Abstract
Quinoa has acquired a great interest due to its high content of nutrients and biomolecules that have nutritional and medicinal properties. The aim of this study was to compare the total phenolic content (TPC), total flavonoids (TF), and the antioxidant capacity of 20 varieties of seeds and sprouts of quinoa extract. Quinoa seeds were germinated for 72 h and dried in an oven at 45 °C. The extracts were obtained by dynamic extraction using methanol. Phytochemical analysis with liquid chromatography coupled with mass spectrometry (LC-ESI-MS/MS), TPC, TF, and the antioxidant capacity was carried out and compared between both extracts. The TPC was determined with Folin-Ciocalteu reagent, TF with AlCl3, and the antioxidant capacity was determined according to the DPPH and ABTS assays. Sprout extracts showed high values of TPC (31.28 ± 0.42 mg GAE/g; Pasankalla variety), TF (14.31 ± 0.50 mg EQ/g; black Coito variety), and antioxidant capacity (IC50 (DPPH): 12.69 ± 0.29 µg/mL and IC50 (ABTS): 3.51 ± 0.04 µg/mL; Pasankalla). The extracts of the Pasankalla variety revealed 93 and 90 phytochemical constituents in the seeds and sprouts, respectively, such as amino acids, phenolic acids, flavonoids, fatty acids, and triterpene saponins, among others. Quinoa sprouts showed a high content of TPC and TF, and high antioxidant capacity compared with seed extracts, especially the Pasankalla variety.
Keywords: Amaranthaceae, free radical, superfoods, phytochemical analysis, flavonoids, phenols, amino acids
1. Introduction
Quinoa (Chenopodium quinoa Willd.) is a pseudocereal belonging to the Amaranthaceae family that is native to the Andean region in South America [1]. Peru is the leading quinoa-exporting country, exporting quinoa with a value of $98.5 million dollars, followed by Bolivia, the Netherlands, the United States, Spain, Germany, Canada, France, Ecuador, and Belgium [2]. Quinoa seeds are known to have a high protein content ranging from 11% to 19%. The seeds are a source of amino acids (isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, valine, histidine, cysteine, tyrosine, glycine, arginine, proline, serine, glutamine, alanine, and aspartic acid), carbohydrates (49% to 68% dry weight), fat (2% to 9.5%), vitamins (thiamine, riboflavin, folic acid, and niacin), and minerals such as iron, zinc, magnesium, and copper (2.4% to 4.8%) [3]. Additionally, some phytochemical constituents such as saponins, phenolic compounds (ferulic, sinapinic and gallic acids, kaempferol, isorhamnetin, and rutin) [4], and peptides with therapeutic activity have been determined, making this crop very attractive for a wide range of food products [5]. Quinoa has been traditionally used in tortillas, pasta, flour, cookies, bread, and soups, among others, and is considered to be a gluten-free superfood and a source of fiber dietary [6]. Thus, quinoa is considered to be an acceptable food worldwide and is highly recommended for vegetarians.
On the other hand, sprouts are obtained by germinating the seeds and provide multiple nutritional and therapeutic benefits to those who consume them in different ways, due to the increase in the availability of nutrients such as fatty acids and carbohydrates, as well as polyphenols and flavonoids, during the germination process, which improves their antioxidant capacity [7]. These changes are due to a multitude of biochemical processes, which generate alterations in the composition of primary and secondary metabolites, producing an intrinsic change in the phenolic compounds and antioxidant activity [8]. Sprouts can improve the nutritional quality of a grain by eliminating or inactivating some antinutritional factors and increasing the digestibility of proteins and starches [9]. During germination, the original composition of the seed changes: the nitrogen-containing proteins move towards smaller protein fractions, oligopeptides, and free amino acids (some increase; others decrease or are not altered). Consequently, the changes increase the biological protein value of the sprouts, and digestibility is higher than in seeds [10].
Studies have reported that quinoa sprouts have high levels of amino acids, peptides, vitamins, and minerals but also include antinutritional components such as tannin, lectin, trypsin inhibitor, and galactoside, although at lower values than in non-germinated seeds [11]. The main enzyme involved in the early phase during the sprouting of quinoa seeds seems to be α-amylase, which leads to the generation of new compounds [12]. Some biological studies in quinoa sprouts have reported hepatoprotective, antioxidant [13], and anti-α-amylase effects in vitro [14], and hypoglycemic effects in diabetic rats [15]. Currently, there are no studies on the antioxidant activity of a wide variety of quinoa sprouts grown in Peru. Thus, as the germination process is a strategy for obtaining sprouts and improving the antioxidant capacity, total phenols, and flavonoids, thereby increasing its nutraceutical value, the main aim of this study was to compare the total phenolic content, flavonoids, and antioxidant capacity of the seeds and sprouts of 20 varieties of quinoa and analyze the phytochemical constituents of varieties with major antioxidant capacity using liquid chromatography-mass spectrometry (LC-ESI-MS/MS). To carry out this study, four phases were developed:
-
(a)
Germinating 20 varieties of quinoa seeds under laboratory conditions and extracting their phytochemical constituents by maceration with methanol.
-
(b)
Determining the total phenolic content (TPC) and total flavonoids (TF) of the seeds and sprouts of quinoa.
-
(c)
Evaluating the antioxidant capacity of the seeds and sprouts of quinoa using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azinobis-(3-ethylbenzothiazoline)-6-sulfonic acid (ABTS) methods.
-
(d)
Analyzing the phytochemical constituents of the seeds and sprouts of the quinoa variety with the best results obtained regarding the antioxidant capacity with liquid chromatography-mass spectrometry (LC-ESI-MS/MS).
2. Results
2.1. Germination Process
Sprouts were obtained in a time of 72 h, and measured between 1.7 and 2.3 cm in length for all varieties. However, the red variety achieved the greatest length among all varieties (2.1–2.3 cm). The other varieties had lengths as follows: White Junín Ayacucho, 1.7–1.9 cm; T-256, 1.8–1.9 cm; Pasankalla, 1.7–1.8 cm; Suano Puno, 1.7–1.9 cm; T-38, 1.8–2.0 cm; Yellow Sacaca, 1.9–2.0 cm; T-45, 1.7–1.9 cm; Santa Ana, 1.7–1.8 cm; T-61 Pomata, 1.8–1.9 cm; CQA-048, 1.8–2.0 cm; Black Collana, 1.7–1.9 cm; T-72 Huancayo, 1.8–1.9 cm; CQA-043, 1.8–1.9 cm; Salcedo, 1.8–2.0 cm; Ayacucho Compuesto, 1.7–1.9 cm; White Choclito, 1.7–1.9 cm; Yellow Maranganí, 1.9–2.1 cm; Black Coito, 1.7–1.9 cm; and Black, 1.8–2.0 cm. Figure 1 shows the 20 varieties of quinoa germinated under standard laboratory conditions of temperature, humidity, and time.
Figure 1.
Twenty varieties of quinoa sprouts. (1), White Junín Ayacucho; (2), T-256; (3), Pasankalla; (4), Suano Puno; (5), T-38; (6), Yellow Sacaca; (7), T-45; (8), Santa Ana; (9), T-61 Pomata; (10), CQA-048; (11), Black Collana; (12), T-72 Huancayo; (13), CQA-043; (14), Salcedo; (15), Ayacucho Compuesto; (16), White Choclito; (17), Red; (18), Yellow Maranganí; (19), Black Coito; (20), Black.
2.2. Total Phenolic Content
The TPC of sprouts was found to range from 19.15 ± 1.54 to 31.28 ± 0.42 mg GAE/g of methanolic extract, being highest in the Pasankalla variety, CQA-048, Black Collana, and Black Coito. On the other hand, in quinoa seed extracts, the variation was from 11.72 ± 0.32 to 28.32 ± 0.49, being greater in the Pasankalla, Black Collana, and Black Coito varieties (Table 1). There was a significant difference between sprout and seed extracts for TPC, (paired sample t-test; p < 0.05), with TPC being higher in sprout extracts than in seed extracts, with an average of 24.57 ± 3.49 mg GAE/g in sprout extracts and 20.12 ± 4.37 mg GAE/g in seed extracts.
Table 1.
Total phenolic content (TPC) and total flavonoids (TF) in the sprouts and seeds of 20 varieties of quinoa.
| Variety | TPC mg EAG/g ME |
TF mg EQ/g ME |
||
|---|---|---|---|---|
| Quinoa Sprouts Mean ± SD |
Quinoa Seeds Mean ± SD |
Quinoa Sprouts Mean ± SD |
Quinoa Seeds Mean ± SD |
|
|
23.32 ± 1.63 | 20.95 ± 0.79 | 11.52 ± 0.26 | 8.77 ± 0.26 * |
|
24.78 ± 0.21 | 13.82 ± 1.04 * | 11.23 ± 0.19 | 10.23 ± 0.95 |
|
31.28 ± 0.42 | 28.32 ± 0.49 * | 13.48 ± 0.38 | 11.52 ± 0.92 * |
|
19.62 ± 0.42 | 17.25 ± 0.66 * | 8.60 ± 0.48 | 8.56 ± 0.38 |
|
21.05 ± 0.40 | 21.75 ± 1.25 | 10.06 ± 0.57 | 9.81 ± 0.25 |
|
24.22 ± 0.31 | 23.58 ± 0.61 | 11.19 ± 0.38 | 8.23 ± 0.29 * |
|
21.02 ± 0.15 | 19.38 ± 2.06 | 11.06 ± 0.21 | 8.39 ± 0.38 * |
|
23.02 ± 0.74 | 18.23 ± 1.01 * | 9.94 ± 0.63 | 7.06 ± 0.33 * |
|
21.12 ± 1.50 | 15.55 ± 0.20 * | 10.94 ± 0.33 | 8.73 ± 0.31 * |
|
28.82 ± 0.67 | 21.32 ± 0.72 * | 7.44 ± 0.50 | 6.23 ± 0.26 * |
|
28.58 ± 1.21 | 26.98 ± 0.25 * | 13.44 ± 0.58 | 8.73 ± 0.14 * |
|
19.15 ± 1.54 | 18.58 ± 0.65 | 12.35 ± 0.48 | 9.81 ± 0.45 * |
|
26.05 ± 0.17 | 11.72 ± 0.32 * | 12.15 ± 0.08 | 11.31 ± 0.50 |
|
20.98 ± 1.99 | 12.38 ± 0.61 * | 11.94 ± 0.13 | 9.81 ± 0.45 * |
|
28.05 ± 0.53 | 21.42 ± 1.17 * | 11.19 ± 0.25 | 10.98 ± 0.40 |
|
24.02 ± 0.78 | 20.78 ± 1.86 | 11.52 ± 0.31 | 9.90 ± 0.26 * |
|
26.05 ± 0.36 | 20.45 ± 0.44 * | 12.31 ± 0.50 | 10.52 ± 0.19 * |
|
27.98 ± 0.70 | 22.82 ± 1.12 * | 13.52 ± 0.44 | 10.98 ± 0.52 * |
|
28.18 ± 0.35 | 24.42 ± 0.75 * | 14.31 ± 0.50 | 9.94 ± 0.13 * |
|
24.12 ± 0.64 | 20.78 ± 0.35 * | 12.31 ± 0.45 | 9.73 ± 0.38 * |
| Total Average ± SD | 24.57 ± 3.49 | 20.12 ± 4.37 * | 11.52 ± 1.67 | 9.46 ± 1.40 * |
* p < 0.05 (paired sample t-test); mg GAE/g ME: mg equivalent to gallic acid per g of methanolic extract.; mg EQ/g ME: mg equivalent to quercetin per g of methanolic extract.
2.3. Total Flavonoids
In sprouts, the flavonoid content varied from 7.44 ± 0.50 to 14.31 ± 0.5 mg EQ/g of extract, being highest in the Black Coito, Yellow Maranganí, Pasankalla, and Black Collana varieties. In seed extract, the variation was from 6.23 ± 0.26 to 11.52 ± 0.92 EQ/g. The results showed a significant increase in the total flavonoids in sprouts compared with seed extracts, but in some varieties, the increase was not significant, such as in T-256, Suano Puno, T-38, CQA-043, and Ayacucho Compuesto (Table 1).
2.4. The Antioxidant Capacity Equivalent to Trolox
Table 2 shows the antioxidant capacity equivalent to Trolox (TEAC) of sprout and seed extracts, with the variation ranging, respectively, from 25.90 to 37.65 and from 25.03 to 29.60 µmol ET/mg of extract for the radical DPPH and from 57.05 to 90.84 and from 45.80 to 67.04 µmol ET/mg of extract for the radical ABTS. The sprouts with the highest antioxidant capacity for the radical DPPH were Pasankalla, White Junín Ayacucho, Yellow Sacaca, Black Collana, and Red; while for the radical ABTS, those with the highest antioxidant capacity were Black Collana, Black, Pasankalla, Suano Puno, Yellow Maranganí, Red, and Black Coito. Furthermore, it was found that in most of the varieties, significant differences appeared (Student’s t-test; p < 0.05), with antioxidant capacity being greater in sprouts than in seed extracts.
Table 2.
Antioxidant capacity equivalent to Trolox (TEAC) of the radical DPPH and ABTS of methanolic extracts of sprouts and seeds of 20 varieties of quinoa.
| Variety | TEAC-DPPH µmol TE/mg MS |
TEAC-ABTS µmol TE/mg MS |
||
|---|---|---|---|---|
| Quinoa Sprouts Mean ± SD |
Quinoa Seeds Mean ± SD |
Quinoa Sprouts Mean ± SD |
Quinoa Seeds Mean ± SD |
|
|
31.26 ± 0.56 | 28.47 ± 1.44 | 64.78 ± 1.63 | 54.68 ± 0.48 * |
|
28.38 ± 0.27 | 25.88 ± 0.72 * | 62.84 ± 1.65 | 61.95 ± 0.96 |
|
37.65 ± 0.88 | 29.60 ± 0.54 * | 78.79 ± 0.86 | 54.19 ± 0.41 * |
|
25.90 ± 0.36 | 25.24 ± 0.22 | 78.66 ± 2.02 | 53.10 ± 1.03 * |
|
27.67 ± 0.30 | 25.03 ± 0.18 * | 59.96 ± 5.62 | 48.88 ± 1.52 * |
|
30.54 ± 1.17 | 29.37 ± 0.82 | 63.21 ± 0.60 | 58.38 ± 2.14 * |
|
25.94 ± 0.29 | 25.54 ± 0.17 * | 60.55 ± 3.46 | 45.80 ± 0.37 * |
|
28.08 ± 0.07 | 26.93 ± 0.23 * | 60.74 ± 1.06 | 53.66 ± 0.55 * |
|
25.92 ± 0.15 | 25.77 ± 0.15 * | 65.40 ± 0.96 | 50.56 ± 2.29 * |
|
26.32 ± 0.12 | 25.64 ± 0.17 | 62.29 ± 2.59 | 64.91 ± 5.06 |
|
29.26 ± 0.40 | 25.90 ± 0.15 * | 90.84 ± 2.22 | 60.56 ± 4.28 * |
|
26.97 ± 0.40 | 25.75 ± 0.20 | 68.67 ± 0.64 | 59.40 ± 0.09 * |
|
26.17 ± 0.55 | 25.89 ± 0.23 | 57.05 ± 2.62 | 56.35 ± 0.34 |
|
26.21 ± 0.24 | 25.96 ± 0.23 * | 64.95 ± 0.83 | 58.21 ± 0.19 * |
|
26.45 ± 0.27 | 26.26 ± 0.20 | 68.91 ± 0.61 | 65.74 ± 0.25 * |
|
27.30 ± 0.23 | 26.88 ± 0.31 * | 58.84 ± 2.73 | 57.92 ± 0.75 |
|
28.60 ± 0.20 | 26.93 ± 0.36 * | 75.79 ± 1.26 | 67.04 ± 0.79 * |
|
27.51 ± 0.29 | 26.20 ± 0.12 * | 78.11 ± 1.69 | 63.76 ± 0.70 * |
|
28.04 ± 0.10 | 26.56 ± 0.16 * | 69.41 ± 0.87 | 63.68 ± 0.93 * |
|
27.67 ± 0.25 | 26.09 ± 0.06 * | 78.79 ± 2.36 | 56.43 ± 0.52 * |
| Total Average ± SD | 28.09 ± 2.68 | 26.50 ± 1.30 * | 68.43 ± 8.96 | 57.71 ± 5.83 * |
* p < 0.05; paired sample t-test.
2.5. The Half Inhibitory Concentration (IC50) of the Methanolic Extracts of Sprouts and Seeds of Quinoa
The half inhibitory concentration (IC50) (Table 3) represents the reduction to 50% of the initial absorbance of the DPPH and ABTS radicals, with the average variation for all varieties ranging from 12.69 to 18.45 mg/mL in sprout extracts and from 16.15 to 19.09 mg/mL in seed extracts using the DPPH assay. In the ABTS assay, the results ranged from 3.05 to 4.71 and from 4.13 to 6.04 mg/mL in sprout and seed extracts, respectively. There was a significant difference (p < 0.05) in the IC50 of the radicals DPPH and ABTS between the sprout and seed extracts, being lower in sprouts than in seed extracts.
Table 3.
Half inhibitory concentration (IC50) of the radicals DPPH and ABTS of methanolic extracts of sprouts and seeds of 20 varieties of quinoa.
| Variety | IC50 (mg/mL) | |||
|---|---|---|---|---|
| DPPH | ABTS | |||
| Quinoa Sprouts Mean ± SD |
Quinoa Seeds Mean ± SD |
Quinoa Sprouts Mean ± SD |
Quinoa Seeds Mean ± SD |
|
|
15.29 ± 0.27 | 16.81 ± 0.83 | 4.27 ± 0.11 | 5.06 ± 0.05 * |
|
16.84 ± 0.16 | 18.47 ± 0.52 * | 4.40 ± 0.12 | 4.47 ± 0.07 |
|
12.69 ± 0.29 | 16.15 ± 0.30 * | 3.51 ± 0.04 | 5.11 ± 0.04 * |
|
18.45 ± 0.26 | 18.93 ± 0.16 | 3.52 ± 0.10 | 5.21 ± 0.10 * |
|
17.27 ± 0.19 | 19.10 ± 0.14 * | 4.64 ± 0.45 | 5.66 ± 0.18 * |
|
15.66 ± 0.60 | 16.28 ± 0.46 | 4.38 ± 0.04 | 4.74 ± 0.17 * |
|
18.42 ± 0.20 | 18.71 ± 0.12 * | 4.58 ± 0.26 | 6.04 ± 0.05 * |
|
17.02 ± 0.04 | 17.75 ± 0.14 * | 4.55 ± 0.08 | 5.16 ± 0.06 * |
|
18.43 ± 0.08 | 18.54 ± 0.11 * | 4.23 ± 0.06 | 5.48 ± 0.25 * |
|
18.15 ± 0.09 | 18.63 ± 0.12 | 4.45 ± 0.18 | 4.28 ± 0.32 |
|
16.33 ± 0.22 | 18.45 ± 0.10 * | 3.05 ± 0.08 | 4.58 ± 0.32 * |
|
17.72 ± 0.27 | 18.55 ± 0.14 | 4.03 ± 0.04 | 4.66 ± 0.01 * |
|
18.26 ± 0.39 | 18.46 ± 0.17 | 4.86 ± 0.22 | 4.91 ± 0.03 |
|
18.23 ± 0.17 | 18.40 ± 0.16 * | 4.26 ± 0.06 | 4.75 ± 0.02 * |
|
18.07 ± 0.18 | 18.20 ± 0.14 | 4.01 ± 0.04 | 4.21 ± 0.02 * |
|
17.51 ± 0.15 | 17.34 ± 0.20 | 4.71 ± 0.21 | 4.78 ± 0.06 |
|
16.71 ± 0.12 | 17.75 ± 0.23 * | 3.95 ± 0.29 | 4.13 ± 0.05 |
|
17.37 ± 0.19 | 18.24 ± 0.09 * | 3.54 ± 0.07 | 4.34 ± 0.05 * |
|
17.04 ± 0.06 | 17.99 ± 0.11 * | 3.99 ± 0.05 | 4.41 ± 0.06 * |
|
17.27 ± 0.15 | 18.31 ± 0.04 * | 3.51 ± 0.11 | 4.90 ± 0.05 |
| Total Average ± SD | 17.14 ± 1.37 | 18.05 ± 0.84 * | 4.12 ± 0.50 | 4.84 ± 0.51 |
* p < 0.05; paired sample t-test.
Table 4 shows positive correlations between antioxidant capacity and both TPC and total flavonoids, and a negative correlation with IC50, with a significant difference in both cases (p < 0.01). This correlation indicates that while the concentration of TPC and TF increased in sprout extracts, their antioxidant capacity also increased and, inversely, as TPC and TF became higher, the IC50 reduced.
Table 4.
Pearson’s correlation coefficients among total phenols, total flavonoids, antioxidant capacity (TEAC), and the half inhibitory concentration (IC50) of the radicals DPPH and ABTS in sprouts and seeds of quinoa.
| Correlations | TEAC-DPPH | TEAC-ABTS | IC50 DPPH | IC50 ABTS | |
|---|---|---|---|---|---|
| TPC of quinoa seeds | Pearson’s correlation | 0.480 ** | 0.352 ** | −0.477 ** | −0.331 ** |
| p-value | <0.0001 | 0.006 | <0.0001 | 0.010 | |
| TF of quinoa sprouts | Pearson’s correlation | 0.372 ** | 0.407 ** | −0.393 ** | −0.404 ** |
| p-value | 0.003 | 0.001 | 0.002 | 0.001 | |
| TPC of quinoa sprouts | Pearson’s correlation | 0.436 ** | 0.106 | −0.433 ** | −0.087 |
| p-value | <0.0001 | 0.421 | 0.001 | 0.508 | |
| TF of quinoa seeds | Pearson’s correlation | 0.092 | 0.202 | −0.098 | −0.214 |
| p-value | 0.483 | 0.121 | 0.455 | 0.100 | |
** The correlation is significant at the 0.01 level (bilateral).
2.6. Phytochemical Analysis of Methanolic Extracts of Sprouts and Seeds of C. quinoa (Pasankalla Variety)
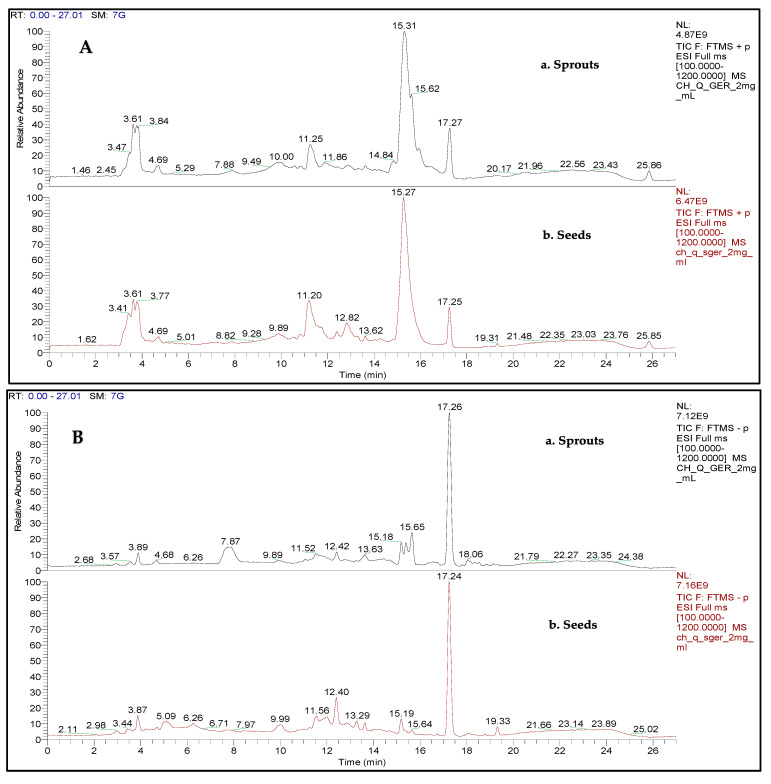
Phytochemical analysis was carried out by LC-ESI-MS/MS for the Pasankalla variety due to its high TPC and TF values and antioxidant capacity, as shown in Table 1, Table 2 and Table 3. Our results indicated that the sprout extract had 90 phytochemical constituents, of which 45 were observed in ESI (−), 33 in ESI (+), and 12 in both modes. In the seed extract, 93 compounds were determined, of which 58 metabolites were observed in ESI (−), 28 in ESI (+), and 7 in both modes, as presented in Table 5. Figure 2 shows the ESI-positive and -negative chromatographic profiles for both sprouts and seeds of the Pasankalla variety.
Table 5.
Number of annotated metabolites (via MS and MS/MS) in each extract according to the ESI (−) and ESI (+) ionization modes.
| C. quinoa (Pasankalla Variety) |
ESI (−) | ESI (+) | ESI (+/−) | Total |
|---|---|---|---|---|
| Seeds | 58 | 28 | 7 | 93 |
| Sprouts | 45 | 33 | 12 | 90 |
Figure 2.
Chromatographic profile (LC-MS) of C. quinoa extracts (Pasankalla variety): (A): ESI (+) ionization mode; (B): ESI (−) ionization mode.
The retention times (Rt), adductions, experimental, and theoretical m/z values, ppm error, MS/MS spectrum (m/z: absolute intensity), SMILES (simplified molecular input line entry system) string, InChIKey (IUPAC international chemical identifier), and tentative compounds are available in the Supplementary Table S1 and Supplementary Table S2.
The phytochemical constituents determined in the extracts of Pasankalla sprouts (Table 6) were classified as (i) primary metabolites, such as amino acids and derivatives (n = 23), organic acids (n = 14), monosaccharide sugar acids and sugar alcohols (n = 8), disaccharides and oligosaccharides (n = 7), lipids (n = 8), and nucleobases/nucleosides (n = 5); and (ii) secondary metabolites, such as phenolic acids (n = 2), triterpenoids (n = 4), O-glycosyl compounds (n = 4), phenolic glycosides (n = 2), flavonoid-O-glycosides (n = 2), alkaloids and derivatives (n = 1), triterpene saponins (n = 4), coumarins (n = 1), and other compounds (n = 13).
Table 6.
Phytochemical constituents of quinoa sprouts (Pasankalla variety) determined by LC-ESI-MS/MS.
| # | Rt (min) | Theoretical Mass (Neutral Form) | Molecular Formula (Neutral Form) | Predicted Metabolite |
Chemical Group |
|---|---|---|---|---|---|
| 1 | 3.85 | 340.1885884 | C18H28O6 | [5-acetyloxy-3-(hydroxymethyl)-2-oxo-6-propan-2-ylcyclohex-3-en-1-yl] 3-methylpentanoate | Menthane monoterpenoids |
| 2 | 3.91 | 130.0266086 | C5H6O4 | Citraconic acid | Organic acids |
| 3 | 4.30 | 313.131408 | C18H19NO4 | Feruloyl tyramine | Ferulic acid and derivatives |
| 4 | 4.40 | 138.031694 | C7H6O3 | Salicylic acid | Salicylic acids |
| 5 | 4.64 | 146.0579087 | C6H10O4 | 2-Methylglutaric acid | Methyl-branched fatty acids |
| 6 | 4.69 | 311.1157579 | C18H17NO4 | Feruloyl dehydrotyramine | Ferulic acid and derivatives |
| 7 | 4.75 | 132.0422586 | C5H8O4 | Glutaric acid | Dicarboxylic acids and derivatives |
| 8 | 5.12 | 118.0266086 | C4H6O4 | Succinic acid (Isomer I) |
Dicarboxylic acids and derivatives |
| 9 | 5.35 | 173.1051933 | C8H15NO3 | n-Acetyl-L-leucine (Isomer I) |
Leucine and derivatives |
| 10 | 5.77 | 118.0266086 | C4H6O4 | Succinic acid (Isomer II) |
Dicarboxylic acids and derivatives |
| 11 | 5.79 | 162.0528233 | C6H10O5 | β-hydroxy-β-methylglutaric acid (Isomer I) |
Hydroxy fatty acids |
| 12 | 5.87 | 123.0320284 | C6H5NO2 | Isonicotinic acid | Pyridinecarboxylic acids |
| 13 | 6.03 | 154.0266086 | C7H6O4 | 2,3-Dihydroxybenzoic acid | Salicylic acids |
| 14 | 6.24 | 173.1051933 | C8H15NO3 | n-Acetyl-L-leucine (Isomer II) |
Leucine and derivatives |
| 15 | 6.31 | 162.0528233 | C6H10O5 | β-hydroxy-β-methylglutaric acid (Isomer II) |
Hydroxy fatty acids |
| 16 | 6.68 | 219.1106725 | C9H17NO5 | Pantothenic acid (Isomer I) |
Vitamin B5 |
| 6.69 | 219.1106725 | C9H17NO5 | |||
| 17 | 7.09 | 219.1106725 | C9H17NO5 | Pantothenic acid (Isomer II) |
Vitamin B5 |
| 7.12 | 219.1106725 | C9H17NO5 | |||
| 18 | 7.84 | 298.1568945 | C19H22O3 | Aurapten | Coumarins |
| 19 | 7.86 | 129.042593 | C5H7NO3 | L-Pyroglutamic acid | Alpha amino acids and derivatives |
| 20 | 10.07 | 480.3087035 | C27H44O7 | NCGC00168839-02!(2S,3R,5R,10R,13R,14S,17S)-2,3,14-trihydroxy-10,13-dimethyl-17-[(2R,3R)-2,3,6-trihydroxy-6-methylheptan-2-yl]-2,3,4,5,9,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-6-one Syn. Phytoecdysteroids |
Phytoecdysteroids |
| 10.08 | 480.3087035 | C27H44O7 | |||
| 21 | 10.52 | 648.3873477 | C36H56O10 | (2S,3S,4S,5R,6R)-6-[[(3S,6aR,6bS,8aS,14bR)-8a-carboxy-4-(hydroxymethyl)-4,6a,6b,11,11,14b-hexamethyl-1,2,3,4a,5,6,7,8,9,10,12,12a,14,14a-tetradecahydropicen-3-yl]oxy]-3,4,5-trihydroxyoxane-2-carboxylic acid Syn. NCGC00381031-01_C36H56O10_Olean-12-en-28-oic acid, 3-(beta-D-glucopyranuronosyloxy)-23-hydroxy-, (3beta,5xi,9xi,18xi)- |
Triterpene saponins |
| 22 | 10.91 | 372.1420321 | C17H24O9 | Syringin | Phenolic glycosides |
| 23 | 11.06 | 356.110732 | C16H20O9 | NCGC00180844-02!(E)-3-[4-methoxy-2-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]prop-2-enoic acid Syn. 2-O-Glucosyloxy-4-methoxycinnamic acid |
Phenolic glycosides |
| 24 | 11.50 | 244.069536 | C9H12N2O6 | Uridine | Pyrimidine nucleosides |
| 25 | 12.30 | 810.440171 | C42H66O15 | NCGC00347541-02_C42H66O15_beta-D-Glucopyranose, 1-O-[(3beta,5xi,9xi,18xi)-3-(beta-D-glucopyranuronosyloxy)-29-hydroxy-28-oxoolean-12-en-28-yl]- | Triterpene saponins |
| 26 | 12.37 | 477.285539 | C23H44NO7P | Lysophosphatidylethanolamine LPE 18:2 | Lipids |
| 27 | 12.42 | 453.285539 | C21H44NO7P | Lysophosphatidylethanolamine LPE 16:0 | Lipids |
| 12.43 | 453.285539 | C21H44NO7P | |||
| 28 | 12.44 | 152.0334253 | C5H4N4O2 | Xanthine (Isomer I) |
Xanthines |
| 28 | 12.86 | 519.3324892 | C26H50NO7P | Lysophosphatidylcholine LPC 18:2 | Lipids |
| 29 | 12.89 | 495.3324892 | C24H50NO7P | Lysophosphatidylcholine LPC 16:0 | Lipids |
| 12.91 | 495.3324892 | C24H50NO7P | |||
| 30 | 12.93 | 131.0946286 | C6H13NO2 | Alanine betaine | Alanine and derivatives |
| 31 | 12.93 | 517.3168391 | C26H48NO7P | Lysophosphatidylcholine LPC 18:3 | Lipids |
| 12.94 | 517.3168391 | C26H48NO7P | |||
| 32 | 12.99 | 956.4980797 | C48H76O19 | 6-[[(3S,6aR,6bS,8aS,14bR)-4,4,6a,6b,11,11,14b-Heptamethyl-8a-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxycarbonyl-1,2,3,4a,5,6,7,8,9,10,12,12a,14,14a-tetradecahydropicen-3-yl]oxy]-3,5-dihydroxy-4-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyoxane-2-carboxylic acid Syn. NCGC00385168-01_C48H76O19_Hexopyranose, 1-O-[(3beta,5xi,9xi,18xi)-3-[(3-O-hexopyranosylhexopyranuronosyl)oxy]-28-oxoolean-12-en-28-yl]- |
Triterpene saponins |
| 33 | 13.20 | 315.2773439 | C18H37NO3 | Dehydrophytosphingosine | Lipids |
| 34 | 13.39 | 456.3603452 | C30H48O3 | Ursolic acid Syn. Isomer I |
Triterpenoids |
| 35 | 13.40 | 956.4980797 | C48H76O19 | 6-[[(3S,6aR,6bS,8aS,14bR)-4,4,6a,6b,11,11,14b-Heptamethyl-8a-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxycarbonyl-1,2,3,4a,5,6,7,8,9,10,12,12a,14,14a-tetradecahydropicen-3-yl]oxy]-3,5-dihydroxy-4-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyoxane-2-carboxylic acidSyn. NCGC00385168-01_C48H76O19_Hexopyranose, 1-O-[(3beta,5xi,9xi,18xi)-3-[(3-O-hexopyranosylhexopyranuronosyl)oxy]-28-oxoolean-12-en-28-yl]- | Triterpene saponins |
| 36 | 13.49 | 152.0684734 | C5H12O5 | Xylitol (Isomer I) |
Sugar alcohols |
| 37 | 13.60 | 291.0954163 | C11H17NO8 | N-fructosyl pyroglutamate | N-fructosyl amino acids |
| 38 | 13.63 | 284.075684 | C10H12N4O6 | Xanthosine | Purine nucleosides |
| 13.64 | 284.075684 | C10H12N4O6 | |||
| 39 | 13.64 | 152.0334253 | C5H4N4O2 | Xanthine (Isomer II) |
Xanthines |
| 40 | 13.72 | 152.0684734 | C5H12O5 | Xylitol (Isomer II) |
Sugar alcohols |
| 41 | 13.80 | 454.3446952 | C30H46O3 | NCGC00380944-01_C30H46O3_(3beta,5xi,9xi,13alpha,17alpha,18xi)-3-Hydroxy-13,28-epoxyurs-11-en-28-oneSyn. 3-Hydroxy-11-ursen-28,13-olide | Triterpenoids |
| 42 | 13.98 | 456.3603452 | C30H48O3 | Ursolic acid (Isomer II) |
Triterpenoids |
| 43 | 14.34 | 267.0967538 | C10H13N5O4 | Adenosine | Purine nucleosides |
| 44 | 14.45 | 180.063388 | C6H12O6 | Psicose | Monosaccharides |
| 45 | 14.69 | 456.3603452 | C30H48O3 | Ursolic acid (Isomer III) |
Triterpenoids |
| 46 | 14.80 | 120.0575148 | C8H8O | Phenylacetaldehyde | Phenylacetaldehydes |
| 47 | 14.80 | 165.0789785 | C9H11NO2 | Phenylalanine | Amino acids |
| 48 | 14.92 | 204.0898776 | C11H12N2O2 | Tryptophan | Amino acids |
| 49 | 15.03 | 131.0946286 | C6H13NO2 | Isoleucine | Isoleucine and derivatives |
| 50 | 15.11 | 756.1901639 | C36H36O18 | NCGC00381212-01![6-[2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxochromen-3-yl]oxy-4,5-dihydroxy-2-[(3,4,5-trihydroxy-6-methyloxan-2-yl)oxymethyl]oxan-3-yl] (E)-3-(4-hydroxyphenyl)prop-2-enoate | Flavonoid-O-glycosides |
| 51 | 15.14 | 756.2112932 | C33H40O20 | 2-(3,4-Dihydroxyphenyl)-3-[(2S,3R,4S,5S,6R)-4,5-dihydroxy-3-[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxy-5,7-dihydroxychromen-4-oneSyn. Quercetin 3-O-rutinoside-(1-2)-O-rhamnoside | Flavonoid-O-glycosides |
| 52 | 15.21 | 182.079038 | C6H14O6 | D-sorbitol | Sugar alcohols |
| 53 | 15.31 | 117.0789785 | C5H11NO2 | Betaine | Alpha amino acids |
| 54 | 15.39 | 180.063388 | C6H12O6 | Mannose (Isomer I) |
Hexoses |
| 55 | 15.41 | 136.0371732 | C4H8O5 | Threonic acid (Isomer I) |
Sugar acids and derivatives |
| 56 | 15.41 | 150.0528233 | C5H10O5 | Xylose | Pentoses |
| 57 | 15.57 | 104.107539 | C5H14NO | Choline | Cholines |
| 58 | 15.61 | 136.0371732 | C4H8O5 | Threonic acid (Isomer II) |
Sugar acids and derivatives |
| 59 | 15.63 | 180.063388 | C6H12O6 | Mannose(Isomer II) | Hexoses |
| 60 | 15.70 | 196.0583026 | C6H12O7 | D-gluconic acid (Isomer I) |
Medium-chain hydroxy acids and derivatives |
| 61 | 15.71 | 137.0476784 | C7H7NO2 | Trigonelline | Alkaloids and derivatives |
| 15.82 | 137.0476784 | C7H7NO2 | |||
| 62 | 15.87 | 145.0851265 | C5H11N3O2 | 4-Guanidinobutyric acid | Gamma amino acids and derivatives |
| 63 | 15.98 | 196.0583026 | C6H12O7 | D-gluconic acid (Isomer II) |
Medium-chain hydroxy acids and derivatives |
| 64 | 15.96 | 181.0738931 | C9H11NO3 | Tyrosine | Tyrosine and derivatives |
| 65 | 16.15 | 165.0789785 | C9H11NO2 | Phenylalanine | Phenylalanine and derivatives |
| 66 | 16.16 | 212.0896027 | C7H16O7 | Volemitol | Sugar alcohols |
| 67 | 16.45 | 293.1474519 | C12H23NO7 | N-fructosyl isoleucine | N-fructosyl amino acids |
| 16.48 | 293.1474519 | C12H23NO7 | |||
| 16.54 | 293.1474519 | C12H23NO7 | |||
| 68 | 16.62 | 103.0633285 | C4H9NO2 | 4-Aminobutyric acid Syn. 4-Aminobutanoic acid/GABA |
Gamma amino acids and derivatives |
| 69 | 17.15 | 147.0531577 | C5H9NO4 | L-Glutamic acid (Isomer I) |
Glutamic acid and derivatives |
| 70 | 17.18 | 342.1162113 | C12H22O11 | Melibiose (Isomer I) |
O-glycosyl compounds |
| 71 | 17.25 | 342.1162113 | C12H22O11 | Isomaltulose | O-glycosyl compounds |
| 72 | 17.26 | 342.1162113 | C12H22O11 | Trehalose | Disaccharide |
| 73 | 17.27 | 129.042593 | C5H7NO3 | Pyroglutamic acid | Alpha amino acids and derivatives |
| 74 | 17.29 | 147.0531577 | C5H9NO4 | L-glutamic acid (Isomer II) |
Glutamic acid and derivatives |
| 75 | 17.38 | 119.0582431 | C4H9NO3 | Threonine(Isomer I) | L-alpha-amino acids |
| 76 | 17.46 | 119.0582431 | C4H9NO3 | Threonine (Isomer II) |
L-alpha-amino acids |
| 77 | 18.02 | 165.0459638 | C5H11NO3S | Methioninesulfoxide | Alpha amino acids |
| 78 | 18.02 | 342.1162113 | C12H22O11 | Melibiose (Isomer II) |
O-glycosyl compounds |
| 79 | 18.10 | 146.0691421 | C5H10N2O3 | Glutamine | D-alpha-amino acids |
| 18.14 | 146.0691421 | C5H10N2O3 | |||
| 80 | 18.12 | 105.042593 | C3H7NO3 | Serine | Serine and derivatives |
| 18.16 | 105.042593 | C3H7NO3 | |||
| 81 | 18.31 | 344.1318613 | C12H24O11 | Maltitol | Fatty acyl glycosides of mono- and disaccharides |
| 82 | 18.40 | 132.053492 | C4H8N2O3 | Asparagine | Asparagine and derivatives |
| 18.41 | 132.053492 | C4H8N2O3 | |||
| 83 | 18.53 | 342.1162113 | C12H22O11 | Melibiose (Isomer III) |
O-glycosyl compounds |
| 84 | 18.84 | 504.1690346 | C18H32O16 | Melezitose (Isomer I) |
Oligosaccharides |
| 18.85 | 504.1690346 | C18H32O16 | |||
| 18.86 | 504.1690346 | C18H32O16 | |||
| 85 | 19.13 | 504.1690346 | C18H32O16 | Melezitose (Isomer II) |
Oligosaccharides |
| 86 | 19.14 | 504.1690346 | C18H32O16 | Maltotriose (Isomer I) |
Oligosaccharides |
| 87 | 19.33 | 504.1690346 | C18H32O16 | Maltotriose (Isomer II) |
Oligosaccharides |
| 88 | 19.34 | 504.1690346 | C18H32O16 | Raffinose | Oligosaccharides |
| 89 | 21.88 | 155.0694765 | C6H9N3O2 | L-Histidine | Histidine and derivatives |
| 90 | 22.28 | 174.1116756 | C6H14N4O2 | L-Arginine | L-alpha-amino acids |
In the seeds (Table 7), the phytochemical constituents were classified as: (i) primary metabolites, such as amino acids and derivatives (n = 11), organic acids (n = 16), monosaccharides sugar acids and sugar alcohols (n = 8), disaccharides and oligosaccharides (n = 7), lipids (n = 14), and nucleobases/nucleosides (n = 5); and (ii) secondary metabolites, such as triterpenoids (n = 3), catechols (n = 3), phenolic glycosides (n = 2), flavonoids (n = 5), alkaloids and derivatives (n = 1), triterpene saponins (n = 1), and other compounds (n = 17).
Table 7.
Phytochemical constituents of quinoa seeds (Pasankalla variety) determined by LC-ESI-MS/MS.
| Rt (min) | Theoretical Mass (Neutral Form) | Molecular Formula (Neutral Form) | Predicted Metabolite |
Chemical Group | |
|---|---|---|---|---|---|
| 1 | 3.19 | 340.1885884 | C18H28O6 | [5-Acetyloxy-3-(hydroxymethyl)-2-oxo-6-propan-2-ylcyclohex-3-en-1-yl] 3-methylpentanoate | Menthane monoterpenoids |
| 2 | 3.72 | 145.0527638 | C9H7NO | 2-Hydroxyquinoline | Hydroquinolones |
| 3 | 3.74 | 122.0367794 | C7H6O2 | 3-Hydroxybenzaldehyde | Phenolic compounds |
| 4 | 3.78 | 206.0579087 | C11H10O4 | Isoeugenitol | Chromones |
| 5 | 3.80 | 152.047344 | C8H8O3 | 4-Hydroxyphenylacetic acid | 1-Hydroxy-2-unsubstituted benzenoids |
| 6 | 3.85 | 168.0422586 | C8H8O4 | 3,4-Dihydroxyphenylacetate (Isomer I) Syn. Homoprotocatechuic acid |
Catechols |
| 7 | 3.89 | 154.0266086 | C7H6O4 | Pyrocatechuic acid (Isomer I) |
Salicylic acids |
| 8 | 3.96 | 130.0266086 | C5H6O4 | Citraconic acid | Methyl-branched fatty acids |
| 9 | 4.13 | 164.047344 | C9H8O3 | 3-Hydroxycinnamic acid | Hydroxycinnamic acids |
| 10 | 4.20 | 132.0786442 | C6H12O3 | 2-Hydroxyisocaproic acid | Hydroxy fatty acids |
| 11 | 4.20 | 160.0735588 | C7H12O4 | 3-Methyladipic acid | Medium-chain fatty acids |
| 12 | 4.22 | 168.0422586 | C8H8O4 | 3,4-Dihydroxyphenylacetate (Isomer II) Syn. Homoprotocatechuic acid |
Catechols |
| 13 | 4.29 | 154.0266086 | C7H6O4 | Pyrocatechuic acid (Isomer II) |
Salicylic acids |
| 14 | 4.43 | 138.031694 | C7H6O3 | Salicylic acid | Salicylic acids |
| 15 | 4.66 | 146.0579087 | C6H10O4 | 2-Methylglutaric acid | Methyl-branched fatty acids |
| 16 | 4.75 | 132.0422586 | C5H8O4 | Glutaric acid (Isomer I) |
Dicarboxylic acids and derivatives |
| 17 | 4.75 | 194.0579087 | C10H10O4 |
trans-4-Hydroxy-3-methoxycinnamate (Isomer I) |
Hydroxycinnamic acids |
| 18 | 4.98 | 134.0215232 | C4H6O5 | Malic acid (Isomer I) |
Beta hydroxy acids and derivatives |
| 19 | 5.07 | 164.047344 | C9H8O3 | 3-Hydroxycinnamic acid (Isomer I) |
Hydroxycinnamic acids |
| 20 | 5.14 | 118.0266086 | C4H6O4 | Succinic acid (Isomer I) |
Dicarboxylic acids and derivatives |
| 21 | 5.45 | 132.0422586 | C5H8O4 | Glutaric acid (Isomer II) |
Dicarboxylic acids and derivatives |
| 22 | 5.75 | 118.0266086 | C4H6O4 | Succinic acid (Isomer II) |
Dicarboxylic acids and derivatives |
| 23 | 5.82 | 194.0579087 | C10H10O4 |
trans-4-Hydroxy-3-methoxycinnamate (Isomer II) |
Hydroxycinnamic acids |
| 24 | 5.83 | 123.0320284 | C6H5NO2 | Isonicotinic acid | Pyridinecarboxylic acids |
| 25 | 5.98 | 110.0367794 | C6H6O2 | Catechol | Catechols |
| 26 | 6.26 | 134.0215232 | C4H6O5 | Malic acid (Isomer II) |
Beta hydroxy acids and derivatives |
| 27 | 6.26 | 164.047344 | C9H8O3 | 3-Hydroxycinnamic acid (Isomer II) |
Hydroxycinnamic acids |
| 28 | 7.05 | 219.1106725 | C9H17NO5 | Pantothenic acid | Secondary alcohols |
| 7.11 | 219.1106725 | C9H17NO5 | |||
| 29 | 7.27 | 516.3298328 | C27H48O9 | MGMG 18:2 | Lipids |
| 30 | 7.40 | 122.0480128 | C6H6N2O | Nicotinamide | Nicotinamides |
| 31 | 7.82 | 264.1110069 | C13H16N2O4 | Phenylacetylglutamine | N-acyl-alpha amino acids |
| 32 | 7.83 | 129.042593 | C5H7NO3 | 5-Oxo-D-proline Syn. D-Pyroglutamic acid |
Proline and derivatives |
| 33 | 8.38 | 514.3141828 | C27H46O9 | NCGC00380867-01_C27H46O9_9,12,15-Octadecatrienoic acid, 3-(hexopyranosyloxy)-2-hydroxypropyl ester, (9Z,12Z,15Z)- | Glycosylmonoacylglycerols |
| 34 | 9.53 | 494.3243536 | C28H46O7 | NCGC00169545-02!(2S,3R,5R,10R,13R,14S,17S)-2,3,14-trihydroxy-10,13-dimethyl-17-[(2R,3R,5R)-2,3,6-trihydroxy-5,6-dimethylheptan-2-yl]-2,3,4,5,9,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-6-one Syn. Makisterone A |
Phytoecdysteroids |
| 35 | 10.03 | 480.3087035 | C27H44O7 | NCGC00168839-02!(2S,3R,5R,10R,13R,14S,17S)-2,3,14-trihydroxy-10,13-dimethyl-17-[(2R,3R)-2,3,6-trihydroxy-6-methylheptan-2-yl]-2,3,4,5,9,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-6-one Syn. Ecdysterone |
Phytoecdysteroids |
| 10.04 | 480.3087035 | C27H44O7 | |||
| 40 | 10.06 | 722.5097847 | C38H75O10P | [3-[[2,3-Dihydroxypropoxy]-hydroxyphosphoryl]oxy-2-hexadecanoyloxypropyl] hexadecanoate Syn. Dipalmitoylphosphatidylglycerol |
Phosphatidylglycerol |
| 41 | 10.90 | 372.1420321 | C17H24O9 | Syringin | Phenolic glycosides |
| 42 | 10.96 | 478.0747403 | C21H18O13 | Quercetin-3-glucuronide | Flavonoid-O-glucuronides |
| 43 | 11.49 | 244.069536 | C9H12N2O6 | Uridine | Pyrimidine nucleosides |
| 44 | 12.03 | 281.1124038 | C11H15N5O4 | 2′-O-methyladenosine | Purine nucleosides |
| 45 | 12.36 | 477.285539 | C23H44NO7P | Lysophosphatidylethanolamine LPE 18:2 | Lipids |
| 46 | 12.40 | 453.285539 | C21H44NO7P | Lysophosphatidylethanolamine LPE 16:0 | Lipids |
| 47 | 12.59 | 131.0946286 | C6H13NO2 | Alanine betaine | Alanine and derivatives |
| 48 | 12.76 | 521.3481393 | C26H52NO7P | Lysophosphatidylcholine LPC 18:1 | Lipids |
| 12.78 | 521.3481393 | C26H52NO7P | |||
| 49 | 12.85 | 519.3324892 | C26H50NO7P | Lysophosphatidylcholine LPC 18:2 | Lipids |
| 50 | 12.88 | 152.0684734 | C5H12O5 | L-arabitol (Isomer I) |
Sugar alcohols |
| 51 | 12.89 | 495.3324892 | C24H50NO7P | Lysophosphatidylcholine LPC 16:0 | Lipids |
| 12.90 | 495.3324892 | C24H50NO7P | |||
| 52 | 12.91 | 517.3168391 | C26H48NO7P | Lysophosphatidylcholine LPC 18:3 | Lipids |
| 12.95 | 517.3168391 | C26H48NO7P | |||
| 53 | 13.11 | 639.3383623 | C29H54NO12P | Hexosyl LPE 18:2 | Lipids |
| 54 | 13.16 | 315.2773439 | C18H37NO3 | Dehydrophytosphingosine Syn. 4-Hydroxy-8-sphingenine |
Lipids |
| 55 | 13.43 | 956.4980797 | C48H76O19 | 6-[[(3S,6aR,6bS,8aS,14bR)-4,4,6a,6b,11,11,14b-heptamethyl-8a-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxycarbonyl-1,2,3,4a,5,6,7,8,9,10,12,12a,14,14a-tetradecahydropicen-3-yl]oxy]-3,5-dihydroxy-4-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyoxane-2-carboxylic acid Syn. NCGC00385168-01_C48H76O19_Hexopyranose, 1-O-[(3beta,5xi,9xi,18xi)-3-[(3-O-hexopyranosylhexopyranuronosyl)oxy]-28-oxoolean-12-en-28-yl]- |
Triterpene saponins |
| 56 | 13.61 | 291.0954163 | C11H17NO8 | N-fructosyl pyroglutamate | N-fructosyl amino acids |
| 57 | 13.61 | 610.1533845 | C27H30O16 | Rutoside Syn. Rutin |
Flavonoid-O-glycosides |
| 58 | 13.61 | 284.075684 | C10H12N4O6 | Xanthosine | Purine nucleosides |
| 13.62 | 284.075684 | C10H12N4O6 | |||
| 59 | 13.62 | 152.0334253 | C5H4N4O2 | Xanthine | Xanthines |
| 60 | 13.73 | 152.0684734 | C5H12O5 | L-arabitol (Isomer II) |
Sugar alcohols |
| 61 | 13.79 | 454.3446952 | C30H46O3 | NCGC00380944-01_C30H46O3_(3beta,5xi,9xi,13alpha,17alpha,18xi)-3-Hydroxy-13,28-epoxyurs-11-en-28-one Syn. 3-Hydroxy-11-Ursen-28,13-Olide |
Triterpenoids |
| 62 | 13.97 | 456.3603452 | C30H48O3 | Ursolic acid (Isomer I) |
Triterpenoids |
| 63 | 14.10 | 770.2269433 | C34H42O20 | 7-Methylquercetin-3-galactoside-6″-rhamnoside-3‴-rhamnoside | Flavonoid -O-glycosides |
| 64 | 14.21 | 221.0899371 | C8H15NO6 | N-acetylmannosamine | N-acyl-alpha-hexosamines |
| 65 | 14.29 | 180.063388 | C6H12O6 | Psicose | Monosaccharides |
| 66 | 14.69 | 456.3603452 | C30H48O3 | Ursolic acid (Isomer II) |
Triterpenoids |
| 67 | 14.76 | 165.0789785 | C9H11NO2 | Phenylalanine | Amino acids |
| 68 | 14.90 | 204.0898776 | C11H12N2O2 | Tryptophan | Indolyl carboxylic acids and derivatives |
| 69 | 14.93 | 283.0916684 | C10H13N5O5 | Guanosine | Purine nucleosides |
| 70 | 14.93 | 742.1956431 | C32H38O20 | NCGC00180410-02!3-[(2S,3R,4S,5S,6R)-6-[[(2R,3R,4R,5R,6S)-3-[(2S,3R,4R)-3,4-dihydroxy-4-(hydroxymethyl)oxolan-2-yl]oxy-4,5-dihydroxy-6-methyloxan-2-yl]oxymethyl]-3,4,5-trihydroxyoxan-2-yl]oxy-2-(3,4-dihydroxyphenyl)-5,7-dihydroxychromen-4-oneSyn. Quercetin 3-(2R-apiosylrutinoside) | Flavonoid-O-glycosides |
| 71 | 15.04 | 131.0946286 | C6H13NO2 | Isoleucine | Isoleucine and derivatives |
| 72 | 15.11 | 104.107539 | C5H14NO | Choline (Isomer I) |
Cholines |
| 73 | 15.14 | 756.2112932 | C33H40O20 | 2-(3,4-Dihydroxyphenyl)-3-[(2S,3R,4S,5S,6R)-4,5-dihydroxy-3-[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxy-5,7-dihydroxychromen-4-one Syn. Quercetin 3-O-rutinoside-(1-2)-O-rhamnoside |
Flavonoid-O-glycosides |
| 74 | 15.19 | 182.079038 | C6H14O6 | D-sorbitol | Sugar alcohols |
| 75 | 15.27 | 117.0789785 | C5H11NO2 | Betaine | Alpha amino acids |
| 76 | 15.39 | 135.0544952 | C5H5N5 | Adenine | 6-Aminopurines |
| 77 | 15.59 | 104.107539 | C5H14NO | Choline (Isomer II) |
Cholines |
| 78 | 15.64 | 180.063388 | C6H12O6 | Mannose | Hexoses |
| 79 | 15.78 | 212.0896027 | C7H16O7 | Perseitol | Sugar alcohols |
| 80 | 15.79 | 137.0476784 | C7H7NO2 | Trigonelline | Alkaloids and derivatives |
| 81 | 15.84 | 145.0851265 | C5H11N3O2 | 4-Guanidinobutanoic acid | Gamma amino acids and derivatives |
| 82 | 16.49 | 293.1474519 | C12H23NO7 | N-fructosyl isoleucine | N-fructosyl amino acids |
| 16.52 | 293.1474519 | C12H23NO7 | |||
| 83 | 17.24 | 342.1162113 | C12H22O11 | Trehalose | Disaccharides |
| 84 | 17.25 | 342.1162113 | C12H22O11 | Maltose | Oligosaccharides |
| 85 | 17.25 | 342.1162113 | C12H22O11 | Isomaltulose | Oligosaccharides |
| 86 | 17.25 | 342.1162113 | C12H22O11 | Melibiose | Oligosaccharides |
| 87 | 17.29 | 147.0531577 | C5H9NO4 | L-glutamic acid | Glutamic acid and derivatives |
| 88 | 18.13 | 146.0691421 | C5H10N2O3 | Glutamine | D-alpha-amino acids |
| 89 | 18.30 | 344.1318613 | C12H24O11 | Maltitol Syn. 4-O-alpha-D-Glucopyranosyl-D-glucitol |
Hexoses |
| 90 | 19.31 | 504.1690346 | C18H32O16 | Maltotriose | Oligosaccharides |
| 91 | 19.33 | 504.1690346 | C18H32O16 | Raffinose | Oligosaccharides |
| 92 | 20.64 | 179.0793724 | C6H13NO5 | D-mannosamine | Hexoses |
| 93 | 20.79 | 666.2218579 | C24H42O21 | Tetrasaccharides (Hex-Hex-Hex-Hex) | Oligosaccharides |
3. Discussion
Polyphenolic compounds are secondary metabolites present in plants, which are divided into flavonoids and non-flavonoids, the first being responsible for the antioxidant capacity, exerting this through various mechanisms such as transition metal chelators, free radical scavengers, and enzyme inhibitors [16]. The antioxidant properties of secondary metabolites are related to vasodilatory, lipid-lowering, antiaging, and anti-inflammatory, modulating apoptosis processes in the vascular endothelium, but these molecules could also be influenced by factors such as the number and position of the phenolic hydroxyl groups, steric effects, and molecular properties [17]. In our results, the content of total phenols and flavonoids found in quinoa sprouts presented differences in each variety analyzed, being influenced by the type of seed, the cultivation site, maturity, storage, and germination conditions, as the flavonoids play an important role in pigmentation [18]. It is known that the phenolic compounds present in plants are formed during their development and under stress conditions; these include simple phenols, phenolic acids, coumarins, flavonoids, stilbenes, hydrolysable and condensed tannins, lignans, and lignins [19]. Additionally, these polyphenols could be altered during the germination process, increasing their content and the antioxidant capacity [20].
In our study, the variation in TPC and TF differed from the studies of Valencia et al., in which the TPC varied from 0.783 to 3437 mg GAE/g in quinoa seeds [21], and that of Carciochi et al. [22], with values of TPC of 39.3 ± 0.9 mg GAE/100 g and TF of 11.06 mg of quercetin/100 g in sprouts. These were higher in our study due to the type of solvent used in the maceration process. In the same way, when the antioxidant activity of the content of polyphenols and flavonoids was evaluated in the red and yellow varieties of quinoa, there was a significant increase after 9 days of germination. In a similar study, the antioxidant capacity in germinated seeds was greater compared with seeds of C. quinoa, increasing up to twofold, similar to the increase in phenolic compounds and antioxidant capacity observed after 72 h of germination [13]. In our study, a wide range of values were observed for phenolic compounds and flavonoids, as well as for the antioxidant activity in each variety of quinoa studied, which can be explained by the characteristics of each seed, variation in the availability of nutrients, and activation of the antioxidant machinery during germination.
Several studies have shown nutritional improvements in quinoa sprouts, such as in crude quinoa flour (CQF) and germinated quinoa flour (GQF), where the CQF/GQF ratio increased the nutritional quality of pasta. Chemical analysis indicated an increase in the proportion of proteins by 37% and a decrease in phytic acid by 77%, which means that the germination process is an effective method to minimize phytic acid content in seeds. Pasta with a high CQF/GQF ratio had an increased content of Ca, K, Fe, Mn, Mg, P, and Zn, and thus using GQF is recommended in the production of bread, cakes, and cookies to take advantage of their nutritional properties, which provide a high content of proteins, minerals, TPC, and amino acids, and a low amount of phytic acid [23]. During germination, quinoa seeds undergo relevant physical and chemical changes; the maximum intensity of macromolecular modification occurs at 48 h. The germinated material contains micronutrients with improved bioavailability. This has a great impact on quinoa, as it improves the technological properties of quinoa, as well as some of its nutritional characteristics, enhancing the use of quinoa sprout flour as an ingredient in food formulation [12]. The germination process of quinoa seeds is an effective technique to enhance the content of total phenols and total flavonoids and to improve the antioxidant capacity, as was demonstrated in quinoa (C. quinoa) and kiwicha (Amaranthus caudatus) [24], where the sprouts had enhanced content of coumaric acid and kaempferol tri-glycoside in quinoa and caffeoylquinic acid in kiwicha. Additionally, a significant increase was observed in the phenolic content and the antioxidant capacity through malting quinoa sprouts [25] and Amaranthus caudatus sprouts [26].
4. Materials and Methods
4.1. Collection of Quinoa Seeds
Fifteen certified varieties were provided by the Agrarian Research Institute (INIA, Ayacucho, Peru) and five varieties were collected between November and December 2019 in the districts of Huamanguilla and Acocro of the province of Huamanga. These are registered with the following names: White Junín Ayacucho, T-256, Pasankalla, Suano Puno, T-38, Yellow Sacaca, T-45, Santa Ana, T-61 Pomata, CQA-048, Black Collana, T-72 Huancayo, CQA-043, Salcedo, Ayacucho Compuesto, White Choclito, Red, Yellow Maranganí, Black Coito, and Black.
4.2. Germination Process
The seeds were washed with hypochlorite 0.02% (w/v) for 20 min, rinsed several times with distilled water, and placed on absorbent paper moistened with distilled water in Technopor containers covered with paper towels and incubated at room temperature (between 18 and 22 °C) for 72 h until good sprouts had been obtained. The sprouts were harvested, dried at 45 °C for 48 h, then crushed and stored under refrigeration [27].
4.3. Preparation of the Methanolic Extract
Ten grams of each sample of sprouts and seeds was subjected to dynamic extraction with 100 mL of methanol (1:10), using a magnetic stirrer for 4 h at room temperature, then filtered with Whatman No. 1 paper and concentrated on a rotary evaporator until dry. Each extract was refrigerated until further use at 4 °C.
4.4. Determination of Total Phenolic Content (TPC)
In total, 50 μL of the methanolic extract (10 mg/mL) was mixed with 1 mL of distilled water, 0.5 mL of 0.2 N Folin-Ciocalteu reagent, and 2.5 mL of 5% sodium carbonate, then the sample was allowed to react in the darkness for 40 min at room temperature (20°C). The absorbance was read at 725 nm using a UV-Vis Genesys 150 Thermo Scientific spectrophotometer. A standard curve was made with a gallic acid solution (50 μg/mL) at concentrations of 10, 20, 30, 40, and 50 μg/mL. The results are presented in mg equivalent to gallic acid per g of methanolic extract (mg GAE/g of extract) [28].
4.5. Determination of Total Flavonoids
In total, 0.5 mL of the extract (10 mg/mL) was mixed with 1 mL with distilled water and 0.15 mL of 5% sodium nitrite; 5 min later, 0.15 mL of 10% aluminum chloride was added, then at 6 min, 2 mL of 4% sodium hydroxide was added. The sample was made up to 5 mL with distilled water, mixed, and allowed to react in the darkness for 15 min at room temperature. The absorbance was read at 510 nm against a blank. A standard curve was made with quercetin (200 μg/mL) at concentrations of 40, 80, 120, 160, and 200 μg/mL. The flavonoid content is presented as mg equivalent to quercetin per g of dry methanolic extract (mg QE/g of extract) [29].
4.6. Determination of the Antioxidant Capacity by the Free Radical Sequestration Method with 2,2-diphenyl-1-picrylhydrazyl
For this assay, 150 μL of extract (10 mg/mL) was mixed with 2850 μL of a methanolic solution of DPPH radicals (20 mg/L) with the absorbance adjusted to 0.6 ± 0.02 nm. After mixing, the sample was incubated in the dark for 30 min and the absorbance was read at 515 nm. The standard curve was elaborated with Trolox at concentrations of 0 to 800 μmol/mL [30]. The antioxidant capacity equivalent to Trolox (TEAC) was calculated with the following formula:
To calculate the half inhibitory concentration (IC50), the percentage of inhibition of the DPPH radical was determined at concentrations of 5, 10, and 20 mg/mL of methanolic extract according to the following equation:
where abscontrol is the absorbance of the control without the sample at t = 0 min, and abssample is the absorbance of the sample at t = 30 min.
4.7. Determination of the Antioxidant Capacity by the Sequestration Method with the Radical Cation of the 2.2′-azinobis-(3-ethylbenzothiazoline)-6-sulfonic acid
A standard solution (ST) was prepared by mixing 10 mL of ABTS (4.06 mg/mL) with 10 mL of potassium persulfate (0.7 mg/mL) and reacted for 12 h. The working solution (ST) was prepared with 1 mL of each extract and 60 mL of methanol. The absorbance was adjusted to 0.7 ± 0.02 with methanol at a wavelength of 734 nm, then 150 μL of the extract (5 mg/mL) was mixed with 2850 μL of the extract solution and incubated in the dark for 7 min, followed by reading the absorbances at 734 nm [31]. The standard curve was made with Trolox at 0–400 μmol/mL. The antioxidant capacity equivalent to Trolox (TEAC) was expressed as µmol ET/mg of the extract.
To calculate the half inhibitory concentrations (IC50), the percentage of inhibition of the ABTS radical was determined at concentrations of 1, 5, and 10 mg/mL as follows:
where abscontrol is the absorbance of the control without the sample at t = 0 min and abssample is the absorbance of the sample at t = 7 min.
4.8. Phytochemical Analysis by LC-ESI-MS/MS of the Main Constituents of Methanolic Extracts of the Sprouts and Seeds of C. quinoa (Pasankalla Variety)
4.8.1. Preparation of the Sample
The methanolic extracts of the sprouts and seeds of C. quinoa were weighed and diluted with methanol until a final concentration of 2 mg/mL had been obtained. Next, each sample was vortexed for 1 min and subsequently centrifuged for 10 min at 10,000 rpm. Finally, 800 µL of the 1 mg/mL solution supernatant (methanol:water, 1:1) was removed in vials for LC-MS analysis in a Dionex UltiMate 3000 liquid chromatograph (Thermo Fisher Scientific, San José, CA, USA) coupled to a Thermo QExactiveTM Plus Orbitrap mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) with an electrospray ionization source.
4.8.2. Chromatographic Conditions
This analysis used a chromatographic column XBridge® Amide BEH water (150 mm × 4.6 mm × 3.5 µm). Solvent A was 0.1% formic acid in water and Solvent B was 0.1% formic acid in ACN. The gradient elution of the method was as follows: 0–2 min, B 95%; 2–17.0 min, B 50%; 17–20.0 min, B 50%; 20.0–21.0 min, B 95%; 21.0–27.0 min, B 95%. The flow rate was 500 µL min−1 with injection of 8 µL and a column oven temperature of 40 °C.
4.8.3. Mass Spectrometry Conditions
A full scan experiment combined with a fragmentation experiment (MS/MS) was performed for both electrospray ionization modes (ESI + and −). The ESI source parameters were as follows: spraying voltage: 3.9 kV (+) and 3.6 kV (−); envelope gas flow rate: 50 (arbitrary values); auxiliary gas flow: 10 (arbitrary values); tube lens voltage: 50 V; probe heater temperature: 400 °C; capillary temperature: 300 °C.
1. (. ESI +) mode: full MS mode parameters: 35,000 resolution; ACG target (automatic gain control): 5e5; maximum IT (injection time): 100 ms; scan range: 100–1200 m/z.
Dd-MS2 (data-dependent acquisition experiment, DDA) mode parameters: 17,500 resolution; ACG objective: 1e5; maximum IT: 50 ms; loop count, 3; isolation window: 1–2 m/z; topN, 3; NCE (stepped normalized collision energy): 15, 30, and 40.
2. (. ESI −) mode: full MS mode parameters: 35,000 resolution; ACG objective: 5e5; maximum IT: 100 ms; range, 100–1200 m/z.
Dd-MS2 (data-dependent acquisition experiment, DDA) mode parameters: 17,500 resolution; ACG objective: 1e5; maximum IT: 50 ms; loop count, 3; isolation window: 1–2 m/z; topN: 3; NCE: 15, 20, and 40.
Data acquisition and processing were performed with Thermo XcaliburTM software version 3.0 (Thermo Fisher Scientific Inc., Waltham, MA, USA) with the Qual Browser, and metabolite annotations were performed with MS-Dial software version 4.70 (Riken, Osaka University, Suita City, Japan) using the MS-Dial metabolomics MPS spectral kit library (available at: http://prime.psc.riken.jp/compms/msdial/main.html; last updated on 13 April 2021).
4.9. Data Analysis
The results are presented as the means plus standard deviation of three repetitions. The differences between the means were analyzed using paired sample t-test for total phenols, flavonoids, antioxidant capacity, and the half inhibitory concentration (IC50), using SPSS software. Pearson’s correlation coefficient was determined to establish the relationships among total phenols and flavonoids, antioxidant capacity (TEAC), and the half inhibitory concentration (IC50), with a p-value less than 0.05 being significant.
5. Conclusions
Based on our results, we concluded that quinoa sprouts germinated for 72 h had higher total phenolic content and total flavonoids compared with seed extracts, and these correlated with its high antioxidant capacity. Furthermore, sprout extracts had better IC50 and TEAC values in the DPPH and ABTS assays. The best variety of quinoa was Pasankalla, which showed a high antioxidant capacity and also contained 90 and 93 phytochemical constituents in the sprout and seed extract, respectively. Some chemical groups highlighted were amino acids, organic acids, phenolic acids, flavonoids, fatty acids, lipids, saponins, and sugars, with a greater diversity of essential amino acids found in sprouts than in seeds.
Acknowledgments
The author thanks the Fondo Nacional de Desarrollo Científico, Tecnológico y de Innovación Tecnológico (FONDECYT) for supporting this project and the Instituto Nacional de Innovación Agraria-INIA, Ayacucho for providing and certifying the quinoa seeds.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/plants10112417/s1, Table S1: List of compounds putatively identified by LC-HRMS/MS in the extract of Chenopodium quinoa sprouts. Table S2: List of compounds putatively identified by LC-HRMS/MS in the extract of Chenopodium quinoa seeds.
Author Contributions
Conceptualization, E.C.E.-R. and E.J.A.-F.; methodology, J.A.T.-J.; validation, E.J.A.-F. and J.L.A.-A.; formal analysis, E.C.E.-R.; investigation, E.C.E.-R.; writing—original draft preparation, E.C.E.-R. and O.H.-C.; writing—review and editing, O.H.-C.; funding acquisition, E.C.E.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fondo Nacional de Desarrollo Científico, Tecnológico y de Innovación Tecnológico (FONDECYT, Peru), grant number 388-2019-FONDECYT.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bazile D., Jacobsen S.-E., Verniau A. The Global Expansion of Quinoa: Trends and Limits. Front. Plant Sci. 2016:622. doi: 10.3389/fpls.2016.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quinoa Export Value by Country Worldwide 2020|Statista. [(accessed on 24 October 2021)]. Available online: https://www.statista.com/statistics/520475/quinoa-export-value-worldwide-by-country/#statisticContainer.
- 3.Angeli V., Silva P.M., Massuela D.C., Khan M.W., Hamar A., Khajehei F., Graeff-Hönninger S., Piatti C. Quinoa (Chenopodium Quinoa Willd.): An Overview of the Potentials of the “Golden Grain” and Socio-Economic and Environmental Aspects of Its Cultivation and Marketization. Foods. 2020;9:216. doi: 10.3390/foods9020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gawlik-Dziki U., Świeca M., Sułkowski M., Dziki D., Baraniak B., Czyz J. Antioxidant and Anticancer Activities of Chenopodium Quinoa Leaves Extracts—In Vitro Study. Food Chem. Toxicol. 2013;57:154–160. doi: 10.1016/j.fct.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Hinojosa L., Leguizamo A., Carpio C., Muñoz D., Mestanza C., Ochoa J., Castillo C., Murillo A., Villacréz E., Monar C., et al. Quinoa in Ecuador: Recent Advances under Global Expansion. Plants. 2021;10:298. doi: 10.3390/plants10020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuktaite R., Repo-Carrasco-Valencia R., de Mendoza C.C., Plivelic T.S., Hall S., Johansson E. Innovatively Processed Quinoa (Chenopodium Quinoa Willd.) Food: Chemistry, Structure and End-Use Characteristics. J. Sci. Food Agric. 2021 doi: 10.1002/jsfa.11214. [DOI] [PubMed] [Google Scholar]
- 7.Jang H.W., Moon J.-K., Shibamoto T. Analysis and Antioxidant Activity of Extracts from Broccoli (Brassica Oleracea L.) Sprouts. J. Agric. Food Chem. 2015;63:1169–1174. doi: 10.1021/jf504929m. [DOI] [PubMed] [Google Scholar]
- 8.Quassinti L., Gianfranceschi G., Lupidi G., Miano A., Bramucci M. Antioxidant and Pro-Oxidant Activities of Savoy Cabbage (Brassica Oleracea L. Var. Sabauda) Sprout Extracts. J. Food Biochem. 2016;40:542–549. doi: 10.1111/jfbc.12247. [DOI] [Google Scholar]
- 9.Niroula A., Khatri S., Khadka D., Timilsina R. Total Phenolic Contents and Antioxidant Activity Profile of Selected Cereal Sprouts and Grasses. Int. J. Food Prop. 2019;22:427–437. doi: 10.1080/10942912.2019.1588297. [DOI] [Google Scholar]
- 10.Benincasa P., Falcinelli B., Lutts S., Stagnari F., Galieni A. Sprouted Grains: A Comprehensive Review. Nutrients. 2019;11:421. doi: 10.3390/nu11020421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le L., Gong X., An Q., Xiang D., Zou L., Peng L., Wu X., Tan M., Nie Z., Wu Q., et al. Quinoa Sprouts as Potential Vegetable Source: Nutrient Composition and Functional Contents of Different Quinoa Sprout Varieties. Food Chem. 2021;357:129752. doi: 10.1016/j.foodchem.2021.129752. [DOI] [PubMed] [Google Scholar]
- 12.Suárez-Estrella D., Bresciani A., Iametti S., Marengo M., Pagani M.A., Marti A. Effect of Sprouting on Proteins and Starch in Quinoa (Chenopodium Quinoa Willd.) Plant Foods Hum. Nutr. 2020;75:635–641. doi: 10.1007/s11130-020-00864-6. [DOI] [PubMed] [Google Scholar]
- 13.Al-Qabba M.M., El-Mowafy M.A., Althwab S.A., Alfheeaid H.A., Aljutaily T., Barakat H. Phenolic Profile, Antioxidant Activity, and Ameliorating Efficacy of Chenopodium Quinoa Sprouts against CCl4-Induced Oxidative Stress in Rats. Nutrients. 2020;12:2904. doi: 10.3390/nu12102904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paśko P., Bartoń H., Zagrodzki P., Gorinstein S., Fołta M., Zachwieja Z. Anthocyanins, Total Polyphenols and Antioxidant Activity in Amaranth and Quinoa Seeds and Sprouts during Their Growth. Food Chem. 2009;115:994–998. doi: 10.1016/j.foodchem.2009.01.037. [DOI] [Google Scholar]
- 15.De Lopes C.O., de Barcelos M.F.P., de Vieira C.N.G., de Abreu W.C., Ferreira E.B., Pereira R.C., Angelis-Pereira M.C. de Effects of Sprouted and Fermented Quinoa (Chenopodium Quinoa) on Glycemic Index of Diet and Biochemical Parameters of Blood of Wistar Rats Fed High Carbohydrate Diet. J. Food Sci. Technol. 2019;56:40. doi: 10.1007/s13197-018-3436-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunyadi A. The Mechanism(s) of Action of Antioxidants: From Scavenging Reactive Oxygen/Nitrogen Species to Redox Signaling and the Generation of Bioactive Secondary Metabolites. Med. Res. Rev. 2019;39:2505–2533. doi: 10.1002/med.21592. [DOI] [PubMed] [Google Scholar]
- 17.Kumar S., Pandey A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013;2013 doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pitzschke A., Fraundorfer A., Guggemos M., Fuchs N. Antioxidative Responses during Germination in Quinoa Grown in Vitamin B-Rich Medium. Food Sci. Nutr. 2015;3:242–251. doi: 10.1002/fsn3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim J.G., Park H., Yoon K.S. Analysis of Saponin Composition and Comparison of the Antioxidant Activity of Various Parts of the Quinoa Plant (Chenopodium Quinoa Willd.) Food Sci. Nutr. 2020;8:694. doi: 10.1002/fsn3.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Q., Xing B., Sun M., Zhou B., Ren G., Qin P. Changes in Bio-Accessibility, Polyphenol Profile and Antioxidants of Quinoa and Djulis Sprouts during in Vitro Simulated Gastrointestinal Digestion. Food Sci. Nutr. 2020;8:4232–4241. doi: 10.1002/fsn3.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valencia Z., Cámara F., Ccapa K., Catacora P., Quispe F. Compuestos bioactivos y actividad antioxidante de semillas de quinua peruana (Chenopodium Quinoa W.) Rev. Soc. Química Perú. 2017;83:16–29. doi: 10.37761/rsqp.v83i1.100. [DOI] [Google Scholar]
- 22.Carciochi R.A., Manrique G., Dimitrov K. Changes in Phenolic Composition and Antioxidant Activity during Germination of Quinoa Seeds (Chenopodium Quinoa Willd.) Int. Food Res. J. 2014;21:767–773. [Google Scholar]
- 23.Demir B., Bilgiçli N. Changes in Chemical and Anti-Nutritional Properties of Pasta Enriched with Raw and Germinated Quinoa (Chenopodium Quinoa Willd.) Flours. J. Food Sci. Technol. 2020;57:3884–3892. doi: 10.1007/s13197-020-04420-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pilco-Quesada S., Tian Y., Yang B., Repo-Carrasco-Valencia R., Suomela J.P. Effects of Germination and Kilning on the Phenolic Compounds and Nutritional Properties of Quinoa (Chenopodium Quinoa) and Kiwicha (Amaranthus Caudatus) J. Cereal Sci. 2020;94:102996. doi: 10.1016/j.jcs.2020.102996. [DOI] [Google Scholar]
- 25.Bhinder S., Kumari S., Singh B., Kaur A., Singh N. Impact of Germination on Phenolic Composition, Antioxidant Properties, Antinutritional Factors, Mineral Content and Maillard Reaction Products of Malted Quinoa Flour. Food Chem. 2021;346:128915. doi: 10.1016/j.foodchem.2020.128915. [DOI] [PubMed] [Google Scholar]
- 26.Aguilar-Felices E.J., Romero-Viacava M., Enciso-Roca E., Herrera-Calderon O., Común-Ventura P., Yuli-Posadas R.Á., Chacaltana-Ramos L., Pari-Olarte B. Antioxidant Activity of the Germinated Seed of Four Varieties of Amaranthus caudatus L. From Peru. Pharmacogn. J. 2019;11 doi: 10.5530/pj.2019.11.93. [DOI] [Google Scholar]
- 27.Aphalo P., Martínez E.N., Añón M.C. Amaranth Sprouts: A Potential Health Promoting and Nutritive Natural Food. Int. J. Food Prop. 2015;18:2688–2698. doi: 10.1080/10942912.2015.1004585. [DOI] [Google Scholar]
- 28.Herrera-Calderon O., Vega R. Optimization of the Extraction of Phenolic Compounds and Antioxidant Activity from the Roots of Waltheria Ovata Using the Response Surface Methodology. Food Res. 2020;4:2095–2102. doi: 10.26656/fr.2017.4(6).211. [DOI] [Google Scholar]
- 29.Herrera-Calderon O., Tinco-Jayo J.A., Franco-Quino C., Chumpitaz-Cerrate V., Castro-Pari W., Pari-Olarte B., Castillo-Romero P., Luis Arroyo-Acevedo J. Antioxidant Activity and Cytotoxic Profile of Chuquiraga Spinosa Lessing on Human Tumor Cell Lines: A Promissory Plant from Peruvian Flora Asian Pacific Journal of Tropical Disease. Asian Pac. J. Trop. Dis. 2017;7:304–308. doi: 10.12980/apjtd.7.2017D6-436. [DOI] [Google Scholar]
- 30.Enciso E.C., Aguilar E.J., Común P.W., Condorhuamán Y.M. Efecto Hepatoprotector y Actividad Antioxidante Del Extracto Hidroalcohólico Del Fruto de Dos Variedades de Opuntia Megacantha “Tuna”. Cienc. Investig. 2020;23 doi: 10.15381/ci.v23i1.18752. [DOI] [Google Scholar]
- 31.González-Palma I., Escalona-Buendía H.B., Ponce-Alquicira E., Téllez-Téllez M., Gupta V.K., Díaz-Godínez G., Soriano-Santos J. Evaluation of the Antioxidant Activity of Aqueous and Methanol Extracts of Pleurotus Ostreatus in Different Growth Stages. Front. Microbiol. 2016;7:1099. doi: 10.3389/fmicb.2016.01099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.