Abstract
Physical exercise may activate a number of important biochemical processes in the human body. The aim of this systematic review and meta-analysis was to identify the long-term effect of physical activity on irisin blood levels. We searched PubMed, Scopus, and Web of Science for articles addressing the long-term effect of physical exercise on irisin blood levels. Fifty-nine articles were included in the final qualitative and quantitative syntheses. A statistically significant within-group effect of exercise on irisin blood levels was in 33 studies; out of them, the irisin level increased 23× and decreased 10×. The significant positive between-groups effect was found 11×. Furthermore, the meta-analysis indicated that physical exercise had a significant positive effect on irisin blood levels (SMD = 0.39 (95% CI 0.27–0.52)). Nevertheless, considerably high heterogeneity was found in all the analyses. This systematic review and meta-analysis indicate that physical exercise might increase irisin blood levels; however, the results of individual studies were considerably inconsistent, which questions the methodological detection of irisin by ELISA kits.
Keywords: physical activity, myokines, health, intervention
1. Background
Description of the Condition
Irisin is a cleavage product of fibronectin type III domain-containing protein 5 (FNDC5) and was first isolated and described by Bostrom, Wu [1] as an exercise-induced hormone in 2012. Since then, many beneficial roles have been ascribed to it. For instance, as an important regulator of energy metabolism, irisin plays a protective role against type 2 diabetes mellitus and obesity [2,3]; maintains cardiovascular health [4]; acts as a behavioural antidepressant in mood regulation [5], and protects against bone loss and muscle atrophy [6]. Irisin has also been linked to the increased expression of brain-derived neurotrophic factor (BDNF) with subsequent beneficial effects on brain health and cognitive function [7]. Most recently, irisin has also shown a positive effect in regulating diverse genes in the adipose tissue, related to the COVID-19 outcome. Precisely, a reduction of genes implicated in elevated viral infection and an increase in genes that block virus-cell cleavage, which indicates a decrease of SARS-CoV-2 infection rate in human cells, has been demonstrated [8].
The irisin-mediated therapeutic effect may, in fact, hold the answers to how physical exercise positively influences the human body. Indeed, it is common knowledge that physical exercise helps to improve health status and can help prevent many diseases such as cardiovascular diseases [9], insulin resistance [10], type 2 diabetes mellitus [11], depression [12], sarcopenia [13] or Alzheimer’s disease [14]. Exercise stimulates PPARγ coactivator-1 α (PGC1α) as a transcriptional coactivator that mediates many biological programs related to energy metabolism. More specifically, it stimulates the expression of FNDC5, which encodes a type I membrane protein that is processed proteolytically, resulting in irisin secretion into the blood [1]. Therefore, any effect of FNDC5 and resulting irisin in regulating the health benefits of exercise is likely dependent upon its induction by exercise.
Nevertheless, contradictory findings have been emerging concerning the function of irisin, its precursor gene, and the relationship between PGC-1α and FNDC5 expression [15,16,17]. For example, Pekkala et al. [15] in 2013 (one year after the first isolation of irisin) found that the upregulation of PGC-1α mRNA expression did not correspond with the FNDC5 mRNA upregulation. Moreover, several experimental studies focused on both the acute and long-term effects of physical exercise on blood irisin levels in different contexts have been recently reviewed, still leaving us with inconclusive results [18]. Therefore, the main aim of this systematic review and meta-analysis was to investigate the long-term effect of physical exercise on blood irisin levels in order to identify a common effect, which could shed a better light on such phenomenon, especially when considering its role in health as a potential therapeutic target and further research in this area.
2. Materials and Methods
This systematic review and meta-analysis were conducted according to the recommendations and criteria outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [19].
2.1. Criteria for Considering Studies for this Review
Studies focused on the influence of physical activity on blood irisin concentration were considered in the analysis. Papers had to be written in English and published in peer-reviewed journals between 2012 and 2021.
2.2. Types of Studies
Randomized control trials (RCT), experimental or semi-experimental studies were considered for this study.
2.3. Types of Participants
All participants, including males and females, were considered for this study without regard to age or health conditions.
2.4. Types of Interventions
All physical activities such as endurance, resistance exercise, walking, dancing, etc., were considered for this study. There were no limitations concerning the duration of the intervention.
2.5. Types of Outcome Measures
The outcome measure was irisin in the bloodstream—a continual scale with physical activities as factors.
2.6. Primary Outcomes
Blood irisin level was measured using a standardized commercial Enzyme-Linked Immunosorbent Assay (ELISA) kit.
2.7. Search Methods for Identification of Studies
Appropriate papers were identified through searches using two electronic databases: PubMed, Scopus, and a metasearch engine: Web of Science. Additionally, the reference lists of eligible papers and several recently published reviews were hand-searched for further studies. The search stream used in all the databases is presented in Table 1.
Table 1.
Search results from electronic databases.
| PubMed Central | Search ((((irisin) OR FNDC5)) AND ((exercise) OR physical)) NOT (((((mice) OR rats) OR mouse) OR rodents) OR animal) | 236 |
| SCOPUS | ((TITLE-ABS-KEY (irisin) OR TITLE-ABS-KEY (FNDC5))) AND ((TITLE-ABS-KEY (exercise) OR TITLE-ABS-KEY (physical))) AND NOT ((TITLE-ABS-KEY (mice) OR TITLE-ABS-KEY (rats) OR TITLE-ABS-KEY (mouse) OR TITLE-ABS-KEY (rodents) OR TITLE-ABS-KEY (animal))) | 386 |
| WoS | TOPIC: (irisin) OR TOPIC: (FNDC5) AND TOPIC: (exercise) OR TOPIC: (physical) NOT TOPIC: (mice) OR TOPIC: (rats) OR TOPIC: (mouse) OR TOPIC: (rodents) OR TOPIC: (animal) | 527 |
2.8. Data Collection and Analysis
All potential papers were first downloaded in EndNote, and then all duplicates were deleted. After removing all the duplicates, all abstracts were explored to identify relevant papers for subsequent selections. If from the abstract the papers seemed suitable, full texts were examined in detail. Additionally, other papers were identified through the reference lists of papers and reviews gained by the database search.
2.9. Assessment of Risk of Bias in Included Studies
A modified version of the Cochrane risk of bias tool (RoB 2) for randomized [20], and risk of bias in non-randomized studies—of interventions (ROBINS-I) for non-randomized comparative studies was used to assess the methodological quality of the included studies [21].
2.10. Measures of Treatment Effect
We calculated the standardized mean difference for each study, and then the Cochran–Mantel–Haenszel statistical method based on a fixed-effect model was used to calculate an effect size [22]. We estimated the heterogeneity using the Cochran Q statistic and I2. A rough guide to the interpretation of I2 is as follows: 0 to 40% might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity, and 75% to 100% represents considerable heterogeneity [23]. Statistics were carried out using Review Manager 5.4.
2.11. Dealing with Missing Data
To calculate the standardized mean difference, in our case Hedges’ g, we needed the sample size for the experimental and control group and the above-mentioned mean differences (after—before) with SD for both groups. In case that they were not available, we calculated them using baseline and follow-up means and SD as a simple post—pre difference; we estimated SD as [24]:
The correlation coefficient—Corr was calculated using this formula:
3. Results
3.1. Description of Studies
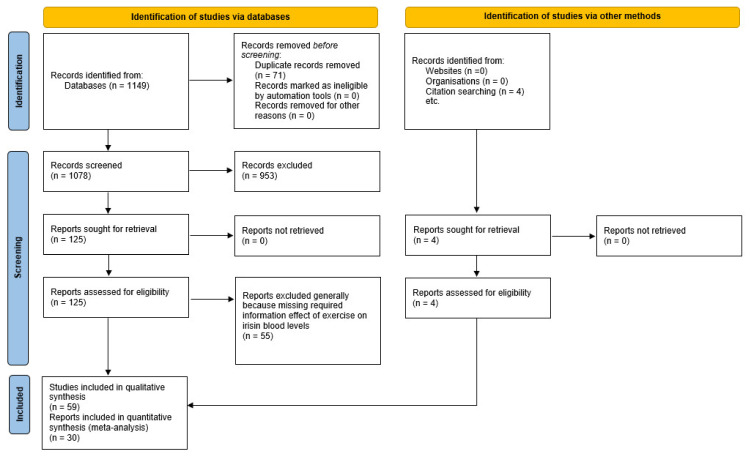
Figure 1 summarises the yield of the search process. Fifty-nine studies were included in this systematic review involving 2164 participants. Healthy participants were included in 32 studies, and three studies were focused on elite or sub-elite athletes. Patients suffering from several different diseases (interstitial lung disease, progressive multiple sclerosis, or type II diabetes) participated in nine studies. Seventeen studies were focused on obese or overweight participants and one on pregnant females. The average age ranged between 9 and 71 years. The basic description of the included studies is presented in Table 2.
Figure 1.
Flowchart illustrating the different phases of the search and study selection.
Table 2.
Studies included in this systematic review.
| Study | Year | Country | Design | Sample Description | Sample Size | Sex | Mean Age (SD) of the Whole Sample |
|---|---|---|---|---|---|---|---|
| Alyami [25] | 2020 | Saudi Arabia | NRT; N-CG | Interstitial lung disease | 10 | T | 30–40+ |
| Amanat [26] | 2020 | Iran | RCT | Obese with metabolic syndrome | 60 | F | 54.5 (6.9) |
| Bagheri [27] | 2020 | Iran | RCT | Obese | 30 | M | 43.8 (3.4) * |
| Bang [28] | 2020 | South Korea | RT; N-CG | Healthy | 16 | M | 29.4 (5.1) |
| Banitalebi [29] | 2019 | Iran | RCT | Obese with type II diabetes | 52 | F | 55.4 (5.9) * |
| Besse-Patin [30] | 2014 | France | SGS | Obese | 11 | M | 35.4 (1.5) |
| Blüher [31] | 2014 | Germany | SGS | Obese | 65 | T | 12.5 (1.6) |
| Boeselt [32] | 2017 | Germany | NRT | Chronic obstructive pulmonary disease | 37 | T | 65.7 (8.3) |
| Bonfante [33] | 2017 | Brazil | RCT | Obese | 22 | M | 49.1 (5.8) |
| Briken [34] | 2016 | Germany | RCT | Progressive multiple sclerosis | 42 | T | 50.0 (7.5) |
| Brinkmann [35] | 2020 | Germany | NRT; N-CG | Obese | 22 | T | 46–74 |
| Damirchi [36] | 2018 | Iran | RCT | Mild cognitive impairment | 20 | F | 68.8 (3.7) * |
| de la Torre-Saldaña [37] | 2019 | Mexico | SGS | Healthy | 38 | F | 23.0 (3.3) |
| Dianatinasab [38] | 2020 | Iran | RCT | Obese with metabolic syndrome | 54 | F | 53.5 (6.5) |
| Dündar [39] | 2019 | Turkey | RCT | Healthy | 34 | M | 14.5 (1.1) |
| Dünnwald [40] | 2019 | Austria | NRT; N-CG | Type II diabetes | 14 | T | 59.6 (5.7) * |
| Eaton [41] | 2017 | Canada | SGS | Healthy | 9 | M | 20.5 (1.5) |
| Ellefsen [42] | 2014 | Germany | SGS | Healthy | 18 | F | 26.0 (6.0) |
| Fernandez-del-Valle [43] | 2018 | US | RCT | Healthy | 26 | T | 21.2 (1.9) * |
| Ghanbari-Niaki [44] | 2018 | Iran | RCT | Healthy | 24 | F | 55.7 (4.9) |
| Hecksteden [45] | 2013 | Germany | RCT | Healthy | 102 | T | 49.0 (7.0) * |
| Huang [46] | 2017 | China | SGS | Obese | 22 | T | 22.1 (2.8) |
| Huh [47] | 2014 | US | SGS | Healthy | 14 | F | 24.3 (2.6) |
| Jawzal [48] | 2020 | Kurdistan | SGS | Healthy | 39 | M | 24 (22–27) |
| Kim [49] | 2016 | South Korea | RCT | Obese | 28 | T | 25.7 (4.1) * |
| Kim [50] | 2018 | South Korea | NRT | Healthy | 26 | F | 71.8 (3.1) * |
| Kim [51] | 2020 | South Korea | SGS | Healthy | 25 | F | 60.3 (5.3) |
| Korkmaz [52] | 2019 | Finland | RCT | Obese | 144 | M | 40–65 |
| Küster [53] | 2017 | Germany | NRT | Mild cognitive impairment | 46 | T | 71.2 (6.0) |
| Micielska [54] | 2019 | Poland | NRT | Healthy | 33 | F | 40.0 (11.0) * |
| Miyamoto-Mikami [55] | 2015 | Japan | RCT | Healthy | 53 | T | 21.0 (1.0)67.0 (8.0) |
| Moienneia [56] | 2016 | Iran | RCT | Healthy | 21 | F | 24.4 (3.0) |
| Moraes [57] | 2013 | Brazil | NRT; N-CG | Hemodialysis patients | 26 | T | 44.8 (14.1) |
| Motahari Rad [58] | 2020 | Iran | RCT | Type II diabetes | 51 | M | 43.9 (2.5) * |
| Murawska-Cialowicz [59] | 2015 | Poland | SGS | Healthy | 12 | T | 26.8 (6.8) * |
| Murawska-Cialowicz [60] | 2020 | Poland | RCT | Healthy | 25 | M | 32.4 (6.6) * |
| Neumayr [61] | 2020 | Austria | SGS | Healthy | 52 | T | 54.3 |
| Norheim [62] | 2013 | Norway | NRT; N-CG | Prediabetes | 26 | M | 40–65 |
| Ozan [63] | 2020 | Turkey | SGS | Elite boxers | 9 | M | 17.2 (3.3) |
| Özbay [64] | 2020 | Turkey | NRT; N-CG | Healthy | 33 | M | 22.6 (1.6) * |
| Palacios-Gonzales [65] | 2015 | Mexico | NRT; N-CG | Obese | 85 | T | 9.0 (0.9) * |
| Pekkala [15] | 2013 | Finland | NRT; N-CG | Healthy | 63 | M | 24–68 |
| Planella-Farrugia [66] | 2019 | Spain | RCT | Healthy | 43 | T | 71.2 (3.3) * |
| Prestes [67] | 2015 | Brazil | NRT | Healthy | 59 | F | 69.2 (6.1) * |
| Rashid [68] | 2020 | Iraq | NRT; N-CG | Obese | 60 | M | 20–43 |
| Rashti [69] | 2019 | Iran | RCT | Healthy | 48 | F | 57.1 (4.1) * |
| Roh [70] | 2020 | South Korea | RCT | Obese | 20 | T | 12.6 (0.5) |
| Sezgin [71] | 2020 | Turkey | NRT; N-CG | Obese | 37 | F | 47.9 (13.2) |
| Shabani [72] | 2018 | Iran | RCT | Healthy | 31 | F | 24.6 (2.5) * |
| Scharhag-Rosenberger [73] | 2014 | Germany | RCT | Healthy | 74 | T | 47.0 (7,0) |
| Śliwicka [74] | 2017 | Poland | SGS | Climbers | 8 | M | 27.0 (2.8) |
| Szumilewicz [75] | 2017 | Poland | NRT; N-CG | Pregnant | 9 | F | 23.0 (3.0) |
| Tibana [76] | 2017 | Brazil | NRT; N-CG | Obese | 49 | F | 61–68 |
| Tsuchiya [77] | 2016 | Japan | RT; N-CG | Healthy | 20 | M | 20.4 (0.8) * |
| Vieira [78] | 2020 | Brazil | RT; N-CG | Healthy | 20 | F | 64.1 (7.0) * |
| Walentukiewicz [79] | 2018 | Poland | RCT | Healthy | 94 | F | 68.0 (5.1) |
| Weber-Rajek [80] | 2019 | Poland | RCT | Obese with stress urinary incontinence | 49 | F | 62.5 (2.0) * |
| Witek [81] | 2016 | Poland | SGS | Tennis players | 12 | M | 16.0 (2.0) |
| Zhao [82] | 2017 | China | RCT | Healthy | 17 | M | 62.3 (3.5) * |
Note: * exercise group; SD = standard deviation; NRT = nonrandomised trial; N-CG = no control group; RCT = randomised controlled trial; RT = randomised trial; SGS = single group design study.
3.2. Risk of Bias and Quality of Reporting Data
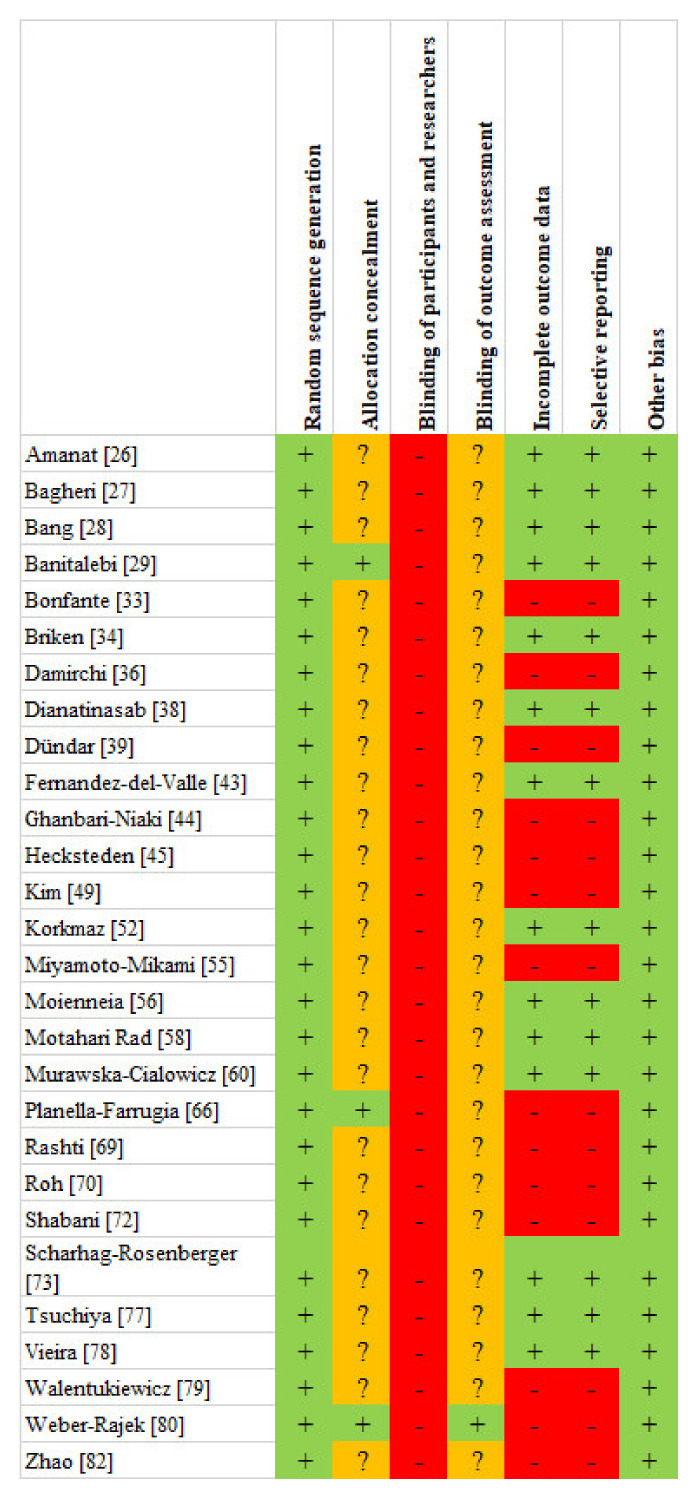
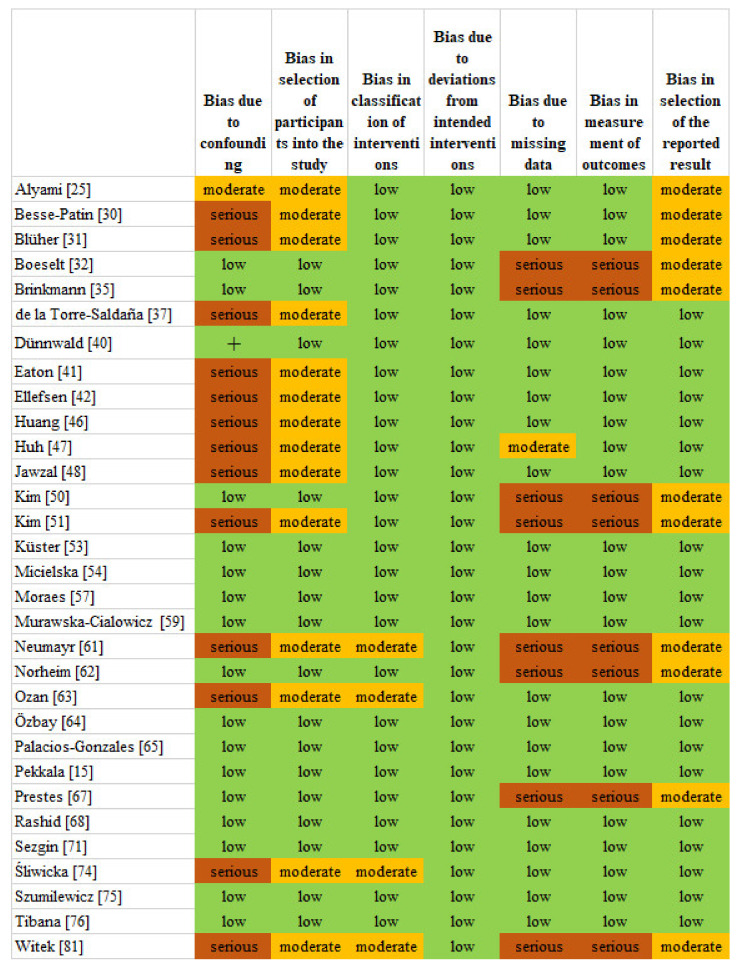
Twenty-eight studies were randomized, and out of these, three did not use any non-exercised control group. The randomized studies showed a relatively low risk of bias according to RoB 2; nevertheless, no studies were without any risk of bias. Almost all of the included studies showed a low risk of bias of “selective reporting” because they reported all the outcomes measured, and all the included studies displayed a low risk of bias in “other bias” (Figure 2). Thirty-one studies were not randomized, and out of these, 14 were a single group design study. The metrological quality of the non-randomized studies was relatively high. The risk of bias assessment of the included papers using the ROBINS-I tool for non-randomized comparative studies is presented in Figure 3.
Figure 2.
Risk of bias assessment using the RoB 2 for randomized control trials. Note: + Low risk; ? Some concerns; - High risk.
Figure 3.
Risk of bias assessment using the ROBINS-I tool for non-randomized comparative studies.
3.3. Systematic Review
Twenty-five studies were randomized control trials (RCT). The rest used different approaches, such as non-randomized trials, often without any non-exercised control group. Several different physical activities (endurance or resistance exercise, walking, swimming, etc.) were used in the studies. A 33× statistically significant within-group effect was found, where the irisin level increased 23× and decreased 10×. The statistically significant between-groups effect was found in 15 studies. A significant positive effect after exercise compared to non-exercise control groups was found in endurance training (ET) 4×, in resistance training (RT) 3×, and in combined training (CT), high-intensity interval training (HIIT), concurrent aerobic-resistance (A-R) as well as in concurrent resistance-aerobic (R-A) training 1×. A significant positive effect was also found after high-intensity interval training (HIIT) compared to continuous moderate-intensity training (CMIT). Long-term moderate physical exercise had a significant positive effect on the irisin blood level in obese compared to normal-weight adults as well as RT in older adults. On the other hand, taekwondo decreased the irisin level in obese children as well as high-repetition resistance training (HRRT) in healthy adults. A lower effect in the irisin level had RT compared to RT with ursolic acid (UA) supplementation in healthy adults. The results of these studies focused on the long-term effect are shown in Table 3.
Table 3.
Studies focused on the long-term effect of physical exercise on blood irisin levels.
| Study | Intervention Description | Length of the Intervention | Weekly Volume | Within-Group Effect Sig. | Between-Groups Effect Sig. | Note |
|---|---|---|---|---|---|---|
| Alyami [25] | Supervised exercise training (SET) | 8 weeks | 2× | - | - | |
| Amanat [26] | Endurance training (ET), resistance training (RT), and combined training (CT) | 12 weeks | 2× to 3× | ↑ * | ↑ ** | * all the EG; ** ET and CT |
| Bagheri [27] | Endurance training (ET) | 8 weeks | 3× | - | - | |
| Bang [28] | Resistance training (RT) vs. resistance training with ursolic acid supplementation (RT + UA) | 8 weeks | 6× | - | ↓ * | * RT |
| Banitalebi [29] | Sprint interval training (SIT), combined endurance and resistance training (A + R) | 10 weeks | 3× | - | - | |
| Besse-Patin [30] | Endurance training | 8 weeks | 5× | - | N/A | |
| Blüher [31] | Exercise and dietary lifestyle program | 1 year | 2× | ↑ | N/A | |
| Boeselt [32] | High-intensity training (HIT) | 12 weeks | 2× | - | - | |
| Bonfante [33] | Combined training (CT) | 24 weeks | 3× | - | ↑ | |
| Briken [34] | Endurance training (ET) | 9 weeks | 2–3× | - | - | |
| Brinkmann [35] | Combined training: males vs. females | 8 weeks | 3× | - | - | |
| Damirchi [36] | Physical training (PT) | 8 weeks | 2× | - | - | |
| de la Torre-Saldaña [37] | Treadmill—6.0–7.9 METs and >8.0 METs | 2 weeks | 5× | ↑ * | N/A | * both |
| Dianatinasab [38] | Endurance training (ET), resistance training (RT), and combined training (CT) | 8 weeks | 3× | - | - | |
| Dündar [39] | Basketball training | 8 weeks | 5× | ↓ | - | |
| Dünnwald [40] | High-intensity interval training (HIIT) vs. continuous moderate-intensity training (CMIT) | 4 weeks | 3× | ↑ * | ↑ * | * HIIT |
| Eaton [41] | High-intensity interval training (HIIT) | 20 days | 2× a day | ↑ | N/A | |
| Ellefsen [42] | Progressive strength training | 12 weeks | 3× | - | N/A | |
| Fernandez-del-Valle [43] | High-intensity interval training (HIIT) | 3 weeks | 3× | ↑ | ↑ | |
| Ghanbari-Niaki [44] | Resistance training (RT) | 9 weeks | 3× | ↑ | - | |
| Hecksteden [45] | Endurance training (ET) and strength training (ST) | 26 weeks | 3× | - | - | |
| Huang [46] | Endurance exercise | 8 weeks | 7× | ↑ | N/A | |
| Huh [47] | Whole-body vibration exercise | 6 weeks | 2× | - | N/A | |
| Jawzal [48] | Military aerobic training | 8 weeks | 7× | ↑ | N/A | |
| Kim [49] | Endurance training (ET), resistance training (RT) | 8 weeks | 5× | ↑ * | ↑ * | * RT |
| Kim [50] | Aquaerobic training (AqT) | 16 weeks | 2× | ↑ | ↑ | |
| Kim [51] | Treadmill walking | 6 weeks | 3× | ↑ | N/A | |
| Korkmaz [52] | Nordic walking (NW), resistance exercise (RE) | 12 weeks | 3× | ↑ * | ↑ * | * Both IG |
| Kuster [53] | Physical training (PT) | 10 weeks | 2× | - | - | |
| Micielska [54] | High-intensity circuit training (HICT) | 5 weeks | 4× | - | - | |
| Miyamoto-Mikami [55] | Endurance training (ET)—healthy young | 8 weeks | 3× | - | - | |
| Endurance training (ET)—middle-aged/older | 8 weeks | 3× | ↑ | ↑ | ||
| Moienneia [56] | Resistance training low (LIRT) vs. high intensity (HIRT) | 8 weeks | 3× | ↓ * | - | * HIRT |
| Moraes [57] | Intradialytic resistance training (IRT) | 6 months | 3× | ↑ | N/A | |
| Motahari Rad [58] | Concurrent aerobic-resistance (A-R) and concurrent resistance-aerobic (R-A) training | 12 weeks | 3× | ↑ * | ↑ * | * Both IG |
| Murawska-Cialowicz [59] | CrossFit training: males vs. females | 3 months | 2× | ↓ * | - | * females |
| Murawska-Cialowicz [60] | High-intensity interval training (HIIT) | 8 weeks | 2× | ↑ * | - | * HIIT |
| Neumayr [61] | Golf vs. Nordic walking or e-biking | 1 week | 7× | ↑ * | N/A | * only golf group |
| Norheim [62] | Combined endurance and strength training: normoglycaemic and normal weight | 12 weeks | 4× | - | - | |
| Ozan [63] | Strength training with thera-band | 8 weeks | 3× | - | N/A | |
| Özbay [64] | Outdoor running (OR) vs. indoor running (IR) | 18 weeks | 4× | ↓ * | - | * IR |
| Palacios-Gonzales [65] | School-based physical activity program: normal weight | 8 months | 5× | - | - | |
| Pekkala [15] | Endurance training (ET) vs. combined endurance and resistance training (ET + RT) | 21 weeks | 2× (ET) or 2× (ET) + 2× (RT) | - | - | |
| Planella-Farrugia [66] | Low-intensity resistance training (LIRT) | 16 weeks | 2× | ↑ * | - | * LIRT |
| Prestes [67] | Resistance training linear periodization (LP) and undulating periodization (UP) | 16 weeks | 2× | - | - | |
| Rashid [68] | Long-term moderate physical exercise: normal weight | 6 months | 7 times | ↑ * | ↑ | * both |
| Rashti [69] | High-intensity interval (HIIT) and moderate-intensity training (MIIT) | 10 weeks | 3× | ↑* | - | * HIIT |
| Roh [70] | Taekwondo in obese children | 16 weeks | 5× | ↓ | ↓ | |
| Sezgin [71] | Endurance training (ET) and personalized nutrition programs: normal weight | 8 weeks | 7× | - | - | |
| Shabani [72] | Resistance training (RT), Endurance training (ET), and concurrent (endurance + resistance) training (CT) | 8 weeks | 3× | ↓ * | - | * RT and CT |
| Scharhag-Rosenberger [73] | High-repetition resistance training (HRRT) | 6 months | 3× | - | ↓ | |
| Śliwicka [74] | Climb 4000 m peaks in the Mont Blanc massif | 14 days | 7× | ↓ | N/A | |
| Szumilewicz [75] | Structured group fitness program—very active (VA) vs. less active (LA) groups | 8 weeks | ≥3× (VA) <3× (LA) | ↓ * | - | * LA |
| Tibana [76] | Resistance training (RT): obese vs. normal weight | 16 weeks | 3× | ↓ * | ↑ ** | * normal weight; ** obese |
| Tsuchiya [77] | Cycle ergometer—sprint training (ST) vs. two consecutive training (TCT) | 4 weeks | 5× (ST) 2–3× (TCT) | ↓ * | - | * both |
| Vieira [78] | Resistance training very high supervision (VHS) vs. high supervision (HS) | 16 weeks | 2× | - | - | |
| Walentukiewicz [79] | Nordic walking (NW) | 12 weeks | 3× | - | - | |
| Weber-Rajek [80] | Pelvic floor muscle training (PMT) | 4 weeks | 3× | ↑ * | - | * PMT |
| Witek [81] | Workload during the competitive season | 8 months | - | - | N/A | |
| Zhao [82] | Resistance training (RT) | 12 weeks | 2× | ↑ * | ↑ | * RT |
Note: ↑ = increased levels; ↓ = decreased levels; N/A not applicable; *, ** = groups’ specification.
3.4. Meta-Analysis
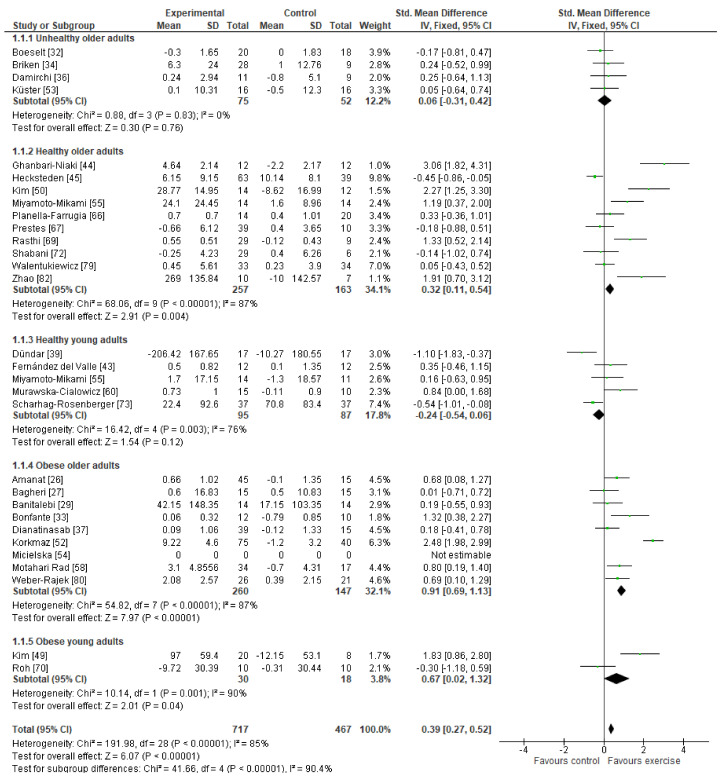
Data from 717 participants in the exercise groups and 467 participants in the non-exercise control groups were included in the overall effect of the meta-analysis. The overall effect was statistically significant, favoring the exercise group (SMD = 0.39 (95% CI 0.27–0.52)). We divided all the control studies into five groups according to the age and diseases presence for the other analyses. A statistically significant positive effect on irisin blood levels was found in healthy older adults (SMD = 0.32 (95% CI 0.11–0.54)), in the obese older adults (SMD = 0.91 (95% CI 0.69–1.13)), and in obese young adults (SMD = 0.67 (95% CI 0.02–1.32)). Nevertheless, there was no effect found in unhealthy older adults. In healthy young adults, the effect tended to be negative (SMD = −0.24 (95% CI −0.54–0.06)). Unfortunately, heterogeneity except in unhealthy older adults was considerably high in all the analyses. The forest plots are shown in Figure 4.
Figure 4.
The forest plots of effect sizes for all the groups.
4. Discussion
4.1. Summary of Main Results and Interpretations
To our knowledge, this is the first systematic review and meta-analysis that focused on the long-term effect of different exercise interventions on blood irisin levels, which included many rigorous studies. The results of this present study indicate that: (a) physical exercise might increase irisin blood levels in specific populations, including healthy and obese older adults as well as obese young adults; however, (b) the results of individual studies exhibit high heterogeneity. More specifically, from the 59 articles included in the final qualitative and quantitative analyses, there was a statistically significant within-group effect of exercise on irisin blood levels in 33 studies (the irisin level increased in 23 and decreased in 10), and the significant positive between-groups effect was found 11 times. These contradictions in the research results of different studies are discussed below.
Considering the results of the studies analyzed in this systematic review and meta-analysis, it seems that long-term physical exercise increases irisin blood levels, especially in obese individuals. More specifically, our study shows that endurance training and combined training increase irisin blood levels in obese older females [26], combined training in obese middle-aged males [33], endurance [46,68] and resistance exercise [49] in young obese adult males, and resistance exercise training in obese older males [52,76]. Moreover, exercise and dietary lifestyle programs demonstrated increases in irisin blood levels in obese children [31], and pelvic floor muscle training increased irisin in obese older females with stress urinary incontinence [80]. As mentioned in the introduction, irisin is secreted from muscles in response to exercise and is believed to positively affect many physiological processes in the human body, such as inhibition of adipogenesis in the adipose tissue [83]. Therefore, for the obese population, irisin could be seen as a possible therapeutic target that positively affects resting energy, glucose tolerance, and insulin sensitivity [84]. In fact, high-intensity interval training increased irisin blood levels in older adults with type II diabetes [40], and concurrent aerobic-resistance training in older adults males with type II diabetes [58]. However, some data also show negative correlations between elevated irisin and adiposity [3]; therefore, for now, the role of irisin in obese and diabetic patients remains unclear. Further investigations are needed to elucidate the complexity of irisin interactions with these metabolic endpoints before considering irisin as a therapeutic target in patients with obesity or diabetes mellitus.
Several kinds of physical exercise increased irisin blood levels among healthy people as well. High-intensity interval training increased irisin in young, healthy males [41,60] and healthy young adults [43], military aerobic training [48] increased irisin blood levels in young, healthy males and one study with a single group design found a significant increase in irisin in young females after treadmill exercise [37]. On the other hand, cycle ergometer—sprint training, consecutive training [77] as well as indoor running [64] led to a decrease of irisin blood levels in young, healthy males, and the same effect was found after basketball training in healthy children [39] or after taekwondo training in obese children [70]. A decrease in irisin blood levels was also found after high-intensity interval training [56], CrossFit training [59], resistance training, and concurrent training [72] in healthy young females as well as after a structured group fitness program in pregnant females [75]. Climbing 4000 m peaks in the Mont Blanc massif led to a decrease of irisin blood levels as well [74]. Currently, no studies are reporting on the beneficial or possible negative effects of increased or decreased irisin levels in the young, healthy population, except for that in athletes, where the irisin level was positively correlated with bone strength [3]. However, we also postulate that irisin may exhibit prophylactic effects against metabolic disorders such as obesity or type II diabetes in this population.
More importantly, exercise including resistance training [44], aquaerobic training [50], endurance training [55], golf [61], low-intensity resistance training [66], high-intensity interval training [69], and treadmill walking [51] increased irisin blood levels in healthy older females and resistance training [82] in healthy older males, which may, in fact, protect against bone loss and muscle atrophy [6]. In this case, irisin might provide a therapeutic choice for treating diseases caused by inactivity (which often is the case for older adults), including osteoporosis and sarcopenia, or it may be used as a useful biomarker for the assessment of bone and muscle health as suggested by Leustean et al. [85]. Especially, the older adult female population would benefit from this protective effect as they are prone to osteoporosis. Cosio et al. [86] and Morteza et al. [87] also showed greater increases of circulating irisin in older adults after resistance training programs. It would also be worth investigating the effect of neuromuscular electrical stimulation (NMES; a passive type of exercise) protocols, such as in the study by Jandova et al. [88], on circulating irisin, which could offer an alternative mode of exercising for people with difficulties maintaining a physically active life. In terms of aerobic training, Morteza et al. [87] reported no significant changes for circulating irisin, which is contrary to our findings. Instead, we report on decreases in circulating irisin in blood following resistance training in normal weighted older males [76] and after high-repetition resistance training in healthy older adults [73]. In any case, the overall effect of the meta-analysis was statistically significant, favouring the exercise group (SMD = 0.39 (95% CI 0.27–0.52)), which indicates that the long-term effect of exercise on blood irisin levels is more positive regardless of the type of training. In that case, qualified physical exercise professionals could use different training strategies based on the needs and preferences of individuals.
Two main caveats must be considered when interpreting the findings of this review and meta-analysis—the higher overall heterogeneity and the methodological aspects of measuring irisin in blood, which may be the actual culprit of the high heterogeneity. Currently, all evidence for irisin in the blood in this review is based on commercial ELISA kits. These kits are based on polyclonal antibodies (pAbs), which have been recently found to have prominent cross-reactivity with non-specific proteins in human and animal sera [89]. Such findings question all previous data obtained with these kits, and until these methodological aspects are resolved, studies relying on these measures should be carefully scrutinized. In summary, this review and meta-analysis may have some evidentiary support for the positive long-term effects of exercise on blood irisin levels, but more importantly, it points to the important methodological issues related to the actual detection of irisin [90], which should be considered when conducting research in this area [85]. In summary, our results confirm the general notion that exercise increases irisin levels in obese and older people. Additionally, irisin may possess protective properties against obesity and possibly also against osteoporosis or sarcopenia, which needs to be investigated further by future studies.
4.2. Quality of the Evidence
The studies included in this review and meta-analysis were comprised of both randomized and non-randomized trials (28 vs. 31, respectively) and were assessed as low risk for bias and quality of reporting data. All included studies assessed effects on the completers only, which may result in an overstatement of the effects.
4.3. Potential Biases in the Review Process
This systematic review and meta-analysis are limited to published research, and no unpublished studies were included in this review. Therefore, our review may be biased due to the possible threat of publication and reporting bias.
4.4. Agreements and Disagreements with Other Studies or Reviews
The current findings are generally consistent with prior reviews [18,86,91,92,93] in that the effects of physical exercise on blood irisin levels vary. Although prior reviews describe the positive effects of both acute and chronic (long-term) effects of physical exercise, they also point to mixed results and the overall lack of evidence [18]. This work, however, represents a more rigorous extension of existing work as a meta-analysis was carried out, demonstrating that the overall effect of exercise on irisin blood levels was statistically significant.
5. Conclusions
In summary, the present systematic review and meta-analysis indicate that long-term physical exercise might increase irisin blood levels in the populations of healthy, obese older adults, and young obese adults. On the other hand, in the population of ill older adults, the results of this study indicate no effect and even a decrease in circulating irisin for healthy young people. These contradictory results and large heterogeneity found in this study, along with many other contradictions brought by previous research in this area, including the methods of irisin detection, call for further investigations. In addition, although many studies in this review and meta-analysis demonstrated a low risk of bias, future research in this area would benefit from more sophisticated and rigorous designs. Larger sample sizes would permit analyses that can account for the heterogeneity, which is an essential factor. Nevertheless, the main focus should be placed on improving current analytical techniques to measure blood irisin levels, which may be the main caveat in this research area before considering irisin as a therapeutic target.
Author Contributions
Conceptualization, T.J., J.C.-I. and M.S.; methodology, T.V. and M.S.; formal analysis, T.V.; investigation, A.B.-R., M.R., H.P., V.H. and J.C.-I.; writing—original draft preparation, T.J., H.P., V.H. and M.S.; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This review was partly funded by grants from Charles University—PRIMUS/19/HUM/012, and SVV 260599, and the Grant Agency of the Charles University grant number 268321. The funding agency played no role in study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available in a publicly accessible repository that does not issue DOIs; Publicly available datasets were analyzed in this study. This data can be found here: https://uloz.to/tamhle/8qgosLnZa8BM/name/Nahrano-25-10-2021-v-12-01-24#!ZGVjBGR2A2V0ZQExLmtjLwOxAGNkA01PBTSFFaO0n2qjpwEwAN== (accessed on 22 June 2021).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Boström P., Wu J., Jedrychowski M.P., Korde A., Ye L., Lo J.C., Rasbach K.A., Boström E.A., Choi J.H., Long J.Z., et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perakakis N., Triantafyllou G.A., Fernández-Real J.M., Huh J.Y., Park K.H., Seufert J., Mantzoros C.S. Physiology and role of irisin in glucose homeostasis. Nat. Rev. Endocrinol. 2017;13:324–337. doi: 10.1038/nrendo.2016.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grygiel-Gorniak B., Puszczewicz M. A review on irisin, a new protagonist that mediates muscle-adipose-bone-neuron con-nectivity. Eur. Rev. Med. Pharmacol. Sci. 2017;21:4687–4693. [PubMed] [Google Scholar]
- 4.Ma C., Ding H., Deng Y., Liu H., Xiong X., Yang Y. Irisin: A New Code Uncover the Relationship of Skeletal Muscle and Cardiovascular Health During Exercise. Front. Physiol. 2021;12:620608. doi: 10.3389/fphys.2021.620608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siteneski A., Cunha M.P., Lieberknecht V., Pazini F.L., Gruhn K., Brocardo P.S., Rodrigues A.L.S. Central irisin admin-istration affords antidepressant-like effect and modulates neuroplasticity-related genes in the hippocampus and prefrontal cortex of mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2018;84:294–303. doi: 10.1016/j.pnpbp.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Mahgoub M., D’souza C., Darmaki R.S.M.H.A., Baniyas M.M., Adeghate E.A. An update on the role of irisin in the regu-lation of endocrine and metabolic functions. Peptides. 2018;104:15–23. doi: 10.1016/j.peptides.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Wrann C.D., White J.P., Salogiannnis J., Laznik-Bogoslavski D., Wu J., Ma D., Lin J.D., Greenberg M.E., Spiegelman B.M. Exercise Induces Hippocampal BDNF through a PGC-1α/FNDC5 Pathway. Cell Metab. 2013;18:649–659. doi: 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Oliveira M., de Sibio M.T., Mathias L.S., Rodrigues B.M., Sakalem M.E., Nogueira C.R. Irisin modulates genes associated with severe coronavirus disease (COVID-19) outcome in human subcutaneous adipocytes cell culture. Mol. Cell. Endocrinol. 2020;515:110917. doi: 10.1016/j.mce.2020.110917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricci N.A., Cunha A.I.L. Physical Exercise for Frailty and Cardiovascular Diseases. Adv. Exp. Med. Biol. 2020;1216:115–129. doi: 10.1007/978-3-030-33330-0_12. [DOI] [PubMed] [Google Scholar]
- 10.Whillier S. Exercise and Insulin Resistance. Adv. Exp. Med. Biol. 2020;1228:137–150. doi: 10.1007/978-981-15-1792-1_9. [DOI] [PubMed] [Google Scholar]
- 11.Balducci S., Sacchetti M., Haxhi J., Orlando G., D’Errico V., Fallucca S., Menini S., Pugliese G. Physical exercise as therapy for type 2 diabetes mellitus. Diabetes/Metab. Res. Rev. 2014;30:13–23. doi: 10.1002/dmrr.2514. [DOI] [PubMed] [Google Scholar]
- 12.Ströhle A. Physical activity, exercise, depression and anxiety disorders. J. Neural Transm. 2009;116:777–784. doi: 10.1007/s00702-008-0092-x. [DOI] [PubMed] [Google Scholar]
- 13.Montero-Fernández N., Serra-Rexach J.A. Role of exercise on sarcopenia in the elderly. Eur. J. Phys. Rehabil. Med. 2013;49:131–143. [PubMed] [Google Scholar]
- 14.De la Rosa A., Olaso-Gonzalez G., Arc-Chagnaud C., Millan F., Salvador-Pascual A., Garcia-Lucerga C., Blasco-Lafarga C., Garcia-Dominguez E., Carretero A., Correas A.G., et al. Physical exercise in the prevention and treatment of Alzheimer’s disease. J. Sport. Health. Sci. 2020;9:394–404. doi: 10.1016/j.jshs.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pekkala S., Wiklund P.K., Hulmi J.J., Ahtiainen J.P., Horttanainen M., Pöllänen E., Mäkelä K.A., Kainulainen H., Häkkinen K., Nyman K., et al. Are skeletal muscle FNDC5 gene expression and irisin release regulated by exercise and related to health? J. Physiol. 2013;591:5393–5400. doi: 10.1113/jphysiol.2013.263707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Timmons J.A., Baar K., Davidsen P.K., Atherton P.J. Is irisin a human exercise gene? Nat. Cell Biol. 2012;488:E9–E10. doi: 10.1038/nature11364. [DOI] [PubMed] [Google Scholar]
- 17.Huh J.Y., Panagiotou G., Mougios V., Brinkoetter M., Vamvini M.T., Schneider B.E., Mantzoros C.S. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentra-tions in response to weight loss and exercise. Metab.Clin.Exp. 2012;61:1725–1738. doi: 10.1016/j.metabol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dinas P.C., Lahart I.M., Timmons J.A., Svensson P.A., Koutedakis Y., Flouris A.D., Metsios G.S. Effects of physical activity on the link between PGC-1a and FNDC5 in muscle, circulating Ιrisin and UCP1 of white adipocytes in humans: A systematic review. F1000Research. 2017;6:286. doi: 10.12688/f1000research.11107.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterne J.A.C., Savovic J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.Y., Corbett M.S., Eldridge S.M., et al. RoB 2: A revised tool for assessing risk of bias in randomized trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 21.Sterne J.A., Hernan M.A., Reeves B.C., Savovic J., Berkman N.D., Viswanathan M., Henry D., Altman D.G., Ansari M.T., Boutron I., et al. ROBINS-I: A tool for assessing risk of bias in non-randomized studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mantel N., Haenszel W. Statistical Aspects of the Analysis of Data From Retrospective Studies of Disease. J. Natl. Cancer Inst. 1959;22:719–748. doi: 10.1093/jnci/22.4.719. [DOI] [PubMed] [Google Scholar]
- 23.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. Br. Med. J. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins J.P.T., Li T., Deeks J.J. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins J., Thomas J., editors. Cochrane Handbook for Systematic Reviews of Interventions Version 6.1. Cochrane; Cochrane, AB, Canada: 2021. [(accessed on 22 June 2021)]. Available online: www.training.cochrane.org/handbook. [Google Scholar]
- 25.Alyami R.M., Alhwaikan A.M., Alharbi A.R., Al-Nafisah G. Impact of supervised exercise training on pulmonary function parameters, exercise capacity and Irisin Biomarker in Interstitial lung disease patients. Pak. J. Med. Sci. 2020;36:1089–1095. doi: 10.12669/pjms.36.5.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amanat S., Sinaei E., Panji M., MohammadporHodki R., Bagheri-Hosseinabadi Z., Asadimehr H., Fararouei M., Dianatinasab A. A Randomized Controlled Trial on the Effects of 12 Weeks of Aerobic, Resistance, and Combined Exercises Training on the Serum Levels of Nesfatin-1, Irisin-1 and HOMA-IR. Front. Physiol. 2020;11:562895. doi: 10.3389/fphys.2020.562895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bagheri R., Rashidlamir A., Ashtary-Larky D., Wong A., Grubbs B., Motevalli M.S., Baker J.S., Laher I., Zouhal H. Effects of green tea extract supplementation and endurance training on irisin, pro-inflammatory cytokines, and adiponectin concentrations in overweight middle-aged men. Eur. J. Appl. Physiol. 2020;120:915–923. doi: 10.1007/s00421-020-04332-6. [DOI] [PubMed] [Google Scholar]
- 28.Bang H.S., Seo D.Y., Chung Y.M., Oh K.-M., Park J.J., Arturo F., Jeong S.-H., Kim N., Han J. Ursolic Acid-Induced Elevation of Serum Irisin Augments Muscle Strength During Resistance Training in Men. Korean J. Physiol. Pharmacol. 2014;18:441–446. doi: 10.4196/kjpp.2014.18.5.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banitalebi E., Kazemi A., Faramarzi M., Nasiri S., Haghighi M.M. Effects of sprint interval or combined aerobic and re-sistance training on myokines in overweight women with type 2 diabetes: A randomized controlled trial. Life Sci. 2019;217:101–109. doi: 10.1016/j.lfs.2018.11.062. [DOI] [PubMed] [Google Scholar]
- 30.Besse-Patin A., Montastier E., Vinel C., Castan-Laurell I., Louche K., Dray C., Daviaud D., Mir L., Marques M.-A., Thalamas C., et al. Effect of endurance training on skeletal muscle myokine expression in obese men: Identification of apelin as a novel myokine. Int. J. Obes. 2014;38:707–713. doi: 10.1038/ijo.2013.158. [DOI] [PubMed] [Google Scholar]
- 31.Blüher S., Panagiotou G., Petroff D., Markert J., Wagner A., Klemm T., Filippaios A., Keller A., Mantzoros C.S. Effects of a 1-year exercise and lifestyle intervention on irisin, adipokines, and inflammatory markers in obese children. Obesity. 2014;22:1701–1708. doi: 10.1002/oby.20739. [DOI] [PubMed] [Google Scholar]
- 32.Boeselt T., Nell C., Lütteken L., Kehr K., Koepke J., Apelt S., Veith M., Beutel B., Spielmanns M., Greulich T., et al. Benefits of High-Intensity Exercise Training to Patients with Chronic Obstructive Pulmonary Disease: A Controlled Study. Respiration. 2017;93:301–310. doi: 10.1159/000464139. [DOI] [PubMed] [Google Scholar]
- 33.Bonfante I.L.P., Chacon-Mikahil M.P.T., Brunelli D.T., Gáspari A.F., Duft R.G., Lopes W.A., Bonganha V., Libardi C.A., Cavaglieri C.R. Combined training, FNDC5/irisin levels and metabolic markers in obese men: A randomised controlled trial. Eur. J. Sport Sci. 2017;17:629–637. doi: 10.1080/17461391.2017.1296025. [DOI] [PubMed] [Google Scholar]
- 34.Briken S., Rosenkranz S.C., Keminer O., Patra S., Ketels G., Heesen C., Hellweg R., Pless O., Schulz K.-H., Gold S.M. Effects of exercise on Irisin, BDNF and IL-6 serum levels in patients with progressive multiple sclerosis. J. Neuroimmunol. 2016;299:53–58. doi: 10.1016/j.jneuroim.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Brinkmann C., Weh-Gray O., Bloch W., Brixius K., Predel H.-G., Kreutz T. Effects of a Combined Endurance/Strength Training Program on Circulating Irisin Levels in Overweight/Obese Men and Women with Type 2 Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes. 2020 doi: 10.1055/a-1284-5428. [DOI] [PubMed] [Google Scholar]
- 36.Damirchi A., Hosseini F., Babaei P. Mental Training Enhances Cognitive Function and BDNF More Than Either Physical or Combined Training in Elderly Women With MCI: A Small-Scale Study. Am. J. Alzheimer’s Dis. Other Dementiasr. 2017;33:20–29. doi: 10.1177/1533317517727068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De la Torre-Saldaña V.A., Gómez-Sámano M.Á., Gómez-Pérez F.J., Rosas-Saucedo J., León-Suárez A., Grajales-Gómez M., Oseguera-Moguel J., Beyhart A.V., Cuevas-Ramos D. Fasting Insulin and Alanine Amino Transferase, but not FGF21, Were Independent Parameters Related with Irisin Increment after Intensive Aerobic Exercising. Rev. Investig. Clin. 2019;71:133–140. doi: 10.24875/RIC.18002764. [DOI] [PubMed] [Google Scholar]
- 38.Dianatinasab A., Koroni R., Bahramian M., Bagheri-Hosseinabadi Z., Vaismoradi M., Fararouei M., Amanat S. The effects of aerobic, resistance, and combined exercises on the plasma irisin levels, HOMA-IR, and lipid profiles in women with metabolic syndrome: A randomized controlled trial. J. Exerc. Sci. Fit. 2020;18:168–176. doi: 10.1016/j.jesf.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dundar A., Kocahan S., Sahin L. Associations of apelin, leptin, irisin, ghrelin, insulin, glucose levels, and lipid parameters with physical activity during eight weeks of regular exercise training. Arch. Physiol. Biochem. 2019;127:291–295. doi: 10.1080/13813455.2019.1635622. [DOI] [PubMed] [Google Scholar]
- 40.Dunnwald T., Melmer A., Gatterer H., Salzmann K., Ebenbichler C., Burtscher M., Schobersberger W., Grander W. Su-pervised Short-term High-intensity Training on Plasma Irisin Concentrations in Type 2 Diabetic Patients. J. Sport. Health. Sci. 2019;40:158–164. doi: 10.1055/a-0828-8047. [DOI] [PubMed] [Google Scholar]
- 41.Eaton M., Granata C., Barry J., Safdar A., Bishop D., Little J.P. Impact of a single bout of high-intensity interval exercise and short-term interval training on interleukin-6, FNDC5, and METRNL mRNA expression in human skeletal muscle. J. Sport Health Sci. 2018;7:191–196. doi: 10.1016/j.jshs.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ellefsen S., Vikmoen O., Slettaløkken G., Whist J.E., Nygaard H., Hollan I., Rauk I., Vegge G., Strand T.A., Raastad T., et al. Irisin and FNDC5: Effects of 12-week strength training, and relations to muscle phenotype and body mass composition in untrained women. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014;114:1875–1888. doi: 10.1007/s00421-014-2922-x. [DOI] [PubMed] [Google Scholar]
- 43.Fernandez-Del-Valle M., Short M.J., Chung E., McComb J., Kloiber S., Naclerio F., Larumbe-Zabala E. Effects of High-Intensity Resistance Training on Circulating Levels of Irisin in Healthy Adults: A Randomized Controlled Trial. Asian J. Sports Med. 2018;9:e13025. doi: 10.5812/asjsm.13025. [DOI] [Google Scholar]
- 44.Ghanbari-Niaki A., Saeidi A., Ahmadian M., Gharahcholo L., Naghavi N., Fazelzadeh M., Mahjoub S., Myers S., Williams A. The combination of exercise training and Zataria multiflora supplementation increase serum irisin levels in postmenopausal women. Integr. Med. Res. 2018;7:44–52. doi: 10.1016/j.imr.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hecksteden A., Wegmann M., Steffen A., Kraushaar J., Morsch A., Ruppenthal S., Kaestner L., Meyer T. Irisin and exercise training in humans—Results from a randomized controlled training trial. BMC Med. 2013;11:235. doi: 10.1186/1741-7015-11-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang J., Wang S., Xu F., Wang D., Yin H., Lai Q., Liao J., Hou X., Hu M. Exercise training with dietary restriction enhances circulating irisin level associated with increasing endothelial progenitor cell number in obese adults: An intervention study. PeerJ. 2017;5:e3669. doi: 10.7717/peerj.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huh J.Y., Mougios V., Skraparlis A., Kabasakalis A., Mantzoros C.S. Irisin in response to acute and chronic whole-body vibration exercise in humans. Metabolism. 2014;63:918–921. doi: 10.1016/j.metabol.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Jawzal K.H., Alkass S.Y., Hassan A.B., Abdulah D.M. The effectiveness of military physical exercise on irisin concentrations and oxidative stress among male healthy volunteers. Horm. Mol. Biol. Clin. Investig. 2020;41:20200007. doi: 10.1515/hmbci-2020-0007. [DOI] [PubMed] [Google Scholar]
- 49.Kim H.-J., Lee H.-J., So B., Son J.S., Yoon D., Song W. Effect of Aerobic Training and Resistance Training on Circulating Irisin Level and Their Association with Change of Body Composition in Overweight/Obese Adults: A Pilot Study. Physiol. Res. 2016;65:271–279. doi: 10.33549/physiolres.932997. [DOI] [PubMed] [Google Scholar]
- 50.Kim J.-H., Kim D.-Y. Aquarobic exercises improve the serum blood irisin and brain-derived neurotrophic factor levels in elderly women. Exp. Gerontol. 2018;104:60–65. doi: 10.1016/j.exger.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 51.Kim S.-J., Yoon E.-S., Jung S.-Y., Kim D.-Y. Effect of uphill walking on browning factor and high molecular weight-adiponectin in postmenopausal women. J. Exerc. Rehab. 2020;16:265–271. doi: 10.12965/jer.2040334.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Korkmaz A., Venojärvi M., Wasenius N., Manderoos S., DeRuisseau K.C., Gidlund E.-K., Heinonen O.J., Lindholm H., Aunola S., Eriksson J.G., et al. Plasma irisin is increased following 12 weeks of Nordic walking and associates with glucose homoeostasis in overweight/obese men with impaired glucose regulation. Eur. J. Sport Sci. 2019;19:258–266. doi: 10.1080/17461391.2018.1506504. [DOI] [PubMed] [Google Scholar]
- 53.Küster O.C., Laptinskaya D., Fissler P., Schnack C., Zügel M., Nold V., Thurm F., Pleiner S., Karabatsiakis A., von Einem B., et al. Novel Blood-Based Biomarkers of Cognition, Stress, and Physical or Cognitive Training in Older Adults at Risk of Dementia: Preliminary Evidence for a Role of BDNF, Irisin, and the Kynurenine Pathway. J. Alzheimers Dis. 2017;59:1097–1111. doi: 10.3233/JAD-170447. [DOI] [PubMed] [Google Scholar]
- 54.Micielska K., Gmiat A., Zychowska M., Kozlowska M., Walentukiewicz A., Lysak-Radomska A., Jaworska J., Rodziewicz E., Duda-Biernacka B., Ziemann E. The beneficial effects of 15 units of high-intensity circuit training in women is modified by age, baseline insulin resistance and physical capacity. Diabetes Res. Clin. Pr. 2019;152:156–165. doi: 10.1016/j.diabres.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 55.Miyamoto-Mikami E., Sato K., Kurihara T., Hasegawa N., Fujie S., Fujita S., Sanada K., Hamaoka T., Tabata I., Iemitsu M. Endurance training-induced increase in circulating irisin levels is associated with reduction of abdominal visceral fat in mid-dle-aged and older adults. PLoS ONE. 2015;10:e0120354. doi: 10.1371/journal.pone.0120354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moienneia N., Hosseini S.R.A. Acute and chronic responses of metabolic myokine to different intensities of exercise in sedentary young women. Obes. Med. 2016;1:15–20. doi: 10.1016/j.obmed.2015.12.002. [DOI] [Google Scholar]
- 57.Moraes C., Leal V.O., Marinho S.M., Barroso S.G., Rocha G.S., Boaventura G.T., Mafra D. Resistance Exercise Training does not Affect Plasma Irisin Levels of Hemodialysis Patients. Horm. Metab. Res. 2013;45:900–904. doi: 10.1055/s-0033-1354402. [DOI] [PubMed] [Google Scholar]
- 58.Rad M.M., Bijeh N., Hosseini S.R.A., Saeb A.R. The effect of two concurrent exercise modalities on serum concentrations of FGF21, irisin, follistatin, and myostatin in men with type 2 diabetes mellitus. Arch. Physiol. Biochem. 2020:1–10. doi: 10.1080/13813455.2020.1829649. [DOI] [PubMed] [Google Scholar]
- 59.Murawska-Cialowicz E., Wojna J., Zuwala-Jagiello J. Crossfit training changes brain-derived neurotrophic factor and irisin levels at rest, after wingate and progressive tests, and improves aerobic capacity and body composition of young physically active men and women. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2015;66:811–821. [PubMed] [Google Scholar]
- 60.Murawska-Cialowicz E., Wolanski P., Zuwala-Jagiello J., Feito Y., Petr M., Kokstejn J., Stastny P., Goliński D. Effect of HIIT with Tabata Protocol on Serum Irisin, Physical Performance, and Body Composition in Men. Int. J. Environ. Res. Public Health. 2020;17:3589. doi: 10.3390/ijerph17103589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neumayr G., Engler C., Lunger L., Lechleitner P. Effects of a one-week vacation with various activity programs on metab-olism and adipokines. Int. J. Sports. Med. 2021;42:703–707. doi: 10.1055/a-1297-4669. [DOI] [PubMed] [Google Scholar]
- 62.Norheim F., Langleite T.M., Hjorth M., Holen T., Kielland A., Stadheim H.K., Gulseth H.L., Birkeland K.I., Jensen J., Drevon C.A. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014;281:739–749. doi: 10.1111/febs.12619. [DOI] [PubMed] [Google Scholar]
- 63.Ozan M., Karakurt S., Aggon E., Agirbas O., Ucan E., Alp H.H. The effects of strength exercises on body composition and irisin. J. Appl. Biomech. 2020;9:178–186. [Google Scholar]
- 64.Ozbay S., Ulupınar S., Şebin E., Altınkaynak K. Acute and chronic effects of aerobic exercise on serum irisin, adropin, and cholesterol levels in the winter season: Indoor training versus outdoor training. Chin. J. Physiol. 2020;63:21–26. doi: 10.4103/CJP.CJP_84_19. [DOI] [PubMed] [Google Scholar]
- 65.Palacios-González B., Vadillo-Ortega F., Polo-Oteyza E., Sánchez T., Ancira-Moreno M., Romero-Hidalgo S., Meráz N., Antuna-Puente B. Irisin levels before and after physical activity among school-age children with different BMI: A direct relation with leptin. Obesity. 2015;23:729–732. doi: 10.1002/oby.21029. [DOI] [PubMed] [Google Scholar]
- 66.Planella-Farrugia C., Comas F., Sabater-Masdeu M., Moreno M., Moreno-Navarrete J.M., Rovira O., Ricart W., Fernández-Real J.M. Circulating Irisin and Myostatin as Markers of Muscle Strength and Physical Condition in Elderly Subjects. Front. Physiol. 2019;10:871. doi: 10.3389/fphys.2019.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prestes J., da Cunha Nascimento D., Tibana R.A., Teixeira T.G., Vieira D.C.L., Tajra V., de Farias D.L., Silva A.O., Funghetto S.S., de Souza V.C., et al. Understanding the individual responsiveness to resistance training periodization. Age. 2015;37:9793. doi: 10.1007/s11357-015-9793-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rashid F.A., Abbas H.J., Naser N.A., Ali H.A. Effect of Long-Term Moderate Physical Exercise on Irisin between Normal Weight and Obese Men. Sci. World J. 2020;2020:1–7. doi: 10.1155/2020/1897027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rashti B.A., Mehrabani J., Damirchi A., Babaei P. The influence of concurrent training intensity on serum irisin and ab-dominal fat in postmenopausal women. Prz. Menopauzalny. 2019;18:166–173. doi: 10.5114/pm.2019.90810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roh H.-T., Cho S.-Y., So W.-Y. Effects of Regular Taekwondo Intervention on Oxidative Stress Biomarkers and Myokines in Overweight and Obese Adolescents. Int. J. Environ. Res. Public Health. 2020;17:2505. doi: 10.3390/ijerph17072505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sezgin G., Kar F., Uslu S. The effect of nutrition and exercise training on irisin and semaphorin-3E levels in obese patients. Arch. Physiol. Biochem. 2020:1–10. doi: 10.1080/13813455.2020.1779310. [DOI] [PubMed] [Google Scholar]
- 72.Shabani R., Izaddoust F. Effects of aerobic training, resistance training, or both on circulating irisin and myostatin in untrained women. Acta Gymnica. 2018;48:47–55. doi: 10.5507/ag.2018.007. [DOI] [Google Scholar]
- 73.Scharhag-Rosenberger F., Meyer T., Wegmann M., Ruppenthal S., Kaestner L., Morsch A., Hecksteden A. Irisin Does Not Mediate Resistance Training–Induced Alterations in Resting Metabolic Rate. Med. Sci. Sports Exerc. 2014;46:1736–1743. doi: 10.1249/MSS.0000000000000286. [DOI] [PubMed] [Google Scholar]
- 74.Śliwicka E., Cisoń T., Kasprzak Z., Nowak A., Pilaczyńska-Szcześniak Ł. Serum irisin and myostatin levels after 2 weeks of high-altitude climbing. PLoS ONE. 2017;12:e0181259. doi: 10.1371/journal.pone.0181259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Szumilewicz A., Worska A., Piernicka M., Kuchta A., Kortas J., Jastrzȩbski Z., Radzimiński Ł., Jaworska J., Micielska K., Ziemann E. The exercise-induced irisin is associated with improved levels of glucose homeostasis markers in pregnant women participating in 8-week prenatal group fitness program: A pilot study. Biomed. Res. Int. 2017;2017:9414525. doi: 10.1155/2017/9414525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tibana R.A., Nascimento D.D.C., de Souza N.M.F., de Souza V.C., Neto I.V.D.S., Voltarelli F.A., Pereira G.B., Navalta J.W., Prestes J. Irisin levels are not associated to resistance training-induced alterations in body mass composition in older untrained women with and without obesity. J. Nutr. Health Aging. 2017;21:241–246. doi: 10.1007/s12603-016-0748-4. [DOI] [PubMed] [Google Scholar]
- 77.Tsuchiya Y., Ijichi T., Goto K. Effect of sprint training on resting serum irisin concentration—Sprint training once daily vs. twice every other day. Metabolism. 2016;65:492–495. doi: 10.1016/j.metabol.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 78.Vieira D.C.L., Nascimento D.C., Tajra V., Teixeira T.G., Farias D.L., Tibana R.A., Silva A.O., Rosa T.S., De Moraes M.R., Voltarelli F.A., et al. High supervised resistance training in elderly women: The role of supervision ratio. Int. J. Exerc. Sci. 2020;13:597–606. doi: 10.70252/WNVF6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Walentukiewicz A., Lysak-Radomska A., Jaworska J., Prusik K., Prusik K., Kortas J.A., Lipiński M., Babinska A., Antosiewicz J., Ziemann E. Vitamin D Supplementation and Nordic Walking Training Decreases Serum Homocysteine and Ferritin in Elderly Women. Int. J. Environ. Res. Public Health. 2018;15:2064. doi: 10.3390/ijerph15102064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weber-Rajek M., Radzimińska A., Strączyńska A., Strojek K., Piekorz Z., Kozakiewicz M., Styczyńska H. A random-ized-controlled trial pilot study examining the effect of pelvic floor muscle training on the irisin concentration in overweight or obese elderly women with stress urinary incontinence. Biomed. Res. Int. 2019;2019:7356187. doi: 10.1155/2019/7356187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Witek K., Zurek P., Zmijewski P., Jaworska J., Lipinska P., Dzedzej-Gmiat A., Antosiewicz J., Ziemann E. Myokines in Response to a Tournament Season among Young Tennis Players. BioMed Res. Int. 2016;2016:1–7. doi: 10.1155/2016/1460892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao J., Su Z., Qu C., Dong Y. Effects of 12 Weeks Resistance Training on Serum Irisin in Older Male Adults. Front. Physiol. 2017;8:171. doi: 10.3389/fphys.2017.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ohtaki H. Chapter 37—Irisin. In: Takei Y., Ando H., Tsutsui K., editors. Handbook of Hormones. Academic Press; San Diego, CA, USA: 2016. pp. 329–330. [Google Scholar]
- 84.Gouveia M.C., Vella J.P., Cafeo F.R., Fonseca F.L.A., Bacci M.R. Association between irisin and major chronic diseases: A review. Eur. Rev. Med. Pharmacol. Sci. 2016;20:4072–4077. [PubMed] [Google Scholar]
- 85.Leustean L., Preda C., Teodoriu L., Mihalache L., Arhire L., Ungureanu M.-C. Role of Irisin in Endocrine and Metabolic Disorders—Possible New Therapeutic Agent? Appl. Sci. 2021;11:5579. doi: 10.3390/app11125579. [DOI] [Google Scholar]
- 86.Cosio P.L., Crespo-Posadas M., Velarde-Sotres A., Pelaez M. Effect of chronic resistance training on circulating irisin: Sys-tematic review and meta-analysis of randomized controlled trials. Int. J. Environ. Res. Public. Health. 2021;18:2476. doi: 10.3390/ijerph18052476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mousavi S.N., Hassani F., Namadian M. Dietary Patterns and the Intake of Trace Elements in People with Hypertension: A Cross-Sectional Study. J. Adv. Med. Biomed. Res. 2020;28:1–10. doi: 10.30699/jambs.28.126.1. [DOI] [Google Scholar]
- 88.Jandova T., Narici M.V., Steffl M., Bondi D., D’Amico M., Pavlu D., Verratti V., Fulle S., Pietrangelo T. Muscle hyper-trophy and architectural changes in response to eight-week neuromuscular electrical stimulation training in healthy older people. Life. 2020;10:184. doi: 10.3390/life10090184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Albrecht E., Norheim F., Thiede B., Holen T., Ohashi T., Schering L., Lee S., Brenmoehl J., Thomas S., Drevon C.A., et al. Irisin—A myth rather than an exercise-inducible myokine. Sci. Rep. 2015;5:8889. doi: 10.1038/srep08889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Atherton P.J., Phillips B.E. Greek goddess or Greek myth: The effects of exercise on irisin/FNDC5 in humans. J. Physiol. 2013;591:5267–5268. doi: 10.1113/jphysiol.2013.265371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cao R.Y., Zheng H., Redfearn D., Yang J. FNDC5: A novel player in metabolism and metabolic syndrome. Biochimie. 2019;158:111–116. doi: 10.1016/j.biochi.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 92.Fox J., Rioux B.V., Goulet E.D.B., Johanssen N.M., Swift D.L., Bouchard D.R., Loewen H., Sénéchal M. Effect of an acute exercise bout on immediate post-exercise irisin concentration in adults: A meta-analysis. Scand. J. Med. Sci. Sports. 2017;28:16–28. doi: 10.1111/sms.12904. [DOI] [PubMed] [Google Scholar]
- 93.Munoz I.Y.M., Camarillo-Romero E.D.S., Garcia J.D.J.G. Irisin a Novel Metabolic Biomarker: Present Knowledge and Future Directions. Int. J. Endocrinol. 2018;2018:1–8. doi: 10.1155/2018/7816806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available in a publicly accessible repository that does not issue DOIs; Publicly available datasets were analyzed in this study. This data can be found here: https://uloz.to/tamhle/8qgosLnZa8BM/name/Nahrano-25-10-2021-v-12-01-24#!ZGVjBGR2A2V0ZQExLmtjLwOxAGNkA01PBTSFFaO0n2qjpwEwAN== (accessed on 22 June 2021).