Abstract
The serodiagnosis of human immunodeficiency virus type 1 (HIV-1) infection primarily relies on the detection of antibodies, most of which are directed against the immunodominant regions (IDR) of HIV-1 structural proteins. Among these, the N-terminal region of gp41 contains cluster I (amino acids [aa] 580 to 623), comprising the cytotoxic T-lymphocyte epitope (AVERYLKDQQLL) and the cysteine loop (CSGKLIC), and cluster II (aa 646 to 682), comprising an ectodomain region (ELDKWA). To delineate the epitope diversity within clusters I and II and to determine whether the diversity affects serologic detection by U.S. Food and Drug Administration (FDA)-licensed enzyme immunoassay (EIA) kits, gp41 Env sequences from 247 seropositive persons infected with HIV-1 group M, subtypes A (n = 42), B (n = 62), B′ (n = 13), C (n = 38), D (n = 41), E (n = 18), F (n = 27), and G (n = 6), and 6 HIV-1-infected but persistently seronegative (HIPS) persons were analyzed. While all IDR were highly conserved among both seropositive and HIPS persons, minor amino acid substitutions (<20% for any one residue, mostly conservative) were observed for all subtypes, except for B′, in comparison with the consensus sequence for each subtype. Most importantly, none of the observed substitutions among the group M plasma specimens affected antibody detection, since all specimens (n = 152) tested positive with all five FDA-licensed EIA kits. Furthermore, all specimens reacted with a group M consensus gp41 peptide (WGIKQLQARVLAVERYLKDQQLLGIWGCSGKLICTTAVPWNASW), and high degrees of cross-reactivity (>80%) were observed with an HIV-1 group N peptide, an HIV-1 group O peptide, and a peptide derived from the homologous region of gp41 from simian immunodeficiency virus from chimpanzee (SIVcpz). Taken together, these data indicate that the minor substitutions observed within the IDR of gp41 of HIV-1 group M subtypes do not affect antibody recognition and that all HIV-1-seropositive specimens containing the observed substitutions react with the FDA-licensed EIA kits regardless of viral genotype and geographic origin.
Human immunodeficiency virus type 1 (HIV-1) is the etiologic agent responsible for the pandemic of AIDS (9, 14). Worldwide, it is estimated that more than 30 million persons are infected with HIV-1 and that 16,000 new cases of HIV-1 infection occur every day. HIV-1 is characterized by an unusually high degree of genetic variability in vivo (14). Analysis of HIV-1 env genes of virus strains from different geographic regions has revealed that HIV-1 can be divided into three main groups: M (major), O (outlier), and N (new) (9, 14, 24). HIV-1 group M has been further subdivided into genetically equidistant clusters of HIV-1 env genes, comprising subtypes A to J (14). Except during the initial acute phase of infection, referred to as the “window period,” which occurs before a persistent antibody response has been established (2, 3), most infected persons produce HIV-1-specific antibodies that can be detected by standard diagnostic tests (2). In addition, several reported patients exhibit a history of HIV-1 seronegativity despite demonstrating clinical AIDS (1, 5, 25). Loss of HIV-1 antibody production concomitant with HIV-1 disease progression has occurred in a small percentage of infected individuals (1).
Since most serologic assays rely on antibody responses to the structural proteins of HIV-1, genetic variability within the envelope protein, particularly gp41, can have an impact on serologic detection (8, 18). Encoded by the env genes of HIV-1 are two heavily glycosylated proteins, the outer membrane gp120 and the carboxyl-terminal transmembrane gp41 (10, 14). gp41 has many functional domains, including the immunodominant region (IDR) in the amino-terminal portion (10). The IDR of gp41 contains cluster I (amino acids [aa] 580 to 623), comprising both the CTL epitope (aa 591 to 602; AVERYLKDQQLL) and the cysteine loop (aa 607 to 613; CSGKLIC), and cluster II (aa 646 to 682), comprising an ectodomain region (aa 671 to 676; ELDKWA). The CTL epitope, cysteine loop, and ectodomain are considered part of the IDR since >99% of HIV-1-infected individuals produce antibodies directed against them (8, 10, 16, 18).
The envelope protein of HIV-1 has an unusually high degree of sequence variability among all subtypes of HIV-1 group M viruses, as well as among group O and group N viruses (14). Since most serologic assays are based on the immunogenicity of gp41, specific mutations in the IDRs of gp41 can potentially alter antibody binding in serologic assays. In this study, we analyzed gp41 sequences from 247 seropositive HIV-1 group M-infected individuals, representing subtypes A to G, and 6 seronegative persons with AIDS to delineate the epitope diversity. In addition, plasma from individuals infected with HIV-1 strains exhibiting amino acid substitutions within the IDR of gp41 were tested with U.S. Food and Drug Administration (FDA)-licensed enzyme immunoassay (EIA) kits as well as a gp41 group M consensus peptide-based EIA to determine whether the observed substitution(s) had an impact on serologic detection.
MATERIALS AND METHODS
Study subjects.
Samples tested in the present study are part of various ongoing studies throughout the world and were selected based on their HIV-1-positive results with various EIA kits. Plasma specimens from 247 HIV-1 group M-infected individuals were selected from Argentina (20 subtype B and 27 subtype F), Cameroon (11 subtype A), Canada (1 subtype B), China (5 subtype B and 1 subtype B′), Egypt (1 subtype B), Ghana (6 subtype A and 5 subtype G), India (3 subtype C), Ivory Coast (7 subtype A, 1 subtype D, and 1 subtype G), Mexico (8 subtype B), Mozambique (5 subtype C), South Africa (1 subtype B and 4 subtype C), Thailand (12 subtype B′ and 18 subtype E), Uganda (17 subtype A, 14 subtype C, and 40 subtype D), the United States (1 subtype A and 26 subtype B), and Zimbabwe (12 subtype C). In addition, six specimens from HIV-1-infected but persistently seronegative (HIPS) persons from the United States (25) were also included. All specimens were subtyped by phylogenetic analysis of the Env region (19, 27).
PCR amplification and sequence analysis.
We recently developed an assay based on a conserved sequence within the gp41 region which is highly sensitive for amplification of viral RNA from plasma of HIV-1-positive specimens representing different subtypes of HIV-1 group M (19, 27). Following amplification, DNA from the nested PCR was cycle sequenced (60 ng of DNA per sequencing reaction) with an ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction Kit according to the manufacturer's protocol (Perkin-Elmer, Foster City, Calif.) and with the nested primers gp46F2 and gp47R2 (27). Sequencing reactions were run in an automated DNA sequencer (model 373; Applied Biosystems, Foster City, Calif.). Sequences were then translated and aligned using DNASIS v.2.1 (Hitachi Software, San Bruno, Calif.). The consensus sequence for each subtype was obtained from the 1997 HIV-1 Molecular Immunology Database (Los Alamos National Laboratory, Los Alamos, N.Mex.).
HIV-1 antibody detection.
Plasma samples were tested using the following FDA-licensed EIA kits: Abbott Laboratories (Chicago, Ill.) HIVAB HIV-1 EIA and HIVAB HIV-1/HIV-2 (recombinant DNA EIA), Genetic Systems (Redmond, Wash.) LAV EIA and HIV-1/HIV-2 Peptide EIA, and Organon Teknika (Durham, N.C.) Vironostika HIV Microelisa System. All assays were performed according to the manufacturers' protocols.
The synthetic peptides derived from the gp41 region representing the consensus sequence for HIV-1 group M as well as the homologous regions for HIV-1 group O and group N and SIVcpzGAB were synthesized by 9-fluorenylmethoxycarbonyl chemistry. The 44-mer peptides representing consensus group M (aa 580 to 623; (WGIKQLQARVLAVERYLKDQQLLGIWGCSGKLICTTAVPWNASW), consensus group O (WGIRQLRARLLALETLIQNQQLLNLWGCKGKLVCYTSVKWNRTW), group N (WGIKQLQAKVLAIERYLRDQQILGSLGCSGKTICYTTVPWNETW), and SIVcpzGAB (WGVKQLQARLLAVERYLQDQQILGLWGCSGKAVCYTTVPWNNSW) were used to develop a peptide-based assay in a manner similar to that described previously (23). Briefly, polyvinyl plates (Immulon II; Dynatech Laboratories, Inc., Alexandria, Va.) were coated with 5 μg of synthetic peptide per ml (100 μl/well) in 0.01 M carbonate buffer (pH 9.6) and incubated overnight at 4°C. The plates were washed six times with phosphate-buffered saline containing 0.05% Tween 20; excess reactive sites were blocked by the addition of 5% bovine serum albumin in phosphate-buffered saline–0.05% Tween 20. This step was followed by the addition of a 1:100 dilution of each test serum. The plates were incubated overnight at 4°C. After six more washes, Fc-specific, alkaline phosphatase-conjugated goat antibody to human immunoglobulin G (Sigma, St. Louis, Mo.) was added, and the plates were left at room temperature for 2 h. This step was followed by the addition of 1 mg of p-nitrophenyl phosphate substrate (Sigma) per ml (50 μl/well). The plates were read after 1 h with an EIA reader (SLT Lab Instruments, Ronkonkome, N.Y.) at 405 nm. The cutoff values were calculated by adding 0.1 to the mean optical densities plus 3 standard deviations of normal control sera in respective peptide-based assays.
RESULTS
Sequence analysis of gp41 cluster I and cluster II.
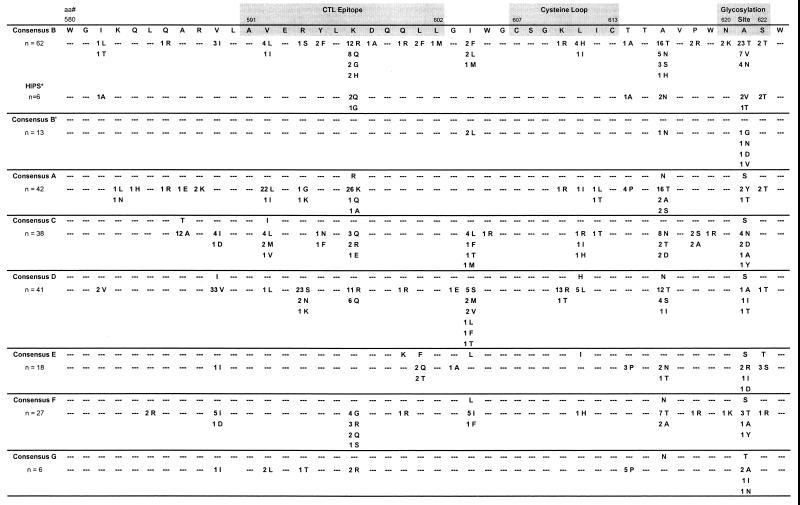
To determine the sequence variability within the immunodominant regions of the gp41 protein, cluster I (aa 580 to 623) and cluster II (aa 646 to 682) sequences derived from the 247 HIV-1 group M-infected individuals and representing subtypes A (n = 42), B (n = 62), B′ (n = 13), C (n = 38), D (n = 41), E (n = 18), F (n = 27), and G (n = 6) were aligned (Fig. 1 and 2). Within cluster I, there were minor amino acid substitutions (<20% for any one residue, mostly conservative) at positions 587, 589, 592, 594, 597, 604, 610, 614, 616, and 621 compared to the consensus sequence for each subtype. Analysis of the CTL epitope and the cysteine loop revealed minor amino acid substitutions in all subtypes except B′. For the CTL epitope, these included V592→ L or I (55%) and R597→K, Q, or A (67%) in subtype A, K587→R, Q, G, or H (39%) in subtype B, R594→S, N, or K (64%) and K597→R or Q (41%) in subtype D, F601→Q or T (22%) in subtype E, K597→G, R, Q, and S (37%) in subtype F, and V592→L (33%) and K597→R (33%) in subtype G. For the cysteine loop, a K610→R or T (34%) substitution in subtype D was observed. Furthermore, a number of samples exhibited amino acid substitutions in both the CTL epitope and the cysteine loop (Fig. 1). While the critical positions of the glycosylation site [N-X-(S,T)] of cluster I were highly conserved (>95%) in all subtypes, significant amino acid substitutions were observed at noncritical position 621 (X) for subtypes B (55%), B′ (31%), C (21%), E (22%), and G (67%) (Fig. 1). Minor amino acid changes (<5%) were observed at critical position N620, S622, or T622.
FIG. 1.
Analysis of sequence divergence in gp41 cluster I (aa 580 to 623) from 247 HIV-1 group M subtype A to G specimens. The CTL epitope (aa 591 to 602), the cysteine loop (aa 607 to 613), and the glycosylation site (aa 620 to 622) are shaded. The left column shows the number of specimens for each subtype examined. The numbers of specimens with amino acid substitutions are shown for each subtype; dashes represent conserved amino acids in all specimens. ∗, specimens from six HIPS AIDS patients infected with subtype B virus.
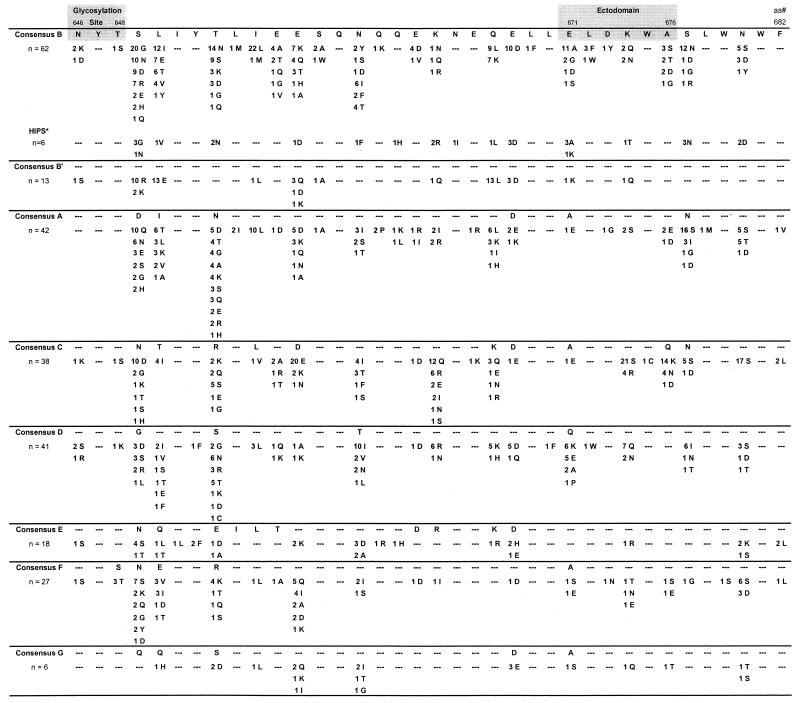
FIG. 2.
Analysis of sequence divergence in gp41 cluster II (aa 646 to 682) from 247 HIV-1 group M subtype A to G specimens. The glycosylation site (aa 646 to 648) and the ectodomain (aa 671 to 676) are shaded. The left column shows the number of specimens for each subtype examined. The numbers of specimens with amino acid substitutions are shown for each subtype; dashes represent conserved amino acids in all specimens. ∗, specimens from six HIPS AIDS patients infected with subtype B virus.
A similar analysis of gp41 cluster II sequences (aa 646 to 682) from group M-infected individuals (subtypes A to G) also revealed minor substitutions (Fig. 2). Overall, minor substitutions occurred at positions 649, 650, 653, 655, 656, 657, 660, 664, 667, 668, 671, 674, 676, 677, and 680. Analysis of the ectodomain revealed minor substitutions, including E671→A, G, D, or S (24%) in subtype B, K674→S or R (66%) and Q676→K, N, or D (50%) in subtype C, and Q671→K, E, A, or P (34%) and K674→Q or N (22%) in subtype D. Furthermore, a number of samples exhibited multiple amino acid substitutions within the ectodomain (Fig. 2). Unlike the glycosylation site within cluster I, the glycosylation site within cluster II did not exhibit significant amino acid substitutions.
We and others have recently described several seronegative AIDS patients who are HIV-1 infected (1, 5, 25). Analysis of gp41 sequences from six of these HIPS persons (all infected with subtype B virus) revealed minor amino acid substitution in both cluster I (Fig. 1) and cluster II (Fig. 2). Specifically, the cysteine loop region within cluster I was conserved; however, K597→Q or G in the CTL epitope and E671→A or K in the ectodomain were observed in half of the specimens.
Effect of amino acid substitutions on serologic detection.
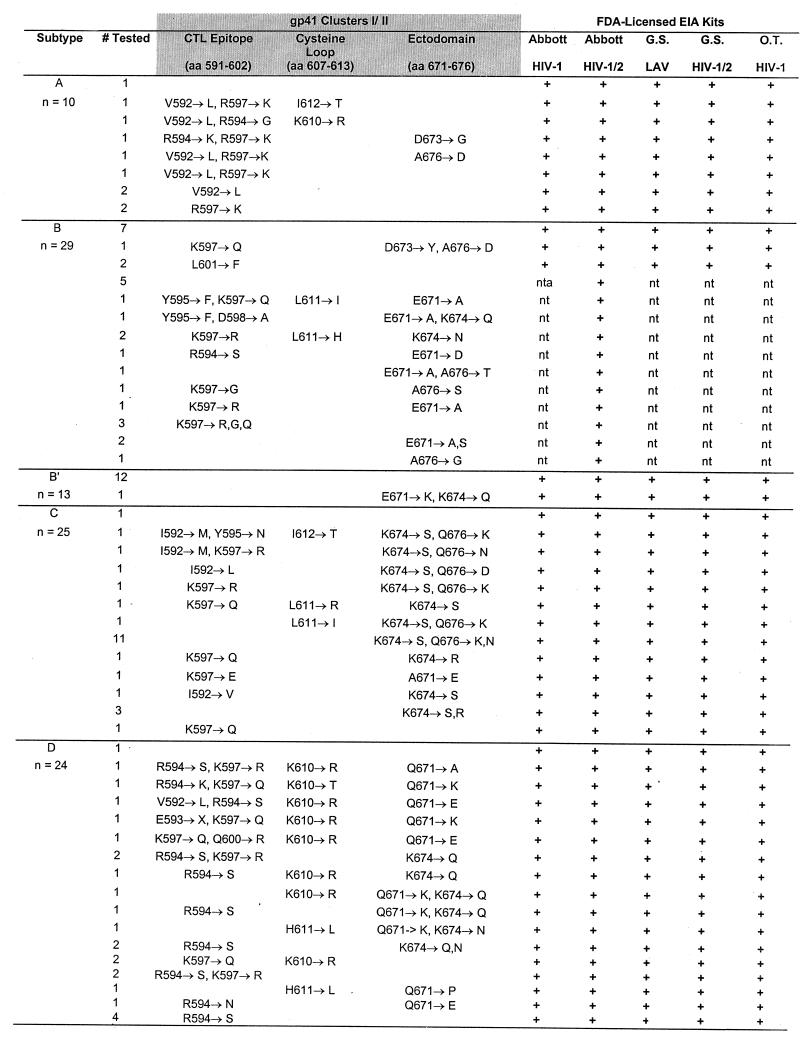
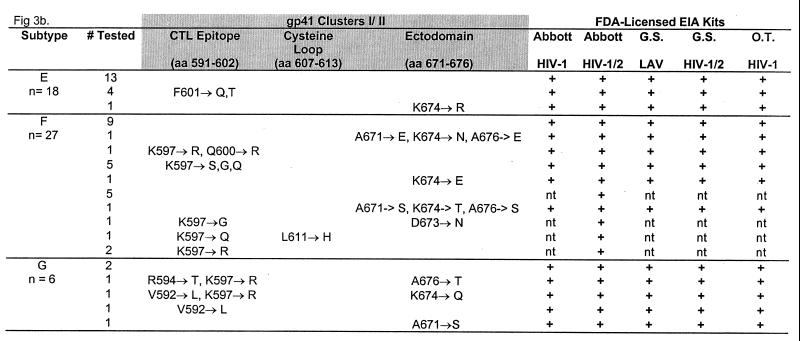
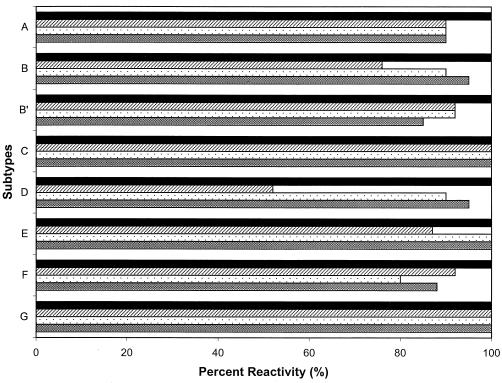
To determine the effect of the amino acid substitutions on serologic detection, a subset of 152 samples (10 subtype A, 29 subtype B, 13 subtype B′, 25 subtype C, 24 subtype D, 18 subtype E, 27 subtype F, and 6 subtype G) representing the cluster I and cluster II sequences with or without amino acid substitutions were tested using the five FDA-licensed EIA kits. All specimens, regardless of single or multiple substitutions in either cluster, were found serologically positive when tested with these kits (Fig. 3). Therefore, the minor amino acid substitutions within clusters I and II had no impact on antibody detection in serum from HIV-1-positive individuals.
FIG. 3.
Reactivity of HIV-1 group M plasma specimens with five FDA-licensed EIA kits (last five columns, from left to right: Abbott HIVAB HIV-1 EIA, Abbott HIVAB HIV-1/HIV-2, Genetic Systems LAV EIA, Genetic Systems HIV-1/HIV-2 Peptide EIA, and Organon Teknika Vironostika HIV Microelisa System). Representative specimens (n = 152) with and without amino acid substitutions in the cluster I and cluster II regions were tested with the EIA kits. Specific mutations within the CTL epitope, the cysteine loop, and the ectodomain are shown for each subtype. nt, not tested.
Since most commercial assays are based on multiple antigens, such as viral lysates, synthetic peptides, and/or recombinant proteins, we determined whether any of the amino acid substitutions would abrogate direct binding to a gp41 consensus peptide. A peptide-based assay containing a 44-mer consensus sequence for HIV-1 group M, subtypes A to H, was used to detect antibodies in specimens with and without mutations. Of the 131 samples (10 subtype A, 21 subtype B, 13 subtype B′, 20 subtype C, 21 subtype D, 15 subtype E, 25 subtype F, and 6 subtype G) tested with the gp41 group M peptide-based EIA, all were reactive with the gp41 consensus peptide, regardless of amino acid substitution or HIV-1 subtype (Fig. 4).
FIG. 4.
Detection of gp41-specific antibodies using synthetic peptides comprising cluster I sequences (aa 580 to 623). The percent reactivities of HIV-1 group M plasma specimens are shown for consensus group M peptide (■) and group O peptide (▨) or homologous regions of group N peptide (░⃞) and SIVcpz peptide (▩). The 44-mer peptides representing consensus sequences include group M (WGIKQLQARVLAVERYLKDQQLLGIWGCSGKLICTTAVPWNASW), group O (WGIRQLRARLLALETLIQNQQLLNLWGCKGKLVCYTSVKWNRTW), group N (WGIKQLQAKVLAIERYLRDQQILGSLGCSGKTICYTTVPWNETW), and SIVcpz (WGVKQLQARLLAVERYLQDQQILGLWGCSGKAVCYTTVPWNNSW). All 131 samples, regardless of substitution in gp41 regions, reacted with the gp41 group M peptide-based EIA, and a high degree of cross-reactivity to HIV-1 group O and N peptides and in SIVcpz peptide was observed.
None of the HIPS specimens had detectable antibody in any of the FDA-licensed EIA kits or gp41 peptide-based assay (data not shown).
Cross-reactive gp41 antibodies for HIV-1 groups M, N, and O.
Recent identification of a new group of HIV-1, group N, as well as research indicating the origin of HIV-1 in Pan troglodytes troglodytes (6, 24), prompted us to examine the cross-reactivity of group M sera with the homologous regions of group O, group N, and SIVcpz gp41 peptides. Of the 131 HIV-1 group M specimens, 120 (91.6%; 9 subtype A, 19 subtype B, 12 subtype B′, 20 subtype C, 19 subtype D, 15 subtype E, 20 subtype F, and 6 subtype G) reacted with the group N peptide and 123 (93.9%; 9 subtype A, 20 subtype B, 11 subtype B′, 20 subtype C, 20 subtype D, 15 subtype E, 22 subtype F, and 6 subtype G) reacted with the SIVcpz peptide, suggesting a high degree of cross-reactivity (Fig. 4). Further, 110 of the 131 group M specimens (84.0%; 9 subtype A, 16 subtype B, 12 subtype B′, 20 subtype C, 11 subtype D, 13 subtype E, 23 subtype F, and 6 subtype G) also reacted with the highly divergent group O peptide (Fig. 4).
DISCUSSION
The genetic diversity within the HIV-1 env gene and within the IDRs of structural proteins directly affects antibody detection. Currently, efforts to improve HIV-1 serologic detection focus on the addition of recombinant and/or synthetic peptides representing the IDRs within the gp41 region of HIV-1 to diagnostic EIA kits. The present study indicates that some variability exists within the IDRs of gp41; however, none of the minor substitutions reported had an impact on serologic detection by five currently approved, FDA-licensed EIA kits or by a gp41 group M peptide-based EIA.
Previous studies have shown that natural sequence variation within cluster I can lead to escape from immune detection, indicating that sequence variation within HIV-1 may contribute to the evolution of viral variants that are no longer recognized by the host immune response (8, 18, 26). Specifically, the cysteine loop, containing an intermolecular disulfide bond, represents the most immunogenic region of gp41 (10). Furthermore, the elimination of loop formation by substitution of S for C at the amino or carboxyl terminus of the peptide abrogates the binding of human monoclonal antibodies, suggesting that the disulfide bridge is necessary to maintain the three-dimensional structure required for antibody recognition (26). Our study demonstrates that the CxxxxxxC domain was conserved in all specimens, regardless of specimen genotype and geographic origin, further confirming the functional importance of this conserved epitope. An L611→H substitution within the cysteine loop has also been shown to affect antibody reactivity (8). However, the 25 specimens in this study (2 subtype B, 22 subtype D and 1 subtype F) containing an L611→H substitution were reactive against whole viral lysates as well as in a gp41 peptide-based assay. While the investigators in the previous study had used a small peptide for epitope delineation (8), we have used a longer peptide that also covers the other immunodominant region and hence may have masked the antibody response specifically directed against only the cysteine loop structure. A recent study has shown that a lack of antibody response to this epitope results in rapid disease progression in infants (4).
Another important aspect of this study was the delineation of sequence variability in the CTL epitope of gp41. The CTL epitope, AVERYLKDQQLL, is restricted by many different class I alleles, including HLA-B8, B-14, A-2, A3.1, and A24 (7, 11, 12, 22). Within the CTL epitope, a K597→R substitution has been shown to abrogate CTL recognition, presumably by interfering with peptide-major histocompatibility complex interactions (12). Many specimens in the present study, ranging from 5% for subtype C to 33% for subtype A, had a natural K597→R mutation. Whether this mutation will result in abrogation of CTL recognition or emergence of CTL escape mutants in these subjects remains to be determined.
The ectodomain within cluster II (ELDKWA) represents an epitope that induces neutralizing antibody activity in humans with HIV-1 infection. A human monoclonal antibody (MAb), 2F5, exhibits broad cross-clade neutralization of primary isolates by binding to the ectodomain region, resulting in an altered confirmation of the gp120-gp41 fusion domain as well as the binding sites for the CD4 cell receptor (13, 16, 17, 20, 21). Variations in this ectodomain region, including D673→N or E and K674→N, almost completely abrogate the binding of MAb 2F5 (20). In the present study, a K674→X substitution in 31 individuals naturally infected with HIV-1 did not affect serologic detection; however, the ability of the substitution to disrupt the neutralizing activity of certain antibodies, such as MAb 2F5, remains to be determined.
N-glycosylated envelope proteins are required for full pathogenic potential, and further evidence suggests that disruption of glycosylation sites on gp41 could abrogate binding of the fusion peptide to the cell membrane (15). Although analysis of the glycosylation sites at positions 620 and 646 revealed a high degree of variability within the second position of the cluster I glycosylation site, less than 5% of these sites exhibited substitutions capable of abrogating glycosylation events.
A comprehensive analysis of the variations in the IDR of gp41 has shown consistent minor variations. However, these minor substitutions in the IDR did not have an impact on overall antibody recognition of HIV-1-seropositive specimens in any of the FDA-licensed EIA kits. One possibility is that antibodies produced against other structural regions, such as p24, are sufficient to elicit a positive response in the EIA kits. However, a strong immune response was detected against the group M gp41 consensus peptide, suggesting that none of these mutations has an impact on gp41-specific antibodies. Further, we demonstrated that serum specimens from HIV-1 group M-infected individuals also have cross-reactive antibodies that recognize a homologous region not only in HIV-1 groups N and O but also in SIVcpz, the SIV closely related to HIV-1 (6). Likewise, we have recently shown that serum specimens from chimpanzees infected with SIV (SIVcpzANT and SIVcpzUS) also reveal cross-reactivity with the HIV-1 group M consensus peptide (S. Masciotra et al., unpublished data). It is interesting to note that gp41 sequences from HIV-1-infected but seronegative cases (5, 25) were similar to the consensus sequences, suggesting that a lack of antibody detection in these specimens was not due to sequence variation in the gp41 region.
Overall, minimal variations appear to occur in the N-terminal region of gp41. It is interesting to note that despite such conservation at the protein level within this region, the nucleotide sequence analysis permits phylogenetic clustering for identification of HIV-1 group M subtypes (19). In conclusion, none of the observed substitutions among the group M (subtypes A to G)-infected individuals had an impact on antibody detection, since the specimens tested positive in both the commercially available FDA-licensed EIA kits and the gp41 group M peptide-based EIA. However, we cannot exclude the possibility that specimens with lower antibody titers may be missed by these assays. While minor substitutions in the gp41 region did not alter the antigenicity of HIV-1, any biological significance of the observed amino acid substitutions within the N-terminal IDRs of gp41 remains to be determined.
ACKNOWLEDGMENTS
We thank J. S. McDougal, C. Schable, B. Parekh, and C. Pau for critical review of the manuscript. We also thank N. Young and K. Limpakarnjanarat for providing some of the specimens tested and R. Moseley for editorial assistance.
REFERENCES
- 1.Binley J M, Klasse P J, Cao Y, Jones I, Markowitz M, Ho D D, Moore J P. Differential regulation of the antibody responses to Gag and Env proteins of human immunodeficiency virus type 1. J Virol. 1997;71:2799–2809. doi: 10.1128/jvi.71.4.2799-2809.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Busch M P, Lee L L, Satten G A, Henrard D R, Farzadegan H, Nelson K E, Read S, Dodd R Y, Peterson L R. Time course of detection of viral and serologic markers preceding human immunodeficiency virus type 1 seroconversion: implications for screening of blood and tissue donors. Transfusion. 1995;35:91–97. doi: 10.1046/j.1537-2995.1995.35295125745.x. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. U.S. Public Health Service guidelines for testing and counseling blood and plasma donors for human immunodeficiency virus type 1 antigen. Morbid Mortal Weekly Rep. 1996;45:1–9. [Google Scholar]
- 4.Cotropia J, Ugen K, Kliks S, Broliden K, Broliden P A, Hoxie J A, Srikantan V, Williams W V, Weiner D B. A human monoclonal antibody to HIV-1 gp41 with neutralizing activity against diverse laboratory isolates. J Acquir Immune Defic Syndr. 1996;12:221–232. doi: 10.1097/00042560-199607000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Ellenberger D L, Sullivan P S, Dorn J, Schable C, Spira T J, Folks T M, Lal R B. Viral and immunologic examination of human immunodeficiency virus type 1-infected, persistently seronegative persons. J Infect Dis. 1999;180:1033–1042. doi: 10.1086/315024. [DOI] [PubMed] [Google Scholar]
- 6.Gao F, Bailes E, Robertson D L, Chen Y, Rodenburg C M, Michael S F, Cummins L B, Arthur L O, Peeters M, Shaw G M, Sharp P M, Hahn B H. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature. 1999;397:546–441. doi: 10.1038/17130. [DOI] [PubMed] [Google Scholar]
- 7.Hammond S A, Obah E, Stanhope P, Monell C R, Strand M, Robbins R M, Bias W B, Karr R W, Koenig S, Siliciano R F. Characterization of a conserved T cell epitope in HIV-1 gp41 recognized by vaccine-induced human cytolytic T cells. J Immunol. 1991;146:1470–1477. [PubMed] [Google Scholar]
- 8.Horal P, Svennerholm B, Jeansson S, Rymo L, Hall W W, Vahlne A. Continuous epitopes of the human immunodeficiency virus type 1 (HIV-1) transmembrane glycoprotein and reactivity of human sera to synthetic peptides representing various HIV-1 isolates. J Virol. 1991;65:2718–2723. doi: 10.1128/jvi.65.5.2718-2723.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu D, Dondero T J, Mastro T D, Gayle H D. Global and molecular epidemiology of HIV. In: Wonnser G P, editor. AIDS and other manifestations of HIV infection. Philadelphia, Pa: Lipponcott-Raven Publishers; 1998. pp. 27–40. [Google Scholar]
- 10.Hunter E. gp41, a multifunctional protein involved in HIV entry and pathogenesis. In: Korber B, Hahn B H, Foley B, Mellors J, Leitner T, Myers G, McCutchan F, Kuiken C, editors. Human retrovirus and AIDS. Los Alamos, N. Mex: Los Alamos National Laboratory; 1997. pp. III55–III73. [Google Scholar]
- 11.Ikeda-Moore Y, Tomiyama H, Miwa K, Oka S, Iwamoto A, Kaneko Y, Takiguchi M. Identification and characterization of multiple HLA-A24-restricted HIV-1 CTL epitopes: strong epitopes are derived from V regions of HIV-1. J Immunol. 1997;159:6241–6252. [PubMed] [Google Scholar]
- 12.Johnson R P, Trocha A, Buchanan T M, Walker B D. Identification of overlapping class I-restricted cytotoxic T cell epitopes in a conserved region of the human immunodeficiency virus type 1 envelope glycoprotein: definition of minimum epitopes and analysis of the effects of sequence variation. J Exp Med. 1992;175:961–971. doi: 10.1084/jem.175.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kessler J A, McKenna P M, Emimi E A, Chan C P, Patel M D, Gupta S K, Mark G E, Barbas C F, Burton D R, Conley A J. Recombinant human monoclonal antibody IgG1b12 neutralizes diverse human immunodeficiency virus type 1 primary isolates. AIDS Res Hum Retrovir. 1997;13:575–582. doi: 10.1089/aid.1997.13.575. [DOI] [PubMed] [Google Scholar]
- 14.Leitner T. Genetic subtypes of HIV-1. In: Myers G, Korber B, Foley B, Jeang K-T, Mellors J, Wain-Hobson S, editors. Human retrovirus and AIDS: a compilation of nucleic acids and amino acid sequences. Los Alamos, N. Mex: Los Alamos National Laboratory; 1996. p. III28. [Google Scholar]
- 15.Montefiori D C, Robinson W E, Jr, Mitchell W M. Role of protein N-glycosylation in pathogenesis of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1988;85:9248–9252. doi: 10.1073/pnas.85.23.9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muster T, Steindl F, Purtscher M, Trkola A, Klima A, Himmler G, Rüker G, Katinger H. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993;67:6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neurath A R, Strick N, Lin K, Jiang S. Multifaceted consequences of anti-gp41 monoclonal antibody 2F5 binding to HIV type 1 virions. AIDS Res Hum Retrovir. 1995;11:687–696. doi: 10.1089/aid.1995.11.687. [DOI] [PubMed] [Google Scholar]
- 18.Oldstone M B, Tishon A, Lewicki H, Kyson H J, Feher V A, Assa-Munt N, Wright P E. Mapping the anatomy of the immunodominant domain of the human immunodeficiency virus gp41 transmembrane protein: peptide conformation analysis using monoclonal antibodies and proton nuclear magnetic resonance spectroscopy. J Virol. 1991;65:1727–1734. doi: 10.1128/jvi.65.4.1727-1734.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pieniazek D, Yang C, Lal R B. Phylogenetic analysis of gp41 envelope of HIV-1 group M, N, and O strains provides an alternate region for subtype determination. In: Korber B, Hahn B H, Foley B, editors. Human retrovirus and AIDS. Los Alamos, N. Mex: Los Alamos National Laboratory; 1998. pp. III112–III117. [Google Scholar]
- 20.Poignard P, Klasse P J, Sattentan Q J. Antibody neutralization of HIV-1. Immunol Today. 1996;17:239–246. doi: 10.1016/0167-5699(96)10007-4. [DOI] [PubMed] [Google Scholar]
- 21.Purtscher M, Trkola A, Grassauer A, Schulz P M, Klima A, Döpper S, Gruber G, Buchacher A, Muster T, Katinger H. Restricted antigenic variability of the epitope recognized by the neutralizing gp41 antibody 2F5. AIDS. 1996;10:587–593. doi: 10.1097/00002030-199606000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Rowland-Jons S, Tan R, McMichael A. Role of cellular immunity in protection against HIV infection. Adv Immunol. 1997;65:277–346. [PubMed] [Google Scholar]
- 23.Rudolph D L, Lal R B. Discrimination of human T lymphotropic virus type-I and type-II infections by synthetic peptides comprising structural epitopes from the envelope glycoproteins. Clin Chem. 1993;39:288–292. [PubMed] [Google Scholar]
- 24.Simon F, Mauclère P, Roques P, Loussert-Ajaka I, Müller-Trutwin M C, Saragosti S, Georges-Coubort M C, Barré-Sinoussi F, Bru-Vézinet F. Identification of a new human immunodeficiency virus type 1 distinct from group M and group O. Nat Med. 1998;4:1032–1037. doi: 10.1038/2017. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan P S, Schable C, Koch W, Do A N, Spira T, Lansky A, Ellenberger D, Lal R B, Hyer C, Davis R, Marx M, Paul S, Kent J, Armor R, McFarland J, Lafontaine J, Mottice S, Cassol S A, Michael N the Seronegative AIDS Clinical Study Group. Persistently negative HIV-1-antibody EIA screening results for patients with HIV-1 infection and AIDS: serologic, clinical, and virologic results. AIDS. 1999;13:89–96. doi: 10.1097/00002030-199901140-00012. [DOI] [PubMed] [Google Scholar]
- 26.Xu J, Gorny M K, Palker T, Karwowska S, Zolla-Pazner S. Epitope mapping of two immunodominant domains of gp41, the transmembrane protein of human immunodeficiency virus type 1, using ten human monoclonal antibodies. J Virol. 1991;65:4832–4838. doi: 10.1128/jvi.65.9.4832-4838.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang C, Pieniazek D, Owen S M, Fridlund C, Nkengasong J, Mastro T D, Rayfield M A, Downing R, Biryawaho B, Tanuri A, Zekeng L, van der Groen G, Gao F, Lal R B. Detection of phylogenetically diverse human immunodeficiency virus type 1 groups M and O from plasma by using highly sensitive and specific generic primers. J Clin Microbiol. 1999;37:2581–2586. doi: 10.1128/jcm.37.8.2581-2586.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]