Abstract
The androgen receptor (AR) is a member of the nuclear receptor superfamily and has three functional domains, namely the N-terminal, DNA binding, and C-terminal domain. The N-terminal domain harbors potent transactivation functions, whereas the C-terminal domain binds to androgens and antiandrogens used to treat prostate cancer. AR has genomic activity being DNA binding-dependent or through interaction with other DNA-bound transcription factors, as well as a number of non-genomic, non-canonical functions, such as the activation of the ERK, AKT, and MAPK pathways. A bulk of evidence indicates that non-coding RNAs have functional interactions with AR. This type of interaction is implicated in the pathogenesis of human malignancies, particularly prostate cancer. In the current review, we summarize the available data on the role of microRNAs, long non-coding RNAs, and circular RNAs on the expression of AR and modulation of AR signaling, as well as the effects of AR on their expression. Recognition of the complicated interaction between non-coding RNAs and AR has practical importance in the design of novel treatment options, as well as modulation of response to conventional therapeutics.
Keywords: androgen receptor, miRNA, lncRNA, circular RNAs, prostate cancer
1. Introduction
The androgen receptor (AR), alternatively named as NR3C4 (nuclear receptor subfamily 3, group C, member 4), is a nuclear receptor [1] that is activated by a number of androgens, such as testosterone and its more active form, dihydrotestosterone [2]. AR has three protein domains, namely the N-terminal transcriptional regulation, DNA binding, and C-terminal ligand binding domain [3]. After being activated by its ligands in the cytoplasm, AR is transferred into the nucleus, where it exerts its main DNA-binding-dependent functions [4]. In fact, AR is a cytoplasmic protein in the absence of its ligands associated with chaperone proteins, such as heat shock proteins and co-chaperones. Androgen binding to the AR results in conformational changes, dissociating it from chaperone proteins [4]. After translocation to the nucleus, the androgen/AR complex dimerizes and binds to androgen response elements (AREs), which are present in the AR target genes. This process is involved in the regulation of the expression of target genes [5]. In addition to this canonical route of action, the androgen/AR complex has other functions that are mediated through non-DNA-binding-dependent routes [6]. Modulation of activity of ERK, AKT, and MAPK pathways are examples of this kind of function [6,7,8].
Notably, specific coregulators have been found to modulate the transcriptional activity of androgen/AR complex. The binding of coregulators with androgen/AR complex can either enhance (via coactivators) or suppress (via corepressors) its transactivation capability. This process is accomplished via the epigenetic changing of chromatin configuration and histone modifications [5].
A bulk of evidence indicates that non-coding RNAs (ncRNAs) have functional interactions with AR. This type of interaction is implicated in the pathogenesis of human malignancies, particularly prostate cancer. In the current review, we summarize the available data on the role of microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs) on expression of AR, as well as the effects of AR on their expression.
2. Effects of miRNAs on AR
2.1. Acting on AR mRNA to Directly Negatively Regulate AR Expression
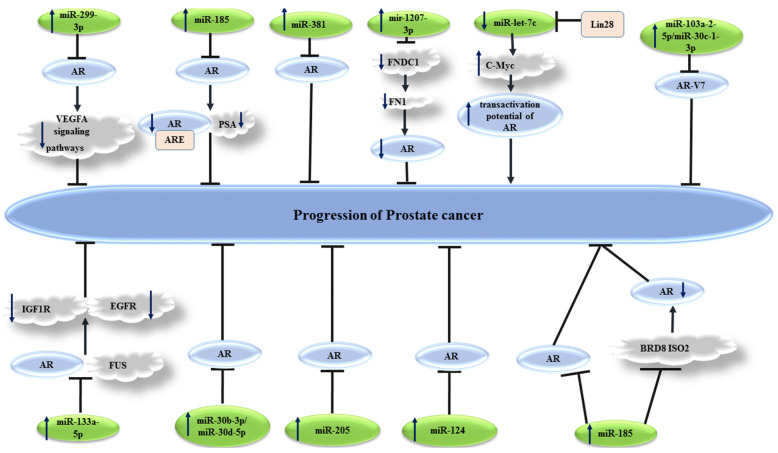
Several miRNAs have been found to suppress the expression of AR. In fact, most of these miRNAs have been shown to bind with 3′UTR of AR transcript, thus inducing its degradation or translation suppression. The interactions between miRNAs and AR mRNA, mainly through binding with its 3′UTR, have been mostly assessed in the context of prostate cancer. MiR-299-3p is one of the AR-interacting miRNAs. Expression of miR-299-3p has been reported to be decreased in prostate cancer samples, compared to noncancerous prostate samples. The restoration of miR-299-3p in prostate cancer cells has led to the induction of cell cycle arrest, reduction of cell proliferation and migration, and enhancement of levels of apoptotic markers. Moreover, the up-regulation of miR-299-3p leads to reduced expressions of AR, PSA, and VEGFA, suppressed epithelial mesenchymal transition (EMT), reduced levels of Slug, TGF-β3, p-AKT, and p-PRAS40, and enhanced E-cadherin levels. Taken together, miR-299-3p exerts anti-tumor effects via affecting activity of AR and VEGFA pathways [9].
Another study in prostate cancer cells has identified miR-185-binding sites in the 3′UTR of the AR transcript. Notably, the suppression of AR expression by miR-185 has compromised the interaction between AR and ARE and decreased the levels of the AR target gene PSA [10]. Moreover, miR-185-mediated inhibition of AR has suppressed the proliferation of prostate cancer cells and enhanced their apoptosis. Thus, the miR-185 has been suggested as a negative regulator of AR signaling and tumor suppressor miRNA in LNCaP cells [10].
MiR-381 is another down-regulated miRNA in prostate cancer. Forced over-expression of miR-381 in LNCaP cells inhibited their proliferation, migratory aptitude, and invasion. Mechanistically, miR-381 suppresses AR mRNA expression by binding to its 3′UTR [11].
MiR-let-7c is another miRNA that decreases expression and activity of AR in prostate cancer cells. It modulates AR transcription through c-Myc. MiR-let-7c-mediated inhibition of AR can reduce the proliferation of prostate cancer cells [12].
Figure 1 depicts a number of tumor suppressor miRNAs that regulate expression of AR in prostate cancer cells.
Figure 1.
Several miRNAs have been shown to affect levels of androgen receptor (AR), thus influencing the progression of prostate cancer. Detailed information about these miRNAs is presented in Table 1. (  reduction or inhibition of,
reduction or inhibition of,  increased levels of,
increased levels of,  decreased levels of).
decreased levels of).
2.2. Indirectly Regulate AR Expression or AR Signal
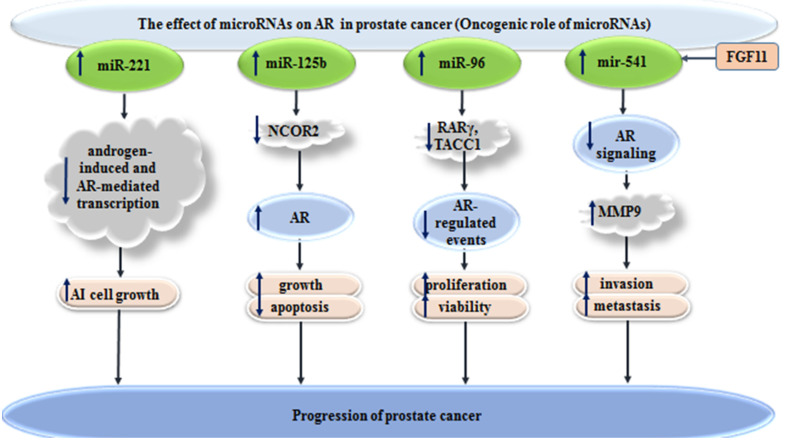
On the other hand, a handful of miRNAs have been found to exert oncogenic roles in prostate cancer, through the regulation of transcription of AR expression or signaling. Following androgen deprivation therapy, hormone-sensitive prostate cancer can evolve to castration-resistant prostate cancer (CRPC). MiRNAs can contribute to this process. For instance, miR-221/-222 has been shown to be up-regulated in bone metastatic CRPC samples. In vitro studies have demonstrated that stable overexpression of miR-221 induces the androgen-independent growth of prostate cancer cells, by releasing these cells from androgen deprivation-related G1 arrest. The up-regulation of this miRNA in LNCaP has led to the reduction of expression of a subclass of androgen-responsive genes, without influencing the expression of AR or integrity of AR-androgen. MiR-221 has been found to regulate the expressions of HECTD2 and RAB1A, two genes being capable of the induction of CRPC phenotype in various prostate cancer cells. Further, the up-regulation of miR-221 has led to alterations in the expression levels of several cell cycle-related genes and the activation of EMT-related pathways. Taken together, it has been hypothesized that miR-221 has a major role in AR signaling reprogramming and the subsequent evolution of the CRPC phenotype [13].
Experiments in mice models have indicated the effect of surgical castration in the induction of an early upsurge in the serum levels of miR-125b. Moreover, bicalutamide-mediated AR blocking has resulted in the prompt release of this miRNA into the media of cultured prostate cancer cells. NCOR2, as a corepressor of AR, has been shown to be targeted by miR-125b. Thus, miR-125b has been suggested as a key regulator of AR, which alters the efficacy of anti-androgen therapies [14].
MiR-96 is another oncogenic miRNA that can target a RARγ network to control AR signaling. Down-regulation of RARγ, a member of the nuclear receptor superfamily, has been shown to significantly affect the viability of prostate cancer cells and gene signature of these cells. A gene network, comprising of numerous RARγ target genes, such as SOX15, has been found to be correlated with poor disease-free survival of prostate cancer patients [15].
MiR-541 is another oncogenic miRNA that can affect prostate cancer course through modulation of AR signaling. In fact, infiltrating CD4(+) T cells, which are associated with poor clinical outcomes in this type of cancer, can increase FGF11 levels. Up-regulation of this growth factor leads to increase levels of miR-541. The subsequent down-regulation of AR signaling regulates MMP9 levels, in favor of tumor metastasis [16]. Figure 2 shows the effects of oncogenic miRNAs in the progression of prostate cancer, through the modulation of AR signaling.
Figure 2.
Effects of oncogenic miRNAs in progression of prostate cancer, through the modulation of AR signaling (  increased levels of,
increased levels of,  decreased levels of).
decreased levels of).
In addition to above-mentioned miRNAs, several miRNAs can directly or indirectly affect AR signaling by binding with 3′UTR, the coding region of AR, or influencing the levels of AR co-activators/co-repressors (Table 1).
Table 1.
The effects of different miRNAs on AR in prostate cancer (prostate cancer (PCa), FUS: fused in sarcoma, AR-V7: androgen receptor variant 7, BCa: breast cancer, BPH: benign prostatic hyperplasia, ANCTs: adjacent non-cancerous tissues, PEITC: phenethyl isothiocyanate, Enz: enzalutamide, CIN: cervical intraepithelial neoplasia, AI: androgen-independent, 5-hmC: 5-Hydroxymethylated cytosine, ↓: decrease in, ↑: increase in).
| miRNAs | Expression of miRNAs in PCa | Target Region of AR mRNA/Effect of miRNAs on AR | Targeted Pathway | Cell Line/Samples/Animal Models | Function of miRNAs in Cancer Cells | References |
|---|---|---|---|---|---|---|
| miR-299-3p | ↓ | ↓ | VEGFA signaling | LNCaP-104S, MDA-PCa-2b, 22Rv-1, C4-2B, PC-3, WPE-1/TCGA PRAD publication: 330 matching tumor and 51 normal samples | ↓ proliferation, EMT process and migration, growth, ↑ cell cycle arrest, apoptosis, and drug sensitivity | [9] |
| miR-185 | ↓ | ↓ 3′UTR | ARE, PSA | LNCaP | ↓ proliferation, ↑ apoptosis | [10] |
| miR-381 | ↓ | ↓ 3′UTR | _ | LNCaP | ↓ proliferation, migration, and invasion | [11] |
| miR-1207-3p | ↓ | ↓ | FNDC1, FN1, AR | WPE1-NA22, MDA PCa 2b, PC-3, E006AA, E006AA-hT, LNCaP, C4-2B, RWPE-1 | ↓ proliferation, migration, ↑ apoptosis | [17] |
| miR-21 | ↑ | ↑ | TGFBR2, Smad2/3 | RWPE-1, MDA-PCa-2b, 22Rv1, PC-3, and LNCaP/male athymic nude mice | ↓ tumor-suppressive activity of TGFβ pathway | [18] |
| miR-let-7c | ↓ | ↓ suppression of AR at the level of transcription | Lin28, c-Myc |
LNCaP, C4-2B/22 PCa samples/nude mice | ↓ proliferation, ↓ transactivation, potential of AR | [12] |
| miR-133a-5p | ↓ | ↓ 3′UTR | FUS, PSA, IGF1R, and EGFR | RWPE-1, VCaP, and LNCaP/TCGA database: 497 tumor tissue samples and 52 non-cancerous tissue samples | ↓ proliferation and viability | [19] |
| miR-103a-2-5p/miR-30c-1-3p | ↓ |
↓ 3′UTR AR-V7 |
_ | VCaP | ↓ cell growth and proliferation | [20] |
| miR-30b-3p and miR-30d-5p | ↓ | ↓ 3′UTR | _ | LNCaP, PC3, LAPC4/15 primary PCa samples, 15 adjacent normal prostate samples, and 15 metastatic CRPC samples | ↓ cell growth | [21] |
| miR-31 | ↓ | ↓ coding region | _ | RWPE-1, VCaP, LNCaP, 22Rv1, PC3, DU145, and HEK293 | ↓ proliferation, cell growth and colony formation, ↑ cell cycle arrest | [22] |
| miR-205 | ↓ | ↓ 3′UTR | _ | DU145, PC3, 22Rv1, LNCaP/49 PCa, and 25 samples without PCa | ↓ proliferation, colony formation and metastases, ↑ cell adhesion, overall survival | [23] |
| miR-124 | ↓ | ↓ 3′UTR | _ | LNCaP, 22Rv1, DU145, PC-3, C4-2/male BALB/C nude mice | ↓ proliferation, migration, and cell growth | [24] |
| miR-145 | ↓ | ↓ | Ago2, PSA, TMPRSS2, KLK2 | PC3, DU145, LNCaP, 22Rv1, VCaP, PNT2/49 PCa, and 25 samples without PCa | ↓ proliferation, ↑ G1 arrest | [25] |
| miR-8080 | _ | ↓ AR-V7 3′-UTR | IGF-1R and NKX3.1 | 22Rv1 and VCaP/male TRAP rats and male nude mice | Luteolin treatment: ↑ MiR-8080: ↓ proliferation, growth and oxidative stress, ↑ apoptosis, and Enz effects under castration conditions | [26] |
| miR-124 | ↓ | ↓ 3′UTR | p53 | RWPE-1, pRNS-1-1, LNCaP, C4-2B, cds2, 22Rv1, and LAPC-4/8 matched pairs of CaP and BPH tissues/male athymic nu/nu mice | ↓ cell growth, ↑ apoptosis | [27] |
| miR-124 | ↓ | ↓ 3′UTRs of AR-V4, -V7 | EZH2 and Src | LNCaP, C4-2B, 22Rv1, and VCaP/male athymic nude mice | ↓ proliferation and cell growth, ↑ apoptosis, sensitivity to Enz | [28] |
| miR-125b | ↑ | ↑ indirectly by decreasing the co-repressor of AR | NCOR2 | HEK293 and LNCAP/male nude mice | ↑ cell growth and survival, ↓ apoptosis | [14] |
| miR-473p | ↑ | _ | MEKK1 | LNCap/38 pairs of tumor tissues and ANCTs | ↑ cell survival, ↓ docetaxel-induced apoptosis in AR+ prostate cancer cells | [29] |
| miR-185 | ↓ | ↓ directly by binding 3′UTRs, ↓ indirectly by suppressing co-activator of AR | BRD8 ISO2 | LNCaP, PC-3/10 pairs of tumor tissues and ANCTs | ↓ proliferation and invasion | [30] |
| miR-449 | _ | ↓ AR-v7 | EZH2 | CWR22Rv1 and VCaP/male nude mice | ↓ cells growth and invasion, Enz resistance | [31] |
| miR-34b | ↓ | ↓ 3′UTR | ETV1 | MDA-PCa-2b, DU-145/143 PCa samples (from 3 different groups), and GEO analysis: GSE21032 |
↓ proliferation, ↑ apoptosis | [32] |
| miR-320a | ↓ | ↓ 3′UTR | _ | 22Rv1, VCaP, and LNCaP/10 PCa samples/SD rats | OBP-801 treatment: ↑ miR-320a: ↓ proliferation and cell growth | [33] |
| miR-17 | ↓ | ↓ indirectly by suppressing co-activator of AR | PCAF | RWPE1, LNCaP, PC-3, DU145, C4–2B, and ALVA31 | PEITC treatment: ↑ miR-17: ↓ cell growth | [34] |
| miR-141 | ↑ | ↑ AR-regulated transcriptional activity | Shp | RWPE-1, LNCaP, DU145, and C4-2B |
PEITC treatment: ↓ miR-141 and AR signaling activation |
[35] |
| miR-449a | _ | ↓ 3′UTR | PSA | C4-2 and LNCaP | capsaicin treatment: ↑ miR-449a: ↓ proliferation, ↑ G0/G1 cell cycle arrest | [36] |
| miR-331-3p | ↓ | ↓ indirectly by regulating ERBB-2 | ERBB-2, PI3K/AKT signaling pathway, PSA | LNCaP, 22RV1, DU145/tumor tissues, and ANCTs | ↓ indirectly AR pathway target genes via cross-talk between ERBB-2 and AR signaling pathways | [37] |
| miR-371 | ↓ | ↓ 3′UTR | KLK3 | LNCaP and PC3/83 PCa samples and 6 BPH as controls/male nude mice | ↓ proliferation and tumor growth | [38] |
| miR-1207-3p | ↓ | ↓ indirectly by regulating FNDC1 | FNDC1, FN1 | RWPE-1, CM, WPE1-NA22, RWPE-1, MDA PCa 2b, PC-3, E006AA, E006AA-hT, LNCaP, C4-2b | ↓ proliferation, migration, ↑ apoptosis | [39] |
| miR-301a | ↑ | ↓ 3′UTR | TGF-β1/Smad/MMP9 signals | CWR22Rv1, 3T3-L1/21 pairs of tumor tissues, and ANCTs/male nude mice | Recruitment of pre-adipocytes: ↑ miR-301a: ↑ invasion and metastasis | [40] |
| miR137 | ↓ | ↓ indirectly by regulating AR cofactor complexes | NCoA2, KDM1A, KDM2A, KDM4A, KDM5B, KDM7A and MED1 | PREC, LNCaP, LNCaP:C4-2, and PC-3/TCGA database | miR137: suppressor of androgen signaling by modulating expression of transcriptional coregulators | [41] |
| miR-361-3p | ↓ | ↓ 3′UTR of ARv7 | _ | CW22Rv1, C4-2, and LNCaP/TCGA analysis/male nude mice | ↑ Enz sensitivity | [42] |
| miR-2909 | ↑ | ↑ | TGFBR2, TGFβ signaling, PSA | PC3 and LNCaP | ↑ cell growth | [43] |
| miR-200a | ↓ | ↓ AR-V7 indirectly by regulating BRD4 | BRD4 | LNCaP and C4-2B/10 ADPC tissue and 10 CRPC tissue samples | ↓ proliferation, ↑ apoptosis | [44] |
| miR-135b | _ | ↓ | MUC1-C | LNCaP | ↑ invasion and EMT process | [45] |
| miR-17-5p | ↓ | ↓ indirectly by regulating co-activator of AR | PCAF, PSA | RWPE1, LNCaP, C4-2B, PC3, and PrEC | ↓ cell growth | [46] |
| miR-3162-5p | ↑ in PCa tissues with higher Gleason grade | ↓ 3′UTR | KLK3, PSA | LNCaP, PC3 | ↓ proliferation, migration, and colony formation | [47] |
| miR-644a | ↓ |
↓ 3′UTR (directly) and indirectly by regulating co-activators of AR |
SRC-1, SRC-2, SRC-3, CCND1, CBP, and ARA24 | LNCaP, LAPC4, and 22RV1/male athymic nude male mice | ↓ invasion, EMT process, metastasis and Warburg effect, ↑ apoptosis | [48] |
| miR-221 | ↑ | ↓ indirectly by regulating co-activators of AR | HECTD2 and RAB1A | LNCaP and LNCaP-Abl, LAPC-4, PC-3, Du145, and 22Rv1 | ↑ AI cell growth, emt process, and metastasis | [13] |
| miR-29b | ↑ | ↑ indirectly by regulating co-activators of AR | TET2, FOXA1, mTOR | LNCaP, BicR, VCaP, and 293T/male BALB/C nude mice | ↑ 5-hmC-mediated tumour progression | [49] |
| miR-141-3p | _ | ↓ 3′UTR | _ | LNCaP | ↓ both mRNA and protein expression levels of AR | [50] |
| miR-96 | ↑ | ↓ indirectly by regulating co-activator of AR | RARγ, TACC1 | RWPE-1, RWPE-2, PNT2, HPr1-AR, LNCaP, LAPC4, EAA006, MDAPCa2b, LNCaP-C42, 22Rv1, PC3 and DU145/36 PCa samples, and MSKCC dataset | ↑ proliferation and viability | [15] |
| miR-185 | ↓ | ↓ indirectly by regulating co-activator of AR | SREBP signaling | LNCaP, C4-2B, RWPE-1/male athymic nude mice | ↓ proliferation, clonogenicit, tumorigenicity, cell growth, migration and invasion, ↑ apoptosis | [51] |
| miR-342 | ↓ | ↓ indirectly by regulating co-activator of AR | SREBP signaling | LNCaP, C4-2B, RWPE-1/male athymic nude mice | ↓ proliferation, clonogenicit, tumorigenicity, cell growth, migration and invasion, ↑ apoptosis | |
| miR-204 | ↓ | ↓ indirectly by regulating XRN1 | XRN1, PSA, miR-34a | LNCaP, 22Rv1 and PC-3 and CL1/171 BPH, plus PCa samples/nude mice and rats | ↓ growth and colony formation of LNCaP and 22Rv1 cells but ↑ growth and colony formation of CL1 and PC-3 cells | [52] |
| miR-541 | ↑ | ↓ | FGF11, MMP9 | LNCaP, CWR22RV1 and C4-2/20 PCa samples/male nude mice | ↑ invasion and metastasis (while infiltrated T cells co-cultured with PCa cells) | [16] |
| miR-205 | ↓ | ↓ indirectly by regulating SQLE | SQLE | LNCaP, C4-2, PC-3, DU145, RWPE-1, HEK293T, VcaP, andLNCaP Abl | ↓ cell growth and de novo cholesterol biosynthesis | [53] |
| miR-130a | ↓ | ↓ indirectly by regulating coregulators of AR | CDK1, PSAP, PSMC3IP, GTF2H1 | LNCaP, PC-3, Du-145 and RWPE-1/5 low Gleason grade PCa samples, 6 high Gleason grade PCa samples, 3 recurrent PCa samples, and 6 nonmalignant samples | ↑ apoptosis | [54] |
| miR-203 | ↓ | ↓ indirectly by regulating coregulators of AR | PARK7, MNAT1, TFIIH, NCOA4, CDK1 | LNCaP, PC-3, Du-145 and RWPE-1/5 low Gleason grade PCa samples, 6 high Gleason grade PCa samples, 3 recurrent PCa samples, and 6 nonmalignant samples | ↑ apoptosis and cell cycle arrest | |
| miR-205 | ↓ | ↓ indirectly by regulating coregulators of AR | PARK7, RAN, KHDRBS1 | LNCaP, PC-3, Du-145 and RWPE-1/5 low Gleason grade PCa samples, 6 high Gleason grade PCa samples, 3 recurrent PCa samples, and 6 nonmalignant samples | ↑ cell cycle arrest | |
| miR-212 | ↓ | ↓ (AR and AR-V7) indirectly by regulating hnRNPH1 | hnRNPH1, SRC-3 | LNCaP, MDA-PCa-2b and C4–2B/13 African American samples, and 17 Caucasian American samples/SCID mice | ↓ cell growth and ↑ sensitivity to bicalutamide | [55] |
| miR-34a | ↓ | ↓ 3′UTR | Notch-1 | C4-2B, CWR22rv1, LNCaP, and VCaP | ↓ proliferation and self-renewal capacity | [56] |
| miR-190a | ↓ | ↓ indirectly by regulating the activator of AR | YB-1 | LNCaP, C4-2, PC-3, DU-145, 22Rv1/mal nude mice | ↓ proliferation and cell growth | [57] |
2.3. Effects of miRNAs on AR in Other Different Cancer Types
In addition to prostate cancer, the effects of miRNAs on AR have been investigated in other cancer types (Table 2). For instance, experiments in two AR-positive bladder cancer cell lines have shown that phenyl glucosamine can inactivate and degrade AR through the restoration of miR-449a expression. Lentivirus-mediated up-regulation of miR-449a has been shown to further suppress the proliferation of these cells via the induction of cell cycle arrest [58].
Table 2.
Effects of miRNAs on AR in different cancer types (↓: decrease in, ↑: increase in).
| Cancer Types | MiRNAs | Expression of miRNAs in Different Cancer Types | Target Region of AR mRNA/How microRNAs Affect AR |
Molecular Mechanisms | Cell Line/Samples/Animal Models | Function of miRNAs in Cancer Cells | References |
|---|---|---|---|---|---|---|---|
| Bladder cancer | miR-449a | _ | ↓ | _ | UMUC3 and TCCSUP | ABDHFA treatment: ↑ miR-449a: ↓ proliferation, viability, ↑ cell cycle arrest |
[58] |
| Breast cancer | miR-9-5p | ↓ | ↓ 3′-UTR | _ | MDA-MB-453, MCF-7, T-47D/11 pairs of tumor tissues and ANCTs | ↓ proliferation and cell growth | [59] |
| Cervical cancer | miR-130a-3p | ↑ | ↓ 3′UTR | _ | 20 CIN I, 20 CIN II, 30 CIN III tissue and 20 healthy tissue samples | ↑ proliferation and invasion | [60] |
| Hepatocellular carcinoma | miR-135b-5p | ↓ | ↓ 3′-UTR | HIF-2α, c-Myc, p27 | SK-hep1, HepG2, SNK, Huh7 and HA22T | ↓ proliferation, colony formation | [61] |
| miR-92a-2-5p | ↑ | ↓ 3′UTR | PHLPP/p-AKT/β-catenin signaling | SK-HEP-1, Hep G2, HEK 293 T, THP-1, Hepa 1-6, HA22T/male nude mice | ↑ invasion | [62] | |
| miR-367-3p | ↓ | ↑ indirectly by regulating MDM2 | MDM2/FKBP5/PHLPP/(pAKT and pERK) signals | SKhep1 and HA22T/126 HCC samples | ↑ Sorafenib chemotherapy efficacy, ↓ invasion and metastasis | [63] | |
| Glioma | miR-599 | ↓ | ↓ 3′UTR | circ-ASPH, SOCS2-AS1 | U251, U87MG, LN229 | ↓ proliferation, migration and invasion | [64] |
In addition, miR-9-5p has been identified as a suppressor of AR expression in breast cancer. A feedback loop has been recognized between these genes in breast cancer cells, in which androgen agonists of AR can increase expression of miR-9-5p. In fact, miR-9-5p can inhibit the proliferation of breast cancer cells in a manner independent from the estrogen receptor (ER) status of these cells. Moreover, miR-9-5p can decrease the activity of AR-downstream signals, even in the conditions that breast cancer cells are induced by AR-agonists [59].
In cervical cancer, the oncogenic miR-130a-3p has been found to target both ERα and AR. MiR-130a-3p silencing, ERα up-regulation, and AR up-regulation have suppressed proliferation and invasion of cervical cancer cells. Besides, antagomiR-130a could decrease tumor bulk in animal models. Taken together, miR-130a-3p has a possible role in the progression of cervical cancer, through the suppression of ERα and AR [60].
In hepatocellular carcinoma, miR-135b-5p has been shown to suppress AR-mediated cell proliferation, through the regulation of HIF-2α/c-Myc/P27 axis [61]. Moreover, macrophage-derived miR-92a-2-5p-containing exosomes could increase the invasiveness of liver cancer cells, through the modulation of AR/PHLPP/p-AKT/β-catenin axis [62]. Finally, miR-367-3p could increase the effectiveness of sorafenib in the suppression of liver cancer metastasis via the modulation of AR signals [63].
In glioma, the circ-ASPH/miR-599/AR/SOCS2-AS1 axis has been identified as a molecular mechanism for cancer progression. In fact, circ-ASPH could mediate this function by sponging miR-599 [64].
3. Regulatory Impact of lncRNAs on AR
3.1. Regulation of AR Expression
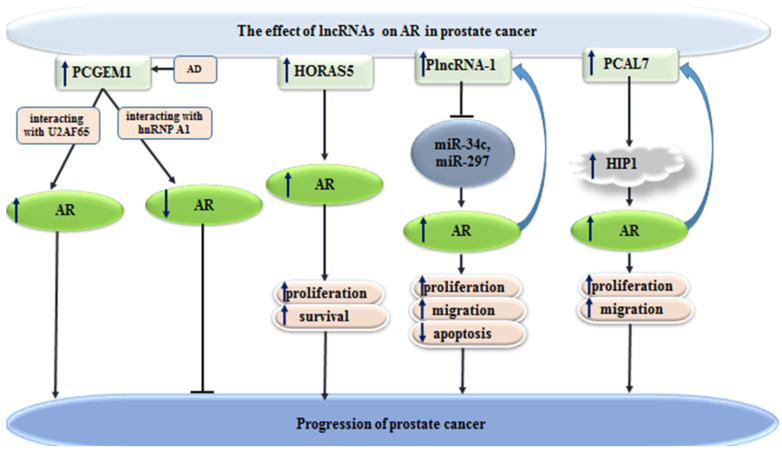
PCGEM1 is a lncRNA with important roles in splicing events. This lncRNA has been found to interact with the splicing factors heterogeneous nuclear ribonucleoprotein (hnRNP) A1 and U2AF65. Experiments have shown correlation between PCGEM1 and AR3, a principal and clinically important alternatively spliced form of AR in prostate cancer. Besides, androgen deprivation leads to enhanced expression of PCGEM1 and its accretion in nuclear speckles. Androgen deprivation-induced PCGEM1 has a role in regulation of the competition between two splicing factors for AR pre-mRNA [65]. Another study has identified HORAS5 as a CRPC-promoting lncRNA through assessment of patient-derived xenografts, clinical information with subsequent in vitro and in vivo confirmation studies. This lncRNA is a cytoplasmic lncRNA, which increases proliferation and viability of prostate cancer cells via sustaining AR activity even in androgen-depleted settings. Notably, HORAS5 silencing has reduced AR expression, as well as expression of oncogenic targets of AR, including KIAA0101. In clinical samples, up-regulation of HORAS5 has been associated with poor survival. Taken together, HORAS5 has been identified as targetable contributor in the induction of CRPC phenotype through maintaining oncogenic activity of AR [66]. Recent investigations identified a novel lncRNASAT1 as AR-interacting partner. The expression of this lncRNA is down-regulated in PCa tumor tissue compared to non-tumor tissue indicating a tumor suppressive function. LncRNASAT1 is up-regulated by the treatment with supraphysiological androgen level (SAL) in PCa cells and human PCa tissue ex vivo and mediates the SAL-induced cellular senescence [67]. Further, it has been shown that lncRNASAT1 interacts with AR on chromatin level regulating AR transactivation and AR target gene expression [68]. Another study has identified a feed-forward regulatory circuit between AR and PlncRNA-1, which enhances progression of prostate cancer [69]. In addition, PCAL7 lncRNA is another lncRNA that enhances progression of this type of cancer through promoting AR signaling [70]. Figure 3 depicts the role of a number of lncRNAs in progression of prostate cancer through modulation of AR signaling.
Figure 3.
Effects of lncRNAs on AR in prostate cancer (  reduction or inhibition of,
reduction or inhibition of,  increased levels of,
increased levels of,  decreased levels of).
decreased levels of).
3.2. Regulation of AR Activity as AR-Interacting Partner
Several other oncogenic lncRNAs have been found to regulate AR signaling. For instance, HOTAIR increases AR-mediated transcriptional program and induces CRPC phenotypes [71]. Moreover, MALAT1 has been shown to suppress cell cycle progression in this type of cancer through regulation of AR signaling [72]. LINC00844 is another lncRNA that affects migration and invasion of prostate cancer cells through modulation of this route [73].
In brief, lncRNAs can affect AR levels through interacting with AR on chromatin level, regulation of AR transactivation and modulation of AR target gene expression. Some lncRNAs can also regulate stability of AR transcripts and preventing its ubiquitination. Table 3 shows the effects of different lncRNAs on AR in prostate cancer.
Table 3.
Effects of different lncRNAs on AR in prostate cancer (ANCT: PCa: prostate cancer, DHT: dihydrotestosterone, CRPC: castration-resistant prostate cancer, ADPC: androgen-dependent prostate cancer, AD: androgen deprivation, DIM: 3,3′-Diindolylmethane, SAM: Synergistic activation mediator, LBD: ligand-binding domain, ↓: decrease in, ↑: increase in).
| LncRNAs | Expression of lncRNAs in PCa | Target Region of AR mRNA/How lncRNAs Affect AR |
Molecular Mechanisms | Cell Lines/Samples/Animal Models | Function of lncRNAs in Cancer Cells | References |
|---|---|---|---|---|---|---|
| ARLNC1 | ↑ | stabilizing AR transcript | _ | VCaP and LNCaP/11 benign prostate samples, 85 localized prostate cancer samples, and 37 from metastatic PCa samples/athymic nude mice | ↑ Proliferation and cell growth, ↓ apoptosis | [74] |
| PRNCR1 and PCGEM1 | ↑ | interact with, and increase its ligand-independent activation | DOT1L | LNCaP, RWPE, WPE, CWR22Rv1/BPH and PCa tissues male athymic Nu/Nu mice | ↑ Proliferation and cell growth | [75] |
| HOTAIR | ↑ | ↑ By preventing AR ubiquitination and blocking its interaction with MDM2 | _ | LNCaP, C4-2B/GEO analysis: GSE35988 and GSE21034 | ↑ cell growth and invasion | [71] |
| MALAT1 | ↑ | ↑ indirectly by inhibiting miR-320 | miR-320 | DU145, 22Rv1, PC3, LNCaP/BALB/cA-nu mice | DHT treatment: ↑ proliferation and cell cycle progression | [72] |
| LINC00844 | ↓ | modulated AR binding to chromatin | NDRG1 | LNCaP, VCaP, and 22Rv1/GEO database: GSE109336 | ↓ migration and invasion | [73] |
| LINC00675 | ↑ | directly modulate AR interaction with MDM2, inhibited AR’s ubiquitination, ↑ indirectly by regulating the co-activator of AR | MDM2, GATA2 | LNCaP-SF, LNCaP-JP, LNCaP, LNCaP-C4-2b, 293T/male BALB/c nude mice | ↑ tumor formation, tumor growth and Enz resistance | [76] |
| CCAT1 | ↑ | ↑ by binding to P68 | DDX5 (P68), mir-28-5P | PC3, Du145, and LNCaP/8 ADPC tissues and 4 CRPC samples/BALB/C nude mice | ↑ proliferation, colony formation, and cell cycle progression, ↓ apoptosis | [77] |
| PCGEM1 | ↑ in AD | ↑ AR3 by interacting with U2AF65, ↓ AR3 by interacting with hnRNP A1 | U2AF65, hnRNP A1 | LNCaP, CWR22Rv1, LNCaP95, HECK293T/male SCID mice | ↑ castration resistance | [65] |
| SOCS2-AS1 | ↑ Castration-resistant Prostate Cancer Cells | ↑ by regulating cofactor recruitment for epigenetic controls | TNFSF10 | LNCaP, VCaP, LTAD | ↑ castration-resistant and cell growth, ↓ apoptosis | [78] |
| HORAS5 | ↑ | ↑ post-transcriptional maintenance of AR mRNA stability | _ | LNCaP and C4-2 male, immunocompromised NOD/SCID mice | ↑ proliferation and survival | [66] |
| PCLN16 | ↑ | ↑ indirectly by regulating HIP1 | HIP1 | NCaP and VCaP/tumor tissues and ANCTs | ↑ proliferation, migration and cell growth | [79] |
| HOTAIR | ↑ in PCa cells after co-culture with HMC-1 cells | ↓ at the transcriptional level | PRC2, MMP9 | LNCaP, CWR22Rv1, C4-2, C4-2B and HMC-1/male nude mice | recruitment of mast cells: ↑ invasion and stem/progenitor cell population | [80] |
| PlncRNA-1 | ↑ | ↑ by sponging AR-targeting microRNAs | miR-34c and miR-297 | RWPE-1, 22RV1, LNCaP, PC3 and DU145/16 PCa tissue samples, 35 biopsy-negative and 37 biopsy-positive blood samples/male nude mice | ↑ proliferation, migration and viability, ↓ apoptosis | [69] |
| PCAL7 | ↑ | ↑ indirectly by regulating HIP1 | HIP1 | 104 tumor tissues and ANCTs | ↑ proliferation, migration | [70] |
| Malat1 | ↑ | ↑ AR-v7 indirectly by interacting with SF2 to splice the AR transcript | SF2 | VCaP and EnzR-PCa C4-2/ 10 CRPC samples before (Pre-Enz) and after (Post-Enz) Enz treatment/nude mice | ↑ Enz resistance | [81] |
| PlncRNA-1 | ↑ | ↑ | NKX3-1 | LNCaP, LNCaP-AI, PC-3, C4-2, RWPE-1 and PWR-1E/16 pairs of PCa tissues and ANCTs, 14 pairs of PCa tissues and BPH tissues | ↑ proliferation and viability, ↓ apoptosis | [82] |
| LBCS | ↓ | ↓ 5′ UTR | hnRNPK | LNCaP, LNCaP-Bic, and LNCaP-AI/130 PCa tissues and 32 BPH tissues plus 70 PCa tissues and 10 BPH | ↓ castration resistance | [83] |
| PCGEM1 | ↑ | upregulation of PCGEM1 by SAM: ↑ AR3 | p54/nrb | LNCaP and CWR22Rv1/male SCID mice | ↑ tumor growth and castration resistance, ↓ apoptosis DIM: ↓ PCGEM1-mediated castration resistance |
[84] |
| PCGEM1 | ↑ | facilitating AR binding to some promoters | c-Myc | LNCaP, | ↑ glucose uptake and glycolysis, ell-cycle progression, proliferation, and survival | [85] |
| LOC283070 | ↑ | ↑ indirectly by inhibiting PHB2 | PHB2 | LNCaP and LNCaP-AI | ↑ proliferation and migration | [86] |
| lnc-OPHN1-5 | _ | ↓ 3′UTR | hnRNPA1 | C4-2R, C4-2BR, C4-2B/75 PCa samples/male NOD CRISPR Prkdc Il2r Gamma triple-immunodeficient mice | ↑ Enz sensitivity | [87] |
| GAS5 | ↓ | ↓ directly by interacting with LBD of AR | _ | C4-2, DU145, 293T/GSE6919 | ↓ proliferation, ↑ apoptosis | [88] |
| GHSROS | ↑ | ↓ | PPP2R2C | PC3, LNCaP, DU145, DUCaP | ↑ proliferation, growth, migration, survival, and resistance to the cytotoxic drug docetaxel | [89] |
| PCA3 | ↑ | PCA3 knock down→ ↑ regulation of AR cofactors | _ | LNCaP | modulating the expression of EMT markers and AR cofactors ∆ PCA3: ↓ cell viability |
[90] |
| PRNCR1 | ↑ | ↑ | _ | LNCaP and C4-2 | ↑ proliferation and invasion, ↓ apoptosis | [91] |
3.3. Effects of lnRNAs on AR in Other Different Cancer Types
The effects of lncRNAs on AR signaling have also been assessed in other types of cancers. In bladder cancer, XIST has been found to be up-regulated parallel with up-regulation of AR. Over-expression of XIST and AR has been correlated with advanced TNM stage in this cancer. XIST silencing has decreased proliferation, invasion, and migratory potential of bladder cancer through modulation of AR signaling. Mechanistically, XIST suppresses expression of miR-124 through direct interaction. Besides, miR-124 has been shown to target 3′UTR of AR [92]. Another experiment in bladder cancer has shown over-expression of LINC00460. LINC00460 levels have been correlated with poor prognosis of these patients. LINC00460 silencing decreased proliferation of 5637 and T24 bladder cancer cells. Based on the observed down-regulation of AR in bladder urothelial cancer tissues, it has been suggested that LINC00460 might exert its oncogenic roles through modulation of AR expression [93]. LINC00278, SLNCR1, SARCC and HOTAIR are other lncRNAs whose effects on AR have been investigated in different cancer types (Table 4).
Table 4.
Effects of lncRNAs on AR in different cancer types (ND: nutrient deprivation, ccRCC: clear cell renal cell carcinoma, ↓: decrease in, ↑: increase in).
| Cancer Types | LncRNAs | Expression of LncRNAs in Different Cancer Types | Target Region of AR mRNA/How lncRNAs Affect AR |
Molecular Mechanisms | Cell Lines/Samples/Animal Models | Function of lncRNAs in Cancer Cells | References |
|---|---|---|---|---|---|---|---|
| Bladder cancer | XIST | ↑ | ↑ by sponging AR-targeting microRNA | miR-124 | TCC-SUP, EJ, SW780 and UM-UC-3, SV-HUC-1 67 pairs of tumor tissues and ANCTs | ↑ proliferation, migration and invasion | [92] |
| LINC00460 | ↑ | ↓ | _ | 5637, T24, J82, TCCSUP, UM-UC-3 and SV-HUC-1/TCGA database | ↑ proliferation and migration | [93] | |
| Esophageal squamous cell carcinoma | LINC00278 | ↓ | indirectly inhibited interaction between YY1 and AR | YY1, eEF2K, YY1BM | DMEM, RPMI1640, FBS, Eca-109, TE-1, and KYSE-30/281 pairs of ESCC tissues and ANCTs, | ND treatment: ↑ LINC00278: ↓ survival, ↑apoptosis | [94] |
| Melanoma | SLNCR1 | ↑ | SLNCR1 binds to AR-binding motifs 1 and 2 | _ | A375, HEK293T, WM1976, | ↓ binding SLNCR1 to AR: ↓ SLNCR1-mediated invasion | [95] |
| SLNCR1 | ↑ | ↑ AR binding to the MMP9 promoter | Brn3a, MMP9 | A375, HEK293T, CY and WM | ↑ invasion | [96] | |
| Renal cell carcinoma | SARCC | ↓ | destabilizing AR protein | miR-143-3p, AKT, MMP-13, K-RAS and P-ERK | SW839, OSRC-2, A498, 769-P, 786-O, Caki-1, Caki-2, HK2/66 ccRCC tissues and 8 metastatic ccRCC tissues/male athymic nude mice | ↓ proliferation, invasion, migration and resistance to Sunitinib | [97] |
| SARCC | Differentially expressed by hypoxia in a VHL-dependent manner | ↓ binding and destablizing AR protein | HIF-2α, C-MYC signals | SW839, OSRC-2, A498, 769-P, and 786-O, Caki-1, Caki-2, HK-2 and 293T/16 ccRCC samples/male athymic nude mice | Differentially modulates proliferation under hypoxia | [98] | |
| HOTAIR | ↑ | ↑ | GLI2 | HK-2, 786-O, ACHN, 769-P, SW839, OSRC-2, HUVEC/male nude mice | ↑ angiogenic phenotype and stemness | [99] |
4. Effects of circRNAs on AR
circZMIZ1 has been shown to be over-expressed in plasma samples of patients with prostate cancer compared with those having benign prostatic hyperplasia (BPH). In vitro studies have shown that circZMIZ1 silencing inhibits cell proliferation and arrests cells at G1. Functionally, circZMIZ1 enhances expression of AR and its splice variant 7 (AR-V7) [100].
On the other hand, expression of cir-ITCH has been shown to be decreased in the tissues and cell lines of prostate cancer compared to corresponding controls. Up-regulation of cir-ITCH could suppress proliferation, migratory potential, and invasiveness of human prostate cancer cells. A reciprocal inhibitory effect has been found between this circRNA and miR-17. Several molecules within Wnt/β-catenin and PI3K/AKT/mTOR cascades have been found to be influenced by cir-ITCH. This circRNA could indirectly reduce expression of AR through regulating the coactivator of this nuclear factor [101]. hsa_circ_0004870 [102] and circRNA17 [103] are two other circRNAs that reduce AR-V7 levels through U2AF65 and miR-181c-5p mediated routes, respectively, thus enhancing efficacy of enzalutamide. Table 5 shows the effects of different circRNAs on AR in prostate cancer.
Table 5.
The effects of different circRNAs on AR in prostate cancer (HCs: healthy controls, Enz: enzalutamide, ↓: decrease in, ↑: increase in).
| circRNAs | Expression of circRNAs in PCa | Target Region of AR mRNA/How circRNAs Affect AR |
Regulated Pathway | Cell Lines/Samples/Animal Models | Function of circRNAs in Cancer Cells | References |
|---|---|---|---|---|---|---|
| circZMIZ1 | ↑ | ↑ AR and AR-V7 | _ | DU145, C4-2, LNCaP, 22RV1, RWPE-1, 14 PCa samples, and 14 HCs | ↑ proliferation, ↓ G1 arrest | [100] |
| circ-ITCH | ↓ | ↓ indirectly by regulating the coactivator of AR | miR-17, Wnt/β-Catenin, and PI3K/AKT/ mTOR Signaling Pathways |
RWPE-1, LNCaP, PC-3/10 pairs of tumor tissues and ANCTs | ↓ migration and invasion | [101] |
| hsa_circ_0004870 | ↓ | ↓ AR-V7 indirectly through U2AF65 | RBM39, U2AF65 | LNCaP, BPH1, 22Rv1 | ↓ Enz resistance | [102] |
| circRNA17 | ↓ | ↓ AR-v7 indirectly by regulating miR-181c-5p | miR-181c-5p | C4–2, CWR22Rv1, and 293T/13 BPH samples, and 14 PCa samples/male nude mice | ↓ Enz resistance and invasion | [103] |
5. Effects of AR on ncRNAs
5.1. AR Responsive miRNA
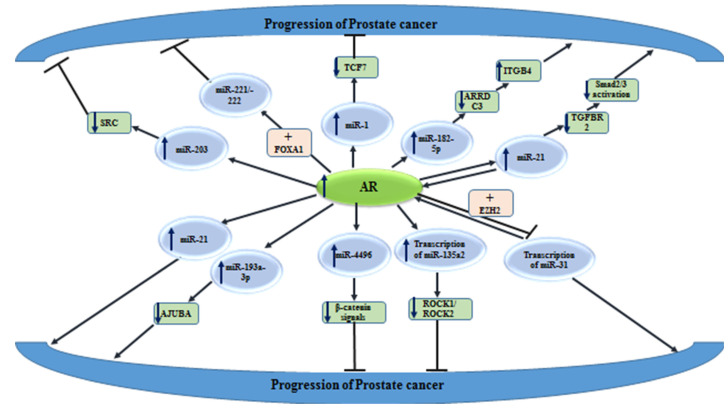
AR has been found to regulate the expression of several ncRNAs. For instance, activated AR has been shown to increase the expression of miR-203 and decrease the expression of SRC kinase in prostate cancer model systems. MiR-203 has a direct interaction with the 3′UTR of SRC and affects its stability following AR activation. A reduction in AR-induced miR-203 levels has been associated with an increased growth and migration potential of prostate cancer cells. The dysregulation of the AR signaling in prostate cancer cells results in the over-expression of SRC and enhancement of metastatic ability of these cells [104].
Another experiment has shown that AR represses the expression of both miR-221/-222. The derepression of their expression after androgen deprivation has enhanced proliferation of prostate cancer cells via facilitating G1/S phase transition. Although this effect might be transient, it has a possible role in the evolution of CRPC. The restoration of AR activity via AR up-regulation could subsequently down-regulate miR-221/-222 [105]. MiR-182-5p is another AR-regulated miRNA that facilitates the progression of prostate cancer by targeting the ARRDC3/ITGB4 axis [106]. Another study has shown the effects of AR on the down-regulation of miR-1 expression and subsequent suppression of TCF7. This process has been shown to participate in the evolution of resistance to androgen deprivation in this type of cancer [107].
AR has been shown to differentially affect the metastasis of prostate and breast cancers, through distinctively changing vasculogenic mimicry (VM) formation. In fact, AR can enhance miR-525-5p transcription in prostate cancer, while decreasing its transcription in breast cancer by binding to different AREs in the precursor promoter of this miRNA. NFIX and HDAC2 have been identified as co-factors of AR in prostate and breast cancer cells, respectively [108]. Figure 4 shows the impact of AR on miRNAs expressions, in the context of prostate cancer.
Figure 4.
Effects of AR on miRNAs expressions, in the context of prostate cancer. Detailed information about these miRNAs is shown in Table 6 (  reduction or inhibition of,
reduction or inhibition of,  increased levels of,
increased levels of,  decreased levels of).
decreased levels of).
5.2. AR Responsive lncRNA and circRNA
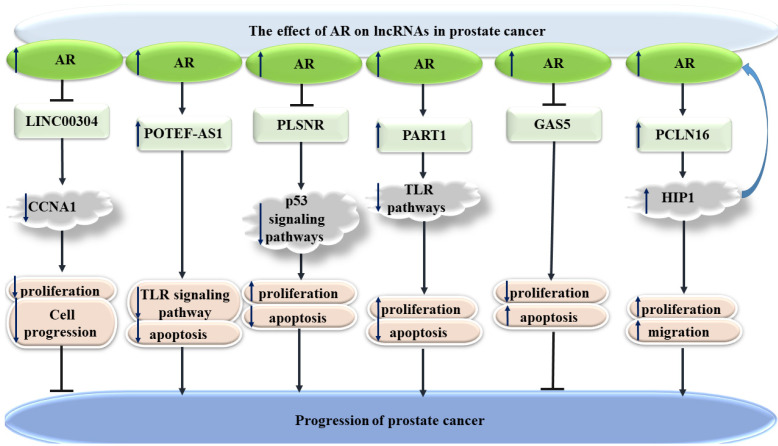
AR has also been shown to affect expression of several lncRNAs. For instance, LINC00304 is an androgen-responsive lncRNA that induces cell cycle transition and increases the proliferation of prostate cancer cells, through the regulation of CCNA1 [109]. Moreover, the androgen-associated up-regulation of POTEF-AS1 has been shown to affect apoptosis-associated pathways, in favor of prostate cancer cells survival [110]. On the other hand, the expression of PSLNR has been shown to be decreased by androgens. This lncRNA suppresses prostate cancer progression partly through regulation of the p53-dependent axis [111]. PART1, as another androgen-regulated lncRNA, can influence the toll-like receptor pathways in this type of cancer. The expression of PART1 has been induced in prostate cancer cells treated with 5α-dihydrotestosterone, indicating that this lncRNA is directly induced by androgen [112]. Figure 5 shows the effects of AR on lncRNAs in prostate cancer.
Figure 5.
Effects of AR on lncRNAs in prostate cancer (  reduction or inhibition of,
reduction or inhibition of,  increased levels of,
increased levels of,  decreased levels of).
decreased levels of).
Other studies, in the context of prostate cancer, have identified AR-regulated miRNAs and lncRNAs. Moreover, a number of circRNAs, such as circRNA-51217, circRNA-ARC1, and circZMIZ1, have been found to be influenced by AR signaling (Table 6). A recent study has identified more than 3000 androgen-responsive circRNAs, using a microarray technique. Notably, the expression of more than 1000 of these circRNAs has been consistent with the expression of their parent genes, suggesting that AR may modulate their expression at the transcriptional level [113].
Table 6.
Effects of AR on different ncRNAs in prostate cancer. (PCa: prostate cancer, CRPC: castration-resistant prostate cancer, HCs: healthy controls, NE: neuroendocrine, BPH: benign prostatic hyperplasia, CRPC: castration-resistant prostate cancer, DOX: doxorubicin, NED: neuroendocrine differentiation, NE: neuroendocrine, Enz: enzalutamide, PRAD: prostate adenocarcinoma, R-2HG: R-2-hydroxyglutarate, ↓: decrease in, ↑: increase in).
| ncRNAs | Regulation by AR | Molecular Mechanisms | Cell Line/Samples/Animal Models | Function of ncRNAs in Cancer Cells | References |
|---|---|---|---|---|---|
| miR-203 | ↑ | SRC | _ | ↓ migration, growth, and metastasis | [104] |
| miR-221/-222 | ↓ | FOXA1 | LNCaP and C4-2B/LuCaP 35 and LuCaP 35CR xenografts | ↑ proliferation and development of CRPC | [105] |
| miR-182-5p | ↑ | ARRDC3, ITGB4 | RWPE-1, 22RV1, LNCaP, DU145/65, pairs of tumor tissues and ANCTs, and 18 pairs of tumor tissues and ANCTs/male nude mice | ↑ proliferation, invasion, migration and growth, ↓ apoptosis | [106] |
| miR-1 | ↑ | TCF7 | PC3, LNCaP/111 PCa samples/nude mice | ↓ proliferation | [107] |
| miR-525-5p | ↑ | SLPI, NFIX | _ | ↓ PCa metastasis | [108] |
| miR-21 | ↑ | TGFBR2, Smad2/3 | RWPE-1, MDA-PCa-2b, 22Rv1, PC-3, and LNCaP/male athymic nude mice | ↓ tumor-suppressive activity of TGFβ pathway | [18] |
| miR-21 | ↑ | _ | LNCaP, LAPC-4, C4-2, CWR22Rv1/10 PCa samples/male athymic Nu/Nu mice | ↑ androgen-dependent and -independent proliferation, tumor growth, and castration resistance | [114] |
| miR-193a-3p | ↑ | AJUBA | LNCaP, C4-2B |
↑ migration and metastasis |
[115] |
| miR-4496 | ↑ | β-catenin signals | C4-2 and PC3 | ↓ invasion | [116] |
| miR-135a | ↑ | ROCK1 and ROCK2 | LNCaP, PC-3/56 pairs of tumor tissues, and ANCTs/chick embryos and adult male mice | ↓ invasion | [117] |
| miR-31 | ↓ | EZH2 | RWPE-1, VCaP, LNCaP, 22Rv1, PC3, DU145, and HEK293 | ↓ proliferation, cell growth and colony formation, ↑ cell cycle arrest | [22] |
| miR-421 | ↓ | NRAS, PRAME, CUL4B, and PFKFB2 | LNCaP, 22Rv1, PC-3 and DU 145/microarray data: GSE21036, GSE45604, GSE38241, and 13 PCa samples 11 samples without PCa | ↓ viability, glycolysis and migration, ↑ cell cycle arrest | [118] |
| miR-1 | ↑ | SRC | LNCaP, DU145RasV12G37, DU145/RasB1/28 HCs, 98 primary tumor, and 13 distant metastasis samples/male nude mice | ↓ proliferation, invasion, and metastasis | [119] |
| miR-32 | ↓ | NSE | RWPE1, LNCaP, and CWR22Rv1/male nude mice | enzalutamide treatment (mast cells) → suppression of AR: ↑ miRNA32: ↑ NE differentiation |
[120] |
| miR-21 promoter |
↑ | PDCD4 | LNCaP and HEK 293, LAPC4/male athymic nu/nu mice | ↑ androgen-dependent and -independent growth and castration resistance, ↓ apoptosis | [121] |
| miR-22 | ↓ | LAMC1 | LNCaP, PC3, DU145, VCaP, CWR22RV1, DUCaP, BPH-1, PC3-AR, LAPC-4, RWPE-1, and EP156T/ 41 pairs of tumor tissues and ANCTs, TCGA analysis: 52 pairs of tumor tissues and ANCTs | ↓ migration | [122] |
| miR-29a | ↓ | MCL1 | LNCaP, PC3, DU145, VCaP, CWR22RV1, DUCaP, BPH-1, PC3-AR, LAPC-4, RWPE-1, and EP156T/ 41 pairs of tumor tissues and ANCTs, TCGA analysis: 52 pairs of tumor tissues and ANCTs | ↓ migration and viability, ↑ apoptosis | |
| miR-99a/let7c/125b-2 cluster | ↓ | IGF1R | LNCaP, C4-2, and PC3 | ↓ proliferation | [123] |
| miR-2909 | ↑ | TGFBR2, TGFβ signaling, PSA | PC3 and LNCaP | ↑ cell growth | [43] |
| miR-32 | ↑ | BTG2 | LNCaP/ 5 BPH and 28 PCs, plus 7 BPH and 14 CRPCs | ↑ cell growth | [124] |
| miR-148a | ↑ | PIK3IP1 | LNCaP/ 5 BPH and 28 PCs, plus 7 BPH and 14 CRPCs | ↑ cell growth and the number of cells in the S phase | |
| miR-194 | ↓ | FOXA1, ERK Signaling | LNCaP, PC3, and 22RV1 | ↑ EMT process, migration, invasion and epithelial-neuroendocrine transdifferentiation | [125] |
| miR-27a (miR-23a27a24-2cluster) |
↑ | PHB | HeLa, Cos-1, LNCaP, DuCaP, VCaP, C42, DU145, PC3, and PC3wtAR | ↑ cell growth | [126] |
| miR-200b |
↑ | _ | PC3/male athymic mice | ↓ proliferation, invasion, cell growth, EMT process and metastasis | [127] |
| miR-19a |
↑ | SUZ12, RAB13, SC4MOL, PSAP, and ABCA1 | LNCaP | ↑ cell viability | [128] |
| miR-27a | ↑ | ABCA1 and PDS5B | LNCaP | ↑ cell viability | |
| miR-133b | ↑ | CDC2L5, PTPRK, RB1CC1, and CPNE3 | LNCaP | ↑ cell viability | |
| miR-22 | ↑ and ↓ during two different mechanisms | IL-6, AR c-MYC, miR-22, PHF8, KDM3A (↑) and AR c-MYC, miR-22, PHF8, KDM3A (↓) |
LNCaP-Abl, LNCaP-IL-6, LNCaP/male mice | ↑ sensitivity LNCaP-Abl cells to the enzalutamide treatment, ↓ proliferation | [129] |
| miR-17-92a | ↑ | ATG7 | NCaP, 22Rv1, DU145, and PC-3 | ↓ autophagy induced by celastrol treatment | [130] |
| miR-204 | ↓ | XRN1, PSA, miR-34a | LNCaP, 22Rv1, PC-3, and CL1/171, BPH, plus PCa samples/nude mice and rats | ↓ growth and colony formation of LNCaP and 22Rv1 cells but ↑ growth and colony formation of CL1 and PC-3 cells | [52] |
| miR-34 | miR-34a ↑ after DOX, but did not change with si-AR, miR-34c↑ after DOX, but to a small extent changed with si-AR |
p53, SPAK, MDC1, and CaMKII | LNCaP, C4-2b, PC3, and DU145 | ↑ caspase activity and apoptosis | [131] |
| miR-135a | ↑ | MMP11, RBAK, PI3K/AKT pathway | LNCaP, 22RV1, DU145, PC-3, and WPMY-1 | ↓ proliferation and migration, ↑ cell cycle arrest, and apoptosis | [132] |
| the miR-200 family, miR-17-92 cluster, and miR-99a/let-7c/miR-125b-2 family | ↓ | HOXC6 and NKX2-2 | RWPE-1 and LNCAP | ↓ metastasis and EMT process | [133] |
| miR-101 | ↑ | _ | LNCaP, 22Rv1, DU145, and PC-3 | ↓ celastrol-induced autophagy | [134] |
| miR-27a | ↓ | MAP2K4, PI3K signalingpathways | TCGA database: GSE45604 andGSE21036 | ↓ proliferation and migration, ↑ apoptosis | [135] |
| miR-190a | ↓ | YB-1 | LNCaP, C4-2, PC-3, DU-145, 22Rv1/mal nude mice | ↓ proliferation and cell growth | [57] |
| ARLNC1 | ↑ | _ | VCaP and LNCaP/11 benign prostate samples, 85 localized prostate cancer samples, and 37 from metastatic PCa samples/athymic nude mice | ↑ Proliferation and cell growth, ↓ apoptosis | [74] |
| PRCAT38 | ↑ | TMPRSS2, FOXA1 | LNCaP, DU145, and VCaP/20 samples (HCs and PCa) | ↑ cell growth and migration | [136] |
| H19 | ↓ | _ | LNCaP | Enzalutamide treatment: ↑ H19 | [137] |
| GAS5 | ↓ | _ | PC3 and 22Rv1 | dexamethasone treatment in AR- PCa cell line PC3: ↑ GAS5: ↓ proliferation, ↑ G0/G1 cell arrest | [138] |
| p21 | ↓ AR binding to the ARE5, ↑ AR binding to the AGRE | EZH2, STAT3 | C4-2, CWR22RV1, NE1.8, NCI-H660, and DU145 | Enz treatment: ↓ AR binding to the ARE5 region of p21: ↑ p21: ↑ NED, NE-like structure | [139] |
| LINC00304 | ↓ | CCNA1 | LNCaP, 22RV1, DU145, PC-3, and WPMY-1/GEO database: GSE38241: 18 PCa samples and 21 HCs | ↑ proliferation and cell cycle progression | [109] |
| POTEF-AS1 | ↑ | TLR signaling pathway | LNCaP, VCaP, LTAD, and VCaP-LTAD | ↑ cell growth, ↓ apoptosis | [110] |
| PLSNR | ↓ | p53 signaling pathway | LNCaP, 22RV1, DU145, PC-3, and WPMY-1/GEO database: GSE55909, 3 pairs of tumor tissues and ANCTs, 13 tumor tissues and ANCTs, plus 20 pairs of PCa sample |
↑ proliferation and cell-cycle progression, ↓ apoptosis |
[111] |
| PART1 | ↑ | TLR pathways | LNCaP and PC3/30 pairs of tumor tissues and ANCTs | ↑ proliferation, ↓ apoptosis | [112] |
| GAS5 | ↓ | _ | LNCaP, 22RV1, DU145, PC3, WPMY-1 14 tumor tissues, and 11 normal tissues | ↑ proliferation, ↓ apoptosis | [140] |
| PCLN16 | ↑ | HIP1 | NCaP and VCaP/tumor tissues and ANCTs | ↑ proliferation, migration, and cell growth | [79] |
| PlncRNA-1 | ↑ | miR-34c and miR-297 | RWPE-1, 22RV1, LNCaP, PC3 and DU145/16 PCa tissue samples, 35 biopsy-negative and 37 biopsy-positive blood samples/male nude mice | ↑ proliferation, migration and viability, ↓ apoptosis | [69] |
| PCAL7 | ↑ | HIP1 | 104 tumor tissues and ANCTs | ↑ proliferation, migration | [70] |
| PCGEM1 | ↑ | _ | 131 primary PCa, 19 metastasized PCa, and 29 normal prostatic tissue samples/intact mice |
↑ PCGEM1 in primary PCa Androgen receptor regulated PCGEM1 in vivo. |
[141] |
| DRAIC | ↓ | _ | VCap, PC3M-luc | ↓ transformation of cuboidal epithelial cells to fibroblast-like morphology, migration, and invasion | [142] |
| PCAT29 | ↓ | _ | VCap, PC3M-luc | ↓ migration and metastasis | |
| a subset of TPCATs, most notably EPCART | ↑ | ERG | LNCaP, VCaP, and DuCaP/87 prostatectomy-treated samples | ↑ proliferation, migration | [143] |
| PCAT29 | ↓ | _ | VCaP, LNCaP, and DU145/GEO database: GSE58397/male nude athymic BALB/c nu/nu mice | ↓ proliferation, migration | [144] |
| Malat1 | ↓ | _ | VCaP and EnzR-PCa C4-2/ 10 CRPC samples, before (Pre-Enz) and after (Post-Enz) Enz treatment/nude mice | Enz treatment: ↑ Malat1 | [81] |
| PlncRNA-1 | ↑ | NKX3-1 | LNCaP, LNCaP-AI, PC-3, C4-2, RWPE-1 and PWR-1E/16 pairs of PCa tissues and ANCTs, 14 pairs of PCa tissues and BPH tissues | ↑ proliferation and viability, ↓ apoptosis | [82] |
| CTBP1-AS | ↑ | PSF, CTBP1 | VCaP, LNCaP, DU145, RWPE and PrEC/105 PCa samples | ↑ castration-resistant tumour growth | [145] |
| RP11-783K16.13, RP11-228B15.4, and CTD-2228K2.7 | ↑ | _ | GEPIA dataset | Higher expression of the lncRNAs were significantly correlated with shorter DFS time in PRAD. | [146] |
| PCAT1 | ↑ rs7463708 increases binding ONECUT2 and AR to the PCAT1 promoter | ONECUT2, LSD1, GNMT, and DHCR24 | LNCaP, LNCaP/shPCGEM1/TCGA dataset | ↑ proliferation and tumor growth | [147] |
| FAM83H-AS1 | ↑ | miR-15a, CCNE2 | LNCaP, LNCaP-AI, and DU145/GEPIA data sets: GSE513217 and GSE55062, plus 20PCa and 8 normal samples | ↑ proliferation, migration, and cell cycle progression | [148] |
| DANCR | ↓ | TIMP2/3 | CWR22Rv1, PC-3, and C4-2B/nude mice | ↑ migration and invasion | [149] |
| GAS5 | ↓ | _ | LNCaP/GSE22606 | ↓ proliferation, ↑ apoptosis | [88] |
| PCAT18 | ↑ | PES | LNCaP, C4-2, BPH/131 PCa, and 29 normal samples/NOD/SCID mice | ↑ proliferation, migration, and invasion | [150] |
| RP1-4514.2, LINC01138, and SUZ12P1 | ↓ | _ | 22RV1, DU145, PC-3 and LNCaP, WMPY-1/3 tumor tissues and 11 ANCTs, plus 14 tumor tissues, and 11 ANCTs and TCGA database | _ | [151] |
| KLKP1 | ↑ | _ | 22RV1, DU145, PC-3 and LNCaP, WMPY-1/3 tumor tissues, 11 ANCTs plus 14 tumor tissues, and 11 ANCTs and TCGA database | _ | |
| TMPO-AS1 | ↓ | _ | LNCaP, DU145, 22Rv1, PC-3, and WPMY-1/54 pairs of PCa samples and TCGA data | ↑ proliferation and migration, ↓ apoptosis | [152] |
| circRNA-51217 | ↓ | R-2HG, miRNA-646, TGFβ1/p-Smad2/3 signaling, ADAR2 | C4-2, PC3, DU145, LNCaP, and HEK293T/TCGA database | IDH1 mutation And R-2HG: ↑ circRNA-51217: ↑ invasion |
[153] |
| circRNA-ARC1 | ↓ | miR-125b-2-3p/miR-4736/PPARγ/MMP-9 signals | CWR22Rv1 and C4-2 | Enz treatment: ↑ invasion | [154] |
| circZMIZ1 | ↑ | _ | DU145, C4-2, LNCaP, 22RV1, RWPE-1, 14 PCa samples, and 14 HCs | ↑ proliferation, ↓ G1 arrest | [100] |
In brief, the effect of AR on the expression of ncRNAs is mainly associated with its role as a transcription factor.
Effects of AR on expression of ncRNAs are also implicated in the pathoetiology of bladder, breast, liver, renal, and gastric cancers (Table 7). Yet, these effects are largely context-dependent. For instance, AR could reduce the expression of miR-21 in breast cancer [155], while inducing its expression in hepatocellular carcinoma [156].
Table 7.
Effects of AR on ncRNAs in different cancer types (Enz: enzalutamide, VM: vasculogenic mimicry, DHEA: dehydroepiandrosterone, RBM: ccRCC bone metastases, ↓: decrease in, ↑: increase in).
| Cancer Types | ncRNAs | Regulation by AR | Molecular Mechanisms | Cell Line/Samples/Animal Models | Function of ncRNAs in Cancer Cells | References |
|---|---|---|---|---|---|---|
| Bladder cancer | miR-525-5p | ↓ | SLPI, HDAC2 | _ | ↑ metastasis | [108] |
| circFNTA | ↑ | ADAR2, miR-370-3p, FNTA pathway, KRAS signaling | SVHUC, T24, J82, 5637, and UMUC3/male athymic BALB/c nude mice | ↑ invasion, metastases, and cisplatin chemo-resistance | [157] | |
| circRNA-ARC1 | ↑ | miR-125b-2-3p/miR-4736/PPARγ/MMP-9 signals | T24 and UMUC3 | Enz treatment: ↓ invasion | [154] | |
| Breast cancer | let-7a | ↑ | CMYC and KRAS | MCF-7, MDA-MB-453, and MDA-MB-231/24 breast cancer samples | ↓ proliferation, cell growth | [158] |
| miR-21 | ↓ | _ | MCF-7, ZR-75-1, MDA-MB-231, SKBR3, and LNCap |
↑ proliferation (miboleron: ↓ miR-21: ↓ proliferation) |
[155] | |
| Triple-negative breast cancer | ARNILA | ↓ | miR-204, Sox4 | MDA-MB-231 and Hs578T, MDA-MB-436/88 TNBC samples/female BALB/c nude mice | ↑ migration, invasion, and EMT process | [159] |
| Early Hepatocarcinogenesis | miR-216a | ↑ | TSLC1 | HepG2/48tumor tissues and 13 non-tumor tissues/male athymic nude mice | ↑ proliferation and migration | [160] |
| Hepatocellular carcinoma | miR-21 | ↑ | PDCD4, ERβ | HepG2, HBEC2-KT/male C57BL/6 mice | DHEA: ↑ miR-21: ↑ proliferation | [156] |
| miR-146a-5p | ↓ | BRCA1 and BCL2 | SK-HEP-1 and HepG2/TCGA database analysis/male nude mice | Enz plus Olaparib treatment: ↑ miR-146a-5p: ↓ proliferation, cell growth, and viability | [161] | |
| circRNA7 | ↓ | miR-7-5p, VE-Cadherin, Notch4 | SKhep1, HA22T | ↑ formation of VM | [162] | |
| circ-LNPEP | ↓ | miR-532-3p, RAB9A | HA22T, SK-HEP-1, and 293/male nude mice | ↑ invasion and metastasis | [163] | |
| circARSP91 | ↓ | ADAR1 | MHCC-97h, LM3 and LO2, HEK-293T/83 pairs of tumor tissues, and ANCTs/nude mice | ↓ tumor growth | [164] | |
| Melanoma | miR-539-3p | ↑ | MITF-AXL signals, USP13 | A375 and WM115 and C32/102 melanoma tissue samples/male nude mice | ↑ invasion and metastasis | [165] |
| SLNCR | SLNCR and AR cooperatively regulate several growth-regulatory genes. | p21, EGR1, MMP9 | WM1976 or A375, SK-MEL-28, WM858 | ↑ proliferation and invasion | [166] | |
| SLNCR1 | ↑ | Brn3a, MMP9 | A375, HEK293T, CY and WM | ↑ invasion | [96] | |
| Cholangiocarcinoma | ZEB1-AS1 | ↑ | miR-133b, HOXB8 | HIBEC, QBC939, CCLP-1, RBE, TFK-1/54 pairs of tumor tissues, and ANCTs/female BALB/c nude mice | ↑ migration, invasion, EMT process, viability, and stemness | [167] |
| Gastric cancer | PART1 | AR interacts with PART1 to stimulatePLZF expression | PLZF, EZH2, PDGFRβ/PI3K/Akt signaling pathway | GES-1, MGC-803, BGC-823, and SGC-7901/GEOdatabase: GSE27342, GSE33335, andGSE3072, plus 136 GC samples | ↓ migration, invasion, and metastasis | [168] |
| Clear cell renal cell carcinoma | TANAR | ↑ | TWIST1 | 786O, SW839, HEK293T/51 ccRCC tissues, and 23 ANCTs/male athymic BALB/c nude mice |
↑ VM formation |
[169] |
| circHIAT1 | ↓ | HIAT1, miR-195-5p/29a-3p/29c-3p, and CDC42 | SRC-2, VHL(þ) Caki-1, SW-839, and ACHN | ↓ migration and invasion | [170] | |
| circEXOC7 | ↓ | DHX9, miR-149-3p, CSF1 | SW839, 786-O, Caki-1, ACHN, HEK293T/4 RCC samples with RBM, and 10 RCC samples without RBM/Balb/c nude mice | ↑ RBM and osteolytic formation | [171] |
6. Discussion
AR has an essential role in the pathogenesis of human cancers, particularly prostate cancer. Since it is required for the development of prostate cancer, androgen deprivation therapy is regarded as a treatment for this type of cancer. Thus, the identification of the regulatory mechanisms of AR signaling is important in the design of treatment options. The importance of this process is further highlighted by the fact that castration resistance might occur during the course of treatment, as a result of expression of constitutively active AR splice variants [172], whose expressions can be modulated by ncRNAs.
Integrative transcriptomic analyses of diverse cancer cell lines and tissues have resulted in the identification of several AR-interacting ncRNAs. In the current study, we have listed ncRNAs that affect expression of AR, as well as those being affected by AR. Notably, mutual interactions have been identified between AR and some of these non-coding transcripts. For instance, the expression of the lncRNA ARLNC1 has been shown to be enhanced by the AR protein. Conversely, ARLNC1 can increase the stability of the AR mRNA through RNA-RNA interaction [74].
AR-targeting miRNAs have been suggested as potent tumor suppressors in prostate cancer. However, a number of other miRNAs have also been found to induce CRPC, by changing the activity of AR signaling. Moreover, AR signaling can affect the expression of miRNAs through different mechanisms, including feedback loops.
LncRNAs and circRNAs that regulate AR signaling have been found to interact with miRNAs. MALAT1/miR-320, CCAT1/miR-28-5P, PlncRNA-1/miR-34c, PlncRNA-1/miR-297, XIST/miR-24, SARCC/miR-143-3p, circ-ITCH/miR-17, and circRNA17/miR-181c-5p are examples of the cooperation between lncRNAs/circRNAs and miRNAs in the regulation of AR signaling. Similarly, AR-regulated lncRNAs and circRNAs have been shown to influence the expression or bioavailability of miRNAs, adding novel layers of complexity in this interaction network.
Taken together, the data presented above indicates the complexity of the transcriptional regulation of miRNAs by AR and the effects of AR on them. Moreover, the interactions between ncRNAs and AR signaling can be context-dependent.
Author Contributions
S.G.-F. and A.B. wrote the draft and revised it. M.T. desigend and supervised the study. T.K., E.J. and J.K. collected the data and adesigend the figures and tables. All authors have read and agreed to the published version of the manuscript.
Funding
This Study was finacially supported by friedrich schiller university jena.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lu N.Z., Wardell S.E., Burnstein K.L., DeFranco D., Fuller P., Giguère V., Hochberg R.B., McKay L., Renoir J.-M., Weigel N.L., et al. International Union of Pharmacology. LXV. The pharmacology and classification of the nuclear receptor superfamily: Glucocorticoid, mineralocorticoid, progesterone, and androgen receptors. Pharmacol. Rev. 2006;58:782–797. doi: 10.1124/pr.58.4.9. [DOI] [PubMed] [Google Scholar]
- 2.Roy A., Lavrovsky Y., Song C., Chen S., Jung M., Velu N., Bi B., Chatterjee B. Regulation of androgen action. Vitam. Horm. 1998;55:309–352. doi: 10.1016/s0083-6729(08)60938-3. [DOI] [PubMed] [Google Scholar]
- 3.MacLean H.E., Warne G.L., Zajac J.D. Localization of functional domains in the androgen receptor. J. Steroid Biochem. Mol. Biol. 1997;62:233–242. doi: 10.1016/S0960-0760(97)00049-6. [DOI] [PubMed] [Google Scholar]
- 4.Davey R.A., Grossmann M. Androgen receptor structure, function and biology: From bench to bedside. Clin. Biochem. Rev. 2016;37:3–15. [PMC free article] [PubMed] [Google Scholar]
- 5.Eder I.E., Culig Z., Putz T., Nessler-Menardi C., Bartsch G., Klocker H. Molecular biology of the androgen receptor: From molecular understanding to the clinic. Eur. Urol. 2001;40:241–251. doi: 10.1159/000049782. [DOI] [PubMed] [Google Scholar]
- 6.Estrada M., Espinosa A., Müller M., Jaimovich E. Testosterone stimulates intracellular calcium release and mitogen-activated protein kinases via a g protein-coupled receptor in skeletal muscle cells. Endocrinology. 2003;144:3586–3597. doi: 10.1210/en.2002-0164. [DOI] [PubMed] [Google Scholar]
- 7.Kang H.-Y., Cho C.-L., Huang K.-L., Wang J.-C., Hu Y.-C., Lin H.-K., Chang C., Huang K.-E. Nongenomic androgen activation of phosphatidylinositol 3-Kinase/Akt signaling pathway in MC3T3-E1 osteoblasts. J. Bone Miner. Res. 2004;19:1181–1190. doi: 10.1359/JBMR.040306. [DOI] [PubMed] [Google Scholar]
- 8.Kousteni S., Bellido T., Plotkin L.I., O’Brien C.A., Bodenner D.L., Han L., Han K., Digregorio G.B., Katzenellenbogen J.A., Katzenellenbogen B.S., et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: Dissociation from transcriptional activity. Cell. 2001;104:719–730. doi: 10.1016/S0092-8674(02)08100-X. [DOI] [PubMed] [Google Scholar]
- 9.Ganapathy K., Staklinski S., Hasan F., Ottman R., Andl T., Berglund A.E., Park J.Y., Chakrabarti R. Multifaceted function of MicroRNA-299-3p fosters an antitumor environment through modulation of androgen receptor and VEGFA signaling pathways in prostate cancer. Sci. Rep. 2020;10:5167. doi: 10.1038/s41598-020-62038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y., Luo F., Xu Y., Wang B., Zhao Y., Xu W., Shi L., Lu X., Liu Q. Epithelial-mesenchymal transition and cancer stem cells, mediated by a long non-coding RNA, HOTAIR, are involved in cell malignant transformation induced by cigarette smoke extract. Toxicol. Appl. Pharmacol. 2015;282:9–19. doi: 10.1016/j.taap.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 11.Rui X., Gu T., Pan H., Shao S., Shao H. MicroRNA-381 suppresses proliferation and invasion of prostate cancer cells through downregulation of the androgen receptor. Oncol. Lett. 2019;18:2066–2072. doi: 10.3892/ol.2019.10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nadiminty N., Tummala R., Lou W., Zhu Y., Zhang J., Chen X., White R.W.D., Kung H.-J., Evans C.P., Gao A.C. MicroRNA let-7c suppresses androgen receptor expression and activity via regulation of Myc expression in prostate cancer cells. J. Biol. Chem. 2012;287:1527–1537. doi: 10.1074/jbc.M111.278705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun T., Wang X., He H.H., Sweeney C.J., Liu S.X., Brown M., Balk S.P., Lee G.-S., Kantoff P.W. MiR-221 promotes the development of androgen independence in prostate cancer cells via downregulation of HECTD2 and RAB1A. Oncogene. 2014;33:2790–2800. doi: 10.1038/onc.2013.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X., Bemis L., Su L.-J., Gao D., Flaig T.W. miR-125b regulation of androgen receptor signaling via modulation of the receptor complex co-repressor NCOR2. BioRes. Open Access. 2012;1:55–62. doi: 10.1089/biores.2012.9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long M., Singh P.K., Russell J.R., Llimos G., Rosario S., Rizvi A., Berg P.V.D., Kirk J., Sucheston-Campbell L.E., Smiraglia D.J., et al. The miR-96 and RARγ signaling axis governs androgen signaling and prostate cancer progression. Oncogene. 2019;38:421–444. doi: 10.1038/s41388-018-0450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu S., Li L., Yeh S., Cui Y., Li X., Chang H.-C. Infiltrating T cells promote prostate cancer metastasis via modulation of FGF11→ miRNA-541→ androgen receptor (AR)→ MMP9 signaling. Mol. Oncol. 2015;9:44–57. doi: 10.1016/j.molonc.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das D.K., Naidoo M., Ilboudo A., Park J.Y., Ali T., Krampis K., Robinson B.D., Osborne J.R., Ogunwobi O.O. miR-1207-3p regulates the androgen receptor in prostate cancer via FNDC1/fibronectin. Exp. Cell Res. 2016;348:190–200. doi: 10.1016/j.yexcr.2016.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishra S., Deng J.J., Gowda P.S., Rao M.K., Lin C.-L., Chen C.L., Huang T., Sun L.-Z. Androgen receptor and microRNA-21 axis downregulates transforming growth factor beta receptor II (TGFBR2) expression in prostate cancer. Oncogene. 2014;33:4097–4106. doi: 10.1038/onc.2013.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng L., Kang Y., Zhang L., Zou W. MiR-133a-5p inhibits androgen receptor (AR)-induced proliferation in prostate cancer cells via targeting FUsed in Sarcoma (FUS) and AR. Cancer Biol. Ther. 2019;21:34–42. doi: 10.1080/15384047.2019.1665393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen W., Yao G., Zhou K. miR-103a-2-5p/miR-30c-1-3p inhibits the progression of prostate cancer resistance to androgen ab-lation therapy via targeting androgen receptor variant 7. J. Cell. Biochem. 2019;120:14055–14064. doi: 10.1002/jcb.28680. [DOI] [PubMed] [Google Scholar]
- 21.Kumar B., Khaleghzadegan S., Mears B., Hatano K., Kudrolli T.A., Chowdhury W., Yeater D.B., Ewing C.M., Luo J., Isaacs W.B., et al. Identification of miR-30b-3p and miR-30d-5p as direct regulators of androgen receptor signaling in prostate cancer by complementary functional microRNA library screening. Oncotarget. 2016;7:72593–72607. doi: 10.18632/oncotarget.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin P.-C., Chiu Y.-L., Banerjee S., Park K., Mosquera J.M., Giannopoulou E., Alves P., Tewari A.-K., Gerstein M.-B., Beltran B., et al. Epigenetic repression of miR-31 disrupts andro-gen receptor homeostasis and contributes to prostate cancer progression. Cancer Res. 2013;73:1232–1244. doi: 10.1158/0008-5472.CAN-12-2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagman Z., Haflidadottir B., Ceder J., Larne O., Bjartell A., Lilja H., Edsjö A., Ceder Y. miR-205 negatively regulates the androgen receptor and is associated with adverse outcome of prostate cancer patients. Br. J. Cancer. 2013;108:1668–1676. doi: 10.1038/bjc.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu M., Chang Y., Guo Y., Wang N., Cui J., Gao W.-Q. Regulation and methylation of tumor suppressor MiR-124 by androgen receptor in prostate cancer cells. PLoS ONE. 2015;10:e0116197. doi: 10.1371/journal.pone.0116197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larne O., Hagman Z., Lilja H., Bjartell A., Edsjö A., Ceder Y. miR-145 suppress the androgen receptor in prostate cancer cells and correlates to prostate cancer prognosis. Carcinogenesis. 2015;36:858–866. doi: 10.1093/carcin/bgv063. [DOI] [PubMed] [Google Scholar]
- 26.Naiki-Ito A., Naiki T., Kato H., Iida K., Etani T., Nagayasu Y., Suzuki S., Yamashita Y., Inaguma S., Onishi M., et al. Recruitment of miR-8080 by luteolin inhibits androgen receptor splice variant 7 expression in castration-resistant prostate cancer. Carcinogenesis. 2020;41:1145–1157. doi: 10.1093/carcin/bgz193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi X.-B., Xue L., Ma A.-H., Tepper C.G., Gandour-Edwards R., Kung H.-J., White R.W.D. Tumor suppressive miR-124 targets androgen receptor and inhibits proliferation of prostate cancer cells. Oncogene. 2013;32:4130–4138. doi: 10.1038/onc.2012.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi X.-B., Ma A.-H., Xue L., Li M., Nguyen H.G., Yang J.C., Tepper C.G., Gandour-Edwards R., Evans C.P., Kung H.J., et al. miR-124 and androgen receptor signaling inhibitors repress pros-tate cancer growth by downregulating androgen receptor splice variants, EZH2, and Src. Cancer Res. 2015;75:5309–5317. doi: 10.1158/0008-5472.CAN-14-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou W., Huang S., Jiang Q., Yuan T. Suppression of miR-4735-3p in androgen receptor-expressing prostate cancer cells in-creases cell death during chemotherapy. Am. J. Transl. Res. 2017;9:3714. [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang C.-Y., Ruan Y., Wang X.-H., Zhao W., Jiang Q., Jing Y.-F., Han B.-M., Xia S.-J., Zhao F.-J. MiR-185 attenuates androgen receptor function in prostate cancer indirectly by targeting bromodomain containing 8 isoform 2, an androgen receptor co-activator. Mol. Cell. Endocrinol. 2016;427:13–20. doi: 10.1016/j.mce.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 31.Lin S.-J., Chou F.-J., Li L., Lin C.-Y., Yeh S., Chang C. Natural killer cells suppress enzalutamide resistance and cell invasion in the castration resistant prostate cancer via targeting the androgen receptor splicing variant 7 (ARv7) Cancer Lett. 2017;398:62–69. doi: 10.1016/j.canlet.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 32.Shiina M., Hashimoto Y., Kato T., Yamamura S., Tanaka Y., Majid S., Saini S., Varahram S., Kulkarni P., Dasgupta P., et al. Differential expression of miR-34b and androgen receptor pathway regulate prostate cancer aggressiveness between African-Americans and Caucasians. Oncotarget. 2016;8:8356–8368. doi: 10.18632/oncotarget.14198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato S., Katsushima K., Shinjo K., Hatanaka A., Ohka F., Suzuki S., Naiki-Ito A., Soga N., Takahashi S., Kondo Y. Histone deacetylase inhibition in prostate cancer triggers miR-320–Mediated suppression of the androgen receptor. Cancer Res. 2016;76:4192–4204. doi: 10.1158/0008-5472.CAN-15-3339. [DOI] [PubMed] [Google Scholar]
- 34.Yu C., Gong A.-Y., Chen D., Leon D.S., Young C.Y.F., Chen X.-M. Phenethyl isothiocyanate inhibits androgen receptor-regulated transcriptional activity in prostate cancer cells through suppressing PCAF. Mol. Nutr. Food Res. 2013;57:1825–1833. doi: 10.1002/mnfr.201200810. [DOI] [PubMed] [Google Scholar]
- 35.Xiao J., Gong A.Y., Eischeid A.N., Chen D., Deng C., Young C.Y., Chen X.M. miR-141 modulates androgen receptor transcriptional activity in human prostate cancer cells through targeting the small heterodimer partner protein. Prostate. 2012;72:1514–1522. doi: 10.1002/pros.22501. [DOI] [PubMed] [Google Scholar]
- 36.Zheng L., Chen J., Ma Z., Liu W., Yang F., Yang Z., Wang K., Wang X., He D., Li L. Capsaicin causes inactivation and degradation of the androgen receptor by inducing the restoration of miR-449a in prostate cancer. Oncol. Rep. 2015;34:1027–1034. doi: 10.3892/or.2015.4055. [DOI] [PubMed] [Google Scholar]
- 37.Epis M.R., Giles K.M., Barker A., Kendrick T.S., Leedman P.J. miR-331-3p Regulates ERBB-2 expression and androgen receptor signaling in prostate cancer. J. Biol. Chem. 2009;284:24696–24704. doi: 10.1074/jbc.M109.030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leite K.R., Morais D.R., Florez M.G., Reis S.T., Iscaife A., Viana N., Moura C.M., Silva I.A., Katz B.S., Pontes J., et al. The role of microRNAs 371 and 34a in androgen receptor control influencing prostate cancer behavior. Urol. Oncol. Semin. Orig. Investig. 2015;33:267.e15–267.e22. doi: 10.1016/j.urolonc.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Das D.K., Ogunwobi O.O. A novel microRNA-1207-3p/FNDC1/FN1/AR regulatory pathway in prostate cancer. RNA Dis. 2017;4:e1503. [PMC free article] [PubMed] [Google Scholar]
- 40.Xie H., Li L., Zhu G., Dang Q., Ma Z., He D., Chang L., Song W., Chang H.C., Krolewski J.J., et al. Infiltrated preadipocytes increase prostate cancer metastasis via modulation of the miR-301a/androgen receptor (AR)/TGF-β1/Smad/MMP9 signals. Oncotarget. 2015;6:12326. doi: 10.18632/oncotarget.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nilsson E.M., Laursen K.B., Whitchurch J., McWilliam A., Ødum N., Persson J., Heery D., Gudas L.J., Mongan N.P. MiR137 is an androgen regulated repressor of an extended network of transcriptional coregulators. Oncotarget. 2015;6:35710–35725. doi: 10.18632/oncotarget.5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu B., Sun Y., Tang M., Liang C., Huang C.-P., Niu Y., Wang Z., Chang C. The miR-361-3p increases enzalutamide (Enz) sensitivity via target-ing the ARv7 and MKNK2 to better suppress the Enz-resistant prostate cancer. Cell Death Dis. 2020;11:807. doi: 10.1038/s41419-020-02932-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ayub S.G., Kaul D., Ayub T. An androgen-regulated miR-2909 modulates TGFβ signalling through AR/miR-2909 axis in pros-tate cancer. Gene. 2017;631:1–9. doi: 10.1016/j.gene.2017.07.037. [DOI] [PubMed] [Google Scholar]
- 44.Guan H., You Z., Wang C., Fang F., Peng R., Mao L., Xu B., Chen M. MicroRNA-200a suppresses prostate cancer progression through BRD4/AR signaling pathway. Cancer Med. 2019;8:1474–1485. doi: 10.1002/cam4.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rajabi H., Ahmad R., Jin C., Joshi M.D., Guha M., Alam M., Kharbanda S., Kufe N. MUC1-C oncoprotein confers androgen-independent growth of human prostate cancer cells. Prostate. 2012;72:1659–1668. doi: 10.1002/pros.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gong A.-Y., Eischeid A.N., Xiao J., Zhao J., Chen D., Wang Z.-Y., Young C.Y., Chen X.-M. miR-17-5p targets the p300/CBP-associated factor and modulates androgen receptor transcriptional activity in cultured prostate cancer cells. BMC Cancer. 2012;12:492. doi: 10.1186/1471-2407-12-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matin F., Jeet V., Srinivasan S., Cristino A.S., Panchadsaram J., Clements J.A., Batra J. MicroRNA-3162-5p-Mediated Crosstalk between Kallikrein Family Members including prostate-specific antigen in prostate cancer. Clin. Chem. 2019;65:771–780. doi: 10.1373/clinchem.2018.295824. [DOI] [PubMed] [Google Scholar]
- 48.Ebron J.S., Shankar E., Singh J., Sikand K., Weyman C.M., Gupta S., Lindner D.-J., Liu X., Campbell M.-J., Shukla G.-C. MiR-644a disrupts oncogenic transformation and war-burg effect by direct modulation of multiple genes of tumor-promoting pathways. Cancer Res. 2019;79:1844–1856. doi: 10.1158/0008-5472.CAN-18-2993. [DOI] [PubMed] [Google Scholar]
- 49.Takayama K.I., Misawa A., Suzuki T., Takagi K., Hayashizaki Y., Fujimura T., Homma Y., Takahashi S., Urano T., Inoue S. TET2 repression by androgen hormone regulates global hydroxymethylation status and prostate cancer progression. Nat. Commun. 2015;6:1–16. doi: 10.1038/ncomms9219. [DOI] [PubMed] [Google Scholar]
- 50.Wang C., Ouyang Y., Lu M., Wei J., Zhang H. miR-141-3p regulates the expression of androgen receptor by targeting its 3’UTR in prostate cancer LNCaP cells. Chin. J. Cell. Mol. Immunol. 2015;31:736–739. [PubMed] [Google Scholar]
- 51.Li X., Chen Y.-T., Josson S., Mukhopadhyay N.K., Kim J., Freeman M.R., Huang W.-C. MicroRNA-185 and 342 inhibit tumorigenicity and induce apoptosis through blockade of the srebp metabolic pathway in prostate cancer cells. PLoS ONE. 2013;8:e70987. doi: 10.1371/journal.pone.0070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ding M., Lin B., Li T., Liu Y., Li Y., Zhou X., Miao M., Gu J., Pan H., Yang F., et al. A dual yet opposite growth-regulating function of miR-204 and its target XRN1 in prostate adenocarcinoma cells and neuroendocrine-like prostate cancer cells. Oncotarget. 2015;6:7686–7700. doi: 10.18632/oncotarget.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalogirou C., Linxweiler J., Schmucker P., Snaebjornsson M.T., Schmitz W., Wach S., Krebs M., Hartmann E., Puhr M., Müller A., et al. MiR-205-driven downregulation of cholesterol biosynthesis through SQLE-inhibition identifies therapeutic vulnerability in aggressive prostate cancer. Nat. Commun. 2021;12:5066. doi: 10.1038/s41467-021-25325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boll K., Reiche K., Kasack K., Mörbt N., Kretzschmar A., Tomm J., Verhaegh G., Schalken J., Bergen M.-V., Horn F., et al. MiR-130a, miR-203 and miR-205 jointly repress key on-cogenic pathways and are downregulated in prostate carcinoma. Oncogene. 2013;32:277–285. doi: 10.1038/onc.2012.55. [DOI] [PubMed] [Google Scholar]
- 55.Yang Y., Jia D., Kim H., Elmageed Z.Y.A., Datta A., Davis R., Srivastav S.K., Moroz K., Crawford B.E., Moparty K., et al. Dysregulation of miR-212 promotes castration resistance through hnRNPH1-Mediated regulation of AR and AR-V7: Implications for racial disparity of prostate cancer. Clin. Cancer Res. 2016;22:1744–1756. doi: 10.1158/1078-0432.CCR-15-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kashat M., Azzouz L., Sarkar S.H., Kong D., Li Y., Sarkar F.H. Inactivation of AR and Notch-1 signaling by miR-34a attenuates prostate cancer aggressiveness. Am. J. Transl. Res. 2012;4:432–442. [PMC free article] [PubMed] [Google Scholar]
- 57.Xu S., Wang T., Song W., Jiang T., Zhang F., Yin Y., Jiang S.-W., Wu K., Yu Z., Wang C., et al. The inhibitory effects of AR/miR-190a/YB-1 negative feedback loop on prostate cancer and underlying mechanism. Sci. Rep. 2015;5:srep13528. doi: 10.1038/srep13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo J., Hu J., Cao R., Chen Q., Li K. Androgen receptor is inactivated and degraded in bladder cancer cells by phenyl glucosa-mine via miR-449a restoration. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018;24:2294. doi: 10.12659/MSM.906836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bandini E., Fanini F., Vannini I., Rossi T., Plousiou M., Tumedei M.M., Limarzi F., Maltoni R., Fabbri F., Hrelia S., et al. miR-9-5p as a regulator of the androgen receptor pathway in breast cancer cell lines. Front. Cell Dev. Biol. 2020;8:579160. doi: 10.3389/fcell.2020.579160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fan Q., Huang T., Sun X., Yang X., Wang J., Liu Y., Ni T., Gu S., Li Y., Wang Y. miR 130a 3p promotes cell proliferation and invasion by targeting estrogen receptor α and androgen receptor in cervical cancer. Exp. Ther. Med. 2021;21:414. doi: 10.3892/etm.2021.9858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bao S.-X., Wang C.-H., Jin S., Hu K.-W., Lu J.-T. miR-135b-5p suppresses androgen receptor-enhanced hepatocellular carcinoma cell proliferation via regulating the HIF-2α/c-Myc/P27 Signals in vitro. OncoTargets Ther. 2020;13:9991. doi: 10.2147/OTT.S268214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu G., Ouyang X., Sun Y., Xiao Y., You B., Gao Y., Yeh S., Li Y., Chang C. The miR-92a-2-5p in exosomes from macrophages increases liver cancer cells invasion via altering the AR/PHLPP/p-AKT/β-catenin signaling. Cell Death Differ. 2020;27:3258–3272. doi: 10.1038/s41418-020-0575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu J., Lin H., Li G., Sun Y., Chen J., Shi L., Cai X., Chang C. The miR-367-3p increases sorafenib chemotherapy efficacy to suppress hepato-cellular carcinoma metastasis through altering the androgen receptor signals. EBioMedicine. 2016;12:55–67. doi: 10.1016/j.ebiom.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qu Y., Qi L., Hao L., Zhu J. Upregulation of circ-ASPH contributes to glioma cell proliferation and aggressiveness by targeting the miR-599/AR/SOCS2-AS1 signaling pathway. Oncol. Lett. 2021;21:388. doi: 10.3892/ol.2021.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Z., Zhou N., Huang J., Ho T.-T., Zhu Z., Qiu Z., Zhou X., Bai C., Wu F., Xu M., et al. Regulation of androgen receptor splice variant AR3 by PCGEM1. Oncotarget. 2016;7:15481–15491. doi: 10.18632/oncotarget.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parolia A., Venalainen E., Xue H., Mather R., Lin D., Wu R., Pucci P., Rogalski J., Evans J.R., Feng F., et al. The long noncoding RNA HORAS 5 mediates castration-resistant prostate cancer survival by activating the androgen receptor transcriptional program. Mol. Oncol. 2019;13:1121–1136. doi: 10.1002/1878-0261.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roediger J., Hessenkemper W., Bartsch S., Manvelyan M., Huettner S.S., Liehr T., Esmaeili M., Foller S., Petersen I., Grimm M.-O., et al. Supraphysiological androgen levels in-duce cellular senescence in human prostate cancer cells through the Src-Akt pathway. Mol. Cancer. 2014;13:214. doi: 10.1186/1476-4598-13-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mirzakhani K., Kallenbach J., Rasa S.M.M., Ribaudo F., Ungelenk M., Ehsani M., Gong W., Gassler N., Leeder M., Grimm M.-O., et al. The androgen recep-tor-lncRNASAT1-AKT-p15 axis mediates androgen-induced cellular senescence in prostate cancer cells. Oncogene. 2021:1–14. doi: 10.1038/s41388-021-02060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fang Z., Xu C., Li Y., Cai X., Ren S., Liu H., Wang Y., Wang F., Chen R., Qu M., et al. A feed-forward regulatory loop between androgen receptor and PlncRNA-1 promotes prostate cancer progression. Cancer Lett. 2016;374:62–74. doi: 10.1016/j.canlet.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 70.Li Z., Teng J., Jia Z., Zhang G., Ai X. The long non-coding RNA PCAL7 promotes prostate cancer by strengthening androgen receptor signaling. J. Clin. Lab. Anal. 2021;35:e23645. doi: 10.1002/jcla.23645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang A., Zhao J.C., Kim J., Fong K.-W., Yang Y.A., Chakravarti D., Mo Y.-Y., Yu J. LncRNA HOTAIR enhances the andro-gen-receptor-mediated transcriptional program and drives castration-resistant prostate cancer. Cell Rep. 2015;13:209–221. doi: 10.1016/j.celrep.2015.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dai X., Liu L., Liang Z., Guo K., Xu S., Wang H. Silencing of lncRNA MALAT1 inhibits cell cycle progression via androgen receptor signaling in prostate cancer cells. Pathol.-Res. Pract. 2019;215:712–721. doi: 10.1016/j.prp.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 73.Lingadahalli S., Jadhao S., Sung Y.Y., Chen M., Hu L., Chen X., Cheung E. Novel lncRNA LINC00844 regulates prostate cancer cell migration and invasion through AR Signaling. Mol. Cancer Res. 2018;16:1865–1878. doi: 10.1158/1541-7786.MCR-18-0087. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Y., Pitchiaya S., Cieślik M., Niknafs Y.S., Tien J.C.-Y., Hosono Y., Iyer M.K., Yazdani S., Subramaniam S., Shukla S., et al. Analysis of the androgen receptor–regulated lncRNA landscape identifies a role for ARLNC1 in prostate cancer progression. Nat. Genet. 2018;50:814–824. doi: 10.1038/s41588-018-0120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang L., Lin C., Jin C., Yang J.C., Tanasa B., Li W., Merkurjev D., Ohgi K.-A., Meng D., Zhang J., et al. lncRNA-dependent mechanisms of androgen-receptor-regulated gene activation programs. Nature. 2013;500:598–602. doi: 10.1038/nature12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yao M., Shi X., Li Y., Xiao Y., Butler W., Huang Y., Du L., Wu T., Bian X., Shi G., et al. LINC00675 activates androgen receptor axis signaling pathway to promote castration-resistant prostate cancer progression. Cell Death Dis. 2020;11:638. doi: 10.1038/s41419-020-02856-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.You Z., Liu C., Wang C., Ling Z., Wang Y., Wang Y., Zhang M., Chen S., Xu B., Guan H., et al. LncRNA CCAT1 promotes prostate cancer cell proliferation by inter-acting with DDX5 and miR-28-5p. Mol. Cancer Ther. 2019;18:2469–2479. doi: 10.1158/1535-7163.MCT-19-0095. [DOI] [PubMed] [Google Scholar]
- 78.Misawa A., Takayama K.-I., Urano T., Inoue S. Androgen-induced long noncoding RNA (lncRNA) SOCS2-AS1 promotes cell growth and inhibits apoptosis in prostate cancer cells. J. Biol. Chem. 2016;291:17861–17880. doi: 10.1074/jbc.M116.718536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shi Z., Chen J., Wumaner A., Li M., Liang C., Li M. A novel long non-coding RNA PCLN16 facilitates androgen receptor sig-naling in prostate cancer. Biochem. Biophys. Res. Commun. 2021;537:78–84. doi: 10.1016/j.bbrc.2020.12.043. [DOI] [PubMed] [Google Scholar]
- 80.Li L., Dang Q., Xie H., Yang Z., He D., Liang L., Song W., Yeh S., Chang C. Infiltrating mast cells enhance prostate cancer invasion via altering LncRNA-HOTAIR/PRC2-androgen receptor (AR)-MMP9 signals and increased stem/progenitor cell population. Oncotarget. 2015;6:14179. doi: 10.18632/oncotarget.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang R., Sun Y., Li L., Niu Y., Lin W., Lin C., Antonarakis E.S., Luo J., Yeh S., Chang C. Preclinical study using Malat1 small interfering RNA or androgen receptor splicing variant 7 degradation enhancer ASC-J9 ® to suppress enzalutamide-resistant prostate cancer progression. Eur. Urol. 2017;72:835–844. doi: 10.1016/j.eururo.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cui Z., Ren S., Lu J., Wang F., Xu W., Sun Y., Wei M., Chen J., Gao X., Xu C., et al. The prostate cancer-up-regulated long noncoding RNA PlncRNA-1 modulates apoptosis and proliferation through reciprocal regulation of androgen receptor. Urol. Oncol. Semin. Orig. Investig. 2013;31:1117–1123. doi: 10.1016/j.urolonc.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 83.Gu P., Chen X., Xie R., Xie W., Huang L., Dong W., Han J., Liu X., Shen J., Huang J., et al. A novel AR translational regulator lncRNA LBCS inhibits castration resistance of prostate cancer. Mol. Cancer. 2019;18:109. doi: 10.1186/s12943-019-1037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ho T.-T., Huang J., Zhou N., Zhang Z., Koirala P., Zhou X., Wu F., Ding X., Mo Y.-Y. Regulation of PCGEM1 by p54/nrb in prostate cancer. Sci. Rep. 2016;6:34529. doi: 10.1038/srep34529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hung C.-L., Wang L.-Y., Yu Y.-L., Chen H.-W., Srivastava S., Petrovics G., Kung H.-J. A long noncoding RNA connects c-Myc to tumor metabolism. Proc. Natl. Acad. Sci. USA. 2014;111:18697–18702. doi: 10.1073/pnas.1415669112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen W.-W., Zhang Y., Wang L.-N., Lin Y.-N., Xing Y.-X., Shi Y., Zhao J., Han B. The novel long noncoding RNA LOC283070 is involved in the transition of LNCaP cells into androgen-independent cells via its interaction with PHB2. Asian J. Androl. 2018;20:511–517. doi: 10.4103/aja.aja_36_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang M., Sun Y., Huang C.-P., Luo J., Zhang L., Meng J., Liang C., Chang C. Targeting the Lnc-OPHN1-5/androgen receptor/hnRNPA1 com-plex increases Enzalutamide sensitivity to better suppress prostate cancer progression. Cell Death Dis. 2021;12:855. doi: 10.1038/s41419-021-03966-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lv S., Pu X., Luo M., Wen H., Xu Z., Wei Q., Dang Q. Long noncoding RNA GAS5 interacts and suppresses androgen receptor ac-tivity in prostate cancer cells. Prostate. 2021;81:893–901. doi: 10.1002/pros.24186. [DOI] [PubMed] [Google Scholar]
- 89.Thomas P.B., Jeffery P., Gahete M.D., Whiteside E., Walpole C., Maugham M., Jovanovic L., Gunter J., Williams E., Nelson C., et al. The long non-coding RNA GHSROS repro-grams prostate cancer cell lines toward a more aggressive phenotype. PeerJ. 2021;9:e10280. doi: 10.7717/peerj.10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lemos A.E.G., Ferreira L.B., Batoreu N.M., de Freitas P.P., Bonamino M.H., Gimba E.R.P. PCA3 long noncoding RNA modulates the expression of key cancer-related genes in LNCaP prostate cancer cells. Tumor Biol. 2016;37:11339–11348. doi: 10.1007/s13277-016-5012-3. [DOI] [PubMed] [Google Scholar]
- 91.Wang L., Shi S., Wang L., Xie Y., Bai E., Zhou X., Li M., Jin G., Zhu Q. Role of PRNCR1 in the castration resistant prostate cancer. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi Chin. J. Cell. Mol. Immunol. 2013;29:789–793. [PubMed] [Google Scholar]
- 92.Xiong Y., Wang L., Li Y., Chen M., He W., Qi L. The long Non-Coding RNA XIST interacted with MiR-124 to modulate bladder cancer growth, invasion and migration by targeting androgen receptor (AR) Cell. Physiol. Biochem. 2017;43:405–418. doi: 10.1159/000480419. [DOI] [PubMed] [Google Scholar]
- 93.Wen L., Zhang X., Bian J., Han L., Huang H., He M., Wei M., Wang P. The long non-coding RNA LINC00460 predicts the prognosis and promotes the proliferation and migration of cells in bladder urothelial carcinoma. Oncol. Lett. 2019;17:3874–3880. doi: 10.3892/ol.2019.10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu S., Zhang L., Deng J., Guo B., Li F., Wang Y., Wu R., Zhang S., Lu J., Zhou Y. A novel micropeptide encoded by Y-Linked LINC00278 links cigarette smoking and AR signaling in male esophageal squamous cell carcinoma. Cancer Res. 2020;80:2790–2803. doi: 10.1158/0008-5472.CAN-19-3440. [DOI] [PubMed] [Google Scholar]
- 95.Schmidt K., Weidmann C.A., Hilimire T.A., Yee E., Hatfield B.M., Schneekloth J.S., Jr., Weeks K.M., Novina C.D. Targeting the oncogenic long non-coding RNA SLNCR1 by blocking its sequence-specific binding to the androgen receptor. Cell Rep. 2020;30:541–554.e5. doi: 10.1016/j.celrep.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schmidt K., Joyce C.E., Buquicchio F., Brown A., Ritz J., Distel R.J., Yoon C.H., Novina C.D. The lncRNA SLNCR1 Mediates Melanoma Invasion through a Conserved SRA1-like Region. Cell Rep. 2016;15:2025–2037. doi: 10.1016/j.celrep.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhai W., Sun Y., Guo C., Hu G., Wang M., Zheng J., Lin W., Huang Q., Li G., Zheng J., et al. LncRNA-SARCC suppresses renal cell carcinoma (RCC) progression via altering the androgen receptor (AR)/miRNA-143-3p signals. Cell Death Differ. 2017;24:1502–1517. doi: 10.1038/cdd.2017.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhai W., Sun Y., Jiang M., Wang M., Gasiewicz T., Zheng J., Chang C. Differential regulation of LncRNA-SARCC suppresses VHL-mutant RCC cell proliferation yet promotes VHL-normal RCC cell proliferation via modulating androgen receptor/HIF-2α/C-MYC axis under hypoxia. Oncogene. 2016;35:4866–4880. doi: 10.1038/onc.2016.19. [DOI] [PubMed] [Google Scholar]
- 99.Bai J.-Y., Jin B., Ma J.-B., Liu T.-J., Yang C., Chong Y., Wang X., He D., Guo P. HOTAIR and androgen receptor synergistically increase GLI2 tran-scription to promote tumor angiogenesis and cancer stemness in renal cell carcinoma. Cancer Lett. 2021;498:70–79. doi: 10.1016/j.canlet.2020.10.031. [DOI] [PubMed] [Google Scholar]
- 100.Jiang H., Lv D.J., Song X.L., Wang C., Yu Y.Z., Zhao S.C. Upregulated circZMIZ1 promotes the proliferation of prostate cancer cells and is a valuable marker in plasma. Neoplasma. 2020;67:68–77. doi: 10.4149/neo_2019_190213N116. [DOI] [PubMed] [Google Scholar]
- 101.Li S., Yu C., Zhang Y., Liu J., Jia Y., Sun F., Zhang P., Li J., Guo L., Xiao H., et al. Circular RNA cir-ITCH is a potential therapeutic target for the treatment of castration-resistant prostate cancer. BioMed Res. Int. 2020;2020:7586521. doi: 10.1155/2020/7586521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Greene J., Baird A.-M., Casey O., Brady L., Blackshields G., Lim M., O’Brien O., Gray S.G., McDermott R., Finn S.P. Circular RNAs are differentially expressed in prostate cancer and are potentially associated with resistance to enzalutamide. Sci. Rep. 2019;9:10739. doi: 10.1038/s41598-019-47189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu G., Sun Y., Xiang Z., Wang K., Liu B., Xiao G., Niu Y., Wu D., Chang C. Preclinical study using circular RNA 17 and micro RNA 181c-5p to suppress the enzalutamide-resistant prostate cancer progression. Cell Death Dis. 2019;10:37. doi: 10.1038/s41419-018-1048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Siu M.K., Chen W.-Y., Tsai H.-Y., Yeh H.-L., Yin J.J., Liu S.-Y., Liu Y.-N. Androgen receptor regulates SRC expression through mi-croRNA-203. Oncotarget. 2016;7:25726. doi: 10.18632/oncotarget.8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gui B., Hsieh C.-L., Kantoff P.W., Kibel A.S., Jia L. Androgen receptor-mediated downregulation of microRNA-221 and -222 in castration-resistant prostate cancer. PLoS ONE. 2017;12:e0184166. doi: 10.1371/journal.pone.0184166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yao J., Xu C., Fang Z., Li Y., Liu H., Wang Y., Xu C., Sun Y. Androgen receptor regulated microRNA miR-182-5p promotes prostate cancer progression by targeting the ARRDC3/ITGB4 pathway. Biochem. Biophys. Res. Commun. 2016;474:213–219. doi: 10.1016/j.bbrc.2016.04.107. [DOI] [PubMed] [Google Scholar]
- 107.Siu M., Chen W., Tsai H., Chen H., Yin J., Chen C., Tsai Y.-C., Liu Y.-N. TCF7 is suppressed by the androgen receptor via microRNA-1-mediated downregulation and is involved in the development of resistance to androgen deprivation in prostate cancer. Prostate Cancer Prostatic Dis. 2017;20:172–178. doi: 10.1038/pcan.2017.2. [DOI] [PubMed] [Google Scholar]
- 108.Yang Z., Chen J., Xie H., Liu T., Chen Y., Ma Z., Pei X., Yang W., Li L. Androgen receptor suppresses prostate cancer metastasis but promotes bladder cancer metastasis via differentially altering miRNA525-5p/SLPI-mediated vasculogenic mimicry formation. Cancer Lett. 2020;473:118–129. doi: 10.1016/j.canlet.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 109.Zhang P., Lu Y., Kong Z., Zhang Y., Fu F., Su X., Huang Y., Wan X., Li Y. Androgen-responsive lncRNA LINC00304 promotes cell cycle and prolif-eration via regulating CCNA1. Prostate. 2019;79:994–1006. doi: 10.1002/pros.23811. [DOI] [PubMed] [Google Scholar]
- 110.Misawa A., Takayama K.-I., Fujimura T., Homma Y., Suzuki Y., Inoue S. Androgen-induced lncRNA POTEF-AS1 regulates apoptosis-related pathway to facilitate cell survival in prostate cancer cells. Cancer Sci. 2016;108:373–379. doi: 10.1111/cas.13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang D., Wan X., Zhang Y., Kong Z., Lu Y., Sun X., Huang Y., Ji C., Li D., Luo J., et al. A novel androgen-reduced prostate-specific lncRNA, PSLNR, inhibits prostate-cancer progression in part by regulating the p53-dependent pathway. Prostate. 2019;79:1379–1393. doi: 10.1002/pros.23840. [DOI] [PubMed] [Google Scholar]
- 112.Sun M., Geng D., Li S., Chen Z., Zhao W. LncRNA PART1 modulates toll-like receptor pathways to influence cell proliferation and apoptosis in prostate cancer cells. Biol. Chem. 2018;399:387–395. doi: 10.1515/hsz-2017-0255. [DOI] [PubMed] [Google Scholar]
- 113.Kong Z., Lu Y., Wan X., Luo J., Li D., Huang Y., Wang C., Li Y., Xu Y. Comprehensive characterization of androgen-responsive circrnas in prostate cancer. Life. 2021;11:1096. doi: 10.3390/life11101096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ribas J., Ni X., Haffner M., Wentzel E.A., Salmasi A.H., Chowdhury W.H., Kudrolli T.-A., Yegnasubramanian S., Luo J., Rodriguez R., et al. miR-21: An androgen receptor–regulated mi-croRNA that promotes hormone-dependent and hormone-independent prostate cancer growth. Cancer Res. 2009;69:7165–7169. doi: 10.1158/0008-5472.CAN-09-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jia L., Gui B., Zheng D., Decker K.F., Tinay I., Tan M., Wang X., Kibel A.S. Androgen receptor-regulated miRNA-193a-3p targets AJUBA to promote prostate cancer cell migration. Prostate. 2017;77:1000–1011. doi: 10.1002/pros.23356. [DOI] [PubMed] [Google Scholar]
- 116.Wang H., Lapek J., Fujimura K., Strnadel J., Liu B., Gonzalez D.J., Zhang W., Watson F., Yu V., Liu C., et al. Pseudopodium-enriched atypical kinase 1 mediates angiogenesis by modulating GATA2-dependent VEGFR2 transcription. Cell Discov. 2018;4:1–24. doi: 10.1038/s41421-018-0024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kroiss A., Vincent S., Decaussin-Petrucci M., Meugnier E., Viallet J., Ruffion A., Chalmel F., Samarut J., Allioli N. Androgen-regulated microRNA-135a de-creases prostate cancer cell migration and invasion through downregulating ROCK1 and ROCK2. Oncogene. 2015;34:2846–2855. doi: 10.1038/onc.2014.222. [DOI] [PubMed] [Google Scholar]
- 118.Meng D., Yang S., Wan X., Zhang Y., Huang W., Zhao P., Wang L., Huang Y., Li T., Li Y. A transcriptional target of androgen receptor, miR-421 regulates proliferation and metabolism of prostate cancer cells. Int. J. Biochem. Cell Biol. 2016;73:30–40. doi: 10.1016/j.biocel.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 119.Liu Y.-N., Yin J., Barrett B., Tillman H., Li D., Casey O.M., Fang L., Hynes P.G., Ameri A.H., Kelly K. Loss of androgen-regulated MicroRNA 1 activates SRC and promotes prostate cancer bone metastasis. Mol. Cell. Biol. 2015;35:1940–1951. doi: 10.1128/MCB.00008-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dang Q., Li L., Xie H., He D., Chen J., Song W., Chang L.-S., Chang H.-C., Yeh S., Chang C. Anti-androgen enzalutamide enhances prostate cancer neuroendocrine (NE) differentiation via altering the infiltrated mast cells→ androgen receptor (AR)→ miRNA32 signals. Mol. Oncol. 2015;9:1241–1251. doi: 10.1016/j.molonc.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zennami K., Choi S.M., Liao R., Li Y., Dinalankara W., Marchionni L., Rafiqi F.H., Kurozumi A., Hatano K., Lupold S.E. PDCD4 is an Androgen-Repressed tumor suppressor that regulates prostate cancer growth and castration resistance. Mol. Cancer Res. 2019;17:618–627. doi: 10.1158/1541-7786.MCR-18-0837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pasqualini L., Bu H., Puhr M., Narisu N., Rainer J., Schlick B., Schäfer G., Angelova M., Trajanoski Z., Börno S.T., et al. miR-22 and miR-29a Are Members of the Androgen Receptor Cistrome Modulating LAMC1 and Mcl-1 in Prostate Cancer. Mol. Endocrinol. 2015;29:1037–1054. doi: 10.1210/me.2014-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sun D., Layer R., Mueller A.C., Cichewicz M.A., Negishi M., Paschal B.M., Dutta A. Regulation of several androgen-induced genes through the repression of the miR-99a/let-7c/miR-125b-2 miRNA cluster in prostate cancer cells. Oncogene. 2013;33:1448–1457. doi: 10.1038/onc.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jalava S.E., Urbanucci A., Latonen L., Waltering K.K., Sahu B., Jänne O.A., Seppälä J., Lähdesmäki H., Tammela T.L.J., Visakorpi T. Androgen-regulated miR-32 targets BTG2 and is overexpressed in castration-resistant prostate cancer. Oncogene. 2012;31:4460–4471. doi: 10.1038/onc.2011.624. [DOI] [PubMed] [Google Scholar]
- 125.Fernandes R.C., Toubia J., Townley S., Hanson A.R., Dredge B.K., Pillman K.A., Bert A.-G., Winter J.-M., Iggo R., Das R., et al. Post-transcriptional gene regulation by mi-crorna-194 promotes neuroendocrine transdifferentiation in prostate cancer. Cell Rep. 2021;34:108585. doi: 10.1016/j.celrep.2020.108585. [DOI] [PubMed] [Google Scholar]
- 126.Fletcher C.E., Dart D.A., Sita-Lumsden A., Cheng H., Rennie P.S., Bevan C.L. Androgen-regulated processing of the oncomir MiR-27a, which targets Prohibitin in prostate cancer. Hum. Mol. Genet. 2012;21:3112–3127. doi: 10.1093/hmg/dds139. [DOI] [PubMed] [Google Scholar]
- 127.Williams L.V., Veliceasa D., Vinokour E., Volpert O. miR-200b inhibits prostate cancer EMT, growth and metastasis. PLoS ONE. 2013;8:e83991. doi: 10.1371/journal.pone.0083991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mo W., Zhang J., Li X., Meng D., Gao Y., Yang S., Wan X., Zhou C., Guo F., Huang Y., et al. Identification of novel AR-targeted microRNAs mediating androgen sig-nalling through critical pathways to regulate cell viability in prostate cancer. PLoS ONE. 2013;8:e56592. doi: 10.1371/journal.pone.0056592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Maina P., Shao P., Liu Q., Fazli L., Tyler S., Nasir M., Dong X., Qi H.H. c-MYC drives histone demethylase PHF8 during neuroendocrine differentiation and in castration-resistant prostate cancer. Oncotarget. 2016;7:75585–75602. doi: 10.18632/oncotarget.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guo J., Mei Y., Li K., Huang X., Yang H. Downregulation of miR-17-92a cluster promotes autophagy induction in response to celastrol treatment in prostate cancer cells. Biochem. Biophys. Res. Commun. 2016;478:804–810. doi: 10.1016/j.bbrc.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 131.Rokhlin O.W., Scheinker V.S., Taghiyev A.F., Bumcrot D., Glover R.A., Cohen M.B. MicroRNA-34 mediates AR-dependent p53-induced apoptosis in prostate cancer. Cancer Biol. Ther. 2008;7:1288–1296. doi: 10.4161/cbt.7.8.6284. [DOI] [PubMed] [Google Scholar]
- 132.Wan X., Pu H., Huang W., Yang S., Zhang Y., Kong Z., Yang Z., Zhao P., Li T. Androgen-induced miR-135a acts as a tumor suppressor through downregulating RBAK and MMP11, and mediates resistance to androgen deprivation therapy. Oncotarget. 2016;7:51284–51300. doi: 10.18632/oncotarget.9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xue M., Liu H., Zhang L., Chang H., Liu Y., Du S., Yang Y., Wang P. Computational identification of mutually exclusive transcriptional drivers dysregulating metastatic microRNAs in prostate cancer. Nat. Commun. 2017;8:14917. doi: 10.1038/ncomms14917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Guo J., Huang X., Wang H., Yang H. Celastrol induces autophagy by targeting AR/miR-101 in prostate cancer cells. PLoS ONE. 2015;10:e0140745. doi: 10.1371/journal.pone.0140745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wan X., Huang W., Yang S., Zhang Y., Zhang P., Kong Z., Li T., Wu H., Jing F., Li Y. Androgen-induced miR-27A acted as a tumor suppressor by targeting MAP2K4 and mediated prostate cancer progression. Int. J. Biochem. Cell Biol. 2016;79:249–260. doi: 10.1016/j.biocel.2016.08.043. [DOI] [PubMed] [Google Scholar]
- 136.Chen Z., Song X., Li Q., Xie L., Guo T., Su T., Tang C., Chang X., Liang B., Huang D., et al. Androgen Receptor-Activated enhancers simultaneously regulate oncogene TMPRSS2 and lncRNA PRCAT38 in prostate cancer. Cells. 2019;8:864. doi: 10.3390/cells8080864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ozgur E., Gezer U. Investigation of lncRNA H19 in prostate cancer cells and secreted exosomes upon androgen stimulation or androgen receptor blockage. Bratisl. Med. J. 2020;121:362–365. doi: 10.4149/BLL_2020_058. [DOI] [PubMed] [Google Scholar]
- 138.Hu J., Deng J., Cao R., Xiong S., Guo J. LncRNA GAS5 participates in the regulation of dexamethasone on androgen recep-tor-negative and-positive prostate cancer cell proliferation. Mol. Cell. Probes. 2020;53:101607. doi: 10.1016/j.mcp.2020.101607. [DOI] [PubMed] [Google Scholar]
- 139.Luo J., Wang K., Yeh S., Sun Y., Liang L., Xiao Y., Xu W., Niu Y., Cheng L., Maity S.-N., et al. LncRNA-p21 alters the antiandrogen enzalutamide-induced prostate cancer neuroendocrine differentiation via modulating the EZH2/STAT3 signaling. Nat. Commun. 2019;10:1–17. doi: 10.1038/s41467-019-09784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang Y., Su X., Kong Z., Fu F., Zhang P., Wang D., Wu H., Wan X., Li Y. An androgen reduced transcript of LncRNA GAS5 promoted prostate cancer proliferation. PLoS ONE. 2017;12:e0182305. doi: 10.1371/journal.pone.0182305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Parolia A., Crea F., Xue H., Wang Y., Mo F., Ramnarine V.R., Liu H.H., Lin D., Saidy N.R.N., Clermont P.-L., et al. The long non-coding RNA PCGEM1 is regulated by androgen receptor activity in vivo. Mol. Cancer. 2015;14:46. doi: 10.1186/s12943-015-0314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sakurai K., Reon B.J., Anaya J., Dutta A. The lncRNA DRAIC/PCAT29 locus constitutes a Tumor-Suppressive nexus. Mol. Cancer Res. 2015;13:828–838. doi: 10.1158/1541-7786.MCR-15-0016-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kohvakka A., Sattari M., Shcherban A., Annala M., Urbanucci A., Kesseli J., Tammela T.L.J., Kivinummi K., Latonen L., Nykter M., et al. AR and ERG drive the expression of prostate cancer specific long noncoding RNAs. Oncogene. 2020;39:5241–5251. doi: 10.1038/s41388-020-1365-6. [DOI] [PubMed] [Google Scholar]
- 144.Malik R., Patel L., Prensner J.R., Shi Y., Iyer M.K., Subramaniyan S., Carley A., Niknafs Y.-S., Sahu A., Han S., et al. The lncRNA PCAT29 inhibits oncogenic phenotypes in prostate cancer. Mol. Cancer Res. 2014;12:1081–1087. doi: 10.1158/1541-7786.MCR-14-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Takayama K.I., Horie-Inoue K., Katayama S., Suzuki T., Tsutsumi S., Ikeda K., Urano T., Fujimura T., Takagi K., Takahashi S., et al. Androgen-responsive long noncoding RNA CTBP1-AS promotes prostate cancer. EMBO J. 2013;32:1665–1680. doi: 10.1038/emboj.2013.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wu Z., Wang S., Li Q., Zhao Q., Shao M. Identification of 10 differently expressed lncRNAs as prognostic biomarkers for pros-tate adenocarcinoma. Math. Biosci. Eng. 2020;17:2037–2047. doi: 10.3934/mbe.2020108. [DOI] [PubMed] [Google Scholar]
- 147.Guo H., Ahmed M., Zhang F., Yao C.Q., Li S., Liang Y., Hua J., Soares F., Sun Y., Langstein J., et al. Modulation of long noncoding RNAs by risk SNPs underlying genetic predispositions to prostate cancer. Nat. Genet. 2016;48:1142–1150. doi: 10.1038/ng.3637. [DOI] [PubMed] [Google Scholar]
- 148.Liu B., Qian D., Zhou W., Jiang H., Xiang Z., Wu D. A novel androgen-induced lncRNA FAM83H-AS1 promotes prostate cancer progression via the miR-15a/CCNE2 axis. Front. Oncol. 2021;10:2943. doi: 10.3389/fonc.2020.620306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jia J., Li F., Tang X.-S., Xu S., Gao Y., Shi Q., Guo W., Wang X., He D., Guo P. Long noncoding RNA DANCR promotes invasion of prostate cancer through epigenetically silencing expression of TIMP2/3. Oncotarget. 2016;7:37868. doi: 10.18632/oncotarget.9350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Crea F., Watahiki A., Quagliata L., Xue H., Pikor L., Parolia A., Wang Y., Lin D., Lam W.-L., Farrar W.-L., et al. Identification of a long non-coding RNA as a novel bi-omarker and potential therapeutic target for metastatic prostate cancer. Oncotarget. 2014;5:764. doi: 10.18632/oncotarget.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wan X., Huang W., Yang S., Zhang Y., Pu H., Fu F., Huang Y., Wu H., Li T., Li Y. Identification of androgen-responsive lncRNAs as diagnostic and prognostic markers for prostate cancer. Oncotarget. 2016;7:60503–60518. doi: 10.18632/oncotarget.11391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Huang W., Su X., Yan W., Kong Z., Wang D., Huang Y., Zhai Q., Zhang X., Wu H., Li Y., et al. Overexpression of AR-regulated lncRNA TMPO-AS1 correlates with tumor progression and poor prognosis in prostate cancer. Prostate. 2018;78:1248–1261. doi: 10.1002/pros.23700. [DOI] [PubMed] [Google Scholar]
- 153.Wang H.-X., Kang L.-J., Qin X., Xu J., Fei J.-W. LINC00460 promotes proliferation and inhibits apoptosis of non-small cell lung cancer cells through targeted regulation of miR-539. Eur Rev. Med. Pharm. Sci. 2020;24:6752–6758. doi: 10.26355/eurrev_202006_21663. [DOI] [PubMed] [Google Scholar]
- 154.Deng G., Wang R., Sun Y., Huang C.-P., Yeh S., You B., Feng C., Li G., Ma S., Chang C. Targeting androgen receptor (AR) with antiandrogen Enzalutamide increases prostate cancer cell invasion yet decreases bladder cancer cell invasion via differentially altering the AR/circRNA-ARC1/miR-125b-2-3p or miR-4736/PPARγ/MMP-9 signals. Cell Death Differ. 2021;28:2145–2159. doi: 10.1038/s41418-021-00743-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Casaburi I., Cesario M.G., Donà A., Rizza P., Aquila S., Avena P., Lanzino M., Pellegrino M., Vivacqua A., Tucci P., et al. Androgens downregulate miR-21 expression in breast cancer cells underlining the protective role of androgen receptor. Oncotarget. 2016;7:12651–12661. doi: 10.18632/oncotarget.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Teng Y., Litchfield L.M., Ivanova M.M., Prough R.A., Clark B.J., Klinge C.M. Dehydroepiandrosterone-induces miR-21 transcrip-tion in HepG2 cells through estrogen receptor β and androgen receptor. Mol. Cell. Endocrinol. 2014;392:23–36. doi: 10.1016/j.mce.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shi Z.-Z., Wang W.-J., Chen Y.-X., Fan Z.-W., Xie X.-F., Yang L.-Y., Chang C., Cai Y., Hao J.-J., Wang M.-R., et al. The miR-1224-5p/TNS4/EGFR axis inhibits tumour progres-sion in oesophageal squamous cell carcinoma. Cell Death Dis. 2020;11:597. doi: 10.1038/s41419-020-02801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lyu S., Yu Q., Ying G., Wang S., Wang Y., Zhang J., Niu Y. Androgen receptor decreases CMYC and KRAS expression by upregu-lating let-7a expression in ER-, PR-, AR+ breast cancer. Int. J. Oncol. 2014;44:229–237. doi: 10.3892/ijo.2013.2151. [DOI] [PubMed] [Google Scholar]
- 159.Yang F., Shen Y., Zhang W., Jin J., Huang D., Fang H., Ji W., Shi Y., Tang L., Chen W., et al. An androgen receptor negatively induced long non-coding RNA ARNILA binding to miR-204 promotes the invasion and metastasis of triple-negative breast cancer. Cell Death Differ. 2018;25:2209–2220. doi: 10.1038/s41418-018-0123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen P.-J., Yeh S.-H., Liu W.-H., Lin C.-C., Huang H.-C., Chen C.-L., Chen D.-S., Chen P.-J. Androgen pathway stimulates MicroRNA-216a transcription to suppress the tumor suppressor in lung cancer-1 gene in early hepatocarcinogenesis. Hepatology. 2012;56:632–643. doi: 10.1002/hep.25695. [DOI] [PubMed] [Google Scholar]
- 161.Zhao J., Sun Y., Lin H., Chou F., Xiao Y., Jin R.A., Cai X., Chang C. Olaparib and enzalutamide synergistically suppress HCC progression via the AR-mediated miR-146a-5p/BRCA1 signaling. FASEB J. 2020;34:5877–5891. doi: 10.1096/fj.201903045RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bao S., Jin S., Wang C., Tu P., Hu K., Lu J. Androgen receptor suppresses vasculogenic mimicry in hepatocellular carcinoma via circRNA7/miRNA7-5p/VE-cadherin/Notch4 signalling. J. Cell. Mol. Med. 2020;24:14110–14120. doi: 10.1111/jcmm.16022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ouyang X., Yao L., Liu G., Liu S., Gong L., Xiao Y. Loss of androgen receptor promotes HCC invasion and metastasis via acti-vating circ-LNPEP/miR-532–3p/RAB9A signal under hypoxia. Biochem. Biophys. Res. Commun. 2021;557:26–32. doi: 10.1016/j.bbrc.2021.02.120. [DOI] [PubMed] [Google Scholar]
- 164.Shi L., Yan P., Liang Y., Sun Y., Shen J., Zhou S., Lin H., Liang X., Cai X. Circular RNA expression is suppressed by androgen receptor (AR)-regulated adenosine deaminase that acts on RNA (ADAR1) in human hepatocellular carcinoma. Cell Death Dis. 2017;8:e3171. doi: 10.1038/cddis.2017.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang Y., Ou Z., Sun Y., Yeh S., Wang X., Long J., Chang C. Androgen receptor promotes melanoma metastasis via altering the miRNA-539-3p/USP13/MITF/AXL signals. Oncogene. 2016;36:1644–1654. doi: 10.1038/onc.2016.330. [DOI] [PubMed] [Google Scholar]
- 166.Schmidt K., Carroll J.S., Yee E., Thomas D.D., Wert-Lamas L., Neier S.C., Sheynkman G., Ritz J., Novina C.D. The lncRNA SLNCR Recruits the Androgen Receptor to EGR1-Bound Genes in Melanoma and Inhibits Expression of Tumor Suppressor p21. Cell Rep. 2019;27:2493–2507.e4. doi: 10.1016/j.celrep.2019.04.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jiang X., Li J., Wang W., Hu Z., Guan C., Zhao Y., Li W., Cui Y. AR-induced ZEB1-AS1 represents poor prognosis in cholangiocarcinoma and facilitates tumor stemness, proliferation and invasion through mediating miR-133b/HOXB8. Aging. 2020;12:1237–1255. doi: 10.18632/aging.102680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Han H., Wang S., Meng J., Lyu G., Ding G., Hu Y., Wang L., Wu L., Yang W., Lv Y., et al. Long noncoding RNA PART1 restrains aggressive gastric cancer through the epigenetic silencing of PDGFB via the PLZF-mediated recruitment of EZH2. Oncogene. 2020;39:6513–6528. doi: 10.1038/s41388-020-01442-5. [DOI] [PubMed] [Google Scholar]
- 169.You B., Sun Y., Luo J., Wang K., Liu Q., Fang R., Liu B., Chou F., Wang R., Meng J., et al. Androgen receptor promotes renal cell carcinoma (RCC) vasculogenic mimicry (VM) via altering TWIST1 nonsense-mediated decay through lncRNA-TANAR. Oncogene. 2021;40:1674–1689. doi: 10.1038/s41388-020-01616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang K., Sun Y., Tao W., Fei X., Chang C. Androgen receptor (AR) promotes clear cell renal cell carcinoma (ccRCC) migration and invasion via altering the circHIAT1/miR-195-5p/29a-3p/29c-3p/CDC42 signals. Cancer Lett. 2017;394:1–12. doi: 10.1016/j.canlet.2016.12.036. [DOI] [PubMed] [Google Scholar]
- 171.Gong D., Sun Y., Guo C., Sheu T., Zhai W., Zheng J., Chang C. Androgen receptor decreases renal cell carcinoma bone metastases via suppressing the osteolytic formation through altering a novel circEXOC7 regulatory axis. Clin. Transl. Med. 2021;11:e353. doi: 10.1002/ctm2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hu R., Dunn T.A., Wei S., Isharwal S., Veltri R.W., Humphreys E., Han M., Partin A.W., Vessella R.L., Isaacs W.B., et al. Ligand-Independent Androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69:16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.