Abstract
Chronic hemodialysis therapy required regular entry into the patient’s blood stream with adequate flow. The use of arteriovenous fistulas and grafts is linked with lower morbidity and mortality than the use of catheters. However, these types of accesses are frequently affected by stenoses, which decrease the flow and lead to both inadequate dialysis and access thrombosis. The idea of duplex Doppler ultrasound surveillance is based on the presumption that in-time diagnosis of an asymptomatic significant stenosis and its treatment prolongs access patency. Details of performed trials are conflicting, and current guidelines do not support ultrasound surveillance. This review article summarizes the trials performed and focuses on the reasons of conflicting results. We stress the need of precise standardized criteria of significant access stenosis and the weakness of the metaanalyses performed.
Keywords: Hemodialysis, hemodialysis vascular access, ultrasonography, arteriovenous fistula, arteriovenous graft
Introduction
Only few other hemodialysis access topics are more passionately debated as ultrasound surveillance. The invention of arteriovenous fistulas (AVFs) and arteriovenous grafts (AVGs) as vascular access for hemodialysis considerably decreased patients’ morbidity and mortality. The patency of AVFs and AVGs is being threatened mostly by the development of a stenosis, which can lead to access dysfunction, inadequate dialysis, and/or access thrombosis. The latter represents an acute risk of access abandonment, and its therapy is more complicated and painful and costlier than the treatment of a (significant) stenosis. It seems, therefore, logical that treating significant stenoses before thrombosis occurs should be beneficial. However, it remains unclear when the risk of acute thrombosis due to stenosis is high enough to justify the preemptive intervention. Moreover, the definition of a hemodynamically significant stenosis varies between centers.
Stenosis is a vessel narrowing that could cause significant pressure drop and flow volume decrease, but also trigger mechanisms of thrombosis due to endothelial cell denudation and both platelet and von Willebrand factor activation in the case of very high shear stress (∼flow velocity). 1,2 The presence of a stenosis can be detected by routine physical examination and/or by signs of dysfunction that manifest themselves during the dialysis session (monitoring). Such stenoses are clinically significant and include edema anatomically distal to the venous stenosis, prolonged bleeding, needling problems, fall of flow volume, decrease of dialysis dose due to higher recirculation, and so forth. However, the accuracy of clinical diagnosis of a stenosis depends on the stenosis location, on vascular access and patient’s characteristics (depth, flow volume, vein branching, and compliance), and highly on the experience of the hemodialysis unit team, which is not always optimal. 3,4 In a recent study by Castro et al., 5 the clinical suspicion on AVF stenosis was not confirmed in 27% of cases. Some stenoses remain clinically asymptomatic, which explains at least part of the “sudden” thrombosis that develops without any warning signs. All these reasons led to the development of surveillance techniques.
Surveillance is defined as the periodic evaluation of the vascular access by using diagnostic tests that may involve special instrumentation and staff and for which an abnormal test result suggests the presence of dysfunction. 6 The rationale of surveillance is based on the hypothesis that corrective intervention of an identified progressive stenosis (such as percutaneous transluminal angioplasty (PTA)) can prevent complete occlusion of AVF/AVG and prolong access lifespan. 7 Besides regular access flow volume measurement using dilutional techniques, Doppler ultrasonography (DUS), is the most investigated and used method. The main advantage of DUS is its ability to provide non-invasive, precise, and reproducible data on the morphology and flow dynamics of the arteriovenous access. 8 However, a number of trials have been published, and the data about DUS surveillance benefits were conflicting. 9 –13 In the absence of precise standardized definition of a significant stenosis or adequate level of experience, DUS (but also angiography) could be an operator-dependent method and, indeed, the indication to preemptive therapy (= significant stenosis) differed from study to study. The absence of strict ultrasound diagnostic criteria of an asymptomatic stenosis could explain the lack of surveillance benefit in some trials, and we describe them in detail below and in Table 1. Moreover, the technology of DUS has improved considerably since the first reports of AVF/AVG ultrasound examination and (negative) trials of surveillance, and its improvement is an ongoing process. The higher working frequency of the ultrasound probes provide better axial spatial resolution, and the higher frame rate corresponds to the higher temporal resolution. Moreover, the size of the ultrasound devices has decreased considerably, which led to their easier transportation even to the patients’ beds and hemodialysis chairs.
Table 1.
Complex criteria of a significant vs borderline stenosis—according to Malik et al. 13 and Ishii et al. 14
| Significant | Borderline |
|---|---|
| Main criteria | |
| Diameter reduction by >50% | |
| Peak systolic velocity increase >2–3× | |
| Additional criteria (≥1): | |
| Residual diameter <1.9–2.0 mm | No additional criterion |
| Flow volume decrease by >25%a) | |
| Flow volume <600 mL/min for AVGs, <500 mL/min for AVFs | |
If only the main criteria are present, the stenosis is borderline and reevaluation is indicated within 6–8 weeks. Significant stenoses are indicated to correction.
a Flow volume decrease by >25% if the previous value was <1000 mL/min.
There is a general agreement that clinically significant stenoses should be treated. In these cases, DUS can help to identify the location of the stenosis, which is important for the appropriate puncture site during the percutaneous procedure. A debate continues about the indication of asymptomatic stenoses diagnosed by any imaging method. We believe that only the usage of strict diagnosis criteria could justify the ultrasound surveillance.
Stenosis definition by DUS
Several criteria have been used for the description of a significant stenosis by DUS.
Percentage of diameter reduction
Percentage of diameter reduction is the oldest morphological assessment of a stenosis, used not only in DUS, but also in angiography. The main problem of this criterion is that the outflow veins have typically irregular lumen, so there is frequently no reference segment for the estimation of stenosis percentage in AVFs. Moreover, many stenoses are asymmetric, and then the estimated severity of the stenosis depends on the direction from which it is viewed. Stenosis visualization in two perpendicular directions could lessen this limitation. Nevertheless, it is diameter narrowing in B-mode (and in color Doppler), which turns the attention of the examiner to the particular vessel segment. Percentage of stenosis (>50%) was used as the single criterion of significance in some trials. 9 –11 Using only this criterion is unreliable because B-mode does not always delineate low-echogenic structures, such as intimal hyperplasia, degenerative venous valves, or recent thrombosis.
Peak systolic velocity
Peak systolic velocity (PSV) increase in comparison to a non-stenosed segment is another widely used criterion. 8 –13 The velocity increase is caused by the stenosis itself and by the recirculation zone due to flow turbulence just behind the stenosis. Sometimes it is expressed as a PSV ratio (PSR). AVG stenosis with PSR 2.0–2.9 had 50%–74% stenosis on angiography, and AVG stenosis with PSR ≥ 3.0 had ≥75% stenosis. 12 The velocities should be always recorded by the same Doppler angle, which is usually set to 60° by the ultrasound manufacturers (Figure 1), but could be changed appropriately by the examiner. It should not exceed 60°. This is related to the formula that calculates the velocity, which includes the cosine of the angle in the numerator. Irregular (asymmetrical) stenoses cause higher pressure drop and velocity increase than symmetrical stenoses of the same area reduction. 15 The velocity increase mirrors the pressure gradient caused by the stenosis according to the Bernoulli equation. This is because the outflow vein is softer or easily collapsible and anatomically proximal to a significant stenosis during palpation or ultrasound evaluation. The use of absolute PSV values as the only criterion is confounding because the increase of PSV does not have the same significance when it is or it is not associated to a drop of the flow rate
Figure 1.
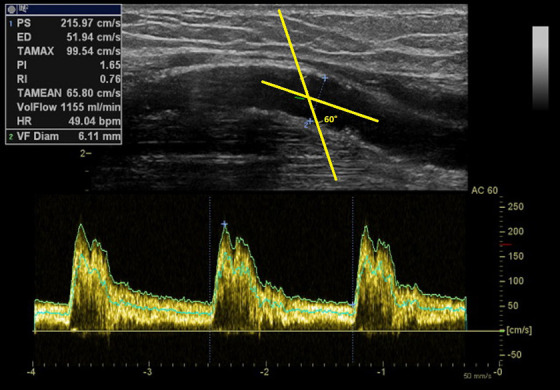
Correct orientation of the Doppler angle in the vascular lumen.
The cosinus of the Doppler angle is a part of the equation transforming the Doppler frequency shift into the velocity. Therefore, it should be always the same for the follow-up. Manufacturers set it usually to 60°.
Flow volume (Qa)
Flow volume (Qa) is frequently considered as the function of the access and is obtainable during DUS examination. For precise values, it has to be measured in a straight vascular segment free of stenoses. In AVFs, Qa is usually measured in the brachial artery, supposing that the vast majority of the flow volume (>90%) enters the AVF. In AVGs, Qa is measured directly in the graft close to its venous anastomosis (Figure 2). Low values are linked to higher thrombosis risk 16 and to increased blood recirculation, with subsequent decrease of the dialysis dose—Kt/V (especially in the popular high-flow regimes). On the contrary, high Qa could be detrimental for the heart or could be responsible for hand ischemia. 17 In considering preemptive correction, studies regarded stenoses as significant either at a flow volume decrease by 20%–25% and/or at a cut-off value of <500–600 mL/min. 13–14,18 –20 However, the problem is that the actual Qa does not depend only on stenosis severity, but also on the actual hydration status and blood pressure similar to and in relation with the cardiac output. Natural/physiologic variation of repeated Qa exceeds 20%. 21 It is advisable to validate Qa values obtained by DUS with dilutional techniques performed during hemodialysis.
Figure 2.
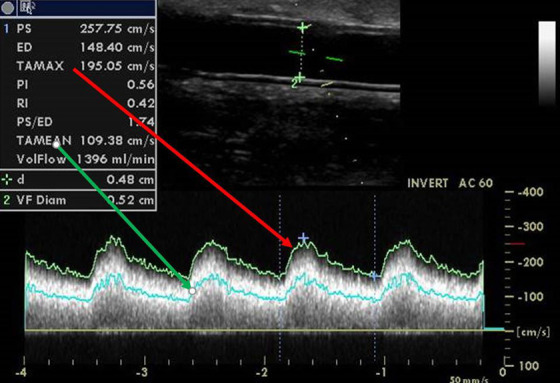
Flow volume measurement in the graft.
The flow volume calculation is based on the following formula: Qa = πr 2 × TAMEAN, where r is the radius of the examined graft and TAMEAN is the time-averaged mean velocity inside. The latter is an integral value during the heart cycle of the average velocity layer (flow velocity is the fastest in the middle and the slowest along the vascular wall). TAMEAN must not be replaced by TAMAX, which is the time velocity integral of the fastest velocity only—using TAMAX would lead to significant overestimation of Qa. Pulse wave Doppler sample size should be wide enough to cover most of the vessel lumen because of the different speeds of blood inside the vessel from the center to the wall.
Residual diameter (RD) of the stenosis could be precisely analyzed by the modern high quality ultrasound devices (Figure 3). 8 The cut-off value <2.0 mm was arbitrarily set and confirmed clinically when used as a part of the “complex stenosis criteria” in AVGs 13,22,23 and validated against angiography. 8 In AVFs, an Australian study showed that the RD < 2.7 mm was associated with 90% sensitivity and 80% specificity of AVF dysfunction. 18 According to a recent study, RD < 2.5 mm is associated with lower Qa. 24 Another current Japanese study with AVFs determined the RD cut-off value to be <1.86 mm. 14 As long as the stenosis is asymmetrical, it should be again visualized by two perpendicular views, and for the diagnosis of a significant stenosis, the RD should be below the cut-off value in both..
Figure 3.
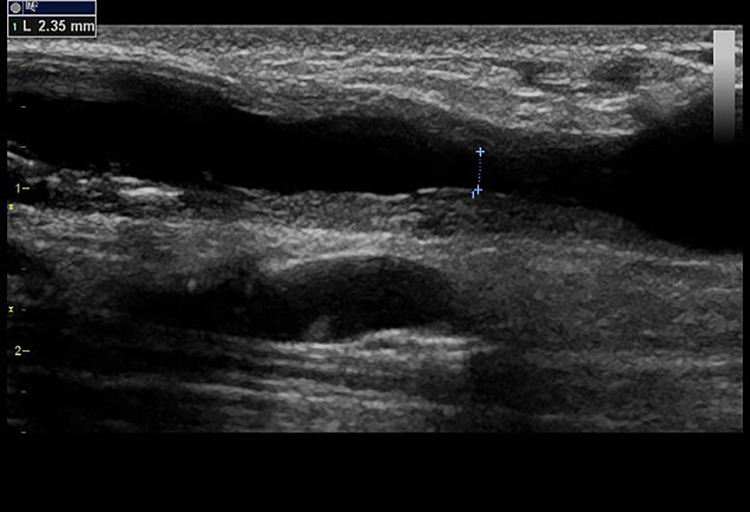
RD measurement.
There is excessive intimal hyperplasia in the outflow vein causing the stenosis. By diameter reduction, this stenosis would be significant if compared with the left or right part of the vein. Nevertheless, the RD is 2.35 mm, so this stenosis was considered borderline, and soon, reevaluation (within 6–8 weeks) was indicated.
The resistive index (RI) of the flow pattern in the feeding artery has been tested in several studies, and although it is used in the assessment of the transplanted kidney, 25 the results in AVFs and AVGs are not conclusive. Values above 0.6–0.7 indicated stenosis. 26,27 The RI is defined by the following equation: RI = (PSV − end diastolic velocity)/PSV. RI increases especially in juxtaanastomotic stenoses.
Some centers and trials have used the complex criteria of a significant stenosis, comprising the combination of two main criteria (>50% diameter reduction + PSR >3) and at least one additional criterion (RD, flow volume decrease, or low Qa—see Table 1). 28 AVG stenoses with a lack of any additional criterion (= borderline stenoses) possess low risk of thrombosis (ca. 1% during 6–8 weeks since the primary diagnosis). 22 Only 54% of borderline AVF stenoses progressed into significant stenosis in the study by Castro et al. 5 The complex stenosis criteria (Table 1) have been developed for the “Watch and wait strategy.” These criteria were used in the largest randomized trial testing ultrasound surveillance and proving its benefit, 13 but also in other trials. 19,28 Unfortunately, the majority of trials used only >50% diameter reduction as the only criterion of stenosis. 9 –11 The second largest randomized controlled trial (RCT) of ultrasound surveillance that did not prove its benefit used complex criteria, but included RD < 4.0 mm. 29 The latter cut-off value is quite high and could be found in many normally functioning AVFs/AVGs.
The key question is this: can ultrasound surveillance provide reliable guidance for the clinician as to which stenosis should be treated and when? The identification of such high-risk stenosis is based on the understanding of stenosis pathophysiology and on their detailed morphological and functional (Qa) evaluation. 22 DUS enables complex evaluation of the stenosis significance, which was, however, used only in a few published trials as mentioned above. Moreover, DUS performed early after AVG creation can also predict future risk of AVG abandonment and thus select high-risk AVGs that could profit from the surveillance most. 30 DUS is even more frequently performed in AVFs after their creation, although the optimal criteria are discussed. 31
AVF/AVG thrombosis, however, can also develop not only because of stenosis progression, but also due to other mechanisms, such as hypotension, dehydration, unwished outer compression during sleep, thrombophilia, and so forth. Moreover, the aforementioned complex stenosis criteria cannot be used mechanically. One such example is a branched AVF—if an outflow vein stenosis is diagnosed, but Qa (measured in the brachial artery) is adequate and the alternative outflow vein (branch) is suitable for hemodialysis needling, the stenosis could be left without intervention. Alternatively, when a decreased Qa is calculated, but there is no clear high-grade stenosis, it is worth to estimate patient’s hydration, for example, by the visualization of the inferior vena cava diameter and collapsibility when the ultrasound device is equipped with abdominal or echocardiography probes. 32 Whole-body bioimpedance spectroscopy is frequently used in hemodialysis units for the assessment of hydration.
Stenoses in AVFs
Anatomically distal AVF is the first access of choice. The more frequent upper extremity fistulas include radio-cephalic, ulno-basilic and brachiocephalic fistulas, Gracz fistula (median cubital vein attached to the brachial or radial artery), and transposed basilic vein. They have a long lifespan, low risk of complications, and their use is associated with the longest life expectancy. 33 The main limitation of AVFs is non-maturation, as up to 40%–60% of AVFs are never suitable for hemodialysis without additional AVF procedures to 34 facilitate maturation and the maturation rate of AVFs is suggested as a surgical quality indicator. 35 Many factors responsible for these unsatisfactory early results have been identified, and they lead to outflow vein or inflow artery stenoses. Early DUS can identify such stenosis and provide an indication to percutaneous or surgical therapy—so-called assisted maturation. 36,37 Later on, stenoses develop mostly in the outflow vein close to the anastomosis in distal forearm AVFs and more proximally in the outflow vein (cephalic arch) in brachiocephalic AVFs. 38
Stenoses in AVGs
The most frequent AVGs are forearm—they begin at the radial or brachial artery and are straight or curved and are attached to the basilic vein. More proximal grafts originate at the brachial or even subclavian artery and are attached to the cephalic or subclavian vein. Lower extremity grafts are created in the case of occluded central veins and are straight (between popliteal artery and some groin vein) or looped (femoral artery to femoral or great saphenous vein). AVGs have a higher rate of complications and a shorter lifespan than AVFs. Therefore, AVG is the vascular access that is created and recommended especially in patients with abandoned subcutaneous veins, with AVF non-maturation, but also in elderly patients, thanks to AVG’s higher maturation rate. 39
Unfortunately, no currently available graft is as good as an AVF that matured without intervention. DUS performed early after AVG creation can predict future complications and thus select patients who would profit more from ultrasound surveillance. 30 AVGs are generally more prone to later stenosis development than AVFs. The typical site of stenosis development is at the venous anastomosis and at the adjacent part of the outflow vein. This is caused by the formation of intimal hyperplasia after creation. Cannulation-related stenoses could also develop in the graft itself, especially when the areal puncture technique is applied instead of the recommended rope-ladder technique. 40 In this case, the graft wall disruption and subsequent healing are responsible. Both development of intimal hyperplasia and puncture healing are slow-acting mechanisms, which give time for surveillance. 22
Ultrasound surveillance in the guidelines
Some published vascular access guidelines support DUS as a screening tool for detecting stenosis and to perform preemptive interventions to prevent the loss of the AV access. The Kidney Disease Outcomes Quality Initiative (KDOQI) clinical practice guideline for vascular access, published in 2006, recommended direct flow measurement and DUS as preferred techniques that may be used in AVF surveillance and preemptive correction of luminal stenosis of >50% when the access flow rate is less than 600 mL/min in AVGs, and ≤400–500 mL/min in AVFs, even if the access is still able to provide adequate hemodialysis. 41
Recent Spanish guidelines have recommended DUS as the first visualization method in the case of clinical suspicion on access dysfunction or stenosis, and regarding surveillance, both DUS and dilution methods for AVFs but not for AVGs. 42 The European Society for Vascular Surgery guidelines also recommended DUS as a non-invasive tool to be the first line imaging method only in patients with suspected vascular access dysfunction. 43 The new European Renal Best Practice (ERBP) guideline on AV access specifies that the evidence for surveillance of AVFs is inconclusive and needs more research. In addition, they recommend not to perform routine surveillance of AVGs with access flow measurements or DUS. 44
Clinical trials on vascular access surveillance
Current statements of the societies are based especially on the results of the metaanalyses. 44,45 Their authors included all RCTs testing the benefit of DUS surveillance and preemptive treatment of stenoses. The conclusions of both metaanalyses are similar: Preemptive stenosis correction of a functional arteriovenous access does not improve access longevity; although results for native AVF are promising, existing evidence is insufficient to guide clinical practice and health policy.
The board and council members of the Vascular Access Society were asked about their practice in DUS surveillance. The results are given in Table 2, and they illustrate the current uncertainty about the clinical value of vascular access surveillance. The results of the largest RCT evidenced the benefit of DUS surveillance and preemptive therapy of carefully assessed stenoses. 13 On the contrary, the metaanalyses had negative results. 45,46 Nevertheless, the number of patients included in the metaanalyses is scarce: six RCTs enrolled 612 patients with the primary endpoint of thrombosis, and four RCTs enrolled 443 patients for access loss. 47 Some of the studies included prevalent patients, while others included solely incident patients at the time of access creation. The most important differences were, however, in the definition of significant stenosis that was indicated to percutaneous or surgical correction (Table 3). Performing a metaanalysis from so different studies leads therefore to significant oversimplification. The final answer to whether to do or not to do DUS surveillance is therefore in including more patients with the use of the complex stenosis criteria and clear indication criteria of percutaneous treatment, ideally in a multicenter study with defined inclusion criteria and endpoints (thrombosis and cumulative patency).
Table 2.
The use of DUS as answered by the VAS council and board members.
| AVF | AVG | |
|---|---|---|
| Do you routinely perform/support ultrasound examination after access creation? | 75% | 75% |
| Do you perform/support duplex Doppler ultrasound in the case of access problems (puncture difficulties, limb edema, decrease of dialysis dose…?) | 100% | 91% |
| Do you check the access by ultrasound regularly? | 55% | 55% |
| Do you indicate PTA if a severe stenosis is found? | 73%a
|
73%a
|
PTA: percutaneous transluminal angioplasty; AVF: arteriovenous fistula; AVG: arteriovenous graft. Percentage of “YES” is depicted.
a Half responded “only if symptomatic.”
Table 3.
Duplex Doppler cut-off criteria of a significant AVG stenosis (used as the indication to angiography and intervention)—from the work by Malik et al. 28
| Trial first author | No of subjects US/control | Diameter reduction in B-mode | Peak velocity increase | Qa decrease | Qa cut-off value (mL/min) |
Residual diameter (mm) |
Prolonged=survival in ultrasound surveillance arm? |
|---|---|---|---|---|---|---|---|
| Mayer et al. 9 | 35/35 | >50% | – | – | – | – | Yes |
| Lumsden et al. 10 | 32/32 | >50% | – | – | – | – | No |
| Ram et al. 11 | 32/34 | >50% | – | – | – | – | No |
| Robbin et al. 29 | 65/61 | >50% | >2fold | <500 | <4.0 | No | |
| Malik et al. 13 | 97/92 | >50% | >2fold | >25% | <600 | <2.0 | Yes |
Qa = access flow volume.
Perspective: is it the time to change the surveillance paradigm?
Although the guidelines are not favorable, the authors of this article are convinced that ultrasound should play a pivotal role in the AVF/AVG management, as it is a precise and highly reproducible method in experienced hands. For the greater distribution of AVF/AVG ultrasound imaging, it is necessary to (1) standardize the examination technique, (2) define the technical demands of the ultrasound devices, and (3) standardize the criteria of significant stenosis in a well-controlled multicenter trial.
Ultrasound should be the first technique of choice in the evaluation of maturation, regardless of physical examination. The goal in the maturation phase should be the search for potentially correcting inflow or outflow defects, often the basis of late stenoses. Hemodynamics and anatomy of AVF and graft vary from case to case, so the examination should be tailored for each patient in order to identify early the sites and causes of stenosis during the maturation phase. Although it has not been proven, it is logical that surveillance is most important in accesses at greater risk. To put things in perspective, top-end ultrasound devices and profound operator training should be a must in dedicated vascular access centers. Nevertheless, as in any field of medicine, clinical judgment is more important than only the results of ultrasonography. It includes also the history of previous thrombosis, thrombophilia, lack of other suitable veins for another arteriovenous access, and so forth.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: JM is supported by the by the grant of the Agency of Health Research of the Czech Republic 17-31796A.
ORCID iDs: Jan Malik  https://orcid.org/0000-0002-2386-3293
https://orcid.org/0000-0002-2386-3293
Mario Meola  https://orcid.org/0000-0002-1330-2820
https://orcid.org/0000-0002-1330-2820
Cora de Bont  https://orcid.org/0000-0003-2176-5275
https://orcid.org/0000-0003-2176-5275
Joris I Rotmans  https://orcid.org/0000-0001-9682-6234
https://orcid.org/0000-0001-9682-6234
Jose Ibeas  https://orcid.org/0000-0002-1292-7271
https://orcid.org/0000-0002-1292-7271
References
- 1. Tsai HM. Shear stress and von Willebrand factor in health and disease. Semin Thromb Hemost 2003; 29(5): 479–488. [DOI] [PubMed] [Google Scholar]
- 2. Remuzzi A, Ene-Iordache B. Novel paradigms for dialysis vascular access: upstream hemodynamics and vascular remodeling in dialysis access stenosis. Clin J Am Soc Nephrol 2013; 8(12): 2186–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maldonado-Carceles AB, Garcia-Medina J, Torres- Cantero AM. Performance of physical examination versus ultrasonography to detect stenosis in haemodialysis arteriovenous fistula. J Vasc Access 2017; 18: 30–34. [DOI] [PubMed] [Google Scholar]
- 4. Malik J, Slavikova M, Malikova H, et al. Many clinically silent access stenoses can be identified by ultrasonography. J Nephrol 2002; 15(6): 661–665. [PubMed] [Google Scholar]
- 5. Castro A, Moreira C, Almeida P, et al. The role of Doppler ultrassonography in significant and borderline stenosis Definition. Blood Purif 2018; 46(2): 94–102. [DOI] [PubMed] [Google Scholar]
- 6. Vachharajani TJ. Diagnosis of arteriovenous fistula dysfunction. Semin Dial 2012; 25(4): 445–450. [DOI] [PubMed] [Google Scholar]
- 7. Paulson WD. Blood flow surveillance of hemodialysis grafts and the dysfunction hypothesis. Semin Dial 2001; 14(3): 175–180. [DOI] [PubMed] [Google Scholar]
- 8. Kudlicka J, Kavan J, Tuka V, et al. More precise diagnosis of access stenosis: ultrasonography versus angiography. J Vasc Access 2012; 13(3): 310–314. [DOI] [PubMed] [Google Scholar]
- 9. Mayer DA, Zingale RG, Tsapogas MJ. Duplex scanning of expanded polytetrafluoroethylene dialysis shunts—impact on patient-management and graft-survival. Vascular Surg 1993; 27: 647–658. [Google Scholar]
- 10. Lumsden AB, MacDonald MJ, Kikeri D, et al. Prophylactic balloon angioplasty fails to prolong the patency of expanded polytetrafluoroethylene arteriovenous grafts: results of a prospective randomized study. J Vasc Surg 1997; 26(3): 382–390; discussion 390–392. [DOI] [PubMed] [Google Scholar]
- 11. Ram SJ, Work J, Caldito GC, et al. A randomized controlled trial of blood flow and stenosis surveillance of hemodialysis grafts. Kidney Int 2003; 64(1): 272–280. [DOI] [PubMed] [Google Scholar]
- 12. Robbin ML, Oser RF, Allon M, et al. Hemodialysis access graft stenosis: US detection. Radiology 1998; 208(3): 655–661. [DOI] [PubMed] [Google Scholar]
- 13. Malik J, Slavikova M, Svobodova J, et al. Regular ultrasonographic screening significantly prolongs patency of PTFE grafts. Kidney Int 2005; 67(4): 1554–1558. [DOI] [PubMed] [Google Scholar]
- 14. Ishii T, Suzuki Y, Nakayama T, et al. Duplex ultrasound for the prediction of vascular events associated with arteriovenous fistulas in hemodialysis patients. J Vasc Access 2016; 17: 499–505. [DOI] [PubMed] [Google Scholar]
- 15. Novakova L, Kolinsky J, Adamec J, et al. Vascular stenosis asymmetry influences considerably pressure gradient and flow volume. Physiol Res 2016; 65(1): 63–69. [DOI] [PubMed] [Google Scholar]
- 16. Quences KB, Oklu R. Hemodialysis access thrombosis. Cardiovasc Diagn Ther 2017; 7: S299–S308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Malik J, Tuka V, Kasalova Z, et al. Understanding the dialysis access steal syndrome. A review of the etiologies, diagnosis, prevention and treatment strategies . J Vasc Access 2008; 9(3): 155–166. [PubMed] [Google Scholar]
- 18. Fahrtash F, Kairaitis L, Gruenewald S, et al. Defining a significant stenosis in an autologous radio-cephalic arteriovenous fistula for hemodialysis. Semin Dial 2011; 24(2): 231–238. [DOI] [PubMed] [Google Scholar]
- 19. Aragoncillo I, Abad S, Caldes S, et al. Adding access blood flow surveillance reduces thrombosis and improves arteriovenous fistula patency: a randomized controlled trial. J Vasc Access 2017; 18: 352–358. [DOI] [PubMed] [Google Scholar]
- 20. Tessitore N, Bedogna V, Poli A, et al. Should current criteria for detecting and repairing arteriovenous fistula stenosis be reconsidered? Interim analysis of a randomized controlled trial . Nephrol Dial Transplant 2014; 29(1): 179–187. [DOI] [PubMed] [Google Scholar]
- 21. Valek M, Lopot F, Dusilova-Sulkova S, et al. Physiologic variability of vascular access blood flow for hemodialysis. Blood Purif 2008; 26(5): 468–472. [DOI] [PubMed] [Google Scholar]
- 22. Tuka V, Slavikova M, Krupickova Z, et al. Short-term outcomes of borderline stenoses in vascular accesses with PTFE grafts. Nephrol Dial Transplant 2009; 24(10): 3193–3197. [DOI] [PubMed] [Google Scholar]
- 23. Malik J, Tuka V, Chytilova E, et al. Low-flow polytetrafluoroethylene accesses: ultrasound surveillance and preemptive interventions ensure long-term patency. Kidney Blood Press Res 2010; 33(3): 181–185. [DOI] [PubMed] [Google Scholar]
- 24. Nassar GM, Beathard G. Exploring correlations between anatomic characteristics of dialysis arteriovenous fistula stenosis and arteriovenous fistula blood flow rate (Qa). J Vasc Access 2020; 21: 60–65. [DOI] [PubMed] [Google Scholar]
- 25. Laranjinha I, Matias P, Oliveira R, et al. The impact of functioning hemodialysis arteriovenous accesses on renal graft perfusion: results of a pilot study. J Vasc Access 2019; 20(5): 482–487. [DOI] [PubMed] [Google Scholar]
- 26. Sanchez MT, Hervas MC, Martainez SE, et al. Value of Doppler ultrasonography in the study of hemodialysis peripheral vascular access dysfunction. Radiologia 2014; 56(5): 420–428. [DOI] [PubMed] [Google Scholar]
- 27. Napoli M, Prudenzano R, Russo F, et al. Juxta-anastomotic stenosis of native arteriovenous fistulas: surgical treatment versus percutaneous transluminal angioplasty. J Vasc Access 2010; 11(4): 346–351. [DOI] [PubMed] [Google Scholar]
- 28. Malik J, Kudlicka J, Novakova L, et al. Surveillance of arteriovenous accesses with the use of duplex Doppler ultrasonography. J Vasc Access 2014; 15(Suppl 7): S28–S32. [DOI] [PubMed] [Google Scholar]
- 29. Robbin ML, Oser RF, Lee JY, et al. Randomized comparison of ultrasound surveillance and clinical monitoring on arteriovenous graft outcomes. Kidney Int 2006; 69(4): 730–735. [DOI] [PubMed] [Google Scholar]
- 30. Kudlicka J, Malik J, Tuka V, et al. Arteriovenous grafts: early ultrasonography tells their fortune. Am J Nephrol 2015; 41: 420–425 [DOI] [PubMed] [Google Scholar]
- 31. Lee T, Magill M, Burke SK, et al. Comparison of postoperative ultrasound criteria to predict unassisted use of arteriovenous fistulas for hemodialysis. J Vasc Access 2018; 19(2): 167–171. [DOI] [PubMed] [Google Scholar]
- 32. Sabaghian T, Hajibaratali B, Samavat S. Which echocardiographic parameter is a better marker of volume status in hemodialysis patients? Ren Fail 2016; 38(10): 1659–1664. [DOI] [PubMed] [Google Scholar]
- 33. Dhingra RK, Young EW, Hulbert-Shearon TE, et al. Type of vascular access and mortality in U.S. hemodialysis patients. Kidney Int 2001; 60(4): 1443–1451. [DOI] [PubMed] [Google Scholar]
- 34. Dember LM, Beck GJ, Allon M, et al. Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: a randomized controlled trial. JAMA 2008; 299: 2164–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fila B, Roca- Tey R, Malik J, et al. Quality assessment of vascular access procedures for hemodialysis: a position paper of the Vascular Access Society based on the analysis of existing guidelines. J Vasc Access 2020; 21: 148–153. [DOI] [PubMed] [Google Scholar]
- 36. Farrington CA, Robbin ML, Lee T, et al. Postoperative ultrasound, unassisted maturation, and subsequent primary patency of arteriovenous fistulas. Clin J Am Soc Nephrol 2018; 13: 1364–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Abreo K, Buffington M, Sachdeva B. Angioplasty to promote arteriovenous fistula maturation and maintenance. J Vasc Access 2018; 19(4): 337–340. [DOI] [PubMed] [Google Scholar]
- 38. Turmel-Rodrigues L, Pengloan J, Baudin S, et al. Treatment of stenosis and thrombosis in haemodialysis fistulas and grafts by interventional radiology. Nephrol Dial Transplant 2000; 15(12): 2029–2036. [DOI] [PubMed] [Google Scholar]
- 39. Lee T, Qian J, Thamer M, et al. Tradeoffs in vascular access selection in elderly patients initiating hemodialysis with a catheter. Am J Kidney Dis 2018; 72(4): 509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Parisotto MT, Schoder VU, Miriunis C, et al. Cannulation technique influences arteriovenous fistula and graft survival. Kidney Int 2014; 86(4): 790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Group VAW. Clinical practice guidelines for vascular access, update 2006—introduction. Am J Kidney Dis 2006; 48: S176–S247. [DOI] [PubMed] [Google Scholar]
- 42. Roca-Tey R, Ibeas J, Moreno T, et al. Dialysis arteriovenous access monitoring and surveillance according to the 2017 Spanish guidelines. J Vasc Access 2018; 19(5): 422–429. [DOI] [PubMed] [Google Scholar]
- 43. Schmidli J, Widmer MK, Basile C, et al. Editor’s choice—vascular access: 2018 clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2018; 55(6): 757–818. [DOI] [PubMed] [Google Scholar]
- 44. Gallieni M, Hollenbeck M, Inston N, et al. Clinical practice guideline on peri- and postoperative care of arteriovenous fistulas and grafts for haemodialysis in adults. Nephrol Dial Transpl 2019; 34: 1–42. [DOI] [PubMed] [Google Scholar]
- 45. Tonelli M, James M, Wiebe N, et al. Ultrasound monitoring to detect access stenosis in hemodialysis patients: a systematic review. Am J Kidney Dis 2008; 51: 630–640. [DOI] [PubMed] [Google Scholar]
- 46. Ravani P, Quinn RR, Oliver MJ, et al. Preemptive correction of arteriovenous access stenosis: a systematic review and meta-analysis of randomized controlled trials. Am J Kidney Dis 2016; 67: 446–460. [DOI] [PubMed] [Google Scholar]
- 47. Tessitore N, Poli A. Pro: vascular access surveillance in mature fistulas: is it worthwhile? Nephrol Dial Transpl 2019; 34: 1102–1106. [DOI] [PubMed] [Google Scholar]


