Abstract
Trichomonas vaginalis is a globally common sexually transmitted human parasite. Many strains of T. vaginalis from around the world have been described to be resistant to the current drug of choice, metronidazole. However, only a few cases of metronidazole resistance have been reported from Europe. The resistant strains cause prolonged infections which are difficult to treat. T. vaginalis infection also increases the risk for human immunodeficiency virus transmission. We present a practical method for determining the resistance of T. vaginalis to 5-nitroimidazoles. The suggested method was developed by determining the MICs and minimal lethal concentrations (MLCs) of metronidazole and ornidazole for T. vaginalis under various aerobic and anaerobic conditions. Using this assay we have found the first three metronidazole-resistant strains from Finland, although the origin of at least one of the strains seems to be Russia. Analysis of the patient-derived and previously characterized isolates showed that metronidazole-resistant strains were also resistant to ornidazole, and MLCs for all strains tested correlated well with the MICs. The suggested MICs of metronidazole for differentiation of sensitive and resistant isolates are >75 μg/ml in an aerobic 24-h assay and >15 μg/ml in an anaerobic 48-h assay.
Trichomonas vaginalis is a flagellated protozoan and is the most common parasite that causes sexually transmitted disease (24). The World Health Organization has estimated that there are annually approximately 170 million infections worldwide (43). The main clinical manifestations of T. vaginalis infections are vaginitis, urethritis, and prostatitis. In addition, infection with trichomonads can increase the risk of pelvic inflammatory disease and tubal infertility (2). Since probably almost 50% of trichomonal infections are asymptomatic (8), the infection can spread easily.
Infection with T. vaginalis may increase the risk of human immunodeficiency virus (HIV) type 1 (HIV-1) transmission. In developing countries T. vaginalis is very common in the areas with the highest prevalence of HIV-1 infections (15). Increased shedding of HIV-1 in the semen in the presence of urethral infection has been described (14). It has been postulated that the increased amount of HIV-infected CD4+ lymphocytes in the walls of the gynecological tract could facilitate congenital infections with HIV-1 (20, 36).
The 5-nitroimidazoles, especially metronidazole, are the most widely used antimicrobial agents for the treatment of trichomoniasis. The mechanism of the protozooicidal effect of the 5-nitroimidazoles is thought to involve generation of nitro radicals, which leads to subsequent DNA damage and cell death. Under normal circumstances T. vaginalis cells use pyruvate-ferrodoxin oxidoreductase (PFOR) and ferrodoxin-linked enzymes to oxidize acetate from pyruvate via acetyl coenzyme A to gain ATP (6). When a nitroimidazole is present, its nitro groups capture electrons from ferrodoxin and nitro radicals are generated, leading to cell damage.
Since the introduction of 5-nitroimidazoles in the 1960s there have been reports of at least 100 metronidazole-resistant strains of T. vaginalis from the United States (5, 9, 12, 16, 21, 23, 29, 31, 32, 35). Only under 20 metronidazole-resistant strains have been described from Europe (3, 7, 12, 16, 19, 26, 40, 41). In addition, some preliminary reports have been published, for example, from Russia (18) and Africa (13). To overcome the clinical problems of metronidazole resistance, higher doses of metronidazole have usually been used to treat the patients (11, 22). Also, despite the cross-resistance with other 5-nitroimidazoles, tinidazole (10) and ornidazole (19) have been used to treat some patients infected with metronidazole-resistant strains of T. vaginalis. Furazolidone, a nitrofuran, has also shown trichomonicidal activity in vitro (30). Paromomycin has been used as a topical treatment for some patients with allergy to metronidazole or infections caused by metronidazole-resistant strains of T. vaginalis (3, 31).
Resistance to nitroimidazoles has been studied by growing T. vaginalis strains in the presence of different drug concentrations under aerobic and anaerobic conditions in vitro. Resistance has been suggested to be dependent on one or more of the following: a reduced PFOR enzyme activity (19), an altered conformation of the hydrogenosome (39), a ferrodoxin with an exceptional redox potential (44), or a reduced amount of intracellular ferrodoxin (27). In the resistant strains the intracellular amounts of ferrodoxin are decreased by over 50% and the rate of ferrodoxin gene transcription is reduced by as much as 40 to 65% compared to those in sensitive isolates (33). Many of the suggested mechanisms lead to fermentation of pyruvate in the cytosol instead of the hydrogenosome, which means that the action of the 5-nitroimidazole drug is inhibited. The aerobic resistance to nitroimidazoles is thought to be enhanced by free O2 in the cytosol, as it is a potent electron acceptor from nitro radicals and thus could inhibit the action of the drug (6). The suggested mechanisms that lead to an excess of intracellular oxygen include decreased amounts of oxidases and a decreased affinity of terminal oxidases to oxygen (39).
In this report we suggest a practical in vitro method for determination of the metronidazole susceptibility of T. vaginalis in advanced clinical microbiology laboratories and report on three clinically metronidazole-resistant and in vitro metronidazole-resistant strains of T. vaginalis from Finland. The susceptibility to metronidazole was tested under various oxygen concentrations. The susceptibilities of the characterized strains of T. vaginalis to metronidazole and ornidazole were compared to those of a set of patient-derived strains.
MATERIALS AND METHODS
Reagents.
Metronidazole and ornidazole were purchased from Sigma Chemical Co. (St. Louis, Mo.) and were dissolved in 0.15 M phosphate buffer (pH 6.4) for the susceptibility assays just before use. Dimethyl sulfoxide (DMSO) was purchased from Merck & Co. (Darmstadt, Germany).
T. vaginalis strains and cultivation.
T. vaginalis strains were cultured in 7-ml Wassermann glass tubes containing 5 ml of Trypticase-yeast extract-maltose (TYM) medium (4) complemented with 10% heat-inactivated horse serum and a combination of 100 IU of streptomycin (Sigma) per ml and penicillin (Orion Diagnostica, Espoo, Finland).
The characterized metronidazole-resistant (28) and -sensitive (29) strains of T. vaginalis were obtained from the American Type Culture Collection (ATCC; ATCC 50143 and ATCC 50148, respectively) and were treated as described below for the patient-derived isolates.
Patient-derived strains of T. vaginalis were obtained from female Finnish patients. The vaginal samples were originally placed into Vagicult tubes (Orion Diagnostica), which have been developed for the diagnostic cultivation of T. vaginalis and yeast. To continue the culture an aliquot of 0.5 ml was taken from each positive culture and was transferred to TYM medium. Aliquots of the culture suspensions containing live T. vaginalis trophozoites (0.2 to 1.0 ml) were transferred to the bottoms of prewarmed new culture tubes three times a week. After 1 to 2 weeks aliquots of each strain were frozen (−70°C) with DMSO by a previously described method (42). For each assay new vials were thawed. After thawing the strains were cultured for approximately 7 days to collect enough trophozoites. The possible presence of bacteria or yeasts was examined by cultivating samples of all T. vaginalis cultures in a thioglycolate medium (37°C for 7 days) and on plates with blood or chocolate agar (CO2 atmosphere) or fastidious anaerobic agar (anaerobic atmosphere). Samples from thioglycolate medium-containing tubes with any cloudy patches were cultivated on the plates. All cultivations were performed by standard bacteriological methods.
Species identification by PCR.
The species of all the strains was analyzed by PCR. In the PCR assay a highly conserved repeated DNA stretch specific for T. vaginalis was amplified with primers TVK3 and TVK4, which have been described elsewhere (17).
5-Nitroimidazole susceptibility assays.
To examine the susceptibilities of the T. vaginalis strains to metronidazole and ornidazole under aerobic and anaerobic conditions, the trophozoites were cultured on 24-well tissue culture plates (Greiner, Gloucestershire, United Kingdom) in airtight jars with adjustable ventilators on the lid. Three plates with duplicate wells for each drug concentration were used in each suspectibility assay. All assays were run twice. Fresh TYM medium (200 μl) was mixed with 200 μl of prediluted 5-nitroimidazole (in 0.15 M phosphate buffer [pH 6.4]) in each well, and subsequently, 100 μl of medium containing live T. vaginalis trophozoites was added to each well. The final concentration of motile trophozoites was adjusted to approximately 105/ml in each well. The drug dilution buffer (phosphate buffer [pH 6.4]) was used as a positive control on each plate.
Anaerobic circumstances were created with the chemical oxygen consumption plate Anaerocult A (Merck & Co.) and were monitored with an anaerobic indicator (Oxoid, Hampshire, England). All incubations were performed at 37°C. The aerobic conditions were created by two different methods. The first assay used the same jars used for the anaerobic assay, but the ventilators were left open for access to room air. In the second assay the susceptibility of T. vaginalis to 5-nitroimidazoles was tested in the presence of different concentrations of oxygen in the culture atmosphere. The amounts of oxygen, nitrogen, and carbon dioxide were adjusted by using bottled gas (AGA Corp., Helsinki, Finland) and separate flow meters (Brooks Instrument B. V., Veeneendaal, The Netherlands). The purities of the gases used were 98, 99, and 97% for N2, O2, and CO2, respectively. The N2 and CO2 were free of O2. To prepare an anaerobic environment, 95% N2 and 5% CO2 were used. To prepare various aerobic environments, different amounts of O2 (0, 5, 10, 20, and 30%) were used to partially replace the N2. The gas mixture was allowed to flow through the inlet and outlet of the anaerobic jar for 30 min at a rate of 1,600 ml/h before the incubation and after the first check at 24 h. Anaerobic circumstances were monitored with an anaerobic indicator (Oxoid).
Determination of MICs and MLCs.
The viabilities of the trophozoites in the culture plate wells were assessed by examining visually the motilities of the cells with an inverted microscope at a ×400 magnification after 24 and 48 h of incubation. The MIC was the lowest concentration of the drug in the well in which no motile cells were detected. To check whether the evaluation of viability (MIC) reflected the effectiveness of the drug at actually killing the cells, the minimal lethal concentrations (MLCs) were also tested after cultivation of the trophozoites in the presence of different oxygen concentrations. The contents of each of the wells were inoculated into the bottoms of tubes containing 5 ml of fresh TYM medium, and the tubes were examined for the presence of motile T. vaginalis trophozoites after 5 and 10 days of cultivation.
Clinical data.
The first (strain 1) and second (strain 2) clinically metronidazole-resistant isolates of T. vaginalis were obtained from female patients living in the Helsinki district in Finland. The third strain (strain 3) was from a female patient who is from the eastern part of Finland and whose husband had often visited Russia. All patients suffered from recurrent trichomonal infections and were treated with several courses (6, 3, and 10 courses for patients 1, 2, and 3, respectively) of metronidazole with a standard or an increased dose. All three patients were hospitalized and were treated with metronidazole intravenously (patient 1 with 1 g a day for 3 days, patient 2 with 500 mg four times a day for 7 days complemented with 100 g of metronidazole vaginally once a day for 10 days, and patient 3 with 750 mg three times a day for 5 days). After the treatment patients were symptomless for a follow-up period of 3 months, and the cultures of their samples were also negative for T. vaginalis.
RESULTS
Anaerobic MICs of metronidazole.
The MICs of two 5-nitroimidazoles (metronidazole and ornidazole) for the different T. vaginalis strains were obtained under both aerobic and anaerobic conditions. The MICs of metronidazole were also determined under various oxygen concentrations. The MLCs were determined by cultivating the trophozoites in fresh nitroimidazole-free medium after incubation with metronidazole.
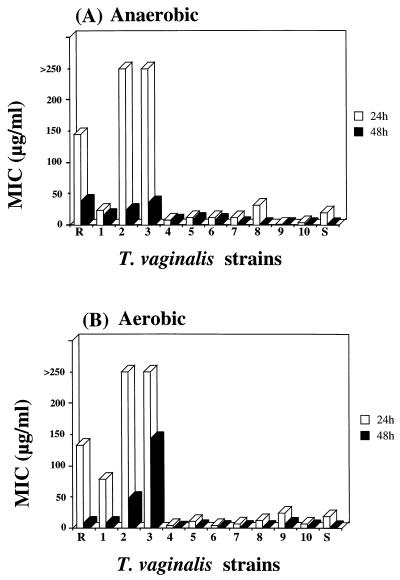
In the anaerobic assay the MICs of metronidazole were determined by cultivating the known resistant isolate (strain R), clinically resistant isolates (strains 1 to 3), the known sensitive isolate (strain S), and clinically sensitive isolates (strains 4 to 10) of T. vaginalis in the presence of different concentrations of metronidazole. Parasite motility was evaluated microscopically after 24 and 48 h. At the 24-h time point the MIC of metronidazole was 145 μg/ml for the known resistant strain (strain R), 23 μg/ml for clinically resistant strain 1, over 250 μg/ml for clinically resistant strains 2 and 3, 20 μg/ml for the known sensitive strain (strain S), and from 8.5 to 12 μg/ml for clinically sensitive strains 4 to 10. At the 48-h time point the MICs were 39 μg/ml (strain R), 16 μg/ml (strain 1), 24 μg/ml (strain 2), 36 μg/ml (strain 3), and from 1.0 to 9.8 μg/ml (strains 4 to 10) (Fig. 1A).
FIG. 1.
Differences in susceptibilities of T. vaginalis strains to metronidazole (MIC) under anaerobic (A) and aerobic (B) conditions in vitro. The known resistant strain is marked R, the known sensitive strain is marked S, strains 1 to 3 are patient-derived clinically resistant strains, and strains 4 to 10 are patient-derived clinically sensitive isolates.
Aerobic MICs.
In the first aerobic assay the sensitivity of T. vaginalis to metronidazole was tested under ordinary room air conditions at 37°C. The MICs of metronidazole at the 24-h time point were 133 μg/ml for the known resistant strain (strain R), 77 μg/ml for clinically resistant strain 1, over 250 μg/ml for strains 2 and 3, 18 μg/ml for the known sensitive strain (strain S), and from 3.5 to 10 μg/ml for strains 4 to 10. After 48 h of cultivation the MICs were 8.4 μg/ml (strain R), 7.8 μg/ml (strain 1), 48 μg/ml (strain 2), 140 μg/ml (strain 3), 1.0 μg/ml (strain S), and from 1.0 to 2.0 μg/ml (strains 4 to 10) (Fig. 1B).
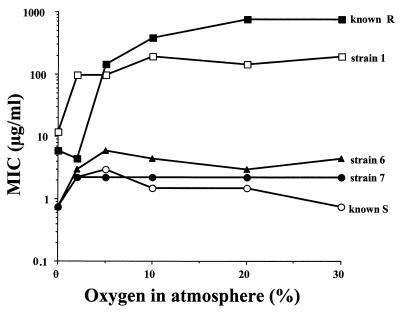
As the results of anaerobic and aerobic resistance for some strains, especially clinically resistant isolate 1, differed, a second aerobic sensitivity assay was performed to examine the effect of oxygen on drug tolerance. The T. vaginalis trophozoites were cultivated in the presence of different amounts of metronidazole under various concentrations of oxygen (0, 5, 10, 20, and 30%) in the atmosphere. It was found that the MIC of metronidazole for known resistant strain R was approximately 100 times higher than that for sensitive strain S when the amount of oxygen in the atmosphere exceeded 2% (Fig. 2). The corresponding MICs of metronidazole for clinically resistant strain 1 were approximately 20 times higher than those for sensitive strains 6 and 7.
FIG. 2.
Effects of different oxygen concentrations in the culture atmosphere on metronidazole susceptibilities of selected T. vaginalis strains. The known resistant strain is marked R, the known sensitive is marked S, strain 1 is a clinically resistant isolate, and strains 6 and 7 are clinically sensitive isolates.
MICs of ornidazole.
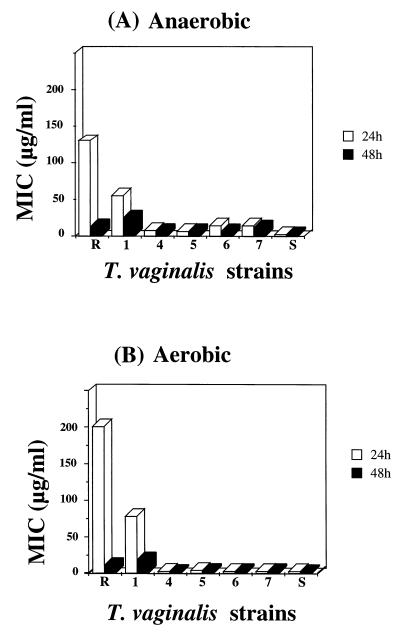
The MICs of ornidazole were determined for the known resistant strain (strain R), clinically resistant strain 1, the known sensitive strain (strain S), and clinically sensitive strains 4 to 7 in both the anaerobic and the aerobic assays. In the anaerobic assay the MICs of ornidazole after 24 h were 133 μg/ml for strain R, 37 μg/ml for strain 1, 2.0 μg/ml for strain S, and from 4.0 to 8.0 μg/ml for strains 4 to 7. After 48 h of cultivation the MICs of ornidazole were 8.4 μg/ml (strain R), 17 μg/ml (strain 1), 2.0 μg/ml (strain S), and from 4.0 to 8.0 μg/ml (strains 4 to 7) (Fig. 3A). In the aerobic assay the MICs were 200 μg/ml (strain R), 78 μg/ml (strain 1), 2.0 μg/ml (strain S), and from 2.0 to 4.0 μg/ml (strains 4 to 7) after 24 h of cultivation and 10 μg/ml (strain R), 18 μg/ml (strain 1), 1.0 μg/ml (strain S), and from 1.0 to 2.0 μg/ml (strains 4 to 7) after 48 h of cultivation (Fig. 3B).
FIG. 3.
Differences in susceptibilities of T. vaginalis to ornidazole (MIC) under anaerobic (A) and aerobic (B) conditions in vitro. The known resistant strain is marked R. Strain 1 is a clinically resistant isolate, and strains 4 to 7 are clinically sensitive isolates.
MLCs of metronidazole.
The MLCs were measured from the second aerobic sensitivity assay. While the MIC was determined by microscopic evaluation on the basis of cell motility at different drug concentrations, the MLC was assayed to reveal the actual lethal concentration on the basis of the growth after 5 and 10 days in fresh medium after exposure to different concentrations of the drug. When no oxygen was present the MLC for strains 1 and R was 6.0 μg/ml and the MLC for the clinically sensitive strains (strains S, 6, and 7) was 0.75 μg/ml (Table 1). The MLCs varied from 6 to 100% of the corresponding MICs. When the concentration of oxygen was ≥10% the sensitive strains died even in the absence of the drug.
TABLE 1.
Effect of oxygen on MLCs of metronidazole for five strains of T. vaginalis after 48 h of incubation in the presence of different oxygen concentrations in the atmosphere
| % O2 | Strain R
|
Strain S
|
Strain 1a
|
Strain 6b
|
Strain 7b
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| MLC (μg/ml) | MLC/MIC (%) | MLC (μg/ml) | MLC/MIC (%) | MLC (μg/ml) | MLC/MIC (%) | MLC (μg/ml) | MLC/MIC (%) | MLC (μg/ml) | MLC/MIC (%) | |
| 0 | 6.0 | 100 | 0.75 | 100 | 6.0 | 50 | 0.75 | 100 | 0.75 | 43 |
| 2 | 1.5 | 33 | 1.5 | 50 | 48 | 50 | 0.75 | 25 | 1.5 | 66 |
| 5 | 24.0 | 16 | 1.5 | 50 | 48 | 50 | <0.75 | 12 | 1.5 | 66 |
| 10 | 48.0 | 13 | 1.5 | 100 | 48 | 25 | <0.75 | 16 | 1.5 | 66 |
| 20 | 48.0 | 6 | C−c | C− | 48 | 33 | C− | C− | 1.75 | 77 |
| 30 | 48.0 | 6 | C− | C− | 24 | 12 | C− | C− | C− | C− |
Treatment-resistant patient-derived strain of T. vaginalis.
Treatment-sensitive patient-derived strains of T. vaginalis.
C−, control cells incubated without metronidazole died.
DISCUSSION
In the present study we have tested different in vitro conditions for determination of the 5-nitroimidazole resistance of T. vaginalis and suggest a relatively simple and easily interpretable method for laboratory testing of strains suspected of being metronidazole resistant. We describe the analysis of the first three metronidazole-resistant T. vaginalis strains, all of which were finally eradicated by intravenous metronidazole therapy.
Testing of drug susceptibility in vitro is necessary to ensure that the long-lasting T. vaginalis infection is really caused by a nonsensitive strain and that the patient is not experiencing a recurrent infection. We tested the susceptibilities of different resistant and sensitive strains of T. vaginalis under various conditions and at various time points. As a conclusion, we suggest that a combination of an aerobic 24-h cultivation and an anaerobic 48-h cultivation of patient-derived strains in the presence of different concentrations of metronidazole can be used to determine whether their susceptibilities to metronidazole are decreased. One of the patient-derived resistant strains (strain 1) described in this report was concluded to be a resistant isolate only from the aerobic assay. Anaerobic 24-h MICs were only slightly elevated for clinically resistant strain 1 and differed from those for the drug-susceptible control (strain S) only by 3 μg/ml.
Aerobic resistance has also been induced in vitro, and these strains were also drug sensitive under anaerobic conditions (38). Thus, both assays are needed in the laboratory analysis (5). A suitable anaerobic susceptibility testing protocol for diagnostics (InPouchTV test) is commercially available (1), but it should be complemented by an aerobic assay.
Interpretation of the MICs or MLCs for T. vaginalis has not been based on any widely accepted scheme. Some investigators have used a method in which the MIC is the lowest concentration of drug at which cell growth takes place and the MLC is the lowest concentration with motile cells. Both definitions are based on visual observation after a 48-h cultivation (23, 25, 28, 29, 38). In some studies the MLC is the original lowest drug concentration at which growth of the parasites in a drug-free medium no longer takes place (1, 12, 19). According to Narcici and Secor (30), the MIC of metronidazole was approximately fourfold higher than the concentration (MLC) that killed the isolates, but no data were presented. It seems that in the MIC assay the trophozoites had suffered irreversible damage but still retained motility. Narcici and Secor (30) used a 48-h cultivation for the determination of possible growth in drug-free medium. However, for some isolates it may take longer to recover from the effect of the drug because we found viable trophozoites after 5 days of cultivation. According to the present study the MIC for T. vaginalis is a suitable indicator of the actual MLC and can be used for diagnostic assessments.
Different threshold concentrations of the 5-nitroimidazole drugs for resistance have been proposed by many investigators. Müller et al. (28) have reported MLCs for 199 T. vaginalis strains and the treatment outcomes for the corresponding patients. According to their interpretation, the MLCs for a treatment-resistant strain were >100 μg/ml in an aerobic assay and >3.1 μg/ml in an anaerobic assay. They also found that treatment success overlapped between susceptible and resistant isolates. We found that following an aerobic 24-h cultivation and an anaerobic 48-h cultivation the MICs for clinically resistant isolates were >75 and >15 μg/ml, respectively. For clinically sensitive strains MICs were considerably lower in both assays (<19 and <10 μg/ml, respectively), and these values did not overlap with the values for the resistant strains. Many different methods are used in laboratories to test for drug susceptibility, and it is not possible to define accurate threshold values for resistant and sensitive strains. There is also uncertainty whether the MIC or MLC can be used as such to determine the suitable drug dosage for the patient (23, 34). We suggest that a patient-derived strain is judged to be resistant after comparing it to a known, highly resistant strain by an easily interpretable method.
In this study we found three patient samples with T. vaginalis strains that were clearly resistant to metronidazole in vitro. The finding correlated well with the clinical data for resistance to treatment. In earlier reports patients who carry resistant strains of T. vaginalis have usually been treated with longer courses of standard doses of metronidazole or with a considerably higher dosage (23). All patients described in this paper were initially treated with several courses of metronidazole per os. Finally, trichomonads were eradicated by using metronidazole intravenously. Some reports have suggested that other 5-nitroimidazoles, like tinidazole, ornidazole (19), or furazolidone (30), are curative for metronidazole-resistant isolates, and some reports have shown the opposite (7). However, oral metronidazole as a single dose of 1.5 g is still the drug of choice for the treatment of trichomoniasis (37), and the susceptibility assay is indicated only when the standard metronidazole dosage is repeatedly incapable of eradicating the infection from the patient and the partner(s). When the MIC for a patient-derived strain is high by a reliable assay, we suggest that the patient be treated with as high a dosage of the drug as possible to treat the infection and to prevent it from spreading.
The origins of our first two treatment-resistant T. vaginalis strains (strains 1 and 2) are not exactly known. The third strain (strain 3) came from a patient in the eastern part of Finland and is likely to have originated from a partner who had visited Russia several times. Tourism between Western Europe and Russia has increased a lot, and part of it is sex tourism in and out of Russia. No statistical data from Russia on how common T. vaginalis infections are and the current status of drug resistance in Russia are available. In Russia self-treatment with the usual doses of metronidazole is possible; thus, the time lag between a new diagnosis and effective treatment is increased. In Europe it is very important to start diagnosing possible resistance in vitro after, for example, 2 months of “recurrent” infections, to report the resistant strains, and to trace the sexual contacts, if possible. Recent epidemiological and in vitro evidence suggests that T. vaginalis infection may enhance the risk of HIV-1 transmission (36). If this connection is confirmed, T. vaginalis susceptibility testing becomes even more important in countries where infections are both frequent and inadequately controlled, for example, in Russia and tropical Africa. Patients infected with a resistant strain of T. vaginalis should be tested for HIV infection.
In summary, using metronidazole sensitivity testing under various oxygen concentrations, we found the first three metronidazole-resistant strains of T. vaginalis from Finland. It appears that the metronidazole resistance of T. vaginalis is an emerging threat in Europe. Thus, testing for metronidazole susceptibility in vitro and introduction of rapid and intense medication should be implemented more often. The in vitro diagnostic assays that we suggest can be relatively simply adopted for use in advanced clinical microbiology laboratories.
ACKNOWLEDGMENTS
We thank all the involved hospitals for providing us with T. vaginalis strains and Suvi-Sirkku Kaukoranta-Tolvanen, Esa Korkeela, and Satu Suhonen for valuable clinical data on the patients.
This study was supported by a state subsidy to the Helsinki University Central Hospital.
REFERENCES
- 1.Borchardt K A, Li Z, Zhang M Z, Shing H. An in vitro metronidazole susceptibility test for trichomoniasis using the InPouch TV test. Genitourin Med. 1996;72:132–135. doi: 10.1136/sti.72.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cates W, Joesoef R J, Goldman M. Atypical pelvic inflammatory disease: can we identify clinical predictors? Am J Obstet Gynecol. 1993;169:341–346. doi: 10.1016/0002-9378(93)90085-w. [DOI] [PubMed] [Google Scholar]
- 3.Coelho D. Metronidazole resistant trichomoniasis successfully treated with paromomysin. Genitourin Med. 1997;73:397–398. doi: 10.1136/sti.73.5.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diamond L. The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol. 1957;43:488–490. [PubMed] [Google Scholar]
- 5.Dombrowski M, Brown W, Bronsteen R. Intravenous therapy of metronidazole-resistant Trichomonas vaginalis. Obstet Gynecol. 1987;69:524–525. [PubMed] [Google Scholar]
- 6.Edwards D. Review. Nitroimidazole drugs—action and resistance mechanisms. I. Mechanisms of action. J Antimicrob Chemother. 1993;31:9–20. doi: 10.1093/jac/31.1.9. [DOI] [PubMed] [Google Scholar]
- 7.Forsgren A, Forssman L. Metronidazole-resistant Trichomonas vaginalis. Br J Vener Dis. 1979;55:351–353. doi: 10.1136/sti.55.5.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fouts A C, Kraus S J. Trichomonas vaginalis: re-evaluation of its clinical presentations and laboratory analysis. J Infect Dis. 1980;141:137–143. doi: 10.1093/infdis/141.2.137. [DOI] [PubMed] [Google Scholar]
- 9.Grossman J, Galask R. Persistent vaginitis caused by metronidazole-resistant trichomonas. Obstet Gynecol. 1990;76:521–522. [PubMed] [Google Scholar]
- 10.Hamed K, Studemeister A. Successful response of metronidazole-resistant trichomonal vaginitis to tinidazole. A case report. Sex Transm Dis. 1992;19:339–340. [PubMed] [Google Scholar]
- 11.Heine P, McGregor J. Trichomonas vaginalis: a re-emerging pathogen. Clin Obstet Gynecol. 1993;36:137–144. doi: 10.1097/00003081-199303000-00019. [DOI] [PubMed] [Google Scholar]
- 12.Heyworth R, Simpson D, McNeillage G, Robertson D, Young H. Isolation of Trichomonas vaginalis resistant to metronidazole. Lancet. 1980;ii:476–478. doi: 10.1016/s0140-6736(80)91911-x. [DOI] [PubMed] [Google Scholar]
- 13.Ikeh E I, Bello C S, Ajayi J A. In vitro susceptibility of Trichomonas vaginalis strains to metronidazole—a Nigerian experience. Genitourin Med. 1993;69:241–242. doi: 10.1136/sti.69.3.241-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson D, Rakwar J, Bwayo J, Kreiss J, Moses S. Urethral Trichomonas vaginalis infection and HIV-1 transmission. Lancet. 1997;350:1076–1077. doi: 10.1016/s0140-6736(05)70456-6. [DOI] [PubMed] [Google Scholar]
- 15.Jackson D, Rakwar J, Kishorchandra Mandaliya B, Bwayo J, Ndinya-Achola J, Nagelkerke N, Kreiss J, Moses S. Urethral infection in a workplace population of East African men: evaluation of strategies for screening and management. J Infect Dis. 1996;175:833–838. doi: 10.1086/513979. [DOI] [PubMed] [Google Scholar]
- 16.Kellock D J, O'Mahoney C P. Sexually acquired metronidazole-resistant trichomoniasis in a lesbian couple. Genitourin Med. 1996;72:60–61. doi: 10.1136/sti.72.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kengne P, Veas F, Vidal N, Rey J, Cuny G. Trichomonas vaginalis: repeated DNA target for highly sensitive and specific polymerase chain reaction diagnosis. Cell Mol Biol. 1994;40:819–831. [PubMed] [Google Scholar]
- 18.Korik L. Strains of Trichomonas vaginalis resistant to metronidazole. Vestnik Dermatol Vanerol. 1971;45:77–80. [PubMed] [Google Scholar]
- 19.Kulda J, Vojtechovska M, Tachezy J, Demes P, Kunzova E. Metronidazole resistance of Trichomonas vaginalis as a cause of treatment failure in trichomoniasis. A case report. Br J Vener Dis. 1982;53:394–399. doi: 10.1136/sti.58.6.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laga M, Manoka A, Kivuvu M, Malele B, Tuliza M, Nzola N, Goeman J, Behets F, Batter V, Alary M, Heyward W L, Ryder R W, Piot P. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS. 1993;7:95–102. doi: 10.1097/00002030-199301000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Livengood C, Lossick J. Resolution of resistant vaginal trichomoniasis associated with the use of intravaginal nonoxynol-9. Obstet Gynecol. 1991;78:954–956. [PubMed] [Google Scholar]
- 22.Lossick J G, Kent H L. Trichominiasis: trends in diagnosis and management. Am J Obstet Gynecol. 1991;165:1217–1222. doi: 10.1016/s0002-9378(12)90730-9. [DOI] [PubMed] [Google Scholar]
- 23.Lossick J G, Müller M, Gorrell T E. In vitro drug susceptibility and doses of metronidazole required for cure in cases of refractory vaginal trichomoniasis. J Infect Dis. 1986;153:948–955. doi: 10.1093/infdis/153.5.948. [DOI] [PubMed] [Google Scholar]
- 24.McLellan R, Spence M, Brockman M, Rattel L, Smith J. The clinical diagnosis of trichomoniasis. Obstet Gynecol. 1982;60:30–34. [PubMed] [Google Scholar]
- 25.Meingassner J G, Mieth H, Czok R, Lindmark D, Müller M. Assay conditions and the demonstration of nitroimidazole resistance in Trichomonas foetus. Antimicrob Agents Chemother. 1978;13:1–3. doi: 10.1128/aac.13.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meingassner J G, Thurner J. Strain of Trichomonas vaginalis resistant to metronidazole and other 5-nitroimidazoles. Antimicr Agents Chemother. 1978;15:254–257. doi: 10.1128/aac.15.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Müller M, Gorrell T E. Metabolism and metronidazole uptake in Trichomonas vaginalis isolates with different metronidazole susceptibilities. Antimicrob Agents Chemother. 1983;24:667–673. doi: 10.1128/aac.24.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Müller M, Lossick J G, Gorrell T E. In vitro susceptibility of Trichomonas vaginalis to metronidazole and treatment outcome in vaginal trichomoniasis. Sex Transm Dis. 1988;15:17–24. doi: 10.1097/00007435-198801000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Müller M, Meingassner J, Miller W, Ledger W. Three metronidazole-resistant strains of Trichomonas vaginalis from the United States. J Obstet Gynecol. 1980;138:808–812. doi: 10.1016/s0002-9378(16)32741-7. [DOI] [PubMed] [Google Scholar]
- 30.Narcici E M, Secor W E. In vitro effect of tinidazole and furazolidone on metronidazole-resistant Trichomonas vaginalis. Antimicrob Agents Chemother. 1996;40:1121–1125. doi: 10.1128/aac.40.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nyirjesy P, Sobel J, Weitz M, Leaman D, Gelone S. Difficult-to-treat trichomoniasis: result with paromomysin cream. Clin Infect Dis. 1998;26:986–988. doi: 10.1086/513951. [DOI] [PubMed] [Google Scholar]
- 32.Nyirjesy P, Weitz M, Gelone S, Fekete T. Paromomycin for nitroimidazole-resistant trichomonisis. Lancet. 1995;346:1110. doi: 10.1016/s0140-6736(95)91788-8. [DOI] [PubMed] [Google Scholar]
- 33.Quon D, d'Oliveira C, Johnson P. Reduced transcription of the ferredoxin gene in metronidazole resistant Trichomonas vaginalis. Proc Natl Acad Sci USA. 1992;89:4402–4406. doi: 10.1073/pnas.89.10.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ralph E, Darwish R, Austin T, Smith E, Pattison F. Susceptibility of Trichomonas vaginalis strains to metronidazole: response to treatment. Sex Transm Dis. 1983;10:119–122. doi: 10.1097/00007435-198307000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Robinson S C. Trichomonal vaginitis resistant to metronidazole. Can Med Assoc J. 1962;86:665. [PMC free article] [PubMed] [Google Scholar]
- 36.Sorvillo F, Kerndt P. Trichomonas vaginalis and amplification of HIV-1 transmission. Lancet. 1998;351:213–214. doi: 10.1016/S0140-6736(05)78181-2. [DOI] [PubMed] [Google Scholar]
- 37.Spence M R, Harwell T S, Davies M C, Smith J L. The minimum single oral metronidazole dose for treating trichomoniasis: a randomized, blinded study. Obstet Gynecol. 1997;89:699–703. doi: 10.1016/s0029-7844(97)81437-8. [DOI] [PubMed] [Google Scholar]
- 38.Tachezy J, Kulda J, Tomkova E. Aerobic resistance of Trichomonas vaginalis to metronidazole induced in vitro. Parasitology. 1993;106:31–37. doi: 10.1017/s0031182000074783. [DOI] [PubMed] [Google Scholar]
- 39.Townson S M, Boreham P F L, Upcroft P, Upcroft J A. Resistance to nitroheterocyclic drugs. Acta Trop. 1994;56:173–194. doi: 10.1016/0001-706x(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 40.van der Weiden R, van der Meijden W, Bogchelman D, Polderman A. Treatment failure in trichomoniasis and persistance of the parasite after Lactobacillus immunotherapy: two case reports. Eur J Obstet Gynecol Reprod Biol. 1990;34:171–178. doi: 10.1016/0028-2243(90)90021-r. [DOI] [PubMed] [Google Scholar]
- 41.Waitkins S, Thomas D. Isolation of Trichomonas vaginalis resistant to metronidazole. Lancet. 1981;8246:590. doi: 10.1016/s0140-6736(81)90984-3. [DOI] [PubMed] [Google Scholar]
- 42.Wasley G, Rayner C. Preservation of Trichomonas vaginalis in liquid nitrogen. Br J Vener Dis. 1970;46:323–325. doi: 10.1136/sti.46.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.World Health Organization. WHO factsheet. Geneva, Switzerland: Office of HIV/AIDS and Sexually Transmitted Disease, World Health Organization; 1997. [Google Scholar]
- 44.Yarlett N, Yarlett N C, Lloyd D. Ferrodoxin-dependent reduction of nitroimidazole derivatives in drug-resistant and susceptible strains of Trichomonas vaginalis. Biochem Pharmacol. 1985;35:1703–1708. doi: 10.1016/0006-2952(86)90327-8. [DOI] [PubMed] [Google Scholar]