Abstract
BAP31 is a 28-kDa integral membrane protein of the endoplasmic reticulum whose cytosolic domain contains two identical caspase recognition sites (AAVD.G) that are preferentially cleaved by initiator caspases, including caspase 8. Cleavage of BAP31 during apoptosis generates a p20 fragment that remains integrated in the membrane and, when expressed ectopically, is a potent inducer of cell death. To examine the consequences of maintaining the structural integrity of BAP31 during apoptosis, the caspase recognition aspartate residues were mutated to alanine residues, and Fas-mediated activation of caspase 8 and cell death were examined in human KB epithelial cells stably expressing the caspase-resistant mutant crBAP31. crBAP31 only modestly slowed the time course for activation of caspases, as assayed by the processing of procaspases 8 and 3 and the measurement of total DEVDase activity. As a result, cleavage of the caspase targets poly(ADP-ribosyl) polymerase and endogenous BAP31, as well as the redistribution of phosphatidylserine and fragmentation of DNA, was observed. In contrast, cytoplasmic membrane blebbing and fragmentation and apoptotic redistribution of actin were strongly inhibited, cell morphology was retained near normal, and the irreversible loss of cell growth potential following removal of the Fas stimulus was delayed. Of note, crBAP31-expressing cells also resisted Fas-mediated release of cytochrome c from mitochondria, and the mitochondrial electrochemical potential was only partly reduced. These results argue that BAP31 cleavage is important for manifesting cytoplasmic apoptotic events associated with membrane fragmentation and reveal an unexpected cross talk between mitochondria and the endoplasmic reticulum during Fas-mediated apoptosis in vivo.
Programmed cell death is characterized by a series of morphological and structural changes culminating in the coordinated packaging of cellular contents into apoptotic bodies, which are ultimately eliminated via phagocytosis by neighboring cells. Early events in this process typically include cell rounding, loss of phospholipid asymmetry in the cell membrane, extensive cytoplasmic membrane blebbing and fragmentation, nuclear pyknosis, and internucleosomal DNA cleavage (26). Although much remains to be learned about the mechanisms underlying these events, programmed cell death is achieved in most cell death pathways as a consequence of the proteolytic cleavage of a diverse array of structural and regulatory proteins by the executors of apoptosis, the caspase family of cysteine proteases (33, 46, 48). Several of the caspase targets are known to have critical roles in at least some of the apoptotic processes. These targets include the DFF40/CAD inhibitor, DFF45/ICAD, which plays a role in the fragmentation of DNA (23, 36), and the actin-associated capping protein gelsolin, which plays a role in cytoplasmic membrane blebbing (17). In addition, the activation of several kinases by caspase cleavage, including PAK2 (20, 34) and the Ste20-related kinases MST1 (14, 19) and SLK (35), contributes to the loss of focal adhesions and the retraction and disassembly of actin stress fibers, events that are associated with elaborate changes to the actomyosin network and membrane remodeling (26).
In contrast to many death-stimulating pathways, the proximal molecular events following activation of Fas (also called CD95) with either Fas ligand or agonistic anti-Fas antibody are well understood. Receptor stimulation results in the assembly of a death-inducing signaling complex that includes the adapter protein FADD and procaspase 8 (4, 5, 7, 28, 40). The resulting activation of this initiator caspase (25) ultimately leads to the processing of effector procaspases, including caspase 3, and apoptosis. A cytosolic target of caspase 8, proapoptotic BID, is cleaved, and the truncated product (tBID) translocates to mitochondria, where it stimulates the release of intermembrane proteins, including cytochrome c (15, 21, 24). BID appears to be a critical effector of this pathway, at least in certain contexts, because death receptor-induced redistribution of cytochrome c was not observed in Bid−/− mouse thymocytes and hepatocytes (47). Released cytochrome c in turn contributes to the activation of initiator caspase 9 (22), which in many death pathways is important for subsequent processing of downstream effector procaspases (22, 39). In contrast, Fas stimulation of certain cell types activates high levels of caspase 8 that are sufficient to process effector procaspases directly, whereas other cell types, at least in culture, activate low levels of procaspase 8 and likely depend on mitochondrial events for amplification of the caspase cascade (37, 38).
Here we show that human epithelial cells expressing a caspase-resistant mutant of BAP31 (crBAP31), a preferred substrate for initiator caspases 8 and 1 (30), are resistant to a number of cytoplasmic changes that typically occur during Fas-mediated apoptosis. BAP31 is a 28-kDa integral membrane protein that is ubiquitously expressed (1, 27; unpublished data) and highly enriched in the endoplasmic reticulum (ER) (3, 30), where it forms a homo-oligomer (31). The protein contains three predicted transmembrane segments within its amino terminus that confer a topology in the ER membrane in which the short hydrophilic amino terminus and an approximately 37-amino-acid loop connecting TM2 and TM3 face the ER lumen and a 14-kDa domain, terminating in a canonical KKXX ER retention signal, faces the cytosol (30). The cytosolic tail is predicted to contain a weak death effector and overlapping coiled-coil (DECC) domain flanked on either side by identical caspase recognition sites. These sites are cleaved in response to diverse death stimuli in vivo (13, 31) and are preferably cleaved by caspases 8 and 1 and only weakly cleaved by effector caspase 3 in vitro (30). The resulting membrane-integrated BAP31 fragment, called p20, is a potent inducer of apoptosis when expressed ectopically, suggesting that BAP31 cleavage may contribute in some manner to the death process. Moreover, the influence of BAP31 may also extend to a role in regulating caspases, which would be consistent with the observation that BAP31 can associate with procaspase 8, BCL-2, and CED-4 in cotransfected cells (30, 31). To investigate the contribution of BAP31 cleavage to apoptosis, therefore, we have created a cell line that expresses a caspase-resistant mutant form of the protein and have examined its influence on the cell death pathway initiated by independent stimulation of caspase 8 activity by the Fas signaling complex.
MATERIALS AND METHODS
Plasmids and transfectants.
cDNA encoding human BAP31 with the Flag peptide epitope sequence inserted between the codons for amino acids 242 and 243 was incorporated into the pcDNA3.1 expression vector, as previously documented (30). Site-directed mutagenesis was performed to convert the codons for aspartate residues at positions 164 and 238 to codons for alanine residues, and the authenticity of the crBAP31-Flag mutant expression vector was confirmed by DNA sequence analysis. pcDNA3.1 vectors expressing CrmA (gift from V. Dixit), BAP31-Flag, and crBAP31-Flag were stably expressed in human KB epithelial cells, as previously described (30).
Antibodies and immunoblots.
The following antibodies were employed: mouse anti-Flag (Sigma), chicken anti-human BAP31 (30), rabbit antibodies against the catalytic subunits of human caspase 8 (gift from D. Nicholson) and caspase 3 (gift from R. Sékaly), mouse anti-poly (ADP-ribosyl) polymerase (anti-PARP) (Biomol), mouse monoclonal antibodies against cytochrome c (2G8.B6 and 7H8.2C12) (gift from R. Jemmerson), and rabbit anti-γ-actin (gift from P. Braun). Cell extracts containing equivalent amounts of protein were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the proteins were transferred to nitrocellulose, incubated with a primary antibody, and visualized with a secondary antibody coupled to enhanced chemiluminescence.
Immunofluorescence.
Cells were fixed in 4% paraformaldehyde and incubated either with mouse monoclonal antibody 2G8.B6 (anti-cytochrome c) followed by goat anti-mouse immunoglobulin G (IgG) conjugated to Texas red or with anti-γ-actin followed by goat anti-rabbit IgG coupled to Alexa 488 (Molecular Probes). Cells were visualized by fluorescence confocal microscopy.
Electron microscopy.
Cells were treated with anti-Fas antibody for various times. After two washes in phosphate-buffered saline (PBS), cell pellets were fixed in 2.5% glutaraldehyde–0.1 M sodium cacodylate for 2 h, washed, treated for 1 h with 0.1% osmium tetroxide, and finally dehydrated in acetone. The pellets were infiltrated with Epon-acetone and embedded in Epon. Thin sections (approximately 100 nm thick) stained with 4% uranyl acetate–lead citrate and thick sections (approximately 0.5 μm thick) stained with toluidine blue were examined by electron and conventional light microscopy, respectively.
Apoptosis assays.
Human KB epithelial cells were maintained in α-minimal essential medium supplemented with 10% fetal bovine serum and treated at approximately 80% confluency with mouse activating anti-Fas antibody (Upstate Biotechnology) and 10 μg of cycloheximide (CHX) per ml. At the times indicated below, cells were collected and analyzed. Cell viability and the structural integrity of the plasma membrane were measured by the ability of cells to exclude trypan blue, as determined microscopically. For assessing phosphatidylserine redistribution and mitochondrial transmembrane potential, cells were incubated for 15 min at 37°C in PBS containing 2% fetal bovine serum and a 40 μM concentration of the mitochondrial-potential-sensitive dye DiOC6 (Molecular Probes) or 0.1 μM fluorescein-conjugated human annexin V (R & D). Following two washes in the same medium, fluorescence was measured by flow cytometry. Caspase activity in whole-cell extracts was obtained by treating the cells with a solution containing 50 mM HEPES (pH 7.4), 1% Triton X-100, 5 mM EDTA, and 2 mM dithiothreitol and incubating them with 50 μM acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin (AcDEVD-amc) for 30 min at 37°C. Fluorescence in the linear range of DEVDase activity was measured with a plate reader (Tecan).
Cytochrome c.
Cells (4 × 106) were washed in PBS and suspended in 0.1 ml of 200 mM mannitol–70 mM sucrose–1 mM EDTA–10 mM HEPES (pH 7.5). After one cycle of freeze-thaw, the cells were homogenized with 35 strokes in a motorized Teflon-glass homogenizer operating at 2,000 rpm and then centrifuged at 800 × g for 10 min to remove nuclei and cell debris. The supernatant was centrifuged at 100,000 × g for 10 min, and the membrane pellet was resuspended in homogenization buffer to the same volume as that of the 100,000 × g supernatant. Equivalent volumes of the pellets and supernatants were subjected to SDS-PAGE and immunoblotting with mouse monoclonal antibody 7H8.2C12 (anti-cytochrome c).
RESULTS
crBAP31.
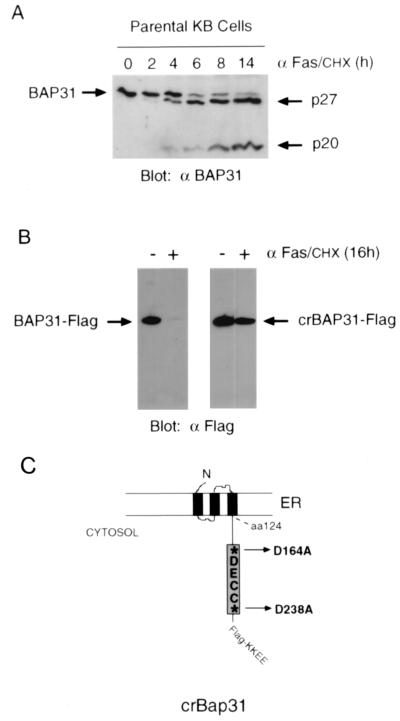
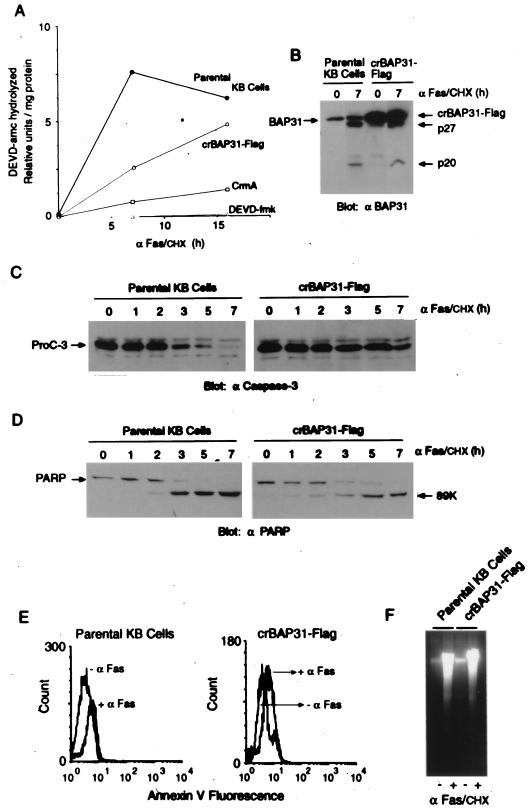
The schematic in Fig. 1 shows the predicted topology of BAP31 in the ER membrane and the location of the caspase recognition sites (AAVD.G) flanking the DECC domain in the cytosolic tail (30). Treatment of human KB epithelial cells with agonistic mouse antibody against Fas (0.5 μg/ml), in the presence of 10 μg of CHX per ml to enhance sensitivity to Fas activation (38), resulted in cleavage of the 246-amino-acid full-length BAP31, generating two products of approximately 27 and 20 kDa (Fig. 1A). These corresponded to cleavages at aspartates 238 and 164, respectively (30). crBAP31 was produced by mutating these residues to alanine residues, and both the construct encoding crBAP31 and that encoding wild-type (wt) BAP31 were further manipulated to include a Flag epitope inserted immediately upstream of the ER retention signal, KKEE, located at the extreme carboxy terminus of the protein. KB cell lines stably expressing wt BAP31 or crBAP31 were then examined by immunoblotting with anti-Flag antibody. crBAP31-Flag, but not BAP31-Flag, was found to remain structurally intact in the face of sustained stimulation with anti-Fas (Fig. 1B). In both cases, the proteins were expressed at approximately three times the level of endogenous BAP31, as assessed using a BAP31 polyclonal antibody (data not shown).
FIG. 1.
Human KB epithelial cells expressing crBAP31. (A) Control (parental) KB cells were stimulated with 0.5 μg of anti-Fas (α Fas) antibody per ml in the presence of 10 μg of CHX per ml for the indicated times, and whole-cell lysates were subjected to SDS-PAGE, immunoblotted with chicken anti-BAP31 (αBAP31) antibody, and visualized by enhanced chemiluminescence (30). The positions of full-length BAP31 and the BAP31 caspase cleavage products, p27 and p20, are indicated. (B) KB cells stably expressing wt BAP31-Flag or crBAP31-Flag were analyzed under the same conditions, but with mouse anti-Flag antibody. (C) Schematic of crBAP31-Flag in the ER (30), in which the caspase recognition aspartate residues (asterisks) at positions 164 and 238 have been mutated to alanine residues. The Flag epitope tag was inserted immediately upstream of the COOH-terminal tetrapeptide ER retrieval signal, KKEE. aa, amino acid.
crBAP31 inhibits Fas-mediated apoptotic membrane blebbing and fragmentation.
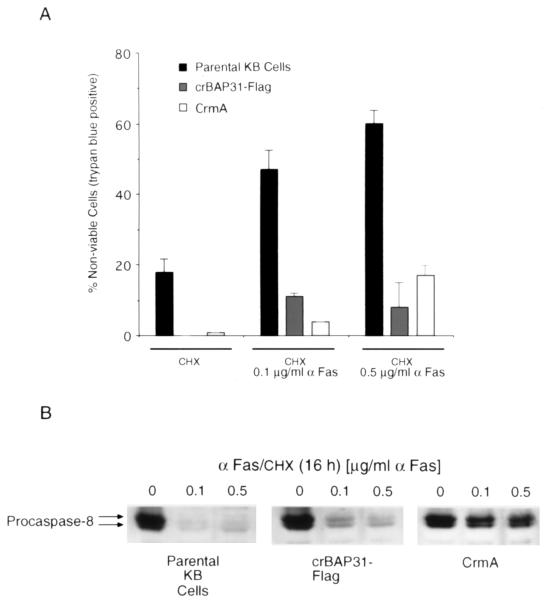
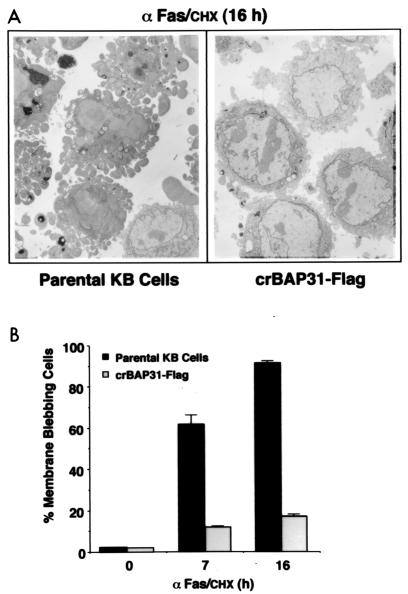
Control (parental) KB epithelial cells and KB cells stably expressing crBAP31-Flag or the cowpox serpin, CrmA, were stimulated for 16 h with anti-Fas and examined by staining with trypan blue to assess the integrity of the plasma membrane (Fig. 2A) and by immunoblotting with an antibody against the catalytic subunit of caspase 8 to assess procaspase 8 processing (Fig. 2B). crBAP31 had little influence on Fas-mediated processing of procaspase 8, as judged by the loss of the 54- to 55-kDa forms of procaspase 8 (25). As expected, CrmA, an inhibitor of caspase 8 that prevents activation of procaspase 8 in vivo (10, 28), significantly retarded procaspase 8 processing (Fig. 2B). Despite the fact that crBAP31 had little or no influence on procaspase 8 processing in response to Fas stimulation, it was as effective as CrmA at blocking the loss of cell membrane integrity, as visualized by staining of cells with trypan blue (Fig. 2A). This finding was investigated further and quantified by examining the appearance of the cells with electron microscopy (Fig. 3). After stimulation by Fas antibody, control cells exhibited extensive membrane blebbing and the formation of apoptotic bodies; crBAP31 cells, on the other hand, remained intact (Fig. 3A). Numerical scoring of these cells in thick sections stained with toluidine blue revealed that more than 60% of control cells had undergone extreme membrane remodeling by 7 h after stimulation by Fas, and this figure increased to more than 90% by 16 h poststimulation (Fig. 3B). Membrane remodeling was inhibited by crBAP31 (Fig. 3B), to an extent similar to that observed for CrmA-expressing cells (data not shown). Note that no difference, in this or other assays, was observed between control cells and cells expressing wt BAP31-Flag, indicating that the observed effects of crBAP31-Flag, which was expressed at a level similar to that of wt BAP31-Flag, were not due to the fact that these cells carried an approximately threefold excess of the BAP31 protein.
FIG. 2.
crBAP31 permits Fas-mediated activation of caspase 8 but inhibits the loss of plasma membrane integrity. (A) Control (parental) KB cells or KB cells stably expressing crBAP31-Flag or CrmA were stimulated with anti-Fas–CHX (αFas/CHX) or CHX alone for 16 h, and the percentage of the cells that were stained with trypan blue was determined. (B) Total cell lysates were subjected to SDS-PAGE and immunoblotted with a rabbit antibody raised against the p18 catalytic subunit of human procaspase 8. The positions of procaspase 8 a and b (25) are indicated with the top and bottom arrows, respectively.
FIG. 3.
crBAP31 inhibits Fas-mediated apoptotic membrane blebbing and fragmentation. (A) Transmission electron microscopy of Fas-stimulated control (parental) KB epithelial cells and KB cells stably expressing crBAP31-Flag. (B) The number of apoptotic membrane-blebbing cells was scored in thick sections and expressed as a percentage of total cells. The averages of three independent determinations, with standard deviations, are presented.
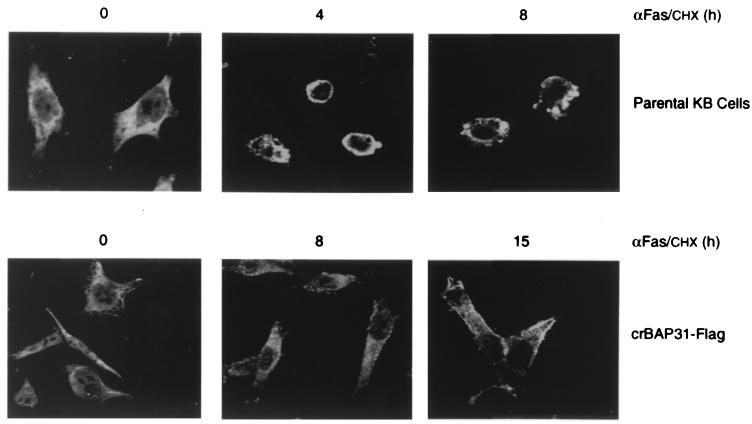
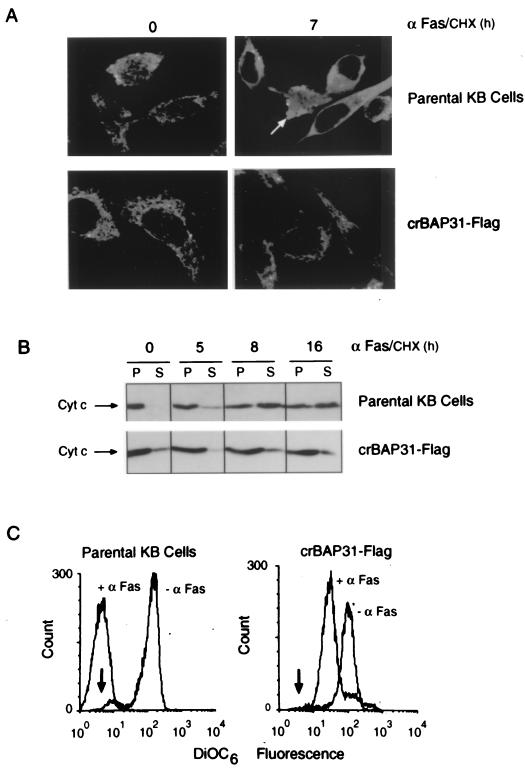
The early stages of apoptotic membrane remodeling have been associated with actin redistribution to the cell periphery during cell rounding (reviewed in reference 26). A similar redistribution of γ-actin was observed 4 h after Fas stimulation of control KB epithelial cells, whereas crBAP31 cells maintained a normal distribution of γ-actin and remained flat and adherent even 15 h poststimulation (Fig. 4). Collectively, these findings reveal that crBAP31 has a strong inhibitory influence on both the cell shape and cytoskeletal changes and the remodeling of membranes into blebs and vesicular bodies that are characteristic of apoptosis.
FIG. 4.
crBAP31 inhibits Fas-mediated redistribution of γ-actin. Control (parental) KB epithelial cells and KB cells stably expressing crBAP31-Flag were stimulated with anti-Fas–CHX (αFas/CHX) for the indicated times and examined by immunofluorescence confocal microscopy using a rabbit anti-γ-actin antibody and goat anti-rabbit IgG coupled to Alexa 488. Representative images are shown.
Activation of caspases in cells expressing crBAP31.
To assess the presence of caspase activity in extracts from control cells and from cells expressing crBAP31 or CrmA, equivalent amounts of extract protein were incubated with the fluorogenic peptide DEVD-amc, which is a general substrate for effector caspases 3 and 7 (10). The presence of crBAP31 appeared to delay the induction of DEVDase activity in response to Fas stimulation, but after 15 h the level was similar to that recorded for control cells (Fig. 5A). In contrast, CrmA significantly inhibited the appearance of DEVDase activity but did not reduce the activity to baseline, which was established in control cell extracts using the noncleavable peptide inhibitor DEVD-fluoromethylketone (DEVD-fmk) (Fig. 5A). This may indicate that the expression level of CrmA in these cells was insufficient to completely abolish all activation of caspase 8 in response to Fas stimulation or that a small amount of DEVDase activity arose in these cells independently of caspase 8 initiation. The retardation of the appearance of DEVDase activity in the presence of crBAP31 was reflected in a slower time course for Fas-stimulated processing of procaspase 3 in crBAP31-expressing cells than in control cells (Fig. 5C), but this was insufficient to significantly influence the cleavage of the caspase 3 and 7 substrate PARP (Fig. 5D). Of note, caspase cleavage of endogenous BAP31 in the crBAP31 cells was also observed (Fig. 5B).
FIG. 5.
Fas-mediated activation of caspases in crBAP31-expressing cells. (A) Total cell lysates were prepared from control (parental) KB epithelial cells or from KB cells stably expressing crBAP31-Flag or CrmA. Equivalent, rate-limiting amounts of protein were assayed for cleavage of DEVD-amc, during which the resulting fluorescence was detected and quantified in the linear time course range with an automated fluorescence plate reader detecting a wavelength of 460 nm. Control cell lysate was also assayed in the presence of a 1 μM concentration of the inhibitor DEVD-fmk. The average of two independent determinations is presented. (B) As described in the legend for Fig. 1, control (parental) and crBAP31-Flag-expressing KB cells were subjected to SDS-PAGE and immunoblotting with chicken anti-BAP31 (αBAP31) (30) under the conditions indicated. (C) The same experiment was performed as for panel B, except that immunoblots were developed using rabbit anti-caspase 3 (6). ProC-3, procaspase 3. (D) Immunoblots were developed using a mouse monoclonal antibody against PARP (αPARP). 89K, 89-kDa caspase cleavage product (6). (E) Cells were treated with or without anti-Fas–CHX (αFas) for 16 h and stained in situ with annexin V, and fluorescence intensity was determined by FACS analysis. (F) Cells received the same treatment as for panel E, except that low-molecular-weight DNA was isolated, resolved by agarose gel electrophoresis, and stained with ethidium bromide (32). The intensely staining fragment migrating toward the top of the gel has an approximate size of 25 kbp.
Other hallmark features of apoptosis that are a consequence of caspase activity include annexin V staining due to caspase-induced redistribution of phosphatidylserine to the outer part of the plasma membrane and DNA fragmentation resulting from caspase-dependent inactivation of DFF45/ICAD. Annexin V staining and fragmentation of DNA were both observed in crBAP31-expressing cells, and by 16 h after stimulation by Fas they occurred to an extent similar to that in control cells (Fig. 5E and F). Therefore, the sustained inhibition of apoptotic cell morphology after Fas stimulation that is conferred over this time period by the presence of crBAP31 (Fig. 3 and 4) occurs in the face of activated caspases, cleavage of the caspase targets PARP and endogenous BAP31, redistribution of phosphatidylserine in the plasma membrane, and fragmentation of nuclear DNA.
Influence of crBAP31 on Fas-induced transformations of mitochondria.
Release of cytochrome c from the mitochondrial intermembrane space is a common response to many death signals and typically occurs at an early step in the apoptotic death pathway (11). Figure 6A presents confocal microscopic images of control and crBAP31-expressing KB epithelial cells before and after stimulation with the activating anti-Fas antibody for 7 h. Fas stimulation caused cytochrome c to redistribute from a mitochondrial location to a diffuse pattern throughout the cytoplasm. This redistribution of cytochrome c was observed in intact, flat, adherent cells prior to the acquisition of the condensed, membrane-fragmented apoptotic morphology (Fig. 6A); this observation is consistent with release of cytochrome c occurring early in the Fas-mediated death pathway. Strikingly, cells expressing crBAP31 resisted cytochrome c redistribution in response to Fas stimulation (Fig. 6A), and this finding was studied further by an analysis of total cell populations by fractionation of cell homogenates and immunoblotting (Fig. 6B). In control cells, prior to Fas stimulation, cytochrome c was recovered in the membrane fraction containing mitochondria but not in the high-speed supernatant fraction. Fas stimulation resulted in a progressive increase in the recovery of cytochrome c in the high-speed supernatant, reaching an apparent maximum after about 8 h. Again, crBAP31 cells generally retained cytochrome c in the mitochondrial fraction, with a constant and low level recovered in the high-speed supernatant throughout the time course of Fas stimulation, up to 16 h (Fig. 6B).
FIG. 6.
crBAP31 inhibits Fas-mediated release of cytochrome c from mitochondria. (A) Control (parental) KB epithelial cells and KB cells stably expressing crBAP31-Flag were stimulated with anti-Fas (αFas/CHX) for the indicated times and examined by immunofluorescence confocal microscopy with mouse monoclonal antibody 2G8.B6 (anti-cytochrome c) and anti-mouse IgG coupled to Texas red. Cytochrome c release from mitochondria can be observed in cells prior to membrane blebbing (arrow indicates an obviously apoptotic cell). (B) At the indicated times of treatment, cells were homogenized and the postnuclear supernatant was separated into membranes (100,000 × g pellet [P]) and supernatant (S), as indicated, and equal aliquots were subjected to SDS-PAGE and immunoblotting with mouse monoclonal antibody 7H8.2C12 (anti-cytochrome c) (Cyt c). (C) Cells were treated with or without anti-Fas (α Fas) for 16 h, stained with DiOC6, and subjected to FACS analysis. The arrow indicates the peak of fluorescence intensity obtained for cells in which the mitochondrial electrochemical potential was collapsed following treatment with 1 μM carbonyl cyanide m-chlorophenylhydrazone.
In addition to releasing cytochrome c, the mitochondria undergo other transformations in response to apoptotic stimuli, including a collapse of the transmembrane electrochemical potential at the inner membrane (inside negative). The status of the mitochondrial electrochemical potential in control and crBAP31-expressing cells with and without Fas stimulation was monitored using the potential-sensitive dye DiOC6 and fluorescence-activated cell sorter (FACS) analysis (Fig. 6C). Whereas control cells exhibited a collapse of the transmembrane potential after 16 h of Fas stimulation equivalent to that seen after treatment with the protonophore carbonyl cyanide m-chlorophenylhydrazone (Fig. 6C), crBAP31-expressing cells responded with a lower but retained potential, indicating that mitochondria in these cells remained at least partly polarized.
Recovery of cell growth potential following removal of the Fas stimulus.
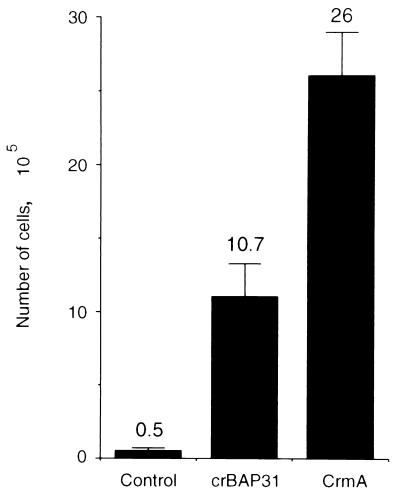
For Fig. 7, control KB cells and KB cells expressing either crBAP31 or CrmA were treated for 7 h with anti-Fas. The cells were collected following trypsinization and washed, and then equivalent numbers of cells were examined for their ability to attach to culture plates and to grow in fresh media lacking the Fas stimulus. Neither CrmA nor crBAP31 conferred any growth difference on these cells in the absence of external treatments (data not shown). However, whereas the treatment of control cells with anti-Fas resulted in a low number of cells recovering and growing on cell plates by 4 days after the removal of the Fas death stimulus, more than 20 times this number was recorded for cells expressing crBAP31 and about 50 times this number was recorded for CrmA-expressing cells. Of note, after 7 h of stimulation by anti-Fas, most of the full-length PARP in crBAP31-expressing cells was cleaved to the 89-kDa apoptotic fragment (Fig. 5D). These results demonstrate, therefore, that a significant number of crBAP31-expressing cells can at least delay the irreversible loss of cell growth potential that is experienced by control cells in response to Fas stimulation, despite the manifestation of caspase activity.
FIG. 7.
crBAP31 inhibits the loss of cell growth potential in response to Fas stimulation. A total of 2 × 105 of the indicated cells were seeded in 12-well dishes overnight and stimulated with anti-Fas for 7 h. All cells were collected after trypsinization, washed twice in PBS, and plated onto 100-mm plates. After 4 days, cell counts were taken. Shown are the average counts from three independent measurements (numerical values above the bars), with standard deviations.
BAP31 association with actomyosin.
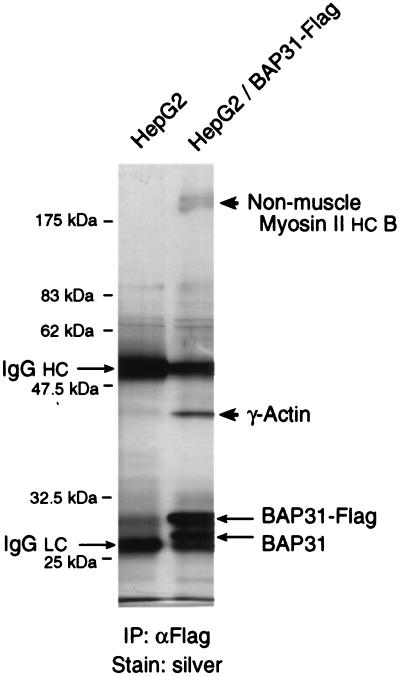
As documented in Discussion, the cytosolic domain of BAP31 exhibits sequence similarities to the coiled coil of heavy-chain myosin. When extracts of human HepG2 cells stably expressing BAP31-Flag were subjected to precipitation with anti-Flag antibody and analysis of the precipitates by SDS-PAGE and silver staining, two prominent bands at approximately 43 and 200 kDa were observed (Fig. 8). In addition, endogenous BAP31 coprecipitated with BAP31-Flag, as confirmed by immunoblotting with anti-BAP31 (data not shown) and consistent with the existence of BAP31 as a homo-oligomer (31). None of these bands was seen in control HepG2 cells. Analysis of the excised 43- and 200-kDa bands by exhaustive tandem mass spectrometry identified them as γ-actin and nonmuscle myosin II heavy-chain B, respectively (A. Ducret, M. Nguyen, D. Breckenridge, and G. Shore, unpublished data). Negligible β-actin was detected in the 43-kDa band, indicating a high degree of specificity for these interactions with BAP31.
FIG. 8.
BAP31 associates with constituents of the actomyosin complex. Extracts of HepG2 cells, either lacking or expressing BAP31-Flag, were subjected to immunoprecipitation with the anti-Flag antibody (α Flag) (31), and the precipitates were analyzed by SDS-PAGE and silver staining. The positions of BAP31-Flag and BAP31 were determined separately by immunoblotting with anti-BAP31 (not shown). Molecular mass markers and the light (LC) and heavy (HC) chains of IgG are also indicated. The bands labeled non-muscle myosin II HC B and γ-actin were identified by tandem mass spectrometry (see Results and Discussion).
DISCUSSION
BAP31 is highly enriched in the ER, as judged by quantitative cryoimmunocytochemistry employing protein A-gold and electron microscopy (30; unpublished data). Interestingly, however, BAP31 has been reported to form associations with distal constituents in the secretory pathway, including IgD (1) and cellubrevin (3), which suggests that BAP31 plays a role in the egress of at least certain proteins out of the ER (3). Its potential role in apoptosis, on the other hand, was derived from the finding that BAP31 can associate with BCL-2, which also localizes to the ER (18) and is a target for caspases (30). Moreover, BAP31 was found to form a complex with procaspase 8 and the Caenorhabditis elegans caspase adapter, CED-4, in cotransfected cells, suggesting that the BAP31 complex plays a role in regulating caspase activity (30, 31). Here we have chosen to investigate BAP31 as a target of caspases by independently activating caspase 8 via the Fas signaling complex in cells that stably express crBAP31. BAP31 is efficiently cleaved by caspase 8 and related caspases, generating a product (p20) that remains integrated in the membrane and, when expressed ectopically, is a potent inducer of apoptosis (30). We have noted in diverse circumstances, however, that p20 readily complexes with epitope-tagged full-length BAP31 (for an example, see reference 31). For the present study, therefore, it is not known if the effects of crBAP31 are due to the preservation of the structural integrity of the protein (preventing the loss of BAP31 function) or to interference with p20 proapoptotic activity by sequestering the cleaved molecule (preventing the gain of p20 function).
Activation of caspase 8 is an important proximal event in the Fas-activated cell death pathway (25, 44), and the ability of caspase 8 to initiate a caspase cascade, either directly or via amplification of mitochondrion-dependent intermediate steps, provides the mechanism for cellular execution by apoptosis (37, 38, 41). One target of caspase 8 in the Fas pathway, proapoptotic BID, is an important, and in certain contexts essential, (47) effector of cytochrome c release from mitochondria (15, 21, 24). Cleavage of BID by caspase 8 or other caspases results in the removal of an inhibitory NH2-terminal segment, rendering the BH3 domain of tBID available for interaction with partner proteins (45). We demonstrate here, however, that another target of caspase 8 (or other caspases) in the Fas pathway, BAP31, may also play an important role both in cytochrome c release from mitochondria and in the extensive membrane remodeling associated with blebbing and formation of apoptotic bodies during the cytoplasmic execution phase of cell death. Interestingly, we observed no inhibition of BID cleavage in Fas-stimulated KB cells expressing crBAP31 (data not shown). Thus, while several studies have shown that incubation of mitochondria with caspase 8-treated naïve cytosolic extracts or with tBID alone can induce egress of cytochrome c from the organelles in vitro (15, 21, 24), the present results argue that the cellular environment or other factors related to the ER may be critical for these events to manifest in vivo. Maintaining the structural integrity of BAP31 in the ER even in the face of sustained caspase activity may preclude these collateral cellular changes from occurring in the Fas death pathway. Emerging evidence, for example, suggests that BID may cooperate with BAX (or BAK) to stimulate cytochrome c release from mitochondria (8) and that a conformational change in BAX in response to a death signal is important for insertion of BAX into the mitochondrial outer membrane (9, 12, 29). While cytosolic factors such as BID and tBID can induce such conformational changes in BAX in vitro (9), additional considerations may apply in vivo. One report, for example, indicates that BAX activation can occur in response to death signal-induced changes in cellular pH (16). BAP31 or its cleavage product may influence this or other ER-regulated conditions that favor a cellular environment in which the proapoptotic activity of tBID and BAX or BAK can be realized.
The release of cytochrome c from mitochondria is an early event in many death signaling pathways and contributes to downstream activation of a caspase cascade. In the human KB epithelial cells studied here, Fas-mediated cytochrome c release was not essential for effector caspase activation, since crBAP31 inhibited the former but not the latter. In this regard, these cells exhibited type I properties (37, 38). Consequently, crBAP31-expressing cells resisted the morphological changes associated with cytoplasmic apoptosis in the face of sustained activity of caspases. The various stages of cytoplasmic apoptosis—release from extracellular matrix attachments and reorganization of focal adhesions (rounding), plasma membrane changes associated with dynamic membrane blebbing, and finally membrane fragmentation and formation of apoptotic bodies (condensation)—are typically associated with changes in the organization of the cellular actomyosin complex, especially in the later stages, which involve caspase-dependent cleavage of actomyosin-associated structural proteins, regulators, and signaling molecules (reviewed in reference 26). It is noteworthy, therefore, that the cytosolic tail of BAP31, from leucine 122 to alanine 236, can be arranged in four segments of four heptads each, in which the frequency of hydrophobic residues at positions 1 and 4 is 71% (27), similar to the frequency observed in myosin heavy-chain coils (42). Moreover, the immunoprecipitation of BAP31-Flag and analysis of associated polypeptides by exhaustive tandem mass spectrometry have identified both γ-actin and nonmuscle myosin II heavy-chain B as proteins that may constitutively interact with the BAP31 complex in vivo (A. Ducret et al., unpublished). These associations are lost for p20 and, therefore, might contribute to the apoptotic cell morphology.
Finally, a limited number of proteins have been identified whose caspase-resistant mutants, like crBAP31, have relatively broad inhibitory influences on the ability of the cell to undergo cytoplasmic apoptosis. These include structural proteins, such as gelsolin (17), and signaling molecules, such as PAK2 (20, 34). Additionally, however, cells expressing crBAP31 exhibited a resistance against Fas-induced release of cytochrome c and the collapse of the mitochondrial electrochemical potential, which presumably preserves or delays the cell from acquiring irreversible mitochondrial dysfunction (43). This may help to explain why at least a fraction of these cells were viable after 7 h of Fas stimulation, whereas control cells were not (Fig. 7). Moreover, the identification of caspase-resistant mutants, such as crBAP31, that have death-inhibiting influences may be relevant to recent suggestions that caspases can play roles in physiological cell stimuli other than apoptosis. For example, cleavage of certain caspase targets but not of others has been noted during stimulation of T lymphocytes in the apparent absence of apoptosis (2). Collateral regulation that preserves the structural integrity of certain key caspase substrates, including BAP31, DFF45/ICAD, and gelsolin, might provide the mechanistic basis for this lack of apoptosis.
ACKNOWLEDGMENTS
We are grateful to D. Nicholson, R. Jemmersen, P. Braun, R. Sékaly, and V. Dixit for providing reagents and to J. Mui for helping with electron microscopy.
D.G.B. was the recipient of a studentship from the Medical Research Council of Canada. This work was supported by the National Cancer Institute and the Medical Research Council of Canada.
REFERENCES
- 1.Adachi T, Schamel W W, Kim K M, Watanabe T, Becker B, Nielsen P J, Reth M. The specificity of association of the IgD molecule with the accessory proteins BAP31/BAP29 lies in the IgD transmembrane sequence. EMBO J. 1996;15:1534–1541. [PMC free article] [PubMed] [Google Scholar]
- 2.Alam A, Cohen L Y, Aouad S, Sékaly R-P. Early activation of caspases during T lymphocyte stimulation results in selective substrate cleavage in non-apoptotic cells. J Exp Med. 1999;190:1879–1890. doi: 10.1084/jem.190.12.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Annaert W G, Becker B, Kistner U, Reth M, Jahn R. Export of cellubrevin from the endoplasmic reticulum is controlled by BAP31. J Cell Biol. 1997;139:1397–1410. doi: 10.1083/jcb.139.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boldin M P, Varfolomeev E E, Pancer Z, Mett I L, Camonis J H, Wallach D. A novel protein that interacts with the death domain of Fas/APO-1 contains a sequence motif related to the death domain. J Biol Chem. 1995;270:7795–7798. doi: 10.1074/jbc.270.14.7795. [DOI] [PubMed] [Google Scholar]
- 5.Boldin M P, Goncharov T M, Goltsev Y V, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 6.Boulakia C A, Chen G, Ng F W H, Teodoro J G, Branton P E, Nicholson D W, Poirier G G, Shore G C. Bcl-2 and adenovirus E1B 19 kDa protein prevent E1A-induced processing of CPP32 and cleavage of poly(ADP-ribose) polymerase. Oncogene. 1996;12:529–535. [PubMed] [Google Scholar]
- 7.Chinnaiyan A M, O'Rourke K, Tewari M, Dixit V M. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 8.Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Mandrell K, Antonsson B, Martinou J-C. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eskes R, Desagher S, Antonsson B, Martinou J-C. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol. 2000;20:929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Calvo M, Peterson E P, Leiting B, Ruel R, Nicholson D W, Thornberry N A. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J Biol Chem. 1998;273:32608–32613. doi: 10.1074/jbc.273.49.32608. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein J C, Waterhouse N J, Juin P, Evan G I, Green D R. The coordinate release of cytochrome c during apoptosis is rapid, complete, and kinetically invariant. Nat Cell Biol. 2000;2:156–167. doi: 10.1038/35004029. [DOI] [PubMed] [Google Scholar]
- 12.Goping I S, Gross A, Lavoie J N, Nguyen M, Jemmerson R, Roth K, Korsmeyer S J, Shore G C. Regulated targeting of Bax to mitochondria. J Cell Biol. 1998;143:207–215. doi: 10.1083/jcb.143.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granville D J, Carthy C M, Jiang H, Ng F W, Shore G C, McManus B M, Hunt D W C. Rapid cytochrome c release, activation of caspases 3, 6, 7 and 8, followed by Bap31 cleavage in HeLa cells treated with photodynamic therapy. FEBS Lett. 1998;437:5–10. doi: 10.1016/s0014-5793(98)01193-4. [DOI] [PubMed] [Google Scholar]
- 14.Graves J D, Gotoh Y, Draves K E, Ambrose D, Han D K, Wright M, Chernoff J, Clark E A, Krebbs E G. Caspase-mediated activation and induction of apoptosis by the mammalian Ste20-like kinase Mst1. EMBO J. 1998;17:2224–2234. doi: 10.1093/emboj/17.8.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gross A, Yin X-M, Wang K, Wei M C, Jockel J, Milliman C, Erdjument-Bromage H, Tempst P, Korsmeyer S J. Caspase-cleaved Bid targets mitochondria and is required for cytochrome c release, while Bcl-XL prevents this release but not tumor necrosis factor-R1/Fas death. J Biol Chem. 1999;274:1156–1163. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]
- 16.Kahled A R, Kim K, Hofmeisterand R, Durum S K. Withdrawal of IL-7 induces Bax translocation from cytosol to mitochondria through a rise in intracellular pH. Proc Natl Acad Sci USA. 1999;96:14476–14481. doi: 10.1073/pnas.96.25.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kothakota S, Azuma T, Reihart C, Klippel A, Tang J, Chu K, McGarry T J, Kirschner M W, Koth K, Kwiatkowski D J, Williams L T. Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis. Science. 1997;278:294–298. doi: 10.1126/science.278.5336.294. [DOI] [PubMed] [Google Scholar]
- 18.Krajewski S, Tanaka S, Takayama S, Schibler M, Fenton W, Reed J C. Investigation of the subcellular distribution of the Bcl-2 oncoprotein: residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res. 1993;53:4701–4714. [PubMed] [Google Scholar]
- 19.Lee K K, Murakawa M, Nishida E, Tsubuki S, Sakamaki K, Yonehara S. Proteolytic activation of MST/Krs, STE20-related protein kinase, by caspase during apoptosis. Oncogene. 1998;16:3029–3037. doi: 10.1038/sj.onc.1201840. [DOI] [PubMed] [Google Scholar]
- 20.Lee N, MacDonald H, Reihard C, Halenbeck R, Roulston A, Shi T, Williams L T. Activation of hPAK65 by caspase cleavage induces some of the morphological and biochemical changes of apoptosis. Proc Natl Acad Sci USA. 1997;94:13642–13647. doi: 10.1073/pnas.94.25.13642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, Zhu H, Xu C-J, Yuan J. Cleavage of Bid by caspase-8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 22.Li P, Nijhawan D, Budihardjo I, Srinivasula S, Ahmad M, Alnemri E S, Wang X. Cytochrome c and dATP-dependent formation of an Apaf-1/caspase-9 complex initiates apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Zou H, Slaughter C, Wang X. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell. 1997;89:175–184. doi: 10.1016/s0092-8674(00)80197-x. [DOI] [PubMed] [Google Scholar]
- 24.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl-2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 25.Medema J P, Scaffidi C, Kischkel F C, Shevchenko A, Mann M, Krammer P H, Peter M E. FLICE is activated by association with the CD95 death inducing signaling complex (DISC) EMBO J. 1997;16:2794–2804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mills J C, Stone N L, Pittman R N. Extranuclear apoptosis: the role of the cytoplasm in the execution phase. J Cell Biol. 1999;146:703–707. doi: 10.1083/jcb.146.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mosser J, Sarde C O, Vicaire S, Yates J R, Mandel J L. A new human gene (DXS1357E) with ubiquitous expression, located at Xq28 adjacent to the adrenoleukodystrophy gene. Genomics. 1994;22:469–471. doi: 10.1006/geno.1994.1413. [DOI] [PubMed] [Google Scholar]
- 28.Muzio M, Chinnaiyan A M, Kischkel F C, O'Rourke F C, Shevchenko A, Ni J, Scaffidi C, Bretz J D, Zhang M, Gentz R, Mann M, Krammer P H, Peter M E, Dixit V M. FLICE, a novel FADD-homologous ICE/Ced-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 29.Nechestan A, Smith C L, Hsu Y-T, Youle R J. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 1999;18:2330–2341. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng F W H, Nguyen M, Kwan T, Branton P E, Nicholson D W, Cromlish J A, Shore G C. p28 Bap31, a Bcl-2/Bcl-XL- and procaspase-8-associated protein in the endoplasmic reticulum. J Cell Biol. 1997;139:327–338. doi: 10.1083/jcb.139.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng F W H, Shore G C. Bcl-XL cooperatively associates with the Bap31 complex in the endoplasmic reticulum, dependent on procaspase-8 and Ced-4 adaptor. J Biol Chem. 1998;139:327–338. doi: 10.1074/jbc.273.6.3140. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen M, Walton P, Branton P E, Korsmeyer S J, Shore G C. Role of membrane anchor domain of Bcl-2 in suppression of apoptosis caused by E1B-defective adenovirus. J Biol Chem. 1994;269:16521–16524. [PubMed] [Google Scholar]
- 33.Nicholson D W. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999;6:1028–1042. doi: 10.1038/sj.cdd.4400598. [DOI] [PubMed] [Google Scholar]
- 34.Rudel T, Bokoch G M. Membrane and morphological changes in apoptotic cells regulated by caspase-mediated activation of PAK2. Science. 1997;276:1571–1574. doi: 10.1126/science.276.5318.1571. [DOI] [PubMed] [Google Scholar]
- 35.Sabourin L A, Seale P, Wagner J, Rudnicki M A. Caspase 3 cleavage of the Ste20-related kinase SLK releases and activates an apoptosis-inducing kinase domain and an actin-disassembling region. Mol Cell Biol. 2000;20:684–696. doi: 10.1128/mcb.20.2.684-696.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakahira H, Enari M, Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998;391:96–99. doi: 10.1038/34214. [DOI] [PubMed] [Google Scholar]
- 37.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli K J, Debatin K-M, Krammer P H, Peter M E. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scaffidi C, Schmitz I, Zha J, Korsmeyer S J, Krammer P H, Peter M E. Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J Biol Chem. 1999;274:22532–22538. doi: 10.1074/jbc.274.32.22532. [DOI] [PubMed] [Google Scholar]
- 39.Slee E A, Harte M T, Kluck R M, Wolf B B, Casiano C A, Newmeyer D D, Wang H-G, Reed J C, Nicholson D W, Alnemri E S, Green D R, Martin S. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases -2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol. 1999;144:281–292. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Srinivasula S M, Ahmad M, Fernandes-Alnemri T, Litwack G, Alnemri E S. Molecular ordering of the Fas-apoptotic pathway: the Fas/APO-1 protease Mch5 is a CrmA-inhibitable protease that activates multiple Ced-3/ICE-like cysteine proteases. Proc Natl Acad Sci USA. 1996;93:14486–14491. doi: 10.1073/pnas.93.25.14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stennicke H R, Jurgensmeier J M, Shin H, Deveraux Q, Wolf B B, Yang X, Zhou Q, Ellerby H M, Bredesen D, Green D R, Reed J C, Froelich C J, Salvesen G S. Pro-caspase-3 is a major physiological target of caspase-8. J Biol Chem. 1998;273:27084–27090. doi: 10.1074/jbc.273.42.27084. [DOI] [PubMed] [Google Scholar]
- 42.Strehler E E, Strehler-Page M A, Perriard J C, Periasamy M, Nadal-Ginard B. Complete nucleotide and encoded amino acid sequence of a mammalian myosin heavy chain gene. J Mol Biol. 1986;190:291–317. doi: 10.1016/0022-2836(86)90003-3. [DOI] [PubMed] [Google Scholar]
- 43.Vander Heiden V G, Thompson C B. Bcl-2 proteins: regulators of apoptosis or of mitochondrial homeostasis. Nat Cell Biol. 1999;1:E209–E216. doi: 10.1038/70237. [DOI] [PubMed] [Google Scholar]
- 44.Varfolomeev E E, Schuchman M, Luria V, Chiannilkulchai N, Beckmann J S, Mett I L, Rebrikov D, Brodianski V M, Kemper O C, Kollet O, Lapidot T, Soffer D, Sobe T, Abraham K B, Goncharov T, Holtmann H, Lonia P, Wallach D. Targeted disruption of the mouse caspase-8 gene ablates cell death induction by the TNF receptors, Fas/APO-1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–276. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 45.Wang K, Yin X-M, Chao D T, Milliman C L, Korsmeyer S J. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 46.Wolf B B, Green D R. Suicidal tendencies: apoptotic cell death by caspase family proteases. J Biol Chem. 1999;274:20049–20052. doi: 10.1074/jbc.274.29.20049. [DOI] [PubMed] [Google Scholar]
- 47.Yin X-M, Wang K, Gross A, Zhao Y, Zinlle S, Klocke B, Roth K A, Korsmeyer S J. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- 48.Zheng T S, Hunot S, Kuida K, Flavell R A. Caspase knockouts: matters of life and death. Cell Death Differ. 1999;6:1043–1053. doi: 10.1038/sj.cdd.4400593. [DOI] [PubMed] [Google Scholar]