Abstract
Since December 2019, over 1.5 million SARS-CoV-2-related fatalities have been recorded in the World Health Organization European Region - 90.2% in people ≥ 60 years. We calculated lives saved in this age group by COVID-19 vaccination in 33 countries from December 2020 to November 2021, using weekly reported deaths and vaccination coverage. We estimated that vaccination averted 469,186 deaths (51% of 911,302 expected deaths; sensitivity range: 129,851–733,744; 23–62%). Impact by country ranged 6–93%, largest when implementation was early.
Keywords: COVID-19, vaccination programs, older age groups, deaths averted, expected mortality, vaccination coverage
Since the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first detected in December 2019 [1], in excess of 1.5 million coronavirus disease (COVID-19) fatalities have been reported in the World Health Organization (WHO) European Region until week 45/2021, with 90.2% deaths in people 60 years and older [2]. In a subset of 33 countries in the Region covered by this study, 442,116 fatalities were reported between December 2020 and November 2021 in this age group. We observed a wide range in cumulative national mortality rate from 5.4 to 1,008.0 per 100,000 population 60 years and older. Here, we estimate the number of deaths averted in this age group since the start of COVID-19 vaccination in those countries of the WHO European Region with available data.
Vaccination roll-out and uptake
The emergence of SARS-CoV-2 was followed by the rapid development, licensure and roll-out of several COVID-19 vaccines from late 2020 onwards, initially targeting select groups including those at higher risk of severe disease, in particular older adults. Fifty-one countries, areas or territories in the Region had reported the weekly number of vaccination doses administered to The European Surveillance System (TESSy) until week 45/2021, of which 33 countries had reported age-specific mortality counts and number of vaccination doses administered. Vaccination uptake (VU) in these countries increased in priority groups such that by week 45, 80% (range: 20–100) of people 60 years and older had received a complete vaccination series and 84% (range: 29–100) had received at least one dose (Table 1, Figures 1, 2, 3).
Table 1. Cumulative number of deaths observed and averted by COVID-19 vaccination, mortality rates and expected mortality rates per 100,000 population aged 60 years and older, using the base vaccine effectiveness scenarioa, by country, WHO European Region, weeks 51/2020–45/2021.
| Country | Vaccines used | Vaccination uptake (%) | Number of deaths | Mortality rate per 100,000 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Partial | Full | Observed | Averted after one dose | Averted after two doses | Averted total | Observed | Total expected | % expected deaths averted by vaccination | ||
| Iceland | AZ-COM-JANSS-MOD | 100 | 100 | 4 | 0 | 52 | 52 | 5.4 | 76.0 | 93 |
| United Kingdom (Scotland) | AZ-COM-MOD | 100 | 100 | 4,585 | 454 | 27,202 | 27,656 | 333.3 | 2,343.8 | 86 |
| Israel | AZ-COM-MOD | 97 | 93 | 3,972 | 925 | 14,737 | 15,662 | 263.1 | 1,300.7 | 80 |
| Norway | AZ-COM-JANSS-MOD | 98 | 97 | 682 | 87 | 2,705 | 2,792 | 54.1 | 275.4 | 80 |
| Ireland | AZ-COM-JANSS-MOD | 100 | 100 | 3,156 | 116 | 8,958 | 9,074 | 325.5 | 1,261.2 | 74 |
| Malta | AZ-COM-JANSS-MOD | 100 | 100 | 305 | 26 | 834 | 860 | 245.4 | 937.3 | 74 |
| Finland | AZ-COM-JANSS-MOD | 95 | 92 | 1,007 | 282 | 2,327 | 2,609 | 62.7 | 225.1 | 72 |
| Spain | AZ-COM-JANSS-MOD | 99 | 97 | 34,032 | 2,102 | 87,413 | 89,515 | 277.1 | 1,006.1 | 72 |
| United Kingdom (England) | UNK | 98 | 97 | 74,354 | 14,918 | 142,686 | 157,604 | 557.1 | 1,738.0 | 68 |
| Cyprus | AZ-COM-JANSS-MOD | 77 | 75 | 530 | 51 | 603 | 654 | 221.8 | 495.5 | 55 |
| Portugal | AZ-COM-JANSS-MOD | 100 | 98 | 12,050 | 503 | 13,719 | 14,222 | 402.4 | 877.3 | 54 |
| Austria | AZ-COM-JANSS-MOD | 88 | 86 | 5,875 | 390 | 6,256 | 6,646 | 254.1 | 541.6 | 53 |
| Greece | AZ-COM-JANSS-MOD | 83 | 81 | 11,703 | 746 | 11,429 | 12,175 | 390.2 | 796.1 | 51 |
| Belgium | AZ-COM-JANSS-MOD | 93 | 92 | 7,708 | 775 | 7,046 | 7,821 | 259.8 | 523.3 | 50 |
| France | AZ-COM-JANSS-MOD | 94 | 86 | 47,681 | 5,732 | 32,983 | 38,715 | 272.2 | 493.1 | 45 |
| Lithuania | AZ-COM-JANSS-MOD | 78 | 75 | 4,155 | 176 | 3,244 | 3,420 | 555.6 | 1,012.9 | 45 |
| Sweden | AZ-COM-MOD | 95 | 93 | 6,612 | 487 | 4,283 | 4,770 | 252.3 | 434.4 | 42 |
| Slovenia | AZ-COM-JANSS-MOD | 82 | 79 | 2,798 | 122 | 1,626 | 1,748 | 485.2 | 788.3 | 38 |
| Italy | AZ-COM-JANSS-MOD | 92 | 88 | 60,898 | 3,900 | 31,588 | 35,488 | 337.5 | 534.2 | 37 |
| Switzerland | COM-JANSS-MOD | 87 | 84 | 4,703 | 167 | 2,476 | 2,643 | 214.9 | 335.6 | 36 |
| Luxembourg | AZ-COM-JANSS-MOD | 88 | 87 | 490 | 39 | 226 | 265 | 392.4 | 604.7 | 35 |
| Estonia | AZ-COM-JANSS-MOD | 75 | 72 | 1,290 | 69 | 604 | 673 | 362.4 | 551.4 | 34 |
| Hungary | AZ-BECNBG-COM-JANSS-MOD-SPU | 82 | 80 | 20,437 | 2,036 | 8,230 | 10,266 | 790.9 | 1,188.1 | 33 |
| North Macedonia | AZ-BECNBG-COM-SIN-SPU | 67 | 65 | 4,030 | 145 | 1,629 | 1,774 | 935.7 | 1,347.6 | 31 |
| Montenegro | AZ-BECNBG-COM-SPU | 64 | 64 | 1,399 | 26 | 555 | 581 | 1,008.0 | 1,426.6 | 29 |
| Latvia | AZ-COM-JANSS-MOD | 72 | 64 | 2,802 | 67 | 894 | 961 | 538.7 | 723.5 | 26 |
| Czechia | AZ-COM-JANSS-MOD | 83 | 82 | 20,292 | 980 | 4,607 | 5,587 | 724.5 | 924.0 | 22 |
| Romania | AZ-COM-JANSS-MOD | 44 | 40 | 30,250 | 299 | 7,223 | 7,522 | 606.3 | 757.0 | 20 |
| Poland | AZ-COM-JANSS-MOD | 74 | 73 | 8,241 | 382 | 1,610 | 1,992 | 83.9 | 104.2 | 19 |
| Croatia | AZ-COM-JANSS-MOD | 73 | 69 | 1,335 | 87 | 164 | 251 | 114.9 | 136.5 | 16 |
| Slovakia | AZ-COM-JANSS-MOD-SPU | 68 | 67 | 9,819 | 303 | 1,302 | 1,605 | 771.0 | 897.0 | 14 |
| Moldova | AZ-BECNBG-COM-JANSS-SIN-SPU | 41 | 41 | 3,584 | 13 | 514 | 527 | 470.3 | 539.4 | 13 |
| Ukraine | AZ-COM-JANSS-MOD-SIICOV-SIN | 29 | 20 | 51,337 | 561 | 2,495 | 3,056 | 496.5 | 526.0 | 6 |
| Total | 84 | 80 | 442,116 | 36,966 | 432,220 | 469,186 | 365.2 | 752.8 | 51 | |
AZ: AstraZeneca Vaxzevria; BECNBG: Beijing CNBG BBIBP-CorV; COM: Pfizer BioNTech Comirnaty; JANSS: Janssen Ad26.COV 2-S; MOD: Moderna mRNA-1273; SPU: Gamaleya Gam-Covid-Vac; SIICOV: SII Covishield; SIN: Sinovac CoronaVac; UNK: unknown product; WHO: World Health Organization.
a VE1 = 60% and VE2 = 95% and time lags of 4 and 3 weeks for first and second vaccination dose, respectively.
Countries are ordered according to the proportion of deaths averted.
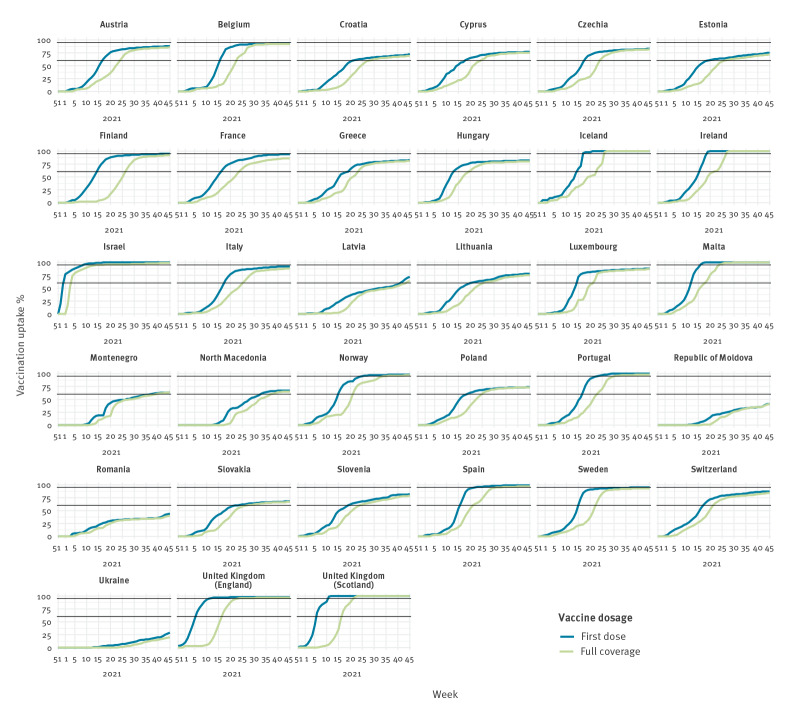
Figure 1.
Cumulative vaccination coverage in the population aged 60 years and older, by country, 33 countries in the WHO European Region, weeks 51/2020–45/2021
WHO: World Health Organization.
Black horizontal lines represent 60% and 95% vaccination uptake respectively.
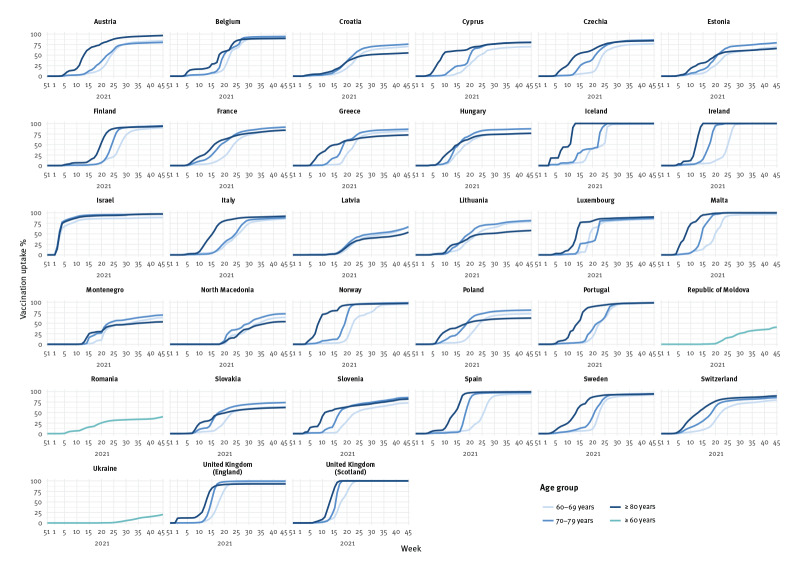
Figure 2.
Complete vaccination coverage, by age group where available, in the population aged 60 years and older, by country, 33 countries in the WHO European Region, weeks 51/2020–45/2021
WHO: World Health Organization.
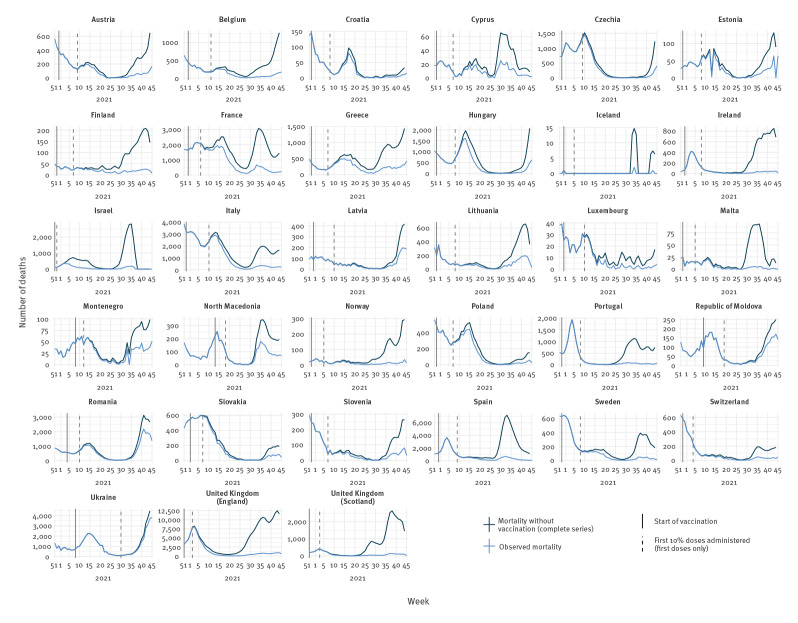
Figure 3.
Observed and expected mortality, using the base vaccine effectiveness scenarioa, together with timing of vaccination in population aged 60 years and older, by country, 33 countries in the WHO European Region, weeks 51/2020–45/2021
WHO: World Health Organization.
a VE1 = 60% and VE2 = 95% and time lags of 4 and 3 weeks for first and second vaccination dose, respectively.
The number of observed deaths plotted here are the true numbers reported by the country and not the rolling average used in calculations.
The impact of such vaccination campaigns on preventing severe disease in terms of averted deaths can be quantified [3,4], although an assessment of lives saved using a standardised methodology across multiple countries in Europe is lacking.
Number of deaths averted as a result of vaccination among older adults
Data from 33 countries reporting both COVID-19 age-specific vaccination and age-aggregated COVID-19 mortality data for people 60 years and older were downloaded from TESSy on 18 November 2021 and restricted to the weeks between 51/2020 and 45/2021. Thirty countries reported more granular age groups (60–69, 70–79 and ≥ 80 years). Population estimates for each age group in year 2020 were drawn from the United Nations [5] for countries not in the European Union (EU) or European Economic Area (EEA) and from Eurostat [6] for EU/EEA countries, except for Israel (age denominators from Israel Central Bureau of Statistics [7]). All population data were downloaded in 2021. All analyses presented here were conducted in R software version 4.0.5 (R Foundation, Vienna, Austria) [8]. We assumed that all countries used the same definition for COVID-19 mortality and that reporting delays were comparable.
The weekly number of deaths averted per country was estimated using methods adapted from Machado et al. [9] (available on GitHub [10]), with the following definitions: VE1 as vaccine effectiveness (VE) (at least one dose), VE2 as vaccine effectiveness (complete series), VU1 being vaccination uptake (at least one dose) and VU2 as vaccination uptake (complete series). The rolling average number of deaths observed over three consecutive weeks centred on the second week was used for the calculations (the true number of weekly fatalities is shown in Tables 1 and 2).
Table 2. Age group and country breakdown of full vaccination uptake, number of deaths observed and averted as well as observed and expected mortality rates per 100,000 population, in the population aged 60 years and older, using the base vaccine effectiveness scenarioa, by country, 30 WHO European Region countries with sufficient data, weeks 51/2020–45/2021.
| Country | 60–69 year-olds | 70–79 year-olds | ≥ 80 year-olds | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Full VU (%) | Deaths observed | Deaths averted | Observed mortality rate per 100,000 | Expected mortality rate per 100,000 | Full VU (%) | Deaths observed | Deaths averted | Observed mortality rate per 100,000 | Expected mortality rate per 100,000 | Full VU (%) | Deaths observed | Deaths averted | Observed mortality rate per 100,000 | Expected mortality rate per 100,000 | |
| Austria | 84 | 676 | 390 | 65.4 | 103.2 | 80 | 1,467 | 675 | 185.6 | 271.0 | 97 | 3,732 | 5,581 | 764.5 | 1,907.6 |
| Belgium | 92 | 900 | 1,016 | 65.7 | 139.9 | 94 | 1,850 | 2,492 | 196.9 | 462.2 | 90 | 4,958 | 4,313 | 753.4 | 1,408.7 |
| Croatia | 71 | 225 | 35 | 39.8 | 46.0 | 76 | 412 | 99 | 113.8 | 141.2 | 55 | 698 | 117 | 298.1 | 348.0 |
| Cyprus | 70 | 108 | 71 | 89.7 | 148.7 | 81 | 171 | 239 | 217.3 | 521.1 | 80 | 251 | 344 | 629.5 | 1,492.3 |
| Czechia | 77 | 3,434 | 347 | 259.2 | 285.4 | 86 | 8,011 | 1,630 | 776.9 | 935.0 | 84 | 8,847 | 3,610 | 1,987.6 | 2,798.6 |
| Estonia | 69 | 197 | 74 | 116.2 | 159.9 | 79 | 362 | 235 | 337.0 | 555.7 | 66 | 731 | 364 | 924.4 | 1,384.6 |
| Finland | 90 | 124 | 216 | 17.4 | 47.7 | 94 | 297 | 671 | 50.9 | 166.0 | 93 | 586 | 1,722 | 188.4 | 742.1 |
| France | 84 | 6,084 | 4,786 | 78.4 | 140.0 | 91 | 11,962 | 13,533 | 208.8 | 445.1 | 84 | 29,635 | 20,396 | 735.9 | 1,242.3 |
| Greece | 82 | 2,167 | 1,998 | 172.4 | 331.3 | 86 | 3,481 | 4,250 | 364.1 | 808.6 | 73 | 6,055 | 5,927 | 770.4 | 1,524.4 |
| Hungary | 77 | 5,110 | 1,772 | 391.1 | 526.8 | 87 | 7,276 | 4,417 | 859.5 | 1,381.2 | 77 | 8,051 | 4,077 | 1,867.4 | 2,813.0 |
| Iceland | 99 | 1 | 14 | 2.7 | 40.0 | 100 | 3 | 38 | 12.8 | 175.1 | 100 | 0 | 0 | 0.0 | 0.0 |
| Ireland | 100 | 376 | 1,189 | 78.2 | 325.5 | 100 | 837 | 2,753 | 253.1 | 1,085.7 | 100 | 1,943 | 5,132 | 1,227.9 | 4,471.3 |
| Israel | 89 | 692 | 1,532 | 93.3 | 299.9 | 98 | 1,123 | 5,271 | 228.8 | 1,302.8 | 97 | 2,157 | 8,859 | 778.6 | 3,976.4 |
| Italy | 86 | 7,318 | 2,661 | 97.8 | 133.3 | 88 | 16,503 | 5,799 | 273.7 | 369.9 | 92 | 37,077 | 27,028 | 818.7 | 1,415.6 |
| Latvia | 67 | 572 | 223 | 228.7 | 317.8 | 67 | 854 | 372 | 524.9 | 753.5 | 54 | 1,376 | 366 | 1,282.5 | 1,623.7 |
| Lithuania | 79 | 709 | 729 | 201.0 | 407.6 | 82 | 1,252 | 1,540 | 560.7 | 1,250.4 | 58 | 2,194 | 1,151 | 1,277.3 | 1,947.4 |
| Luxembourg | 85 | 52 | 14 | 83.7 | 106.2 | 87 | 106 | 49 | 279.9 | 409.3 | 90 | 332 | 202 | 1,335.1 | 2,147.3 |
| Malta | 96 | 54 | 140 | 93.6 | 336.3 | 100 | 88 | 167 | 194.8 | 564.6 | 100 | 163 | 553 | 760.2 | 3,339.4 |
| Montenegro | 63 | 367 | 142 | 477.0 | 661.5 | 70 | 537 | 277 | 1,328.0 | 2,013.0 | 53 | 495 | 162 | 2,311.7 | 3,068.2 |
| North Macedonia | 64 | 1,368 | 522 | 559.2 | 772.5 | 72 | 1,693 | 933 | 1,264.5 | 1,961.4 | 54 | 969 | 319 | 1,857.9 | 2,469.6 |
| Norway | 95 | 102 | 180 | 17.4 | 48.1 | 98 | 181 | 707 | 40.5 | 198.9 | 97 | 399 | 1,905 | 174.4 | 1,007.3 |
| Poland | 73 | 2,049 | 242 | 39.6 | 44.3 | 81 | 2,733 | 747 | 94.2 | 120.0 | 62 | 3,459 | 1,003 | 198.5 | 256.0 |
| Portugal | 98 | 1,185 | 1,253 | 91.6 | 188.4 | 99 | 2,750 | 3,070 | 270.1 | 571.5 | 98 | 8,115 | 9,899 | 1,189.2 | 2,639.8 |
| Slovakia | 64 | 2,626 | 284 | 377.7 | 418.6 | 74 | 3,693 | 720 | 926.2 | 1,106.8 | 62 | 3,500 | 601 | 1,948.5 | 2,283.0 |
| Slovenia | 73 | 345 | 155 | 121.1 | 175.5 | 86 | 672 | 474 | 378.6 | 645.7 | 82 | 1,781 | 1,119 | 1,558.5 | 2,537.7 |
| Spain | 95 | 4,107 | 5,722 | 76.9 | 184.0 | 98 | 8,107 | 14,387 | 201.9 | 560.2 | 99 | 21,818 | 69,406 | 746.2 | 3,120.0 |
| Sweden | 92 | 564 | 291 | 51.6 | 78.2 | 95 | 1,678 | 1,142 | 168.8 | 283.6 | 94 | 4,370 | 3,337 | 821.3 | 1,448.5 |
| Switzerland | 80 | 402 | 211 | 41.1 | 62.7 | 86 | 991 | 495 | 131.8 | 197.6 | 90 | 3,310 | 1,937 | 720.8 | 1,142.6 |
| United Kingdom (England) | 96 | 9,721 | 24,719 | 164.9 | 584.3 | 100 | 18,722 | 65,338 | 402.1 | 1,805.5 | 93 | 45,911 | 67,547 | 1,641.7 | 4,057.0 |
| United Kingdom (Scotland) | 100 | 683 | 5,038 | 106.6 | 893.3 | 100 | 1,294 | 8,174 | 276.2 | 2,021.2 | 100 | 2,608 | 14,444 | 977.9 | 6,393.6 |
| Total | 86 | 52,318 | 55,966 | 110.4 | 228.5 | 91 | 99,106 | 140,694 | 285.1 | 689.7 | 88 | 205,521 | 261,421 | 901.3 | 2,047.8 |
VU: vaccine uptake; WHO: World Health Organization.
a VE1 = 60% and VE2 = 95% and time lags of 4 and 3 weeks for first and second vaccination dose, respectively.
The weekly number of deaths averted for each dose was calculated as below for each age group, setting the base scenario for VE against COVID-19 mortality at 60% and 95% for VE1 and VE2, respectively [11-13]. For single-dose vaccines, the VE of the complete vaccination series was equal to the first dose.
Here, w represents the time delays of 2 and 1 weeks for the development of a full immune response after, respectively, the first and second vaccine dose [14] and the median time from infection to death of 2 weeks [15,16]. The number of deaths averted each week was then added to the number of observed deaths to calculate the total expected number of deaths and cumulative mortality rate per 100,000 population per country.
Using the base VE scenario, we calculated that 51% (n=469,186) of total expected deaths (n=911,302) were averted by vaccination over the study period; ranging from 93% of deaths averted in Iceland to 6% in Ukraine (Table 1). All three countries with 60% or less of their population 60 years and older fully vaccinated by week 45/2021 (Moldova, Romania and Ukraine; Table 1) had a maximum of only 20% expected deaths averted over the study period (Figure 3). On the contrary, all four countries (Israel, Malta, United Kingdom (UK)-England and UK-Scotland) that achieved very high complete vaccination coverage (above 90%) already by week 23/2021 (Figure 1) averted over 65% of total expected deaths by week 45 (Table 1 and Figure 3).
In the 30 countries with more detailed data on age groups allowing for inclusion in age-stratified analyses, the largest number of fatalities were averted after vaccination among people 80 years and older (261,421 fatalities, 57% of total averted deaths). A total of 140,694 (31%) fatalities were averted among 70–79 year-olds and 55,966 (12% of averted deaths) among 60–69 year-olds in the base scenario (Table 2).
Sensitivity analyses investigating assumed immune response delays and vaccine effectiveness
To investigate the parameters used in the base scenario using VE1 (60%) and VE2 (95%), we ran sensitivity analyses varying the time to protection and time from infection to death using shorter (3 and 2 weeks, respectively) and longer (5 and 4 weeks, respectively) time ranges.
A sensitivity analysis using median time lags of 4 and 3 weeks was also run using lower bound VE (50% and 70% for first dose and complete series, respectively) and upper bound VE values (70% and 97.5%, respectively). We deemed this range of values representative of the observational studies for the vaccines most frequently used in the countries in this study (Table 1), namely Vaxzevria (AstraZeneca, Oxford, United Kingdom), mRNA-1273 (Moderna, Cambridge (Massachusetts), United States) and Comirnaty (BioNTech-Pfizer, Mainz, Germany/New York, United States) [2].
Using lower and upper bound VE values, we estimated that the number of deaths averted ranged between 129,851 and 733,744, respectively, in people 60 years and older in the 33 countries (Table 1, Supplementary Table S1). The proportion of total estimated deaths averted by vaccination ranged between 23% and 62% according to the VE sensitivity analysis (Supplementary Table S2, where also country-specific estimates are available). There was minimal variation in deaths averted by varying the time lag (Table 1, Supplementary Table S1) .
Discussion
Overall, we estimate the widespread implementation of COVID-19 vaccination programmes for older people has averted a median of 469,186 deaths (sensitivity range: 129,851–733,744) in people 60 years and older in 33 countries (51% of 911,302 expected deaths; sensitivity range: 23–62%). However, this direct impact has been heterogenous (median impact range: 6–93% by country) because of the speed and extent of the vaccination in these eligible groups. Countries with high early uptake (e.g. Israel, Malta, UK-England and UK-Scotland) have substantially reduced predicted mortality, especially in people 80 years and older. Other countries have experienced more limited impact of vaccination to date. Possible explanations are that their programme was implemented more slowly (e.g. Moldova, Romania and Ukraine) or that they achieved higher vaccination levels mainly after a wave of SARS-CoV-2 transmission earlier in 2021 (e.g. Croatia, Czechia and Poland).
A small number of other studies have estimated the number of lives saved from COVID-19 vaccination in older age groups [3,4,17,18], or estimated the difference in life expectancy in three countries [19]. All showed the positive impact of vaccination in saving lives. Our analysis adds to these studies by using a standard approach to compare the estimated direct impact of differential roll-out of COVID-19 vaccine programmes across 33 diverse countries in older adults across the European region.
The analysis contains several limitations and assumptions. Our estimate is conservative as we did not estimate the indirect effect of vaccination or the impact of public health and social measures on mortality by reduction in transmission. The capacity of healthcare systems to respond to the pandemic were not considered here. These primary findings are derived using use a base scenario of vaccine effectiveness and time lag. Using more extreme vaccine effectiveness estimates against mortality in particular scenarios can vary the number of deaths averted as shown in the sensitivity analysis. VE was not differentiated by vaccine manufacturer or type. Based on current, limited data, we assumed that VE against mortality was similar for the SARS-CoV-2 Alpha and Delta variants (Phylogenetic Assignment of Named Global Outbreak (Pango) lineage designation B.1.1.7 and B.1.617.2), the predominant circulating variants of concern during our study period. In addition, we assumed that there has not been any waning in protection against severe disease to date [2]. Differential reporting in mortality surveillance systems was another potential limiting factor, which we assumed to be comparable between countries. Additional third doses of vaccination have not been considered here, as countries had only started to implement them. Many of these limitations and assumptions are likely to have under-estimated the true number of deaths in each country to varying extents. We attempted to account for this by undertaking an 'observed over expected' analysis, estimating the proportion of deaths averted by vaccination to enable a more robust comparison of direct vaccine impact between countries.
Conclusion
We show that since the start of COVID-19 vaccination in Europe, the lives of many older adults have been saved through immunisation. Our results highlight large differences between countries in the direct impact of vaccination on COVID-19-related mortality. Early and complete implementation of vaccination of older adults was associated with the largest reduction in expected deaths. It is critically important that all countries rapidly achieve high coverage for these priority groups to prevent further morbidity and mortality, particularly with SARS-CoV-2 transmission rates increasing as Europe moves into the 2021/22 winter period.
Acknowledgements
We gratefully acknowledge the input of national public health staff involved in surveillance activities and data submission to TESSy.
The authors would like to thank all the countries for the provision of mortality and vaccine uptake data including: Verovchuk Bogdan (Ukraine), Lukyanov Anfisa (Ukraine), Oksana Koshalko (Ukraine), Wioleta Kitowska (Poland), Magdalena Rosinska (Poland), Miroslaw Czarkowski (Poland), Marija Oniščuka (Latvia), Patrizio Pezzotti (Italy), Antonino Bella (Italy), Alberto Mateo Urdiales (Italy), Chiara Sacco (Italy), Martina Del Manso (Italy), Massimo Fabiani (Italy), Matteo Sputi (Italy), Daniele Petrone (Italy), Maria Fenicia Vascio (Italy), Marco Bressi (Italy), Stefano Boros (Italy), Marco Tallon (Italy), Kirsten Konsmo (Norway), Magnus Øgle (Norway), Amparo Larrauri (Spain), Carmen Olmedo (Spain), Aurora Limia (Spain), Katerina Fabianova (Czechia), Hana Orlikova (Czechia), Pavel Slezak (Czechia), Helena Sebestova (Czechia), Iva Vlckova (Czechia), Patrik Lenz (Czechia), Alena Fialova (Czechia), Marek Maly (Czechia), Jan Zofka (Czechia), Jiri Jarkovsky (Czechia), Directorate of Epidemiological Surveillance and Intervention for Infectious Diseases, National Public Health Organization, Athens, (Greece), Josie Murray (United Kingdom (Scotland)), Diane Stockton (United Kingdom (Scotland)), Kimberly Marsh (United Kingdom (Scotland)), A-lan Banks (United Kingdom (Scotland)), David Yirrell (United Kingdom (Scotland)), Mary Sinnathamby (United Kingdom (England)), Hester Allen (United Kingdom (England)), Laura Coughlan (United Kingdom (England)), Camille Tsang (United Kingdom (England)), Gavin Dabrera (United Kingdom (England)).
Disclaimer: The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated.
Supplementary Data
Conflict of interest: None declared.
Authors’ contributions: MMIM and JB conducted the analysis; MMIM wrote the manuscript. RGP and PM conceptualised the analysis. All authors (MMIM, PM, JH, NB, GS, GR, NN, NA, TD, JM, MS, RN, FR, HM, CM, JK, JM, TM, SK, DLB, FH, RR, MK, DN, CS, RP, RGP) were involved in the review and development of the manuscript.
References
- 1.World Health Organization Regional Office for Europe (WHO/Europe). Coronavirus disease (COVID-19) pandemic. Copenhagen: WHO/Europe. [Accessed: 20 Jul 2021]. Available from: https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/novel-coronavirus-2019-ncov#
- 2.World Health Organization (WHO). Weekly epidemiological update on COVID-19 - 16 November 2021. Edition 66. Geneva: WHO; 2021. Available from: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---16-november-2021
- 3.Galvani A, Moghadas S, Schneider E. Deaths and hospitalizations averted by rapid U.S. vaccination rollout. New York: Commonwealth Fund; 2021. Available from: https://www.commonwealthfund.org/publications/issue-briefs/2021/jul/deaths-and-hospitalizations-averted-rapid-us-vaccination-rolloutb [Google Scholar]
- 4.Public Health England (PHE). Impact of COVID-19 vaccines on mortality in England. December 2020 to March 2021. London: PHE; 2021.Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/977249/PHE_COVID-19_vaccine_impact_on_mortality_March.pdf
- 5.United Nations Department of Economic and Social Affairs Population Division. World population prospects 2019. New York: United Nations. [Accessed: 10 Mar 2021]. Available from: https://population.un.org/wpp/Download/Standard/Population
- 6.Eurostat. Demo_pjan. Luxembourg: Eurostat; 2020. [Accessed: 22 Feb 2021]. Available from: https://ec.europa.eu/eurostat/estat-navtree-portlet-prod/BulkDownloadListing?dir=data&sort=1&sort=2&start=d
- 7.Israeli Central Bureau of Statistics (CBS). Statistical Abstract of Israel 2021 - No.72. Jerusalem: CBS; 2021. [Accessed: 11 Oct 2021]. Hebrew. Available from: https://www.cbs.gov.il/he/publications/Pages/2021/%D7%A9%D7%A0%D7%AA%D7%95%D7%9F-%D7%A1%D7%98%D7%98%D7%99%D7%A1%D7%98%D7%99-%D7%9C%D7%99%D7%A9%D7%A8%D7%90%D7%9C-2021-%D7%9E%D7%A1%D7%A4%D7%A8-72.aspx
- 8.R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation of Statistical Computing; 2016. Available from: https://www.R-project.org
- 9. Machado A, Mazagatos C, Dijkstra F, Kislaya I, Gherasim A, McDonald SA, et al. Impact of influenza vaccination programmes among the elderly population on primary care, Portugal, Spain and the Netherlands: 2015/16 to 2017/18 influenza seasons. Euro Surveill. 2019;24(45):1900268. 10.2807/1560-7917.ES.2019.24.45.1900268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization (WHO). COVID-Deaths-Averted-analysis. GitHub Repository. GitHub. [Accessed: 14 Sep 2021]. Available from: https://github.com/whocov/COVID-Deaths-Averted-analysis
- 11.Goldberg Y, Mandel M, Woodbridge Y, Fluss R, Novikov I, Yaari R, et al. Protection of previous SARS-CoV-2 infection is similar to that of BNT162b2 vaccine protection: A three-month nationwide experience from Israel. MedRxiv. 2021.04.20.21255670. Preprint. 10.1101/2021.04.20.21255670 [DOI] [PMC free article] [PubMed]
- 12.Andrews N, Tessier E, Stowe J, Gower C, Kirsebom F, Simmons R, et al. Vaccine effectiveness and duration of protection of Comirnaty, Vaxzevria and Spikevax against mild and severe COVID-19 in the UK. MedRxiv. 2021.09.15.21263583. Preprint. https://doi.org/ 10.1101/2021.09.15.21263583 [DOI]
- 13. Ranzani OT, Hitchings MDT, Dorion M, D’Agostini TL, de Paula RC, de Paula OFP, et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of covid-19 in Brazil: test negative case-control study. BMJ. 2021;374:n2015. 10.1136/bmj.n2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-15. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hauser A, Counotte MJ, Margossian CC, Konstantinoudis G, Low N, Althaus CL, et al. Estimation of SARS-CoV-2 mortality during the early stages of an epidemic: A modeling study in Hubei, China, and six regions in Europe. PLoS Med. 2020;17(7):e1003189. 10.1371/journal.pmed.1003189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. García-García D, Vigo MI, Fonfría ES, Herrador Z, Navarro M, Bordehore C. Retrospective methodology to estimate daily infections from deaths (REMEDID) in COVID-19: the Spain case study. Sci Rep. 2021;11(1):11274. 10.1038/s41598-021-90051-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bartsch SM, Wedlock PT, O’Shea KJ, Cox SN, Strych U, Nuzzo JB, et al. Lives and costs saved by expanding and expediting coronavirus disease 2019 vaccination. J Infect Dis. 2021;224(6):938-48. 10.1093/infdis/jiab233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Victora PC, Castro PMC, Gurzenda S, Medeiros AC, França GVA, Barros PAJD. Estimating the early impact of vaccination against COVID-19 on deaths among elderly people in Brazil: Analyses of routinely-collected data on vaccine coverage and mortality. EClinicalMedicine. 2021;38:101036. 10.1016/j.eclinm.2021.101036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goldstein JR, Cassidy T, Wachter KW. Vaccinating the oldest against COVID-19 saves both the most lives and most years of life. Proc Natl Acad Sci USA. 2021;118(11):e2026322118. 10.1073/pnas.2026322118 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.