Abstract
Cleaning wastewater has become one of the most serious issues for a number of scientists and researchers in recent years, as water is the most basic need for the daily life of humans. There has been a focus on the removal of noxious pollutants from wastewater effluents by using nanocatalysts owing to their unique physicochemical actions and stability. Herein we manufactured TiO2 nanoparticles supported by activated carbon (AC-TiO2) using a cost-effective sonochemical method. The band structures of the AC-TiO2 and TiO2 were modified from 3.2 to 3.1 eV, thus increasing the catalytic activity. The structural, optical and anatase crystal phase properties, with morphological confirmation, were studied by applying UV-DRS, PL, FESEM, XRD, along with HRTEM, respectively. The specific surface area, calculated by BET analysis, was found to be ~241 m2/gm and ~46 m2/gm for AC-TiO2 and TiO2. The degradation efficiency of the as-prepared nanocatalysts against the very toxic but rarely studied organic textile dye pollutant RO 84 was investigated and 97% efficiency were found for the AC-TiO2 as compared to pure TiO2, which is a highly appreciated finding in the catalytic dye degradation application domain. Such surface-modified nanocatalysts could be further implemented for the treatment of wastewaters/waste effluents released from chemical industries, laboratories and other sources.
Keywords: nanocatalysts, activated carbon, effluent, titania, anatase
1. Introduction
Organic or inorganic molecule-based contaminants present in wastewater have been found with change into various chemical states, compositional ratios or other heterocyclic structures such as aniline intermediate species [1,2,3]. Dye components and their derivatives affect the health of the environment by contaminating water, soil, and air. Thus, there has been a motivation for researchers to design effective technologies to resolve such environmental issues, especially in the dye and textile industries. From this point of view, dye fabrication, textile research, and production plants are now required to treat their effluents for the purpose of ensuring the safety of human life and an ecofriendly environment. A number of studies have been carried out in academic and industrial sectors to minimize the high load of organic and solid contents of discharged wastewaters [4,5], which mainly consist of biological toxic, organic, and inorganic impurities which threaten water scarcity for both humans and the ecosystem. In catalysis applications, the decomposition of effluents/contaminants from wastewater using photocatalytic processes are appreciated by the dye and textile sectors. Photochemical reactions using nanocatalysts and technologies have become the simplest route to treat the organic (heterocyclic/aromatic)-based wastes and toxic substances. Doping of metal oxides and their hybrid structures with suitable metals, ions, or atomic layered structures is applied to improve the catalytic performance. Such modified structures are widely explored due to the efficient redox reactions with higher electrons and hole recombination rates. The formation of reactive species such as transient hydroxyls (•OH) and holes often regulate the catalytic process owing to the delayed recombination of free charges [4,6]. Fundamentally, photodegradation can be understood to depend on two classes of catalysts, homogeneous and heterogeneous. Homogeneous processes are often conducted using continuous UV light irradiation in conjunction with specific oxidants, e.g., ozone and hydrogen peroxides. In the case of heterogeneous catalysis, oxidants in conjunction with crystalline photoactive catalysts are required to trigger the catalytic mechanism. Light-based degradation effects depend on different reaction parameters, including the reaction medium (pH), amount of catalyst and the nature of the targeted chemical contaminants or organic effluents [7,8]. By following a chemical treatment approach various pollutants, especially dye contents, pathogens and toxic heavy metals, could be mineralized and therefore the photocatalysis chemistry is promoted by using appropriate chemicals (catalysts). Photocatalytic reactions can take place under certain experimental parameters which felicitate a feasible approach to regulate the catalytic actions and appreciated for wastewater cleaning and hence solar radiation or UV irradiation may be employed as a light source to activate the catalyst [8]. Among the various catalysts, TiO2 nanoparticles are semiconductor metal oxide photocatalysts that promise to offer an inexpensive, desirable method for wastewater purification [4,8]. It has been mentioned that TiO2 shows stronger catalytic action under a certain light intensity when in the form of anatase, while other phases like rutile exhibit a comparably weak photoactive catalytic activity and hence the anatase form of TiO2 is more appreciated. Systems to improve the photoactivity of TiO2 have been formulated following several preparation procedures to control the shape and surface modification of anatase phase titania [9,10,11]. The pairing of electrons-holes could be considered a limiting factor for TiO2 semiconducting nanocatalysts. Therefore, various efforts have been undertaken to make TiO2 a more successful photocatalyst by doping it with different additives such as metal oxides or carbon composites. The altered structures of the nanodimensional hybrid substances open multidirectional options due to the larger surface and adjustable morphological properties [12,13,14]. Optoelectronic and photoelectrochemical processes under light modulation could be regulated by inserting multivalent ions at a fixed stoichiometric concentration [15,16]. Metamaterials can also be constructed in order to achieve desirable properties by introducing suitable chemical constituents or elements. This represents an advanced technique with wider uses in material modification [2,11]. Carbon-containing compounds can be classified into different types according to their functionalities, such as 2D graphene, its reduced derivatives, carbon nanotubes (CNTs) or fiber-like shapes including activated carbon [2,17]. Among these, activated carbons are highly functionalized carbon allotropes that can be derived from any natural carbon source. These activated carbons have a greater tendency to display enhanced adsorption properties due to their porosity and active surface area. The changes in elemental ions in TiO2 with structural tuning represent an outstanding research area in the photocatalysis sector [17,18]. Particularly, the bandwidth of the TiO2 could be changed by the adding elements such as C, Br and others which changes the band position of the conduction and valance bands allowing single or multivalent electron transformations [19]. The optimized band values allow a discrete level of light into the semiconducting materials like TiO2, especially in catalytic systems. In the semiconducting nanomaterial or metamaterials, the band structures and physicochemical aspects are modulated by appropriate experimental schemes to ensure efficient catalytic activity [20,21]. Non-metal elements, mainly tetravalent carbon allotropes, can be used to boost the physical and chemical properties of TiO2 for the mineralization and elemental degradation of hazardous molecules [22]. That is why heterogeneous catalysts have been highly studied for the field of degradation for their environment impact in the dye and textile sectors [22,23]. In contrast, different classes of metal-oxide compositions are employed for the decomposition of various types of dye and the purification of water by removing heavy elements/compounds [15,21]. Generally, electromagnetic intensity responds to the catalyst at a certain power density or energy whereas the TiO2 responds to UV light in the range under 370–415 nm. However, the visible range of the electromagnetic spectra can also be used to induce specific photocatalysis processes by manipulating other catalytic factors such as bandgap energy, catalyst morphologies and the elemental constituents. The photocatalytic decompositions of dyes using TiO2 can be classified on the basis of the redox reactions taking place, the chemical composition and the nature of co-product(s) which could be explained as follows: first, photo decolorization involves chemically active redox process in terms of photo-oxidation or reduction, resulting in initial chemical phases based on reduction or oxidation redox mechanisms [24,25]. Second, light-treated dye components, which means non-reactive co-products, are produced by photodegradation approaches. This has been a highly preferred term for the catalytic chemistry on dye molecules and their derivatives. Lastly, the total breakdown of the dye components into possible preferred chemical species including water, nitrogen-containing gases, and this process is called photomineralization [24,26,27]. In this work, a high photocatalytic active TiO2 (anatase) was modified in terms of band position by doping with active carbon (AC-TiO2) NPs and used to degrade the rarely studied organic dye molecule RO84. The structures with altered structural and surface properties were then confirmed as an efficient photocatalyst to remove pollutants, especially for the degradation of RO8 in industrial wastewaters. These structural and surface studies of the pure and supported TiO2 are focused on the possible environmental impact.
2. Materials and Methods
All reagents were of analytical grade. Titanium isopropoxide, was purchased from Sigma Aldrich (Taufkirchen, Germany), while acetic acid (SRL, Ahemdabad, India), nitric acid (SRL, Ahemdabad, India, 69%) and ethanol (Schinzen, China) and activated carbon (HiMedia, New Delhi, India) were also used.
2.1. Preparation of Titanium Oxide Powder
Titanium isopropoxide was the selected starting material. A 1 M solution in ethanol (50 mL) was prepared. About 30 mL of this as-prepared precursor solution was taken and acetic acid (0.1 M) and nitric acid (0.1 M) were added dropwise under constant stirring to moderate the chemical reaction under ultra-sonication at a fixed frequency of 40 kHz. The reaction was carried out for 4 h at 70 °C at an open atmosphere. The white cake formed was separated and dried under vacuum for a night at 25 °C. Then the obtained solid powder was calcined at 700 °C for complete phase transformation.
2.2. TiO2 Supported on Activated Carbon
The carbon source, namely activated carbon, was purchased in the form of pellets which were crushed manually and then mixed with distilled water. The carbon suspension was added into the precursor Ti [OCH (CH3)2]4 which was further reduced by acetic acid. The grey colored product was further dried and calcined to obtain the TiO2 NPs. The obtained activated carbon TiO2 (C/TiO2) was further characterized.
2.3. Characterization Techniques
The techniques used for the characterization were ultraviolet (UV) spectroscopy for the optical properties and bandgap studies, X-ray diffraction (XRD) for the structural phases, the structural morphology and elemental composition was performed using a field emission scanning electron microscope (FESEM) and high-resolution transmission electron microscopy (HR-TEM), respectively, while the characteristic vibrational modes for the expected functional groups were identified using Fourier-transform infrared spectroscopy (FTIR). Brunner-Emmett-Teller (BET), photoluminescence (PL) was used for surface area and luminescent property measurements.
2.4. Photocatalytic Experiments
The photocatalytic studies of the synthesized catalysts against the organic textile dye RO84 were carried under a mercury lamp using visible light. Firstly, 5, 10 and 15 ppm stock solutions of RO8 dye were prepared and then 0.1 to 0.3 g of photocatalyst was mixed with 20 mL of the stock solutions separately. The catalytic activity was determined by turning on the mercury lamp and the light-treated material was sampled at 10 min intervals. The treated samples were centrifuged and then their UV-Vis spectra were recorded to follow the degradation. The degradation % of the RO84 was calculated by applying the following equation:
The rate constant (k) was calculated by finding the first-order kinetics of the pure and AC-TiO2. The formula used for the kinetic rate is given below:
where C0 is the initial concentration of the RO8 dye solution, and C is the concentration of dye after light exposure for a certain time (t).
3. Results and Discussion
3.1. XRD Spectrometry: Phase Confirmations
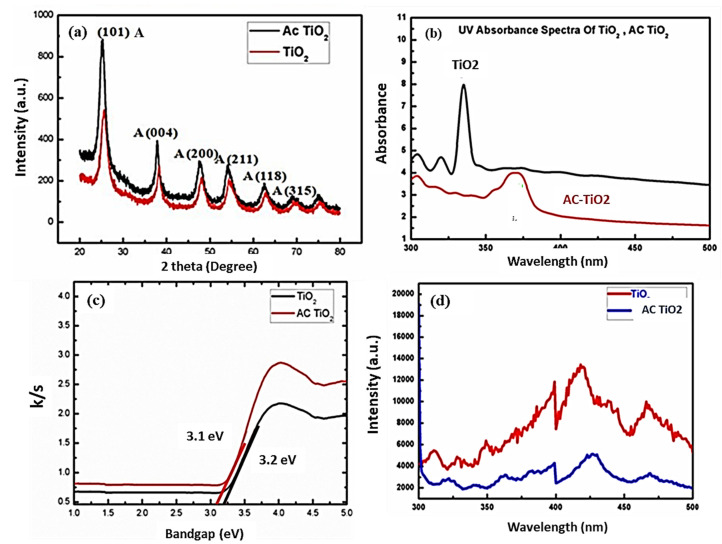
The unsupported and carbon-based TiO2 NPs were analyzed in order to confirm their crystalline structure. The crystalline profiles of TiO2 and doped samples were recorded using XRD and are depicted in Figure 1a. The main peaks at the positions of 25.3°, 37.8°, 48.0°, 53.8°, 55.1°, and 62.8° indicate formation of TiO2 anatase with respect to the 101, 004, 200, 211, 118 and 315 crystallographic planes (JCPDS number 75-15437) [26,28]. The XRD results thus confirm that an anatase crystalline phase of TiO2 is formed after the heat treatment at 500 °C in an open atmosphere environment. The diffraction peak intensity increased in the case of AC-TiO2 nanocomposites after adding the AC in a certain amount. Remarkably, no further peaks were observed in the AC-TiO2 composites at the 24.6° location, which is assigned to the loaded AC showing diffracted peaks corresponding to the (002) crystalline plane. This could be because this characteristic peak of AC would be masked by the anatase crystal faces of TiO2 located at 25.3°. Furthermore, the crystalline sizes of the formed TiO2 photocatalyst could be estimated using Scherer’s formula, especially for the characteristic diffraction peak and crystallographic planes and a range of 26.9 and 29.3 nm (crystalline sizes) for pure and AC-TiO2 was observed [26,29].
Figure 1.
(a) XRD profile for unsupported TiO2 and AC-TiO2, (b) optical absorption (UV-Vis) curve for TiO2 and AC-TiO2, (c) UV-Visible study (DRS) of TiO2 and AC-TiO2 and (d) photoluminescence response for pure TiO2 and AC-TiO2 structure.
3.2. UV-Absorbance Spectra
Figure 1b displays the UV optical absorbance for TiO2 and AC-TiO2 NPs, displaying a prominent absorption hump at around λ < 400 nm. The main optical absorption features were found at around 200–400 nm, and are due to the electron transitions from the valence to the conduction band of the TiO2. These optical ranges confirmed the formation of the TiO2 in anatase form.
TiO2 surrounded by carbon layered structures absorbs a higher range of light below the visible zone of the electromagnetic spectrum (≈400 nm). Basically, the spectral intensities shift for the AC-TiO2 as near to the TiO2 energy zone [29,30]. The carbon often allows Ti to sustain the trivalent state along with the O2 vacancy state, depending upon the conduction and valence band positions. Activated C compounds present a larger absorption of the visible spectral range, confirmed by the change in grey color [2,31]. There was a significant difference reported for the light absorption capacity for the pure and carbon-loaded TiO2. Here the bandgap modification concept was introduced to degrade the particular RO84 organic dye molecules. The modified band gap energies (3.1 and 3.2 eV) for pure and AC-TiO2 are not often studied for organic textile dye effluents, thus, the obtained band position responds actively for the catalytic destruction of RO84 dye.
3.3. UV-DRS Spectra
UV-DRS results for the powdered AC-TiO2 and TiO2 are depicted in Figure 1c. The absorption peak at around 400–450 nm was assigned to the AC-TiO2 along with the TiO2. The resulting spectra revealed that the optimized experimental route is practical for producing the anatase state with the aim of absorbing visible light in the electromagnetic spectrum. Bandgaps values for both samples were determined, and found to be 3.1 and 3.2 eV, corresponding to the doped AC-TiO2 and TiO2 nanopowders, respectively [2,32]. Notably, the bandgap of AC-TiO2 was found to be less than that of the pure TiO2 which could be due to the interference of the activated carbon. This modified AC-TiO2 band was found to be highly desirable for the catalysis of RO84 textile organic dye decomposition, which is a new finding for particular wastewater treatment applications.
3.4. Photoluminescence Study
A photoluminescence study was done for all the samples under normal conditions (RT) and the emission peak response is displayed in Figure 1d. The anatase phase of the respective materials emits light in the green luminescent band and therefore the study has been carried out to confirm the crystal phase. The luminescence process mainly occurs due to the electron transitions from the trapping levels of conduction bands to the valance band. Certain intensities of the light allow the transit of free electrons, resulting in emission bands (luminescent bands). [32]. There is a significant change in the peak intensity of the unsupported and supported TiO2 because of the doped carbon in certain experimental setups. Thus, the used method was found to be very appropriate for the synthesis of the anatase phase in both the samples [31,32].
3.5. BET Analysis: Adsorption Spectra
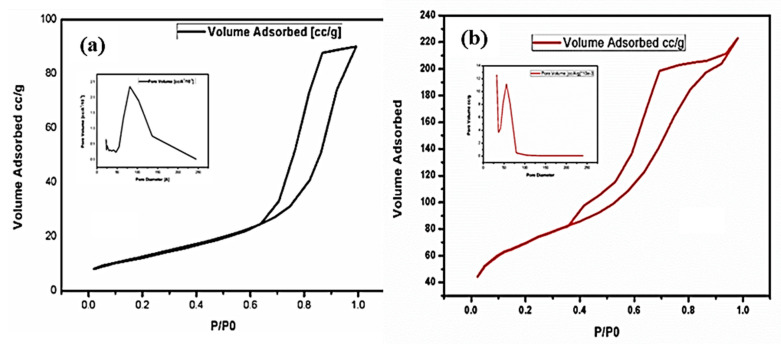
Figure 2 illustrates the N2 adsorption with the desorption isotherm, distribution of pore sizes of anataseTiO2 and AC-TiO2, respectively (Figure 2a,b). Type-II isotherm character was shown by the TiO2, whereas a type-IV isotherm is demonstrated for the AC-TiO2 samples in this scenario, Type-II could be confirmed based on the disappearance of hysteresis in the obtained graph and a valid difference was noticed between the adsorption and desorption areas for TiO2 samples above/Po > 0.6 pressure. The occurrence of the gap revealed that larger pores are available on the surfaces with voids. The closed adsorption-desorption curve indicated by its narrow size mesoporous (2–50 nm as per IUPAC) structures on the surface at P/Po ~ 0.4 relative pressure. Here the hysteresis nature is assigned to the capillary condensation character for both structures considering mesoporous and micropores. The calculated specific surface area was ~241 m2/gm for AC-TiO2 solid powder which was larger than that of the TiO2 powder (~46 m2/gm), so the change in the surface area also supports the modification conditions applied during the surface alterations and morphological changes caused by doping [25,27].
Figure 2.
BET absorption spectra of (a) unsupported TiO2 and (b) supported AC/TiO2.
3.6. Morphological Studies: HRTEM Analysis
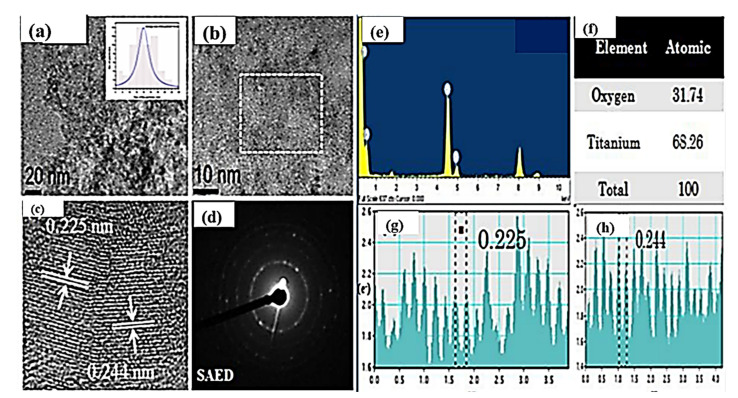
Figure 3a–h present the HRTEM images of pure TiO2 NPs after the calcination at 500 °C. The average particle size was found to be 24.5 nm and the interplanar distance (d) was observed as 0.244 nm with crystalline behaviour, evidenced by the SAED pattern. Furthermore, the prepared powdered structures of the samples were assigned to the Ti and O atomic %, respectively.
Figure 3.
HRTEM images of synthesized TiO2 NPs, (a) TEM micrographs with histogram, (b) HRTEM images, (c) lattice parameter and (d) SAED pattern of TiO2. (e) EDX spectra (f) atomic % of Ti and O, (g,h) particle d-spacing analysis.
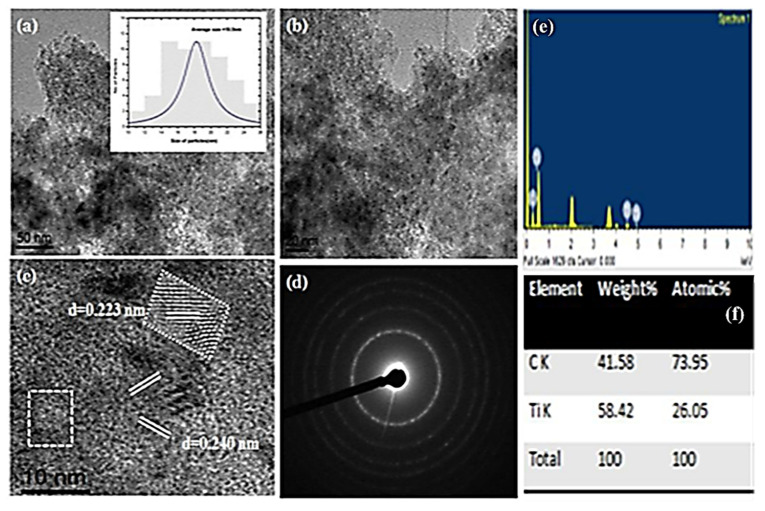
Figure 4a–f show HRTEM and EDX images of the AC-TiO2. Micrographs of the analysed samples show powdered carbon substances surrounding the open face of TiO2.
Figure 4.
HRTEM and EDX images of synthesized AC-TiO2 NPs, Image (a,b) are TEM images, with histogram, lattice parameter (c,d) SAED pattern of AC-TiO2, (e,f) shows the EDX image with atomic percentages.
The average size displayed by the histogram is found to be 25.4 nm and crystalline behaviour was estimated by the analysing the SAED which shows a higher solid-state for the AC- supported TiO2. The obtained values for the pure and supported TiO2 particles were found to be smaller than the XRD sizes as refers to crystallite sizes. Figure 4e,f confirmed the elemental compositions for the doped carbon along with the Ti percentage, studied by EDX peak analysis in terms of atomic weight %.
4. Removal of Organic Pollutants
The characteristics of the band positions, opacity and the surface of the anatase form of TiO2 nanostructures revealed acceptable potentials for photocatalytic activity. Among all three crystalline states, anatase is widely adopted along with hybrid structures doped with lightweight atoms including anions (halogens) because of their liquid synthesis procedures [33,34]. The doping has been studied and extensively explored towards catalytic applications of TiO2 semiconductors [35,36,37,38]. In this work, we manufactured TiO2 and carbon-supported TiO2 and characterized them. The degradation of the textile dye molecule RO84 by the as-designed photocatalyst was further studied as a new approach to dye degradation studies. The as-modified band structure was influenced by visible range of electromagnetic radiation, hence demonstrating catalytic activity against the RO84 organic structure.
Photocatalytic Degradation of RO84
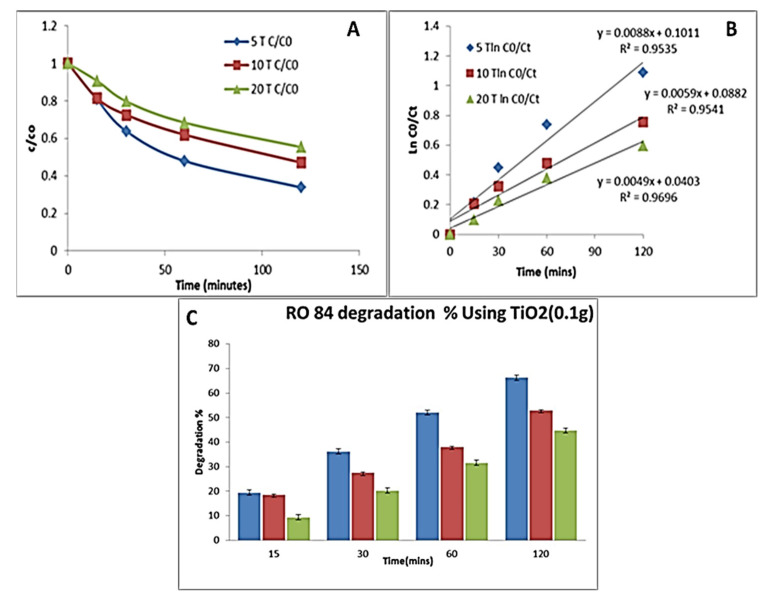
Initially, 0.1 g of sample was used, which was then varied up to 0.3 g to optimize the degradation performance (as detailed in the Materials and Methods section). Five, 10, and 20 ppm concentration of the RO84 was taken and firstly reacted with 0.1 g of catalysts (TiO2 and ACTiO2). The TiO2 shown 66% degradation at a low concentration of dye, determined by evaluating the first-order reaction rate as shown in the kinetics graphs (Figure 5A,B).
Figure 5.
(A,B) Kinetic degradation plot of RO84 with TiO2 (0.1 g). (C) Degradation % of RO84 using TiO2 (0.1 g).
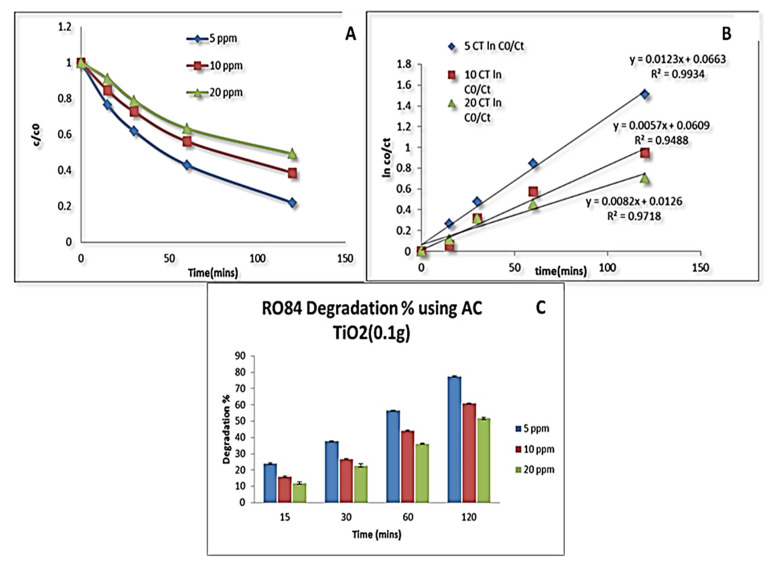
A 44% degradation was found at a higher concentration for the same amount of catalyst. The kinetic plot was obtained for the TiO2 (0.1 g) followed by first-order fitting. The rate constants were 0.008, 0.0059 and 0.0049 min−1 with respect to 5, 10, 20 ppm of dye with R2 equal to 0.95. The degradation kinetics curves with respect to time (min) are shown in Figure 5C. However, when the same concentration of the dye was used for the AC-TiO2 nanocrystals to check the degradation rate, it was found to be 78% and 51% for the lower and higher concentrations, respectively. The k values were found on the order of 0.01, 0.005, and 0.008 min−1 for the used concentration of RO84 based on first order fits. Figure 6A–C display the kinetic studies of the degradation % vs. time of RO84 using 0.1 g of AC-TiO2. AC with 10–20 nm sizes was decorated on a TiO2 surface by Xing et al., [39] using a sol-gel technique and around 93% degradation of RhB was observed using UV light. They proposed a dual mechanism for the absorption and degradation activity for the removal of RhB in water. The adsorption efficiency was increased by the availability of the open surfaces mainly due to the activated carbon on the TiO2 structure.
Figure 6.
(A,B) Degradation kinetics of RO84 with AC-TiO2 (0.1 g). (C) Degradation percentage of RO84 using AC-TiO2 (0.1 g).
Nearly two-fold better catalytic efficiency was reported by Amran and co-authors [40] by using a modified TiO2 surface. Carbon was used to alter the bandgap of TiO2 which was reduced to 2.38 eV and employed to enhance the performance in the visible light range. The methylene blue molecule was degraded using this AC-doped TiO2 and 82.67% efficiency was recorded due to a larger absorption of visible light by carbon.
To minimize the loss of designed composites (AC/TiO2) the polymer fiber was immobilized and that was the reason for the better recyclability performance during the catalytic measurements.
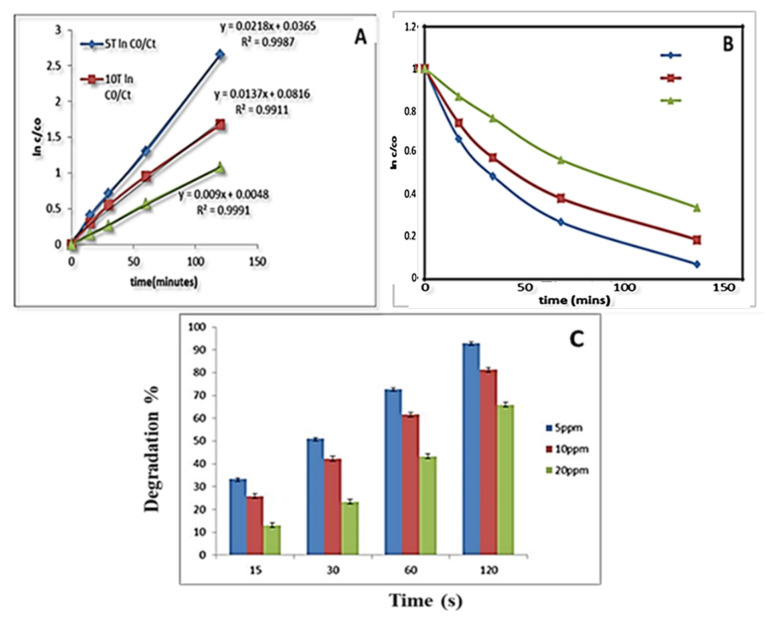
A certain amount of catalyst was found to be much more effective in the degradation of the RO84 dye as we changed the amount from 0.1–0.3 g. The experimental conditions also played a key factor to enhance the reaction rate and thus the route employed is found to be very useful for this particular catalyst (TiO2, and AC-TiO2). By increasing the concentration of catalyst (TiO2) to 0.3 g a degradation of up to 92% was achieved for a lower concentration of dye in which the reaction follows first-order kinetics. The R2 values are above 0.95 and the k values are found to be 0.02, 0.01 and 0.009 min−1 for 5, 10, 20 ppm of RO84, respectively, as depicted in Figure 7A–C. The degradation percentage was found to be 65% at a higher concentration of dye. Significantly, the kinetic rate of the photocatalysis is regulated by the recombination time of electron-holes of the semiconductors. TiO2 has already been implemented as one of the finest catalysts for degradation of contaminants due to its characteristic band structures and crystal phases [41,42]. In our study it has been modified structurally and applied to achieve an enhanced functionality for the decomposition of RO84.
Figure 7.
(A,B) RO84 degradation kinetics using TiO2 at 0.3 g. (C) Degradation % of RO84 using TiO2 (0.3).
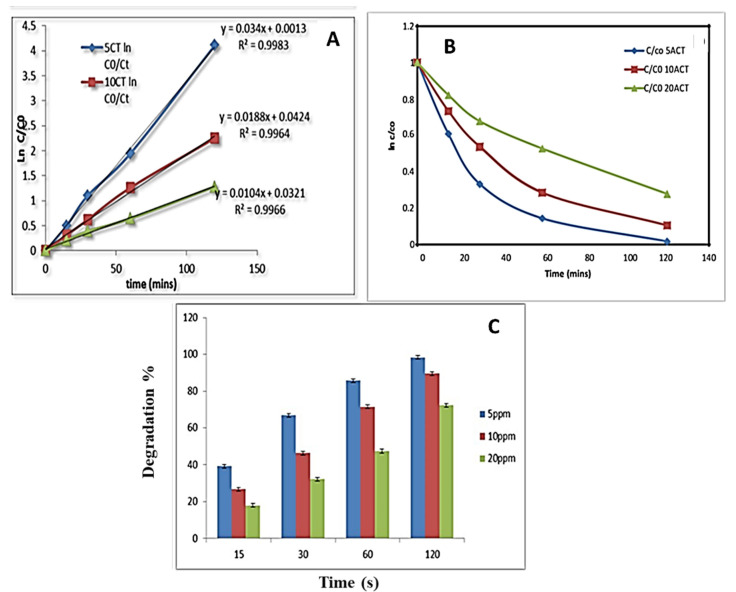
The larger amount of AC-TiO2 (0.3 g) induces a higher degradation rate of RO84 and the corresponding kinetic plots are shown in Figure 8. The kinetic rate could be determined in the form of k values, found as 0.034, 0.01 and 0.010 min−1 for 5, 10, 20 ppm of RO84, respectively. A 97% degradation efficiency was found for the lower concentration of taken dye using a 0.3 g amount of photocatalyst, as shown in Figure 8A–C, whereas 72% decomposition efficiency was obtained for a higher concentration of the dye for the same catalyst. Therefore, the activated carbon provided a larger area to adsorb RO84 molecules with strong binding onto the designed catalyst surface [42,43]. The bandgap engineering of anatase TiO2 supported by AC also provides a feasible environment to degrade the pollutants in our case. The crystalline and stabilized AC/TiO2 was thus found to be very effective based on the catalysis improvement caused by the bigger surface and the controlled structural properties. The adsorption and light absorption at a certain light intensity was modulated by the morphological changes in the TiO2 and therefore it has been expressed as an enriched catalytic activity for RO84.
Figure 8.
(A,B) RO84 degradation kinetics using AC-TiO2 at 0.3 g, (C) Degradation % of RO84 using AC-TiO2 (0.3 g).
5. Conclusions
A crystalline form of TiO2 anatase supported by activated carbon was prepared. Its structural, optical, and luminescence properties were confirmed. Narrow-sized AC-TiO2 and TiO2 NPs were observed by the use of XRD and HRTEM, whereas the surface area was studied by BET experiments. The surface areas for TiO2 and AC-TiO2 were found to be ~241 m2/gm and ~46 m2/gm, respectively. The atomic percentage elemental compositions of pure TiO2 and AC-TiO2 were also determined by an EDX study. An enhanced degradation rate of the pollutant RO84 was obtained using TiO2 and AC-TiO2 nanocatalysts which showed a maximum of 92% and 97% efficiency with the optimized amount (0.3 g) of photocatalyst, respectively. The activated carbon possesses a larger active area to facilitate the outstanding catalytic action of the TiO2 owing to its chemical stability with unique physicochemical functionalities. The modified bandgap of the as-prepared photocatalyst was found to be highly desirable to decompose the molecular structure of the target RO84, which is a major finding in wastewater remediation. These modified nanostructures could be further applied to multiple species in environmental applications.
Author Contributions
Conceptualization, G.K.I., V.K.Y., S.R., and M.H.A.; Data curation, N.C., S.I., and S.R., methodology, G.K.I., N.C., M.H.A., S.R. and B.-H.J.; validation, K.K.Y., S.I., and M.B.S.; formal analysis, N.C., N.G., and M.B.S.; resources, K.K.Y., and B.-H.J.; writing—original draft preparation, G.K.I., S.R., writing—review and editing, G.K.I., S.R., and V.K.Y., supervision, S.I., G.K.I. and B.-H.J.; project administration V.K.Y., N.C., K.K.Y., and N.G.; Funding acquisition, B.-H.J., S.I., N.G., and M.H.A.; Investigation, V.K.Y., B.-H.J., and M.B.S., Software, M.B.S., G.K.I., and M.H.A., Visualization, N.G., V.K.Y., K.K.Y., and B.-H.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by University Researchers, supporting project number TURSP-2020/91, Taif University, Taif, Saudi Arabia. This study was also supported by the Korea Environment Industry & Technology Institute (KEITI) through Subsurface Environment Management (SEM) Projects, funded by Korea Ministry of Environment (MOE) (No.2020002480007).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Khoshnood M., Azizian S. Adsorption of 2,4-dichlorophenoxyacetic acid pesticide by graphitic carbon nanostructures prepared from biomasses. J. Ind. Eng. Chem. 2012;18:1796–1800. doi: 10.1016/j.jiec.2012.04.007. [DOI] [Google Scholar]
- 2.Kukovecza A., Kordás K., Kissd J., Kónya Z. Atomic scale characterization and surface chemistry of metal modified titanate nanotubes and nanowires. Surf. Sci. Rep. 2016;71:473–546. doi: 10.1016/j.surfrep.2016.06.001. [DOI] [Google Scholar]
- 3.Aronzon C.M., Sandoval M.T., Herkovits J., Pérez-Coll C.S. Stage-dependent toxicity of 2, 4-dichlorophenoxyacetic on the embryonic development of a South American toad, Rhinella arenarum. Environ. Toxicol. 2011;26:373–381. doi: 10.1002/tox.20564. [DOI] [PubMed] [Google Scholar]
- 4.Huy B.T., Jung D.-S., Phuong N.T.K., Lee Y.-I. Enhanced photodegradation of 2,4-dichlorophenoxyacetic acid using a novel TiO2@MgFe2O4 core@shell structure. Chemosphere. 2017;184:849–856. doi: 10.1016/j.chemosphere.2017.06.069. [DOI] [PubMed] [Google Scholar]
- 5.Singh R.K., Philip L., Ramanujam S. Removal of 2,4-dichlorophenoxyacetic acid in aqueous solution by pulsed corona discharge treatment: Effect of different water constituents, degradation pathway and toxicity assay. Chemosphere. 2017;184:207–214. doi: 10.1016/j.chemosphere.2017.05.134. [DOI] [PubMed] [Google Scholar]
- 6.Yao Y., Chen H., Qin J., Wu G., Lian C., Zhang J., Wang S. Iron encapsulated in boron and nitrogen codoped carbon nanotubes as synergistic catalysts for Fenton-like reaction. Water Res. 2016;101:281–291. doi: 10.1016/j.watres.2016.05.065. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y.-S., Lin L.-Y. Synthesis of monoclinic BiVO4 nanorod array for photoelectrochemical water oxidation: Seed layer effects on growth of BiVO4 nanorod array. Electrochim. Acta. 2018;285:164–171. doi: 10.1016/j.electacta.2018.07.232. [DOI] [Google Scholar]
- 8.Gomes J.F., Leal I., Bednarczyk K., Gmurek M., Stelmachowski M., Diak M., Quinta-Ferreira M.E., Costa R., Quinta-Ferreira R.M., Martins R. Photocatalytic ozonation using doped TiO2 catalysts for the removal of parabens in water. Sci. Total Environ. 2017;609:329–340. doi: 10.1016/j.scitotenv.2017.07.180. [DOI] [PubMed] [Google Scholar]
- 9.Rosal R., Rodríguez A., Melon J.A.P., Petre A., Calvo E.G., Gómez M.J., Agüera A., Fernández-Alba A.R. Occurrence of emerging pollutants in urban wastewater and their removal through biological treatment followed by ozonation. Water Res. 2010;44:578–588. doi: 10.1016/j.watres.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Fujishima A., Rao T.N., Tryk D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000;1:1–21. doi: 10.1016/S1389-5567(00)00002-2. [DOI] [Google Scholar]
- 11.Carp O., Huisman C.L., Reller A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004;32:33–177. doi: 10.1016/j.progsolidstchem.2004.08.001. [DOI] [Google Scholar]
- 12.Kumar P., Inwati G.K., Mathpal M.C., Ghosh S., Roos W., Swart H. Defects induced Enhancement of Antifungal activities of Zn doped CuO nanostructures. Appl. Surf. Sci. 2021;560:150026. doi: 10.1016/j.apsusc.2021.150026. [DOI] [Google Scholar]
- 13.Inwati G.K., Kumar P., Roos W.D., Swart H.C. Thermally induced structural metamorphosis of ZnO:Rb nanostructures for antibacterial impacts. Colloids Surf. B Biointerfaces. 2020;188:110821. doi: 10.1016/j.colsurfb.2020.110821. [DOI] [PubMed] [Google Scholar]
- 14.Makarova O.V., Rajh T., Thurnauer M.C., Martin A., Kemme P.A., Cropek D. Surface Modification of TiO2 Nanoparticles for Photochemical Reduction of Nitrobenzene. Environ. Sci. Technol. 2000;34:4797–4803. doi: 10.1021/es001109+. [DOI] [Google Scholar]
- 15.Inwati G., Rao Y., Singh M. Thermodynamically induced in situ and tunable Cu plasmonic behaviour. Sci. Rep. 2018;8:3006. doi: 10.1038/s41598-018-20478-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inwati G., Kumar P., Roos W., Swart H., Singh M. UV-irradiation effects on tuning LSPR of Cu/Ag nanoclusters in ion exchanged glass matrix and its thermodynamic behaviour. J. Alloys Compd. 2020;823:153820. doi: 10.1016/j.jallcom.2020.153820. [DOI] [Google Scholar]
- 17.Jiang G., Lin Z., Chen C., Zhu L., Chang Q., Wang N., Wei W., Tang H. TiO2 nanoparticles assembled on graphene oxide nanosheets with high photocatalytic activity for removal of pollutants. Carbon. 2011;49:2693–2701. doi: 10.1016/j.carbon.2011.02.059. [DOI] [Google Scholar]
- 18.Pu X., Zhang D., Gao Y., Shao X., Ding G., Li S., Zhao S. One-pot microwave-assisted combustion synthesis of graphene oxide-TiO 2 hybrids for photodegradation of methyl orange. J. Alloys Compd. 2013;551:382–388. doi: 10.1016/j.jallcom.2012.11.028. [DOI] [Google Scholar]
- 19.Pan S., Liu X. CdS–Graphene nanocomposite: Synthesis, adsorption kinetics and high photocatalytic performance under visible light irradiation. New J. Chem. 2012;36:1781–1787. doi: 10.1039/c2nj40301c. [DOI] [Google Scholar]
- 20.Inwati G., Rao Y., Singh M. In situ growth of low-dimensional silver nanoclusters with their tunable plasmonic and thermodynamic behavior. ACS Omega. 2017;2:5748–5758. doi: 10.1021/acsomega.7b00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inwati G., Rao Y., Singh M. Single step aqueous synthesis of unsupported PtNi nanoalloys using flower extract as reducing agent and their compositional role to enhance electrocatalytic activity. AIP Conf. Proc. 2017;1837:040048. [Google Scholar]
- 22.Frank S.N., Bard A.J. Semiconductor Electrodes. 12. Photoassisted Oxidations and Photoelectrosynthesis at Polycrystalline TiO2 Electrodes. J. Am. Chem. Soc. 1977;99:4667–4675. doi: 10.1021/ja00456a024. [DOI] [Google Scholar]
- 23.Wang Y., Huang Y., Ho W., Zhang L., Zou Z., Lee S.-C. Biomolecule-controlled hydrothermal synthesis of C–N–S-tridoped TiO2 nanocrystalline photocatalysts for NO removal under simulated solar light irradiation. J. Hazard. Mater. 2009;169:77–87. doi: 10.1016/j.jhazmat.2009.03.071. [DOI] [PubMed] [Google Scholar]
- 24.Su C., Tseng C.-M., Chen L.-F., You B.-H., Hsu B.-C., Chen S.-S. Sol–hydrothermal preparation and photocatalysis of titanium dioxide. Thin Solid Films. 2006;498:259–265. doi: 10.1016/j.tsf.2005.07.123. [DOI] [Google Scholar]
- 25.Torres S.M., Pastrana-Martínez L.M., Figueiredo J., Faria J.L., Silva A. Design of graphene-based TiO2 photocatalysts—a review. Environ. Sci. Pollut. Res. 2012;19:3676–3687. doi: 10.1007/s11356-012-0939-4. [DOI] [PubMed] [Google Scholar]
- 26.Leary R., Westwood A. Carbonaceous nanomaterials for the enhancement of TiO2 photocatalysis. Carbon. 2011;49:741–772. doi: 10.1016/j.carbon.2010.10.010. [DOI] [Google Scholar]
- 27.Endo M., Janczarek M., Wei Z., Wang K., Markowska-Szczupak A., Ohtani B., Kowalska E. Bactericidal Properties of Plasmonic Photocatalysts Composed of Noble Metal Nanoparticles on Faceted Anatase Titania. J. Nanosci. Nanotechnol. 2019;19:442–452. doi: 10.1166/jnn.2019.15780. [DOI] [PubMed] [Google Scholar]
- 28.Hejazi S., Mohajernia S., Wu Y., Andryskova P., Zoppellaro G., Hwang I., Tomanec O., Zboril R., Schmuki P. Intrinsic Cu nanoparticle decoration of TiO2 nanotubes: A platform for efficient noble metal free photocatalytic H2 production. Electrochem. Commun. 2019;98:82–86. doi: 10.1016/j.elecom.2018.11.020. [DOI] [Google Scholar]
- 29.Yang H.G., Zeng H.C. Preparation of Hollow Anatase TiO2 Nanospheres via Ostwald Ripening. J. Phys. Chem. B. 2004;108:3492–3495. doi: 10.1021/jp0377782. [DOI] [PubMed] [Google Scholar]
- 30.Sacco O., Vaiano V., Han C., Sannino D., Dionysiou D.D. Photocatalytic removal of atrazine using N-doped TiO2 supported on phosphors. Appl. Catal. B Environ. 2015;164:462–474. doi: 10.1016/j.apcatb.2014.09.062. [DOI] [Google Scholar]
- 31.Sandeep S., Nagashree K., Maiyalagan T., Keerthiga G. Photocatalytic degradation of 2,4-dichlorophenoxyacetic acid—A comparative study in hydrothermal TiO2 and commercial TiO2. Appl. Surf. Sci. 2018;449:371–379. [Google Scholar]
- 32.Lu Z., Chen F., He M., Song M., Ma Z., Shi W., Yan Y., Lan J., Li F., Xiao P. Microwave synthesis of a novel magnetic imprinted TiO2 photocatalyst with excellent transparency for selective photodegradation of enrofloxacin hydrochloride residues solution. Chem. Eng. J. 2014;249:15–26. doi: 10.1016/j.cej.2014.03.077. [DOI] [Google Scholar]
- 33.Kandiel T.A., Dillert R., Feldhoff A., Bahnemann D.W. Direct Synthesis of Photocatalytically Active Rutile TiO2 Nanorods Partly Decorated with Anatase Nanoparticles. J. Phys. Chem. C. 2010;114:4909–4915. doi: 10.1021/jp912008k. [DOI] [Google Scholar]
- 34.Natarajan K., Natarajan T.S., Bajaj H.C., Tayade R.J. Rutile phase dominant TiO2 formed by thermal treatment and its high photocatalytic activity under narrow spectrum ultraviolet light emitting diodes. Mater. Res. Express. 2018;6:015049. doi: 10.1088/2053-1591/aae861. [DOI] [Google Scholar]
- 35.Shankar M.V., Anandan S., Venkatachalam N., Arabindoo B., Department V.M. Fine route for an efficient removal of 2,4-dichlorophenoxyacetic acid (2,4-D) by zeolite-supported TiO2 M.V. Bull. Iran. Math. Soc. 2013;39:347–353. doi: 10.1016/j.chemosphere.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 36.Piera E., Calpe J.C., Brillas E., Domènech X., Peral J. 2,4-Dichlorophenoxyacetic acid degradation by catalyzed ozonation: TiO2/UVA/O3 and Fe(II)/UVA/O3 systems. Appl. Catal. B Environ. 2000;27:169–177. doi: 10.1016/S0926-3373(00)00149-1. [DOI] [Google Scholar]
- 37.Iliev V., Tomova D., Bilyarska L. Journal of Photochemistry and Photobiology A: Chemistry Promoting the oxidative removal rate of 2,4-dichlorophenoxyacetic acid on gold-doped WO3/TiO2/reduced graphene oxide photocatalysts under UV light irradiation. J. Photochem. Photobiol. A Chem. 2018;351:69–77. doi: 10.1016/j.jphotochem.2017.10.022. [DOI] [Google Scholar]
- 38.Bian X., Chen J., Ji R. Degradation of 2,4-Dichlorophenoxyacetic Acid (2,4-D) by Novel Photocatalytic Material of Tourmaline-Coated TiO2 Nanoparticles: Kinetic Study and Model. Materials. 2013;6:1530–1542. doi: 10.3390/ma6041530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xing B., Shi C., Zhang C., Yi G., Chen L., Guo H., Huang G., Cao J. Preparation of TiO2/Activated Carbon Composites for Photocatalytic Degradation of RhB under UV Light Irradiation. J. Nanomater. 2016;2016:8393648. doi: 10.1155/2016/8393648. [DOI] [Google Scholar]
- 40.Saiful Amran S.N.B., Wongso V., Abdul Halim N.S., Husni M.K., Sambudi N.S., Wirzal M.D.H. Immobilized carbon-doped TiO2 in polyamide fibers for the degradation of methylene blue. J. Asian Ceram. Soc. 2019;7:321–330. doi: 10.1080/21870764.2019.1636929. [DOI] [Google Scholar]
- 41.Zhao Y.-X., Li X.-Y., Tian C., Wang J.-X. Production of carbon-doped titanium dioxide (C–TiO2) from polytitanium-coagulated sludge as an adsorbent or photocatalyst for pollutant removals. J. Clean. Prod. 2020;267:121979. doi: 10.1016/j.jclepro.2020.121979. [DOI] [Google Scholar]
- 42.Kuriechen S.K., Murugesan S. Carbon-Doped Titanium Dioxide Nanoparticles Mediated Photocatalytic Degradation of Azo Dyes Under Visible Light. Water Air Soil Pollut. 2013;224:1671. doi: 10.1007/s11270-013-1671-5. [DOI] [Google Scholar]
- 43.Zhou J., Zhu B., Wang L., Li Y., Qiao Q. Enhanced photocatalytic activity of Fe-doped TiO2 coated on N-doped activated carbon composites for photocatalytic degradation of dyeing wastewater. AIP Conf. Proc. 2017;1890:020009. [Google Scholar]