Abstract
The purpose of this study was to determine the sensitivity and specificity of three different methods of cytomegalovirus (CMV) detection for AIDS patients at risk for CMV retinitis. Patients with CD4+ counts of <100/μl and negative baseline screening eye examinations were tested for CMV infection by (i) pp65 antigenemia expression in leukocytes, (ii) the Digene Hybrid Capture CMV DNA System, and (iii) the Roche Amplicor Qualitative PCR Test. The incidence of CMV retinitis in our study of 296 patients at the Medical Center of Louisiana—New Orleans HIV Outpatient Clinic was 7.2 per 100 person-years (a total of 20 episodes in 18 patients from April 1997 to February 1999). Receiver operating characteristic curves were calculated for each assay to determine optimal cutoff points which maximized the sensitivity and specificity of each assay. The sensitivities of the assays compared to the eye examinations were 80% for the pp65 antigenemia assay (cutoff, >0 cell per 1.5 × 105 leukocytes), 85% for the Digene assay (cutoff, 1,400 genome copies/ml of whole blood), and 60% for the Amplicor assay. The specificities of the assays were 84, 84, and 87%, respectively. The Digene assay with a cutoff of ≥1,400 genome copies/ml gave optimal sensitivity and specificity and was found to have predictive values equal to those of the more technically cumbersome antigenemia assay.
Human cytomegalovirus (CMV) is a ubiquitous opportunistic pathogen in human immunodeficiency virus (HIV)-infected individuals (17). Prior to the era of highly active antiretroviral treatment (HAART), 20 to 40% of persons with AIDS developed CMV end-organ disease, including retinitis, esophagitis, colitis, or encephalitis (3, 20). HIV- and CMV-coinfected persons with CD4+ cell counts below 50/μl are at greatest risk for the development of CMV disease (10, 11, 13). Recent data indicate that immune reconstitution following HAART is associated with a decreased incidence of opportunistic infections, including CMV retinitis (4, 18). There are also reported cases of patients recovering from CMV retinitis after initiation of HAART (21).
Nonetheless, CMV retinitis still occurs in patients who cannot tolerate HAART or those who fail treatment due to nonadherence or development of HIV drug resistance. Retinitis is the most common manifestation of CMV infection in AIDS patients, accounting for 75 to 80% of CMV disease (8). Patients with CMV retinitis often are asymptomatic or manifest only vague visual complaints (20, 24). Therefore, the disease is frequently diagnosed during ophthalmologic screening of AIDS patients with normal vision. If not recognized and treated early, CMV retinitis may later evolve to profound complications, including retinal detachment and blindness (20, 24).
For these reasons, early detection of active CMV infection through noninvasive laboratory tests has been proposed as a way of predicting the subsequent development of retinitis (5, 7, 24). Several molecular diagnostic assays have been developed for the detection of CMV antigen expression or nucleic acid in peripheral blood. Furthermore, some studies suggest that initiation of CMV therapy when certain threshold levels of CMV antigenemia and/or DNA load are present may delay the development of retinitis (1, 5, 7). In this study, we used three molecular diagnostic tests for the detection of CMV viremia: (i) detection of CMV pp65 antigen in leukocytes, (ii) the Digene Hybrid Capture CMV DNA System, and (iii) qualitative plasma CMV DNA detection with the Roche Amplicor Qualitative PCR Test. The study population included patients who received screening and follow-up eye examinations for CMV retinitis. The virologic assays were used with the goal of identifying patients at risk for the development of CMV retinitis.
MATERIALS AND METHODS
Patients.
All patients received their diagnostic evaluations at the Medical Center of Louisiana—New Orleans HIV Outpatient Clinic between April 1997 and February 1999. A total of 296 patients were evaluated. Clinic patients with CD4+ cell counts of <100/μl were referred by their primary care providers for eye examinations. All of these patients were included in this study. The following clinical and diagnostic variables were obtained: (i) a complete eye examination performed by the staff ophthalmologist; (ii) plasma HIV load, determined by the Roche Amplicor method; (iii) T-cell subsets; and (iv) three CMV assays described in detail below. Patients with normal retinal examinations were reexamined at intervals of 3 to 6 months and retested using the assays described below. Repeat T-cell subset and viral load determinations were obtained at the discretion of the primary care provider. When available, the data were included in the statistical analysis (described below).
Specimen collection and processing.
Blood samples (about 7 to 10 ml) were collected in EDTA tubes at the baseline eye examination and were processed within 4 h of collection. For each specimen, 3.5 ml of EDTA-treated whole blood was used for the Digene assay, and plasma was separated from the remaining blood sample for the Amplicor assay. Then, the remaining plasma-free blood was resuspended with phosphate-buffered saline (PBS) to its original volume for the CMV pp65 antigenemia assay.
Assay methods. (i) CMV pp65 antigenemia assay.
Detection of CMV pp65 lower matrix protein in peripheral blood leukocytes was performed by a modification of previous methods (1, 5) as follows. Leukocytes were separated from 4 to 5 ml of PBS-resuspended, plasma-free, EDTA-treated blood with a 4:1 (blood/dextran) volume of 5% dextran solution. The leukocytes then were washed and resuspended in PBS to yield 1.5 × 106 cells/ml. Slides were prepared using 100 μl of the leukocyte suspension per slide and a cytocentrifuge (Cytospin-2; Shandon Scientific, Pittsburgh, Pa.). Slides were air dried overnight. A duplicate slide was kept at −70°C in case of the need for sample retesting. The other was fixed in 5% formaldehyde and permeabilized with 0.5% Nonidet P-40 (Boehringer GmbH, Mannheim, Germany). Slides were then stained by an indirect immunofluorescence technique with a mouse monoclonal antibody directed to CMV pp65 lower matrix protein and fluorescein isothiocyanate-labeled goat monoclonal antibodies to mouse antigen according to the manufacturer's specifications (Chemicon International, Inc., Temecula, Calif.). The number of antigen-positive cells per slide was counted and expressed as the number of positive cells per 1.5 × 105 leukocytes examined.
(ii) Hybrid capture CMV DNA assay.
The Digene Hybrid Capture DNA System (version 2; Digene Corp., Silver Spring, Md.) was used according to the manufacturer's instructions. The assay detects CMV DNA in leukocyte-rich cell pellets obtained from whole blood by use of a red blood cell lysis procedure followed by centrifugation and DNA extraction. Specimens containing the target DNA hybridize with a specific CMV RNA probe cocktail. Multiple conjugated antibodies bound to each captured hybrid react with a chemiluminescent substrate, resulting in light emission measured as relative light units on a luminometer. The analytical sensitivity of the version 2 assay at the positive cutoff, generated from a set of three standards supplied with the assay, is equivalent to 675 CMV genome copies per ml of whole blood. A positive result was defined as ≥1,400 genome copies/ml of whole blood. Values between 675 and 1,400 copies/ml (considered equivocal, according to the manufacturer's package insert) were considered negative in this study (14, 16, 22).
(iii) PCR for CMV DNA detection.
The Roche Amplicor Qualitative PCR Test (Roche Diagnostics Systems, Inc., Branchburg, N.J.) was performed according to the manufacturer's instructions for qualitative detection of CMV DNA per 50 μl of plasma. The PCR was performed with a Perkin-Elmer model 9600 thermal cycler, and amplicons were detected by an enzyme-linked immunoassay. Samples with a CMV optical density (OD) of ≥0.25 and an internal control (IC) OD of ≥0.25 or <0.25 were interpreted as positive, while samples with a CMV OD of <0.25 and an IC OD of ≥0.25 were interpreted as negative. Samples with a CMV OD of <0.25 and an IC OD of <0.25 were noninterpretable, and additional testing would be required due to the possibility of the presence of PCR inhibitors in the samples (2, 12).
Statistical analysis.
A cross-sectional assessment for the following demographic and clinical characteristics of the study population was conducted: gender, race, CD4 cell count, CD8 cell count, HIV load, and administration of HAART (defined as the use of three or more antiretroviral drugs given in combination). These characteristics were then compared for positive versus negative episodes of CMV retinitis using Fisher's exact test.
Two analyses were then conducted. The first analysis used nonparametric receiver operating characteristic (ROC) curve comparisons (9) to determine the most predictive test of CMV retinitis. The test determined as most predictive in this step was then entered into a multivariate generalized estimating equation (GEE) model (26) to determine significant factors associated with CMV retinitis, as described below. To assess the validity of the different tests (antigenemia, Digene, and Amplicor), appropriate cutoff points for those tests were determined; to determine which test is most appropriate, ROC curves were computed, plotted, and compared. Computations of all sensitivities and specificities were made using SPSS 9.0 for Windows statistical software. The sensitivites and specificities for every distinct value for the continuous outcome tests (antigenemia and Digene) and for the qualitative (Amplicor) test were calculated. The sensitivity was then plotted against 1 − specificity in order to obtain the ROC curves. The area under the curve (AUC) for each test was then computed and compared to the area that would have been expected if the diagnostic test had no predictive value whatsoever. The AUC was defined as the probability that a randomly selected diseased patient had a test value greater than that of a randomly selected nondiseased patient. If the AUC expected by chance alone was 0.50, the nonparametric Mann-Whitney U statistic was used to determine the difference between the actual AUC and 0.50. With a value significantly above 0.50, it was established that the diagnostic test would positively predict the disease state correctly (G. Campbell, unpublished data).
The point at which both the sensitivity and the specificity were mutually maximized was determined from the ROC curve plot. Determination of diagnostic cutoff points using the ROC curve analysis was conducted by calculating the maximum sensitivity times specificity. These points defined the appropriate laboratory cutoff points for the diagnostic tests with continuous outcomes. Sensitivities, specificities, positive predictive values, and negative predictive values were then calculated based on the determined cutoff points. Finally, the AUCs were compared to each other using pairwise comparisons. Z statistics were computed based on the AUCs and their standard errors. Since a total of three comparisons were made and it was determined a priori which comparisons were made, Bonferroni adjustments (25) were made for the significance level in order to control type 1 error. To determine this level, the traditional α level of 0.05 was divided by 3 to result in an adjusted α level of 0.01667 for significance.
In order to form a predictive model with CMV retinitis as the outcome, GEEs were used (26). This method is used to analyze longitudinal data (with repeated measures on each individual subject) when there is a likelihood that those repeated measures will be correlated with each other or when some of those values are missing. It is a modification of the techniques used in logistic regression and linear regression, where the quasi-likelihood estimates are used to estimate the equation coefficients. The use of these equations allows for increased power, and model selection is more robust (27). GEEs were calculated using the statistical module PROC GENMOD in SAS statistical software. Initially, bivariate GEEs were modeled in order to determine which variables should be entered into a multivariate model. Based on these models, HAART therapy status, months of follow-up time, CD4+ T-helper lymphocyte count, and Digene test result (based on a cutoff point previously determined by ROC curve analysis) were all entered into a multivariate GEE. Odds ratios (OR) and 95% confidence intervals (CI) were computed. This modeling technique allowed for both correlated measures (as in the longitudinal data used here) and missing values (useful since some viral load measurements were missing for some patients).
RESULTS
Laboratory results for 512 samples taken from 296 AIDS patients receiving screening eye examinations for CMV retinitis at the Medical Center of Louisiana—New Orleans HIV Outpatient Clinic were included in the statistical analysis. All samples were tested using the three assays described above. The cross-sectional demographic and clinical characteristics of the patient population are shown in Table 1. The majority of patients were male (80%). A total of 72.6% of patients were African-American, 23.6% were Caucasian, and the rest were Hispanic (3.4%) and Asian (0.3%). The patients had a mean age of 38.04 years (standard deviation, 7.83); they ranged from 18 to 65 years old. The mean and median CD4+ T-lymphocyte cell counts were 67.09 cells/μl (standard deviation, 94.7) and 26.5 cells/μl, respectively. Of patients who had a recent CD4+ T-lymphocyte cell count, 66.2% had a count of ≤50 cells/μl. The mean and median CD8+ T-lymphocyte cell counts were 631 cells/μl (standard deviation, 464.9) and 492 cells/μl, respectively. The mean and median HIV loads were 218,433 copies/ml (standard deviation, 258,738) and 104,500 copies/ml, respectively. The vast majority (97%) of patients had recent HIV loads of greater than 10,000 copies/ml. Of the 230 patients (78%) whose charts were available for antiretroviral regimen review, 147 (64%) were on HAART. It should be noted, however, that the duration of HAART treatment for most of these patients was less than 6 months.
TABLE 1.
Demographic and clinical characteristics of the study sample (n = 296)
| Variable | No. (%) |
|---|---|
| Gender | |
| Male | 238 (80.4) |
| Female | 58 (19.6) |
| Race | |
| Black | 215 (72.6) |
| Other | 81 (27.4) |
| CD4+ cells/μl | |
| ≤50 | 196 (66.2) |
| >50 | 100 (33.8) |
| CD8+ cells/μl | |
| <125a | 18 (6.1) |
| ≥125 | 278 (93.9) |
| HIV load (copies/dl) | |
| ≤10,000 | 9 (3.0) |
| >10,000 | 287 (97.0) |
| Antiretroviral regimen (n = 230)b | |
| HAART | 147 (63.9) |
| Non-HAART | 83 (36.1) |
| CMV diagnosis (eye examination) | 18c (6.7) |
Medical Center of Louisiana normal reference range is 228 to 2,290 cells/μl.
Information was incomplete for some patients.
Twenty cases in 18 patients.
Fisher's exact test was used to determine which variables were associated with CMV disease at the bivariate level. This analysis showed that CD4+ T-lymphocyte cell counts, CD8+ T-lymphocyte cell counts, and HAART were the factors most statistically associated with CMV disease (Table 2). There were 20 cases of retinitis in 18 patients. Two patients had more than one episode of CMV retinitis. There was also one patient who had a relapse of CMV retinitis while on oral ganciclovir after a ganciclovir eye implant.
TABLE 2.
Comparison of patients with and without CMV retinitis (n = 296)a
| Variable | %
|
Pd | |
|---|---|---|---|
| With retinitis (n = 18) | Without retinitis (n = 278) | ||
| Gender | |||
| Male | 88.9 | 79.9 | 0.227 |
| Female | 11.1 | 20.1 | |
| Race | |||
| Black | 61.1 | 73.4 | 0.193 |
| Other | 38.9 | 26.6 | |
| Age (yr) | |||
| ≤40 | 50.0 | 34.5 | 0.142 |
| >40b | 50.0 | 65.5 | |
| CD4+ cells/μl | |||
| ≤50 | 88.9 | 64.7 | 0.026 |
| >50 | 11.1 | 35.3 | |
| CD8+ cells/μl | |||
| <125c | 22.2 | 5.0 | 0.017 |
| ≥125 | 77.8 | 95.0 | |
| HIV load (copies/dl) | |||
| ≤10,000 | 5.6 | 2.9 | 0.436 |
| >10,000 | 94.4 | 97.1 | |
| Antiretroviral regimen (n = 230) | |||
| HAART | 61.1 | 34.0 | 0.022 |
| Non-HAART | 38.9 | 66.0 | |
| Positive antigenemia assay | |||
| Yes | 66.7 | 15.5 | <0.001 |
| No | 33.3 | 84.5 | |
| Positive Digene assay | |||
| Yes | 66.7 | 16.9 | <0.001 |
| No | 33.3 | 83.1 | |
| Positive Amplicor assay | |||
| Yes | 50.0 | 16.9 | <0.001 |
| No | 50.0 | 83.1 | |
All values were assessed from the patient's last visit.
These ages correlated with more serious manifestations of some viral infections.
Reference range, 228 to 2,290 cells/μl.
Significant values are shown in bold type.
ROC curve analysis.
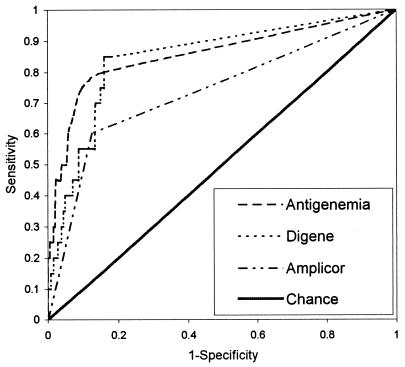
The ROC curves generated for the assays are shown in Fig. 1; the analysis of the data is summarized in Table 3. This analysis demonstrates the diagnostic values of the three assays compared to that of the eye examination, which is the “gold standard.” The areas under the ROC curves were significantly larger than the area expected by chance alone for all three types of assays (P, <0.001). Maximum sensitivity and specificity were determined for each assay, and diagnostic cutoff points were defined based on these points on the curves. These cutoff points for the pp65 antigenemia and Digene assays were determined to be the presence of any pp65 antigen-positive cells/1.5 × 105 cells and 1.4 × 103 CMV genomes/ml, respectively. Since the Amplicor assay is qualitative, a positive result was designated as the cutoff point. Using these cutoff points, diagnostic value was assessed. A total of 93 samples positive in the antigenemia assay were analyzed; 16 (17.2%) were positive for CMV retinitis using a cutoff of the presence of any antigen-positive cells. A total of 106 samples positive in the Digene assay were analyzed; 17 (16%) were positive for CMV retinitis using a cutoff of 1,400 genome copies/ml. A total of 74 positive Amplicor samples were available; 12 (16.2%) were positive for CMV retinitis. Of the patients diagnosed with CMV retinitis, two tested negative in all three assays used.
FIG. 1.
Nonparametric ROC curves for different tests of CMV retinitis.
TABLE 3.
Results of ROC curve analysis (n = 512)
| Assaya | % of area under ROC curveb | 95% CI | Determined cutoff | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|
| Antigenemia | 85.5 | 0.747–0.962 | >0 cells | 0.800 | 0.843 | 0.172 | 0.990 |
| Digene | 84.6 | 0.752–0.941 | 1,400 genome copies/ml | 0.850 | 0.839 | 0.177 | 0.993 |
| Amplicor | 73.7 | 0.607–0.867 | Positive (qualitative) | 0.600 | 0.874 | 0.162 | 0.982 |
For comparisons of the antigenemia and Digene assays, antigenemia and Amplicor assays, and Digene and Amplicor assays, P values were 0.09, <0.001, and <0.001, respectively. The level of significance was set at 0.01667 (Bonferroni adjustment).
The areas under the ROC curves were significantly larger than the area expected by chance alone for all three types of assays (P, <0.001).
Using a cutoff point of the presence of any cells in the antigenemia assay, the sensitivity was calculated to be 0.80 and the specificity was calculated to be 0.843. For the Digene assay with a cutoff point of 1,400 genome copies/ml, the sensitivity was 0.85 and the specificity was 0.839. Finally, for the Amplicor assay, the sensitivity and specificity were determined to be 0.60 and 0.874, respectively. The positive predictive values were all quite low, ranging from 16 to 18%. However, the negative predictive values were all above 98%. The high negative predictive value is partly a result of the low prevalence of retinitis. Pairwise comparisons of the AUCs resulted in the determination that no significant difference was seen between the antigenemia and Digene assays in their validity for accurately diagnosing CMV retinitis. Significant differences were seen between the Amplicor assay and both the antigenemia and the Digene assays (P, <0.001).
Multivariate GEE analysis.
The GEE was used to determine which variables were significantly associated with the development of CMV retinitis in a multivariate analysis. With GEE at the bivariate level, only HAART naivete (P, 0.0053), longer follow-up time in months (P, <0.001), CD4+ T-helper lymphocyte cell counts less than or equal to 50 cells/μl (P, 0.0088), and a positive Digene assay result (P, <0.001) were determined to be significantly associated with CMV retinitis. These factors were entered into a multivariate GEE analysis, which yielded OR and 95% CI that are presented in Table 4. Significantly associated factors in this model were a 1-month increase in follow-up time (OR, 1.01; 95% CI, 1.01 to 1.02) and a positive Digene assay result (OR, 1.19; 95% CI, 1.10 to 1.28).
TABLE 4.
Results of fitting a GEE for the prediction of CMV retinitis (n = 512)
| Predictor | OR | 95% CI | Pa |
|---|---|---|---|
| On HAART | 1.03 | 0.98–1.07 | 0.232 |
| One-month increase in follow-up time | 1.01 | 1.01–1.02 | <0.001 |
| CD4+ cell count of ≤50 cells/mm3 | 0.99 | 0.97–1.02 | 0.5920 |
| Digene assay positive | 1.19 | 1.10–1.28 | <0.001 |
Significant values are shown in bold type.
DISCUSSION
The incidence of CMV retinitis has declined since the availability of protease inhibitors (4, 18). Our patient population had an incidence of 7.2 per 100 person-years, slightly higher than the national reported incidence of approximately 5 per 100 person-years in the first quarter of 1997 (18). Adherence to treatment and follow-up are significant problems in our clinic and may contribute to this higher rate.
To date, ours is the largest study using the three assays for a large cohort of AIDS patients undergoing routine ophthalmologic examinations. This study was aimed at evaluating the diagnostic values of the three assays compared to the gold standard, eye examinations by an ophthalmologist, for AIDS patients at risk for CMV retinitis. The dilated eye examination for the diagnosis of CMV retinitis is noninvasive but lacks sensitivity when retinitis is early in its evolution (24). Therefore, CMV detection assays may assist clinicians in the diagnosis of CMV retinitis in its early stages in patients at high risk for developing CMV retinitis (24).
The previous studies reporting the diagnostic value of the pp65 antigenemia assay for CMV disease in AIDS patients are summarized in Table 5. Our study had the largest sample size. There were wide ranges in the sensitivity, specificity, and positive predictive value among the studies. There are several reasons for the different findings in these studies. First, the patient populations had different CD4+ T-lymphocyte cell counts at entry into the studies. Second, there was some variability in the antigenemia assay technique used, including the number of peripheral blood leukocytes per slide and the reagents used. Also, the interpretation of this assay is reader dependent. Third, some of the studies included CMV disease other than retinitis. CMV retinitis is a more localized disease than other CMV end-stage organ diseases; therefore, the level of CMV antigenemia may be lower.
TABLE 5.
Comparison of the diagnostic values of the pp65 antigenemia assay for CMV disease in AIDS patientsa
| Reference or source | Study population (CD4+ cells/μl) | Threshold (cells) | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|
| 1 | 174 AIDS patients (<100) | >0/2 × 105 | 0.91 | 0.65 | 0.47 | 0.95 |
| >5/2 × 105 | 0.67 | 0.81 | 0.63 | 0.84 | ||
| 5 | 200 AIDS patients (<100) | >0/1 × 105 | 0.92 | 0.88 | ||
| 7 | 49 AIDS patients (<150, or 10%) | >0/2 × 105 | 0.85 | 0.64 | 0.46 | 0.92 |
| ≥20 | 0.77 | 0.97 | 0.91 | 0.92 | ||
| 6 | 62 AIDS patients | >0/2 × 105 | 0.81 | 0.72 | ||
| 14 | 238 HIV-infected patients | >0/1 × 105 | 0.92 | 0.89 | ||
| 15 | 36 AIDS patients | >0/2 × 105 | 1.00 | 0.86 | ||
| 19 | 24 AIDS patients with CMV retinitis and 24 without CMV disease | >0/3 × 105 | 0.33 | 1.00 | ||
| 22 | 138 AIDS patients (<50) | >0/1 × 105 | 1.00 | 0.50 | 0.29 | 1.00 |
| 23 | 22 AIDS patients | >0/2 × 105 | 1.00 | 0.64 | 0.73 | 1.00 |
| This study | 296 AIDS patients (<100) | >0/1.5 × 105 | 0.80 | 0.84 | 0.17 | 0.99 |
PPV and NPV, positive and negative predictive values, respectively.
Our clinic population may differ from other study populations. The New Orleans HIV Outpatient Clinic serves a high proportion of African-American males, many of whom obtain treatment in advanced stages of HIV infection. Although Table 2 suggests a relatively large number of patients receiving HAART, it must be considered that the vast majority of these individuals had been on treatment for less than 6 months.
Our study indicates that all three of these assays have low positive predictive values, in the range of 16 to 17%. Therefore, one should view the application of these assays as sensitive but nonspecific indicators of CMV disease. When the cutoff for a positive test was increased, the specificity and positive predictive value were better (1, 7). One study suggested that preemptive anti-CMV therapy be initiated if antigenemia is greater than 20 cells (7).
An additional finding in this study was that all three methods had high negative predictive values (above 98% for each assay). These findings would therefore favor the use of these assays for ruling out patients at high risk for retinitis. Nonetheless, it should be pointed out that despite the high negative predictive values, 2 of the 20 cases of active retinitis were associated with negative results. Also, the relatively low disease prevalence in the study cohort may partially explain the high negative predictive values for the assays used.
The Digene Hybrid Capture CMV DNA System is advantageous compared to the antigenemia method because it is technically less cumbersome and operator dependent. In previous studies (14, 22) and in this study (Table 6), the Digene assay (version 2) had high diagnostic values and much narrower ranges of sensitivity, specificity, and positive predictive value than the pp65 antigenemia assay. The sensitivities in all studies were higher than 0.85 (0.85 to 0.94). As for the antigenemia assay, all studies demonstrated a very high negative predictive value (97 to 99%). Theoretically, the Digene assay (version 2) may be more sensitive than the antigenemia assay due to presence of a signal amplification step for CMV DNA detection. Our study confirmed the findings of Mazzulli et al. (14). Our study also showed that a cutoff point of 1,400 genome copies/ml rendered a higher specificity than the cutoff points provided by the standards supplied in the assay kits (approximately 675 genome copies/ml) and did not reduce the sensitivity (Table 6). This higher cutoff point may be worth being adopted if further studies support its value in AIDS patients at risk for CMV retinitis.
TABLE 6.
Comparison of the diagnostic values of the Digene assay for CMV disease in AIDS patientsa
| Reference or source | Study population (CD4+ cells/μl) | Cutoff (genome copies/ml) | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|
| 14 | 238 HIV-infected patients | 675 | 0.94 | 0.95 | ||
| 22 | 138 AIDS patients (<50) | 675 | 0.91 | 0.64 | 0.34 | 0.97 |
| This study | 296 AIDS patients (<100) | 1,400 | 0.85 | 0.84 | 0.18 | 0.99 |
PPV and NPV, positive and negative predictive values, respectively.
PCR techniques for CMV DNA detection have been developed in different laboratories and tested with patient samples, including those of HIV-infected persons, in many centers. We found the diagnostic value of the Amplicor assay to be significantly lower than that of the antigenemia and Digene assays for AIDS patients at risk for CMV retinitis. This result may be explained by the use of a different blood compartment (plasma) for CMV DNA detection. Theoretically, plasma contains fewer viral genome copies than the cellular compartment. Another explanation is that the positive cutoff of the Amplicor assay could be too high for the setting of CMV retinitis, which is a localized disease. However, when peripheral blood leukocytes were used for CMV DNA detection by PCR techniques, the sensitivity increased to 1.00, while the specificity decreased to 0.61 (2). PCR techniques may provide faster CMV detection for a larger number of specimens but need further standardization before being used in this clinical setting.
The data from our study showed that the pp65 antigenemia assay at a cutoff point of >0 cells and the Digene assay at a cutoff point of ≥1,400 genome copies/ml were significantly more effective than the Amplicor assay. The Digene assay gave the optimal diagnostic value at this cutoff point. These assays will help to select subgroups of patients who need more frequent follow-up when CMV viremia is detected. Patients should continue their follow-up with the eye clinic, since our study demonstrated that a 1-month increase in follow-up time was a risk factor associated with CMV retinitis at the multivariate analysis level. Finally, since all three assays have high negative predictive values, clinicians should consider deferring empiric or prophylactic anti-CMV treatment when patients have a negative test result.
ACKNOWLEDGMENTS
We thank Bruce Barron (Louisiana State University, Department of Opthalmology) and Newton E. Hyslop, Jr. (Tulane University Medical Center, Section of Infectious Diseases) for eye screening examinations and consultative advice.
This project was supported through institutional resources without financial support from the companies manufacturing the CMV assays used.
REFERENCES
- 1.Bek B, Boeckh M, Lepenies J, Bieniek B, Arasteh K, Heise W, Deppermann K-M, Bornhoft G, Stoffler-Meilicke M, Schuller I, Hoffken G. High-level sensitivity of quantitative pp65 cytomegalovirus (CMV) antigenemia assay for diagnosis of CMV disease in AIDS patients and follow-up. J Clin Microbiol. 1996;34:457–459. doi: 10.1128/jcm.34.2.457-459.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boivin G, Handfield J, Toma E, Murray G, Lalonde R, Tevere V J, Sun R, Bergeron M G. Evaluation of the AMPLICOR cytomegalovirus test with specimens from human immunodeficiency virus-infected subjects. J Clin Microbiol. 1998;36:2509–2513. doi: 10.1128/jcm.36.9.2509-2513.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Britt W J. Betaherpesviruses: cytomegalovirus and human herpesviruses 6 and 7. In: Topley W W L, Wilson G S, editors. Topley & Wilson's microbiology and microbial infections. 9th ed. 1. Virology. New York, N.Y: The Oxford University Press, Inc.; 1998. pp. 339–350. [Google Scholar]
- 4.Casado J L, Perez-Elias M J, Marti-Belda P, Antela A, Suarez M, Ciancas E, Frutos B, Perez M D, Guerrero A. Improved outcome of cytomegalovirus retinitis in AIDS patients after introduction of protease inhibitors. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:130–134. doi: 10.1097/00042560-199810010-00005. [DOI] [PubMed] [Google Scholar]
- 5.Dodt K K, Jacobsen P H, Hofmann B, Meyer C, Kolmos H J, Skinhoj P, Norrild B, Mathiesen L. Development of cytomegalovirus (CMV) disease may be predicted in HIV-infected patients by CMV polymerase chain reaction and the antigenemia test. AIDS. 1997;11:F21–F28. doi: 10.1097/00002030-199703110-00001. [DOI] [PubMed] [Google Scholar]
- 6.Fox E F, Smith N A, Rice P, Dunn H, Peters B S. Cytomegalovirus pp65 antigenaemia as an indicator of end-organ disease in AIDS. Int J STD AIDS. 1998;9:545–547. doi: 10.1258/0956462981922809. [DOI] [PubMed] [Google Scholar]
- 7.Francisci D, Tosti A, Baldelli F, Stagni G, Pauluzzi S. The pp65 antigenemia test as a predictor of cytomegalovirus-induced end-organ disease in patients with AIDS. AIDS. 1997;11:1341–1345. doi: 10.1097/00002030-199711000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Gallant J E, Moore R D, Richman D D, Keruly J, Chaisson R E the Zidovudine Epidemiology Study Group. Incidence and natural history of cytomegalovirus disease in patients with advanced human immunodeficiency virus disease treated with zidovudine. J Infect Dis. 1992;166:1223–1227. doi: 10.1093/infdis/166.6.1223. [DOI] [PubMed] [Google Scholar]
- 9.Hanley J A, McNeil B J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 10.Jacobson M A, Zegans M, Pavan P R, O'Donnell J J, Sattler F, Rao N, Owens S, Pollard R. Cytomegalovirus retinitis after initiation of highly active antiretroviral therapy. Lancet. 1997;349:1443–1445. doi: 10.1016/S0140-6736(96)11431-8. [DOI] [PubMed] [Google Scholar]
- 11.Kuppermann B D, Petty J G, Richman D D, Mathews W C, Fullerton S C, Richman L S, Freeman W R. Correlation between CD4+ counts and prevalence of cytomegalovirus retinitis and human immunodeficiency virus-related noninfectious retinal vasculopathy in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 1993;115:575–582. doi: 10.1016/s0002-9394(14)71453-9. [DOI] [PubMed] [Google Scholar]
- 12.Long C M, Drew L, Miner R, Jekic-McMullen D, Impraim C, Kao S Y. Detection of cytomegalovirus in plasma and cerebrospinal fluid specimens from human immunodeficiency virus-infected patients by the AMPLICOR CMV test. J Clin Microbiol. 1998;36:2434–2438. doi: 10.1128/jcm.36.9.2434-2438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacGregor R R, Pakola S J, Graziani A L, Montzka D P, Hodinka R L, Nichols C W, Friedman H M. Evidence of active cytomegalovirus infection in clinically stable HIV-infected individuals with CD4+ lymphocyte counts below 100/μl of blood: features and relation to risk of subsequent CMV retinitis. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:324–330. [PubMed] [Google Scholar]
- 14.Mazzulli T, Drew L W, Yen-Lieberman B, Jekic-McMullen D, Kohn D J, Isada C, Moussa G, Chua R, Walmsley S. Multicenter comparison of the digene hybrid capture CMV DNA assay (version 2.0), the pp65 antigenemia assay, and cell culture for detection of cytomegalovirus viremia. J Clin Microbiol. 1999;37:958–963. doi: 10.1128/jcm.37.4.958-963.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazzulli T, Rubin R H, Ferraro M J, D'Aquila R T, Doveikis S A, Smith B R, The T H, Hirsch M S. Cytomegalovirus antigenemia: clinical correlations in transplant recipients and in persons with AIDS. J Clin Microbiol. 1993;31:2824–2827. doi: 10.1128/jcm.31.10.2824-2827.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazzulli T, Wood S, Chua R, Walmsley S. Evaluation of the Digene Hybrid Capture System for detection and quantitation of human cytomegalovirus viremia in human immunodeficiency virus-infected patients. J Clin Microbiol. 1996;34:2959–2962. doi: 10.1128/jcm.34.12.2959-2962.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mocarski E S., Jr . Cytomegaloviruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2447–2523. [Google Scholar]
- 18.Palella F J, Jr, Delaney K M, Moorman A C, Loveless M O, Fuhrer J, Satten G A, Aschman D J, Holmberg S D the HIV Outpatient Study Investigators. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 19.Pannuti C S, Kallas E G, Muccioli C, Roland R K, Ferreira E C, Bueno S M H S, Do Canto C L M, Villas Boas L S, Belfort R., Jr Cytomegalovirus antigenemia in acquired immunodeficiency syndrome patients with untreated cytomegalovirus retinitis. Am J Ophthalmol. 1996;122:847–852. doi: 10.1016/s0002-9394(14)70381-2. [DOI] [PubMed] [Google Scholar]
- 20.Polis M A, Masur H. Textbook of AIDS medicine. 2nd ed. Baltimore, Md: The Williams & Wilkins Co.; 1999. Cytomegalovirus infection in patients with HIV infection; pp. 373–389. [Google Scholar]
- 21.Reed J B, Schwab I R, Gordon J, Morse L S. Regression of cytomegalovirus retinitis associated with protease-inhibitor treatment in patients with AIDS. Am J Ophthalmol. 1997;124:199–205. doi: 10.1016/s0002-9394(14)70784-6. [DOI] [PubMed] [Google Scholar]
- 22.Walmsley S, O'Rourke K, Mortimer C, Rachlis A, Fong I, Mazzulli T. Predictive value of cytomegalovirus (CMV) antigenemia and digene hybrid capture DNA assays for CMV disease in human immunodeficiency virus-infected patients. Clin Infect Dis. 1998;27:573–581. doi: 10.1086/514703. [DOI] [PubMed] [Google Scholar]
- 23.Wetherill P E, Landry M L, Alcabes P, Friedland G. Use of a quantitative cytomegalovirus (CMV) antigenemia test in evaluating HIV+ patients with and without CMV disease. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12:33–37. doi: 10.1097/00042560-199605010-00005. [DOI] [PubMed] [Google Scholar]
- 24.Whitley R J, Jacobson M A, Friedberg D N, Holland G N, Jabs D A, Dieterich D T, Hardy W D, Polis M A, Deutsch T A, Feinberg J, Spector S A, Walmsley S, Drew W L, Powderly W G, Griffiths P D, Benson C A, Kessler H A. Guidelines for the treatment of cytomegalovirus diseases in patients with AIDS in the era of potent antiretroviral therapy: recommendations of an international panel. International AIDS Society-USA Arch Intern Med. 1998;158:957–969. doi: 10.1001/archinte.158.9.957. [DOI] [PubMed] [Google Scholar]
- 25.Winer, B. J., D. R. Brown, and K. M. Michaels (ed.). Statistical principles in experimental design3rd ed. McGraw-Hill, Inc., New York, N.Y.
- 26.Zeger S L, Liang K Y. Longitudinal data analysis for discrete and continuous outcome. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 27.Zeger S L, Liang K Y. An overview of methods for the analysis of longitudinal data. Stat Med. 1992;11:1825–1839. doi: 10.1002/sim.4780111406. [DOI] [PubMed] [Google Scholar]