Abstract
In animal cells, capacitative calcium entry (CCE) mechanisms become activated specifically in response to depletion of calcium ions (Ca2+) from secretory organelles. CCE serves to replenish those organelles and to enhance signaling pathways that respond to elevated free Ca2+ concentrations in the cytoplasm. The mechanism of CCE regulation is not understood because few of its essential components have been identified. We show here for the first time that the budding yeast Saccharomyces cerevisiae employs a CCE-like mechanism to refill Ca2+ stores within the secretory pathway. Mutants lacking Pmr1p, a conserved Ca2+ pump in the secretory pathway, exhibit higher rates of Ca2+ influx relative to wild-type cells due to the stimulation of a high-affinity Ca2+ uptake system. Stimulation of this Ca2+ uptake system was blocked in pmr1 mutants by expression of mammalian SERCA pumps. The high-affinity Ca2+ uptake system was also stimulated in wild-type cells overexpressing vacuolar Ca2+ transporters that competed with Pmr1p for substrate. A screen for yeast mutants specifically defective in the high-affinity Ca2+ uptake system revealed two genes, CCH1 and MID1, previously implicated in Ca2+ influx in response to mating pheromones. Cch1p and Mid1p were localized to the plasma membrane, coimmunoprecipitated from solubilized membranes, and shown to function together within a single pathway that ensures that adequate levels of Ca2+ are supplied to Pmr1p to sustain secretion and growth. Expression of Cch1p and Mid1p was not affected in pmr1 mutants. The evidence supports the hypothesis that yeast maintains a homeostatic mechanism related to CCE in mammalian cells. The homology between Cch1p and the catalytic subunit of voltage-gated Ca2+ channels raises the possibility that in some circumstances CCE in animal cells may involve homologs of Cch1p and a conserved regulatory mechanism.
The secretory compartments of eukaryotic cells require high concentrations of calcium ions (Ca2+) for the activities of numerous enzymes that catalyze the folding, modification, processing, and trafficking of secretory proteins. Typically, Ca2+ is pumped from the cytosol directly into the endoplasmic reticulum and related secretory compartments by the ATP-dependent SERCA-type Ca2+ pumps. Depending on the inherent leakiness of each compartment to Ca2+, inhibitors of SERCA pumps can lead to depletion of Ca2+ in the secretory pathway and a variety of secretory defects. Most cells express Ca2+ release channels in the endoplasmic reticulum that can be activated by second messengers during responses to extracellular stimuli. Rapid Ca2+ release lowers Ca2+ in the endoplasmic reticulum and elevates free Ca2+ concentrations in the cytosol ([Ca2+]c), which then can activate various signaling transduction pathways. Because Ca2+ pumps in the plasma membrane (PMCAs) compete with SERCA pumps for substrates, [Ca2+]c can return to basal levels prior to refilling of secretory compartments. Thus, in the absence of Ca2+ influx into the cell, repetitive or continuous activation of Ca2+ release channels will lead to only transient elevation of [Ca2+]c and sustained depletion of the secretory Ca2+ reservoir. To offset the detrimental effects of Ca2+ efflux, most cell types employ a regulatory mechanism known as capacitative calcium entry (CCE) which stimulates Ca2+ influx specifically in response to depletion of Ca2+ from the endoplasmic reticulum (45, 46). Thus, CCE increases the magnitude and duration of calcium signals and also helps replenish the secretory pathway when signaling ceases. Despite the apparent ubiquity and importance of the CCE mechanism, the molecular mechanisms by which secretory organelles communicate with plasma membrane Ca2+ channels remains controversial because the critical lumenal, cytoplasmic, and membrane factors have not yet been firmly established in any cell type (see Discussion). Consequently, we sought to develop the budding yeast Saccharomyces cerevisiae as a model system for studies of CCE.
Like animal cells, yeast cells employ a compartmentalized secretory system containing numerous homologs of Ca2+-dependent enzymes, including a furin-like protease (Kex2p) in the trans-Golgi network (16), mannosidase I (Mns1p) (27), calnexin (Cne1p), UDP-glucose:glucosyltransferase (Kre5p), protein disulfide isomerases (Pdi1p and Eug1p), and BiP (Kar2p) in the endoplasmic reticulum (12, 14, 34, 41, 48, 56, 57). Yeast lacks a true homolog of SERCA-type Ca2+ pumps but does express a related secretory pathway Ca2+ pump termed Pmr1p, localized predominantly to the Golgi complex (2, 49). Mutants lacking Pmr1p are viable in standard growth media but concentrate only half as much calcium in the endoplasmic reticulum (55) and exhibit a number of phenotypes attributed to secretory Ca2+ depletion, such as decreased retention of foreign proteins in the endoplasmic reticulum by the quality control machinery (49) and defects in pro-alpha-factor processing by Kex2p in the Golgi complex (2). All of these defects can be reversed by elevating Ca2+ in the culture medium and in some cases by expression of animal SERCA pumps or by overexpression of Pmc1p (13), the yeast homolog of PMCA, which is localized to the vacuole (7) but may function to some degree in pmr1 mutants while transiting through earlier secretory compartments (32, 38). In spite of some differences in the types and localization of Ca2+ pumps, yeast and mammalian cells maintain a similar need and means for concentrating Ca2+ in secretory compartments.
Although yeast lacks any proteins related to the inositol triphosphate receptor or the ryanodine receptor, yeast does retain a full repertoire of factors involved in sensing and transducing calcium signals. Yeast homologs of calmodulin, calmodulin-dependent protein kinases, and calmodulin-dependent protein phosphatases (also called PP2B or calcineurin) have been characterized previously (8–10, 26, 29, 37, 43, 62). Growth in very high Ca2+ conditions elevates [Ca2+]c and stimulates expression of Pmr1p and Pmc1p through a mechanism requiring Tcn1p/Crz1p, a calcineurin-dependent transcription factor (33, 54). This transcription-dependent response serves to eliminate excess Ca2+ from the cytoplasm, permitting growth in high-Ca2+ environments. Calcineurin appears to posttranslationally inhibit the function of Vcx1p/Hum1p, an H+/Ca2+ exchanger in the vacuole that also promotes growth in high-Ca2+ conditions (6, 44). Together, Pmr1p, Pmc1p, and Vcx1p control [Ca2+]c and very likely serve to dissipate calcium signals generated in response to external stimuli and conditions.
Sequencing of the yeast genome revealed a gene termed CCH1 that encodes a homolog of voltage-gated Ca2+ channels (VGCCs) found in the plasma membrane of electrically excitable animal cells. Recently, cch1 mutants and mid1 mutants were recovered in two separate genetic screens and shown to be partially deficient in Ca2+ influx that is stimulated by either pheromone treatment or stress associated with cdc1 mutants (15, 20, 39). MID1 encodes a plasma membrane glycoprotein with no significant similarity to any animal proteins in current databases. How these stimuli evoke Ca2+ influx by Cch1p and Mid1p and how the resulting calcium signals are utilized by responding cells remain unanswered.
Here we show that Cch1p and Mid1p are both required for a high-affinity Ca2+ influx system that can be stimulated up to 25-fold in situations causing depletion of secretory Ca2+ pools. We detected high levels of Cch1p- and Mid1p-dependent Ca2+ uptake in pmr1 mutants and in wild-type strains overexpressing either Pmc1p or Vcx1p. Cch1p and Mid1p were also required for growth in low-calcium environments. Therefore, yeast may employ a regulatory mechanism related to CCE in animal cells in order to ensure Ca2+ homeostasis over a wide range of environmental conditions.
MATERIALS AND METHODS
Genetic methods.
All yeast strains used in this study were derived from strain W303-1A (59) by standard molecular and genetic methods (51). The pmc1::TRP1, pmr1::HIS3, pmr1::LEU2, tcn1::G418r, vcx1Δ, and VCX1-D1 mutations have been described elsewhere (6, 33). The mid1::LEU2 null mutation was introduced using plasmid pFB457 (39). The cch1::TRP1 null mutation, which deletes 95% of the CCH1/YGR217w gene, was introduced by transformation of yeast with pKC289 after linearization by EcoRI digestion. pKC289 was constructed using standard procedures (50) by ligating into the XhoI and BamHI sites of pRS304 (52) two segments of DNA flanking CCH1 (from nucleotides −212 to +300 and +6120 to +6900 relative to the predicted start codon) which had been amplified by PCR using primers specific to yeast genomic DNA. DNA encoding 13 repeats of the Myc epitope were inserted into the genome just before the stop codon of CCH1 by homologous recombination of PCR-generated sequences (31). A centromere-based plasmid expressing a hemagglutinin (HA) epitope-tagged Mid1p protein (YCplacMID1-23CA5x2) was a gift from H. Iida (20). Overexpression of PMR1, PMC1, or rabbit SERCA1a was achieved in various strains by transformation with plasmid pKC152 (pRS316-Gal containing a cDNA from PMR1 [28]), pKC302 (a 2μm LEU2 derivative of pKC47 [7]), or pRS316PSA (13), respectively, using empty vectors as controls.
To identify mutants specifically deficient in CCE, strains K837 (MATa pmr1::HIS3 PMC1-lacZ::URA3) and K842 (MATα pmr1::LEU2 PMC1-lacZ::URA3) were mutagenized to ∼3% viability by 1 h of treatment with 3.3% methanesulfonic acid ethyl ester and then plated for single colonies on SC-uracil agar medium supplemented with 10 mM MgCl2. After growth overnight at 30°C on the same medium, a total of 33,000 mutagenized colonies were screened for PMC1-lacZ expression by permeabilizing and staining the cells with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) as described previously (33). Colonies exhibiting lower than normal expression of PMC1-lacZ were selected, retested, and screened again on medium containing 150 mM CaCl2 to identify mutants deficient in calmodulin, calcineurin, Tcn1p, PMC1-lacZ, or other factors. The remaining 267 mutants were analyzed for complementation after pairwise mating among each other. Two recessive complementation groups were identified by this approach. The larger group (30 mutants) failed to complement cch1 pmr1 null mutants, and the smaller group (6 mutants) failed to complement mid1 pmr1 null mutants. The ungrouped mutants may define other components of the yeast CCE-like mechanism.
Other methods.
All assays were performed on log-phase yeast cultures grown in standard synthetic (SC or SCGal) or rich (YPD or YPGal) culture medium containing 2% glucose or galactose (51). For 45Ca2+ accumulation assays, log-phase yeast cells were collected by centrifugation, washed, resuspended in fresh medium containing tracer amounts of 45CaCl2 (Amersham), incubated at 30°C for 15 s to 4 h with intermittent mixing, then harvested onto Whatman GFF filters, washed, and processed for liquid scintillation counting (6). Total cell-associated Ca2+ was determined from specific activities of the media: standard SC and YPD media contained 0.7 and 0.14 mM total Ca2+, respectively, as determined by atomic absorption spectroscopy. Extracellular Ca2+ concentrations were varied in some experiments by first treating 2×-concentrated YPD medium adjusted to pH 10 using NaOH with 4% (vol/vol) Chelex-100 resin (Bio-Rad) for 1 h at 20°C, removing the resin by sterile filtration, and then adjusting the volume to 1×, the pH to 6.5 with HCl, and MgCl2 to 1 or 10 mM. This procedure depleted Ca2+ to less than 0.5 μM as determined by atomic absorption spectroscopy, allowing accurate determination of 45Ca2+ uptake over the range from 15 μM to 8.0 mM (Fig. 1). For 45Ca2+ release assays, log-phase yeast cells were grown for 6 to 9 h in YPD medium supplemented with tracer 45CaCl2, harvested by centrifugation at 4°C, washed four times in ice-cold YPD medium, and then diluted 11-fold into prewarmed YPD medium at 30°C. The cell-free supernatant was collected at intervals by rapid filtration through type HA 0.45-μm-pore-size filters (Millipore), and aliquots were removed for measurement of radioactivity by liquid scintillation counting.
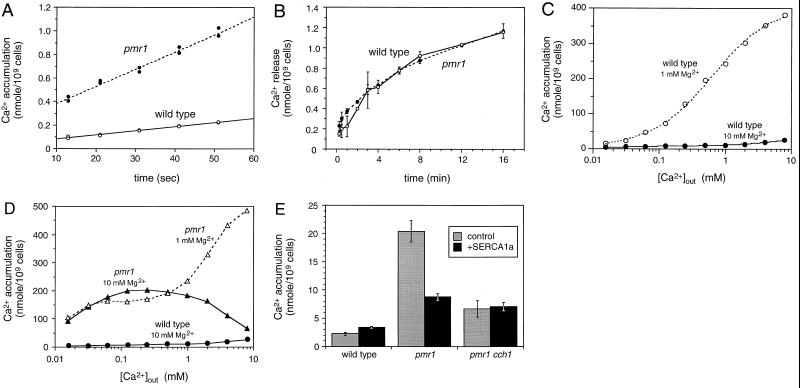
FIG. 1.
Stimulation of a high-affinity Ca2+ influx system in pmr1 mutants. Initial rates of 45Ca2+ influx (A) and efflux (B) were determined for wild-type and pmr1 mutant cells growing in YPD medium supplemented with 0.16 and 0.016 μM 45Ca2+ as described in Materials and Methods. (C and D) Accumulation of 45Ca2+ in wild-type and pmr1 mutant cells was determined after 2.5 h of growth in YPD culture medium treated with Chelex-100 resin to remove divalent cations and supplemented with either 1 or 10 mM MgCl2 and the indicated concentrations of 45CaCl2. For the wild type, the data were fit to standard Michaelis-Menten equations for two enzymes by nonlinear regression. The low-affinity Ca2+ uptake systems predominated in 1 mM Mg2+ (Km ∼ 500 μM, Vmax ∼ 390 nmol/109 cells) but largely disappeared in 10 mM Mg2+, exposing a second high-affinity Ca2+ uptake system (apparent Km ∼ 10 μM, Vmax ∼ 9 nmol/109 cells). The high-affinity Mg2+-resistant uptake system was enhanced up to 25-fold in pmr1 mutants relative to wild type. Some data from panel C are repeated in panel D for comparison. (E) SERCA1a functionally replaces Pmr1p. Expression of rabbit SERCA1a from plasmid pRS316PSA (13) suppressed Ca2+ uptake in pmr1 mutants but not pmr1 cch1 double mutants or the wild type. Uptake of 45Ca2+ was monitored as described for panel A after 4 h of growth of log-phase cells in YPD medium (0.14 mM Ca2+) supplemented with 10 mM MgCl2.
Determination of [Ca2+]c using the fluorescent Ca2+ indicator Indo-1 (Molecular Probes) was performed in triplicate as described previously (18). Quantitative β-galactosidase assays were performed on permeabilized cells as described previously (33). Growth assays were performed in 96-well culture dishes as described previously (6), using YPD medium supplemented with 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid, tetrapotassium salt (BAPTA). Total cell lysates were prepared after glass bead lysis in 10% trichloroacetic acid followed by solubilization in sample buffer containing 8 M urea and 15% sodium dodecyl sulfate (SDS) (22). SDS-polyacrylamide gel electrophoresis (PAGE) and Western blot analysis were performed as described previously (6), using monoclonal antibodies 12CA5 (Boehringer Mannheim) for the HA epitope, 9E10 (Santa Cruz Biotechnology) for the Myc epitope, and 16G9-E6 for mitochondrial porin (Molecular Probes).
For immunofluorescence microscopy, cells were grown to mid-log phase in YPD medium, fixed at room temperature in 4% formaldehyde (2 h in 1 M potassium phosphate [pH 6.5] and then overnight in 0.1 M potassium phosphate [pH 6.5]), converted to spheroplasts with 45 μg of Zymolyase 100T per ml (30 min, 30°C) in 1 ml of SHA buffer (1 M sorbitol, 0.1 M Na-HEPES [pH 7.5], 5 mM NaN3, 0.2% β-mercaptoethanol, protease inhibitor cocktail), permeabilized with 1% SDS (10 min, room temperature in SHA buffer), washed twice in SHA buffer, and then placed on coverslips coated with poly-d-lysine. Coverslips with cells were treated with WT buffer (1% nonfat dry milk, 0.5 mg of bovine serum albumin/ml, 150 mM NaCl, 50 mM HEPES [pH 7.5], 0.1% Tween 20, 1 mM NaN3) for 15 min, then incubated at 4°C overnight in WT buffer plus monoclonal antibody 9E10 (Santa Cruz Biotechnology), washed five times with WT buffer, and incubated at room temperature for 45 min with donkey anti-mouse F(ab′)2 fragment conjugated to R-phycoerythrin (Jackson Immunoresearch). After five washes with WT buffer, coverslips were mounted on glass slides with 15 μl of mounting medium (0.1% DABCO in 90% glycerol) and sealed with fingernail polish. Fluorescence images were taken at 580 nm on a Zeiss Axiovert microscope using a 100× objective after excitation at 488 nm.
For immunoprecipitation experiments, log-phase yeast cultures were harvested, washed, and resuspended in BB buffer (0.3 M sorbitol, 0.1 M NaCl, 5 mM MgCl2, 10 mM Tris-HCl [pH 7.5], protease inhibitors), broken by vortexing with acid-washed glass beads (425- to 600-μm diameter, 2 min, 4°C), collected, and then centrifuged at 135,000 × g for 30 min at 4°C. The crude membrane pellet was resuspended in 1.0 ml of immunoprecipitation buffer (50 mM Tris-HCl [pH 8.0], 1.0% Triton X-100, 150 mM NaCl, 2 mM EDTA, protease inhibitors) and recentrifuged. The clear supernatant containing essentially all cellular Pma1p, Cch1p, and Mid1p was then incubated with 5 μg of monoclonal antibody 12CA5 for 2 h at 4°C and rocked with 100 μl of Sepharose CL-4B beads conjugated with protein A (100 mg/ml; Sigma) for 2 h at 4°C. Beads were collected by brief centrifugation, washed three times with 1 ml of radioimmunoprecipitation assay buffer (50 mM Tris-HCl [pH 7.5], 200 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS), and then heated in urea sample buffer prior to SDS-PAGE and immunoblotting.
RESULTS
Stimulation of high-affinity Ca2+ influx system in pmr1 mutants.
If a CCE-like mechanism operates in yeast, we reasoned that mutants lacking one or more of the intracellular Ca2+ transporters would accumulate higher levels of 45Ca2+ from the growth medium. In standard growth media, mutants lacking either Pmc1p, Vcx1p, or both vacuolar transporters accumulated less 45Ca2+ in the vacuole (6, 7) but exhibited initial rates of 45Ca2+ influx and [Ca2+]c levels that were indistinguishable from those of the wild-type control strain (35, 36). Depletion of Ca2+ from the vacuole, therefore, did not stimulate a Ca2+ influx mechanism to a significant degree.
In contrast, pmr1 mutants lacking the Golgi-localized Ca2+ pump accumulated extracellular 45Ca2+ at an initial rate that was approximately 4.1-fold greater than the wild-type rate (Fig. 1A), 14.7 pmol/s/109 cells for pmr1 mutants and 3.56 pmol/s/109 cells for the wild type. The rates of Ca2+ efflux from the wild type and pmr1 mutants were identical (Fig. 1B), ∼2.1 pmol/s/109 cells averaged over the first 3 min of incubation. Halachmi and Eilam reported contradictory findings (19); however, these authors measured 45Ca2+ influx and efflux using nongrowing cells suspended in buffer lacking inorganic ions rather than cells growing in YPD culture medium as performed here. Therefore, our data suggest that one or more Ca2+ influx pathways may be stimulated in growing pmr1 mutant cells.
Similar differences were observed in long-term 45Ca2+ accumulation experiments. In one such experiment, we monitored 45Ca2+ accumulation after 2.5 h of incubation in the presence of various concentrations of extracellular Ca2+. For wild-type cells, the data were fitted to standard Michaelis-Menten equations using nonlinear regression (Fig. 1C). The analysis showed that wild-type yeast cells accumulated 45Ca2+ from the growth medium using primarily a low-affinity Ca2+ uptake system (apparent Km ∼ 500 μM, Vmax ∼ 390 nmol/109 cells). Raising extracellular Mg2+ from 1 to 10 mM strongly inhibited the low-affinity Ca2+ uptake system (Fig. 1C) and exposed a second high-affinity Mg2+-resistant Ca2+ uptake system (apparent Km ∼ 10 μM) operating at a much lower level (Vmax ∼ 9 nmol/109 cells). In an experiment parallel to that of Fig. 1A, we found that addition of 10 mM MgCl2 diminished the initial rate of 45Ca2+ influx into wild-type cells 2.6-fold (to 1.28 pmol/s/109 cells [data not shown]). These and other results (3, 19) suggest that Mg2+ competitively inhibits the low-affinity Ca2+ uptake system.
When analyzed in identical conditions, the isogenic pmr1 mutant exhibited up to 25-times-higher activity of the high-affinity Mg2+-resistant Ca2+ uptake system, whereas the low-affinity Mg2+-sensitive Ca2+ uptake activity was not significantly changed (Fig. 1D). The high-affinity Ca2+ uptake in pmr1 mutants appeared to saturate around 250 μM extracellular Ca2+ and then declined to near wild-type levels as extracellular Ca2+ was increased toward the 10 mM range. This decline in pmr1 mutants correlates with replenishment of Ca2+ into secretory organelles (2, 13). Taken together, these data suggest that a high-affinity Mg2+-resistant Ca2+ uptake system is specifically stimulated in pmr1 mutants when secretory compartments are depleted of Ca2+.
Expression of a mammalian SERCA Ca2+ pump in the endoplasmic reticulum of pmr1 mutants replenished lumenal Ca2+ and reversed many of the secretory defects attributed to Ca2+ depletion (11). We found that expression of rabbit SERCA1a also abolished the high-affinity Ca2+ uptake system in pmr1 mutants but had no detectable effect in wild-type cells (Fig. 1E). Reversal of the pmr1 phenotypes by either expression of a SERCA pump or supplementing Ca2+ to ≥10 mM indicates that the high-affinity Ca2+ uptake activity responds to depletion of Ca2+ from a secretory compartment. This evidence supports the notion that Ca2+ influx in yeast can be coupled to Ca2+ deficiency in secretory compartments, much like the process of CCE in mammalian cells.
Elevation of [Ca2+]c in pmr1 mutants.
CCE in mammalian cells promotes sustained elevation of [Ca2+]c. To test whether [Ca2+]c becomes elevated in pmr1 mutant cells, the fluorescent Ca2+ indicator Indo-1 was loaded into the cytoplasm as described previously (18), and the loaded cells were resuspended in synthetic growth medium (∼0.7 mM Ca2+) for fluorescence measurements. Under these conditions, pmr1 mutants maintained [Ca2+]c at 1.6 ± 0.3 μM, whereas the isogenic wild-type strain maintained [Ca2+]c at 0.1 ± 0.05 μM (n = 3). Similar differences between wild-type and pmr1 mutants were noted previously for nongrowing cells suspended in a glucose buffer (19).
Elevated [Ca2+]c can activate the calcineurin-dependent transcription factor Tcn1p/Crz1p (33, 54). To test whether this response pathway was stimulated in pmr1 mutants, a PMC1-lacZ reporter gene was transformed into wild-type and pmr1 mutant strains and examined for calcineurin-dependent expression using FK506, a specific inhibitor of calcineurin. Calcineurin-dependent expression of PMC1-lacZ was approximately five times greater in pmr1 mutants than in wild-type cells (Fig. 2A). Calcineurin-dependent expression of PMC1-lacZ was completely blocked by addition of BAPTA to chelate extracellular Ca2+ (not shown). Thus, two independent methods show that [Ca2+]c becomes elevated in pmr1 mutants as a consequence of Ca2+ influx.
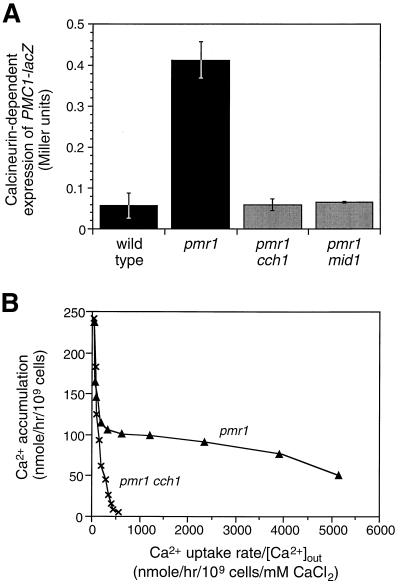
FIG. 2.
Involvement of Cch1p and Mid1p function in the pmr1-stimulated high-affinity Ca2+ uptake and signaling system. (A) Expression of the calcineurin-dependent PMC1-lacZ reporter gene in wild-type cells and pmr1 mutants carrying cch1 or mid1 null mutations was monitored after 4 h of growth in YPD medium. Mean ± standard deviation is shown for three replicates. (B) High-affinity Ca2+ uptake in pmr1 mutants requires Cch1p function. 45Ca2+ uptake was monitored after 1 h of growth in Chelex-100-treated YPD medium containing 1 mM MgCl2 as in Fig. 1 except that data were plotted in Eadie-Hofstee format (y intercept = Vmax, slope = −Km).
Identification of Cch1p and Mid1p as essential components of the high-affinity Ca2+ uptake system stimulated in pmr1 mutants.
To identify factors specifically required for high-affinity Ca2+ uptake in yeast, pmr1 null mutants were mutagenized and screened for variants that fail to express PMC1-lacZ (see Materials and Methods). Mutants deficient in calmodulin, calcineurin, or Tcn1p/Crz1p function were discarded, and the remaining ∼250 variants were subjected to complementation tests. This method revealed 30 and 6 independent mutations in two genes, CCH1 and MID1, which had been reported previously to be important for pheromone-stimulated Ca2+ influx (15, 20, 39). MID1 encodes a plasma membrane glycoprotein (20) related to uncharacterized proteins expressed in other fungi. CCH1 encodes a 2,039-amino-acid protein containing four repeated membrane domains, each showing strong similarity to the α1 pore-forming subunit of VGCCs (E value = 10−54 by BLAST2.0 [1]). Deletion of either CCH1 or MID1 in pmr1 mutants specifically abolished the high-affinity Ca2+ uptake system (Fig. 2B and 3) and decreased calcineurin-dependent PMC1-lacZ expression to wild-type levels (Fig. 2A). Simultaneous disruption of both CCH1 and MID1 in pmr1 mutants diminished 45Ca2+ uptake to the same extent as the individual disruptions (Fig. 3C), demonstrating that both Cch1p and Mid1p were required for the stimulated Ca2+ uptake. Thus, Cch1p and Mid1p are necessary components of a high-affinity Ca2+ uptake system that can be stimulated in pmr1 mutants grown in moderate- to low-calcium environments. Additional results (see below) suggest that these proteins serve as catalytic and regulatory subunits of a heteromeric Ca2+ influx channel.
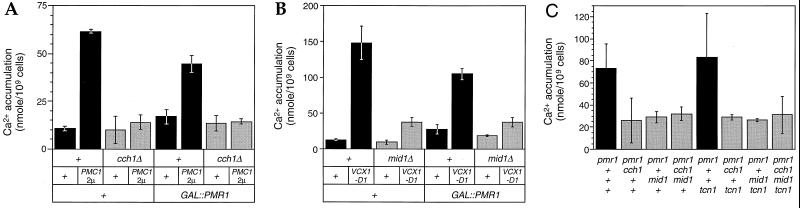
FIG. 3.
Stimulation of the Cch1p- and Mid1p-dependent Ca2+ uptake system by increasing the activity of vacuolar Ca2+ transporters. (A) Accumulation of 45Ca2+ in wild-type and cch1 mutant strains bearing plasmids that overexpress either PMC1, PMR1, or both transporter genes as indicated was determined after 4 h of growth in SCGal-uracil medium (0.7 mM Ca2+). Mean of three replicates (± standard deviation) is shown. Cch1p-dependent Ca2+ uptake was stimulated by overexpression of Pmc1p and partially reversed by simultaneous overexpression of Pmr1p (P < 0.02). (B) Accumulation of 45Ca2+ into wild-type, VCX1-D1 (hyperactive), mid1, and mid1 VCX1-D1 strains (all pmc1 null mutants [6]) bearing plasmids that overexpress PMR1 was determined as for panel A. Overexpression of Pmr1p partially reversed Mid1p-dependent uptake stimulated by overexpression of Vcx1p (P < 0.04). (C) Accumulation of 45Ca2+ into pmr1 and pmr1 tcn1 strains with or without cch1 and mid1 mutations was determined as for Fig. 1B. Mean of three replicates (± standard deviation) is shown.
Excessive Ca2+ sequestration into the vacuole stimulates Ca2+ accumulation through a Cch1p- and Mid1-dependent process.
Depletion of Ca2+ from secretory organelles might occur as a consequence of excessive Ca2+ sequestration into the vacuole. To test this possibility, 45Ca2+ accumulation was quantitated in strains either expressing a hyperactive Vcx1p variant or overexpressing Pmc1p. Overexpression of Pmc1p from a high-dosage plasmid greatly stimulated 45Ca2+ accumulation in wild-type cells but had no significant effect on 45Ca2+ accumulation in cch1 null mutants (Fig. 3A) or mid1 mutants (not shown). Similarly, expression of a hyperactive Vcx1p mutant termed Vcx1p-D1 (6) strongly stimulated 45Ca2+ uptake in wild-type strains but not in mid1 mutants (Fig. 3B). In both cases, stimulation of the Ca2+ uptake system was partially reversed by simultaneous overexpression of Pmr1p (P < 0.04), suggesting that the vacuolar Ca2+ transporters competed with Pmr1p in these conditions. Thus, the Cch1p- and Mid1p-dependent Ca2+ uptake system could be stimulated either by Pmr1p inactivation or by Pmr1p insufficiency due to competition with vacuolar Ca2+ transporters.
The possibility that increased 45Ca2+ accumulation in pmr1 mutants was due to increased Ca2+ sequestration by Pmc1p or Vcx1p was not supported by the results of several experiments. First, 45Ca2+ accumulation and PMC1-lacZ expression were the same in pmr1 vcx1 double mutants as in pmr1 single mutants (data not shown), showing that Vcx1p was not required for the effect. Second, pmr1 tcn1 double mutants which lack the transcription factor required for Pmc1p induction showed levels of 45Ca2+ accumulation that were similar to or even greater than those for pmr1 mutants and still depended on Cch1p and Mid1p (Fig. 3C). Finally, pmr1 pmc1 double mutants grown in the presence of FK506 to maintain viability (4) exhibited higher levels of 45Ca2+ accumulation than similarly grown pmr1 mutants (data not shown), consistent with previous inferences that Pmc1p may partially refill secretory compartments during its trafficking to the vacuole in pmr1 mutants (13, 32). Thus, the stimulation of the Cch1p- and Mid1p-dependent Ca2+ uptake system did not correlate with the abundance of any particular Ca2+ transporter or the level of [Ca2+]c but instead correlated inversely with Ca2+ transport into secretory compartments.
Expression and localization of Cch1p in pmr1 mutants.
A possible mechanism for the observed increase in Ca2+ influx and accumulation upon depletion of Ca2+ stores is increased expression of either Cch1p or Mid1p or both proteins. To test this possibility, quantitative Western blot analyses were performed using epitope-tagged variants of Cch1p and Mid1p. Thirteen repeats of the Myc epitope were inserted at the extreme C terminus of Cch1p by homologous recombination into the chromosomal CCH1 gene (see Materials and Methods). The resulting Cch1p-Myc fusion protein was fully functional in pmr1 mutants (data not shown) but was expressed at identical levels in wild-type and pmr1 mutant strains grown in standard medium (Fig. 4A, top panel). In this experiment, mitochondrial porin was used as a loading control (bottom panel). Furthermore, a functional Mid1p-HA fusion protein expressed from a low-dosage plasmid from its own promoter (20) also accumulated at similar levels in wild-type and pmr1 mutant strains (Fig. 4B). In this case, nonspecific cross-reacting bands served as internal loading controls. These results rule out the possibility that depletion-stimulated Ca2+ uptake system involves significant up-regulation of Cch1p or Mid1p.
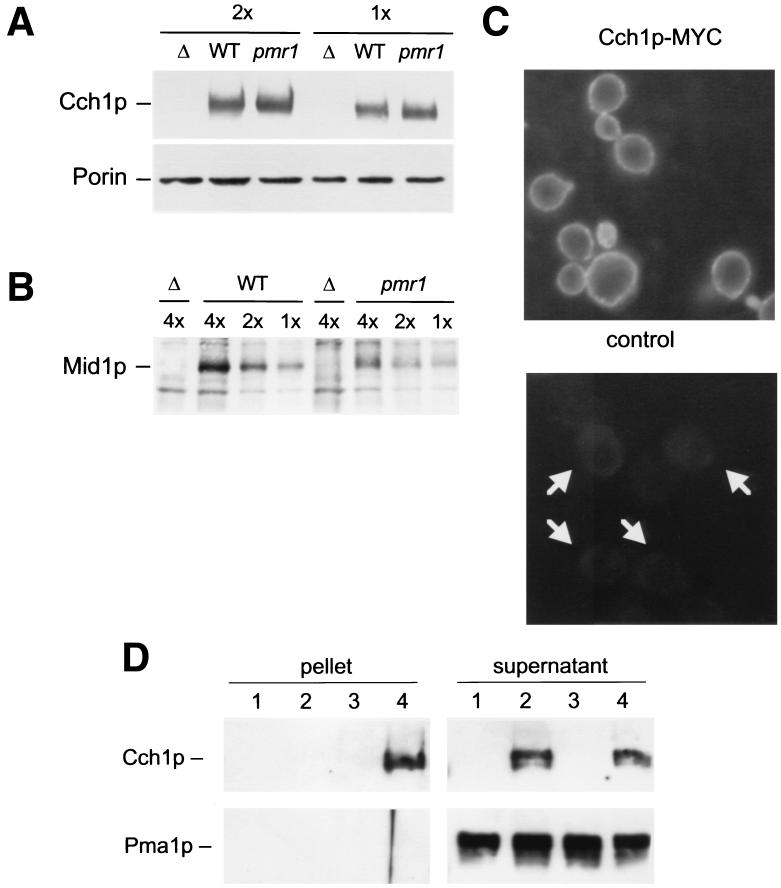
FIG. 4.
Expression, localization, and interaction between Cch1p and Mid1p are unaffected in pmr1 mutants. (A and B) Western blot analysis of epitope-tagged Cch1p and Mid1p variants was performed on total cell protein extracted from wild-type (WT), pmr1 mutant (pmr1), or untagged control (Δ) strains after growth in YPD medium to mid-log phase. Various amounts of each sample (either 1, 2, or 4 cell equivalents) were loaded and compared to endogenous cross-reacting proteins or mitochondrial porin as standards to control for slight variations in sample preparation or loading. (C) Immunofluorescence microscopy was performed on wild-type strains containing (top) or lacking (bottom) the epitope-tagged Cch1p-Myc variant. (D) Coimmunoprecipitation of Mid1p and Cch1p. Crude membranes were isolated from wild-type cells carrying Mid1p-HA (lanes 3 and 4) or Cch1p-Myc (lanes 2 and 4), solubilized in buffer containing Triton X-100, and immunoprecipitated using monoclonal antibodies to the HA epitope. Equal portions of the immunoprecipitated pellet and supernatant were then analyzed by Western blotting using either monoclonal antibodies to the Myc epitope (top) or polyclonal antibodies to the Pma1p protein (bottom). Cch1p but not Pma1p coprecipitated with Mid1p-HA (lane 4).
It is possible that depletion of Ca2+ from secretory organelles promotes the relocalization of Cch1p and/or Mid1p from internal compartments to the cell surface or their interaction in the plasma membrane, thereby allowing their function as a high-affinity Ca2+ influx channel. Such a mechanism has been proposed recently for vertebrate cells (61). A functional epitope-tagged variant of Mid1p was localized to the plasma membrane of wild-type yeast cells in nonsignaling conditions (20). To determine whether Cch1p might undergo regulated trafficking, the epitope-tagged Cch1p-Myc variant was localized by immunofluorescence microscopy. As observed previously for Mid1p, Cch1p was detectable only at the surface rim in wild-type cells (Fig. 4C). No significant staining was evident in intracellular structures aside from a faint cytoplasmic staining that was also evident in the nontagged control strain. A similar staining pattern was observed in pmr1 mutants (data not shown). To determine whether Cch1p physically interacts with Mid1p in the plasma membrane, wild-type cells expressing Cch1p-Myc and/or Mid1p-HA were lysed in the presence of nondenaturing detergent and subjected to immunoprecipitation with anti-HA antibodies followed by Western blotting with anti-Myc antibodies. Cch1p was efficiently coprecipitated with Mid1p in the tagged strain (Fig. 4D, lane 4) but not in control strains lacking the tag (lane 2). The plasma membrane marker protein Pma1p was not precipitated in any conditions (bottom panels). Thus, Cch1p and Mid1p both localize to the plasma membrane of unstimulated cells, where they physically interact. Stimulation of Cch1p and Mid1p function in pmr1 mutants may therefore involve some other type of regulatory mechanism such as a diffusible messenger (5).
Cch1p and Mid1p supply essential Ca2+ to Pmr1p during ion starvation.
Previous studies showed that wild-type cells cultured in media supplemented with BAPTA, a potent chelator of Ca2+ and other ions, exhibit defects in protein sorting and secretion similar to those observed in pmr1 mutants in standard medium (13). Very high levels of BAPTA even inhibited the growth of wild-type cells, whereas much lower chelator concentrations inhibited the growth of pmr1 mutants (11, 13). It can be inferred from these and other experiments that an essential role of Pmr1p is to supply secretory organelles with the Ca2+ necessary to sustain secretion and growth in low-calcium environments. The studies presented above predict that Cch1p and Mid1p would also be important for growth in low calcium conditions due to their ability to provide substrate to Pmr1p. This prediction was tested by comparing the growth of various mutants lacking Cch1p, Mid1p, and/or Pmr1p in media supplemented with increasing amounts of BAPTA.
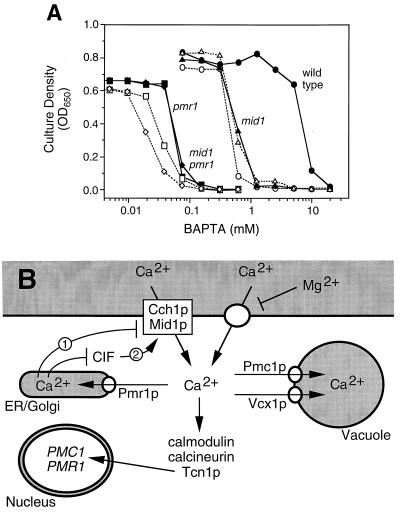
Interestingly, cch1 and mid1 mutants each failed to grow at intermediate concentrations of BAPTA in between the concentrations effective for wild-type and pmr1 mutant strains (Fig. 5A). The cch1 mid1 double mutant was as sensitive to BAPTA as the single mutants, suggesting again that Cch1p and Mid1p function within a single high-affinity Ca2+ uptake pathway. If the major function of these proteins is to provide sufficient Ca2+ to the cytoplasm for concentration by Pmr1p in secretory organelles, Cch1p and Mid1p would be expected to have little or no effect on BAPTA tolerance in pmr1 mutants due to their inability to refill the secretory stores. Indeed, mid1 pmr1 double mutants were just as sensitive to BAPTA as pmr1 single mutants, showing that Mid1p confers BAPTA tolerance only when Pmr1p also functions. Both cch1 pmr1 double mutants and cch1 mid1 pmr1 triple mutants were slightly more sensitive to BAPTA than the respective CCH1 strains, but this effect was relatively small and very likely a consequence of slightly reduced growth rates of all cch1 strains independent of Ca2+ (K. W. Cunningham, unpublished observation). Similar results were obtained when EGTA was used in place of BAPTA and when a fixed concentration of BAPTA (20 mM) was used with various concentrations of added Ca2+ (data not shown), which argues against nonspecific or indirect effects of the chelators. Thus Cch1p, Mid1p, and Pmr1p all appear to function within a single pathway that promotes the acquisition and concentration of essential Ca2+ into secretory organelles. This pathway closely parallels the CCE pathway of animal cells.
FIG. 5.
A physiological role for the CCE-like mechanism in yeast. (A) Cch1p and Mid1p are essential for growth in low-Ca2+ environments. Yeast strains carrying combinations of cch1, mid1, or pmr1 null mutations were grown in YPD culture medium supplemented with the indicated concentrations of sodium BAPTA for either 24 h (all PMR1 strains) or 48 h (all pmr1 mutant strains). Optical density at 650 nm (OD650) was averaged for three independent cultures. Strains containing the cch1 mutation are shown with open symbols and dashed lines. (B) A working model of Ca2+ homeostasis and signaling in yeast. Depletion of secretory Ca2+ stores during growth in low-Ca2+ environments activates the high-affinity Mg2+-resistant Ca2+ channel composed of Cch1p and Mid1p through a CCE mechanism possibly involving CIF (arrow 1 or 2). Cch1p and Mid1p activation provides more substrate to Pmr1p for transport into secretory compartments and elevate [Ca2+]c, which can stimulate expression of Pmr1p and Pmc1p. Refilling of secretory pathway Ca2+ stores by Pmr1p (and possibly Pmc1p in transit to the vacuole) prevents CIF accumulation activation of Cch1p and Mid1p but does not affect a low-affinity Mg2+-sensitive Ca2+ uptake system. Secretory Ca2+ depletion and stimulation of Ca2+ influx also can be achieved genetically by decreasing Pmr1p activity or increasing Pmc1p or Vcx1p activities.
DISCUSSION
The data reported here collectively suggest that depletion of Ca2+ from secretory compartments in yeast cells stimulates the activity of a high-affinity Ca2+ influx channel composed of Cch1p and Mid1p which helps replenish the depleted organelles (Fig. 5B). We report evidence that the initial rate of Ca2+ influx is stimulated in pmr1 mutants and the rate of Ca2+ efflux is unaffected, leading eventually to elevated [Ca2+]c, elevated expression of Pmc1p, and elevated accumulation of Ca2+ in the vacuole. An earlier study concluded that Ca2+ influx was normal in pmr1 mutants but that Ca2+ efflux was drastically inhibited, leading to similar long-term effects (19). In that report, Ca2+ influx was measured using nongrowing cells incubated in a minimal buffer lacking Mg2+, conditions which might have disfavored detection of Cch1p and Mid1p activity. Ca2+ efflux was also measured in nongrowing cells over extremely long periods of time in the absence of extracellular Ca2+ (up to 30 h) although, like us, they found similar rates of Ca2+ efflux in wild-type and pmr1 mutant strains when Ca2+ was added to the buffer (19). The results of our long-term 45Ca2+ accumulation experiments suggested that a novel high-affinity Mg2+-resistant Ca2+ uptake system was specifically stimulated in pmr1 mutants relative to a low-affinity Mg2+-sensitive Ca2+ uptake system present at similar levels in both wild-type and pmr1 mutant cells. Remarkably, this high-affinity system in pmr1 mutants depended on the function of both Cch1p and Mid1p and could be suppressed by overexpression of mammalian SERCA pumps or simply raising extracellular Ca2+ to ≥10 mM, conditions known to replenish Ca2+ in the secretory pathway independent of Pmr1p function (13). Thus, the stimulation of the high-affinity Cch1p- and Mid1p-dependent Ca2+ uptake system appeared to correlate inversely with Ca2+ accumulation in secretory compartments, much like CCE in mammalian cells.
Further support for the functional coupling between Ca2+ stores and Ca2+ influx derives from studies of the vacuolar Ca2+ transporters. Abnormal elevation of either Pmc1p or Vcx1p activity stimulated Ca2+ accumulation in wild-type cells but not in cells lacking Cch1p or Mid1p (Fig. 3), an effect that was significantly reversed by overexpression of Pmr1p. It could be argued that the elevated vacuolar Ca2+ transport decreased Ca2+ efflux, but if this were the case, one would predict greater Ca2+ accumulation independent of Cch1p and Mid1p since these factors contribute very little to the overall Ca2+ influx in wild-type cells. The simplest model consistent with the data is that the vacuolar Ca2+ transporters compete with Pmr1p for substrate and can effectively deplete the secretory pathway of Ca2+ which stimulates Ca2+ influx via Cch1p and Mid1p. This model helps explain why Pmc1p expression and Vcx1p function are so tightly regulated in wild-type cells (6, 33); excessive vacuolar Ca2+ transport may deplete Ca2+ from secretory organelles especially under conditions of low Ca2+ availability from the environment. However, direct measurement of secretory Ca2+ concentrations would be necessary to confirm this hypothesis.
Our data also support the hypothesis that Cch1p and Mid1p function together as catalytic and regulatory/accessory subunits of a single high-affinity Ca2+ influx channel. In BAPTA tolerance experiments, Ca2+ accumulation experiments, and experiments that monitor [Ca2+]c, the cch1 and mid1 single mutants exhibited phenotypes quantitatively similar to those of cch1 mid1 double mutants, suggesting that neither Cch1p nor Mid1p can function without the other. Additionally, mutations in both of these genes were recovered in two genetic screens distinct from ours (20, 39). Cch1p strongly resembles the pore-forming α1 subunit of VGCCs (15, 39) characterized extensively in vertebrate cells. In animals, VGCCs typically comprise several subunits in addition to α1 (4). Mid1p shows no significant homology to any animal proteins in current databases, but surprisingly, expression of Mid1p in CHO cells resulted in the appearance of a nonselective cation channel that responded to membrane stretch (21). Overexpression of Mid1p in either pmr1 mutants (data not shown) or wild-type cells under conditions of membrane stretch (20) had no effect on Ca2+ influx rates, as if another factor was limiting for Mid1p activity in yeast. Finally, we show that Cch1p and Mid1p can physically interact in the plasma membrane of wild-type cells.
How closely does CCE in animals resemble the CCE-like process in yeast? Homologs of Pmr1p, the secretory Ca2+ ATPases, are expressed ubiquitously in mammalian cells but they have not yet been characterized functionally (17). Their involvement in Ca2+ homeostasis and potential for coupling to Ca2+ influx mechanisms therefore remain uninvestigated. On the other hand, SERCA-type Ca2+ pumps are well known to supply Ca2+ to the endoplasmic reticulum and to prevent stimulation of CCE pathway in mammalian cells. Specific inhibitors of SERCA selectively deplete the endoplasmic reticulum of Ca2+ and concomitantly enhance Ca2+ influx through CCE channels in the plasma membrane. Physiological stimulation of CCE in animal cells occurs after release of Ca2+ from the endoplasmic reticulum through the activation of Ca2+ release channels such as the IP3 receptors, RyR receptors, or other unidentified channels. Although the Ca2+ influx channels activated during CCE have not been conclusively identified, recent evidence suggests that certain members of the TRP family of ion channels (TRPCs) respond to depletion of Ca2+ from the endoplasmic reticulum at least when expressed in heterologous systems (46). TRPCs are only distantly related to VGCCs, and the yeast genome contains no clear homologues of TRPC. At least 10 distinct genes encoding VGCC catalytic subunits have been identified in humans, and few of these proteins have been ruled out as CCE channels. Indeed, specific VGCC inhibitors can block Ca2+ influx in response to SERCA inhibition in at least some vertebrate cell types (30, 60). Given that the need for Ca2+ in secretory organelles is conserved among eukaryotes, it is plausible that a mechanism to ensure Ca2+ homeostasis in the secretory pathway arose prior to the divergence of animals and fungi and is largely conserved in both groups of organisms today. In this view, the primary differences between fungal and animal cells would be the types of Ca2+ pumps used to supply the secretory organelles and the types of Ca2+ channels that release secretory Ca2+. The significance of these differences may be relatively minor because stimulation of CCE-like processes in pmr1 mutants could be suppressed by expression of rabbit SERCA1a (Fig. 1D). However, the available data do not yet exclude the alternative possibility of independent origins of CCE in animals and yeast. Mutants lacking Pmr1p exhibit only a twofold decrease of free Ca2+ in the endoplasmic reticulum from a level that is already much lower than that of mammalian cells (55), raising the possibility that another secretory organelle such as the Golgi complex couples to the CCE-like process in yeast.
Current hypotheses for the coupling between Ca2+ influx channels in the plasma membrane and Ca2+ depletion in the endoplasmic reticulum include secretory mechanisms (61), docking mechanisms involving proteins in the plasma membrane and endoplasmic reticulum (42), conformational coupling mechanisms involving IP3 receptors and certain TRPCs (24, 25), various forms of retrograde signal transduction (46), and the production or release of small diffusible molecules that serve as intracellular messengers (40, 47). One such messenger of CCE termed CIF (calcium influx factor) has been described as a membrane-impermeant molecule that rapidly accumulates in human T cells treated with SERCA inhibitors and triggers Ca2+ influx in the absence of Ca2+ release from the endoplasmic reticulum (23, 58). A molecule with identical properties was recently shown to accumulate in pmr1 mutants but not wild-type yeast cells (5). The contribution of this molecule to Ca2+ influx in yeast cells has not been tested because no methods to introduce it into the cytoplasm or to genetically manipulate its biosynthesis have been developed. Additionally, the kinetics of Cch1p stimulation and CIF accumulation after depletion of secretory Ca2+ stores could not be determined in yeast because Pmr1p is resistant to the known SERCA inhibitors (53) and no other means of rapidly triggering Ca2+ depletion from secretory organelles have been developed. Despite these limitations, the yeast system affords genetic technologies useful for the identification of factors involved in the response to depletion of secretory Ca2+ stores. Characterization of the corresponding animal factors together with more mechanistic studies of the yeast system should help resolve the questions of whether the CCE mechanism evolved prior to the divergence of fungi and animals, which features of the mechanism are conserved today, and how the mechanism functions and varies in diverse cell types.
ACKNOWLEDGMENTS
We are grateful to Steve Garrett, Hidetoshi Iida, and Hans Rudolph for plasmids and advice, to Fujisawa USA Inc. for a gift of FK506, and to members of our laboratory and department for advice and technical support.
This work was supported by grants from the Searle Scholars Program/The Chicago Community Trust and the National Institutes of Health (grant GM53082 to K.W.C.).
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antebi A, Fink G R. The yeast Ca2+-ATPase homologue, PMR1, is required for normal Golgi function and localizes in a novel Golgi-like distribution. Mol Biol Cell. 1992;3:633–654. doi: 10.1091/mbc.3.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beeler T, Gable K, Zhao C, Dunn T. A novel protein, Csg2p, is required for Ca2+ regulation in Saccharomyces cerevisiae. J Biol Chem. 1994;269:7279–7284. [PubMed] [Google Scholar]
- 4.Catterall W A. Molecular properties of sodium and calcium channels. J Bioenerg Biomembr. 1996;28:219–230. doi: 10.1007/BF02110697. [DOI] [PubMed] [Google Scholar]
- 5.Csutora P, Su Z, Kim H Y, Bugrim A, Cunningham K W, Nuccitelli R, Keizer J E, Hanley M R, Blalock J E, Marchase R B. Calcium influx factor is synthesized by yeast and mammalian cells depleted of organellar calcium stores. Proc Natl Acad Sci USA. 1999;96:121–126. doi: 10.1073/pnas.96.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham K W, Fink G R. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in yeast. Mol Cell Biol. 1996;16:2226–2237. doi: 10.1128/mcb.16.5.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham K W, Fink G R. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J Cell Biol. 1994;124:351–363. doi: 10.1083/jcb.124.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cyert M S, Kunisawa R, Kaim D, Thorner J. Yeast has homologs (CNA1 and CNA2 gene products) of mammalian calcineurin, a calmodulin-regulated phosphoprotein phosphatase. Proc Natl Acad Sci USA. 1991;88:7376–7380. doi: 10.1073/pnas.88.16.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cyert M S, Thorner J. Regulatory subunit (CNB1 gene product) of yeast Ca2+/calmodulin-dependent phosphoprotein phosphatases is required for adaptation to pheromone. Mol Cell Biol. 1992;12:3460–3469. doi: 10.1128/mcb.12.8.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis T N, Urdea M S, Masiarz F R, Thorner J. Isolation of the yeast calmodulin gene: calmodulin is an essential protein. Cell. 1986;47:423–431. doi: 10.1016/0092-8674(86)90599-4. [DOI] [PubMed] [Google Scholar]
- 11.Degand I, Catty P, Talla E, Thinès-Sempoux D, De Kerchove D'Exaerde A, Goffeau A, Ghislain M. Rabbit sarcoplasmic reticulum Ca2+-ATPase replaces yeast PMC1 and PMR1 Ca2+-ATPases for cell viability and calcineurin-dependent regulation of calcium tolerance. Mol Microbiol. 1999;31:545–556. doi: 10.1046/j.1365-2958.1999.01195.x. [DOI] [PubMed] [Google Scholar]
- 12.de Virgilio C, Burckert N, Neuhaus J M, Boller T, Wiemken A. CNE1, a Saccharomyces cerevisiae homologue of the genes encoding mammalian calnexin and calreticulin. Yeast. 1993;9:185–188. doi: 10.1002/yea.320090209. [DOI] [PubMed] [Google Scholar]
- 13.Dürr G, Strayle J, Plemper R, Elbs S, Klee S K, Catty P, Wolf D H, Rudolph H K. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol Biol Cell. 1998;9:1149–1162. doi: 10.1091/mbc.9.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farquhar R, Honey N, Murant S J, Bossier P, Schultz L, Montgomery D, Ellis R W, Freedman R B, Tuite M F. Protein disulfide isomerase is essential for viability in Saccharomyces cerevisiae. Gene. 1991;108:81–89. doi: 10.1016/0378-1119(91)90490-3. [DOI] [PubMed] [Google Scholar]
- 15.Fischer M, Schnell N, Chattaway J, Davies P, Dixon G, Sanders D. The Saccharomyces cerevisiae CCH1 gene is involved in calcium influx and mating. FEBS Lett. 1997;419:259–262. doi: 10.1016/s0014-5793(97)01466-x. [DOI] [PubMed] [Google Scholar]
- 16.Fuller R S, Brake A, Thorner J. Yeast prohormone processing enzyme (KEX2 gene product) is a Ca2+-dependent serine protease. Proc Natl Acad Sci USA. 1989;86:1434–1438. doi: 10.1073/pnas.86.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gunteski-Hamblin A M, Clarke D M, Shull G E. Molecular cloning and tissue distribution of alternatively spliced mRNAs encoding possible mammalian homologues of the yeast secretory pathway calcium pump. Biochemistry. 1992;31:7600–7608. doi: 10.1021/bi00148a023. [DOI] [PubMed] [Google Scholar]
- 18.Halachmi D, Eilam Y. Calcium homeostasis in yeast cells exposed to high concentrations of calcium. Roles of vacuolar H+-ATPase and cellular ATP. FEBS Lett. 1993;316:73–78. doi: 10.1016/0014-5793(93)81739-m. [DOI] [PubMed] [Google Scholar]
- 19.Halachmi D, Eilam Y. Elevated cytosolic free Ca2+ concentrations and massive Ca2+ accumulation within vacuoles, in yeast mutant lacking PMR1, a homolog of Ca2+-ATPase. FEBS Lett. 1996;392:194–200. doi: 10.1016/0014-5793(96)00799-5. [DOI] [PubMed] [Google Scholar]
- 20.Iida H, Nakamura H, Ono T, Okumura M S, Anraku Y. MID1, a novel Saccharomyces cerevisiae gene encoding a plasma membrane protein, is required for Ca2+ influx and mating. Mol Cell Biol. 1994;14:8259–8271. doi: 10.1128/mcb.14.12.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanzaki M, Nagasawa M, Kojima I, Sato C, Naruse K, Sokabe M, Iida H. Molecular identification of a eukaryotic, stretch-activated nonselective cation channel. Science. 1999;285:882–886. doi: 10.1126/science.285.5429.882. [DOI] [PubMed] [Google Scholar]
- 22.Katzmann D J, Epping E A, Moye-Rowley W S. Mutational disruption of plasma membrane trafficking of Saccharomyces cerevisiae Yor1p, a homologue of mammalian multidrug resistance protein. Mol Cell Biol. 1999;19:2998–3009. doi: 10.1128/mcb.19.4.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim H Y, Thomas D, Hanley M R. Chromatographic resolution of an intracellular calcium influx factor from thapsigargin-activated Jurkat cells. Evidence for multiple activities influencing calcium elevation in Xenopus oocytes. J Biol Chem. 1995;270:9706–9708. doi: 10.1074/jbc.270.17.9706. [DOI] [PubMed] [Google Scholar]
- 24.Kiselyov K, Mignery G A, Zhu M X, Muallem S. The N-terminal domain of the IP3 receptor gates store-operated hTrp3 channels. Mol Cell. 1999;4:423–429. doi: 10.1016/s1097-2765(00)80344-5. [DOI] [PubMed] [Google Scholar]
- 25.Kiselyov K, Xu X, Mozhayeva G, Kuo T, Pessah I, Mignery G, Zhu X, Birnbaumer L, Muallem S. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature. 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- 26.Kuno T, Tanaka H, Mukai H, Chang C D, Hiraga K, Miyakawa T, Tanaka C. cDNA cloning of a calcineurin B homolog in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1991;180:1159–1163. doi: 10.1016/s0006-291x(05)81188-x. [DOI] [PubMed] [Google Scholar]
- 27.Lipari F, Herscovics A. Calcium binding to the class I alpha-1,2-mannosidase from Saccharomyces cerevisiae occurs outside the EF hand motif. Biochemistry. 1999;38:1111–1118. doi: 10.1021/bi981643i. [DOI] [PubMed] [Google Scholar]
- 28.Liu H, Krizek J, Bretscher A. Construction of a GAL1-regulated yeast cDNA expression library and its application to the identification of genes whose overexpression causes lethality in yeast. Genetics. 1992;132:665–673. doi: 10.1093/genetics/132.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Ishii S, Tokai M, Tsutsumi H, Ohki O, Akada R, Tanaka K, Tsuchiya E, Fukui S, Miyakawa T. The Saccharomyces cerevisiae genes (CMP1 and CMP2) encoding calmodulin-binding proteins homologous to the catalytic subunit of mammalian protein phosphatase 2B. Mol Gen Genet. 1991;227:52–59. doi: 10.1007/BF00260706. [DOI] [PubMed] [Google Scholar]
- 30.Lomax R B, Herrero C J, Garcia-Palomero E, Garcia A G, Montiel C. Capacitative Ca2+ entry into Xenopus oocytes is sensitive to omega-conotoxins GVIA, MVIIA and MVIIC. Cell Calcium. 1998;23:229–239. doi: 10.1016/s0143-4160(98)90121-x. [DOI] [PubMed] [Google Scholar]
- 31.Longtine M S, McKenzie A, III, Demarini D J, Shah N G, Wach A, Brachat A, Philippsen P, Pringle J R. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 32.Marchi V, Sorin A, Wei Y, Rao R. Induction of vacuolar Ca2+-ATPase and H+/Ca2+ exchange activity in yeast mutants lacking Pmr1, the Golgi Ca2+-ATPase. FEBS Lett. 1999;454:181–186. doi: 10.1016/s0014-5793(99)00803-0. [DOI] [PubMed] [Google Scholar]
- 33.Matheos D P, Kingsbury T J, Ahsan U S, Cunningham K W. Tcn1p/Crz1p, a calcineurin-dependent transcription factor that differentially regulates gene expression in Saccharomyces cerevisiae. Genes Dev. 1997;11:3445–3458. doi: 10.1101/gad.11.24.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meaden P, Hill K, Wagner J, Slipetz D, Sommer S S, Bussey H. The yeast KRE5 gene encodes a probable endoplasmic reticulum protein required for (1→6)-β-d-glucan synthesis and normal cell growth. Mol Cell Biol. 1990;10:3013–3019. doi: 10.1128/mcb.10.6.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miseta A, Fu L, Kellermayer R, Buckley J, Bedwell D M. The Golgi apparatus plays a significant role in the maintenance of Ca2+ homeostasis in the vps33Δ vacuolar biogenesis mutant of Saccharomyces cerevisiae. J Biol Chem. 1999;274:5939–5947. doi: 10.1074/jbc.274.9.5939. [DOI] [PubMed] [Google Scholar]
- 36.Miseta A, Kellermayer R, Aiello D P, Fu L, Bedwell D M. The vacuolar Ca2+/H+ exchanger Vcx1p/Hum1p tightly controls cytosolic Ca2+ levels in S. cerevisiae. FEBS Lett. 1999;451:132–136. doi: 10.1016/s0014-5793(99)00519-0. [DOI] [PubMed] [Google Scholar]
- 37.Ohya Y, Kawasaki H, Suzuki K, Londesborough J, Anraku Y. Two yeast genes encoding calmodulin-dependent protein kinases. Isolation, sequencing and bacterial expressions of CMK1 and CMK2. J Biol Chem. 1991;266:12784–12794. [PubMed] [Google Scholar]
- 38.Okorokov L A, Lehle L. Ca2+-ATPases of Saccharomyces cerevisiae: diversity and possible role in protein sorting. FEMS Microbiol Lett. 1998;162:83–91. doi: 10.1111/j.1574-6968.1998.tb12982.x. [DOI] [PubMed] [Google Scholar]
- 39.Paidhungat M, Garrett S. A homolog of mammalian, voltage-gated calcium channels mediates yeast pheromone-stimulated Ca2+ uptake and exacerbates the cdc1(Ts) growth defect. Mol Cell Biol. 1997;17:6339–6347. doi: 10.1128/mcb.17.11.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parekh A B, Terlau H, Stuhmer W. Depletion of InsP3 stores activates a Ca2+ and K+ current by means of a phosphatase and a diffusible messenger. Nature. 1993;364:814–818. doi: 10.1038/364814a0. [DOI] [PubMed] [Google Scholar]
- 41.Parlati F, Dominguez M, Bergeron J J, Thomas D Y. Saccharomyces cerevisiae CNE1 encodes an endoplasmic reticulum (ER) membrane protein with sequence similarity to calnexin and calreticulin and functions as a constituent of the ER quality control apparatus. J Biol Chem. 1995;270:244–253. doi: 10.1074/jbc.270.1.244. [DOI] [PubMed] [Google Scholar]
- 42.Patterson R L, van Rossum D B, Gill D L. Store-operated Ca2+ entry: evidence for a secretion-like coupling model. Cell. 1999;98:487–499. doi: 10.1016/s0092-8674(00)81977-7. [DOI] [PubMed] [Google Scholar]
- 43.Pausch M H, Kaim D, Kunisawa R, Admon A, Thorner J. Multiple Ca2+/calmodulin-dependent protein kinase genes in a unicellular eukaryote. EMBO J. 1991;10:1511–1522. doi: 10.1002/j.1460-2075.1991.tb07671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pozos T C, Sekler I, Cyert M S. The product of HUM1, a novel yeast gene, is required for vacuolar Ca2+/H+ exchange and is related to mammalian Na+/Ca2+ exchangers. Mol Cell Biol. 1996;16:3730–3741. doi: 10.1128/mcb.16.7.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Putney J W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 46.Putney J W, Jr, McKay R R. Capacitative calcium entry channels. Bioessays. 1999;21:38–46. doi: 10.1002/(SICI)1521-1878(199901)21:1<38::AID-BIES5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 47.Randriamampita C, Tsien R Y. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993;364:809–814. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- 48.Rose M D, Misra L M, Vogel J P. KAR2, a karyogamy gene, is the yeast homolog of the mammalian BiP/GRP78 gene. Cell. 1989;57:1211–1221. doi: 10.1016/0092-8674(89)90058-5. [DOI] [PubMed] [Google Scholar]
- 49.Rudolph H K, Antebi A, Fink G R, Buckley C M, Dorman T E, LeVitre J, Davidow L S, Mao J I, Moir D T. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell. 1989;58:133–145. doi: 10.1016/0092-8674(89)90410-8. [DOI] [PubMed] [Google Scholar]
- 50.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 51.Sherman F, Hicks J B, Fink G R. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1986. [Google Scholar]
- 52.Sikorski R S, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sorin A, Rosas G, Rao R. PMR1, a Ca2+-ATPase in yeast Golgi, has properties distinct from sarco/endoplasmic reticulum and plasma membrane calcium pumps. J Biol Chem. 1997;272:9895–9901. doi: 10.1074/jbc.272.15.9895. [DOI] [PubMed] [Google Scholar]
- 54.Stathopoulos A M, Cyert M S. Calcineurin acts through the CRZ1/TCN1 encoded transcription factor to regulate gene expression in yeast. Genes Dev. 1997;11:3432–3444. doi: 10.1101/gad.11.24.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strayle J, Pozzan T, Rudolph H K. Steady-state free Ca2+ in the yeast endoplasmic reticulum reaches only 10 μM and is mainly controlled by the secretory pathway pump Pmr1. EMBO J. 1999;18:4733–4743. doi: 10.1093/emboj/18.17.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tachibana C, Stevens T H. The yeast EUG1 gene encodes an endoplasmic reticulum protein that is functionally related to protein disulfide isomerase. Mol Cell Biol. 1992;12:4601–4611. doi: 10.1128/mcb.12.10.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tachikawa H, Miura T, Katakura Y, Mizunaga T. Molecular structure of a yeast gene, PDI1, encoding protein disulfide isomerase that is essential for cell growth. J Biochem. 1991;110:306–313. doi: 10.1093/oxfordjournals.jbchem.a123576. [DOI] [PubMed] [Google Scholar]
- 58.Thomas D, Hanley M R. Evaluation of calcium influx factors from stimulated Jurkat T-lymphocytes by microinjection into Xenopus oocytes. J Biol Chem. 1995;270:6429–6432. doi: 10.1074/jbc.270.12.6429. [DOI] [PubMed] [Google Scholar]
- 59.Wallis J W, Chrebet G, Brodsky G, Rolfe M, Rothstein R. A hyper-recombination mutation in S. cerevisiae identifies a novel eukaryotic topoisomerase. Cell. 1989;58:409–419. doi: 10.1016/0092-8674(89)90855-6. [DOI] [PubMed] [Google Scholar]
- 60.Willmott N J, Choudhury Q, Flower R J. Functional importance of the dihydropyridine-sensitive, yet voltage-insensitive store-operated Ca2+ influx of U937 cells. FEBS Lett. 1996;394:159–164. doi: 10.1016/0014-5793(96)00939-8. [DOI] [PubMed] [Google Scholar]
- 61.Yao Y, Ferrer-Montiel A V, Montal M, Tsien R Y. Activation of store-operated Ca2+ current in Xenopus oocytes requires SNAP-25 but not a diffusible messenger. Cell. 1999;98:475–485. doi: 10.1016/s0092-8674(00)81976-5. [DOI] [PubMed] [Google Scholar]
- 62.Ye R R, Bretscher A. Identification and molecular characterization of the calmodulin-binding subunit gene (CMP1) of protein phosphatase 2B from Saccharomyces cerevisiae. An alpha-factor inducible gene. Eur J Biochem. 1992;204:713–723. doi: 10.1111/j.1432-1033.1992.tb16686.x. [DOI] [PubMed] [Google Scholar]