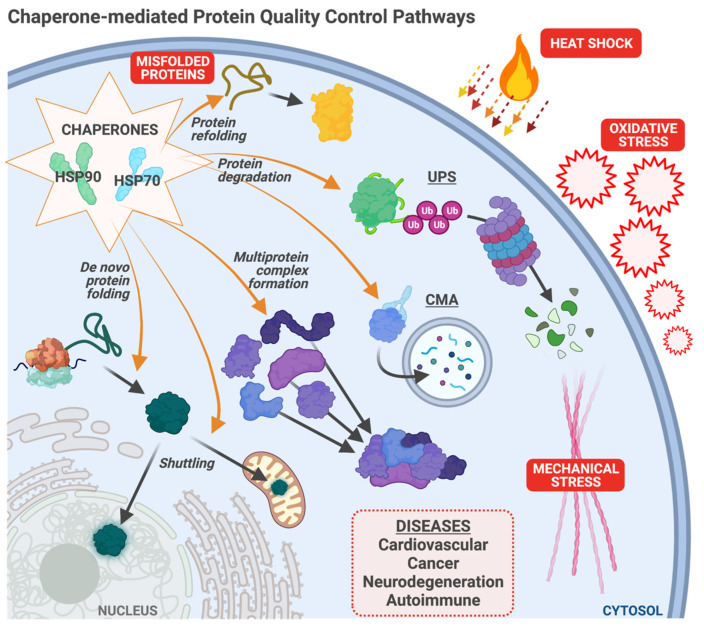
Figure 1.
Chaperone-mediated protein quality control. Chaperones, including HSP70 and HSP90, maintain cellular homeostasis through multiple pathways: assisting with de novo protein folding; multiprotein complex formation; protein shuttling throughout the cell; degradation of terminally misfolded proteins (via the ubiquitin-proteasome system (UPS) and chaperone-mediated autophagy (CMA); and refolding of misfolded proteins damaged by cellular stress. The chaperone system responds to multiple stressors, including the accumulation of misfolded proteins, heat shock, oxidative stress, and mechanical stress. Chaperone dysfunction contributes to numerous diseases discussed in this review.