Dear Editor,
Since the SARS-CoV-2 pandemic started, severe COVID-19 has weighed heavily on all intensive care units (ICUs) worldwide. Ventilator-associated pneumonia (VAP) is a severe complication that increases morbidity and mortality. In a recent study, Rouzé et al. demonstrated that SARS-CoV-2 pneumonia is associated with a higher incidence of VAP than influenza or non-viral pneumonia [1]. SARS-CoV-2 pneumonia increases the risk of VAP, which is in turn associated with higher 28-day mortality and longer duration of mechanical ventilation.
Multiple meta-analyses and randomised controlled trials (RCTs) found that the use of selective decontamination of the digestive tract (SDD) with topical antibiotics (colistin-tobramycin-amphotericin B) combined with intravenous cefotaxime reduced the incidence of VAP and ICU mortality, especially in ICUs with low multidrug-resistant Gram-negative bacteria (MDRGNB) prevalence rates [2]. Current French guidelines have recommended the use of SDD for the prevention of ICU-associated pneumonia in ICUs with low MDRGNB rates and broad-spectrum antibiotic consumption [3].
We performed a retrospective observational study in two French ICUs (Rennes and Angers University Hospitals) with low baseline MDRGNB rates to compare the incidence of VAP with or without routine use of SDD. All patients with documented COVID-19 who were mechanically ventilated for longer than 48 h between the 1st of January and the 31st of December 2020 were included. In one centre (Rennes), SDD was routinely used: intravenous cefotaxime course was limited to 5 days, whereas oropharyngeal and digestive topical antibiotics were administrated during the whole duration of mechanical ventilation. SDD was combined with intranasal mupirocin and one daily chlorhexidine washing, as long as patients remained intubated. In the other centre (Angers), no topical antibiotics were used, and systemic antibiotics were prescribed only if bacterial infection was suspected, at the discretion of the attending physician. Empirical treatment of bacterial pneumonia or superinfection was guided by serum procalcitonin levels and/or large consolidation on chest CT scan.
Diagnosis of VAP was based on guidelines in both centres, using fever, radiological findings, and microbiological culture on bronchoalveolar lavage or endotracheal aspirate. The first episode of ICU-acquired pneumonia was the only one considered. The primary endpoint was VAP incidence. Secondary endpoint was 28-day mortality. Data were collected from medical files on a standardised questionnaire. The study was approved by local ethics committee (approval number 21.120). Patients or their relatives were informed as part of requirements for institutional research. Results were expressed as median and interquartile range (IQR) for quantitative variables, and n (%) for qualitative variables. Kaplan-Meier plot was used to represent probability of remaining free from VAP across strata and a log-rank test was performed. We used a multivariate Cox regression model to evaluate risk factors associated with the occurrence of VAP and with 28-day mortality. The final model included age, gender, SAPS-2 score, extracorporeal membrane oxygenation support and SDD. The proportional hazard assumption was tested using Schoenfeld’s test. P-values <.05 were considered as statistically significant. Statistical analyses were carried out using R-Studio 2020, Integrated Development for R.
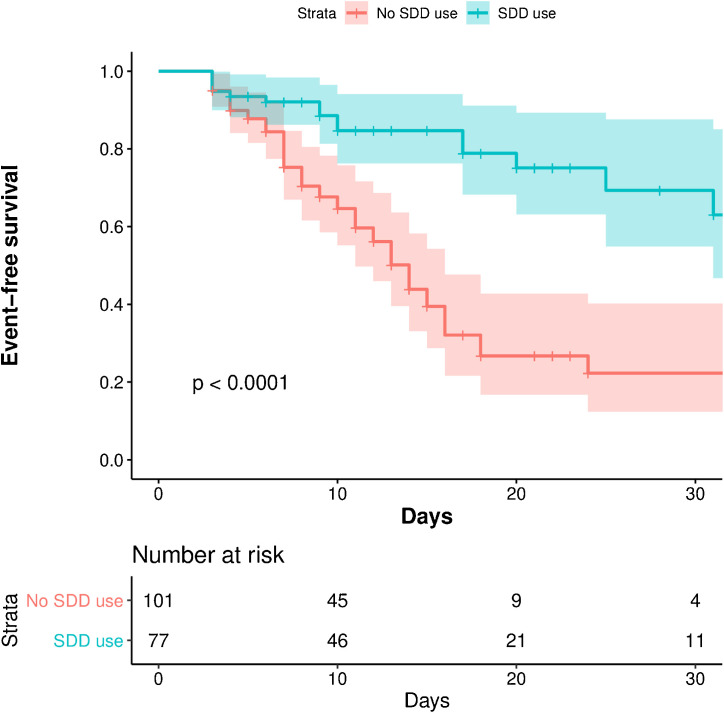
We included 178 consecutive patients with critical COVID-19 who required mechanical ventilation in ICU longer than 48 h. Compared to the group without SDD, patients receiving SDD were younger (66 years [55−72] vs. 68 [62−74], P = 0.03) and had a lower simplified acute physiology score (SAPS II) at admission (37 [28−45] vs. 42 [33−50], P = 0.03). The bloodstream infection rate was similar between the two groups (13% vs. 17%). VAP incidence was lower in the SDD group than in the group without SDD (9.4 vs. 23.5 per 1000 ventilator days, P < 0.001). Main pathogens were Gram-negative bacilli, especially Enterobacteriaceae and Pseudomonas aeruginosa (Table 1 ). Log-rank test showed significant difference of probability of VAP between the two groups (P < 0.001) (Fig. 1 ). In multivariate analysis, SDD was independently associated with a decreased risk of VAP (adjusted-HR 0.36; 95% CI [0.20−0.63]) (Table 2 ). Mortality at day-28 was lower in the SDD group (aHR 0.33, 95% CI [0.12−0.87]; P = 0.03).
Table 1.
Baseline characteristics and clinical course according to SDD use.
| Characteristics | SDD group, n = 77 | No SDD group, n = 101 | P-value |
|---|---|---|---|
| Age, median [IQR] | 66 [55−72] | 68 [62−74] | 0.03 |
| Male gender, n (%) | 60 (78) | 75 (74) | 0.73 |
| SAPS-2 score at admission, median [IQR] | 37 [28−45] | 42 [33−50] | 0.03 |
| Inter-hospital transferred patients, n (%) | 18 (23) | 18 (18) | 0.45 |
| Extracorporeal membrane oxygenation support, n (%) | 8 (10) | 19 (19) | 0.14 |
| At least one VAP, n (%) | 16 (21) | 50 (50) | < 0.001 |
| VAP incidence (per 1000 ventilator days) | 9.4 | 23.5 | < 0.001 |
| VAP microbiological documentation, n | |||
| Enterobacteriaceae | 14 | 29 | |
| H. influenzae | 0 | 7 | |
| P. aeruginosa | 4 | 6 | |
| A. baumannii | 0 | 2 | |
| S. aureus | 0 | 11 | |
| Other | 1 | 3 | |
| Bloodstream infection, n (%) | 10 (13) | 17 (17) | 0.53 |
| Length of mechanical ventilation, days | 14 (8−28) | 15 (9−29) | 0.75 |
| 28-day mortality, n (%) | 5 (6.5) | 22 (21.8) | 0.01a |
After adjustment for age, gender, SAPS-2 and extracorporeal membrane oxygenation support, the hazard ratio for death in the SDD group was 0.33, 95% CI [0.12−0.87]; P = 0.03.
Fig. 1.
Kaplan-Meier estimate of the probability of remaining free from VAP in patients with critical COVID-19.
Table 2.
Multivariate analysis of predictive factors of ventilator-acquired pneumonia.
| Ventilator-acquired pneumonia | Patients with VAP (n = 66) | Patients w/o VAP (n = 112) | P-value | Adjusted HR (95% CI) | P-value |
|---|---|---|---|---|---|
| Age,a median [IQR] | 67 [59−72] | 67 [58−75] | 0.71 | 1.01 (0.99−1.04) | 0.41 |
| Male gender,a n (%) | 58 (88) | 76 (68) | 0.01 | 2.70 (1.27−5.74) | 0.01 |
| SAPS-2 score at admission, median [IQR] | 40 [33−49] | 38 [30−48] | 0.82 | ||
| ECMO support,a n (%) | 18 (27) | 9 (8) | < 0.001 | 2.3 (1.24−4.10) | 0.008 |
| Inter-hospital transferred patients, n (%) | 12 (18) | 24 (21) | 0.52 | ||
| SDD use, a n (%) | 16 (24) | 61 (54) | < 0.001 | 0.36 (0.20−0.63) | < 0.001 |
SAPS-2 = simplified acute physiology score; ECMO = extracorporeal membrane oxygenation; SDD = Selective digestive decontamination.
Variables included in the multivariate analysis.
To date, the use of SDD is controversial and remains limited in ICUs around the world. The main reason was the negative result of a recent trial which failed to demonstrate a reduction in the incidence of bloodstream infection with MDRGN and in mortality in ICUs with intermediate/high multidrug resistance rate [4]. However, in that trial, i) no systemic antibiotics was used in the SDD regimen due to an intermediate to high MDRGN, ii) VAP was not assessed. In contrast, we found a lower incidence of VAP in critically ill COVID-19 patients who received SDD. Of note, a meta-analysis found that only the combination of topical and systemic antibiotics was associated with reduced mortality [2]. The second reason that precludes broader use of SDD relies in the fear that it may promote the emergence of MDR in ICU patients. However, there are no robust data in the literature suggesting the emergence of bacterial resistance with the use of SDD [5]. Indeed, several studies showed that SDD was associated with a significant decrease of systemic antibiotic use during ICU stay. For all these reasons, the use of SDD in critical COVID-19 patients deserves more consideration and may be particularly favourable in this population, due to the high incidence of VAP.
This work has limitations. First, this observational study suffers from potential biases inherent to its retrospective and observational design. Second, the radiological diagnosis of VAP can be subjective and differ from one physician to another, which may lead to a measurement bias. Third, as the study was performed in two centres with low MDRGNB prevalence rates, these findings may not be generalisable to other settings. Fourth, the observed difference for 28-day mortality could be due to regional differences in ICU admission policy, case mix, constraints on surge capacity and COVID-19 management. Unfortunately, we do not have data to examine this.
To our knowledge, this work is the first to assess SDD for the prevention of VAP in critical COVID-19. Our results suggest a substantial decrease in the occurrence of VAP with the use of SDD. Randomised controlled trials are needed to better assess the efficacy of SDD for the prevention of VAP in critical COVID-19 patients.
Disclosure of interest
DLP, PT, PJ, FR, AK and CC have no conflict of interest to disclose.
Funding
This work was not funded.
Authors' contributions
DLP, CC and PT wrote the original draft. DLP did the analysis. PJ, FR, AK and CC contributed to the investigation and data collection. PT, PJ, FR, AK and CC critically reviewed the manuscript.
Ethical approval and consent to participate
This study was approved by ethics committee of Rennes University Hospital (approval number 21.120). Patients or their relatives were informed as part of requirements for institutional research.
Consent for publication
All authors will sign a statement releasing the copyright should the manuscript be accepted for publication.
Acknowledgements
None.
References
- 1.Rouzé A., Martin-Loeches I., Povoa P., Makris D., Artigas A., Bouchereau M., et al. Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: a European multicenter cohort study. Intensive Care Med. 2021;47:188–198. doi: 10.1007/s00134-020-06323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minozzi S., Pifferi S., Brazzi L., Pecoraro V., Montrucchio G., D’Amico R. Topical antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving mechanical ventilation. Cochrane Database Syst Rev. 2021;1 doi: 10.1002/14651858.CD000022.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leone M., Bouadma L., Bouhemad B., Brissaud O., Dauger S., Gibot S., et al. Hospital-acquired pneumonia in ICU. Anaesth Crit Care Pain Med. 2018;37:83–98. doi: 10.1016/j.accpm.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Wittekamp B.H., Plantinga N.L., Cooper B.S., Lopez-Contreras J., Coll P., Mancebo J., et al. Decontamination strategies and bloodstream infections with antibiotic-resistant microorganisms in ventilated patients: a randomized clinical trial. JAMA. 2018;320:2087. doi: 10.1001/jama.2018.13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daneman N., Sarwar S., Fowler R.A., Cuthbertson B.H. Effect of selective decontamination on antimicrobial resistance in intensive care units: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13:328–341. doi: 10.1016/S1473-3099(12)70322-5. [DOI] [PubMed] [Google Scholar]