Abstract
Objectives
In streptococci, the type M resistance to macrolides is due to the mef(A)–msr(D) efflux transport system of the ATP-Binding cassette (ABC) superfamily, where it is proposed that mef(A) codes for the transmembrane channel and msr(D) for the two ATP-binding domains. Phage ϕ1207.3 of Streptococcus pyogenes, carrying the mef(A)–msr(D) gene pair, is able to transfer the macrolide efflux phenotype to Streptococcus pneumoniae. Deletion of mef(A) in pneumococcal ϕ1207.3-carrying strains did not affect erythromycin efflux. In order to identify candidate genes likely involved in complementation of mef(A) deletion, the Mef(A) amino acid sequence was used as probe for database searching.
Results
In silico analysis identified 3 putative candidates in the S. pneumoniae R6 genome, namely spr0971, spr1023 and spr1932. Isogenic deletion mutants of each candidate gene were constructed and used in erythromycin sensitivity assays to investigate their contribution to mef(A) complementation. Since no change in erythromycin sensitivity was observed compared to the parental strain, we produced double and triple mutants to assess the potential synergic activity of the selected genes. Also these mutants did not complement the mef(A) function.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13104-021-05856-6.
Keywords: Mef(A), Msr(D), Macrolide efflux, Streptococcus pyogenes, Streptococcus pneumoniae, ABC-transporter, ϕ1207.3, Prophage
Introduction
Macrolide resistance in streptococci is usually associated with two major mechanisms: (i) target-site modification, mediated by the erythromycin ribosomal methylase (erm) family genes responsible for 23S rRNA methylation; (ii) active drug efflux, mediated by the mef family genes which confer the M phenotype, characterized by low level resistance to 14- and 15-membered macrolides [1–6]. The two mef major allelic variants, mef(A) and mef(E), were originally described in Streptococcus pyogenes and in Streptococcus pneumoniae, respectively [7, 8]. These variants are highly homologous and are also found in other streptococcal species, gram-positive and gram-negative genera [3, 5, 9–12] (for an updated list see the Dr. Marylin Roberts’s website https://faculty.washington.edu/marilynr). The mef alleles are associated to different chromosomal genetic elements. In S. pneumoniae, we found Tn1207.1, a 7244-bp non-conjugative element carrying mef(A), whereas the 5532-bp pneumococcal genetic element (mega) was found to carry mef(E) [13–17]. In S. pyogenes, we described the 52,491-bp prophage Φ1207.3 carrying mef(A) whose left 7244-bp sequence is identical to Tn1207.1 [18–20]. In clinical isolates of S. pyogenes other mef(A)-carrying prophages were found, including Φ10394.4, Φm46.1 and its variant VP_00501.1 [21–23]. In the mef-carrying genetic elements, the msr(D) gene was always associated to and co-transcribed with the mef gene and contributes to macrolide efflux resistance [23–28]. In our previous work, genome database search showed that in 33 out of 37 genomes, mef(A) was associated in tandem to msr(D), while bioinformatic analysis showed that the Mef(A) protein was predicted to form six transmembrane helices and the Msr(D) protein to have two Nucleotide Binding Domains (NBDs) typical of ATP-binding transporters [27]. We hypothesized that mef(A) and msr(D) constitute a two-gene ATP-Binding Cassette efflux transport system, where mef(A) encodes the transmembrane channel, and msr(D) the two ATP-binding domains. A functional analysis of the relative contribution of mef(A) and msr(D) to macrolide resistance supported this hypothesis, showing that deletion of msr(D) abolishes erythromycin resistance, whereas deletion of mef(A) causes only a twofold reduction of MIC value [27]. It is likely that in absence of Mef(A), Msr(D) utilizes an alternative transmembrane channel for macrolide efflux. In the present work, a pneumococcal genome homology search was used to investigate the presence of transmembrane proteins homologous to Mef(A), which could complement the Mef(A) function. Three genes encoding transmembrane proteins were identified and their role as alternative Msr(D) cognate transmembrane channel was investigated through site specific mutagenesis and functional studies.
Main text
Methods
Bacterial strains, growth and mating condition
All pneumococcal strains used in this work and their relevant properties are reported in Table 1. Bacterial strains were grow grown in Tryptic Soy Broth (TSB) or Tryptic Soy Agar (TSA) supplemented with 3% defibrinated horse blood [29]. Transfer of Φ1207.3 or Φ1207.3Δmef(A) from strains FR183 and FP40 to the deletion mutants was obtained through a mating protocol as already reported [30]. Briefly, donor and recipient cells were grown separately in TSB in the presence of the appropriate antibiotics. Upon reaching the end of exponential phase, cells were mixed at a donor-recipient 1:10 ratio, centrifuged, and pellet was plated on TSA plates supplemented with 5% blood. Plates were incubated at 37 °C in the presence of 5% CO2 for 4 h and cells were recovered with a cotton swab and resuspended in TSB. To select for recombinants, cell suspension was plated following a multilayer plating procedure [30].
Table 1.
Bacterial strains
| Strain | Propertiesa | References |
|---|---|---|
| Rx1 | Unencapsulated D39 S. pneumoniae derivative | [36, 44] |
| DP1004 | Rx1 derivative, str-41, SmR | [29, 45] |
| FR183 | DP1004 derivative, carrying φ1207.3, SmR, EmR | [27] |
| FP40 | FR183 derivative, carrying φ1207.3Δmef(A), SmR, EmR, CmR | [27] |
| FR323 | DP1004 derivative, spr0971ΔSpe, SmR, SpeR | This study |
| FR324 | DP1004 derivative, spr1023ΔKm, SmR, KmR | This study |
| FR325 | DP1004 derivative, spr1932ΔKm, SmR, KmR | This study |
| FR358 | FR323 derivative, carrying φ1207.3, SmR, EmR, SpeR | This study |
| FR359 | FR324 derivative, carrying φ1207.3, SmR, EmR, SpeR | This study |
| FR360 | FR325 derivative, carrying φ1207.3, SmR, EmR, SpeR | This study |
| FR355 | FR323 derivative, carrying φ1207.3Δmef(A), SmR,EmR, CmR, SpeR | This study |
| FR356 | FR324 derivative, carrying φ1207.3Δmef(A), SmR, EmR, CmR, SpeR | This study |
| FR357 | FR325 derivative, carrying φ1207.3Δmef(A), SmR, EmR, CmR, SpeR | This study |
| FR335 | FR323 derivative, spr0971ΔSpe, spr1023ΔKm, SmR, SpeR, KmR | This study |
| FR336 | FR323 derivative, spr0971ΔSpe, spr1932ΔKm, SmR, SpeR, KmR | This study |
| FR337 | FR325 derivative, spr1932ΔKm, Δspr1023 (in-frame), SmR, KmR | This study |
| FR344 | FR335 derivative, carrying φ1207.3, SmR, EmR, SpeR, KmR | This study |
| FR345 | FR336 derivative, carrying φ1207.3, SmR, EmR, SpeR, KmR | This study |
| FR349 | FR337 derivative, carrying φ1207.3, SmR, EmR, KmR | This study |
| FR346 | FR335 derivative, carrying φ1207.3Δmef(A), SmR, EmR, CmR, SpeR, KmR | This study |
| FR347 | FR336 derivative, carrying φ1207.3Δmef(A), SmR, EmR, CmR, SpeR, KmR | This study |
| FR348 | FR337 derivative, carrying φ1207.3Δmef(A), SmR, EmR, CmR, KmR | This study |
| FR338 | FR337 derivative, spr1932ΔKm, Δspr1023 (in-frame), spr0971ΔSpe, SmR, KmR, SpeR | This study |
| FR351 | FR338 derivative, carrying φ1207.3, SmR, EmR, KmR, SpeR | This study |
| FR350 | FR338 derivative, carrying φ1207.3Δmef(A), SmR, EmR, CmR, KmR, SpeR | This study |
Sm streptomycin, Em erythromycin, Cm chloramphenicol, Spe spectinomycin, Km kanamycin
astr-41 indicates a point mutation conferring resistance to streptomycin
Bioinformatic analysis
Homology searches of the pneumococcal genome R6 available at the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/genome/microbes/) was performed using Microbial BLAST with the Megablast algorithm (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastp&PAGE_TYPE=BlastSearch&BLAST_SPEC=MicrobialGenomes&LINK_LOC=blasttab&LAST_PAGE=blastp). Default parameters were used and only alignments with significant e-values (< 0.001) were considered. Protein sequence analysis was carried out with the softwares TMpred and Phyre2 [31–34].
Gene SOEing PCR mutagenesis
Isogenic deletion mutants were obtained transforming S. pneumoniae Rx1 derivative recipients with mutagenic constructs assembled by Gene Splicing by Overlap Extension (Gene SOEing) [29]. The oligonucleotide primers used for mutagenesis, sequencing and PCR selection of the recombinants strain are reported in Additional file 1: Table S1. Deletion of spr0971 coding sequence (CDS) was obtained with a mutagenic construct containing the ami/aad9 spectinomycin resistance cassette (894 bp) [35] flanked by the upstream (601 bp) and downstream (459 bp) spr0971 flanking fragments, respectively. The spr1023 CDS was deleted with a mutagenic construct containing the ami/aphIII kanamycin resistance cassette (1033 bp) [36] joined to the left (696 bp) and right (658 bp) spr1023 flanking fragments. The spr1932 mutagenic construct contained the kanamycin resistance cassette flanked by the upstream (724 bp) and downstream (694 bp) spr1932 flanking fragments. The mutagenic construct for spr1023 in-frame deletion was obtained assembling the DNA fragments located upstream (749 bp) and downstream (773 bp) of spr1023 CDS. Linear PCR constructs were used directly as donor DNA in transformation experiments. Recombinant strains were selected for acquisition of spectinomycin or kanamycin resistance, while deletion of spr1023 was selected by selective PCR analysis [29]. The correct integration of constructs was confirmed by PCR and sequencing.
Minimal inhibitory concentration (MIC) determination
The minimal inhibitory concentration (MIC) was assessed by microdilution method, according to the Clinical and Laboratory Standards Institute guideline (CLSI, 2020) as already reported [27]. Briefly, bacteria were grown in TSB until reaching the exponential phase (OD590 = 0.3, corresponding to approximately 108 CFU/ml), then culture aliquots were taken and frozen at − 70 °C in 10% glycerol. Frozen cultures were then thawed, diluted 1:100 in TSB (106 CFU/ml) and 100 µl were added to a 96-wells microplate containing 100 µl of serial twofold dilutions of erythromycin, reaching a final concentration of 5 × 105 CFU/ml in each well. Plates were incubated at 37 °C and visually analyzed after 18 h. Bacterial growth was assessed using the microplate ELISA reader VERSAmax (Molecular Devices). The S. pneumoniae ATCC49619 strain was used as a quality control. MIC assays were performed in quintuplicate with at least two technical replicates per experiment.
Results
Identification and sequence analysis of a candidate genes encoding a Mef(A) homologous protein
The 405-aa Mef(A) sequence (GenBank accession no. AAT72347) was used as a query to conduct a BLAST homology search of S. pneumoniae R6 genome. Homology analysis revealed the presence of three genes coding for proteins with a significant homology (e-value < 0.001) to Mef(A): (i) spr0971 (GenBank accession number NP_358565.1); (ii) spr1023 (GenBank accession number NP_358617.1); (iii) spr1932 (GenBank accession number NP_359523.1). The spr0971 gene, annotated as “ABC transporter membrane-spanning permease—macrolide efflux”, codes for a 403 aa protein displaying 23% identity to Mef(A). The spr1023 gene, annotated as “macrolide ABC transporter permease”, codes for a 392 aa protein with 24% identity to Mef(A). The spr1932 gene, annotated as “hypothetical protein”, codes for a 415 aa protein with 21% identity to Mef(A). Analysis of the transmembrane domains of all deduced amino acid products predicted the presence of up to 12 transmembrane helices.
Investigation of the role of the candidate genes on Mef(A) complementation
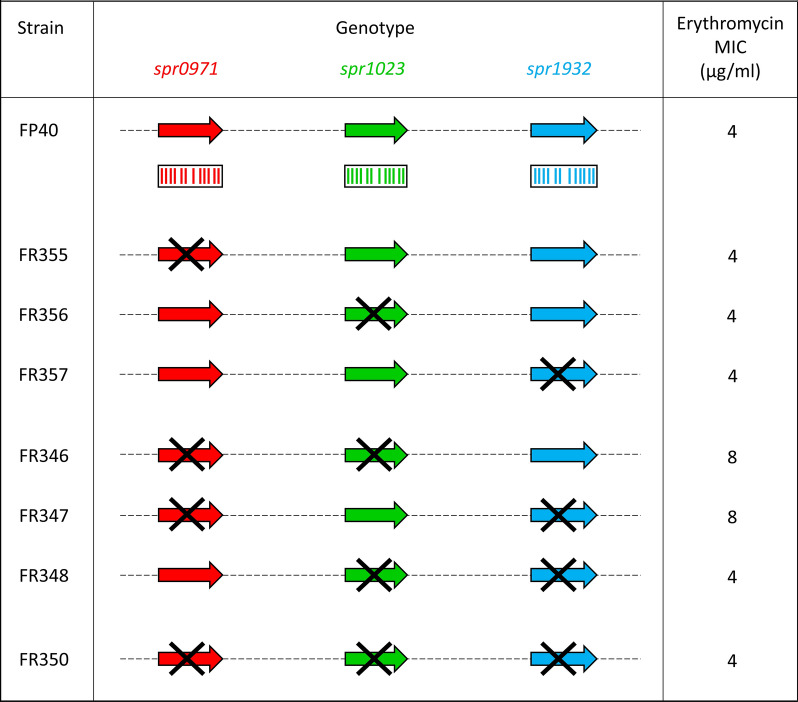
To define if the selected candidate genes could complement the mef(A) function, we constructed three isogenic deletion mutants in S. pneumoniae DP1004 background (Table 1). The 1209 bp spr0971 CDS was deleted and replaced by the 894-bp ami/aad9 cassette, whereas the 1176 bp spr1023 and the 1245 bp spr1932 CDSs were deleted by allelic replacement with the 1033-bp ami/aphIII cassette. These mutants were used as recipients to obtain derivative strains harboring the recombinant Φ1207.3Δmef(A). Sensitivity to erythromycin of the isogenic mutants was assessed by determining the MIC. In our previous study, we reported an erythromycin MIC of 4 µg/ml for the Φ1207.3Δmef(A)-carrying strain FP40, consistent with the presence of an alternative transmembrane channel able to complement the mef(A) function [27]. Deletion of spr0971, spr1023 and spr1932, did not affect erythromycin sensitivity (Fig. 1 and Table 2). Then, to investigate if the Mef(A) complementation is due to a synergic action of these genes, we constructed double deletion mutants. The spr0971-spr1023 and spr0971-spr1932 double deletion mutants were obtained transforming strain FR323 (spr0971ΔSpe) with the spr1023 and spr1932 kanamycin mutagenic constructs, respectively, while the spr1023–spr1932 double mutant was obtained by transforming the strain FR325 (spr1932ΔKm) with a mutagenic construct designed to produce spr1023 in frame deletion. Deletion of spr1023 and spr1932, did not affect erythromycin sensitivity, whereas the spr0971–spr1023 and spr0971–spr1932 deletions produce a twofold increase of erythromycin MIC (8 µg/ml). Finally, a triple spr0971–spr1023–spr1932 mutant was constructed using the spr1023–spr1932 double mutant FR337 as recipient and the spr0971 spectinomycin mutagenic construct as donor DNA. No change in erythromycin sensitivity (MIC = 4 µg/ml) was observed also for the triple mutant. As control strains we used: (i) the parental strain DP1004; (ii) strain FR183 carrying the Φ1207.3 phage; (iii) strain FP40 carrying the Φ1207.3Δmef(A) recombinant phage; (iv) deletion mutants without Φ1207.3; (v) deletion mutants carrying Φ1207.3.
Fig. 1.
Schematic representation of genotype and phenotype of S. pneumoniae isogenic deletion mutants. Macrolide efflux in Streptococci is associated to the mef(A)–msr(D) macrolide efflux system. The contribution of spr0971, spr1023, spr1932 genes to mef(A) complementation was investigated by constructing isogenic deletion mutants, carrying the recombinant Φ1207.3Δmef(A) phage. Single, double and triple mutants were obtained by PCR gene SOEing and transformation. Genes are reported as arrows, while the cross indicates gene deletions. Spr0971, Spr1023 and Spr1932 proteins are reported as not scaled boxes and the 12 transmembrane domains as bars
Table 2.
Erythromycin sensitivity
| Strain | Genotype | Phenotypeb | Erythromycin MIC (µg/ml) |
||||
|---|---|---|---|---|---|---|---|
| Chromosome | Φ1207.3a | ||||||
| spr0971 | spr1023 | spr1932 | mef(A) | msr(D) | |||
| DP1004 | + | + | + | np | np | Sensitive | 0.06 |
| FR183 | + | + | + | + | + | Resistant | 8 |
| FP40 | + | + | + | − | + | Resistant | 4 |
| FR323 | − | + | + | np | np | Sensitive | 0.06 |
| FR324 | + | − | + | np | np | Sensitive | 0.06 |
| FR325 | + | + | − | np | np | Sensitive | 0.06 |
| FR358 | − | + | + | + | + | Resistant | 8 |
| FR359 | + | − | + | + | + | Resistant | 8 |
| FR360 | + | + | − | + | + | Resistant | 8 |
| FR355 | − | + | + | − | + | Resistant | 4 |
| FR356 | + | − | + | − | + | Resistant | 4 |
| FR357 | + | + | − | − | + | Resistant | 4 |
| FR335 | − | − | + | np | np | Sensitive | 0.06 |
| FR336 | − | + | − | np | np | Sensitive | 0.06 |
| FR337 | + | − | − | np | np | Sensitive | 0.06 |
| FR344 | − | − | + | + | + | Resistant | 8 |
| FR345 | − | + | − | + | + | Resistant | 8 |
| FR349 | + | − | − | + | + | Resistant | 8 |
| FR346 | − | − | + | − | + | Resistant | 8 |
| FR347 | − | + | − | − | + | Resistant | 8 |
| FR348 | + | − | − | − | + | Resistant | 4 |
| FR338 | − | − | − | np | np | Sensitive | 0.06 |
| FR351 | − | − | − | + | + | Resistant | 8 |
| FR350 | − | − | − | − | + | Resistant | 4 |
anp: Φ1207.3 phage not present
bMIC interpretative standards: sensitive ≤ 0.25 µg/ml, intermediate = 0.5 µg/ml, and resistant ≥ 1 µg/ml
Conclusions
Our previous findings and those of other research groups [23–28] reported that the macrolide efflux in Streptococci relies on the presence of the mef(A)–msr(D) operon. Based on bioinformatic analysis of the Mef(A) and Msr(D) proteins and their functional characterization, we proposed that mef(A) and msr(D) constitute a two-gene ATP-Binding Cassette efflux transport system, where mef(A) encodes the transmembrane channel, and msr(D) the two ATP-binding domains. Msr(D) is a member of the ACB-F family of ABC transporters, which are widespread among both bacteria and eukaryotes [37]. In contrast to other members of this family, which were shown to interact with the ribosome and protect it from antibiotics [38, 39], Msr(D) (i) is always associated to a cognate transmembrane domain encoded by the mef(A) gene, (ii) was shown to be involved in the erythromycin efflux from the bacterial cell [27] and (iii) was shown to be associated to Mef(A) and localized on the membrane [25].
Since deletion of msr(D) abolishes erythromycin resistance, whereas deletion of mef(A) causes only a twofold reduction of MIC value, we hypothesized that in absence of Mef(A), Msr(D) recruits an alternative transmembrane partner. In this work, to determine if the mef(A) deletion is complemented by pneumococcal chromosomal genes, we investigated the presence of genes encoding transmembrane proteins homologous to Mef(A). Homology search identified three candidate genes, namely spr0971, spr1023, spr1932. Isogenic single, double and triple deletion mutants were constructed and the single and synergic contribution of these genes to mef(A) complementation was assessed by erythromycin sensitivity assays. The expected decrease of erythromycin MIC, due to the absence of a putative alternative Mef(A) channel, was not observed, suggesting that these genes are not involved in the complementation of mef(A) deletion. For the two spr0971–spr1023 and spr0971–spr1932 double mutants, we observed a twofold increase of the erythromycin MIC, which was not seen in the triple mutant. These results are unexpected, as the MIC value in absence of mef(A) and other alternative transmembrane channels would be predicted to decrease. The same increase was observed also following the deletion of a fourth gene, spr0875 (data not shown). This gene, which encodes a protein homologous to Mef(A), was previously characterized and associated to the efflux of other compounds including fusidic acid and sodium dodecyl sulfate [40, 41]. For this reason and because its deletion resulted in the increase of erythromycin MIC value, we excluded it from further investigations. We hypothesise that this increase could be due a possible “unspecific” permease activity which allows erythromycin entrance in the bacterial cell through one or more of the pores encoded by these genes. In conclusion the quest to identify the alternative Msr(D) cognate transmembrane channel remains open.
Limitations
Investigation of putative candidate genes, responsible for complementing mef(A) deletion in the mef(A)–msr(D) macrolide efflux system in S. pneumoniae, was performed using a targeted approach based on the homology to Mef(A). Nonetheless, it is possible that the proteins involved in this complementation may not display significant homology to Mef(A). A genome-wide approach based on the creation and screening of a library of random mariner transposon mutants [42, 43], allowing for random mutagenesis of the whole pneumococcal genome, could be used to investigate the possible effect of other chromosomal genes in mef(A) complementation.
Supplementary Information
Additional file 1: Table S1. Oligonucleotides primer used to construct the isogenic deletion mutants.
Acknowledgements
Not applicable.
Abbreviations
- ABC
ATP-Binding cassette
- erm
Erythromycin ribosomal methylase
- Gene SOEing
Gene Splicing by Overlap Extension
- NBDs
Nucleotide Binding Domains
- MIC
Minimal inhibitory concentration
- TSB
Tryptic Soy Broth
- TSA
Tryptic Soy Agar
Authors’ contributions
VF, FS, GP, FI conceived and designed the study; VF carried out the experiments; VF, FS, FI performed data analysis; FS, FI, GP supervised the work; VF and FI drafted the first version of the manuscript; FS reviewed the manuscript; GP received funds for the study. All authors read and approved the final manuscript.
Funding
This research was funded by the Italian Ministry of Education, University and Research (MIUR-Italy) under Grant Number 20177J5Y3P (call “Progetti di Ricerca di Rilevante Interesse Nazionale – Bando 2017”).
Availability of data and materials
All data generated or analysed during this study are included in this published article. The pneumococcal genome R6 is available at the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/genome/microbes/) under accession number AE007317.1. Protein search was performed with Microbial BLAST using the Megablast algorithm (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastp&PAGE_TYPE=BlastSearch&BLAST_SPEC=MicrobialGenomes&LINK_LOC=blasttab&LAST_PAGE=blastp). The Mef(A) protein sequence is available at the GenBank accession no. AAT72347; spr0971, spr1023, and spr1932 protein sequences are available under accession numbers NP_358565.1, NP_358617.1, and NP_359523.1, respectively.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weisblum B. Erythromycin resistance by ribosome modification. Antimicrob Agents Chemother. 1995;39(3):577–585. doi: 10.1128/AAC.39.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sutcliffe J, Grebe T, Tait-Kamradt A, Wondrack L. Detection of erythromycin-resistant determinants by PCR. Antimicrob Agents Chemother. 1996;40(11):2562–2566. doi: 10.1128/aac.40.11.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts MC, Sutcliffe J, Courvalin P, Jensen LB, Rood J, Seppala H. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob Agents Chemother. 1999;43(12):2823–2830. doi: 10.1128/aac.43.12.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leclercq R, Courvalin P. Resistance to macrolides and related antibiotics in Streptococcus pneumoniae. Antimicrob Agents Chemother. 2002;46(9):2727–2734. doi: 10.1128/AAC.46.9.2727-2734.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts MC. Update on macrolide-lincosamide-streptogramin, ketolide, and oxazolidinone resistance genes: MLSKO genes. FEMS Microbiol Lett. 2008;282(2):147–159. doi: 10.1111/j.1574-6968.2008.01145.x. [DOI] [PubMed] [Google Scholar]
- 6.Fyfe C, Grossman TH, Kerstein K, Sutcliffe J. Resistance to macrolide antibiotics in public health pathogens. Cold Spring Harb Perspect Med. 2016;6(10):a025395. doi: 10.1101/cshperspect.a025395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clancy J, Petitpas J, Dib-Hajj F, Yuan W, Cronan M, Kamath AV, Bergeron J, Retsema JA. Molecular cloning and functional analysis of a novel macrolide-resistance determinant, mefA, from Streptococcus pyogenes. Mol Microbiol. 1996;22(5):867–879. doi: 10.1046/j.1365-2958.1996.01521.x. [DOI] [PubMed] [Google Scholar]
- 8.Tait-Kamradt A, Clancy J, Cronan M, Dib-Hajj F, Wondrack L, Yuan W, Sutcliffe J. mefE is necessary for the erythromycin-resistant M phenotype in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41(10):2251–2255. doi: 10.1128/aac.41.10.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luna VA, Coates P, Eady EA, Cove JH, Nguyen TTH, Roberts MC. A variety of Gram-positive bacteria carry mobile mef genes. J Antimicrob Chemother. 1999;44(1):19–25. doi: 10.1093/jac/44.1.19. [DOI] [PubMed] [Google Scholar]
- 10.Luna VA, Heiken M, Judge K, Ulep C, Van Kirk N, Luis H, Bernardo M, Leitao J, Roberts MC. Distribution of mef (A) in Gram-positive bacteria from healthy Portuguese children. Antimicrob Agents Chemother. 2002;46(8):2513–2517. doi: 10.1128/AAC.46.8.2513-2517.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts MC, Soge OO, No DB. Characterization of macrolide resistance genes in Haemophilus influenzae isolated from children with cystic fibrosis. J Antimicrob Chemother. 2011;66(1):100–104. doi: 10.1093/jac/dkq425. [DOI] [PubMed] [Google Scholar]
- 12.Klaassen CHW, Mouton JW. Molecular detection of the macrolide efflux gene: to discriminate or not to discriminate between mef (A) and mef (E) Antimicrob Agents Chemother. 2005;49(4):1271–1278. doi: 10.1128/AAC.49.4.1271-1278.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santagati M, Iannelli F, Oggioni MR, Stefani S, Pozzi G. Characterization of a genetic element carrying the macrolide efflux gene mef(A) in Streptococcus pneumoniae. Antimicrob Agents Chemother. 2000;44(9):2585–2587. doi: 10.1128/aac.44.9.2585-2587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gay K, Stephens DS. Structure and dissemination of a chromosomal insertion element encoding macrolide efflux in Streptococcus pneumoniae. J Infect Dis. 2001;184(1):56–65. doi: 10.1086/321001. [DOI] [PubMed] [Google Scholar]
- 15.Del Grosso M, Iannelli F, Messina C, Santagati M, Petrosillo N, Stefani S, Pozzi G, Pantosti A. Macrolide efflux genes mef(A) and mef(E) are carried by different genetic elements in Streptococcus pneumoniae. J Clin Microbiol. 2002;40(3):774–778. doi: 10.1128/JCM.40.3.774-778.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Grosso M, Scotto d’Abusco A, Iannelli F, Pozzi G, Pantosti A. Tn2009, a Tn916-like element containing mef(E) in Streptococcus pneumoniae. Antimicrob Agents Chemother. 2004;48(6):2037–2042. doi: 10.1128/AAC.48.6.2037-2042.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Grosso M, Camilli R, Iannelli F, Pozzi G, Pantosti A. The mef(E)-carrying genetic element (mega) of Streptococcus pneumoniae: insertion sites and association with other genetic elements. Antimicrob Agents Chemother. 2006;50(10):3361–3366. doi: 10.1128/AAC.00277-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santagati M, Iannelli F, Cascone C, Campanile F, Oggioni MR, Stefani S, Pozzi G. The novel conjugative transposon Tn1207.3 carries the macrolide efflux gene mef(A) in Streptococcus pyogenes. Microb Drug Resist. 2003;9(3):243–247. doi: 10.1089/107662903322286445. [DOI] [PubMed] [Google Scholar]
- 19.Pozzi G, Iannelli F, Oggioni M, Santagati M, Stefani S. Genetic elements carrying macrolide efflux genes in Streptococci. Curr Drug Target Infect Disord. 2004;4(3):203–206. doi: 10.2174/1568005043340641. [DOI] [PubMed] [Google Scholar]
- 20.Iannelli F, Santagati M, Santoro F, Oggioni MR, Stefani S, Pozzi G. Nucleotide sequence of conjugative prophage Φ1207.3 (formerly Tn1207.3) carrying the mef(A)/msr(D) genes for efflux resistance to macrolides in Streptococcus pyogenes. Front Microbiol. 2014;5:687. doi: 10.3389/fmicb.2014.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banks DJ, Porcella SF, Barbian KD, Martin JM, Musser JM. Structure and distribution of an unusual chimeric genetic element encoding macrolide resistance in phylogenetically diverse clones of group A Streptococcus. J Infect Dis. 2003;188(12):1898–1908. doi: 10.1086/379897. [DOI] [PubMed] [Google Scholar]
- 22.Brenciani A, Bacciaglia A, Vignaroli C, Pugnaloni A, Varaldo PE, Giovanetti E. Φm46.1, the main Streptococcus pyogenes element carrying mef (A) and tet (O) genes. Antimicrob Agents Chemother. 2010;54(1):221–229. doi: 10.1128/AAC.00499-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vitali LA, Di Luca MC, Prenna M, Petrelli D. Correlation between genetic features of the mef (A)–msr (D) locus and erythromycin resistance in Streptococcus pyogenes. Diagn Microbiol Infect Dis. 2016;84(1):57–62. doi: 10.1016/j.diagmicrobio.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Ambrose KD, Nisbet R, Stephens DS. Macrolide efflux in Streptococcus pneumoniae is mediated by a dual efflux pump (mel and mef) and is erythromycin inducible. Antimicrob Agents Chemother. 2005;49(10):4203–4209. doi: 10.1128/AAC.49.10.4203-4209.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nunez-Samudio V, Chesneau O. Functional interplay between the ATP binding cassette Msr(D) protein and the membrane facilitator superfamily Mef(E) transporter for macrolide resistance in Escherichia coli. Res Microbiol. 2013;164(3):226–235. doi: 10.1016/j.resmic.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Tatsuno I, Okada R, Hata N, Matsumoto M, Isaka M, Isobe K, Hasegawa T. Predominant role of msr(D) over mef(A) in macrolide resistance in Streptococcus pyogenes. Microbiology. 2016;162(1):46–52. doi: 10.1099/mic.0.000206. [DOI] [PubMed] [Google Scholar]
- 27.Iannelli F, Santoro F, Santagati M, Docquier J-D, Lazzeri E, Pastore G, Cassone M, Oggioni MR, Rossolini GM, Stefani S, Pozzi G. Type M resistance to macrolides is due to a two-gene efflux transport system of the ATP-binding cassette (ABC) superfamily. Front Microbiol. 2018;31(9):1670. doi: 10.3389/fmicb.2018.01670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tatsuno I, Isaka M, Masuno K, Hata N, Matsumoto M, Hasegawa T. Functional predominance of msr (D), which is more effective as mef(A)-associated than mef(E)-associated, over mef (A)/mef (E) in macrolide resistance in Streptococcus pyogenes. Microb Drug Resist. 2018;24(8):1089–1097. doi: 10.1089/mdr.2017.0277. [DOI] [PubMed] [Google Scholar]
- 29.Iannelli F, Pozzi G. Method for introducing specific and unmarked mutations into the chromosome of Streptococcus pneumoniae. Mol Biotechnol. 2004;26(1):81–86. doi: 10.1385/MB:26:1:81. [DOI] [PubMed] [Google Scholar]
- 30.Iannelli F, Santoro F, Fox V, Pozzi G. A mating procedure for genetic transfer of integrative and conjugative elements (ICEs) of Streptococci and Enterococci. Methods Protoc. 2021;4(3):59. doi: 10.3390/mps4030059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikeda M, Arai M, Okuno T, Shimizu T. TMPDB: a database of experimentally-characterized transmembrane topologies. Nucleic Acids Res. 2003;31(1):406–409. doi: 10.1093/nar/gkg020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santoro F, Oggioni MR, Pozzi G, Iannelli F. Nucleotide sequence and functional analysis of the tet (M)-carrying conjugative transposon Tn5251 of Streptococcus pneumoniae: Tn5251 of Streptococcus pneumoniae. FEMS Microbiol Lett. 2010;308(2):150–158. doi: 10.1111/j.1574-6968.2010.02002.x. [DOI] [PubMed] [Google Scholar]
- 33.Iannelli F, Santoro F, Oggioni MR, Pozzi G. Nucleotide sequence analysis of integrative conjugative element Tn5253 of Streptococcus pneumoniae. Antimicrob Agents Chemother. 2014;58(2):1235–1239. doi: 10.1128/AAC.01764-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJE. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10(6):845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santoro F, Romeo A, Pozzi G, Iannelli F. Excision and circularization of integrative conjugative element Tn5253 of Streptococcus pneumoniae. Front Microbiol. 2018;31(9):1779. doi: 10.3389/fmicb.2018.01779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pearce BJ, Iannelli F, Pozzi G. Construction of new unencapsulated (rough) strains of Streptococcus pneumoniae. Res Microbiol. 2002;153(4):243–247. doi: 10.1016/s0923-2508(02)01312-8. [DOI] [PubMed] [Google Scholar]
- 37.Murina V, Kasari M, Takada H, Hinnu M, Saha CK, Grimshaw JW, Seki T, Reith M, Putrinš M, Tenson T, Strahl H, Hauryliuk V, Atkinson GC. ABCF ATPases involved in protein synthesis, ribosome assembly and antibiotic resistance: structural and functional diversification across the tree of life. J Mol Biol. 2019;431(18):3568–3590. doi: 10.1016/j.jmb.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murina V, Kasari M, Hauryliuk V, Atkinson GC. Antibiotic resistance ABCF proteins reset the peptidyl transferase centre of the ribosome to counter translational arrest. Nucleic Acids Res. 2018;46(7):3753–3763. doi: 10.1093/nar/gky050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su W, Kumar V, Ding Y, Ero R, Serra A, Lee BST, Wong ASW, Shi J, Sze SK, Yang L, Gao Y-G. Ribosome protection by antibiotic resistance ATP-binding cassette protein. Proc Natl Acad Sci. 2018;115(20):5157–5162. doi: 10.1073/pnas.1803313115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gill MJ, Brenwald NP, Wise R. Identification of an efflux pump gene, pmrA, associated with fluoroquinolone resistance in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1999;43(1):187–189. doi: 10.1128/aac.43.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tocci N, Iannelli F, Bidossi A, Ciusa ML, Decorosi F, Viti C, Pozzi G, Ricci S, Oggioni MR. Functional analysis of pneumococcal drug efflux pumps associates the MATE DinF transporter with quinolone susceptibility. Antimicrob Agents Chemother. 2013;57(1):248–253. doi: 10.1128/AAC.01298-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akerley BJ, Rubin EJ, Camilli A, Lampe DJ, Robertson HM, Mekalanos JJ. Systematic identification of essential genes by in vitro mariner mutagenesis. Proc Natl Acad Sci. 1998;95(15):8927–8932. doi: 10.1073/pnas.95.15.8927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lampe DJ, Akerley BJ, Rubin EJ, Mekalanos JJ, Robertson HM. Hyperactive transposase mutants of the Himar1 mariner transposon. Proc Natl Acad Sci. 1999;96(20):11428–11433. doi: 10.1073/pnas.96.20.11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith MD, Guild WR. A plasmid in Streptococcus pneumoniae. J Bacteriol. 1979;137(2):735–739. doi: 10.1128/jb.137.2.735-739.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salles C, Créancier L, Claverys JP, Méjean V. The high level streptomycin resistance gene from Streptococcus pneumoniae is a homologue of the ribosomal protein S12 gene from Escherichia coli. Nucleic Acids Res. 1992;20(22):6103. doi: 10.1093/nar/20.22.6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Oligonucleotides primer used to construct the isogenic deletion mutants.
Data Availability Statement
All data generated or analysed during this study are included in this published article. The pneumococcal genome R6 is available at the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/genome/microbes/) under accession number AE007317.1. Protein search was performed with Microbial BLAST using the Megablast algorithm (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastp&PAGE_TYPE=BlastSearch&BLAST_SPEC=MicrobialGenomes&LINK_LOC=blasttab&LAST_PAGE=blastp). The Mef(A) protein sequence is available at the GenBank accession no. AAT72347; spr0971, spr1023, and spr1932 protein sequences are available under accession numbers NP_358565.1, NP_358617.1, and NP_359523.1, respectively.