Abstract
We aimed to compare the effectiveness of some different acupuncture modalities on motor function using the unified Parkinson disease rating scale (UPDRS)-III scores of idiopathic Parkinson’s disease (PD) via pairwise and network meta-analyses (NMA) of randomized controlled trials (RCTs). The Cochrane risk of bias assessment tool was used to assess the methodological quality of the included RCTs. A frequentist approach-based random effect model NMA was performed. Seventeen RCTs with 1071 participants were included. The five following modalities were identified: combination of conventional medication (levodopa) with (1) electroacupuncture (ELEC), (2) manual acupuncture (MANU), (3) bee venom acupuncture (BEEV), (4) sham acupuncture (SHAM), and (5) conventional medication alone (CONV). In NMA on UPDRS-III, BEEV was the best modality compared to CONV (mean difference [MD]) −7.37, 95% confidence interval [−11.97, −2.77]). The comparative ranking assessed through NMA was suggested to be BEEV, MANU, ELEC, SHAM, and CONV. Regarding daily activity assessment (UPDRS-II), the magnitude of effectiveness was in the order of BEEV, ELEC, MANU, SHAM, and CONV. Combination treatment with BEEV (MANU or ELEC) and CONV can be recommended to improve motor function in PD patients. Due to the limited number of included RCTs, further NMA with more rigorous RCTs are warranted.
Keywords: network meta-analysis, meta-analysis, Parkinson’s disease, motor symptom, systematic review, acupuncture
1. Introduction
Parkinson’s disease (PD) is a degenerative neurological disorder associated with dopaminergic cell loss in the substantia nigra and other brain structures characterized by several movement symptoms, such as tremor, rigidity, tremor at rest, and postural instability [1]. PD is the second most common neurodegenerative disorder after Alzheimer’s dementia. The prevalence of PD is increasing faster than in other neurological diseases [1,2]. The prevalence increases with age, and in most cases, the cause is unknown [3,4]. Approximately 6.1 million people worldwide were diagnosed with PD in 2016, which is more than double that of 1990 [5]. The movement symptoms of PD are managed using a combination of conventional medications, such as levodopa, carbidopa, dopamine agonists, and monoamine oxidase B inhibitors [5]. On the other hand, if levodopa is administered for a long period, treatment may not be continued due to side effects, such as the on-off phenomenon [6]. Levodopa-induced dyskinesia also impairs the quality of life of patients with PD, making effective treatment difficult [7]. In a previous study, more than 40% of patients with PD experienced wear-off and levodopa-induced dyskinesia, which lowered drug adherence [8]. If conventional drugs are ineffective, several surgical strategies, such as deep brain stimulation (DBS) or radiofrequency ablation, could be considered [9]. However, there are a number of complications associated with a surgical approach, and patient expectations after surgery are sometimes not fulfilled [10]. In addition, as the disease progresses, the burden on caregivers increases because of frequent nursing home visits, longer hospital stays, and higher rates of emergency room visitation [11]. Therefore, in addition to conventional management, alternative therapeutic options are needed to manage various symptoms considering the characteristics of PD, which has a long disease duration.
Recently, various complementary and integrative medicine (CIM) therapies, such as acupuncture, herbal medicine, qi-gong, massage, yoga, meditation, and music therapy, have been widely utilized in clinical practice for PD symptom management [12]. Acupuncture is one of the most commonly used CIM interventions for the management of patients with PD [13]. In previous studies, acupuncture improved motor symptoms, non-motor symptoms, quality of life, and disease progression, and decreased the adverse events and dosage of anti-parkinsonian medication [13]. Several clinical studies and systematic reviews have shown the effects of various types of acupuncture treatment combined with conventional medication on motor symptom improvement using the unified PD rating scale (UPDRS) [14,15,16,17].
However, it is unknown which type of acupuncture treatment is the preferred option because the acupuncture treatment applied in each study is different. Most clinical research and systematic reviews have compared two interventions at a time. Multiple intervention comparison research designs are not common. However, in clinical practice, physicians are curious about which treatment is more effective among the various widely used treatments. However, in terms of time and cost, it is difficult to conduct direct comparative (head-to-head) studies on various acupuncture treatments, and the need to compare multiple interventions at a time is increasing. Nowadays, a novel methodology named ‘network meta-analysis (NMA)’ is used to simultaneously estimate the relative effect of various interventions [18,19]. NMA results help stakeholders to make decisions by providing a combined quantitative effect size acquired from direct and indirect comparisons of different interventions [20].
This study aimed to compare the effect on movement symptom improvement in patients with PD about several acupuncture types combined with conventional medication (CM), such as manual acupuncture (MA), electroacupuncture (EA), and bee venom acupuncture (BVA), compared with placebo acupuncture or conventional medication only. We adopted conventional systematic review, pairwise meta-analysis (PMA), and NMA methodology to compare the effect size of various acupuncture types to help with decision making regarding the management of patients with PD.
2. Materials and Methods
We followed the preferred reporting items for systematic reviews and meta-analyses for network meta-analysis checklist (PRISMA-NMA) [21]. This review protocol was registered with the Open Science Framework on 7 August 2021 (https://osf.io/q8n7z/).
2.1. Search Strategy
Eligible studies were systematically searched from their inception to June 2021 using Medline (via PubMed), Cochrane Library, Embase (via Elsevier), China National Knowledge Infrastructure, Korea Citation Index (KCI), NDSL, Research Information Sharing Service, and Oriental Medicine Advanced Searching Integrated System. A mixture of free words and medical subject headings were used for PD and acupuncture. There were no language restrictions. The search strategy in Medline (via PubMed) is as follows:
#1 parkinson disease [Mesh] OR parkinson disease OR Idiopathic Parkinson’s Disease OR Lewy Body Parkinson’s Disease OR Parkinson’s Disease, Idiopathic OR Parkinson’s Disease, Lewy Body OR Parkinson Disease, Idiopathic OR Parkinson’s Disease OR Idiopathic Parkinson Disease OR Lewy Body Parkinson Disease OR Primary Parkinsonism OR Parkinsonism, Primary OR Paralysis Agitans.
#2 acupuncture [Mesh] OR Acupuncture OR Pharmacopuncture OR Acupuncture Treatment OR Acupuncture Treatments OR Treatment, Acupuncture OR Therapy, Acupuncture OR Pharmacoacupuncture Treatment OR Treatment, Pharmacoacupuncture OR Pharmacoacupuncture Therapy OR Therapy, Pharmacoacupuncture OR Acupotomy OR Acupotomies OR Electroacupuncture OR Bee Venoms OR Venoms, Bee OR Bee Venom OR Venom, Bee OR Apis Venoms OR Venoms, Apis OR Apitoxin OR Honeybee Venoms OR Venoms, Honeybee OR Honeybee Venom OR Venom, Honeybee OR Fire needle therapy OR Fire acupuncture.
#3 #1 AND #2: A detailed explanation of the search terms used in each database is provided in Supplementary Materials Digital Content 1.
2.2. Eligible Criteria
2.2.1. Type of Studies
Only randomized controlled clinical trials (RCTs) were included. We did not include cluster randomized clinical trials. Other study designs, such as animal studies, uncontrolled tests, or case reports, were excluded. Multi-armed trials (≥ three arms) were included if they did not violate the eligibility criteria.
2.2.2. Type of Participants
Patients diagnosed with idiopathic PD were included without limitation of age, sex, race, severity, or duration of disease. Patients other than those with idiopathic PD, such as Parkinson’s syndrome, were excluded.
2.2.3. Type of Intervention Used in the Experimental and Control Groups
The experimental group intervention consisted of different types of acupuncture treatment combined with CM. In the control group, we selected L-dopa, which has been an effective gold standard dopamine-based medication for movement symptom management for approximately 60 years, as an essential medication for the control group (CM) [22,23]. Studies were included if the combination of L-dopa and other drugs was equally applied to the acupuncture and control groups. However, studies in which treatment medication therapy was performed only with other drugs without L-dopa were excluded. Acupuncture treatments included electroacupuncture (EA), MA, or BVA. We excluded combined acupuncture treatments, such as EA + BVA or MA + BVA, to evaluate the therapeutic effect of each acupuncture intervention type. The intervention in the control group was defined as CM therapy alone or CM + sham acupuncture treatment. We did not restrict the duration, dosage, or frequency of treatment.
2.2.4. Type of Outcome Measure
The primary outcome of our study was the motor function of patients with PD evaluated using the UPDRS-III scale [24]. The secondary outcomes were daily life activity scores using the UPDRS-II [24]. The Movement Disorder Society UPDRS (MDS-UPDRS) was excluded because it is different from UPDRS [25]. The timing of the outcome assessment was selected immediately after the end of the acupuncture treatment session. Data acquired during the follow-up assessment were not considered.
2.3. Study Selection and Data Extraction
Two reviewers (M.K. and J.L.) independently conducted the study selection and data extraction.
Disagreement between the two researchers was resolved by discussion with a third independent reviewer (M.J.). Duplicate publications, patients diagnosed with Parkinsonism syndrome, and cases in combination with other treatments were excluded. A standardized data collection form developed during the pilot process using Excel was utilized during the data extraction process. The extracted items were as follows: sample size and the number of dropouts, first author, year of publication, location, age, sex, disease severity, disease duration, treatment intervention, control group intervention, treatment period, and outcome variables. We contacted the corresponding author to acquire sufficient data if there was insufficient information in the published article via e-mail. EndNote X9 (EndNote version X9, Thomson Reuters, CA, USA) was used for article selection and management.
2.4. Risk of Bias Assessment
Two independent researchers (J.L., M.K.) used the Cochrane risk of bias assessment tool to evaluate the quality of the research methods of the included studies [26]. Random sequence generation; allocation concealment; blinding of participants, personnel, and outcome assessors; incomplete outcome data; selective outcome reporting; and other sources of bias were graded as low, unclear, and high. Disagreement between the two researchers was resolved by discussion with a third independent reviewer (M.J.). Review Manager (RevMan) version 5.4 software was used to illustrate the risk of bias.
2.5. PMA
In the PMA, we conducted a conventional direct comparison of the two study arms. Data synthesis was performed using the Review Manager (RevMan) ([Computer program]. Version 5.4, The Cochrane Collaboration, 2020). The random effect model was adopted because it was judged that there was heterogeneity due to differences in the study design, such as baseline characteristics, number of interventions, and methods among the included studies. The mean difference (MD) for the continuous variables and 95% confidence interval (CI) were used to assess the effect size of the intervention on UPDRS-III and II. Heterogeneity was determined by both the chi-square (χ2) test and Higgins’ statistic. The heterogeneity interpretation based on the statistic is considered not to be important (0 to 40%), moderate heterogeneity (30% to 60%), substantial heterogeneity (50% to 90%), and considerable heterogeneity (75% to 100%) [27]. A p-value of ≤0.1 was considered to indicate significant heterogeneity [28].
2.6. NMA
2.6.1. Assumptions of the NMA
The frequentist model was utilized for the NMA, combining direct and indirect evidence using R version 4.1.0 (A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria)) using the Netmeta package [29]. There are several assumptions for NMA, such as connectivity, homogeneity, transitivity, and consistency [30]. Connectivity was visually verified by connecting each network node with a line using a network plot. Homogeneity was assessed using the Cochrane Q statistic or the I2 score. In our study, a random effect model was applied, as it was judged that there was heterogeneity between studies due to differences in study design or interventions [30,31]. When evaluating transitivity, it is necessary to explore the distribution of effect modifiers and determine their effects on the effect size. In our study, we qualitatively compared the sample size, age, sex, disease duration, severity, treatment dosage, and period for transitivity assessment [30]. Consistency is a quantitative statistical evaluation of transitivity. Consistency was statistically evaluated using the net-splitting method [32].
2.6.2. Statistical Assessment
The network forest plot presented with MD and 95% CI of each intervention was used to rank each treatment strategy for visual and statistical verification. The P-score was also used to rank treatment, which assesses certainty that a specific intervention is better than competing inventions. The P-score is nearly identical to the numerical values of SUCRA in the Bayesian model NMA [33]. For the consistency assumption, we checked both global (network level) and local approaches (particular contrast of intervention level) [21]. In the global approach, we used the ‘decomp.design’ function of R software to assess consistency under the assumption of a full design-by-treatment interaction random effect model [34]. Q statistics were used to assess inconsistency in the global approach. If the p-value for the Q statistics was below 0.05, it was assumed that significant inconsistency (disagreement) existed in the global network. In the local approach, we adopted the net-splitting method to split the network estimation of the effect size on each intervention into direct and indirect evidence using the Facenetsplit function of R software. It calculates the difference between direct and indirect estimates and assesses whether the difference is statistically significant [34]. Net-split plots were also provided for visual inspection of inconsistencies between direct and indirect comparisons. If the p-value for the net-split analysis was below 0.05, it was assumed that significant inconsistency (disagreement) existed in a specific local loop, which indicates a considerable difference between indirect and direct effect size estimation. If there were significant disagreements in the local or global approach, we conducted a sensitivity analysis by sequentially excluding studies one by one. If we identified which studies were inconsistent, we excluded studies from the NMA. A net league table is also presented. The upper right triangle presents the effect size estimated by only direct comparison, which is similar to the pairwise comparison. As direct comparison does not exist in all treatment comparisons, there are several blanks in the upper triangle. The lower left triangle provides a pooled estimation of the direct and indirect comparisons of the effect size.
2.6.3. Sensitivity Analysis
NMA was performed by sequentially removing each study one by one to confirm whether a specific study excessively affected the overall result. The results were visually and statistically checked to determine whether the results were consistent with the overall trend.
2.7. Publication Bias
We used a conventional funnel plot for visual inspection of the publication bias. We also used Egger’s test to statistically assess publication bias [35]. If the p-value for Egger’s test was greater than 0.05, it indicated no evidence of publication bias.
3. Results
3.1. Characteristics of the Included Studies and Network Geometry
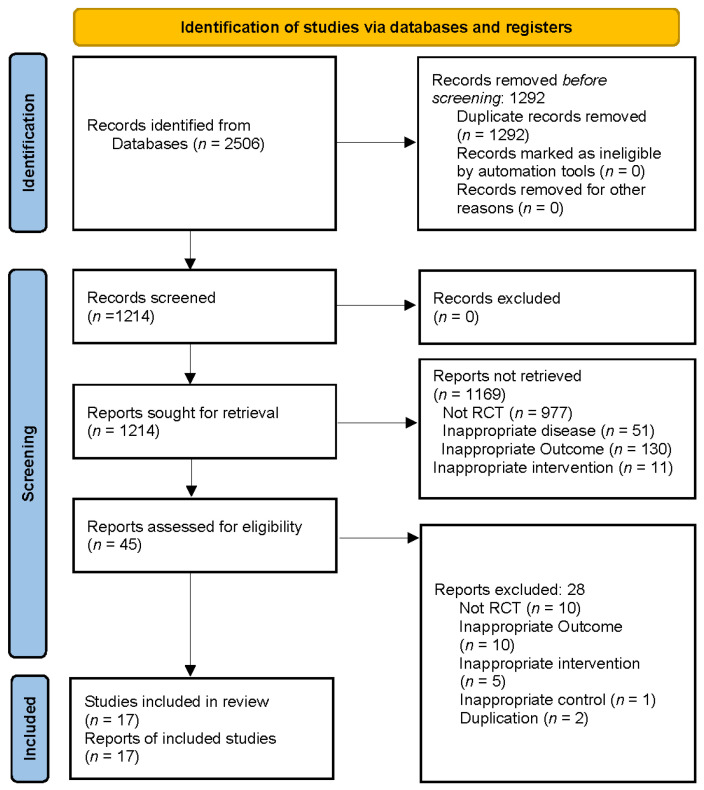
A total of 2505 articles were screened from eight databases. After careful review of the title and abstract, 17 articles were finally included (Figure 1). In 17 RCTs, 1071 participants were included. A list of the 28 studies excluded after reviewing the full text is provided in Supplementary Materials Digital Content 2. Eight articles were written in English [36,37,38,39,40,41,42,43], one article was written in Japanese [44], two in Korean [15,45], and six articles were written in Chinese [14,46,47,48,49,50]. Detailed characteristics of the included studies including publication year, first author, country, sample size (initial and final), age, sex, disease severity, disease duration, CM dosage (mg/day), treatment and control group intervention, and treatment period are described in Table 1.
Figure 1.
PRISMA flow diagram.
Table 1.
Characteristics of the included studies.
| First Author, Year (Location) |
Sample Size (A:B) (Initial →Final) |
Age (Year), Mean ± SD |
Sex (M:F) |
Disease Severity : H-Y Stage |
Disease Duration (Year) |
(A) Treatment Intervention (Conventional Drug Therapy Dosage, mg/d) |
(B) Control Intervention (Conventional Drug Therapy Dosage, mg/day) |
Treatment/Follow-Up Period (Week) |
|---|---|---|---|---|---|---|---|---|
| Electroacupuncture + Conventional drug therapy | ||||||||
| Chen 2012 [14] (China) |
30:30 →30:30 |
(A) 65.60 ± 3.79 (B) 61.93 ± 3.67 |
(A) 19:11 (B) 17:13 |
(A) 2.18 ± 0.26 (B) 2.04 ± 0.30 |
(A) 5.40 ± 1.75 (B) 6.40 ± 2.15 |
ELEC (432 ± 139) |
CONV (435 ± 154) |
6/None |
| Gu 2013 [48] (China) |
23:25 →23:25 |
(A) 66 ± 8 (B) 70 ± 8 |
(A) 10:13 (B) 15:10 |
NR | (A) 4.44 ± 3.32 (B) 4.56 ± 3.11 |
ELEC (250) |
CONV (250) |
12/None |
| Huang 2009 [46] (China) |
15:15 →15:15 |
(A) 65.60 ± 3.78 (B) 60.80 ± 3.63 |
(A) 8:7 (B) 6:9 |
(A) 2.18 ± 0.26 (B) 2.04 ± 0.30 |
(A) 5.40 ± 1.75 (B) 6.4 ± 2.14 |
ELEC (375−750) |
CONV (375−750) |
5/None |
| Lei 2016 [36] (USA) |
10:5 →10:5 |
(A) 69.8 ± 4.5 (B) 71.0 ± 11.7 |
(A) 6:4 (B) 2:3 |
(A) 3.0 ± 1.0 (B) 2.9 ± 0.7 |
(A) 6.2 ± 5.9 (B) 5.2 ± 4.7 |
ELEC (614 ± 381) |
SHAM (324 ± 295) |
3/None |
| Liu 2016 [49] (China) |
39:35 →39:35 |
(A) 65.65 ± 4.15 (B) 65.59 ± 4.18 |
(A) 21:18 (B) 19:16 |
NR | (A) 4.41 ± 2.01 (B) 4.33 ± 2.04 |
ELEC (NR) |
CONV (NR) |
12/None |
| Wang 2015 [40] (China) |
30:20 →28:20 |
(A) 62.1 ± 8.7 (B) 59.1 ± 12.4 |
(A) 13:15 (B) 9:11 |
(A) 2.0 ± 0.7 (B) 2.0 ± 0.8 |
(A) 2.9 ± 2.9 (B) 2.7 ± 2.3 |
ELEC (104.1 ± 253.2) |
CONV (160.6 ± 260.0) |
Two months/ None |
| Xu 2020 [42] (China) |
38:38 →33:37 |
(A) 61.73 ± 10.28 (B) 61.95 ± 9.77 |
(A) 15:18 (B) 21:16 |
Stage 1 (A) 9 (B) 8 Stage 1.5 (A) 4 (B) 12 Stage 2 (A) 5 (B) 6 Stage 2.5 (A) 9 (B) 5 Stage 3 (A) 4 (B) 4 Stage 4 (A) 2 (B) 2 |
(A) 3.52 ± 2.78 (B) 3.26 ± 2.32 |
ELEC (187.5−375) |
CONV (187.5−375) |
8/4 |
| MA + Conventional drug therapy | ||||||||
| Jung 2006 [15] (Korea) |
NR →16:21 |
(A) 59.69 ± 9.6 (B) 61.00 ± 9.7 |
(A) 11:5 (B) 10:11 |
NR | (A) 5.66 ± 4.23 (B) 6.07 ± 4.82 |
MANU (NR) |
SHAM (NR) |
4/None |
| Kluger 2016 [43] (USA) |
47:47 →47:47 |
(A) 64.4 ± 10.3 (B) 63.0 ± 13.0 |
(A) 30:17 (B) 29:18 |
Stage 1 (A) 4 (B) 2 Stage 1.5 (A) 6 (B) 3 Stage 2 (A) 11 (B) 17 Stage 2.5 (A) 18 (B) 12 Stage 3 (A) 6 (B) 10 Stage 4 (A) 0 (B) 4 |
NR | MANU (558.9 ± 379.3) |
SHAM (628.6 ± 482.9) |
6/None |
| Kong 2017 [37] (Singapore) |
20:20 →19:17 |
(A) 66.4 ± 6.5 (B) 62.9 ± 9.7 |
(A) 6:14 (B) 7:13 |
NR |
(A) 87.2 ± 53.2 (B) 50.1 ± 26.4 |
MANU (637.8 ± 394.3) |
SHAM (592.6 ± 303.1) |
5/4 |
| Li 2018 [38] (China) |
14:13:14 →14:12:11 |
(A) 62.17 ± 7.66 (B) 65.79 ± 6.07 (C) 62.85 ± 5.00 |
(A) 9:3 (B) 8:6 (C) 7:6 |
NR | NR | (A) MANU (367.86 ± 146.24) | (B) SHAM(338.46 ± 112.09) (C) CONV (345.83 ± 173.81) |
12/None |
| Lu 2020 [50] (China) |
20:20 →20:20 |
(A) 66.50 ± 8.81 (B) 65.90 ± 8.92 |
(A) 10:10 (B) 12:8 |
NR | (A) 15.10 ± 1.72 (B) 15.25 ± 2.04 |
MANU (250) |
CONV (250) |
4/None |
| Mizushima 2011 [44] (Japan) |
NR →103:95 |
(A) 63.9 ± 8.2 (B) 64.7 ± 9.8 |
(A) 45:58 (B) 50:45 |
NR | (A) 1.6 ± 0.6 (B) 1.8 ± 1.2 |
MANU (186.0 ± 134.0) |
CONV (251.0 ± 172.8) |
Five years/5 years |
| Park 2007 [45] (Korea) |
NR →21:13 |
(A) 60.00 ± 9.0 (B) 61.26 ± 9.81 |
(A) 12:9 (B) 2:11 |
(A) 1.7619 ± 0.95 (B) 1.8846 ± 0.68 |
(A) 5.63 ± 5.29 (B) 5.84 ± 3.3 |
MANU (NR) |
SHAM (NR) |
4/None |
| Ren 2011 [47] (China) |
90:90 →90:90 |
(A) 59.1 ± 12.1 (B) 58.2 ± 11.9 |
(A) 52:38 (B) 49:41 |
NR | (A) 1.8 ± 0.3 (B) 1.9 ± 0.4 |
MANU (750) |
CONV (750) |
30 days/None |
| BVA + Conventional drug therapy | ||||||||
| Cho 2012 [41] (Korea) |
18:17:14→13:13:9 |
(A) 57.0 (B) 55.0 (C) 57.0 |
(A) 5:8 (B) 5:8 (C) 3:6 | NR |
(A) 5.0 (B) 6.0 (C) 5.0 |
(A) BEEV (NR) (B) MANU (NR) |
(C) CONV (NR) |
8/None |
| Hartmann 2016 [39] (France) |
20:20 →15:20 |
(A) 60.3 ± 15 (B) 63.3 ± 8 |
(A) 8:12 (B) 12:8 |
Stage 2 (A) 6 (B) 7 Stage 2.5(A) 14 (B) 11 Stage 3 (A) 0 (B) 2 |
(A) 6.2 ± 5 (B) 6.3 ± 5.1 |
BEEV (391→64 ± 127) Baseline median→Result mean, SD |
SHAM (512→98 ± 156) Baseline median→Result mean, SD |
11 months |
(A) Treatment intervention; (B) Control intervention (in 2 arm design); (C) Control intervention in 3 arm design; SD, standard deviation; H-Y stage, Hoehn and Yahr stage; NR, not reported. Intervention: ELEC, electroacupuncture + conventional drug therapy; SHAM, sham acupuncture + conventional drug therapy; MANU, manual acupuncture + conventional drug therapy; CONV, single conventional drug therapy; BEEV, bee-venom acupuncture + conventional drug therapy. Outcomes) UPDRS: Unified PD rating scale; AE: Rate of the number of participants with adverse events between groups; DR, dropout rate. Li 2018: MANU vs. SHAM vs. CONV; Cho 2012: BEEV vs. MANU vs. CONV.
Five types of arms were identified: (1) manual acupuncture + conventional drug (MANU), (2) electroacupuncture + conventional drug (ELEC), (3) BVA + conventional drug (BEEV), (4) sham acupuncture + conventional drug (SHAM), and (5) conventional drug alone (CONV). Fifteen RCTs had two armed designs, and only two RCTs had three armed designs (one RCT included MANU vs. SHAM vs. CONV [38], and one RCT included BEEV vs. MANU vs. CONV [41]). Seven trials included the ELEC arm, nine trials included the MANU arm, and two included the BEEV arm. Therefore, a total of 18 comparisons (36 treatment arms) were included in the 17 RCTs. Six RCTs compared ELEC vs. CONV [14,40,42,46,48,49]; five RCTs compared MANU vs. CONV [38,41,44,47,50]; one RCT compared ELEV vs. SHAM [36]; five RCTs compared MANU vs. SHAM [15,37,38,43,45]; and one RCT compared BEEV vs. SHAM [39]. Detailed descriptions of each intervention, including the acupuncture point, needle stimulation, retention time, treatment duration, and frequency, are described in Table 2.
Table 2.
Detailed description of the acupuncture treatment.
| First Author, Year (Location) |
Acupuncture Point | Depth of Insertion | Needle Stimulation, Electrical Stimulation |
Needle Retention Time | Treatment Frequency, Total Number of Treatment Session |
Duration of Treatment Sessions |
|---|---|---|---|---|---|---|
| Electroacupuncture + Conventional drug therapy | ||||||
| Chen 2012 [14] (China) |
GV20, EX-HN1, EX-HN3 | NR | 2 Hz frequency | 1 h | 3 times a week, 18 total sessions |
6 weeks |
| Gu 2013 [48] (China) |
Bilateral anterior parietal-temporal oblique lines (motor areas) GB20, LI11, LI4, LR3, KI3, GB34 |
NR | 2 Hz frequency, The strength the patient can tolerate |
20 min | 3 times a week, 36 total sessions |
12 weeks |
| Huang 2009 [46] (China) |
MS6, MS4, MS8, MS9, MS14 | NR | Continuous waves, 100 Hz frequency, 2−4 mA | 30 min | 6 times a week, 30 total sessions |
5 weeks |
| Lei 2016 [36] (USA) |
Foot motor sensory area, balance area, GV20, GV14, LI4, ST36, GB34, BL40, SP6, KI3, LR3 | NR | Amplitude (intensity) 3.5 and 4.5, Frequency 100 Hz or 4 Hz |
30 min | Once a week, 3 total sessions |
3 weeks |
| Liu 2016 [49] (China) |
Anterior parietal and temporal oblique lines (motor areas) on both sides, LR3, KI3, LI11, GB20, GB34, LI4 |
NR | 2 Hz frequency, The strength the patient can tolerate |
20 min | Three times a week, 36 total sessions |
12 weeks |
| Wang 2015 [40] (China) |
Bilateral GB20, LI4, Central Du14, Du16 |
2−2.5 cm | Pulses of 9 V, 1 A, 9 W, 100 Hz | 30 min | Once every three days, 20 total sessions |
2 months |
| Xu 2020 [42] (China) |
GV17, GB19, Sishenzhen, and temporal three-needle | 0.8−1.5 cm | Twisting, lifting and thrusting, continuous waves at alternating low 100 Hz frequency |
30 min | Four days per week, 32 total sessions |
8 weeks |
| MA + Conventional drug therapy | ||||||
| Cho 2012 [41] (Korea) |
Bilateral GB20, LI11, GB34, ST36, LR3 | 1.0−1.5 cm | Twisting at 2 Hz for 10 s | 20 min | Twice a week, 16 total sessions |
8 weeks |
| Jung 2006 [15] (Korea) |
Bilateral LR3, GB34 | NR | None | 15 min | Twice a week, 8 total sessions |
4weeks |
| Kluger 2016 [43] (USA) |
GV20, GV24, CV6, Bilateral LI10, HT7, ST36, SP6 |
0.5−1 cm | Twisting three times in a clockwise direction | 30 min | Twice a week (at least one day apart), 12 total sessions |
6 weeks |
| Kong 2017 [37] (Singapore) |
Bilateral PC6, LI4, ST36, SP6, KI3, CV6 |
0.5−1 inch | None | 20 min | Twice a week (at least three days apart), 10 total sessions |
5 weeks |
| Li 2018 [38] (China) |
DU20, Bilateral GB20, Chorea-Tremor Controlled Zone | 2−3 cm | Twist every 10 min | 30 min | Twice a week, 24 total sessions |
12 weeks |
| Lu 2020 [50] (China) |
LR3, LR2, LR8, KI3, KI7, KI10, SP6, ST36, LI11, PC6, LI4 | 20−30 mm | After 15 min, the needle is lifted, inserted, and twisted once | 30 min | Once a day, 28 total sessions |
28 days |
| Mizushima 2011 [44] (Japan) |
Individualized point according to meridian diagnosis | Individualized depth | Individualized way according to diagnosis | NR | Two to four times a month, NR |
5 years |
| Park 2007 [45] (Korea) |
One side LR3, GB34, ST36 | NR | None | 15 min | Twice a week, 8 total sessions |
4 weeks |
| Ren 2011 [47] (China) |
Bilateral BL18, BL23, GB20, LI11, LI4, GB34, KI3, LR3 | NR | Flattening and relieving | 30 min | Once a day, 30 total sessions |
30 days |
| BVA + Conventional drug therapy | ||||||
| Cho 2012 [41] (Korea) |
Bilateral GB20, LI11, GB34, ST36, LR3 | NR | 0.1 mL BEEV diluted to 0.005% in distilled water | - | Twice a week, 16 total sessions |
8 weeks |
| Hartmann 2016 [39] (France) |
NR | s.c | BEEV (Alyostal® 100 μg in 1 mL of NaCl 0.9%) | - | Once a month, 11 total sessions |
11 months |
NR, Not reported; SC, subcutaneous; The control group, including the placebo acupuncture group, received the same frequency and total number of acupuncture treatments as the treatment group.
3.2. Risk of Bias of the Included Studies
In random sequence generation, five studies were graded as unclear [36,40,46,48,49]. In allocation concealment, 10 studies were graded as unclear [14,15,36,40,44,45,46,48,49,50]. Nine studies were graded as high in blinding of participants because several articles were add-on study designs that cannot blind participants [14,38,41,42,43,46,48,49,50]. Furthermore, four studies were graded as unclear. In the blinding of outcome assessment, 11 studies were graded as unclear [14,15,39,43,44,45,46,47,48,49,50]. In incomplete outcome data, two studies were graded as unclear [15,44]. Six studies were graded as high as the DR was more than 10% [36,37,38,39,41,45]. One study was graded as high in selective reporting because it did not report the outcome variables previously described in the protocol [36]. Detailed visualization of each study with the risk of bias graph is presented in Supplementary Materials Digital Content 3.
3.3. Primary Outcome (Movement Function, UPDRS-III): PMA
In PMA of movement function evaluated by UPDRS-III, statistical significance was shown between the following comparisons presented with MD and 95% CI (in favor of bold marks): (1) electroacupuncture + CM (ELEC) vs. CM (CONV) (MD −3.63, 95% CI −6.05 to −1.21); (2) manual acupuncture + CM (MANU) vs. CONV (MD −3.90, 95% CI −6.24 to −1.57); (3) electroacupuncture + CM (ELEC) vs. sham acupuncture + CM (SHAM) (MD −18.10, 95% CI −30.31 to −5.89). In other comparisons, such as BVA + CM (BEEV) vs. CONV, MANU vs. SHAM, and BEEV vs. SHAM, the acupuncture modality tended to be more effective than the control group, but the difference was not statistically significant. Detailed descriptions of the effect size and each trial-based forest plot are provided in Supplementary Materials Digital Content 4.
3.4. Secondary Outcome (Daily Life Activity, UPDRS-II): PMA
In PMA of daily life activity evaluated by UPDRS-II, statistical significance was shown between the following comparisons presented with MD and 95% CI (in favor of bold marks): (1) ELEC vs. CONV (MD −4.50, 95% CI −6.19 to −2.80); (2) MANU vs. CONV (MD −4.07, 95% CI −4.87 to −3.27). In other comparisons, such as BEEV vs. CONV, ELEC vs. SHAM, and MANU vs. SHAM, the acupuncture modality showed a tendency to be more effective than the control group, but this was not statistically significant. Detailed descriptions of the effect size and each trial-based forest plot are provided in Supplementary Materials Digital Content 4.
3.5. Primary Outcome (Movement Function, UPDRS-III): NMA
3.5.1. Assumption of NMA and Network Geometry
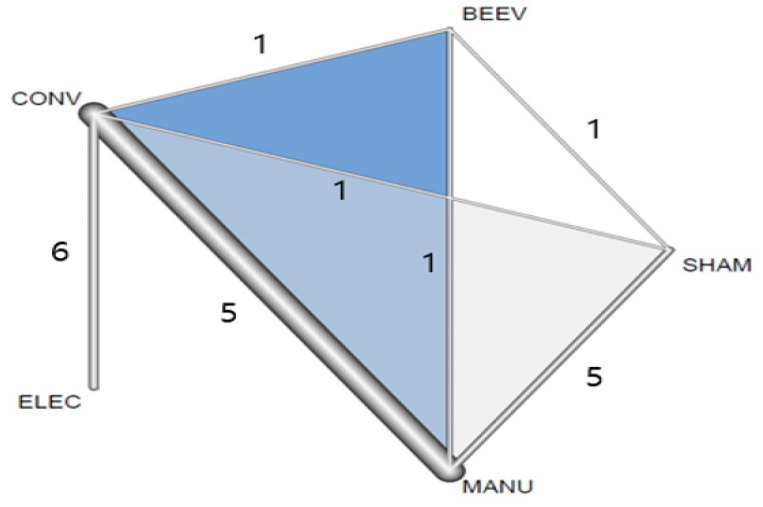
As explained in the Methods section, we decided to adopt a random effect model in the homogeneity assumption. In the transitivity assumption, the research team agreed on the transitivity of the included studies using Table 1 and Table 2. We assessed the consistency assumption using a global and local approach. In the global approach, we found significant inconsistencies (p < 0.05). In the local approach, we found inconsistency due to a study that compared ELEC and CONV (Lei 2016 [36]). After we excluded the study (Lei 2016 [36]) according to the study protocol, the consistency assumption was satisfied at the local and global levels. Net-split graphs that include direct estimates, indirect estimates, and network estimates for consistency assessment are provided in Supplementary Materials Digital Content 5. The connectivity assumption was confirmed through network geometry (net graph), which is a visual presentation of the links in the included studies (Figure 2). After excluding the study (Lei 2016 [36]), in the network analysis of the primary outcome, there were five nodes (ELEC, BEEV, MANU, CONV, SHMA) from 16 studies and 20 pairwise comparisons from seven types of comparison pairs (edges). The number of included comparisons in each edge is shown in Figure 2.
Figure 2.
Network geometry of the included studies on UPDRS-III (Net graph). BEEV, bee venom acupuncture + conventional drug therapy; CONV, single conventional drug therapy; ELEC, electroacupuncture + conventional drug therapy; MANU, manual acupuncture + conventional drug therapy; SHAM, sham acupuncture + conventional drug therapy; UPDRS, Unified PD rating scale.
3.5.2. Comparative Effectiveness of the Acupuncture Modality in UPDRS-III
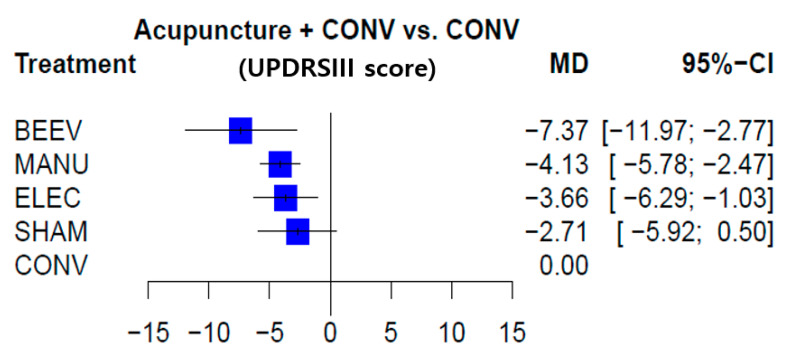
The probabilities of treatment ranking (P-score) among the included interventions were as follows: BEEV (0.9509), MANU (0.6325), ELEC (0.5349), SHAM (0.3685), and CONV (0.0132). According to the P-score, BEEV is most likely the best acupuncture modality for movement function assessed by the UPDRS-III (Figure 3 and Table 3). Mixed effect estimates (combining direct and indirect estimates) for each intervention compared with CONV were as follows (in favor of bold marks): BEEV (MD −7.37, 95% CI −11.97 to −2.77); MANU (MD −4.13, 95% CI −5.78 to −2.47); ELEC (MD −3.66, 95% CI −6.29 to −1.03); SHAM (MD −2.71, 95% CI −5.92 to 0.50). BEEV, MANU, and ELEC were superior to CONV in UPDRS-III. However, SHAM was not statistically significant. No difference was observed in the comparison between the different acupuncture modalities (Table 3).
Figure 3.
Treatment level network meta-analysis forest plot (UPDRS-III). BEEV, bee venom acupuncture + conventional drug therapy; CONV, single conventional drug therapy; ELEC, electroacupuncture + conventional drug therapy; MANU, manual acupuncture + conventional drug therapy; SHAM, sham acupuncture + conventional drug therapy; UPDRS, Unified PD rating scale.
Table 3.
League table on UPDRS-III.
| BEEV | −3.31(−8.91;2.29) | −7.13(−16.98;2.72) | −5.44(−12.53;1.65) | |
| −3.24(−7.72;1.23) | MANU | −1.41(−4.38;1.57) | −4.07(−5.72;−2.41;) | |
| −3.71(−9.01;1.59) | −0.47(−3.57;2.64) | ELEC | - | −3.66(−6.29;−1.03) |
| −4.66(−9.63;0.31) | −1.42(−4.24;1.41) | −0.95(−5.10;3.20) | SHAM | −7.48(−17.54;2.58) |
| −7.37(−11.97;−2.77) | −4.13(−5.78;−2.47) | −3.66(−6.29;−1.03) | −2.71(−5.92;0.50) | CONV |
BEEV, bee venom acupuncture + conventional drug therapy; CONV, single conventional drug therapy; ELEC, electroacupuncture + conventional drug therapy; MANU, manual acupuncture + conventional drug therapy; SHAM, sham acupuncture + conventional drug therapy; UPDRS, Unified PD rating scale.
The part highlighted in BOLD with underlining is a comparison with statistically significant results. The upper right triangle presents the effect size estimated using only direct comparison. As direct comparison does not exist in all treatment comparisons, there are several blanks in the upper right triangle. The lower left triangle provides a pooled estimation of the direct and indirect comparisons of the effect size.
3.5.3. Sensitivity Analysis
After excluding one study in the sensitivity analysis, (1) BEEV showed a tendency to be most effective in all 16 analyses; (2) in three sensitivity analyses (when excluding [40,44,47]), the ranking between MANU and ELEC was changed, with ELEC showing a better effect; and (3) CONV tended to have the smallest effect size throughout the analysis (Supplementary Materials Digital Content 6).
3.6. Secondary Outcome (Daily Life Activity, UPDRS-II): NMA
3.6.1. Assumption of NMA and Network Geometry
Homogeneity and transitivity assumptions are the same as those described in Section 3.5.1. We assessed the consistency assumption via a global and local approach and found no evidence of inconsistency after excluding the study by Lei [36]. The connectivity assumption was confirmed through network geometry (Supplementary Materials Digital Content 7). There were five nodes (ELEC, BEEV, MANU, CONV, and SHMA) from 10 studies and 14 pairwise comparisons from six types of comparison pairs (edges).
3.6.2. Comparative Effectiveness of the Acupuncture Modality in UPDRS-II
The probability of treatment as the best treatment option was presented through a measure called the P-score. The P-scores of the included modalities were as follows: BEEV (0.8971), ELEC (0.6685), MANU (0.5527), SHAM (0.3801), and CONV (0.0016). According to the P-score, BEEV was found to most likely be the best acupuncture modality for activities of daily life assessed by the UPDRS-II. The estimated effect size of each acupuncture modality compared to CONV via the NMA is presented in a treatment level forest plot and league table (Supplementary Materials Digital Content 7). In the treatment level forest plot and league table, the network estimate of the effect size (combining direct and indirect estimates) compared to CONV was as follows (in favor of bold marks): BEEV (MD −6.07, 95% CI −9.41 to −2.72); ELEC (MD −4.50, 95% CI −6.19 to −2.80); MANU (MD −4.08, 95% CI −4.84 to −3.32); SHAM (MD −3.21, 95% CI −5.72 to −0.70). BEEV, MANU, ELEC, and SHAM were superior to CONV in the UPDRS-II. As UPDRS-II is a secondary outcome, we did not conduct an additional sensitivity analysis.
3.7. Adverse Events (AEs)
AEs were also assessed in the present study. Based on the comparisons, AE rates are summarized as follows. Reported AEs according to RCT design are as follows: (1) ELEC vs. CONV design: 3/30 AEs in the ELEC group vs. 12/30 AEs in the CONV group were reported (Chen 2012 [14]); (2) MANU vs. CONV: not reported; (3) BEEV vs. CONV: 1/18 AEs in the BEEV group were reported (Cho 2012 [41]); (4) ELEC vs. SHAM: not reported; (5) MANU vs. SHAM: 1/47 in the MANU group was reported (Kluger 2016 [43]); and (6) BEEV vs. SHAM: 4/20 AEs in the BEEV group were reported (Hartmann 2016 [39]).
3.8. Publication Bias
A network funnel plot of the primary outcome (UPDRS-III) was constructed. There was no significant asymmetry seen in the visual inspection of the funnel plot (Figure 4). The Egger’s test did not find any significant evidence of publication bias (p = 0.269). In the secondary outcome (UPDRS-II), there was no evidence of publication bias (Supplementary Materials Digital Content 7).
Figure 4.
Network funnel plot: UPDRS-III score.
4. Discussion
4.1. Summary of Findings
The purpose of this PMA and NMA was to explore which acupuncture treatment modality combined with conventional drug therapy is more effective than conventional drug therapy alone for the improvement of motor symptoms (UPDRS-III) and activity of daily living (UPDRS-II) in PD. In NMA on motor symptoms (UPDRS-III), the order of effect size was BEEV, MANU, ELEC, SHAM, and CONV. BVA combination therapy is most likely the best modality for movement symptoms. In NMA on activities of daily living (UPDRS-II), the order of the effect size was BEEV, ELEC, MANU, SHAM, and CONV. BVA combination therapy is most likely to be the best modality for activities of daily living. No serious AEs were observed.
4.2. Implications for Clinical Practice and Suggestions for Further Research
The mechanism and therapeutic effect of acupuncture on PD have been elucidated in several studies. In a PD animal model, the expression of tropomyosin receptor kinase B (trkB) was increased in the ipsilateral substantia nigra, and a neuroprotective effect on neuronal cell death was revealed [51]. It also exhibits dopaminergic neuroprotective effects by inducing hypothalamic melanin-concentrating hormone biosynthesis [52]. As a result, it is possible to improve motor behavior while reducing the loss of dopaminergic neurons [51]. In a mechanistic study with functional MRI, acupuncture treatment for patients with PD demonstrated that the putamen and primary motor cortex were activated, and motor function was improved [51]. The mechanism of BVA has also been studied. Apamin toxin contained in BEEV is a polypeptide neurotoxin that blocks Ca2+ activated K + (SK) channels and induces hyperpolarization of dopaminergic neurons, thereby partially rescuing dopaminergic neurons in dissociated midbrain cell cultures [53]. BVA increases the size and number of neurons and striatal dopamine and protects dopaminergic neurons. Therefore, when BEEV is used alone or in combination with conventional drugs for PD, neuronal degeneration is alleviated, and movement disorders are reduced [54]. Several systematic reviews and meta-analyses of RCTs have also been published about the effect and safety of several acupuncture treatment modalities on PD [13,16,17,55].
However, it is unclear which acupuncture modality has a better effect and should be considered in clinical practice and research on PD. Therefore, we performed this NMA to help clinicians and researchers decide which acupuncture modality to use for PD. Although several NMA studies on acupuncture for various diseases have been reported [56,57,58], this is the first NMA study of acupuncture on PD. In our study, BEEV seems to be the best therapeutic option for motor symptoms and activities of daily living in patients with PD. However, the 95% CI overlapped different acupuncture modalities. Therefore, caution should be exercised when applying the results of this study to clinical practice and clinical research. In terms of effect size, the minimal clinical important differences (MCIDs) of the UPDRS motor scores were 2.5 points (minimal effect), 5.2 points (moderate effect), and 10.8 points (large effect) [59]. It was similar (approximately 5–7) in other MCID studies on the UPDRS III scores in patients with PD [60,61,62]. Considering the previous results of the MCID study, our results for the BEEV group showed a clinically significant moderate effect. The effect sizes of ELEC and MANU existed between minimal and moderate effects.
From a clinical perspective, even though BEEV might be the best option for motor symptoms and activities of daily living, MANU/ELEC might be an appropriate option for several motor symptoms [63]. In the presence of severe tremors, it may be difficult to use ELECs in the distal extremities. Therefore, physicians can try electroacupuncture treatment using acupuncture points on the scalp. BEEV might be inappropriate in some cases due to the risk of AEs, such as anaphylaxis [64]. In our results, MANU and ELEC had the best effect after BEEV in UPDRS-II and III. Therefore, if it is difficult to apply BEEV due to Aes, MANU or ELEC could be used as an alternative approach. However, the superiority between MANU and ELEC could not be determined in our study. In the sensitivity analysis, after excluding a long-term follow-up manual acupuncture study [44], ELEC was found to be better than MANU in UPDRS-III. Therefore, it might be possible that the treatment dose (number of sessions) might be an important factor for the therapeutic effect, but as the number of RCTs included in this study was relatively small, we could not conduct further analysis. As head-to-head comparison studies on ELEC and MANU are not common, meta-regression analysis or real-world evidence-based research with health insurance data are needed to address this issue. In summary, when deciding on the acupuncture treatment strategy for patients with PD in clinical practice, we need to consider several factors, such as applicability, adherence, AEs, and target symptoms. In real-world clinical practice, as an overlap of 95% CI of the effect size is clearly visible, it is recommended that BEEV combined with MA with/without electrical stimulation is recommended. Based on the results of this study, in clinical practice, we recommend using electroacupuncture on GB20 (Fengchi) and GB34 (Yanglingquan) for approximately 20–30 min in patients with PD from a clinical point of view. Since bee venom is a natural toxin, in terms of safety, therapeutic dosage is very important. In our study, the total amount of BEEV per session and total number of treatment sessions applied in our review were 100 μg (in 1 mL of NaCl 0.9%) for 11 sessions [39] and 50 μg (in 1 mL of NaCl 0.9%) for 16 sessions [41], respectively. With regard to safety, attention should be paid to side effects (such as anaphylaxis) when higher doses of BEEV than those reported in this study are applied. In addition to predictable dose-dependent side effects, non-predictable side effects due to individual sensitivity should also be considered.
Interestingly, the combined treatment of sham acupuncture with conventional medicine group (SHAM) was superior to the conventional medicine alone group (CONV). Placebo acupuncture is known to have a larger non-specific effect than other physical and pharmacological placebo modalities [65]. Sham acupuncture is known to be more effective than usual care or wait-list control groups for musculoskeletal diseases, such as non-specific low back pain [66]. Our study suggests that sham acupuncture might also have considerable non-specific effects on degenerative neurological diseases, such as PD. Therefore, a sham acupuncture-controlled design might underestimate the effect of acupuncture treatment. A pragmatic clinical study on comprehensive acupuncture treatment (combining ELEC, MANU, and BEEV) compared to an active control group (such as rehabilitation, medication, qi-gong) might be a more appropriate design to address physicians’ questions about which intervention should be added to CM.
4.3. Strengths and Limitations
Our study had several strengths. This is the first NMA acupuncture study for PD in an area that is difficult to conduct clinical trials due to resource limitations and research priorities. We included studies across multiple databases without language restrictions. The assumptions for performing the network meta-analysis were systemically reviewed, and there was a methodological advantage in that a sensitivity analysis was performed to confirm the robustness of the NMA results. We provided the NMA results with MD (not standardized MD) for applicability and interpretability in clinical practice.
However, this study has several limitations. First, the number of included studies and types of acupuncture modalities were relatively small. Heterogeneity exists between acupuncture regimens, even though we adopted a random-effects model. Therefore, further acupuncture RCTs on PD are needed to ensure the robustness of our results. In further NMA studies with more clinical RCTs, we can focus on more specific clinical questions, such as responders to acupuncture treatment in terms of severity, age, sex, disease duration, and accompanying symptoms [67]. In terms of dosage, we could not conduct a subgroup analysis of treatment duration, frequency, or needle retention time due to the lack of relevant studies. Since it is an important factor for the therapeutic effect of acupuncture [68,69], we need further subgroup analysis or meta-regression studies for detailed treatment regimens and dosages in acupuncture treatment. Second, in the sensitivity analysis, although this is largely consistent with the results of the primary analysis, the order of the effect sizes of ELEC and MANU was reversed in some cases. This suggests that it is difficult to differentiate between ELECs and MANUs. Further research is needed on this issue from an academic perspective. However, from a clinical perspective, it is recommended to combine electroacupuncture and MA simultaneously based on CM, as a commercial electroacupuncture device usually covers less than 12 acupuncture points. Third, we excluded combined acupuncture strategies, such as BVA combined with electroacupuncture, to explore the effect of a single acupuncture modality. However, in real-world clinical practice, each acupuncture modality is combined with other types of acupuncture. Therefore, we could not assess the synergetic effects of acupuncture modalities. Moreover, we might have underestimated the effects of acupuncture. Because the number of relevant RCTs was insufficient, further NMA studies are also needed on combined acupuncture modalities in the future. Next, the methodological quality of the included RCTs was relatively poor. Therefore, caution should be exercised when interpreting these results. Caution is also required when interpreting our results, as the reference group (CONV) of NMA had considerable heterogeneity. Finally, we included only CM in the reference (control) group. However, there are various standard treatments, such as surgical intervention and rehabilitation. As we used pharmacologic treatment as a control group, it might provide different results when using non-pharmacological intervention as a control group in the further NMA study.
5. Conclusions
We conducted a PMA and NMA to evaluate the effects of various acupuncture modalities on patients with idiopathic PD. The probability of comparative effectiveness in motor symptoms of patients with idiopathic PD was assumed to be in the order of BEEV, MANU, ELEC, SHAM, and CONV. However, more rigorous RCTs are needed for further NMA, including non-motor symptoms of PD. Along with conventional levodopa therapy, BVA, electroacupuncture, and MA could be more effective in clinical practice than single-drug therapy.
Acknowledgments
This article is based on the doctorate thesis of Miri Kwon.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/healthcare9111502/s1. Supplemental Digital Content 1. Search strategies used on each database. Supplemental Digital Content 2. Articles excluded after the full text review with reasons. Supplemental Digital Content 3. Risk of bias graph. Supplemental Digital Content 4. Forest plot of PMA (UPDRS-III and UPDRS-II). Supplemental Digital Content 5. Net-split plot: UPDRS-III score. Supplemental Digital Content 6. Sensitivity analysis. Supplemental Digital Content 7. Network plot, forest plot, league table, net-split plot, and funnel plot on the secondary outcome (UPDRS-II).
Author Contributions
Conceptualization, J.L. and T.-h.K.; methodology, M.J.C.; software, J.L.; validation, T.-h.K.; formal analysis, J.L. and M.K.; investigation, M.K.; resources, J.L.; data curation, M.J.C.; writing—original draft preparation, M.K.; writing—review and editing, J.L. and T.-h.K.; visualization, J.L.; supervision, T.-h.K.; project administration, J.L.; funding acquisition, J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. NRF-2019R1F1A1059310).
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available on request to the corresponding author. The study protocol is available at the open science platform (https://osf.io/q8n7z/).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Armstrong M.J., Okun M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA. 2020;323:548–560. doi: 10.1001/jama.2019.22360. [DOI] [PubMed] [Google Scholar]
- 2.Tolosa E., Wenning G., Poewe W. The Diagnosis of Parkinson’s Disease. Lancet Neurol. 2006;5:75–86. doi: 10.1016/S1474-4422(05)70285-4. [DOI] [PubMed] [Google Scholar]
- 3.Tysnes O.-B., Storstein A. Epidemiology of Parkinson’s Disease. J. Neural Transm. 2017;124:901–905. doi: 10.1007/s00702-017-1686-y. [DOI] [PubMed] [Google Scholar]
- 4.Abbas M.M., Xu Z., Tan L.C.S. Epidemiology of Parkinson’s Disease—East Versus West. Mov. Disord. Clin. Pract. 2017;5:14–28. doi: 10.1002/mdc3.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong M.J., Okun M.S. Choosing a Parkinson Disease Treatment. JAMA. 2020;323:1420. doi: 10.1001/jama.2020.1224. [DOI] [PubMed] [Google Scholar]
- 6.Schapira A.H.V., Chaudhuri K.R., Jenner P. Non-Motor Features of Parkinson Disease. Nat. Rev. Neurosci. 2017;18:435–450. doi: 10.1038/nrn.2017.62. [DOI] [PubMed] [Google Scholar]
- 7.Freitas M.E., Hess C.W., Fox S.H. Motor Complications of Dopaminergic Medications in Parkinson’s Disease. Semin. Neurol. 2017;37:147–157. doi: 10.1055/s-0037-1602423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun B., Wang T., Li N., Qiao J. Analysis of Motor Complication and Relative Factors in a Cohort of Chinese Patients with Parkinson’s Disease. Park. Dis. 2020;2020:e8692509. doi: 10.1155/2020/8692509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abosch A., Gross R.E. Surgical Treatment of Parkinson’s Disease: Deep Brain Stimulation versus Radiofrequency Ablation. Clin. Neurosurg. 2004;51:296–303. [PubMed] [Google Scholar]
- 10.Rossi M., Bruno V., Arena J., Cammarota Á., Merello M. Challenges in PD Patient Management After DBS: A Pragmatic Review. Mov. Disord. Clin. Pract. 2018;5:246–254. doi: 10.1002/mdc3.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sveinbjornsdottir S. The Clinical Symptoms of Parkinson’s Disease. J. Neurochem. 2016;139:318–324. doi: 10.1111/jnc.13691. [DOI] [PubMed] [Google Scholar]
- 12.Ghaffari B.D., Kluger B. Mechanisms for Alternative Treatments in Parkinson’s Disease: Acupuncture, Tai Chi, and Other Treatments. Curr. Neurol. Neurosci. Rep. 2014;14:451. doi: 10.1007/s11910-014-0451-y. [DOI] [PubMed] [Google Scholar]
- 13.Lee S.-H., Lim S. Clinical Effectiveness of Acupuncture on Parkinson Disease: A PRISMA-Compliant Systematic Review and Meta-Analysis. Medicine. 2017;96:e5836. doi: 10.1097/MD.0000000000005836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y., Feng W., Zhang X. Parkinson’s disease combined with overactive bladder syndrome treated with acupuncture and medication. Chin. Acupunct. Moxibustion. 2012;32:215–218. [PubMed] [Google Scholar]
- 15.Jung J., Kim K., Park Y., Kim H., Lee S., Chang D., Lee Y. The Study on the Effect of Acupuncture on UPDRS and Heart Rate Variability in the Patients with Idiopathic Parkinson’s Disease. J. Korean Acupunct. Moxibustion Soc. 2006;23:143–153. [Google Scholar]
- 16.Noh H., Kwon S., Cho S.-Y., Jung W.-S., Moon S.-K., Park J.-M., Ko C.-N., Park S.-U. Effectiveness and Safety of Acupuncture in the Treatment of Parkinson’s Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Complement. Ther. Med. 2017;34:86–103. doi: 10.1016/j.ctim.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Liu H., Chen L., Zhang Z., Geng G., Chen W., Dong H., Chen L., Zhan S., Li T. Effectiveness and Safety of Acupuncture Combined with Madopar for Parkinson—s Disease: A Systematic Review with Meta-Analysis. Acupunct. Med. 2017;35:404–412. doi: 10.1136/acupmed-2016-011342. [DOI] [PubMed] [Google Scholar]
- 18.Rouse B., Chaimani A., Li T. Network Meta-Analysis: An Introduction for Clinicians. Intern. Emerg. Med. 2017;12:103–111. doi: 10.1007/s11739-016-1583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pratt M., Wieland S., Ahmadzai N., Butler C., Wolfe D., Pussagoda K., Skidmore B., Veroniki A., Rios P., Tricco A.C., et al. A Scoping Review of Network Meta-Analyses Assessing the Efficacy and Safety of Complementary and Alternative Medicine Interventions. Syst. Rev. 2020;9 doi: 10.1186/s13643-020-01328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanters S., Ford N., Druyts E., Thorlund K., Mills E.J., Bansback N. Use of Network Meta-Analysis in Clinical Guidelines. Bull. World Health Organ. 2016;94:782–784. doi: 10.2471/BLT.16.174326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutton B., Salanti G., Caldwell D.M., Chaimani A., Schmid C.H., Cameron C., Ioannidis J.P.A., Straus S., Thorlund K., Jansen J.P., et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 22.Tambasco N., Romoli M., Calabresi P. Levodopa in Parkinson’s Disease: Current Status and Future Developments. Curr. Neuropharmacol. 2018;16:1239–1252. doi: 10.2174/1570159X15666170510143821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fahn S., Oakes D., Shoulson I., Kieburtz K., Rudolph A., Lang A., Olanow C.W., Tanner C., Marek K., Parkinson Study Group Levodopa and the Progression of Parkinson’s Disease. N. Engl. J. Med. 2004;351:2498–2508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- 24.Ramaker C., Marinus J., Stiggelbout A.M., Hilten B.J. van Systematic Evaluation of Rating Scales for Impairment and Disability in Parkinson’s Disease. Mov. Disord. 2002;17:867–876. doi: 10.1002/mds.10248. [DOI] [PubMed] [Google Scholar]
- 25.Goetz C.G., Fahn S., Martinez-Martin P., Poewe W., Sampaio C., Stebbins G.T., Stern M.B., Tilley B.C., Dodel R., Dubois B., et al. Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Process, Format, and Clinimetric Testing Plan. Mov. Disord. Off. J. Mov. Disord. Soc. 2007;22:41–47. doi: 10.1002/mds.21198. [DOI] [PubMed] [Google Scholar]
- 26.Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; Hoboken, NJ, USA: 2019. [Google Scholar]
- 27.Egger M., Davey Smith G., Schneider M., Minder C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borenstein M., Hedges L.V., Higgins J.P.T., Rothstein H.R. A Basic Introduction to Fixed-Effect and Random-Effects Models for Meta-Analysis. Res. Synth. Methods. 2010;1:97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 29.Shim S.R., Kim S.-J., Lee J., Rücker G. Network Meta-Analysis: Application and Practice Using R Software. Epidemiol. Health. 2019;41 doi: 10.4178/epih.e2019013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watt J., Tricco A.C., Straus S., Veroniki A.A., Naglie G., Drucker A.M. Research Techniques Made Simple: Network Meta-Analysis. J. Investig. Dermatol. 2019;139:4–12.e1. doi: 10.1016/j.jid.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 31.Biondi-Zoccai G., Abbate A., Benedetto U., Palmerini T., D’Ascenzo F., Frati G. Network Meta-Analysis for Evidence Synthesis: What Is It and Why Is It Posed to Dominate Cardiovascular Decision Making? Int. J. Cardiol. 2015;182:309–314. doi: 10.1016/j.ijcard.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 32.Tonin F.S., Rotta I., Mendes A.M., Pontarolo R. Network Meta-Analysis: A Technique to Gather Evidence from Direct and Indirect Comparisons. Pharm. Pract. 2017;15:943. doi: 10.18549/PharmPract.2017.01.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rücker G., Schwarzer G. Ranking Treatments in Frequentist Network Meta-Analysis Works without Resampling Methods. BMC Med. Res. Methodol. 2015;15:58. doi: 10.1186/s12874-015-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higgins J.P.T., Jackson D., Barrett J.K., Lu G., Ades A.E., White I.R. Consistency and Inconsistency in Network Meta-Analysis: Concepts and Models for Multi-Arm Studies. Res. Synth. Methods. 2012;3:98–110. doi: 10.1002/jrsm.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bowden J., Davey Smith G., Burgess S. Mendelian Randomization with Invalid Instruments: Effect Estimation and Bias Detection through Egger Regression. Int. J. Epidemiol. 2015;44:512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lei H., Toosizadeh N., Schwenk M., Sherman S., Karp S., Sternberg E., Najafi B. A Pilot Clinical Trial to Objectively Assess the Efficacy of Electroacupuncture on Gait in Patients with Parkinson’s Disease Using Body Worn Sensors. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0155613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kong K.H., Ng H.L., Li W., Ng D.W., Tan S.I., Tay K.Y., Au W.L., Tan L.C.S. Acupuncture in the Treatment of Fatigue in Parkinson’s Disease: A Pilot, Randomized, Controlled, Study. Brain Behav. 2017;8 doi: 10.1002/brb3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Z., Chen J., Cheng J., Huang S., Hu Y., Wu Y., Li G., Liu B., Liu X., Guo W., et al. Acupuncture Modulates the Cerebello-Thalamo-Cortical Circuit and Cognitive Brain Regions in Patients of Parkinson’s Disease with Tremor. Front. Aging Neurosci. 2018;10 doi: 10.3389/fnagi.2018.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hartmann A., Müllner J., Meier N., Hesekamp H., van Meerbeeck P., Habert M.-O., Kas A., Tanguy M.-L., Mazmanian M., Oya H., et al. Bee Venom for the Treatment of Parkinson Disease—A Randomized Controlled Clinical Trial. PLoS ONE. 2016;11:e0158235. doi: 10.1371/journal.pone.0158235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang F., Sun L., Zhang X., Jia J., Liu Z., Huang X., Yu S., Zuo L., Cao C., Wang X., et al. Effect and Potential Mechanism of Electroacupuncture Add-On Treatment in Patients with Parkinson’s Disease. Evid.-Based Complement. Altern. Med. ECAM. 2015;2015 doi: 10.1155/2015/692795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho S.-Y., Shim S.-R., Rhee H.Y., Park H.-J., Jung W.-S., Moon S.-K., Park J.-M., Ko C.-N., Cho K.-H., Park S.-U. Effectiveness of Acupuncture and Bee Venom Acupuncture in Idiopathic Parkinson’s Disease. Parkinsonism Relat. Disord. 2012;18:948–952. doi: 10.1016/j.parkreldis.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 42.Xu Y., Cai X., Qu S., Zhang J., Zhang Z., Yao Z., Huang Y., Zhong Z. Madopar Combined with Acupuncture Improves Motor and Non-Motor Symptoms in Parkinson’s Disease Patients: A Multicenter Randomized Controlled Trial. Eur. J. Integr. Med. 2020;34:101049. doi: 10.1016/j.eujim.2019.101049. [DOI] [Google Scholar]
- 43.Kluger B.M., Rakowski D., Christian M., Cedar D., Wong B., Crawford J., Uveges K., Berk J., Abaca E., Corbin L., et al. Randomized, Controlled Trial of Acupuncture for Fatigue in Parkinson’s Disease. Mov. Disord. 2016;31:1027–1032. doi: 10.1002/mds.26597. [DOI] [PubMed] [Google Scholar]
- 44.Takeo M. Treatment Results between Matched Pair of L-Dopa Medication Treatment and Acupuncture Treatment Combination on Parkinson Disease. Kampo Med. 2011;62:691–694. doi: 10.3937/kampomed.62.691. (In Japanese) [DOI] [Google Scholar]
- 45.Park Y.-C., Chang D.-I., Lee Y.-H., Park D.-S. The Study on the Effect of Acupuncture Treatment in Patients with Idiopathic Parkinson’s Disease. J. Acupunct. Res. 2007;24:43–54. [Google Scholar]
- 46.Yong H., Ying Z., XueMei J., AnWu T., DongJiang L., Ming S., ZhouHua W. Effect of scalp acupuncture on regional cerebral blood flow in Parkinson’s disease patients. China J. Tradit. Chin. Med. Pharm. 2009;24:305–308. [Google Scholar]
- 47.Ren X., Shi Y., Song S., Hu X., Han Z. Clinical Study on Acupuncture Tonifying Liver and Kidney in the Treatment of Parkinson Disease (in Chinese) Chin. Arch. Tradit. Chin. Med. 2011;29:2470–2473. [Google Scholar]
- 48.Gu K., Liu K., Lu Z., Fan X., Zong L. Clinical Observations on Combined Treatment of Parkinson’s Disease Using Acupuncture and Medicine. Shanghai J. Acu-Mox. 2013;32:993–995. [Google Scholar]
- 49.Liu B. Clinical Study on Acupuncture Treatment of Parkinson’s Disease. China Health Stand. Manag. 2016;7:128–129. [Google Scholar]
- 50.Lu Y.-K., Wang X.-Z., Yang G.-F., Yang H.-Y. Clinical Observation of Liver-Calming and Kidney-Nourishing Acupuncture Therapy Combined with Acupuncture at Starting and Ending Points of Muscles in Treating Middle and Late Stages of Parkinson’s Disease. J. Guangzhou Univ. Tradit. Chin. Med. 2020;37:1907–1912. [Google Scholar]
- 51.Park H.-J., Lim S., Joo W.-S., Yin C.-S., Lee H.-S., Lee H.-J., Seo J.-C., Leem K., Son Y.-S., Kim Y.-J., et al. Acupuncture Prevents 6-Hydroxydopamine-Induced Neuronal Death in the Nigrostriatal Dopaminergic System in the Rat Parkinson’s Disease Model. Exp. Neurol. 2003;180:93–98. doi: 10.1016/S0014-4886(02)00031-6. [DOI] [PubMed] [Google Scholar]
- 52.Park J.-Y., Kim S.-N., Yoo J., Jang J., Lee A., Oh J.-Y., Kim H., Oh S.T., Park S.-U., Kim J., et al. Novel Neuroprotective Effects of Melanin-Concentrating Hormone in Parkinson’s Disease. Mol. Neurobiol. 2017;54:7706–7721. doi: 10.1007/s12035-016-0258-8. [DOI] [PubMed] [Google Scholar]
- 53.Salthun-Lassalle B., Hirsch E.C., Wolfart J., Ruberg M., Michel P.P. Rescue of Mesencephalic Dopaminergic Neurons in Culture by Low-Level Stimulation of Voltage-Gated Sodium Channels. J. Neurosci. 2004;24:5922–5930. doi: 10.1523/JNEUROSCI.5668-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Badawi H.M., Abdelsalam R.M., Abdel-Salam O.M., Youness E.R., Shaffie N.M., Eldenshary E.-E.D.S. Bee Venom Attenuates Neurodegeneration and Motor Impairment and Modulates the Response to L-Dopa or Rasagiline in a Mice Model of Parkinson’s Disease. Iran. J. Basic Med. Sci. 2020;23:1628–1638. doi: 10.22038/ijbms.2020.46469.10731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang J., Qin X., Cai X., Huang Y. Effectiveness of Acupuncture in the Treatment of Parkinson’s Disease: An Overview of Systematic Reviews. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tan X., Pan Y., Su W., Gong S., Zhu H., Chen H., Lu S. Acupuncture Therapy for Essential Hypertension: A Network Meta-Analysis. Ann. Transl. Med. 2019;7 doi: 10.21037/atm.2019.05.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang J., Liu Y., Huang X., Chen Y., Hu L., Lan K., Yu H. Efficacy Comparison of Different Acupuncture Treatments for Functional Dyspepsia: A Systematic Review with Network Meta-Analysis. Evid.-Based Complement. Altern. Med. ECAM. 2020;2020 doi: 10.1155/2020/3872919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu H., Shi Y., Xiao Y., Liu P., Wu S., Pang P., Deng L., Chen X. Efficacy Comparison of Different Acupuncture Treatments for Primary Insomnia: A Bayesian Analysis. Evid.-Based Complement. Altern. Med. ECAM. 2019;2019 doi: 10.1155/2019/8961748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shulman L.M., Gruber-Baldini A.L., Anderson K.E., Fishman P.S., Reich S.G., Weiner W.J. The Clinically Important Difference on the Unified Parkinson’s Disease Rating Scale. Arch. Neurol. 2010;67:64–70. doi: 10.1001/archneurol.2009.295. [DOI] [PubMed] [Google Scholar]
- 60.Hauser R.A., Gordon M.F., Mizuno Y., Poewe W., Barone P., Schapira A.H., Rascol O., Debieuvre C., Fräßdorf M. Minimal Clinically Important Difference in Parkinson’s Disease as Assessed in Pivotal Trials of Pramipexole Extended Release. Park. Dis. 2014;2014:e467131. doi: 10.1155/2014/467131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Horváth K., Aschermann Z., Ács P., Deli G., Janszky J., Komoly S., Balázs É., Takács K., Karádi K., Kovács N. Minimal Clinically Important Difference on the Motor Examination Part of MDS-UPDRS. Parkinsonism Relat. Disord. 2015;21:1421–1426. doi: 10.1016/j.parkreldis.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 62.Sánchez-Ferro Á., Matarazzo M., Martínez-Martín P., Martínez-Ávila J.C., Gómez de la Cámara A., Giancardo L., Arroyo Gallego T., Montero P., Puertas-Martín V., Obeso I., et al. Minimal Clinically Important Difference for UPDRS-III in Daily Practice. Mov. Disord. Clin. Pract. 2018;5:448–450. doi: 10.1002/mdc3.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zeng B.-Y., Zhao K. Effect of Acupuncture on the Motor and Nonmotor Symptoms in Parkinson’s Disease—A Review of Clinical Studies. CNS Neurosci. Ther. 2016;22:333–341. doi: 10.1111/cns.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cherniack E.P., Govorushko S. To Bee or Not to Bee: The Potential Efficacy and Safety of Bee Venom Acupuncture in Humans. Toxicon Off. J. Int. Soc. Toxinol. 2018;154:74–78. doi: 10.1016/j.toxicon.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 65.Linde K., Niemann K., Meissner K. Are Sham Acupuncture Interventions More Effective than (Other) Placebos? A Re-Analysis of Data from the Cochrane Review on Placebo Effects. Complement. Med. Res. 2010;17:259–264. doi: 10.1159/000320374. [DOI] [PubMed] [Google Scholar]
- 66.Xiang Y., He J., Li R. Appropriateness of Sham or Placebo Acupuncture for Randomized Controlled Trials of Acupuncture for Nonspecific Low Back Pain: A Systematic Review and Meta-Analysis. J. Pain Res. 2017;11:83–94. doi: 10.2147/JPR.S152743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leem J. Acupuncture for Motor Symptom Improvement in Parkinson’s Disease and the Potential Identification of Responders to Acupuncture Treatment. Integr. Med. Res. 2016;5:332–335. doi: 10.1016/j.imr.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bauer M., McDonald J.L., Saunders N. Is Acupuncture Dose Dependent? Ramifications of Acupuncture Treatment Dose within Clinical Practice and Trials. Integr. Med. Res. 2020;9:21–27. doi: 10.1016/j.imr.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leem J. Does Acupuncture Reduce the Risk of Acute Myocardial Infarction? Integr. Med. Res. 2016;5:165–168. doi: 10.1016/j.imr.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on request to the corresponding author. The study protocol is available at the open science platform (https://osf.io/q8n7z/).