Abstract
Background
The prevalence of bacterial infection in patients with COVID-19 is low, however, empiric antibiotic use is high. Risk stratification may be needed to minimize unnecessary empiric antibiotic use.
Objective
To identify risk factors and microbiology associated with respiratory and bloodstream bacterial infection in patients with COVID-19.
Data sources
We searched MEDLINE, OVID Epub and EMBASE for published literature up to 5 February 2021.
Study eligibility criteria
Studies including at least 50 patients with COVID-19 in any healthcare setting.
Methods
We used a validated ten-item risk of bias tool for disease prevalence. The main outcome of interest was the proportion of COVID-19 patients with bloodstream and/or respiratory bacterial co-infection and secondary infection. We performed meta-regression to identify study population factors associated with bacterial infection including healthcare setting, age, comorbidities and COVID-19 medication.
Results
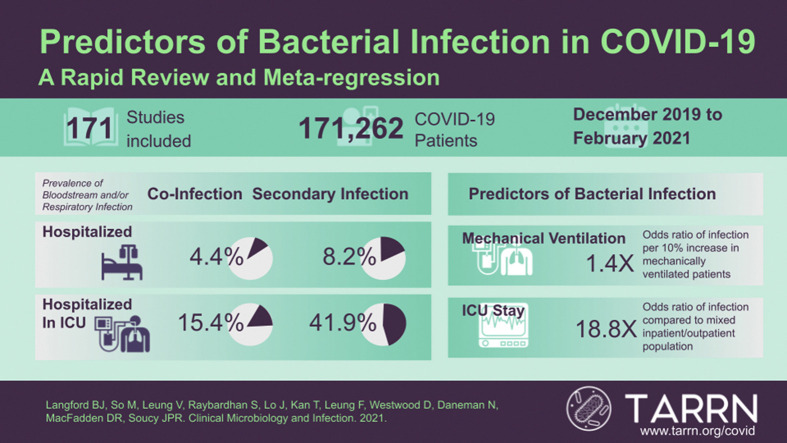
Out of 33 345 studies screened, 171 were included in the final analysis. Bacterial infection data were available from 171 262 patients. The prevalence of co-infection was 5.1% (95% CI 3.6–7.1%) and secondary infection was 13.1% (95% CI 9.8–17.2%). There was a higher odds of bacterial infection in studies with a higher proportion of patients in the intensive care unit (ICU) (adjusted OR 18.8, 95% CI 6.5–54.8). Female sex was associated with a lower odds of secondary infection (adjusted OR 0.73, 95% CI 0.55–0.97) but not co-infection (adjusted OR 1.05, 95% CI 0.80–1.37). The most common organisms isolated included Staphylococcus aureus, coagulase-negative staphylococci and Klebsiella species.
Conclusions
While the odds of respiratory and bloodstream bacterial infection are low in patients with COVID-19, meta-regression revealed potential risk factors for infection, including ICU setting and mechanical ventilation. The risk for secondary infection is substantially greater than the risk for co-infection in patients with COVID-19. Understanding predictors of co-infection and secondary infection may help to support improved antibiotic stewardship in patients with COVID-19.
Keywords: COVID-19, Bacterial infection, Co-infection, Secondary infection, Risk factors, Antimicrobial stewardship
Graphical abstract
Introduction
There is rising concern about the long-term impact of COVID-19 on antimicrobial resistance. While bacterial co-infection rates in patients presenting with COVID-19 have been found to be low (<5%) [1,2], the majority (50–75%) of hospitalized patients with COVID-19 receive antibiotics [3,4]. The rates of secondary bacterial infection, those that occur later on in illness, tend to be higher (10–20%), but are typically related to healthcare-associated infections involving invasive devices, such as ventilators and central venous access [1,5,6]. There are limited data describing the causative pathogens and their susceptibility patterns among COVID-19 patients with bacterial co-infections.
Despite the low rate of infection early in illness, a large observational study found that 57% of patients with COVID-19 received early empiric antibiotics and 46% of these patients had their antibiotics continued after a positive COVID-19 PCR test. Of those who had antibiotics continued despite no confirmed bacterial infection, 64% had antibiotics continued for 5 or more days [3].
The vast discrepancy between the rate of proven bacterial infection and antibiotic use suggests an urgent need for antimicrobial stewardship. However, there are several diagnostic challenges that drive antibiotic overuse in COVID-19, most notably the difficulties in (a) differentiating viral from bacterial infection prior to the COVID-19 test result, and (b) definitively ruling out bacterial co-infection in those after a positive COVID-19 test.
A better understanding of the population-level predictors of bacterial co-infection and secondary infection can help identify situations where antibiotics are more likely to provide benefit, and uncover opportunities for antimicrobial stewardship. Additionally, a characterization of the common organisms identified in early versus late bacterial infection in a COVID-19 patient may help clinicians optimize prescribing, particularly regarding when and which empiric treatment may be beneficial. As the standard of care for COVID-19 infection evolved rapidly over the course of the pandemic, including the life-saving measures of respiratory support and use of immunotherapy based on emerging evidence, it was deemed important to update our analysis reflecting this change in practice. Our objective was to build upon our previously published rapid review to describe the prevalence and microbiological characteristics of respiratory and bloodstream co-infection and secondary infection and to use meta-regression to identify study-level predictors of bacterial infections in patients with COVID-19.
Materials and methods
We conducted a rapid systematic review guided by the Cochrane Rapid Reviews Methods Group [7] to evaluate concomitant bacterial infection in patients with COVID-19. We included studies of humans with COVID-19, across all healthcare settings (i.e., hospital, community, long-term care) and age groups (paediatric and adult patients).
We included cohort studies, case series with 50 or more patients and randomized controlled trials that did not evaluate antibiotic use as an intervention, but excluded reviews, editorials, letters and case studies. We excluded studies that (a) included patients with non-laboratory-proven COVID-19, (b) tested for co-infection using only nasopharyngeal swabs, (c) did not differentiate between bacterial infection and other co-infections or secondary infections (e.g., fungal), (d) indicated bacterial infection was presumed or suspected (e.g., use of white blood cell count, biomarkers) and (e) used serology as a bacterial infection diagnostic approach. This protocol was registered under PROSPERO, the international registry of systematic reviews (ID CRD42021241098).
Data sources
We performed systematic searches of MEDLINE, OVID Epub and EMBASE databases for published literature in any language from 1 January 2019–5 February 2021 with assistance from a medical library information specialist. The expanded timeline aimed to capture new publications since the systematic search we performed for our earlier review (1 January 2019–16 April 2020) [1]. The current search was structured to include COVID-19 terms co-infection, bacterial infection, respiratory infection, epidemiology or descriptive cohort study terms. The complete search strategy is described in the Online Supplement. The results of the search were imported into Covidence (Covidence, Melbourne, Australia), an online software tool for systematic reviews. Duplicate records were removed using Covidence.
Study selection
Initial screening of titles and abstracts from the search were shared by two authors (B.L. or V.L.) who independently identified studies that met all inclusion criteria and none of the exclusion criteria. All full text studies meeting initial criteria were then reviewed by one of the authors (B.L., M.S., V.L., S.R., T.K., J.L. or D.M.) for final inclusion in the rapid review. Single screening was utilized given the high volume of citations and the substantial kappa agreement (0.62–0.68) from our team for previous rapid reviews in which duplicate screening was performed [9].
Data extraction
One of seven authors (B.L., M.S., V.L., S.R., T.K., J.L. or D.M.) independently extracted data from included studies using a standardized data collection form. For quality assurance, one of three authors (F.L., V.L. or B.L.) then verified all extracted data to confirm accuracy and completeness. We collected data on the following variables for demographics and setting: author; country of study; start and end dates; study design; healthcare setting (inpatient intensive care unit (ICU) vs. non-ICU outpatient); sample size; age group; patient population; mean or median age; and number of female patients. Regarding clinical characteristics, we collected information on COVID-19 severity; number of patients requiring mechanical ventilation; number of patients that were smokers; number of patients with comorbidities (chronic obstructive pulmonary disease, cardiovascular disease or diabetes); and number of patients who were prescribed corticosteroids, interleukin-6 (IL-6) inhibitors (i.e., tocilizumab or sarilumab) and antibacterial agents. We extracted respiratory and bloodstream microbiology data, including specimen source and identification, if the information was available.
Assessment of bias
We used a validated ten-item risk of bias tool for disease prevalence [10]. Risk of bias was categorized as low (score ≥8), moderate (score 5–7) or high (score 0–4). A sensitivity analysis was performed stratifying proportion of bacterial infection by study risk of bias. Additional sensitivity analyses were performed to (a) remove studies where bacteriological testing method and bacterial infection source were not specified and (b) to stratify studies based on whether they used a clinical definition for bacterial infection (e.g., differentiated infection from colonization or contamination using clinical criteria).
Data synthesis
The main outcome of interest was the overall prevalence of respiratory and bloodstream bacterial co-infection or secondary infection among patients with COVID-19. We evaluated the number of patients developing a bacterial infection at any point during the course of their illness while under study observation as a proportion of all patients with COVID-19.
First, studies were categorized based on whether they evaluated co-infection (authors used the term co-infection and/or specified that infection was assessed on admission or at the time of health care visit), secondary infection (authors used the term secondary infection, super-infection, healthcare-associated infection and/or indicated that infection was identified >48 hr after initial assessment). Studies that did not specify co-infection or secondary infection were grouped with co-infection. To estimate overall bacterial infection, for studies reporting both co-infection and secondary infection, we selected the higher proportion of the two. Second, the source of infection was categorized as respiratory, bloodstream, both respiratory and bloodstream or not specified. Infections specified from other sources (e.g., urine, skin) were not included. For the purposes of this study, both co-infection and secondary infection were limited to respiratory and/or bloodstream specimens to estimate the prevalence of infections closely linked to COVID-19 while limiting bias due to variability in diagnosis of other less relevant conditions (e.g., skin and soft tissue infections and urinary tract infections). Third, studies were categorized based on the bacteriological testing method (culture, nucleic acid amplification, a combination of approaches, or not specified).
We pooled proportion data across studies via a random-effects meta-analysis using a generalized linear mixed model (GLMM) with logit link approach [11,12]. Results were illustrated using forest plots stratified by healthcare setting and infection type. Heterogeneity was assessed by the I2 statistic [13]. All analyses were carried out using R version 4.1.1 with the packages metafor and meta. The statistical code for this analysis is made available online [14].
Meta-regression
To predict the effect of specific population-level variables on bacterial infection, we performed univariable meta-regression evaluating patient characteristics (age, sex, comorbidities, use of medication for treatment of COVID-19), markers of severity (e.g., mechanical ventilation), healthcare setting, geographic region (Asia, Middle East, Europe, North America, South America) and end-month of study. Differences in prevalence of antibiotic prescribing for each variable were described in terms of the prevalence odds ratio (OR) compared with reference, per 10% increase for continuous variables and per 10-year increase for age. Multi-variable meta-regression was then performed to account for key factors mediating the risk of infection including mean/median patient age [15] and severity of COVID-19 (an index including the highest value of % of patients with ARDS; % of ICU patients; % of mechanical ventilation patients) [16]. One model per variable was constructed, and only studies where both the variable of interest and the adjustment variables were reported were included. We used the same subset of studies with available data for both the unadjusted and adjusted analyses. Given studies that did not report whether they evaluated co-infection versus secondary infection were grouped with co-infection, a sensitivity analysis was performed to remove these studies with unspecified outcome to determine the robustness of our findings.
Results
Of 56 767 studies identified via searching, after duplicate removal, we reviewed a total of 33 345 studies via title and abstract screening, 1798 of which were assessed via full-text screening. We included 171 studies in the final analysis[1], [3], [4], [5], [6], [7], [9], [10], [11], [12], [13], [14], [15], [16], [[17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45]], [[46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77]], [[78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108]], [[109], [110], [111], [112], [113], [114], [115], [116], [117], [118], [119], [120], [121], [122], [123], [124], [125], [126], [127], [128], [129], [130]], [[131], [132], [133], [134], [135], [136], [137], [138], [139], [140], [141], [142], [143], [144], [145], [146], [147], [148], [149], [150], [151], [152], [153], [154], [155], [156]], [[157], [158], [159], [160], [161], [162], [163], [164], [165], [166], [167], [168], [169], [170], [171], [172], [173], [174], [175], [176], [177], [178], [179], [180], [181], [182], [183], [184], [185]] (Fig. 1 ). Study design was primarily retrospective in nature (n = 149), with fewer prospective cohorts (n = 19), or randomized controlled trials (n = 3). COVID-19 study data was collected from December 2019 to October 2020.
Fig. 1.
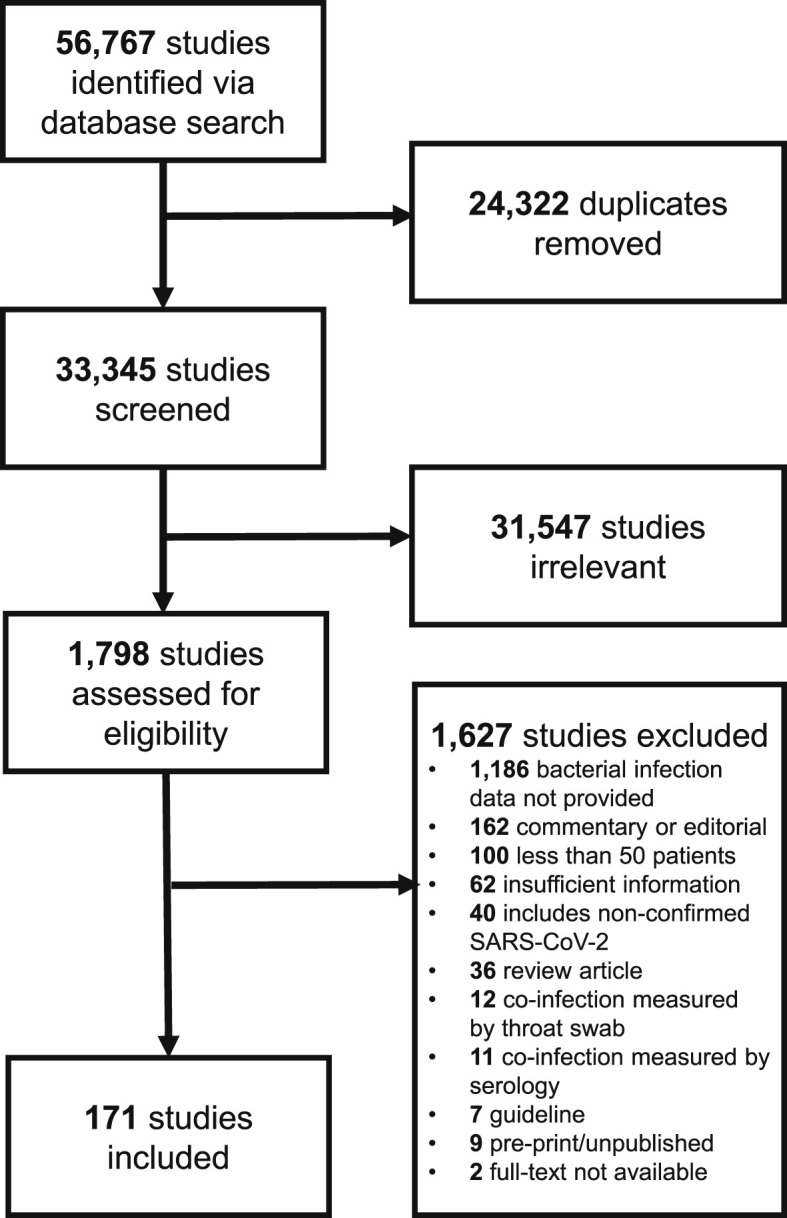
PRISMA study flow diagram.
Study geography
The majority of studies took place in Europe (67, 39%), followed by Asia (55, 32%), North America (34, 20%), Middle East (10, 6%), South and Central America (3, 2%) and multiple regions (2, 1%).
Healthcare setting
Most studies took place in a general hospital setting (129, 75%), followed by only ICU patients (33, 19%), mixed hospitalized patients and outpatients (9, 5%) and a single study limited to outpatients (1, 1%).
Patient characteristics
A total of 171 262 patients were evaluated for bacterial infection across the 171 studies. A median 41% of participants were female (interquartile range (IQR) 33–48%). The median age was 61 years (IQR 53–64, range 5–85). Only four studies included a population with a median age less than 18 years. Studies included a median of 25% ICU patients (IQR 13–100%) and a median of 21% (IQR 10–52%) patients receiving mechanical ventilation. Smokers comprised a median of 9% (IQR 5–22%) of the study populations. Common comorbidities included chronic respiratory disease (median 6%, IQR 4–12%), cardiovascular disease (median 14%, IQR 6–20%) and diabetes (median 21%, IQR 12–32%).
Bacterial infection assessment in COVID-19
Most studies did not specify how bacterial infection was detected (82, 48%), followed by studies using culture methods (72, 42%), combined approach using culture with nucleic acid amplification and/or urinary antigen testing (16, 9%) and nucleic acid amplification alone (1, 1%). Sources assessed for infection included a combination of respiratory and bloodstream isolates (75, 44%), respiratory only (38, 22%), blood only (22, 13%) and unclear site of infection (35, 20%). The minority of studies (66, 39%) employed a clinical definition for diagnosis of bacterial infection. A total of 86 studies (59 explicitly used co-infection terminology, and 27 not specified) including 137 325 patients evaluated for co-infection, and 95 studies including 40 843 patients evaluated for secondary infection. This includes ten studies that assessed patients for both co-infection and secondary infection.
Microbiological characteristics of bacterial infections in COVID-19
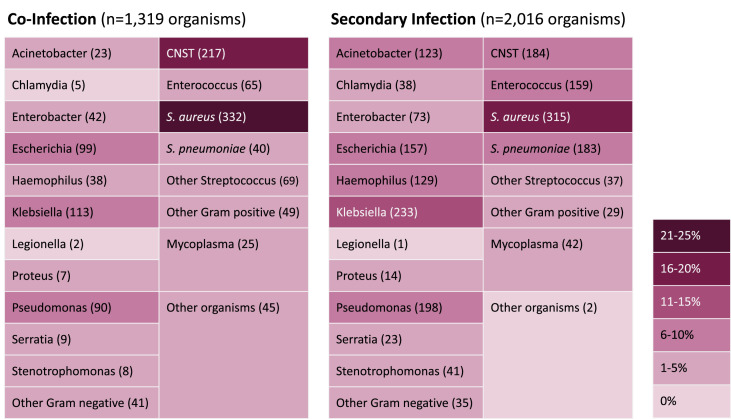
A total of 76 studies specified organism identification for bacterial infections (co-infections n = 1319 and secondary infections n = 2016). For co-infections, the most common bacterial organisms identified included Staphylococcus aureus (332, 25%), coagulase-negative Staphylococcus (CNST) (217, 16%) and Klebsiella species (113, 9%). For secondary infections, identified organisms were more diverse but the most common organisms were similar including S. aureus (315, 16%) and Klebsiella species (233, 11%). Fig. 2 contains complete details for all organisms identified.
Fig. 2.
Microbiological characteristics of bacterial infection in patients with COVID-19. CNST, coagulase negative Staphylococcus spp.
Prevalence of bacterial infection in patients with COVID-19
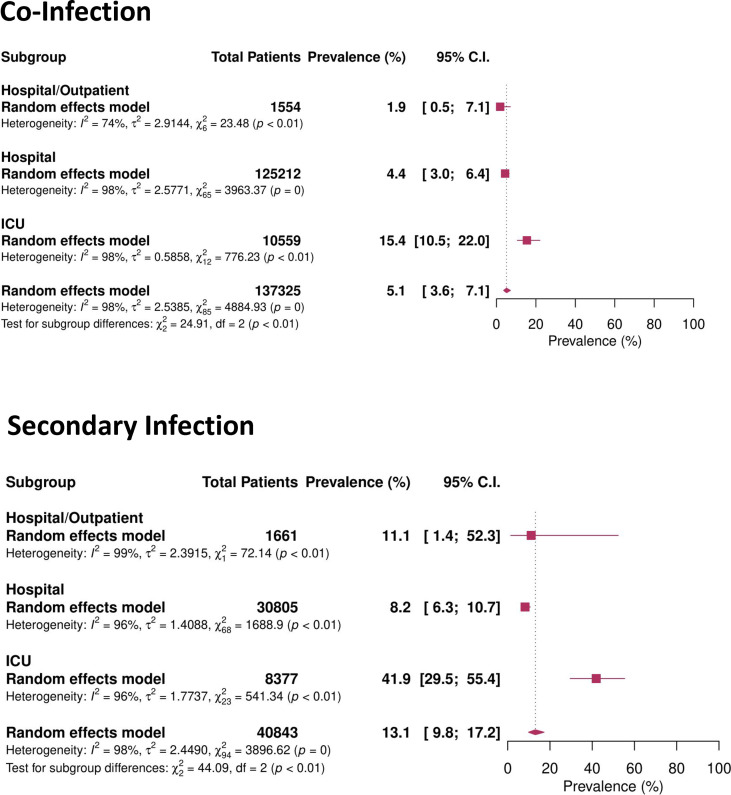
When pooling bacterial infection across all 171 262 patients with COVID-19, the prevalence of overall respiratory and/or bloodstream bacterial infection was 8.8% (95% CI 6.9–11.1%). Bacterial co-infection occurred in 5.1% (95% CI 3.6–7.1%) of patients and increased with the level or intensity of care, from mixed hospital/outpatient populations (1.9%, 95% CI 0.5–7.1%), to hospitalized patients (4.4%, 95% CI 3.0–6.4%), to intensive care only (15.4%, 95% CI 10.5–22.0%). Secondary bacterial infection was more common at 13.1% (95% CI 9.8–17.2%). While mixed hospital/outpatient populations (11.1%, 95% CI 1.4–52.3%) and hospitalized populations (8.2%, 95% CI 6.3–10.7%) experienced a lower risk of secondary infection, secondary bacterial infection was more common in ICU populations (41.9%, 95% CI 29.5–55.4). The degree of heterogeneity in the prevalence of bacterial co-infection and secondary infection was substantial (I2 = 98%) (Fig. 3 ).
Fig. 3.
Forest plot of co-infection and secondary infection prevalence based on healthcare setting co-infection.
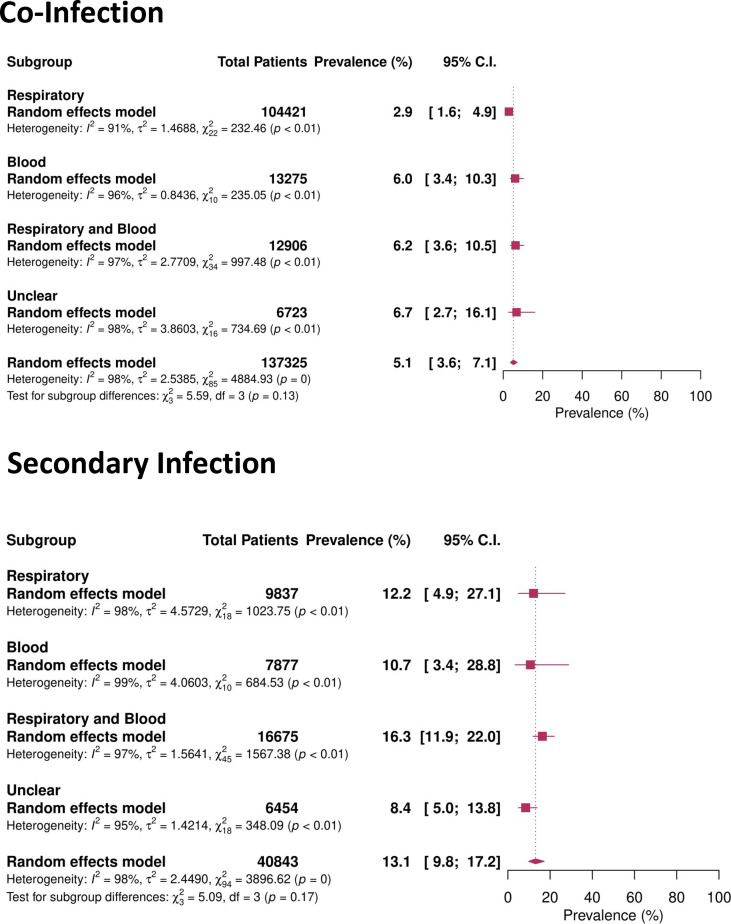
The prevalence of bacterial infection differed depending on the site of infection reported: respiratory and blood isolates (co-infection 6.2%, 95% CI 3.6–10.5%; secondary infection 16.3%, 11.9–22.0%), blood alone (co-infection 6.0%, 95% CI 3.4–10.3%; secondary infection 10.7%, 95% CI 3.4–28.8%) and respiratory isolates alone (co-infection 2.9%, 95% CI 1.6–4.9%; secondary infection 12.2%, 95% CI 4.9–27.1%) (Fig. 4 ).
Fig. 4.
Forest plot of co-infection and secondary infection prevalence based on specimen source.
Meta-regression: predictors of co-infection and secondary infection in COVID-19
COVID-19 co-infection rates were higher in studies with a higher proportion of ICU patients (adjusted OR 6.96, 95% CI 2.14–22.65) and secondary infection (adjusted OR 32.10, 95% CI 2.66–387.30) than those not admitted to an ICU. Studies with a higher proportion of mechanically ventilated patients were associated with a greater odds of both co-infection (adjusted OR 1.24, 95% CI 1.11–1.40) and secondary infection (adjusted OR 1.44, 95% CI 1.32–1.57). Advanced study population age did not appear to be associated with a greater odds of co-infection (adjusted OR 1.06, 95% CI 0.87–1.28 per 10-year increase) or secondary infection (adjusted OR 1.24, 95% CI 0.93–1.66 per 10-year increase). A higher proportion of female patients appeared to be protective for secondary infection (adjusted OR 0.73, 95% CI 0.55–0.97) but not co-infection (adjusted OR 1.05, 95% CI 0.80–1.37). There was no association between the proportion of study corticosteroid use and bacterial infections (co-infection adjusted OR 1.07, 95% CI 0.88–1.29, secondary infection adjusted OR 0.99, 95% CI 0.87–1.12) or IL-6 inhibitor use (co-infection adjusted OR 1.06, 95% CI 0.85–1.31, secondary infection adjusted OR 0.98, 95% CI 0.87–1.10) and bacterial infection in patients with COVID-19 (Table 1 ).
Table 1.
Study-level predictors of bacterial co-infection, secondary infection and overall bacterial infection in patients with Covid-19 (prevalence OR, 95% CI)
| Characteristic | Co-infection (n = 86) |
Secondary infection (n = 95) |
Bacterial infection (n = 171) |
No. of studies |
|||
|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | Overall | |
| Setting (reference = Hospital/Outpatient) | Reference | Reference | Reference | Reference | Reference | Reference | 8 |
| Hospital | 1.74 (0.64–4.71) | 1.49 (0.54–4.12) | 3.53 (0.28–44.19) | 4.16 (0.36–48.82) | 2.89 (1.07–7.78) | 2.31 (0.86–6.24) | 120 |
| ICU | 8.40 (2.65–26.65) | 6.96 (2.14–22.65) | 28.63 (2.22–369.50) | 32.10 (2.66–387.30) | 24.79 (8.57–71.74) | 18.83 (6.48–54.77) | 32 |
| Study end month (reference = Jan to May 2020) | Reference | Reference | Reference | Reference | Reference | Reference | 134 |
| Jun to Oct 2020 | 1.66 (0.53–5.15) | 1.28 (0.48–3.44) | 1.14 (0.43–2.98) | 1.14 (0.54–2.41) | 1.48 (0.67–3.25) | 1.18 (0.63–2.20) | 19 |
| Region (reference = North America) | Reference | Reference | Reference | Reference | Reference | Reference | 29 |
| Europe | 1.35 (0.57–3.20) | 1.03 (0.48–2.18) | 1.81 (0.76–4.34) | 1.46 (0.72–2.96) | 1.68 (0.82–3.41) | 1.28 (0.72–2.27) | 62 |
| Asia | 0.96 (0.39–2.36) | 1.51 (0.67–3.41) | 0.69 (0.27–1.76) | 0.99 (0.46–2.12) | 0.80 (0.38–1.69) | 1.18 (0.64–2.18) | 49 |
| Middle East | 0.98 (0.14–7.05) | 2.39 (0.37–15.39) | 0.70 (0.17–2.90) | 0.66 (0.21–2.08) | 0.96 (0.27–3.39) | 1.01 (0.36–2.85) | 8 |
| South and Central America | 1.52 (0.20–11.45) | 2.04 (0.35–11.82) | 2.37 (0.11–49.18) | 0.71 (0.06–8.03) | 1.31 (0.19–9.07) | 1.07 (0.22–5.13) | 3 |
| Multiple | 11.08 (0.84–145.78) | 3.81 (0.39–37.42) | 0.03 (0.00–0.92) | 0.08 (0.00–1.30) | 0.67 (0.07–6.94) | 0.47 (0.07–3.13) | 2 |
| Risk of bias (reference = Low) | Reference | Reference | Reference | Reference | Reference | Reference | 52 |
| Moderate | 1.17 (0.59–2.33) | 1.57 (0.87–2.86) | 1.20 (0.58–2.49) | 1.02 (0.58–1.79) | 1.22 (0.69–2.15) | 1.20 (0.76–1.89) | 82 |
| High | 1.72 (0.64–4.61) | 2.45 (1.05–5.72) | 0.60 (0.18–2.00) | 0.67 (0.26–1.71) | 0.91 (0.38–2.15) | 1.13 (0.57–2.26) | 19 |
| Age (10-year increase) | 1.20 (0.97–1.48) | 1.06 (0.87–1.28) | 1.53 (1.07–2.18) | 1.24 (0.93–1.66) | 1.37 (1.12–1.69) | 1.12 (0.94–1.34) | 153 |
| % Female (10% increase) | 0.74 (0.58–0.93) | 1.05 (0.80–1.37) | 0.50 (0.37–0.67) | 0.73 (0.55–0.97) | 0.58 (0.47–0.71) | 0.87 (0.71–1.07) | 152 |
| % Mech. ventilation (10% increase) | 1.26 (1.13–1.41) | 1.24 (1.11–1.40) | 1.45 (1.33–1.58) | 1.44 (1.32–1.57) | 1.42 (1.32–1.53) | 1.41 (1.30–1.52) | 124 |
| % Smoker (10% increase) | 0.89 (0.58–1.37) | 0.72 (0.46–1.14) | 0.85 (0.52–1.37) | 0.79 (0.55–1.12) | 0.89 (0.63–1.27) | 0.73 (0.54–0.98) | 58 |
| % COPD (10% increase) | 1.02 (0.55–1.90) | 0.86 (0.49–1.51) | 1.27 (0.71–2.29) | 1.02 (0.64–1.64) | 1.39 (0.89–2.16) | 1.08 (0.74–1.57) | 106 |
| % CVD (10% increase) | 0.98 (0.67–1.45) | 0.86 (0.57–1.31) | 1.27 (0.90–1.77) | 1.11 (0.80–1.52) | 1.18 (0.90–1.54) | 1.00 (0.77–1.30) | 100 |
| % Diabetes (10% increase) | 1.18 (0.97–1.45) | 1.10 (0.88–1.37) | 1.33 (1.00–1.76) | 1.00 (0.77–1.28) | 1.24 (1.03–1.50) | 1.03 (0.86–1.23) | 126 |
| % Corticosteroid (10% increase) | 1.12 (0.91–1.39) | 1.07 (0.88–1.29) | 1.11 (0.95–1.30) | 0.99 (0.87–1.12) | 1.14 (0.99–1.30) | 1.01 (0.91–1.14) | 103 |
| % IL-6 inhibitor (10% increase) | 1.27 (1.00–1.61) | 1.06 (0.85–1.31) | 1.05 (0.89–1.23) | 0.98 (0.87–1.10) | 1.12 (0.97–1.28) | 0.99 (0.89–1.10) | 65 |
Adjusted analysis accounts for median/mean study age, and severity index (% of patients with severe COVID-19).
Setting and mechanical ventilation are only adjusted for age.
Sensitivity analyses
Although the overall estimate of bacterial infection was similar when stratified by study risk of bias, studies with a moderate risk of bias reported numerically higher rates of bacterial infections in ICU patients (44.2%, 95% CI 28.8–60.8) than the same population in studies with high (18.8%, 95% CI 13.9–25.0%) and low risk of bias (24.5%, 95% CI 15.2–36.9%) (Fig. S1). However, this difference was not statistically significant upon meta-regression in either unadjusted or adjusted analyses. Studies with a high risk of bias had a greater odds of co-infection (adjusted OR 2.45, 95% CI 1.05–5.72) but not secondary infection (adjusted OR 0.67, 95% CI 0.26–1.71) (Table 1).
Estimates of proportion of bacterial infection were similar when removing studies where bacteriological testing method and bacterial infection source were not specified (Fig. S2). Studies that employed a clinical definition for bacterial infection appeared to report prevalence of infection similar to those without this definition (Fig. S3).
When removing studies that did not explicitly state whether they evaluated co-infection versus secondary infection, the findings were similar, with one key exception. ICU setting did not appear to be associated with co-infection risk after removing these studies (adjusted OR 3.61; 95% CI 0.46–28.36); however, the association between ICU setting and secondary infection risk persisted, (adjusted OR 32.10; 95% CI 2.66–387.30) (see Supplementary Table 2).
Antibiotic prescribing in patients with COVID-19
Out of 31 802 patients evaluated for antibiotic prescribing, the prevalence of antibiotic use in patients with COVID-19 was 76.2% (95% CI 67.3–83.2%). There did not appear to be an appreciable change in prescribing over time during the period January to September 2020. There was substantial heterogeneity between studies in the prevalence of antibiotic prescribing (I2 = 99%) (Fig. S4).
Discussion
We previously conducted a rapid review/meta-analysis on the prevalence of bacterial co-infection and another on the antibiotic prescribing based on literature published in the first few months of the pandemic [1,4]. The current update included literature published up to the beginning of February 2021—over a year since SARS-CoV-2 was first identified. In this analysis, the pooled estimated prevalence of bacterial co-infection in 5.1%, and increased with the severity of COVID-19 infection. The prevalence of secondary infections was estimated to be 13%, more than double that of co-infection. With meta-regression, this updated analysis provides a more comprehensive and granular picture on the predictors of bacterial infections in COVID-19 while confirming our earlier findings. Furthermore, compared with pandemic influenza which has been associated with a risk of bacterial co-infection of at least 19% [186,187] the risks of bacterial co-infection and secondary infections from SARS-CoV-2 appear to be lower.
A series of pivotal studies established two classes of immunotherapy, systemic corticosteroids and IL-6 inhibitors, as standards of care for patients with moderate to severe acute respiratory distress syndrome requiring hospitalization requiring respiratory support [[188], [189], [190], [191]]. Given their immunomodulatory effects, concern has been raised regarding their role in secondary infections in COVID-19. Our analyses did not identify a significantly higher odds of secondary bacterial infections among patients exposed to corticosteroids or IL-6 inhibitors. These findings may reflect the fact that other drivers, such as the inflammatory response to SARS-CoV-2 and invasive devices, are more significant contributing factors of bacterial infections in patients with COVID-19 [192] and as such there may be limited benefit of empiric antibiotics in the absence of proven infection for patients receiving these agents. Alternatively, our study may not be adequately powered to detect the impact of these immunomodulatory agents on secondary infections, given the relatively low rate of bacterial infection events.
The most common organisms identified in patients with both co-infection and secondary infection are S. aureus, CNST and Klebsiella species. These pathogens reflect those seen in hospital-acquired pneumonia and bloodstream infections also seen outside of the context of COVID-19 [193,194]. However, given most studies did not attempt to differentiate infection from contamination, CNST may represent contamination in many cases [174].
At 77%, the prevalence of antibiotic prescribing was high and incongruent with the relatively low prevalence of bacterial infections. This outcome remained consistent with our previous publication [4]. Of note, clinical guidelines for COVID-19 recommendations varied with respect to the use of empiric antibiotics, particularly in critically ill patients [[195], [196], [197], [198]]. Differences in the recommended approach to antibiotic prescribing may explain why the use of antibiotics remained higher than prevalence of bacterial infections. Our findings support a more judicious approach, particularly in patients outside of the ICU.
A limitation of this review is that these findings are constrained by the quality of the underlying primary literature, as less than one third of included studies were considered to be at low risk of bias. We also noted a high degree of heterogeneity across studies, of which it is not clear whether this reflects a difference in clinical characteristics, diagnostic approaches and/or clinical practice across settings. However, our findings are relatively consistent across study risk of bias rating and align with those in recent large high-quality observational cohort studies [83,161]. A second limitation is our focus on microbiological identification of bacterial infection. There may be a risk of under-estimating the true prevalence of co-infection and secondary infection given that only a proportion of patients receive microbiological testing to confirm the presence of bacterial infection. On the other hand, a microbiological definition alone may overestimate infection in the setting of colonization without evidence of a true respiratory infection. Finally, the distinction between bacterial co-infection and secondary infection is not always consistent across the literature [199]. Terms may be used interchangeably or not at all, leading to a risk of misclassification bias. To address this risk, we reported both an estimate of co-infection and secondary infection, as well as overall bacterial infection, the latter of which reduces the impact of imprecision associated with terminology chosen by study authors.
Over a year into the pandemic, these findings provide further supporting evidence for the relatively low rate of bacterial infection in COVID-19 and may serve an impetus for antimicrobial stewardship initiatives, such as risk stratification for early empiric antibiotics, and early discontinuation in patients with COVID-19 and lack of confirmed concomitant bacterial infection.
Conclusion
While the odds of respiratory and bloodstream bacterial infection are low in patients with COVID-19, potential risk factors for infection include ICU setting and mechanical ventilation. The risk for secondary infection is substantially greater than the risk for co-infection in patients with COVID-19. Antibiotics appear to continue to be overprescribed in COVID-19. Understanding predictors of co-infection and secondary infection may help to support improved antibiotic stewardship in patients with COVID-19.
Transparency declaration
The authors have no conflicts of interest to declare. Funding: This project was carried out as part of routine work.
Author contributions
Concept and design: All authors. Acquisition, analysis or interpretation of data: All authors. Drafting of the manuscript: Langford, So. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Soucy, Langford. Administrative, technical or material support: All authors.
Acknowledgements
The authors would like to thank: Ashley Farrell, BA, MLIS, AHIP, Information Specialist for her support with formulating and executing the search strategy for this review and Parmvir Parmar MD, FRCPC for his support in full text screening for this review. We thank the Toronto Antimicrobial Resistance Research Network (TARRN), an inter-professional forum of clinicians and researchers that acted as a venue for bringing together this research team, and a repository for our rapid review.
Editor: L Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.11.008.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Langford B.J., So M., Raybardhan S., Leung V., Westwood D., MacFadden D.R., et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26:1622–1629. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russell C.D., Fairfield C.J., Drake T.M., Turtle L., Seaton R.A., Wootton D.G., et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. Lancet Microbe. 2021;2:e354–e365. doi: 10.1016/S2666-5247(21)00090-2. S2666524721000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaughn V.M., Gandhi T.N., Petty L.A., Patel P.K., Prescott H.C., Malani A.N., et al. Empiric antibacterial therapy and community-onset bacterial coinfection in patients hospitalized with coronavirus disease 2019 (covid-19): a multi-hospital cohort study. Clin Infect Dis. 2021;72:e533–e541. doi: 10.1093/cid/ciaa1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langford B.J., So M., Raybardhan S., Leung V., Soucy J.-P.R., Westwood D., et al. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clin Microbiol Infect. 2021;27:520–531. doi: 10.1016/j.cmi.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chong W.H., Saha B.K., Ramani Ananthakrishnan, Chopra A. State-of-the-art review of secondary pulmonary infections in patients with COVID-19 pneumonia. Infection. 2021;49:591–605. doi: 10.1007/s15010-021-01602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grasselli G., Scaravilli V., Mangioni D., Scudeller L., Alagna L., Bartoletti M., et al. Hospital-acquired infections in critically ill patients with COVID-19. Chest. 2021;160:454–465. doi: 10.1016/j.chest.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garritty C., Gartlehner G., Kamel C., King V.J., Nussbaumer-Streit B., Stevens A., et al. Cochrane rapid reviews. Interim guidance from the Cochrane rapid reviews methods Group. 2020. https://methods.cochrane.org/rapidreviews/sites/methods.cochrane.org.rapidreviews/files/public/uploads/cochrane_rr_-_guidance-23mar2020-final.pdf Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viera A.J., Garrett J.M. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37:360–363. [PubMed] [Google Scholar]
- 10.Hoy D., Brooks P., Woolf A., Blyth F., March L., Bain C., et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65:934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Lin L., Xu C. Arcsine-based transformations for meta-analysis of proportions: pros, cons, and alternatives. Health Sci Rep. 2020;3:e178. doi: 10.1002/hsr2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin L., Chu H. Meta-analysis of proportions using generalized linear mixed models. Epidemiology. 2020;31:713–717. doi: 10.1097/EDE.0000000000001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deeks J., Higgins J., Altman D. 2019. Cochrane handbook for systematic reviews of interventions.https://training.cochrane.org/handbook Available from: [Google Scholar]
- 14.Soucy J.-P. 2021. Predictors and microbiology of co-infection in patients with COVID-19: living rapid review update and meta-regression statistical code.https://github.com/jeanpaulrsoucy/covid-19-coinfection-metaregression Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ongrádi J., Kövesdi V. Factors that may impact on immunosenescence: an appraisal. Immun Ageing A. 2010;7:7. doi: 10.1186/1742-4933-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levine S.A., Niederman M.S. The impact of tracheal intubation on host defenses and risks for nosocomial pneumonia. Clin Chest Med. 1991;12:523–543. [PubMed] [Google Scholar]
- 17.Alharthy A., Aletreby W., Faqihi F., Balhamar A., Alaklobi F., Alanezi K., et al. Clinical characteristics and predictors of 28-day mortality in 352 critically ill patients with COVID-19: a retrospective study. J Epidemiol Glob Health. 2021;11:98–104. doi: 10.2991/jegh.k.200928.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angelis V., Tippu Z., Joshi K., Reis S., Gronthoud F., Fribbens C., et al. Defining the true impact of coronavirus disease 2019 in the at-risk population of patients with cancer. Eur J Cancer. 2020;136:99–106. doi: 10.1016/j.ejca.2020.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Annane D., Heming N., Grimaldi-Bensouda L., Fremeaux-Bacchi V., Vigan M., Roux A.-L., et al. Eculizumab as an emergency treatment for adult patients with severe COVID-19 in the intensive care unit: a proof-of-concept study. EClinicalMedicine. 2020;28:100590. doi: 10.1016/j.eclinm.2020.100590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armann J.P., Diffloth N., Simon A., Doenhardt M., Hufnagel M., Trotter A., et al. Hospital admission in children and adolescents with COVID-19. Dtsch Arzteblatt Int. 2020;117:373–374. doi: 10.3238/arztebl.2020.0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azoulay E., Fartoukh M., Darmon M., Geri G., Voiriot G., Dupont T., et al. Increased mortality in patients with severe SARS-CoV-2 infection admitted within seven days of disease onset. Intensive Care Med. 2020;46:1714–1722. doi: 10.1007/s00134-020-06202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bahl A., Van Baalen M.N., Ortiz L., Chen N.-W., Todd C., Milad M., et al. Early predictors of in-hospital mortality in patients with COVID-19 in a large American cohort. Intern Emerg Med. 2020;15:1485–1499. doi: 10.1007/s11739-020-02509-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balena F., Bavaro D.F., Fabrizio C., Bottalico I.F., Calamo A., Santoro C.R., et al. Tocilizumab and corticosteroids for covid-19 treatment in elderly patients. J Gerontol Geriatr. 2020;68:197–203. [Google Scholar]
- 24.Balkhair A., Al-Zakwani I., Al Busaidi M., Al-Khirbash A., Al Mubaihsi S., BaTaher H., et al. Anakinra in hospitalized patients with severe COVID-19 pneumonia requiring oxygen therapy: results of a prospective, open-label, interventional study. Int J Infect Dis. 2021;103:288–296. doi: 10.1016/j.ijid.2020.11.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbaro R.P., MacLaren G., Boonstra P.S., Iwashyna T.J., Slutsky A.S., Fan E., et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396:1071–1078. doi: 10.1016/S0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bardi T., Pintado V., Gomez-Rojo M., Escudero-Sanchez R., Azzam Lopez A., Diez-Remesal Y., et al. Nosocomial infections associated to COVID-19 in the intensive care unit: clinical characteristics and outcome. Eur J Clin Microbiol Infect Dis. 2021;40:495–502. doi: 10.1007/s10096-020-04142-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barry M., Al Mohaya A., Al Hijji A., Akkielah L., Al Rajhi A., Almajid F., et al. Clinical characteristics and outcome of hospitalized COVID-19 patients in a MERS-CoV endemic area. J Epidemiol Glob Health. 2020;10:214–221. doi: 10.2991/jegh.k.200806.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartoletti M., Marconi L., Scudeller L., Pancaldi L., Tedeschi S., Giannella M., et al. Efficacy of corticosteroid treatment for hospitalized patients with severe COVID-19: a multicentre study. Clin Microbiol Infect. 2021;27:105–111. doi: 10.1016/j.cmi.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Becchetti C., Zambelli M.F., Pasulo L., Donato M.F., Invernizzi F., Detry O., et al. COVID-19 in an international European liver transplant recipient cohort. Gut. 2020;69:1832–1840. doi: 10.1136/gutjnl-2020-321923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhatt P.J., Shiau S., Brunetti L., Xie Y., Solanki K., Khalid S., et al. Risk factors and outcomes of hospitalized patients with severe Covid-19 and secondary bloodstream infections: a multicenter, case-control study. Clin Infect Dis. 2021;72:e995–e1003. doi: 10.1093/cid/ciaa1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonazzetti C., Morena V., Giacomelli A., Oreni L., Casalini G., Galimberti L.R., et al. Unexpectedly high frequency of enterococcal bloodstream infections in coronavirus disease 2019 patients admitted to an Italian ICU: an Observational Study. Crit Care Med. 2021;49:e31–e40. doi: 10.1097/CCM.0000000000004748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boren H.K., Thaulow C.M., Quist-Paulsen E., Waehre T., Akselsen P.E., Tonby K. Tidsskr den nor laegeforening tidsskr. Prakt Med Ny Raekke. 2020;140 doi: 10.4045/tidsskr.20.0443. [DOI] [PubMed] [Google Scholar]
- 33.Borobia A.M., Carcas A.J., Arnalich F., Alvarez-Sala R., Monserrat-Villatoro J., Quintana M., et al. A cohort of patients with COVID-19 in a major teaching hospital in Europe. J Clin Med. 2020;9:1–10. doi: 10.3390/jcm9061733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai Q., Huang D., Ou P., Yu H., Zhu Z., Xia Z., et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;39c:7804028. doi: 10.1111/all.14309. [DOI] [PubMed] [Google Scholar]
- 35.Campochiaro C., Della-Torre E., Cavalli G., De Luca G., Ripa M., Boffini N., et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med. 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cataldo M.A., Tetaj N., Selleri M., Marchioni L., Capone A., Caraffa E., et al. Incidence of bacterial and fungal bloodstream infections in COVID-19 patients in intensive care: an alarming “collateral effect. J Glob Antimicrob Resist. 2020;23:290–291. doi: 10.1016/j.jgar.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cates J., Lucero-Obusan C., Dahl R.M., Schirmer P., Garg S., Oda G., et al. Risk for in-hospital complications associated with covid-19 and influenza - veterans health administration, United States, october 1, 2018-may 31, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1528–1534. doi: 10.15585/mmwr.mm6942e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cattelan A.M., Di Meco E., Trevenzoli M., Frater A., Ferrari A., Villano M., et al. Clinical characteristics and laboratory biomarkers changes in COVID-19 patients requiring or not intensive or sub-intensive care: a comparative study. BMC Infect Dis. 2020;20:934. doi: 10.1186/s12879-020-05647-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chamorro-de-Vega E., Rodriguez-Gonzalez C.-G., Manrique-Rodriguez S., Lobato-Matilla E., Garcia-Moreno F., Olmedo M., et al. Clinical course of severe patients with COVID-19 treated with tocilizumab: report from a cohort study in Spain. Expert Rev Clin Pharmacol. 2021;14:249–260. doi: 10.1080/17512433.2021.1875819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaudhary W.A., Chong P.L., Mani B.I., Asli R., Momin R.N., Abdullah M.S., et al. Primary respiratory bacterial coinfections in patients with COVID-19. Am J Trop Med Hyg. 2020;103:917–919. doi: 10.4269/ajtmh.20-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen L., Yu J., He W., Chen L., Yuan G., Dong F., et al. Risk factors for death in 1859 subjects with COVID-19. Leukemia. 2020;34:2173–2183. doi: 10.1038/s41375-020-0911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen T., Dai Z., Mo P., Li X., Ma Z., Song S., et al. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China (2019): a single-centered, retrospective study. J Gerontol A Biol Sci Med Sci. 2020:75. doi: 10.1093/gerona/glaa089. 1788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng K., He M., Shu Q., Wu M., Chen C., Xue Y. Analysis of the risk factors for nosocomial bacterial infection in patients with COVID-19 in a tertiary hospital. Risk Manag Healthc Policy. 2020;13:2593–2599. doi: 10.2147/RMHP.S277963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng L.S.-K., Chau S.K.-Y., Tso E.Y.-K., Tsang S.W.-C., Li I.Y.-F., Wong B.K.-C., et al. Bacterial co-infections and antibiotic prescribing practice in adults with COVID-19: experience from a single hospital cluster. Ther Adv Infect Dis. 2020;7 doi: 10.1177/2049936120978095. 2049936120978095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cobb N.L., Sathe N.A., Duan K.I., Seitz K.P., Thau M.R., Sung C.C., et al. Comparison of clinical features and outcomes in critically ill patients hospitalized with COVID-19 versus influenza. Ann Am Thorac Soc. 2020;18:632–640. doi: 10.1513/AnnalsATS.202007-805OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cobos-Siles M., Cubero-Morais P., Arroyo-Jimenez I., Rey-Hernandez M., Hernandez-Gomez L., Vargas-Parra D.J., et al. Cause-specific death in hospitalized individuals infected with SARS-CoV-2: more than just acute respiratory failure or thromboembolic events. Intern Emerg Med. 2020;15:1533–1544. doi: 10.1007/s11739-020-02485-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Contou D., Claudinon A., Pajot O., Micaelo M., Longuet Flandre P., Dubert M., et al. Bacterial and viral co-infections in patients with severe SARS-CoV-2 pneumonia admitted to a French ICU. Ann Intensive Care. 2020;10:119. doi: 10.1186/s13613-020-00736-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cordeanu E.-M., Jambert L., Severac F., Lambach H., Tousch J., Heitz M., et al. Outcomes of COVID-19 hospitalized patients previously treated with renin-angiotensin system inhibitors. J Clin Med. 2020;9:1–16. doi: 10.3390/jcm9113472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47:60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.D’Onofrio V., Van Steenkiste E., Meersman A., Waumans L., Cartuyvels R., Van Halem K., et al. Differentiating influenza from COVID-19 in patients presenting with suspected sepsis. Eur J Clin Microbiol Infect Dis. 2021;40:987–995. doi: 10.1007/s10096-020-04109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Della-Torre E., Campochiaro C., Cavalli G., De Luca G., Napolitano A., La Marca S., et al. Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: an open-label cohort study. Ann Rheum Dis. 2020;79:1277–1285. doi: 10.1136/annrheumdis-2020-218122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Di Domenico S.L., Coen D., Bergamaschi M., Albertini V., Ghezzi L., Cazzaniga M.M., et al. Clinical characteristics and respiratory support of 310 COVID-19 patients, diagnosed at the emergency room: a single-center retrospective study. Intern Emerg Med. 2021:1051–1060. doi: 10.1007/s11739-020-02548-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Di Nisio M., Potere N., Candeloro M., Spacone A., Pieramati L., Ferrandu G., et al. Interleukin-6 receptor blockade with subcutaneous tocilizumab improves coagulation activity in patients with COVID-19. Eur J Intern Med. 2021;83:34–38. doi: 10.1016/j.ejim.2020.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dreher M., Kersten A., Bickenbach J., Balfanz P., Hartmann B., Cornelissen C., et al. The characteristics of 50 hospitalized COVID-19 patients with and without ARDS. Dtsch Arzteblatt Int. 2020;117:271. doi: 10.3238/arztebl.2020.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dupuis C., Bouadma L., de Montmollin E., Goldgran-Toledano D., Schwebel C., Reignier J., et al. Association between early invasive mechanical ventilation and day-60 mortality in acute hypoxemic respiratory failure related to coronavirus disease-2019 pneumonia. Crit Care Explor. 2021;3 doi: 10.1097/CCE.0000000000000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elabbadi A., Turpin M., Gerotziafas G.T., Teulier M., Voiriot G., Fartoukh M. Bacterial coinfection in critically ill COVID-19 patients with severe pneumonia. Infection. 2021;49:559–562. doi: 10.1007/s15010-020-01553-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elghoudi A., Aldhanhani H., Ghatasheh G., Sharif E., Narchi H. Covid-19 in children and young adolescents in Al Ain, United Arab Emirates – a retrospective cross-sectional Study. Front Pediatr. 2020;8:603741. doi: 10.3389/fped.2020.603741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Falcone M., Tiseo G., Giordano C., Leonildi A., Menichini M., Vecchione A., et al. Predictors of hospital-acquired bacterial and fungal superinfections in COVID-19: a prospective observational study. J Antimicrob Chemother. 2021;76:1078–1084. doi: 10.1093/jac/dkaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feng G., Huang W.-Q., Liu M.-L., Lin S.-C., Zhang X.-Z., Zhang Y., et al. Clinical features of COVID-19 patients in xiaogan city. SN Compr Clin Med. 2020;2:1717–1723. doi: 10.1007/s42399-020-00465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feng Y., Ling Y., Bai T., Xie Y., Huang J., Li J., et al. COVID-19 with different severity: a multi-center study of clinical features. Am J Respir Crit Care Med. 2020;201:1380–1388. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feng Z., Yu Q., Yao S., Luo L., Zhou W., Mao X., et al. Early prediction of disease progression in COVID-19 pneumonia patients with chest CT and clinical characteristics. Nat Commun. 2020;11:4968. doi: 10.1038/s41467-020-18786-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferguson J., Rosser J.I., Quintero O., Scott J., Subramanian A., Gumma M., et al. Characteristics and outcomes of coronavirus disease patients under nonsurge conditions, Northern California, USA, March-April 2020. Emerg Infect Dis. 2020;26:1679–1685. doi: 10.3201/eid2608.201776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferrando C., Mellado-Artigas R., Gea A., Arruti E., Aldecoa C., Bordell A., et al. Patient characteristics, clinical course and factors associated to ICU mortality in critically ill patients infected with SARS-CoV-2 in Spain: a prospective, cohort, multicentre study. Rev Esp Anestesiol Reanim. 2020;67:425–437. doi: 10.1016/j.redar.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fisler G., Izard S.M., Shah S., Lewis D., Kainth M.K., Hagmann S.H.F., et al. Characteristics and risk factors associated with critical illness in pediatric COVID-19. Ann Intensive Care. 2020;10:171. doi: 10.1186/s13613-020-00790-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Galvez-Romero J.L., Palmeros-Rojas O., Real-Ramirez F.A., Sanchez-Romero S., Tome-Maxil R., Ramirez-Sandoval M.P., et al. Cyclosporine A plus low-dose steroid treatment in COVID-19 improves clinical outcomes in patients with moderate to severe disease: a pilot study. J Intern Med. 2021;289:906–920. doi: 10.1111/joim.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garazzino S., Montagnani C., Dona D., Meini A., Felici E., Vergine G., et al. Multicentre Italian study of SARS-CoV-2 infection in children and adolescents, preliminary data as at 10 April 2020. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.18.2000600. 2000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garcia-Vidal C., Sanjuan G., Moreno-Garcia E., Puerta-Alcalde P., Garcia-Pouton N., Chumbita M., et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021;27:83–88. doi: 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gavin W., Campbell E., Zaidi S.-A., Gavin N., Dbeibo L., Beeler C., et al. Clinical characteristics, outcomes and prognosticators in adult patients hospitalized with COVID-19. Am J Infect Control. 2021;49:158–165. doi: 10.1016/j.ajic.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giacobbe D.R., Battaglini D., Ball L., Brunetti I., Bruzzone B., Codda G., et al. Bloodstream infections in critically ill patients with COVID-19. Eur J Clin Invest. 2020;50 doi: 10.1111/eci.13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goncalves Mendes Neto A., Lo K.B., Wattoo A., Salacup G., Pelayo J., DeJoy R., et al. Bacterial infections and patterns of antibiotic use in patients with COVID-19. J Med Virol. 2021;93:1489–1495. doi: 10.1002/jmv.26441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gottlieb M., Sansom S., Frankenberger C., Ward E., Hota B. Clinical course and factors associated with hospitalization and critical illness among COVID-19 patients in Chicago, Illinois. Acad Emerg Med. 2020;27:963–973. doi: 10.1111/acem.14104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M., et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2:e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo L., Xiong W., Liu D., Feng Y., Wang P., Dong X., et al. The MNCP-SPI score predicting risk of severe covid-19 among mild-pneumonia patients on admission. Infect Drug Resist. 2020;13:3593–3600. doi: 10.2147/IDR.S263157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.He F., Ding X.-F., Cao M., Gong H.-Y., Fu X.-Z., Luo J., et al. Comparative analysis of 95 patients with different severity in the early outbreak of COVID-19 in Wuhan, China. Can J Infect Dis Med Microbiol. 2020;2020:4783062. doi: 10.1155/2020/4783062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hughes S., Troise O., Donaldson H., Mughal N., Moore L.S.P. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect. 2020;26:1395–1399. doi: 10.1016/j.cmi.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ignatius E.H., Wang K., Karaba A., Robinson M., Avery R.K., Blair P., et al. Tocilizumab for the treatment of COVID-19 among hospitalized patients: a matched retrospective cohort analysis. Open Forum Infect Dis. 2021;8:ofaa598. doi: 10.1093/ofid/ofaa598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ip A., Berry D.A., Hansen E., Goy A.H., Pecora A.L., Sinclaire B.A., et al. Hydroxychloroquine and tocilizumab therapy in COVID-19 patients-An observational study. PLoS One. 2020;15 doi: 10.1371/journal.pone.0237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jang J.G., Hur J., Choi E.Y., Hong K.S., Lee W., Ahn J.H. Prognostic factors for severe coronavirus disease 2019 in Daegu, Korea. J Korean Med Sci. 2020;35 doi: 10.3346/jkms.2020.35.e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Javanian M., Bayani M., Shokri M., Sadeghi-Haddad-Zavareh M., Babazadeh A., Yeganeh B., et al. Clinical and laboratory findings from patients with COVID-19 pneumonia in Babol North of Iran: a retrospective cohort study. Rom J Intern Med. 2020;58:161–167. doi: 10.2478/rjim-2020-0013. [DOI] [PubMed] [Google Scholar]
- 81.Javanian M., Bayani M., Shokri M., Sadeghi-Haddad-Zavareh M., Babazadeh A., Ghadimi R., et al. Risk factors for mortality of 557 adult patients with COVID 19 in Babol, Northern Iran: a retrospective cohort study. Bratisl Lek Listy. 2021;122:34–38. doi: 10.4149/BLL_2021_003. [DOI] [PubMed] [Google Scholar]
- 82.Jimenez E., Fontan-Vela M., Valencia J., Fernandez-Jimenez I., Alvaro-Alonso E.A., Izquierdo-Garcia E., et al. Characteristics, complications and outcomes among 1549 patients hospitalised with COVID-19 in a secondary hospital in Madrid, Spain: a retrospective case series study. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Karaba S.M., Jones G., Helsel T., Smith L.L., Avery R., Dzintars K., et al. Prevalence of co-infection at the time of hospital admission in COVID-19 patients, a multicenter study. Open Forum Infect Dis. 2021;8:ofaa578. doi: 10.1093/ofid/ofaa578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Karami Z., Knoop B.T., Dofferhoff A.S.M., Blaauw M.J.T., Janssen N.A., van Apeldoorn M., et al. Few bacterial co-infections but frequent empiric antibiotic use in the early phase of hospitalized patients with COVID-19: results from a multicentre retrospective cohort study in The Netherlands. Infect Dis. 2021;53:102–110. doi: 10.1080/23744235.2020.1839672. [DOI] [PubMed] [Google Scholar]
- 85.Karmen-Tuohy S., Carlucci P.M., Zervou F.N., Zacharioudakis I.M., Rebick G., Klein E., et al. Outcomes among HIV-positive patients hospitalized with COVID-19. J Acquir Immune Defic Syndr. 2020;85:6–10. doi: 10.1097/QAI.0000000000002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kewan T., Covut F., Al-Jaghbeer M.J., Rose L., Gopalakrishna K.V., Akbik B. Tocilizumab for treatment of patients with severe COVID-19: a retrospective cohort study. EClinicalMedicine. 2020;24:100418. doi: 10.1016/j.eclinm.2020.100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kimmig L.M., Wu D., Gold M., Pettit N.N., Pitrak D., Mueller J., et al. IL-6 Inhibition in critically ill COVID-19 patients is associated with increased secondary infections. Front Med. 2020;7:583897. doi: 10.3389/fmed.2020.583897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kolenda C., Ranc A.-G., Boisset S., Caspar Y., Carricajo A., Souche A., et al. Assessment of respiratory bacterial coinfections among severe acute respiratory syndrome Coronavirus 2-positive patients hospitalized in intensive care units using conventional culture and BioFire, FilmArray pneumonia panel plus assay. Open Forum Infect Dis. 2020;7 doi: 10.1093/ofid/ofaa484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kumar G., Adams A., Hererra M., Rojas E.R., Singh V., Sakhuja A., et al. Predictors and outcomes of healthcare-associated infections in COVID-19 patients. Int J Infect Dis. 2021;104:287–292. doi: 10.1016/j.ijid.2020.11.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lardaro T., Wang A.Z., Bucca A., Croft A., Glober N., Holt D.B., et al. Characteristics of COVID-19 patients with bacterial co-infection admitted to the hospital from the emergency department in a large regional healthcare system. J Med Virol. 2021;93:2883–2889. doi: 10.1002/jmv.26795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee S.-I., Koh J.S., Kim Y.J., Kang D.H., Park D., Park H.S., et al. Secondary infection among hospitalized COVID-19 patients: a retrospective cohort study in a tertiary care setting. Respirol Carlton Vic. 2021;26:277–278. doi: 10.1111/resp.13992. [DOI] [PubMed] [Google Scholar]
- 92.Lehmann C.J., Pho M.T., Pitrak D., Ridgway J.P., Pettit N.N. Community acquired co-infection in COVID-19: a retrospective observational experience. Clin Infect Dis. 2020;72:1450–1452. doi: 10.1093/cid/ciaa902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lendorf M.E., Boisen M.K., Kristensen P.L., Lokkegaard E.C.L., Krog S.M., Brandi L., et al. Characteristics and early outcomes of patients hospitalised for covid-19 in North Zealand, Denmark. Dan Med J. 2020;67:1–11. [PubMed] [Google Scholar]
- 94.Li J., Li M., Zheng S., Li M., Zhang M., Sun M., et al. Plasma albumin levels predict risk for nonsurvivors in critically ill patients with COVID-19. Biomark Med. 2020:827–837. doi: 10.2217/bmm-2020-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li J., Wang J., Yang Y., Cai P., Cao J., Cai X., et al. Etiology and antimicrobial resistance of secondary bacterial infections in patients hospitalized with COVID-19 in Wuhan, China: a retrospective analysis. Antimicrob Resist Infect Control. 2020;9:153. doi: 10.1186/s13756-020-00819-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y., et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li Y., Deng W., Xiong H., Li H., Chen Z., Nie Y., et al. Immune-related factors associated with pneumonia in 127 children with coronavirus disease 2019 in Wuhan. Pediatr Pulmonol. 2020;55:2354–2360. doi: 10.1002/ppul.24907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lian J., Jin X., Hao S., Cai H., Zhang S., Zheng L., et al. Analysis of epidemiological and clinical features in older patients with corona virus disease 2019 (COVID-19) out of Wuhan. Clin Infect Dis. 2020;28:740–747. doi: 10.1093/cid/ciaa242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu W., Tao Z.-W., Lei W., Ming-Li Y., Kui L., Ling Z., et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl) 2020;133:1032–1038. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu Z., Li X., Fan G., Zhou F., Huang L., Yu J., et al. Low-to-moderate dose corticosteroids treatment in hospitalized adults with COVID-19. Clin Microbiol Infect. 2021;27:112–117. doi: 10.1016/j.cmi.2020.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Llitjos J.-F., Bredin S., Lascarrou J.-B., Soumagne T., Cojocaru M., Leclerc M., et al. Increased susceptibility to intensive care unit-acquired pneumonia in severe COVID-19 patients: a multicentre retrospective cohort study. Ann Intensive Care. 2021;11:20. doi: 10.1186/s13613-021-00812-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Luduena M.G., Labato M., Chiaradia V., Yamuni J., Finocchietto P., Pisarevsky A.A. [Analysis of the first 100 patients with COVID-19 admitted to internal medicine wards at the hospital de Clinicas jose de San martin, buenos aires university] Medicina (Mex) 2020;80:48–55. [PubMed] [Google Scholar]
- 103.Lui G.C.-Y., Yip T.C.-F., Wong V.W.-S., Chow V.C.-Y., Ho T.H.-Y., Li T.C.-M., et al. Significantly lower case-fatality ratio of coronavirus disease 2019 (COVID-19) than severe acute respiratory syndrome (SARS) in Hong Kong – a territory-wide cohort study. Clin Infect Dis. 2021;72:e466–e475. doi: 10.1093/cid/ciaa1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Luyt C.-E., Sahnoun T., Gautier M., Vidal P., Burrel S., Pineton de Chambrun M., et al. Ventilator-associated pneumonia in patients with SARS-CoV-2-associated acute respiratory distress syndrome requiring ECMO: a retrospective cohort study. Ann Intensive Care. 2020;10:158. doi: 10.1186/s13613-020-00775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lv Z., Cheng S., Le J., Huang J., Feng L., Zhang B., et al. Clinical characteristics and co-infections of 354 hospitalized patients with COVID-19 in Wuhan, China: a retrospective cohort study. Microbe. Infect. 2020;22:195–199. doi: 10.1016/j.micinf.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mady A., Aletreby W., Abdulrahman B., Lhmdi M., Noor A.M., Alqahtani S.A., et al. Tocilizumab in the treatment of rapidly evolving COVID-19 pneumonia and multifaceted critical illness: a retrospective case series. Ann Med Surg. 2020;60:417–424. doi: 10.1016/j.amsu.2020.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maes M., Higginson E., Pereira-Dias J., Curran M.D., Parmar S., Khokhar F., et al. Ventilator-associated pneumonia in critically ill patients with COVID-19. Crit Care. 2021;25:25. doi: 10.1186/s13054-021-03460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mahmoudi H. Bacterial co-infections and antibiotic resistance in patients with COVID-19. GMS Hyg Infect Control. 2020;15:Doc35. doi: 10.3205/dgkh000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McCracken J.A., Nakeshbandi M., Arace J., Riley W.J., Sharma R. COVID-19 related deaths in an urban academic medical center in Brooklyn – a descriptive case series. Transl Med Commun. 2020;5:12. doi: 10.1186/s41231-020-00065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mendizabal M., Pinero F., Ridruejo E., Anders M., Silveyra M.D., Torre A., et al. Prospective Latin American cohort evaluating outcomes of patients with COVID-19 and abnormal liver tests on admission. Ann Hepatol. 2021;21:100298. doi: 10.1016/j.aohep.2020.100298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mo P., Xing Y., Xiao Y., Deng L., Zhao Q., Wang H., et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. 2020;73:e4208–e4213. doi: 10.1093/cid/ciaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morena V., Milazzo L., Oreni L., Bestetti G., Fossali T., Bassoli C., et al. Off-label use of tocilizumab for the treatment of SARS-CoV-2 pneumonia in Milan, Italy. Eur J Intern Med. 2020;76:36–42. doi: 10.1016/j.ejim.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moreno-Perez O., Andres M., Leon-Ramirez J.-M., Sanchez-Paya J., Rodriguez J.C., Sanchez R., et al. Experience with tocilizumab in severe COVID-19 pneumonia after 80 days of follow-up: a retrospective cohort study. J Autoimmun. 2020;114:102523. doi: 10.1016/j.jaut.2020.102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Morrison A.R., Johnson J.M., Griebe K.M., Jones M.C., Stine J.J., Hencken L.N., et al. Clinical characteristics and predictors of survival in adults with coronavirus disease 2019 receiving tocilizumab. J Autoimmun. 2020;114:102512. doi: 10.1016/j.jaut.2020.102512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Munoz P., Galar A., Catalan P., Valerio M., Aldamiz-Echevarria T., Colliga C., et al. The first 100 cases of covid-19 in a hospital in Madrid with a 2-month follow-up. Rev Esp Quimioter. 2020;33:369–378. doi: 10.37201/req/072.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nebreda-Mayoral T., Miguel-Gomez M.A., March-Rossello G.A., Puente-Fuertes L., Canton-Benito E., Martinez-Garcia A.M., et al. Bacterial/fungal infection in hospitalized patients with COVID-19 in a tertiary hospital in the Community of Castilla y Leon, Spain. Enferm Infecc Microbiol Clin. 2020 doi: 10.1016/j.eimc.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nicolas D., Camos-Carreras A., Spencer F., Arenas A., Butori E., Maymo P., et al. A prospective cohort of SARS-CoV-2-infected health care workers: clinical characteristics, outcomes, and follow-up strategy. Open Forum Infect Dis. 2021;8 doi: 10.1093/ofid/ofaa592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nori P., Cowman K., Chen V., Bartash R., Szymczak W., Madaline T., et al. Bacterial and fungal coinfections in COVID-19 patients hospitalized during the New York City pandemic surge. Infect Control Hosp Epidemiol. 2021;42:84–88. doi: 10.1017/ice.2020.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Obata R., Maeda T., Do D.R., Kuno T. Increased secondary infection in COVID-19 patients treated with steroids in New York City. Jpn J Infect Dis. 2020;74:307–315. doi: 10.7883/yoken.JJID.2020.884. [DOI] [PubMed] [Google Scholar]
- 120.Odille G., Girard N., Sanchez S., Lelarge S., Mignot A., Putot S., et al. Should we prescribe antibiotics in older patients presenting COVID-19 pneumonia? J Am Med Dir Assoc. 2021;22:258–259. doi: 10.1016/j.jamda.2020.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Papamanoli A., Yoo J., Grewal P., Predun W., Hotelling J., Jacob R., et al. High-dose methylprednisolone in nonintubated patients with severe COVID-19 pneumonia. Eur J Clin Invest. 2021;51:e13458. doi: 10.1111/eci.13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pereira A., Cruz-Melguizo S., Adrien M., Fuentes L., Marin E., Perez-Medina T. Clinical course of coronavirus disease-2019 in pregnancy. Acta Obstet Gynecol Scand. 2020;99:839–847. doi: 10.1111/aogs.13921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pereira M.R., Aversa M.M., Farr M.A., Miko B.A., Aaron J.G., Mohan S., et al. Tocilizumab for severe COVID-19 in solid organ transplant recipients: a matched cohort study. Am J Transplant [Internet] 2020;20:3198–3205. doi: 10.1111/ajt.16314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pettit N.N., Nguyen C.T., Mutlu G.M., Wu D., Kimmig L., Pitrak D., et al. Late onset infectious complications and safety of tocilizumab in the management of COVID-19. J Med Virol. 2021;93:1459–1464. doi: 10.1002/jmv.26429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Piroth L., Cottenet J., Mariet A.-S., Bonniaud P., Blot M., Tubert-Bitter P., et al. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir Med. 2021;9:251–259. doi: 10.1016/S2213-2600(20)30527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pongpirul W.A., Wiboonchutikul S., Charoenpong L., Panitantum N., Vachiraphan A., Uttayamakul S., et al. Clinical course and potential predictive factors for pneumonia of adult patients with coronavirus disease 2019 (COVID-19): a retrospective observational analysis of 193 confirmed cases in Thailand. PLoS Negl Trop Dis. 2020;14:1–17. doi: 10.1371/journal.pntd.0008806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Price C.C., Altice F.L., Shyr Y., Koff A., Pischel L., Goshua G., et al. Tocilizumab treatment for cytokine release syndrome in hospitalized patients with coronavirus disease 2019: survival and clinical outcomes. Chest. 2020;158:1397–1408. doi: 10.1016/j.chest.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Quartuccio L., Sonaglia A., Pecori D., Peghin M., Fabris M., Tascini C., et al. Higher levels of IL-6 early after tocilizumab distinguish survivors from non-survivors in COVID-19 pneumonia: a possible indication for deeper targeting IL-6. J Med Virol. 2020;92:2852–2856. doi: 10.1002/jmv.26149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rahmani H., Davoudi-Monfared E., Nourian A., Khalili H., Hajizadeh N., Jalalabadi N.Z., et al. Interferon beta-1b in treatment of severe COVID-19: a randomized clinical trial. Int Immunopharmacol [ 2020;88:106903. doi: 10.1016/j.intimp.2020.106903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ramadan H.K.-A., Mahmoud M.A., Zakaria M., Aburahma, Elkhawaga A.A., El-Mokhtar M.A., et al. Predictors of severity and co-infection resistance profile in COVID-19 patients: first report from upper Egypt. Infect Drug Resist. 2020;13:3409–3422. doi: 10.2147/IDR.S272605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Razazi K., Arrestier R., Haudebourg A.F., Benelli B., Carteaux G., Decousser J.-W., et al. Risks of ventilator-associated pneumonia and invasive pulmonary aspergillosis in patients with viral acute respiratory distress syndrome related or not to Coronavirus 19 disease. Crit Care. 2020;24:699. doi: 10.1186/s13054-020-03417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rinaldi M., Bartoletti M., Bussini L., Pancaldi L., Pascale R., Comai G., et al. COVID-19 in solid organ transplant recipients: No difference in survival compared to general population. Transpl Infect Dis. 2020;23 doi: 10.1111/tid.13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ripa M., Galli L., Poli A., Oltolini C., Spagnuolo V., Mastrangelo A., et al. Secondary infections in patients hospitalized with COVID-19: incidence and predictive factors. Clin Microbiol Infect. 2021;27:451–457. doi: 10.1016/j.cmi.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rodriguez-Garcia J.L., Sanchez-Nievas G., Arevalo-Serrano J., Garcia-Gomez C., Jimenez-Vizuete J.M., Martinez-Alfaro E. Baricitinib improves respiratory function in patients treated with corticosteroids for SARS-CoV-2 pneumonia: an observational cohort study. Rheumatology. 2021;60:399–407. doi: 10.1093/rheumatology/keaa587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rojas-Marte G., Khalid M., Mukhtar O., Hashmi A.T., Waheed M.A., Ehrlich S., et al. Outcomes in patients with severe COVID-19 disease treated with tocilizumab: a case-controlled study. QJM. 2020;113:546–550. doi: 10.1093/qjmed/hcaa206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rossotti R., Travi G., Ughi N., Corradin M., Baiguera C., Fumagalli R., et al. Safety and efficacy of anti-IL6-receptor tocilizumab use in severe and critical patients affected by coronavirus disease 2019: a comparative analysis. J Infect. 2020;81:e11–e17. doi: 10.1016/j.jinf.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rothe K., Feihl S., Schneider J., Wallnofer F., Wurst M., Lukas M., et al. Rates of bacterial co-infections and antimicrobial use in COVID-19 patients: a retrospective cohort study in light of antibiotic stewardship. Eur J Clin Microbiol Infect Dis. 2021;40:859–869. doi: 10.1007/s10096-020-04063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rouze A., Martin-Loeches I., Povoa P., Makris D., Artigas A., Bouchereau M., et al. Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: a European multicenter cohort study. Intensive Care Med. 2021;47:188–198. doi: 10.1007/s00134-020-06323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Salama C., Han J., Yau L., Reiss W.G., Kramer B., Neidhart J.D., et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;384:20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schmidt M., Hajage D., Lebreton G., Monsel A., Voiriot G., Levy D., et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: a retrospective cohort study. Lancet Respir Med. 2020;8:1121–1131. doi: 10.1016/S2213-2600(20)30328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schmidt M., Hajage D., Demoule A., Pham T., Combes A., Dres M., et al. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47:60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sepulveda J., Westblade L.F., Whittier S., Satlin M.J., Greendyke W.G., Aaron J.G., et al. Bacteremia and blood culture utilization during COVID-19 surge in New York City. J Clin Microbiol. 2020;58:e00875–e00920. doi: 10.1128/JCM.00875-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shang Y., Liu T., Wei Y., Li J., Shao L., Liu M., et al. Scoring systems for predicting mortality for severe patients with COVID-19. EClinicalMedicine. 2020;24:100426. doi: 10.1016/j.eclinm.2020.100426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sharov K.S. SARS-CoV-2-related pneumonia cases in pneumonia picture in Russia in March-May 2020: secondary bacterial pneumonia and viral co-infections. J Glob Health. 2020;10 doi: 10.7189/jogh.10.-020504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shi P., Ren G., Yang J., Li Z., Deng S., Li M., et al. Clinical characteristics of imported and second-generation coronavirus disease 2019 (COVID-19) cases in Shaanxi outside Wuhan, China: a multicentre retrospective study. Epidemiol Infect. 2020;148:e238. doi: 10.1017/S0950268820002332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sim B.L.H., Chidambaram S.K., Wong X.C., Pathmanathan M.D., Peariasamy K.M., Hor C.P., et al. Clinical characteristics and risk factors for severe COVID-19 infections in Malaysia: a nationwide observational study. Lancet Reg Health West Pac. 2020;4:100055. doi: 10.1016/j.lanwpc.2020.100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sinha P., Mostaghim A., Bielick C.G., McLaughlin A., Hamer D.H., Wetzler L.M., et al. Early administration of interleukin-6 inhibitors for patients with severe COVID-19 disease is associated with decreased intubation, reduced mortality, and increased discharge. Int J Infect Dis. 2020;99:28–33. doi: 10.1016/j.ijid.2020.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sogaard K.K., Baettig V., Osthoff M., Marsch S., Leuzinger K., Schweitzer M., et al. Community-acquired and hospital-acquired respiratory tract infection and bloodstream infection in patients hospitalized with COVID-19 pneumonia. J Intensive Care. 2021;9:10. doi: 10.1186/s40560-021-00526-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Somers E.C., Eschenauer G.A., Troost J.P., Golob J.L., Gandhi T.N., Wang L., et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin Infect Dis. 2021;73:e445–e454. doi: 10.1093/cid/ciaa954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Soriano M.C., Vaquero C., Ortiz-Fernandez A., Caballero A., Blandino-Ortiz A., de Pablo R. Low incidence of co-infection, but high incidence of ICU-acquired infections in critically ill patients with COVID-19. J Infect. 2021;82:e20–e21. doi: 10.1016/j.jinf.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Stevens R.W., Jensen K., O’Horo J.C., Shah A. Antimicrobial prescribing practices at a tertiary-care center in patients diagnosed with COVID-19 across the continuum of care. Infect Control Hosp Epidemiol. 2021;42:89–92. doi: 10.1017/ice.2020.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tomazini B.M., Maia I.S., Cavalcanti A.B., Berwanger O., Rosa R.G., Veiga V.C., et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324:1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Townsend L., Hughes G., Kerr C., Kelly M., O’Connor R., Sweeney E., et al. Bacterial pneumonia coinfection and antimicrobial therapy duration in SARS-CoV-2 (COVID-19) infection. JAC-Antimicrob Resist. 2020;2:dlaa071. doi: 10.1093/jacamr/dlaa071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tran V.-T., Mahevas M., Bani-Sadr F., Robineau O., Perpoint T., Perrodeau E., et al. Corticosteroids in patients hospitalized for COVID-19 pneumonia who require oxygen: observational comparative study using routine care data. Clin Microbiol Infect. 2021;27:603–610. doi: 10.1016/j.cmi.2020.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vanhomwegen C., Veliziotis I., Malinverni S., Konopnicki D., Dechamps P., Claus M., et al. Procalcitonin accurately predicts mortality but not bacterial infection in COVID-19 patients admitted to intensive care unit. Ir J Med Sci. 2021;190:1649–1652. doi: 10.1007/s11845-020-02485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vena A., Giacobbe D.R., Di Biagio A., Mikulska M., Taramasso L., De Maria A., et al. Clinical characteristics, management and in-hospital mortality of patients with coronavirus disease 2019 in Genoa, Italy. Clin Microbiol Infect. 2020;26:1537–1544. doi: 10.1016/j.cmi.2020.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang A., Gao G., Wang S., Chen M., Qian F., Tang W., et al. Clinical characteristics and risk factors of acute respiratory distress syndrome (ARDS) in COVID-19 patients in beijing, China: a retrospective study. Med Sci Monit Int Med J Exp Clin Res. 2020;26 doi: 10.12659/MSM.925974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang J.-B., Wang H.-T., Wang L.-S., Li L.-P., Xv J., Xv C., et al. Epidemiological and clinical characteristics of fifty-six cases of COVID-19 in Liaoning Province, China. World J Clin Cases. 2020;8:5188–5202. doi: 10.12998/wjcc.v8.i21.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang K., Qiu Z., Liu J., Fan T., Liu C., Tian P., et al. Analysis of the clinical characteristics of 77 COVID-19 deaths. Sci Rep. 2020;10:16384. doi: 10.1038/s41598-020-73136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang Z., Ma W., Zheng X., Wu G., Zhang R. Household transmission of SARS-CoV-2. J Infect. 2020;81:179–182. doi: 10.1016/j.jinf.2020.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang L., Amin A.K., Khanna P., Aali A., McGregor A., Bassett P., et al. An observational cohort study of bacterial co-infection and implications for empirical antibiotic therapy in patients presenting with COVID-19 to hospitals in North West London. J Antimicrob Chemother. 2021;76:796–803. doi: 10.1093/jac/dkaa475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang S., Chen Z., Lin Y., Lin L., Lin Q., Fang S., et al. Clinical characteristics of 199 discharged patients with COVID-19 in Fujian Province: a multicenter retrospective study between January 22nd and February 27th, 2020. PLoS One. 2020;15 doi: 10.1371/journal.pone.0242307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wu B., Lei Z.-Y., Wu K.-L., He J.-R., Cao H.-J., Fu J., et al. Compare the epidemiological and clinical features of imported and local COVID-19 cases in Hainan, China. Infect Dis Poverty. 2020;9:143. doi: 10.1186/s40249-020-00755-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wu C., Chen X., Cai Y., Xia J., Xu S., Huang H., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wu J., Li W., Shi X., Chen Z., Jiang B., Liu J., et al. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19) J Intern Med. 2020;288:128–138. doi: 10.1111/joim.13063. [DOI] [PubMed] [Google Scholar]
- 166.Wu J., Huang Y., Tu C., Bi C., Chen Z., Luo L., et al. Household transmission of SARS-CoV-2, Zhuhai, China, 2020. Clin Infect Dis. 2020;71:2099–2108. doi: 10.1093/cid/ciaa557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Xiao L.-S., Zhang W.-F., Gong M.-C., Zhang Y.-P., Chen L.-Y., Zhu H.-B., et al. Development and validation of the HNC-LL score for predicting the severity of coronavirus disease 2019. EBioMedicine. 2020;57:102880. doi: 10.1016/j.ebiom.2020.102880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xu J., Yang X., Yang L., Zou X., Wang Y., Wu Y., et al. Clinical course and predictors of 60-day mortality in 239 critically ill patients with COVID-19: a multicenter retrospective study from Wuhan, China. Crit Care. 2020;24:394. doi: 10.1186/s13054-020-03098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Xu R., Hou K., Zhang K., Xu H., Zhang N., Fu H., et al. Performance of two risk-stratification models in hospitalized patients with coronavirus Disease. Front Med. 2020;7:518. doi: 10.3389/fmed.2020.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xu W., Sun N.-N., Gao H.-N., Chen Z.-Y., Yang Y., Ju B., et al. Risk factors analysis of COVID-19 patients with ARDS and prediction based on machine learning. Sci Rep. 2021;11:2933. doi: 10.1038/s41598-021-82492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yao T., Gao Y., Cui Q., Peng B., Chen Y., Li J., et al. Clinical characteristics of a group of deaths with COVID-19 pneumonia in Wuhan, China: a retrospective case series. BMC Infect Dis. 2020;20:695. doi: 10.1186/s12879-020-05423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yi H., Lu F., Jin X., Chen R., Liu B., Dong X., et al. Clinical characteristics and outcomes of coronavirus disease 2019 infections among diabetics: a retrospective and multicenter study in China. J Diabetes. 2020;12:919–928. doi: 10.1111/1753-0407.13098. [DOI] [PubMed] [Google Scholar]
- 174.Yu D., Ininbergs K., Hedman K., Giske C.G., Stralin K., Ozenci V. Low prevalence of bloodstream infection and high blood culture contamination rates in patients with COVID-19. PLoS ONE. 2020;15 doi: 10.1371/journal.pone.0242533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yu Y., Xu D., Fu S., Zhang J., Yang X., Xu L., et al. Patients with COVID-19 in 19 ICUs in Wuhan, China: a cross-sectional study. Crit Care. 2020;24:219. doi: 10.1186/s13054-020-02939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zachariah P., Johnson C.L., Halabi K.C., Ahn D., Sen A.I., Fischer A., et al. Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID-19) in a children’s hospital in New York City, New York. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zangrillo A., Beretta L., Scandroglio A.M., Monti G., Fominskiy E., Colombo S., et al. Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit Care Resusc J Australas Acad Crit Care Med. 2020;22:200–211. doi: 10.1016/S1441-2772(23)00387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zeng Q.-L., Li G.-M., Ji F., Ma S.-H., Zhang G.-F., Xu J.-H., et al. Clinical course and treatment efficacy of COVID-19 near Hubei Province, China: a multicentre, retrospective study. Transbound Emerg Dis. 2020;67:2971–2982. doi: 10.1111/tbed.13674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhang G., Hu C., Luo L., Fang F., Chen Y., Li J., et al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol. 2020;127:104364. doi: 10.1016/j.jcv.2020.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhang J., Kong W., Xia P., Xu Y., Li L., Li Q., et al. Impaired fasting glucose and diabetes are related to higher risks of complications and mortality among patients with coronavirus disease 2019. Front Endocrinol. 2020;11:525. doi: 10.3389/fendo.2020.00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhang Y., Xiao L.-S., Li P., Hu C., Zhang W.-F., Sun Q.-C., et al. Clinical characteristics of patients with progressive and non-progressive coronavirus disease 2019: evidence from 365 hospitalised patients in Honghu and Nanchang, China. Front Med. 2020;7:556818. doi: 10.3389/fmed.2020.556818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang L., Fan T., Yang S., Feng H., Hao B., Lu Z., et al. Comparison of clinical characteristics of COVID-19 between elderly patients and young patients: a study based on a 28-day follow-up. Aging. 2020;12:19898–19910. doi: 10.18632/aging.104077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhang P., He Z., Yu G., Peng D., Feng Y., Ling J., et al. The modified NUTRIC score can be used for nutritional risk assessment as well as prognosis prediction in critically ill COVID-19 patients. Clin Nutr. 2020;40:534–541. doi: 10.1016/j.clnu.2020.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhao K., Li R., Wu X., Zhao Y., Wang T., Zheng Z., et al. Clinical features in 52 patients with COVID-19 who have increased leukocyte count: a retrospective analysis. Eur J Clin Microbiol Infect Dis. 2020;39:2279–2287. doi: 10.1007/s10096-020-03976-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Klein E.Y., Monteforte B., Gupta A., Jiang W., May L., Hsieh Y.-H., et al. The frequency of influenza and bacterial coinfection: a systematic review and meta-analysis. Influenza Other Respir Virus. 2016;10:394–403. doi: 10.1111/irv.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.MacIntyre C.R., Chughtai A.A., Barnes M., Ridda I., Seale H., Toms R., et al. The role of pneumonia and secondary bacterial infection in fatal and serious outcomes of pandemic influenza a(H1N1)pdm09. BMC Infect Dis. 2018;18:637. doi: 10.1186/s12879-018-3548-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.RECOVERY Collaborative Group Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.The RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.The REMAP-CAP Investigators Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Osuchowski M.F., Winkler M.S., Skirecki T., Cajander S., Shankar-Hari M., Lachmann G., et al. The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir Med. 2021;9:622–642. doi: 10.1016/S2213-2600(21)00218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Luyt C.-E., Hékimian G., Koulenti D., Chastre J. Microbial cause of ICU-acquired pneumonia: hospital-acquired pneumonia versus ventilator-associated pneumonia. Curr Opin Crit Care. 2018;24:332–338. doi: 10.1097/MCC.0000000000000526. [DOI] [PubMed] [Google Scholar]
- 194.Dudeck M.A., Allen-Bridson K., Edwards J.R. Pathogens associated with repeat versus single central-line–associated bloodstream infections, acute-care hospitals, NHSN. Infect Control Hosp Epidemiol. 2020;41:s343–s344. doi: 10.1017/ice.2019.303. [DOI] [PubMed] [Google Scholar]
- 195.National Institute for Health and Care Excellence (NICE) 2020. COVID-19 rapid guideline: antibiotics for pneumonia in adults in hospital.https://www.nice.org.uk/guidance/ng173/chapter/4-Assessing-the-ongoing-need-for-antibiotics Available from: [PubMed] [Google Scholar]
- 196.COVID-19 Treatment Guidelines Panel COVID-19 treatment guidelines. National institutes of health. https://www.covid19treatmentguidelines.nih.gov/ Available from: [PubMed]
- 197.World Health Organization Clinical management of COVID-19 interim guidance. Geneva, Switzerland: world health organization. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected Available from:
- 198.Sieswerda E., de Boer M.G.J., Bonten M.M.J., Boersma W.G., Jonkers R.E., Aleva R.M., et al. Recommendations for antibacterial therapy in adults with COVID-19 – an evidence based guideline. Clin Microbiol Infect. 2021;27:61–66. doi: 10.1016/j.cmi.2020.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Feldman C., Anderson R. The role of co-infections and secondary infections in patients with COVID-19. Pneumonia. 2021;13:5. doi: 10.1186/s41479-021-00083-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.