Abstract
Green light photoactive Ru-based coordination polymer nanoparticles (CPNs), with chemical formula [[Ru(biqbpy)]1.5(bis)](PF6)3 (biqbpy = 6,6′-bis[N-(isoquinolyl)-1-amino]-2,2′-bipyridine; bis = bis(imidazol-1-yl)-hexane), were obtained through polymerization of the trans-[Ru(biqbpy)(dmso)Cl]Cl complex (Complex 1) and bis bridging ligands. The as-synthesized CPNs (50 ± 12 nm diameter) showed high colloidal and chemical stability in physiological solutions. The axial bis(imidazole) ligands coordinated to the ruthenium center were photosubstituted by water upon light irradiation in aqueous medium to generate the aqueous substituted and active ruthenium complexes. The UV-Vis spectral variations observed for the suspension upon irradiation corroborated the photoactivation of the CPNs, while High Performance Liquid Chromatography (HPLC) of irradiated particles in physiological media allowed for the first time precisely quantifying the amount of photoreleased complex from the polymeric material. In vitro studies with A431 and A549 cancer cell lines revealed an 11-fold increased uptake for the nanoparticles compared to the monomeric complex [Ru(biqbpy)(N-methylimidazole)2](PF6)2 (Complex 2). After irradiation (520 nm, 39.3 J/cm2), the CPNs yielded up to a two-fold increase in cytotoxicity compared to the same CPNs kept in the dark, indicating a selective effect by light irradiation. Meanwhile, the absence of 1O2 production from both nanostructured and monomeric prodrugs concluded that light-induced cell death is not caused by a photodynamic effect but rather by photoactivated chemotherapy.
Keywords: coordination polymer nanoparticles, nanoparticles, photoactivated chemotherapy, prodrug, drug delivery, ruthenium-based drug
1. Introduction
Ruthenium-based drugs have raised interest over the last years as an alternative to Pt drugs for oncotherapy, with an increasing number of them entering clinical trials, such as NAMI-A, KP 1019, KP 1339, or TLD–1433 [1,2,3,4,5,6,7]. Especially relevant has been the development of ruthenium prodrug molecular complexes bearing photolabile ligands for photoactivated chemotherapy (PACT) applications [8,9,10,11,12]. Interestingly, these complexes generally exhibit low toxicity in the dark but become toxic once activated by visible light irradiation. The mechanism of prodrug activation is related to the specific ligand photosubstitution by water molecules, to afford activated aqua photoproducts able to induce a therapeutic action [10,13,14,15,16,17]. Moreover, PACT is an oxygen-independent activation mechanism that works even under hypoxic conditions. This feature makes it potentially more versatile than type II photodynamic therapy (PDT), which requires the presence of a significant amount of dioxygen to generate enough reactive singlet oxygen species to induce cytotoxicity [10,11,14,18,19,20,21,22,23,24].
However, before reaching clinical use, photoactivated Ru complexes must face key challenges such as water solubility, preferential accumulation in tumors [25], precise controlled release of the drug [26,27,28], increase in the biocompatibility while minimizing residual toxicity in the dark [29,30,31], and improvement of their fast clearance from the bloodstream [32]. To overcome most of these limitations, photolabile complexes [33,34], and specifically Ru-based complexes [35], can be incorporated in nanoparticles (NPs) for their application in photoinduced therapies. For instance, Wu et al. have reported the covalent link of Ru to block copolymers [10,36] that stabilize photoactivatable ruthenium complexes under physiological conditions [9]. This strategy includes ruthenium-containing block copolymer units that self-assembled into nanoparticles in aqueous solution with excellent uptake in vitro and in vivo results. The inhibition of cancer cells was related to the generation of singlet oxygen (1O2) upon irradiation with red light [37,38]. Other approaches involve the conjugation of photocleavable Ru complexes to the surface of upconverting NPs [39,40,41,42]. Even so, the encapsulation of photoactive Ru-based complexes is in its fledgling stage, so there is a growing interest to develop novel NPs that allow a proper fine-tune structure/function correlations and adapt it for their use in photoactivated chemotherapy [43].
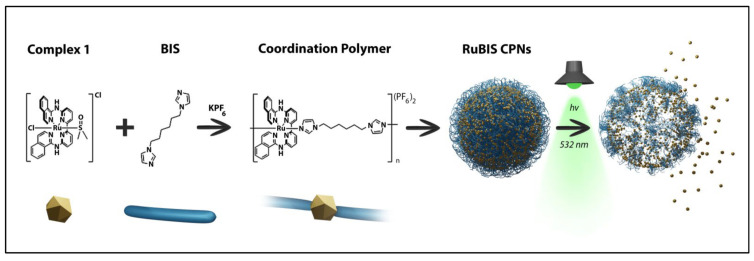
Herein, we hypothesize that coordination polymer nanoparticles (CPNs) bearing Ru-active complexes as constitutive building blocks and a photocleavable bridging ligand may represent a step forward for PACT applications (the schematic representation of the proposed system is shown in Figure 1). In addition to achieve smart NPs with high payloads, CPNs have already been successfully demonstrated to be highly performing as biocompatible contrast agents and antitumoral application, including Pt (IV)-based CPNs [44,45]. These nanoformulations offer good colloidal stability, scalability, cellular internalization, and even more noticeably high payloads, as the prodrug constitutes the backbone of the nanoparticles polymer itself [46]. All these advantages turn out to be really helpful to reduce the dose, the irradiation intensity required to activate the anticancer drug diffusion, and therefore any side effect. Though, as far as we know, the number of ruthenium-based coordination polymers with antitumor applications is rather limited, none of them being photoactivable as far as we know.
Figure 1.
Scheme of synthesis and photoactivation process of Ru-based coordination polymer nanoparticles (RuBIS CPNs).
We have achieved this challenge with the synthesis of CPNs containing [Ru(biqbpy)(dmso)Cl]Cl monomer (complex 1, where biqbpy = stands for 6,6′-bis[N-(isoquinolyl)-1-amino]-2,2′-bipyridine), which is known to form cytotoxic aqueous active species [Ru(biqbpy)(H2O)2]2+ upon blue or green light activation [14]. The polymerization process was performed using the photocleavable bis(imidazol-1-yl)-hexane (BIS) ligand and following a methodology previously described for the synthesis of non-photoactive CPNs of relevance in biological applications [47,48,49,50].
2. Materials and Methods
2.1. Reagents and Instrumentation
Solvents were purchased from Sigma–Aldrich (Merck KGaA, Darmstadt, Alemania) and used as received, and complex 1 was synthesized and characterized according to previously reported methodology [14]. Fourier transform infrared (FTIR) spectra were carried out with a Tensor 27/PMA50FTIR Spectrometer (Bruker Optics GmbH, Ettlingen, Germany) in a range of 4000–400 cm−1. Determination of the particle-size distributions and the zeta potential values were measured by dynamic light scattering (DLS) using a ZetaSizer nano ZS (ZEN3600, Malvern Instruments, Ltd., Malvern, UK). Scanning Electron Microscopy (SEM) images were obtained on a scanning electron microscope (FEI Quanta 650 FEG, Thermo Fisher Scientific, Eindhoven, The Netherlands). The samples were casted on aluminum holders following by evaporation, and later, a thin platinum layer was sprayed to increase the conductivity of samples. Ultraviolet–visible spectroscopy (UV-vis) study was carried out in the Agilent Cary 60 spectrophotometer (Agilent Technologies, Santa Clara, CA, USA) in the dark using a 1 cm quartz cuvette with a stirring bar, containing 2 mL of RuBIS CPNs (20 μg/mL). Time-dependent UV-Vis spectra during irradiation were recorded at regular time intervals (specified in the spectra) after irradiation of the stirred sample with a continuous beam of a green (λ = 532 nm, 30 mW, 0.42 mW/cm2) or blue laser (450 nm, 100 mW, 0.35 mW/cm2) set in front of the cuvette.
2.2. Synthesis and Characterization of the Photoactive Materials
2.2.1. Synthesis of Complex 2
[Ru(biqbpy)(dmso)Cl]Cl (50.0 mg, 0.072 mmol) was dissolved in EtOH and H2O (5 mL, v/v = 1/1). Then, 1-methylimidazole (60.0 mg, 0.73 mmol) was injected in the mixture and refluxed for 12 h under N2 protection. The reaction mixture was cooled to room temperature. Then, ice-cold water (5 mL) and KPF6 (20 mg) were added to the reaction mixture; thus, dark brown precipitate was formed and filtered. After column chromatography (SiO2, Ethyl acetate/MeOH = 10:1), 2 (50 mg, 80%) [Ru(biqbpy)(N-methylimidazole)2] (PF6)2 complex (complex 2, Figure 2a) was obtained as a dark brown solid. For NMR and mass spectrometry characterization, see Figures S1–S4.
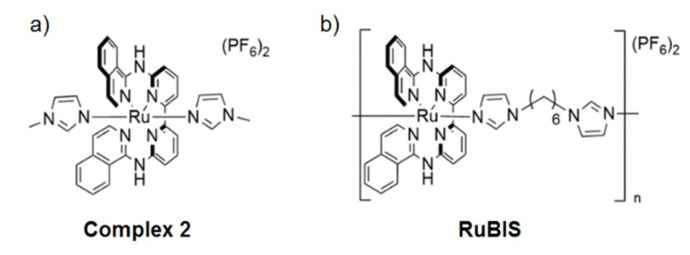
Figure 2.
Chemical representation of complex 2 (a) and RuBIS CPNs (b).
1H NMR (360 MHz, (CD3)2SO) δ 10.73 (s, 2H), 8.93 (d, J = 8.1 Hz, 2H), 8.46 (d, J = 7.7 Hz, 2H), 8.21 (d, J = 6.7 Hz, 2H), 8.12 (t, J = 8.0 Hz, 2H), 7.93 (ddd, J = 23.1, 18.0, 7.3 Hz, 8H), 7.41 (d, J = 6.6 Hz, 2H), 7.32 (s, 2H), 6.86 (s, 2H), 6.08 (s, 2H), 3.35 (s, 6H). 13C NMR (91 MHz, (CD3)2SO) δ 156.28, 151.20, 150.01, 145.50, 140.25, 135.81, 135.58, 131.80, 129.28, 128.82, 127.86, 123.77, 122.69, 120.21, 118.81, 116.06, 115.79, 34.22. MS-ESI (m/z): [M]+ Calcd. for C36H32N10Ru+ 705.8, found 705.2. Elem. Anal. Calcd. for C36H32F12N10P2Ru·3H2O: C, 40.55; H, 3.60; N, 13.51 Found: C, 40.72; H, 3.62; N, 12.95.
2.2.2. Synthesis of Coordination Polymer Nanoparticles RuBIS
Ru complex ([Ru(biqbpy)(dmso)Cl]Cl) (10.9 mg, 15.8 μmol) was firstly dissolved in a 2-necked round-bottom flask (10 mL) in 1 mL of Milli-Q® water under reflux and N2 atmosphere for 10 min. BIS (3.4 mg, 15.8 μmol) was dissolved in 1 mL of Milli-Q® water and injected to the reaction slowly, and the color of the reaction changed from orange to dark brown. The reaction was stirred at 80 °C at 600 rpm for 1 h in dark conditions. After 1 h, 0.5 mL of saturated KPF6 water solution was added to the mixture, causing the precipitation of a solid. The solid was purified through three times centrifugation (10 min, 4300 rpm) and washed with Milli-Q® water. Finally, the as-obtained solid was freeze-dried and stored as a powder (13.0 mg, yield = 92.8 wt %). Chemical analysis, detailed in the results and discussion section, enabled us to propose the chemical formula [[Ru(biqbpy)]1.5(bis)](PF6)3 (Figure 2b).
2.3. HPLC Methodology for RuBIS CPNs Releasing Quantification
Time-dependent HPLC evolution under irradiation of a RuBIS colloidal suspension (200 µg/mL) was performed with stirring, and aliquots at different irradiation times were taken, filtered, and analyzed by HPLC. A calibration curve was performed using different concentrations (0.1, 0.5, 1, 5, 10, and 20 μg/mL) of a stock solution in PBS buffer of the [Ru(biqbpy)(H2O)2]2+ activated complex. The stock solution was obtained from the irradiation with green light (100 mW, 1.1 mW/cm2) of complex 1 (1 mg/mL) dissolved in PBS solution for 20 h to make sure that Ru complex was fully converted to the active form through the photocleavage and photosubstitution process. The measurements were carried out using an HPLC Waters 2695 separation module (Waters Corp., Milford, MA, USA) coupled to a Waters 2487 UV-Vis detector (Waters Corp., Milford, MA, USA) and using a Restek® C-18 (250 mm × 4.6 mm) column (Restek Corp., Bellefonte, PA, USA). Eluent A was a 0.1% (v/v) H3PO4 aqueous solution, and eluent B was acetonitrile absolute (HPLC grade). Before the analysis, the column was pre-equilibrated using the starting conditions (99% A (v/v)) for 10 min, followed by a gradual decrease in A from 100% to 40% (v/v) in the first 20 min and lasting 5 min. Then, the mobile phase reduced to 20% A (v/v) in 1 min and lasted 4 min. At the end, mobile phase was increased to 100% A (v/v) in 1 min to elute residues, and this ratio was kept for additional 5 min. For the next injection, the mobile phase was reset to the initial conditions (A:B) 100:0 (v/v) and kept for 10 min to equilibrate. The flow rate was set at 1.0 mL/min at temperature. This method was used for both the calibration curve and quantification of an active complex release from RuBIS CPNs.
2.4. Quantitative 1H NMR and 19F NMR for Component Analysis of RuBIS CPNs
The slow rotational correlation time in NMR of these nanoparticles in a colloidal solution makes it difficult to obtain a quantitative NMR spectrum. To accomplish this, the RuBIS CPNs were dissolved in deuterated dimethyl sulfoxide solvent ((CD3)2SO) containing a minimum quantity of deuterium chloride (DCl) to decompose the nanoparticles into the molecular entities and thus obtain sharp signals that allow quantifying the ligand-to-ligand ratio. To make sure that different spectra are comparable, the same ratio DCl/(CD3)2SO was used (50 µL DCl/mL (CD3)2SO). In addition, the internal reference (CH2FCN) that has a hydrogen and fluorine atom was chosen to increase the accuracy. The formula shown below was used to calculate the amount of components.
where S = Integrated area of the peak, N = Number of protons atoms in the functional group), m = Weighted mass, M = Molecular weight, and P = Purity.
2.5. In Vitro Studies
2.5.1. Cell Culturing
Human epidermoid carcinoma A431 and human lung carcinoma A549 cancer cell lines were tested. These cell lines were distributed by the European Collection of Cell Cultures (ECACC, Salisbury, UK) and purchased through Sigma Aldrich (Merck KGaA, Darmstadt, Alemania). Cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) “complete” (i.e., DMEM with phenol red, supplemented with Fetal Calf Serum (FCS, 10.0% v/v), Penicillin–Streptomycin (PS solution; 0.2% v/v), and GlutaMax (GM, 0.9% v/v)). Both cell lines were cultured under humidified conditions (37 °C atmosphere containing 7.0% CO2) in 75 cm2 flasks and sub-cultured (1:3–1:6) upon reaching 70–80% confluency (once per week). Media were refreshed every 3 days; cells were passaged for 4–8 weeks maximum.
2.5.2. Cell-Irradiation Setup
The same cell-irradiation system was used as published previously from our group [51] that consisted of a Ditabis thermostat (980923001) fitted with two flat-bottomed microplate thermoblocks (800010600) and a 96-LED array fitted to a standard 96-well plate. The λexc = 520 nm LED with power density (10.9 mW/cm2) (OVL-3324), fans (40 mm, 24 V DC, 9714839), and power supply (EA-PS 2042-06B) were obtained from Farnell.
2.5.3. Cytotoxicity Assay
Cells were seeded at a density of 5 × 103/mL for A549 and 8 × 103/mL for A431 in 96-well plates at t = 0 h using Opti-MEM complete without phenol red (100 μL) and incubated for 24 h at 37 °C, under 7% of CO2. Subsequently, aliquots (100 μL) of six different concentrations between 50 ng/mL and 50 µg/mL (0.7 ng/mL to 2.83 µg/mL based on metal content) of freshly prepared stock RuBIS CPNs suspension or complex 2 solution in Opti-MEM were added to three adjacent wells as a triplicate. A minimum amount of DMSO (<0.5%) was used to dissolve the compounds, which does not harm the cells in each well, including in the control wells. After incubation in the dark for an additional 24 h, the plates were irradiated for 60 min with green light (λexc = 520 nm, power density 10.92 mW/cm2, light dose = 39.3 J/cm2). After irradiation, the plates were incubated in the dark for an additional 48 h either in normoxia or hypoxic incubator. Then, the cells were fixed using cold (4 °C) TCA (10% w/v; 100 μL). Subsequently, TCA was removed from the wells, and the plates were washed with water (×5), stained with SRB (0.6% w/v in acetic acid (1% v/v; 100 μL) for 30 min, washed with acetic acid (1% v/v; ≈300 μL), and air dried overnight. After solubilizing the SRB dye with Tris-base (10 mm; 200 μL), the absorbance was read in each well at λ = 510 nm by using a M1000 Tecan Reader.
The fraction of viable cells in each well was calculated using SRB absorbance (Excel and GraphPad Prism software) obtained from triplicates for each concentration. The relative cell viabilities were obtained by dividing the average absorbance of the treated wells by that observed in the untreated wells for three independent biological replicates (three different passage numbers per cell line). The average cell viability was plotted versus log (concentration) (μM), including the standard deviation error of each point. The effective concentration (EC50) was calculated by using the dose–response curve for each cell line (dark vs. irradiated conditions), by fitting the curves to a non-linear regression function, as relative cell viability, and obtaining a variable Hill slope from Equation (1)
| Y = 100/(1+10((log10EC50−X) ⋅ Hill Slope)). | (1) |
2.5.4. Cellular Uptake Measurements
A431 cells (5 × 105) were seeded in 12-well plates, incubated for 24 h under normoxic conditions, and treated with RuBIS CPNs (25 μg/mL) or complex 2 (19 μg/mL) for 2 h. Then, cells were washed thrice with cold (4 °C) PBS (3 × 2 mL) to remove any compound attached outside the cells. Then, the cells were trypsinized and collected into a 2 mL Eppendorf tube in Opti-MEM media. Cells were counted on a BioRad cell-counting board and carefully washed once with cold PBS to remove trypsin. Then, collected cells were centrifuged at 3000 rpm for 5 min. The resulting cell pellet was digested using 65% HNO3 at 100 °C overnight in a hot air oven. Once back to room temperature, the total volume was completed to 10 mL using Milli-Q® water. The ruthenium content and the standard deviation values of these solutions were analyzed on the duplicate experimental results using the Perkin Elmer NexION 2000 (PerkinElmer, Shelton, CT, USA) of an inductively coupled plasma mass spectrometer (ICP-MS, PerkinElmer, Shelton, CT, USA).
2.5.5. Endocytosis Inhibition Studies
A431 cells (5 × 105 cells) were seeded in 12-well plates, incubated for 24 h under normoxic conditions, and then treated with NaN3 (active uptake inhibitor, 15.4 mM), NH4Cl (20 mM), or Dynasore (dynamin-dependent endocytosis inhibitor, 80 μM) for 1 h; alternatively, the cells were incubated at 4 °C for 1 h. After that, the cells were incubated with either RuBIS CPNs (50 μg/mL) or complex 2 (38 μg/mL) for 3 h in the regular incubator for the inhibitor samples or at 4 °C for the low temperature samples. Then, the cells were treated as in the normal cellular uptake study.
2.5.6. ICP-MS Analysis
The sample was digested in nitric acid (65%, Suprapur®, Merck, Darmstadt, Germany), while diluted (1%) nitric acid was used as a carrying solution. NIST-traceable 1000 mg/L elemental standards (TraceCERT®, Fluka Chemie GmbH, Buchs, Switzerland) were used for the calibration and as internal standards. Calibration standards were prepared in a Secuflow fume hood (SCALA, Wangen, Germany) to prevent contamination, and MiliQ® was used in all sample preparation and analysis steps. The measurements were analyzed using the NexION® 2000 ICP-MS (PerkinElmer, Shelton, CT, USA) equipped with a concentric glass nebulizer and Peltier-cooled glass spray chamber. An SC2 DX autosampler (PerkinElmer, Shelton, CT, USA) was used for sample introduction. Data recording and processing was done by using SyngistixTM Software (v.2.5, PerkinElmer, Shelton, CT, USA). Trace elemental calibration standards were prepared at 0, 1, 4, 20, and 100 µg/L using an NIST-traceable 1000 mg/L Ru standard. An additional set of calibration for Ru (0, 0.1, 0.5, 2.5, and 10 µg/L) was prepared for samples that were anticipated to contain low-level Ru. Samples were analyzed without dilution to minimize the possibility of contamination, using 10 μg/L Rh and In as the internal standard. To check the calibration, samples were analyzed through a repeated measurement of one of the calibration standards and a blank measurement. Curves with correlation coefficient higher than 0.999 were accepted for the calibration.
2.5.7. Singlet Oxygen (1O2) Production Studies
Singlet oxygen generation measurements were conducted in cell-growing medium using 9, 10-anthracenediyl-bis(methylene) dimalonic acid (ABMDMA) as an 1O2-specific probe. ABMDMA is a hydrophilic anthracene derivative that reacts with 1O2 to produce the corresponding endoperoxide [52], thereby lowering the absorbance at 400 nm. For the experiment, 0.1 mM of ABMDMA (in Opti-MEM) was mixed with RuBIS CPNs (25 μg/mL), which was previously dispersed in Opti-MEM cell culture media. The resulting samples were taken into a 3 mL quartz cuvette to record the absorbance in the dark or following green light irradiation (λexc = 520 nm, 39.3 J/cm2). Absorption spectra were recorded initially every 30 s during the first 1 min of continuous light irradiation and successively every 1 min interval during 6 min. The reference rose Bengal dye caused significant changes to the absorption spectra of ABMDMA at 400 nm, which indicated the production of 1O2 with a quantum yield of ΦΔ = 0.68 [53].
3. Results and Discussion
3.1. Synthesis and Characterization
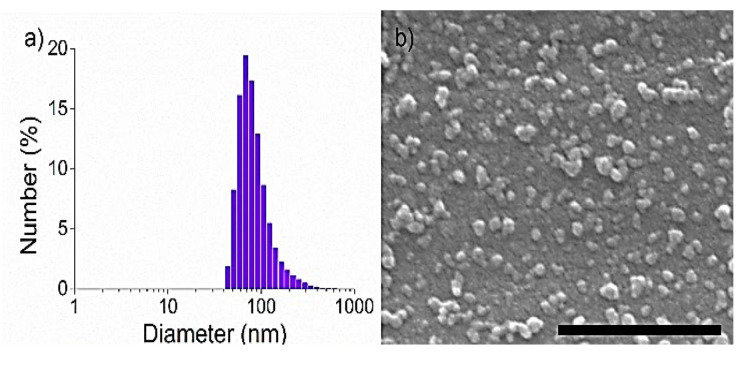
In a typical synthesis, complex 1 was refluxed in 1 mL of MilliQ® water under N2 atmosphere. Subsequently, 1 mL of ethanol solution containing one equivalent of BIS ligand was slowly injected in the Ru complex solution, and the reflux was maintained for 1 h. Afterwards, the solution was cooled down to room temperature, and a saturated KPF6 solution was added to the mixture, resulting in a brown precipitate. The solid was centrifuged, washed with cold ethanol, and freeze-dried for subsequent storage and characterization. Fourier-transform infrared spectroscopy (FTIR) of the freeze-dried solid showed the presence of the typical peaks of both BIS (3000 cm−1, 1509 cm−1 and 1472 cm−1) [54] and of complex 1 (1532 cm−1 and 1098 cm−1). More importantly, new bands at 839 cm−1 and 429 cm−1 assigned to antisymmetric stretching of PF6 and Ru-N stretching modes, respectively, confirmed the coordination of the BIS ligand to complex 1 and the presence of PF6− as a counterion (Figure S5) [55]. Dynamic light scattering (DLS) analysis of colloidal CPNs in Milli-Q® water showed an average hydrodynamic diameter of 93 ± 46 nm (Figure 3a) in agreement with the value of 50 ± 12 nm of average size found using scanning electron microscopy (SEM) (Figure 3b). Moreover, the colloidal stability of the nanoparticles for at least 24 h in the dark was also corroborated by DLS analysis in a 20 mg/mL BSA-containing PBS solution, which was used as a physiological media model (Figure S6). The absence of the diffraction pattern observed from the X-ray powder diffraction (XRD) indicated the amorphous nature of as-obtained RuBIS CPNs (Figure S7). Finally, inductively coupled plasma mass spectrometry (ICP-MS) analysis of the nanoparticles showed 6.9 ± 0.2 wt % of Ru content (Figure S8) and 1H and 19F nuclear magnetic resonance (NMR) of dissolved CPNs in acidic solvent, using CH2FCN as the internal reference (see the procedure in the Materials and methods section and NMR spectra in Figure S9a,b), which allowed us to propose the chemical formula [[Ru(biqbpy)]1.5(bis)](PF6)3. The stoichiometric deviation from the theoretical expected ratio of the components for a linear polymer ([Ru(biqbpy)]:BIS = 1:1) is quite archetypal for CPNs. This is attributed to the out-of-equilibrium synthetic conditions that lead to the fast precipitation process of oligomeric species with different stoichiometry [45,46,48,49,56,57]. In any case, the Ru complex payload value of 41 wt % is more than four-fold higher than most conventional metallodrug-loaded polymer carriers known to date (typically less than 10%) [58]. It is worth mentioning that the characterization analysis was successfully performed for at least three different batches to assure the reproducibility of the synthetic methodology.
Figure 3.
(a) Size distribution of RuBIS CPNs, measured using dynamic light scattering in PBS solution (average diameters of 50 ± 12 nm). (b) Representative SEM image of RuBIS CPNs, scale bar: 500 nm.
3.2. Photoreactivity of RuBIS CPNs
3.2.1. Monitorization by UV-Vis
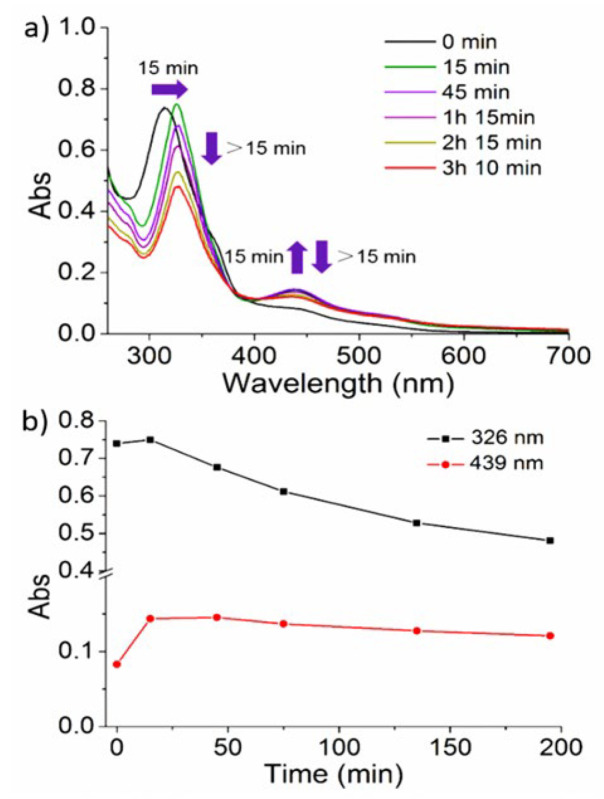
UV-Vis spectroscopy of fresh-made PBS suspensions of the nanoparticles showed an absorption band with a maximum at λmax = 315 nm and two shoulders at λabs = 365 nm and 439 nm lengthening up to 600 nm (Figure 4, black curve, time = 0 min). No spectral changes were observed in the dark upon time (3 h and 10 min, Figure S10) while, as expected, significant changes were found under irradiation. For these experiments, two different irradiation wavelengths were selected: (i) 450 nm, close to the CPNs absorption maximum in the visible region (Figure S11) and (ii) 532 nm, taking advantage of the tail of the broad absorption band (Figure 4). This last wavelength should be not only suitable for triggering the photosubstitution reactions but it also less harmful than blue light to living cells with also deeper penetration in biological tissues.
Figure 4.
(a) Evolution of the UV-Vis spectra of a PBS suspension of RuBIS CPNs upon green light (λexc = 532 nm); (b) plot of absorption changes at 326 nm (black) and 439 nm (red).
Irradiation with 450 nm (0.35 W/cm2) for the first 10 min showed a red shift of the main band, from λmax = 315 to 326 nm and an intensity increase in the shoulder at λabs = 439 nm. Irradiation for longer periods (up to 2.5 h) evidenced an intensity decrease in both bands, including the shoulder at λabs = 365 nm until its disappearance (Figure S11) and an isosbestic point at λiso = 406 nm. This time-dependent two-step process was tentatively explained by an initial fast formation of photoinduced intermediate species (tirradiation ≤ 10 min) and a subsequent slower photochemical reaction (tirradiation > 10 min) leading to the formation of the final photoproduct. Irradiation with green light (λexc = 532 nm, 0.42 W/cm2) yielded similar results, although the spectral changes occurred more slowly due to the lower absorption of the RuBIS CPNs at the used wavelength. A red shift from λmax = 315 to 326 nm and an increase in intensity at λabs = 439 nm was observed for the first 15 min of irradiation, while longer irradiation periods (up to 3 h) induced a decrease in the two bands (326 nm and 439 nm) and the formation of an isosbestic point (Figure 4). Interestingly, similar evolution in UV-Vis spectra was observed for the molecular complex 1 under both blue and green light irradiation (Figure S12) [14], which suggests a similar photo-induced process toward the active photoproduct.
3.2.2. HPLC Studies
Complementary high-performance liquid chromatography (HPLC) studies were done to study the photoproducts resulting upon RuBIS CPNs green light irradiation. Its intrinsic selectivity and sensitivity compared to 1H-NMR or UV-Vis spectroscopy allows the precise quantification of photoproducts as well as the ability to differentiate final Ru-containing by-products more specifically than inductively coupled plasma mass spectrometry (ICP-MS). Before any measurement with RuBIS CPNs, a stock solution in PBS buffer of complex 1 (1 mg/mL), was irradiated with green light (1.1 W/cm2) for 20 h (Figure S13). Elution of the irradiated sample resulted in a single peak at 18.3 min assigned to the active complex [Ru(biqbpy)(H2O)2]2+, as confirmed by ESI mass spectrometry (Figure S14) with the corresponding m/z = 271.0 (calc. m/z = 270.8). As complex 1 to active form conversion was quantitative under such experimental conditions [14], a calibration curve of the fully activated complex was obtained at different concentrations (R2 = 0.996) (Figure S15).
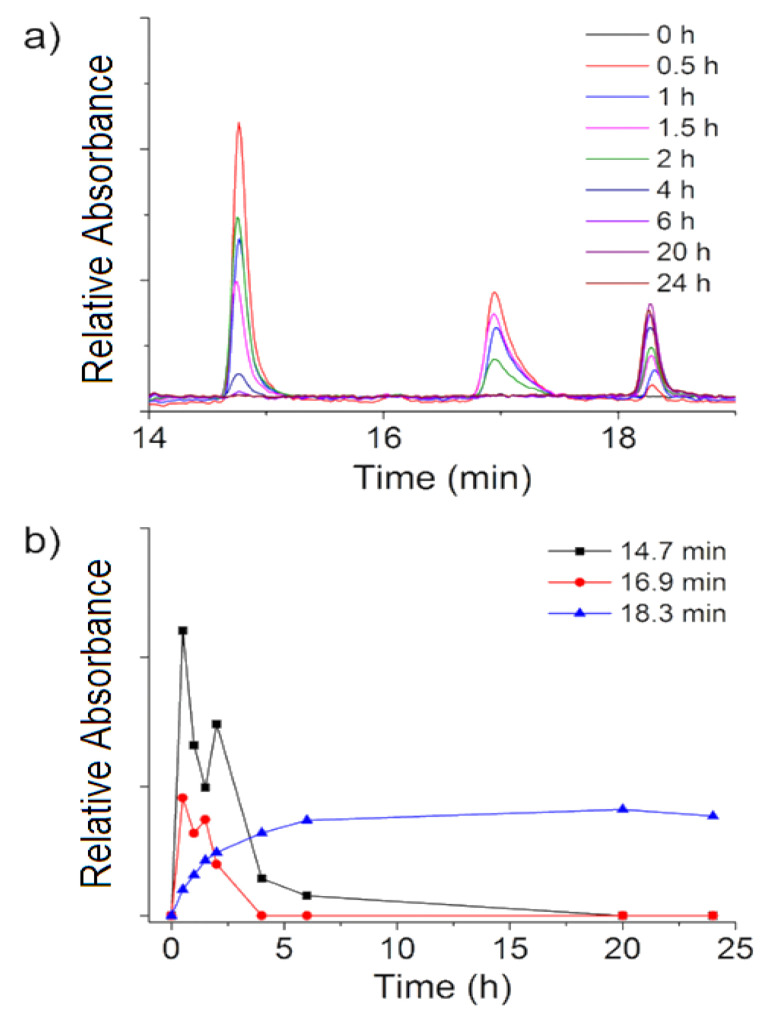
Subsequently, a colloidal suspension of RuBIS CPNs in PBS buffer was irradiated (532 nm, 1.1 W/cm2 and 24 h), and aliquots at different irradiation times were taken, filtered, and analyzed by HPLC. To guarantee the detection of the complex released, the concentration of the initial suspension was increased with respect to that used in UV-Vis experiments up to 200 µg/mL and the irradiation time was enlarged. The results are shown in Figure 5. After the first 30 min, three different peaks with retention times of 14.7, 16.9, and 18.3 min, associated to different species, appeared after 30 min (Figure 5a and Figure S16). Further irradiation up to 6 h induced a notable increase in the peak at 18.3 min, while the two other decreased until almost complete disappearance after 20 h (Figure 5b). The formation of intermediate species at shorter irradiation times and their decrease upon prolonged irradiation to form a final product resembles the behavior observed with UV-Vis experiments (the different conversion times observed by UV-Vis vs. HPLC for the intermediates and final photoproduct were ascribed to the different CPNs concentrations used in each case). To get more detailed information on the intermediate as well as the final photoproduct chemical composition, mass spectrometry was used. Analysis of intermediate fractions at 14.7 and 16.9 min revealed oligomeric species that may come from larger fragments, such as {[Ru2(biqbpy)2bis(MeOH)2](PF6)2}2+ (found m/z = 828.8 calc. m/z = 828.7) (Figure S17), while the final photoproduct obtained at 18.3 min was identified as the target active complex [Ru(biqbpy)(H2O)2]2+ (Figure S14). Using the calibration curve previously obtained with this bis-aqua product, it was possible to quantitatively determine that after 24 h of green light irradiation of a 200 µg/mL RuBIS CPNs suspension (PBS buffer release), 1.1 µg/mL solution of the activated complex was obtained.
Figure 5.
(a) Time-dependent HPLC chromatogram evolution under irradiation of a RuBIS CPNs colloidal suspension (200 µg/mL). (b) Plot of the time-dependent variation of relative UV absorption of each component (at given retention times) upon irradiation. Detection wavelength: λ = 305 nm.
3.3. Cellular Uptake Measurements
Skin non-melanoma A431 cells were incubated in the dark for 2 h with RuBIS CPNs, and the amount of Ru in the cells was quantified using ICP-MS. To better define the role of the nanostructuration on the internalization and phototherapy, we repeated these experiments with a related molecular complex [Ru(biqbpy)(N-methylimidazole)2](PF6)2 (complex 2), which was especially synthesized as a reference model. Complex 2, a mononuclear analogue of complex 1 coordinated with 2 axial methylimidazole ligands, was selected as a reference molecular complex for RuBIS CPNs due to the analogous ruthenium coordination sphere in both systems, which facilitates and makes more suitable the comparative studies. Complex 2 was obtained upon the reaction of complex 1 with an excess of 1-methylimidazole (ratio 1:10) in EtOH under reflux and N2 atmosphere (for more information, see the Materials and Methods section). Remarkably, the time-dependent UV-Vis evolution of complex 2 under green light irradiation (Figure S18) is very similar to that previously observed for complex 1 (Figure S12) [14], validating its use for further biological studies.
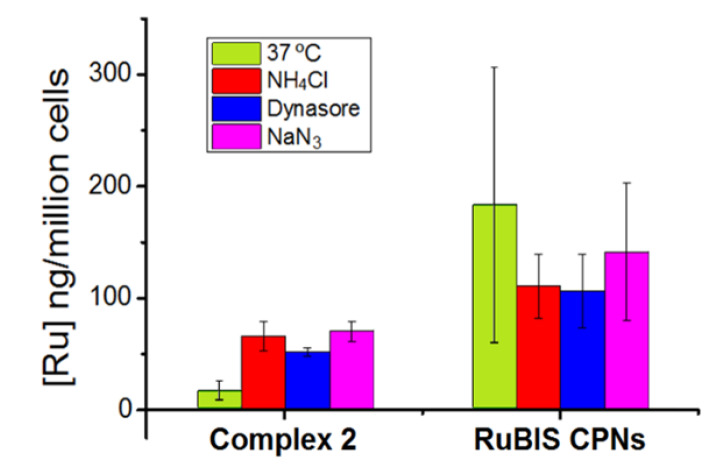
As can be seen in Figure 6, while complex 2 uptake (16 ng Ru/million cell) is comparable to similar molecular Ru complexes previously reported [14], RuBIS CPNs were taken up by cells up to 11 times more (183 ng Ru/million cell). Such difference was tentatively assigned to the different internalization mechanisms. To confirm it, additional Ru quantification studies were performed co-incubating RuBIS CPNs or complex 2 with the endocytosis inhibitors NaN3, NH4Cl, or Dynasore, which are inhibitors of active uptake, endocytosis, and dynamin-dependent endocytosis, respectively. Indeed, cellular uptake of RuBIS CPNs was slightly decreased in the presence of dynasore (96.3 ng Ru/million cells), ammonium chloride (101 ng Ru/million cells), and to a lesser extent of sodium azide (138 ng Ru/million cells), compared to treatment with RuBIS CNPs alone (183 ng Ru/million cells). For the mononuclear complex 2, the opposite result was observed, with a higher uptake in the presence of those inhibitors compared to treatment with 2 alone. Overall, these results suggested that endocytosis-related processes may be involved in cellular uptake of RuBIS CNPs, as reported previously for other nanoparticles [59], while for complex 2, different mechanisms probably take place [60].
Figure 6.
Cellular uptake quantification for Ru content in A431 cells treated with complex 2 (19 µg/mL) or RuBIS CPNs (25 µg/mL) nanoparticles for 2 h in the presence or absence of uptake inhibitors: ammonium chloride, dynasore, or sodium azide endocytosis.
3.4. (Photo)cytotoxicity Studies
RuBIS CPNs dispersions (from 0.7 ng/mL to 2.83 µg/mL based on Ru content) or complex 2 solutions (from 1.0 ng/mL to 5.0 ug/mL based on Ru content) in Opti-MEM medium (with minimum amounts of DMSO < 0.5% to fully dissolve complex 2) were seeded in human skin (A431) and lung cancer (A549) cell lines. As described in previous reports [61], the cells were seeded at t = 0, treated at t = 24 h, irradiated with green light (λexc = 520 nm, 39.3 J/cm2) for 1 h at 48 h, and its cell viability was quantified at t = 96 h using a standard sulforhodamine B (SRB) assay. Half-effective growth inhibition concentration (EC50 in µM) values at t = 96 h are shown in Table 1, and dose–response curves are shown in Figure S19. Cisplatin was also tested under the same conditions and used as control.
Table 1.
Cytotoxicity of RuBIS CPNs and complex 2 in A431 and A549 cancer cell lines in the dark and under green light (λexc = 520 nm, 39.3 J/cm2) irradiation.
| Cell Type | Light DoseJ/cm2 | RuBIS CPNs | Complex 2 | Cisplatin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EC50 (µM) | CI [a] | PI [b] | EC50(µM) | CI [a] | PI [b] | EC50(µM) | CI [a] | PI [b] | ||
| A431 | 0 | 11.9 | +0.46 −n.a. |
2.4 | 28.1 | +0.06 −0.60 |
1.7 | 3.0 | +0.45 −0.41 |
1.1 |
| 39.3 | 5.0 | +0.04 −0.04 |
16.3 | +0.55 −0.32 |
3.3 | +0.31 −0.28 |
||||
| A549 | 0 | 9.1 | +0.09 −0.08 |
1.8 | 28.3 | +1.16 −0.74 |
1.0 | 3.0 | +0.15 −0.15 |
1.0 |
| 39.3 | 5.0 | +0.02 −0.02 |
27.5 | +0.43 −0.37 |
3.0 | +0.17 −0.17 |
||||
[a] Confidence interval, [b] photo indices. EC50 values are expressed in μM as half-maximal effective concentration (95% confidence interval are also given in μM).
The EC50 values obtained for RuBIS CPNs in the dark (EC50,dark) of 11.88 µM and 9.10 µM decrease under irradiation (EC50,light) to 4.95 µM and 5.04 µM for A431 and A549 cells, respectively (Table 1). Therefore, there is a remarkable difference in the EC50 values for the RuBIS CPNs with and without irradiation, showing cell-dependent phototoxicity index (PI) values of 2.4 and 1.8 toward A431 and A549 cells, respectively. In both cases, the PI values of RuBIS CPNs are higher than those of the molecular complex 2 (1.7 and 1.0, respectively). From these data, the following considerations deserve to be mentioned. First, the EC50,dark of RuBIS CPNs was higher than that of complex 2, which was expected, as it is possibly related to the significant higher uptake. Second, the EC50,light values of model complex 2 were 16.33 µM and 27.52 µM in A431 and A549 cells, respectively, which are up to 3–5 five times higher than those found for RuBIS CPNs, confirming the positive nanostructuration effect on the enhancement of phototoxicity. These results imply that less Ru is required to photoinduce an efficient chemotherapeutic effect, minimizing the cells death for non-irradiated cells. Moreover, the obtained EC50,light values are similar to previously described monomeric complexes [14] but using almost a half-irradiation dose, which is also an additional advantage given the possible undesirable side effects that may appear from using a high irradiation doses. Last but not least, EC50,dark values for RuBIS CPNs are three to four times higher than those of cisplatin (3.01 and 3.04 µM), i.e., less toxic; while EC50,light values of RuBIS CPNs were close to those obtained for the photo-independent gold-standard cisplatin (EC50 = 3.01 µM and 3.04 µM in A431 and A549, respectively), which equate their effectiveness to drugs commercially used today. Cytotoxicity of the bis-imidazol (bis) and methylimidazole ligands are considered negligible, since a previous evaluation in our group using concentrations ranging from 0 to 100 μg/mL in different cell lines (Figure S20) corroborated their very low cytotoxic effect at the highest concentration assayed (100 μg/mL).
3.5. Singlet Oxygen Production
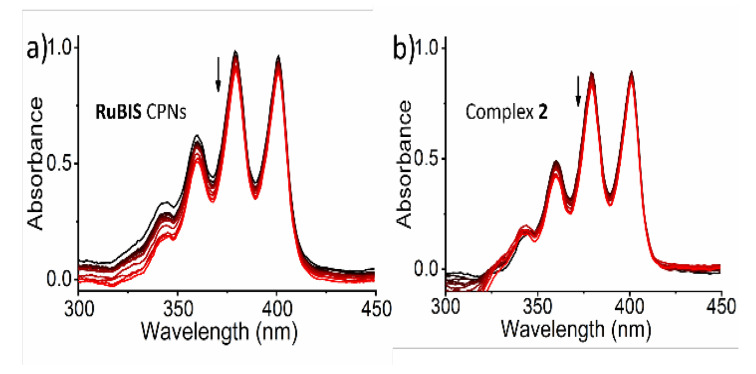
To discard PDT as a possible origin of the photoactivity instead of PACT, the production of singlet oxygen (1O2) upon green light irradiation of RuBIS CPNs was quantified. For this, the common method is to measure the near-infrared emission intensity of 1O2 (1270 nm) in CD3OD; though in this case and to mimic the cell culture conditions, the value of singlet oxygen quantum yield (ΦΔ) was indirectly determined in Opti-MEM medium using a selective water-soluble 1O2 probe (9,10-anthracenediyl-bis(methylene)-dimalonic acid, ABMDMA). In the dark, this dye absorbs light at 378 nm while in the presence of photo-generated 1O2, a less conjugated endoperoxide is formed, leading to a decrease in the absorbance at 378 nm [62]. The rose Bengal dye was used as reference, as it produces 1O2 with a known quantum yield φΔ = 0.76 [53] under green light irradiation. When RuBIS CPNs (25 μg/mL) was mixed with ABMDMA (100 μM) in Opti-MEM, no changes in the absorption spectra were observed with or without green light irradiation (λexc = 520 nm, see Figure 7), contrary to rose Bengal (Figure S21) [63]. The same study was performed in the same way for complex 2. In both cases, the results excluded 1O2 production, as expected for photosubstituted active ruthenium compounds.
Figure 7.
The negligible absorption spectral changes of ABMDMA upon green light irradiation in the presence of (a) RuBIS CPNs (25 μg/mL), (b) complex 2 (19 μg/mL). The 1O2 generation is studied in Opti-MEM medium. The arrows indicate the evolution of the spectra with time.
4. Conclusions
We have successfully designed and synthetized light-sensitive coordination polymer nanoparticles (CPNs) based on the polymerization of a Ru(II) polypyridyl prodrug 1 with a photocleavable bis-imidazole linking ligand BIS. Precise control of the reaction conditions led to the reproducible synthesis of narrow size distribution (50 ± 12 nm) CPNs with remarkable drug encapsulation yields well over those already described for other nanoencapsulation systems. The photoactivation of the RuBIS CPNs showed controlled release of the anticancer Ru complex [Ru(biqbpy)(H2O)2]2+ upon green (532 nm) irradiation, while they were stable in cell-growing medium in the dark, reducing the cell dead population and side effects in its inactivated form. Interestingly, the dose of light necessary to obtain enough cytotoxic complex from RuBIS CPNs in vitro (39.3 J/cm2) is notably lower compared to previous values published for similar green light photoactivated ruthenium systems (75 J/cm2) [14]. Moreover, in vitro studies demonstrated that RuBIS CNPs have an 11-fold increased uptake in comparison to related monomeric complexes thanks to the energy-dependent endocytosis uptake pathway triggered by the CNPs formulation. This fact determined a substantial increase in phototoxicity index in comparison with monomeric species and a light-selective cytotoxic effect close to the gold standard cisplatin. All in all, RuBIS CPNs demonstrates the potential of photoactivated CPNs for PACT anticancer treatments.
Acknowledgments
The authors thanks Jose Bolanos Cardet for the design of the Figure 1 and Graphical Abstract.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nano11113089/s1, Figure S1: 1H-NMR spectrum of complex 2 in (CD3)2SO, Figure S2: COSY spectrum of complex 2 in (CD3)2SO, Figure S3: 13C-NMR spectrum of complex 2 in (CD3)2SO, Figure S4: Mass spectrum of complex 2, Figure S5: FTIR spectra of complex 1, BIS ligand and RuBIS CPNs, Figure S6: Time-dependent DLS measurements RuBIS CPNs, Figure S7: X-ray Diffractometry of BIS ligand, KPF6, complex 1, and RuBIS CPNs, Figure S8: Calibration curve of the Ru for inductively coupled plasma mass spectrometry (ICP-MS), Figure S9a: 1H NMR spectra of RuBIS CPNs, complex 1, CH2FCN (internal reference) and BIS ligand, Figure S9b: 19F NMR spectra of RuBIS CPNs and CH2FCN (internal reference), Figure S10: (a) UV-vis absorption spectra of a PBS suspension of RuBIS CPNs in the dark over time. (b) Plot of absorbance at λabs = 315 and 439 nm of the RuBIS CPNs suspension in the dark. (c) DLS tracing results of the RuBIS CPNs suspension in PBS solution recorded at 0 min and 3 h 10 min, Figure S11: (a) Evolution of the UV-vis spectra of a PBS suspension of RuBIS CPNs, upon blue light irradiation (λex = 450 nm). (b) Plot of absorbance changes at λabs = 326 nm and 439 nm, Figure S12: Evolution of the UV-vis absorbance spectra of a solution of complex 1, upon 450 nm blue or 530 nm green light irradiation under argon. Reprinted with permission from Ref. [14]. Copyright 2016 the Royal Society of Chemistry. Figure S13: The whole chromatogram of fully activated drug [Ru(biqbpy)(H2O)2]2+ converted from complex 1 at different concentrations, Figure S14: Mass spectrometry of the fractions at different retention times), Figure S15: The drug ([Ru(biqbpy)(H2O)2]2+) calibration curve, Figure S16: The whole chromatogram of the drug release of RuBIS CPNs (0–35 min), Figure S17: Mass spectra of samples with retention time, Figure S18: UV-vis absorbance spectra of a complex 2 solution after green light irradiation, Figure S19: Cytotoxicity assays with and without green light (520 nm) irradiation, Figure S20: Cytotoxicity of Bis and 1-methylimidazole free ligands, Figure S21: Absorption spectral changes of ABMDMA in the dark (a) or following green light irradiation with the light of dose 39.3 J/cm2, (b) in the presence of Rose Bengal (0.1 µM), Figure S22: Determination of required irradiation time for RuBIS CPNs and complex 2 activation.
Author Contributions
J.Z. synthesized the materials, performed all the physicochemical characterizations. V.R. conducted in vitro studies and X.-Q.Z. conducted singlet oxygen generation studies. C.F. conducted preliminary studies and generated proof of concept results, D.R.-M. participated in the design of the experiments and discussion of the results. S.B. supervised the in vitro results and participated in the discussion results. C.R. and F.N. supervised the whole research work and coordinated the different studies. The manuscript was written through contributions of all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grant RTI2018-098027-B-C21 funded by MCIN/AEI/10.13039/501100011033 and by ERDF A way of making Europe. With the support from “Metalfármacos multifuncionales para el diagnóstico y la terapia” with grant RED2018-102471-T funded by MCIN/AEI/10.13039/501100011033. The ICN2 is funded by the CERCA programme/Generalitat de Catalunya. The ICN2 is supported by the Severo Ochoa Centres of Excellence programme, grant SEV-2017-0706 funded by MCIN/AEI/10.13039/501100011033. J.D. Zhang thanks the BIST PhD Fellowship Programme (This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 754558). NWO is kindly acknowledged for financial support to SB via a VICI grant. COST is kindly acknowledged for stimulating scientific discussion and financial support via the Cost Action CA 17140 “Cancer nanomedicine from the bench to the bedside”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available on the request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kladnik J., Kljun J., Burmeister H., Ott I., Romero-Canelón I., Turel I. Towards Identification of Essential Structural Elements of Organoruthenium(II)-Pyrithionato Complexes for Anticancer Activity. Chem. A Eur. J. 2019;25:14169–14182. doi: 10.1002/chem.201903109. [DOI] [PubMed] [Google Scholar]
- 2.Zhao Z., Gao P., You Y., Chen T. Cancer-Targeting Functionalization of Selenium-Containing Ruthenium Conjugate with Tumor Microenvironment-Responsive Property to Enhance Theranostic Effects. Chem. A Eur. J. 2018;24:3289–3298. doi: 10.1002/chem.201705561. [DOI] [PubMed] [Google Scholar]
- 3.Hartinger C.G., Zorbas-Seifried S., Jakupec M.A., Kynast B., Zorbas H., Keppler B.K. From bench to bedside–preclinical and early clinical development of the anticancer agent indazolium trans-[tetrachlorobis(1H-indazole)ruthenate(III)] (KP1019 or FFC14A) J. Inorg. Biochem. 2006;100:891–904. doi: 10.1016/j.jinorgbio.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Gransbury G.K., Kappen P., Glover C.J., Hughes J.N., Levina A., Lay P.A., Musgrave I.F., Harris H.H. Comparison of KP1019 and NAMI-A in tumour-mimetic environments. Metallomics. 2016;8:762–773. doi: 10.1039/C6MT00145A. [DOI] [PubMed] [Google Scholar]
- 5.Mital M., Ziora Z. Biological applications of Ru(II) polypyridyl complexes. Coord. Chem. Rev. 2018;375:434–458. doi: 10.1016/j.ccr.2018.02.013. [DOI] [Google Scholar]
- 6.Zeng L., Gupta P., Chen Y., Wang E., Ji L., Chao H., Chen Z.-S. The development of anticancer ruthenium(ii) complexes: From single molecule compounds to nanomaterials. Chem. Soc. Rev. 2017;46:5771–5804. doi: 10.1039/C7CS00195A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jakupec M.A., Kandioller W., Schoenhacker-Alte B., Trondl R., Berger W., Keppler B.K. Ruthenium Complexes: Photochemical and Biomedical Applications. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2017. Trends and Perspectives of Ruthenium Anticancer Compounds (Non-PDT) pp. 271–291. [DOI] [Google Scholar]
- 8.Smith N.A., Zhang P., Greenough S.E., Horbury M.D., Clarkson G.J., McFeely D., Habtemariam A., Salassa L., Stavros V.G., Dowson C.G., et al. Combatting AMR: Photoactivatable ruthenium(ii)-isoniazid complex exhibits rapid selective antimycobacterial activity. Chem. Sci. 2016;8:395–404. doi: 10.1039/C6SC03028A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen M., Sun W., Kretzschmann A., Butt H.-J., Wu S. Nanostructured polymer assemblies stabilize photoactivatable anticancer ruthenium complexes under physiological conditions. J. Inorg. Biochem. 2020;207:111052. doi: 10.1016/j.jinorgbio.2020.111052. [DOI] [PubMed] [Google Scholar]
- 10.Sun W., Wen Y., Thiramanas R., Chen M., Han J., Gong N., Wagner M., Jiang S., Meijer M., Bonnet S., et al. Red-Light-Controlled Release of Drug-Ru Complex Conjugates from Metallopolymer Micelles for Phototherapy in Hypoxic Tumor Environments. Adv. Funct. Mater. 2018;28:1804227. doi: 10.1002/adfm.201804227. [DOI] [Google Scholar]
- 11.Imberti C., Zhang P., Huang H., Sadler P.J. New Designs for Phototherapeutic Transition Metal Complexes. Angew. Chem. Int. Ed. 2019;59:61–73. doi: 10.1002/anie.201905171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lameijer L.N., Ernst D., Hopkins S.L., Meijer M.S., Askes S.H.C., Le Dévédec S.E., Bonnet S. A Red-Light-Activated Ruthenium-Caged NAMPT Inhibitor Remains Phototoxic in Hypoxic Cancer Cells. Angew. Chem. Int. Ed. 2017;56:11549–11553. doi: 10.1002/anie.201703890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Betanzos-Lara S., Salassa L., Habtemariam A., Sadler P.J. Photocontrolled nucleobase binding to an organometallic RuII arene complex. Chem. Commun. 2009;43:6622–6624. doi: 10.1039/b914153g. [DOI] [PubMed] [Google Scholar]
- 14.Van Rixel V.H.S., Siewert B., Hopkins S.L., Askes S.H.C., Busemann A., Siegler M.A., Bonnet S. Green light-induced apoptosis in cancer cells by a tetrapyridyl ruthenium prodrug offering two trans coordination sites. Chem. Sci. 2016;7:4922–4929. doi: 10.1039/C6SC00167J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Havrylyuk D., Deshpande M., Parkin S., Glazer E.C. Ru(ii) complexes with diazine ligands: Electronic modulation of the coordinating group is key to the design of “dual action” photoactivated agents. Chem. Commun. 2018;54:12487–12490. doi: 10.1039/C8CC05809A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuello-Garibo J.-A., Meijer M.S., Bonnet S. To cage or to be caged? The cytotoxic species in ruthenium-based photoactivated chemotherapy is not always the metal. Chem. Commun. 2017;53:6768–6771. doi: 10.1039/C7CC03469E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howerton B.S., Heidary D.K., Glazer E.C. Strained Ruthenium Complexes Are Potent Light-Activated Anticancer Agents. J. Am. Chem. Soc. 2012;134:8324–8327. doi: 10.1021/ja3009677. [DOI] [PubMed] [Google Scholar]
- 18.Burke C.S., Byrne A., Keyes T.E. Targeting Photoinduced DNA Destruction by Ru(II) Tetraazaphenanthrene in Live Cells by Signal Peptide. J. Am. Chem. Soc. 2018;140:6945–6955. doi: 10.1021/jacs.8b02711. [DOI] [PubMed] [Google Scholar]
- 19.Shi G., Monro S., Hennigar R., Colpitts J., Fong J., Kasimova K., Yin H., DeCoste R., Spencer C., Chamberlain L., et al. Ru(II) dyads derived from α-oligothiophenes: A new class of potent and versatile photosensitizers for PDT. Coord. Chem. Rev. 2015;282:127–138. doi: 10.1016/j.ccr.2014.04.012. [DOI] [Google Scholar]
- 20.Zhang C., Guan R., Liao X., Ouyang C., Rees T., Liu J., Chen Y., Ji L., Chao H. A mitochondria-targeting dinuclear Ir–Ru complex as a synergistic photoactivated chemotherapy and photodynamic therapy agent against cisplatin-resistant tumour cells. Chem. Commun. 2019;55:12547–12550. doi: 10.1039/C9CC05998A. [DOI] [PubMed] [Google Scholar]
- 21.Farrer N.J., Salassa L., Sadler P.J. Photoactivated chemotherapy (PACT): The potential of excited-state d-block metals in medicine. Dalton Trans. 2009;48:10690–10701. doi: 10.1039/b917753a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong D.Y.Q., Ong W.W.F., Ang W.H. Induction of Immunogenic Cell Death by Chemotherapeutic Platinum Complexes. Angew. Chem. Int. Ed. 2015;54:6483–6487. doi: 10.1002/anie.201500934. [DOI] [PubMed] [Google Scholar]
- 23.Lv W., Zhang Z., Zhang K.Y., Yang H., Liu S., Xu A., Guo S., Zhao Q., Huang W. A Mitochondria-Targeted Photosensitizer Showing Improved Photodynamic Therapy Effects Under Hypoxia. Angew. Chem. Int. Ed. 2016;55:9947–9951. doi: 10.1002/anie.201604130. [DOI] [PubMed] [Google Scholar]
- 24.Lameijer L.N., Hopkins S.L., Brevé T.G., Askes S.H.C., Bonnet S. d-Versus l-Glucose Conjugation: Mitochondrial Targeting of a Light-Activated Dual-Mode-of-Action Ruthenium-Based Anticancer Prodrug. Chem. A Eur. J. 2016;22:18484–18491. doi: 10.1002/chem.201603066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rad A.T., Chen C.-W., Aresh W., Xia Y., Lai P.-S., Nieh M.-P. Combinational Effects of Active Targeting, Shape, and Enhanced Permeability and Retention for Cancer Theranostic Nanocarriers. ACS Appl. Mater. Interfaces. 2019;11:10505–10519. doi: 10.1021/acsami.8b21609. [DOI] [PubMed] [Google Scholar]
- 26.Mari C., Pierroz V., Ferrari S., Gasser G. Combination of Ru(ii) complexes and light: New frontiers in cancer therapy. Chem. Sci. 2015;6:2660–2686. doi: 10.1039/C4SC03759F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barry N.P.E., Sadler P.J. Challenges for Metals in Medicine: How Nanotechnology May Help to Shape the Future. ACS Nano. 2013;7:5654–5659. doi: 10.1021/nn403220e. [DOI] [PubMed] [Google Scholar]
- 28.Mackay F.S., Woods J.A., Heringová P., Kašpárková J., Pizarro A.M., Moggach S.A., Parsons S., Brabec V., Sadler P.J. A potent cytotoxic photoactivated platinum complex. Proc. Natl. Acad. Sci. USA. 2007;104:20743–20748. doi: 10.1073/pnas.0707742105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mulcahy S.P., Li S., Korn R., Xie X., Meggers E. Solid-Phase Synthesis of Tris-heteroleptic Ruthenium(II) Complexes and Application to Acetylcholinesterase Inhibition. Inorg. Chem. 2008;47:5030–5032. doi: 10.1021/ic800080b. [DOI] [PubMed] [Google Scholar]
- 30.Vyas N.A., Bhat S., Kumbhar A.S., Sonawane U.B., Jani V., Joshi R.R., Ramteke S., Kulkarni P., Joshi B. Ruthenium(II) polypyridyl complex as inhibitor of acetylcholinesterase and Aβ aggregation. Eur. J. Med. Chem. 2014;75:375–381. doi: 10.1016/j.ejmech.2014.01.052. [DOI] [PubMed] [Google Scholar]
- 31.Alatrash N., Narh E.S., Yadav A., Kim M.-J., Janaratne T., Gabriel J., MacDonnell F.M. Synthesis, DNA Cleavage Activity, Cytotoxicity, Acetylcholinesterase Inhibition, and Acute Murine Toxicity of Redox-Active Ruthenium(II) Polypyridyl Complexes. ChemMedChem. 2017;12:1055–1069. doi: 10.1002/cmdc.201700240. [DOI] [PubMed] [Google Scholar]
- 32.Koch J.H., Rogers W.P., Dwyer F.P., Gyarfas E.C. The Metabolic Fate of Tris-1,10-Phenanthroline 106Ruthenium (II) Perchlorate, a Compound With Anticholinesterase and Curare-Like Activity. Aust. J. Biol. Sci. 1957;10:342. doi: 10.1071/BI9570342. [DOI] [Google Scholar]
- 33.Poynton F.E., Bright S.A., Blasco S., Williams D.C., Kelly J.M., Gunnlaugsson T. The development of ruthenium(ii) polypyridyl complexes and conjugates for in vitro cellular and in vivo applications. Chem. Soc. Rev. 2017;46:7706–7756. doi: 10.1039/C7CS00680B. [DOI] [PubMed] [Google Scholar]
- 34.Villemin E., Ong Y.C., Thomas C.M., Gasser G. Polymer encapsulation of ruthenium complexes for biological and medicinal applications. Nat. Rev. Chem. 2019;3:261–282. doi: 10.1038/s41570-019-0088-0. [DOI] [Google Scholar]
- 35.Karges J., Li J., Zeng L., Chao H., Gasser G. Polymeric Encapsulation of a Ruthenium Polypyridine Complex for Tumor Targeted One- and Two-Photon Photodynamic Therapy. ACS Appl. Mater. Interfaces. 2020;12:54433–54444. doi: 10.1021/acsami.0c16119. [DOI] [PubMed] [Google Scholar]
- 36.Sun W., Zeng X., Wu S. Photoresponsive ruthenium-containing polymers: Potential polymeric metallodrugs for anticancer phototherapy. Dalton Trans. 2017;47:283–286. doi: 10.1039/C7DT03390G. [DOI] [PubMed] [Google Scholar]
- 37.Sun W., Parowatkin M., Steffen W., Butt H.-J., Mailänder V., Wu S. Ruthenium-Containing Block Copolymer Assemblies: Red-Light-Responsive Metallopolymers with Tunable Nanostructures for Enhanced Cellular Uptake and Anticancer Phototherapy. Adv. Healthc. Mater. 2015;5:467–473. doi: 10.1002/adhm.201500827. [DOI] [PubMed] [Google Scholar]
- 38.Sun W., Li S., Haupler B., Liu J., Jin S., Steffen W., Schubert U.S., Butt H.J., Liang X.J., Wu S. An Amphiphilic Ruthenium Polymetallodrug for Combined Photodynamic Therapy and Photochemotherapy In Vivo. Adv. Mater. 2017;29:1603702. doi: 10.1002/adma.201603702. [DOI] [PubMed] [Google Scholar]
- 39.Zhang C., Guo X., Da X., Yao Y., Xiao H., Wang X., Zhou Q. UCNP@BSA@Ru nanoparticles with tumor-specific and NIR-triggered efficient PACT activity in vivo. Dalton Trans. 2021;50:7715–7724. doi: 10.1039/D1DT00777G. [DOI] [PubMed] [Google Scholar]
- 40.Meijer M.S., Natile M.M., Bonnet S. 796 nm Activation of a Photocleavable Ruthenium(II) Complex Conjugated to an Upconverting Nanoparticle through Two Phosphonate Groups. Inorg. Chem. 2020;59:14807–14818. doi: 10.1021/acs.inorgchem.0c00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y., Jiang G., Zhou Q., Zhang Y., Li K., Zheng Y., Zhang B., Wang X. An upconversion nanoparticle/Ru(ii) polypyridyl complex assembly for NIR-activated release of a DNA covalent-binding agent. RSC Adv. 2016;6:23804–23808. doi: 10.1039/C6RA03396B. [DOI] [Google Scholar]
- 42.Ruggiero E., Habtemariam A., Yate L., Mareque-Rivas J.C., Salassa L. Near infrared photolysis of a Ru polypyridyl complex by upconverting nanoparticles. Chem. Commun. 2013;50:1715–1718. doi: 10.1039/c3cc47601d. [DOI] [PubMed] [Google Scholar]
- 43.Soliman N., McKenzie L.K., Karges J., Bertrand E., Tharaud M., Jakubaszek M., Guérineau V., Goud B., Hollenstein M., Gasser G., et al. Ruthenium-Initiated polymerization of lactide: A route to remarkable cellular uptake for photodynamic therapy of cancer. Chem. Sci. 2020;11:2657–2663. doi: 10.1039/C9SC05976H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suárez-García S., Solórzano R., Alibés R., Busqué F., Novio F., Ruiz-Molina D. Antitumour activity of coordination polymer nanoparticles. Coord. Chem. Rev. 2021;441:213977. doi: 10.1016/j.ccr.2021.213977. [DOI] [Google Scholar]
- 45.Adarsh N., Frias C., Lohidakshan T.P., Lorenzo J., Novio F., Garcia-Pardo J., Ruiz-Molina D. Pt(IV)-based nanoscale coordination polymers: Antitumor activity, cellular uptake and interactions with nuclear DNA. Chem. Eng. J. 2018;340:94–102. doi: 10.1016/j.cej.2018.01.058. [DOI] [Google Scholar]
- 46.Novio F., Lorenzo J., Nador F., Wnuk K., Ruiz-Molina D. Carboxyl Group (-CO2H) Functionalized Coordination Polymer Nanoparticles as Efficient Platforms for Drug Delivery. Chem. A Eur. J. 2014;20:15443–15450. doi: 10.1002/chem.201403441. [DOI] [PubMed] [Google Scholar]
- 47.Imaz I., Rubio-Martínez M., García-Fernández L., García F., Ruiz-Molina D., Hernando J., Puntes V., Maspoch D. Coordination polymer particles as potential drug delivery systems. Chem. Commun. 2010;46:4737–4739. doi: 10.1039/c003084h. [DOI] [PubMed] [Google Scholar]
- 48.Borges M., Yu S., Laromaine A., Roig A., Suárez-García S., Lorenzo J., Ruiz-Molina D., Novio F. Dual T1/T2 MRI contrast agent based on hybrid SPION@coordination polymer nanoparticles. RSC Adv. 2015;5:86779–86783. doi: 10.1039/C5RA17661A. [DOI] [Google Scholar]
- 49.Nador F., Wnuk K., Garcia-Pardo J., Lorenzo J., Solorzano R., Ruiz-Molina D., Novio F. Dual-Fluorescent Nanoscale Coordination Polymers via a Mixed-Ligand Synthetic Strategy and Their Use for Multichannel Imaging. ChemNanoMat. 2017;4:183–193. doi: 10.1002/cnma.201700311. [DOI] [Google Scholar]
- 50.Lee S., Lee J.H., Kim J.C., Lee S., Kwak S.K., Choe W. Porous Zr6L3 Metallocage with Synergetic Binding Centers for CO2. ACS Appl. Mater. Interfaces. 2018;10:8685–8691. doi: 10.1021/acsami.7b18836. [DOI] [PubMed] [Google Scholar]
- 51.Hopkins S.L., Siewert B., Askes S.H.C., Veldhuizen P., Zwier R., Heger M., Bonnet S. An in vitro cell irradiation protocol for testing photopharmaceuticals and the effect of blue, green, and red light on human cancer cell lines. Photochem. Photobiol. Sci. 2016;15:644–653. doi: 10.1039/C5PP00424A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Z.-A., Kuthati Y., Kankala R.K., Chang Y.-C., Liu C.-L., Weng C.-F., Mou C.-Y., Lee C.-H. Encapsulation of palladium porphyrin photosensitizer in layered metal oxide nanoparticles for photodynamic therapy against skin melanoma. Sci. Technol. Adv. Mater. 2015;16:54205. doi: 10.1088/1468-6996/16/5/054205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lutkus L.V., Rickenbach S., McCormick T.M. Singlet oxygen quantum yields determined by oxygen consumption. J. Photochem. Photobiol. A Chem. 2019;378:131–135. doi: 10.1016/j.jphotochem.2019.04.029. [DOI] [Google Scholar]
- 54.Barsukova M., Goncharova T., Samsonenko D., Dybtsev D., Potapov A. Synthesis, Crystal Structure, and Luminescent Properties of New Zinc(II) and Cadmium(II) Metal-Organic Frameworks Based on Flexible Bis(imidazol-1-yl)alkane Ligands. Crystals. 2016;6:132. doi: 10.3390/cryst6100132. [DOI] [Google Scholar]
- 55.Elsayed S., Jean-Claude B.J., Butler I.S., Mostafa S.I. Synthesis, structural characterization and anticancer activity of some new complexes of 6-amino-4-hydroxy-2-thiopyrimidine. J. Mol. Struct. 2012;1028:208–214. doi: 10.1016/j.molstruc.2012.05.073. [DOI] [Google Scholar]
- 56.García-Pardo J., Novio F., Nador F., Cavaliere I., Suárez-García S., Lope-Piedrafita S., Candiota A.P., Romero-Gimenez J., Rodríguez-Galván B., Bové J., et al. Bioinspired Theranostic Coordination Polymer Nanoparticles for Intranasal Dopamine Replacement in Parkinson’s Disease. ACS Nano. 2021;15:8592–8609. doi: 10.1021/acsnano.1c00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Solórzano R., Tort O., García-Pardo J., Escribà T., Lorenzo J., Arnedo M., Ruiz-Molina D., Alibés R., Busqué F., Novio F. Versatile iron-catechol-based nanoscale coordination polymers with antiretroviral ligand functionalization and their use as efficient carriers in HIV/AIDS therapy. Biomater. Sci. 2018;7:178–186. doi: 10.1039/C8BM01221K. [DOI] [PubMed] [Google Scholar]
- 58.Aryal S., Hu C.-M.J., Zhang L. Polymer—Cisplatin Conjugate Nanoparticles for Acid-Responsive Drug Delivery. ACS Nano. 2009;4:251–258. doi: 10.1021/nn9014032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manzanares D., Ceña V. Endocytosis: The Nanoparticle and Submicron Nanocompounds Gateway into the Cell. Pharmaceutics. 2020;12:371. doi: 10.3390/pharmaceutics12040371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Z., Zhang Y., Zhu D., Li S., Yu X., Zhao Y., Ouyang X., Xie Z., Li L. Transporting carriers for intracellular targeting delivery via non-endocytic uptake pathways. Drug Deliv. 2017;24:45–55. doi: 10.1080/10717544.2017.1391889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vichai V., Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006;1:1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- 62.Yu G., Zhang M., Saha M.L., Mao Z., Chen J., Yao Y., Zhou Z., Liu Y., Gao C., Huang F., et al. Antitumor Activity of a Unique Polymer That Incorporates a Fluorescent Self-Assembled Metallacycle. J. Am. Chem. Soc. 2017;139:15940–15949. doi: 10.1021/jacs.7b09224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peterson J.C., Arrieta E., Ruggeri M., Silgado J.D., Mintz K.J., Weisson E.H., Leblanc R.M., Kochevar I., Manns F., Parel J.-M. Detection of singlet oxygen luminescence for experimental corneal rose bengal photodynamic antimicrobial therapy. Biomed. Opt. Express. 2020;12:272–287. doi: 10.1364/BOE.405601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available on the request from the corresponding author.