The foregoing discussion has considered many of the main concerns over the rise in the use of the synthetic cathinones, including their potential for drug abuse and addiction, as well as the long-term consequences based on their potential for neurotoxicity. Although far from safe, most synthetic cathinones appear to have reduced potential for neurotoxicity compared to METH and MDMA. On the other hand, it has very commonly been thought that synthetic cathinones may present a greater threat of overdose than their non-β-ketone relatives. When discussing the potential adverse effects of these compounds, researchers have consistently noted increases in the number of emergency room admissions associated with overdoses as their use has risen (American Association of Poison Control Centers, 2011; Wood et al., 2015, 2013). These emergency room admissions are consistent with the sorts of effects with which psychostimulant drugs have long been associated in terms of acute overdose, including acute anxiety, insomnia, fatigue, mydriasis, agitation, aggression, combative behavior, panic attacks, disorientation, confusion, memory loss, blackouts, myoclonus, excited delirium, paranoia, hallucinations, increased suicidal ideation, pyrexia, chest pain, tachycardia, cardiac arrhythmias and hypertension (Beck et al., 2018; Marinetti and Antonides, 2013). The majority of synthetic cathinone intoxication or overdose cases initially displayed signs of increased agitation, aggression, violent behavior and psychosis (CDC, 2011; Imam et al., 2013; James et al., 2011), although there may certainly be an over-estimation of the prevalence of such outcomes. These symptoms include primarily adverse neurological and cardiac effects, but cases of lethal overdose often end in multiple organ failure as part of the “Excited Delirium” syndrome previously reported to occur with other psychostimulant drugs (Marinetti and Antonides, 2013; Mash, 2016).
One factor that may contribute to the number of synthetic cathinone overdoses is the condition under which these drugs are taken in which individuals often do not know exactly what drug or drug dose they are taking. A study of electronic dance music scene patrons (from the summer of 2016) found that 27.8% tested positive for one or more synthetic cathinones (including butylone, ethylone, pentylone and α-PVP). Half of these individuals tested positive for a drug that they did not know they had taken (Palamar et al., 2017). These uncertainties may contribute to the likelihood of overdose, but they also contribute to uncertainties about the risks of overdose with the cathinones, since it is often not clear what drugs were taken, how much was taken and what combination of drugs was used – the majority of overdose cases involved ingestion of multiple drugs (when there is a complete toxicological assessment, which is not done on a routine basis) (Papaseit et al., 2017b). Nonetheless, case reports that the synthetic cathinones also produce renal, hepatic and cardiac injury indicate that peripheral toxicity may be a primary cause of cathinone-induced lethality.
Renal symptoms of synthetic cathinone overdose include high blood urea nitrogen (BUN) levels, hyperkalemia, dehydration and hyponatremia (Benzer et al., 2013; Borek and Holstege, 2012; Eiden et al., 2013; Imam et al., 2013; Murray et al., 2012; Penders et al., 2012; Sutamtewagul et al., 2014; Thirakul et al., 2017) and increases in creatine phosphokinase levels, indicative of rhabdomyolysis, which may lead to acute renal injury (Benzer et al., 2013; Eiden et al., 2013; Imam et al., 2013; Kramer et al., 2016; Levine et al., 2013; Regunath et al., 2012; Sutamtewagul et al., 2014; Young et al., 2013). One study reported a rate of rhabdomyolysis of 63% in hospital admissions for synthetic cathinone overdoses (O’Connor et al., 2015), although this is certainly at the high end of reports. That these compounds can cause renal toxicity is not entirely surprising since other psychostimulants can cause renal injury through effects on vascular reactivity and hemodynamics mediated by sympathomimetic effects (Chiueh and Kopin, 1978; Karch, 1987), oxidative stress (Kapasi et al., 1997) and myoglobinuria (Brannan et al., 2004; Vearrier et al., 2012).
Hospital admissions from synthetic cathinone overdose are also associated with cardiac injury, which has been reported as a cause of death. On initial hospital admission, patients present with elevated respiration, heart rate and blood pressure, which consequently lead to the presence of sinus tachycardia, cardiac arrest and reduced cardiac output. These symptoms are observed for a range of synthetic cathinones, including MDPV (Borek and Holstege, 2012), methylone (Barrios et al., 2016; Carbone et al., 2013), 3-methylmethcathinone (3-MMC) (Adamowicz et al., 2014) and mephedrone (Wood et al., 2010). Again, this is similar to effects of classic psychostimulants (Jacobs, 2006). Although peripheral in nature, cardiac symptoms may result from overstimulation of adrenoreceptors in the CNS, indirectly triggering alterations in cardiovascular function (for a review, see Carvalho et al., 2012). Rhabdomyolysis may contribute to myocardial damage (Benzer et al., 2013; Eiden et al., 2013; Imam et al., 2013; Sivagnanam et al., 2013; Sutamtewagul et al., 2014), as is the case for other psychostimulants (Counselman et al., 1997).
Hepatoxicity has been frequently reported in cases of synthetic cathinone overdose. Most cases have elevations in aspartate and alanine aminotransferase levels, common indicators of liver damage (Borek and Holstege, 2012; Kramer et al., 2016; Murray et al., 2012; Thirakul et al., 2017). Additionally, post mortem histological analysis of methylone overdose victims have indicated hepatic portal and lobular inflammation (Benzer et al., 2013; Carbone et al., 2013). Although the initial neurological and psychiatric symptoms associated with Excited Delirium Syndrome generally abate within about 24 h, hepatic failure associated with synthetic cathinone overdose often emerges in the days following initial hospitalization. For instance, one case report of “bath salt” use indicated mildly elevated aspartate and alanine transaminase levels upon admission, but levels increased close to 50-fold two days later (Kramer et al., 2016). Other psychostimulants such as MDMA and METH have also frequently produced hepatotoxic effects, which have been suggested to involve increased efflux of monoamines, oxidation of biogenic amines, hyperthermia, production of reactive metabolites, mitochondrial impairment and apoptosis (for a review, see Carvalho et al., 2012). Moreover, impairments in liver function, resulting in increased levels of plasma ammonia and brain glutamate, have been suggested to play an important role in the neurotoxic effects of METH (Halpin et al., 2014b).
7.2. Preclinical Findings
Although it would seem that the potential toxic and lethal effects of synthetic cathinones are well-documented in the clinical literature, it is very difficult to draw firm conclusions about particular drugs due to the uncontrolled nature of the clinical circumstance. Although there are many clinical cases with confirmed toxicity, this is not generally the case for all overdoses, making epidemiology unreliable. Since new synthetic cathinones are constantly emerging, this would be especially difficult, even if there were a greater societal investment in identifying the precise causes of drug overdose in these cases. This is in addition to the usual difficulties with post mortem studies, e.g., varying times since exposure, varying qualities of sample collection and clinical observations, etc. It has already been noted that the vast majority of overdose cases involve the ingestion of multiple drugs, including various combinations of synthetic cathinones with each other and with other licit and illicit drugs. This makes identifying the precise risks of particular synthetic cathinones nearly impossible based on these data alone. Preclinical investigations should help clarify the risks of individual cathinones, but surprisingly little work has been dedicated to this problem.
Most preclinical work has used in vitro approaches. In one such study, Lantz et al. (2017) reported that cathinone phthalimide decreases membrane integrity and alters mitochondrial function in pheochromocytoma cells. Studies have also demonstrated hepatotoxic effects of MDPV (Silva et al., 2016), that are at least partially dependent on temperature (Valente et al., 2016). Another study found a rank order of hepatotoxicity of MDPV > MDMA > methylone (Valente et al., 2015). Further study found that pentedrone has even greater hepatotoxic and cytotoxic potential than MDPV, which was greater than MDMA, 4-MEC and methylone (Araujo et al., 2015; Valente et al., 2016). Mephedrone was more cytotoxic in SH-SY5Y cells than METH, while methylone showed less toxicity than either METH or MDMA. Mephedrone cytotoxicity was partially prevented by DAT blockade or overexpression of mitochondria-protective anti-apoptotic Bcl-2. Luethi et al. (2017b) showed that MDPV, mephedrone and naphyrone are mitochondrial toxicants that impair the function of the electron transport chain and deplete cellular ATP stores in hepatocytes. Finally, recent studies comparing the toxicity of α-pyrrolidinooctanophenone (α-POP) and α-pyrrolidinoheptiophenone (α-PHPP) and other pyrrolidinophenones for several human cell types found that cells of the vascular, respiratory and central nervous systems were most sensitive to these synthetic cathinones. They established a structure activity relationship (SAR): toxicity depended on alkyl chain length (α-POP > α-PHPP > pyrrolidinophenones with shorter alkyl chains), and the 4-fluoro and 3,4-methylenedioxy modifications on α-POP and α-PHPP also increased cytotoxicity. Toxicity involved the production of reactive oxygen species that was prevented by pretreatment with N-acetyl-L-cysteine (Matsunaga et al., 2017a, 2017b). Additional studies are certainly needed to evaluate SAR for synthetic cathinone-induced toxicity, as well as the mechanisms that underlie these effects which may provide better treatments for cathinone overdose. It is also clear that there is variability in their toxic potential, an understanding of which will contribute to overdose treatment, as well as regulatory efforts.
In vivo examination of synthetic cathinone lethality is limited to a single study to date which examined the lethal effects of methylone (Piao et al., 2015). This study found that methylone has an LD50 value that is slightly lower than METH or MDMA. Moreover, methylone lethality was substantially reduced in DAT knockout mice, but not in SERT knockout mice. This was very similar to a result obtained previously with METH (Numachi et al., 2007). Importantly, both of these studies found a dissociation between the effects of these drugs on temperature and lethality. This is not to say that temperature is not an important factor, but rather that there is certainly toxicity that does not require hyperthermia or that can be reduced even in the presence of hyperthermia. Overall, the role of hyperthermia in cathinone-induced toxicity and lethality should be further explored. The potential of synthetic cathinones for toxicity and lethality (as well as the mechanisms underlying these effects) remain one of the least understood aspects of their use and abuse.
8. Conclusions
The present review summarizes past and current research on the behavioral and physiological effects of the synthetic cathinones. While until recently very little was known about even the most commonly used cathinones, there is now an understanding of a variety of issues concerning this group of drugs. For example, like other psychostimulants, they are used and abused, although some of these compounds may exhibit even greater potential for abuse than these older drugs. Synthetic cathinones also have the potential to produce neurotoxic effects, although for many of these drugs this may be reduced compared to METH and MDMA, and the clinical and pre-clinical toxicity of these compounds are well established. However, more research is needed to examine the neurotoxicity and general toxicity of the synthetic cathinones, the underlying mechanisms of this toxicity and the conditions under which these effects may occur. A better understanding of the abuse potential, neurotoxicity/toxicity and lethality of the synthetic cathinones will be important for future efforts aimed at limiting the societal impact of the use and abuse of these drugs as well as for the prevention and treatment of its toxicological effects and potential overdose.
Acknowledgements
Requests for reprints should be sent to A.L. Riley, Psychopharmacology Laboratory, American University, Washington, DC 20016. The work summarized in the review was supported in part from The Mellon Foundation (ALR), the National Institute on Drug Abuse (DMK), the Department of Veterans Affairs (DMK) and The National Key Research and Development Program of China (No. 2017YFC0803605) (PX and HWS).
References
- Aarde SM, Angrish D, Barlow DJ, Wright MJ Jr., Vandewater SA, Creehan KM, Taffe MA, 2013a. Mephedrone (4-methylmethcathinone) supports intravenous self-administration in Sprague-Dawley and Wistar rats. Addict. Biol 18, 786–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA, 2013b. The novel recreational drug 3, 4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology 71, 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Creehan KM, Vandewater SA, Dickerson TJ, Taffe MA, 2015a. In vivo potency and efficacy of the novel cathinone α-pyrrolidinopentiophenone and 3, 4-methylenedioxypyrovalerone: self-administration and locomotor stimulation in male rats. Psychopharmacology 232, 3045–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Huang PK, Dickerson TJ, Taffe MA, 2015b. Binge-like acquisition of 3,4-methylenedioxypyrovalerone (MDPV) self-administration and wheel activity in rats. Psychopharmacology 232, 1867–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamowicz P, Zuba D, Byrska B, 2014. Fatal intoxication with 3-methyl-N-methylcathinone (3-MMC) and 5-(2-aminopropyl)benzofuran (5-APB). Forensic Sci. Int 245, 126–132. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, 2012. The science of making drug-addicted animals. Neuroscience 211, 107–125. [DOI] [PubMed] [Google Scholar]
- American Association of Poison Control Centers, 2011. Us Poison Centers Raise Alarm about Toxic Substance Marketed as Bath Salts. AAPCC Press Release. http://www.aapcc.org/press/22/. [Google Scholar]
- Angoa-Perez M, Kane MJ, Francescutti DM, Sykes KE, Shah MM, Mohammed AM, Thomas DM, Kuhn DM, 2012. Mephedrone, an abused psychoactive component of “bath salts” and methamphetamine congener, does not cause neurotoxicity to dopamine nerve endings of the striatum. J. Neurochem 120, 1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angoa-Perez M, Kane MJ, Briggs DI, Francescutti DM, Sykes CE, Shah MM, Thomas DM, Kuhn DM, 2013. Mephedrone does not damage dopamine nerve endings of the striatum, but enhances the neurotoxicity of methamphetamine, amphetamine, and MDMA. J. Neurochem 125, 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angoa-Perez M, Kane MJ, Herrera-Mundo N, Francescutti DM, Kuhn DM, 2014. Effects of combined treatment with mephedrone and methamphetamine or 3,4-methylenedioxymethamphetamine on serotonin nerve endings of the hippocampus. Life Sci. 97, 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angoa-Perez M, Anneken JH, Kuhn DM, 2017. Neurotoxicology of synthetic cathinone analogs. Curr. Top. Behav. Neurosci 32, 209–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anneken JH, Angoa-Perez M, Kuhn DM, 2015. 3,4-Methylenedioxypyrovalerone (MDPV) prevents while methylone enhances methamphetamine-induced damage to dopamine nerve endings: beta-ketoamphetamine modulation of neurotoxicity by the dopamine transporter. J. Neurochem 133, 211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anneken JH, Angoa-Perez M, Sati GC, Crich D, Kuhn DM, 2017a. Assessing the role of dopamine in the differential neurotoxicity patterns of methamphetamine, mephedrone, methcathinone and 4-methylmethamphetamine. Neuropharmacology Epub 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anneken JH, Angoa-Perez M, Sati GC, Crich D, Kuhn DM, 2017b. Dissecting the influence of two structural substituents on the differential neurotoxic effects of acute methamphetamine and mephedrone treatment on dopamine nerve endings with the use of 4-methylmethamphetamine and methcathinone. J. Pharmacol. Exp. Ther 360, 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo AM, Valente MJ, Carvalho M, da Silva DD, Gaspar H, Carvalho F, Bastos MD, de Pinho PG, 2015. Raising awareness of new psychoactive substances: chemical analysis and in vitro toxicity screening of’ legal high’ packages containing synthetic cathinones. Arch. Toxicol 89, 757–771. [DOI] [PubMed] [Google Scholar]
- Assi S, Gulyamova N, Kneller P, Osselton D, 2017. The effects and toxicity of cathinones from the users’ perspectives: a qualitative study. Hum. Psychopharmacol. Clin. Exp 32, 1–7. [DOI] [PubMed] [Google Scholar]
- Ator NA, Griffiths RR, 1987. Self-administration of barbiturates and benzodiazepines: a review. Pharmacol. Biochem. Be 27, 391–398. [DOI] [PubMed] [Google Scholar]
- Ator NA, Griffiths RR, 2003. Principles of drug abuse liability assessment in laboratory animals. Drug Alcohol Depen 70, S52–S72. [DOI] [PubMed] [Google Scholar]
- Balster RL, Lukas SE, 1985. Review of self-administration. Drug Alcohol Depen. 14, 249–261. [DOI] [PubMed] [Google Scholar]
- Banks ML, Worst TJ, Rusyniak DE, Sprague JE, 2014. Synthetic cathinones (“bath salts”). J. Emerg. Med 46, 632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Valone JM, Bevins RA, 1999. Locomotion and conditioned place preference produced by acute intravenous amphetamine: role of dopamine receptors and individual differences in amphetamine self-administration. Psychopharmacology 143, 39–46. [DOI] [PubMed] [Google Scholar]
- Barrios L, Grison-Hernando H, Boels D, Bouquie R, Monteil-Ganiere C, Clement R, 2016. Death following ingestion of methylone. Int. J. Legal Med 130, 381–385. [DOI] [PubMed] [Google Scholar]
- Baumann MH, 2014. Awash in a sea of “bath salts”: implications for biomedical research and public health. Addiction 109, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Wang X, Rothman RB, 2007. 3,4-methylenedioxymethamphetamine (MDMA) neurotoxicity in rats: a reappraisal of past and present findings. Psychopharmacology (Berl) 189, 407–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV, 2012. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacol. 37, 1192–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, 2013a. Psychoactive “bath salts”: not so soothing. Eur. J. Pharmacol 698, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, Brandt SD, Tella SR, Cozzi NV, Schindler CW, 2013b. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive “Bath Salts” products. Neuropsychopharmacology 38, 552–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Solis E, Watterson LR, Marusich JA, Fantegrossi WE, Wiley JL, 2014. Baths salts, spice, and related designer drugs: the science behind the headlines. J. Neurosci 34, 15150–15158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Bukhari MO, Lehner KR, Anizan S, Rice KC, Concheiro M, Huestis MA, 2016. Neuropharmacology of 3,4-methylenedioxypyrovalerone (MDPV), its metabolites, and related analogs. Curr. Topics Behav. Neurosci 32, 93–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck O, Backberg M, Signell P, Helander A, 2018. Intoxications in the STRIDA project involving a panorama of psychostimulant pyrovalerone derivatives, MDPV copycats. Clin. Toxicol 56, 256–263. [DOI] [PubMed] [Google Scholar]
- Bedingfield JB, King DA, Holloway FA, 1998. Cocaine and caffeine: conditioned place preference, locomotor activity, and additivity. Pharmacol. Biochem. Be 61, 291–296. [DOI] [PubMed] [Google Scholar]
- Benschop A, Bujalski M, Dabrowska K, Demetrovics Z, Egger D, Felinczi K, Henriques S, Kalo Z, Kamphausen G, Korf DJ, Nabben T, Silver JP, van Hout MC, Werse B, Wells J, Wieczorek Lukasz; Wouters M New psychoactive substances: transnational project on different user groups, user characteristics, extent and patterns of use, market dynamics, and best practices in prevention. NPS-transnational Project; (HOME/2014/JDRU/AG/DRUG/7077) 2017. [Google Scholar]
- Benzer TI, Nejad SH, Flood JG, 2013. Case 40–2013 - A 36-year-old man with agitation and paranoia. New England J. Med. Surg. Collat. Branches Sci 369, 2536–2545. [DOI] [PubMed] [Google Scholar]
- Berquist II MD, Baker LE, 2017. Characterization of the discriminative stimulus effects of 3,4-methylenedioxypyrovalerone in male Sprague-Dawley rats. Behav. Pharmacol 28, 394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berquist MD II, Peet MM, Baker LE, 2015. Behavioral sensitization following concurrent exposure to mephedrone and D-amphetamine in female mice. Behav. Pharmacol 26, 180–183. [DOI] [PubMed] [Google Scholar]
- Berquist MD II, Traxler HK, Mahler AM, Baker LE, 2016. Sensitization to the locomotor stimulant effects of “bath salt” constituents, 4-methylmethcathinone (4-MMC) and 3,4-methylenedioxypyrovalerone (MDPV), in male Sprague-Dawley rats. Drug Alcohol Depen. 164, 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biezonski DK, Meyer JS, 2011. The nature of 3, 4-methylenedioxymethamphetamine (MDMA)-induced serotonergic dysfunction: evidence for and against the neurodegeneration hypothesis. Curr. Neuropharmacol 9, 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilsky EJ, Hui Y, Hubbell CL, Reid LD, 1990. Methylenedioxymethamphetamine’s capacity to establish place preferences and modify intake of an alcoholic beverage. Pharmacol. Biochem. Be 37, 633–638. [DOI] [PubMed] [Google Scholar]
- Bonano JS, Glennon RA, De Felice LJ, Banks ML, Negus SS, 2014a. Abuse-related and abuse-limiting effects of methcathinone and the synthetic “bath salts” cathinone analogs methylenedioxypyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology 231, 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonano JS, Runyon SP, Hassler C, Glennon RA, Negus SS, 2014b. Effects of the neuropeptide S receptor antagonist RTI-118 on abuse-related facilitation of intracranial self-stimulation produced by cocaine and methylenedioxypyrovalerone (MDPV) in rats. Eur. J. Pharmacol 743, 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borek HA, Holstege CP, 2012. Hyperthermia and multiorgan failure after abuse of “bath salts” containing 3,4-methylenedioxypyrovalerone. Ann. Emerg. Med 60, 103–105. [DOI] [PubMed] [Google Scholar]
- Botanas CJ, Yoon SS, de la Peña JB, dela Peña IJ, Kim M, Woo T, Seo JW, Jang CG, Park KT, Lee YH, Lee YS, Kim HJ, Cheong JH, 2017a. The abuse potential of two novel synthetic cathinones with modification on the alpha-carbon position, 2-cyclohexyl-2-(methylamino)-1-phenylethanone (MACHP) and 2-(methylamino)-1-phenyloctan-1-one (MAOP), and their effects on dopaminergic activity. Pharmacol. Biochem. Be 153, 160–167. [DOI] [PubMed] [Google Scholar]
- Botanas CJ, Yoon SS, de la Peña JB, dela Peña IJ, Kim M, Woo T, Seo JW, Jang CG, Park KT, Lee YH, Lee YS, Kim HJ, Cheong JH, 2017b. A novel synthetic cathinone, 2-(methylamino)-1-(naphthalen-2-yl) propan-1-one (BMAPN), produced rewarding effects and altered striatal dopamine-related gene expression in mice. Behav. Brain Res 317, 494–501. [DOI] [PubMed] [Google Scholar]
- Botanas CJ, Yoon SS, de la Peña JB, dela Peña IJ, Kim M, Woo T, Seo JW, Jang CG, Park KT, Young HL, Yong YS, Kim HJ, Cheong JH, 2017c. The abuse potential of α-piperidinopropiophenone (PIPP) and α-piperidinopentiothiophenone (PIVT), two new synthetic cathinones with piperidine ring substituent. Biomol. Ther. (Seoul) 25, 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozarth MA, 1987. Neuroanatomical boundaries of the reward-relevant opiate-receptor field in the ventral tegmental area as mapped by the conditioned place preference method in rats. Brain Res. 414, 77–84. [DOI] [PubMed] [Google Scholar]
- Brandt SD, Sumnall HR, Measham F, Cole J, 2010. Analyses of second-generation ‘legal highs’ in the UK: initial findings. Drug Test. Anal. 2, 377–382. [DOI] [PubMed] [Google Scholar]
- Brannan TA, Soundararajan S, Houghton BL, 2004. Methamphetamine-associated shock with intestinal infarction. Med. Gen. Med 6, 6. [PMC free article] [PubMed] [Google Scholar]
- Brockwell NT, Eikelboom R, Beninger RJ, 1991. Caffeine-induced place and taste conditioning: production of dose-dependent preference and aversion. Pharmacol. Biochem. Be 38, 513–517. [DOI] [PubMed] [Google Scholar]
- Brunt TM, Atkinson AM, Nefau T, Martinez M, Lahaie E, Malzcewski A, Pazitny M, Belackova V, Brandt SD, 2017. Online test purchased new psychoactive substances in 5 different European countries: a snapshot study of chemical compositions and price. Int. J. Drug Policy 44, 105–114. [DOI] [PubMed] [Google Scholar]
- Buenrostro-Jàuregui M, Ciudad-Roberts A, Moreno J, Muñoz-Villegas P, Lòpez-Arnau R, Pubill D, Escubedo E, Camarasa J, 2016. Changes in CREB, and deltaFosB are associated with the behavioural sensitization induced by methylenedioxypyrovalerone. J. Psychopharmacol. (Oxford) 30, 707–712. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, Jayanthi S, Lyles J, 2007. Neurotoxicity of substituted amphetamines: molecular and cellular mechanisms. Neurotox. Res 11, 183–202. [DOI] [PubMed] [Google Scholar]
- Cameron KN, Kolanos R, Solis E Jr., Glennon RA, De Felice LJ, 2013a. Bath salts components mephedrone and methylenedioxypyrovalerone (MDPV) act synergistically at the human dopamine transporter. Brit. J. Pharmacol. 168, 1750–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron K, Kolanos R, Vekariya R, De Felice L, Glennon RA, 2013b. Mephedrone and methylenedioxypyrovalerone (MDPV), major constituents of “bath salts”, produce opposite effects at the human dopamine transporter. Psychopharmacology 227, 493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappell H, LeBlanc AE, 1971. Conditioned aversion to saccharin by single administrations of mescaline and d-amphetamine. Psychopharmacologia 22, 352–356. [DOI] [PubMed] [Google Scholar]
- Cappon GD, Morforda LL, Vorheesa CV, 1998. Enhancement of cocaine-induced hyperthermia fails to elicit neurotoxicity. Neurotoxicol. Taratol 20, 531–535. [DOI] [PubMed] [Google Scholar]
- Capriola M, 2013. Synthetic cathinone abuse. Clin. Pharmacol 5, 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone PN, Carbone DL, Carstairs SD, Luzi SA, 2013. Sudden cardiac death associated with methylone use. Am. J. Foren. Med. Path 34, 26–28. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, King LA, Nutt DJ, 2011. A web-based survey on mephedrone. Drug Alcohol Depen. 118, 19–22. [DOI] [PubMed] [Google Scholar]
- Carvalho M, Carmo H, Costa VM, Capela JP, Pontes H, Remião F, Carvalho F, de Lourdes Bastos M, 2012. Toxicity of amphetamines: an update. Arch. Toxicol 86, 1167–1231. [DOI] [PubMed] [Google Scholar]
- Cason AM, Grigson PS, 2013. Prior access to a sweet is more protective against cocaine self-administration in female rats than in male rats. Physiol. Behav 112, 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control, 2011. Emergency department visits after use of a drug sold as “Bath Salts”–Michigan, November 13, 2010 - March 31, 2011. Morbidity and mortality weekly report 60, 624–627. [PubMed] [Google Scholar]
- Chambers KC, Sengstake CB, 1976. Sexually dimorphic extinction of a conditioned taste aversion in rats. Anim. Learn. Behav 4, 181–185. [DOI] [PubMed] [Google Scholar]
- Cheong JH, Choi MJ, Jang C, Lee YS, Lee S, Kim HJ, Seo J, Yoon SS, 2017. Behavioral evidence for the abuse potential of the novel synthetic cathinone alphapyrrolidinopentiothiophenone (PVT) in rodents. Psychopharmacology 234, 857–867. [DOI] [PubMed] [Google Scholar]
- Chiueh CC, Kopin IJ, 1978. Centrally mediated release by cocaine of endogenous epinephrine and norepinephrine from the sympathoadrenal medullary system of unanesthetized rats. J. Pharmacol. Exp. Ther 205, 148–154. [PubMed] [Google Scholar]
- Ciudad-Roberts A, Camarasa J, Ciudad CJ, Pubill D, Escubedo E, 2015. Alcohol enhances the psychostimulant and conditioning effects of mephedrone in adolescent mice: postulation of unique roles of D3 receptors and BDNF in place preference acquisition. Br. J. Pharmacol 172, 4970–4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobuzzi JL, Siletti KA, Hurwitz ZE, Wetzell B, Baumann MH, Riley AL, 2014. Age differences in (±) 3,4-methylenedioxymethamphetamine (MDMA)-induced conditioned taste aversions and monoaminergic levels. Dev. Psychobiol 56, 635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colado MI, Camarero J, Mechan AO, Sanchez V, Esteban B, Elliott JM, Green AR, 2001. A study of the mechanisms involved in the neurotoxic action of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) on dopamine neurones in mouse brain. Brit. J. Pharmacol. 134, 1711–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Abbott M, Galindo K, Rush EL, Rice KC, France CP, 2016. Discriminative stimulus effects of binary drug mixtures: studies with cocaine, MDPV, and caffeine. J. Pharmacol. Exp. Ther 359, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colpaert FC, 1987. Drug discrimination: methods of manipulation, measurement, and analysis. In: Bozarth MA (Ed.), Methods of Assessing the Reinforcing Properties of Abused Drugs. Springer, NY, pp. 341–372. [Google Scholar]
- Coppola M, Mondola R, 2012. 3,4-methylenedioxypyrovalerone (MDPV): chemistry, pharmacology, and toxicology of a new designer drug of abuse marketed online. Toxicol. Lett (208), 12–15. [DOI] [PubMed] [Google Scholar]
- Counselman FL, McLaughlin EW, Kardon EM, Bhambhani-Bhavnani AS, 1997. Creatine phosphokinase elevation in patients presenting to the emergency department with cocaine-related complaints. Am. J. Emerg. Med 15, 221–223. [DOI] [PubMed] [Google Scholar]
- Cozzi NV, Sievert MK, Shulgin AT, Jacob P 3rd, Ruoho AE, 1999. Inhibition of plasma membrane monoamine transporters by beta-ketoamphetamines. Eur. J. Pharmacol 381, 63–69. [DOI] [PubMed] [Google Scholar]
- Creehan KM, Vandewater SA, Taffe MA, 2015. Intravenous self-administration of mephedrone, methylone and MDMA in female rats. Neuropharmacology 92, 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Niehus JS, Bachtold JF, 1992. Ambient temperature effects on taste aversion conditioned by ethanol: contribution of ethanol-induced hypothermia. Alcohol. Clin. Exp. Res 16, 1117–1124. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Tull LE, Rindal KE, Meyer PJ, 2002. Distal and proximal preexposure to ethanol in the place conditioning task: tolerance to aversive effect, sensitization to activating effect, but no change in rewarding effect. Psychopharmacology 160, 414–424. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA, 2009. Genetic influences on conditioned taste aversion. In: Reilly S, Schachtman TR (Eds.), Conditioned Taste Aversions: Behavioral and Neural Processes. Oxford University Press, NY, pp. 387–421. [Google Scholar]
- Dafters RI, Lynch E, 1998. Persistent loss of thermoregulation in the rat induced by 3, 4-methylenedioxymethamphetamine (MDMA or “Ecstasy”) but not by fenfluramine. Psychopharmacology 138, 207–212. [DOI] [PubMed] [Google Scholar]
- Daskalopoulou M, Rodger A, Philips AN, Sherr L, Speakman A, Collins S, Elford J, Johnson MA, Gilson R, Fisher M, Wilkins E, Anderson J, McDonnell J, Edwards S, Perry N, O’Connell R, Lascar M, Jones M, Johnson AM, Hart G, Miners A, Geretti AM, Burman WJ, Lampe FC, 2014. Recreational drug use, polydrug use and sexual behavior in HIV-diagnosed men who have sex with men in the UK: results from the cross-sectional ASTRA study. Lancet HIV 1, e22–e31. [DOI] [PubMed] [Google Scholar]
- Davis CM, Riley AL, 2010. Conditioned taste aversion learning. Ann. NY. Acad. Sci 1187, 247–275. [DOI] [PubMed] [Google Scholar]
- Davis CM, Roma PG, Dominguez JM, Riley AL, 2007. Morphine-induced place conditioning in Fischer and Lewis rats: acquisition and dose-response in a fully biased procedure. Pharmacol. Biochem. Be 86, 516–523. [DOI] [PubMed] [Google Scholar]
- Davis S, Blakey K, Rands-Trevor K, 2012. GC-MS and GC-IRD analysis of 2-, 3- and 4-methylmethamphetamine and 2-, 3- and 4-methylamphetamine. Forensic Sci. Int 220, 67–73. [DOI] [PubMed] [Google Scholar]
- den Hollander B, Rozov S, Linden AM, Uusi-Oukari M, Ojanperä I, Korpi ER, 2013. Long-term cognitive and neurochemical effects of “bath salt” designer drugs methylone and mephedrone. Pharmacol. Biochem. Behav 103, 501–509. [DOI] [PubMed] [Google Scholar]
- DeRuiter J, Hayes L, Valaer A, Clarj CR, 1994. Methcathinone and designer analogues: synthesis, stereochemical analysis, and analytical properties. J. Chromatogr. Sci 32, 552–564. [Google Scholar]
- Dolan SB, Chen Z, Huang R, Gatch MB, 2018. “Ecstasy” to addiction: mechanisms and reinforcing effects of three synthetic cathinone analogs of MDMA. Neuropharmacology 133, 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolengevich-Segal H, Rodríguez-Salgado B, Gómez-Arnau J, Sánchez-Mateos D, 2016. Severe psychosis, drug dependence, and hepatitis C related to slamming mephedrone. Case Rep. Psychiatry 2016, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP, 2010. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 72, 114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug Enforcement Administration, United States Department of Justice, 2011. Schedules of controlled substances: temporary placement of three synthetic cathinones into schedule I. Fed. Regist 76, 65371–65375. [PubMed] [Google Scholar]
- Drug Enforcement Administration, United States Department of Justice, 2013. Establishment of drug codes for 26 substances. Fed. Regist 78, 664–666. [PubMed] [Google Scholar]
- Drug Enforcement Administration, United States Department of Justice, 2014. Schedules of controlled substances: temporary placement of 10 synthetic cathinones into schedule I. Fed. Regist 79, 12938–12943. [PubMed] [Google Scholar]
- Drug Enforcement Administration, United States Department of Justice, 2016. Schedules of controlled substances: extension of temporary placement of 10 synthetic cathinones in schedule I of the Controlled Substances Act. Fed. Regist 81, 11429–11431. [PubMed] [Google Scholar]
- Drug Enforcement Administration, United States Department of Justice, 2017. Schedules of controlled substances: placement of 10 synthetic cathinones into schedule I. Fed. Regist 82, 12171–12177. [PubMed] [Google Scholar]
- Eiden C, Mathieu O, Cathala P, Debruyne D, Baccino E, Petit P, Peyriere H, 2013. Toxicity and death following recreational use of 2-pyrrolidino valerophenone. Clin. Toxicol 51, 899–903. [DOI] [PubMed] [Google Scholar]
- Elsmore TF, Fletcher GV, 1972. Δ9-tetrahydrocannabinol: aversive effects in rat at high doses. Science 175, 911–912. [DOI] [PubMed] [Google Scholar]
- Escubedo E, Guitart L, Sureda FX, Jiménez A, Pubill D, Pallàs M, Camins A, Camarasa J, 1998. Microgliosis and downregulation of adenosine transporter induced by methamphetamine in rats. Brain Res. 814, 120–126. [DOI] [PubMed] [Google Scholar]
- Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A, 2013. Substituted methcathinones differ in transporter and receptor interactions. Biochem. Pharmacol 85, 1803–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshleman AJ, Forster MJ, Wolfrum KM, Johnson RA, Janowsky A, Gatch MB, 2014. Behavioral and neurochemical pharmacology of six psychoactive substituted phenethylamines: mouse locomotion, rat drug discrimination and in vitro receptor and transporter binding and function. Psychopharmacology 231, 875–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Fomenko V, Kaganovsky K, Shelton K, Wenzel JM, 2015. On the positive and negative affective responses to cocaine and their relation to drug self-administration in rats. Psychopharmacology 232, 2363–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshleman AJ, Wolfrum KM, Reed JF, Kim SO, Swanson T, Johnson RA, Janowsky A, 2017. Structure-activity relationships of substituted cathinones, with transporter binding, uptake, and release. J. Pharmacol. Exp. Ther 360, 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction, 2014. Injection of synthetic cathinones in Europe. Perspectives on Drugs series. Lisbon: EMCCDA; May 2014; updated June 2015. (emcdda.europa.eu/topics/pods/synthetic-cathinonesinjection). [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction, 2016. European Drug Report 2016: Trends and Developments, Publications Office of the European Union, Luxembourg. (http://www.emcdda.europa.eu/edr2016). [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction, 2017. European Drug Report 2017: Trends and Developments, Publications Office of the European Union, Luxembourg. (http://www.emcdda.europa.eu/edr2017). [Google Scholar]
- Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC, 2013. In vivo effects of abused “bath salt” constituent 3, 4-methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacol. 38, 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischman M, 1988. Behavioral pharmacology of cocaine. J. Clin. Psychiat 49, 7–10. [PubMed] [Google Scholar]
- Fleckenstein AE, Haughey HM, Metzger RR, Kokoshka JM, Riddle EL, Hanson JE, Gibb JW, Hanson GR, 1999. Differential effects of psychostimulants and related agents on dopaminergic and serotonergic transporter function. Eur. J. Pharmacol 382, 45–49. [DOI] [PubMed] [Google Scholar]
- Fouriezos G, Nawiesniak E, 1987. A comparison of two methods designed to rapidly estimate thresholds of rewarding brain stimulation. In: Bozarth MA (Ed.), Methods of Assessing the Reinforcing Properties of Abused Drugs. Springer, New York, pp. 447–462. [Google Scholar]
- Fratantonio J, Andrade L, Febo M, 2015. Designer drugs: a synthetic catastrophe. J. Reward Defic. Syndr 1, 82–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman KB, Riley AL, 2005. Cocaine-induced conditioned taste avoidance over extended CS-US intervals. Behav. Pharmacol 16, 591–595. [DOI] [PubMed] [Google Scholar]
- Freeman KB, Riley AL, 2009. The origins of conditioned taste aversion learning: an historical analysis. In: Reilly S, Schachtman T (Eds.), Conditioned Taste Aversion: Behavioral and Neural Processes. Academic Press, NY, pp. 9–33 2009. [Google Scholar]
- Freeman KB, Rice KC, Riley AL, 2005. Assessment of monoamine transporter inhibition in the mediation of cocaine-induced conditioned taste aversions. Pharmacol. Biochem. Be. 82, 583–589. [DOI] [PubMed] [Google Scholar]
- Fukumura M, Cappon GD, Pu C, Broening HW, Vorhees CV, 1998. A single dose model of methamphetamine-induced neurotoxicity in rats: effects on neostriatal monoamines and glial fibrillary acidic protein. Brain Res. 806, 1–7. [DOI] [PubMed] [Google Scholar]
- Gannon BM, Fantegrossi WE, 2016. Cocaine-like discriminative stimulus effects of mephedrone and naphyrone in mice. J. Drug Alcohol Res 5, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Williamson A, Suzuki M, Rice KC, Fantegrossi WE, 2016. Stereoselective effects of abused “bath salt” constituent 3,4-methylenedioxypyrovalerone in mice: drug discrimination, locomotor activity, and thermoregulation. J. Pharmacol. Exp. Ther 356, 615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Galindo KI, Mesmin MP, Rice KC, Collins GT, 2017a. Relative reinforcing effects of second-generation synthetic cathinones: acquisition of self-administration and fixed ratio dose-response curves in rats. Neuropharmacol 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Galindo KI, Rice KC, Collins GT, 2017b. Individual differences in the relative reinforcing effects of 3,4-methylenedioxypyrovalerone under fixed and progressive ratio schedules of reinforcement in rats. J. Pharmacol. Exp. Ther 361, 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Rice KC, Collins GT, 2017c. Reinforcing effects of abused “bath salts” constituents 3,4-methylenedioxypyrovalerone and α-pyrrolidinopentiophenone and their enantiomers. Behav. Pharmacol 28, 578–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Russell LN, Modi MS, Rice KC, Fantegrossi WE, 2017d. Effects of orally self-administered bath salt constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice. Drug Alcohol Depen. 179, 408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Galindo KI, Mesmin MP, Rice KC, Collins GT, 2018. Reinforcing effects of binary mixtures of common bath salt constituents: studies with 3,4-methylenedioxypyrovalerone (MDPV), 3,4-methylenedioxymethcathinone (methylone), and caffeine in rats. Neuropsychopharmacol. 43, 761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J, Ervin F, 1968. Gustatory-visceral and telereceptor-cutaneous conditioning: adaptation in internal and external milieus. Commun. Behav. Biol 1, 389–415. [Google Scholar]
- Garcia J, Koelling RA, 1967. A comparison of aversions induced by X rays, toxins, and drugs in the rat. Radiat. Res Supplement 7, 439–450. [PubMed] [Google Scholar]
- Garcia J, Kimeldorf DJ, Koelling RA, 1955. Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science 122, 157–158. [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ, 2013. Locomotor stimulant and discriminative stimulus effects of “bath salt” cathinones. Behav. Pharmacol 24, 437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Dolan SB, Forster MJ, 2015a. Comparative behavioral pharmacology of three pyrrolidine-containing synthetic cathinone derivatives. J. Pharmacol. Exp. Ther 354, 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Rutledge MA, Forster MJ, 2015b. Discriminative and locomotor effects of five synthetic cathinones in rats and mice. Psychopharmacology 232, 1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Dolan SB, Forster MJ, 2017. Locomotor activity and discriminative stimulus effects of a novel series of synthetic cathinone analogs in mice and rats. Psychopharmacology 234, 1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German CL, Fleckenstein AE, Hanson GR, 2014. Bath salts and synthetic cathinones: an emerging designer drug phenomenon. Life Sci. 97, 2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA, 2014. Bath salts, mephedrone, and methylenedioxypyrovalerone as emerging illicit drugs that will need targeted therapeutic intervention. Adv. Pharmacol 69, 581–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA, Young R, 2016. Neurobiology of 3,4-methylenedioxypyrovalerone (MDPV) and α-pyrrolidinovalerophenone (α-PVP). Brain Res. Bull 126, 111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowa JR, Shaw AE, Riley AL, 1994. Cocaine-induced conditioned taste aversions: comparisons between effects in LEW/N and F344/N rat strains. Psychopharmacology 114, 229–232. [DOI] [PubMed] [Google Scholar]
- Gore-Langton JK, Flax SM, Pomfrey RL, Wetzell BB, Riley AL, 2015. Measures of the aversive effects of drugs: a comparison of conditioned taste and place aversions. Pharmacol. Biochem. Be 134, 99–105. [DOI] [PubMed] [Google Scholar]
- Gosnell BA, Yracheta JM, Bell SM, Lane KE, 1996. Intravenous self-administration of cathinone by rats. Behav. Pharmacol 7, 526–531. [PubMed] [Google Scholar]
- Goudie A, 1979. Aversive stimulus properties of drugs. Neuropharmacology 18, 971–979. [DOI] [PubMed] [Google Scholar]
- Goudie A, Dickins D, Thornton E, 1978. Cocaine-induced conditioned taste aversions in rats. Pharmacol. Biochem. Be 8, 757–761. [DOI] [PubMed] [Google Scholar]
- Grecco GG, Sprague JE, 2016. Impact of functional group modifications on designer phenethylamine induced hyperthermia. Chem. Res. Toxicol 29, 871–878. [DOI] [PubMed] [Google Scholar]
- Gregg RA, Tallarida CS, Reitz A, McCurdy C, Rawls SM, 2013a. Mephedrone (4-methylmethcathinone), a principal constituent of psychoactive bath salts, produces behavioral sensitization in rats. Drug Alcohol Depen. 133, 746–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RA, Tallarida CS, Reitz AB, Rawls SM, 2013b. Mephedrone interactions with cocaine: prior exposure to “bath salt” constituent enhances cocaine-induced locomotor activation in rats. Behav. Pharmacol 24, 684–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RA, Baumann MH, Partilla JS, Bonano JS, Vouga A, Tallarida CS, Velvadapu V, Smith GR, Peet MM, Reitz AB, Negus SS, Rawls SM, 2015. Stereochemistry of mephedrone neuropharmacology: enantiomer-specific behavioural and neurochemical effects in rats. Br. J. Pharmacol 172, 883–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RA, Hicks C, Nayak SU, Tallarida CS, Nucero P, Smith GR, Reitz AB, Rawls SM, 2016. Synthetic cathinone MDPV downregulates glutamate transporter subtype I (GLT-1) and produces rewarding and locomotor-activating effects that are reduced by a GLT-1 activator. Neuropharmacology 108, 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigson PS, Freet CS, 2000. The suppressive effects of sucrose and cocaine, but not lithium chloride, are greater in Lewis than in Fischer rats: evidence for the reward comparison hypothesis. Behav. Neurosci 114, 353–363. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Twining RC, 2002. Cocaine-induced suppression of saccharin intake: a model of drug-induced devaluation of natural rewards. Behav. Neurosci 116, 321–333. [PubMed] [Google Scholar]
- Gyarmathy VA, Peterfi A, Figeczki T, Kiss J, Medgyesi-Frank K, Posta J, Csorba J, 2017. Diverted medications and new psychoactive substances - A chemical network analysis of discarded injecting paraphernalia in Hungary. Int. J. Drug Policy 46, 61–65. [DOI] [PubMed] [Google Scholar]
- Gygi MP, Gibb JW, Hanson GR, 1996. Methcathinone: an initial study of its effects on monoaminergic systems. J. Pharmacol. Exp. Ther 276, 1066–1072. [PubMed] [Google Scholar]
- Gygi MP, Fleckenstein AE, Gibb JW, Hanson GR, 1997. Role of endogenous dopamine in the neurochemical deficits induced by methcathinone. J. Pharmacol. Exp. Ther 283, 1350–1355. [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Wen Chu P, Ellis JD, Allen SC, Andrenyak DM, Vieira-Brock PL, German CL, Conrad KM, Hoonakker AJ, Gibb JW, Wilkins DG, Hanson GR, Fleckenstein AE, 2011. 4-methylmethcathinone (mephedrone): neuropharmacological effects of a designer stimulant of abuse. J. Pharmacol. Exp. Ther 339, 530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin LE, Collins SA, Yamamoto BK, 2014a. Neurotoxicity of methamphetamine and 3,4-methylenedioxymethamphetamine. Life Sci. 97, 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin LE, Northrop NA, Yamamoto BK, 2014b. Ammonia mediates methamphetamine-induced increases in glutamate and excitotoxicity. Neuropsychopharmacol. 39, 1031–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey EL, Baker LE, 2016. Differential effects of 3, 4-methylenedioxypyrovalerone (MDPV) and 4-methylmethcathinone (mephedrone) in rats trained to discriminate MDMA or a d-amphetamine+MDMA mixture. Psychopharmacology 233, 673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey EL, Burroughs RL, Baker LE, 2017. Effects of D1 and D2 receptor antagonists on the discriminative stimulus effects of methylendioxypyrovalerone and mephedrone in male Sprague-Dawley rats trained to discriminate D-amphetamine. Behav. Pharmacol 28, 586–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hataoka K, Kaizaki-Mitsumoto A, Numazawa S, 2017. Alpha-PVP induces the rewarding effect via activating dopaminergic neuron. J. Toxicol. Sci 42, 539–543. [DOI] [PubMed] [Google Scholar]
- Hicks C, Huang P, Ramos L, Nayak SU, Caro Y, Reitz AB, Smith GR, Lee DYW, Rawls SM, Liu-Chen L-Y, 2018. Dopamine D1-like receptor agonist and D2like receptor antagonist (−)-stepholidine reduces reinstatement of drug-seeking behavior for 3,4-methylenedioxypyrovalerone (MDPV) in rats. ACS Chem. Neurosci 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope VD, Cullen KJ, Smith J, 2016. Is the recent emergence of mephedrone injecting in the United Kingdom associated with elevated risk behaviours and blood borne virus infection? Euro Surveill. 21, 1–9. [DOI] [PubMed] [Google Scholar]
- Horan B, Smith M, Gardner EL, Lepore M, Ashby CR, 1997. (−)-Nicotine produces conditioned place preference in Lewis, but not Fischer 344 rats. Synapse 26, 93–94. [DOI] [PubMed] [Google Scholar]
- Horger BA, Shelton K, Schenk S, 1990. Preexposure sensitizes rats to the rewarding effects of cocaine. Pharmacol. Biochem. Be. 37, 707–711. [DOI] [PubMed] [Google Scholar]
- Horger BA, Giles MK, Schenk S, 1992. Preexposure to amphetamine and nicotine predisposes rats to self-administer a low dose of cocaine. Psychopharmacology 107, 271–276. [DOI] [PubMed] [Google Scholar]
- Hunt T, Amit Z, 1987. Conditioned taste aversion induced by self-administered drugs: paradox revisited. Neurosci. Biobehav. R 11, 107–130. [DOI] [PubMed] [Google Scholar]
- Hurwitz ZE, Merluzzi AP, Riley AL, 2013. Age-dependent differences in morphine-induced taste aversions. Dev. Psychobiol 55, 415–428. [DOI] [PubMed] [Google Scholar]
- Huskinson SL, Naylor JE, Townsend EA, Rowlett JK, Blough BE, Freeman KB, 2017. Self-administration and behavioral economics of second-generation synthetic cathinones in male rats. Psychopharmacology 234, 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam SF, Patel H, Mahmoud M, Prakash NA, King MS, Fremont RD, 2013. Bath salts intoxication: a case series. J. Emerg. Med 45, 361–365. [DOI] [PubMed] [Google Scholar]
- Imperio CG, Grigson PS, 2015. Greater avoidance of a heroin-paired taste cue is associated with greater escalation of heroin self-administration in rats. Behav. Neurosci 129, 380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infurna RN, Spear LP, 1979. Developmental changes in amphetamine-induced taste aversions. Pharmacol. Biochem. Be 11, 31–35. [DOI] [PubMed] [Google Scholar]
- Jablonski SA, Williams MT, Vorhees CV, 2016. Mechanisms involved in the neurotoxic and cognitive effects of developmental methamphetamine exposure. Birth Defects Res. (Part C) 108, 131–141. [DOI] [PubMed] [Google Scholar]
- Jacobs W, 2006. Fatal amphetamine-associated cardiotoxicity and its medicolegal implications. Am. J. Foren. Med. Path 27, 156–160. [DOI] [PubMed] [Google Scholar]
- James D, Adams RD, Spears R, Cooper G, Lupton DJ, Thompson JP, Thomas SH, 2011. Clinical characteristics of mephedrone toxicity reported to the UK National Poisons Information Service. J. Emerg. Med 28, 686–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järbe TUC, 1986. State-dependent learning and drug discriminative control of behaviour: an overview. Acta Neurol. Scand 74 (S109), 37–60. [DOI] [PubMed] [Google Scholar]
- Javadi-Paydar M, Nguyen JD, Vanewater SA, Dickerson TJ, Taffe MA, 2017. Locomotor and reinforcing effects of pentedrone, pentylone and methylone in rats. Neuropharmacology Epub 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE, Schuster CR, 1981. A comparison of the behavioral effects of l- and dl-cathinone and d-amphetamine. J. Pharmacol. Exp. Ther 219, 355–362. [PubMed] [Google Scholar]
- Johnson DW, Glick SD, 1993. Dopamine release and metabolism in nucleus accumbens and striatum of morphine-tolerant and nontolerant rats. Pharmacol. Biochem. Behav 46, 341–347. [DOI] [PubMed] [Google Scholar]
- Johnson PS, Johnson MW, 2014. Investigation of “bath salts” use patterns within an online sample of users in the United States. J. Psychoactive Drugs 46, 369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalasinsky KS, Hugel J, Kish SJ, 2004. Use of MDA (the “love drug”) and methamphetamine in Toronto by unsuspecting users of ecstasy (MDMA). J. Forensic Sci 49, 1106–1112. [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, 1990. Effect of acute and daily cocaine treatment on extracellular dopamine in the nucleus accumbens. Synapse. 5, 48–58. [DOI] [PubMed] [Google Scholar]
- Kalix P, 1992. Cathinone, a natural amphetamine. Pharmacol. Toxicol 70, 77–86. [DOI] [PubMed] [Google Scholar]
- Kamińska K, Noworyta-Sokołowska K, Górska A, Rzemieniec J, Wnuk A, Wojtas A, Kreiner G, Kajta M, Gołembiowska K, 2018. The effects of exposure to mephedrone during adolescence on brain neurotransmission and neurotoxicity in adult rats. Neurotox. Res 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski BJ, Griffiths RR, 1994. Intravenous self-injection of methcathinone in the baboon. Pharmacol. Biochem. Be 47, 981–983. [DOI] [PubMed] [Google Scholar]
- Kapasi A, Mattana J, Wagner J, 1997. Morphine amplifies cocaine-induced renal cortical expression of tissue inhibitors of metalloproteinase (TIMP)-2. J. Am. Soc. Nephrol 6 (528A). [Google Scholar]
- Karch S, 1987. Serum catecholamines in cocaine-intoxicated patients with cardiac symptoms. Ann. Emerg. Med 16, 481. [Google Scholar]
- Karch SB, 2015. Cathinone neurotoxicity (“the “3Ms”). Curr. Neuropharmacol 13, 21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karila L, Megarbane B, Cottencin O, Lejoyeux M, 2015. Synthetic cathinones: a new public health problem. Curr. Neuropharmacol 13, 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karila L, Billieux J, Benyamina A, Lancon C, Cottencin O, 2016. The effects and risks associated to mephedrone and methylone in humans: a review of the preliminary evidences. Brain Res. Bull 126, 61–67. [DOI] [PubMed] [Google Scholar]
- Karila L, Lafaye E, Scocard A, Cottencin O, Benyamina A, 2017. MDPV and α-PVP use in humans: the twisted sisters. Neuropharmacology Epub 1–8. [DOI] [PubMed] [Google Scholar]
- Karlsson L, Andersson M, Kronstrand R, Kugelberg FC, 2014. Mephedrone, methylone and 3, 4-methylenedioxypyrovalerone (MDPV) induce conditioned place preference in mice. Basic Clinical Pharmacol. Toxicol 115, 411–416. [DOI] [PubMed] [Google Scholar]
- Kelly JP, 2011. Cathinone derivatives: a review of their chemistry, pharmacology and toxicology. Drug Test. Anal 3, 439–453. [DOI] [PubMed] [Google Scholar]
- Kelly MA, Low MJ, Rubinstein M, Phillips TJ, 2008. Role of dopamine D1-like receptors in methamphetamine locomotor responses of D2 receptor knockout mice. Genes Brain Behav. 7, 568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly BC, Wells BE, Pawson M, Leclair A, Parsons JT, Golub SA, 2013. Novel psychoactive drug use among younger adults involved in US nightlife scenes. Drug Alcohol Rev. 32, 588–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King HE, Riley AL, 2013. A history of morphine-induced taste aversion learning fails to affect morphine-induced place preference conditioning in rats. Learn. Behav 41, 433–442. [DOI] [PubMed] [Google Scholar]
- King HE, Riley AL, 2016. The affective properties of synthetic cathinones: role of reward and aversion in their abuse. Curr. Topics Behav. Neurosci 32, 165–182. [DOI] [PubMed] [Google Scholar]
- King HE, Wetzell B, Rice KC, Riley AL, 2014. 3,4-methylenedioxypyrovalerone (MDPV)-induced conditioned taste avoidance in the F344/N and LEW rat strains. Pharmacol. Biochem. Be (126), 163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King HE, Wakeford A, Taylor W, Wetzell B, Rice KC, Riley AL, 2015a. Sex differences in 3, 4-methylenedioxypyrovalerone (MDPV)-induced taste avoidance and place preferences. Pharmacol. Biochem. Be 137, 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King HE, Wetzell B, Rice KC, Riley AL, 2015b. An assessment of MDPV-induced place preference in adult Sprague-Dawley rats. Drug Alcohol Depen. 146, 116–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby T, Thornber-Dunwell M, 2013. High-risk drug practices tighten grip on London gay scene. Lancet 381, 101–102. [DOI] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L, 2006. Escalation of methamphetamine self-administration in rats: a dose–effect function. Psychopharmacology 186, 48–53. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Kim AH, Wakabayashi KT, Baumann MH, Shaham Y, 2015. Effects of social interaction and warm ambient temperature on brain hyperthermia induced by the designer drugs methylone and MDPV. Neuropsychopharmacol. 40, 436–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolanos R, Partilla JS, Baumann MH, Hutsell BA, Banks ML, Negus SS, Glennon RA, 2015a. Stereoselective actions of methylenedioxypyrovalerone (MDPV) to inhibit dopamine and norepinephrine transporters and facilitate intracranial self-stimulation in rats. ACS Chem. Neuorsci 6, 771–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolanos R, Sakloth F, Jain AD, Partilla JS, Baumann MH, Glennon RA, 2015b. Structural modification of the designer stimulant α‑pyrrolidinovalerophenone (α-PVP) influences potency at dopamine transporters. ACS Chem. Neurosci 6, 1726–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2016. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiat. 3, 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Arends MA, Le Moal M, 2014. Drugs, Addiction and the Brain. Erlbaum, Academic Press, Waltham, MA. [Google Scholar]
- Kosten TA, Miserendino M, Chi S, Nestler EJ, 1994. Fischer and Lewis rat strains show differential cocaine effects in conditioned place preference and behavioral sensitization but not in locomotor activity or conditioned taste aversion. J. Pharmacol. Exp. Ther 269, 137–144. [PubMed] [Google Scholar]
- Kramer C, Wetzel D, Wijdicks E, 2016. Devastating delayed leukoencephalopathy associated with bath salt inhalation. Neurocrit. Care 24, 454–458. [DOI] [PubMed] [Google Scholar]
- Lancellotti D, Bayer BM, Glowa JR, Houghtling RA, Riley AL, 2001. Morphine-induced conditioned taste aversions in the LEW/N and F344/N rat strains. Pharmacol. Biochem. Be 68, 603–610. [DOI] [PubMed] [Google Scholar]
- Lantz SM, Rosas-Hernandez H, Cuevas E, Robinson B, Rice KC, Fantegrossi WE, Imam SZ, Paule MG, Ali SF, 2017. Monoaminergic toxicity induced by cathinone phthalimide: an in vitro study. Neurosci. Lett 655, 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester D, Nachman M, Le Magnen J, 1970. Aversive conditioning by ethanol in the rat. Q. J. Stud. Alcohol 31, 578–586. [PubMed] [Google Scholar]
- Levine RR, 1990. Pharmacology: Drug Actions and Reactions, 4th edition. Little, Brown and Co., Boston, MA. [Google Scholar]
- Levine M, Levitan R, Skolnik A, 2013. Compartment syndrome after “bath salts” use: a case series. Ann. Emerg. Med 61, 480–483. [DOI] [PubMed] [Google Scholar]
- Lin JY, Arthurs J, Reilly S, 2015. Gustatory insular cortex, aversive taste memory and taste neophobia. Neurobiol. Learn. Mem 119, 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisek R, Xu W, Yuvasheva E, Chiu YT, Reitz AB, Liu-Chen LY, Rawls SM, 2012. Mephedrone (“bath salt”) elicits conditioned place preference and dopamine-sensitive motor activation. Drug Alcohol Depen. 126, 257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listos J, Talarek S, Gryzinska M, Listos P, Kedzierska E, Orzelska-Gorka J, Dylewska M, Lupina M, Kotlinska JH, 2017. Mephedrone exposure in adolescent rats alters the rewarding effect of morphine in adults. Eur. J. Pharmacol 810, 63–69. [DOI] [PubMed] [Google Scholar]
- López-Arnau R, Martínez-Clemente J, Pubill D, Escubedo E, Camarasa J, 2014b. Serotonergic impairment and memory deficits in adolescent rats after binge exposure of methylone. J. Psychopharmacol. (Oxford) 28, 1053–1063. [DOI] [PubMed] [Google Scholar]
- López-Arnau R, Martínez-Clemente J, Rodrigo T, Pubill D, Camarasa J, Escubedo E, 2015. Neuronal changes and oxidative stress in adolescent rats after repeated exposure to mephedrone. Toxicol. Appl. Pharmacol 286, 27–35. [DOI] [PubMed] [Google Scholar]
- López-Arnau R, Buenrostro-Jáuregui M, Muñoz-Villegas P, Rodríguez-Morató J, Ciudad-Roberts A, Duart L, Camarasa J, De la Torre R, Pubill D, Escubedo E, 2017a. The combination of MDPV and ethanol results in decreased cathinone and increased alcohol levels. Study of such pharmacological interaction. Prog. Neuropsychopharmacol. Biol. Psychiatry 76, 19–28. [DOI] [PubMed] [Google Scholar]
- López-Arnau R, Lujan MA, Duart-Castells L, Pubill D, Camarasa J, Valverse O, Escubedo E, 2017b. Exposure of adolescent mice to 3,4-methylenedioxypyrovalerone increases the psychostimulant, rewarding and reinforcing effects of cocaine in adulthood. Br. J. Pharmacol 174, 1161–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Arnau R, Buenrostro-Jáuregui M, Camarasa J, Pubill D, Escubedo E, 2018. Effect of the combination of mephedrone plus ethanol on serotonin and dopamine release in the nucleus accumbens and medial prefrontal cortex of awake rats. N-S. Arch. Pharmacol 391, 247–254. [DOI] [PubMed] [Google Scholar]
- López-Arnau R, Martínez-Clemente J, Pubill D, Escubedo E, Camarasa J, 2012. Comparative neuropharmacology of three psychostimulant cathinone derivatives: butylone, mephedrone and methylone. Brit. J. Pharmacol 167, 407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Arnau R, Martínez-Clemente J, Abad S, Pubill D, Camarasa J, Escubedo E, 2014a. Repeated doses of methylone, a new drug of abuse, induce changes in serotonin and dopamine systems in the mouse. Psychopharmacology 231, 3119–3129. [DOI] [PubMed] [Google Scholar]
- Luethi D, Kolaczynska KE, Docci L, Krähenbühl S, Hoener MC, Liechti ME, 2017a. Pharmacological profile of mephedrone analogs and related new psychoactive substances. Neuropharmacology 134, 4–12. [DOI] [PubMed] [Google Scholar]
- Luethi D, Liechti ME, Krahenbuhl S, 2017b. Mechanisms of hepatocellular toxicity associated with new psychoactive synthetic cathinones. Toxicology 387, 57–66. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Seiden LS, 1998. Small changes in ambient temperature cause large changes in 3, 4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J. Neurosci 18, 5086–5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinetti LJ, Antonides HM, 2013. Analysis of synthetic cathinones commonly found in bath salts in human performance and postmortem toxicology: method development, drug distribution and interpretation of results. J. Anal. Toxicol 37, 135–146. [DOI] [PubMed] [Google Scholar]
- Martin JC, Ellinwood EH, 1973. Conditioned aversion to a preferred solution following methamphetamine injections. Psychopharmacologia 29, 253–261. [DOI] [PubMed] [Google Scholar]
- Martinez-Clemente J, López -Arnau R, Abad S, Pubill D, Escubedo E, Camarasa J, 2014. Dose and time-dependent selective neurotoxicity induced by mephedrone in mice. PLoS ONE 9, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Clemente J, Escubedo E, Pubill D, Camarasa J, 2012. Interaction of mephedrone with dopamine and serotonin targets in rats. Eur. Neuropsychopharmacol 22, 231–236. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Grant KR, Blough BE, Wiley JL, 2012. Effects of synthetic cathinones contained in “bath salts” on motor behavior and a functional observational battery in mice. Neurotoxicology 33, 1305–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Antonazzo KR, Wiley JL, Blough RE, Partilla JS, Baumann MH, 2014. Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3,4-methylenedioxypyrovalerone (MDPV). Neuropharmacology 87, 206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JC, Lefever TW, Blough BE, Thomas BF, Wiley JL, 2016. Pharmacological effects of methamphetamine and alpha-PVP vapor and injection. Neurotoxicology 55, 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mash DC, 2016. Excited delirium and sudden death: a syndromal disorder at the extreme end of the neuropsychiatric continuum. Front. Physiol 7, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastropaolo JP, Moskowitz KH, Dacanay RJ, Riley AL, 1989. Conditioned taste aversions as a behavioral baseline for drug discrimination learning: an assessment with phencyclidine. Pharmacol. Biochem. Be 32, 1–8. [DOI] [PubMed] [Google Scholar]
- Matsunaga T, Morikawa Y, Kamata K, Shibata A, Miyazono H, Sasajima Y, Suenami K, Sato K, Takekoshi Y, Endo S, El-Kabbani O, Ikari A, 2017a. Alphapyrrolidinononanophenone provokes apoptosis of neuronal cells through alterations in antioxidant properties. Toxicology 386, 93–102. [DOI] [PubMed] [Google Scholar]
- Matsunaga T, Morikawa Y, Tanigawa M, Kamata K, Shibata A, Sasajima Y, Suenami K, Sato K, Takekoshi Y, Endo S, El-Kabbani O, Ikari A, 2017b. Structure-activity relationship for toxicity of alpha-pyrrolidinophenones in human aortic endothelial cells. Forensic Toxicol. 35, 309–316. [Google Scholar]
- Matthews A, Sutherland R, Peacock A, Buskirk JV, Whittaker E, Burns L, Bruno R, 2017. I like the old stuff better than the new stuff? Subjective experiences of new psychoactive substances. Int. J. Drug Policy 40, 44–49. [DOI] [PubMed] [Google Scholar]
- McCall H, Adams N, Mason D, Willis J, 2015. What is chemsex and why does it matter? BMJ 351, 1–2. [DOI] [PubMed] [Google Scholar]
- Mead AN, Rocha BA, Donovan DM, Katz JL, 2002. Intravenous cocaine inducedactivity and behavioral sensitization in norepinephrine-, but not dopamine-transporter knockout mice. Eur. J. Neurosci 16, 514–520. [DOI] [PubMed] [Google Scholar]
- Merluzzi AP, Hurwitz ZE, Briscione MA, Cobuzzi JL, Wetzell B, Rice KC, Riley AL, 2014. Age-dependent MDPV-induced taste aversions and thermoregulation in adolescent and adult rats. Dev. Psychobiol 56, 943–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BL, Stogner JM, 2014. Not-so-clean fun: a profile of bath salt users among a college sample in the United States. J. Psychoactive Drugs 46, 147–153. [DOI] [PubMed] [Google Scholar]
- Miner NB, O’Callaghan JP, Phillips TJ, Janowsky A, 2017. The combined effects of 3,4-methylenedioxymethamphetamine (MDMA) and selected substituted methcathinones on measures of neurotoxicity. Neurotoxicol. Teratol 61, 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa M, Kojima T, Nakaji S, 2011. Behavioral and rewarding effects of methylone, an analog of MDMA in mice. Hirosaki Med. J 62, 56–71. [Google Scholar]
- Mohr ALA, Friscia M, Yeakel JK, Logan BK, 2018. Use of synthetic stimulants and hallucinogens in a cohort of electronic dance music festival attendees. Forensic Sci. Int 282, 168–178. [DOI] [PubMed] [Google Scholar]
- Morice E, Denis C, Giros B, 2010. Evidence of long-term expression of behavioral sensitization to both cocaine and ethanol in dopamine transporter knockout mice. Psychopharmacology 208, 57–66. [DOI] [PubMed] [Google Scholar]
- Motbey CP, Hunt GE, Bowen MT, Artiss S, McGregor IS, 2012a. Mephedrone (4-methylmethcathinone, ‘meow’): acute behavioural effects and distribution of Fos expression in adolescent rats. Addict. Biol 17, 409–422. [DOI] [PubMed] [Google Scholar]
- Motbey CP, Karanges E, Li KM, Wilkinson S, Winstock AR, Ramsay J, Hicks C, Kendig MD, Wyatt N, Callaghan PD, McGregor IS, 2012b. Mephedrone in adolescent rats: residual memory impairment and acute but not lasting 5-HT depletion. PLoS ONE 7, e45473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motbey CP, Clemens KJ, Apetz N, Winstock AR, Ramsey J, Li KM, Wyatt N, Callaghan PD, Bowen MT, Cornish JL, McGregor IS, 2013. High levels of intravenous mephedrone (4-methylmethcathinone) self-administration in rats: neural consequences and comparison with methamphetamine. J. Psychopharmacol. (Oxford) 27, 823–836. [DOI] [PubMed] [Google Scholar]
- Murray BL, Murphy CM, Beuhler MC, 2012. Death following recreational use of designer drug “bath salts” containing 3,4-methylenedioxypyrovalerone (MDPV). J. Med. Toxicol 8, 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai F, Nonaka R, Satoh Hisashi Kamimura K, 2007. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur. J. Pharmacol 559, 132–137. [DOI] [PubMed] [Google Scholar]
- Naseri G, Fazel A, Golalipour MJ, Haghir H, Sadeghian H, Mojarrad M, Hosseini M, Shahrokhi Sabzevar S, Beheshti F, Ghorbani A, 2018. Exposure to mephedrone during gestation increases the risk of stillbirth and induces hippocampal neurotoxicity in mice offspring. Neurotoxicol. Teratol 67, 10–17. [DOI] [PubMed] [Google Scholar]
- Naylor JE, Freeman KB, Blough BE, Woolverton WL, Huskinson SL, 2015. Discriminative-stimulus effects of second generation synthetic cathinones in methamphetamine-trained rats. Drug Alcohol Depen. 149, 280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Banks ML, 2016. Decoding the structure of abuse potential for new psychoactive substances: structure-activity relationships for abuse-related effects of 4-substituted methcathinone analogs. Curr. Topics Behav. Neurosci 32, 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson KH, Hempel BJ, Clasen MM, Rice KC, Riley AL, 2017. Conditioned taste avoidance, conditioned place preference and hyperthermia induced by the second generation “bath salt” α-pyrrolidinopentiophenone (α-PVP). Pharmacol. Biochem. Be 156, 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JD, Grant Y, Creehan KM, Vandewater SA, Taffe MA, 2017. Escalation of intravenous self-administration of methylone and mephedrone under extended access conditions. Addict. Biol 22, 1160–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novellas J, López-Arnau R, Carbó ML, Pubill D, Camarasa J, Escubedo E, 2015. Concentrations of MDPV in rat striatum correlate with the psychostimulant effect. J. Psychopharmacol. (Oxford) 29, 1209–1218. [DOI] [PubMed] [Google Scholar]
- Numachi Y, Ohara A, Yamashita M, Fukushima S, Kobayashi H, Hata H, Watanabe H, Hall FS, Lesch KP, Murphy DL, Uhl GR, Sora I, 2007. Methamphetamine-induced hyperthermia and lethal toxicity: role of the dopamine and serotonin transporters. Eur. J. Pharmacol 572, 120–128. [DOI] [PubMed] [Google Scholar]
- O’Connor EC, Chapman K, Butler P, Mead AN, 2011. The predictive validity of the rat self-administration model for abuse liability. Neurosci. Biobehav. R 35, 912–938. [DOI] [PubMed] [Google Scholar]
- O’Connor AD, Padilla-Jones A, Gerkin RD, Levine M, 2015. Prevalence of rhabdomyolysis in sympathomimetic toxicity: a comparison of stimulants. J. Med. Toxicol 11, 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsolini L, Papanti GD, Francesconi G, Schifano F, 2015. Mind navigators of chemicals’ experimenters? A web-based description of e-psychonauts. Cyberpsych. Beh. Soc. N 18, 296–300. [DOI] [PubMed] [Google Scholar]
- Overton DA, 1991. Historical context of state dependent learning and discriminative drug effects. Behav. Pharmacol 2, 253–264. [PubMed] [Google Scholar]
- Paillet-Loilier M, Cesbron A, Le Boisselier R, Bourgine J, Debruyne D, 2014. Emerging drugs of abuse: current perspectives on substituted cathinones. Subt. Abuse Rehabilitation 5, 37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamar JJ, 2015. “Bath salt” use among a nationally representative sample of high school seniors in the United States. Am. J. Addiction 24, 488–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamar JJ, Salomone A, Vincenti M, Cleland CM, 2016. Detection of “bath salts” and other novel psychoactive substances in hair samples of ecstasy/MDMA/“Molly” users. Drug Alcohol Depend. 161, 200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamar JJ, Salomone A, Gerace E, Di Corcia D, Vincenti M, Cleland CM, 2017. Hair testing to assess both known and unknown use of drugs amongst ecstasy users in the electronic dance music scene. Int. J. Drug Policy 41, 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Wilkinson JL, Metschke DM, Bevins RA, 2005. Stimulus properties of nicotine, amphetamine, and chlordiazepoxide as positive features in a Pavlovian appetitive discrimination task in rats. Neuropsychopharmacol. 30, 731–741. [DOI] [PubMed] [Google Scholar]
- Pantano F, Tittarelli R, Mannocchi G, Pacifici R, di Luca A, Busardò FP, Marinelli E, 2017. Neurotoxicity induced by mephedrone: an up-to-date review. Curr. Neuropharmacol 15, 738–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaseit E, Molto J, Muga R, Torrens M, de la Torre R, Farre M, 2017a. Clinical pharmacology of the synthetic cathinone mephedrone. Curr. Top. Behav. Neurosci 32, 313–331. [DOI] [PubMed] [Google Scholar]
- Papaseit E, Olesti E, de la Torre R, Torrens M, Farre M, 2017b. Mephedrone concentrations in cases of clinical intoxication. Curr. Pharm. Des 23, 5511–5522. [DOI] [PubMed] [Google Scholar]
- Parker LA, 2003. Taste avoidance and taste aversion: evidence for two different processes. Anim. Learn. Behav 31, 165–172. [DOI] [PubMed] [Google Scholar]
- Parrott AC, 2004. MDMA (3,4-methylenedioxymethamphetamine) or ecstasy: the neuropsychobiological implications of taking it at dances and raves. Neuropsychobiology 50, 329–335. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Justice JB Jr., 1993. Serotonin and dopamine sensitization in the nucleus accumbens, ventral tegmental area, and dorsal raphe nucleus following repeated cocaine administration. J. Neurochem 61, 1611–1619. [DOI] [PubMed] [Google Scholar]
- Penders TM, Gestring RE, Vilensky DA, 2012. Excited delirium following use of synthetic cathinones (bath salts). Gen. Hosp. Psychiat 34, 647–650. [DOI] [PubMed] [Google Scholar]
- Perrone D, 2016. Designer drugs. In: Brownstein HH (Ed.), The Handbook of Drugs and Society. John Wiley & Sons, NY, pp. 149–175. [Google Scholar]
- Pescatore KA, Glowa JR, Riley AL, 2005. Strain differences in the acquisition of nicotine-induced conditioned taste aversion. Pharmacol. Biochem. Be 82, 751–757. [DOI] [PubMed] [Google Scholar]
- Péterfi A, Tarján A, Horváth GC, Csesztregi T, Nyírády A, 2014. Changes in patterns of injecting drug use in Hungary: a shift to synthetic cathinones. Drug Test. Anal 6, 825–831. [DOI] [PubMed] [Google Scholar]
- Peyrière H, Jacquet JM, Eiden C, Tuaillon E, Psomas C, Reynes J, 2013. Viral and bacterial risks associated with mephedrone abuse in HIV-infected men who have sex with men. AIDS 27, 2971–2972. [DOI] [PubMed] [Google Scholar]
- Piao YS, Hall FS, Moriya Y, Ito M, Ohara A, Kikura-Hanajiri R, Goda Y, Lesch KP, Murphy DL, Uhl GR, 2015. Methylone-induced hyperthermia and lethal toxicity: role of the dopamine and serotonin transporters. Behav. Pharmacol 26, 345–352. [DOI] [PubMed] [Google Scholar]
- Picetti R, Ho A, Butelman ER, Kreek MJ, 2010. Dose preference and dose escalation in extended-access cocaine self-administration in Fischer and Lewis rats. Psychopharmacology 211, 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picetti R, Caccavo JA, Ho A, Kreek MJ, 2012. Dose escalation and dose preference in extended-access heroin self-administration in Lewis and Fischer rats. Psychopharmacology 220, 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW, 1997. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res. Rev 25, 192–216. [DOI] [PubMed] [Google Scholar]
- Pifl C, Reither H, Hornykiewicz O, 2015. The profile of mephedrone on human monoamine transporters differs from 3,4-methylenedioxymethamphetamine primarily by lower potency at the vesicular monoamine transporter. Eur. J. Pharmacol 755, 119–126. [DOI] [PubMed] [Google Scholar]
- Pirona A, Bo A, Hedrich D, Ferri M, van Gelder N, Giraudon I, Montanari L, Simon R, Mounteney J, 2017. New psychoactive substances: current health-related practices and challenges in responding to use and harms in Europe. Int. J. Drug Policy 40, 84–92. [DOI] [PubMed] [Google Scholar]
- Pomfrey RL, Bostwick TA, Wetzell BB, Riley AL, 2015. Adolescent nicotine exposure fails to impact cocaine reward, aversion and self-administration in adult male rats. Pharmacol. Biochem. Be 137, 30–37. [DOI] [PubMed] [Google Scholar]
- Prosser JM, Nelson LS, 2012. The toxicology of bath salts: a review of synthetic cathinones. J. Med. Toxicol. 8, 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prus AJ, James JR, Rosecrans JA, 2009. Conditioned place preference. In: Buccafusco JJ (Ed.), Methods of Behavior Analysis in Neuroscience. CRC Press/Taylor & Francis, Boca Raton, FL, pp. 59–76. [Google Scholar]
- Pu C, Fisher JE, Cappon GD, Vorhees CV, 1994. The effects of amfonelic acid, a dopamine uptake inhibitor, on methamphetamine-induced dopaminergic terminal degeneration and astrocytic response in rat striatum. Brain Res 649, 217–224. [DOI] [PubMed] [Google Scholar]
- Pubill D, Canudas AM, Pallàs M, Camins A, Camarasa J, Escubedo E, 2003. Different glial response to methamphetamine- and methylenedioxymethamphetamine-induced neurotoxicity. Naunyn Schmiedebergs Arch. Pharmacol 367, 490–499. [DOI] [PubMed] [Google Scholar]
- Ramoz L, Lodi S, Bhatt P, Reitz AB, Tallarida C, Tallarida RJ, Raffa RB, Rawls SM, 2012. Mephedrone (“bath salt”) pharmacology: insights from invertebrates. Neuroscience 208, 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regunath H, Ariyamuthu VK, Dalal P, Misra M, 2012. Bath salt intoxication causing acute kidney injury requiring hemodialysis. Hemodial. Int 16, 47–49. [DOI] [PubMed] [Google Scholar]
- Reid LD, 1987. Tests involving pressing for intracranial stimulation as an early procedure for screening likelihood of addiction of opioids and other drugs. In: Bozarth MA (Ed.), Methods of Assessing the Reinforcing Properties of Abused Drugs. Springer, NY, pp. 391–420. [Google Scholar]
- Revusky S, Garcia J, 1970. Learned associations over long delays. Psychol. Learn. Motiv 4, 1–84. [Google Scholar]
- Riley AL, 2011. The paradox of drug taking: the role of the aversive effects of drugs. Physiol. Behav 103, 69–78. [DOI] [PubMed] [Google Scholar]
- Riley AL, Davis CM, Roma PG, 2009. Strain differences in taste aversion learning: implications for animal models of drug abuse. In: Reilly S, Schachtman T (Eds.), Conditioned Taste Aversion: Behavioral and Neural Processes. Academic Press, NY, pp. 226–261 2009. [Google Scholar]
- Riley AL, Clasen MM, Friar M, 2016. Conditioned taste aversion baseline of drug discrimination learning: assessments and applications. In: Porter J, Prus A (Eds.), Drug Discrimination: A Behavioral Approach for Understanding Subjective Drug Effects. Springer-Verlag, NY. [Google Scholar]
- Riley AL, Hempel BJ, Clasen MM, 2018. Sex as a biological variable: drug use and abuse. Physiol Behav Epub 1–18. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC, 1993. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. Brain Res. Rev 18, 247–291. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC, 2008. Review. The incentive sensitization theory of addiction: some current issues. Philos. Trans. R. Soc. Lond., B, Biol. Sci 363, 3137–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Jurson PA, Bennett JA, Bentgen KM, 1988. Persistent sensitization of dopamine neurotransmission in ventral striatum (nucleus accumbens) produced by prior experience with (+)-amphetamine: a microdialysis study in freely moving rats. Brain Res. 462, 211–222. [DOI] [PubMed] [Google Scholar]
- Robinson JE, Agoglia AE, Fish EW, Krouse MC, Malanga C, 2012. Mephedrone (4-methylmethcathinone) and intracranial self-stimulation in C57BL/6J mice: comparison to cocaine. Behav. Brain Res 234, 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roma PG, Flint WW, Higley JD, Riley AL, 2006. Assessment of the aversive and rewarding effects of alcohol in Fischer and Lewis rats. Psychopharmacology 189, 187–199. [DOI] [PubMed] [Google Scholar]
- Roma PG, Davis CM, Kohut SJ, Huntsberry ME, Riley AL, 2008. Early maternal separation and sex differences in the aversive effects of amphetamine in adult rats. Physiol. Behav 93, 897–904. [DOI] [PubMed] [Google Scholar]
- Ross EA, Watson M, Goldberger B, 2011. “Bath salts” intoxication. N. Engl. J. Med 365, 967–968. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME, 2004. Sex differences in the escalation of intravenous cocaine intake following long-or short-access to cocaine self-administration. Pharmacol. Biochem. Be 78, 199–207. [DOI] [PubMed] [Google Scholar]
- Rozin P, Kalat JW, 1971. Specific hungers and poison avoidance as adaptive specializations of learning. Psychol. Rev. 78, 459–486. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Jenab S, Fabian SJ, Festa ED, Kemen LM, Quinones-Jenab V, 2003. Sex differences in the conditioned rewarding effects of cocaine. Brain Res. 970, 214–220. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R, 2006. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict. Biol. 11, 2–38. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B, 1997. Sensitization and tolerance in psychostimulant self-administration. Pharmacol. Biochem. Be 57, 543–550. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Thorndike EB, Goldberg SR, Lehner KR, Cozzi NV, Brandt SD, Baumann MH, 2016. Reinforcing and neurochemical effects of the “bath salts” constituents 3, 4-methylenedioxypyrovalerone (MDPV) and 3,4-methylenedioxy-N-methylcathinone (methylone) in male rats. Psychopharmacology 233, 1981–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CJ, Gibb JW, 1985. Role of the dopamine uptake carrier in the neurochemical response to methamphetamine: effects of amfonelic acid. Eur. J. Pharmacol 109, 73–80. [DOI] [PubMed] [Google Scholar]
- Schneir A, Ly BT, Casagrande K, Darracq M, Offerman SR, Thornton S, Smollin C, Vohra R, Rangun C, Tomaszewski C, Gerona RR, 2014. Comprehensive analysis of “bath salts” purchased from California stores and the internet. Clin. Toxicol 52, 651–658. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Morris RW, Kuhn CM, 2006. Adolescent rats are protected from the conditioned aversive properties of cocaine and lithium chloride. Pharmacol. Biochem. Be 84, 344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Francis R, MacDonald A, Keistler C, O’Neill L, Kuhn CM, 2014. Effect of sex on ethanol consumption and conditioned taste aversion in adolescent and adult rats. Psychopharmacology 231, 1831–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, Iwata Y, Tsuchiya KJ, Suda S, Suzuki K, Kawai M, Takebayashi K, Yamamoto S, Matsuzaki H, Ueki T, Mori N, Gold MS, Cadet JL, 2008. Methamphetamine causes microglial activation in the brains of human abusers. J. Neurosci 28, 5756–5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafine KM, Briscione MA, Rice KC, Riley AL, 2012a. Dopamine mediates cocaine-induced conditioned taste aversions as demonstrated within cross-drug preexposure to GBR12909. Pharmacol. Biochem. Be 102, 269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafine KM, Briscione MA, Riley AL, 2012b. The effects of haloperidol on cocaine-induced taste aversions. Physiol. Behav 105, 1161–1167. [DOI] [PubMed] [Google Scholar]
- Sewalia K, Watterson LR, Hryciw A, Belloc A, Ortiz JB, 2017. Neurocognitive dysfunction following repeated binge-like self-administration of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV). Neuropharmacology 134, 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman JE, Roberts T, Roskam SE, Holman EW, 1980. Temporal properties of the rewarding and aversive effects of amphetamine in rats. Pharmcol. Biochem. Be 13, 597–599. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Benwell ME, Akbar MT, Stolerman IP, Balfour DJ, 1994. Behavioural and neurochemical adaptations to nicotine in rats: influence of NMDA antagonists. Br. J. Pharmacol 111, 1073–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortall SE, Macerola AE, Swaby RTR, Jayson R, Korsah C, Pillidge KE, Wigmore PM, Ebling FJP, Green AR, Fone KCF, King MV, 2013. Behavioural and neurochemical comparison of chronic intermittent cathinone, mephedrone and MDMA administration to the rat. Eur. Neuropsychopharmacol 23, 1085–1095. [DOI] [PubMed] [Google Scholar]
- Shortall SE, Spicer CH, Ebling FJ, Green AR, Fone KC, King MV, 2016. Contribution of serotonin and dopamine to changes in core body temperature and locomotor activity in rats following repeated administration of mephedrone. Addict. Biol 21, 1127–1139. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Lê AD, 2006. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology 186, 201–208. [DOI] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Ferris MJ, Jones SR, 2015. Adaptations of presynaptic dopamine terminals induced by psychostimulant self-administration. ACS Chem. Neurosci 6, 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva B, Fernandes C, Tiritan ME, Pinto MM, Valente MJ, Carvalho M, de Pinho PG, Remião F, 2016. Chiral enantioresolution of cathinone derivatives present in “legal highs”, and enantioselectivity evaluation on cytotoxicity of 3,4-methylenedioxypyrovalerone (MDPV). Forensic Toxicol. 34, 372–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler LD, Liechti ME, 2016. Interactions of cathinone NPS with human transporters and receptors in transfected cells. Curr. Topics Behav. Neurosci 32, 49–72. [DOI] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME, 2013. Pharmacological characterization of designer cathinones in vitro. Brit. J. Pharmacol 169, 458–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler LD, Rickli A, Hoener MC, Liechti ME, 2014. Monoamine transporter and receptor interaction profiles of a new series of designer cathinones. Neuropharmacology 79, 152–160. [DOI] [PubMed] [Google Scholar]
- Simmler LD, Buchy D, Chaboz S, Hoener MC, Liechti ME, 2016. In vitro characterization of psychoactive substances at rat, mouse, and human trace amine-associated receptor 1. J. Pharmacol. Exp. Ther 357, 134–144. [DOI] [PubMed] [Google Scholar]
- Simmons SJ, Gregg RA, Tran FH, Mo L, von Weltin E, Barker DJ, Gentile TA, Watterson LR, Rawls SM, Muschamp JW, 2016. Comparing rewarding and reinforcing properties between “bath salt” 3,4-methylenedioxypyrovalerone (MDPV) and cocaine using ultrasonic vocalizations in rats. Addict. Biol 23, 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons SJ, Martorana R, Philogene-Khalid H, Tran FH, Gentile TA, Xu X, Su S, Rawls SM, Muschamp JW, 2017. Role of hypocretin/orexin receptor blockade on drug-taking and ultrasonic vocalizations (USVs) associated with low-effort self-administration of cathinone-derived 3,4-methylenedioxypyrovalerone (MDPV) in rats. Psychopharmacology 234, 3207–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GR, Riley AL, 2005. Morphine preexposure facilitates morphine place preference and attenuates morphine taste aversion. Pharmcol. Biochem. Be 80, 471–479. [DOI] [PubMed] [Google Scholar]
- Sitte HH, Freissmuth M, 2015. Amphetamines, new psychoactive drugs and the monoamine transporter cycle. Trends Pharmacol. Sci 36, 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivagnanam K, Chaudari D, Lopez P, Sutherland ME, Ramu VK, 2013. “Bath salts” induced severe reversible cardiomyopathy. Am. J. Case Rep 14, 288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DA, Negus SS, Poklis JL, Blough BE, Banks ML, 2017. Cocaine-like discriminative stimulus effects of alpha-pyrrolidinovalerophenone, methcathinone and their 3,4-methylenedioxy or 4-methyl analogs in rhesus monkeys. Addict. Biol 22, 1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Justinova Z, Yasar S, Goldberg SR, 2006. Using drug-discrimination techniques to study the abuse-related effects of psychoactive drugs in rats. Nat. Protoc 1, 1194–1206. [DOI] [PubMed] [Google Scholar]
- Sparago M, Wlos J, Yuan J, Hatzidimitriou G, Tolliver J, Dal Cason TA, Katz J, Ricaurte G, 1996. Neurotoxic and pharmacologic studies on enantiomers of the Nmethylated analog of cathinone (methcathinone): a new drug of abuse. J. Pharmacol. Exp. Ther 279, 1043–1052. [PubMed] [Google Scholar]
- Spear LP, 2011. Rewards, aversions and affect in adolescence: emerging convergences across laboratory animal and human data. Dev. Cogn. Neuros-Neth 1, 390–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, Jansen J, 2011. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin. Toxicol 49, 499–505. [DOI] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW, 2011. Drug wanting: behavioral sensitization and relapse to drug-seeking Behavior. Pharmacol. Rev 63, 348–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöhr T, Wermeling DS, Weiner I, Feldon J, 1998. Rat strain differences in open-field behavior and the locomotor stimulating and rewarding effects of amphetamine. Pharmacol. Biochem. Be 59, 813–818. [DOI] [PubMed] [Google Scholar]
- Stolerman I, D’Mello G, 1981. Oral self-administration and the relevance of conditioned taste aversions. Adv. Behav. Pharmacol 3, 169–214. [Google Scholar]
- Sulzer D, 2011. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron 69, 628–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Maidment NT, Rayport S, 1993. Amphetamine and other weak bases act to promote reverse transport of dopamine in ventral midbrain neurons. J. Neurochem 60, 527–535. [DOI] [PubMed] [Google Scholar]
- Sutamtewagul G, Sood V, Nugent K, 2014. Sympathomimetic syndrome, choreoathetosis, and acute kidney injury following “bath salts” injection. Clin. Nephrol 81, 63–66. [DOI] [PubMed] [Google Scholar]
- Sutherland R, Bruno R, Peacock A, Lenton S, Matthews A, Salom C, Dietze P, Butler K, Burns L, Barratt MJ, 2017. Motivations for new psychoactive substance use among regular psychostimulant users in Australia. Int. J. Drug Policy 43, 23–32. [DOI] [PubMed] [Google Scholar]
- Thirakul P, Hair LS, Bergen KL, Pearson JM, 2017. Clinical presentation, autopsy results and toxicology findings in an acute N-ethylpentylone fatality. J. Anal. Toxicol 41, 342–346. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Dowgiert J, Geddes TJ, Francescutti-Verbeem D, Liu X, Kuhn DM, 2004a. Microglial activation is a pharmacologically specific marker for the neurotoxic amphetamines. Neurosci. Lett 367, 349–354. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM, 2004b. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J. Pharmacol. Exp. Ther 311, 1–7. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM, 2008. The newly synthesized pool of dopamine determines the severity of methamphetamine-induced neurotoxicity. J. Neurochem 105, 605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA Jr, 2004. Effects of κ-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology 172, 463–470. [DOI] [PubMed] [Google Scholar]
- Torres OV, Natividad LA, Tejeda HA, Van Weelden SA, O’Dell LE, 2009. Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharmacology 206, 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turenne SD, Miles C, Parker LA, Siegel S, 1996. Individual differences in reactivity to the rewarding/aversive properties of drugs: assessment by taste and place conditioning. Pharmacol. Biochem. Be 53, 511–516. [DOI] [PubMed] [Google Scholar]
- Twining RC, Bolan M, Grigson PS, 2009. Yoked delivery of cocaine is aversive and protects against the motivation for drug in rats. Behav. Neurosci 123, 913–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM, 1998. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog. Neurobiol 56, 613–672. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, 2007. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict. Biol 12, 227–462. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime, 2014. Global Synthetic Drugs Assessment. United Nations publication, Sales No. E.14.XI.6. [Google Scholar]
- US Department of Justice, 2011. Synthetic Cathinones (Bath Salts): an Emerging Domestic Threat. Product Number 2011-S0787–004, National Drug Intelligence Center. [Google Scholar]
- Valente M, Araújo A, Silva R, Bastos M, Carvalho F, de Pinho PG, Carvalho M, 2015. Hepatic oxidative stress induced by methylone and MDPV: a comparison to MDMA. Toxicol. Lett 2, S265. [Google Scholar]
- Valente MJ, Araujo AM, Bastos MD, Fernandes E, Carvalho F, de Pinho PG, Carvalho M, 2016. Editor’s highlight: characterization of hepatotoxicity mechanisms triggered by designer cathinone drugs (beta-keto amphetamines). Toxicol. Sci 153, 89–102. [DOI] [PubMed] [Google Scholar]
- Vandewater SA, Creehan KM, Taffe MA, 2015. Intravenous self-administration of entactogen-class stimulants in male rats. Neuropharmacology 99, 538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varner KJ, Daigle K, Weed PF, Lewis PB, Mahne SE, Sankaranarayanan A, Winsauer PJ, 2013. Comparison of the behavioral and cardiovascular effects of mephedrone with other drugs of abuse in rats. Psychopharmacology 225, 675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vearrier D, Greenberg MI, Miller SN, Okaneku JT, Haggerty DA, 2012. Methamphetamine: history, pathophysiology, adverse health effects, current trends, and hazards associated with the clandestine manufacture of methamphetamine. DM-Dis. Mon 58, 38–89. [DOI] [PubMed] [Google Scholar]
- Verendeev A, Riley AL, 2011. Relationship between the rewarding and aversive effects of morphine and amphetamine in individual subjects. Learn. Behav 39, 399–408. [DOI] [PubMed] [Google Scholar]
- Verendeev A, Riley AL, 2012. Conditioned taste aversion and drugs of abuse: history and interpretation. Neurosci. Biobehav. R 36, 2193–2205. [DOI] [PubMed] [Google Scholar]
- Verendeev A, Riley AL, 2013. The role of the aversive effects of drugs in self-administration: assessing the balance of reward and aversion in drug-taking behavior. Behav. Pharmacol 24, 363–374. [DOI] [PubMed] [Google Scholar]
- Vlachou S, Nomikos GG, Panagis G, 2005. CB1 cannabinoid receptor agonists increase intracranial self-stimulation thresholds in the rat. Psychopharmacology 179, 498–508. [DOI] [PubMed] [Google Scholar]
- Vouga A, Gregg RA, Haidery M, Ramnath A, Al-Hassani HK, Tallarida CS, Grizzanti D, Raffa RB, Smith GR, Reitz AB, Rawls SM, 2015. Stereochemistry and neuropharmacology of a “bath salt” cathinone: S-enantiomer of mephedrone reduces cocaine-induced reward and withdrawal in invertebrates. Neuropharmacology 91, 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Olive MF, 2014. Synthetic cathinones and their rewarding and reinforcing effects in rodents. Adv. Neurosci. (Hindawi) 209875, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Olive MF, 2016. Reinforcing effects of cathinone NPS in the intravenous drug self-administration paradigm. Curr. Topics Behav. Neurosci 32, 133–144. [DOI] [PubMed] [Google Scholar]
- Watterson LR, Hood L, Sewalia K, Tomek SE, Yahn S, Johnson CT, Wegner S, Blough BE, Marusich JA, Olive MF, 2012. The reinforcing and rewarding effects of methylone, a synthetic cathinone commonly found in “bath salts”. J. Addict. Res. Ther. Suppl 9, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF, 2014a. Potent rewarding and reinforcing effects of the synthetic cathinone 3, 4-methylenedioxypyrovalerone (MDPV). Addict. Biol 19, 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF, 2014b. Effects of α-pyrrolidinopentiophenone and 4-methyl-n-ethylcathinone, two synthetic cathinones commonly found in second-generation “bath salts”, on intracranial self-stimulation thresholds in rats. Int. J. Neuropsychoph 18, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Taylor SB, Nemirovsky NE, Olive MF, 2016. Sensitization to the motor stimulant effects of 3,4-methylenedioxypyrovalerone (MDPV) and cross-sensitization to methamphetamine in rats. J. Drug Alcohol Res 5 pii: 235967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks JR, 1962. Experimental morphine addiction: method for automatic intravenous injections in unrestrained rats. Science 138, 143–144. [DOI] [PubMed] [Google Scholar]
- White N, Sklar L, Amit Z, 1977. The reinforcing action of morphine and its paradoxical side effect. Psychopharmacology 52, 63–66. [DOI] [PubMed] [Google Scholar]
- Winstock A, Mitcheson L, Ramsey J, Davies S, Puchnarewicz M, Marsden J, 2011. Mephedrone: use, subjective effects and health risks. Addiction 106, 1991–1996. [DOI] [PubMed] [Google Scholar]
- Woloshchuk CJ, Nelson KH, Rice KC, Riley AL, 2016. Effects of 3,4-methylenedioxypyrovalerone (MDPV) pre-exposure on the aversive effects of MDPV, cocaine and lithium chloride: implications for abuse. Drug Alcohol Depen. 167, 121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DM, Davies S, Puchnarewicz M, Button J, Archer R, Ovaska H, Ramsey J, Lee T, Holt DW, Dargan PI, 2010. Recreational use of mephedrone (4-methylmethcathinone, 4-MMC) with associated sympathomimetic toxicity. J. Med. Toxicol. 6, 327–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DM, Greene SL, Dargan PI, 2013. Five-year trends in self-reported recreational drugs associated with presentation to a UK emergency department with suspected drug-related toxicity. Eur. J. Emerg. Med 20, 263–267. [DOI] [PubMed] [Google Scholar]
- Wood DM, Dines AM, Heyerdahl F, Yates C, Giraudon I, Hovda KE, Dargan PI, European Drug Emergencies Network Research Group, 2015. The cathinones are the most commonly reported novel psychoactive substances (NPS) associated with emergency department presentations with acute drug toxicity reported to the European Drug Emergencies Network (Euro-DEN). Clin. Toxicol 53, 355–356. [Google Scholar]
- Woolverton WL, Johanson CE, 1984. Preference in rhesus monkeys given a choice between cocaine and d,l-cathinone. J. Exp. Anal. Behav 41, 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MJ Jr., Angrish D, Aarde SM, Barlow DJ, Buczynski MW, Creehan KM, Vandewater SA, Parsons LH, Houseknecht KL, Dickerson TJ, Taffe MA, 2012. Effect of ambient temperature on the thermoregulatory and locomotor stimulant effects of 4-methylmethcathinone in Wistar and Sprague-Dawley rats. PLoS ONE 7, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Hu XT, Cooper DC, Moratalla R, Graybiel AM, White FJ, Tonegawa S, 1994. Elimination of cocaine-induced hyperactivity and dopamine-mediated neurophysiological effects in dopamine D1 receptor mutant mice. Cell 79, 945–955. [DOI] [PubMed] [Google Scholar]
- Xu P, Qiu Y, Zhang Y, Yanping B, Pengfei X, Liu Y, Kim JH, Shen HW, 2016. The effects of 4-methylethcathinone on conditioned place preference, locomotor sensitization, and anxiety-like behavior: a comparison with methamphetamine. Int. J. Neuropsychoph 19, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao WD, Gainetdinov RR, Arbuckle MI, Sotnikova TD, Cyr M, Beaulieu JM, Torres GE, Grant SGN, Caron MG, 2004. Identification of PSD-95 as a regulator of dopamine-mediated synaptic and behavioral plasticity. Neuron 41, 625–638. [DOI] [PubMed] [Google Scholar]
- Yates JR, Beckmann JS, Meyer AC, Bardo MT, 2013. Concurrent choice for social interaction and amphetamine using conditioned place preference in rats: effects of age and housing condition. Drug Alcohol Depen. 129, 240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AC, Schwarz ES, Velez LI, Gardner M, 2013. Two cases of disseminated intravascular coagulation due to “bath salts” resulting in fatalities, with laboratory confirmation. Am. J. Emerg. Med 31 445.e3–445.e5. [DOI] [PubMed] [Google Scholar]
- Zakharova E, Wade D, Izenwasser S, 2009. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol. Biochem. Be 92, 131–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawilska JB, Andrzejczak D, 2015. Next generation of novel psychoactive substances on the horizon – a complex problem to face. Drug Alcohol Depen. 157, 1–17. [DOI] [PubMed] [Google Scholar]
- Zawilska JB, Wojcieszak J, 2017. α-pyrrolidinophenones: a new wave of designer cathinones. Forensic Toxicol. 35, 201–216. [Google Scholar]
- Zernig G, Ahmed SH, Cardinal RN, Morgan D, Acquas E, Foltin RW, Vezina P, Negus SS, Cresp JA, Stöckl P, Grubinger P, Madlung E, Haring C, Kurz M, Saria A, 2007. Explaining the escalation of drug use in substance dependence: models and appropriate animal laboratory tests. Pharmacology 80 (80), 65–119. [DOI] [PubMed] [Google Scholar]