Abstract
Background. A number of recent randomized controlled trials reported the efficacy of brain–computer interface (BCI) for upper-limb stroke rehabilitation compared with other therapies. Despite the encouraging results reported, there is a significant variance in the reported outcomes. This paper aims to investigate the effectiveness of different BCI designs on poststroke upper-limb rehabilitation. Methods. The effect sizes of pooled and individual studies were assessed by computing Hedge’s g values with a 95% confidence interval. Subgroup analyses were also performed to examine the impact of different BCI designs on the treatment effect. Results. The study included 12 clinical trials involving 298 patients. The analysis showed that the BCI yielded significant superior short-term and long-term efficacy in improving the upper-limb motor function compared to the control therapies (Hedge’s g = 0.73 and 0.33, respectively). Based on our subgroup analyses, the BCI studies that used the intention of movement had a higher effect size compared to those used motor imagery (Hedge’s g = 1.21 and 0.55, respectively). The BCI studies using band power features had a significantly higher effect size than those using filter bank common spatial patterns features (Hedge’s g = 1.25 and − 0.23, respectively). Finally, the studies that used functional electrical stimulation as the BCI feedback had the highest effect size compared to other devices (Hedge’s g = 1.2). Conclusion. This meta-analysis confirmed the effectiveness of BCI for upper-limb rehabilitation. Our findings support the use of band power features, the intention of movement, and the functional electrical stimulation in future BCI designs for poststroke upper-limb rehabilitation.
Keywords: brain–computer interface, meta-analysis, mental tasks, randomized clinical trials, stroke rehabilitation
Introduction
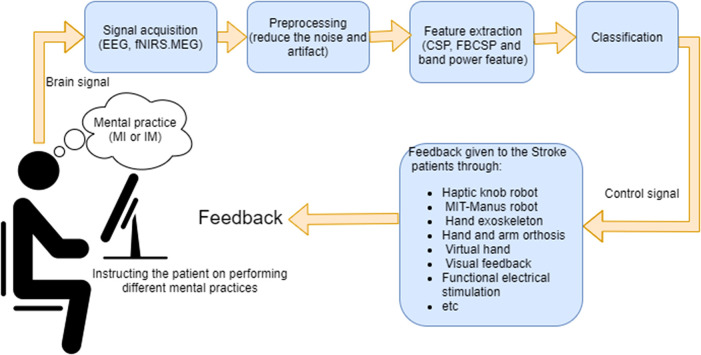
As a novel rehabilitation method, BCI has attracted a lot of attention. A BCI records, analyzes, and decodes brain signals and translates them into commands for communication and control.1,2 The BCI system for stroke rehabilitation usually consists of 6 stages, as shown in Figure 1:
Signal acquisition: A number of modalities for acquisition of brain signals are suitable for the BCI in stroke rehabilitation, namely electroencephalography (EEG), functional near-infrared spectroscopy (fNIRS), and magnetoencephalography (MEG). Due to its lower cost, higher temporal resolution, and portability, EEG is the most commonly used modality in BCI-based stroke rehabilitation. 3
Mental practice: In the motor imagery (MI)-based BCI studies, the patients are instructed to imagine moving the impaired hand without any physical movements, whereas in the intention of the movement (IM)-based BCI studies, the patients attempt to perform physical movement of the impaired hand if possible. The MI or IM produces brain waves, called movement-related cortical potentials (MRCPs) and event-related desynchronization/synchronization (ERD/ERS).4,5 MRCP and ERD/ERS are distinct movement-related brain patterns. MRCP is characterized as slow changes of the brain signals in the time domain. ERD and ERS are, respectively, described as a suppression and an enhancement in the power of the sensorimotor rhythms. For example, the power of beta rhythm (13-30 Hz) recorded over the sensorimotor regions has been shown to decrease before the motor task, reaches its minimum during the movement execution (ERD), and then recovers sharply after the end of the motor task (ERS). 6 In the application of upper-limb stroke rehabilitation, the BCI is used to detect either MRCP or ERD\ERS in brain signals when the patient performs the relevant mental practice.
Preprocessing: The recorded brain signals can be contaminated with artifacts caused by blinking, muscle activity, and other sources of noise. In the preprocessing stage, different spectral, temporal, and spatial algorithms are applied on the measured brain signals to reduce these artifacts. Among different preprocessing algorithms, the threshold-based artifact rejection, and 8 to 30 Hz band-pass filtering have been widely used in many BCI-based stroke rehabilitation studies. 7
Feature extraction: In this step, to detect movement-related brain patterns, a set of informative, nonredundant, and distinctive characteristics, named features are extracted from the preprocessed brain signals. The previous BCI-based stroke rehabilitation studies have often used one of the following 3 types of features, namely, common spatial patterns (CSP) features, 8 filter bank CSP (FBCSP) features,9,10 and band power features.11–13 CSP is a feature extraction algorithm that assigns different weights to different EEG channels, such that the weighted sum of the powers of brain signals is maximized for one class and minimized for the other class.14,15 In MI- and IM-based BCIs, the CSP features are the weighted sum of the powers of 8 to 30 Hz band-pass filtered brain signals, whereas FBCSP features are multiple CSP features extracted from a bank of brain signals filtered using different small band-pass filters. 16
Classification: The extracted features are fed to a classifier to detect whether or not the recorded brain signals prominently represent the movement-related brain patterns associated with the performed mental practice. If the movement-related brain patterns are detected, a control signal is sent to an external device to provide the feedback.
Feedback: The patient is presented with feedback indicating whether the classification algorithm accurately interpreted their motor intention/imagination. The commonly used type of BCI feedback in stroke rehabilitation is kinesthetic, whereby following the detection of the movement-related brain patterns, the impaired hand is moved along a predefined trajectory. For instance, Ang et al 9 and Biasiucci et al, 17 respectively, used an Massachusetts Institute of Technology (MIT)–Manus robot and functional electrical stimulation (FES) in order to facilitate the movement of the impaired hand as the BCI feedback. The MIT-Manus robot is a wearable robot that has been extensively studied for providing individualized rehabilitation after stroke. During the intervention, the patient is instructed to move the affected arm towards a target. If needed, the robot facilitates the movement of the affected arm by providing assistive forces based on the patient’s speed and the direction of the movement. Although rehabilitation with the MIT-Manus robot can be potentially effective,18,19 if the patient does not engage well in generating voluntary attempts, the movements of the affected arm turn out to be completely passive. This leads to a great decrease in the possible benefits of the therapy.
Figure 1.
Components of brain–computer interface commonly used for upper-limb stroke rehabilitation.
Abbreviations: CSP, common spatial patterns; EEG, electroencephalography; FBCSP, filter bank common spatial patterns; fNIRS, functional near-infrared spectroscopy; IM, intention of movement; MI, motor imagery; MEG, magnetoencephalography.
To enhance neuroplasticity in the poststroke upper-limb rehabilitation, the BCI links the movement-related brain patterns (generated during either MI or the IM of the affected arm) with feedback such as robotic-based movements, neuromuscular stimulation, virtual reality, etc. 20 In other words, the BCI is coupled with the existing therapies to enhance their efficacy by making the rehabilitation more active. 21
Recently, a number of randomized controlled trials (RCTs) have investigated the efficacy of the BCI for poststroke upper-limb rehabilitation, and compared the outcomes with those obtained from other existing therapies.11,13,17,22 Despite the encouraging results in many of these RCTs, there is a significant variance in their reported BCI outcomes.9,10,13,17 This issue might be due to the heterogeneity among their BCI study designs, 23 including differences in the performed mental practice, the extracted brain features, the type of feedback given to the patients, and the level of stroke chronicity in the participants. A meta-analysis conducted by Cervera et al 24 reported positive effects of the BCI on upper-limb stroke rehabilitation in a short term. Another meta-analysis conducted by Bai et al 25 considered the long-term efficacy of the BCI on upper-limb stroke rehabilitation. However, given the considerable heterogeneity in the motor function improvement among the BCI RCTs, there is a need for an extensive meta-analysis to assess the impact of different BCI designs on the treatment efficacy.
This study conducts a systematic review and meta-analysis of the short-term and long-term effects of BCI on upper-limb rehabilitation after stroke. Importantly, we also study the impact of different BCI design characteristics on the efficacy of the poststroke upper-limb rehabilitation. The findings of this meta-analysis aim to improve future clinical trials by providing evidence-based information about different designs of the BCI used for rehabilitation.
Method
This study was conducted in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) checklist for systematic review and meta-analysis. 26 PRISMA aims to help researchers effectively report the findings of systematic reviews and meta-analyses. 27 The PRISMA checklist contains 27 items, which should be reported to ensure the transparency and completeness of the report. The 27 items are divided into 7 categories, including title, abstract, introduction, methods, results, discussion, and funding.
We systematically searched PubMed, Physiotherapy Evidence-Based Database (PEDro), and Cochrane Library for the studies that published up until 25 April 2020. Supplemental Appendix 1 provides the detailed electronic search strategy that we used. The identified studies were included in this meta-analysis only if they met the following inclusion criteria:
The study is written in English.
The study design is a randomized controlled trial of upper-limb BCI rehabilitation, in which the 2 groups (ie, the experimental group and the control) are all stroke patients;
The study reported the results of the Fugl-Mayer assessment for upper extremity (FMA-UE) before and after the intervention.
We chose the FMA-UE, because it is the most commonly used outcome measure in the upper-limb BCI rehabilitation studies. 28 The FMA-UE is widely used to evaluate and measure the upper-limb motor function impairment in patients after the stroke. 29 The FMA-UE score mainly ranges from a minimum of 0 (hemiplegia) to a maximum of 66 (normal motor function). We excluded studies without a control group, studies with healthy subjects, studies with a feedback mechanism not combined with BCI, or studies without FMA-UE. Two reviewers independently evaluated the eligibility of the included articles, and disagreements were resolved through consensus during a meeting.
We extracted the following details from each included studies: surname of the first author, year of the publication, aim of the study, brain imaging modality, number of participants, phase of the stroke (ie, chronic or subacute), length and frequency of the interventions, outcome measures, type of performed mental practice during the BCI intervention (ie, MI or IM), BCI feature extraction method, type of BCI feedback, and length of follow-up assessments after the intervention. The corresponding investigators were contacted if the included studies lacked some details.
The PEDro scale is commonly used to measure the methodological quality of a clinical trial by considering 11 criteria (ie, eligibility criteria specified, random allocation, concealed allocation, baseline comparability, blinded subjects, blinded therapist, blinded assessor, adequate follow-up, intention to treat analysis, between-group statistical comparison for at least 1 key outcome, point and variability measures). 30 The PEDro score is a score ranging from 0 to 10, which represents the total number of criteria, excluding the first one that has been satisfied in the clinical trial. A clinical trial with a score from 6 to 10 is considered as high quality, 4 to 5 as fair quality, and ≤3 as poor quality. In this study, 2 reviewers independently applied the PEDro scale to assess the methodological quality of the included studies. In the case of disagreement, a third reviewer was consulted and an agreement was reached.
We conducted the meta-analysis using Comprehensive Meta-Analysis (CMA) version 3.0 software. 31 CMA is a tool to perform meta-analysis, create forest plots, calculate effect sizes, and much more. We calculated the effect sizes for the pooled and individual studies using Hedge’s equation with correction for small studies. 32 Due to considerable variations in characteristics of the included studies, random-effects models were used to estimate the pooled effect sizes and their 95% confidence intervals (CIs). 33 In addition, we performed subgroup analyses to investigate the impact of different BCI design characteristics (ie, performed mental practice, extracted BCI features, type of the given BCI feedback, and the stroke phase) on treatment efficacy.
We used the Higgins’ I2 statistic to assess heterogeneity across the included studies. 34 Generally, I2 > 50% could be considered as substantial heterogeneity. Finally, the probability of publication bias in our meta-analysis was assessed by plotting the funnel plot and applying Egger's regression test.35,36
Results
Literature Search and Characteristics of the Selected Studies
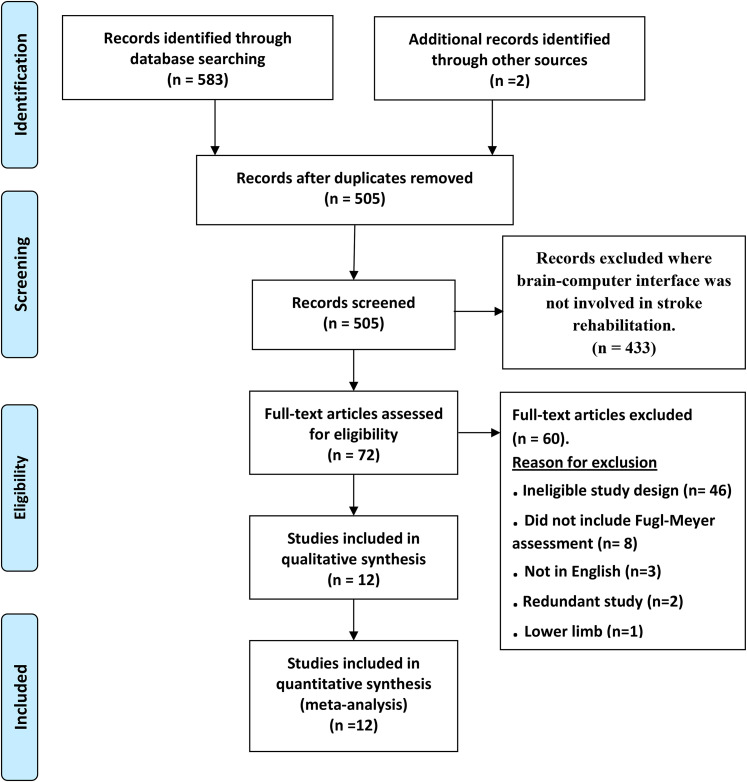
Figure 2 shows the flowchart of the search strategy and the selection steps taken in this review. We initially identified 585 articles, 12 of which met the inclusion criteria. The study by Ang et al 37 had 2 control groups, 1 control group used the standard arm therapy and the second control group used the haptic knob. Thus, we combined the 2 control groups into a single control group as recommended by the Cochrane handbook for systematic reviews of interventions. 38 Table 1 provides the main characteristics of the included studies.
Figure 2.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flowchart illustrating the process for the selection of the included studies in this meta-analysis.
Table 1.
Main Characteristics of the Included Studies.
| Study | EG/CG | BCI modality | Experimental intervention | Control intervention | Intervention period | Feature | Stroke phase | MI/IM | Outcome measures | Follow-up (weeks) |
|---|---|---|---|---|---|---|---|---|---|---|
| Ang et al 37 | 6/8 HK/7SAT | EEG | BCI-HK 1 h BCI + 30 min TAAM | HK:1 h HK + 30 min TAAM; SAT:1.5 h TAAM | 6 weeks, 18 sessions | FBCSP | Chronic | MI | FMA-UE | 24 |
| Ang et al 9 | 11/14 | EEG | BCI-MIT-Manus robot 1.5 h 136 repetitions | MIT-Manus robot 1.5 h 1040 repetitions | 4 weeks, 12 sessions | FBCSP | Chronic | MI | FMA-UE | 12 |
| Ang et al. 10 | 10/9 | EEG | 20 min tDCS + 1 h BCI- MIT-Manus robot | 20 min sham tDCS + 1 h sham BCI | 2 weeks, 10 sessions | FBCSP | Chronic | MI | FMA-UE | 4 |
| Biasiucci et al 17 | 14/13 | EEG | BCI-FES 1 h | Sham BCI-FES 1 h | 5 weeks, 10 sessions | Band power | Chronic | IM | FMA-UE, ESS, MRC, MAS | 36 |
| Cheng et al 42 | 5/5 | EEG | BCI-assisted soft robotic glove 90 min + 30 min SAT | Soft robotic glove 90 min + 30 min SAT | 6 weeks, 18 sessions | FBCSP | Chronic | MI | FMA-UE, ARAT | 24 |
| Frolov et al 39 | 36/11 | EEG | BCI-exoskeleton 30 min + SPT | Sham BCI 30 min | 2 weeks, 10 sessions | Band power | Chronic | MI | FMA-UE, ARAT | N/A |
| Kim et al 40 | 15/15 | EEG | BCI-FES 30 min + 30 min AOT | AOT 30 min | 4 weeks, 12 sessions BCI. 20 sessions AOT | Band power | Chronic | IM | FMA-UE, MAL, MBI | N/A |
| Li et al 8 | 7ne;7nc | EEG | BCI-FES 1–1.5 h + CON | FES 20 min + CON | 8 weeks, 24 sessions BCI/FES and 40 sessions CON | CSP | Subacute | MI | FMA-UE, ARAT | N/A |
| Mihara et al 12 | 10/10 | fNIRS | BCI-visual feedback 20 min + 120 min NDT | Sham BCI 20 min + 120 min NDT | 2 weeks, 6 sessions BCI/Sham, 14 sessions NDT | Band power | Subacute | MI | FMA-UE, ARAT, MAL | 2 |
| Pichiorri et al 41 | 14/14 | EEG | BCI-virtual hand 1 h | MI, 1 h | 4 weeks, 12 sessions | Band power | Subacute | MI | FMA-UE | N/A |
| Ramos-Murguialday et al 11 | 16/16 | EEG | BCI-orthosis 1 h + 1 h BPT | Sham BCI-orthosis 1 h + 1 h BPT | 4 weeks, 20 sessions | Band power | Chronic | IM | FMA-UE GAS, MAL | 26 |
| Wu et al 13 | 14/11 | EEG | BCI-exoskeleton 1 h + 1 h routine training | Routine training 2 h | 4 weeks, 20 sessions | Band power | Subacute | MI | FMA-UE ARAT, WMFT | N/A |
Abbreviations: AOT, action observational training; ARAT, action research arm test; BCI, brain–computer Interface; BPT, behavioral physical therapy; CG, control group; CON, conventional therapy; CSP, common spatial pattern; EG, experimental group; EES, European stroke scale score; GAS, goal attainment scale; FBCSP, filter bank common spatial pattern; fNIRS, functional near-infrared spectroscopy; FES, functional electrical stimulation; FMA-UE, Fugl-Meyer assessment upper extremity; IM, intention of movement; HK, haptic knob; MAL, motor activity long; MAS, modified Ashworth scale; MBI, modified Barthel index; MI, motor imagery; MRC, medical research council; N/A, not available; NDT, neurodevelopmental treatment; SAT, standard activity therapy; SPT, standard physical therapy; TAAM, therapist-assisted arm mobilization; tDCS, transcranial direct-current stimulation; WMFT, Wolf motor function test.
Supplemental Table S1 presents the PEDro scores for the 12 included studies. It can be seen that according to the PEDro scores, none of the selected studies are considered to have low methodological quality.
Supplemental Table S2 presents the mean and standard deviation of the changes in FMA-UE scores between the pre and postintervention in the selected studies, while the Supplemental Table S3 shows the mean and standard deviation of the changes in FMA-UE scores between the preintervention and the follow-up session.
Short-term and Long-term Efficacy of BCI
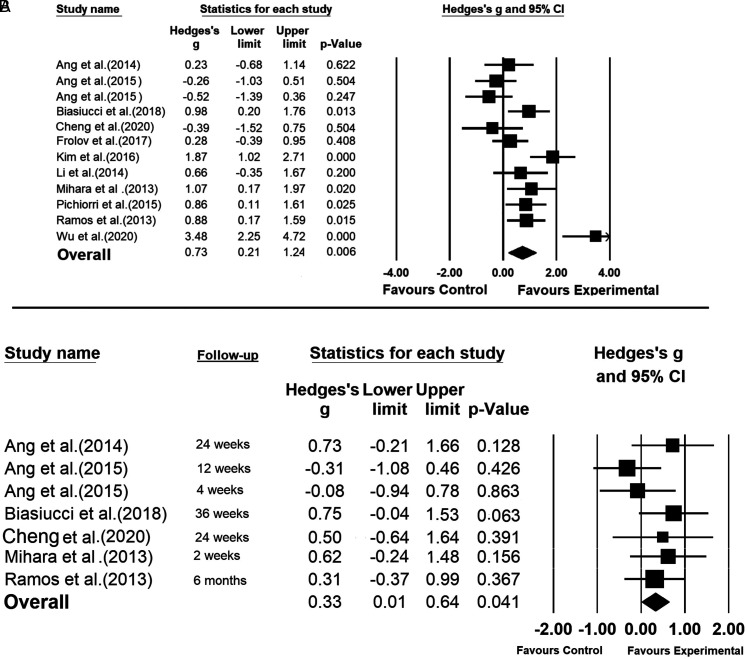
The pooled results showed that according to the short-term assessments immediately after finishing the intervention, the BCI is significantly more effective than the control interventions in post-stroke upper-limb rehabilitation (Hedge’s g = 0.73; P = .006) (Figure 3A). In 9 out of 12 studies, the BCI resulted in higher improvements in FMA-UE, compared to the control interventions (Ang et al, 37 Biasiucci et al, 17 Frolov et al, 39 Kim et al, 40 Li et al, 8 Mihara et al, 12 Pichiorri et al, 41 Ramos-Murguialday et al, 11 and Wu et al 13 ). The highest BCI intervention effect size was reported by Wu et al 13 (Hedge’s g = 3.48; P < .001). In 6 studies, namely Biasiucci et al, 17 Kim et al, 40 Mihara et al, 12 Pichiorri et al, 41 Ramos-Murguialday et al, 11 and Wu et al, 13 the effect size was significantly favoring BCI. There was substantial heterogeneity among the included studies (I2 = 77.12%; Q = 48.077; df = 11; P = .000).
Figure 3.
Evaluating effects of brain–computer interface, compared to control interventions, in improving upper-limb motor functions after stroke: (A) assessed immediately after finishing the intervention and (B) assessed in the follow-up session a number of weeks after finishing the intervention.
There is no evidence that the short-term effects of BCI are subject to publication bias. As shown in Supplemental Figure 1, the included studies have a relatively symmetric distribution across the overall effect size in the funnel plot. Moreover, the P value for Egger’s test is not significant (P = .3795).
The overall effect size, shown in Figure 3B, indicates the effectiveness of the BCI intervention in long term (Hedge’s g = 0.33; P = .041) with no heterogeneity among the included studies (I2 = 0.000%; Q = 5.839; df = 6; P = .442). Specifically, in 5 out of 7 studies, the FMA-UE changes between the follow-up session and the preintervention were in favor of the BCI group. According to the funnel plot shown in Supplemental Figure 2, and the Egger’s test (P = .541), there is no evidence of publication bias in the outcome of analyzing the long-term efficacy of the BCI.
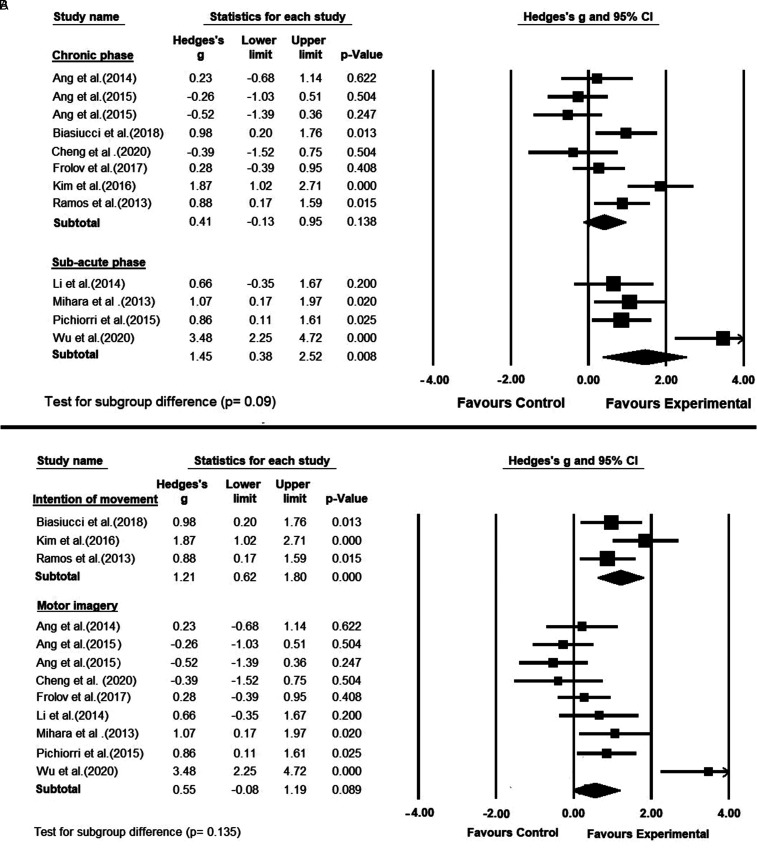
Chronic Versus Subacute
Eight studies recruited stroke patients in the chronic phase (>6 months from stroke onset)9–11,17,37,39,40,42 and the remaining 4 studies recruited stroke patients in the subacute phase (1-6 months from stroke onset).8,12,13,41 For both groups, the pooled effect size on motor recovery was in favor of BCI compared to the control interventions. However, the pooled effect size was higher for the patients in the subacute phase than those in the chronic group (Hedge’s g = 1.45; P = .008 vs Hedge’s g = 0.41; P = .138) (Figure 4A). The observed effect sizes tended to be significantly different between the 2 subgroups (P = .09). Furthermore, still a substantial heterogeneity was observed between the studies in the subacute phase (I2 = 80.17%; Q = 15.128; df = 3; P = .002) as well as in the chronic phase (I2 = 71.634%; Q = 24.577; df = 7; P = .001).
Figure 4.
(A) A subgroup meta-analysis comparing the efficacy of brain–computer interface in improving upper-limb motor functions, between 2 different phases of stroke. (B) A subgroup meta-analysis comparing the efficacy of brain–computer interfaces with different mental practices on poststroke upper-limb motor recovery; (ie, motor imagery vs intention of movement).
MI Versus IM
In the included studies, the performed BCI mental practices were different (Figure 4B). Nine studies instructed the BCI group to imagine the movement of the affected hand,8–10,12,13,37,39,41,42 whereas 3 studies asked the BCI group to attempt moving the affected hand.11,17,40 The effect size on motor function recovery was higher for the studies using the IM (Hedge’s g = 1.21; P < .001) compared with those using the MI (Hedge’s g = 0.55; P = .089). However, the difference between the 2 subgroups was not statistically significant (P = .135). The heterogeneity among the studies using the IM was moderate (I2 = 42.38%; Q = 3.471; df = 2; P = .176), whereas there was a substantial heterogeneity among the MI studies (I2 = 78.348%; Q = 37.01; df = 8; P = .000).
BCI Classification Features
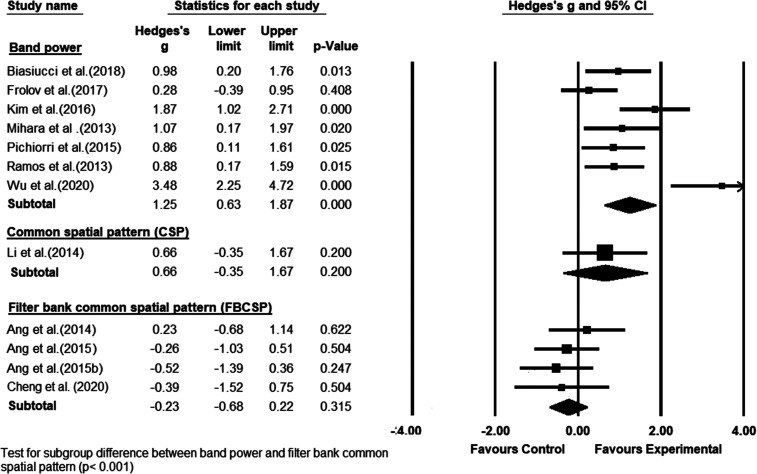
The included studies were also different in BCI features that they used. Seven studies used the band power features to detect movement-related brain patterns in BCI.11–13,17,39–41 The CSP features were used only in 1 study 8 and the FBCSP features were used in 4 studies.9,10,37,42 The group of studies that used band power features had the highest significant effect size on motor function recovery in favor of the BCI intervention (Hedge’s g = 1.25; P < .001) (Figure 5), with substantial heterogeneity among the studies (I2 = 75.208%; Q = 24.201; df = 6; P = .000). Conversely, the effect size on motor function recovery was in favor of the control group in the studies using the FBCSP features in the BCI (Hedge’s g = −0.23; P = .315) with no heterogeneity. The difference between the studies with the band power features and the studies with the FBCSP features was statistically significant (P<.001).
Figure 5.
A subgroup meta-analysis comparing the efficacy of brain–computer interface, grouped based on different classification features, on poststroke upper-limb motor recovery.
Only 1 study used the CSP features in their BCI model, yielding the effect size in favor of the BCI group (Hedge’s g = 0.66; P = .2).
Type of BCI Feedback
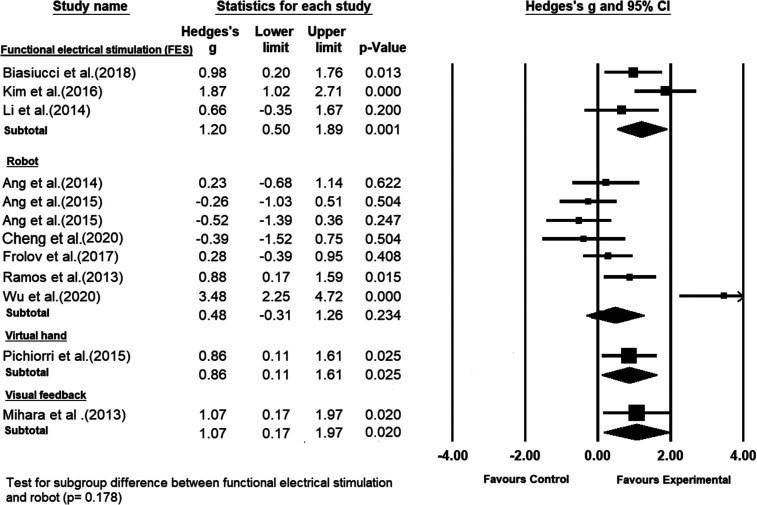
The type of BCI feedback used to move the affected hand was different across the studies. As can be seen in Figure 6, functional electrical stimulation (FES) was used in 3 studies,8,17,40 a hand exoskeleton robot was used in 2 studies.13,39 The MIT-Manus robot was used in 2 studies,9,10 and haptic knob, an assisted soft robotic glove, and orthosis (hand and arm) robot were used in 1 each.11,37,42 One study provided only visual feedback to the patients. 12 Finally, the study conducted by Pichiorri et al 41 used a virtual hand to provide the BCI feedback to the patients.
Figure 6.
A subgroup meta-analysis comparing the efficacy of brain–computer interface, grouped based on different types of feedbacks, on poststroke upper-limb recovery.
Compared to the control interventions, the highest statistically significant effect size on upper extremity recovery was obtained by the group of studies that used FES as the BCI feedback (Hedge’s g = 1.2; P = .001), with moderate heterogeneity among the studies (I2 = 47.369%; Q = 3.8; df = 2; P = .15). However, the effect size of the group studies with the FES-based feedback was not significantly higher than the effect sizes of the other groups of studies with the other types of BCI feedback.
Discussion
This study was conducted according to the recommendations of the PRISMA checklist for meta-analyses and systematic reviews. 26 Our meta-analysis studied changes in the FMA-UE scores between pre and postintervention, and showed that BCI had a significantly higher effect size in improving upper extremity functions following stroke, when compared with control therapies. These findings are consistent with the results of the previous meta-analysis, 24 and support the short-term efficacy of BCI. Importantly, our study analyzed 12 randomized controlled trials involving 298 stroke patients, while the previous study 24 covered 9 randomized controlled trials with 235 stroke patients.
We also analyzed the results of 7 out of 12 included studies that reported the FMA-UE scores of the patients in a follow-up session held a number of weeks after the cession of the intervention. Our results showed that the BCI effects in restoring upper extremity functions are persistent over long term with a pooled effect size significantly better than the control interventions. As an example, the upper-limb improvements were almost maintained at 36 weeks after the intervention in the study conducted by Biasiucci et al. 17 However, the recent meta-analysis conducted by Bai et al 25 did not observe long-term efficacy of BCI compared to conventional therapies. The reason might be because they considered a smaller number of randomized clinical trials (5 studies). In addition, we combined the 2 control groups in the study conducted by Ang et al 37 to create a single control group, 38 whereas Bai et al 25 selected the haptic knob group and excluded the standard arm therapy group.
Interestingly, the most recent randomized controlled trial conducted by Wu et al 13 showed the highest effect size in improving upper extremity functions in favor of BCI (ie, Hedge’s g = 3.48). 13 As can be seen in Figure 3A, the BCI effect size of this study is much larger than the effect sizes of the other included studies. In this study, unlike the other studies, the MI instruction was given to the patients by displaying a video of a hand using different tools. Then, the patients were asked to repeat the presented hand movement using mental imagery. The authors emphasized that the given instruction played an important role in the observed motor function recovery, possibly by linking the brain's visual and motor system.
Our subgroup meta-analysis showed that for both subacute and chronic patients, BCI is more effective than conventional therapies in improving upper-limb function (see Figure 4A). Our results also showed that the BCI studies performing intention/attempting of movement of the impaired hand, often followed by real movement if available, achieved a higher overall effect size than those that performed only MI (although not statistically significant). As a possible reason, we would argue that intending to move rather than just imaging the movement may lead to higher activity in neural circuits and better patient engagement and attention.43,44 Blokland et al 45 showed that for both groups of paralyzed and healthy participants, the accuracy of the BCI system that focused on the IM was significantly higher than that of MI. Moreover, among healthy participants, the IM and motor execution had more similar brain spectral responses and BCI performance than the results of MI. Considering the evidence provided here, future BCI-based stroke rehabilitation studies are encouraged to focus on intending rather than imagining of moving impaired hands. Further studies are required to confirm this observation.
Our subgroup meta-analysis grouped the included studies according to the BCI features that they used, further revealing that the use of band power features yielded the highest effect size in favor of the BCI compared to the control interventions. Indeed, the BCI studies using the band power features achieved a significantly greater upper-limb motor function recovery than those using the FBCSP features (P < .001). Previous studies on healthy and stroke participants suggested that FBCSP could lead to a higher BCI accuracy than the band power features. In addition, some studies have reported that there is a correlation between the BCI accuracy and motor function improvement after a BCI intervention.44,46 Thus, someone may initially assume that using FBCSP should produce a higher BCI effect size on motor recovery. However, the long-term effectiveness of BCI for stroke rehabilitation greatly involves human learning. The results of our meta-analysis suggest that in long term the use of band power features potentially helps patients better learn to self-regulate their brain patterns, leading to more motor recovery as compared to more complex features such as FBCSP. In FBCSP, the patients may not easily find a connection between their mental practice and what they observe as the output BCI.
The randomized control trials that coupled the BCI with FES had the largest significant effect size in restoring upper-limb function. This improvement may be due to the positive impact of FES on cortical excitability as reported by several studies.47,48
In the study conducted by Ang et al, 9 the effect size was in favor of the control group. This may be due to the relatively small number of training repetitions in the BCI group compared to the control group (136 vs 1040 repetitions). In addition to the number of training repetitions, the use of MI and FBCSP may have contributed to the negative results, as discussed in this study. Another study by the same research group also showed an effect size in favor of the control group. 10 This finding might be because of the short period of the rehabilitation intervention (2 weeks). Interestingly, this study reported a slight improvement in the BCI outcomes at the follow-up session held 4 weeks postintervention. However, it would be difficult to distinguish if this observed slight improvement was as a result of the BCI intervention or the transcranial direct current stimulation (tDCS). Typically, a longer intervention, such as 6 weeks of rehabilitation with 3 sessions per week is recommended. 49
Limitations
In this meta-analysis, we observed large variations in the BCI intervention effect sizes across the included clinical trials. As discussed previously, these variations can be potentially due to differences in the BCI design, including differences in the BCI feedback, performed mental practices, extracted classification features, and the phase of the stroke in the participants, among others. This finding further confirms that there is a need to optimize the BCI design for upper-limb stroke rehabilitation in order to maximize the potential motor function improvement in patients.
Only 12 randomized clinical trials (298 patients) were available to analyze in this study. Hence, more studies with a larger number of patients are required to increase the reliability and generalizability of the results. Moreover, in order to have a reliable subgroup meta-analysis, it has been recommended to have at least 5 clinical trials in each subgroup. 50 In some of our subgroup analyses, this condition was not met. Moreover, we did not consider the variations among the included clinical trials in terms of the intensity of BCI intervention (see Table 1).
Conclusion
This study showed that BCI has significant immediate and long-term effects in improving upper-limb motor functions after stroke, compared to conventional therapies. Our results support using “intention of movement of the impaired hand” as the BCI mental practice, the band power features as the BCI classification features, and the functional electrical stimulation as the BCI feedback in future BCI-based stroke rehabilitation studies.
Supplementary Material
Acknowledgments
We would like to thank Maria A. Cervera, Gangadhar Grappelli and Joshua Giles for their help and advice in data collection and extraction of clinical trial information.
Footnotes
Author Contributions: SM and MA contributed to the study concept and design, independently searching and evaluating study eligibility, extracting the data and drafting the manuscript. The statistical analysis was performed by SM, and the research was supervised by MA. KKA, KPSN, and KSP all contributed significantly to the interpretation of the findings and the writing of the manuscript. The manuscript was revised by all authors, and they all gave their approval to the final version.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the University of Sheffield.
ORCID iDs: Salem Mansour https://orcid.org/0000-0002-0942-5780
Krishnan P.S. Nair https://orcid.org/0000-0002-4004-2315
Supplemental material: Supplemental material for this article is available online.
References
- 1.Mak JN, Wolpaw JR. Clinical applications of brain-computer interfaces: current state and future prospects. IEEE Rev Biomed Eng. 2009;2:187–199. doi:10.1109/RBME.2009.2035356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soekadar SR, Birbaumer N, Slutzky MW, Cohen LG. Brain–machine interfaces in neurorehabilitation of stroke. Neurobiol Dis. 2015;83:172–179. doi:10.1016/j.nbd.2014.11.025 [DOI] [PubMed] [Google Scholar]
- 3.Nicolas-Alonso LF, Gomez-Gil J. Brain computer interfaces, a review. Sensors. 2012;12(2):1211–1279. doi:10.3390/s120201211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shakeel A, Navid MS, Anwar MN, Mazhar S, Jochumsen M, Niazi IK. A review of techniques for detection of movement intention using movement-related cortical potentials. Comput Math Meth Med. 2015;2015:346217. doi:10.1155/2015/346217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolpaw JR, Birbaumer N, McFarland DJ, Pfurtscheller G, Vaughan TM. Brain–computer interfaces for communication and control. Clin Neurophysiol. 2002;113(6):767–791. doi:10.1016/S1388-2457(02)00057-3 [DOI] [PubMed] [Google Scholar]
- 6.Nakayashiki K, Saeki M, Takata Y, Hayashi Y, Kondo T. Modulation of event-related desynchronization during kinematic and kinetic hand movements. J Neuroeng Rehabil. 2014;11(1):90. doi:10.1186/1743-0003-11-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Renard Y, Lotte F, Gibert G, et al. Openvibe: an open-source software platform to design, test, and use brain–computer interfaces in real and virtual environments. Presence: Teleoperators and Virtual Environments. 2010;19(1):35–53. doi:10.1162/pres.19.1.35 [Google Scholar]
- 8.Li M, Liu Y, Wu Y, Liu S, Jia J, Zhang L. Neurophysiological substrates of stroke patients with motor imagery-based brain-computer interface training. Int J Neurosci. 2014;124(6):403–415. doi:10.3109/00207454.2013.850082 [DOI] [PubMed] [Google Scholar]
- 9.Ang KK, Chua KSG, Phua KS, et al. A randomized controlled trial of EEG-based motor imagery brain-computer interface robotic rehabilitation for stroke. Clin EEG Neurosci. 2015;46(4):310–320. doi:10.1177/1550059414522229 [DOI] [PubMed] [Google Scholar]
- 10.Ang KK, Guan C, Phua KS, et al. Facilitating effects of transcranial direct current stimulation on motor imagery brain-computer interface with robotic feedback for stroke rehabilitation. Arch Phys Med Rehabil. 2015;96(3):S79–S87. doi:10.1016/j.apmr.2014.08.008 [DOI] [PubMed] [Google Scholar]
- 11.Ramos-Murguialday A, Broetz D, Rea M, et al. Brain–machine interface in chronic stroke rehabilitation: a controlled study. Ann Neurol. 2013;74(1):100–108. doi:10.1002/ana.23879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mihara M, Hattori N, Hatakenaka M, et al. Near-infrared spectroscopy-mediated neurofeedback enhances efficacy of motor imagery-based training in poststroke victims: a pilot study. Stroke. 2013;44(4):1091–1098. doi:10.1161/STROKEAHA.111.674507 [DOI] [PubMed] [Google Scholar]
- 13.Wu Q, Yue Z, Ge Y, et al. Brain functional networks study of subacute stroke patients with upper limb dysfunction after comprehensive rehabilitation including BCI training. Front Neurol. 2020;10:1419. doi:10.3389/fneur.2019.01419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramoser H, Muller-Gerking J, Pfurtscheller G. Optimal spatial filtering of single trial EEG during imagined hand movement. IEEE Trans Rehabil Eng. 2000;8(4):441–446. doi:10.1109/86.895946 [DOI] [PubMed] [Google Scholar]
- 15.Blankertz B, Tomioka R, Lemm S, Kawanabe M, Muller K-R. Optimizing spatial filters for robust EEG single-trial analysis. IEEE Signal Process Mag. 2007;25(1):41–56. doi:10.1109/MSP.2008.4408441 [Google Scholar]
- 16.Ang KK, Chin ZY, Wang C, Guan C, Zhang H. Filter bank common spatial pattern algorithm on BCI competition IV datasets 2a and 2b. Front Neurosci. 2012;6:39. doi:10.3389/fnins.2012.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biasiucci A, Leeb R, Iturrate I, et al. Brain-actuated functional electrical stimulation elicits lasting arm motor recovery after stroke. Nat Commun. 2018;9(1):1–13. doi:10.1038/s41467-018-04673-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwakkel G, Kollen BJ, Krebs HI. Effects of robot-assisted therapy on upper limb recovery after stroke: a systematic review. Neurorehabil Neural Repair. 2008;22(2):111–121. doi:10.1177/1545968307305457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veerbeek JM, Langbroek-Amersfoort AC, Van Wegen EE, Meskers CG, Kwakkel G. Effects of robot-assisted therapy for the upper limb after stroke: a systematic review and meta-analysis. Neurorehabil Neural Repair. 2017;31(2):107–121. doi:10.1177/1545968316666957 [DOI] [PubMed] [Google Scholar]
- 20.Sabathiel N, Irimia DC, Allison BZ, Guger C, Edlinger G. Paired associative stimulation with brain-computer interfaces: a new paradigm for stroke rehabilitation. Springer; 2016:261–272. doi:10.1007/978-3-319-39955-3_25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Dokkum L, Ward T, Laffont I. Brain computer interfaces for neurorehabilitation–its current status as a rehabilitation strategy post-stroke. Ann Phys Rehabil Med. 2015;58(1):3–8. doi:10.1016/j.rehab.2014.09.016 [DOI] [PubMed] [Google Scholar]
- 22.Buch E, Weber C, Cohen LG, et al. Think to move: a neuromagnetic brain-computer interface (BCI) system for chronic stroke. Stroke. 2008;39(3):910–917. doi:10.1161/STROKEAHA.107.505313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shu X, Chen S, Yao L, et al. Fast recognition of BCI-inefficient users using physiological features from EEG signals: a screening study of stroke patients. Front Neurosci. 2018;12:93. doi:10.3389/fnins.2018.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cervera MA, Soekadar SR, Ushiba J, et al. Brain-computer interfaces for post-stroke motor rehabilitation: a meta-analysis. Ann Clin Transl Neurol. 2018;5(5):651–663. doi:10.1002/acn3.544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bai Z, Fong KN, Zhang JJ, Chan J, Ting K. Immediate and long-term effects of BCI-based rehabilitation of the upper extremity after stroke: a systematic review and meta-analysis. J Neuroeng Rehabil. 2020;17:1–20. doi:10.1186/s12984-020-00686-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Reprint—preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther. 2009;89(9):873–880. doi:10.1093/ptj/89.9.873 [PubMed] [Google Scholar]
- 27.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–e34. doi:10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 28.Coscia M, Wessel MJ, Chaudary U, et al. Neurotechnology-aided interventions for upper limb motor rehabilitation in severe chronic stroke. Brain. 2019;142(8):2182–2197. doi:10.1093/brain/awz181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gladstone DJ, Danells CJ, Black SE. The Fugl-Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16(3):232–240. doi:10.1177/154596802401105171 [DOI] [PubMed] [Google Scholar]
- 30.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83(8):713–721. doi:10.1093/ptj/83.8.713 [PubMed] [Google Scholar]
- 31.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to meta-analysis. John Wiley & Sons; 2011. [Google Scholar]
- 32.Hedges LV. Distribution theory for glass’s estimator of effect size and related estimators. J Educ Stat. 1981;6(2):107–128. doi:10.3102/10769986006002107 [Google Scholar]
- 33.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45:139–145. doi:10.1016/j.cct.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. 2003;327(7414):557–560. doi:10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haidich A-B. Meta-analysis in medical research. Hippokratia. 2010;14(Suppl 1):29. [PMC free article] [PubMed] [Google Scholar]
- 36.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046–1055. doi:10.1016/S0895-4356(01)00377-8 [DOI] [PubMed] [Google Scholar]
- 37.Ang KK, Guan C, Phua KS, et al. Brain-computer interface-based robotic end effector system for wrist and hand rehabilitation: results of a three-armed randomized controlled trial for chronic stroke. Front Neuroeng. 2014;7:30. doi:10.3389/fneng.2014.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higgins JP, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons; 2019. [Google Scholar]
- 39.Frolov AA, Mokienko O, Lyukmanov R, et al. Post-stroke rehabilitation training with a motor-imagery-based brain-computer interface (BCI)-controlled hand exoskeleton: a randomized controlled multicenter trial. Front Neurosci. 2017;11:400. doi:10.3389/fnins.2017.00400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim T, Kim S, Lee B. Effects of action observational training plus brain–computer interface-based functional electrical stimulation on paretic arm motor recovery in patient with stroke: a randomized controlled trial. Occup Ther Int. 2016;23(1):39–47. doi:10.1002/oti.1403 [DOI] [PubMed] [Google Scholar]
- 41.Pichiorri F, Morone G, Petti M, et al. Brain–computer interface boosts motor imagery practice during stroke recovery. Ann Neurol. 2015;77(5):851–865. doi:10.1002/ana.24390 [DOI] [PubMed] [Google Scholar]
- 42.Cheng N, Phua KS, Lai HS, et al. Brain-computer interface-based soft robotic glove rehabilitation for stroke. IEEE Trans Biomed Eng. 2020. 67(12):3339–3351. doi:10.1109/TBME.2020.2984003 [DOI] [PubMed] [Google Scholar]
- 43.Chaudhary U, Birbaumer N, Ramos-Murguialday A. Brain–computer interfaces for communication and rehabilitation. Nat Rev Neurology. 2016;12(9):513. doi:10.1038/nrneurol.2016.113 [DOI] [PubMed] [Google Scholar]
- 44.Chowdhury A, Meena YK, Raza H, et al. Active physical practice followed by mental practice using BCI-driven hand exoskeleton: a pilot trial for clinical effectiveness and usability. IEEE J Biomed Health Inf. 2018;22(6):1786–1795. doi:10.1109/JBHI.2018.2863212 [DOI] [PubMed] [Google Scholar]
- 45.Blokland Y, Spyrou L, Bruhn J, Farquhar J. Why BCI researchers should focus on attempted, not imagined movement. 2016: [DOI] [PubMed]
- 46.Bundy DT, Wronkiewicz M, Sharma M, Moran DW, Corbetta M, Leuthardt EC. Using ipsilateral motor signals in the unaffected cerebral hemisphere as a signal platform for brain–computer interfaces in hemiplegic stroke survivors. J Neural Eng. 2012;9(3):036011. doi:10.1088/1741-2560/9/3/036011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ridding MC, McKay DR, Thompson PD, Miles TS. Changes in corticomotor representations induced by prolonged peripheral nerve stimulation in humans. Clin Neurophysiol. 2001;112(8):1461–1469. doi:10.1016/S1388-2457(01)00592-2 [DOI] [PubMed] [Google Scholar]
- 48.Barsi GI, Popovic DB, Tarkka IM, Sinkjær T, Grey MJ. Cortical excitability changes following grasping exercise augmented with electrical stimulation. Exp Brain Res. 2008;191(1):57. doi:10.1007/s00221-008-1495-5 [DOI] [PubMed] [Google Scholar]
- 49.Ang KK, Guan C. Brain–computer interface for neurorehabilitation of upper limb after stroke. Proc IEEE. 2015;103(6):944–953. doi:10.1109/JPROC.2015.2415800 [Google Scholar]
- 50.Richardson M, Garner P, Donegan S. Interpretation of subgroup analyses in systematic reviews: a tutorial. Clin Epidemiol Glob Health. 2019;7(2):192–198. doi:10.1016/j.cegh.2018.05.005 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.