Abstract
Gelatin, a denatured form of collagen, is an attractive biomaterial for biotechnology. In particular, gelatin particles have been noted due to their attractive properties as drug carriers. The drug release from gelatin particles can be easily controlled by the crosslinking degree of gelatin molecule, responding to the purpose of the research. The gelatin particles capable of drug release are effective in wound healing, drug screening models. For example, a sustained release of growth factors for tissue regeneration at the injured sites can heal a wound. In the case of the drug screening model, a tissue-like model composed of cells with high activity by the sustained release of drug or growth factor provides reliable results of drug effects. Gelatin particles are effective in drug delivery and the culture of spheroids or cell sheets because the particles prevent hypoxia-derived cell death. This review introduces recent research on gelatin microparticles-based strategies for regenerative therapy and drug screening models.
Keywords: biotechnology, drug delivery, drug research model, gelatin, regenerative medicine
1. Introduction
As representative biomaterials, chitosan [1,2], alginate [3,4], hyaluronic acid [5,6], collagen [7,8], gelatin [9,10], polylactic acid [11], polyglycolic acid [12,13], poly (lactic-co-glycolic acid) [14,15,16], or polyethylene glycol [17,18] are well known. Among the biomaterials, gelatin is often used for medical [19,20] or cosmetics [21] because gelatin is water-soluble [22], low inflammatory [23], and promotes high cell adhesion [24]. Gelatin formulation, such as a scaffold, has been investigated for cell transplantation [25,26,27]. Moreover, it has been reported that gelatin fiber supports the culture of cell sheets [28,29]. In addition to these non-spherical shape types, gelatin particles, especially micro size, have been investigated in the field of in vivo therapy or in vitro cell culture. This paper is a short review of recent research on gelatin microparticles-based biotechnology strategies for regenerative therapy and drug screening.
2. Protocol for the Preparation of Gelatin Microparticles
An aqueous gelatin solution is added to the olive oil by stirring for 10 min at 40 °C to prepare the water-in-oil emulsion. The emulsion temperature is decreased at 4 °C for the natural gelation of gelatin solution to obtain non-crosslinked hydrogel microspheres. The resulting gelatin microparticles (GMs) are washed a few times with cold acetone to exclude the residual oil completely. Next, GMs are fractionated by appropriate size using sieves [30]. Note that it is better to perform this protocol on ice because the non-crosslinked GMs are easily degraded at room temperature.
3. Crosslinking Methods
Non-crosslinked GMs cannot be used in cell culture or animal experiments because of the quick degradation. To obtain the formulation with appropriate degradation, chemical or dehydrothermal crosslinking processes are needed. The comparison of the two methods is shown in Table 1.
Table 1.
Comparison of features between chemical and dehydrothermal crosslinking methods.
| Points Compared | Crosslinking Method | |
|---|---|---|
| Chemical | Dehydrothermal | |
| Instrument needed | Nothing | Oven |
| Temperature (°C) | 40 | 140~160 |
| Particle condition under process | Liquid | solid |
| Crosslinking reagent added | Aldehyde, isocyanates, acyl azides, or carbodiimide [31,32,33,34] | Nothing |
| Stop reagent added | Glycine [35] | Nothing |
| Time required (days) | 1 | 2~5 |
| Merit |
|
|
| Demerit |
|
|
Among the chemical crosslinking reagents, it has been reported that there are some differences. For example, when the cells were cultured on the gelatin formulations crosslinked by genipin, cell seeding efficiency was significantly lower than aldehyde or carbodiimide. In addition, when the carbodiimide was used for crosslinking reagent, the gelatin formulations presented poor anti-hydrolysis ability [40]. Due to the reports, the aldehyde is often selected for crosslinking. Recently, dehydrothermal crosslinking has been noted because of the ease of handling [23]. If the machine for vacuum heating can be obtained, dehydrothermal crosslinking is the most appropriate choice.
4. Gelatin-Based Drug Delivery Systems
Growth factors are needed to enhance cell activity or function [41,42,43]. Therefore, the delivery of growth factors to cells would be a promising strategy for treating diseases. However, growth factors are quickly degraded, so the carrier for growth factors contained is essential. Gelatin molecules can interact with growth factors by electronic interaction because gelatin is a denatured form of collagen, a major extracellular matrix (ECM) component [44]. When the collagenase degrades the gelatin particles, the growth factors are released with gelatin molecule debris (Figure 1) [44,45]. This drug release mechanism is effective in tissue regeneration. When the gelatin particles containing growth factors are injected into the damaged tissues, growth factors are rapidly released, leading to tissue regeneration. This is due to the high secretion level of collagenase (e.g., vascular endothelial growth factor or matrix metalloproteinase) in the damaged tissues. In addition, the release speed of growth factors can be controlled by changing the crosslinking degree of gelatin molecules [46,47]. For example, when gelatin particles with the slow release of growth factors are needed, you should introduce a higher concentration of crosslinking reagents or a longer time for dehydrothermal crosslinking. Taken together, the mechanism of matrix-degradation-based drug release characterization is one of the attractive properties of gelatin [22,44].
Figure 1.
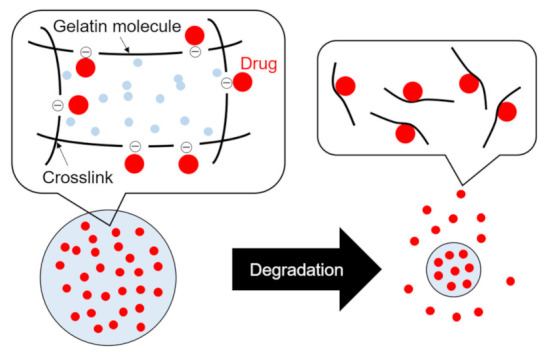
A schematic representation of drug release from gelatin particles (when the isoelectric point of gelatin is negative.). The gelatin used for sustained drug release can be selected considering the isoelectric point of the drug (If the drug to be released is basic, gelatin with a negative charge is preferable.). Drugs and gelatin molecules interact by physicochemical interaction (e.g., ionic or hydrogen interaction). When the gelatin particles are degraded, the drugs with gelatin molecule debris are rapidly released with time.
5. Applications of Gelatin Microparticles
In regenerative therapy and drug research models, enhanced cell activity or function is one of the most important concepts [48]. To achieve regenerative therapy, cells in the damaged tissue should proliferate by obtaining high cell activity. In the case of drug screening models, the cell activity or function of models should be close to that of natural tissues. To assist the enhancement of cell activity or function, GMs are often used. In this chapter, regenerative therapy and drug research model using GMs are introduced.
5.1. Regenerative Therapy
Table 2 summarizes some recent reports on regenerative therapy using gelatin microparticles.
Table 2.
Examples of regenerative therapy and tissue regeneration strategies using gelatin microparticles.
| Ref. | Date | Tissue Regenerated |
In Vitro (Cell Type)/In Vivo (Animal Type) |
Growth Factors Released | Main Results |
|---|---|---|---|---|---|
| [49] | 2015 | Cardiac | In vitro (human cardiac cells derived from iPS cells)/In vivo (mouse) | - | The survival rate of stacked cell sheets was improved by incorporating gelatin microparticles between each cell sheet. |
| [50] | 2017 | Blood vessels | In vitro (human umbilical vein endothelial cells and human dermal fibroblast cells)/In vivo (mouse) | Platelet-rich plasm A(PRP) |
Gelatin microparticles containing PRP promoted the formation of capillaries and microvascular networks. |
| [51] | 2018 | Sternal | In vivo (rabbit) | PRP | PRP-gelatin microparticles injection showed a significantly higher indicator of sternal healing than only gelatin microparticles injection. |
| [52] | 2018 | Bone | In vitro (mouse mesenchymal stem cells and mouse macrophages) | Bone morphogenic protein-2 (BMP-2) |
The gelatin microparticles were prepared to be preferentially degraded by pro-inflammatory macrophages, leading to the spatiotemporal BMP-2 release. The strategy enabled to achieve the efficient bone differentiation of stem cells. |
| [53] | 2018 | Cardiac | In vivo (rat) | Basic fibroblast growth factor (bFGF) |
Gelatin microparticles capable of bFGF control release showed the improvement of cell sheets’ viability. |
| [54] | 2019 | Cartilage | In vitro (human periosteum derived cells) |
Transforming growth factor-β1 (TGF-β1) |
TGF-β1 release from gelatin microparticles promotes the chondrogenic differentiation of human periosteum-derived cells. |
| [55] | 2019 | Bone | In vitro (rabbit mesenchymal stem cells)/In vivo (rabbit) |
BMP-2 | BMP-2 release system of gelatin microparticles is effective in bone regeneration of X-ray-radius defects. |
| [56] | 2021 | Cartilage and disk | In vitro (human stem cells)/In vivo (rat) | Matrilin3 and TGF-β3 | Chondrogenic differentiation was promoted when gelatin particles containing Matrilin-3 and TGF-β3 were incorporated into stem cell spheroids while preventing hypertrophy. |
| [57] | 2021 | Masseter muscle | In vitro (rat stem cells) |
bFGF and PRP | The combination of cell transplantation and the drug release system efficiently differentiated stem cells towards muscle lineage. |
There are two important factors for the achievement of tissue regeneration using materials transplantation into the damaged tissues. One is the speed of material degradation. To regenerate the tissue damaged, cells should actively migrate and proliferate in the defective site. Therefore, the speed of cell migration and material degradation should be linked and synchronized [22]. As mentioned above, the degradation profile of gelatin particles can be easily modified by the crosslinking reagent concentration or the dehydrothermal crosslink period. Therefore, gelatin particles are suitable for tissue regeneration in terms of degradation control. The second is the disappearance of the material. The remaining materials are unnecessary after the tissue regeneration is completed. Even though wound healing and tissue regeneration are achieved, the permanent existence of materials would induce inflammation [58]. Gelatin particles are materials capable of solving this problem because they are degraded into harmless amino acids to the body.
5.2. Drug Research Model
Table 3 summarizes the research on the GMs-based spheroids for drug research.
Table 3.
In vitro drug research studies using 3D cell/tissue spheroids combined with gelatin microparticles.
| Ref. | Date | Tissue or Disease | Cells Used | Growth Factors or Drugs Released | Main Results |
|---|---|---|---|---|---|
| [59] | 2017 | Epithelial | Mammary epithelial cells | - | β-casein expression of epithelial spheroids incorporating gelatin microparticles coated with Matrigel was higher than microparticles-free spheroids. |
| [60] | 2017 | Cancer | Cancer-associated fibroblasts and cancer cells | - | Cancer cells and cancer-associated fibroblasts (CAF) spheroids combined with gelatin particles showed a stromal matrix rich in collagen deposition and expressed the desmoplastic reaction markers. |
| [61] | 2017 | Epithelial | Mammary epithelial cells and preadipocyte cells | - | Epithelial-preadipocytes multicellular spheroids incorporating gelatin microparticles showed the enhancement of β-casein expression compared to spheroids in the absence of the gelatin microparticles. |
| [62] | 2017 | Bone | Pre-osteoblast cells | Bone morphogenic proteins-2 (BMP-2) | When spheroids incorporating gelatin microparticles containing BMP-2 were prepared, efficient osteogenic differentiation was observed compared to spheroids incorporating gelatin microparticles. |
| [63] | 2018 | Cancer | Cancer-associated fibroblasts and cancer cells | - | Cancer cells and CAF spheroids embedded gelatin particles enabled the evaluation of the anti-cancer drug effects efficiently. |
| [64] | 2018 | Pancreas | Insulinoma cells | - | The insulinoma spheroids incorporating gelatin microparticles prompted the secretion of insulin. |
| [65] | 2018 | Cancer | Cancer cells, endothelial cells, and fibroblasts | - | 3D tissue model consisting of cancer cells, endothelial cells, and fibroblasts was prepared. In this model, aberrant capillary-like structures were observed, which are important events of breast cancer progression. |
| [39] | 2019 | Cancer | Cancer-associated fibroblasts and cancer cells | p53 inhibitor | CAF spheroids incorporating gelatin microparticles containing a p53 inhibitor were prepared to activate the CAF function in vitro, similar to in vivo. The activated CAF spheroids can promote the invasion ability of cancer cells. |
| [66] | 2020 | Cancer | Cancer-associated fibroblasts and cancer cells | Transforming growth factor-β (TGF-β) |
CAF spheroids incorporating gelatin microparticles containing TGF-β enabled increased invasion rate of cancer cells, responding to TGF-β concentration. |
| [67] | 2020 | Cancer | Cancer-associated fibroblasts, macrophages, and cancer cells | Adenosine and TGF-β | 3D tumor-associated macrophages incorporating gelatin microparticles containing adenosine and 3D CAF incorporating gelatin microparticles containing TGF-β were combined. This system can mimic the tumor microenvironment, responding to the tissue region. |
Drug discovery is one of the most promising strategies to treat intractable diseases. Several hard processes should be passed to develop new drugs: drug screening using cells, preclinical study, and clinical study [68]. However, the drug efficacy of drug screening is often different from that of a preclinical or clinical study, leading to drug development failure [69,70]. This is mainly due to the difference in environmental conditions between in vitro and in vivo [71,72]. Cells are usually cultured by a two-dimensional culture system of a dish or plate. However, cells in the body environment tend to interact with each other in a three-dimensional (3D) manner. The interaction leads to an enhanced cell function, such as proliferation [73,74], differentiation [75,76], or metabolism [77]. Based on the characteristics, 3D tissue-like models, such as spheroids [78,79,80,81,82], organoids [83,84,85,86], or microfluidics systems [87,88,89], have been recently demonstrated. However, hypoxia is induced in the center of spheroids, leading to cell death [90,91]. Due to cell death, it is difficult to culture the spheroids for a long period to investigate the cell function. GMs have been incorporated into the spheroids to tackle the issues because oxygen or nutrients can be permeated through the water phase of gelatin gels [30]. The function of spheroids incorporating GMs is higher than that without GMs incorporation [23,30]. For example, when the insulinoma spheroids are prepared, the insulin secretion is enhanced. The model is useful as a tool for type 1 diabetes drug research [64].
In addition, the drug delivery system technology of GMs is effective in the drug research model. To enhance the cell function in vitro, similar to in vivo, the release of drugs, which enhance the cell function or activity, is important. Based on this reason, spheroids incorporating GMs containing drugs have been demonstrated for the anti-cancer drug research model [39,66,67]. Under the tumor environment, cancer cells interact with cancer cells and stromal cells of cancer-associated fibroblasts (CAF) [92,93]. Because CAF are always activated in vivo, it is important to activate CAF in vitro to mimic the tumor environment [94]. Therefore, to enhance and activate the CAF, CAF spheroids incorporating GMs containing drugs have been prepared. In addition, when the activated CAF spheroids and cancer cells are co-cultured via model basement membrane, cancer cells are effectively migrated with the penetration through the membrane. This CAF spheroids/cancer cells co-culture model is a promising tool to evaluate the invasion ability of cancer cells in vitro; therefore, the effect of candidate anti-invasion drugs can be investigated using the model [39,66].
6. Future Perspective and Conclusions
Biomaterial usage for in vivo therapy or in vitro research has been noted because the biomaterial enables the enhancement of cell potentials, such as proliferation, differentiation, or metabolism. For further development of the field, it is essential to use material of low inflammatory induction. Because gelatin is a denatured form of collagen, a major component of proteins, gelatin is a suitable material for patient-friendly therapy. In addition, gelatin can support cell viability by providing collagen proteins to the cells. However, ECM components consist not only of collagen but also polysaccharides [95]. Based on this cell characteristic, polysaccharides-based biomaterials, such as alginate, chitosan, or hyaluronic acid, are also essential to enhance cell activity or function. Therefore, the combination of polysaccharides-based biomaterials and gelatin materials would further develop regenerative therapy or drug research models.
In this review, regenerative therapy and drug research models using gelatin microparticles (GMs) are introduced. In both two applications, collagenase-triggered drug release is the common keyword. In the case of regenerative therapy, the higher secretion of collagenase in the injured site is utilized. Because the drug is released from GMs only on injured sites, it is possible to enhance the drug effects or reduce the side effects. When the GMs are incorporated into the spheroids for drug research models, collagenase secretion by the 3D cell-cell interaction can enhance the drug release. This on-off drug release would also be effective in other applications in the future, such as vaccines. The allergen must be administered to antigen-presenting cells (APC), such as dendritic cells. When the allergen is diffused, severe anaphylaxis will occur. Therefore, to achieve efficient vaccines, allergen should be intensively administered to APC. To tackle this issue, GMs-based allergen release would be promising. Because the sites of allergen administration are healthy, the allergen is not leaked from gelatin microparticles after the injection. After the GMs are selectively up taken into the APC by the APC-specific ligand coating, the allergen is released from GMs “inside” the APC. This is because the collagenase exists as the intracellular enzyme. Therefore, GMs are attractive drug carriers for many applications.
Funding
This research was funded by JPSP KAKENHI Grant-in-Aid for Young Scientists (Start-up), grant number 21K20517.
Conflicts of Interest
The author declares no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bakshi P.S., Selvakumar D., Kadirvelu K., Kumar N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020;150:1072–1083. doi: 10.1016/j.ijbiomac.2019.10.113. [DOI] [PubMed] [Google Scholar]
- 2.Arca H.Ç., Şenel S. Chitosan based systems for tissue engineering part II: Soft tissues. Fabad J. Pharm. Sci. 2008;33:211–216. [Google Scholar]
- 3.Uyen N.T.T., Hamid Z.A.A., Tram N.X.T., Ahmad N. Fabrication of alginate microspheres for drug delivery: A review. Int. J. Biol. Macromol. 2020;153:1035–1046. doi: 10.1016/j.ijbiomac.2019.10.233. [DOI] [PubMed] [Google Scholar]
- 4.Cheng D., Jiang C., Xu J., Liu Z., Mao X. Characteristics and applications of alginate lyases: A review. Int. J. Biol. Macromol. 2020;164:1304–1320. doi: 10.1016/j.ijbiomac.2020.07.199. [DOI] [PubMed] [Google Scholar]
- 5.Necas J., Bartosikova L., Brauner P., Kolar J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008;53:397–411. doi: 10.17221/1930-VETMED. [DOI] [Google Scholar]
- 6.Rayahin J.E., Buhrman J.S., Zhang Y., Koh T.J., Gemeinhart R.A. High and Low Molecular Weight Hyaluronic Acid Differentially Influence Macrophage Activation. ACS Biomater. Sci. Eng. 2015;1:481–493. doi: 10.1021/acsbiomaterials.5b00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avila Rodríguez M.I., Rodríguez Barroso L.G., Sánchez M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018;17:20–26. doi: 10.1111/jocd.12450. [DOI] [PubMed] [Google Scholar]
- 8.Bhagwat P.K., Dandge P.B. Collagen and collagenolytic proteases: A review. Biocatal. Agric. Biotechnol. 2018;15:43–55. doi: 10.1016/j.bcab.2018.05.005. [DOI] [Google Scholar]
- 9.Huang T., Tu Z.-C., Shangguan X., Sha X., Wang H., Zhang L., Bansal N. Fish gelatin modifications: A comprehensive review. Trends Food Sci. Technol. 2019;86:260–269. doi: 10.1016/j.tifs.2019.02.048. [DOI] [Google Scholar]
- 10.Bin Sulaiman S., Idrus R.B.H., Hwei N.M. Gelatin microsphere for cartilage tissue engineering: Current and future strategies. Polymers. 2020;12:2404. doi: 10.3390/polym12102404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singhvi M.S., Zinjarde S.S., Gokhale D.V. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019;127:1612–1626. doi: 10.1111/jam.14290. [DOI] [PubMed] [Google Scholar]
- 12.Budak K., Sogut O., Aydemir Sezer U. A review on synthesis and biomedical applications of polyglycolic acid. J. Polym. Res. 2020;27:208. doi: 10.1007/s10965-020-02187-1. [DOI] [Google Scholar]
- 13.Jem K.J., Tan B. The development and challenges of poly (lactic acid) and poly (glycolic acid) Adv. Ind. Eng. Polym. Res. 2020;3:60–70. doi: 10.1016/j.aiepr.2020.01.002. [DOI] [Google Scholar]
- 14.Nii T., Takeuchi I., Kimura Y., Makino K. Effects of the conformation of PLGA molecules in the organic solvent on the aerodynamic diameter of spray dried microparticles. Colloids Surf. A Physicochem. Eng. Asp. 2018;539:347–353. doi: 10.1016/j.colsurfa.2017.12.042. [DOI] [Google Scholar]
- 15.Ghitman J., Biru E.I., Stan R., Iovu H. Review of hybrid PLGA nanoparticles: Future of smart drug delivery and theranostics medicine. Mater. Des. 2020;193:108805. doi: 10.1016/j.matdes.2020.108805. [DOI] [Google Scholar]
- 16.Xu Y., Kim C.S., Saylor D.M., Koo D. Polymer degradation and drug delivery in PLGA-based drug–polymer applications: A review of experiments and theories. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2017;105:1692–1716. doi: 10.1002/jbm.b.33648. [DOI] [PubMed] [Google Scholar]
- 17.Chen J., Spear S.K., Huddleston J.G., Rogers R.D. Polyethylene glycol and solutions of polyethylene glycol as green reaction media. Green Chem. 2005;7:64–82. doi: 10.1039/b413546f. [DOI] [Google Scholar]
- 18.Topchiyeva I.N. Synthesis of biologically active polyethylene glycol derivatives. A review. Polym. Sci. U.S.S.R. 1990;32:833–851. doi: 10.1016/0032-3950(90)90214-Q. [DOI] [Google Scholar]
- 19.Bello A.B., Kim D., Kim D., Park H., Lee S.H. Engineering and functionalization of gelatin biomaterials: From cell culture to medical applications. Tissue Eng. Part. B Rev. 2020;26:164–180. doi: 10.1089/ten.teb.2019.0256. [DOI] [PubMed] [Google Scholar]
- 20.Tabata Y., Ikada Y. Vascularization effect of basic fibroblast growth factor released from gelatin hydrogels with different biodegradabilities. Biomaterials. 1999;20:2169–2175. doi: 10.1016/S0142-9612(99)00121-0. [DOI] [PubMed] [Google Scholar]
- 21.Mitura S., Sionkowska A., Jaiswal A. Biopolymers for hydrogels in cosmetics: Review. J. Mater. Sci. Mater. Med. 2020;31:50. doi: 10.1007/s10856-020-06390-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nii T., Makino K., Tabata Y. Three-Dimensional culture system of cancer cells combined with biomaterials for drug screening. Cancers. 2020;12:2754. doi: 10.3390/cancers12102754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nii T., Makino K., Tabata Y. Influence of shaking culture on the biological functions of cell aggregates incorporating gelatin hydrogel microspheres. J. Biosci. Bioeng. 2019;128:606–612. doi: 10.1016/j.jbiosc.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z., Tian Z., Jin X., Holzman J.F., Menard F., Kim K. Visible light-based stereolithography bioprinting of cell-adhesive gelatin hydrogels. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. EMBS. 2017:1599–1602. doi: 10.1109/EMBC.2017.8037144. [DOI] [PubMed] [Google Scholar]
- 25.Li G., Che M.T., Zhang K., Qin L.N., Zhang Y.T., Chen R.Q., Rong L.M., Liu S., Ding Y., Shen H.Y., et al. Graft of the NT-3 persistent delivery gelatin sponge scaffold promotes axon regeneration, attenuates inflammation, and induces cell migration in rat and canine with spinal cord injury. Biomaterials. 2016;83:233–248. doi: 10.1016/j.biomaterials.2015.11.059. [DOI] [PubMed] [Google Scholar]
- 26.Xu X., Hu J., Lu H. Histological observation of a gelatin sponge transplant loaded with bone marrow-derived mesenchymal stem cells combined with platelet-rich plasma in repairing an annulus defect. PLoS ONE. 2017;12:e0171500. doi: 10.1371/journal.pone.0171500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu B., Zhang H., Du L., Yuan Q., Zhang K., Xu H., Ma X., Liu Y., Jiang H., Li N. Selective Retention of Bone Marrow Stromal Cells with Gelatin Sponge for Repair of Intervertebral Disc Defects after Microendoscopic Discectomy: A Prospective Controlled Study and 2-Year Follow-Up. Biomed. Res. Int. 2021;2021:4822383. doi: 10.1155/2021/4822383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura K., Nobutani K., Shimada N., Tabata Y. Gelatin hydrogel-fragmented fibers suppress shrinkage of cell sheet. Tissue Eng. Part. C Methods. 2020;26:216–224. doi: 10.1089/ten.tec.2019.0348. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura K., Saotome T., Shimada N., Matsuno K., Tabata Y. A Gelatin Hydrogel Nonwoven Fabric Facilitates Metabolic Activity of Multilayered Cell Sheets. Tissue Eng. Part. C Methods. 2019;25:344–352. doi: 10.1089/ten.tec.2019.0061. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi K., Tabata Y. Preparation of stem cell aggregates with gelatin microspheres to enhance biological functions. Acta Biomater. 2011;7:2797–2803. doi: 10.1016/j.actbio.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 31.Campiglio C.E., Negrini N.C., Farè S., Draghi L. Cross-Linking strategies for electrospun gelatin scaffolds. Materials. 2019;12:2476. doi: 10.3390/ma12152476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuijpers A.J., Engbers G.H.M., Feijen J., De Smedt S.C., Meyvis T.K.L., Demeester J., Krijgsveld J., Zaat S.A.J., Dankert J. Characterization of the network structure of carbodiimide cross-linked gelatin gels. Macromolecules. 1999;32:3325–3333. doi: 10.1021/ma981929v. [DOI] [Google Scholar]
- 33.Bigi A., Cojazzi G., Panzavolta S., Rubini K., Roveri N. Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials. 2001;22:763–768. doi: 10.1016/S0142-9612(00)00236-2. [DOI] [PubMed] [Google Scholar]
- 34.Cheng N.C., Estes B.T., Young T.H., Guilak F. Genipin-Crosslinked cartilage-derived matrix as a scaffold for human adipose-derived stem cell chondrogenesis. Tissue Eng. Part. A. 2013;19:484–496. doi: 10.1089/ten.tea.2012.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murata Y., Jo J.-I., Tabata Y. Preparation of cationized gelatin nanospheres incorporating molecular beacon to visualize cell apoptosis. Sci. Rep. 2018;8:14839. doi: 10.1038/s41598-018-33231-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshimoto Y., Jo J.-I., Tabata Y. Preparation of antibody-immobilized gelatin nanospheres incorporating a molecular beacon to visualize the biological function of macrophages. Regen. Ther. 2020;14:11–18. doi: 10.1016/j.reth.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adamiak K., Sionkowska A. Current methods of collagen cross-linking: Review. Int. J. Biol. Macromol. 2020;161:550–560. doi: 10.1016/j.ijbiomac.2020.06.075. [DOI] [PubMed] [Google Scholar]
- 38.Sri Ramakrishnan L., PS U., Sabu C.K., Krishnan A.G., Nair M.B. Effect of wheat gluten on improved thermal cross-linking and osteogenesis of hydroxyapatite-gelatin composite scaffolds. Int. J. Biol. Macromol. 2021;183:1200–1209. doi: 10.1016/j.ijbiomac.2021.04.181. [DOI] [PubMed] [Google Scholar]
- 39.Nii T., Makino K., Tabata Y. A Cancer Invasion Model Combined with Cancer-Associated Fibroblasts Aggregates Incorporating Gelatin Hydrogel Microspheres Containing a p53 Inhibitor. Tissue Eng. Part. C Methods. 2019;25:711–720. doi: 10.1089/ten.tec.2019.0189. [DOI] [PubMed] [Google Scholar]
- 40.Yang G., Xiao Z., Long H., Ma K., Zhang J., Ren X., Zhang J. Assessment of the characteristics and biocompatibility of gelatin sponge scaffolds prepared by various crosslinking methods. Sci. Rep. 2018;8:1616. doi: 10.1038/s41598-018-20006-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flaumenhaft R., Rifkin D.B. Extracellular matrix regulation of growth factor and protease activity. Curr. Opin. Cell Biol. 1991;3:817–823. doi: 10.1016/0955-0674(91)90055-4. [DOI] [PubMed] [Google Scholar]
- 42.Vlodavsky I., Ishai-Michaeli R., Bashkin P., Levi E., Korner G., Bar-Shavit R., Fuks Z., Klagsbrun M. Extracellular matrix-resident basic fibroblast growth factor: Implication for the control of angiogenesis. J. Cell. Biochem. 1991;45:167–176. doi: 10.1002/jcb.240450208. [DOI] [PubMed] [Google Scholar]
- 43.Jones J.I., Gockerman A., Busby W.H., Camacho-Hubner C., Clemmons D.R. Extracellular matrix contains insulin-like growth factor binding protein-5: Potentiation of the effects of IGF-I. J. Cell Biol. 1993;121:679–687. doi: 10.1083/jcb.121.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tabata Y., Ikada Y. Protein release from gelatin matrices. Adv. Drug Deliv. Rev. 1998;31:287–301. doi: 10.1016/S0169-409X(97)00125-7. [DOI] [PubMed] [Google Scholar]
- 45.Young S., Wong M., Tabata Y., Mikos A.G. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J. Control. Release. 2005;109:256–274. doi: 10.1016/j.jconrel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 46.Murata Y., Jo J.I., Tabata Y. Intracellular controlled release of molecular beacon prolongs the time period of mRNA visualization. Tissue Eng. Part. A. 2019;25:1527–1537. doi: 10.1089/ten.tea.2019.0017. [DOI] [PubMed] [Google Scholar]
- 47.Tajima S., Tabata Y. Preparation and functional evaluation of cell aggregates incorporating gelatin microspheres with different degradabilities. J. Tissue Eng. Regen. Med. 2013;7:801–811. doi: 10.1002/term.1469. [DOI] [PubMed] [Google Scholar]
- 48.Nii T., Katayama Y. Biomaterial-Assisted Regenerative Medicine. Int. J. Mol. Sci. 2021;22:8657. doi: 10.3390/ijms22168657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsuo T., Masumoto H., Tajima S., Ikuno T., Katayama S., Minakata K., Ikeda T., Yamamizu K., Tabata Y., Sakata R., et al. Efficient long-term survival of cell grafts after myocardial infarction with thick viable cardiac tissue entirely from pluripotent stem cells. Sci. Rep. 2015;5:1–14. doi: 10.1038/srep16842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kakudo N., Morimoto N., Ogawa T., Hihara M., Notodihardjo P.V., Matsui M., Tabata Y., Kusumoto K. Angiogenic effect of platelet-rich plasma combined with gelatin hydrogel granules injected into murine subcutis. J. Tissue Eng. Regen. Med. 2017;11:1941–1948. doi: 10.1002/term.2091. [DOI] [PubMed] [Google Scholar]
- 51.Shibata M., Takagi G., Kudo M., Kurita J., Kawamoto Y., Miyagi Y., Kanazashi M., Sakatani T., Naito Z., Tabata Y., et al. Enhanced Sternal Healing Through Platelet-Rich Plasma and Biodegradable Gelatin Hydrogel. Tissue Eng. Part. A. 2018;24:1406–1412. doi: 10.1089/ten.tea.2017.0505. [DOI] [PubMed] [Google Scholar]
- 52.Annamalai R.T., Turner P.A., Carson W.F., Levi B., Kunkel S., Stegemann J.P. Harnessing macrophage-mediated degradation of gelatin microspheres for spatiotemporal control of BMP2 release. Biomaterials. 2018;161:216–227. doi: 10.1016/j.biomaterials.2018.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Z., Masumoto H., Jo J.I., Yamazaki K., Ikeda T., Tabata Y., Minatoya K. Sustained release of basic fibroblast growth factor using gelatin hydrogel improved left ventricular function through the alteration of collagen subtype in a rat chronic myocardial infarction model. Gen. Thorac. Cardiovasc. Surg. 2018;66:641–647. doi: 10.1007/s11748-018-0969-z. [DOI] [PubMed] [Google Scholar]
- 54.Kudva A.K., Dikina A.D., Luyten F.P., Alsberg E., Patterson J. Gelatin microspheres releasing transforming growth factor drive in vitro chondrogenesis of human periosteum derived cells in micromass culture. Acta Biomater. 2019;90:287–299. doi: 10.1016/j.actbio.2019.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xia P., Wang S., Qi Z., Zhang W., Sun Y. BMP-2-releasing gelatin microspheres/PLGA scaffolds for bone repairment of X-ray-radiated rabbit radius defects. Artif. Cells Nanomed. Biotechnol. 2019;47:1662–1673. doi: 10.1080/21691401.2019.1594852. [DOI] [PubMed] [Google Scholar]
- 56.Bello A.B., Kim Y., Park S., Muttigi M.S., Kim J., Park H., Lee S. Matrilin3/TGFβ3 gelatin microparticles promote chondrogenesis, prevent hypertrophy, and induce paracrine release in MSC spheroid for disc regeneration. NPJ Regen. Med. 2021;6:1–13. doi: 10.1038/s41536-021-00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitsui R., Matsukawa M., Nakagawa K., Isomura E., Kuwahara T., Nii T., Tanaka S., Tabata Y. Efficient cell transplantation combining injectable hydrogels with control release of growth factors. Regen. Ther. 2021;18:372–383. doi: 10.1016/j.reth.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anderson J.M. Chapter 4 Mechanisms of inflammation and infection with implanted devices. Cardiovasc. Pathol. 1993;2:33–41. doi: 10.1016/1054-8807(93)90045-4. [DOI] [Google Scholar]
- 59.Tajima S., Tabata Y. Preparation of epithelial cell aggregates incorporating matrigel microspheres to enhance proliferation and differentiation of epithelial cells. Regen. Ther. 2017;7:34–44. doi: 10.1016/j.reth.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brancato V., Comunanza V., Imparato G., Corà D., Urciuolo F., Noghero A., Bussolino F., Netti P.A. Bioengineered tumoral microtissues recapitulate desmoplastic reaction of pancreatic cancer. Acta Biomater. 2017;49:152–166. doi: 10.1016/j.actbio.2016.11.072. [DOI] [PubMed] [Google Scholar]
- 61.Tajima S., Tabata Y. Preparation of EpH4 and 3T3L1 cells aggregates incorporating gelatin hydrogel microspheres for a cell condition improvement. Regen. Ther. 2017;6:90–99. doi: 10.1016/j.reth.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tajima S., Tabata Y. Preparation of cell aggregates incorporating gelatin hydrogel microspheres containing bone morphogenic protein-2 with different degradabilities. J. Biomater. Sci. Polym. Ed. 2017;29:775–792. doi: 10.1080/09205063.2017.1358547. [DOI] [PubMed] [Google Scholar]
- 63.Brancato V., Gioiella F., Imparato G., Guarnieri D., Urciuolo F., Netti P.A. 3D breast cancer microtissue reveals the role of tumor microenvironment on the transport and efficacy of free-doxorubicin in vitro. Acta Biomater. 2018;75:200–212. doi: 10.1016/j.actbio.2018.05.055. [DOI] [PubMed] [Google Scholar]
- 64.Inoo K., Bando H., Tabata Y. Enhanced survival and insulin secretion of insulinoma cell aggregates by incorporating gelatin hydrogel microspheres. Regen. Ther. 2018;8:29–37. doi: 10.1016/j.reth.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mazio C., Casale C., Imparato G., Urciuolo F., Netti P.A. Recapitulating spatiotemporal tumor heterogeneity in vitro through engineered breast cancer microtissues. Acta Biomater. 2018;73:236–249. doi: 10.1016/j.actbio.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 66.Nii T., Makino K., Tabata Y. A cancer invasion model of cancer-associated fibroblasts aggregates combined with TGF-β1 release system. Regen. Ther. 2020;14:196–204. doi: 10.1016/j.reth.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nii T., Kuwahara T., Makino K., Tabata Y. A co-culture system of three-dimensional tumor-associated macrophages and three-dimensional cancer-associated fibroblasts combined with biomolecule release for cancer cell migration. Tissue Eng. Part. A. 2020;26:1272–1282. doi: 10.1089/ten.tea.2020.0095. [DOI] [PubMed] [Google Scholar]
- 68.Rao V.S., Srinivas K. Modern drug discovery process : An in silico approach. J. Bioinforma. Seq. Anal. 2011;2:89–94. [Google Scholar]
- 69.Breslin S., O’Driscoll L. Three-Dimensional cell culture: The missing link in drug discovery. Drug Discov. Today. 2013;18:240–249. doi: 10.1016/j.drudis.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 70.Hait W.N. Anticancer drug development: The grand challenges. Nat. Rev. Drug Discov. 2010;9:253–254. doi: 10.1038/nrd3144. [DOI] [PubMed] [Google Scholar]
- 71.Brancato V., Oliveira J.M., Correlo V.M., Reis R.L., Kundu S.C. Could 3D models of cancer enhance drug screening? Biomaterials. 2020;232:119744. doi: 10.1016/j.biomaterials.2019.119744. [DOI] [PubMed] [Google Scholar]
- 72.Kim M.J., Chi B.H., Yoo J.J., Ju Y.M., Whang Y.M., Chang I.H. Structure establishment of three-dimensional (3D) cell culture printing model for bladder cancer. PLoS ONE. 2019;14:e0223689. doi: 10.1371/journal.pone.0223689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ng K.W., Leong D.T.W., Hutmacher D.W. The challenge to measure cell proliferation in two and three dimensions. Tissue Eng. 2005;11:182–191. doi: 10.1089/ten.2005.11.182. [DOI] [PubMed] [Google Scholar]
- 74.Shamekhi M.A., Mirzadeh H., Mahdavi H., Rabiee A., Mohebbi-Kalhori D., Baghaban Eslaminejad M. Graphene oxide containing chitosan scaffolds for cartilage tissue engineering. Int. J. Biol. Macromol. 2019;127:396–405. doi: 10.1016/j.ijbiomac.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 75.Kurosawa H. Methods for inducing embryoid body formation: In vitro differentiation system of embryonic stem cells. J. Biosci. Bioeng. 2007;103:389–398. doi: 10.1263/jbb.103.389. [DOI] [PubMed] [Google Scholar]
- 76.Zhang B., Wang L., Song P., Pei X., Sun H., Wu L., Zhou C., Wang K., Fan Y., Zhang X. 3D printed bone tissue regenerative PLA/HA scaffolds with comprehensive performance optimizations. Mater. Des. 2021;201:109490. doi: 10.1016/j.matdes.2021.109490. [DOI] [Google Scholar]
- 77.Rodríguez-Enríquez S., Gallardo-Pérez J.C., Avilés-Salas A., Marín-Hernández A., Carreño-Fuentes L., Maldonado-Lagunas V., Moreno-Sánchez R. Energy metabolism transition in multi-cellular human tumor spheroids. J. Cell. Physiol. 2008;216:189–197. doi: 10.1002/jcp.21392. [DOI] [PubMed] [Google Scholar]
- 78.Nunes A.S., Barros A.S., Costa E.C., Moreira A.F., Correia I.J. 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs. Biotechnol. Bioeng. 2019;116:206–226. doi: 10.1002/bit.26845. [DOI] [PubMed] [Google Scholar]
- 79.Han K., Pierce S.E., Li A., Spees K., Anderson G.R., Seoane J.A., Lo Y.H., Dubreuil M., Olivas M., Kamber R.A., et al. CRISPR screens in cancer spheroids identify 3D growth-specific vulnerabilities. Nature. 2020;580:136–141. doi: 10.1038/s41586-020-2099-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tchoryk A., Taresco V., Argent R.H., Ashford M., Gellert P.R., Stolnik S., Grabowska A., Garnett M.C. Penetration and uptake of nanoparticles in 3D tumor spheroids. Bioconjug. Chem. 2019;30:1371–1384. doi: 10.1021/acs.bioconjchem.9b00136. [DOI] [PubMed] [Google Scholar]
- 81.Park Y., Franz C.K., Ryu H., Luan H., Cotton K.Y., Kim J.U., Chung T.S., Zhao S., Vazquez-Guardado A., Yang D.S., et al. Three-Dimensional, multifunctional neural interfaces for cortical spheroids and engineered assembloids. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abf9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Birey F., Andersen J., Makinson C.D., Islam S., Wei W., Huber N., Fan H.C., Metzler K.R.C., Panagiotakos G., Thom N., et al. Assembly of functionally integrated human forebrain spheroids. Nature. 2017;545:54–59. doi: 10.1038/nature22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Drost J., Clevers H. Organoids in cancer research. Nat. Rev. Cancer. 2018;18:407–418. doi: 10.1038/s41568-018-0007-6. [DOI] [PubMed] [Google Scholar]
- 84.Kim J., Koo B.K., Knoblich J.A. Human organoids: Model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 2020;21:571–584. doi: 10.1038/s41580-020-0259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hofer M., Lutolf M.P. Engineering organoids. Nat. Rev. Mater. 2021;6:402–420. doi: 10.1038/s41578-021-00279-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takebe T., Wells J.M. Organoids by design. Science. 2019;364:956–959. doi: 10.1126/science.aaw7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Meyvantsson I., Beebe D.J. Cell culture models in microfluidic systems. Annu. Rev. Anal. Chem. 2008;1:423–449. doi: 10.1146/annurev.anchem.1.031207.113042. [DOI] [PubMed] [Google Scholar]
- 88.Castiaux A.D., Spence D.M., Martin R.S. Review of 3D cell culture with analysis in microfluidic systems. Anal. Methods. 2019;11:4220–4232. doi: 10.1039/C9AY01328H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Trujillo-de Santiago G., Flores-Garza B.G., Tavares-Negrete J.A., Lara-Mayorga I.M., González-Gamboa I., Zhang Y.S., Rojas-Martínez A., Ortiz-López R., Álvarez M.M. The tumor-on-chip: Recent advances in the development of microfluidic systems to recapitulate the physiology of solid tumors. Materials. 2019;12:2945. doi: 10.3390/ma12182945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kellner K., Liebsch G., Klimant I., Wolfbeis O.S., Blunk T., Schulz M.B., Göpferich A. Determination of oxygen gradients in engineered tissue using a fluorescent sensor. Biotechnol. Bioeng. 2002;80:73–83. doi: 10.1002/bit.10352. [DOI] [PubMed] [Google Scholar]
- 91.Compañ V., Guzmán J., Riande E. A potentiostatic study of oxygen transmissibility and permeability through hydrogel membranes. Biomaterials. 1998;19:2139–2145. doi: 10.1016/S0142-9612(98)00113-6. [DOI] [PubMed] [Google Scholar]
- 92.Shiga K., Hara M., Nagasaki T., Sato T., Takahashi H., Takeyama H. Cancer-Associated fibroblasts: Their characteristics and their roles in tumor growth. Cancers. 2015;7:2443–2458. doi: 10.3390/cancers7040902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kalluri R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer. 2016;16:582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 94.Li H., Fan X., Houghton J.M. Tumor microenvironment: The role of the tumor stroma in cancer. J. Cell. Biochem. 2007;101:805–815. doi: 10.1002/jcb.21159. [DOI] [PubMed] [Google Scholar]
- 95.Scott J.E. Supramolecular organization of extracellular matrix glycosaminoglycans, in vitro and in the tissues. FASEB J. 1992;6:2639–2645. doi: 10.1096/fasebj.6.9.1612287. [DOI] [PubMed] [Google Scholar]


