Abstract
(1) Background: The incidence of papillary thyroid cancers is increasing. Papillary neoplasm metastasizes to the central and lateral lymph nodes of the neck. The recurrence rate is less than 30%. The gold standard of treatment for lymph node recurrences is surgery, but surgery is burdened by a high rate of complications. Therefore, laser ablation of recurrent lymph nodes has been recognized as an alternative treatment with minimal invasiveness, a low complication rate and a curative effect. (2) Methods: We analyzed 10 patients who underwent a total thyroidectomy and metabolic radiotherapy and who developed a lymph node recurrence in the laterocervical compartment in the following 12–18 months. (3) Results: Patients developed lymph node recurrence at IV and Vb levels in 70% and 30% of cases, respectively. All patients were treated with a single laser ablative session. Hydrodissection was performed in all patients. The energy delivered was 1120 ± 159.3 Joules and 3–4 Watts in 362 ± 45.7 s. No complications were reported. All patients underwent a 6-month follow-up. A volumetric reduction of 40.12 ± 2.2%, 49.1 ± 2.13% and 59.8 ± 3.05%, respectively at 1-, 3- and 6-months of follow-up was reported. (4) Conclusions: At 6 months, a fine needle aspiration was performed, which was negative for malignant cells and negative for a dosage of Thyroglobulin in eluate. The laser ablation is an effective alternative to surgical treatment.
Keywords: thyroid carcinoma, laser ablation, papillary thyroid carcinoma, lymph nodes metastases, recurrence
1. Introduction
In the last decade, we have seen an exponential increase in the incidence of microcarcinomas (PTMC) and papillary thyroid carcinoma (PTC) due to easier access to imaging methods. This is associated with an increase in the diagnosis of synchronous and metachronous lymph node metastases. This event has opened new clinical and therapeutic scenarios [1,2,3]. PTC spreads by the lymphatic route; in 30% of cases it can be associated with synchronous lymph node metastases and in 30% of cases with metachronous lymph node metastases [4]. The gold standard treatment of both synchronous and metachronous lymph node metastases is surgery, which involves an extensive lymphectomy of the laterocervical and/or central compartments or a lymphectomy of one or more compartments. Surgery is burdened by a higher rate of major complications in the case of reoperations and in patients treated with metabolic radiotherapy, greater than in the primary intervention [4,5]. In recent decades, due to complications, several minimally invasive techniques have been perfected such as percutaneous ethanol injection (PEI), percutaneous laser ablation (LA), microwave ablation and radiofrequency ablation (RFA) [6,7,8,9]. US-guided LA has played a prominent role due to the ability to perform ablations of small lesions safely [7].
In 1960 the first handcrafted laser with a ruby was made [10]. Since then, the laser technique has undergone considerable developments and it has been applied in various medical fields. In 1962 the ruby laser was used for the first time in medicine, retinal surgery and dentistry [11,12,13]. Today, there are various types of lasers. The LA is a type of minimally invasive procedure. The use of LA must always be supported by scientific evidence, due to the complications and the side effects deriving from incorrect use.
LA was first used in the treatment of thyroid disease in the 2000s. Today it is routinely used in the ablation of symptomatic benign thyroid nodules, recurrence, and lymph node metastases. LA is used in the treatment of metastatic lymph nodes lesions due to the possibility of inducing small volumes of well-defined and predictable tissues necrosis. This feature makes LA suitable for the ablative treatment of metachronous metastases, adjacent to delicate anatomical structures.
Our study analyzes retrospectively the first 10 cases that underwent LA of the metastatic lymph nodes after a total thyroidectomy and radiometabolic therapy for PTC. The treated lymph node metastases occurred in the 12–18 months following the radiometabolic treatment with I131. Recurrence was diagnosed by serum thyroglobulin (Tg) elevation, a positive ultrasound, a fine needle aspiration positive for malignant cells and positive Tg in the eluate.
2. Materials and Methods
We retrospectively collected data from 10 consecutive patients undergoing LA treatment of metachronous lymph node metastases at the Department of Endocrine and Ultrasound-Guided Surgery of the “Ospedale del Mare”, Naples, Italy. The patients enrolled in the study were referred to our department in the period between December 2017 and June 2018 for the surgical treatment and in the period between June 2019 and December 2020 for the LA treatment. All included patients underwent a total thyroidectomy and subsequent metabolic radiotherapy (RAI) for the diagnosis of PTC, according to the American Thyroid Association (ATA) guidelines [4]. During oncological follow-up, all patients had an increase in serum Tg levels > 20 μg/L. Patients referred for LA treatment had a high surgical risk of comorbidities and/or had refused further surgery or were ineligible for a second RAI treatment. All cases were evaluated in the multidisciplinary oncological group prior to treatment.
The Campania Centro ethics committee approved the protocol and all patients signed a written informed consent. All procedures were performed in accordance with the Helsinki Declaration.
We included patients over 18 years with a histological diagnosis of PTC in the 12–18 months prior to ablative treatment. All patients were treated with a total thyroidectomy and RAI. During the follow-up, all patients showed an increase in serum Tg (values > 20 μg/L) and ultrasound evidence of pathological lymph nodes in the laterocervical compartment. All patients underwent a fine needle aspiration cytology (FNAC) of the lesion, which showed the presence of malignant cells and Tg values in the eluate >5000 μg/L. The volume of the lesion was calculated during the ultrasound examination. A complete history and preoperative hematological evaluation were collected in an electronic database. We evaluated patients’ hematological tests: complete blood cells count, thyroid function and thyroid antibodies. The B-mode ultrasound examination was performed to evaluate the lymph node level, the lesion’s volume and the presence of further metastases. Clinical and demographic data (age, sex, volume of nodal metastasis, level of metastasis) were collected in an electronic database.
Patients followed an ultrasound follow-up program after laser ablative treatment, with a timing of 1-, 3- and 6-months. During the follow-up, we calculated the treated lesion’s volume in ml, the volumetric reduction in percentage compared to the pre-treatment volume and the serum Tg value. At the end of the 6-month follow-up, we performed a lesion’s FNAC. In all treated cases we obtained a negative cytology for malignant cells and absence of Tg in the eluate.
The ultrasound examination was conducted with a 7.5–12 MHz linear probe (MyLab™ ClassC and MyLab™ 9 Platform, Esaote Biomedica, Genova, Italy). The basal volume of the lymph node lesion was calculated using the software. Vascularity was studied by Color Doppler (CD) examination and slow flow analysis.
We analyzed the number of laser fibers used, the Watts, the Joules and the time expressed in seconds. We used a continuous wave multi-source laser system with a length of 1064 nm (EchoLaser ModiLiteTM, Elesta SpA, Calenzano, Italy). The procedures were performed with ultrasound guidance and with a free hand technique. Currently, EchoLaser can now be used with a recently released new software (available with the ESI, EchoLaser Smart Interface) for a more precise and safer planning of the procedure. The laser generator, connected to the optical fiber, produces an illumination of the optical fiber which generates heat in tissues adjacent to the tip by. The temperature increase induces the denaturation of the proteins and irreversible cell damage. The energy produced decreases exponentially distancing from the tip of the optical fiber.
The patients underwent a single LA session. The procedure was performed by placing the patients in a supine position with a moderate hyperextension of the neck. We performed a pericapsular local anesthesia with 2% Lidocaine.
The applicators used were 21 Gauche (G) needles. The output power varied from 3 to 5 Watts (W) and in our population we used an output power of 3 W in nine cases and a power of 4 W in only one case, due to the lymph node’s volume. The fibers used were quartz optical fibers with a flat tip and a diameter of 300 microns. The applicators were inserted into the target lesion through guides applied to the probe with different angles of incidence depending on the pre-treatment planning. The procedure began with the insertion of the 21G introducer into the target lesion along its major axis. The treatment was performed with a prefixed power, while the lighting time of the optical fiber varied according to the volume to be treated. The 21G applicators allow atraumatic, precise and multiple positioning of the fibers. More optical fibers can be used, as in one of the cases presented, with a distance of at least 0.8 cm. The tip of the fiber must have a minimum safety distance of 5–10 mm from the anatomical structures of the neck. We performed a preliminary hydrodissection with a 5% glucose solution to ward off the lymph node metastases from the anatomical structures of the neck [14,15,16].
We performed the “pullback” technique in one case. The “pullback” technique can be used in the treatment of lesions with a major axis greater than 2 cm [7]. The technique allows the enlargement of the area to be ablated and the planning made at the beginning of the procedure to be respected. On the ultrasound, the ablated area appears hyperechoic due to the micro bubbles created by the evaporation of liquids. The hyperechoic area increases its size as the volume of the necrosis area increases. The treatment can be considered concluded when the volume of the hyperechoic area remains stable. The evaluation of the treatment’s effectiveness is performed through the Color Doppler (CD) analysis [14,15,16,17]. The real volume of the area subjected to ablation can be determined 72 h after the end of the treatment, as the cell damage becomes permanent [14].
At the end of the procedure the functionality of the vocal cords was evaluated with an ecolaryngoscopy.
All patients were discharged two hours after the procedure in the absence of any type of symptoms. There were no cases of major or minor complications.
Statistical analysis was performed with SPSS version 23 (SPSS©, Chicago, IL, USA). Continuous variables were described as mean, standard deviation (SD) and range, while categorical variables were described as number of cases and the percentage.
3. Results
We retrospectively enrolled 10 patients (5 males and 5 females) treated with a single LA session for a single lymph node recurrence. All patients had a diagnosis of classic variant PTC that had been previously treated with a total thyroidectomy and RAI. The demographic characteristics are showed in Table 1. The mean age was 40.2 years (±17.98 SD). All patients were treated with suppressive therapy with L-thyroxine. During the oncological follow-up, all patients had an increase in the serum Tg value greater than 20 μg/L. The increase in serum Tg was followed by an ultrasound of the neck which revealed the presence of disease. There were seven metastatic lymph nodes at level IV and three at level Vb with a baseline volume of 1.82 mL (±3.45 SD). In all cases, a FNAC of the suspected lesion was performed with a Tg assay on the eluate. Data are reported in Table 1 and Table 2.
Table 1.
Demographic and clinical data of the study population (FNAC, fine needle aspiration cytology; Tg, thyroglobulin; mL, milliliter).
| Data | Population |
|---|---|
| Gender | |
| Male | 5 (50%) |
| Female | 5 (50%) |
| Age | 40.2 ± 17.98 |
| FNAC | Positive for malignant cell |
| Eluate Tg | >5000 μg/L |
| Lymph node level | |
| IV | 7 (70%) |
| Vb | 3 (30%) |
| Side | |
| Right | 4 (40%) |
| Left | 6 (60%) |
| Lymph node volume | 1.82 ± 3.45 |
| Fiber optic | 1.01 ± 0.31 |
| Watt | 3.1 ± 0.31 |
| Time in second | 531.86 ± 109.5 |
| Joule | 1256 ± 396 |
| 1-month volume in ml | 1.12 ± 2.18 |
| % reduction at 1 month | 40.12 ± 2.2 |
| 3-months volume in ml | 0.88 ± 1.65 |
| % reduction at 3 months | 49.1 ± 2.13 |
| 6-months volume in ml | 0.706 ± 1.30 |
| % reduction at 6 months | 59.8 ± 3.05 |
Table 2.
Demographic and clinical data of the included patients.
| Patient | Gender | Age | FNAC | Eluate Tg (μg/L) | Lymph Node Level | Side | Lymph Node Volume (mL) | Fiber Optic | Watts | Time (Seconds) | Joules |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 20 | Positive | >5000 | IV | Right | 0.7 | 1 | 3 | 270 | 810 |
| 2 | Male | 19 | Positive | >5000 | IV | Right | 0.35 | 1 | 3 | 365 | 1095 |
| 3 | Male | 19 | Positive | >5000 | IV | Right | 0.25 | 1 | 3 | 365 | 1095 |
| 4 | Female | 59 | Positive | >5000 | Vb | Left | 11.58 | 2 | 4 | 340 | 2720 |
| 5 | Female | 59 | Positive | >5000 | IV | Left | 0.46 | 1 | 3 | 335 | 1005 |
| 6 | Female | 63 | Positive | >5000 | IV | Left | 1.27 | 1 | 3 | 420 | 1260 |
| 7 | Male | 28 | Positive | >5000 | IV | Right | 1.32 | 1 | 3 | 400 | 1200 |
| 8 | Female | 35 | Positive | >5000 | VB | Right | 1.05 | 1 | 3 | 395 | 1185 |
| 9 | Female | 48 | Positive | >5000 | VB | Left | 0.79 | 1 | 3 | 325 | 975 |
| 10 | Female | 52 | Positive | >5000 | IV | Left | 0.98 | 1 | 3 | 405 | 1215 |
The size and margins of the metastases determined the number of laser fibers used. In metastases of less than 1 cm of maximum diameter (nine cases), only one fiber was used, while in metastases greater than 1 cm of maximum diameter (only one case), two laser fibers were used.
The treatment protocol, W and Joules, was planned before the start of the procedure by the expert operator (S.S.). An output power of 3 W was used in nine cases, while a power of 4 W was used in only one case for a mean of 531.86 s (±109.5 SD) and a mean of 1256 Joules (±396 SD). The data relating to individual cases analyzed are shown in Table 2.
After laser ablation treatment, the mean volume of the nodule was 1.12 ± 2.18 mL with a 40.12% reduction in volume 1 month after treatment. After 3 months the mean volume was 0.88 ± 1.65 mL with a 49.1% reduction in volume and after 6 months the mean volume was 0.706 ± 1.30 mL with a 59.8% reduction in volume (Table 3). The data showed a volume reduction of 60% after 6 months, but the most significant data were the absence of malignant cells at the FNAC performed after 6 months, the absence of Tg in the eluate and absent serum Tg values.
Table 3.
Clinical data of volume reduction in the lymph nodes metastases treated with laser ablation.
| Patients | 1 Month Volume (mL) | % Reduction at 1 Month | 3 Months Volume (mL) | % Reduction at 3 Months | 6 Months Volume (mL) | % Reduction at 6-Months |
|---|---|---|---|---|---|---|
| 1 | 0.1 | 42 | 0.08 | 50 | 0.07 | 60 |
| 2 | 0.22 | 37.2 | 0.19 | 45 | 0.16 | 55 |
| 3 | 0.15 | 40 | 0.13 | 48 | 0.11 | 56 |
| 4 | 7.3 | 37 | 5.56 | 52 | 4.4 | 62 |
| 5 | 0.22 | 43 | 0.2 | 47 | 0.26 | 57 |
| 6 | 0.74 | 42 | 0.62 | 51 | 0.48 | 62 |
| 7 | 0.76 | 42 | 0.64 | 51 | 0.49 | 63 |
| 8 | 0.61 | 41 | 0.53 | 50 | 0.38 | 64 |
| 9 | 0.48 | 39 | 0.4 | 49 | 0.32 | 59 |
| 10 | 0.61 | 38 | 0.51 | 48 | 0.39 | 60 |
The ablative techniques were conducted by one skilled operator (S.S.) of a tertiary thyroid center. There were no major complications or minor complications.
4. Discussion
The treatment of PTC is a total thyroidectomy with or without central lymphadenectomy followed or not followed by RAI therapy depending on the histotype and the presence of mutations [4]. Lymph node recurrence can occur in about 1–2% of patients after surgical and RAI therapy [4]. Lymph node recurrences can be treated with surgery or with additional RAI therapy, even if reoperations are burdened by a very high rate of major complications (25% of cases of vocal cord palsy) and metabolic radiotherapy is burdened by a very high failure rate when repeated [4]. In fact, various studies have shown that neoplastic cells subjected to repeated RAI therapies lose the ability to pick up radioactive iodine [4]. Ultrasound-guided mini-interventional procedures are an effective and safe alternative [6,7,8,9]. The minimally invasive ultrasound-guided procedures have been developed and validated. They can be performed repeatedly without an increased complication rate, without hospitalization and general anesthesia; all characteristics well accepted by patients. PEI was the first treatment used but was soon abandoned due to the unpredictable spread of alcohol [18]. Subsequently, RFA took over due to its ability to generate a complete necrosis of the lesion, even if burdened by high complication rates [19]. LA appears to be the safest and the most effective technique.
The LA technique of body tissues was proposed in 1983 by Bown and has undergone numerous revisions, standardizations and experiments [20]. Numerous data have emerged from the literature in support of minimally invasive ablative techniques. Dupuy et al. [21] treated eight cases of lymph node metastases with RFA; all healed with a single application but two cases of major complications were reported. Kim et al. [22] reported a series of 73 patients with lymph node recurrence, 27 treated with RFA and 46 treated with surgery. The study did not show statistical differences between the two groups in relation to disease free survival at 1 and 3 years. Unlike other techniques, LA is preferred in the treatment of thyroid pathologies due to the size of the introducers, which are reduced, easily reaching the target tissue with a low risk of tissue injury and bleeding. The thermic energy used allows a precise ablation of small volumes, reducing heat dissipation and the subsequent necrosis of the surrounding tissues. In cases of large lesions, multiple needles can be used simultaneously to increase the volume of the necrosis area. Mauri et al. [3] presented a series of 24 patients treated with LA for PTC’s lymph node recurrence. The study showed that 86.9% of patients had a complete ablation 30 months after treatment and in 79.1% of patients no disease was detected on imaging methods. In only five cases a second session was required and no increase in the complications rate was reported. Zhou et al. [23] published a study on 81 patients with PTMC, 36 treated with LA and 45 treated with a lobectomy with a unilateral sixth compartment lymphectomy. The study showed that, with a mean follow-up of 49.2 months and 48.5 months, respectively, no patients developed lymph node recurrences. Therefore, LA can be considered an alternative treatment for patients who are unsuitable for or who refuse surgery.
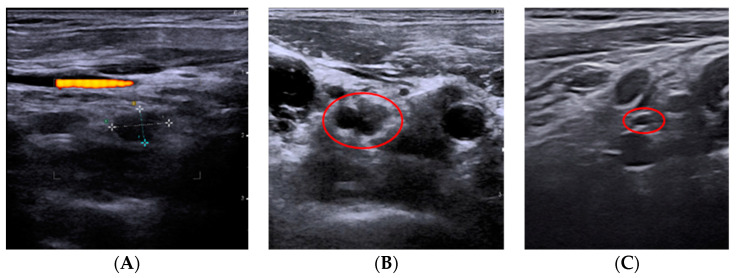
Our series of 10 patients showed that a normalization of serum thyroglobulin levels, the absence of neoplastic cells in the control FNC and the absence of Tg in the eluate were recorded 6 months after the ablative treatment. In only one case we used two laser fibers due to the large size of the lesion and neither major nor minor complications were reported. The risk of ablative treatment in relation to lymph node recurrences is thermic injury to the nerve structures; we reduced this risk by practicing a hydrodissection, with a 5% glucose solution, of the lesion and reducing the power and increasing the application time. Figure 1 shows one of the cases included in our case series. Image A shows the lymph node recurrence prior to the treatment. Image B shows the tip of the laser fiber in the center of the lesion before the ablative treatment was started. Image C shows the ultrasound at 6 months. The lymph node lesion appears as a hypoechoic area with a hyperechoic halo (image of scarring fibrosis).
Figure 1.
The images (A) show a lymph node recurrence to treat with laser ablation. Image (B) shows the tip of the laser fiber in the center of the lesion before the start of the ablative treatment. Image (C) shows the ultrasound follow-up at 6 months. The lymph node recurrence appears as a hypoechoic area with a hyperechoic halo (image of scarring fibrosis).
Our data demonstrate that LA of PTC’s lymph node recurrence is a viable treatment. This technique allows a reduction in the surgical treatment of metachronous metastases from PTC with the reduction in morbidity associated with a reoperation.
It is useful in cases of multiple lymph node recurrences occurring during the follow-up, even after previous treatment. All these patients would be candidates for a lymphadenectomy with an exponential increase in complications.
However, our study had limitations: the sparse case history, the absence of randomization of the population, the impossibility of comparison with other techniques and the lack of follow-up at 5 and 10 years. This is a preliminary study; further analyses are needed.
5. Conclusions
LA represents a safe, effective and minimally invasive alternative to surgery in the treatment of lymph node recurrences from papillary thyroid carcinoma treated with surgical therapy and metabolic radiotherapy. This technique allows a total necrosis of the metastatic lesion associated with a reduced complications rate compared to surgical treatment.
Author Contributions
Conceptualization, C.O. and S.S.; Methodology, C.O. and S.S.; Software, C.O. and S.S.; Validation, C.O. and S.S.; Formal Analysis, C.O. and S.S.; Investigation, C.O. and S.S.; Resources, C.O. and S.S.; Data Curation, C.O. and S.S.; Writing—Original Draft Preparation, C.O. and S.S.; Writing—Review and Editing, C.O. and S.S.; Visualization, C.O., C.M., G.A., M.G.E., U.B. and S.S.; Supervision, C.O. and S.S.; Project Administration, C.O. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by Elesta SpA, Calenzano, Italy.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee “Campania Centro” (protocol code 0170338/E of 15 July 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are deposited in an electronic database at the Department of Endocrine and Ultrasound-guided Surgery, “Ospedale del Mare”, Naples, Italy.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lim H., Devesa S.S., Sosa J.A., Check D., Kitahara C.M. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA. 2017;317:1338–1348. doi: 10.1001/jama.2017.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.La Vecchia C., Malvezzi M., Bosetti C., Garavello W., Bertuccio P., Levi F., Negri E. Thyroid cancer mortality and incidence: A global overview. Int. J. Cancer. 2015;136:2187–2195. doi: 10.1002/ijc.29251. [DOI] [PubMed] [Google Scholar]
- 3.Mauri G., Cova L., Ierace T., Baroli A., Di Mauro E., Pacella C.M., Goldberg S.N., Solbiati L. Treatment of Metastatic Lymph Nodes in the Neck from Papillary Thyroid Carcinoma with Percutaneous Laser Ablation. Cardiovasc. Interv. Radiol. 2016;39:1023–1030. doi: 10.1007/s00270-016-1313-6. [DOI] [PubMed] [Google Scholar]
- 4.Haugen B.R. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: What is new and what has changed? Cancer-Am. Cancer Soc. 2017;123:372–381. doi: 10.1002/cncr.30360. [DOI] [PubMed] [Google Scholar]
- 5.Ito Y., Miyauchi A. Lateral and mediastinal lymph node dissection in differentiated thyroid carcinoma: Indications, benefits, and risks. World J. Surg. 2007;31:905–915. doi: 10.1007/s00268-006-0722-0. [DOI] [PubMed] [Google Scholar]
- 6.Fontenot T.E., Deniwar A., Bhatia P., Al-Qurayshi Z., Randolph G.W., Kandil E. Percutaneous ethanol injection vs reoperation for locally recurrent papillary thyroid cancer: A systematic review and pooled analysis. JAMA Otolaryngol. Head Neck Surg. 2015;141:512–518. doi: 10.1001/jamaoto.2015.0596. [DOI] [PubMed] [Google Scholar]
- 7.Papini E., Bizzarri G., Bianchini A., Valle D., Misischi I., Guglielmi R., Salvatori M., Solbiati L., Crescenzi A., Pacella C.M., et al. Percutaneous ultrasound-guided laser ablation is effective for treating selected nodal metastases in papillary thyroid cancer. J. Clin. Endocrinol. Metab. 2013;98:E92–E97. doi: 10.1210/jc.2012-2991. [DOI] [PubMed] [Google Scholar]
- 8.Yue W., Chen L., Wang S., Yu S. Locoregional control of recurrent papillary thyroid carcinoma by ultrasoundguided percutaneous microwave ablation: A prospective study. Int. J. Hyperth. 2015;31:403–408. doi: 10.3109/02656736.2015.1014433. [DOI] [PubMed] [Google Scholar]
- 9.Wang L., Ge M., Xu D., Chen L., Qian C., Shi K., Liu J., Chen Y. Ultrasonography-guided percutaneous radiofrequency ablation for cervical lymph node metastasis from thyroid carcinoma. J. Cancer. Res. 2014;10:C144–C149. doi: 10.4103/0973-1482.145844. [DOI] [PubMed] [Google Scholar]
- 10.Maiman T.H. Optical and microwave-optical experiments in ruby. Phys. Rev. Lett. 1960;4:564–566. doi: 10.1103/PhysRevLett.4.564. [DOI] [Google Scholar]
- 11.Zaret M.M., Breinin G.M., Schmidt H., Ripps H., Siegel I.M., Solon L.R. Ocular lesions produced by an optical maser (laser) Science. 1961;134:1525–1526. doi: 10.1126/science.134.3489.1525. [DOI] [PubMed] [Google Scholar]
- 12.Goldman L., Hornby P., Meyer R., Goldman B. Impact of the laser on dental caries. Nature. 1964;25:203–417. doi: 10.1038/203417a0. [DOI] [PubMed] [Google Scholar]
- 13.Schena E., Saccomandi P., Fong Y. Laser ablation for cancer: Past, present and future. J. Funct. Biomater. 2017;8:19. doi: 10.3390/jfb8020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed M., Brace C.L., Lee F.T., Jr., Goldberg S.N. Principles of and advances in percutaneous ablation. Radiology. 2011;258:351–369. doi: 10.1148/radiol.10081634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomsen S. Pathologic analysis of photothermal and photomechanical effects of laser-tissue interactions. Photochem. Photobiol. 1991;53:825–835. doi: 10.1111/j.1751-1097.1991.tb09897.x. [DOI] [PubMed] [Google Scholar]
- 16.Jacques S.L. Laser-tissue interactions. Photochemical, photothermal and photomechanical. Surg. Clin. North Am. 1992;72:531–558. doi: 10.1016/S0039-6109(16)45731-2. [DOI] [PubMed] [Google Scholar]
- 17.Stafford R.J., Fuentes D., Elliott A.A., Weinberg J.S., Ahrar K. Laser-induced thermal therapy for tumor ablation. Crit. Rev. Biomed. Eng. 2010;38:79–100. doi: 10.1615/CritRevBiomedEng.v38.i1.70. [DOI] [PubMed] [Google Scholar]
- 18.Solbiati L., Giangrande A., De Pra L., Bellotti E., Cantù P., Ravetto C. Percutaneous ethanol injection of parathyroid tumors under US guidance: Treatment for secondary hyperparathyroidism. Radiology. 1985;155:607–610. doi: 10.1148/radiology.155.3.3889999. [DOI] [PubMed] [Google Scholar]
- 19.Shin J.E., Baek J.H., Lee J.H. Radiofrequency and ethanol ablation for the treatment of recurrent thyroid cancers: Current status and challenges. Curr. Opin. Oncol. 2013;25:14–19. doi: 10.1097/CCO.0b013e32835a583d. [DOI] [PubMed] [Google Scholar]
- 20.Bown S.G. Phototherapy in tumors. World J. Surg. 1983;7:700–709. doi: 10.1007/BF01655209. [DOI] [PubMed] [Google Scholar]
- 21.Dupuy D.E., Monchik J.M., Decrea C., Pisharodi L. Radiofrequency ablation of regional recurrence from well-differentiated thyroid malignancy. Surgery. 2001;130:971–977. doi: 10.1067/msy.2001.118708. [DOI] [PubMed] [Google Scholar]
- 22.Kim J., Yoo W.S., Park Y.J., Park D.J., Yun T.J., Choi S.H., Sohn C.H., Lee K.E., Sung M.W., Youn Y.K., et al. Efficacy and safety of radiofrequency ablation for treatment of locally recurrent thyroid cancers smaller than 2 cm. Radiology. 2015;276:909–918. doi: 10.1148/radiol.15140079. [DOI] [PubMed] [Google Scholar]
- 23.Zhou W., Ni X., Xu S., Zhang L., Chen L., Zhan W. Ultrasound-guided laser ablation versus surgery for solitary papillary thyroid microcarcinoma: A retrospective study. Int. J. Hyperth. 2019;36:897–904. doi: 10.1080/02656736.2019.1649475. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are deposited in an electronic database at the Department of Endocrine and Ultrasound-guided Surgery, “Ospedale del Mare”, Naples, Italy.