Abstract
The multifunctional role of the human skin is well known. It acts as a sensory and immune organ that protects the human body from harmful environmental impacts such as chemical, mechanical, and physical threats, reduces UV radiation effects, prevents moisture loss, and helps thermoregulation. In this regard, skin disorders related to skin integrity require adequate treatment. Lipid nanoparticles (LN) are recognized as promising drug delivery systems (DDS) in treating skin disorders. Solid lipid nanoparticles (SLN) together with nanostructured lipid carriers (NLC) exhibit excellent tolerability as these are produced from physiological and biodegradable lipids. Moreover, LN applied to the skin can improve stability, drug targeting, occlusion, penetration enhancement, and increased skin hydration compared with other drug nanocarriers. Furthermore, the features of LN can be enhanced by inclusion in suitable bases such as creams, ointments, gels (i.e., hydrogel, emulgel, bigel), lotions, etc. This review focuses on recent developments in lipid nanoparticle systems and their application to treating skin diseases. We point out and consider the reasons for their creation, pay attention to their advantages and disadvantages, list the main production techniques for obtaining them, and examine the place assigned to them in solving the problems caused by skin disorders.
Keywords: skin diseases, lipid-based nanosystems, solid lipid nanoparticles, nanostructured lipid carriers, cream, ointment, gel
1. Introduction
Skin diseases cause significant discomfort to millions of people around the world daily. Various studies show that between 30 and 70% of the world’s population suffers from skin diseases [1]. In most cases, skin diseases are caused mainly by various infectious pathogens (bacteria, fungi, viruses) or inflammatory processes of various etiologies [2,3]. The majority of skin diseases are acute and have a significant psychological impact on individuals. Despite substantial advances in dermatological treatment, many infectious skin problems remain complex and persistent in treatment. These problems depend on the type of pathogen, the integrity of the skin layers, and especially on the patient’s medical status [4]. A number skin diseases such as atopic dermatitis, allergic contact dermatitis, and psoriasis are chronic and represent a complex result of infiltration of inflammatory T cells and increased production of cytokines in the lesions [5]. The success of topical treatment of skin diseases requires a timely, accurate diagnosis, as well as an effective, simple, and not very invasive targeted topical treatment. In addition, it depends on the type of dosage form (the type of delivery system used to supply the drug to the skin) and the application method [6].
This review focuses on the recent advances of lipid-based nanosystems in the treatment of skin disorders. We discuss the most common types of lipid-based nanocarriers researched for dermal drug delivery and used for the treatment of skin disorders by incorporation into appropriate dosage forms, namely: Nanovesicular carriers, lipid nanoparticulate carriers, microemulsions, and nanoemulsions. The considered compositions are intended principally for local treatment and are an attempt to reflect the current picture of development in this field.
2. Skin
The skin is the largest metabolically active organ of the human body. Its vital functions include protection from external environmental threats, vitamin D synthesis, and the maintenance of the body’s dynamic balance [7,8,9]. Moreover, the protective function is expressed by limiting the direct invasion of microorganisms as a physical barrier [10]. This defensive mechanism provides physical and immunological, metabolic, and UV protection [11]. Good knowledge of the barrier properties of the skin and the assessment of changes in the barrier functions as a result of skin diseases can be used to successfully develop new effective drug delivery systems, especially for the diseased skin and for the application of therapeutic agents such as drugs or vaccines [12,13]. Topical pharmaceuticals for the treatment of skin disorders can reach the problematic site without the risk of massive systemic absorption or other lateral effects [14].
In short, the skin consists of three main layers—epidermis, dermis, and hypodermis (subcutaneous fat tissue) [15].
The superficial part of the skin is called the epidermis. In essence, it is a laminar, squamous corneal epithelium composed mostly of two types of cells: Keratinocytes and dendritic cells (antigen-presenting cells) [16].
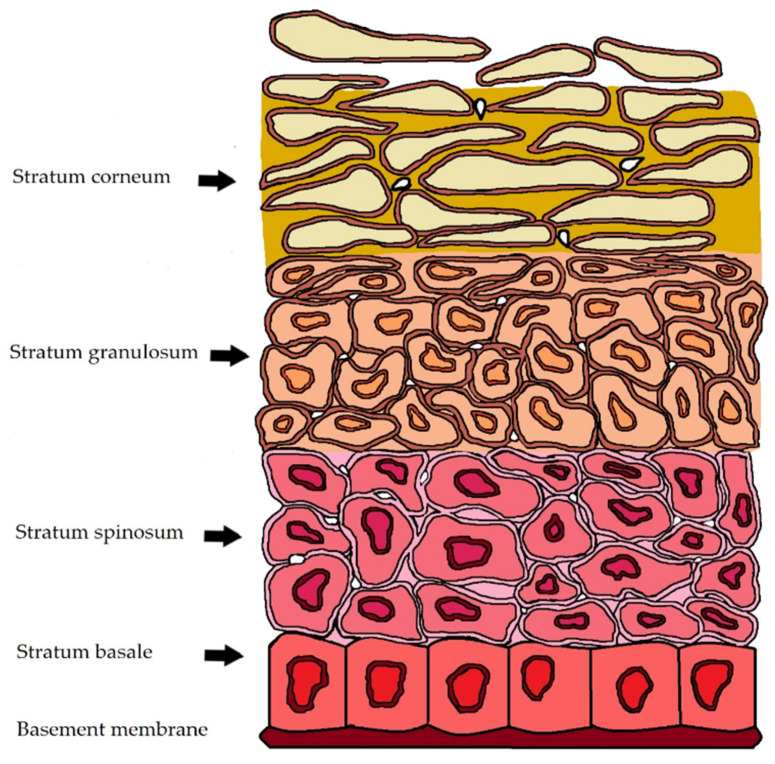
The epidermis consists of five layers—the stratum corneum, stratum lucidum, stratum granulosum (granular ply), stratum spinosum (spinous ply), and stratum germinativum (basal ply) [17]. No blood vessels were found in the epidermis [18]. The structure of the epidermis is schematically represented in Figure 1.
Figure 1.
Schematic representation of the epidermis. Epidermis has a thickness of 0.1–0.2 mm. Stratum corneum (10–20 μm) and the viable epidermis (50–150 μm).
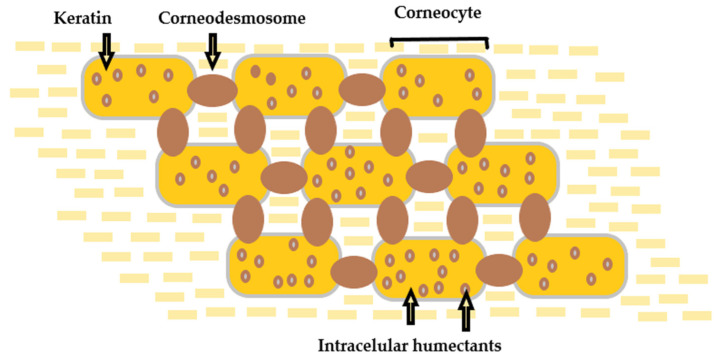
The outermost sublayer of the epidermis, named stratum corneum (SC) (10–20 µm), plays a fundamental role as the body’s first and main physical skin barrier from external menaces [19,20,21]. It consists of corneocytes—specific cells that are the essential limiting factor for permeation through the skin, restricting the passage of molecules significantly larger than 500 Da [22]. The SC functions as a two-compartment system organized in a “brick and mortar” formation, with an extracellular matrix of lamellar membranes [23]. The structure of the stratum corneum is schematically represented in Figure 2.
Figure 2.
Structure of the stratum corneum—“brick and mortar” formation. The pathway between the stacked corneocytes is filled with “mortar” lipids.
The permeation of matter through the SC is possible primarily through passive diffusion in three ways: Transcellular (the bulk of flux), intercellular, and appendageal [24,25,26]. For all of the substances permeating the skin, diffusion through the SC is a rate-limiting stage [27]. In this regard, significant efforts are being made to establish and improve skin nanoparticle delivery systems that can supply and sustainably release active substances of varying lipophilicity and molecular weights to and through the skin, as well as ensure their protection against skin metabolism [28].
Keratinocytes, melanocytes (melanin producers), Merkel cells (sensory receptors), and Langerhans cells (immunocompetent cells) are epidermal formations—essential for the skin’s vitality [29]. In addition, it is crucial to consider the barrier nature of the diseased skin, since there are substantial differences in barrier abilities between healthy and diseased skin. Therefore, we have reason to say that the skin reflects a person’s health.
3. Skin Disorders
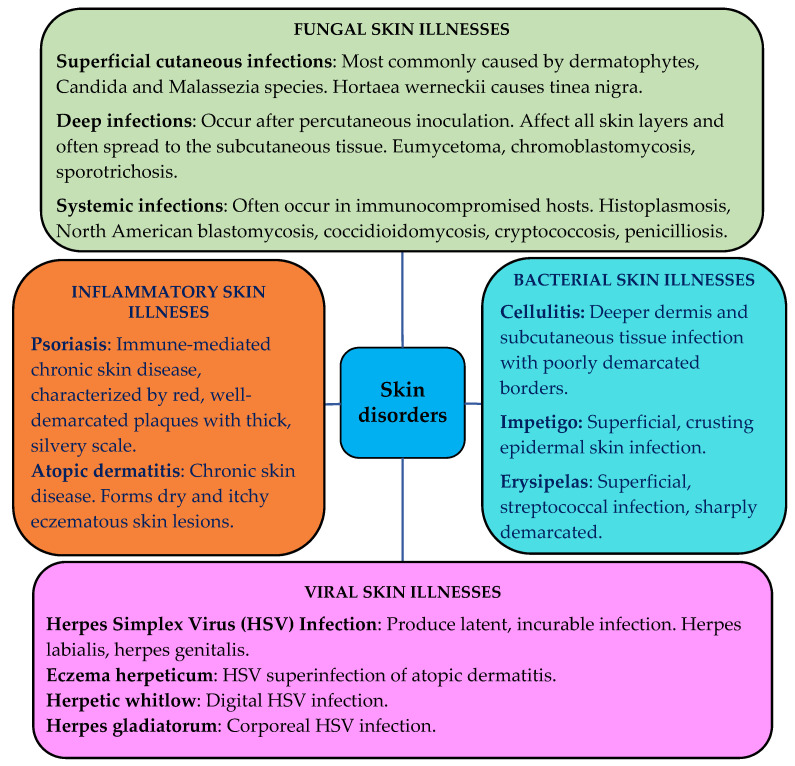
The skin can be affected by various pathological changes, i.e., inflammatory, neoplastic, traumatic, hormonal, degenerative, and even hereditarily determined [30]. Infectious skin diseases such as bacterial, fungal or viral affect people and cause various dermatological problems. Chronic inflammatory skin diseases such as atopic dermatitis, allergic contact dermatitis, psoriasis, etc., are a consequence of infiltration of inflammatory T cells [31]. The schematic representation of various skin disorders is shown in Figure 3 [32,33,34,35,36,37,38,39,40,41,42,43].
Figure 3.
Representation of the most common skin disorders [32,33,34,35,36,37,38,39,40,41,42,43].
In practice, most of the skin disorders are complicated, polygenic, and multifactorial [44]. This indicates that multiple factors, lifestyle, and the environment play a fundamental role in the clinical picture of the diseases [45].
Treatment of Skin Disorders with Conventional Topical Delivery Systems
The human skin creates a vast opportunity for drug delivery application. In general, dermal (topical) and transdermal skin drug delivery can be differentiated. Dermal delivery is the application of the drug directly at the place of action—on the skin’s surface. Transdermal drug delivery is an alternative, painless, and non-invasive approach used to deliver drugs for therapeutic use [46].
Many conventional topical preparations are intended for topical delivery of the drug and not for systemic action. This skin preparation delivers a concentrated amount of the active ingredient for absorption via the application layer [47]. In addition, the use of penetration enhancers in conventional formulations increases the delivery rate through the epidermis, but can also cause unwanted side effects [48].
Some of the commonly used therapeutic solutions are listed in Table 1.
Table 1.
Mostly used therapeutic solutions for T cell-mediated skin disorders.
| Drug Substance | Main Action | References |
|---|---|---|
| Corticosteroids | For local application. Manifests a slight immunosuppressive effect. Ineffectiveness in severe cases. | [49] |
| Retinoids | Retinoids can be synthetic or natural derivatives of vitamin A. Probable mechanisms of action: Facilitate the transport of cytoplasmic retinoid-binding proteins; influence angiogenesis; modulate T cell responses. | [50,51] |
| Vitamin D3 metabolites | Metabolites of vitamin D3 are included in ointments and creams for the treatment of psoriasis. Good effects in milder diseases. Not fully understood effects. The 1,25(OH)2D3 enhances the suppressive activity of CD4(+)CD25(+) cells in draining lymph nodes. | [52,53,54] |
| UVB treatment | Multiple effects and effectiveness for several T cell-mediated skin diseases. Not equally effective in the different disorders. Unspecific and generally immunosuppressive. | [55,56,57] |
| Methotrexate | Immunosuppressive effect. It does not target specific T cell groups. Still not a fully understood therapeutic effect. | [49,51] |
| Cyclosporine A | Affects IL-2 producing cells, in particular CD4(+) T cells. General immunosuppressive effect. | [49,51] |
For chronic inflammatory skin diseases, a topical treatment is often not too efficient. Therefore, more efficacious medicinal products (MP) are given systemically, where they can be immunosuppressive, and their long-term usage is not recommended as they suppress the affected area [58].
A large part of therapeutic indications is treated by MP, which are intended for topical administration [59]. These MP feature different molecular structures, but retain some common physicochemical characteristics such as high lipophilicity and poor aqueous solubility. For them, the rate and extent of drug delivery have to be sufficient to achieve local therapeutic concentrations in an acceptable term and provide an effective pharmacological action [60].
The barrier function of the targeted biologic membrane provides a significant challenge for optimal therapy. The efficacy of drug delivery and the therapeutic effect depend mainly on the diffusion affinity of the drug substance and the interaction between the excipients of the formulation and the membrane components. Therefore, the conducive balance between potency and deliverability has to be ensured through the design and development of a delivery system to reach optimum therapeutic levels at the site of infection [60].
Biologics have become increasingly popular as a targeted treatment. Biologics are products composed of sugars, proteins, nucleic acids or complex combinations of these substances. Several biologics that target specific subgroups of cells in the skin have been tested [61].
Empirical experience shows that conventional topical preparations suffer from certain limitations and are compromised in patient compliance, safety, and efficacy of therapy [62]. Against this background, the need for advanced carriers that could effectively improve skin penetration and reduce drug-related side effects is more than necessary [63].
An inventive strategy for improving the penetration of molecules through the epidermal barrier is the application of nanocarriers due to the advantage of their lipophilicity, which mediates the passage through the intact lipid layer. The use of lipid nanoparticles in dermal formulations provides several benefits: Chemical protection of the incorporated drug molecules, application to the skin of labile drug substances, improved bioavailability of drugs, and the ability for better release by provision penetration and retention in the skin [64].
Lipid-based nanosystems have proven to be suitable for dermal carriers due to their biocompatibility, efficient delivery of active ingredients, and stability. In addition, their enhanced surface leads to the improved penetration of active ingredients [65].
4. Lipid-Based Drug Delivery Systems
Lipid-based drug delivery systems (LBDDS) are formulations containing a dissolved or suspended drug substance in lipidic excipients [66]. LBDDS are a progressive strategy to formulate pharmaceuticals for topical delivery [67]. Liposomes, which are “pioneers” among lipid DDS, have been used to improve drug solubility and traditionally for topical and transdermal drug delivery.
Table 2 presents a brief overview of lipid-based drug delivery systems.
Table 2.
Presentation of some lipid-based delivery systems.
| Lipid-Based Delivery System |
Description | Advantages | Disadvantages |
|---|---|---|---|
| Nanovesicular carriers | |||
| Liposomes [68] | Conventional single or multilayer vesicles. Formed by contact of biodegradable lipids with an aqueous medium. Widely used as drug carriers for hydrophilic and lipophilic molecules. | Biocompatible and biodegradable lipids. Conventional production processes. Improved local delivery. Suitable for loading both hydrophobic and hydrophilic substances. | Insufficient chemical and physical stability. Short half-life. Inadequate penetration into the viable epidermis and dermis. High production costs. Difficulties in scalability. |
| Transfersomes [69,70,71] |
Highly deformable, elastic or ultra-flexible liposomes. Vesicles, similar to conventional liposomes in terms of preparation and structure. Claimed to permeate as intact vesicles through the skin layers. Functionally deformed due to the presence of an edge activator. | Smaller vesicle size, higher elasticity. Compared with conventional liposomes—better penetration through the skin. High membrane hydrophilicity and elasticity allow them to avoid aggregation and fusion under osmotic stress, unlike the conventional liposomes. | Elasticity of these vesicles can be compromised by hydrophobic drug loading. Occlusive application and complete skin hydration limit transdermal delivery due to inhibition of transdermal hydration. Relatively high production costs. Absence of well-established regulatory guidance for skin delivery. |
| Ethosomes [72,73] | Lipid vesicles are composed of phospholipids, ethanol, and water. Similar to liposomes in terms of their preparation techniques and structure. Concentration of ethanol 20–45%. Their size decreases with an increase in the ethanol concentration. Exhibit high encapsulation efficiency. | Appropriate for both hydrophobic and hydrophilic drug loading. Enhanced skin delivery under both occlusive and nonocclusive conditions. Higher elasticity, smaller vesicle size, and higher entrapment efficiency than conventional liposomes. | High ethanol content can lead to skin irritation and toxicity. Possible structural and chemical instability during long-term storage. Need to optimize the concentration of lipids and ethanol for improved physicochemical properties and stability of ethosomes. |
| Lipid nanoparticulate carriers | |||
| Solid lipid nanoparticles [74,75] | Colloidal lipid nanoparticles are composed of a physiological biodegradable solid lipophilic matrix (solid at room temperature and body temperature), in which the drug molecules can be incorporated. | Increased drug stability. High drug payload. Incorporation of lipophilic and hydrophilic drugs. Avoidance of organic solvents. Lack of biotoxicity of the carrier. Relatively cost-effective. | SLN are incorporated into semisolid carriers such as ointments and gels due to the high water content. Potential expulsion of active compounds during storage. Cost-effective manufacturing process. |
| Nanostructure Lipid Carriers [76,77] |
Colloidal lipid nanoparticles composed of physiological mixing liquid lipid (oils) with the solid lipids, in which the liquid lipid is incorporated into the solid matrix or localized at the surface of solid particles | Improved drug loading compared with SLN. Lower water content compared with SLN. Firmly incorporates the drug substance during storage. Biodegradable and biocompatible. Large-scale production is easily possible. | Tendency to unpredictable gelation. Polymorphic transition. Low drug incorporation due to the crystalline structure of solid lipids. Lack of long-term stability data. |
| Lipospheres [78,79,80] |
Microspheres, composed of solid hydrophobic lipid core and stabilized by a monolayer of a phospholipid embedded on the surface. | Improved drug stability, especially for photo-labile drugs. Possibility for controlled drug release. Controlled particle size. High drug loading. Biodegradable and biocompatible. | Larger particle size and poor skin permeation compared with lipid-based vesicular carriers, SLN, and NLC. Poor drug loading for hydrophilic compounds. |
5. Nanovesicular Carriers
Liposomes (further accepted as conventional liposomes) are considered to be the first generation of nanovesicular carriers. They are small artificial vesicles of the spherical shape created from cholesterol and natural, non-toxic phospholipids with an enclosed inner aqueous core [81]. There are several methods for liposome preparation such as thin-film hydration, reverse phase evaporation, and microfluidic mixing [82]. In 1980, Mezei and Gulasekharam reported the first generation of liposomes as drug delivery systems for topical administration [83]. Since then, liposomes are widely applied as dermal drug carriers [84].
The second generation of nanovesicular carriers—transfersomes were developed in 1992 by Cevc et al. They are modified liposomes with an average diameter below 300 nm and contain an edge activator that makes transfersomes nearly eight times more flexible than the conventional liposomes [85,86,87]. Edge activators can be sorbitan esters, sodium cholate, polysorbates, dipotassium glycyrrhizinate, etc. [88]. The competitive advantage of transfersomes is their ability to squeeze through tiny holes, five to ten times smaller than the vesicles dimensions [89,90,91,92]. In 1996, Touitou designed the ethosomes—the next step for improving permeation abilities [93]. These contain a fluid bilayer in their structure due to the high concentration of ethanol—20 to 45 wt%. Ethosomes potentiate the penetration effect through the clogged and unlocked skin and lead to drug penetration to a depth of about 200 µm [94]. Numerous studies have been devoted to the efficacy of ethosomes for dermal drug delivery in both occlusive and non-occlusive applications [95,96]. They demonstrate an excellent ability to improve the skin permeation of drugs, both highly lipophilic and highly hydrophilic active ingredients [97,98,99,100,101]. A certain number of studies are reported for the superior skin delivery of ethosomes compared with liposomes, transferosomes, and commercial formulations [102]. For example, psoralen-loaded ethosomes (an antipsoriasis drug) have shown 3.50 and 2.15 times higher permeation flux and skin deposition, respectively, compared with liposomes [103].
Table 3 summarizes some of the patents for the application of ethosomal drug delivery.
Table 3.
Presentation of some of the patents for the application of ethosomes in skin formulations.
| Application | Title/Inventors | Year | Results |
|---|---|---|---|
| CN103006562 (A) | Daptomycin ethosome preparation/ Li Chong, Liu Xia, Yin Qikun, Wang Xiaoying, Chen Zhangbao |
2013 | Stable translucent dispersion system with a small and uniform particle size. High entrapment efficiency. Excellent transdermal performance. Simple and convenient preparation method. |
| EP 2810642 A1 | Chitosan-modified ethosome structure/ Chin-Tung Lee, Po-Liang Chen |
2013 | The chitosan-modified ethosome structure contains different active substances. Improved storage and transportation. |
| CN103800277 (A) | Leflunomide ethosome composition and its preparation method/ Zhang Tao, Ding Yanji, Deng Jie, Luo Jing, Zhong Xiaodong |
2014 | Improves the transdermal rate of leflunomide. Improves curative effects. |
| CN103536700 A | Chinese medicinal ethosome gel patch for treating herpes zoster and preparation method thereof/Bu Ping, Hu Rong, Chen Lin, Wei Rong, Wu Huanhuan, Huang Xiaoli | 2014 | Easy in medication. Convenient to use. Good therapeutic effect. Strong analgesic action. No adverse reaction. |
| CN 104706571 A | Preparation method of ethosome/ natural material/polyvinyl alcohol composite hydrogel/Yang Xingxing, Lynn, Chen Mengxia, Fanlin Peng |
2015 | Addition of the polyvinyl alcohol, which improves the properties of the hydrogel. |
| CN106474065A | A kind of tetracaine ethosome and its preparation technology/Zhu Xiaoliang, Wu Dongze, Ma Xiaodong | 2017 | Stable in terms of component and proportion. Preferable percutaneous permeation. |
5.1. Emerging Lipid Nanovesicular Carriers
The usefulness of different lipid vesicles prompted researchers to experiment with modifications to give them specific structural or application properties [104]. However, the current research on these vesicles is somewhat limited, especially for topical drug delivery. Table 4 lists the emerging lipid vesicles developed for skin drug delivery, in the recent past.
Table 4.
Emerging lipid nanovesicular carriers for skin drug delivery.
| Emerging Lipid Vesicles | Description | Reference |
|---|---|---|
| Niosomes | Nonionic surfactant and cholesterol (or its derivatives)—based vesicle with improved stability (especially oxidative stability). | [105,106] |
| Cubosomes | Submicron, nanostructured particles, composed of bicontinuous cubic liquid crystalline phase. | [107,108,109] |
| Hexosomes | Constructed of hexagonal liquid crystalline phases dispersed in a continuous aqueous medium. | [110] |
| Aquasomes | Self-assembled nanovesicles, composed of three layers. | [111] |
| Colloidosomes | Hollow shell microcapsules composed of coagulated particles. | [112] |
| Sphingosomes | Contained sphingolipids such as sphingosine, ceramide, sphingomyelin or glycosphingolipid; and are concentric, bilayered nanovesicles with an acidic pH inside. | [113] |
| Ufasomes | Lipid carriers attach to the surface of the skin and support the lipid exchange between the outermost layers of the SC. | [114,115] |
| Archeosomes | Consisted of archebacteria lipids, chemically distinct from eukaryotic and prokaryotic species. Less sensitive to high temperature, alkaline pH, and oxidative stress. | [116,117] |
| Lipoplexes | Cationic lipid-DNA complexes. Efficient carriers for cell transfection. Toxic effects arising from either cationic lipids or nucleic acids. | [118] |
| Proliposomes | Dry, free-flowing particles that immediately form a liposomal dispersion in contact with water. | [119,120] |
Another nanovesicular carrier widely used for dermal drug delivery is called niosomes, with an average particle size between 50 and 200 nm [106]. These are based on nonionic surfactants and cholesterol and are considered more stable and less expensive than liposomes. Several methods can be used for niosomes preparation such as high-pressure homogenization, extrusion or sonication. Due to the small size of these vesicles, the drug loading and stability decrease. The problem can be solved by adding a stabilizer [121] that can be specified, as well as other nanovesicular carriers that are being increasingly explored in recent years for dermal delivery such as cubosomes, hexasomes, aquasomes, colloidosomes, sphingosomes, ufasomes, archeosomes, lipoplexes, proliposomes, etc. [111,122]. Cubosomes are bicontinuous cubic liquid crystalline phases with two different hydrophilic areas separated by a lipid bilayer [123]. Their preparation requires the usage of high-energy dispersion techniques [124,125]. Hexosomes are built from hexagonal liquid crystalline phases dispersed in a continuous aqueous medium [126]. Cubosomes and hexosomes can incorporate hydrophilic, hydrophobic, and amphiphilic drugs, increased drug loading, and good stability. It has recently been shown that incorporating bile salt edge activators into hexosomes can significantly improve their skin penetration properties [127]. Aquasomes are composed of three layers: A solid nanocrystalline core, an oligomeric shell, and a layer of a bioactive substance absorbed onto the shell [111]. They are produced via colloidal precipitation, plasma condensation, and inverted magnetron sputtering. Aquasomes have high drug loading capacity and can protect fragile drug molecules from degradation [128]. Colloidosomes are typically used to encapsulate sensitive bioactive compounds and are hollow shell microcapsules created of coagulated particles [111]. Sphingosomes are comprised of sphingolipids (sphingosine, ceramide, etc.) and are concentric, bilayered nanovesicles with an acidic pH inside [113]. The resultant vesicular systems can be unilamellar, multilamellar, oligolamellar or multivesicular. Different preparation methods such as reverse phase evaporation, mechanical dispersion, solvent injection, sonication, and microfluidization can be used for the production of sphingosomes. They are characterized by the enhanced drug loading efficiency and stability [129]. Ufasomes are composed of lipid bilayers derived from unsaturated fatty acids and ionic surfactants [130]. In parallel with the conventional liposomes, they are more stable and have a better drug loading efficiency, but are more susceptible to oxidation [131].
5.2. Nanovesicular Carriers in the Treatment of Skin Disorders
5.2.1. Antipsoriatic Effect
Psoriasis is a skin disorder characterized by impaired epidermal differentiation, commonly treated by systemic methotrexate, an effective cytotoxic drug. Abdelbary and Abou Ghaly generated topical methotrexate-loaded niosomes for the influence on psoriasis [132]. A thin-film hydration technique is used for the preparation by the inclusion of a surfactant (Span 60) and cholesterol. In comparison with the free drug solution, an increased drug deposition in the skin of rats is monitored.
5.2.2. Antifungal Effect
Perez et al. prepared ultra-deformable liposomes containing amphotericin B to treat cutaneous fungal infections and leishmaniasis [133]. Liposomes containing Tween 80 as an edge activator had maximal deformability and the highest drug/phospholipid ratio. Amphotericin B was encapsulated at 75% encapsulation efficiency in their bilayer. However, drug-loaded liposomes were more toxic to fungal strains than to mammalian cells.
5.2.3. Anti-Vitiligo Effect
Garg et al. developed ethosome-based nanohydrogel formulations of methoxsalen to effectively treat vitiligo with enhanced topical delivery [134]. The formulation contained approximately 28% of ethanol. Ethosomes are incorporated into Carbopol gels and showed substantially skin permeation (on rats), accumulating in epidermal and dermal layers. In addition, there are observed erythema and reduced skin phototoxicity in comparison with a conventional cream.
5.2.4. Anti-Acne Effect
The two main processes that are typical for acne vulgaris include:
The proliferation of propionibacterium acnes bacteria in pilosebaceous units of the skin;
Local inflammation [135].
Traditional topical anti-acne compositions mainly cause burning, erythema, photosensitivity, scaling, and bacterial resistance [136]. In 2008, Touitou et al. developed an ethosomal gel system containing clindamycin phosphate and salicylic acid for an efficient acne treatment and enhanced topical tolerability [135]. Recently, Apriani et al. developed an azelaic acid ethosome-based cream against propionibacterium acne [137]. The ethosomal cream demonstrated a superior antibactericidal activity compared with the marketed cream Zelface®.
5.2.5. Antiviral Effect
Acyclovir has been investigated for the topical treatment of viral infections for more than four decades [138]. In 1999, Horwitz et al. reported an ethosomal cream system for acyclovir—commercial Zovirax® [139]. Recently, Shukla et al. designed an ethosomal gel with acyclovir, where the ethosomes were prepared by the cold method [140]. The survey shows the in vitro drug release of 82% over 8 h with a zero-order release profile.
5.2.6. Local Anesthetic Effect
In the experimental work, Babaie et al. prepared lidocaine-loaded nanoethosomes for penetration into the deep strata of the skin with a particle size around 100 nm [141]. Increased ethanol concentration from 10 to 40% leads to the production of ethosomes with four-times larger particle sizes.
5.2.7. Antibiotic Effect
In 2005, Godin et al. developed an ethosomal system for the dermal delivery of antibiotics to improve their penetration through the SC and the bacterial membrane/cell wall [142,143]. In addition, Zahid et al. formulated ethosomes containing clindamycin phosphate in a recently announced report using a cold method [144]. The optimized formulation demonstrated an excellent in vitro drug release.
5.2.8. Anticarcinogenic Effect
A report by Cosco et al. represented the formation of transfersomes for the combined delivery of resveratrol and 5-fluorouracil. The co-encapsulation of the drugs synergistically improved their anti-cancer activity on skin cancer cells [145].
Table 5 presents the practical implementation of nanovesicular carriers in dermal drug delivery systems.
Table 5.
Application of nanovesicular carriers in dermal DDS for the treatment of skin disorders.
| LNP Type | API/Drug | Application | Reference |
|---|---|---|---|
| Conventional liposomes |
Licorice | Licorice-loaded liposomes included in the formulation for the treatment of oxidative stress injuries. | [146] |
| Conventional liposomes |
Quercetin and resveratrol | Quercetin- and resveratrol-loaded liposomes for the treatment of inflammatory/oxidative responses associated with skin cancer. | [147] |
| Liposomes | Tretinoin | A tretinoin-loaded liposomal formulation for the treatment of acne. | [148] |
| Liposomes | Benzoyl peroxide | Benzoyl peroxide and chloramphenicol encapsulation in liposomes for the treatment of acne. | [149] |
| Liposomes | Benzoyl peroxide/Adapalene | Benzoyl peroxide- and adapalene-loaded modified liposomal gel for the treatment of acne. | [150] |
| Transfersomes | Indocyanine green | Indocyanine green-loaded transfersomes for the treatment of acne vulgaris. | [151] |
| Transfersomes | 5-Fluorouracil | 5-Fluorouracil-loaded transfersomes for the treatment of skin cancer. | [152] |
| Transfersomes | Resveratrol and 5-fluorouracil | Transfersomes containing resveratrol and 5-fluorouracil for the treatment of skin cancer. | [145] |
| Transfersomes | Amphotericin B | Development of amphotericin B-loaded transfersomes for antifungal and antileishmanial treatment. | [133] |
| Transfersomes | siRNA | Transfersomes containing siRNA developed for delivery to the human basal epidermis for the treatment of melanoma. | [153] |
| Transfersomes | RNAi | Transfersomes containing RNAi, formulated for the treatment of psoriasis. | [154] |
| Transfersomes | Indocyanine | Indocyanine-loaded transfersomes for the treatment of basal cell carcinoma. | [155] |
| Transfersomes | Clindamycin | Development of clindamycin-loaded transfersomes for the treatment of acne. | [156] |
| Transfersomes | Paclitaxel | Paclitaxel containing transfersomes, modified by a cell-penetrating-peptide embedded in oligopeptide hydrogel for the topical treatment of melanoma. | [157] |
| Transfersomes | Sodium stibogluconate | Transfersomes loaded with sodium stibogluconate for the treatment of leishmaniasis. | [158] |
| Transfersomes | Lidocaine | Lidocaine transferosomal gel, containing permeation enhancers for local anesthetic action. | [159] |
| Transfersomes | Sulforaphane | Transfersomes comprising sulforaphane for the treatment of skin cancer. | [160] |
| Transfersomes | Miltefosine polyphenol | Formulation of miltefosine polyphenol-loaded transfersomes for the topical treatment of leishmaniasis. | [161] |
| Transfersome | N-acetylcysteine | N-acetylcysteine-loaded transfersomes for antioxidant activity in anti-aging therapy. | [162] |
| Ethosomes | Methoxsalen | Formulation of ethosomes containing methoxsalen for the topical treatment against vitiligo. | [134] |
| Ethosomes | Griseofulvin | Design of griseofulvin-loaded ethosomes for enhanced antifungal treatment. | [163] |
| Ethosomes | Cryptotanshinone | Cryptotanshinone-loaded ethosomes for anti-acne treatment. | [164] |
| Ethosomes | Epigallocatechin-3-gallate | Epigallocatechin-3-gallate-loaded ethosomes for the treatment of skin cancer. | [165] |
| Ethosomes | Thymoquinone | Thymoquinone-loaded ethosomes for the topical treatment of acne. | [166] |
| Ethosomes | Clobetasol propionate | Ethosomes of clobetasol propionate for the treatment of eczema. | [167] |
| Ethosomes | Tretinoin | Gel containing tretinoin-loaded ethosomes for anti-acne treatment. | [168] |
| Ethosomes | Azelaic acid | Azelaic acid-loaded ethosomes for anti-acne treatment. | [137] |
| Niosomes | Resveratrol | Resveratrol-loaded niosomes for the treatment of psoriasis. | [169] |
| Niosomes | Diacerein | Niosomes for the topical diacerein delivery and treatment of psoriasis. | [170] |
| Niosomes | Celastrol | Celastrol-loaded niosomes for the treatment of psoriasis. | [171] |
| Cubosomes | Paclitaxel | Paclitaxel-loaded cubosomes against skin cancer. | [172] |
| Cubosomes | Erythromycin | Erythromycin-loaded cubosomes for the treatment of acne. | [173] |
| Hexosomes, cubosomes | Ketoconazole | Ketoconazole-loaded hexosomes for antifungal treatment. | [174] |
| Ufasomes | Minoxidil | Minoxidil-loaded ufasomes for the treatment of hair loss. | [175] |
6. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers
Solid lipid nanoparticles (SLN), as well as nanostructured lipid carriers (NLC), are extensively employed in cutaneous delivery systems. Since their creation in the nineties, lipid nanoparticles (SLN and NLC) are well known by the research and pharma technology community. Easily available raw materials, relatively simple production methods, biocompatibility, and non-toxicity as their advantages over other colloidal carriers can be mentioned as the main reasons for this [67].
Therefore, these two types of lipid nanoparticles—SLN and NLC, are classified according to their structure. First, SLN are developed with a composition of solid lipids only. Then, to upgrade to SLN, NLC were created by representing a mixture of solid and liquid lipids, with a predominant solid lipid [176].
The dermal use of SLN and NLC is proving to be one of the most convenient for therapeutic and cosmetic purposes, despite various applications to date. Lipid nanoparticles are aqueous dispersions with low viscosity for successful direct application to the skin, which implies their incorporation in semisolid systems based on SLN or NLC. One of the first successful administered and marketed products based on NLC is Cutanova® from Dr. Rimpler GmbH (Wedemark, Germany) and Nanobase® from Yamanouchi (Tokyo, Japan) [64].
6.1. Solid Lipid Nanoparticles
Generally speaking, SLN are nanometric colloidal carriers composed of a solid lipid core with an incorporated active pharmaceutical ingredient(s) (API) and a surfactant-stabilized shell [177,178,179]. SLN are composed in the 1990s and have unique properties such as biodegradability, impressive toxicity profile, protection of the API against degradation, high load capacity, sterilization ability, and scalability [180,181]. The interaction between the lipid core of SLN and the waxy lipids in SC leads to a significant permeation enhancement of the encapsulated drug into the skin, which determines their successful cutaneous application [182].
6.2. Nanostructured Lipid Carriers
The second generation of lipid nanoparticles—NLC, are composed of a mixture of solid lipids and liquid lipids in the nanocore, usually in a ratio of 7:3 to 9:1 [183]. This leads to a more significant disorder of the core of the lipid matrix, and accordingly decreases the melting point to stop the recrystallization of solid lipids [184]. NLC are considered to be an improved variety of SLN, holding the same unique properties, but with an optimized core composition, resulting in a higher drug loading capacity, better stability, and ability to act at lower temperatures. Of note is the fact that NLCs are still solid at body temperature [185].
6.3. Preparation of SLN and NLC
The literature describes a significant number of production methods and many different combinations of lipids to obtain SLN and NLC. Nevertheless, the most common technique used today is high-pressure homogenization (HPH). The procedure is divided into two stages:
Hot homogenization—the lipids are heated above their melting point;
Cold homogenization—takes place at low temperatures and is suitable for hydrophilic and temperature-sensitive API [186,187].
Other commonly used techniques are: Sonication/ultra-sonication [188,189], membrane contactor technique [190], phase inversion [191], solvent injection [192], emulsification [193], the microemulsion method [194], etc.
6.4. SLN and NLC in the Treatment of Skin Disorders
In dermal applications, SLN and NLC create a thin hydrophobic monolayer during skin contact, which has a pointed occlusive effect that settles the API penetration and prevents water loss from the skin [195].
When applied topically, the lipid nanoparticles interact with the sebum and specific skin lipids, provoking a change in the natural arrangement of corneocytes. As a result of this interaction, the encapsulated molecules are released, and their penetration into the lower layers of the epidermis and dermis is potentiated, depending on their lipophilicity, of course [196].
6.4.1. Antioxidant Effect
Okonogi and Riangjanapatee formulated NLC loaded with lycopene through a hot HPH. It has been found that the NLC with the highest concentration of lycopene had the slowest release rate and better antioxidant activity [197].
In another study, Shrotriya et al. reported the development of SLN loaded with resveratrol (entrapment efficiency of 86–89%) to treat irritant contact dermatitis (chronic skin disorder with eczematous injuries). The composition was realized by incorporation into a Carbopol gel and showed increased antioxidant activity compared with a conventional resveratrol gel [198].
Furthermore, Montenegro et al. designed a novel Idebenone (IDE)-loaded NLC containing tocopheryl acetate (VitE) as a liquid component to obtain a synergic effect between IDE and VitE [199].
6.4.2. Anti-Inflammatory Effect
Pivetta et al. formulated NLC with thymol for the local treatment of inflammatory skin diseases (entrapment efficiency of 89%). The NLC were incorporated into a gel and showed anti-inflammatory activity and healing of induced psoriasis in mice [200].
Gad et al. reported the encapsulation of chamomile oil in SLN for the local treatment of wounds. The composition contained stearic acid and chamomile oil and was prepared by the method of hot homogenization. Wound reduction was shown in the topical application in rats [201].
6.4.3. Antifungal Effect
Butani et al. developed a stable SLN system, containing amphotericin B with an enhancing antifungal activity (entrapment efficiency of 94%). The formulation indicated higher drug permeation and drug accumulation in the skin than the conventional gel in rats. A solvent diffusion technique was used for the preparation of the SLN [202].
NLC, for the treatment of candidiasis with Mediterranean essential oils and clotrimazole, were designed by Carbone et al. As a result, they are obtained as a stable NLC, without an initial burst effect and with prolonged release of clotrimazole, as well as an enhanced antifungal activity [203].
6.4.4. Anti-Acne Effect
Tretinoin-loaded NLC with anti-aging and anti-acne activities were reported by Ghate et al. The hot melt probe sonication and hot melt microemulsion methods were used to prepare the NLC. The tretinoin-loaded NLC in Carbopol gels showed no irritation or erythema after the application in rats [204].
Malik and Kaur developed the azelaic acid-loaded NLC, prepared by the melt emulsification and ultra-sonication method (entrapment efficiencies greater than 80%). NLC were incorporated into aloe-vera-based Carbopol hydrogels and demonstrated a deeper skin penetration than the commercial product (Aziderm 10%). Furthermore, the in vivo experiment in mice showed a higher effect of NLC incorporated into a gel than the plain drug suspended in the gel [205].
Table 6 presents the practical implementation of SLN and NLC in dermal drug delivery systems.
Table 6.
Application of SLN and NLC DDS for the treatment of skin disorders.
| LNP Type | API/Drug | Application | Reference |
|---|---|---|---|
| SLN | Doxorubicin | Doxorubicin-loaded SLN for the treatment of skin cancer. | [206] |
| SLN | Adapalene | Adapalene-loaded SLN in the gel for anti-acne treatment. | [207] |
| SLN | Triamcinolone acetonide | Triamcinolone acetonide-loaded SLN for the topical treatment of psoriasis. | [208] |
| SLN | Resveratrol, vitamin E, and epigallocatechin gallate |
SLN containing resveratrol, vitamin E, and epigallocatechin gallate for antioxidant benefits. | [209] |
| SLN | Silybin | Silybin-loaded SLN enriched gel for irritant contact dermatitis. | [210] |
| SLN | Fluconazole | Fluconazole-loaded SLN topical gel for the treatment of pityriasis versicolor. | [211] |
| SLN | Tazarotene | Tazarotene-loaded SLN for the treatment of psoriasis. | [212] |
| SLN | Miconazole nitrate | Miconazole nitrate-loaded SLN for antifungal activity. | [213] |
| SLN | Adapalene | Adapalene-loaded SLN for anti-acne therapy. | [214] |
| SLN | Isotretinoin and α-tocopherol |
SLN loaded with retinoic acid and lauric acid for the topical treatment of acne vulgaris. | [215] |
| NLC | Spironolactone | Spironolactone-loaded NLC-based gel for the effective treatment of acne vulgaris. | [216] |
| NLC | Clobetasol propionate | NLC-based topical gel of clobetasol propionate for the treatment of eczema. | [217] |
| NLC | Tacrolimus and tumor necrosis factor α siRNA |
NLC co-delivering tacrolimus and tumor necrosis factor α siRNA for the treatment of psoriasis. | [218] |
| NLC | Itraconazole | Topical NLC containing itraconazole for the treatment of fungal infections. | [219] |
| NLC | Apremilast | NLC for topical delivery of apremilast for the treatment of psoriasis. | [220] |
| NLC | Dithranol | Dithranol-loaded NLC-based gel for the treatment of psoriasis. | [221] |
| NLC | Voriconazole | Voriconazole-loaded NLC for antifungal applications. | [222] |
| NLC | Mometasone furoate | NLC-based hydrogel of mometasone furoate for the treatment of psoriasis. | [223] |
| NLC | Antimicrobial peptide nisin Z |
Antimicrobial peptide nisin Z with conventional antibiotic-loaded NLC to enhance antimicrobial activity. | [224] |
| NLC | Adapalene and vitamin C | Adapalene- and vitamin C-loaded NLC for acne treatment. | [225] |
7. Microemulsions and Nanoemulsions
In general, microemulsions and nanoemulsions are dispersion systems composed of two immiscible liquid phases that can penetrate deeper levels of the skin [226]. Evidence suggests that they may disrupt the SC lipid structural order, resulting in the loss of skin barrier properties [227]. Despite the apparent similarities between these two systems representing low-viscosity colloidal dispersions, they are classified as entirely different formulations [228].
7.1. Microemulsions
It has been found that microemulsions are spontaneously formed, transparent, and isotropic thermodynamically stable dispersion systems. The composition of the droplets is carried out by the precise mixing of volumes of immiscible liquids (usually oil and water) and the interfacial film of stabilizing surfactants at specific pressures and temperatures [229]. Short alkyl chain alcohols such as co-surfactants are typically chosen to potent the spontaneous formation of microemulsions. The isotropic and visually mono-phasic transparent system, in which the droplet size is usually below 100 nm, creates a flexible interfacial film characterized by ultra-low surface tension values [230]. The microemulsion as a formulation can improve the delivery of skin MP with both hydrophilic and lipophilic active substances compared with conventional carriers. In this regard, Patel et al. reported that the microemulsion with ketoconazole penetrated more efficiently than the saturated aqueous solution [231].
Three various structural types of microemulsions can be formed:
Oil-in-water (O/W) microemulsion;
Water-in-oil (W/O) microemulsion;
Bicontinuous microemulsion [232].
7.2. Nanoemulsions
Nanoemulsions typically contain 20–500 nm large droplets and have a different appearance depending on their size. Traditionally, they are stabilized by surfactants and do not change in the long term [233]. However, they are non-equilibrium structures, and an energetic input has to be typically applied (often from an emulsion) to form the droplet size according to the nanoscale [234]. The basic methods for preparing nanoemulsions are high-energy emulsifying methods such as HPH, ultrasound, jet spraying, microfluidization, and low energy emulsifying methods such as solvent displacement, spontaneous emulsification, phase inversion [235].
7.3. Microemulsions and Nanoemulsions in the Treatment of Skin Disorders
7.3.1. Antipsoriatic Effect
The clobetasol propionate- and calcipotriol-loaded nanoemulsion gel for the topical treatment of psoriasis is reported, developed, and optimized by Kaur et al. The spontaneous emulsification method was used for the preparation. Compared with the other commercial MP [236], the nanoemulsion containing gel showed higher antipsoriatic activity in mice.
Recently, Rajitha et al. reported the preparation of loaded nanoemulsion based on chaulmoogra oil, which is based on the self-emulsification method. Compared with the conventional methotrexate solution, the nanoemulsion showed enhanced skin permeation and retention of methotrexate in the deep skin layers [237].
7.3.2. Antifungal Effect
In another study, Coneac et al. reported the development of microemulsion-loaded hydrogels for the topical delivery of fluconazole. Nonionic surfactants have been used to stabilize the microemulsions, which then were incorporated in Carbopol gels. Compared with the conventional hydrogel and Nizoral® cream, the optimized microemulsion-loaded hydrogels showed higher in vitro flux values, higher release rate, and higher in vitro antifungal activity against Candida albicans [238].
7.3.3. Anti-Inflammatory Effect
Two nanoemulsion systems for the dermal application of natural or synthetic mixtures of pentacyclic triterpenes, with an anti-inflammatory effect, were reported by Alvarado et al. [239]. Slightly different permeation profiles of natural and synthetic triterpene-containing formulations are stated. The nanoemulsion, containing a natural triterpene mixture, demonstrated a more significant anti-inflammatory activity due to the slower permeation through the mouse skin [239].
In another study, Goindi et al. reported an ionic liquid-in-water microemulsion formulation that can solubilize etodolac, a poorly water-soluble anti-inflammatory drug. An effective permeation profile, as well as anti-arthritic and anti-inflammatory activities are evaluated in vivo in different models compared with a marketed formulation of etodolac (Proxym gel®) [240].
7.3.4. Antioxidant Effect
Lv et al. reported the preparation of essential oil-based microemulsions for topical application in order to improve the solubility, photostability, and skin permeation of quercetin. First, self-micro emulsifying DDS were prepared and then formed microemulsions. The microemulsions protected quercetin from degradation in an alkaline environment and under UV radiation. In these formulations, the in vitro skin permeation study on rats showed 2.5–3 times enhanced permeation capacity of quercetin compared with the conventional aqueous solution [241].
7.3.5. Local Anesthetic Effect
To optimize the percutaneous absorption of lidocaine and prilocaine, Negi et al. formed nanoemulsions using the high-shear mixing method followed by the HPH. The optimized nanoemulsion systems showed higher permeation rates and permeability coefficient values in parallel with the marketed cream and were further incorporated into a Carbopol hydrogel. In addition, the nanoemulsions and the nanoemulsion gel had a stronger anesthetic effect in vivo than the commercial product [242].
7.3.6. Anticarcinogenic Effect
In the last few years, Pham et al. developed a nano-emulsification approach to optimize the incorporation of Tocomin® for the accompanying therapy of skin carcinomas. Different preparation methods were used. The technique, combining a single-phase in-version temperature homogenization method with ultrasonication, produced a stable Tocomin®-loaded nanoemulsion, which demonstrated an exceeding cytotoxic profile against two human cutaneous carcinoma cell models [243].
Table 7 presents the practical implementation of microemulsions and nanoemulsions in dermal drug delivery systems.
Table 7.
Application of microemulsions and nanoemulsions DDS for the treatment of skin disorders.
| Type | API/Drug | Application | Reference |
|---|---|---|---|
| Microemulsion | Tazarotene | Tazarotene-loaded microemulsion for the treatment of psoriasis. | [244] |
| Microemulsion | Methotrexate | Methotrexate-loaded microemulsion for the treatment of psoriasis. | [245] |
| Microemulsion | Retinoid | Retinoid-loaded microemulsion for the treatment of psoriasis. | [246] |
| Microemulsion | Clotrimazole | Microemulsion coated with chitosan and containing clotrimazole for antifungal activity. | [247] |
| Microemulsion | Griseofulvin | Griseofulvin-loaded microemulsion for the antifungal treatment. | [248] |
| Microemulsion | Boswellia carterii oleo-gum-resin | Boswellia carterii oleo-gum resin-loaded microemulsion for the treatment of acne and eczema. | [249] |
| Microemulsion | Indian pennywort, walnut, and turmeric | Topical dosage microemulsion of Indian pennywort, walnut, and turmeric for the treatment of eczema. | [250] |
| Microemulsion | Triamcinolone | Microemulsion containing triamcinolone for transdermal delivery for the treatment of eczema. | [251] |
| Microemulsion | Retinyl palmitate | Microemulsion containing retinyl palmitate for the treatment of acne, aging, and psoriasis. | [252] |
| Nanoemulsion | Triptolide | Triptolide nanoemulsion gels for the treatment of eczema. | [253] |
| Nanoemulsion | Ivermectin | Nanoemulsion containing ivermectin for the treatment of different types of parasite infestations. | [254] |
| Nanoemulsion | Cyclosporine | Cyclosporine-loaded nanoemulsion for the treatment of psoriasis. | [255] |
| Nanoemulsion | Coumestrol /Hydroxyethylcellulose | Nanoemulsion containing coumestrol and hydroxyethylcellulose for the treatment of antiherpes. | [256] |
| Nanoemulsion | 8-Methoxypsoralen | 8-Methoxypsoralenloaded nanoemulsion for the treatment of vitiligo and psoriasis. | [257] |
| Nanoemulsion | Coenzyme Q10 | Coenzyme Q10-loaded nanoemulsion as an antioxidant agent. | [258] |
| Nanoemulsion | Psoralen | Psoralen-loaded nanoemulsion for the treatment of psoriasis and vitiligo. | [259] |
| Nanoemulsion | Isotretinoin | Isotretinoin-loaded nanoemulsion for the treatment of acne. | [260] |
| Nanoemulsion | Amphotericin B | Amphotericin B-loaded nanoemulsion for the antifungal treatment. | [261] |
| Nanoemulsion | Zinc phthalocyanine | Zinc phthalocyanine-loaded nanoemulsion for use in photodynamic therapy for leishmaniasis. | [262] |
8. Topical Dosage Forms with Lipid Nanoparticulate DDS for the Treatment of Skin Disorders
The size of Global Topical Drug Delivery Market was estimated at USD 95.08 billion in 2020 and expected to reach USD 101.10 billion in 2021 and USD 140.01 billion by 2026. The current market situation (USA, EUR, JP, AUS) for the topical skin products, shows domination of generic products—about 74% of all the approved topical products are generic equivalents of reference medicines (RLD). According to the collected data, gels, creams, ointments, lotions, and solutions dominate the market for both topical reference and topical generic products. The available semi-solid, solid, and liquid topical products contain different combinations of surfactants, oils, water, colloidal, and solid ingredients in solutions or dispersions [263].
Specific needs of skin, affected by inflammation, acne, or infections require adequate drug therapy with appropriate topical dosage forms. For example, the adhesive patch can provide a sustained and controlled release, while the gel can provide a faster and more intense action. On the other hand, some topical dosage forms may not be most suitable for application to certain areas of the skin, around the eyes, for example [264].
One of the most important considerations in the development of the topical dosage forms is the patient need. The second consideration is the drug’s physicochemical properties. In general, regulatory procedures for the registration of topical products are slow, as clinical equivalence studies (clinical trials) involve a high number of participants, require time and significant costs to ensure a sufficiently objective assessment of the final therapeutic effect. Technological or cost problems are among the reasons for additional difficulties in the implementation of promising dermatological products [265].
Table 8 summarizes some of the literature available on topical nano-based topical dosage forms for skin diseases.
Table 8.
Topical nanoformulations used in the treatment of various skin conditions.
| Type | API/Drug | Disease | Reference |
|---|---|---|---|
| Nanoemulsion gel | Clobetasol propionate | Treatments of psoriasis. | [266] |
| Nanoethogel | Amphotericin B | Dermatophytes and fungal infections. | [236] |
| Nanoemulsion gel | 5-Fluorouracil | Non-melanoma skin cancers. | [267] |
| Hydrogel | Zinc oxide | Wound healing effect on fibroblast cells. | [268] |
| Nanoemulgel, NLC | Vitamin E | Skin hydration. | [269] |
| Hydrogel | Cyclosporine and calcipotriol | Treatments of psoriasis. | [270] |
9. Future Prospects of Lipid Nanoparticulate DDS for the Treatment of Skin Disorders
It can be generalized that the main challenges in the development of cutaneous lipid nanometric delivery systems include:
Precise delivery across the skin and to certain skin strata, depending on the final target;
Successful elimination of lipid nanomaterial toxicity threats in topical medical formulations and cosmetics;
Ensuring improved permeation and low skin irritability as a result of the use of lipid nanocarriers;
Improved cutaneous release of incorporated API with a broad spectrum of physiological and physicochemical properties.
Lipid nanoparticulate DDS can be employed intensively for the delivery of phytomedicines intended for topical administration. The approach can be promising in this regard, considering the difficulties in their delivery which is caused by their physicochemical properties.
The formulation of phytopreparations with lipid nanoparticles would find a useful application in nanomedicine at the desired targeted delivery, for example, in cancer treatments.
10. Conclusions
Skin disorders represent a progressively emerging clinical public health problem. Treatment strategies based on conventional formulations are non-specific and can lead to considerable systemic toxicity. The progressive approach of the use of lipid nano-formulations as skin drug delivery systems can provide an incomparable prospect for the application of highly competent and safe treatments with the improved benefit-risk ratio.
The use of lipid nanoparticlulate DDS is favored recently due to the GRAS status of the excipients. Lipid nanocarriers can effectively protect the API from degradation on the skin’s surface, increase their concentration gradient in the upper skin layers, and enable gradual release. Lipid nanoparticles for topical application could be formulated with the high content of lipid matrix or dispersed in different foundations.
Lipid nanosystems provide a promising, flexible platform for the safe, effective, and biocompatible topical delivery of the API, as they do not cause cytotoxicity or morphological changes in the skin layers. The interest shown by pharmaceutical scientists, in the development of lipid nanoparticle delivery systems, may offer a future that provides sufficiently efficient lipid nanoparticle products for needy users.
Acknowledgments
The authors would like to express their acknowledgement to the supporters—the Medical University of Varna “Paraskev Stoyanov,” Varna, Bulgaria.
Author Contributions
Conceptualization, V.Y.A. and S.R.S.; writing—original draft preparation, S.R.S.; writing—review and editing, V.Y.A. and S.R.S.; visualization, S.R.S.; supervision, V.Y.A.; project administration, V.Y.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Medical University of Varna “Prof. Dr. Paraskev Stoyanov,” Fund “Nauka” Project No. 18027.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sanclemente G., Burgos C., Nova J., Hernández F., González C., Reyes M.I., Córdoba N., Arévalo Á., Meléndez E., Colmenares J., et al. The impact of skin diseases on quality of life: A multicenter study. Actas Dermosifiliogr. 2017;108:244–252. doi: 10.1016/j.ad.2016.11.008. (In English, Spanish) [DOI] [PubMed] [Google Scholar]
- 2.Seth D., Cheldize K., Brown D., Freeman E.F. Global burden of skin disease: Inequities and innovations. Curr. Dermatol. Rep. 2017;6:204–210. doi: 10.1007/s13671-017-0192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain S., Barambhe M.S., Jain J., Jajoo U.N., Pandey N. Prevalence of skin diseases in rural Central India: A community-based, cross-sectional, observational study. J. Mahatma Gandhi Inst. Med. Sci. 2016;21:111–115. doi: 10.4103/0971-9903.189537. [DOI] [Google Scholar]
- 4.Yew Y.W., Kuan A.H.Y., Ge L., Yap C.W., Heng B.H. Psychosocial impact of skin diseases: A population-based study. PLoS ONE. 2020;15:e0244765. doi: 10.1371/journal.pone.0244765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jee M.H., Mraz V., Geisler C., Bonefeld C.M. γδ T cells and inflammatory skin diseases. Immunol. Rev. 2020;298:61–73. doi: 10.1111/imr.12913. [DOI] [PubMed] [Google Scholar]
- 6.Akhtar N., Singh V., Yusuf M., Khan R.A. Non-invasive drug delivery technology: Development and current status of transdermal drug delivery devices, techniques and biomedical applications. Biomed. Eng. Biomed. Tech. 2020;65:243–272. doi: 10.1515/bmt-2019-0019. [DOI] [PubMed] [Google Scholar]
- 7.Holick M.F. Photobiology of vitamin D. In: Feldman D., editor. Vitamin D. 4th ed. Academic Press; London, UK: 2018. pp. 45–55. [Google Scholar]
- 8.Graham H.K., Eckersley A., Ozols M., Mellody K.T., Sherratt M.J. Human Skin: Composition, structure and visualisation methods. In: Limbert G., editor. Skin Biophysics. 1st ed. Springer; Cham, Switzerland: 2019. pp. 1–18. [Google Scholar]
- 9.Lundborg M., Narangifard A., Wennberg C.L., Lindahl E., Daneholt B., Norlén L. Human skin barrier structure and function analyzed by cryo-EM and molecular dynamics simulation. J. Struct. Biol. 2018;203:149–161. doi: 10.1016/j.jsb.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Sguizzato M., Esposito E., Cortesi R. Lipid-based nanosystems as a tool to overcome skin barrier. Int. J. Mol. Sci. 2021;22:8319. doi: 10.3390/ijms22158319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodby B., Penta K., Pecorelli A., Lila M.A., Valacchi G. Skin health from the inside out. Annu. Rev. Food Sci. Technol. 2020;11:235–254. doi: 10.1146/annurev-food-032519-051722. [DOI] [PubMed] [Google Scholar]
- 12.Lademann J., Schanzer S., Richter H., Meinke M.C., Weigmann H.J., Patzelt A. Stripping procedures for penetration measurements of topically applied substances. In: Dragicevic N., Maibach H., editors. Percutaneous Penetration Enhancers Drug Penetration into/through the Skin: Methodology and General Considerations. 1st ed. Springer; Berlin, Germany: 2017. pp. 205–214. [Google Scholar]
- 13.Gorzelanny C., Mess C., Schneider S.W., Huck V., Brandner J.M. Skin Barriers in Dermal Drug Delivery: Which Barriers Have to Be Overcome and How Can We Measure Them? Pharmaceutics. 2020;12:684. doi: 10.3390/pharmaceutics12070684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wohlrab J. Topical preparations and their use in dermatology. J. Dtsch. Dermatol. Ges. 2016;14:1061–1069. doi: 10.1111/ddg.13151. [DOI] [PubMed] [Google Scholar]
- 15.Ribeiro C.S., Leal F., Jeunon T. Skin anatomy, histology, and physiology. In: Issa M., Tamura B., editors. Daily Routine in Cosmetic Dermatology. Clinical Approaches and Procedures in Cosmetic Dermatology. Springer; Cham, Switzerland: 2017. pp. 1–12. [Google Scholar]
- 16.Lavers I. Exploring skin anatomy, function and site-specific treatment options. J. Aesthet. Nurs. 2017;6:172–180. doi: 10.12968/joan.2017.6.4.172. [DOI] [Google Scholar]
- 17.Nafisi S., Maibach H.I. Chapter 3—Skin penetration of nanoparticles. In: Shegokar R., Souto E.B., editors. Micro and Nano Technologies, Emerging Nanotechnologies in Immunology. Elsevier; Amsterdam, The Netherlands: 2018. pp. 47–88. [Google Scholar]
- 18.Montagna W., Ebling F.J.G. Human Skin. Encyclopedia Britannica. 2021. [(accessed on 19 October 2021)]. Available online: https://www.britannica.com/science/human-skin.
- 19.Osseiran S., Dela Cruz J., Jeong S., Wang H., Fthenakis C., Evans C. Characterizing stratum corneum structure, barrier function, and chemical content of human skin with coherent Raman scattering imaging. Biomed. Opt. Express. 2018;9:6425–6443. doi: 10.1364/BOE.9.006425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yokose U., Ishikawa J., Morokuma Y., Naoe A., Inoue Y., Yasuda Y., Tsujimura H., Fujimura T., Murase T., Hatamochi A. The ceramide [NP]/[NS] ratio in the stratum corneum is a potential marker for skin properties and epidermal differentiation. BMC Dermatol. 2020;20:1–12. doi: 10.1186/s12895-020-00102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desmet E., van Gele M., Lambert J. Topically applied lipid- and surfactant-based nanoparticles in the treatment of skin disorders. Expert Opin. Drug Del. 2017;14:109–122. doi: 10.1080/17425247.2016.1206073. [DOI] [PubMed] [Google Scholar]
- 22.Das C., Olmsted P.D. The physics of stratum corneum lipid membranes. Phil. Trans. R. Soc. A. 2016;374:1–16. doi: 10.1098/rsta.2015.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsui T., Amagai M. Dissecting the formation, structure and barrier function of the stratum corneum. Int. Immunol. 2017;29:243–244. doi: 10.1093/intimm/dxv013. [DOI] [PubMed] [Google Scholar]
- 24.Ezati N., Roberts M.S., Zhang Q., Moghimi H.R. Measurement of Hansen Solubility Parameters of Human Stratum Corneum. Iran. J. Pharm. Res. 2020;19:572–578. doi: 10.22037/ijpr.2019.112435.13755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruela A.L.M., Perissinato A.G., Lino M.E., Mudrik P.S., Pereira G.R. Evaluation of skin absorption of drugs from topical and transdermal formulations. Braz. J. Pharm. Sci. 2016;52:527–544. doi: 10.1590/s1984-82502016000300018. [DOI] [Google Scholar]
- 26.Zsikó S., Csányi E., Kovács A., Budai-Szűcs M., Gácsi A., Berkó S. Methods to Evaluate Skin Penetration in Vitro. Sci. Pharm. 2019;87:19. doi: 10.3390/scipharm87030019. [DOI] [Google Scholar]
- 27.Walicka A., Iwanowska-Chomiak B. Drug Diffusion Transport Through Human Skin. Int. J. Appl. Mech. Eng. 2018;23:977–988. doi: 10.2478/ijame-2018-0055. [DOI] [Google Scholar]
- 28.Sala M., Diab R., Elaissari A., Fessi H. Lipid nanocarriers as skin drug delivery systems: Properties, mechanisms of skin interactions and medical applications. Int. J. Pharm. 2018;535:1–17. doi: 10.1016/j.ijpharm.2017.10.046. [DOI] [PubMed] [Google Scholar]
- 29.Abd E., Yousef S.A., Pastore M.N., Telaprolu K., Mohammed Y.H., Namjoshi S., Grice J.E., Roberts M.S. Skin models for the testing of transdermal drugs. Clin. Pharmacol. 2016;8:163–176. doi: 10.2147/CPAA.S64788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tabassum N., Hamdani M. Plants used to treat skin diseases. Pharmacogn. Rev. 2014;8:52–60. doi: 10.4103/0973-7847.125531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferreira K.C.B., Valle A.B.C.d.S., Paes C.Q., Tavares G.D., Pittella F. Nanostructured Lipid Carriers for the Formulation of Topical Anti-Inflammatory Nanomedicines Based on Natural Substances. Pharmaceutics. 2021;13:1454. doi: 10.3390/pharmaceutics13091454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolff K., Johnson R.A., Saavedra A.P., Roh E.K. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; New York, NY, USA: 2017. [(accessed on 19 October 2021)]. pp. 693–759. Available online: https://accessmedicine.mhmedical.com/content.aspx?bookid=2043§ionid=154893575. [Google Scholar]
- 33.Urban K., Chu S., Scheufele C., Giesey R.L., Mehrmal S., Uppal P., Delost G.R. The global, regional, and national burden of fungal skin diseases in 195 countries and territories: A cross-sectional analysis from the Global Burden of Disease Study 2017. JAAD Int. 2020;2:22–27. doi: 10.1016/j.jdin.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Craddock L.N., Schieke S.M. Superficial fungal infection. In: Kang S., Amagai M., Bruckner A.L., Enk A.H., Margolis D.J., McMichael A.J., Orringer J.S., editors. Fitzpatrick’s Dermatology. 9th ed. McGraw-Hill; New York, NY, USA: 2019. [(accessed on 19 October 2021)]. pp. 1–48. Chapter 160. Available online: https://accessmedicine.mhmedical.com/content.aspx?sectionid=210432218&bookid=2570&Resultclick=2. [Google Scholar]
- 35.Silverberg B. A Structured Approach to Skin and Soft Tissue Infections (SSTIs) in an Ambulatory Setting. Clin. Pract. 2021;11:65–74. doi: 10.3390/clinpract11010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pearson D.R., Margolis D.J. Cellulitis and erysipelas. In: Kang S., Amagai M., Bruckner A.L., Enk A.H., Margolis D.J., McMichael A.J., Orringer J.S., editors. Fitzpatrick’s Dermatology. 9th ed. McGraw-Hill; New York, NY, USA: 2019. [(accessed on 19 October 2021)]. pp. 1–19. Chapter 151. Available online: https://accessmedicine.mhmedical.com/content.aspx?bookid=2570§ionid=210413306. [Google Scholar]
- 37.Sartelli M., Guirao X., Hardcastle T., Kluger Y., Boermeester M.A., Rasa K., Ansaloni L., Coccolini F., Montravers P., Abu-Zidan F.M., et al. 2018 WSES/SIS-E consensus conference: Recommendations for the management of skin and soft tissue infections. World J. Emerg. Surg. 2018;13:58. doi: 10.1186/s13017-018-0219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Falcone M., Concia E., Giusti M., Mazzone A., Santini C., Stefani S., Violi F. Acute bacterial skin and skin structure infections in internal medicine wards: Old and new drugs. Inter. Emerg. Med. 2016;11:637–648. doi: 10.1007/s11739-016-1450-6. [DOI] [PubMed] [Google Scholar]
- 39.Khadr L., Harfouche M., Omori R., Schwarzer G., Chemaitelly H., Abu-Raddad L.J. The Epidemiology of Herpes Simplex Virus Type 1 in Asia: Systematic Review, Meta-analyses, and Meta-regressions. Clin. Infect. Dis. 2019;68:757–772. doi: 10.1093/cid/ciy562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poole C.L., Kimberlin D.W. Antiviral Approaches for the Treatment of Herpes Simplex Virus Infections in Newborn Infants. Annu. Rev. Virol. 2018;5:407–425. doi: 10.1146/annurev-virology-092917-043457. [DOI] [PubMed] [Google Scholar]
- 41.Mustafa M., Illzam E.M., Muniandy R.K., Sharifah A.M., Nang M.K., Ramesh B. Herpes simplex virus infections, Pathophysiology and Management. IOSR J. Dent. Med. Sci. 2016;15:85–91. doi: 10.9790/0853-150738591. [DOI] [Google Scholar]
- 42.Schwingen J., Kaplan M., Kurschus F.C. Review-Current Concepts in Inflammatory Skin Diseases Evolved by Transcriptome Analysis: In-Depth Analysis of Atopic Dermatitis and Psoriasis. Int. J. Mol. Sci. 2020;21:699. doi: 10.3390/ijms21030699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ho A.W., Kupper T.S. T cells and the skin: From protective immunity to inflammatory skin disorders. Nat. Rev. Immunol. 2019;19:490–502. doi: 10.1038/s41577-019-0162-3. [DOI] [PubMed] [Google Scholar]
- 44.Golan Y. Current Treatment Options for Acute Skin and Skin-structure Infections. Clin. Infect. Dis. 2019;68:206–212. doi: 10.1093/cid/ciz004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aktas E., Esin M.N. Skin disease symptoms and related risk factors among young workers in high-risk jobs. Contact Dermat. 2016;75:96–105. doi: 10.1111/cod.12606. [DOI] [PubMed] [Google Scholar]
- 46.Münch S., Wohlrab J., Neubert R.H.H. Dermal and transdermal delivery of pharmaceutically relevant macromolecules. Eur. J. Pharm. Biopharm. 2017;119:235–242. doi: 10.1016/j.ejpb.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 47.Jain K.K. Transdermal Drug Delivery-Technologies, Markets and Companies. Jain PharmaBiotech; Basel, Switzerland: 2021. pp. 1–302. [Google Scholar]
- 48.Alany R. Topical and Transdermal Formulation and Drug Delivery. Pharm. Dev. Technol. 2017;22:457. doi: 10.1080/10837450.2017.1310175. [DOI] [PubMed] [Google Scholar]
- 49.Brunaugh A.D., Smyth H.D.C., Williams R.O., III . Essential Pharmaceutics. Springer; Cham, Switzerland: 2019. Topical and transdermal drug delivery; pp. 131–147. AAPS Introductions in the Pharmaceutical Sciences. [Google Scholar]
- 50.Zasada M., Budzisz E. Retinoids: Active molecules influencing skin structure formation in cosmetic and dermatological treatments. Postep. Derm. Alergol. 2019;36:392–397. doi: 10.5114/ada.2019.87443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chien A. Retinoids in Acne Management: Review of Current Understanding, Future Considerations, and Focus on Topical Treatments. J. Drugs Dermatol. 2018;17:51–55. [PubMed] [Google Scholar]
- 52.Temova Rakuša Ž., Pišlar M., Kristl A., Roškar R. Comprehensive Stability Study of Vitamin D3 in Aqueous Solutions and Liquid Commercial Products. Pharmaceutics. 2021;13:617. doi: 10.3390/pharmaceutics13050617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arita R., Kawashima M., Ito M., Tsubota K. Clinical safety and efficacy of vitamin D3 analog ointment for treatment of obstructive meibomian gland dysfunction. BMC Ophthalmol. 2017;17:84. doi: 10.1186/s12886-017-0482-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mahajan H., Deshmukh G., Dhoot D., Mamadi R., Barkate H. Comparative Clinical Assessment of Effectiveness and Safety of Calcitriol and Calcipotriol in Mild Plaque Psoriasis. Int. J. Clin. Dermatol. 2020;3:28–31. [Google Scholar]
- 55.Singh R.K., Lee K.M., Jose M.V., Nakamura M., Ucmak D., Farahnik B., Abrouk M., Zhu T.H., Bhutani T., Liao W. The Patient’s Guide to Psoriasis Treatment. Part 1: UVB Phototherapy. Dermatol. Ther. 2016;6:307–313. doi: 10.1007/s13555-016-0129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rossi M., Rovati C., Arisi M., Tomasi C., Calzavara-Pinton I., Venturini M., Calzavara-Pinton P. A Short Cycle of Narrow-Band UVB Phototherapy in the Early Phase of Dupilumab Therapy Can Provide a Quicker Improvement of Severe Atopic Dermatitis. Dermatology. 2021;237:407–415. doi: 10.1159/000512456. [DOI] [PubMed] [Google Scholar]
- 57.Lossius A.H., Sundnes O., Ingham A.C., Edslev S.M., Bjørnholt J.V., Lilje B., Bradley M., Asad S., Haraldsen G., Skytt-Andersen P., et al. Shifts in the Skin Microbiota after UVB Treatment in Adult Atopic Dermatitis. Dermatology. 2021:1–12. doi: 10.1159/000515236. [DOI] [PubMed] [Google Scholar]
- 58.Eicher L., Knop M., Aszodi N., Senner S., French L., Wollenberg A. A systematic review of factors influencing treatment adherence in chronic inflammatory skin disease-strategies for optimizing treatment outcome. J. Eur. Acad. Dermatol. Venereol. 2019;33:2253–2263. doi: 10.1111/jdv.15913. [DOI] [PubMed] [Google Scholar]
- 59.Leppert W., Malec–Milewska M., Zajaczkowska R., Wordliczek J. Transdermal and Topical Drug Administration in the Treatment of Pain. Molecules. 2018;23:681. doi: 10.3390/molecules23030681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ray P., Singh S., Gupta S. Topical antimicrobial therapy: Current status and challenges. Indian J. Med. Microbiol. 2019;37:299–308. doi: 10.4103/ijmm.IJMM_19_443. [DOI] [PubMed] [Google Scholar]
- 61.Wohlrab J. Neue Entwicklungen bei Topika. Hautarzt. 2019;70:953–959. doi: 10.1007/s00105-019-04505-1. [DOI] [PubMed] [Google Scholar]
- 62.Garg A., Sharma G.S., Goyal A.K., Ghosh G., Si S.C., Rath G. Recent advances in topical carriers of anti-fungal agents. Heliyon. 2020;6:e04663. doi: 10.1016/j.heliyon.2020.e04663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elmowafy M. Skin penetration/permeation success determinants of nanocarriers: Pursuit of a perfect formulation. Colloids Surf. B. 2021;203:111748. doi: 10.1016/j.colsurfb.2021.111748. [DOI] [PubMed] [Google Scholar]
- 64.Chella N., Shastri N.R. Lipid Carriers: Role and Applications in Nano Drug Delivery. In: Jana S., Jana S., editors. Particulate Technology for Delivery of Therapeutics. Springer; Singapore: 2017. pp. 253–289. [Google Scholar]
- 65.Garcês A., Amaral M.H., Sousa Lobo J.M., Silva A.C. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: A review. Eur. J. Pharm. Sci. 2018;112:159–167. doi: 10.1016/j.ejps.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 66.Filipczak N., Yalamarty S.S.K., Li X., Khan M.M., Parveen F., Torchilin V. Lipid-Based Drug Delivery Systems in Regenerative Medicine. Materials. 2021;14:5371. doi: 10.3390/ma14185371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.LePree J.M. Lipid based delivery—Are lipid-based drug delivery systems in your formulation toolbox? [(accessed on 19 October 2021)];Drug Dev. Deliv. 2017 17:20–25. Available online: https://drug-dev.com/lipid-based-delivery-are-lipid-based-drug-delivery-systems-in-your-formulation-toolbox/ [Google Scholar]
- 68.Carita A.C., Eloy J.O., Chorilli M., Lee R.J., Leonardi G.R. Recent Advances and Perspectives in Liposomes for Cutaneous Drug Delivery. Curr. Med. Chem. 2018;25:606–635. doi: 10.2174/0929867324666171009120154. [DOI] [PubMed] [Google Scholar]
- 69.Garg V., Singh H., Bimbrawh S., Singh S.K., Gulati M., Vaidya Y., Kaur P. Ethosomes and Transfersomes: Principles, Perspectives and Practices. Curr. Drug. Deliv. 2017;14:613–633. doi: 10.2174/1567201813666160520114436. [DOI] [PubMed] [Google Scholar]
- 70.Opatha S.A.T., Titapiwatanakun V., Chutoprapat R. Transfersomes: A Promising Nanoencapsulation Technique for Transdermal Drug Delivery. Pharmaceutics. 2020;12:855. doi: 10.3390/pharmaceutics12090855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chaurasiya P., Ganju E., Upmanyu N., Ray S.K., Jain P. Transfersomes: A novel technique for transdermal drug delivery. J. Drug Deliv. Ther. 2019;9:279–285. doi: 10.22270/jddt.v9i1.2198. [DOI] [Google Scholar]
- 72.Nainwal N., Jawla S., Singh R., Saharan V.A. Transdermal applications of ethosomes—A detailed review. J. Liposome Res. 2019;29:103–113. doi: 10.1080/08982104.2018.1517160. [DOI] [PubMed] [Google Scholar]
- 73.Natsheh H., Vettorato E., Touitou E. Ethosomes for Dermal Administration of Natural Active Molecules. Curr. Pharm. Des. 2019;25:2338–2348. doi: 10.2174/1381612825666190716095826. [DOI] [PubMed] [Google Scholar]
- 74.Paliwal R., Paliwal S.R., Kenwat R., Kurmi B.D., Sahu M.K. Solid lipid nanoparticles: A review on recent perspectives and patents. Expert Opin. Ther. Pat. 2020;30:179–194. doi: 10.1080/13543776.2020.1720649. [DOI] [PubMed] [Google Scholar]
- 75.Liu M., Wen J., Sharma M. Solid Lipid Nanoparticles for Topical Drug Delivery: Mechanisms, Dosage Form Perspectives, and Translational Status. Curr. Pharm. Des. 2020;26:3203–3217. doi: 10.2174/1381612826666200526145706. [DOI] [PubMed] [Google Scholar]
- 76.Geng Q., Zhao Y., Wang L., Xu L., Chen X., Han J. Development and Evaluation of Astaxanthin as Nanostructure Lipid Carriers in Topical Delivery. AAPS Pharm. Sci. Tech. 2020;21:318. doi: 10.1208/s12249-020-01822-w. [DOI] [PubMed] [Google Scholar]
- 77.Araujo V.H.S., Delello Di Filippo L., Duarte J.L., Spósito L., de Camargo B.A.F., da Silva P.B., Chorilli M. Exploiting solid lipid nanoparticles and nanostructured lipid carriers for drug delivery against cutaneous fungal infections. Crit. Rev. Microbiol. 2020;47:79–90. doi: 10.1080/1040841X.2020.1843399. [DOI] [PubMed] [Google Scholar]
- 78.Jain A., Pooladanda V., Bulbake U., Doppalapudi S., Rafeeqi T.A., Godugu C., Khan W. Liposphere mediated topical delivery of thymoquinone in the treatment of psoriasis. Nanomedicine. 2017;13:2251–2262. doi: 10.1016/j.nano.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 79.Esposito E., Sguizzato M., Bories C., Nastruzzi C., Cortesi R. Production and Characterization of a Clotrimazole Liposphere Gel for Candidiasis Treatment. Polymers. 2018;10:160. doi: 10.3390/polym10020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Waghule T., Gorantla S., Rapalli V.K., Shah P., Dubey S.K., Saha R.N., Singhvi G. Emerging Trends in Topical Delivery of Curcumin Through Lipid Nanocarriers: Effectiveness in Skin Disorders. AAPS Pharm. Sci. Tech. 2020;21:284. doi: 10.1208/s12249-020-01831-9. [DOI] [PubMed] [Google Scholar]
- 81.Abu Lila A.S., Ishida T. Liposomal Delivery Systems: Design Optimization and Current Applications. Biol. Pharm. Bull. 2017;40:1–10. doi: 10.1248/bpb.b16-00624. [DOI] [PubMed] [Google Scholar]
- 82.Has C., Sunthar P. A comprehensive review on recent preparation techniques of liposomes. J. Liposome Res. 2020;30:336–365. doi: 10.1080/08982104.2019.1668010. [DOI] [PubMed] [Google Scholar]
- 83.Mezei M., Gulasekharam V. Liposomes—A selective drug delivery system for the topical route of administration I. Lotion dosage form. Life Sci. 1980;26:1473–1477. doi: 10.1016/0024-3205(80)90268-4. [DOI] [PubMed] [Google Scholar]
- 84.Lucia M. Lipid-Based Nanoparticles as Carriers for Dermal Delivery of Antioxidants. Curr. Drug. Metab. 2017;18:469–480. doi: 10.2174/1389200218666170222152038. [DOI] [PubMed] [Google Scholar]
- 85.Yang M., Gu Y., Tang X., Wang T., Liu J. Advancement of Lipid-Based Nanocarriers and Combination Application with Physical Penetration Technique. Curr. Drug. Deliv. 2019;16:312–324. doi: 10.2174/1567201816666190118125427. [DOI] [PubMed] [Google Scholar]
- 86.Rai S., Pandey V., Rai G. Transfersomes as versatile and flexible nano-vesicular carriers in skin cancer therapy: The state of the art. Nano Rev. Exp. 2017;8:1325708. doi: 10.1080/20022727.2017.1325708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Matos C., Lobão P. Non-Steroidal Anti-Inflammatory Drugs Loaded Liposomes for Topical Treatment of Inflammatory and Degenerative Conditions. Curr. Med. Chem. 2020;27:3809–3829. doi: 10.2174/0929867326666190227233321. [DOI] [PubMed] [Google Scholar]
- 88.Natsheh H., Touitou E. Phospholipid Vesicles for Dermal/Transdermal and Nasal Administration of Active Molecules: The Effect of Surfactants and Alcohols on the Fluidity of Their Lipid Bilayers and Penetration Enhancement Properties. Molecules. 2020;25:2959. doi: 10.3390/molecules25132959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Avadhani K.S., Manikkath J., Tiwari M., Chandrasekhar M., Godavarthi A., Vidya S.M., Hariharapura R.C., Kalthur G., Udupa N., Mutalik S. Skin delivery of epigallocatechin-3-gallate (EGCG) and hyaluronic acid loaded nano-transfersomes for antioxidant and anti-aging effects in UV radiation induced skin damage. Drug Deliv. 2017;24:61–74. doi: 10.1080/10717544.2016.1228718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chacko I.A., Ghate V.M., Dsouza L., Lewis S.A. Lipid vesicles: A versatile drug delivery platform for dermal and transdermal applications. Colloids Surf. B Biointerfaces. 2020;195:111262. doi: 10.1016/j.colsurfb.2020.111262. [DOI] [PubMed] [Google Scholar]
- 91.Lai F., Caddeo C., Manca M.L., Manconi M., Sinico C., Fadda A.M. What’s new in the field of phospholipid vesicular nanocarriers for skin drug delivery. Int. J. Pharm. 2020;583:119398. doi: 10.1016/j.ijpharm.2020.119398. [DOI] [PubMed] [Google Scholar]
- 92.Bnyan R., Khan I., Ehtezazi T., Saleem I., Gordon S., O’Neill F., Roberts M. Formulation and optimisation of novel transfersomes for sustained release of local anaesthetic. J. Pharm. Pharmacol. 2019;71:1508–1519. doi: 10.1111/jphp.13149. [DOI] [PubMed] [Google Scholar]
- 93.Touitou E. Compositions for Applying Active Substances to or through the Skin. 5,716,638. US Patent. 1998 February 10;
- 94.Ita K. Current Status of Ethosomes and Elastic Liposomes in Dermal and Transdermal Drug Delivery. Curr. Pharm. Des. 2016;22:5120–5126. doi: 10.2174/1381612822666160511150228. [DOI] [PubMed] [Google Scholar]
- 95.Lin H., Lin L., Choi Y., Michniak-Kohn B. Development and in-vitro evaluation of co-loaded berberine chloride and evodiamine ethosomes for treatment of melanoma. Int. J. Pharm. 2020;15:119278. doi: 10.1016/j.ijpharm.2020.119278. [DOI] [PubMed] [Google Scholar]
- 96.Arora D., Nanda S. Quality by design driven development of resveratrol loaded ethosomal hydrogel for improved dermatological benefits via enhanced skin permeation and retention. Int. J. Pharm. 2019;567:118448. doi: 10.1016/j.ijpharm.2019.118448. [DOI] [PubMed] [Google Scholar]
- 97.Moolakkadath T., Aqil M., Ahad A., Imam S.S., Praveen A., Sultana Y., Mujeeb M., Iqbal Z. Fisetin loaded binary ethosomes for management of skin cancer by dermal application on UV exposed mice. Int. J. Pharm. 2019;560:78–91. doi: 10.1016/j.ijpharm.2019.01.067. [DOI] [PubMed] [Google Scholar]
- 98.Lalotra A.S., Singh V., Khurana B., Agrawal S., Shrestha S., Arora D. A Comprehensive Review on Nanotechnology-Based Innovations in Topical Drug Delivery for the Treatment of Skin Cancer. Curr. Pharm. Des. 2020;26:5720–5731. doi: 10.2174/1381612826666200819202821. [DOI] [PubMed] [Google Scholar]
- 99.Pandey K. An Overview on Promising Nanotechnological Approaches for the Treatment of Psoriasis. Recent Pat. Nanotechnol. 2020;14:102–118. doi: 10.2174/1872210514666200204124130. [DOI] [PubMed] [Google Scholar]
- 100.Iqbal B., Ali J., Baboota S. Recent advances and development in epidermal and dermal drug deposition enhancement technology. Int. J. Dermatol. 2018;57:646–660. doi: 10.1111/ijd.13902. [DOI] [PubMed] [Google Scholar]
- 101.Pathak K., Vaidya A., Sharma V. Confronting Penetration Threshold via Fluidic Terpenoid Nanovesicles. Curr. Drug. Deliv. 2018;15:765–776. doi: 10.2174/1567201815666180108162110. [DOI] [PubMed] [Google Scholar]
- 102.Kumar L., Verma S., Singh M., Chalotra T., Utreja P. Advanced Drug Delivery Systems for Transdermal Delivery of Non-Steroidal Anti-Inflammatory Drugs: A Review. Curr. Drug. Deliv. 2018;15:1087–1099. doi: 10.2174/1567201815666180605114131. [DOI] [PubMed] [Google Scholar]
- 103.Zhang Y.T., Shen L.N., Wu Z.H., Zhao J.H., Feng N.P. Comparison of ethosomes and liposomes for skin delivery of psoralen for psoriasis therapy. Int. J. Pharm. 2014;471:449–452. doi: 10.1016/j.ijpharm.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 104.Shabbir M., Nagra U., Zaman M., Mahmood A., Barkat K. Lipid Vesicles and Nanoparticles for Non-invasive Topical and Transdermal Drug Delivery. Curr. Pharm. Des. 2020;26:2149–2166. doi: 10.2174/1381612826666200114090659. [DOI] [PubMed] [Google Scholar]
- 105.Sun M.C., Xu X.L., Lou X.F., Du Y.Z. Recent Progress and Future Directions: The Nano-Drug Delivery System for the Treatment of Vitiligo. Int. J. Nanomed. 2020;15:3267–3279. doi: 10.2147/IJN.S245326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Soleymani S., Iranpanah A., Najafi F., Belwal T., Ramola S., Abbasabadi Z., Momtaz S., Farzaei M.H. Implications of grape extract and its nanoformulated bioactive agent resveratrol against skin disorders. Arch. Dermatol. Res. 2019;311:577–588. doi: 10.1007/s00403-019-01930-z. [DOI] [PubMed] [Google Scholar]
- 107.Boge L., Hallstensson K., Ringstad L., Johansson J., Andersson T., Davoudi M., Larsson P.T., Mahlapuu M., Håkansson J., Andersson M. Cubosomes for topical delivery of the antimicrobial peptide LL-37. Eur. J. Pharm. Biopharm. 2019;134:60–67. doi: 10.1016/j.ejpb.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 108.Baveloni F.G., Riccio B.V.F., Di Filippo L.D., Fernandes M.A., Meneguin A.B., Chorilli M. Nanotechnology-based Drug Delivery Systems as Potential for Skin Application: A Review. Curr. Med. Chem. 2021;28:3216–3248. doi: 10.2174/0929867327666200831125656. [DOI] [PubMed] [Google Scholar]
- 109.Kurangi B., Jalalpure S., Jagwani S. Formulation and Evaluation of Resveratrol Loaded Cubosomal Nanoformulation for Topical Delivery. Curr. Drug. Deliv. 2021;18:607–619. doi: 10.2174/1567201817666200902150646. [DOI] [PubMed] [Google Scholar]
- 110.Badie H., Abbas H. Novel small self-assembled resveratrol-bearing cubosomes and hexosomes: Preparation, charachterization, and ex vivo permeation. Drug Dev. Ind. Pharm. 2018;44:2013–2025. doi: 10.1080/03639045.2018.1508220. [DOI] [PubMed] [Google Scholar]
- 111.Ahmad U., Ahmad Z., Khan A.A., Akhtar J., Singh S.P., Ahmad F.J. Strategies in Development and Delivery of Nanotechnology Based Cosmetic Products. Drug Res. 2018;68:545–552. doi: 10.1055/a-0582-9372. [DOI] [PubMed] [Google Scholar]
- 112.Sun Q., Chen J.F., Routh A.F. Coated colloidosomes as novel drug delivery carriers. Exp. Opin. Drug Del. 2019;16:903–906. doi: 10.1080/17425247.2019.1652594. [DOI] [PubMed] [Google Scholar]
- 113.Bieberich E. Sphingolipids and lipid rafts: Novel concepts and methods of analysis. Chem. Phys. Lipids. 2018;216:114–131. doi: 10.1016/j.chemphyslip.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Neupane Y.R., Mahtab A., Siddiqui L., Singh A., Gautam N., Rabbani S.A., Goel H., Talegaonkar S. Biocompatible Nanovesicular Drug Delivery Systems with Targeting Potential for Autoimmune Diseases. Curr. Pharm. Des. 2020;26:5488–5502. doi: 10.2174/1381612826666200523174108. [DOI] [PubMed] [Google Scholar]
- 115.Shetty K., Sherje A.P. Nano intervention in topical delivery of corticosteroid for psoriasis and atopic dermatitis-a systematic review. J. Mat. Sci. Mater. Med. 2021;32:88. doi: 10.1007/s10856-021-06558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vazzana M., Fangueiro J.F., Faggio C., Santini A., Souto E.B. Archaeosomes for Skin Injuries. In: Ascenso A., Ribeiro H., Simões S., editors. Carrier-Mediated Dermal Delivery: Applications in the Prevention and Treatment of Skin Disorders. Pan Stanford Publishing; Singapore: 2017. pp. 323–355. [Google Scholar]
- 117.Sallam M.A., Prakash S., Kumbhojkar N., Shields C.W., Mitragotri S. Formulation-based approaches for dermal delivery of vaccines and therapeutic nucleic acids: Recent advances and future perspectives. Bioeng. Transl. Med. 2021;6:e10215. doi: 10.1002/btm2.10215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lin Y.L., Chen C.H., Wu H.Y., Tsai N.M., Jian T.Y., Chang Y.C., Lin C.H., Wu C.H., Hsu F.T., Leung T.K., et al. Inhibition of breast cancer with transdermal tamoxifen-encapsulated lipoplex. J. Nanobiotechnol. 2016;14:11. doi: 10.1186/s12951-016-0163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Singh N., Kushwaha P., Ahmad U., Abdullah M. Proliposomes: An Approach for the Development of Stable Liposome. Ars. Pharm. 2019;60:231–240. [Google Scholar]
- 120.Hiremath N., Gowda D.V., Anusha R., Shamant B.S., Srivastava A., Moin A. Proliposomes: A novel approach to carrier drug delivery system. J. Chem. Pharm. Res. 2016;8:348–354. [Google Scholar]
- 121.Aziz D.E., Abdelbary A.A., Elassasy A.I. Investigating superiority of novel bilosomes over niosomes in the transdermal delivery of diacerein: In vitro characterization, ex vivo permeation and in vivo skin deposition study. J. Liposome Res. 2019;29:73–85. doi: 10.1080/08982104.2018.1430831. [DOI] [PubMed] [Google Scholar]
- 122.Kotla N.G., Chandrasekar B., Rooney P., Sivaraman G., Larrañaga A., Krishna K.V., Pandit A., Rochev Y. Biomimetic Lipid- Based Nanosystems for Enhanced Dermal Delivery of Drugs and Bioactive Agents. ACS Biomater. Sci. Eng. 2017;3:1262–1272. doi: 10.1021/acsbiomaterials.6b00681. [DOI] [PubMed] [Google Scholar]
- 123.Karami Z., Hamidi M. Cubosomes: Remarkable drug delivery potential. Drug Discov. Today. 2016;21:789–801. doi: 10.1016/j.drudis.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 124.Duarah S., Durai R.D., Narayanan V.B. Nanoparticle-in-gel system for delivery of vitamin C for topical application. Drug Deliv. Transl. Res. 2017;7:750–760. doi: 10.1007/s13346-017-0398-z. [DOI] [PubMed] [Google Scholar]
- 125.Sureka S., Gupta G., Agarwal M., Mishra A., Singh S.K., Singh R.P., Sah S.K., de Jesus A., Pinto T., Dua K. Formulation, In-Vitro and Ex-Vivo Evaluation of Tretinoin Loaded Cubosomal Gel for the Treatment of Acne. Recent Pat. Drug Deliv. Formul. 2018;12:121–129. doi: 10.2174/1872211312666180213121117. [DOI] [PubMed] [Google Scholar]
- 126.Magana J.R., Esquena J., Solans C., Rodriguez-Abreu C. Deconstruction of technical grade diglycerol isostearate enables the controlled preparation of hexosomes and liposomes. J. Molec. Liq. 2021;343:117594. doi: 10.1016/j.molliq.2021.117594. [DOI] [Google Scholar]
- 127.Fornasier M., Pireddu R., Del Giudice A., Sinico C., Nylander T., Schillén K., Galantini L., Murgia S. Tuning lipid structure by bile salts: Hexosomes for topical administration of catechin. Colloids Surf. B. Biointerfaces. 2021;199:111564. doi: 10.1016/j.colsurfb.2021.111564. [DOI] [PubMed] [Google Scholar]
- 128.Gururaj S.S., Ashok V.B., Swati S.M., Nilesh R.B., Prashant H.K., Nishant P.K., Sagar T. An overview on nanocarrier technology Aquasomes. J. Pharm. Res. 2009;2:1174–1177. [Google Scholar]
- 129.Lopez C., Mériadec C., David-Briand E., Dupont A., Bizien T., Artzner F., Riaublanc A., Anton M. Loading of lutein in egg-sphingomyelin vesicles as lipid carriers: Thermotropic phase behaviour, structure of sphingosome membranes and lutein crystals. Food Res Int. 2020;138:109770. doi: 10.1016/j.foodres.2020.109770. [DOI] [PubMed] [Google Scholar]
- 130.Cristiano M.C., Froiio F., Mancuso A., Cosco D., Dini L., Di Marzio L., Fresta M., Paolino D. Oleuropein-Laded Ufasomes Improve the Nutraceutical Efficacy. Nanomaterials. 2021;11:105. doi: 10.3390/nano11010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fan Y., Fang Y., Ma L. The self-crosslinked ufasome of conjugated linoleic acid: Investigation of morphology, bilayer membrane and stability. Colloids Surf. B. Biointerfaces. 2014;123:8–14. doi: 10.1016/j.colsurfb.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 132.Abdelbary A.A., AbouGhaly M.H. Design and optimization of topical methotrexate loaded niosomes for enhanced management of psoriasis: Application of Box–Behnken design, in-vitro evaluation and in-vivo skin deposition study. Int. J. Pharm. 2015;485:235–243. doi: 10.1016/j.ijpharm.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 133.Perez A.P., Altube M.J., Schilrreff P., Apezteguia G., Celes F.S., Zacchino S., de Oliveira C.I., Romero E.L., Morilla M.J. Topical amphotericin B inultradeformable liposomes: Formulation, skin penetration study, antifungal and antileishmanial activity in vitro. Colloids Surf. B Biointerfaces. 2016;139:190–198. doi: 10.1016/j.colsurfb.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 134.Garg B.J., Garg N.K., Beg S., Singh B., Katare O.P. Nanosized ethosomes-based hydrogel formulations of methoxsalen for en- hanced topical delivery against vitiligo: Formulation optimization, in vitro evaluation and preclinical assessment. J. Drug Target. 2016;24:233–246. doi: 10.3109/1061186X.2015.1070855. [DOI] [PubMed] [Google Scholar]
- 135.Oge L.K., Broussard A., Marshall M.D. Acne Vulgaris: Diagnosis and Treatment. Am. Fam. Physician. 2019;100:475–484. [PubMed] [Google Scholar]
- 136.Sevimli Dikicier B. Topical treatment of acne vulgaris: Efficiency, side effects, and adherence rate. J. Int. Med. Res. 2019;47:2987–2992. doi: 10.1177/0300060519847367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Apriani E.F., Rosana Y., Iskandarsyah I. Formulation, characterization, and in vitro testing of azelaic acid ethosome-based cream against Propionibacterium acnes for the treatment of acne. J. Adv. Pharm. Technol. Res. 2019;10:75. doi: 10.4103/japtr.JAPTR_289_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schaeffer H.J., Beauchamp L., de Miranda P., Elion G.B., Bauer D.J., Collins P. 9-(2-Hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature. 1978;272:583–585. doi: 10.1038/272583a0. [DOI] [PubMed] [Google Scholar]
- 139.Horwitz E., Pisanty S., Czerninski R., Helser M., Eliav E., Touitou E. A clinical evaluation of a novel liposomal carrier for acyclovir in the topical treatment of recurrent herpes labialis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1999;87:700–705. doi: 10.1016/S1079-2104(99)70164-2. [DOI] [PubMed] [Google Scholar]
- 140.Shukla K.V., Sharma A., Yadav M. Formulation development and evaluation of ethosomal gel of acyclovir for the treatment of herpes zoster. J. Nanosci. Nanotechnol. 2019;9((Suppl. 2)):664–668. [Google Scholar]
- 141.Babaie S., Ghanbarzadeh S., Davaran S., Kouhsoltani M., Hamishehkar H. Nanoethosomes for Dermal Delivery of Lidocaine. Adv. Pharm. Bull. 2015;5:549–556. doi: 10.15171/apb.2015.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Godin B., Touitou E., Rubinstein E., Athamna A., Athamna M. A new approach for treatment of deep skin infections by an ethosomal antibiotic preparation: An in vivo study. J. Antimicrob. Chemother. 2005;55:989–994. doi: 10.1093/jac/dki125. [DOI] [PubMed] [Google Scholar]
- 143.Godin B., Touitou E. Erythromycin ethosomal systems: Physicochemical characterization and enhanced antibacterial activity. Curr. Drug Deliv. 2005;2:269–275. doi: 10.2174/1567201054367931. [DOI] [PubMed] [Google Scholar]
- 144.Zahid S.R., Dangi S., Shende S.M., Upmanyu N. Formulation and evaluation of clindamycin phosphate ethosomal gel. World J. Pharm. Pharm. Sci. 2020;9:1804–1813. [Google Scholar]
- 145.Cosco D., Paolino D., Maiuolo J., Di Marzio L., Carafa M., Ventura C.A., Fresta M. Ultradeformable liposomes as multidrug carrier of resveratrol and 5-fluorouracil for their topical delivery. Int. J. Pharm. 2015;489:1–10. doi: 10.1016/j.ijpharm.2015.04.056. [DOI] [PubMed] [Google Scholar]
- 146.Castangia I., Caddeo C., Manca M.L., Casu L., Latorre A.C., Diez-Sales O., Ruiz-Sauri A., Bacchetta G., Fadda A.M., Manconi M. Delivery of liquorice extract by liposomes and hyalurosomes to protect the skin against oxidative stress injuries. Carbohydr. Polym. 2015;134:657–663. doi: 10.1016/j.carbpol.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 147.Caddeo C., Nacher A., Vassallo A., Armentano M.F., Pons R., Fernandez-Busquets X., Carbone C., Valenti D., Fadda A.M., Manconi M. Effect of quercetin and resveratrol co-incorporated in liposomes against inflammatory/oxidative response associated with skin cancer. Int. J. Pharm. 2016;513:153–163. doi: 10.1016/j.ijpharm.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 148.Rahman S.A., Abdelmalak N.S., Badawi A., Elbayoumy T., Sabry N., El Ramly A. Tretinoin-loaded liposomal formulations: From lab to comparative clinical study in acne patients. Drug Deliv. 2016;23:1184–1193. doi: 10.3109/10717544.2015.1041578. [DOI] [PubMed] [Google Scholar]
- 149.Ingebrigtsen S.G., Škalko-Basnet N., Jacobsen C.d.A.C., Holsæter A.M. Successful co-encapsulation of benzoyl peroxide and chloramphenicol in liposomes by a novel manufacturing method-dual asymmetric centrifugation. Eur. J. Pharm. Sci. 2017;97:192–199. doi: 10.1016/j.ejps.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 150.Jain S., Kale D.P., Swami R., Katiyar S.S. Codelivery of benzoyl peroxide & adapalene using modified liposomal gel for improved acne therapy. Nanomedicine. 2018;13:1481–1493. doi: 10.2217/nnm-2018-0002. [DOI] [PubMed] [Google Scholar]
- 151.Sarwa K.K., Mazumder B., Rudrapal M., Verma V.K. Potential of capsaicin-loaded transfersomes in arthritic rats. Drug Deliv. 2015;22:638–646. doi: 10.3109/10717544.2013.871601. [DOI] [PubMed] [Google Scholar]
- 152.Khan M.A., Pandit J., Sultana Y., Sultana S., Ali A., Aqil M., Chauhan M. Novel carbopol-based transfersomal gel of 5-fluorouracil for skin cancer treatment: In vitro characterization and in vivo study. Drug Deliv. 2015;22:795–802. doi: 10.3109/10717544.2014.902146. [DOI] [PubMed] [Google Scholar]
- 153.Dorrani M., Garbuzenko O.B., Minko T., Michniak-Kohn B. Development of edge-activated liposomes for siRNA delivery to human basal epidermis for melanoma therapy. J. Control Release. 2016;228:150–158. doi: 10.1016/j.jconrel.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 154.Desmet E., Bracke S., Forier K., Taevernier L., Stuart M.C.A., De Spiegeleer B., Raemdonck K., Van Gele M., Lambert J. An elastic liposomal formulation for RNAi-based topical treatment of skin disorders: Proof-of-concept in the treatment of psoriasis. Int. J. Pharm. 2016;500:268–274. doi: 10.1016/j.ijpharm.2016.01.042. [DOI] [PubMed] [Google Scholar]
- 155.Fadel M., Samy N., Nasr M., Alyoussef A.A. Topical colloidal indocyanine green-mediated photodynamic therapy for treatment of basal cell carcinoma. Pharm. Dev. Technol. 2017;22:545–550. doi: 10.3109/10837450.2016.1146294. [DOI] [PubMed] [Google Scholar]
- 156.Gupta M., Prajapati R.N., Irchhaiya R., Singh N., Prajapati S.K. Novel clindamycin loaded transfersomes formulation for effective management of acne. World J. Pharm. Res. 2017;6:765–773. doi: 10.20959/wjpr20176-8494. [DOI] [Google Scholar]
- 157.Jiang T., Wang T., Li T., Ma Y., Shen S., He B., Mo R. Enhanced Transdermal Drug Delivery by Transfer-some-Embedded Oligopeptide Hydrogel for Topical Chemotherapy of Melanoma. ACS Nano. 2018;12:9693–9701. doi: 10.1021/acsnano.8b03800. [DOI] [PubMed] [Google Scholar]
- 158.Dar M.J., Din F.U., Khan G.M. Sodium stibogluconate loaded nano-deformable liposomes for topical treatment of leishmaniasis: Macrophage as a target cell. Drug Deliv. 2018;25:1595–1606. doi: 10.1080/10717544.2018.1494222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Omar M.M., Hasan O.A., El Sisi A.M. Preparation and optimization of lidocaine transferosomal gel containing permeation enhancers: A promising approach for enhancement of skin permeation. Int. J. Nanomed. 2019;14:1551–1562. doi: 10.2147/IJN.S201356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cristiano M.C., Froiio F., Spaccapelo R., Mancuso A., Nisticò S.P., Udongo B.P., Fresta M., Paolino D. Sulforaphane-Loaded Ultradeformable Vesicles as A Potential Natural Nanomedicine for the Treatment of Skin Cancer Diseases. Pharmaceutics. 2020;12:6. doi: 10.3390/pharmaceutics12010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dar M.J., McElroy C.A., Khan M.I., Satoskar A.R., Khan G.M. Development and evaluation of novel miltefosine-polyphenol co-loaded second generation nano-transfersomes for the topical treatment of cutaneous leishmaniasis. Expert Opin. Drug Deliv. 2020;17:97–110. doi: 10.1080/17425247.2020.1700227. [DOI] [PubMed] [Google Scholar]
- 162.Harmita H., Iskandarsyah I., Afifah S.F. Effect of transfersome formulation on the stability and antioxidant activity of N- acetylcysteine in anti-aging cream. Int. J. Appl. Pharm. 2020;12:156–162. doi: 10.22159/ijap.2020.v12s1.FF034. [DOI] [Google Scholar]
- 163.Marto J., Vitor C., Guerreiro A., Severino C., Eleuterio C., Ascenso A., Simoes S. Ethosomes for enhanced skin delivery of griseofulvin. Colloids Surf. B Biointerfaces. 2016;146:616–623. doi: 10.1016/j.colsurfb.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 164.Yu Z., Lv H., Han G., Ma K. Ethosomes loaded with cryptotanshinone for acne treatment through topical gel formulation. PLoS ONE. 2016;11:e0159967. doi: 10.1371/journal.pone.0159967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.El-Kayal M., Nasr M., Elkheshen S., Mortada N. Colloidal (-)-epigallocatechin-3-gallate vesicular systems for prevention and treatment of skin cancer: A comprehensive experimental study with preclinical investigation. Eur. J. Pharm. Sci. 2019;137:104972. doi: 10.1016/j.ejps.2019.104972. [DOI] [PubMed] [Google Scholar]
- 166.Kausar H., Mujeeb M., Ahad A., Moolakkadath T., Aqil M., Ahmad A., Akhter H.M. Optimization of ethosomes for topical thymoquinone delivery for the treatment of skin acne. J. Drug Deliv. Sci. Technol. 2019;49:177–187. doi: 10.1016/j.jddst.2018.11.016. [DOI] [Google Scholar]
- 167.Richa P., Kumar S.D., Kumar S.A. Formulation and evaluation of ethosomes of clobetasol propionate. World J. Pharm. Res. 2016;5:1183–1197. [Google Scholar]
- 168.Mishra R., Shende S., Jain P.K., Jain V. Formulation and evaluation of gel containing ethosomes entrapped with tretinoin. J. Drug Deliv. Ther. 2018;8:315–321. doi: 10.22270/jddt.v8i5-s.1982. [DOI] [Google Scholar]
- 169.Pando D., Matos M., Gutierrez G., Pazos C. Formulation of resveratrol entrapped niosomes for topical use. Colloids Surf. B Biointerfaces. 2015;128:398–404. doi: 10.1016/j.colsurfb.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 170.Moghddam S.R.M., Ahad A., Aqil M., Imam S.S., Sultana Y. Formulation and optimization of niosomes for topical diacerein delivery using 3-factor, 3-level Box-Behnken design for the management of psoriasis. Mater. Sci. Eng. C. 2016;69:789–797. doi: 10.1016/j.msec.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 171.Meng S., Sun L., Wang L., Lin Z., Liu Z., Xi L., Wang Z., Zheng Y. Loading of water-insoluble celastrol into niosome hydrogels for improved topical permeation and anti-psoriasis activity. Colloids Surf. B Biointerfaces. 2019;182:110352. doi: 10.1016/j.colsurfb.2019.110352. [DOI] [PubMed] [Google Scholar]
- 172.Zhai J., Tan F.H., Luwor R.B., Srinivasa Reddy T., Ahmed N., Drummond C.J., Tran N. In Vitro and in Vivo Toxicity and Biodistribution of Paclitaxel-Loaded Cubosomes as a Drug Delivery Nanocarrier: A Case Study Using an A431 Skin Cancer Xenograft Model. ACS Appl. Biol. Mater. 2020;3:4198–4207. doi: 10.1021/acsabm.0c00269. [DOI] [PubMed] [Google Scholar]
- 173.Khan S., Jain P., Jain S., Jain R., Bhargava S., Jain A. Topical Delivery of Erythromycin through Cubosomes for Acne. Pharm. Nanotechnol. 2018;6:38–47. doi: 10.2174/2211738506666180209100222. [DOI] [PubMed] [Google Scholar]
- 174.Rapalli V.K., Banerjee S., Khan S., Jha P.N., Gupta G., Dua K., Hasnain M.S., Nayak A.K., Dubey S.K., Singhvi G. QbD-driven formulation development and evaluation of topical hydrogel containing ketoconazole loaded cubosomes. Mater. Sci. Eng. C. 2021;119:111548. doi: 10.1016/j.msec.2020.111548. [DOI] [PubMed] [Google Scholar]
- 175.Kumar P., Singh S.K., Handa V., Kathuria H. Oleic Acid Nanovesicles of Minoxidil for Enhanced Follicular Delivery. Medicines. 2018;5:103. doi: 10.3390/medicines5030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Silva A.C., Amaral M.H., Lobo J.M., Lopes C.M. Lipid nanoparticles for the delivery of biopharmaceuticals. Curr. Pharm. Biotechnol. 2015;16:291–302. doi: 10.2174/1389201015666141229103709. [DOI] [PubMed] [Google Scholar]
- 177.Trombino S., Mellace S., Cassano R. Solid lipid nanoparticles for antifungal drugs delivery for topical applications. Ther. Deliv. 2016;7:639–647. doi: 10.4155/tde-2016-0040. [DOI] [PubMed] [Google Scholar]
- 178.Xing H., Wang H., Wu B., Zhang X. Lipid nanoparticles for the delivery of active natural medicines. Curr. Pharm. Des. 2017;23:6705–6713. doi: 10.2174/1381612824666171128105853. [DOI] [PubMed] [Google Scholar]
- 179.De Souza M.L., Dos Santos W.M., de Sousa A.L.M.D., de Albuquerque Wanderley Sales V., Nóbrega F.P., de Oliveira M.V.G., Rolim-Neto P.J. Lipid Nanoparticles as a Skin Wound Healing Drug Delivery System: Discoveries and Advances. Curr. Pharm. Des. 2020;26:4536–4550. doi: 10.2174/1381612826666200417144530. [DOI] [PubMed] [Google Scholar]
- 180.Naseri N., Valizadeh H., Zakeri-Milani P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015;5:305–313. doi: 10.15171/apb.2015.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gratieri T., Krawczyk-Santos A.P., da Rocha P.B., Gelfuso G.M., Marreto R.N., Taveira S.F. SLN- and NLC-Encapsulating Antifungal Agents: Skin Drug Delivery and their Unexplored Potential for Treating Onychomycosis. Curr. Pharm. Des. 2017;23:6684–6695. doi: 10.2174/1381612823666171115112745. [DOI] [PubMed] [Google Scholar]
- 182.Pham D.T.T., Tran P.H.L., Tran T.T.D. Development of solid dispersion lipid nanoparticles for improving skin delivery. Saudi Pharm. J. 2019;27:1019–1024. doi: 10.1016/j.jsps.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Waghule T., Rapalli V.K., Gorantla S., Saha R.N., Dubey S.K., Puri A., Singhvi G. Nanostructured Lipid Carriers as Potential Drug Delivery Systems for Skin Disorders. Curr. Pharm. Des. 2020;26:4569–4579. doi: 10.2174/1381612826666200614175236. [DOI] [PubMed] [Google Scholar]
- 184.Czajkowska-Kośnik A., Szekalska M., Winnicka K. Nanostructured lipid carriers: A potential use for skin drug delivery systems. Pharmacol. Rep. 2019;71:156–166. doi: 10.1016/j.pharep.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 185.Gordillo-Galeano A., Mora-Huertas C.E. Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release. Eur. J. Pharm. Biopharm. 2018;133:285–308. doi: 10.1016/j.ejpb.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 186.Rajpoot K. Solid Lipid Nanoparticles: A Promising Nanomaterial in Drug Delivery. Curr. Pharm. Des. 2019;25:3943–3959. doi: 10.2174/1381612825666190903155321. [DOI] [PubMed] [Google Scholar]
- 187.Ganesan P., Narayanasamy D. Lipid nanoparticles: Different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain. Chem. Pharm. 2017;6:37–56. doi: 10.1016/j.scp.2017.07.002. [DOI] [Google Scholar]
- 188.Trivino A., Gumireddy A., Chauhan H. Drug-Lipid-Surfactant Miscibility for the Development of Solid Lipid Nanoparticles. AAPS Pharm. Sci. Tech. 2019;20:46. doi: 10.1208/s12249-018-1229-3. [DOI] [PubMed] [Google Scholar]
- 189.Soleimanian Y., Goli S.A.H., Varshosaz J., Sahafi S.M. Formulation and characterization of novel nanostructured lipid carriers made from beeswax, propolis wax and pomegranate seed oil. Food Chem. 2018;244:83–92. doi: 10.1016/j.foodchem.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 190.Sebaaly C., Charcosset C., Stainmesse S., Fessi H., Greige-Gerges H. Clove essential oil-in-cyclodextrin-in-liposomes in the aqueous and lyophilized states: From laboratory to large scale using a membrane contactor. Carbohydr. Polym. 2016;138:75–85. doi: 10.1016/j.carbpol.2015.11.053. [DOI] [PubMed] [Google Scholar]
- 191.Shah M.K., Khatri P., Vora N., Patel N.K., Jain S., Lin S. Lipid nanocarriers: Preparation, characterization and absorption mechanism and applications to improve oral bioavailability of poorly water-soluble drugs. In: Grumezescu A.M., editor. Biomedical Applications of Nanoparticles. William Andrew Publishing; Norwich, NY, USA: 2019. pp. 117–147. [Google Scholar]
- 192.Duong V.-A., Nguyen T.-T.-L., Maeng H.-J. Preparation of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Drug Delivery and the Effects of Preparation Parameters of Solvent Injection Method. Molecules. 2020;25:4781. doi: 10.3390/molecules25204781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chaudhary S.A., Patel D.M., Patel J.K., Patel D.H. Solvent Emulsification Evaporation and Solvent Emulsification Diffusion Techniques for Nanoparticles. In: Patel J.K., Pathak Y.V., editors. Emerging Technologies for Nanoparticle Manufacturing. Springer; Cham, Switzerland: 2021. pp. 287–300. [Google Scholar]
- 194.Singh D., Tiwary A.K., Bedi N. Self-microemulsifying Drug Delivery System for Problematic Molecules: An Update. Recent Pat. Nanotech. 2019;13:92–113. doi: 10.2174/1872210513666190619102521. [DOI] [PubMed] [Google Scholar]
- 195.Desai P., Patlolla R.R., Singh M. Interaction of nanoparticles and cell-penetrating peptides with skin for transdermal drug delivery. Mol. Membr. Biol. 2010;27:247–259. doi: 10.3109/09687688.2010.522203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jensen L.B., Petersson K., Nielsen H.M. In vitro penetration properties of solid lipid nanoparticles in intact and barrier-impaired skin. Eur. J. Pharm. Biopharm. 2011;79:68–75. doi: 10.1016/j.ejpb.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 197.Okonogi S., Riangjanapatee P. Physicochemical characterization of lycopene-loaded nanostructured lipid carrier formula-tions for topical administration. Int. J. Pharm. 2015;478:726–735. doi: 10.1016/j.ijpharm.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 198.Shrotriya S.N., Ranpise N.S., Vidhate B.V. Skin targeting of resveratrol utilizing solid lipid nanoparticle-engrossed gel for chemically induced irritant contact dermatitis. Drug Deliv. Transl. Res. 2017;7:37–52. doi: 10.1007/s13346-016-0350-7. [DOI] [PubMed] [Google Scholar]
- 199.Montenegro L., Messina C.M., Manuguerra S., Santagati L.M., Pasquinucci L., Turnaturi R., Parenti C., Arena R., Santulli A. In Vitro Antioxidant Activity and In Vivo Topical Efficacy of Lipid Nanoparticles Co-Loading Idebenone and Tocopheryl Acetate. Appl. Sci. 2019;9:845. doi: 10.3390/app9050845. [DOI] [Google Scholar]
- 200.Pivetta T.P., Simões S., Araújo M.M., Carvalho T., Arruda C., Marcato P.D. Development of nanoparticles from natural lipids for topical delivery of thymol: Investigation of its anti-inflammatory properties. Colloids Surf. B Biointerfaces. 2018;164:281–290. doi: 10.1016/j.colsurfb.2018.01.053. [DOI] [PubMed] [Google Scholar]
- 201.Gad H.A., El-Rahman F.A.A., Hamdy G.M. Chamomile oil loaded solid lipid nanoparticles: A naturally formulated remedy to enhance the wound healing. J. Drug Deliv. Sci. Technol. 2019;50:329–338. doi: 10.1016/j.jddst.2019.01.008. [DOI] [Google Scholar]
- 202.Butani D., Yewale C., Misra A. Topical Amphotericin B solid lipid nanoparticles: Design and development. Colloids Surf. B Biointerfaces. 2016;139:17–24. doi: 10.1016/j.colsurfb.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 203.Carbone C., do Céu Teixeira M., do Céu Sousa M., Martins-Gomes C., Silva A.M., Souto E.M.B., Musumeci T. Clotrima- zole-Loaded Mediterranean Essential Oils NLC: A Synergic Treatment of Candida Skin Infections. Pharmaceutics. 2019;11:231. doi: 10.3390/pharmaceutics11050231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ghate V.M., Lewis S.A., Prabhu P., Dubey A., Patel N. Nanostructured lipid carriers for the topical delivery of tretinoin. Eur. J. Pharm. Biopharm. 2016;108:253–261. doi: 10.1016/j.ejpb.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 205.Malik D.S., Kaur G. Exploring therapeutic potential of azelaic acid loaded NLCs for the treatment of acne vulgaris. J. Drug Deliv. Sci. Technol. 2020;55:101418. doi: 10.1016/j.jddst.2019.101418. [DOI] [Google Scholar]
- 206.Tupal A., Sabzichi M., Ramezani F., Kouhsoltani M., Hamishehkar H. Dermal delivery of doxorubicin-loaded solid lipid nanoparticles for the treatment of skin cancer. J. Microencapsul. 2016;33:372–380. doi: 10.1080/02652048.2016.1200150. [DOI] [PubMed] [Google Scholar]
- 207.Harde H., Agrawal A.K., Katariya M., Kale D., Jain S. Development of a topical adapalene-solid lipid nanoparticle load-ed gel with enhanced efficacy and improved skin tolerability. RSC Adv. 2015;5:43917–43929. doi: 10.1039/C5RA06047H. [DOI] [Google Scholar]
- 208.Pradhan M., Singh D., Singh M.R. Influence of selected variables on fabrication of Triamcinolone acetonide loaded solid lipid nanoparticles for topical treatment of dermal disorders. Artif. Cells Nanomed. Biotechnol. 2016;44:392–400. doi: 10.3109/21691401.2014.955105. [DOI] [PubMed] [Google Scholar]
- 209.Wang W., Chen L., Huang X., Shao A. Preparation and Characterization of Minoxidil Loaded Nanostructured Lipid Car-riers. AAPS Pharm. Sci. Tech. 2017;18:509–516. doi: 10.1208/s12249-016-0519-x. [DOI] [PubMed] [Google Scholar]
- 210.Shrotriya S.N., Vidhate B.V., Shukla M.S. Formulation and development of Silybin loaded solid lipid nanoparticle enriched gel for irritant contact dermatitis. J. Drug Deliv. Sci. Technol. 2017;41:164–173. doi: 10.1016/j.jddst.2017.07.006. [DOI] [Google Scholar]
- 211.El-Housiny S., Shams Eldeen M.A., El-Attar Y.A., Salem H.A., Attia D., Bendas E.R., El-Nabarawi M.A. Flucona-zole-loaded solid lipid nanoparticles topical gel for treatment of pityriasis versicolor: Formulation and clinical study. Drug Deliv. 2018;25:78–90. doi: 10.1080/10717544.2017.1413444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Aland R., Ganesan M., Rajeswara R.P. Development and optimization of tazarotene loaded solid lipid nanoparticles for topical delivery. Asian J. Pharm. Clin. Res. 2019;12:63–77. doi: 10.22159/ajpcr.2019.v12i10.31755. [DOI] [Google Scholar]
- 213.Al-Maghrabi P.M., Khafagy E.-S., Ghorab M.M., Gad S. Influence of formulation variables on miconazole nitrate-loaded lipid based nanocarrier for topical delivery. Colloids Surf. B Biointerfaces. 2020;193:111046. doi: 10.1016/j.colsurfb.2020.111046. [DOI] [PubMed] [Google Scholar]
- 214.Bhalekar M., Upadhaya P., Madgulkar A. Formulation and evaluation of Adapalene-loaded nanoparticulates for epidermal localization. Drug Deliv. Transl. Res. 2015;5:585–595. doi: 10.1007/s13346-015-0261-z. [DOI] [PubMed] [Google Scholar]
- 215.Gupta S., Wairkar S., Bhatt L.K. Isotretinoin and α-tocopherol acetate-loaded solid lipid nanoparticle topical gel for the treatment of acne. J. Microencapsul. 2020;37:1–9. doi: 10.1080/02652048.2020.1823499. [DOI] [PubMed] [Google Scholar]
- 216.Kelidari H.R., Saeedi M., Hajheydari Z., Akbari J., Morteza-Semnani K., Akhtari J., Valizadeh H., Asare-Addo K., Nokhodchi A. Spironolactone loaded nanostructured lipid carrier gel for effective treatment of mild and moderate ac-ne vulgaris: A randomized, double-blind, prospective trial. Colloids Surf. B Biointerfaces. 2016;146:47–53. doi: 10.1016/j.colsurfb.2016.05.042. [DOI] [PubMed] [Google Scholar]
- 217.Nagaich U., Gulati N. Nanostructured lipid carriers (NLC) based controlled release topical gel of clobetasol propionate: Design and in vivo characterization. Drug Deliv. Transl. Res. 2016;6:289–298. doi: 10.1007/s13346-016-0291-1. [DOI] [PubMed] [Google Scholar]
- 218.Viegas J.S.R., Praça F.G., Caron A.L., Suzuki I., Silvestrini A.V.P., Medina W.S.G., Del Ciampo J.O., Kravicz M., Bentley M.V.L.B. Nanostructured lipid carrier co-delivering tacrolimus and TNF-α siRNA as an innovate approach to psoriasis. Drug Deliv. Transl. Res. 2020;10:646–660. doi: 10.1007/s13346-020-00723-6. [DOI] [PubMed] [Google Scholar]
- 219.Passos J.S., De Martino L.C., Dartora V.F.C., De Araujo G.L.B., Ishida K., Lopes L.B. Development, skin targeting and antifungal efficacy of topical lipid nanoparticles containing itraconazole. Eur. J. Pharm. Sci. 2020;149:105296. doi: 10.1016/j.ejps.2020.105296. [DOI] [PubMed] [Google Scholar]
- 220.Madan J.R., Khobaragade S., Dua K., Awasthi R. Formulation, optimization, and in vitro evaluation of nanostructured lipid carriers for topical delivery of Apremilast. Dermatol. Ther. 2020;33:e13370. doi: 10.1111/dth.13370. [DOI] [PubMed] [Google Scholar]
- 221.Sathe P., Saka R., Kommineni N., Raza K., Khan W. Dithranol-loaded nanostructured lipid carrier-based gel ameliorate psoriasis in imiquimod-induced mice psoriatic plaque model. Drug Dev. Ind. Pharm. 2019;45:826–838. doi: 10.1080/03639045.2019.1576722. [DOI] [PubMed] [Google Scholar]
- 222.Waghule T., Rapalli V.K., Singhvi G., Manchanda P., Hans N., Dubey S.K., Hasnain M.S., Nayak A.K. Voriconazole loaded nanostructured lipid carriers based topical delivery system: QbD based designing, characterization, in-vitro and ex-vivo evaluation. J. Drug Deliv. Sci. Technol. 2019;52:303–315. doi: 10.1016/j.jddst.2019.04.026. [DOI] [Google Scholar]
- 223.Kaur N., Sharma K., Bedi N. Topical Nanostructured Lipid Carrier Based Hydrogel of Mometasone Furoate for the Treatment of Psoriasis. Pharm. Nanotechnol. 2018;6:133–143. doi: 10.2174/2211738506666180523112513. [DOI] [PubMed] [Google Scholar]
- 224.Lewies A., Wentzel J.F., Jordaan A., Bezuidenhout C., Du Plessis L.H. Interactions of the antimicrobial peptide nisin Z with conventional antibiotics and the use of nanostructured lipid carriers to enhance antimicrobial activity. Int. J. Pharm. 2017;526:244–253. doi: 10.1016/j.ijpharm.2017.04.071. [DOI] [PubMed] [Google Scholar]
- 225.Jain A., Garg N.K., Jain A., Kesharwani P., Jain A.K., Nirbhavane P., Tyagi R.K. A synergistic approach of adapalene-loaded nanostructured lipid carriers, and vitamin C co-administration for treating acne. Drug Dev. Ind. Pharm. 2016;42:897–905. doi: 10.3109/03639045.2015.1104343. [DOI] [PubMed] [Google Scholar]
- 226.Baroli B. Penetration of nanoparticles and nanomaterials in the skin: Fiction or reality? J. Pharm. Sci. 2009;99:21–50. doi: 10.1002/jps.21817. [DOI] [PubMed] [Google Scholar]
- 227.Basavaraj K.H. Nanotechnology in medicine and relevance to dermatology: Present concepts. Indian J. Dermatol. 2012;57:169–174. doi: 10.4103/0019-5154.96186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Tadros T., Izquierdo P., Esquena J., Solans C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004;108–109:303–318. doi: 10.1016/j.cis.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 229.Lee V.H.L. Nanotechnology: Challenging the limit of creativity in targeted drug delivery. Adv. Drug Deliv. Rev. 2004;56:1527–1528. doi: 10.1016/j.addr.2004.07.003. [DOI] [Google Scholar]
- 230.Heuschkel S., Goebel A., Neubert R.H.H. Microemulsions-modern colloidal carrier for dermal and transdermal drug delivery. J. Pharm. Sci. 2007;97:603–631. doi: 10.1002/jps.20995. [DOI] [PubMed] [Google Scholar]
- 231.Patel M.R., Patel R.B., Parikh J.R., Solanki A.B., Patel B.G. Investigating effect of microemulsion components: In vitro permeation of ketoconazole. Pharm. Dev. Technol. 2011;16:250–258. doi: 10.3109/10837451003610845. [DOI] [PubMed] [Google Scholar]
- 232.Kogan A., Garti N. Microemulsions as transdermal drug delivery vehicles. Adv. Colloid Interface Sci. 2006;123–126:369–385. doi: 10.1016/j.cis.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 233.Anton N., Benoit J.-P., Saulnier P. Design and production of nanoparticles formulated from nanoemulsion templates—A review. J. Control Release. 2008;128:185–199. doi: 10.1016/j.jconrel.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 234.McClements D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter. 2012;8:1719–1729. doi: 10.1039/C2SM06903B. [DOI] [Google Scholar]
- 235.Badnjevic A. CMBEBIH 2017: Proceedings of the International Conference on Medical and Biological Engineering 2017. Springer; New York, NY, USA: 2017. p. 825. [Google Scholar]
- 236.Kaur A., Katiyar S.S., Kushwah V., Jain S. Nanoemulsion loaded gel for topical co-delivery of clobitasol propionate and calcipotriol in psoriasis. Nanomedicine. 2017;13:1473–1482. doi: 10.1016/j.nano.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 237.Rajitha P., Shammika P., Aiswarya S., Gopikrishnan A., Jayakumar R., Sabitha M. Chaulmoogra oil based methotrexate loaded topical nanoemulsion for the treatment of psoriasis. J. Drug Deliv. Sci. Technol. 2019;49:463–476. doi: 10.1016/j.jddst.2018.12.020. [DOI] [Google Scholar]
- 238.Coneac G., Vlaia V., Olariu I., Mut A.M., Anghel D.F., Ilie C., Popoiu C., Lupuleasa D., Vlaia L. Development and Evaluation of New Microemulsion-Based Hydrogel Formulations for Topical Delivery of Fluconazole. AAPS PharmSciTech. 2015;16:889–904. doi: 10.1208/s12249-014-0275-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Alvarado H.L., Abrego G., Souto E.B., Garduno-Ramirez M.L., Clares B., Garcia M.L., Calpena A.C. Nanoemulsions for dermal controlled release of oleanolic and ursolic acids: In vitro, ex vivo and in vivo characterization. Colloids Surf. B Biointerfaces. 2015;130:40–47. doi: 10.1016/j.colsurfb.2015.03.062. [DOI] [PubMed] [Google Scholar]
- 240.Goindi S., Kaur R., Kaur R. An ionic liquid-in-water microemulsion as a potential carrier for topical delivery of poorly water soluble drug: Development, ex-vivo and in-vivo evaluation. Int. J. Pharm. 2015;495:913–923. doi: 10.1016/j.ijpharm.2015.09.066. [DOI] [PubMed] [Google Scholar]
- 241.Lv X., Liu T., Ma H., Tian Y., Li L., Li Z., Gao M., Zhang J., Tang Z. Preparation of Essential Oil-Based Microemulsions for Improving the Solubility, pH Stability, Photostability, and Skin Permeation of Quercetin. AAPS PharmSciTech. 2017;18:3097–3104. doi: 10.1208/s12249-017-0798-x. [DOI] [PubMed] [Google Scholar]
- 242.Negi P., Singh B., Sharma G., Beg S., Katare O.P. Biocompatible lidocaine and prilocaine loaded-nanoemulsion system for enhanced percutaneous absorption: QbD-based optimisation, dermatokinetics and in vivo evaluation. J. Microencapsul. 2015;32:419–431. doi: 10.3109/02652048.2015.1046513. [DOI] [PubMed] [Google Scholar]
- 243.Pham J., Nayel A., Hoang C., Elbayoumi T. Enhanced effectiveness of tocotrienol-based nano-emulsified system for topical delivery against skin carcinomas. Drug Deliv. 2016;23:1514–1524. doi: 10.3109/10717544.2014.966925. [DOI] [PubMed] [Google Scholar]
- 244.Nasr M., Abdel-Hamid S. Optimizing the dermal accumulation of a tazarotene microemulsion using skin deposition modeling. Drug Dev. Ind. Pharm. 2016;42:636–643. doi: 10.3109/03639045.2015.1062512. [DOI] [PubMed] [Google Scholar]
- 245.Amarji B., Garg N.K., Singh B., Katare O.P. Microemulsions mediated effective delivery of methotrexate hydrogel: More than a tour de force in psoriasis therapeutics. J. Drug Target. 2016;24:147–160. doi: 10.3109/1061186X.2015.1058804. [DOI] [PubMed] [Google Scholar]
- 246.Nasr M., Abdel-Hamid S., Moftah N.H., Fadel M., Alyoussef A.A. Jojoba Oil Soft Colloidal Nanocarrier of a Synthetic Retinoid: Preparation, Characterization and Clinical Efficacy in Psoriatic Patients. Curr. Drug Deliv. 2017;14:426–432. doi: 10.2174/1567201813666160513132321. [DOI] [PubMed] [Google Scholar]
- 247.Kumari B., Kesavan K. Effect of chitosan coating on microemulsion for effective dermal clotrimazole delivery. Pharm. Dev. Technol. 2017;22:617–626. doi: 10.1080/10837450.2016.1230629. [DOI] [PubMed] [Google Scholar]
- 248.Moghimipour E., Salimi A., Changizi S. Preparation and microstructural characterization of Griseofulvin microemulsions using different experimental methods: SAXS and DSC. Adv. Pharm. Bull. 2017;7:281–289. doi: 10.15171/apb.2017.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Omar A.M., Ammar N.M., Hussein R.A., Mostafa D.M., Basha M., Abdel Hamid M.F. Boswellia carterii Birdwood topical microemulsion for the treatment of inflammatory dermatological conditions; a prospective study. Trop. J. Nat. Prod. Res. 2020;4:372–377. [Google Scholar]
- 250.Khiljee S., Ur Rehman N., Khiljee T., Loebenberg R., Ahmad R.S. Formulation and clinical evaluation of topical dosage forms of Indian Penny Wort, walnut and turmeric in eczema. Pak. J. Pharm. Sci. 2015;28:2001–2007. [PubMed] [Google Scholar]
- 251.Jagdale S., Chaudhari B. Optimization of microemulsion based transdermal gel of triamcinolone. Recent Pat. Anti-Infect. Drug Discov. 2017;12:61–78. doi: 10.2174/1574891X12666170426092911. [DOI] [PubMed] [Google Scholar]
- 252.Algahtani M.S., Ahmad M.Z., Ahmad J. Nanoemulgel for improved topical delivery of retinyl palmitate: Formulation design and stability evaluation. Nanomaterials. 2020;10:848. doi: 10.3390/nano10050848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Yang M., Gu Y., Yang D., Tang X., Liu J. Development of triptolide-nanoemulsion gels for percutaneous administration: Physicochemical, transport, pharmacokinetic and pharmacodynamic characteristics. J. Nano Biotechnol. 2017;15:88. doi: 10.1186/s12951-017-0323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Das S., Lee S.H., Chia V.D., Chow P.S., MacBeath C., Liu Y., Shlieout G. Development of microemulsion based topical ivermectin formulations: Pre-formulation and formulation studies. Colloids Surf. B Biointerfaces. 2020;189:110823. doi: 10.1016/j.colsurfb.2020.110823. [DOI] [PubMed] [Google Scholar]
- 255.Pandey S.S., Maulvi F.A., Patel P.S., Shukla M.R., Shah K.M., Gupta A.R., Joshi S.V., Shah D.O. Cyclosporine laden tailored microemulsion-gel depot for effective treatment of psoriasis: In vitro and in vivo studies. Colloids Surf. B Biointerfaces. 2020;186:110681. doi: 10.1016/j.colsurfb.2019.110681. [DOI] [PubMed] [Google Scholar]
- 256.Argenta D.F., Bidone J., Koester L.S., Bassani V.L., Simoes C.M.O., Teixeira H.F. Topical Delivery of Coumestrol from Lipid Nanoemulsions Thickened with Hydroxyethylcellulose for Antiherpes Treatment. AAPS PharmSciTech. 2018;19:192–200. doi: 10.1208/s12249-017-0828-8. [DOI] [PubMed] [Google Scholar]
- 257.Barradas T.N., Senna J.P., Cardoso S.A., de Holanda e Silva K.G., Elias Mansur C.R. Formulation characterization and in vitro drug release of hydrogel-thickened nanoemulsions for topical delivery of 8-methoxypsoralen. Mater Sci. Eng. C. 2018;92:245–253. doi: 10.1016/j.msec.2018.06.049. [DOI] [PubMed] [Google Scholar]
- 258.Kaci M., Belhaffef A., Meziane S., Dostert G., Menu P., Velot E., Desobry S., Arab-Tehrany E. Nanoemulsions and topical creams for the safe and effective delivery of lipophilic antioxidant coenzyme Q10. Colloids Surf. B Biointerfaces. 2018;167:165–175. doi: 10.1016/j.colsurfb.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 259.Barradas T.N., Senna J.P., Cardoso S.A., Nicoli S., Padula C., Santi P., Rossi F., de Holanda e Silva K.G., Mansur C.R.E. Hydrogel-thickened nanoemulsions based on essential oils for topical delivery of psoralen: Permeation and stability studies. Eur. J. Pharm. Sci. 2017;116:38–50. doi: 10.1016/j.ejpb.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 260.Miastkowska M., Sikora E., Ogonowski J., Zielina M., Ludzik A. The kinetic study of isotretinoin release from nanoemulsion. Colloids Surf. A Physicochem. Eng. Asp. 2016;510:63–68. doi: 10.1016/j.colsurfa.2016.07.060. [DOI] [Google Scholar]
- 261.Hussain A., Samad A., Singh S.K., Ahsan M.N., Haque M.W., Faruk A., Ahmed F.J. Nanoemulsion gel-based topical delivery of an antifungal drug: In vitro activity and in vivo evaluation. Drug Deliv. 2016;23:642–657. doi: 10.3109/10717544.2014.933284. [DOI] [PubMed] [Google Scholar]
- 262.Betzler de Oliveira de Siqueira L., da Silva Cardoso V., Rodrigues I.A., Vazquez-Villa A.L., Pereira dos Santos E., da Costa Leal Ribeiro Guimaraes B., dos Santos Cerqueira Coutinho C., Vermelho A.B., Ricci E., Jr. Development and evaluation of zinc phthalocyanine nanoemulsions for use in photodynamic therapy for Leishmania spp. Nanotechnology. 2017;28:65101. doi: 10.1088/1361-6528/28/6/065101. [DOI] [PubMed] [Google Scholar]
- 263.Global Topical Drug Delivery Industry. GLOBE NEWSWIRE; New York, NY, USA: 2021. [(accessed on 19 October 2021)]. Topical Drug Delivery Market Research Report by Product (Liquid Formulations, Semi-Solid Formulations, and Solid Formulations), by Rout to Administration (Dermal Drug Delivery, Nasal Drug Delivery, and Ophthalmic Drug Delivery), by End-User, by Region (Americas, Asia-Pacific, and Europe, Middle East & Africa)—Global Forecast to 2026—Cumulative Impact of COVID-19. Available online: https://www.reportlinker.com/p06033143/?utm_source=GNW. [Google Scholar]
- 264.Proksch E., Berardesca E., Misery L., Engblom J., Bouwstra J. Dry skin management: Practical approach in light of latest research on skin structure and function. J. Dermatolog. Treat. 2020;31:716–722. doi: 10.1080/09546634.2019.1607024. [DOI] [PubMed] [Google Scholar]
- 265.Roberts M.S., Mohammed Y., Pastore M.N., Namjoshi S., Yousef S., Alinaghi A., Haridass I.N., Abd E., Leite-Silva V.R., Benson H.A.E., et al. Topical and cutaneous delivery using nanosystems. J. Control Release. 2017;247:86–105. doi: 10.1016/j.jconrel.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 266.Kaur L., Singh K., Paul S., Singh S., Singh S., Jain S.K. A Mechanistic Study to Determine the Structural Similarities between Artificial Membrane Strat-M™ and Biological Membranes and Its Application to Carry Out Skin Permeation Study of Amphotericin B Nanoformulations. AAPS PharmSciTech. 2018;19:1606–1624. doi: 10.1208/s12249-018-0959-6. [DOI] [PubMed] [Google Scholar]
- 267.Ahmad N., Ahmad R., Buheazaha T.M., AlHomoud H.S., Al-Nasif H.A., Sarafroz M. A comparative ex vivo permeation evaluation of a novel 5-Fluorocuracil nanoemulsion-gel by topically applied in the different excised rat, goat, and cow skin. Saudi J. Biol. Sci. 2020;27:1024–1040. doi: 10.1016/j.sjbs.2020.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Raguvaran R., Manuja B.K., Chopra M., Thakur R., Anand T., Kalia A., Manuja A. Sodium alginate and gum acacia hydrogels of ZnO nanoparticles show wound healing effect on fibroblast cells. Int. J. Biol. Macromol. 2017;96:185–191. doi: 10.1016/j.ijbiomac.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 269.Eiras F., Amaral M.H., Silva R., Martins E., Sousa Lobo J.M., Silva A.C. Characterization and biocompatibility evaluation of cutaneous formulations containing lipid nanoparticles. Int. J. Pharm. 2017;519:373–380. doi: 10.1016/j.ijpharm.2017.01.045. [DOI] [PubMed] [Google Scholar]
- 270.Arora R., Katiyar S.S., Kushwah V., Jain S. Solid lipid nanoparticles and nanostructured lipid carrier-based nanotherapeutics in treatment of psoriasis: A comparative study. Expert Opin. Drug Deliv. 2017;14:165–177. doi: 10.1080/17425247.2017.1264386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.