Abstract
Background
COVID-19 stay-at-home orders may reduce access to substance use treatment and naloxone, an opioid overdose reversal drug. The objective of this analysis was to compare monthly trends in pharmacy-based dispensing rates of medications for opioid use disorder (MOUD) (buprenorphine and extended-release [ER] naltrexone) and naloxone in the United States during March 2019–December 2020 by age and sex.
Methods
We calculated monthly prescription dispensing rates per 100,000 persons using IQVIA New to Brand. We used Joinpoint regression to calculate monthly percent change in dispensing rates and Wilcoxon Rank Sum tests to examine differences in median monthly rates overall, and by age and sex between March 2019–December 2019 and March 2020–December 2020.
Results
Buprenorphine dispensing increased among those aged 40–64 years and ≥ 65 years from March 2019 to December 2020. Median rates of total ER naltrexone dispensing were lower in March 2020–December 2020 compared to March 2019–December 2019 for the total population, and for females and males. From March 2019 to December 2020, ER naltrexone dispensing decreased and naloxone dispensing increased for those aged 20–39 years.
Conclusions
Dispensing ER naltrexone declined during the study period. Given the increase in substance use during the COVID-19 pandemic, maintaining equivalent access to MOUD may not be adequate to accommodate rising numbers of new patients with opioid use disorder. Access to all MOUD and naloxone could be further expanded to meet potential needs during and after the public health emergency, given their importance in preventing opioid overdose-related harms.
Keywords: Medication for opioid use disorder, COVID-19, Naloxone, Buprenorphine, Extended-release naltrexone
1. Introduction
Persons with substance use disorder (SUD) have faced unique challenges during the COVID-19 pandemic. Stress and worry may lead to increased substance use (McKay and Asmundson, 2020, Rehm et al., 2020), and stay-at-home orders and office closures may restrict access to treatment and recovery (Leppla and Gross, 2020). Overdose risk may be increased as more persons may be using drugs alone due to social distancing and reduced access to overdose prevention resources such as naloxone, an opioid overdose reversal drug (Holloway et al., 2020, Ostrach et al., 2021). Preliminary data indicate that fatal overdoses have been increasing during the COVID-19 pandemic (Centers for Disease Control and Prevention, 2020), highlighting the urgency of ensuring access to SUD treatment and naloxone.
Despite the effectiveness of medications for opioid use disorder (MOUD) to decrease opioid use, death from overdose, and other related sequelae (Degenhardt et al., 2011, Martin et al., 2013, Sordo et al., 2017), MOUD were underused prior to the COVID-19 pandemic (Beetham et al., 2019). Unlike methadone, a long-acting full opioid agonist only available from certified opioid treatment programs (OTPs) (Substance Abuse and Mental Health Services Administration, 2020c), buprenorphine can be prescribed by qualified clinicians in various settings. Buprenorphine is a partial opioid agonist that helps to lower physical dependency on opioids and withdrawal symptoms for patients with opioid use disorder (OUD) while providing lower doses of the euphoric and respiratory depression effects of opioids (Substance Abuse and Mental Health Services Administration, 2021). However, recent data indicate that 40% of U.S. counties do not contain any buprenorphine providers, and an additional 24% of counties have low capacity to treat patients (a rate less than or equal to 473.8 per 100,000 people) (Grimm, 2020).
Extended-release (ER) naltrexone is an MOUD that can be administered by any clinician licensed to prescribe medications (Substance Abuse and Mental Health Services Administration, 2020d). It is available as a once-monthly intramuscular injection and is approved to treat both OUD and alcohol use disorder (Substance Abuse and Mental Health Services Administration, 2020d). While the injection offers advantages over oral medications in treatment adherence and diversion, disadvantages are the treatment must be administered in a clinic setting and patients must wait 14 days after taking opioids before starting on ER naltrexone (Substance Abuse and Mental Health Services Administration, 2020d), thereby undergoing symptoms associated with opioid detoxification.
In addition to MOUD, robust naloxone access is a critical strategy to reduce opioid overdose morbidity and mortality. Community- and pharmacy-dispensed take-home naloxone has been expanding in recent years as opioid overdose deaths continue to rise. Between 2016 and 2018, naloxone dispensing rates from retail pharmacies increased from 41.5 to 170.2 per 100,000 persons (Guy et al., 2019). However, naloxone access varies widely geographically and among demographic subgroups, and overall dispensing rates remain low (Guy et al., 2019) despite drug overdose deaths reaching record highs (Ahmad et al., 2021).
On March 11, 2020, the World Health Organization declared COVID-19 a pandemic—8 days later, the first U.S. state (California) issued a stay-at-home order, followed by most other states between March 21, 2020, and April 7, 2020 (Kaiser Family Foundation, 2020). A rapid survey of syringe service programs (SSPs) during these early weeks of the pandemic showed that 43% of respondents indicated decreased availability to services such as MOUD treatment, and 25% reported that one or more of their SSP sites had closed during the pandemic, potentially reducing access to safe injection supplies and naloxone (Glick et al., 2020). Following the issuance of stay-at-home orders, the Substance Abuse and Mental Health Services Administration (SAMHSA) released guidance on emergency exemptions allowing the use of telehealth to maintain existing patients on methadone and maintain or initiate patients on buprenorphine treatment (Substance Abuse and Mental Health Services Administration, 2020b). Although these exemptions can expand access to MOUD treatment during the pandemic, research on national treatment access and coverage during this timeframe is limited. Tracking prescription dispensing patterns of pharmacy-based MOUD (buprenorphine and ER naltrexone) and naloxone is one method of identifying whether gaps in treatment and overdose prevention have occurred during the COVID-19 pandemic. One report showed that patients who were dispensed naloxone and buprenorphine remained within forecasted levels from March 2020 to May 2020, but patients who were dispensed ER naltrexone fell below (Jones et al., 2021a, Jones et al., 2021b). However, no reports to-date have examined MOUD and naloxone dispensing stratified by age or sex.
The objective of this analysis was to compare monthly trends in pharmacy-based dispensing rates of buprenorphine, ER naltrexone, and naloxone in the United States during March 2019–December 2020 by age and sex. Because the focus of this study was on pharmacy-based dispensing, we did not examine methadone prescribing, which is not included in pharmacy claims data.
2. Methods
2.1. Data source
We obtained buprenorphine, ER naltrexone, and naloxone dispensing data from a retail pharmacy dataset (IQVIA New to Brand) which includes prescriptions dispensed from 50,400 retail pharmacies, covering roughly 92% of dispensed prescriptions in the United States. We calculated monthly prescription dispensing rates per 100,000 persons for each medication. We used quarterly population data from the U.S. Census Bureau to estimate monthly populations. In May 2020, New Jersey implemented a policy mandating co-prescribing of naloxone with opioids prescribed for managing chronic pain (New Jersey Office of the Attorney General, 2020). This resulted in uncharacteristically high dispensing of naloxone so we removed New Jersey from our analysis of naloxone dispensing, which focuses on national patterns.
2.2. Statistical analysis
Joinpoint models analyze trends across time by connecting lines at “joinpoints” to determine if and when significant changes in trends occur. We calculated the monthly percent change (MPC) using Joinpoint regression from March 2019 to December 2020. When using Joinpoint, MPC refers to the estimated MPC over a time period, such as in between two joinpoints or across the entire study period when the model contains no joinpoints. MPCs for each variable are assumed to change at a constant percentage (National Cancer Institute, 2021a, National Cancer Institute, 2021b) and are expressed as the percentage change with a 95% confidence interval (CI). Monte Carlo permutations (4499 permutations) are used to test significance of the models (Kim et al., 2000).
We used Wilcoxon Rank Sum tests to examine the difference in median rates between March 2019–December 2019 and March 2020–December 2020 for prescribing rates overall and stratified by sex and age group. We chose March 2020–December 2020 to represent trends in dispensing during the pandemic because most U.S. stay-at-home orders were initiated in March. We then compared dispensing trends with the same time in the previous year to account for seasonality. We used R version 3.6.1 for analyses. CDC determined that this study was a non-research activity and no IRB approval was required.
3. Results
3.1. Buprenorphine
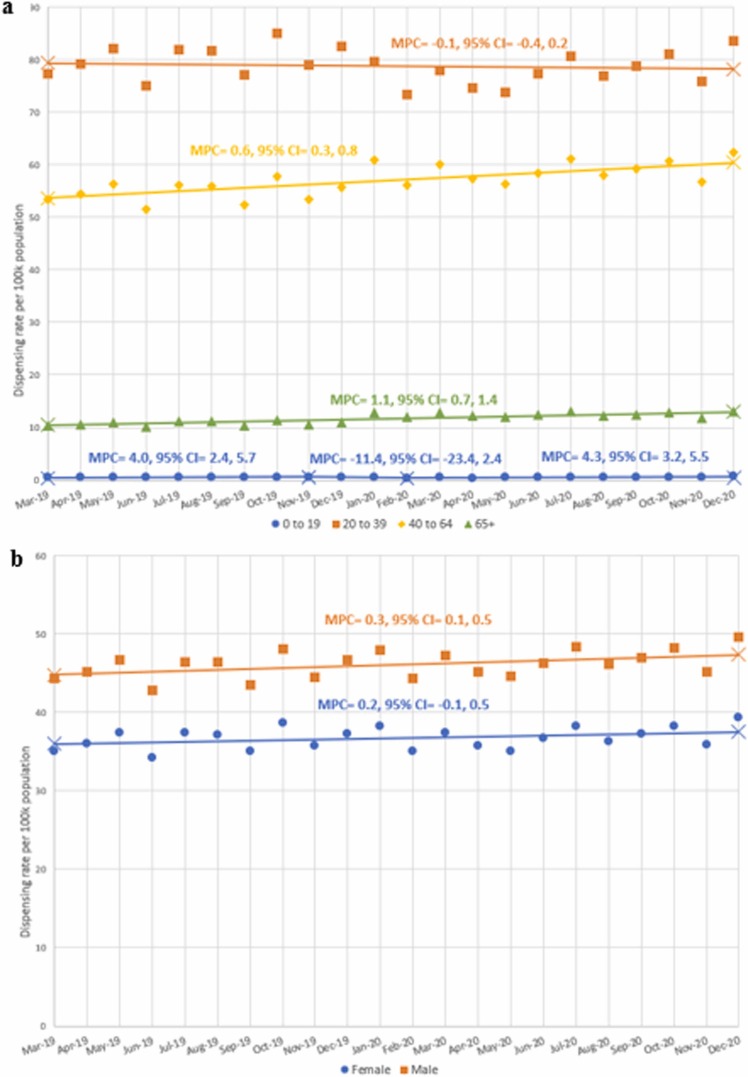
We found no statistically significant monthly trends of overall buprenorphine dispensing from March 2019 through December 2020 (MPC = 0.2, 95% CI = −0.1, 0.5 [ Fig. 1]); however, dispensing for those aged 40–64 (MPC = 0.6, 95% CI = 0.3, 0.8) and ≥ 65 (MPC = 1.1, 95% CI = 0.7, 1.4) did significantly increase ( Fig. 2a). In addition, the trend for males increased over the study period (MPC = 0.03, 95% CI = 0.1, 0.5 [Fig. 2b]); however, the median dispensing rates for males during March 2019–December 2019 and March 2020–December 2020 did not show a statistically significant difference.
Fig. 1.
Monthly total dispensing rates per 100,000 persons from March 2019 to December 2020 for buprenorphine, naloxone, and extended-release (ER) naltrexone. There is a break in the y-axis, creating a jump in dispensing rate per 100k population from 3 to 35. The monthly percent change (MPC) is calculated by Joinpoint. A change in trend (inflection point) is denoted by X. New Jersey is not included in naloxone dispensing rates due to unusually high dispensing rates because of a new policy in May 2020 co-prescribing naloxone with opioids prescribed for managing chronic pain. We obtained dispensing data for buprenorphine, ER naltrexone, and naloxone from IQVIA New to Brand. We used quarterly population data from the United States Census Bureau to estimate monthly populations and to calculate monthly dispensing rates.
Fig. 2.
a. Monthly buprenorphine dispensing rates per 100,000 persons from March 2019 to December 2020 by years of age group. The monthly percent change (MPC) is calculated by Joinpoint. A change in trend (inflection point) is denoted by X. We obtained dispensing data for buprenorphine from IQVIA New to Brand. We used quarterly population data from the United States Census Bureau to estimate monthly populations and to calculate monthly dispensing rates. b. Monthly buprenorphine dispensing rates per 100,000 persons from March 2019 to December 2020 by sex. Changes in monthly percent change (MPC) determined by Joinpoint are shown by different line patterns. A change in trend (inflection point) is denoted by X. Dispensing data for buprenorphine was obtained from IQVIA New to Brand. We used quarterly population data from the United States Census Bureau to estimate monthly populations and to calculate monthly dispensing rates.
3.2. ER naltrexone
From March 2019 to December 2020, total monthly ER naltrexone dispensing had a decreasing trend (MPC = −0.7, 95% CI = −1.1, −0.3). The median dispensing rate for March 2020–December 2020 was significantly lower than in March 2019–December 2019 (0.54 vs. 0.61, p = 0.001). Trends in dispensing for those aged 0–19 varied across the study period, significantly increasing February 2020 to December 2020 (MPC = 6.6, 95% CI = 2.8, 10.5). The median dispensing rate of ER naltrexone among those aged 0–19 for March 2020–December 2020 was significantly lower than for March 2019–December 2019 (0.011 vs. 0.015, p = 0.03). Those aged 20–34 also had a significantly lower median dispensing rate for March 2020–December 2020 compared to March 2019–December 2019 (1.03 vs. 1.21, p < 0.001 [ Fig. 3a]) and a decreasing trend across the study period (MPC = −1.1, 95% CI = −1.5, −0.7). In addition, both females (MPC = −0.7, 95% CI = −1.2, −0.3 & 0.44 vs. 0.49, p = 0.001) and males (MPC = −0.7, 95% CI = −1.1, −0.3 & 0.65 vs. 0.71, p = 0.002) had decreasing trends across the study period (Fig. 3b) and lower median dispensing rates in March 2020–December 2020 compared to March 2019–December 2019.
Fig. 3.
a. Monthly extended-release (ER) naltrexone dispensing rates per 100,000 persons from March 2019 to December 2020 by years of age group. The monthly percent change (MPC) is calculated by Joinpoint. A change in trend (inflection point) is denoted by X. We obtained dispensing data for ER naltrexone from IQVIA New to Brand. We used quarterly population data from the United States Census Bureau to estimate monthly populations and to calculate monthly dispensing rates. b. Monthly extended-release (ER) naltrexone dispensing rates per 100k persons from March 2019 to December 2020 by sex. The monthly percent change (MPC) is calculated by Joinpoint. A change in trend (inflection point) is denoted by X. We obtained dispensing data for ER naltrexone from IQVIA New to Brand. We used quarterly population data from the United States Census Bureau to estimate monthly populations and to calculate monthly dispensing rates.
3.3. Naloxone
Total naloxone dispensing was stable over the study period; however, the median dispensing rate for March 2020–December 2020 was significantly higher than for March 2019–December 2019 (2.60 vs. 2.35, p = 0.04). Persons aged 20–39 had a statistically significant increasing trend from March 2019 to December 2020 (MPC = 1.3, 95% CI = 0.9, 1.8 [ Fig. 4a]), and the median dispensing rate in March 2020–December 2020 was significantly higher than in March 2019–December 2019 (2.50 vs. 2.11, p = 0.01). While those aged 40–64 had a statistically significant decreasing trend from March 2019 to April 2020 (MPC = −0.9, 95% CI = −1.6, −0.2) and July 2020 to December 2020 (MPC = −3.1, 95% CI = −6.0, −0.1), a non-significant increasing trend occurred from April 2020 to July 2020 (MPC = 8.6, 95%CI = −5.1, 24.2) and an overall increase occurred in naloxone dispensing for this age group when comparing median dispensing rates from March 2020–December 2020 to March 2019–December 2019 (2.12 vs. 1.78, p < 0.001). Persons aged 65 and older had a statistically significant decreasing trend from March 2019 to April 2020 (MPC = −1.0, 95% CI = −1.9, −0.1) and from July 2020 to December 2020 (MPC = −4.1, 95% CI = −7.9, −0.01). In addition, although not statistically significant, naloxone dispensing sharply increased from April 2020 to July 2020 (MPC = 9.5, 95% CI = −8.7, 31.2). The median dispensing rates from March 2019-December 2019 and from March 2020 to December 2020 did not differ significantly. While neither females nor males had statistically significant changes in monthly trends (Fig. 4b), both females (2.91 vs. 2.64, p = 0.04) and males (2.27 vs. 2.00, p = 0.01) had higher median naloxone dispensing in March 2020–December 2020 compared with March 2019– December 2019. ( Table 1).
Fig. 4.
a. Monthly naloxone dispensing rates per 100,000 persons from March 2019 to December 2020 by years of age group. The monthly percent change (MPC) is calculated by Joinpoint. A change in trend (inflection point) is denoted by X. New Jersey is not included in naloxone dispensing rates due to unusually high dispensing rates because of a new policy in May 2020 co-prescribing naloxone with opioids prescribed for managing chronic pain. We obtained dispensing data for naloxone from IQVIA New to Brand. We used quarterly population data from the United States Census Bureau to estimate monthly populations and to calculate monthly dispensing rates. b. Monthly naloxone dispensing rates per 100,000 persons from March 2019 to December 2020 by sex. The monthly percent change (MPC) is calculated by Joinpoint. A change in trend (inflection point) is denoted by X. New Jersey is not included in naloxone dispensing rates due to unusually high dispensing rates because of a new policy in May 2020 co-prescribing naloxone with opioids prescribed for managing chronic pain. We obtained dispensing data for naloxone from IQVIA New to Brand. We used quarterly population data from the United States Census Bureau to estimate monthly populations and to calculate monthly dispensing rates.
Table 1.
Comparison of median dispensing rates per 100k persons of buprenorphine, naloxone, extended-release (ER) naltrexone for March 2019–December 2019 and March-December 2020 using Wilcoxon Rank Sum test.
|
Buprenorphine |
ER Naltrexone |
Naloxonea |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| March 2019– December 2019 | March 2020– December 2020 | p-value | March 2019– December 2019 | March 2020– December 2020 | p-value | March 2019– December 2019 | March 2020– December 2020 | p-value | |
| Total | 41.12 | 41.79 | 0.19 | 0.61 | 0.54 | 0.001 | 2.35 | 2.60 | 0.04 |
| Age Group | |||||||||
| 0–19 | 0.51 | 0.54 | 0.85 | 0.015 | 0.011 | 0.03 | 0.11 | 0.13 | 0.13 |
| 20–39 | 80.39 | 77.7 | 0.14 | 1.21 | 1.03 | < 0.001 | 2.11 | 2.50 | 0.01 |
| 40–64 | 55.04 | 58.75 | < 0.001 | 0.81 | 0.78 | 0.21 | 1.78 | 2.12 | < 0.001 |
| 65 + | 10.79 | 12.40 | < 0.001 | 0.10 | 0.10 | 0.52 | 4.00 | 2.23 | 0.10 |
| Sex | |||||||||
| Female | 36.56 | 37.01 | 0.38 | 0.49 | 0.44 | 0.001 | 2.64 | 2.91 | 0.04 |
| Male | 45.73 | 46.6 | 0.14 | 0.71 | 0.65 | 0.002 | 2.00 | 2.27 | 0.01 |
We obtained dispensing data for buprenorphine, ER naltrexone, and naloxone from IQVIA New to Brand. We used quarterly population data from the United States Census Bureau to estimate monthly populations and to calculate monthly dispensing rates.
New Jersey is not included in naloxone dispensing rates due to unusually high dispensing rates because of a new policy in May 2020 co-prescribing naloxone with opioids prescribed for managing chronic pain.
4. Discussion
Based on pharmacy dispensing data, patients may have experienced changes in access to MOUD treatment and to naloxone during and after COVID-19 stay-at-home orders between March 2020 and December 2020. In some cases, those changes were positive: rates of monthly buprenorphine dispensing were significantly higher for persons aged 40 and above, and total median naloxone dispensing rates were significantly higher compared with the same timeframe in 2019. However, other findings from this analysis, coupled with reports of increased substance use and fatal overdoses (Centers for Disease Control and Prevention, 2020, Czeisler et al., 2020), show worrying signs that access to treatment for some patients may have been reduced during the pandemic. This is shown particularly for patients receiving ER naltrexone who had significantly lower median dispensing rates during March 2020–December 2020 compared with March 2019–December 2019. These findings have important implications for prescribers, policymakers, public health agencies, and community organizations as both the COVID-19 pandemic and the opioid overdose crisis continue to cause significant morbidity and mortality in the United States.
Although total buprenorphine dispensing did not differ between March-December 2020 and March-December 2019, significant increases occurred for those aged 40 and above. The significant increasing trends in both the Joinpoint analysis and the Wilcoxon Rank-Sum analysis lends support to an overall increase in buprenorphine dispensing for these age groups, despite office closures and other potential disruptions in access to treatment during the COVID-19 pandemic. Similar to our findings, a recent study of healthcare use of buprenorphine in Texas showed no significant changes during the early months of the pandemic (Thornton et al., 2020), while another study using data from the Texas Prescription Dispensing Monitoring Program showed a significant increase in outpatient buprenorphine prescriptions filled during the first 90 days after COVID-19 was declared a national emergency (Cance and Doyle, 2020). The expanded allowance for telehealth to initiate and maintain persons on buprenorphine treatment (Substance Abuse and Mental Health Services Administration, 2020b) may have facilitated access to buprenorphine during the pandemic (Jones et al., 2021a, Jones et al., 2021b). Still, the increase in substance use during the COVID-19 pandemic (Czeisler et al., 2020) and overdose reportedly at an all-time high (Centers for Disease Control and Prevention, 2020) should be noted; therefore, maintaining equivalent access in buprenorphine dispensing may not be adequate to accommodate rising numbers of new patients with OUD. Indeed, a study using IQVIA dispensing data showed that although buprenorphine prescriptions filled for existing patients remained relatively unchanged, prescribing for new patients was below expected values during March–May 2020 and continued to fall below 2019 numbers through September 2020 (Currie et al., 2021). Further, prior research indicates that even before the COVID-19 pandemic, 96% of states and the District of Columbia had inadequate buprenorphine treatment capacity compared to rates of OUD (Jones et al., 2015). These findings support both continuing telehealth exemptions for buprenorphine treatment beyond the pandemic and expanding access to this treatment for the growing number of patients who would benefit from it. Recent HHS policy updates eliminating the DATA waiver training requirements and expanding the types of providers who can prescribe buprenorphine (Department of Health and Human Services, 2021) are important steps to ensuring this treatment becomes more accessible.
The declines in ER naltrexone dispensing observed in this analysis, particularly for those aged 20–39, could indicate reduced access to this form of treatment during the COVID-19 pandemic. The pandemic may have impacted access to this medication more than other types of MOUD given it is provided as a once-a-month injection during an office visit. News and media sources have reported that office closures and diverted healthcare resources during the early months of the pandemic forced some providers to pivot from offering ER naltrexone once every 30 days to once every 45 days, possibly leaving patients vulnerable to return to opioid use and overdose (Hawryluk, 2020). The downward trend of ER naltrexone dispensing after most stay-at-home orders were lifted in May is a worrying indicator that barriers to this treatment continued even after doctor’s offices and outpatient clinics returned to more typical patient visit volumes. Additionally, because ER naltrexone is the only MOUD that can also treat co-morbid alcohol and OUD, patients diagnosed with both conditions may have greater vulnerability in accessing treatment during the pandemic. This is concerning given reports of increased alcohol consumption and return to use during the pandemic (Kim et al., 2020), in addition to increases in opioid overdoses (Centers for Disease Control and Prevention, 2020). While estimates of ER naltrexone use for each type of SUD are limited, one recent study of Veterans prescribed ER naltrexone showed 55% were being treated for OUD and 45% for alcohol use disorder, based on their primary drug of choice (Chang et al., 2018). Access to this medication for either type of SUD may have been negatively impacted by the COVID-19 pandemic.
Despite declining trends initially and in the latter months of 2020, overall naloxone dispensing rates increased during March–December 2020 compared to March–December 2019. Additionally, sharp increases in naloxone dispensing were observed in the early months of the pandemic (April–July 2020) among persons aged 40 years and older. The lack of statistical significance for these MPCs is likely due to the short timespan for the increase and subsequent lack of statistical power to determine significance (National Cancer Institute, 2021a, National Cancer Institute, 2021b). These trends provide positive signs that interruptions in services during the pandemic did not necessarily reduce access to naloxone, although others have noted declines in the number of persons filling naloxone prescriptions beginning in March 2020 (O’Donoghue et al., 2021). The fluctuations observed for persons aged 40 years and older in naloxone dispensing, particularly at the beginning of the pandemic, may reflect state-level policy changes and targeted initiatives by harm reduction and overdose prevention programs to ensure key populations had continued access to naloxone during periods of lockdown. Some of these focused efforts to reach populations at risk of overdose may have declined following lifting stay-at-home orders based on the trends observed in this analysis. However, naloxone dispensing remained elevated during the pandemic period overall compared to the same timeframe in 2019. Our analysis found increases in median naloxone dispensing rates for both sexes during March–December 2020 compared to March–December 2019. However, during both the pandemic and pre-pandemic, females had higher naloxone dispensing rates than males, despite the prevalence of substance use and overdose being higher among males (Hedegaard et al., 2020, Ho, 2020). Thus, additional strategies may be needed to ensure males who are at risk of opioid overdose have access to naloxone. Further research is needed to determine the reasons behind this gender disparity in naloxone dispensing.
In July 2020, the U.S. Food and Drug Administration announced a policy change requiring new labeling for prescription opioids recommending that healthcare professionals talk with their patients about naloxone availability and consider prescribing it alongside prescription opioid pain medications and MOUD (U.S. Food and Drug Administration). This may have contributed to the overall increase in naloxone dispensing. An earlier study showed that changes in state laws mandating naloxone co-prescription led to an increase in naloxone dispensing rates (Sohn et al., 2019). Therefore, state policy changes for naloxone distribution may have facilitated the increase in naloxone dispensing during the COVID-19 pandemic. For example, after Ohio changed its dispensing law in 2015 to allow pharmacists to dispense naloxone without a prescription, dispensing increased by 2238% among the Medicaid population (Gangal et al., 2020). A similar trend in our dataset was observed for New Jersey following the implementation of a policy in May 2020 mandating co-prescribing of naloxone with prescription opioids for patients with chronic pain (New Jersey Office of the Attorney General, 2020). Because this change was so sizeable, New Jersey was excluded from the naloxone analysis in this study to not unduly influence national patterns. Further analysis is needed to examine changes in naloxone dispensing policies at the state-level during the COVID-19 pandemic.
Despite the observed increases in buprenorphine and naloxone dispensing during the COVID-19 pandemic, concerns remain about their overall adequacy to reach the patients in need of these prescriptions. For example, a study of Texas pharmacies conducted in the spring of 2020 showed that only 34.1% had both buprenorphine/naloxone films and naloxone nasal spray available to be dispensed in a timely manner (Hill et al., 2020). Similarly, a 2017 study of community pharmacists in West Virginia showed that half stocked buprenorphine, 74.8% stocked buprenorphine/naloxone, and only 20.4% sold naloxone over the counter (Thornton et al., 2017). Naloxone prescription fills are also limited for patients receiving Medicaid in 10 states, which cover 20% of the Medicaid population nationwide (Roberts et al., 2020). Additionally, a study showed that 1 in 5 DATA-waivered physicians in Ohio were not active MOUD prescribers, and only half of active prescribers accepted health insurance (Parran et al., 2017). Furthermore, recent reports indicate a current shortage of injectable naloxone, widely used by SSPs due to its affordability, raising concerns about the adequacy of current naloxone prescribing rates to address the increase in fatal overdose that has occurred during the COVID-19 pandemic (Centers for Disease Control and Prevention, 2020, Godvin, 2021).
Providers, policymakers, and public health departments can use numerous strategies to ensure access to and uptake of MOUD and naloxone during the COVID-19 pandemic and beyond. Clinics that prescribe MOUD can increase appointment hours, stagger appointment scheduling, and continue or expand use of telehealth for buprenorphine prescribing. New guidance from the U.S. Department of Health and Human Services has removed the DATA waiver training requirements for prescribing buprenorphine and expanded the list of approved prescribers to include other medical practitioners beyond physicians (Department of Health and Human Services, 2021). Providers who have not completed the DATA waiver can take advantage of this policy change, in combination with the telehealth prescribing exemptions, to increase availability and accessibility of this medication. Evidence-based treatment delivery via telehealth, especially videoconferencing, is as effective as in-person treatment and in some cases, even more effective for treatment retention (Oesterle et al., 2020). Continuing telehealth prescribing allowances beyond the pandemic will be critical for widespread adoption of MOUD. Health insurance coverage that includes the use of buprenorphine and ER naltrexone is also necessary for ensuring that cost is not a barrier to obtaining treatment (Andraka-Christou and Capone, 2018). Greater integration of MOUD, particularly buprenorphine, into harm reduction organizations and other community-based organizations that serve people who use drugs is another strategy that may increase the accessibility of treatment during and after the COVID-19 pandemic (Collins et al., 2020). Additionally, providers should screen patients for SUD so those who are currently prescribed opioids for pain management, or otherwise at risk of opioid misuse and overdose, have access to naloxone. Implementing standing orders are important for leveraging pharmacies for naloxone access. Over time, this will increase the availability of naloxone for distribution in community pharmacies (Eldridge et al., 2020). Finally, enhanced implementation of community-based, take-home naloxone programs can make medication more accessible (Collins et al., 2020, Courser and Raffle, 2021) and address some of the disparities observed in this analysis, such as lower rates of prescription dispensing among males.
This analysis is subject to at least four limitations. First, methadone was not evaluated because it is only administered through OTPs and is not captured in pharmacy-based dispensing data. Methadone is the leading MOUD prescribed for patients with OUD at substance use treatment facilities (Alderks, 2017), although buprenorphine prescribing has been increasing at a faster rate in recent years. Since emergency exemptions also created greater flexibilities for methadone prescribing (Substance Abuse and Mental Health Services Administration, 2020a), it is important to also consider our findings alongside the potential impact of methadone availability on overall access to MOUD during the COVID-19 pandemic. Second, data on race and ethnicity were not captured in this analysis. Longstanding racial and ethnic disparities exist in access to MOUD (Nguemeni Tiako, 2020); although telehealth and longer take-home dose exemptions during the pandemic can reduce these disparities (Nguemeni Tiako, 2020), we could not capture racial and ethnic differences in rates of medication dispensing. Third, for buprenorphine, we were unable to assess prescriptions written by physicians and not filled by the patient, or whether prescriptions were used by the intended recipient. Likewise, many states have laws allowing persons to obtain naloxone from a standing order (Davis and Carr, 2017) and bystanders who may intervene by administering naloxone when an overdose occurs. Fourth, we did not have data on prescription dosage, duration, or patient diagnoses. Thus, we were unable to discern whether patients receiving ER naltrexone were being treated for OUD or alcohol use disorder, and if changes in prescription characteristics (i.e. days’ supply) impacted dispensing rates.
5. Conclusions
Persons prescribed ER naltrexone may have experienced gaps in treatment during the COVID-19 pandemic. Although buprenorphine and naloxone dispensing rates increased during March-December 2020 among persons aged 40 and above, rising substance use and overdose rates during the pandemic raise concerns that these increases may not be sufficient. In a worsening overdose epidemic, expanding access to buprenorphine, ER naltrexone, and naloxone are critical both during and after the pandemic (Davis and Samuels, 2020, López-Pelayo et al., 2020). Providers, policymakers, and public health officials can ensure continued and increased access to naloxone and MOUD through use of telehealth, partnerships with community-based organizations, standing orders for pharmacy-based naloxone dispensing, and take-home naloxone programs. Policies and prescribing practices may benefit from paying special attention to groups that may be underserved, such as males for naloxone access.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Role of funding source
Nothing declared.
Authorship contributions
All authors contributed to the conceptualization and writing of this manuscript. All authors were involved in the review and edition of this manuscript. Guy additional assisted with acquiring the data used for analysis.
Conflict of interest
No conflicts declared.
References
- Ahmad, F., Rossen, LM, Sutton, P., 2021. Provisional drug overdose death counts. 〈https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm〉. (Accessed October 15, 2021).
- Alderks, C.E., 2017. Trends in the Use of Methadone, Buprenorphine, and Extended-Release Naltrexone at Substance Abuse Treatment Facilities: 2003–2015. 〈https://www.samhsa.gov/data/sites/default/files/report_3192/ShortReport-3192.html〉. (Accessed October 15, 2021). [PubMed]
- Andraka-Christou B., Capone M.J. A qualitative study comparing physician-reported barriers to treating addiction using buprenorphine and extended-release naltrexone in U.S. office-based practices. Int. J. Drug Policy. 2018;54:9–17. doi: 10.1016/j.drugpo.2017.11.021. [DOI] [PubMed] [Google Scholar]
- Beetham T., Saloner B., Wakeman S.E., Gaye M., Barnett M.L. Access to office-based buprenorphine treatment in areas with high rates of opioid-related mortality: an audit study. Ann. Intern. Med. 2019;171(1):1–9. doi: 10.7326/M18-3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cance J.D., Doyle E. Changes in outpatient buprenorphine dispensing during the COVID-19 pandemic. Jama. 2020;324(23):2442–2444. doi: 10.1001/jama.2020.22154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2020. Increase in fatal drug overdoses across the United States driven by synthetic opioids before and during the COVID-19 pandemic. 〈https://emergency.cdc.gov/han/2020/han00438.asp〉.
- Chang G., Crawford M., Pitts M., Schein A.Z., Goodwin K., Enggasser J.L. Adherence to extended release naltrexone: Patient and treatment characteristics. Am. J. Addict. 2018;27(6):524–530. doi: 10.1111/ajad.12786. [DOI] [PubMed] [Google Scholar]
- Collins A.B., Ndoye C.D., Arene-Morley D., Marshall B.D.L. Addressing co-occurring public health emergencies: The importance of naloxone distribution in the era of COVID-19. Int. J. Drug Policy. 2020;83 doi: 10.1016/j.drugpo.2020.102872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courser M.W., Raffle H. With crisis comes opportunity: unanticipated benefits resulting from pivots to take-home naloxone (THN) programs during the COVID-19 pandemic. J. Subst. Abus. Treat. 2021;122 doi: 10.1016/j.jsat.2020.108220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie J.M., Schnell M.K., Schwandt H., Zhang J. Prescribing of opioid analgesics and buprenorphine for opioid use disorder during the COVID-19 pandemic. JAMA Netw. Open. 2021;4(4) doi: 10.1001/jamanetworkopen.2021.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeisler M.E., Lane R.I., Petrosky E., Wiley J.F., Christensen A., Njai R., Weaver M.D., Robbins R., Facer-Childs E.R., Barger L.K., Czeisler C.A., Howard M.E., Rajaratnam S.M.W. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic - United States, June 24-30, 2020. Mmwr. Morb. Mortal. Wkly. Rep. 2020;69(32):1049–1057. doi: 10.15585/mmwr.mm6932a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C., Carr D. State legal innovations to encourage naloxone dispensing. J. Am. Pharm. Assoc.: JAPhA. 2017;57(2s) doi: 10.1016/j.japh.2016.11.007. S180-s184. [DOI] [PubMed] [Google Scholar]
- Davis C.S., Samuels E.A. Continuing increased access to buprenorphine in the United States via telemedicine after COVID-19. Int. J. Drug Policy. 2020;93 doi: 10.1016/j.drugpo.2020.102905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L., Bucello C., Mathers B., Briegleb C., Ali H., Hickman M., McLaren J. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addict. (Abingdon, Engl. ) 2011;106(1):32–51. doi: 10.1111/j.1360-0443.2010.03140.x. [DOI] [PubMed] [Google Scholar]
- Department of Health and Human Services, 2021. HHS Releases New Buprenorphine Practice Guidelines, Expanding Access to Treatment for Opioid Use Disorder, EIN Presswire.
- Eldridge L.A., Agley J., Meyerson B.E. Naloxone availability and dispensing in Indiana pharmacies 2 years after the implementation of a statewide standing order. J. Am. Pharm. Assoc.: JAPhA. 2020;60(3):470–474. doi: 10.1016/j.japh.2019.11.024. [DOI] [PubMed] [Google Scholar]
- Gangal N.S., Hincapie A.L., Jandarov R., Frede S.M., Boone J.M., MacKinnon N.J., Koechlin K., DeFiore-Hyrmer J., Holthusen A., Heaton P.C. Association between a state law allowing pharmacists to dispense naloxone without a prescription and naloxone dispensing rates. JAMA Netw. Open. 2020;3(1) doi: 10.1001/jamanetworkopen.2019.20310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick S.N., Prohaska S.M., LaKosky P.A., Juarez A.M., Corcorran M.A., Des Jarlais D.C. The impact of COVID-19 on syringe services programs in the United States. AIDS Behav. 2020;24(9):2466–2468. doi: 10.1007/s10461-020-02886-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godvin M. The US faces a naloxone shortage at the worst possible time. Filter. 2021 〈https://filtermag.org/us-naloxone-shortage/〉 [Google Scholar]
- Grimm, C., 2020. Geographic Disparities Affect Access to Buprenorphine Services for Opioid Use Disorder. 〈https://oig.hhs.gov/oei/reports/oei-12–17-00240.pdf〉.
- Guy G.P., Jr., Haegerich T.M., Evans M.E., Losby J.L., Young R., Jones C.M. Vital signs: pharmacy-based naloxone dispensing - United States, 2012-2018. Mmwr. Morb. Mortal. Wkly. Rep. 2019;68(31):679–686. doi: 10.15585/mmwr.mm6831e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawryluk M. Broomfield woman’s suspected overdose spotlights hidden deaths of the COVID-19 pandemic. Denver Post. 2020 〈https://www.denverpost.com/2020/06/28/covid-deaths-mental-health-suicide-addiction/〉 [Google Scholar]
- Hedegaard H., Miniño A.M., Warner M. Drug overdose deaths in the United States, 1999-2018. NCHS Data Brief. 2020;356:1–8. [PubMed] [Google Scholar]
- Hill, L.G., Loera, L.J., Evoy, K.E., Renfro, M.L., Torrez, S.B., Zagorski, C.M., Perez, J.C., Jones, S.M., Reveles, K.R., 2020. Availability of buprenorphine/naloxone films and naloxone nasal spray in community pharmacies in Texas, USA. Addiction (Abingdon, England). [DOI] [PubMed]
- Ho J.Y. Cycles of gender convergence and divergence in drug overdose mortality. Popul. Dev. Rev. 2020;46(3):443–470. doi: 10.1111/padr.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway, I.W., C Spaulding, A., Miyashita Ochoa, A., A Randall, L., R King, A., Team, T.H.S., Frew, P.M., 2020. COVID-19 vulnerability among people who use drugs: recommendations for global public health programmes and policies. 23(7), e25551. [DOI] [PMC free article] [PubMed]
- Jones C.M., Campopiano M., Baldwin G., McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am. J. Public Health. 2015;105(8):e55–e63. doi: 10.2105/AJPH.2015.302664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C.M., Diallo M.M., Vythilingam M., Schier J.G., Eisenstat M., Compton W.M. Characteristics and correlates of U.S. clinicians prescribing buprenorphine for opioid use disorder treatment using expanded authorities during the COVID-19 pandemic. Drug Alcohol Depend. 2021;225 doi: 10.1016/j.drugalcdep.2021.108783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C.M., Guy G.P., Board A. Comparing actual and forecasted numbers of unique patients dispensed select medications for opioid use disorder, opioid overdose reversal, and mental health, during the COVID-19 pandemic, United States, January 2019 to May 2020. Drug Alcohol Depend. 2021;219 doi: 10.1016/j.drugalcdep.2020.108486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser Family Foundation, 2020. When State Stay-at-Home Orders Due to Coronavirus Went into Effect. 〈https://www.kff.org/other/slide/when-state-stay-at-home-orders-due-to-coronavirus-went-into-effect/〉. (Accessed April 19, 2021).
- Kim H.J., Fay M.P., Feuer E.J., Midthune D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000;19(3):335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Kim J.U., Majid A., Judge R., Crook P., Nathwani R., Selvapatt N., Lovendoski J., Manousou P., Thursz M., Dhar A., Lewis H., Vergis N., Lemoine M. Effect of COVID-19 lockdown on alcohol consumption in patients with pre-existing alcohol use disorder. Lancet Gastroenterol. Hepatol. 2020;5(10):886–887. doi: 10.1016/S2468-1253(20)30251-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppla I.E., Gross M.S. Optimizing medication treatment of opioid use disorder during COVID-19 (SARS-CoV-2) J. Addict. Med. 2020;14(4):e1–e3. doi: 10.1097/ADM.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Pelayo H., Aubin H.-J., Drummond C., Dom G., Pascual F., Rehm J., Saitz R., Scafato E., Gual A. “The post-COVID era”: challenges in the treatment of substance use disorder (SUD) after the pandemic. BMC Med. 2020;18(1):241. doi: 10.1186/s12916-020-01693-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin N.K., Hickman M., Hutchinson S.J., Goldberg D.J., Vickerman P. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clin. Infect. Dis. 2013;57(Suppl 2) doi: 10.1093/cid/cit296. (Suppl 2), S39-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay D., Asmundson G.J.G. COVID-19 stress and substance use: current issues and future preparations. J. Anxiety Disord. 2020;74 doi: 10.1016/j.janxdis.2020.102274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute, Annual Percent Change (APC) and Confidence Interval. 〈https://surveillance.cancer.gov/help/joinpoint/setting-parameters/method-and-parameters-tab/apc-aapc-tau-confidence-intervals/estimate-average-percent-change-apc-and-confidence-interval〉. (Accessed June 21, 2021a).
- National Cancer Institute, Average Annual Percent Change (AAPC) and Confidence Interval. 〈https://surveillance.cancer.gov/help/joinpoint/setting-parameters/method-and-parameters-tab/apc-aapc-tau-confidence-intervals/average-annual-percent-change-aapc〉. (Accessed June 21, 2021b).
- New Jersey Office of the Attorney General, 2020. AG Grewal Announces Action to Reduce Risk of Fatal Overdoses During COVID-19 Crisis.
- Nguemeni Tiako M.J. Addressing racial & socioeconomic disparities in access to medications for opioid use disorder amid COVID-19. J. Subst. Abus. Treat. 2020;122 doi: 10.1016/j.jsat.2020.108214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterle T.S., Kolla B., Risma C.J., Breitinger S.A., Rakocevic D.B., Loukianova L.L., Hall-Flavin D.K., Gentry M.T., Rummans T.A., Chauhan M., Gold M.S. Substance use disorders and telehealth in the COVID-19 pandemic era: a new outlook. Mayo Clin. Proc. 2020;95(12):2709–2718. doi: 10.1016/j.mayocp.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrach B., Buer L.-M., Armbruster S., Brown H., Yochym G., Zaller N. COVID-19 and rural harm reduction challenges in the US Southern Mountains. J. Rural Health: Off. J. Am. Rural Health Assoc. Natl. Rural Health Care Assoc. 2021;37(1):252–255. doi: 10.1111/jrh.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donoghue A.L., Biswas N., Dechen T., Anderson T.S., Talmor N., Punnamaraju A., Stevens J.P. Trends in filled naloxone prescriptions before and during the COVID-19 pandemic in the United States. JAMA Health Forum. 2021;2(5) doi: 10.1001/jamahealthforum.2021.0393. e210393-e210393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parran T.V., Muller J.Z., Chernyak E., Adelman C., Delos Reyes C.M., Rowland D., Kolganov M. Access to and payment for office-based buprenorphine treatment in Ohio. Subst. Abus.: Res. Treat. 2017;11 doi: 10.1177/1178221817699247. 1178221817699247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J., Kilian C., Ferreira-Borges C., Jernigan D., Monteiro M., Parry C.D.H., Sanchez Z.M., Manthey J. Alcohol Use COVID 19: Implic. Monit. Policy. 2020;39(4):301–304. doi: 10.1111/dar.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A.W., Look K.A., Trull G., Carpenter D.M. Medicaid prescription limits and their implications for naloxone accessibility. Drug Alcohol Depend. 2020;218 doi: 10.1016/j.drugalcdep.2020.108355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn M., Talbert J.C., Huang Z., Lofwall M.R., Freeman P.R. Association of naloxone coprescription laws with naloxone prescription dispensing in the United States. JAMA Netw. Open. 2019;2(6) doi: 10.1001/jamanetworkopen.2019.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordo L., Barrio G., Bravo M.J., Indave B.I., Degenhardt L., Wiessing L., Ferri M., Pastor-Barriuso R. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. Bmj. 2017;357:j1550. doi: 10.1136/bmj.j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2020a. FAQs: Provision of methadone and buprenorphine for the treatment of Opioid Use Disorder in the COVID-19 emergency. 〈https://www.samhsa.gov/sites/default/files/faqs-for-oud-prescribing-and-dispensing.pdf〉. (Accessed April 19, 2021).
- Substance Abuse and Mental Health Services Administration, 2020b. Medication-Assisted Treatment: Methadone. 〈https://www.samhsa.gov/medication-assisted-treatment/medications-counseling-related-conditions/methadone〉. (Accessed April 19, 2021).
- Substance Abuse and Mental Health Services Administration, 2020c. Medication-Assisted Treatment: Naltrexone. 〈https://www.samhsa.gov/medication-assisted-treatment/medications-counseling-related-conditions/naltrexone〉. (Accessed April 19, 2021).
- Substance Abuse and Mental Health Services Administration, 2021. Medication-Assisted Treatment: Buprenorphine. 〈https://www.samhsa.gov/medication-assisted-treatment/medications-counseling-related-conditions/buprenorphine〉. (Accessed April 19, 2021).
- Thornton J.D., Lyvers E., Scott V.G.G., Dwibedi N. Pharmacists’ readiness to provide naloxone in community pharmacies in West Virginia. J. Am. Pharm. Assoc.: JAPhA. 2017;57(2s):S12–S18. doi: 10.1016/j.japh.2016.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton J.D., Varisco T.J., Bapat S.S., Downs C.G., Shen C. Impact of COVID-19 related policy changes on buprenorphine dispensing in Texas. J. Addict. Med. 2020;14(6):e372–e374. doi: 10.1097/ADM.0000000000000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration, 2020 FDA Requiring Labeling Changes for Opioid Pain Medicines, Opioid Use Disorder Medicines Regarding Naloxone. 〈https://www.fda.gov/news-events/press-announcements/fda-requiring-labeling-changes-opioid-pain-medicines-opioid-use-disorder-medicines-regarding〉.