Abstract
Immunotherapy has revolutionized the treatment landscape for many cancer types. The treatment for renal cell carcinoma (RCC) has especially evolved in recent years, from cytokine-based immunotherapies to immune checkpoint inhibitors. Although clinical benefit from immunotherapy is limited to a subset of patients, many combination-based approaches have led to improved outcomes. The success of such approaches is a direct result of the tumor immunology knowledge accrued regarding the RCC microenvironment, which, while highly immunogenic, demonstrates many unique characteristics. Ongoing translational work has elucidated some of the mechanisms of response, as well as primary and secondary resistance, to immunotherapy. Here, we provide a comprehensive review of the RCC immunophenotype with a specific focus on how preclinical and clinical data are shaping the future of immunotherapy.
Keywords: renal cell carcinoma, tumor microenvironment, immunology, immunotherapy
1. Introduction
Renal cell carcinoma (RCC) is the eighth most prevalent cancer in the U.S with a lifetime risk of 1.7% and an estimated incidence of approximately 76,000 cases and 13,800 deaths in 2021 [1]. Interestingly, the disease displays a high degree of both inter- and intra-tumoral heterogeneity [2]. Histopathologically, RCC is divided primarily into the clear cell (ccRCC, 75%), papillary (pRCC, 15%), and chromophobe (chRCC, 5%) subsets. For ccRCC, although VHL alterations or chromosome 3p loss are the most common genetic alterations, there are many additional frequently observed alterations implicated in disease pathogenesis [3]. Genomic and transcriptomic analyses have highlighted this heterogeneity, defining distinct molecular RCC subtypes by various classification schemes [4,5,6], many of which carry unique characteristics, prognoses, and associations with response to specific treatments.
In parallel with a deeper understanding of RCC biology, management of the disease has significantly evolved in recent years, leading to improved patient outcomes [7]. This is, in large part, due to the resurgence of cancer immunotherapy. Previously, RCC was managed with cytokine-based therapies including IL-2 [8] and IFNα [9]; however, clinical benefit was largely limited to a very small subset of patients [8,10]. Subsequently, multiple tyrosine kinase inhibitors (TKIs) with anti-angiogenic properties provided an improved benefit to toxicity profile, leading to broader benefit for more patients. However, the duration of treatment response proved to be relatively modest [11]. Most recently, immune checkpoint inhibitors (ICIs) have emerged as an important modality for treating RCC (Table 1). These monoclonal antibodies are designed to antagonize inhibitory pathways that promote immunosuppression and tumor immune evasion. Specifically, ICIs targeting the PD-1/PD-L1 and CTLA-4/B7 axes have yielded great clinical success in many cancer types. PD-1 is a transmembrane protein expressed primarily on activated T cells, with additional expression on natural killer (NK) cells, B cells, dendritic cells (DCs), and macrophages. PD-1 binds to its ligand, PD-L1, which is often expressed on tumor cells and various antigen-presenting cells, leading to subsequent inhibition of T cell expansion and activity [12]. CTLA-4 is expressed on T cells after initial activation, as well as on regulatory T cells (Tregs), and serves to disrupt the crucial costimulatory signaling between CD28 and B7-1/CD80 and B7-1/CD86 needed for T cell activity [13,14]. While these interactions serve physiologic roles in dampening the adaptive immune response in chronic inflammatory conditions, in the setting of malignancy, they lead to an immunosuppressive microenvironment promoting tumor immune escape.
Table 1.
Approved immunotherapies for RCC.
| Treatment(s) | Class | Setting | Indication | Key Data | Clinical Trial |
|---|---|---|---|---|---|
| Monotherapy | |||||
| Nivolumab | ICI | Second line | Advanced RCC after prior anti-angiogenic therapy | Nivolumab vs. Everolimus: • mOS: 25.0 mo (95% CI, 21.8–NE) vs. 19.6 mo (95% CI, 17.6–23.1) [HR 0.73; 98.5% CI, 0.57–0.93; p = 0.002] • mPFS: 4.6 mo (95% CI, 3.7–5.4) vs. 4.4 mo (95% CI, 3.7–5.5) [HR 0.88; 95% CI, 0.75–1.03; p = 0.11] • ORR: 25% vs. 5% [OR 5.98; 95% CI, 3.68–9.72; p < 0.001] |
CheckMate 025 [18] |
| High-Dose IL-2 | Cytokine | First line | Metastatic RCC | Proleukin: • ORR: 14% (90% CI, 10–19) • CR: 12 (5%) CR • PR: 24 (9%, median response duration 19.0 mo) |
[8] |
| Combination Therapy | |||||
| Ipilimumab + Nivolumab | ICI + ICI | First line | Intermediate/poor-risk advanced RCC | Ipilimumab + Nivolumab vs. Sunitinib: • mOS: NR (95% CI, 28.2–NE) vs. 26 mo (95% CI, 22.1–NE) [HR 0.63; 99.8%, CI 0.44–0.89; p < 0.001] • mPFS: 11.6 mo (95% CI, 8.7–15.5) vs. 8.4 mo (95% CI, 7.0–10.8) [HR 0.82; 99% CI, 0.64–1.05; p = 0.03] • ORR: 42% (95% CI, 37–47) vs. 27% (95% CI, 22–31) [p < 0.001] |
CheckMate 214 [24] |
| Nivolumab + Cabozantinib | ICI + TKI | First line | Advanced RCC | Nivolumab + Cabozantinib vs. Sunitinib: • Probability of OS at 12 mo: 85.7% (95% CI, 81.3–89.1) vs. 75.6% (95% CI, 70.5–80.0) [HR 0.60; 98.89% CI, 0.40–0.89; p = 0.001] • mPFS: 16.6 mo (95% CI, 12.5–24.9) vs. 8.3 mo (95% CI, 7.0–9.7) [HR 0.51; 95% CI, 0.41–0.64; p < 0.001] • ORR: 55.7% (95% CI, 50.1–61.2) vs. 27.1% (95% CI, 22.4–32.3) [p < 0.001] |
CheckMate 9ER [31] |
| Pembrolizumab + Lenvatinib | ICI + TKI | First line | Advanced RCC | Pembrolizumab + Lenvatinib vs. Sunitinib: • mOS: NR (33.6–NE) vs. NR (NE–NE) [HR 0.66; 95% CI, 0.49–0.88; p = 0.0049] • mPFS: 23.9 mo (95% CI, 20.8–27.7) vs. 9.2 mo (95% CI, 6.0–11.0) [HR 0.39; 95% CI, 0.32–0.49; p < 0.0001] • ORR: 71% (95% CI, 66–76) vs. 36% (95% CI, 31–41) [p < 0.0001] |
KEYNOTE-581/CLEAR [30] |
| Pembrolizumab + Axitinib | ICI + TKI | First line | Advanced RCC | Pembrolizumab + Axitinib vs. Sunitinib: • mOS: NR vs. NR [HR 0.53; 95% CI, 0.38–0.74; p < 0.0001] • mPFS: 15.1 mo (95% CI, 12.6–17.7) vs. 11.1 mo (95% CI, 8.7–12.5) [HR 0.69; 95% CI, 0.57–0.84; p < 0.001) • ORR: 59.3% (95%, CI 54.5–63.9) vs. 35.7% (95% CI, 31.1–40.4) [p < 0.001] |
KEYNOTE-426 [27] |
| Avelumab + Axitinib | ICI + TKI | First line | Advanced RCC | Avelumab + Axitinib vs. Sunitinib: • 12 mo OS (PD-L1+): 86% vs. 83% (HR 0.78; 95% CI, 0.55–1.08; p = 0.14) • mPFS (PD-L1+): 13.8 mo vs. 7.2 mo [HR 0.61; 95% CI, 0.47–0.79; p < 0.001] • mPFS (overall population): 13.8 mo (95% CI, 11.1–NE) vs. 8.4 mo (95% CI, 6.9–11.1) [HR 0.69; 95% CI, 0.56–0.84; p < 0.001] • ORR (PD-L1+): 55.2% (95% CI, 49.0–61.2) vs. 25.5% (95% CI, 20.6–30.9) |
JAVELIN Renal 101 [28] |
| IFNα-2a + Bevacizumab | Cytokine + VEGF inhibitor | First line | Metastatic RCC | IFNα-2a + Bevacizumab vs. IFNα-2a + Placebo: • mOS: 23.3 mo vs. 21.3 mo [HR 0.91; 95% CI, 0.76 to 1.10; p = 0.3360] • mPFS: 10.2 mo vs. 5.4 mo [HR 0.63; 95% CI, 0.52 to 0.75; p = 0.0001] • ORR: 31% vs. 12% [p < 0.001] |
AVOREN [9] |
Immune checkpoint inhibitor (ICI), tyrosine kinase inhibitor (TKI), median overall survival (mOS), months (mo), confidence interval (CI), not estimable (NE), hazard ratio (HR), median progression free survival (mPFS), overall response rate (ORR), complete response (CR), partial response (PR), and not reached (NR).
For patients presenting with localized RCC, surgery (either partial or radical nephrectomy) is standard of care and potentially curative. However, some will inevitably develop disease recurrence, which is associated with a poor prognosis [3]. In 2017, sunitinib was approved by the Federal Drug Administration in the adjuvant setting for patients at high risk for recurrence after nephrectomy, after demonstrating disease free survival (DFS) benefit in the S-TRAC trial [15]. However, utilization of such treatment approach has been largely underutilized, with lack of overall survival (OS) benefit in S-TRAC, lack of definitive benefit in other similar randomized studies [16], and the barrier of potential treatment toxicities. In the recently published phase III KEYNOTE-564 trial, adjuvant pembrolizumab (anti-PD-1) also demonstrated definitive DFS benefit in patients at high risk of recurrence after nephrectomy [17], offering another promising treatment option in this setting.
ICIs have also transformed the treatment landscape for patients with advanced and metastatic RCC. For patients previously treated with anti-angiogenic therapy, nivolumab (anti-PD-1) monotherapy proved superior to everolimus in patients with metastatic ccRCC who had progressed from prior anti-angiogenic therapy [18]. Pembrolizumab monotherapy has also shown promise as a first-line option, in both advanced ccRCC [19] as well as non-ccRCC [20]. While this single-armed approach provides clinical benefit for a subset of patients, many others do not benefit with meaningful or durable response.
Combination-based immunotherapy approaches have proven to be very effective for many cancer types, including RCC [21]. The synergy seen with combination ICIs may be due to the differential functions of the immunosuppressive pathways they antagonize. Dual ICI therapy results in a unique gene expression profile [22] and the expansion of specific T cell subsets [23] not seen with either PD-1/PD-L1- or CTLA-4-based monotherapies. In CheckMate 214, International Metastatic RCC Database Consortium (IMDC) intermediate/poor-risk ccRCC patients treated with nivolumab plus ipilimumab (anti-CTLA-4) had significant benefit in OS, PFS, quality of life, and subsequent treatment-free survival compared to those treated with sunitinib [24,25]. The median OS for those treated with the combination was 55.7 months, an unprecedented outcome that has not been previously seen with other therapies. However, 20% of patients experience primary progression, and, additionally, the durability of response varies widely. Thus, it is critical to better understand the unique properties of the tumor microenvironment (TME) to both identify mechanisms of resistance and develop optimal synergistic and novel combinatorial approaches.
Another effective combination approach is the use of an ICI concurrently with a TKI. Approved TKIs are generally multi-targeted, with predominant inhibitory activity on angiogenesis via the VEGF pathway. While these inhibitors have direct tumor cytotoxic activity by impacting the endothelium, they also display numerous immunomodulatory properties. VEGF functions in decreasing T cell infiltration and anti-tumor activity, while increasing intra-tumoral levels of Tregs, tumor-associated macrophages (TAMs), and myeloid-derived suppressor cells (MDSCs). Interestingly, transcriptomic analysis of sunitinib- or pazopanib-treated patients identified enrichment of PD-L1 and M2 TAMs in the patient cluster with the worst outcomes [26]. These preclinical findings make the ICI/TKI combination a promising therapeutic approach in patients with advanced ccRCC, leading to clinical benefit that has now been validated in several clinical trials [27,28,29,30,31].
2. Targeting the Tumor Microenvironment
In order to optimize the treatment approach for RCC, it is critical to understand the makeup of the TME that will determine the impact of each therapy. Transcriptomic analysis of the RCC immune infiltrate from The Cancer Genome Atlas (TCGA) database identified ccRCC as having the highest degree of total immune infiltration and T cell infiltration out of 19 cancer types [32], with an immunologically “cold” RCC TME rarely observed [33]. There are also various cell signaling pathways that serve to enhance or inhibit immune infiltration and function, including immune checkpoints such as PD-L1 and CTLA-4. Tumor mutational burden (TMB) has become another area of focus, as the generation of sporadic non-synonymous tumor-specific mutations, termed neo-antigens, can serve as the basis for enhanced tumor immunogenicity. RCC is unique in many of these regards, with a tumor immune composition and mutational patterns dissimilar to what are expected with an immunotherapy-responsive tumor type.
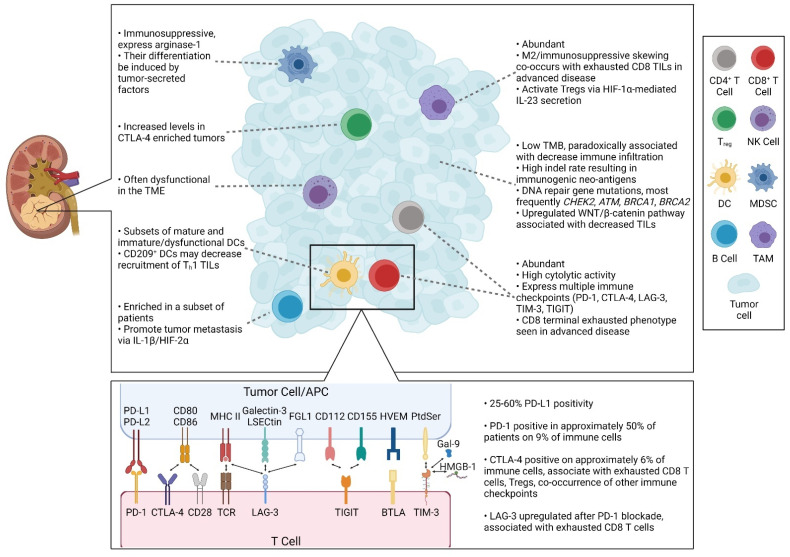
Importantly, in vivo, RCC typically exhibits a large degree of inter- and intra-tumoral heterogeneity [2]. Among the RCC subtypes, ccRCC demonstrates an increased immune response-related gene expression signature compared to pRCC and chRCC [34]. A separate taxonomic TCGA analysis that stratified RCC into nine distinct subtypes similarly found the ccRCC clusters to contain the greatest levels of total immune and T cell infiltration, as well as increased expression of the genes for CD3, PD-1, PD-L2, CTLA-4, CD134, and CD137 [5]. Mass cytometry-based clustering further highlighted the diversity of the immune makeup, identifying 22 distinct T cell and 17 distinct TAM phenotypes [35]. Here, we review the current understanding regarding the immunophenotype and relevant components that comprise the RCC TME (Figure 1).
Figure 1.
Representation of the RCC TME. Text highlighting key data regarding the infiltrating immune cells, tumor cells, and immune checkpoint molecules.
2.1. Cellular Targets
2.1.1. Tumor-Infiltrating Lymphocytes: CD8 T Cells, CD4 T Cells, Regulatory T Cells, and B Cells
Tumor-infiltrating lymphocytes (TILs) represent an integral and diverse component of the tumor immune infiltrate. Generally, CD8 T cells and Th1-differentiated CD4 T cells function to promote anti-tumor immunity, while Tregs and Th2 T cells are associated with immune evasion. Th17 CD4 T cells display mixed roles in the TME, and their differentiation exists within a homeostatic balance with Tregs. B cells also play conflicting roles, with the regulatory B cell subset specifically acting to modulate the anti-tumor immune response. RCC display a strong abundance of intra-tumoral T cells compared to other cancer types. Multiple TCGA analyses have identified ccRCC as having both the highest T cell infiltration score among 9 cancer types [32] and the highest proportion of “T cell-inflamed tumors” among 31 malignancies [36]. CD8 T cells, in particular, are enriched in the majority of cases [37,38]. Among the CD4 T cells, studies have reported an overall low level of Th2 T cells within RCC [32,37]. However, circulating CD4 T cells in the peripheral bloodstream appear to be skewed towards a Th2/Th17 phenotype, especially in higher stage tumors [39]. Among the RCC subsets, there may be a Th17 and Th2 predominance in chRCC and pRCC, respectively [34].
TILs are not only numerous within these tumors, they also appear to be highly functional. In one study quantitating immune cytolytic activity based on the expression level of the CD8 T cell cytolytic effector molecules granzyme A (GZMA) and perforin (PRF1), ccRCC ranked highest among 15 cancer types, and the scoring was markedly increased compared to pRCC and benign renal tissue [40]. Distinct CD8 and CD4 T cell populations display differential expression of the genes for activation and costimulatory markers including CD28, CD69, CD38, HLA-DR, OX-40, 4-1BB, ICOS, and CXCR3 and checkpoint markers such as PD-1, CTLA-4, and Tim-3 [35,37]. However, in more advanced and metastatic disease, RCC CD8 TILs shift towards a terminal exhausted phenotype, expressing multiple immune checkpoint molecules with restricted T cell receptor (TCR) diversity [41].
Tregs serve a physiologic role in promoting self-tolerance and maintaining immunological homeostasis under benign conditions. However, within the TME, they inhibit anti-tumor immunity through multiple mechanisms including via CTLA-4 and the production of immunosuppressive cytokines TGF-β and IL-10 [42]. The data regarding Tregs in RCC have been mixed. Compared to other cancer types, the RCC TME appears to contain a lower level of Tregs [32]. However, in the periphery, Treg levels have been observed in both greater [43] and lower [39] levels in RCC patients compared to healthy controls. Notably, patients with low levels of circulating Tregs appear to have these immunosuppressive cells comprise a greater proportion of TIL compartment within their tumors [43]. A similar pattern of decreased peripheral Tregs and increased tumor-infiltrating Tregs has been associated with higher tumor stage [39].
B cells play conflicting roles in modulating the tumor immune response. B cells and plasma cells are able participate in and promote antigen presentation, and produce antibodies and cytokines that enhance the anti-tumor immune response. However, subsets such as regulatory B cells can suppress the immune response through production of TGF-β, IL-10, and IL-35, as well as via antibodies that form tumorigenic immune-complexes [44]. While many RCCs do not contain a significant degree of B cells, a subset are highly infiltrated [45], with overall B cell levels documented at a frequency of approximately 4% in ccRCC [35]. Functionally, B cells recruited into renal tumors have been implicated in promoting tumor migration and metastatic potential via IL-1β/HIF-2α signaling [46]. B cell infiltration and B cell receptor (BCR) diversity may also hold poor prognostic implications for RCC [47].
2.1.2. Dendritic Cells
Among the DC subsets, plasmacytoid DCs may be overrepresented in ccRCC [32,34]. Similar to Tregs, both myeloid DCs and plasmacytoid DCs have been observed in lower levels in the periphery but higher levels within the RCC TME, and many display an immature dysfunctional phenotype [48]. CD209+ DCs, in particular, may play a role in promoting tumor progression by decreasing the recruitment of Th1 T cells into the tumor [49]. Intra-tumoral DCs have similarly been linked as a prognostic marker for immunotherapy [50].
2.1.3. Tumor-Associated Macrophages
Macrophages represent a diverse immune cell type, exemplified by the 17 distinct TAM phenotypes observed in RCC [35]. Immunophenotyping analysis identified M2-polarized TAMs, in addition to CD8 T cells, as the most abundant immune cell type within the RCC TME [51]. Discrepancies can exist between disease sites, as RCC metastatic lesions displayed a greater number of TAMs but a decreased immunosuppressive M2-skewing compared to the primary tumor [52], and intra-tumoral macrophages appear to be decreased after treatment with bevacizumab [53]. TAMs are intricately involved in crosstalk with many other cell types, and, in RCC, evidence suggests that they activate Tregs via HIF-1α-mediated IL-23 secretion triggered by tumor glutamine consumption [54]. A more M2-skewed TAM phenotype appears to be enriched in advanced and metastatic RCC, co-occurring with terminally exhausted CD8 TILs in a hypothesized “immune dysfunction circuit” [41].
2.1.4. NK Cells
NK cells are another abundant and cytotoxic immune cell type in RCC [55], documented at a frequency of approximately 9%. Among the RCC subtypes, pRCC [34] may contain the strongest proportion of NK cells in both the primary tumor and metastatic lesions [56]. Renal tumor cells may induce NK cell dysfunction through multiple mechanisms involving the diacylglycerol kinase [57], mitogen-activated protein kinase (MAPK/WEK) [57], and TGF-β/SMAD [58] signaling pathways.
2.1.5. Myeloid-Derived Suppressor Cells
MDSCs, characterized as granulocytic/polymorphonuclear (G/PMN-MDSCs) or monocytic (M-MDSCs) based on their cell lineage of origin, further suppress the anti-tumor immune response through multiple mechanisms including the production of arginase-1, reactive oxygen species, TGF-β, and IL-10 [59]. In RCC, total MDSCs, G-MDSCs, and immature-MDSCs have been correlated with increasing tumor grade and stage [60], and are functionally immunosuppressive through arginase-1 production [61]. MDSC generation may be tumor-induced, as exposure to conditioned media from RCC cell lines resulted in functionally immunosuppressive M-MDSC differentiation [62]. Interestingly, preclinical RCC data have demonstrated that inhibitors to CXCR2 [63], IL-1β [63], class I histone deacetylase (HDAC) [64], and HMGB1 [65] can slow tumor growth and potentially synergize with anti-PD-1 blockade in an MDSC inhibition-dependent manner. Finally, anti-angiogenesis TKIs such as sunitinib can also suppress MDSC activity [66], but this immunomodulatory ability may be mitigated by intra-tumoral GM-CSF [67].
2.2. Extracellular Targets
2.2.1. Tumor Mutational Burden
High TMB is a common feature shared among the majority of tumor types known to respond to ICIs [68,69,70,71]. RCC is unique in that despite its high degree of immune infiltration, these tumors have proven to contain a relatively low mutational burden. RCC has a mutation rate of approximately 1.1 mutations/megabase (mut/Mb), even lower (<1 mut/Mb) in chRCC [72,73]. That places RCC lower than many of its ICI-approved counterparts, including melanoma, non-small-cell lung cancer, urothelial carcinoma, colorectal cancer, cervical cancer, head and neck cancer, endometrial cancer, and hepatocellular carcinoma. ccRCC demonstrates a paradoxical correlation in which higher TMB is associated with decreased survival and decreased levels of intra-tumoral CD8 T cells, M1 and M2 macrophages, CD4 memory resting T cells, and DCs [74]. Separate database analysis correlated high TMB in ccRCC with lower gene expression for TILs, immune checkpoints, cytokines, and other pro-inflammatory genes, and high TMB in pRCC was associated with low PD-L1 levels, Tregs, and expression of pro-inflammatory genes [75].
2.2.2. Neo-Antigens
Somatic tumor alterations occasionally result in the production of neo-antigen peptides that contain unique tumor-specific and immunogenic epitopes. Neo-antigens strongly correlate with TMB, and represent a mechanistic bridge linking TMB with tumor immunogenicity. One reason RCC may not abide by this trend involves the patterns in which RCC undergoes genetic alterations. RCC (including ccRCC, pRCC, and chRCC) demonstrated the highest proportion and number of insertion-deletion (indel) mutations among a pan-cancer study, 2.4-fold greater than the average cancer rate. Indels, especially those that create open reading frames, are associated with a higher rate of neo-antigen formation than single nucleotide variants (SNVs), and may be highly immunogenic, with RCC-specific neo-antigens associated with higher expression of genes for antigen presentation and CD8 [76].
Another explanation for this discordance may be due to tumor immunity within the RCC TME. In an analysis of predicted neo-antigen burden based on non-silent point mutations, ccRCC demonstrated one of the lowest ratios of observed:predicted neo-epitopes. Authors hypothesized ccRCC to represent an “immune-susceptible” tumor type. Given its known high cytolytic activity, the lower than expected neo-antigen load may represent spontaneous tumor surveillance resulting in clonal elimination of neo-epitope expressing cancer cells [40].
2.2.3. DNA Repair
Aberrations in DNA repair pathways is a hallmark of malignancy [77]. DNA repair deficiencies lead to a hypermutable state resulting in increased formation of neo-antigens, and may serve as a biomarker of ICI efficacy [78,79]. In RCC, DNA repair gene alterations have been documented at frequencies of approximately 19–25% [80,81], with CHEK2, ATM, and BRCA1, and BRCA2 most commonly implicated [80,82]. Up to 26 DNA repair genes have displayed differential expression in ccRCC compared to benign tissue, six of which were identified as potential prognostic biomarkers (ISG15, RAD51AP1, SFRP2, SLFN11 as high-risk genes; SPATA18, VAV as low risk genes). After risk stratifying patients based on DNA repair gene expression, high-risk tumors contained increased expression of immune checkpoints (genes for PD-1, LAG-3, CTLA-4, and TIGIT) and were enriched for neutrophil activity-related gene expression pathways [83].
2.2.4. Tumor and Immune Metabolism
Cancer and immune cell metabolism is another significant component of the TME capable of modulating anti-tumor immunity and impacting response to immunotherapy [84]. RCC is a particularly metabolically-driven disease process, with genetic alterations resulting in metabolic changes allowing tumor cells to survive in hypoxic conditions [85]. Metabolomic analyses have uncovered specific metabolic reprogramming that occurs within RCC tumor cells relating to glucose uptake and glycolysis, amino acid utilization, and fatty acid metabolism [85,86,87,88,89], with prognostic and therapeutic implications [34,87,90,91,92]. Specifically, the kynurenine/tryptophan ratio negatively correlated with response to nivolumab, representing a potential mechanism of ICI resistance with implications for combination therapy with indoleamine 2,3-dioxygenase 1 (IDO1) inhibition [93]. Single-cell transcriptomic analysis identified a distinct RCC tumor cell subset with metabolic plasticity as determined by increased glycolysis, oxidative phosphorylation, and fatty acid metabolism, which was associated with 9p21.3 deletion and differential expression of immune checkpoint molecules [94]. Regarding immune cell metabolism, TILs from RCC patients may display metabolic dysfunction with aberrant glucose utilization, increased reactive oxygen species, and a distinct mitochondrial morphology [95].
2.3. Immune Checkpoints
ICIs targeting the PD-1/PD-L1 interaction have become a mainstay of treatment for many cancers. Reported PD-L1 positivity in RCC ranges from 25% up to 60% [96], with approximately 32% of patients having ≥5% positivity [97]. PD-1 positivity is seen on approximately 9% of RCC-infiltrating mononuclear cells [98] and in 57% of patients, commonly co-occurring in tumors expressing PD-L1 [99]. When comparing the primary renal tumor to metastatic lesions, site-based discrepancies for PD-L1 expression was observed in approximately 20% of cases, primarily involving PD-L1 positive primary tumors losing expression at distant sites [100]. Overall, PD-L1 positivity has been associated with more aggressive disease, increased tumor size and grade, and poor prognosis [101,102,103].
A number of additional immune checkpoints have been prime targets for therapeutic investigation, including CTLA-4, PD-L2, LAG-3, TIM-3, and TIGIT, which are upregulated in ccRCC [104]. CTLA-4, most notably, can be found on approximately 6% of RCC-infiltrating mononuclear cells [98]. Tumors with increased CTLA-4 levels have been linked to a more immune enriched TME with differential cytokine and chemokine expression [104], but those immune cells appear to comprise an exhausted CD8 T cell and Treg predominant phenotype, with increased expression of the checkpoints PD-L1, PD-1, LAG-3, IDO1, and TIGIT [105]. Interestingly, CTLA-4 expression may be linked to TMB via BAP1 mutations [105], and the miRNA miR-20b-5p was identified as a potential target of CTLA-4 with positive prognostic associations in RCC [104]. Importantly, CTLA-4 blockade via ipilimumab has been approved for the treatment of ccRCC in combination with nivolumab [24].
LAG-3 is upregulated in RCC compared to benign tissue [106]. Its expression appears to be under epigenetic regulation by DNA methylation, and levels correlated with TIL- and IFNγ-related gene expression [107]. TIM-3 is also associated with immune infiltration and cytokine expression in ccRCC [104]. LAG-3 and TIM-3 are seen on both CD4 and CD8 TILs in RCC. LAG-3, in particular, is often co-expressed on TILs with PD-1, with PD-1 blockade upregulating LAG-3 and in vitro data suggesting promise for this therapeutic combination [108]. TIGIT is detectable in approximately 75% of ccRCC tumors, primarily on tumor-infiltrating T cells and NK cells [109]. TIGIT expression is greater in tumor regions than adjacent normal tissue, with levels higher than those quantified for PD-1, and its expression correlated with that of CD3ε [110] but not with tumor grade or stage [109]. Further, an inhibitory TIGIT antibody demonstrated preclinical efficacy in a murine kidney tumor model in a T cell-dependent manner [111]. A histological analysis of LAG-3, TIM-3, and TIGIT identified distinct intra-tumoral phenotypes dominated primarily by a single checkpoint. In this study, the LAG-3 cluster was associated with a CD39+ exhausted CD8 T cell- and macrophage-dominant phenotype, and the TIGIT cluster with higher CTLA-4 expression [112]. Finally, multiple immune checkpoints, including PD-L1, PD-1, CTLA-4, LAG-3, TIM-3, and TIGIT, have been associated with genomic instability [113], long non-coding RNAs [114], TNFRSF9 expression and TNFRSF9+ CD8 TILs [115], and CXCL13+ CD8 TILs [116] in RCC.
3. Current State of Biomarkers for Immunotherapy
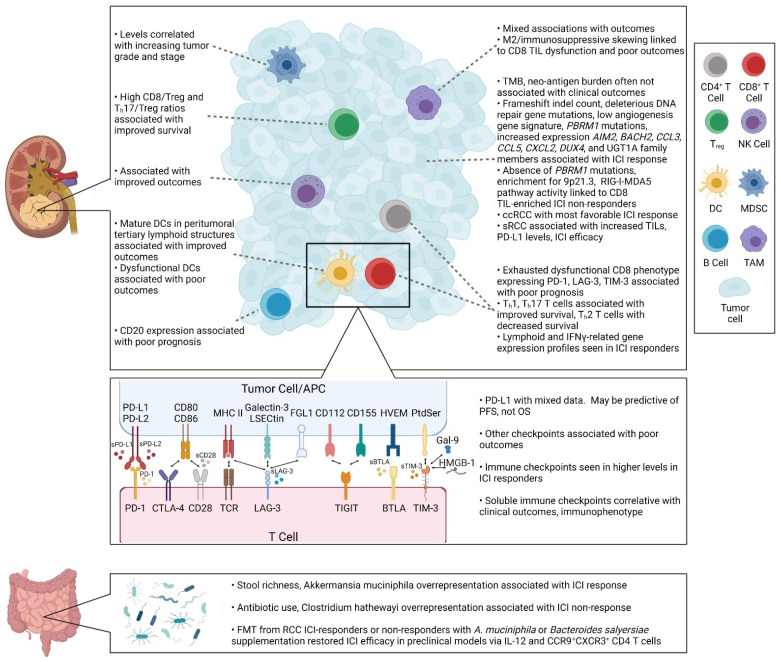
Clinically validated biomarkers are critical for optimizing immunotherapy use with accurate patient selection. Generally, biomarkers are either prognostic or predictive, with the former used to identify the risk of an outcome irrespective of treatment, and the latter of which may be utilized to identify responding patients to a specific treatment. One of the challenges in identifying biomarkers, especially predictive biomarkers, is the great deal of both inter- and intra-tumoral heterogeneity in RCC, as discussed above. Single biomarkers may not be generalizable to the entire RCC population or even to the entirety of a single patient’s disease. More accurate and clinically meaningful biomarkers may require novel molecular- or immunological-based stratifications that account for the complexity of the TME. Here, we provide an overview of the existing data regarding RCC immune-related biomarkers (Figure 2). It is important to keep in mind that these biomarkers predominantly fall within the prognostic grouping, and have not been validated for patient selection in the clinical setting.
Figure 2.
Biomarkers for immunotherapy in RCC. Text highlighting key biomarker-related data regarding immune cells, tumor cells, immune checkpoint molecules, and the microbiome.
3.1. Single Immune Checkpoints
PD-L1 expression is utilized for patient selection for ICIs in multiple cancers. However, its role as a biomarker remains controversial, with confounding variables including differential sensitivities between the multiple immunohistochemistry assays, differences in PD-L1 positivity cutoff values, cell types included in the scoring (tumor ± immune cells), intra-tumoral heterogeneity, and biopsy timing and specimen quality [117]. Given the lack of definitive data to support its use in RCC, routine use of PD-L1 expression to select patients for ICI treatment is currently not standard of care. In CheckMate 214, nivolumab and ipilimumab provided OS and ORR benefit among intermediate- and poor-risk patients regardless of PD-L1 expression. However, increased PFS was seen among patients with PD-L1 ≥1% [24]. Further, for atezolizumab and bevacizumab, increased PFS trended with PD-L1 expression level in IMmotion150 [118], and clinical benefit increased in a PD-L1 level-dependent manner ranging from <1% to ≥10% in IMmotion151 [29]. On the contrary, ICI efficacy was independent of PD-L1 status in KEYNOTE-426 [27], JAVELIN Renal 101 [28,119], and CheckMate 025 [18]. A meta-analysis comparing these six trials concluded that PD-L1 expression may be predictive of PFS but not OS [96].
Although not clinically validated, several other immune checkpoints have also been investigated as potential biomarkers to select for ICI use for RCC patients. Increased expression of the genes for PD-L2, PD-1, CTLA-4, and HHLA2 have been associated with worse OS, PFS and DFS [98,104,105,120,121], with concurrently high CTLA-4 and PD-1 expression conferring an especially high-risk state [98]. Conversely, low expression of PD-L1 and TIM-3 have been linked to worse prognosis [104]. Specifically on tumor-infiltrating mononuclear cells, PD-1 and CTLA-4 expression were associated decreased OS [98], with PD-1 linked to higher tumor grade, tumor stage, and cancer-specific death [99]. In terms of response to ICI, higher levels of PD-1, PD-L1, LAG-3, IDO1, ICOS, and BTLA were associated with patients responding to nivolumab treatment [122]. When analyzing the soluble form of immune checkpoints in the periphery of ccRCC patients, correlations were observed between soluble BTLA and TIM-3 and decreased survival, PD-L2 with recurrence, TIM-3 and LAG-3 with advanced stage, and LAG-3 and CD28 negatively correlated with T cell cytolytic activity [123]. Finally, tumor stratification based on DNA repair gene expression identified high-risk tumors with increased expression of the checkpoints PD-1, LAG-3, CTLA-4, TIGIT [83], and classifying tumors by high expression of the immune checkpoints PD-1, PD-L1, PD-L2, and LAG-3, as well as the absence of mature DCs, was associated with poor prognosis even in the setting of CD8 T cell infiltration [121].
3.2. Immune Cells and Immune Gene Signatures
An overarching pattern across cancer types is the association between increased TILs and improved outcomes and response to immunotherapy [124]. However, the data for TILs in RCC have been mixed, with multiple studies linking infiltration with poor [37,56,74,125,126,127,128] and improved [32] outcomes. Deeper analyses have identified that poor prognosis was specifically associated with exhausted polyclonal CD8 T cells expressing immune checkpoints such as PD-1, TIM-3, and LAG-3 and displaying decreased cytotoxic functionality [121,127]. In addition, these associations must be considered in the context of the disease state being studies, as increasing TILs are seen in higher grade tumors [37,128], with terminally exhausted CD8 TILs with restricted TCR diversity populating more advanced and metastatic RCC [41]. Among the T cell subsets, Th1 T cells, Th17 T cells, and high Th17/Treg and CD8/Treg ratios have been associated with improved survival [32,34,37], while Tregs (circulating, tumor infiltrating, and specifically ICOS+ Tregs) and Th2 T cells were associated with decreased survival [32,34,43,121,127].
In CheckMate 009, pre-treatment analysis of nivolumab responders demonstrated 311 differentially expressed genes, including higher immune response in silico (IRIS) transcripts of myeloid and lymphoid lineages, IFNγ and IFNα response gene expression, and a higher T cell CD3TCR expression score [129]. IMmotion150 and IMmotion151 biomarker analyses identified myeloid- and angiogenesis-related gene expression patterns, as well as the expression of T effector/IFNγ response genes to be associated with PD-L1 expression and PFS after ICI [118,130]. In JAVELIN Renal 101, CD8 T cells correlated with PFS by gene expression deconvolution, but not by IHC [119]. Interestingly, among circulating immune cells, a low baseline level of PD-1 + CD69+ exhausted activated CD4 T cells and a high baseline level of CD244+ exhausted CD4 T cells were associated with higher risk of progression after nivolumab [131].
Among the other immune cell types, NK cells, CD11c+ TAMs, and mature DCs have been associated with improved outcomes [37,56,74,121,132], and CD206+ TAMs, CD20+ B cells, and DC-LAMP+ and dysfunctional DCs with decreased prognosis [45,56,121,127,132]. Immunosuppressive M2-phenotype macrophages in advanced disease may exist in a immune dysfunction circuit with terminally exhausted CD8 TILs, which together was linked to poor outcomes [41]. In an analysis of 14 cancer types, poor survival with high B cell infiltration was specifically associated with high expression of MS4A1 (CD20) for ccRCC [45]. With respect to DCs, mature functional DCs that aggregate in peritumoral tertiary lymphoid structures have been associated with better prognosis [121], in contrast to poorer outcomes seen tumors with dysfunctional DCs [127]. Further, utilizing patient-derived xenograft models, an inflamed TME RCC subtype, with enrichment for the stromal compartment and multiple immune-infiltrating cell types (CD8 T cells, Th1 T cells, Tregs, NK cells, neutrophils, and TAMs), was associated with decreased survival [133].
3.3. TMB, Neo-Antigens, and DNA Repair
As described above, biomarkers such as TMB and neo-antigen burden do not hold the same prognostic associations in RCC as they do in other cancer types. In general, these correlations with clinical outcomes are strongest in tumor types in which CD8 TILs directly correlate with neo-antigen load [134]. In the IMmotion150 and JAVELIN Renal 101 trials, there was no association with TMB or neo-antigen burden and PFS [118,119]. This has been corroborated in additional studies [33], but, interestingly, an abstract reported that frameshift indel count did correlate with OS after PD-1 blockade [51]. DNA damage repair deficiency has also been associated with improved OS after immunotherapy for RCC [80].
3.4. Genomic Profiles
Genomic analyses of the ICI trials have also elucidated gene expression patterns that may have biomarker implications for RCC going forward. Response to anti-PD-1 treatment has been associated with loss of function mutations in PBRM1, a member of the SWI/SNF chromatin remodeling complex and one of the most commonly altered ccRCC genes seen in up to 50% of cases [135], resulting in aberrant expression of immune-related, JAK/STAT, and hypoxia pathways [136,137]. An immune-related prognostic model with PBRM1 mutations identified a high-risk subgroup with increased Tregs, TAMs, and immune checkpoints including PD-1, CTLA-4, LAG-3, TIGIT, and CD47 [138]. Interestingly, absence of PBRM1 mutations and enrichment for loss of 9p21.3, which contains antigen presenting machinery and HLA class II genes, were observed in many tumors with high levels of CD8 TILs, and may explain some of paradoxical correlations and ICI resistance seen in these patients [33]. Mechanistically, the lesser immunogenicity associated with loss of PBRM1 may be attributed to decreased IFNγ-STAT1 signaling impacting the expression of IFNγ target genes [139]. As a biomarker, PBRM1 mutations may prove useful for predicting response to nivolumab, especially in the second- or later-line setting for ccRCC patients [140].
Response to nivolumab has also been linked to increased expression of AIM2 (inflammasome) [129], BACH2 (CD4 differentiation regulator), CCL3, and UGT1A family members (metabolic and solute transport pathway) [141]. The JAVELIN Renal 101 biomarker analysis identified that PFS was associated with a 26-gene panel, termed “Renal 101 Immuno signature”, including genes involved in T cell activation, NK cell-mediated toxicity, the chemokines CCL5 and CXCL2, and DUX4 which is involved in HLA suppression and antigen presentation [119]. In addition a low angiogenesis gene signature correlated with increased PFS after atezolizumab plus bevacizumab [130]. The immunosuppressive WNT/β-catenin signaling pathway also appears active in RCC, and was associated with decreased TILs [36,129] and higher TMB tumors with poor outcomes [74]. Interestingly, some of the anti-tumorigenic immunomodulatory properties of the TKI pazopanib may be due to its inhibitory activity on the β-catenin pathway [142].
Molecular subtypings have also been identified that associate with immunotherapy response. Taxonomic analysis from TCGA stratified RCC into nine distinct subtypes based on DNA mutations, copy number variations (CNVs), DNA methylation, and gene expression. Among the three ccRCC subtypes, high expression of the genes for PD-1, CTLA-4, and TLR9 were associated with decreased survival, while PD-L1 was linked with better outcomes (though per authors this may be confounded by the loss of 9p, which contains the PD-L1 locus, leading to more aggressive disease) [5]. The BIONIKK trial is implementing a 35-gene expression panel that will stratify patients in one of four molecular subtypes for treatment assignment with either ICI-, ICI/ICI-, or ICI/TKI-based regimens [143]. Prospective trials such as this may prove fruitful for identifying predictive biomarkers to influence patient selection.
3.5. Microbiome
The microbiome plays an integral role in tumorigenesis, systemic immunity, and the host anti-tumor immune response in the setting of immunotherapies. In RCC, antibiotic use surrounding ICI administration has been associated with decreased PFS, leading to overrepresentation of species including Clostridium hathewayi [144,145]. TKI use can also alter the microbiome composition in RCC patients [144]. Metagenomic sequencing of RCC fecal samples linked stool richness by gene count of metagenomic species levels with ICI response, specifically in patients with overrepresentation of the commensal Akkermansia muciniphila [144,145,146]. Interestingly, fecal microbiota transplantation (FMT) from RCC ICI responders, or FMT from non-responders with additional A. muciniphila or Bacteroides salyersiae supplementation, restored ICI activity in antibiotic-treated and germ-free mice in an IL-12- and CCR9 + CXCR3+ CD4 T cell-dependent manner [144,145]. Further, RCC patients given probiotic supplementation including Bifidobacterium animalis, which is depleted in RCC patients, led to increased levels of the bacteria within the gut microbiome. While there was no effect on outcomes for the probiotic with TKI treatment, responding patients had higher levels of A. muciniphila and Barnesiella intestinihominis [147].
3.6. Clinical Phenotypes
Clinical features associated with response to ICI in RCC include a high neutrophil-to-lymphocyte ratio (NLR), both at baseline and after treatment [148], IMDC poor/intermediate-risk patients, and the Memorial Sloan Kettering Cancer Center (MSKCC) poor-risk group [149,150], although between the MSKCC risk groups there was no difference in TMB or the expression cytolytic or immune checkpoint genes [151]. On the contrary, high systemic immune-inflammation index (SII), low BMI < 25 kg/m2, and older age ≥ 70 years old have been linked with decreased OS with nivolumab [152].
As expected based on the immune profile of non-ccRCCs, these tumors demonstrate lesser responses to ICI treatment than ccRCC [153]. Among the RCC subtypes, sarcomatoid dedifferentiation (sRCC) is seen in approximately 4% patients, and is associated with more aggressive disease [154]. sRCC tends to have higher PD-1/PD-L1 levels and CD8 TIL density in comparison to non-sarcomatoid RCC [99,155], the former of which may be attributed to 9p24.1 amplification seen in approximately 6% of sRCC [156]. Similarly, trials have consistently demonstrated increased PD-L1 positivity and expression of IFNγ- and T cell effector-related genes for sRCC [130,157,158,159]. Additionally, first-line ICI in patients with metastatic ccRCC with sarcomatoid differentiation has been associated with an excellent clinical response [160].
4. Future Directions: How Can We Develop Better Treatments by Creating a More Favorable Anti-Tumor Immune Microenvironment?
The future of immunotherapy for RCC (Table 2) must build upon the foundational knowledge of the TME and the mechanisms of both response and primary and secondary resistance. While immunotherapy is now an established treatment option for many RCC patients, only a minority of patients respond to monotherapy ICI. The future lies in a combination-based multi-modal approach to best optimize the immunomodulation required for clinical benefit. As such, the combinations of PD-1 with CTLA-4 blockade and ICIs with TKIs have already garnered approval for RCC, and many more approaches are currently under active investigation.
Table 2.
Ongoing and completed clinical trials for non-approved immunotherapies for RCC.
| Treatment(s) | Phase | Setting, Patient Population | Key Data * | Clinical Trial |
|---|---|---|---|---|
| ICIs | ||||
| Nivo ± Ipi | III | First-line, intermediate/poor-risk advanced ccRCC | NCT03873402 | |
| Pembro | III | Adjuvant, ccRCC with high-risk, intermediate–high-risk, or M1 NED | Pembro vs. Placebo: • 24 mo DFS: 77.3% vs. 68.1% [HR 0.68; 95% CI, 0.53–0.87; p = 0.002] |
KEYNOTE-564, NCT03142334 [17] |
| Nivo ± Ipi | III | Adjuvant, high-risk ccRCC | CheckMate 914, NCT03138512 [161] | |
| Durva ± Treme | III | Adjuvant, RCC with high/intermediate risk of relapse | RAMPART, NCT03288532 [162] | |
| Pembro | II | First-line, advanced ccRCC or non-ccRCC | • mOS: NR • mPFS: 7.1 mo (95% CI, 5.6–11.0) • ORR: 36.4% |
KEYNOTE-427, NCT02853344 [19,20] |
| Nivo followed by Nivo + Ipi | II | First-line and salvage, advanced ccRCC | • mPFS: 7.4 mo (5.5–10.9) • ORR: 35% |
HCRN GU16-260, NCT03117309 [164] |
| Nivo ± followed by Ipi | II | First- or second-line and salvage, advanced ccRCC and non-ccRCC | Nivo with salvage Ipi (69% of patients in arm B): • Conversion from SD/PD to PR: 4% (90% CI, 1–11) |
OMNIVORE, NCT03203473 [166] |
| Nivo ± followed by Nivo + Ipi | II | First- or second-line and salvage, metastatic or unresectable non-ccRCC | • mPFS: 4.0 mo (95% CI, 3.6–7.4) • ORR: 17% |
UNISON/ANZUP 1602, NCT03177239 [167] |
| Nivo followed by Nivo + Ipi “boost” | II | First- and second-line, intermediate/high-risk advanced RCC | Nivo first-line vs. second-line: • mOS: 27.2 mo (95 % CI, 19.9–NE) vs. 20.2 mo (95 % CI, 15.6–NE) • ORR: 28 % vs. 17 % |
TITAN-RCC, NCT02917772 [165] |
| Durva + Treme | II | ICI/CD137/cMet-naïve and VEGF treatment-refractory (advanced ccRCC) or VEGF treatment naïve or -refractory (advanced pRCC) | • mPFS: 4.9 months (95% CI, 2.5–10.0) | CALYPSO, NCT02819596 |
| Durva + Treme | Ib | Neoadjuvant and adjuvant, locally advanced RCC | NCT02762006 [163] | |
| MEDI0680 (PD-1) + Durva vs. Nivo | I/II | ICI-naïve, advanced ccRCC | MEDI0680 + Durva vs. Nivo: • mPFS 3.6 mo vs. 3.6 mo • ORR: 14.3% vs. 19.0% |
NCT02118337 [171] |
| Nivo + Ipi, Relatlimab (LAG-3), BMS-986205 (IDO1), or BMS-813160 (CCR2/CCR5) | II | First- or second-line, advanced RCC | Nivo + Ipi: • ORR: 15.2% |
FRACTION-RCC, NCT02996110 [168] |
| Relatlimab ± Nivo | I/II | ICI-naïve, RCC | NCT01968109 | |
| LAG525 (LAG-3) ± Spartalizumab (PD-1) | I/II | Second- or later-line, advanced RCC | NCT02460224 [169] | |
| Sabatolimab (TIM-3) ± Spartalizumab | I-Ib/II | ICI-naïve or pre-treated, advanced RCC | NCT02608268 [170] | |
| INCAGN02390 (TIM-3) | I | Later-line, advanced RCC | NCT03652077 | |
| CA-170 (PD-L1, PD-L2, VISTA) | I | ICI-ineligible, advanced RCC | NCT02812875 [172] | |
| TKIs + ICIs | ||||
| Bev + Atezo | III | First-line, PD-L1+ metastatic RCC | Bev + Atezo vs. Sunitinib: • mPFS (PD-L1+): 11.2 mo vs. 7.7 mo [HR 0.74; 95% CI, 0.57–0.96; p = 0.0217] • mOS (ITT): [HR 0.93; 95% CI, 0.76–1.14] |
IMmotion151, NCT02420821 [29] |
| Cabo + Atezo | III | Second- or third-line, ICI-refractory advanced RCC | CONTACT-03, NCT04338269 [180] | |
| Nivo + Ipi ± Cabo | III | First-line, intermediate/poor-risk advanced ccRCC | COSMIC-313, NCT03937219 [178] | |
| Nivo + Ipi followed by maintenance Nivo (CR), Cabo (PD), or Nivo ± Cabo (non-CR/PD) | III | First-line, intermediate/poor-risk advanced ccRCC | A031704/ PDIGREE, NCT03793166 [179] |
|
| Nivo ± Ipi ± followed by Nivo or Sunitinib/Pazopanib | II | First-line, metastatic ccRCC stratified into one of four molecular subtypes | BIONIKK, NCT02960906 [143] | |
| Savolitinib + Durva | II | ICI/CD137/cMet naïve and VEGF treatment refractory (advanced ccRCC) or VEGF treatment naïve or refractory (advanced pRCC) | • mPFS (pRCC): 4.9 mo (95% CI, 2.5–10.0) • mPFS (pRCC, MET-driven disease): 10.5 mo (95% CI, 2.9–15.7) • mOS (pRCC, MET-driven disease: 27.4 mo (95% CI, 7.3–NR) • ORR (pRCC): 29% • ORR (pRCC, MET-driven disease): 57% |
CALYPSO, NCT02819596 [181] |
| Sitravatinib + Nivo | II | Neoadjuvant, ccRCC | • ORR 11.8% (33.3% for 120 mg Sitravatinib) | NCT03680521 [174] |
| Cabo + Atezo | I/II | First-line (ccRCC) or prior VEGF TKI treatment (non-ccRCC) | 40 mg Cabo + Atezo vs. 60 mg Cabo + Atezo: • mPFS (ccRCC): 19.5 mo (95% CI, 11.0 –NE) vs. 15.1 mo (95% CI, 8.2–22.3) • ORR (ccRCC): 53% (80% CI, 41–65) vs. 58% (80% CI, 46–70) 40 mg Cabo: • ORR (non-ccRCC): 31% (80% CI, 20–44) • mPFS (non-ccRCC): 9.5 mo |
COSMIC-021, NCT03170960 [175] |
| Cabo + Pembro | I/II | First- or later-line Pembro/Cabo-naïve, advanced RCC | • mPFS: 10.4 mo (95% CI, 6.3–NR) • mOS: NR • 17.8 mo ORR: 60% (95% CI, 0.458–1.00) |
NCT03149822 [177] |
| Lenvatinib + Pembro | Ib/II | First- or later-line, metastatic ccRCC | ICI-pre-treated population: • mOS: NR (95% CI, NR–NR) • mPFS: 12.2 mo (95% CI, 9.5–17.7) • 24 wk ORR: 55.8% (95% CI, 45.7–65.5) |
KEYNOTE-146, NCT02501096 [182] |
| Ibrutinib + Nivo | Ib/II | Second- or later-line, advanced RCC | NCT02899078 [173] | |
| Cabo + Nivo ± Ipi | I | Later-line, advanced ccRCC | NCT02496208 [176] | |
| Cellular Therapies | ||||
| VEGFR2 CAR T cells | I/II | Second- or later-line, metastatic RCC | NCT01218867 | |
| Anti-c-Met CAR T cells | I/II | PR/NR/recurrency if prior ICI, c-Met+ RCC | NCT03638206 | |
| ROR2, AXL CAR T cells | I/II | ROR2+ or AXL+ relapsed and refractory stage IV metastatic RCC | NCT03393936 | |
| CD70 CAR T cells | I/II | Second- or later-line, CD70+ ccRCC | NCT02830724 † | |
| D-CIK + Axitinib | II | First-line or after progression on anti-angiogenesis or cytokine therapy, advanced RCC | NCT03736330 | |
| HIF-2α + ICI | ||||
| PT2385 + Nivo or Cabo | I | Second- or later-line, advanced ccRCC | PT2385 + Nivo: • mPFS: 7.3 mo • ORR: 22% |
NCT02293980 [184] |
| Vaccines | ||||
| IMA901 + Sunitinib | III | First-line, advanced ccRCC | IMA901 + Sunitinib vs. Sunitinib: • mOS: 33.17 mo (95% CI, 27.8–41.4) vs. NR (33.7–NR) [HR 1.34; 95% CI, 0.96–1.86; p = 0.087] |
IMPRINT, NCT01265901 [187] |
| Rocapuldencel-T + Sunitinib | III | First-line, advanced RCC | Rocapuldencel-T + Sunitinib vs. SOC: • mOS: 27.7 mo (95% CI, 23.0–35.9) vs. 32.4 mo (95% CI, 22.5-NE) [HR 1.10; 95% CI, 0.83–1.40] • mPFS: 6.0 mo vs. 7.83 mo [HR 1.15; 95% CI, 0.92–1.44] • ORR: 42.7% (95% CI, 37.1–48.4) vs. 39.4% (95% CI, 31.6–47.5) |
ADAPT, NCT01582672 † [189] |
| AGS-003 + Sunitinib | II | First-line, intermediate/poor-risk metastatic ccRCC | • mOS: 30.2 mo (95% CI, 9.4–57.1) • mPFS: 11.2 mo (95% CI, 6.0–19.4) |
NCT00678119 [188] |
| INTUVAX/Ilixadencel (intra-tumoral) + Sunitinib | II | Neoadjuvant + adjuvant first-line, synchronous metastatic RCC | INTUVAX + Sunitinib vs. Sunitinib: • mPFS 11.8 mo vs. 11.0 mo • ORR 42.4% vs. 24.0% • mDOR 7.1 mo vs. 2.9 mo |
MERECA, NCT02432846 [191] |
| GEN-009 Adjuvanted Vaccine + Nivo or Pembro | I/II | First-line (intermediate/poor-risk beginning Nivo + Ipi) or after anti-angiogenic therapy (beginning nivolumab), advanced RCC | NCT03633110 [192] | |
| VB10.NEO + Bempegaldesleukin | I/IIa | PR/SD/PD on ICI, advanced ccRCC | DIRECT-01, NCT03548467 [193] | |
| Pexastimogene Devacirepvec + Cemiplimab | I/II | First- or later-line ICI-naïve, advanced ccRCC | • ORR: 37.5% | NCT03294083 [194] |
| DC Tumor Fusion + GM-CSF | I/II | Chemotherapy- and biological therapy-naïve, stage IV RCC | NCT00458536 | |
| Autologous or Allogeneic tumor cells | I/II | Chemotherapy-refractory, metastatic RCC | NCT00722228 | |
| Treme + Durva + PolyICLC | I/II | Dual ICI-naïve, biopsy-accessible advanced RCC | NCT02643303 [195] | |
| COMBIG-DC/INTUVAX | I | Intermediate/poor-risk metastatic RCC | • mOS: NR | NCT01525017 [190] |
| Neovax ± Ipi | I | First- or later-line ICI-naïve, stage III/IV resectable ccRCC | NCT02950766 | |
| Cevumeran ± Atezo | Ia/Ib | First- or later-line ICI-naïve, advanced RCC | NCT03289962 | |
| PSMA plasmid DNA vaccine | I | Favorable-risk RCC | NCT00096629 | |
| IL-2 + ICI | ||||
| Bempegaldesleukin + Nivo | III | First-line, advanced ccRCC | PIVOT-09, NCT03729245 [199] | |
| High Dose IL-2 + Pembro | II | First- or later-line ICI-naïve, metastatic RCC | • Projected ORR: 69% | NCT02964078 [196] |
| High Dose IL-2 + Nivo | Ib/II | First-, second-, or third-line, IL-2-, IFN-, PD-1/PD-L1-ICI-naïve, metastatic ccRCC | NCT02989714 [197] | |
| Bempegaldesleukin + Nivo ± Ipi | I/II | First-, second-, or third-line IL-2-naïve, advanced RCC | PIVOT-02, NCT02983045 [198] | |
| Microbiome | ||||
| ± Deferred cytoreductive nephrectomy following Nivo + Ipi (microbiome analysis) | III | First-line, intermediate/poor-risk synchronous metastatic RCC | NORDIC-SUN, NCT03977571 | |
| Nivo or Pembro ± Metformin or Rosiglitazone (microbiome analysis) | II | PD-1/L1 ICI-naïve, advanced RCC | NCT04114136 | |
| Nivo + Ipi ± SBRT (microbiome analysis) | II | First-line, intermediate/poor-risk metastatic RCC | CYTOSHRINK, NCT04090710 | |
| FMT from ICI-responding donors + ICI | I/II | Receiving or eligible for ICI, advanced RCC | TACITO, NCT04758507 | |
| FMT + Nivo + Ipi (irAEs analysis) | I | First-line, intermediate/poor-risk advanced RCC | PERFORM, NCT04163289 | |
| MRx0518 (Enterococcus) | I | No therapy in past 2 years, RCC | MICROBIOME, NCT03934827 | |
| ICI/s ± other systemic therapy (microbiome analysis) | N/A | Eligible for ICI, stage I–IV RCC | PARADIGM, NCT05037825 | |
| Nivo ± Ipi, Durva ± Treme (microbiome analysis) | N/A | ICI-naïve, advanced RCC | NCT04107168 | |
| Other Immunomodulators | ||||
| Epacadostat (IDO1) + Pembro | III | First-line, advanced ccRCC | KEYNOTE-679/ECHO-302, NCT03260894 | |
| Entinostat (HDAC) + Nivo + Ipi | II | Nivo + Ipi-refractory, metastatic RCC | NCT03552380 | |
| High Dose IL-2 ± Entinostat | II | Second- or third-line (including ICI), advanced ccRCC | NCT03501381 | |
| Atezo + Bev + Entinostat | I/II | ICI-naïve (II Cohort A) or ICI-refractory (II Cohort B), metastatic RCC | • mPFS: 7.6 mo (95% CI, 1.6–16.3) • ORR: 47.1% (95% CI, 23.0–72.2) |
NCT03024437 [203] |
| Aldesleukin + Entinostat | I/II | First-, second-, or third-line, metastatic ccRCC | • ORR: 37% (90% CI, 24–51) [p = 0.010] • mPFS 13.8 mo (95% CI, 6.0–18.8) • mOS 65.3 mo (95% CI, 52.6–65.3) • Decreased Tregs associated with response |
NCT01038778 [204] |
| HBI-8000 (HDAC) + Nivo | I/II | Advanced RCC | NCT02718066 | |
| NIR178 (A2AR) + Spartalizumab | II | Later-line, TKI-refractory, advanced RCC | NCT03207867 | |
| Dalantercept (ALK1/TGF-β) + Axitinib | II | Second- or third-line (including VEGF inhibitor) ICI-naïve, advanced ccRCC | Dalantercept + Axitinib vs. Axitinib + Placebo: • mOS: NR vs. NR [HR 1.39; 95% CI, 0.70–2.77; p = 0.349] • mPFS: 6.8 mo vs. 5.6 mo [HR 1.11; 95% CI, 0.71–1.73; p = 0.670] • ORR: 19.0% (95% CI, 9.9–31.4) vs. 24.6% (95% CI, 14.5–37.3) |
DART, NCT01727336 [200,201] |
| Oleclumab (CD73) + Durva | II | No prior CD73/CD39/innate immune agonist, advanced RCC | DOMINATION, NCT04262375 | |
| Axitinib ± Ivuxolimab (OX40) | II | Second- (one prior TKI + ICI) or third-line (one prior non-axitinib TKI, one ICI), metastatic RCC | NCT03092856 | |
| INBRX-106 (OX40) ± Pembro | I | Later-line OX40 agonist-naïve, advanced RCC | NCT04198766 | |
| Feladilimab (ICOS) + Treme | I/II | CTLA-4/ICOS-treatment-naïve, advanced ccRCC | NCT03693612 [209] | |
| Varlilumab (CD27) + Nivo | I/II | Anti-angiogenic therapy-experienced, ICI-naïve, no CTLA-4/CD27 therapy in past 3 mo, advanced ccRCC | NCT02335918 | |
| INCAGN01876 (GITR) + Nivo or Ipi or Nivo + Ipi | I/II | Later-line, advanced RCC | NCT03126110 | |
| Axitinib ± Carotuximab (Endoglin) | I/II | Later-line, one prior TKI (other than axitinib) ± one prior ICI, advanced ccRCC | NCT01806064 [202] | |
| Telaglenastat (glutaminase) + Nivo | I/II | Later-line ± ICI-naïve, advanced ccRCC | NCT02771626 | |
| Utomilumab (4-1BB) + Pembro | I | Advanced RCC | KEYNOTE-0036, NCT02179918 [208] | |
| Mogamulizumab (CCR4) | I | RCC | NCT02946671 | |
| Dostarlimab (PD-1) + Niraparib (PARP) Cobolimab (TIM-3), Bev, or Platinum-based doublet chemotherapy | I | Advanced RCC | IOLite, NCT03307785 [205] | |
| Valemetostat (EZH1/2) + Ipi | I | Later-line ICI- and anti-angiogenic therapy-refractory, advanced ccRCC | NCT04388852 | |
| Ciforadenant (A2AR) | I | Second- or third-line ICI-refractory, ccRCC | NCT02655822 [206,207] | |
| Siremadlin (MDM2) + Spartalizumab | I | Later-line, advanced RCC | NCT02890069 | |
| Nivo + Stereotactic Body Radiotherapy | II | Second- or third-line PD-1/L1/L2-naïve, advanced RCC | • mOS: 22.1 mo (95% CI, 18.1-NR) • mPFS: 4 mo (95% CI, 2.8–7.1) • ORR: 19% |
NIVES, NCT03469713 [211] |
| Guadecitabine + Durva | I/II | ICI-naïve (Cohort 1) or PD-1/L2-regractory (Cohort 2), advanced ccRCC | • mOS: NR • mPF: 17 mo |
NCT03308396 [210] |
| Treme ± Cryoablation | N/A | CTLA-4 ICI-naïve, advanced ccRCC or non-ccRCC | NCT02626130 | |
Immune checkpoint inhibitor (ICI), tyrosine kinase inhibitor (TKI), nivolumab (Nivo), ipilimumab (Ipi), pembrolizumab (Pembro), atezolizumab (Atezo), durvalumab (Durva), tremelimumab (Treme), bevacizumab (Bev), cabozantinib (Cabo), stereotactic body radiation therapy (SBRT), standard of care (SOC), no evidence of disease (NED), months (mo), weeks (wk), disease free survival (DFS), median overall survival (mOS), confidence interval (CI), not estimable (NE), hazard ratio (HR), median progression free survival (mPFS), overall response rate (ORR), median duration of response (mDOR), complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD), not reached (NR), intention-to-treat (ITT), immune-related adverse events (irAE), not applicable (N/A), and milligrams (mg). * Data from published literature or abstracts. † Trials discontinued or suspended.
4.1. ICIs
Regarding the ICIs, there are numerous clinical questions yet to be answered. That includes the context in which to best administer ICIs, as it remains to be seen whether immunotherapy has a role in the neoadjuvant and adjuvant setting. The KEYNOTE-564 trial recently reported increased DFS with adjuvant pembrolizumab in patients at high risk of recurrence [17]. Ongoing investigations are assessing the efficacy of adjuvant nivolumab ± ipilimumab (CheckMate 914) [161], adjuvant durvalumab (PD-L1) ± tremelimumab (CTLA-4) (RAMPART) [162], as well as durvalumab and tremelimumab in the neoadjuvant and adjuvant setting [163].
Another ongoing question regards the use of ICI or combination therapy as first-line versus second-line treatment. In KEYNOTE-427, first-line pembrolizumab demonstrated promising activity in both advanced ccRCC [19] and non-ccRCC [20]. While nivolumab is approved in the post-anti-angiogenic therapy setting, its role as a first-line treatment is currently under investigation in multiple trials [164,165].
The optimal duration of therapy, potential maintenance dosing, and subsequent treatment regimens is also unknown. In the OMNIVORE trial, nivolumab responders will undergo observation without active treatment, while non-responders will be switched to ipilimumab. Preliminary reports suggests a low rate of response conversion after subsequent ipilimumab therapy [166]. In HCRN GU16-260, salvage nivolumab plus ipilimumab dual ICI after non-response to first-line nivolumab demonstrated an 11% PR rate [164]. The sequential regimen of nivolumab followed by combination nivolumab plus ipilimumab is being evaluated in UNISON/ANZUP 1602 [167]. TITAN-RCC is testing the potential efficacy of a nivolumab and ipilimumab “boost” after nivolumab monotherapy [165].
PD-1/PD-L1 blockade is also in combination trials with other checkpoint inhibitors, including those targeting CTLA-4 [168], LAG-3 [169], and TIM-3 [170], as well as the combination of concurrent PD-1 (MEDI0680) and PD-L1 (durvalumab) antagonism [171]. In another approach, RCC is among multiple cancer types in trial for CA-170, an oral small molecular inhibitor of PD-L1 and VISTA [172]. Finally, a more biomarker driven approach may ultimately prove necessary to best select patient subpopulations most appropriate for specific treatment, and the ongoing BIONIKK trial is utilizing a 35-gene expression panel to stratify patients into one of four molecular subtypes, based on which patients will be treated with either nivolumab monotherapy, combination nivolumab and ipilimumab, or combination ICI followed by sunitinib or pazopanib [143].
4.2. TKIs and HIF-2α Inhibitors
Anti-angiogenesis TKIs have a clear role in the treatment of RCC and display multi-faceted immunomodulatory properties. Current clinical combination approaches in development include: TKI with a single ICI in multiple disease settings [31,173,174,175,176,177], triple therapy with TKI plus dual ICI/ICI [176,178], adding cabozantinib to nivolumab as maintenance in patients who had clinical benefit to first-line ipilimumab plus nivolumab [179], and using ICI/TKI as a second-line treatment for patients who previously received ICI [180]. In addition, TKI combination approaches are also in development for those with non-ccRCC, including combining the MET-TKI savolitinib with durvalumab [181]. The combination of lenvatinib and pembrolizumab has definitively demonstrated superiority to sunitinib in the first-line setting for metastatic ccRCC [30], and the same combination is also under investigation as a salvage option after progression with anti PD-1/PD-L1 treatment [182].
HIF-2α inhibitors have recently gained traction as a promising avenue for RCC treatment. VHL inactivation leads to constitutive HIF-2α activity, thereby promoting tumorigenesis and immune evasion with upregulation of various downstream targets, including VEGF. HIF-2α inhibitors, such as PT2385 and belzutifan (MK6482/PT2977) have now reached phase III clinical trials for RCC [183], and are being tested in combination with ICIs [184]. HIF-2α inhibition may modulate the immune microenvironment, decreasing immunosuppressive myeloid cells while increasing infiltration by mature DCs [185].
4.3. Cellular Therapies
Adoptive cell therapies, which have yielded clinical success in multiple cancer types, are also in trial for RCC. Chimeric antigen receptor (CAR) T cell therapy involves autologous administration of patient-derived T cells engineered ex vivo with synthetic receptors allowing them to specifically target and eliminate tumor cells. CAR T cells under investigation for RCC include those targeting VEGFR2, c-Met, Ror2, and CD70 (trial currently suspended), as well as the previous target carboxy-anhydrase-IX (terminated due to hepatotoxicity [186]). Another cellular approach involves dendritic cell stimulation of cytokine-induced killer (D-CIK) cells with ex vivo expansion of T cells exposed to pembrolizumab, which is in trial in combination with axitinib.
4.4. Vaccines
Vaccines have thus far demonstrated limited efficacy for RCC. In the IMPRINT trial, the multi-peptide vaccine IMA901 did not confer added benefit to sunitinib as a first-line therapy [187]. Rocapuldencel-T (AGS-003), an autologous dendritic cell-based vaccine, demonstrated benefit in early phase trials [188], but the phase III ADAPT trial in combination with sunitinib was terminated early after interim analysis suggested no benefit for OS [189]. Ilixadencel (INTUVAX), in which activated allogeneic DCs are injected intra-tumorally, induced an anti-tumor immune response in early phase trial [190], with promising results from the interim analysis of the phase II MERECA trial involving pre-nephrectomy vaccine administration with adjuvant sunitinib [191]. Personalized neo-antigen vaccines based on patient-specific mutations have also been of interest, and related trials with GEN-009 [192], NeoVax (with and without ipilimumab), VB10.NEO (with the IL-2 agonist bempegaldesleukin) [193], and cevumeran (with atezolizumab) are currently underway for RCC. Additional vaccine approaches include a DC tumor fusion with GM-CSF, a recombinant vaccinia virus (with cemiplimab, PD-1) [194], a whole cell autologous and allogenic approach, the TLR3 agonist polyICLC (with tremelimumab and durvalumab) [195], and a prostate-specific membrane antigen (PSMA)-based vaccine.
4.5. Cytokine Stimulation: IL-2
IL-2 has long been tested as an immunotherapy for RCC, given its ability to promote expansion of T cells and NK cells. The combination of high dose IL-2 and pembrolizumab has shown preliminary activity [196], and is also under investigation with nivolumab [197]. Later-generation IL-2 therapies, such as the bempegaldesleukin (BEMPEG/NKTR-214), preferentially act through the CD122 pathway, thereby limiting the Treg-promoting function of IL-2. In the PIVOT-02 trial, the combination of bempegaldesleukin plus nivolumab conferred ORRs of 71.4% as a first-line and 28.6% as a second-line treatment for immunotherapy-naïve RCC, leading to increased levels of CD8 TILs [198]. The phase III PIVOT-09 trial comparing this combination with TKIs in the first-line setting is currently active [199].
4.6. Microbiome
The microbiome is another potential target for immunomodulation. In RCC, antibiotic use has been associated with poorer outcomes with ICIs [144,145], with A. muciniphila and B. salyersiae identified as potential biomarkers or therapeutic targets [144,145,146]. Regarding clinical trials, probiotic supplementation of B. animalis in RCC patients altered the makeup of the microbiome but did not impact TKI efficacy [147]. Ongoing trials are further investigating the evolution of the microbiome of RCC patients over the course of various treatments, the efficacy of FMT from RCC ICI responders, and the ability to use FMT to mitigate the toxicity from combination ICI treatment.
4.7. Other Immunomodulators
Investigators have also attempted to target the immunosuppressive TGF-β pathway, using the ALK1 inhibitor dalantercept combined with axitinib [200,201], but the drug was subsequently discontinued after the DART study failed to demonstrate clinical benefit. TRC105, which targets endoglin, an accessory receptor for TGF-β, is currently being tested with axitinib [202]. Other immunomodulatory targets in clinical trial with ICIs include inhibitors to IDO1 (including the phase III KEYNOTE-679/ECHO-302 trial), CCR4, CCR2/5, CD73, HDAC [203,204], histone methyltransferase EZH1/2, PARP [205], mTOR, glutaminase, the adenosine A2A receptor [206] (an Adenosine Signature biomarker was identified in responders [207]), and the p53-MDM2 interaction. Immunomodulatory agonists in combination with ICIs are targeting 4-1BB [208], OX-40, CD27, ICOS [209], and GITR. Lastly, ICIs are being evaluated with platinum-based chemotherapy [205] and guadecitabine (with reported decreased MDSCs and Tregs in responders, and Th17 T cells associated with immune-mediated toxicity [210]), stereotactic body radiotherapy [211], and cryoablation.
5. Conclusions
To optimize the therapeutic landscape and future clinical trial design for RCC patients, it is essential to understand the basic and translational tumor immunology science that laid the foundation for these treatment breakthroughs [212]. CD8 and CD4 TILs infiltrate the majority of RCC tumors, and while cytolytic activity may be high, they often display an exhausted phenotype with expression of immune checkpoints extending past PD-1 and CTLA-4. TAMs are also abundant within the TME, often with an immunosuppressive skewing, activating Tregs and causing T cell dysfunction. Tumor cells themselves promote immune escape, upregulating WNT/β-catenin signaling, inducing MDSC differentiation and NK cell dysfunction, and exhibiting metabolic reprogramming. Many ongoing trials target these players, combining PD-1/PD-L1 or CTLA-4 ICIs with various other checkpoint-based antagonists, immunostimulatory agonists, chemokines/cytokines, and genetic and metabolic modulators, among other agents. Data on TKIs have revealed numerous immunomodulatory roles, with the specific effects between different TKIs not yet well characterized. Also underappreciated is the impact of the gut microbiome on distal anti-tumor immunity, and further delineation of the responsible mechanisms may provide more specific targets for RCC immunomodulation. In addition, there are multiple emerging techniques that may aid in further characterizing the immune response and relevant biomarkers in RCC, including analysis of circulating tumor cells, single-cell sequencing, and ex vivo organoid modeling [213].
Analyses of post-ICI treatment specimens have provided insight into the immunomodulatory effects of these therapies, and the potential mechanisms of both response and resistance. Extreme ICI responders have been noted to have strong CD8 T cell infiltration both in pre- and post-treatment tissue [214], and data from trials have demonstrated ICIs linked to increased TILs, expression of IFNγ-stimulated and Th1 genes, MHC-I levels, and chemokines such as CXCL9, CXCL10, and CX3CL1 [97,215]. Responding patients had differentially increased expression of lymphoid and myeloid gene sets, IFNγ response genes, and checkpoints including TIGIT, CTLA-4, PD-L2, as well as decreased TGF-β signaling and MMP3 expression, with RIG-I-MDA5 pathway activity noted in non-responders with a high degree of TILs [129]. Single-cell transcriptomic analysis identified increased activated and terminally exhausted CD8 T cells and pro-inflammatory TAM skewing in ICI responders, as well as two distinct tumor cell populations with differential responses to ICI [94]. Clinical trials that incorporate post-treatment biopsies will continue to aid in our understanding of the mechanisms of response and resistance to immunotherapy.
Many uncertainties still remain within RCC, including the surprisingly low mutational burden for an immunotherapy-responsive cancer. The high indel rate resulting in the generation of more immunogenic neo-epitopes partially addresses this phenomenon, yet the number of observed neo-antigens remains lower than expected, and the paradoxical correlations between TMB, TILs, and immunotherapy response are still not well understood. Potential genetic and molecular links, such as the lack of PBRM1 mutations, enrichment for 9p21.3 loss, and increased RIG-I-MDA5 pathway activity may explain ICI non-response in CD8 T cell-infiltrated tumors. Moving forward, these concepts may prove crucial for understanding the interactions in tumor immune microenvironment, optimizing response to current therapies, and innovating the next generation of immunomodulatory agents personalized to individual patient characteristics.
Acknowledgments
Figures created with BioRender.com (accessed on 25 October 2021).
Author Contributions
Conceptualization, J.A. and C.-K.T.; writing—original draft preparation, J.A.; writing—review and editing, J.A., C.-K.T. and N.K.; figure and table creation, J.M., N.T. and J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
C.-K.T. has consulting roles with Eisai, Exelixis.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA. Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Gerlinger M., Rowan A.J., Horswell S., Larkin J., Endesfelder D., Gronroos E., Martinez P., Matthews N., Stewart A., Tarpey P., et al. Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. N. Engl. J. Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsieh J.J., Purdue M.P., Signoretti S., Swanton C., Albiges L., Schmidinger M., Heng D.Y., Larkin J., Ficarra V. Renal Cell Carcinoma. Nat. Rev. Dis. Primer. 2017;3:1–19. doi: 10.1038/nrdp.2017.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brannon A.R., Reddy A., Seiler M., Arreola A., Moore D.T., Pruthi R.S., Wallen E.M., Nielsen M.E., Liu H., Nathanson K.L., et al. Molecular Stratification of Clear Cell Renal Cell Carcinoma by Consensus Clustering Reveals Distinct Subtypes and Survival Patterns. Genes Cancer. 2010;1:152–163. doi: 10.1177/1947601909359929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen F., Zhang Y., Şenbabaoğlu Y., Ciriello G., Yang L., Reznik E., Shuch B., Micevic G., De Velasco G., Shinbrot E., et al. Multilevel Genomics-Based Taxonomy of Renal Cell Carcinoma. Cell Rep. 2016;14:2476–2489. doi: 10.1016/j.celrep.2016.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499:43–49. doi: 10.1038/nature12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dutcher J.P., Flippot R., Fallah J., Escudier B. On the Shoulders of Giants: The Evolution of Renal Cell Carcinoma Treatment—Cytokines, Targeted Therapy, and Immunotherapy. Am. Soc. Clin. Oncol. Educ. Book. 2020:418–435. doi: 10.1200/EDBK_280817. [DOI] [PubMed] [Google Scholar]
- 8.Fyfe G., Fisher R.I., Rosenberg S.A., Sznol M., Parkinson D.R., Louie A.C. Results of Treatment of 255 Patients with Metastatic Renal Cell Carcinoma Who Received High-Dose Recombinant Interleukin-2 Therapy. J. Clin. Oncol. 1995;13:688–696. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 9.Escudier B., Pluzanska A., Koralewski P., Ravaud A., Bracarda S., Szczylik C., Chevreau C., Filipek M., Melichar B., Bajetta E., et al. Bevacizumab plus Interferon Alfa-2a for Treatment of Metastatic Renal Cell Carcinoma: A Randomised, Double-Blind Phase III Trial. Lancet Lond. Engl. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 10.Minasian L.M., Motzer R.J., Gluck L., Mazumdar M., Vlamis V., Krown S.E. Interferon Alfa-2a in Advanced Renal Cell Carcinoma: Treatment Results and Survival in 159 Patients with Long-Term Follow-Up. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1993;11:1368–1375. doi: 10.1200/JCO.1993.11.7.1368. [DOI] [PubMed] [Google Scholar]
- 11.Sharma R., Kadife E., Myers M., Kannourakis G., Prithviraj P., Ahmed N. Determinants of Resistance to VEGF-TKI and Immune Checkpoint Inhibitors in Metastatic Renal Cell Carcinoma. J. Exp. Clin. Cancer Res. 2021;40:186. doi: 10.1186/s13046-021-01961-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han Y., Liu D., Li L. PD-1/PD-L1 Pathway: Current Researches in Cancer. Am. J. Cancer Res. 2020;10:727–742. [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz J.C., Zhang X., Fedorov A.A., Nathenson S.G., Almo S.C. Structural Basis for Co-Stimulation by the Human CTLA-4/B7-2 Complex. Nature. 2001;410:604–608. doi: 10.1038/35069112. [DOI] [PubMed] [Google Scholar]
- 14.Stamper C.C., Zhang Y., Tobin J.F., Erbe D.V., Ikemizu S., Davis S.J., Stahl M.L., Seehra J., Somers W.S., Mosyak L. Crystal Structure of the B7-1/CTLA-4 Complex That Inhibits Human Immune Responses. Nature. 2001;410:608–611. doi: 10.1038/35069118. [DOI] [PubMed] [Google Scholar]
- 15.Ravaud A., Motzer R.J., Pandha H.S., George D.J., Pantuck A.J., Patel A., Chang Y.-H., Escudier B., Donskov F., Magheli A., et al. Adjuvant Sunitinib in High-Risk Renal-Cell Carcinoma after Nephrectomy. N. Engl. J. Med. 2016;375:2246–2254. doi: 10.1056/NEJMoa1611406. [DOI] [PubMed] [Google Scholar]
- 16.Haas N.B., Manola J., Uzzo R.G., Flaherty K.T., Wood C.G., Kane C., Jewett M., Dutcher J.P., Atkins M.B., Pins M., et al. Adjuvant Sunitinib or Sorafenib for High-Risk, Non-Metastatic Renal-Cell Carcinoma (ECOG-ACRIN E2805): A Double-Blind, Placebo-Controlled, Randomised, Phase 3 Trial. Lancet. 2016;387:2008–2016. doi: 10.1016/S0140-6736(16)00559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choueiri T.K., Tomczak P., Park S.H., Venugopal B., Ferguson T., Chang Y.-H., Hajek J., Symeonides S.N., Lee J.L., Sarwar N., et al. Adjuvant Pembrolizumab after Nephrectomy in Renal-Cell Carcinoma. N. Engl. J. Med. 2021;385:683–694. doi: 10.1056/NEJMoa2106391. [DOI] [PubMed] [Google Scholar]
- 18.Motzer R.J., Escudier B., McDermott D.F., George S., Hammers H.J., Srinivas S., Tykodi S.S., Sosman J.A., Procopio G., Plimack E.R., et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. [(accessed on 27 May 2021)]. Available online: https://www.nejm.org/doi/10.1056/NEJMoa1510665.
- 19.McDermott D.F., Lee J.-L., Bjarnason G.A., Larkin J.M.G., Gafanov R.A., Kochenderfer M.D., Jensen N.V., Donskov F., Malik J., Poprach A., et al. Open-Label, Single-Arm Phase II Study of Pembrolizumab Monotherapy as First-Line Therapy in Patients With Advanced Clear Cell Renal Cell Carcinoma. J. Clin. Oncol. 2021;39:1020–1028. doi: 10.1200/JCO.20.02363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDermott D.F., Lee J.-L., Ziobro M., Suarez C., Langiewicz P., Matveev V.B., Wiechno P., Gafanov R.A., Tomczak P., Pouliot F., et al. Open-Label, Single-Arm, Phase II Study of Pembrolizumab Monotherapy as First-Line Therapy in Patients With Advanced Non-Clear Cell Renal Cell Carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021;39:1029–1039. doi: 10.1200/JCO.20.02365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rotte A. Combination of CTLA-4 and PD-1 Blockers for Treatment of Cancer. J. Exp. Clin. Cancer Res. 2019;38:255. doi: 10.1186/s13046-019-1259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das R., Verma R., Sznol M., Boddupalli C.S., Gettinger S.N., Kluger H., Callahan M., Wolchok J.D., Halaban R., Dhodapkar M.V., et al. Combination Therapy with Anti–CTLA-4 and Anti–PD-1 Leads to Distinct Immunologic Changes In Vivo. J. Immunol. 2015;194:950–959. doi: 10.4049/jimmunol.1401686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei S.C., Anang N.-A.A.S., Sharma R., Andrews M.C., Reuben A., Levine J.H., Cogdill A.P., Mancuso J.J., Wargo J.A., Pe’er D., et al. Combination Anti–CTLA-4 plus Anti–PD-1 Checkpoint Blockade Utilizes Cellular Mechanisms Partially Distinct from Monotherapies. Proc. Natl. Acad. Sci. USA. 2019;116:22699–22709. doi: 10.1073/pnas.1821218116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Motzer R.J., Tannir N.M., McDermott D.F., Frontera O.A., Melichar B., Choueiri T.K., Plimack E.R., Barthélémy P., Porta C., George S., et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Regan M.M., Jegede O.A., Mantia C.M., Powles T., Werner L., Motzer R.J., Tannir N.M., Lee C.-H., Tomita Y., Voss M.H., et al. Treatment-Free Survival after Immune Checkpoint Inhibitor Therapy versus Targeted Therapy for Advanced Renal Cell Carcinoma: 42-Month Results of the CheckMate 214 Trial. Clin. Cancer Res. 2021 doi: 10.1158/1078-0432.CCR-21-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hakimi A.A., Voss M.H., Kuo F., Sanchez A., Liu M., Nixon B.G., Vuong L., Ostrovnaya I., Chen Y.-B., Reuter V., et al. Transcriptomic Profiling of the Tumor Microenvironment Reveals Distinct Subgroups of Clear Cell Renal Cell Cancer: Data from a Randomized Phase III Trial. Cancer Discov. 2019;9:510–525. doi: 10.1158/2159-8290.CD-18-0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rini B.I., Plimack E.R., Stus V., Gafanov R., Hawkins R., Nosov D., Pouliot F., Alekseev B., Soulières D., Melichar B., et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019;380:1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 28.Motzer R.J., Penkov K., Haanen J., Rini B., Albiges L., Campbell M.T., Venugopal B., Kollmannsberger C., Negrier S., Uemura M., et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019;380:1103–1115. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rini B.I., Powles T., Atkins M.B., Escudier B., McDermott D.F., Suarez C., Bracarda S., Stadler W.M., Donskov F., Lee J.L., et al. Atezolizumab plus Bevacizumab versus Sunitinib in Patients with Previously Untreated Metastatic Renal Cell Carcinoma (IMmotion151): A Multicentre, Open-Label, Phase 3, Randomised Controlled Trial. Lancet. 2019;393:2404–2415. doi: 10.1016/S0140-6736(19)30723-8. [DOI] [PubMed] [Google Scholar]
- 30.Motzer R., Alekseev B., Rha S.-Y., Porta C., Eto M., Powles T., Grünwald V., Hutson T.E., Kopyltsov E., Méndez-Vidal M.J., et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N. Engl. J. Med. 2021;384:1289–1300. doi: 10.1056/NEJMoa2035716. [DOI] [PubMed] [Google Scholar]
- 31.Choueiri T.K., Powles T., Burotto M., Escudier B., Bourlon M.T., Zurawski B., Oyervides Juárez V.M., Hsieh J.J., Basso U., Shah A.Y., et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2021;384:829–841. doi: 10.1056/NEJMoa2026982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Şenbabaoğlu Y., Gejman R.S., Winer A.G., Liu M., Van Allen E.M., de Velasco G., Miao D., Ostrovnaya I., Drill E., Luna A., et al. Tumor Immune Microenvironment Characterization in Clear Cell Renal Cell Carcinoma Identifies Prognostic and Immunotherapeutically Relevant Messenger RNA Signatures. Genome Biol. 2016;17:231. doi: 10.1186/s13059-016-1092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braun D.A., Hou Y., Bakouny Z., Ficial M., Sant’ Angelo M., Forman J., Ross-Macdonald P., Berger A.C., Jegede O.A., Elagina L., et al. Interplay of Somatic Alterations and Immune Infiltration Modulates Response to PD-1 Blockade in Advanced Clear Cell Renal Cell Carcinoma. Nat. Med. 2020;26:909–918. doi: 10.1038/s41591-020-0839-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ricketts C.J., De Cubas A.A., Fan H., Smith C.C., Lang M., Reznik E., Bowlby R., Gibb E.A., Akbani R., Beroukhim R., et al. The Cancer Genome Atlas Comprehensive Molecular Characterization of Renal Cell Carcinoma. Cell Rep. 2018;23:313–326.e5. doi: 10.1016/j.celrep.2018.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chevrier S., Levine J.H., Zanotelli V.R.T., Silina K., Schulz D., Bacac M., Ries C.H., Ailles L., Jewett M.A.S., Moch H., et al. An Immune Atlas of Clear Cell Renal Cell Carcinoma. Cell. 2017;169:736–749.e18. doi: 10.1016/j.cell.2017.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luke J.J., Bao R., Sweis R., Spranger S., Gajewski T.F. WNT/β-Catenin Pathway Activation Correlates with Immune Exclusion across Human Cancers. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019;25:3074–3083. doi: 10.1158/1078-0432.CCR-18-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geissler K., Fornara P., Lautenschläger C., Holzhausen H.-J., Seliger B., Riemann D. Immune Signature of Tumor Infiltrating Immune Cells in Renal Cancer. Oncoimmunology. 2015;4:e985082. doi: 10.4161/2162402X.2014.985082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ock C.-Y., Keam B., Kim S., Lee J.-S., Kim M., Kim T.M., Jeon Y.K., Kim D.-W., Chung D.H., Heo D.S. Pan-Cancer Immunogenomic Perspective on the Tumor Microenvironment Based on PD-L1 and CD8 T-Cell Infiltration. Clin. Cancer Res. 2016;22:2261–2270. doi: 10.1158/1078-0432.CCR-15-2834. [DOI] [PubMed] [Google Scholar]
- 39.Li L., Yang C., Zhao Z., Xu B., Zheng M., Zhang C., Min Z., Guo J., Rong R. Skewed T-Helper (Th)1/2- and Th17/T Regulatory-cell Balances in Patients with Renal Cell Carcinoma. Mol. Med. Rep. 2015;11:947–953. doi: 10.3892/mmr.2014.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rooney M.S., Shukla S.A., Wu C.J., Getz G., Hacohen N. Molecular and Genetic Properties of Tumors Associated with Local Immune Cytolytic Activity. Cell. 2015;160:48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braun D.A., Street K., Burke K.P., Cookmeyer D.L., Denize T., Pedersen C.B., Gohil S.H., Schindler N., Pomerance L., Hirsch L., et al. Progressive Immune Dysfunction with Advancing Disease Stage in Renal Cell Carcinoma. Cancer Cell. 2021;39:632–648.e8. doi: 10.1016/j.ccell.2021.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Togashi Y., Shitara K., Nishikawa H. Regulatory T Cells in Cancer Immunosuppression—Implications for Anticancer Therapy. Nat. Rev. Clin. Oncol. 2019;16:356–371. doi: 10.1038/s41571-019-0175-7. [DOI] [PubMed] [Google Scholar]
- 43.Griffiths R.W., Elkord E., Gilham D.E., Ramani V., Clarke N., Stern P.L., Hawkins R.E. Frequency of Regulatory T Cells in Renal Cell Carcinoma Patients and Investigation of Correlation with Survival. Cancer Immunol. Immunother. CII. 2007;56:1743–1753. doi: 10.1007/s00262-007-0318-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharonov G.V., Serebrovskaya E.O., Yuzhakova D.V., Britanova O.V., Chudakov D.M. B Cells, Plasma Cells and Antibody Repertoires in the Tumour Microenvironment. Nat. Rev. Immunol. 2020;20:294–307. doi: 10.1038/s41577-019-0257-x. [DOI] [PubMed] [Google Scholar]
- 45.Sjöberg E., Frödin M., Lövrot J., Mezheyeuski A., Johansson M., Harmenberg U., Egevad L., Sandström P., Östman A. A Minority-Group of Renal Cell Cancer Patients with High Infiltration of CD20+B-Cells Is Associated with Poor Prognosis. Br. J. Cancer. 2018;119:840–846. doi: 10.1038/s41416-018-0266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li S., Huang C., Hu G., Ma J., Chen Y., Zhang J., Huang Y., Zheng J., Xue W., Xu Y., et al. Tumor-Educated B Cells Promote Renal Cancer Metastasis via Inducing the IL-1β/HIF-2α/Notch1 Signals. Cell Death Dis. 2020;11:1–14. doi: 10.1038/s41419-020-2355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iglesia M.D., Parker J.S., Hoadley K.A., Serody J.S., Perou C.M., Vincent B.G. Genomic Analysis of Immune Cell Infiltrates Across 11 Tumor Types. JNCI J. Natl. Cancer Inst. 2016;108 doi: 10.1093/jnci/djw144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gigante M., Blasi A., Loverre A., Mancini V., Battaglia M., Selvaggi F.P., Maiorano E., Napoli A., Castellano G., Storkus W.J., et al. Dysfunctional DC Subsets in RCC Patients: Ex Vivo Correction to Yield an Effective Anti-Cancer Vaccine. Mol. Immunol. 2009;46:893–901. doi: 10.1016/j.molimm.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Figel A.-M., Brech D., Prinz P.U., Lettenmeyer U.K., Eckl J., Turqueti-Neves A., Mysliwietz J., Anz D., Rieth N., Muenchmeier N., et al. Human Renal Cell Carcinoma Induces a Dendritic Cell Subset That Uses T-Cell Crosstalk for Tumor-Permissive Milieu Alterations. Am. J. Pathol. 2011;179:436–451. doi: 10.1016/j.ajpath.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kobayashi M., Suzuki K., Yashi M., Yuzawa M., Takayashiki N., Morita T. Tumor Infiltrating Dendritic Cells Predict Treatment Response to Immmunotherapy in Patients with Metastatic Renal Cell Carcinoma. Anticancer Res. 2007;27:1137–1141. [PubMed] [Google Scholar]
- 51.Voss M.H., Buros Novik J., Hellmann M.D., Ball M., Hakimi A.A., Miao D., Margolis C., Horak C., Wind-Rotolo M., De Velasco G., et al. Correlation of Degree of Tumor Immune Infiltration and Insertion-and-Deletion (Indel) Burden with Outcome on Programmed Death 1 (PD1) Therapy in Advanced Renal Cell Cancer (RCC) J. Clin. Oncol. 2018;36:4518. doi: 10.1200/JCO.2018.36.15_suppl.4518. [DOI] [Google Scholar]
- 52.Motoshima T., Miura Y., Wakigami N., Kusada N., Takano T., Inoshita N., Okaneya T., Sugiyama Y., Kamba T., Takeya M., et al. Phenotypical Change of Tumor-Associated Macrophages in Metastatic Lesions of Clear Cell Renal Cell Carcinoma. Med. Mol. Morphol. 2018;51:57–63. doi: 10.1007/s00795-017-0174-7. [DOI] [PubMed] [Google Scholar]
- 53.Tamma R., Rutigliano M., Lucarelli G., Annese T., Ruggieri S., Cascardi E., Napoli A., Battaglia M., Ribatti D. Microvascular Density, Macrophages, and Mast Cells in Human Clear Cell Renal Carcinoma with and without Bevacizumab Treatment. Urol. Oncol. 2019;37:355.e11–355.e19. doi: 10.1016/j.urolonc.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 54.Fu Q., Xu L., Wang Y., Jiang Q., Liu Z., Zhang J., Zhou Q., Zeng H., Tong S., Wang T., et al. Tumor-Associated Macrophage-Derived Interleukin-23 Interlinks Kidney Cancer Glutamine Addiction with Immune Evasion. Eur. Urol. 2019;75:752–763. doi: 10.1016/j.eururo.2018.09.030. [DOI] [PubMed] [Google Scholar]
- 55.Schleypen J.S., Von Geldern M., Weiss E.H., Kotzias N., Rohrmann K., Schendel D.J., Falk C.S., Pohla H. Renal Cell Carcinoma-Infiltrating Natural Killer Cells Express Differential Repertoires of Activating and Inhibitory Receptors and Are Inhibited by Specific HLA Class I Allotypes. Int. J. Cancer. 2003;106:905–912. doi: 10.1002/ijc.11321. [DOI] [PubMed] [Google Scholar]
- 56.Remark R., Alifano M., Cremer I., Lupo A., Dieu-Nosjean M.-C., Riquet M., Crozet L., Ouakrim H., Goc J., Cazes A., et al. Characteristics and Clinical Impacts of the Immune Environments in Colorectal and Renal Cell Carcinoma Lung Metastases: Influence of Tumor Origin. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013;19:4079–4091. doi: 10.1158/1078-0432.CCR-12-3847. [DOI] [PubMed] [Google Scholar]
- 57.Prinz P.U., Mendler A.N., Brech D., Masouris I., Oberneder R., Noessner E. NK-Cell Dysfunction in Human Renal Carcinoma Reveals Diacylglycerol Kinase as Key Regulator and Target for Therapeutic Intervention. Int. J. Cancer. 2014;135:1832–1841. doi: 10.1002/ijc.28837. [DOI] [PubMed] [Google Scholar]
- 58.Xia Y., Zhang Q., Zhen Q., Zhao Y., Liu N., Li T., Hao Y., Zhang Y., Luo C., Wu X. Negative Regulation of Tumor-Infiltrating NK Cell in Clear Cell Renal Cell Carcinoma Patients through the Exosomal Pathway. Oncotarget. 2017;8:37783–37795. doi: 10.18632/oncotarget.16354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Veglia F., Sanseviero E., Gabrilovich D.I. Myeloid-Derived Suppressor Cells in the Era of Increasing Myeloid Cell Diversity. Nat. Rev. Immunol. 2021;21:485–498. doi: 10.1038/s41577-020-00490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guan X., Liu Z., Zhang J., Jin X. Myeloid-Derived Suppressor Cell Accumulation in Renal Cell Carcinoma Is Correlated with CCL2, IL-17 and IL-18 Expression in Blood and Tumors. Adv. Clin. Exp. Med. Off. Organ Wroclaw Med. Univ. 2018;27:947–953. doi: 10.17219/acem/70065. [DOI] [PubMed] [Google Scholar]
- 61.Rodriguez P.C., Ernstoff M.S., Hernandez C., Atkins M., Zabaleta J., Sierra R., Ochoa A.C. Arginase I-Producing Myeloid-Derived Suppressor Cells in Renal Cell Carcinoma Are a Subpopulation of Activated Granulocytes. Cancer Res. 2009;69:1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okada S.L., Simmons R.M., Franke-Welch S., Nguyen T.H., Korman A.J., Dillon S.R., Gilbertson D.G. Conditioned Media from the Renal Cell Carcinoma Cell Line 786.O Drives Human Blood Monocytes to a Monocytic Myeloid-Derived Suppressor Cell Phenotype. Cell. Immunol. 2018;323:49–58. doi: 10.1016/j.cellimm.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 63.Najjar Y.G., Rayman P., Jia X., Pavicic P.G., Rini B.I., Tannenbaum C., Ko J., Haywood S., Cohen P., Hamilton T., et al. Myeloid-Derived Suppressor Cell Subset Accumulation in Renal Cell Carcinoma Parenchyma Is Associated with Intratumoral Expression of IL1β, IL8, CXCL5, and Mip-1α. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017;23:2346–2355. doi: 10.1158/1078-0432.CCR-15-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Orillion A., Hashimoto A., Damayanti N., Shen L., Adelaiye-Ogala R., Arisa S., Chintala S., Ordentlich P., Kao C., Elzey B., et al. Entinostat Neutralizes Myeloid-Derived Suppressor Cells and Enhances the Antitumor Effect of PD-1 Inhibition in Murine Models of Lung and Renal Cell Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017;23:5187–5201. doi: 10.1158/1078-0432.CCR-17-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li J., Sun J., Rong R., Li L., Shang W., Song D., Feng G., Luo F. HMGB1 Promotes Myeloid-Derived Suppressor Cells and Renal Cell Carcinoma Immune Escape. Oncotarget. 2017;8:63290–63298. doi: 10.18632/oncotarget.18796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ko J.S., Zea A.H., Rini B.I., Ireland J.L., Elson P., Cohen P., Golshayan A., Rayman P.A., Wood L., Garcia J., et al. Sunitinib Mediates Reversal of Myeloid-Derived Suppressor Cell Accumulation in Renal Cell Carcinoma Patients. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009;15:2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 67.Ko J.S., Rayman P., Ireland J., Swaidani S., Li G., Bunting K.D., Rini B., Finke J.H., Cohen P.A. Direct and Differential Suppression of Myeloid-Derived Suppressor Cell Subsets by Sunitinib Is Compartmentally Constrained. Cancer Res. 2010;70:3526–3536. doi: 10.1158/0008-5472.CAN-09-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rizvi N.A., Hellmann M.D., Snyder A., Kvistborg P., Makarov V., Havel J.J., Lee W., Yuan J., Wong P., Ho T.S., et al. Cancer Immunology. Mutational Landscape Determines Sensitivity to PD-1 Blockade in Non-Small Cell Lung Cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Snyder A., Makarov V., Merghoub T., Yuan J., Zaretsky J.M., Desrichard A., Walsh L.A., Postow M.A., Wong P., Ho T.S., et al. Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. N. Engl. J. Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van Allen E.M., Miao D., Schilling B., Shukla S.A., Blank C., Zimmer L., Sucker A., Hillen U., Foppen M.H.G., Goldinger S.M., et al. Genomic Correlates of Response to CTLA-4 Blockade in Metastatic Melanoma. Science. 2015;350:207–211. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yarchoan M., Hopkins A., Jaffee E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017;377:2500–2501. doi: 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alexandrov L.B., Nik-Zainal S., Wedge D.C., Aparicio S.A.J.R., Behjati S., Biankin A.V., Bignell G.R., Bolli N., Borg A., Børresen-Dale A.-L., et al. Signatures of Mutational Processes in Human Cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lawrence M.S., Stojanov P., Polak P., Kryukov G.V., Cibulskis K., Sivachenko A., Carter S.L., Stewart C., Mermel C.H., Roberts S.A., et al. Mutational Heterogeneity in Cancer and the Search for New Cancer-Associated Genes. Nature. 2013;499:214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang C., Li Z., Qi F., Hu X., Luo J. Exploration of the Relationships between Tumor Mutation Burden with Immune Infiltrates in Clear Cell Renal Cell Carcinoma. Ann. Transl. Med. 2019;7 doi: 10.21037/atm.2019.10.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang X., Li M. Correlate Tumor Mutation Burden with Immune Signatures in Human Cancers. BMC Immunol. 2019;20:4. doi: 10.1186/s12865-018-0285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Turajlic S., Litchfield K., Xu H., Rosenthal R., McGranahan N., Reading J.L., Wong Y.N.S., Rowan A., Kanu N., Al Bakir M., et al. Insertion-and-Deletion-Derived Tumour-Specific Neoantigens and the Immunogenic Phenotype: A Pan-Cancer Analysis. Lancet Oncol. 2017;18:1009–1021. doi: 10.1016/S1470-2045(17)30516-8. [DOI] [PubMed] [Google Scholar]
- 77.Chae Y.K., Anker J.F., Carneiro B.A., Chandra S., Kaplan J., Kalyan A., Santa-Maria C.A., Platanias L.C., Giles F.J. Genomic Landscape of DNA Repair Genes in Cancer. Oncotarget. 2016;7:23312–23321. doi: 10.18632/oncotarget.8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Le D.T., Uram J.N., Wang H., Bartlett B.R., Kemberling H., Eyring A.D., Skora A.D., Luber B.S., Azad N.S., Laheru D., et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Le D.T., Durham J.N., Smith K.N., Wang H., Bartlett B.R., Aulakh L.K., Lu S., Kemberling H., Wilt C., Luber B.S., et al. Mismatch-Repair Deficiency Predicts Response of Solid Tumors to PD-1 Blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ged Y., Chaim J.L., DiNatale R.G., Knezevic A., Kotecha R.R., Carlo M.I., Lee C.-H., Foster A., Feldman D.R., Teo M.Y., et al. DNA Damage Repair Pathway Alterations in Metastatic Clear Cell Renal Cell Carcinoma and Implications on Systemic Therapy. J. Immunother. Cancer. 2020;8:e000230. doi: 10.1136/jitc-2019-000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Na J.C., Nagaya N., Rha K.H., Han W.K., Kim I.Y. DNA Damage Response Pathway Alteration in Locally Advanced Clear-Cell Renal-Cell Carcinoma Is Associated with a Poor Outcome. Clin. Genitourin. Cancer. 2019;17:299–305.e1. doi: 10.1016/j.clgc.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 82.Hartman T.R., Demidova E.V., Lesh R.W., Hoang L., Richardson M., Forman A., Kessler L., Speare V., Golemis E.A., Hall M.J., et al. Prevalence of Pathogenic Variants in DNA Damage Response and Repair Genes in Patients Undergoing Cancer Risk Assessment and Reporting a Personal History of Early-Onset Renal Cancer. Sci. Rep. 2020;10:13518. doi: 10.1038/s41598-020-70449-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guo E., Wu C., Ming J., Zhang W., Zhang L., Hu G. The Clinical Significance of DNA Damage Repair Signatures in Clear Cell Renal Cell Carcinoma. Front. Genet. 2021;11 doi: 10.3389/fgene.2020.593039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leone R.D., Powell J.D. Metabolism of Immune Cells in Cancer. Nat. Rev. Cancer. 2020;20:516–531. doi: 10.1038/s41568-020-0273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lucarelli G., Loizzo D., Franzin R., Battaglia S., Ferro M., Cantiello F., Castellano G., Bettocchi C., Ditonno P., Battaglia M. Metabolomic Insights into Pathophysiological Mechanisms and Biomarker Discovery in Clear Cell Renal Cell Carcinoma. Expert Rev. Mol. Diagn. 2019;19:397–407. doi: 10.1080/14737159.2019.1607729. [DOI] [PubMed] [Google Scholar]
- 86.Ragone R., Sallustio F., Piccinonna S., Rutigliano M., Vanessa G., Palazzo S., Lucarelli G., Ditonno P., Battaglia M., Fanizzi F.P., et al. Renal Cell Carcinoma: A Study through NMR-Based Metabolomics Combined with Transcriptomics. Diseases. 2016;4:7. doi: 10.3390/diseases4010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lucarelli G., Rutigliano M., Sallustio F., Ribatti D., Giglio A., Lepore Signorile M., Grossi V., Sanese P., Napoli A., Maiorano E., et al. Integrated Multi-Omics Characterization Reveals a Distinctive Metabolic Signature and the Role of NDUFA4L2 in Promoting Angiogenesis, Chemoresistance, and Mitochondrial Dysfunction in Clear Cell Renal Cell Carcinoma. Aging. 2018;10:3957–3985. doi: 10.18632/aging.101685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bombelli S., Torsello B., De Marco S., Lucarelli G., Cifola I., Grasselli C., Strada G., Bovo G., Perego R.A., Bianchi C. 36-KDa Annexin A3 Isoform Negatively Modulates Lipid Storage in Clear Cell Renal Cell Carcinoma Cells. Am. J. Pathol. 2020;190:2317–2326. doi: 10.1016/j.ajpath.2020.08.008. [DOI] [PubMed] [Google Scholar]
- 89.Netti G.S., Lucarelli G., Spadaccino F., Castellano G., Gigante M., Divella C., Rocchetti M.T., Rascio F., Mancini V., Stallone G., et al. PTX3 Modulates the Immunoflogosis in Tumor Microenvironment and Is a Prognostic Factor for Patients with Clear Cell Renal Cell Carcinoma. Aging. 2020;12:7585–7602. doi: 10.18632/aging.103169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bianchi C., Meregalli C., Bombelli S., Di Stefano V., Salerno F., Torsello B., De Marco S., Bovo G., Cifola I., Mangano E., et al. The Glucose and Lipid Metabolism Reprogramming Is Grade-Dependent in Clear Cell Renal Cell Carcinoma Primary Cultures and Is Targetable to Modulate Cell Viability and Proliferation. Oncotarget. 2017;8:113502–113515. doi: 10.18632/oncotarget.23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lucarelli G., Galleggiante V., Rutigliano M., Sanguedolce F., Cagiano S., Bufo P., Lastilla G., Maiorano E., Ribatti D., Giglio A., et al. Metabolomic Profile of Glycolysis and the Pentose Phosphate Pathway Identifies the Central Role of Glucose-6-Phosphate Dehydrogenase in Clear Cell-Renal Cell Carcinoma. Oncotarget. 2015;6:13371–13386. doi: 10.18632/oncotarget.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lucarelli G., Rutigliano M., Ferro M., Giglio A., Intini A., Triggiano F., Palazzo S., Gigante M., Castellano G., Ranieri E., et al. Activation of the Kynurenine Pathway Predicts Poor Outcome in Patients with Clear Cell Renal Cell Carcinoma. Urol. Oncol. 2017;35:461.e15–461.e27. doi: 10.1016/j.urolonc.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 93.Li H., Bullock K., Gurjao C., Braun D., Shukla S.A., Bossé D., Lalani A.-K.A., Gopal S., Jin C., Horak C., et al. Metabolomic Adaptations and Correlates of Survival to Immune Checkpoint Blockade. Nat. Commun. 2019;10:4346. doi: 10.1038/s41467-019-12361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bi K., He M.X., Bakouny Z., Kanodia A., Napolitano S., Wu J., Grimaldi G., Braun D.A., Cuoco M.S., Mayorga A., et al. Tumor and Immune Reprogramming during Immunotherapy in Advanced Renal Cell Carcinoma. Cancer Cell. 2021;39:649–661.e5. doi: 10.1016/j.ccell.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beckermann K., Siska P., Mason F., Rathmell K., Rathmell J.C. Metabolic Barriers to Immunotherapy in Renal Cell Carcinoma. J. Clin. Oncol. 2017;35:11560. doi: 10.1200/JCO.2017.35.15_suppl.11560. [DOI] [Google Scholar]
- 96.Carretero-González A., Lora D., Martín Sobrino I., Sáez Sanz I., Bourlon M.T., Anido Herranz U., Martínez Chanzá N., Castellano D., de Velasco G. The Value of PD-L1 Expression as Predictive Biomarker in Metastatic Renal Cell Carcinoma Patients: A Meta-Analysis of Randomized Clinical Trials. Cancers. 2020;12:1945. doi: 10.3390/cancers12071945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Choueiri T.K., Fishman M.N., Escudier B., McDermott D.F., Drake C.G., Kluger H., Stadler W.M., Perez-Gracia J.L., McNeel D.G., Curti B., et al. Immunomodulatory Activity of Nivolumab in Metastatic Renal Cell Carcinoma. Clin. Cancer Res. 2016;22:5461–5471. doi: 10.1158/1078-0432.CCR-15-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kahlmeyer A., Stöhr C.G., Hartmann A., Goebell P.J., Wullich B., Wach S., Taubert H., Erlmeier F. Expression of PD-1 and CTLA-4 Are Negative Prognostic Markers in Renal Cell Carcinoma. J. Clin. Med. 2019;8:743. doi: 10.3390/jcm8050743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thompson R.H., Dong H., Lohse C.M., Leibovich B.C., Blute M.L., Cheville J.C., Kwon E.D. PD-1 Is Expressed by Tumor-Infiltrating Immune Cells and Is Associated with Poor Outcome for Patients with Renal Cell Carcinoma. Clin. Cancer Res. 2007;13:1757–1761. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 100.Callea M., Albiges L., Gupta M., Cheng S.-C., Genega E.M., Fay A.P., Song J., Carvo I., Bhatt R.S., Atkins M.B., et al. Differential Expression of PD-L1 between Primary and Metastatic Sites in Clear-Cell Renal Cell Carcinoma. Cancer Immunol. Res. 2015;3:1158–1164. doi: 10.1158/2326-6066.CIR-15-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thompson R.H., Kuntz S.M., Leibovich B.C., Dong H., Lohse C.M., Webster W.S., Sengupta S., Frank I., Parker A.S., Zincke H., et al. Tumor B7-H1 Is Associated with Poor Prognosis in Renal Cell Carcinoma Patients with Long-Term Follow-Up. Cancer Res. 2006;66:3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 102.Thompson R.H., Gillett M.D., Cheville J.C., Lohse C.M., Dong H., Webster W.S., Krejci K.G., Lobo J.R., Sengupta S., Chen L., et al. Costimulatory B7-H1 in Renal Cell Carcinoma Patients: Indicator of Tumor Aggressiveness and Potential Therapeutic Target. Proc. Natl. Acad. Sci. USA. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xu F., Xu L., Wang Q., An G., Feng G., Liu F. Clinicopathological and Prognostic Value of Programmed Death Ligand-1 (PD-L1) in Renal Cell Carcinoma: A Meta-Analysis. Int. J. Clin. Exp. Med. 2015;8:14595–14603. [PMC free article] [PubMed] [Google Scholar]
- 104.Liao G., Wang P., Wang Y. Identification of the Prognosis Value and Potential Mechanism of Immune Checkpoints in Renal Clear Cell Carcinoma Microenvironment. Front. Oncol. 2021;11:720125. doi: 10.3389/fonc.2021.720125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu S., Wang F., Tan W., Zhang L., Dai F., Wang Y., Fan Y., Yuan M., Yang D., Zheng Y., et al. CTLA4 Has a Profound Impact on the Landscape of Tumor-Infiltrating Lymphocytes with a High Prognosis Value in Clear Cell Renal Cell Carcinoma (CcRCC) Cancer Cell Int. 2020;20:519. doi: 10.1186/s12935-020-01603-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang M., Du Q., Jin J., Wei Y., Lu Y., Li Q. LAG3 and Its Emerging Role in Cancer Immunotherapy. Clin. Transl. Med. 2021;11:e365. doi: 10.1002/ctm2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Klümper N., Ralser D.J., Bawden E.G., Landsberg J., Zarbl R., Kristiansen G., Toma M., Ritter M., Hölzel M., Ellinger J., et al. LAG3 (LAG-3, CD223) DNA Methylation Correlates with LAG3 Expression by Tumor and Immune Cells, Immune Cell Infiltration, and Overall Survival in Clear Cell Renal Cell Carcinoma. J. Immunother. Cancer. 2020;8:e000552. doi: 10.1136/jitc-2020-000552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zelba H., Bedke J., Hennenlotter J., Mostböck S., Zettl M., Zichner T., Chandran A., Stenzl A., Rammensee H.-G., Gouttefangeas C. PD-1 and LAG-3 Dominate Checkpoint Receptor–Mediated T-Cell Inhibition in Renal Cell Carcinoma. Cancer Immunol. Res. 2019;7:1891–1899. doi: 10.1158/2326-6066.CIR-19-0146. [DOI] [PubMed] [Google Scholar]
- 109.Hong X., Wang X., Wang T., Zhang X. Correlation of T Cell Immunoglobulin and ITIM Domain (TIGIT) and Programmed Death 1 (PD-1) with Clinicopathological Characteristics of Renal Cell Carcinoma May Indicate Potential Targets for Treatment. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018;24:6861–6872. doi: 10.12659/MSM.910388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Johnston R.J., Comps-Agrar L., Hackney J., Yu X., Huseni M., Yang Y., Park S., Javinal V., Chiu H., Irving B., et al. The Immunoreceptor TIGIT Regulates Antitumor and Antiviral CD8 + T Cell Effector Function. Cancer Cell. 2014;26:923–937. doi: 10.1016/j.ccell.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 111.Park A.I., Srivastava M., Mayes E., Jie H.-B., Yun R., Murriel C., Xie M., Lam A., Ji M., Axelrod F., et al. Abstract 2003: Antibody against TIGIT (T Cell Immunoreceptor with Ig and ITIM Domains) Induces Anti-Tumor Immune Response and Generates Long-Term Immune Memory. Cancer Res. 2017;77:2003. doi: 10.1158/1538-7445.AM2017-2003. [DOI] [Google Scholar]
- 112.Takamatsu K., Tanaka N., Hakozaki K., Takahashi R., Teranishi Y., Murakami T., Kufukihara R., Niwa N., Mikami S., Shinojima T., et al. Profiling the Inhibitory Receptors LAG-3, TIM-3, and TIGIT in Renal Cell Carcinoma Reveals Malignancy. Nat. Commun. 2021;12:5547. doi: 10.1038/s41467-021-25865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu S., Li X. A Genomic Instability-Derived Risk Index Predicts Clinical Outcome and Immunotherapy Response for Clear Cell Renal Cell Carcinoma. Bioengineered. 2021;12:1642–1662. doi: 10.1080/21655979.2021.1922330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Qiu Y., Wang X., Fan Z., Zhan S., Jiang X., Huang J. Integrated Analysis on the N6-Methyladenosine-Related Long Noncoding RNAs Prognostic Signature, Immune Checkpoints, and Immune Cell Infiltration in Clear Cell Renal Cell Carcinoma. Immun. Inflamm. Dis. 2021;9:1596–1612. doi: 10.1002/iid3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li Y., Wang Z., Jiang W., Zeng H., Liu Z., Lin Z., Qu Y., Xiong Y., Wang J., Chang Y., et al. Tumor-Infiltrating TNFRSF9+ CD8+ T Cells Define Different Subsets of Clear Cell Renal Cell Carcinoma with Prognosis and Immunotherapeutic Response. Oncoimmunology. 2020;9:1838141. doi: 10.1080/2162402X.2020.1838141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dai S., Zeng H., Liu Z., Jin K., Jiang W., Wang Z., Lin Z., Xiong Y., Wang J., Chang Y., et al. Intratumoral CXCL13+CD8+T Cell Infiltration Determines Poor Clinical Outcomes and Immunoevasive Contexture in Patients with Clear Cell Renal Cell Carcinoma. J. Immunother. Cancer. 2021;9:e001823. doi: 10.1136/jitc-2020-001823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Doroshow D.B., Bhalla S., Beasley M.B., Sholl L.M., Kerr K.M., Gnjatic S., Wistuba I.I., Rimm D.L., Tsao M.S., Hirsch F.R. PD-L1 as a Biomarker of Response to Immune-Checkpoint Inhibitors. Nat. Rev. Clin. Oncol. 2021;18:345–362. doi: 10.1038/s41571-021-00473-5. [DOI] [PubMed] [Google Scholar]
- 118.McDermott D.F., Huseni M.A., Atkins M.B., Motzer R.J., Rini B.I., Escudier B., Fong L., Joseph R.W., Pal S.K., Reeves J.A., et al. Clinical Activity and Molecular Correlates of Response to Atezolizumab Alone or in Combination with Bevacizumab versus Sunitinib in Renal Cell Carcinoma. Nat. Med. 2018;24:749–757. doi: 10.1038/s41591-018-0053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Motzer R.J., Robbins P.B., Powles T., Albiges L., Haanen J.B., Larkin J., Mu X.J., Ching K.A., Uemura M., Pal S.K., et al. Avelumab plus Axitinib versus Sunitinib in Advanced Renal Cell Carcinoma: Biomarker Analysis of the Phase 3 JAVELIN Renal 101 Trial. Nat. Med. 2020;26:1733–1741. doi: 10.1038/s41591-020-1044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen D., Chen W., Xu Y., Zhu M., Xiao Y., Shen Y., Zhu S., Cao C., Xu X. Upregulated Immune Checkpoint HHLA2 in Clear Cell Renal Cell Carcinoma: A Novel Prognostic Biomarker and Potential Therapeutic Target. J. Med. Genet. 2019;56:43–49. doi: 10.1136/jmedgenet-2018-105454. [DOI] [PubMed] [Google Scholar]
- 121.Giraldo N.A., Becht E., Pagès F., Skliris G., Verkarre V., Vano Y., Mejean A., Saint-Aubert N., Lacroix L., Natario I., et al. Orchestration and Prognostic Significance of Immune Checkpoints in the Microenvironment of Primary and Metastatic Renal Cell Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015;21:3031–3040. doi: 10.1158/1078-0432.CCR-14-2926. [DOI] [PubMed] [Google Scholar]
- 122.de Velasco G., Miao D., Shukla S., Voss M.H., Wu C., Murray B., Meyerson M., Signoretti S., Motzer R.J., Van Allen E.M., et al. Integrated Genomic Correlates of Response to PD-1 Inhibitor Nivolumab in Metastatic Renal Cell Carcinoma (MRCC) J. Clin. Oncol. 2016;34:545. doi: 10.1200/jco.2016.34.2_suppl.545. [DOI] [Google Scholar]
- 123.Wang Q., Zhang J., Tu H., Liang D., Chang D.W., Ye Y., Wu X. Soluble Immune Checkpoint-Related Proteins as Predictors of Tumor Recurrence, Survival, and T Cell Phenotypes in Clear Cell Renal Cell Carcinoma Patients. J. Immunother. Cancer. 2019;7 doi: 10.1186/s40425-019-0810-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li F., Li C., Cai X., Xie Z., Zhou L., Cheng B., Zhong R., Xiong S., Li J., Chen Z., et al. The Association between CD8+ Tumor-Infiltrating Lymphocytes and the Clinical Outcome of Cancer Immunotherapy: A Systematic Review and Meta-Analysis. EClinicalMedicine. 2021;41 doi: 10.1016/j.eclinm.2021.101134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Becht E., Giraldo N.A., Beuselinck B., Job S., Marisa L., Vano Y., Oudard S., Zucman-Rossi J., Laurent-Puig P., Sautès-Fridman C., et al. Prognostic and Theranostic Impact of Molecular Subtypes and Immune Classifications in Renal Cell Cancer (RCC) and Colorectal Cancer (CRC) Oncoimmunology. 2015;4:e1049804. doi: 10.1080/2162402X.2015.1049804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bromwich E.J., McArdle P.A., Canna K., McMillan D.C., McNicol A.-M., Brown M., Aitchison M. The Relationship between T-Lymphocyte Infiltration, Stage, Tumour Grade and Survival in Patients Undergoing Curative Surgery for Renal Cell Cancer. Br. J. Cancer. 2003;89:1906–1908. doi: 10.1038/sj.bjc.6601400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Giraldo N.A., Becht E., Vano Y., Petitprez F., Lacroix L., Validire P., Sanchez-Salas R., Ingels A., Oudard S., Moatti A., et al. Tumor-Infiltrating and Peripheral Blood T-Cell Immunophenotypes Predict Early Relapse in Localized Clear Cell Renal Cell Carcinoma. Clin. Cancer Res. 2017;23:4416–4428. doi: 10.1158/1078-0432.CCR-16-2848. [DOI] [PubMed] [Google Scholar]
- 128.Nakano O., Sato M., Naito Y., Suzuki K., Orikasa S., Aizawa M., Suzuki Y., Shintaku I., Nagura H., Ohtani H. Proliferative Activity of Intratumoral CD8(+) T-Lymphocytes as a Prognostic Factor in Human Renal Cell Carcinoma: Clinicopathologic Demonstration of Antitumor Immunity. Cancer Res. 2001;61:5132–5136. [PubMed] [Google Scholar]
- 129.Ross-Macdonald P., Walsh A.M., Chasalow S.D., Ammar R., Papillon-Cavanagh S., Szabo P.M., Choueiri T.K., Sznol M., Wind-Rotolo M. Molecular Correlates of Response to Nivolumab at Baseline and on Treatment in Patients with RCC. J. Immunother. Cancer. 2021;9:e001506. doi: 10.1136/jitc-2020-001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rini B.I., Huseni M., Atkins M.B., McDermott D.F., Powles T.B., Escudier B., Banchereau R., Liu L.-F., Leng N., Fan J., et al. Molecular Correlates Differentiate Response to Atezolizumab (Atezo) + Bevacizumab (Bev) vs Sunitinib (Sun): Results from a Phase III Study (IMmotion151) in Untreated Metastatic Renal Cell Carcinoma (MRCC) Ann. Oncol. 2018;29:viii724–viii725. doi: 10.1093/annonc/mdy424.037. [DOI] [Google Scholar]
- 131.Desnoyer A., Larive A., Drubay D., Lanoy E., Vano Y., Rioux-Leclercq N., Chouaib S., Beuselinck B., Tantot F., Escudier B., et al. Fresh Blood Immune Cell Monitoring in Patients Treated with Nivolumab in the GETUG-AFU26 NIVOREN Study: Association with Toxicity and Treatment Outcome. Ann. Oncol. 2019;30:v394. doi: 10.1093/annonc/mdz249.068. [DOI] [Google Scholar]
- 132.Xu L., Zhu Y., Chen L., An H., Zhang W., Wang G., Lin Z., Xu J. Prognostic Value of Diametrically Polarized Tumor-Associated Macrophages in Renal Cell Carcinoma. Ann. Surg. Oncol. 2014;21:3142–3150. doi: 10.1245/s10434-014-3601-1. [DOI] [PubMed] [Google Scholar]
- 133.Wang T., Lu R., Kapur P., Jaiswal B.S., Hannan R., Zhang Z., Pedrosa I., Luke J.J., Zhang H., Goldstein L.D., et al. An Empirical Approach Leveraging Tumorgrafts to Dissect the Tumor Microenvironment in Renal Cell Carcinoma Identifies Missing Link to Prognostic Inflammatory Factors. Cancer Discov. 2018;8:1142–1155. doi: 10.1158/2159-8290.CD-17-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McGrail D.J., Pilié P.G., Rashid N.U., Voorwerk L., Slagter M., Kok M., Jonasch E., Khasraw M., Heimberger A.B., Lim B., et al. High Tumor Mutation Burden Fails to Predict Immune Checkpoint Blockade Response across All Cancer Types. Ann. Oncol. 2021;32:661–672. doi: 10.1016/j.annonc.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Carril-Ajuria L., Santos M., Roldán-Romero J.M., Rodriguez-Antona C., de Velasco G. Prognostic and Predictive Value of PBRM1 in Clear Cell Renal Cell Carcinoma. Cancers. 2019;12:16. doi: 10.3390/cancers12010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Braun D.A., Ishii Y., Walsh A.M., Van Allen E.M., Wu C.J., Shukla S.A., Choueiri T.K. Clinical Validation of PBRM1 Alterations as a Marker of Immune Checkpoint Inhibitor Response in Renal Cell Carcinoma. JAMA Oncol. 2019;5:1631–1633. doi: 10.1001/jamaoncol.2019.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Miao D., Margolis C.A., Gao W., Voss M.H., Li W., Martini D.J., Norton C., Bossé D., Wankowicz S.M., Cullen D., et al. Genomic Correlates of Response to Immune Checkpoint Therapies in Clear Cell Renal Cell Carcinoma. Science. 2018;359:801–806. doi: 10.1126/science.aan5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen J., Yao C., Qiao N., Ge Y., Li J., Lin Y., Yao S. Development and Validation of a PBRM1-Associated Immune Prognostic Model for Clear Cell Renal Cell Carcinoma. Cancer Med. 2021;10:6590–6609. doi: 10.1002/cam4.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu X.-D., Kong W., Peterson C.B., McGrail D.J., Hoang A., Zhang X., Lam T., Pilie P.G., Zhu H., Beckermann K.E., et al. PBRM1 Loss Defines a Nonimmunogenic Tumor Phenotype Associated with Checkpoint Inhibitor Resistance in Renal Carcinoma. Nat. Commun. 2020;11:2135. doi: 10.1038/s41467-020-15959-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dias Carneiro A.P.C., Marques Monteiro F.S., Soares A. PBRM1 Mutations as a Predictive Biomarker for Immunotherapy in Metastatic Renal Cell Carcinoma: A Systematic Review. Kidney Cancer. 2021;5:79–92. doi: 10.3233/KCA-210111. [DOI] [Google Scholar]
- 141.Ascierto M.L., McMiller T.L., Berger A.E., Danilova L., Anders R.A., Netto G.J., Xu H., Pritchard T.S., Fan J., Cheadle C., et al. The Intratumoral Balance between Metabolic and Immunologic Gene Expression Is Associated with Anti–PD-1 Response in Patients with Renal Cell Carcinoma. Cancer Immunol. Res. 2016;4:726–733. doi: 10.1158/2326-6066.CIR-16-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zizzari I.G., Napoletano C., Botticelli A., Caponnetto S., Calabrò F., Gelibter A., Rughetti A., Ruscito I., Rahimi H., Rossi E., et al. TK Inhibitor Pazopanib Primes DCs by Downregulation of the β-Catenin Pathway. Cancer Immunol. Res. 2018;6:711–722. doi: 10.1158/2326-6066.CIR-17-0594. [DOI] [PubMed] [Google Scholar]
- 143.Epaillard N., Simonaggio A., Elaidi R., Azzouz F., Braychenko E., Thibault C., Sun C.-M., Moreira M., Oudard S., Vano Y.-A. BIONIKK: A Phase 2 Biomarker Driven Trial with Nivolumab and Ipilimumab or VEGFR Tyrosine Kinase Inhibitor (TKI) in Naïve Metastatic Kidney Cancer. Bull. Cancer. 2020;107:eS22–eS27. doi: 10.1016/S0007-4551(20)30283-6. [DOI] [PubMed] [Google Scholar]
- 144.Derosa L., Hellmann M.D., Spaziano M., Halpenny D., Fidelle M., Rizvi H., Long N., Plodkowski A.J., Arbour K.C., Chaft J.E., et al. Negative Association of Antibiotics on Clinical Activity of Immune Checkpoint Inhibitors in Patients with Advanced Renal Cell and Non-Small-Cell Lung Cancer. Ann. Oncol. 2018;29:1437–1444. doi: 10.1093/annonc/mdy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Routy B., Chatelier E.L., Derosa L., Duong C.P.M., Alou M.T., Daillère R., Fluckiger A., Messaoudene M., Rauber C., Roberti M.P., et al. Gut Microbiome Influences Efficacy of PD-1–Based Immunotherapy against Epithelial Tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 146.Salgia N.J., Bergerot P.G., Maia M.C., Dizman N., Hsu J., Gillece J.D., Folkerts M., Reining L., Trent J., Highlander S.K., et al. Stool Microbiome Profiling of Patients with Metastatic Renal Cell Carcinoma Receiving Anti–PD-1 Immune Checkpoint Inhibitors. Eur. Urol. 2020;78:498–502. doi: 10.1016/j.eururo.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 147.Dizman N., Hsu J., Bergerot P.G., Gillece J.D., Folkerts M., Reining L., Trent J., Highlander S.K., Pal S.K. Randomized Trial Assessing Impact of Probiotic Supplementation on Gut Microbiome and Clinical Outcome from Targeted Therapy in Metastatic Renal Cell Carcinoma. Cancer Med. 2020;10:79–86. doi: 10.1002/cam4.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lalani A.-K.A., Xie W., Martini D.J., Steinharter J.A., Norton C.K., Krajewski K.M., Duquette A., Bossé D., Bellmunt J., Allen E.M.V., et al. Change in Neutrophil-to-Lymphocyte Ratio (NLR) in Response to Immune Checkpoint Blockade for Metastatic Renal Cell Carcinoma. J. Immunother. Cancer. 2018;6:5. doi: 10.1186/s40425-018-0315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Escudier B., Sharma P., McDermott D.F., George S., Hammers H.J., Srinivas S., Tykodi S.S., Sosman J.A., Procopio G., Plimack E.R., et al. CheckMate 025 Randomized Phase 3 Study: Outcomes by Key Baseline Factors and Prior Therapy for Nivolumab Versus Everolimus in Advanced Renal Cell Carcinoma. Eur. Urol. 2017;72:962–971. doi: 10.1016/j.eururo.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 150.Escudier B., Motzer R.J., Tannir N.M., Porta C., Tomita Y., Maurer M.A., McHenry M.B., Rini B.I. Efficacy of Nivolumab plus Ipilimumab According to Number of IMDC Risk Factors in CheckMate 214. Eur. Urol. 2020;77:449–453. doi: 10.1016/j.eururo.2019.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.de Velasco G., Miao D., Voss M.H., Hakimi A.A., Hsieh J.J., Tannir N.M., Tamboli P., Appleman L.J., Rathmell W.K., Van Allen E.M., et al. Tumor Mutational Load and Immune Parameters across Metastatic Renal Cell Carcinoma Risk Groups. Cancer Immunol. Res. 2016;4:820–822. doi: 10.1158/2326-6066.CIR-16-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Giorgi U.D., Procopio G., Giannarelli D., Sabbatini R., Bearz A., Buti S., Basso U., Mitterer M., Ortega C., Bidoli P., et al. Association of Systemic Inflammation Index and Body Mass Index with Survival in Patients with Renal Cell Cancer Treated with Nivolumab. Clin. Cancer Res. 2019;25:3839–3846. doi: 10.1158/1078-0432.CCR-18-3661. [DOI] [PubMed] [Google Scholar]
- 153.McKay R.R., Bossé D., Xie W., Wankowicz S.A.M., Flaifel A., Brandao R., Lalani A.-K.A., Martini D.J., Wei X.X., Braun D.A., et al. The Clinical Activity of PD-1/PD-L1 Inhibitors in Metastatic Non-Clear Cell Renal Cell Carcinoma. Cancer Immunol. Res. 2018;6:758–765. doi: 10.1158/2326-6066.CIR-17-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Blum K.A., Gupta S., Tickoo S.K., Chan T.A., Russo P., Motzer R.J., Karam J.A., Hakimi A.A. Sarcomatoid Renal Cell Carcinoma: Biology, Natural History and Management. Nat. Rev. Urol. 2020;17:659–678. doi: 10.1038/s41585-020-00382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kawakami F., Sircar K., Rodriguez-Canales J., Fellman B.M., Urbauer D.L., Tamboli P., Tannir N.M., Jonasch E., Wistuba I.I., Wood C.G., et al. Programmed Cell Death Ligand 1 and Tumor-Infiltrating Lymphocyte Status in Patients with Renal Cell Carcinoma and Sarcomatoid Dedifferentiation. Cancer. 2017;123:4823–4831. doi: 10.1002/cncr.30937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gupta S., Cheville J.C., Jungbluth A.A., Zhang Y., Zhang L., Chen Y.-B., Tickoo S.K., Fine S.W., Gopalan A., Al-Ahmadie H.A., et al. JAK2/PD-L1/PD-L2 (9p24.1) Amplifications in Renal Cell Carcinomas with Sarcomatoid Transformation: Implications for Clinical Management. Mod. Pathol. 2019;32:1344–1358. doi: 10.1038/s41379-019-0269-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Choueiri T.K., Larkin J.M.G., Pal S.K., Motzer R.J., Venugopal B., Alekseev B.Y., Miyake H., Gravis G., Bilen M.A., Chudnovsky A., et al. Efficacy and Biomarker Analysis of Patients (Pts) with Advanced Renal Cell Carcinoma (ARCC) with Sarcomatoid Histology (SRCC): Subgroup Analysis from the Phase III JAVELIN Renal 101 Trial of First-Line Avelumab plus Axitinib (A + Ax) vs Sunitinib (S) Ann. Oncol. 2019;30:v361. doi: 10.1093/annonc/mdz249.009. [DOI] [Google Scholar]
- 158.McDermott D.F., Choueiri T.K., Motzer R.J., Aren O.R., George S., Powles T., Donskov F., Harrison M.R., Rodriguez Cid J.R.R., Ishii Y., et al. CheckMate 214 Post-Hoc Analyses of Nivolumab plus Ipilimumab or Sunitinib in IMDC Intermediate/Poor-Risk Patients with Previously Untreated Advanced Renal Cell Carcinoma with Sarcomatoid Features. J. Clin. Oncol. 2019;37:4513. doi: 10.1200/JCO.2019.37.15_suppl.4513. [DOI] [Google Scholar]
- 159.Rini B.I., Plimack E.R., Stus V., Gafanov R., Hawkins R., Nosov D., Pouliot F., Soulieres D., Melichar B., Vynnychenko I., et al. Pembrolizumab (Pembro) plus Axitinib (Axi) versus Sunitinib as First-Line Therapy for Metastatic Renal Cell Carcinoma (MRCC): Outcomes in the Combined IMDC Intermediate/Poor Risk and Sarcomatoid Subgroups of the Phase 3 KEYNOTE-426 Study. J. Clin. Oncol. 2019;37:4500. doi: 10.1200/JCO.2019.37.15_suppl.4500. [DOI] [Google Scholar]
- 160.Iacovelli R., Ciccarese C., Bria E., Bracarda S., Porta C., Procopio G., Tortora G. Patients with Sarcomatoid Renal Cell Carcinoma–Re-Defining the First-Line of Treatment: A Meta-Analysis of Randomised Clinical Trials with Immune Checkpoint Inhibitors. Eur. J. Cancer. 2020;136:195–203. doi: 10.1016/j.ejca.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 161.Bex A., Russo P., Tomita Y., Grünwald V., Ramirez L.-M., McHenry B.M., Motzer R.J. A Phase III, Randomized, Placebo-Controlled Trial of Nivolumab or Nivolumab plus Ipilimumab in Patients with Localized Renal Cell Carcinoma at High-Risk of Relapse after Radical or Partial Nephrectomy (CheckMate 914) J. Clin. Oncol. 2020;38:TPS5099. doi: 10.1200/JCO.2020.38.15_suppl.TPS5099. [DOI] [Google Scholar]
- 162.Oza B., Frangou E., Smith B., Bryant H., Kaplan R., Choodari-Oskooei B., Powles T., Stewart G.D., Albiges L., Bex A., et al. RAMPART: A Phase III Multi-Arm Multi-Stage Trial of Adjuvant Checkpoint Inhibitors in Patients with Resected Primary Renal Cell Carcinoma (RCC) at High or Intermediate Risk of Relapse. Contemp. Clin. Trials. 2021;108:106482. doi: 10.1016/j.cct.2021.106482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ornstein M.C., Zabell J., Wood L.S., Hobbs B., Devonshire S., Martin A., Allman K.D., Rao A., Gilligan T.D., Campbell S., et al. A Phase Ib Trial of Neoadjuvant/Adjuvant Durvalumab +/- Tremelimumab in Locally Advanced Renal Cell Carcinoma (RCC) J. Clin. Oncol. 2020;38:5021. doi: 10.1200/JCO.2020.38.15_suppl.5021. [DOI] [Google Scholar]
- 164.Atkins M.B., Jegede O., Haas N.B., McDermott D.F., Bilen M.A., Drake C.G., Sosman J.A., Alter R.S., Plimack E.R., Rini B.I., et al. Phase II Study of Nivolumab and Salvage Nivolumab + Ipilimumab in Treatment-Naïve Patients (Pts) with Advanced Renal Cell Carcinoma (RCC) (HCRN GU16-260) J. Clin. Oncol. 2020;38:5006. doi: 10.1200/JCO.2020.38.15_suppl.5006. [DOI] [Google Scholar]
- 165.Grimm M.-O., Esteban E., Barthélémy P., Schmidinger M., Busch J., Valderrama B.P., Schmitz M., Schumacher U., Baretton G.B., Duran I., et al. Efficacy of Nivolumab/Ipilimumab in Patients with Initial or Late Progression with Nivolumab: Updated Analysis of a Tailored Approach in Advanced Renal Cell Carcinoma (TITAN-RCC) J. Clin. Oncol. 2021;39:4576. doi: 10.1200/JCO.2021.39.15_suppl.4576. [DOI] [Google Scholar]
- 166.McKay R.R., McGregor B.A., Xie W., Braun D.A., Wei X., Kyriakopoulos C.E., Zakharia Y., Maughan B.L., Rose T.L., Stadler W.M., et al. Optimized Management of Nivolumab and Ipilimumab in Advanced Renal Cell Carcinoma: A Response-Based Phase II Study (OMNIVORE) J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020;38:4240–4248. doi: 10.1200/JCO.20.02295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gedye C., Pook D.W., Krieger L.E.M., Harris C.A., Goh J.C., Kichenadasse G., Gurney H., Underhill C., Parnis F., Joshua A.M., et al. UNISON: Nivolumab Then Ipilimumab + Nivolumab in Advanced Nonclear Cell Renal Cell Carcinoma (ANZUP 1602) J. Clin. Oncol. 2020;38:TPS768. doi: 10.1200/JCO.2020.38.6_suppl.TPS768. [DOI] [Google Scholar]
- 168.Choueiri T.K., Kluger H.M., George S., Tykodi S.S., Kuzel T.M., Perets R., Nair S., Procopio G., Carducci M.A., Castonguay V., et al. FRACTION-RCC: Innovative, High-Throughput Assessment of Nivolumab + Ipilimumab for Treatment-Refractory Advanced Renal Cell Carcinoma (ARCC) J. Clin. Oncol. 2020;38:5007. doi: 10.1200/JCO.2020.38.15_suppl.5007. [DOI] [Google Scholar]
- 169.Hong D.S., Schoffski P., Calvo A., Sarantopoulos J., Ochoa De Olza M., Carvajal R.D., Prawira A., Kyi C., Esaki T., Akerley W.L., et al. Phase I/II Study of LAG525 ± Spartalizumab (PDR001) in Patients (Pts) with Advanced Malignancies. J. Clin. Oncol. 2018;36:3012. doi: 10.1200/JCO.2018.36.15_suppl.3012. [DOI] [Google Scholar]
- 170.Abstract CT183: Phase (Ph) I/II Study of MBG453± Spartalizumab (PDR001) in Patients (Pts) with Advanced Malignancies|Cancer Research. [(accessed on 2 October 2021)]. Available online: https://cancerres.aacrjournals.org/content/79/13_Supplement/CT183.
- 171.Voss M.H., Azad A.A., Hansen A.R., Gray J.E., Welsh S.J., Achour I., Hu H., Lewis L., Walcott F.L., Oosting S.F. Results from a Randomised Phase I/II Trial Evaluating the Safety and Antitumour Activity of Anti-PD-1 (MEDI0680)/Anti-PD-L1 (Durvalumab) vs Anti-PD-1 (Nivolumab) Alone in Metastatic Clear Cell Renal Cell Carcinoma (CcRCC) Ann. Oncol. 2019;30:v516. doi: 10.1093/annonc/mdz253.094. [DOI] [Google Scholar]
- 172.Sasikumar P.G., Sudarshan N.S., Adurthi S., Ramachandra R.K., Samiulla D.S., Lakshminarasimhan A., Ramanathan A., Chandrasekhar T., Dhudashiya A.A., Talapati S.R., et al. PD-1 Derived CA-170 Is an Oral Immune Checkpoint Inhibitor That Exhibits Preclinical Anti-Tumor Efficacy. Commun. Biol. 2021;4:1–12. doi: 10.1038/s42003-021-02191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lara P., Parikh M., Robles D., Lara F., Meyers F.J., Pan C. Pilot Trial of Ibrutinib plus Nivolumab in Patients (Pts) with Metastatic Renal Cell Cancer (MRCC): Results from a Dose-Finding Cohort. J. Clin. Oncol. 2018;36:600. doi: 10.1200/JCO.2018.36.6_suppl.600. [DOI] [Google Scholar]
- 174.Karam J.A., Msaouel P., Matin S.F., Campbell M.T., Zurita A.J., Shah A.Y., Wistuba I.I., Haymaker C.L., Marmonti E., Duose D.Y., et al. A Phase II Study of Sitravatinib (Sitra) in Combination with Nivolumab (Nivo) in Patients (Pts) Undergoing Nephrectomy for Locally-Advanced Clear Cell Renal Cell Carcinoma (AccRCC) J. Clin. Oncol. 2021;39:312. doi: 10.1200/JCO.2021.39.6_suppl.312. [DOI] [Google Scholar]
- 175.Pal S.K., McGregor B., Suárez C., Tsao C.-K., Kelly W., Vaishampayan U., Pagliaro L., Maughan B.L., Loriot Y., Castellano D., et al. Cabozantinib in Combination With Atezolizumab for Advanced Renal Cell Carcinoma: Results From the COSMIC-021 Study. J. Clin. Oncol. 2021;39:3725–3736. doi: 10.1200/JCO.21.00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Apolo A.B., Nadal R., Girardi D.M., Niglio S.A., Ley L., Cordes L.M., Steinberg S.M., Sierra Ortiz O., Cadena J., Diaz C., et al. Phase I Study of Cabozantinib and Nivolumab Alone or With Ipilimumab for Advanced or Metastatic Urothelial Carcinoma and Other Genitourinary Tumors. J. Clin. Oncol. 2020;38:3672–3684. doi: 10.1200/JCO.20.01652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kessler E.R., Hu J., Srivastava G., Kemme D.J., Iruku P., Rana V., Schuster S.R., Amirault M., Callihan E., Flaig T.W., et al. Phase I/II Trial of Pembrolizumab and Cabozantinib in the Treatment of Metastatic Renal Cell Carcinoma (MRCC) J. Clin. Oncol. 2021;39:4544. doi: 10.1200/JCO.2021.39.15_suppl.4544. [DOI] [Google Scholar]
- 178.Choueiri T.K., Albiges L., Powles T., Scheffold C., Wang F., Motzer R.J. A Phase III Study (COSMIC-313) of Cabozantinib (C) in Combination with Nivolumab (N) and Ipilimumab (I) in Patients (Pts) with Previously Untreated Advanced Renal Cell Carcinoma (ARCC) of Intermediate or Poor Risk. J. Clin. Oncol. 2020;38:TPS767. doi: 10.1200/JCO.2020.38.6_suppl.TPS767. [DOI] [Google Scholar]
- 179.Zhang T., Ballman K.V., Choudhury A.D., Chen R.C., Watt C., Wen Y., Shergill A., Zemla T.J., Emamekhoo H., Vaishampayan U.N., et al. PDIGREE: An Adaptive Phase III Trial of PD-Inhibitor Nivolumab and Ipilimumab (IPI-NIVO) with VEGF TKI Cabozantinib (CABO) in Metastatic Untreated Renal Cell Cancer (Alliance A031704) J. Clin. Oncol. 2020;38:TPS5100. doi: 10.1200/JCO.2020.38.15_suppl.TPS5100. [DOI] [Google Scholar]
- 180.Pal S.K., Albiges L., Suarez Rodriguez C., Liu B., Doss J., Khurana S., Scheffold C., Voss M.H., Choueiri T.K. CONTACT-03: Randomized, Open-Label Phase III Study of Atezolizumab plus Cabozantinib versus Cabozantinib Monotherapy Following Progression on/after Immune Checkpoint Inhibitor (ICI) Treatment in Patients with Advanced/Metastatic Renal Cell Carcinoma. J. Clin. Oncol. 2021;39:TPS370. doi: 10.1200/JCO.2021.39.6_suppl.TPS370. [DOI] [Google Scholar]
- 181.Suarez Rodriguez C., Larkin J., Patel P.M., Valderrama B.P., Rodriguez-Vida A., Glen H., Thistlethwaite F., Ralph C., Srinivasan G., Mendez-Vidal M.J., et al. Clinical Activity of Durvalumab and Savolitinib in MET-Driven, Metastatic Papillary Renal Cancer. J. Clin. Oncol. 2021;39:4511. doi: 10.1200/JCO.2021.39.15_suppl.4511. [DOI] [PubMed] [Google Scholar]
- 182.Lee C.-H., Shah A.Y., Rasco D., Rao A., Taylor M.H., Di Simone C., Hsieh J.J., Pinto A., Shaffer D.R., Girones Sarrio R., et al. Lenvatinib plus Pembrolizumab in Patients with Either Treatment-Naive or Previously Treated Metastatic Renal Cell Carcinoma (Study 111/KEYNOTE-146): A Phase 1b/2 Study. Lancet Oncol. 2021;22:946–958. doi: 10.1016/S1470-2045(21)00241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Choi W.S.W., Boland J., Lin J. Hypoxia-Inducible Factor-2α as a Novel Target in Renal Cell Carcinoma. J. Kidney Cancer VHL. 2021;8:1–7. doi: 10.15586/jkcvhl.v8i2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rini B.I., Appleman L.J., Figlin R.A., Plimack E.R., Merchan J.R., Wang K., Thamake S., Zojwalla N.J., Choueiri T.K., McDermott D.F. Results from a Phase I Expansion Cohort of the First-in-Class Oral HIF-2α Inhibitor PT2385 in Combination with Nivolumab in Patients with Previously Treated Advanced RCC. J. Clin. Oncol. 2019;37:558. doi: 10.1200/JCO.2019.37.7_suppl.558. [DOI] [Google Scholar]
- 185.Wong T.W., Shrimali R., Contreras C., Cheng T., Czerwinski R.M., Dixon D.D., Du X., Fett C., Goree J., Grina J.A., et al. Abstract B140: PT2977, a Novel HIF-2a Antagonist, Has Potent Antitumor Activity and Remodels the Immunosuppressive Tumor Microenvironment in Clear Cell Renal Cell Cancer. Mol. Cancer Ther. 2018;17:B140. doi: 10.1158/1535-7163.MCT-17-0471. [DOI] [Google Scholar]
- 186.Lamers C.H.J., Klaver Y., Gratama J.W., Sleijfer S., Debets R. Treatment of Metastatic Renal Cell Carcinoma (MRCC) with CAIX CAR-Engineered T-Cells-a Completed Study Overview. Biochem. Soc. Trans. 2016;44:951–959. doi: 10.1042/BST20160037. [DOI] [PubMed] [Google Scholar]
- 187.Rini B.I., Stenzl A., Zdrojowy R., Kogan M., Shkolnik M., Oudard S., Weikert S., Bracarda S., Crabb S.J., Bedke J., et al. IMA901, a Multipeptide Cancer Vaccine, plus Sunitinib versus Sunitinib Alone, as First-Line Therapy for Advanced or Metastatic Renal Cell Carcinoma (IMPRINT): A Multicentre, Open-Label, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2016;17:1599–1611. doi: 10.1016/S1470-2045(16)30408-9. [DOI] [PubMed] [Google Scholar]
- 188.Amin A., Dudek A.Z., Logan T.F., Lance R.S., Holzbeierlein J.M., Knox J.J., Master V.A., Pal S.K., Miller W.H., Karsh L.I., et al. Survival with AGS-003, an Autologous Dendritic Cell-Based Immunotherapy, in Combination with Sunitinib in Unfavorable Risk Patients with Advanced Renal Cell Carcinoma (RCC): Phase 2 Study Results. J. Immunother. Cancer. 2015;3:14. doi: 10.1186/s40425-015-0055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Figlin R.A., Tannir N.M., Uzzo R.G., Tykodi S.S., Chen D.Y.T., Master V., Kapoor A., Vaena D., Lowrance W., Bratslavsky G., et al. Results of the ADAPT Phase 3 Study of Rocapuldencel-T in Combination with Sunitinib as First-Line Therapy in Patients with Metastatic Renal Cell Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020;26:2327–2336. doi: 10.1158/1078-0432.CCR-19-2427. [DOI] [PubMed] [Google Scholar]
- 190.Laurell A., Lönnemark M., Brekkan E., Magnusson A., Tolf A., Wallgren A.C., Andersson B., Adamson L., Kiessling R., Karlsson-Parra A. Intratumorally Injected Pro-Inflammatory Allogeneic Dendritic Cells as Immune Enhancers: A First-in-Human Study in Unfavourable Risk Patients with Metastatic Renal Cell Carcinoma. J. Immunother. Cancer. 2017;5:52. doi: 10.1186/s40425-017-0255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lindskog M., Laurell A., Kjellman A., Melichar B., Niezabitowski J., Maroto P., Zieliński H., Villacampa F., Bigot P., Bajory Z., et al. A Randomized Phase II Study with Ilixadencel, a Cell-Based Immune Primer, plus Sunitinib versus Sunitinib Alone in Synchronous Metastatic Renal Cell Carcinoma. J. Clin. Oncol. 2020;38:11. doi: 10.1200/JCO.2020.38.5_suppl.11. [DOI] [Google Scholar]
- 192.Cohen R.B., Twardowski P., Johnson M.L., Gillison M.L., Stein M.N., Vaishampayan U.N., McNeil L., Shainheit M., DeOliveira D., Jain M., et al. GEN-009, a Neoantigen Vaccine Containing ATLAS Selected Neoantigens, to Generate Broad Sustained Immunity against Immunogenic Tumor Mutations and Avoid Inhibitory Peptides. J. Clin. Oncol. 2020;38:3107. doi: 10.1200/JCO.2020.38.15_suppl.3107. [DOI] [Google Scholar]
- 193.Krauss J., Krackhardt A., Jager E., Williams A., Wold H., Gerner L., Sekelja M., Schjetne K., Fredriksen A.B., Axelsen M. Abstract CT217: An Open-Label, Phase I/IIa Study of VB10.NEO (DIRECT-01) in Combination with Checkpoint Blockade in Patients with Locally Advanced or Metastatic Solid Tumors Including Melanoma, NSCLC, Renal Cell Carcinoma, Urothelial Cancer or SSCHN. Cancer Res. 2019;79:CT217. doi: 10.1158/1538-7445.AM2019-CT217. [DOI] [Google Scholar]
- 194.Rha S.Y., Merchan J., Oh S.Y., Kim C., Bae W.K., Lee H.W., Dillon M., Deering R., Kroog G., Ha K.S. Abstract CT121: A Phase Ib Study of Recombinant Vaccinia Virus in Combination with Immune Checkpoint Inhibition (ICI) in Advanced Renal Cell Carcinoma (RCC) Cancer Res. 2020;80:CT121. doi: 10.1158/1538-7445.AM2020-CT121. [DOI] [Google Scholar]
- 195.Slingluff C.L., Dasilva D., Schwarzenberger P., Ricciardi T., Macri M.J., Ryan A., Venhaus R.R., Bhardwaj N. Phase 1/2 Study of in Situ Vaccination with Tremelimumab + Intravenous (IV) Durvalumab + Poly-ICLC in Patients with Select Relapsed, Advanced Cancers with Measurable, Biopsy-Accessible Tumors. J. Clin. Oncol. 2017;35:TPS3106. doi: 10.1200/JCO.2017.35.15_suppl.TPS3106. [DOI] [Google Scholar]
- 196.Chatzkel J.A., Swank J., Ludlow S., Lombardi K., Croft C., Artigas Y., Rodriguez Y., Terraciano T., Hart S., Rembisz J., et al. Overall Responses with Coordinated Pembrolizumab and High Dose IL-2 (5-in-a-Row Schedule) for Therapy of Metastatic Clear Cell Renal Cancer: A Single Center, Single Arm Trial. J. Clin. Oncol. 2019;37:657. doi: 10.1200/JCO.2019.37.7_suppl.657. [DOI] [Google Scholar]
- 197.Yentz S., Alva A. Phase Ib/II Trial of Interleukin-2 (IL-2) and Nivolumab in Metastatic Clear Cell Renal Cell Cancer (RCC) Ann. Oncol. 2017;28:v328–v329. doi: 10.1093/annonc/mdx371.080. [DOI] [Google Scholar]
- 198.Diab A., Tannir N.M., Bentebibel S.-E., Hwu P., Papadimitrakopoulou V., Haymaker C., Kluger H.M., Gettinger S.N., Sznol M., Tykodi S.S., et al. Bempegaldesleukin (NKTR-214) plus Nivolumab in Patients with Advanced Solid Tumors: Phase I Dose-Escalation Study of Safety, Efficacy, and Immune Activation (PIVOT-02) Cancer Discov. 2020;10:1158–1173. doi: 10.1158/2159-8290.CD-19-1510. [DOI] [PubMed] [Google Scholar]
- 199.Tannir N.M., Agarwal N., Pal S.K., Cho D.C., Formiga M., Guo J., George D.J., Tagliaferri M.A., Singel S.M., O’Keeffe B.A., et al. PIVOT-09: A Phase III Randomized Open-Label Study of Bempegaldesleukin (NKTR-214) plus Nivolumab versus Sunitinib or Cabozantinib (Investigator’s Choice) in Patients with Previously Untreated Advanced Renal Cell Carcinoma (RCC) J. Clin. Oncol. 2020;38:TPS763. doi: 10.1200/JCO.2020.38.6_suppl.TPS763. [DOI] [Google Scholar]
- 200.Voss M.H., Bhatt R.S., Plimack E.R., Rini B.I., Alter R.S., Beck J.T., Wilson D., Zhang X., Mutyaba M., Glasser C., et al. The DART Study: Results from the Dose-Escalation and Expansion Cohorts Evaluating the Combination of Dalantercept plus Axitinib in Advanced Renal Cell Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017;23:3557–3565. doi: 10.1158/1078-0432.CCR-16-2395. [DOI] [PubMed] [Google Scholar]
- 201.Voss M.H., Bhatt R.S., Vogelzang N.J., Fishman M., Alter R.S., Rini B.I., Beck J.T., Joshi M., Hauke R., Atkins M.B., et al. A Phase 2, Randomized Trial Evaluating the Combination of Dalantercept plus Axitinib in Patients with Advanced Clear Cell Renal Cell Carcinoma. Cancer. 2019;125:2400–2408. doi: 10.1002/cncr.32061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Choueiri T.K., Michaelson M.D., Posadas E.M., Sonpavde G.P., McDermott D.F., Nixon A.B., Liu Y., Yuan Z., Seon B.K., Walsh M., et al. An Open Label Phase Ib Dose Escalation Study of TRC105 (Anti-Endoglin Antibody) with Axitinib in Patients with Metastatic Renal Cell Carcinoma. Oncologist. 2019;24:202–210. doi: 10.1634/theoncologist.2018-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Pili R., Adra N., Damayanti N., Logan T.F., Narayan V., Monk P., Dropcho S., Sego L.M., Liu H., Althouse S.K., et al. Immunomodulation by HDAC Inhibition: Results from a Phase I Study with Entinostat in Combination with Atezolizumab and Bevacizumab in Metastatic Renal Cell Carcinoma Patients. J. Clin. Oncol. 2020;38:5064. doi: 10.1200/JCO.2020.38.15_suppl.5064. [DOI] [Google Scholar]
- 204.Pili R., Quinn D.I., Hammers H.J., Monk P., George S., Dorff T.B., Olencki T., Shen L., Orillion A., Lamonica D., et al. Immunomodulation by Entinostat in Renal Cell Carcinoma Patients Receiving High-Dose Interleukin 2: A Multicenter, Single-Arm, Phase I/II Trial (NCI-CTEP#7870) Clin. Cancer Res. 2017;23:7199–7208. doi: 10.1158/1078-0432.CCR-17-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gabrail N.Y., Bessudo A., Hamilton E.P., Sachdev J.C., Patel M.R., Rodon Ahnert J., Evilevitch L., Duncan M., Guo W., Lu S., et al. IOLite: Multipart, Phase 1b, Dose-Finding Study of the PD-1 Inhibitor Dostarlimab in Combination with the PARP Inhibitor Niraparib ± Bevacizumab (Bev), or with Platinum-Based Chemotherapy ± Bev for Advanced Cancer. J. Clin. Oncol. 2019;37:2560. doi: 10.1200/JCO.2019.37.15_suppl.2560. [DOI] [Google Scholar]
- 206.Fong L., Forde P.M., Powderly J.D., Goldman J.W., Nemunaitis J.J., Luke J.J., Hellmann M.D., Kummar S., Doebele R.C., Mahadevan D., et al. Safety and Clinical Activity of Adenosine A2a Receptor (A2aR) Antagonist, CPI-444, in Anti-PD1/PDL1 Treatment-Refractory Renal Cell (RCC) and Non-Small Cell Lung Cancer (NSCLC) Patients. J. Clin. Oncol. 2017;35:3004. doi: 10.1200/JCO.2017.35.15_suppl.3004. [DOI] [Google Scholar]
- 207.Hotson A., Willingham S., Fong L., Powderly J., II, Luke J., Sznol M., George S., Choueiri T., Giannakis M., Rini B., et al. P54: Adenosine Signature Genes Associate with Tumor Regression in Renal Cell Carcinoma (RCC) Patients Treated with the Adenosine A2A Receptor (A2AR) Antagonist, CPI-444. [(accessed on 1 November 2021)]. Available online: https://www.corvuspharma.com/file.cfm/23/docs/SITC2018_%20Hotson.pdf.
- 208.Tolcher A.W., Sznol M., Hu-Lieskovan S., Papadopoulos K.P., Patnaik A., Rasco D.W., Gravio D.D., Huang B., Gambhire D., Chen Y., et al. Phase Ib Study of Utomilumab (PF-05082566), a 4-1BB/CD137 Agonist, in Combination with Pembrolizumab (MK-3475) in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2017;23:5349–5357. doi: 10.1158/1078-0432.CCR-17-1243. [DOI] [PubMed] [Google Scholar]
- 209.Hansen A., Abdul-Karim R., Rizvi N., Rischen D., Hilton J., Li Z., Ott P., Karpinich-Fedoriw N., Yadavilli S., Wang X., et al. Abstract CT166: A Phase I/II, Open-Label, Two Part Study of GSK3359609 in Combination with Tremelimumab in Participants with Selected, Advanced Solid Tumors. Cancer Res. 2019;79:CT166. doi: 10.1158/1538-7445.AM2019-CT166. [DOI] [Google Scholar]
- 210.Zakharia Y., Singer E.A., Ross R., Joshi M., Abern M., Garje R., Park J.J., Kryczek I., Zou W., Alva A.S. Phase Ib/II Study of Durvalumab and Guadecitabine in Advanced Kidney Cancer Big Ten Cancer Research Consortium BTCRC GU16-043. J. Clin. Oncol. 2021;39:328. doi: 10.1200/JCO.2021.39.6_suppl.328. [DOI] [Google Scholar]
- 211.Masini C., Iotti C., De Giorgi U., Bellia R.S., Buti S., Salaroli F., Zampiva I., Mazzarotto R., Mucciarini C., Baldessari C., et al. Nivolumab (NIVO) in Combination with Stereotactic Body Radiotherapy (SBRT) in Pretreated Patients (Pts) with Metastatic Renal Cell Carcinoma (MRCC): First Results of Phase II NIVES Study. J. Clin. Oncol. 2020;38:613. doi: 10.1200/JCO.2020.38.6_suppl.613. [DOI] [Google Scholar]
- 212.Vuong L., Kotecha R.R., Voss M.H., Hakimi A.A. Tumor Microenvironment Dynamics in Clear-Cell Renal Cell Carcinoma. Cancer Discov. 2019;9:1349–1357. doi: 10.1158/2159-8290.CD-19-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Deleuze A., Saout J., Dugay F., Peyronnet B., Mathieu R., Verhoest G., Bensalah K., Crouzet L., Laguerre B., Belaud-Rotureau M.-A., et al. Immunotherapy in Renal Cell Carcinoma: The Future Is Now. Int. J. Mol. Sci. 2020;21:2532. doi: 10.3390/ijms21072532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Shirotake S., Kaneko G., Nagata K., Oyama M., Nishimoto K. Histological Complete Response with Nivolumab for Renal Cell Carcinoma with Multiple Metastases: A Case Report. Mol. Clin. Oncol. 2019;10:244–248. doi: 10.3892/mco.2018.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wallin J.J., Bendell J.C., Funke R., Sznol M., Korski K., Jones S., Hernandez G., Mier J., He X., Hodi F.S., et al. Atezolizumab in Combination with Bevacizumab Enhances Antigen-Specific T-Cell Migration in Metastatic Renal Cell Carcinoma. Nat. Commun. 2016;7:12624. doi: 10.1038/ncomms12624. [DOI] [PMC free article] [PubMed] [Google Scholar]