Abstract
In the liver, glucose induces the expression of a number of genes involved in glucose and lipid metabolism, e.g., those encoding L-type pyruvate kinase and fatty acid synthase. Recent evidence has indicated a role for the AMP-activated protein kinase (AMPK) in the inhibition of glucose-activated gene expression in hepatocytes. It remains unclear, however, whether AMPK is involved in the glucose induction of these genes. In order to study further the role of AMPK in regulating gene expression, we have generated two mutant forms of AMPK. One of these (α1312) acts as a constitutively active kinase, while the other (α1DN) acts as a dominant negative inhibitor of endogenous AMPK. We have used adenovirus-mediated gene transfer to express these mutants in primary rat hepatocytes in culture in order to determine their effect on AMPK activity and the transcription of glucose-activated genes. Expression of α1312 increased AMPK activity in hepatocytes and blocked completely the induction of a number of glucose-activated genes in response to 25 mM glucose. This effect is similar to that observed following activation of AMPK by 5-amino-imidazolecarboxamide riboside. Expression of α1DN markedly inhibited both basal and stimulated activity of endogenous AMPK but had no effect on the transcription of glucose-activated genes. Our results suggest that AMPK is involved in the inhibition of glucose-activated gene expression but not in the induction pathway. This study demonstrates that the two mutants we have described will provide valuable tools for studying the wider physiological role of AMPK.
In the presence of insulin, high levels of glucose stimulate the transcription of a number of genes involved in the conversion of carbohydrates to lipids in the liver (15, 45). In primary rat hepatocytes in culture, the levels of mRNA encoding L-type pyruvate kinase (L-PK), fatty acid synthase (FAS), and spot 14 (S14) increase with increasing concentrations of glucose (5 to 25 mM) (10, 24, 33). The mechanism by which this occurs remains unclear, but metabolism of glucose to glucose-6-phosphate appears to be an essential step in the process (33). In this respect, insulin is required to increase the expression of glucokinase in the liver to allow conversion of glucose to glucose-6-phosphate (15). There is growing evidence to suggest that protein phosphorylation plays an important role in the regulation of glucose-activated gene expression. For example, okadaic acid, an inhibitor of protein phosphatase types 1 and 2A, has been shown to inhibit the glucose stimulation of S14 (40) and FAS (13) gene expression in cultured hepatocytes.
The AMP-activated protein kinase (AMPK) provides a potential candidate for a protein kinase involved in the regulation of glucose-activated genes. A significant clue regarding a possible role for AMPK in the regulation of gene transcription came from the finding that it is structurally and functionally related to the yeast protein kinase complex SNF1 (1, 40, 52). In yeast, the transcription of a number of genes is repressed by high concentrations of glucose (46). The kinase activity of SNF1 is essential for the derepression of these genes in yeast grown under conditions of glucose limitation (3). AMPK and SNF1 both form heterotrimeric complexes consisting of a catalytic subunit and two regulatory subunits. The amino acid sequences of the mammalian AMPK subunits are highly related to their counterparts in the SNF1 complex (1, 32, 40, 51), and the kinases show functional similarities (9, 50, 52). Taken together, these findings led us, and others (31), to speculate that AMPK may be involved in regulating gene transcription in mammals. Evidence that this may be the case came from studies in which AMPK in hepatocytes was activated by incubation with 5-amino-imidazolecarboxamide (AICA) riboside, leading to the inhibition of FAS, L-PK, and S14 gene expression by glucose (13, 31). These results imply that AMPK is involved in the repression of glucose-activated genes. However, AICA riboside is not a specific activator of AMPK (16, 28), and therefore the results obtained in these studies cannot be taken as unequivocal proof that AMPK was mediating this response. Furthermore, neither of these studies provided any information as to whether AMPK was involved in the activation process.
In order to investigate further the role of AMPK in the regulation of glucose-activated gene expression, we have developed two mutant forms of the kinase: one that acts as a constitutively active kinase (α1312) and one that acts as a dominant negative inhibitor (α1DN) of endogenous AMPK. We used adenovirus-mediated gene transfer to express these mutants at high levels in primary rat hepatocytes in culture. Expression of α1312 results in a significant increase in AMPK activity and blocks the glucose activation of the FAS, L-PK, S14, and acetyl coenzyme A (acetyl-CoA) carboxylase (ACC) genes. In contrast, expression of α1DN reduces endogenous AMPK activity by up to 75% but does not have any effect on the transcription of these genes. Taken together, these results point to a role for AMPK in the down-regulation of glucose-activated genes but suggest that it is not involved in their activation. To our knowledge this is the first study to use molecular reagents to modulate AMPK activity in order to determine the effects of the kinase on a downstream pathway. In addition to helping characterize the role of AMPK in the regulation of gene expression, these reagents should provide valuable tools for the further elucidation of the physiological role of AMPK.
MATERIALS AND METHODS
Animals.
Animal studies were conducted according to French guidelines for the care and use of experimental animals. Female Wistar rats (200 to 300 g) were used for the isolation of hepatocytes.
Isolation and primary culture of rat hepatocytes and adenovirus infection.
Hepatocytes were isolated exactly as described previously (13) using the collagenase method. After cell attachment, hepatocytes were cultured for 16 to 18 h in the presence of 5 mM glucose. Hepatocytes were infected with various titers of adenovirus (0 to 100 PFU/cell) and incubated in either 5 mM glucose and 100 nM insulin or 25 mM glucose and 100 nM insulin for up to 90 h, as indicated. The adenoviral infection protocol was carried out as previously described (14). In some cases, at the end of this period, AICA riboside (250 or 500 μM) was added to the media, and the cells were incubated for a further 1 to 4 h, as indicated. Approximately 8 × 106 cells on a 10-cm plate were lysed by the direct addition of 1.5 ml of 5× buffer A to the culture medium (6 ml) to give a final concentration of 50 mM Tris-HCl (pH 7.5), 50 mM NaF, 5 mM sodium pyrophosphate, 1 mM EDTA, 1 mM dithiothreitol, 10% glycerol, and 1% Triton X-100 (buffer A). Cellular debris was removed by centrifugation at 14,000 × g for 10 min at 4°C, and the resulting supernatant (cell lysate) was used for analysis of AMPK activity or Western blotting.
Construction of recombinant adenoviruses.
cDNA encoding residues 1 to 312 of α1, containing a mutation that alters threonine 172 to an aspartic acid (T172D) (41), was used to construct the recombinant adenovirus Ad.α1312 as described previously (22). Briefly, the cDNA of α1312 was subcloned into the shuttle vector pAdTrack-CMV. The resultant plasmid was linearized by the restriction endonuclease PmeI and cotransformed with the supercoiled adenoviral vector pAd-Easy1 into Escherichia coli strain BJ5183. Recombinants were selected by kanamycin resistance and screened by restriction endonuclease digestion. The recombinant adenoviral construct was cleaved with PacI and transfected into the packaging cell line HEK293.
cDNA encoding α1, containing a mutation that alters aspartic acid residue 157 to alanine (41), was used to construct Ad.α1DN. The cDNA was subcloned into the EcoRI/XhoI-linearized pDK6 shuttle vector (11) under the control of the cytomegalovirus IE promoter. The pDK6-α1DN plasmid was cotransfected in HEK293 cells together with the ClaI-cut DNA of the E1a− adenovirus vector Ad.gal-nls (36). Recombinant Ad.α1DN plaques were detected by amplification of viral DNA using α1-specific primers, and one clone was further amplified in HEK293 cells. The adenovirus vector Ad.null, in which the expression cassette contains the major late promoter with no exogenous gene, was used as a control (30). Adenoviruses were propagated in HEK293 cells, purified by cesium chloride density centrifugation, and stored as previously described (30).
Antibodies and immunological reagents.
An anti-Myc monoclonal antibody (clone 9E10 [12]) was used to detect the recombinant α1 mutant proteins, which both contain the sequence EQKLISEEDL immediately after the initiating methionine residue (41). Sheep antibodies against the rat α1 and α2 subunits (53) and against the rat γ1 subunit (4) and rabbit antibodies against the rat β1 subunit (51) were produced as described previously. Anti-mouse, anti-rabbit, and anti-sheep antibodies conjugated to horseradish peroxidase and protein A and protein G conjugated to horseradish peroxidase were obtained from Bio-Rad. Protein G-Sepharose was from Sigma.
Immunoprecipitation and AMPK assays.
AMPK was immunoprecipitated from 0.5 to 1.5 ml of cell lysate by incubation with either anti-α1, anti-α2, or anti-γ1 antibody bound to protein G-Sepharose for 2 h at 4°C. Recombinant α1 proteins were immunoprecipitated from hepatocyte lysates using an anti-Myc (clone 9E10) antibody bound to protein G-Sepharose. Immune complexes were collected by brief centrifugation and washed extensively in buffer A. AMPK activity in the immune complex was determined by phosphorylation of the SAMS (full sequence: HMRSAMSGLHLVKRR) synthetic peptide substrate (53). At the end of the assay period, the immune complex was washed with buffer A to remove unreacted ATP, and proteins within the complex were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by Western blotting.
Western blotting.
Samples were boiled in SDS sample buffer, resolved by SDS-PAGE, and transferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was incubated for 1 h at room temperature in 10 mM Tris-HCl (pH 7.4) 0.5 M NaCl, and 0.5% (vol/vol) Tween 20 (TBST) containing 5% (wt/vol) low-fat milk powder. Following a 2-h incubation with primary antibody diluted in TBST containing 5% milk powder, blots were washed extensively with TBST at room temperature. Blots of crude lysates were incubated with the appropriate secondary antibody conjugated to horseradish peroxidase, whereas blots of immune complexes were probed with protein A or protein G conjugated to horseradish peroxidase. After further washing with TBST, the blots were developed using enhanced chemiluminescence (Boehringer Mannheim).
Isolation of total RNA and Northern blot analysis.
Total cellular RNAs were extracted from cultured hepatocytes using guanidine thiocyanate (5) and prepared for Northern blot hybridization as previously described (7). Labeling of each cDNA probe with [α-32P]dCTP was performed by random priming. cDNA probes for albumin, ACC, FAS, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), L-PK, and S14 were used as previously described (14).
Statistical analysis.
Results, expressed as the mean ± the standard error of the mean (SEM), were analyzed using a two-tailed unpaired Student t test.
RESULTS
Expression of a constitutively active form of AMPK in hepatocytes.
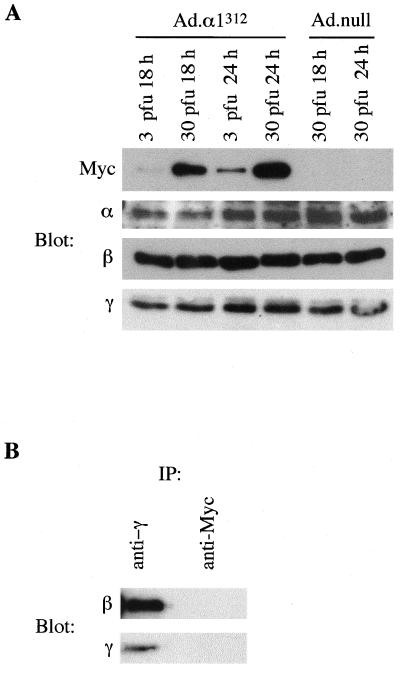
In a recent study, Crute et al. reported that truncation of AMPKα1 at residue 312 yielded a polypeptide that no longer associated with the β and γ subunits but retained significant kinase activity (8). We subsequently showed that mutation of threonine 172 within the α subunit, the major site phosphorylated by AMPK kinase (20), to an aspartic acid residue within this truncated protein prevented its inactivation by protein phosphatases (41). These findings indicated the potential of this mutant to act as a constitutively active kinase. In order to determine the effect of high-level expression of this mutant in primary rat hepatocytes, we constructed a recombinant adenovirus (Ad.α1312). Following infection with Ad.α1312, hepatocyte lysates were analyzed by Western blotting for expression of α1312 and endogenous AMPK subunits. The recombinant α1312 protein contains a Myc epitope tag at the N terminus, allowing detection with an anti-Myc antibody. The mutant protein was just detectable after 18 h with 3 PFU/cell, and the expression increased markedly with time and adenoviral titer (Fig. 1A). Expression of α1312 did not have any significant effect on the amount of endogenous AMPK subunits present in total cell extracts. The antibodies used for detecting the α subunit cross-react with both the α1 and α2 isoforms but do not recognize the truncated α1312 mutant. In order to determine whether the truncated α1312 protein associates in a complex with the β and γ subunits in hepatocytes, complex formation in anti-γ or anti-Myc immunoprecipitates was analyzed by Western blotting (Fig. 1B). In immune complexes isolated with an anti-Myc antibody, which immunoprecipitates α1312, the β and γ subunits were not detected, confirming that this mutant does not associate with the regulatory subunits. In contrast, immune complexes isolated with an anti-γ antibody, which immunoprecipitates the endogenous AMPK complexes, contained readily detectable levels of both the β and γ subunits.
FIG. 1.
Expression of α1312 in hepatocytes. (A) Primary rat hepatocytes in culture were infected with Ad.α1312 or Ad.null at either 3 or 30 PFU/cell. Cell lysates from hepatocytes harvested 18 or 24 h postinfection were analyzed by Western blotting for expression of the recombinant α1312 protein (using an anti-Myc antibody) or the endogenous AMPK subunits (using either an anti-α antibody which recognizes both α1 and α2 isoforms, but not the truncated α1312 protein, or an anti-β or anti-γ antibody). (B) Cell lysates from hepatocytes infected with Ad.α1312 (30 PFU/cell for 24 h) were immunoprecipitated (IP) with either an anti-γ antibody or an anti-Myc antibody. Immune complexes were resolved by SDS-PAGE, and the presence of the β and γ subunits was determined by Western blotting. In each case, a representative blot from two independent experiments is shown.
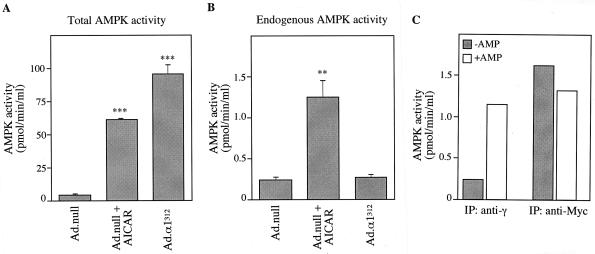
Expression of α1312 resulted in significantly increased levels of AMPK activity present in cell lysates compared to those for control-infected cells (Ad.null). This increase in activity was greater than that detected following treatment of cells with AICA riboside (Fig. 2A). In order to measure the endogenous activity of AMPK, cell lysates were immunoprecipitated with an anti-γ antibody, which immunoprecipitates native AMPK complexes but not recombinant α1312 (Fig. 2B). Endogenous AMPK activity was unaffected by expression of α1312, whereas the endogenous activity was stimulated approximately sixfold following treatment with 250 μM AICA riboside. Figure 2C shows that AMPK activity in immune complexes isolated using an anti-γ antibody, i.e., endogenous AMPK, was stimulated four- to fivefold by 200 μM AMP and that the activity of α1312, present in an anti-Myc immune complex, was not dependent on AMP. α1312 activity was resistant to inactivation by treatment with protein phosphatase 2C (data not shown), confirming our previous finding that α1312 is resistant to dephosphorylation (41). These results show that the expressed α1312 protein acts as a constitutively active kinase, significantly increasing AMPK activity within primary rat hepatocytes.
FIG. 2.
Activity of α1312 in hepatocytes. Hepatocytes were grown in the presence of 25 mM glucose and 100 nM insulin and infected with either Ad.null or Ad.α1312 (30 PFU/cell). Twenty-four hours postinfection, hepatocytes were incubated in the presence or absence of 250 μM AICA riboside (AICAR) for 1 h before harvesting. (A) Total activity (endogenous AMPK and expressed α1312) was measured in cell lysates, without prior immunoprecipitation, using the SAMS peptide assay. (B) Endogenous AMPK activity was measured in immune complexes isolated by immunoprecipitation using an anti-γ antibody bound to protein G-Sepharose. (C) AMPK activity present in either anti-γ (endogenous) or anti-Myc (expressed α1312) immunoprecipitates was measured in the absence (shaded bars) or presence (open bars) of 0.2 mM AMP. Activities shown are the mean ± the SEM from four experiments (A and B) or the average of two independent experiments assayed in duplicate (C). ∗∗∗ denotes a significant difference from the Ad.null value (P < 0.0005), and ∗∗ indicates that P was <0.005. Activities are plotted as picomoles of 32P incorporation/minutes per milliliter of lysate.
Inhibition of glucose-activated gene expression by AMPK.
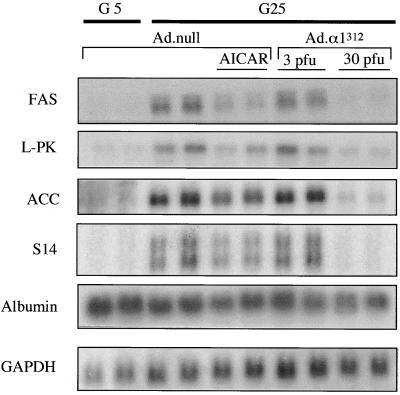
Members of our group and others have previously shown that activation of AMPK by AICA riboside blocks the glucose activation of a number of genes in hepatocytes (13, 31). We therefore determined the effect of expression of α1312 on the transcription of glucose-activated genes. Figure 3 shows a Northern blot analysis of RNA from hepatocytes infected with either Ad.α1312 or Ad.null. The expression of genes encoding FAS, L-PK, S14, and ACC was increased by incubating the cells in 25 mM glucose compared to results obtained with 5 mM glucose (Fig. 3). Consistent with the results of earlier studies, AICA riboside antagonized glucose activation of these genes. At 30 PFU/cell, expression of α1312 almost totally abolished the increase in gene expression by 25 mM glucose, although this effect was far less apparent in cells where the mutant protein was expressed at a low level (3 PFU/cell). The expression of control genes (those for albumin and GAPDH) was not significantly affected by any of the treatments.
FIG. 3.
Expression of α1312 inhibits the transcription of glucose-activated genes in cultured hepatocytes. Hepatocytes were grown in medium containing either 5 mM glucose and 100 nM insulin (G5) or 25 mM glucose and 100 nM insulin (G25) and were infected with Ad.α1312 (3 or 30 PFU/cell) or Ad.null (30 PFU/cell) and incubated for 18 h. At the end of this period, hepatocytes were treated with or without AICA riboside (AICAR) (250 μM) and incubated for 4 h. Total RNA was extracted, electrophoresed on a 1% agarose gel, and subjected to Northern blot analysis using cDNA probes encoding either FAS, L-PK, ACC, S14, albumin, or GAPDH. Blots were washed extensively and exposed to autoradiographic film at −70°C for 1 to 2 days. The blot shown is representative of two independent experiments.
Expression of a dominant negative form of AMPK in hepatocytes.
Considering that an increase in AMPK activity, caused either by AICA riboside or by overexpression of α1312, results in the inhibition of transcription of glucose-activated genes, it is tempting to speculate that in hepatocytes, inhibition of AMPK is the mechanism by which glucose induces the expression of these genes. To date, however, no specific inhibitors of AMPK have been reported, and so it has not been possible to test this hypothesis directly. In an attempt to address this problem, we undertook to develop a dominant negative mutant of AMPK, which would allow us to determine directly whether AMPK is involved in the induction of glucose-activated gene expression in hepatocytes.
Aspartate 157 within the α subunit lies in the conserved DFG motif (subdomain VII in protein kinase catalytic subunits), which has been shown to be essential for MgATP binding in all protein kinases (26). Mutation of this residue to alanine, in either α1 or α2, yields an inactive kinase but does not have any effect on the binding of the β and γ subunits within the complex (41). Since formation of the heterotrimeric complex is essential for AMPK activity, we reasoned that overexpression of the inactive α1 subunit (α1DN) would act as a dominant negative inhibitor by competing with the native α subunit for binding with β and γ. We therefore used adenovirus-mediated gene transfer in primary rat hepatocytes to test this hypothesis.
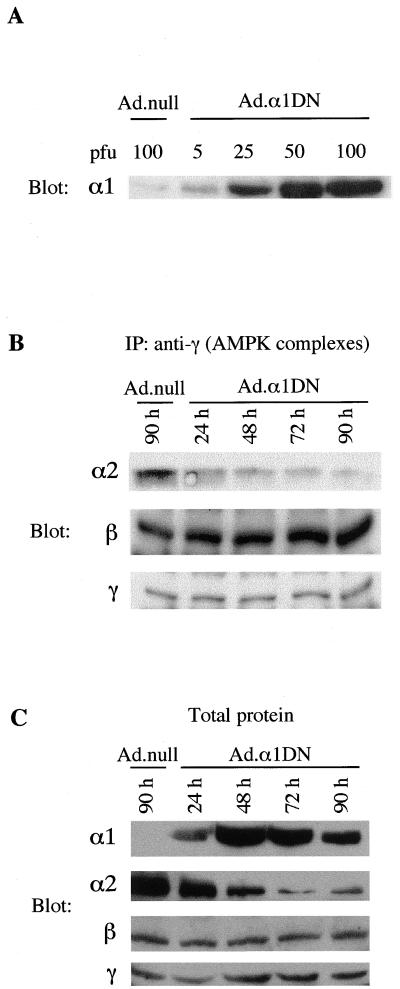
Infection of primary rat hepatocytes with Ad.α1DN led to the marked expression of the mutant α1 subunit, detected by Western blotting of cell lysates using an anti-α1 antibody, which was dependent on the adenoviral titer used for infection (Fig. 4A). Endogenous α1 in control-infected cells (Ad.null) was barely detectable due to the low sensitivity of this antibody. Western blot analysis of immune complexes isolated using an anti-γ antibody showed that as the expression of α1DN increased, there was a concomitant decrease in the amount of α2 present in AMPK complexes (Fig. 4B). In contrast, the levels of the β and γ subunits remained constant. These results imply that α1DN competes with the native α subunits for the binding of the β and γ subunits. Western blot analysis of the total level of AMPK subunits present in cell lysates following a time course of α1DN expression showed that increasing expression of the inactive α1 subunit had no significant effect on the expression of the endogenous β and γ subunits. Interestingly, however, the expression of α2 decreased markedly with time after infection (Fig. 4C). These results suggest that as the endogenous α2 subunit (and presumably the endogenous α1 subunit) is displaced from the complex, it becomes relatively unstable and is removed from the cell.
FIG. 4.
Expression of α1DN in hepatocytes. (A) Hepatocytes in culture were infected with varying titers of Ad.α1DN or Ad.null. Twenty-four hours after infection, cell lysates were resolved by SDS-PAGE, transferred to a PVDF membrane, and probed with an anti-α1 antibody. The α1-specific antibody used for detection recognizes both the endogenous α1 subunit and the recombinant α1 mutant protein. (B) Hepatocytes were infected with Ad.α1DN (10 PFU/cell), and at varying times after infection, AMPK complexes were isolated by immunoprecipitation (IP) with an anti-γ antibody. Proteins within the immune complex were resolved by SDS-PAGE, transferred to a PVDF membrane, and probed with either α2-, β-, or γ-specific antibodies. A control lane shows the expression of the subunits in an immune complex isolated from hepatocytes infected for 90 h with Ad.null (10 PFU/cell). (C) Hepatocytes were infected with either Ad.α1DN (10 PFU/cell) or Ad.null (10 PFU/cell). At various times after infection, total protein in cell lysates was analyzed for expression of α1 and the endogenous α2, β, and γ subunits, using subunit-specific AMPK antibodies. In each case, a representative blot from two independent experiments is shown.
Effect of inactive α1 on AMPK activity in hepatocytes.
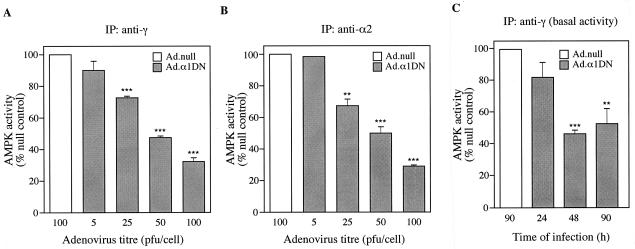
Hepatocytes that had been preincubated with AICA riboside to activate AMPK were used to study the effect of expression of α1DN on AMPK activity. Using an anti-γ antibody, which immunoprecipitates both the α1 and α2 isoforms (38), there was a decrease in kinase activity following expression of α1DN. The degree of inhibition correlated with increasing expression of α1DN (Fig. 5A). At 100 PFU/cell, there was approximately 70 to 75% inhibition of the AICA riboside-stimulated AMPK activity. Similar results were obtained after measuring AMPK activity in crude cell lysates or in a partially purified polyethylene glycol fraction of the kinase (data not shown). At titers of Ad.α1DN that were greater than 100 PFU/cell, the hepatocytes became unviable. Virtually identical results were obtained when AMPK was immunoprecipitated with an α2-specific antibody (Fig. 5B). We were not able to measure the effect on endogenous α1 activity directly due to competition of the immunoprecipitating α1-specific antibody by the high levels of recombinant α1 subunit. Previously, however, it has been shown that α1 and α2 contribute almost equally to total AMPK activity in rat liver (38, 53). Since the total activity of AMPK falls by the same amount as the α2-specific activity, our results imply that expression of α1DN inhibits both α1- and α2-containing complexes to a similar extent. Expression of α1DN also led to a decrease in AMPK activity of up to 60% in hepatocytes that had not been incubated with AICA riboside, i.e., under conditions which would reflect basal AMPK activity (Fig. 5C).
FIG. 5.
Expression of α1DN inhibits AMPK activity. (A and B) Hepatocytes were infected with varying titers of Ad.α1DN or Ad.null. Forty-four hours after infection, AICA riboside (500 μM) was added to the culture medium, and the hepatocytes were incubated for a further 1 h. AMPK complexes were isolated from cell lysates by immunoprecipitation (IP) with either an anti-γ antibody (A) or an anti-α2 antibody (B). Activity present in the immune complexes was measured by phosphorylation of the SAMS peptide. For panel C, hepatocytes were infected with either Ad.α1DN (10 PFU/cell) or Ad.null (10 PFU/cell) in the absence of AICA riboside. At various times postinfection, AMPK activity present in immune complexes isolated using an anti-γ antibody was determined as described above. In each case, results are plotted as a percentage of the activity present in hepatocytes infected with Ad.null and are the mean ± the SEM of four independent experiments. ∗∗∗ denotes a significant difference from the Ad.null value (P < 0.0005), and ∗∗ indicates that P was <0.005.
Effect of inhibition of AMPK on glucose-activated gene expression.
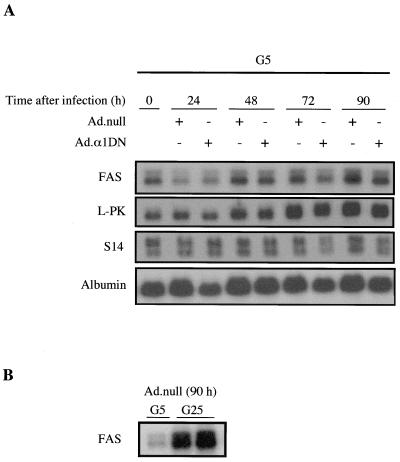
In order to determine whether AMPK plays a role in the induction of glucose-activated gene expression, we examined the effect of inhibiting AMPK by expression of α1DN. The expression of glucose-activated genes in hepatocytes grown in 5 mM glucose is very low but increases with increasing glucose concentrations (13). Figure 6A shows that there was no increase in the mRNA levels of FAS, L-PK, or S14 following expression of α1DN in hepatocytes grown in 5 mM glucose, even though basal AMPK activity was reduced by up to 60% under these conditions. In contrast, however, the level of FAS mRNA was markedly increased by 25 mM glucose in hepatocytes maintained under these conditions (Fig. 6B). These results indicate that partial inhibition of AMPK is not sufficient for induction of glucose-activated gene expression in hepatocytes.
FIG. 6.
Overexpression of α1DN has no effect on the transcription of glucose-activated genes. (A) Hepatocytes were incubated in the presence of 5 mM glucose and 100 nM insulin (G5) for 18 h before infection with either Ad.α1DN (10 PFU/cell) or Ad.null (10 PFU/cell). At various times after infection, total RNA was extracted from the cells and subjected to Northern blotting with cDNA probes encoding either FAS, L-PK, ACC, S14, or albumin. Blots were washed extensively and exposed to autoradiographic film at −70°C for 1 to 2 days. (B) Northern blot analysis of RNA isolated from hepatocytes 90 h after infection with Ad.null (10 PFU/cell) cultured in medium containing 100 nM insulin and either 5 mM glucose (G5) or 25 mM glucose (G25). In each case, a representative blot from at least four independent experiments is shown.
Effect of glucose concentration on endogenous AMPK activity.
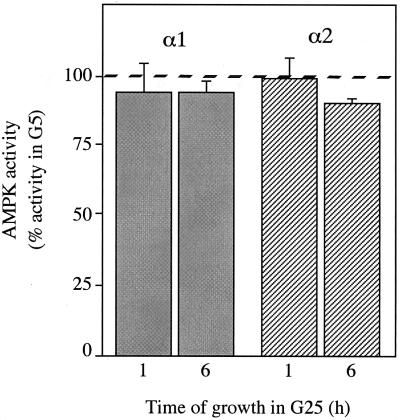
A caveat to our finding that inhibition of AMPK has no effect on the induction of gene expression in response to glucose is that we were unable to inhibit AMPK activity completely. It remained possible, therefore, that high concentrations of glucose could activate gene expression by inhibiting AMPK activity to a greater extent than we have observed using the dominant negative approach. In order to test this, we measured AMPK activity present in anti-α1 and anti-α2 immune complexes that were isolated from hepatocytes incubated in the presence of 100 nM insulin with either 5 mM glucose or 25 mM glucose. Although in every case AMPK activity was very low, we were unable to detect any significant reduction in the activity of either α1 or α2 complexes from hepatocytes incubated in 25 mM glucose compared to results with 5 mM glucose (Fig. 7). This result rules out the possibility that high concentrations of glucose activate gene expression in hepatocytes by directly inhibiting AMPK.
FIG. 7.
Effect of extracellular glucose concentration on AMPK activity in hepatocytes. Hepatocytes were cultured overnight in medium containing 5 mM glucose and 100 nM insulin (G5). Medium was removed, and fresh G5 medium or medium containing 25 mM glucose and 100 nM insulin (G25) was added. Hepatocytes were harvested following incubation for a further 1 or 6 h. AMPK activity present in immune complexes isolated using either an anti-α1 (shaded bars) or anti-α2 (hatched bars) antibody was determined by phosphorylation of the SAMS peptide. Activities are plotted as a percentage of AMPK activity present in hepatocytes maintained in G5 and are the mean ± the SEM of three experiments. The 100% value for the activity in G5 is indicated by the dashed line.
DISCUSSION
AMPK was originally identified through its phosphorylation and inhibition of key enzymes involved in biosynthetic pathways, such as ACC (fatty acid synthesis) and 3-hydroxy-3-methylglutaryl CoA reductase (isoprenoid and cholesterol biosynthesis) (18). It has now become clear that AMPK plays a much wider role in cellular regulation, e.g., in the regulation of fatty acid oxidation in the liver (34, 48, 49) and muscles (29, 47), in the activation of glucose uptake in muscles (21), and in the inhibition of glucose-activated gene expression in the liver (13, 31). Virtually all of the studies examining the physiological role of AMPK have involved experiments using AICA riboside to activate the kinase. AICA riboside is a cell-permeative compound which is phosphorylated within the cell by adenosine kinase to form AICA ribotide, or ZMP (37), which mimics the effect of AMP on activation of AMPK (6, 43, 44). A limitation when using AICA riboside to study the function of AMPK is that its effects are not restricted to activation of AMPK (16, 28), while in some cell types, e.g., cardiomyocytes, ZMP does not accumulate to high levels (25). Results obtained with AICA riboside, therefore, should be interpreted with caution and treated only as preliminary evidence for the involvement of AMPK in a particular pathway.
Convincing evidence of a role for AMPK in a particular cellular pathway could be gained by using alternative methods for modulating AMPK activity within the cell. Until now, however, there have been no reports of other well-characterized activators or inhibitors of AMPK. In order to address this issue, we have developed a constitutively active form of AMPK to increase activity and a dominant negative mutant of AMPK to decrease activity. To determine the use of these reagents as molecular tools to study the function of AMPK, we chose to investigate the effects of their overexpression on the transcription of glucose-activated genes. In the present study, we used primary rat hepatocytes in culture, a system which has been used extensively as a model for studying glucose-activated gene expression (15, 45), coupled with adenovirus-mediated gene transfer. Using this approach, we were able to express high levels of both α1312, the constitutively active form of AMPK, and α1DN, the dominant negative form. At 30 PFU/cell, the activity contributed by α1312 in hepatocyte lysates was greater than that of endogenous AMPK following stimulation by AICA riboside. Expression of α1312 blocked the induction of four glucose-responsive genes (FAS, L-PK, ACC, and S14) but had no effect on the expression of control genes (albumin and GAPDH), which are not induced by glucose.
In the liver, insulin acts indirectly in stimulating glucose-activated gene expression by inducing glucokinase expression (15, 45). In the present study, insulin (100 nM) was included throughout the incubation of the hepatocytes, and the concentration of glucose was varied between 5 and 25 mM. We are unable to distinguish, therefore, among the inhibitory effects of AMPK in a glucose pathway, an insulin pathway, or both. In addition, it is possible that the effect of overexpression of α1312 on gene expression is due to its mimicking a closely related kinase rather than being the direct consequence of AMPK activity. However, the effect of α1312 is similar to that observed following stimulation of AMPK with AICA riboside, providing strong evidence that AMPK per se inhibits glucose-activated gene expression. To our knowledge this is the first study to use a constitutively active form of AMPK to alter a cellular response.
Having obtained convincing evidence that AMPK inhibits glucose-activated gene expression, we investigated next whether AMPK could also be involved in the glucose activation pathway. Expression of α1DN in hepatocytes led to a marked decrease in AMPK activity. The magnitude of this effect was the same regardless of whether anti-γ or anti-α2 antibodies were used to immunoprecipitate AMPK. In liver, the anti-γ antibody immunoprecipitates complexes containing both the α1 and α2 isoforms (38), implying that the activities of the α1 and α2 isoforms are reduced to a similar extent by expression of inactive α1. The most likely explanation for the reduction in AMPK activity is that α1DN competes with the endogenous α subunit for the binding of the β and γ subunits. It is possible that the unassociated α subunit is subject to an increased turnover rate and is depleted from the cell. Consistent with this hypothesis are the results of a previous study which showed that the turnover rate of α1 in transiently transfected COS cells is decreased by coexpression of the β and γ subunits (8). Alternatively, it is possible that the decrease in α2 expression is due to feedback inhibition of α2 caused by overexpression of the α1DN protein. Further experiments will be required to address this issue.
Since an increase in AMPK activity inhibits glucose-activated gene transcription, it follows that a decrease in AMPK activity could stimulate gene transcription. This does not appear to be the case, since inhibition of AMPK activity by more than 50% in hepatocytes grown in 5 mM glucose had no detectable effect on the expression of any of the glucose-activated genes we examined. In a recent study, α2-containing complexes, but not α1 complexes, were shown to be present within the nucleus (38). This result suggests that the effect of AMPK on gene expression may be mediated specifically by α2-containing complexes. However, we found that expression of α1DN inhibited both α1 and α2 complexes to the same extent, arguing against this scenario. Expression of α1DN did not completely abolish AMPK activity, and it remained feasible that high concentrations of glucose could reduce activity by a greater amount than the dominant negative inhibitor. However, we were unable to detect any decrease in the activity of endogenous AMPK in cultured hepatocytes after changing the glucose concentration in the medium from 5 to 25 mM. In contrast, the expression of glucose-activated genes is significantly increased under these conditions. Taken together, these results rule out the possibility that glucose exerts its effects on gene expression by directly inhibiting AMPK.
In a previous study, Salt et al. observed a correlation between AMPK activity and extracellular glucose concentration in cell lines derived from pancreatic β cells (39). In this case, however, AMPK activity was only increased by very low concentrations of glucose (below 1 mM). This is reminiscent of the activation of SNF1 in yeast following removal of glucose from the medium (50, 52). The increase in AMPK activity upon glucose removal in β cells correlated with an increase in both the ADP/ATP ratio and AMP/ATP ratio. Increasing the glucose concentration above 10 mM had no obvious effect on either AMPK activity or the ratio of ADP to ATP or AMP to ATP (39). The results obtained with pancreatic β cell lines are consistent with our finding for hepatocytes that AMPK is not inhibited by high concentrations of glucose.
A specific role of AMPK in the inhibition of glucose-activated gene expression, rather than in the induction process, is compatible with the idea that AMPK acts as a low-energy sensor within the cell (16, 17). Under optimal conditions the AMP/ATP ratio is maintained at a level below that required to lead to activation of AMPK. Consistent with this is the finding that the basal activity of AMPK isolated from hepatocytes grown in the presence of 5 mM glucose is very low. Many stress conditions lead to depletion of ATP and a concomitant rise in the ratio of AMP to ATP, resulting in activation of AMPK (19). In the case of glucose-activated gene expression, activation of AMPK would inhibit transcription, an energy-utilizing pathway, thereby conserving the energy within the cell. It is less clear whether any physiological conditions would lead to inhibition of basal AMPK activity. From our current understanding of the regulation of AMPK, inhibition of basal activity would require a decrease in the AMP/ATP ratio, although it is possible that some other, unrelated process could be involved. However, we have not been able to measure any reduction of basal AMPK activity in hepatocytes, and others have failed to detect significant inhibition of AMPK in pancreatic β cell lines (39), by high levels of glucose. Based on these findings, we propose that activation of AMPK is a physiologically relevant process, whereas a reduction of basal AMPK activity is unlikely to occur in vivo.
Comparing the role of AMPK in inhibiting glucose-activated gene expression with the role of SNF1 in yeast reveals some intriguing details. SNF1 is activated in the presence of low glucose levels (derepressing conditions). Although the metabolic signal leading to activation remains enigmatic, it is analogous to the activation of AMPK following a rise in the AMP/ATP ratio. Once activated, SNF1 switches on transcription of glucose-repressed genes, such as SUC2, the gene coding for invertase (2, 3). This is the mirror image of the situation for AMPK, which switches off transcription of glucose-activated genes. With high glucose levels (glucose-repressing conditions), SNF1 is maintained in an inactive state and does not appear to play a role in the glucose induction pathway (27), analogous to the situation with AMPK. It is possible that the repressive role of AMPK in gene expression is not limited to glucose-activated genes but is a more global response. Inhibition of gene expression following a fall in the energy status of the cell would prevent further utilization of ATP, which would be beneficial to the cell. To date we have identified only genes which are inactivated by AMPK. Could AMPK also be involved in activation of gene transcription, as is the case for SNF1 in yeast? In a recent paper, Holmes et al. reported that chronic activation of AMPK by subcutaneous injection of AICA riboside over a 5-day period increased the expression of GLUT4 as well as the total activity of hexokinase in the skeletal muscle (23). The GLUT4 gene provides a particularly attractive candidate for activation by AMPK, since previous studies have shown that GLUT4 mRNA is increased by exercise training (35). It will be interesting, therefore, to determine whether AMPK is involved in the activation of GLUT4 gene expression in muscles.
The results reported here describe the expression of mutant forms of AMPK that act either as a constitutively active kinase or as a dominant negative inhibitor of endogenous AMPK in primary cells. The constitutively active form of AMPK provides a specific way to increase AMPK activity. Previously, AICA riboside has often been used to activate AMPK. However, as has been noted by others, AICA riboside is not a specific activator of AMPK and therefore should not be used in isolation to identify downstream targets of AMPK (16, 28). In addition to describing a more specific method for increasing AMPK activity, we also describe a specific inhibitor of AMPK, the α1DN mutant. Using these reagents, we have been able to obtain convincing evidence that AMPK is involved in inhibiting expression of glucose-activated genes but does not play a role in their induction. It will be possible to use adenovirus-mediated gene transfer to express these mutants in cultured cells and in vivo in order to alter AMPK activity. These mutants will provide valuable tools for studying the wider physiological role of AMPK.
ACKNOWLEDGMENTS
This study was supported by the Medical Research Council (MRC), London (D.C.), the Algerian state (D.A.-M.), and the Centre National de la Recherche Scientifique (P.F.). Part of this work was funded by an Intermediate Research Fellowship from the British Heart Foundation (A.W.) and from a European Union FAIR contract (97/3011). S.C.S. was supported by an MRC-CASE Ph.D. studentship (in collaboration with AstraZeneca Pharmaceuticals).
A.W. and D.A.-M. contributed equally to this study.
REFERENCES
- 1.Carling D, Aguan K, Woods A, Verhoeven A J M, Beri R K, Brennan C H, Sidebottom C, Davison M D, Scott J. Mammalian AMP-activated protein kinase is homologous to yeast and plant protein kinases involved in the regulation of carbon metabolism. J Biol Chem. 1994;269:11442–11448. [PubMed] [Google Scholar]
- 2.Celenza J L, Carlson M. Mutational analysis of the Saccharomyces cerevisiae SNF1 protein kinase and evidence for functional interaction with the SNF4 protein. Mol Cell Biol. 1989;9:5034–5044. doi: 10.1128/mcb.9.11.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Celenza J L, Carlson M. A yeast gene that is essential for release from glucose repression encodes a protein kinase. Science. 1986;233:1175–1180. doi: 10.1126/science.3526554. [DOI] [PubMed] [Google Scholar]
- 4.Cheung P C F, Salt I P, Davies S P, Hardie D G, Carling D. Characterization of AMP-activated protein kinase γ-subunit isoforms and their role in AMP binding. Biochem J. 2000;346:659–669. [PMC free article] [PubMed] [Google Scholar]
- 5.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 6.Corton J M, Gillespie J G, Hawley S A, Hardie D G. 5-Aminoimidazole-4-carboxamide ribonucleoside—a specific method for activating AMP-activated protein kinase in intact cells. Eur J Biochem. 1995;229:558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- 7.Coupe C, Perdereau D, Ferre P, Hitier Y, Narkewicz M, Girard J. Lipogenic enzyme activities and mRNA in rat adipose tissue at weaning. Am J Physiol. 1990;258:E126–E133. doi: 10.1152/ajpendo.1990.258.1.E126. [DOI] [PubMed] [Google Scholar]
- 8.Crute B E, Seefeld K, Gamble J, Kemp B E, Witters L A. Functional domains of the α1 catalytic subunit of the AMP-activated protein kinase. J Biol Chem. 1998;273:35347–35354. doi: 10.1074/jbc.273.52.35347. [DOI] [PubMed] [Google Scholar]
- 9.Dale S, Wilson W A, Edelman A M, Hardie D G. Similar substrate recognition motifs for mammalian AMP-activated protein kinase, higher-plant HMG-CoA reductase kinase-A, yeast Snf1, and mammalian calmodulin-dependent protein kinase-I. FEBS Lett. 1995;361:191–195. doi: 10.1016/0014-5793(95)00172-6. [DOI] [PubMed] [Google Scholar]
- 10.Decaux J F, Antoine B, Kahn A. Regulation of the expression of the L-type pyruvate kinase gene in adult rat hepatocytes in primary culture. J Biol Chem. 1989;264:11584–11590. [PubMed] [Google Scholar]
- 11.Emtage P C R, Wan Y, Bramson J L, Graham F L, Gauldie J. A double recombinant adenovirus expressing the costimulatory molecule B7-1 (murine) and human IL-2 induces complete tumor regression in a murine breast adenocarcinoma model. J Immunol. 1998;160:2531–2538. [PubMed] [Google Scholar]
- 12.Evan G I, Lewis G K, Ramsay G, Bishop J M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foretz M, Carling D, Guichard C, Ferre P, Foufelle F. AMP-activated protein kinase inhibits the glucose-activated expression of fatty acid synthase gene in rat hepatocytes. J Biol Chem. 1998;272:14767–14771. doi: 10.1074/jbc.273.24.14767. [DOI] [PubMed] [Google Scholar]
- 14.Foretz M, Pacot C, Dugail I, Lemarchand P, Guichard C, Berthelier-Lubrano C, Spiegelman B, Kim J B, Ferre P, Foufelle F. ADD1/SREBP1c is required in the activation of hepatic lipogenic gene expression by glucose. Mol Cell Biol. 1999;19:3760–3768. doi: 10.1128/mcb.19.5.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girard J, Ferre P, Foufelle F. Mechanisms by which carbohydrates regulate expression of genes for glycolytic and lipogenic enzymes. Annu Rev Nutr. 1997;17:325–352. doi: 10.1146/annurev.nutr.17.1.325. [DOI] [PubMed] [Google Scholar]
- 16.Hardie D G, Carling D. The AMP-activated protein kinase: fuel gauge of the mammalian cell? Eur J Biochem. 1997;246:259–273. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- 17.Hardie D G, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- 18.Hardie D G, Carling D, Sim A T R. The AMP-activated protein kinase—a multisubstrate regulator of lipid metabolism. Trends Biochem Sci. 1989;14:20–23. [Google Scholar]
- 19.Hardie D G, Salt I P, Hawley S A, Davies S P. AMP-activated protein kinase: an ultrasensitive system for monitoring cellular energy charge. Biochem J. 1999;338:717–722. [PMC free article] [PubMed] [Google Scholar]
- 20.Hawley S A, Davison M D, Woods A, Davies S P, Beri R K, Carling D, Hardie D G. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi T, Hirshman M F, Kurth E J, Winder W W, Goodyear L J. Evidence for AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes. 1998;47:1369–1373. doi: 10.2337/diab.47.8.1369. [DOI] [PubMed] [Google Scholar]
- 22.He T C, Zhou S, da Costa L T, Yu J, Kinzler K W, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmes B F, Kurth-Kraczec E J, Winder W W. Chronic activation of 5′-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J Appl Physiol. 1999;87:1990–1995. doi: 10.1152/jappl.1999.87.5.1990. [DOI] [PubMed] [Google Scholar]
- 24.Jacoby D B, Zilz N D, Towle H C. Sequences within the 5′-flanking region of the S14 gene confer responsiveness to glucose in primary hepatocytes. J Biol Chem. 1989;264:17623–17626. [PubMed] [Google Scholar]
- 25.Javaux F, Vincent M F, Wagner D R, Van den Berghe G. Cell-type specificity of inhibition of glycolysis by 5-amino-4 imidazolecarboxamide riboside. Lack of effect in rabbit cardiomyocytes and human erythrocytes, and inhibition in FTO-2B rat hepatoma cells. Biochem J. 1995;305:913–919. doi: 10.1042/bj3050913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson L N, Noble M E M, Owen D J. Active and inactive protein kinases: structural basis for regulation. Cell. 1996;85:149–158. doi: 10.1016/s0092-8674(00)81092-2. [DOI] [PubMed] [Google Scholar]
- 27.Johnston M. Feasting, fasting and fermenting: glucose sensing in yeast and other cells. Trends Genet. 1999;15:29–33. doi: 10.1016/s0168-9525(98)01637-0. [DOI] [PubMed] [Google Scholar]
- 28.Kemp B E, Mitchelhill K I, Stapleton D, Michell B J, Chen Z-P, Witters L A. Dealing with energy demand: the AMP-activated protein kinase. Trends Biochem Sci. 1999;24:22–25. doi: 10.1016/s0968-0004(98)01340-1. [DOI] [PubMed] [Google Scholar]
- 29.Kudo N, Barr A J, Barr R L, Desai S, Lopaschuk G D. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5′-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J Biol Chem. 1995;270:17513–17520. doi: 10.1074/jbc.270.29.17513. [DOI] [PubMed] [Google Scholar]
- 30.Lafont A, Loirand G, Pacaud P, Vilde F, Lemarchand P, Escande D. Vasomotor dysfunction early after exposure of normal rabbit arteries to an adenoviral vector. Hum Gene Ther. 1997;8:1033–1040. doi: 10.1089/hum.1997.8.9-1033. [DOI] [PubMed] [Google Scholar]
- 31.Leclerc I, Kahn A, Doiron B. The AMP-activated protein kinase inhibits the transcriptional stimulation by glucose in liver cells, acting through the glucose response complex. FEBS Lett. 1998;431:180–184. doi: 10.1016/s0014-5793(98)00745-5. [DOI] [PubMed] [Google Scholar]
- 32.Mitchelhill K I, Stapleton D, Gao G, House C, Michell B, Kateis F, Witters L A, Kemp B E. Mammalian AMP-activated protein kinase shares structural and functional homology with the catalytic domain of yeast Snf1 protein kinase. J Biol Chem. 1994;269:2361–2364. [PubMed] [Google Scholar]
- 33.Mourrieras F, Foufelle F, Foretz M, Morin J, Bouche S, Ferre P. Induction of fatty acid synthase and S14 gene expression by glucose, xylitol and dihydroxyacetone in cultured rat hepatocytes is closely correlated with glucose 6-phosphate concentrations. Biochem J. 1997;323:345–349. doi: 10.1042/bj3260345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muoio D M, Seefeld K, Witters L A, Coleman R. AMP-activated protein kinase reciprocally regulates triacylglycerol synthesis and fatty acid oxidation in liver and muscle: evidence that sn-glycerol-3-phosphate acyltransferase is a novel target. Biochem J. 1999;338:783–791. [PMC free article] [PubMed] [Google Scholar]
- 35.Neufer P D, Dohm G L. Exercise induces a transient increase in transcription of the GLUT4 gene in skeletal muscle. Am J Physiol. 1993;265:C1597–C1603. doi: 10.1152/ajpcell.1993.265.6.C1597. [DOI] [PubMed] [Google Scholar]
- 36.Oualikene W, Gonin P, Eloit M. Lack of evidence of phenotypic complementation of E1A/E1B-deleted adenovirus upon superinfection by wild-type virus in the cotton rat. J Virol. 1995;69:6518–6524. doi: 10.1128/jvi.69.10.6518-6524.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabina R L, Patterson D, Holmes E W. 5-Amino-4-imidazolecarboxamide riboside (Z-riboside) metabolism in eukaryotic cells. J Biol Chem. 1985;260:6107–6114. [PubMed] [Google Scholar]
- 38.Salt I P, Celler J W, Hawley S A, Prescott A, Woods A, Carling D, Hardie D G. AMP-activated protein kinase: greater AMP dependence, and preferential nuclear localization, of complexes containing the α2 isoform. Biochem J. 1998;334:177–187. doi: 10.1042/bj3340177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salt I P, Johnson G, Ashcroft S J H, Hardie D G. AMP-activated protein kinase is activated by low glucose in cell lines derived from pancreatic β cells, and may regulate insulin release. Biochem J. 1998;335:533–539. doi: 10.1042/bj3350533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stapleton D, Gao G, Mitchell B J, Widmer J, Mitchelhill K, Teh T, House C M, Witters L A, Kemp B E. Mammalian 5′-AMP-activated protein kinase non-catalytic subunits are homologs of proteins that interact with yeast Snf1 protein kinase. J Biol Chem. 1994;269:29343–29346. [PubMed] [Google Scholar]
- 41.Stein S C, Woods A, Jones N A, Davison M D, Carling D. The regulation of AMP-activated protein kinase by phosphorylation. Biochem J. 2000;345:437–443. [PMC free article] [PubMed] [Google Scholar]
- 42.Sudo Y, Mariash C N. Two glucose signalling pathways in S14 gene transcription in primary hepatocytes: a common role of protein phosphorylation. Endocrinology. 1994;134:2532–2540. doi: 10.1210/endo.134.6.8194479. [DOI] [PubMed] [Google Scholar]
- 43.Sullivan J E, Brocklehurst K J, Marley A E, Carey F, Carling D, Beri R K. Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett. 1994;353:33–36. doi: 10.1016/0014-5793(94)01006-4. [DOI] [PubMed] [Google Scholar]
- 44.Sullivan J E, Carey F, Carling D, Beri R K. Characterization of AMP-activated protein kinase in human liver using specific peptide substrates and the effects of AMP analogs on enzyme activity. Biochem Biophys Res Commun. 1994;200:1551–1556. doi: 10.1006/bbrc.1994.1627. [DOI] [PubMed] [Google Scholar]
- 45.Towle H C, Kaytor E N, Shih H M. Regulation of the expression of lipogenic enzymes by carbohydrates. Annu Rev Nutr. 1997;17:405–433. doi: 10.1146/annurev.nutr.17.1.405. [DOI] [PubMed] [Google Scholar]
- 46.Trumbly R J. Glucose repression in the yeast Saccharomyces cerevisiae. Mol Microbiol. 1992;6:15–21. doi: 10.1111/j.1365-2958.1992.tb00832.x. [DOI] [PubMed] [Google Scholar]
- 47.Vavvas D, Apazidis A, Saha A K, Gamble J, Patel A, Kemp B E, Witters L A, Ruderman N B. Contraction-induced changes in acetyl-CoA carboxylase and 5′-AMP-activated kinase in skeletal muscle. J Biol Chem. 1997;272:13255–13261. doi: 10.1074/jbc.272.20.13255. [DOI] [PubMed] [Google Scholar]
- 48.Velasco G, Geelen M J H, Guzman M. Control of hepatic fatty acid oxidation by 5′-AMP-activated protein kinase involves a malonyl-CoA-dependent and a malonyl-CoA-independent mechanism. Arch Biochem Biophys. 1997;337:169–175. doi: 10.1006/abbi.1996.9784. [DOI] [PubMed] [Google Scholar]
- 49.Velasco G, Gomez del Pulgar T, Carling D, Guzman M. Evidence that the AMP-activated protein kinase stimulates rat liver carnitine palmitoyltransferase I by phosphorylating cytoskeletal components. FEBS Lett. 1998;439:317–320. doi: 10.1016/s0014-5793(98)01400-8. [DOI] [PubMed] [Google Scholar]
- 50.Wilson W A, Hawley S A, Hardie D G. Glucose repression/derepression in budding yeast: SNF1 protein kinase is activated by phosphorylation under derepressing conditions, and this correlates with a high AMP:ATP ratio. Curr Biol. 1996;6:1426–1434. doi: 10.1016/s0960-9822(96)00747-6. [DOI] [PubMed] [Google Scholar]
- 51.Woods A, Cheung P C F, Smith F C, Davison M D, Scott J, Beri R K, Carling D. Characterization of AMP-activated protein kinase β subunit and γ subunit—assembly of the heterotrimeric complex in vitro. J Biol Chem. 1996;271:10282–10290. doi: 10.1074/jbc.271.17.10282. [DOI] [PubMed] [Google Scholar]
- 52.Woods A, Munday M R, Scott J, Yang X L, Carlson M, Carling D. Yeast Snf1 is functionally related to mammalian AMP-activated protein kinase and regulates acetyl-CoA carboxylase in vivo. J Biol Chem. 1994;269:19509–19515. [PubMed] [Google Scholar]
- 53.Woods A, Salt I, Scott J, Hardie D G, Carling D. The α1 and α2 isoforms of the AMP-activated protein kinase have similar activities in rat liver but exhibit differences in substrate specificity in vitro. FEBS Lett. 1996;397:347–351. doi: 10.1016/s0014-5793(96)01209-4. [DOI] [PubMed] [Google Scholar]