Abstract
Introduction
Cold atmospheric plasma (CAP) has positive effects on wound healing and antimicrobial properties. However, an ongoing challenge is the development of specific modes of application for different clinical indications.
Objectives
We investigated in a prospective pilot study the response and tolerability of a newly developed CAP wound dressing for the acute healing of split skin graft donor sites compared to conventional therapy.
Methods
We applied both treatments to each patient (n = 10) for 7 days and measured 4 parameters of wound healing every other day (i.e., 1,440 measurements) using a hyperspectral imaging camera. Additionally, we evaluated the clinical appearance and pain levels reported by the patients.
Results
The CAP wound dressing was superior to the control (p < 0.001) in the improvement of 3 wound parameters, that is, deep tissue oxygen saturation, hemoglobin distribution, and tissue water distribution. CAP was well tolerated, and pain levels were lower in CAP-treated wound areas.
Conclusion
CAP wound dressing is a promising new tool for acute wound healing.
Keywords: Cold atmospheric plasma, Plasma therapy, Plasma medicine, Wound dressing, Acute wound healing, Split skin graft donor site, Hyperspectral imaging
Introduction
In addition to solid, liquid, and gas, the plasma forms the fourth state of matter, which consists of a mixture of different biologically highly active agents. The plasma contains radical and nonradical reactive oxygen and nitrogen species, electrons and ions, visible light, UV radiation, and electromagnetic fields. The synergistic interaction between these agents is accompanied by immense heat generation [1]. In recent years, much effort has been devoted to developing plasma sources with temperatures below 40°C and comparable properties, that is, cold atmospheric plasma (CAP), which can be applied directly to biological tissues. Over the last decade, CAP has emerged as a new treatment option for different medical indications. Interdisciplinary research groups demonstrated its great potential in several studies for the use in disinfection, wound therapy, reduction of pruritus, pain, and improvement of specific skin diseases [2, 3, 4, 5, 6, 7, 8]. Significant antibacterial, virucidal, fungicidal, and anti-inflammatory effects of the plasma have been reported and supported its use for the sterilization of medical equipment and transplants, and reduction of biofilms on implants. Additionally, CAP significantly reduced the germ load in wounds and dental root canals [4, 9, 10, 11, 12]. Importantly, CAP positively influenced the healing process of chronic and acute wounds [9, 13, 14, 15]. In the future, the plasma could even reduce treatment costs by eliminating the need for lengthy conventional therapies [6, 10, 16, 17, 18].
In our study, we investigated whether a novel and innovative CAP wound dressing could positively affect healing of acute split skin graft donor sites compared to conventional therapy over a 1-week period and how it was tolerated. Every other day, during replacement of wound dressings, we measured deep tissue oxygen saturation (i.e., near-infrared [NIR] perfusion), superficial hemoglobin oxygen saturation (StO2), hemoglobin distribution (i.e., tissue hemoglobin index [THI]), and tissue water distribution (i.e., tissue water index [TWI]) using a modern hyperspectral imaging (HSI) camera device [19, 20, 21]. These parameters provide insights into the biological mechanisms of the healing process and, thus, allow a quantification in the context of the 3 stages of wound healing: inflammation, proliferation, and remodeling [22, 23, 24].
Materials and Methods
Study Design
This study was conducted as an investigator-initiated, prospective, longitudinal, controlled, two-armed, monocenter cohort study in patients who received a split skin graft at the Clinic for Dermatology and Venereology of the University Medical Center Rostock, Germany, from December 2018 to June 2020. Exclusion criteria were pregnancy, age below 18 years, implants (especially if electroconductive), arrhythmia, heart failure during the last 6 months, or suffering from epileptic seizures as mentioned by the CINOGY manual [25]. Split skin graft sides were surgically produced by removal of the epidermis and the uppermost layer of the dermis with a dermatome at a thickness of 0.5 mm. Subsequently, the patients remained in the hospital for 7 days and received a total of 18 CAP applications during their stay (3 per day).
Wound Therapy and Assessment of Pain Intensity
The split skin graft donor site (rectangle) was divided into two comparably large areas. The distal half was treated with CAP using an innovative, sterile, disposable silicone wound dressing (PlasmaDerm® Dress, 10 × 10 cm, CINOGY System GmbH, Duderstadt, Germany), which was applied directly to the wound. PlasmaDerm® Dress is a large, square plaster that can be repetitively connected to an electrical power supply. The applied voltage to create the plasma is pulsed in the range of 300 Hz. The patient leakage current is below the threshold defined by IEC 60601-1 and IEC 60601-1-11. The direct barrier discharge source within the plaster allows treatment of larger wound areas without the need for gas supply using ambient air. It consists of 2 electrodes, of which one is shielded by an insulating layer, formed by the dressing. The skin tissue serves as the counter electrode creating a discharge gap filled with atmospheric air containing filamentous micro-discharges (online suppl. Fig. S1; for all online suppl. material, see www.karger.com/doi/10.1159/000517524). The proximal half served as the control and was treated with our clinic standard practice, a polyhexanide wound gel (Lavanid®, Serag-Wiessner, Neila, Germany) and fatty gauze (Cuticell®, BSN Medical GmbH, Hamburg, Germany). Both dressings were renewed every other day (days 3, 5, and 7; shown in Fig. 1). A flap protruding from the PlasmaDerm® Dress allowed the plasma source to be connected without removing or replacing the dressing. With a distance to the tissue of at least 5 mm, CAP was applied using a single dose of 100–240 V and 1.3–8 VA. We performed the treatment 90 s 3 times a day, with a minimum interval of 5 h, for a total of 7 days in a row. Pain intensity was assessed with a visual analog scale ([VAS]; score 0–10; 0, no pain; 10, maximal pain) separately for the proximal and distal wound half at each day after the third treatment.
Fig. 1.
Study design overview. a Ten Patients received a split skin removal. The site was divided into 2 comparably large areas after the surgery. The distal half was treated with CAP using an innovative, sterile, disposable silicone wound dressing (PlasmaDerm® Dress), which was activated 3 times a day for 90 s. A flap protruding from the PlasmaDerm® Dress allowed the plasma source to be connected without removing or replacing the dressing. The proximal half served as the control and was treated conventionally with a wound gel (Lavanid®) and fatty gauze (Cuticell®). b Both dressings were renewed every 2 days (days 1, 3, 5, and 7). In this process, we measured different wound parameters (superficial and deep blood circulation, TWI, and oxygenation) using a state-of-the-art HSI camera. CAP, cold atmospheric plasma; TWI, tissue water index; HSI, hyperspectral imaging.
Hyperspectral Imaging
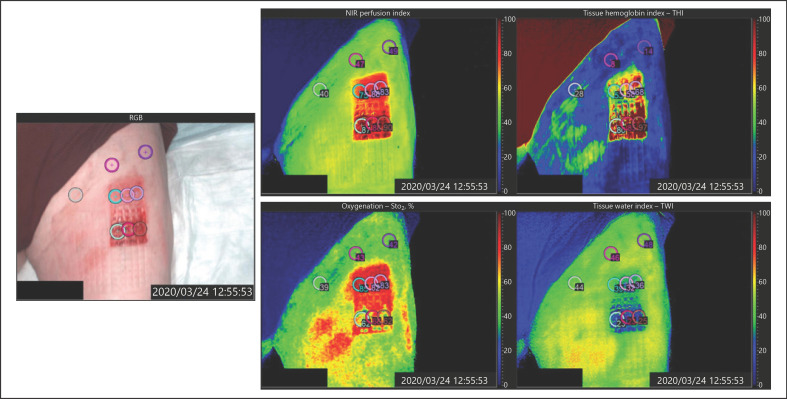
Every other day, during the dressing replacements, the healing of both wound areas was documented using a HSI camera (TIVITA® Wound, Diaspective Vision GmbH, Pepelow, Germany). Notably, the first HSI measurements on day 1 were performed before the start of CAP treatment. The camera measures wound parameters simultaneously, contact-free, noninvasive, and nonionizing. Multiple HSI camera channels measured the intensity of electromagnetic waves emitted by the wound in the range of 500–1,000 nm. Specific wavelength spikes corresponded to biological and molecular conditions at defined tissue spots and depths, and it provided information on the respective wound healing. This way, the HSI camera measured tissue parameters of wound healing such as oxygen saturation in deeper (4–6 mm) tissue layers (NIR-perfusion; index value: 0–100), hemoglobin oxygen saturation (StO2, 1 mm deep; in %), hemoglobin distribution in the microcirculatory system (THI; index value: 1–100), and tissue water distribution (TWI; index value: 0–100) [19, 20, 26]. Hyperspectral images were captured in a dark room at a distance of 50 cm from the wound. If a visible fibrin layer was present in the wound, it was debrided with a ring curette before each capture. The HSI camera generated red, green, and blue standard and spectral images, which quantified values of the 4 different wound healing parameters from blue (low values) to red (high values).
In order to perform spectral analysis with the TIVITA® software, we defined certain circular areas (marks) in the red, green, and blue images, and within these areas, the exact values for the 4 specific parameters in their respective spectral images were then calculated by software. We placed 3 marks on each control and CAP-treated wound area, respectively, and additionally 3 on the nonlesional normal thigh skin, as illustrated in Figure 2. Each parameter was quantified independently and without further offsetting. Altogether, this resulted in 9 measurement points for each of the 4 parameters and 10 patients on 4 days, that is, a total of 1,440 measurements were performed.
Fig. 2.
Images captured by the HSI camera. Spectral images highlighting exact values of the NIR, StO2, THI, and TWI on a scale from 0 (blue) to 100 (red). NIR, near-infrared; StO2, superficial hemoglobin oxygen saturation; THI, tissue hemoglobin index; TWI, tissue water index; HSI, hyperspectral imaging.
Statistics
IBM SPSS Statistics for Windows (version 25, IBM Corp., Armonk, NY, USA) was used for statistical analysis. A linear mixed model was implemented to compare HSI and VAS values for CAP and conventional therapy. Day of treatment, therapeutic modality, age, anticoagulation therapy, and diabetes were considered as fixed, whereas the individual patients were used as random effects. Statistical analysis of individual days was performed using the linear mixed model by splitting data and using patients as random, and the treatment as fixed effects. The normal distribution of the error terms in the linear mixed models was checked with the help of Q−Q plots and histograms of the residuals. A p value of p < 0.05 was regarded as statistically significant in each method. The p values were not adjusted for multiple testing.
Results
Patient Characteristics
Ten patients, who received a split skin removal on the ventral thigh for either covering a tumor excision site, burn wound, or chronic venous leg ulcer, were included in this study (Table 1). The mean age was 74.4 years. Five of them were females and 5 males. One additional patient had to be excluded from the study due to technical reasons and her results were not further considered. The study flow chart and design are depicted in Figure 1.
Table 1.
Patient characteristics
| Variable | Patients (n = 10) |
|---|---|
| Age (mean ± SD), years | 74.4±8.1 |
| Gender, male/female, n | 5/5 |
| Anticoagulation, yes/no, n | 6/4 |
| Diabetes, yes/no, n | 3/7 |
| Arterial hypertension, yes/no, n | 9/1 |
SD, standard deviation.
Hyperspectral Imaging
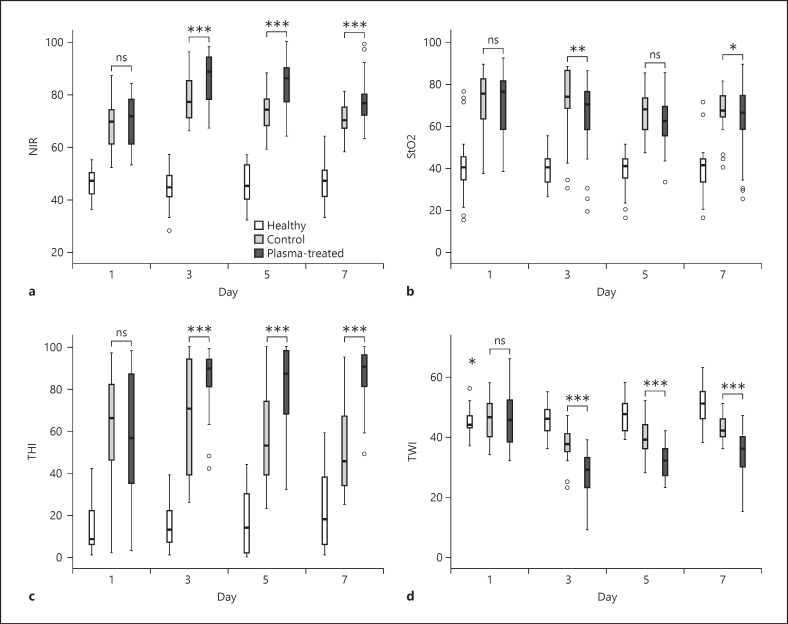
HSI was used to evaluate the healing of both parts of the skin graft donor site, respectively (shown in Fig. 2). Importantly, on day 1 and before the start of the CAP treatment, no differences were observed in any of the HSI parameters. From day 3 onwards (i.e., the second measurements), deep tissue oxygenation (NIR) and hemoglobin distribution (THI) were significantly higher in the CAP-treated wound areas than the standard-treated areas (shown in Fig. 3a, c). Notably, NIR reached its maximum after 3 days in the CAP and control wound region. THI remained increased in the CAP area on days 5 and 7, while it declined in the control area. The linear mixed regression model calculated a difference of +6.83 for NIR (p < 0.001; [4.9, 8.8]) and +19.93 for THI (p < 0.001; [14.8, 25.1]) in CAP-treated areas compared to control areas. TWI decreased by 6.47 (p < 0.001; [−8.1, −4.8]) in the CAP area showing the lowest values after 3 days (shown in Fig. 3d). StO2 was reduced by 4.14 (p = 0.002; [−6.8, −1.5]; shown in Fig. 3b).
Fig. 3.
a–d Effect of plasma treatment over 7 days on the 4 HSI parameters (NIR, StO2, THI, and TWI) of acute skin wounds. Patients with acute split skin graft donor site wounds were treated for 7 days with a conventional wound dressing and a CAP applying dressing on comparably large areas of the wound. Every other day, HSI measured parameter indices (scale 1–100) on both areas. Statistics were analyzed using a linear mixed model on each day separately (*p < 0.05; **p < 0.01; ***p < 0.001). CAP, cold atmospheric plasma; HSI, hyperspectral imaging; NIR, near-infrared perfusion; StO2, superficial hemoglobin oxygen saturation; THI, tissue hemoglobin index; TWI, tissue water index.
Pain Assessment
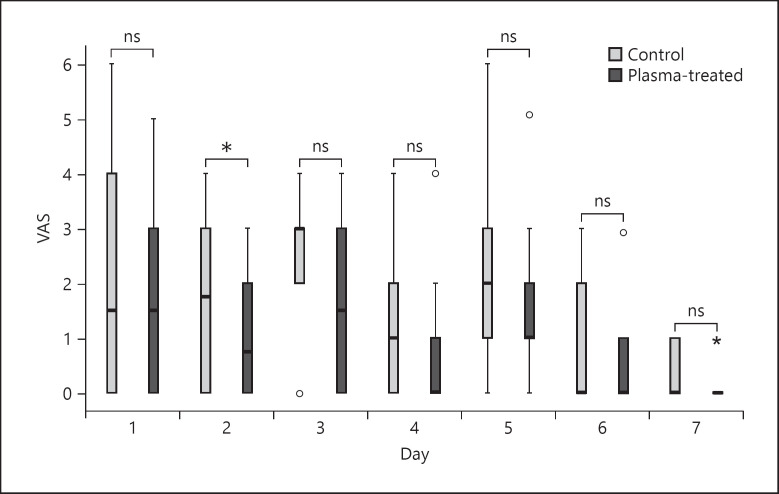
Pain levels were assessed daily and separately for the CAP and conventionally treated areas using VAS (shown in Fig. 4). A significant difference was observed on day 2, with a reduction of 0.7 VAS points (p = 0.025; [0.11, 1.29]). Notably, the first 2 dressing changes (at days 3 and 5) led to a transient increase in pain levels in both wound areas. As expected for the healing of acute wounds, the lowest values of pain in both areas were documented on day 7.
Fig. 4.
Reported pain levels by the patients according to VAS. Statistics were analyzed using a linear mixed model on each day separately. Score 0–10; 0, no pain; 10, maximal pain. *p < 0.05. VAS, visual analog scale.
Clinical Evaluation
All patients in this study showed no signs of irritation, infection, or inflammation in either wound area. In the conventionally treated wound halves, hair follicles were more prominent, and little to no exudate was visible. The CAP-treated area appeared to be more exudative and bloodier, and in some patients, a thick plate of blood clots formed, which was carefully removed with a curette during dressing changes. Nevertheless, wound edges treated with CAP showed earlier signs of reepithelialization than the control area.
Association with Anticoagulant Therapy and Other Patient Parameters
Six patients received anticoagulation therapies before and during the study and 3 patients suffered from type 1 or 2 diabetes (Table 1). Statistical evaluation revealed an association only for age (−0.45 per year; p = 0.018; [−0.79; −0.11]) and anticoagulation therapy (+5.95; p = 0.043; [−0.25; 11.65]) on NIR. Diabetes did not show association with the HSI parameters, and the VAS score was not affected at all (data not shown).
Discussion
We conducted a clinical pilot study with CAP using an innovative wound dressing (PlasmaDerm® Dress) for the acute healing of split skin removal sites on the thigh and revealed that CAP wound dressings significantly increased NIR and THI, and reduced TWI, indicating improved healing of acute skin wounds. Measurements of early wound healing parameters were performed by HSI, using novel spectral analysis software (TIVITA®). HSI provides a standardized, comparable, noninvasive, and fast method to measure tissue parameters and has already been used in other studies for the assessment of the wound healing process [27, 28, 29].
Differences in both treatments were observed from day 3 to 7, which are part of the second phase of wound healing [22]. This phase lasts from day 2 to 10 after injury and is characterized by the formation of a new tissue and especially new blood vessels, called angiogenesis, stimulated by growth factors such as FGF2 or VEGF, which additionally promote proliferation, migration, lymphangiogenesis, and cell survival. CAP contains reactive oxygen species, such as H2O2, OH, and ROO−, which have been shown to stimulate the release of growth hormones and cytokines, including FGF2 [22, 30, 31, 32, 33, 34]. Additionally, multiple studies have demonstrated that CAP activates the endothelial nitric oxide synthase increasing the synthesis of nitric oxide, which has stimulatory effects on wound healing by promoting angiogenesis [35, 36, 37]. Accordingly, previous studies demonstrated increased porcine endothelial cell proliferation and elevated reepithelialization and neovascularization in mice after CAP treatment [21, 38]. These mechanisms are likely reflected by increased NIR and THI in our study.
We also observed a reduction of TWI in the CAP wound dressing treated areas, that is, a lower amount of water in the deeper tissues, which has been associated with increased lymphatic drainage. The removal of tissue water from the wound ground and lymphangiogenesis is a representative of improved wound healing, possibly due to the removal of old and unneeded materials [39, 40, 41].
StO2 was significantly lower in the CAP-treated areas. This observation is contradictory to the results of another study, which showed increased postcapillary blood oxygenation (1–2 mm depth) in the healthy skin after CAP treatment with the PlasmaDerm® FLEX9060-device, that applies no relevant pressure to the upper skin surface [42]. Presumably, due to the superficial skin pressure of the new wound dressing made of a nonabsorbent plastic layer, diminished drainage of wound exudate and reduced StO2 resulted. Further development of the CAP wound dressing composition is warranted to reduce superficial pressure points and might, thus, be also beneficial to enhance StO2 in the future.
As a control treatment, we used an antiseptic wound gel and a fatty gauze, which represents the standard treatment at our clinic for acute split skin graft donor sites. Because of the distinct compositions of the control and CAP wound dressing, the observed differences cannot undoubtedly be attributed to the CAP application itself. However, it is unlikely that the plastic layer of the CAP wound dressing per se had considerable positive effects on the measured HSI parameters.
CAP treatment was well tolerated. Pain decreased in both wound areas during the study treatment and was significantly lower in the CAP-treated areas on day 2. These results are consistent with previous studies, in which CAP was able to reduce pain in skin infections [43].
Remarkably, the novel CAP wound dressing is compact, portable, easily applicable, and could even be performed by patients themselves at home. This kind of treatment can be applied many times over several days without removing the wound dressing and thereby possibly disturbing the healing process.
Although a faster reepithelialization of the wound margins in CAP-treated areas was subjectively noted, our study lasted only 7 days and is therefore not able to quantify changes in the wound size. However, the positive role of CAP on different early wound healing parameters in our study is consistent with other studies, which have evaluated the effect of CAP on acute and chronic wounds and have shown predominately beneficial results [6, 9, 10, 14, 44, 45, 46, 47].
Limitations of our study include its small size and design as a monocentric, prospective, longitudinal cohort study. This study provides positive results of CAP for the healing of acute wounds after surgery as applied through a novel, innovative wound dressing, which is easily applicable and patient-friendly. Further studies with a larger sample size and longer duration are necessary to confirm our findings and thereby further promote the implementation of CAP in clinical practice for the treatment of acute wounds.
Statement of Ethics
This study was approved by the Ethics Committee of the Medical faculty, University of Rostock (study reference: 2018-011 6). Written informed consent was obtained from each patient before participation, and the principles of the Helsinki Declaration were applied. This cohort study is reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline [48].
Conflict of Interest Statement
The hyperspectral camera used for the measurements in this publication was developed by Diaspective Vision GmbH. Philip Wahl is an employee of this company. Diaspective Vision GmbH had no influence in the study design and analysis or interpretation of the data. The other authors have no conflicts of interest to declare.
Funding Sources
This work was supported by the European Social Fund (ESF), reference: ESF/14-BM-A55-0001/18, and the Ministry of Education, Science, and Culture of Mecklenburg-West Pomerania, the Deutsche Forschungsgemeinschaft (DFG EM 63/13-1), the Damp foundation, the Hiege foundation, the “Forschungsförderung der Universitätsmedizin Rostock” (FORUN), and CINOGY System GmbH, Duderstadt, Germany. The funders had no role in the study design, collection, analysis or interpretation of the data, writing of the manuscript, or the decision to submit the paper for publication.
Author Contributions
The study was concepted and designed by S.E. A.W., S.R., J.T., P.W., and S.E. performed the study. A.W., M.H,. F.W., L.B., S.E., and A.T. were responsible for the data analysis and interpretation. A.W., M.H., and F.W. performed the statistical analysis. Administrative, technical, and material support was provided by A.W., P.W., S.R., J.T., S.E., and A.T. A.W. provided the first manuscript draft. M.H. and A.T. reviewed and edited the script. All authors have participated in the critical revision of the manuscript with regard to important intellectual content.
Supplementary Material
Supplementary data
Supplementary data
Acknowledgements
We would like to thank Gundula Jung, Katrin Froh, Christina Henneke, and Stephanie Wollgarten for patient acquisition and support, as well as the entire team of nurses and doctors of the Clinic and Policlinic for Dermatology and Venereology of the University Medical Center Rostock, Germany. Special thanks to Jana Schätzel, who initially helped to implement the study.
References
- 1.Heinlin J, Isbary G, Stolz W, Morfill G, Landthaler M, Shimizu T, et al. Plasma applications in medicine with a special focus on dermatology. J Eur Acad Dermatol Venereol. 2011 Jan;25((1)):1–11. doi: 10.1111/j.1468-3083.2010.03702.x. [DOI] [PubMed] [Google Scholar]
- 2.Tiede R, Emmert S, Plasmabehandlung von Wunden . In: Plasmamedizin. Metelmann HR, von Woedtke T, Weltmann KD, editors. Berlin, Heidelberg: Springer; 2016. pp. p. 73–89. [Google Scholar]
- 3.Fridman G, Friedman G, Gutsol A, Shekhter AB, Vasilets VN, Fridman A. Applied plasma medicine. Plasma Process Polym. 2008 Aug;5((6)):503–33. [Google Scholar]
- 4.Stoffels E, Sakiyama Y, Graves DB. Cold atmospheric plasma: charged species and their interactions with cells and tissues. IEEE Trans Plasma Sci. 2008 Aug;36((4)):1441–57. [Google Scholar]
- 5.Boeckmann L, Bernhardt T, Schäfer M, Semmler ML, Kordt M, Waldner AC, et al. [Current indications for plasma therapy in dermatology] Hautarzt. 2020 Feb;71((2)):109–13. doi: 10.1007/s00105-019-04530-0. [DOI] [PubMed] [Google Scholar]
- 6.Bernhardt T, Semmler ML, Schäfer M, Bekeschus S, Emmert S, Boeckmann L. Plasma medicine: applications of cold atmospheric pressure plasma in dermatology. Oxid Med Cell Longev. 2019 Sep;2019:10. doi: 10.1155/2019/3873928. Article ID 3873928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stratmann B, Costea TC, Nolte C, Hiller J, Schmidt J, Reindel J, et al. Effect of cold atmospheric plasma therapy vs standard therapy placebo on wound healing in patients with diabetic foot ulcers: a randomized clinical trial. JAMA Netw Open. 2020 Jul;3((7)):e2010411. doi: 10.1001/jamanetworkopen.2020.10411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gelker M, Müller-Goymann CC, Viöl W. Plasma permeabilization of human excised full-thickness skin by µs- and ns-pulsed DBD. Skin Pharmacol Physiol. 2020 Jan;33((2)):69–76. doi: 10.1159/000505195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heinlin J, Zimmermann JL, Zeman F, Bunk W, Isbary G, Landthaler M, et al. Randomized placebo-controlled human pilot study of cold atmospheric argon plasma on skin graft donor sites. Wound Repair Regen. 2013 Nov;21((6)):800–7. doi: 10.1111/wrr.12078. [DOI] [PubMed] [Google Scholar]
- 10.Isbary G, Heinlin J, Shimizu T, Zimmermann JL, Morfill G, Schmidt HU, et al. Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: results of a randomized controlled trial. Br J Dermatol. 2012 Aug;167((2)):404–10. doi: 10.1111/j.1365-2133.2012.10923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong MG, Kroesen G, Morfill G, Nosenko T, Shimizu T, Van Dijk J, et al. Plasma medicine: an introductory review. New J Phys. 2009 Nov;11((11)):115012. [Google Scholar]
- 12.Nosenko T, Shimizu T, Morfill GE. Designing plasmas for chronic wound disinfection. New J Phys. 2009 Nov;11((11)):115013. [Google Scholar]
- 13.Chuangsuwanich A, Assadamongkol T, Boonyawan D. The healing effect of low-temperature atmospheric-pressure plasma in pressure ulcer: a randomized controlled trial. Int J Low Extrem Wounds. 2016 Dec;15((4)):313–9. doi: 10.1177/1534734616665046. [DOI] [PubMed] [Google Scholar]
- 14.Metelmann H-R, Vu TT, Do HT, Le TNB, Hoang THA, Phi TTT, et al. Scar formation of laser skin lesions after cold atmospheric pressure plasma (CAP) treatment: a clinical long term observation. Clin Plasma Med. 2013 Jun;1((1)):30–5. [Google Scholar]
- 15.Isbary G, Stolz W, Shimizu T, Monetti R, Bunk W, Schmidt H-U, et al. Cold atmospheric argon plasma treatment may accelerate wound healing in chronic wounds: results of an open retrospective randomized controlled study in vivo. Clin Plasma Med. 2013 Dec;1((2)):25–30. [Google Scholar]
- 16.Rutkowski R, Daeschlein G, von Woedtke T, Smeets R, Gosau M, Metelmann HR. Long-term risk assessment for medical application of cold atmospheric pressure plasma. Diagnostics. 2020 Apr;10((4)):210. doi: 10.3390/diagnostics10040210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmermann JL, Shimizu T, Schmidt H-U, Li Y-F, Morfill GE, Isbary G. Test for bacterial resistance build-up against plasma treatment. New J Phys. 2012 Jul;14((7)):073037. [Google Scholar]
- 18.Weltmann K-D, von Woedtke T. Basic requirements for plasma sources in medicine. Eur Phys J Appl Phys. 2011 Jul;55((1)):13807. [Google Scholar]
- 19.Sucher R, Athanasios A, Köhler H, Wagner T, Brunotte M, Lederer A, et al. Hyperspectral Imaging (HSI) in anatomic left liver resection. Int J Surg Case Rep. 2019 Aug;62:108–11. doi: 10.1016/j.ijscr.2019.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daeschlein G, Rutkowski R, Lutze S, von Podewils S, Sicher C, Wild T, et al. Hyperspectral imaging: innovative diagnostics to visualize hemodynamic effects of cold plasma in wound therapy. Biomed Tech. 2018 Oct;63((5)):603–8. doi: 10.1515/bmt-2017-0085. [DOI] [PubMed] [Google Scholar]
- 21.Kalghatgi S, Friedman G, Fridman A, Clyne AM. Endothelial cell proliferation is enhanced by low dose non-thermal plasma through fibroblast growth factor-2 release. Ann Biomed Eng. 2010 Mar;38((3)):748–57. doi: 10.1007/s10439-009-9868-x. [DOI] [PubMed] [Google Scholar]
- 22.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008 May;453((7193)):314–21. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 23.Guo JW, Pu CM, Liu CY, Lo SL, Yen YH. Curcumin-loaded self-microemulsifying gel for enhancing wound closure. Skin Pharmacol Physiol. 2021 Feb;33((6)):300–8. doi: 10.1159/000512122. [DOI] [PubMed] [Google Scholar]
- 24.Stuermer EK, Besser M, Terberger N, Bachmann HS, Severing AL. Side effects of frequently used antihypertensive drugs on wound healing in vitro. Skin Pharmacol Physiol. 2019 May;32((3)):162–72. doi: 10.1159/000499433. [DOI] [PubMed] [Google Scholar]
- 25.CINOGY System GmbH, PlasmaDerm [Internet] [cited 2021 Mar 14] Available from: https://plasmaderm.de/faq/
- 26.Lendeckel D, Eymann C, Emicke P, Daeschlein G, Darm K, O'Neil S, et al. Proteomic changes of tissue-tolerable plasma treated airway epithelial cells and their relation to wound healing. Biomed Res Int. 2015 Oct;2015:506059–17. doi: 10.1155/2015/506059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu G, Fei B. Medical hyperspectral imaging: a review. J Biomed Opt. 2014 Jan;19((1)):10901. doi: 10.1117/1.JBO.19.1.010901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wild T, Becker M, Winter J, Schuhschenk N, Daeschlein G, Siemers F. Hyperspectral imaging of tissue perfusion and oxygenation in wounds: assessing the impact of a micro capillary dressing. J Wound Care. 2018 Jan;27((1)):38–51. doi: 10.12968/jowc.2018.27.1.38. [DOI] [PubMed] [Google Scholar]
- 29.Daeschlein G, Langner I, Wild T, Von Podewils S, Sicher C, Kiefer T, et al. Hyperspectral imaging as a novel diagnostic tool in microcirculation of wounds. Clin Hemorheol Microcirc. 2017 Dec;67((3–4)):467–74. doi: 10.3233/CH-179228. [DOI] [PubMed] [Google Scholar]
- 30.Arjunan KP, Friedman G, Clyne AM. ASME 2011 Summer Bioengineering Conference, SBC. American Society of Mechanical Engineers; 2011. Jun, Non-thermal dielectric barrier discharge plasma promotes vascularization through reactive oxygen species; pp. p. 611–2. [Google Scholar]
- 31.Yu Y, Tan M, Chen H, Wu Z, Xu L, Li J, et al. Non-thermal plasma suppresses bacterial colonization on skin wound and promotes wound healing in mice. J Huazhong Univ Sci Technolog Med Sci. 2011 Jun;31((3)):390–4. doi: 10.1007/s11596-011-0387-2. [DOI] [PubMed] [Google Scholar]
- 32.Weich HA, Iberg N, Klagsbrun M, Folkman J. Transcriptional regulation of basic fibroblast growth factor gene expression in capillary endothelial cells. J Cell Biochem. 1991 Oct;47((2)):158–64. doi: 10.1002/jcb.240470209. [DOI] [PubMed] [Google Scholar]
- 33.de Araújo R, Lôbo M, Trindade K, Silva DF, Pereira N. Fibroblast growth factors: a controlling mechanism of skin aging. Skin Pharmacol Physiol. 2019 Jul;32((5)):275–82. doi: 10.1159/000501145. [DOI] [PubMed] [Google Scholar]
- 34.Luengas-Martinez A, Hardman-Smart J, Rutkowski D, Purba TS, Paus R, Young HS. Vascular endothelial growth factor blockade induces dermal endothelial cell apoptosis in a clinically relevant skin organ culture model. Skin Pharmacol Physiol. 2020 Jul;33((3)):110–8. doi: 10.1159/000508344. [DOI] [PubMed] [Google Scholar]
- 35.Duchesne C, Banzet S, Lataillade JJ, Rousseau A, Frescaline N. Cold atmospheric plasma modulates endothelial nitric oxide synthase signalling and enhances burn wound neovascularisation. J Pathol. 2019 Nov;249((3)):368–80. doi: 10.1002/path.5323. [DOI] [PubMed] [Google Scholar]
- 36.Suschek CV, Opländer C. The application of cold atmospheric plasma in medicine: the potential role of nitric oxide in plasma-induced effects. Clin Plasma Med. 2016 Jul;4((1)):1–8. [Google Scholar]
- 37.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008 Feb;7((2)):156–67. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 38.Arjunan KP, Friedman G, Fridman A, Clyne AM. Non-thermal dielectric barrier discharge plasma induces angiogenesis through reactive oxygen species. J R Soc Interface. 2012 Jan;9((66)):147–57. doi: 10.1098/rsif.2011.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Komatsu E, Nakajima Y, Mukai K, Urai T, Asano K, Okuwa M, et al. Lymph drainage during wound healing in a hindlimb lymphedema mouse model. Lymphat Res Biol. 2017 Mar;15((1)):32–8. doi: 10.1089/lrb.2016.0026. [DOI] [PubMed] [Google Scholar]
- 40.Cho CH, Sung HK, Kim KT, Cheon HG, Oh GT, Hong HJ, et al. COMP-angiopoietin-1 promotes wound healing through enhanced angiogenesis, lymphangiogenesis, and blood flow in a diabetic mouse model. Proc Natl Acad Sci U S A. 2006 Mar;103((13)):4946–51. doi: 10.1073/pnas.0506352103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tammela T, Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 2010 Feb;140((4)):460–76. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 42.Borchardt T, Ernst J, Helmke A, Tanyeli M, Schilling AF, Felmerer G, et al. Effect of direct cold atmospheric plasma (diCAP) on microcirculation of intact skin in a controlled mechanical environment. Microcirculation. 2017 Nov;24((8)):e12399. doi: 10.1111/micc.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Isbary G, Shimizu T, Zimmermann JL, Heinlin J, Al-Zaabi S, Rechfeld M, et al. Randomized placebo-controlled clinical trial showed cold atmospheric argon plasma relieved acute pain and accelerated healing in herpes zoster. Clin Plasma Med. 2014 Dec;2((2)):50–5. [Google Scholar]
- 44.Isbary G, Zimmermann JL, Shimizu T, Li Y-F, Morfill GE, Thomas HM, et al. Non-thermal plasma: more than five years of clinical experience. Clin Plasma Med. 2013 Jun;1((1)):19–23. [Google Scholar]
- 45.Brehmer F, Haenssle HA, Daeschlein G, Ahmed R, Pfeiffer S, Görlitz A, et al. Alleviation of chronic venous leg ulcers with a hand-held dielectric barrier discharge plasma generator (PlasmaDerm ® VU-2010): results of a monocentric, two-armed, open, prospective, randomized and controlled trial ( NCT01415622) J Eur Acad Dermatol Venereol. 2015 Jan;29((1)):148–55. doi: 10.1111/jdv.12490. [DOI] [PubMed] [Google Scholar]
- 46.Zhang J-P, Guo L, Chen Q-L, Zhang K-Y, Wang T, An G-Z, et al. Effects and mechanisms of cold atmospheric plasma on skin wound healing of rats. Contrib Plasma Phys. 2019 Jan;59((1)):92–101. [Google Scholar]
- 47.Kubinova S, Zaviskova K, Uherkova L, Zablotskii V, Churpita O, Lunov O, et al. Non-thermal air plasma promotes the healing of acute skin wounds in rats. Sci Rep. 2017 May;7((1)):45183. doi: 10.1038/srep45183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007 Oct;147((8)):W163. doi: 10.7326/0003-4819-147-8-200710160-00010-w1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data