Abstract
Background:
Galectin-3 (gal-3) is a β-galactoside-binding lectin associated tissue fibrosis and inflammation. There is limited understanding of the relationship between gal-3 and vascular health. Our aim was to assess the association between gal-3 and arterial stiffness in older adults.
Methods:
We conducted a cross-sectional study of 4275 participants (mean age of 75 years) from the Atherosclerosis Risk in Communities (ARIC) Study. Central arterial stiffness was measured by carotid-femoral pulse wave velocity (cfPWV). We evaluated the association of gal-3 with cfPWV using multivariable linear regression.
Results:
The median (interquartile range) gal-3 concentration was 16.5 (13.8, 19.8) ng/mL and mean cfPWV was 1163±303 cm/s. Higher gal-3 concentration was associated with greater central arterial stiffness after adjustment for age, sex, race-center, heart rate, systolic blood pressure, anti-hypertensive medication use, and current smoking status (β 36.4 cm/s change in cfPWV per log unit change in gal-3; 95% CI: 7.2, 65.5, p=0.015). The association was attenuated after adjusting for additional cardiovascular risk factors (β 17.3, 95% CI −14.4, 49.0).
Conclusion:
In community-dwelling older adults, gal-3 concentration was associated with central arterial stiffness, likely sharing common pathways with traditional cardiovascular risk factors.
Keywords: Galectin-3, arterial stiffness, aging
Introduction
Galectin-3 (gal-3) is a β-galactoside-binding lectin that is associated with pathophysiologic processes including inflammation, fibrosis and metabolic disorders. Recently, there has been an increased interest in gal-3 as a biomarker for cardiovascular disease as mounting evidence suggests that increased concentrations are associated with heart failure (HF), atrial fibrillation, atherosclerosis, pulmonary hypertension, and adverse cardiovascular outcomes (1–7). The role of gal-3 in modulating pathways of inflammation and fibrosis makes it an interesting candidate in the study of myocardial and vascular remodeling, especially pertaining to patients with HF and hypertension (8, 9). While elevated biomarker levels are known to be associated with these conditions, the relationship between gal-3 and arterial stiffness is less well understood (10).
Progression of arterial stiffness reflects the complex interplay of age-related vessel remodeling and oxidative stress, inflammation and vascular strain from hypertension or metabolic derangement (11, 12). Central arterial stiffness as measured by aortic pulse wave velocity has been associated with an increased risk for cardiovascular events and all-cause mortality (13–15). There have been a few studies assessing the association between gal-3 and arterial stiffness; Libhaber et. al. showed that gal-3 was independently associated with aortic stiffness as measured by carotid-femoral pulse wave velocity (cfPWV) in 966 black community participants from South Africa with a mean age of 43.4 years (16). Smaller studies have also shown significant associations between gal-3 and arterial stiffness in patients with prevalent HF and those on hemodialysis (17, 18). Data is limited on the relationship between gal-3 and arterial stiffness in older age, when vascular pathologies most often manifests. In this analysis of participants in the Atherosclerosis Risk in Communities (ARIC) study, we aim to evaluate the cross-sectional relationship between gal-3 and arterial stiffness in a community-dwelling older white and black adults without prevalent HF in order to better understand the role of gal-3 in vascular aging as well as to assess gal-3 as a surrogate biomarker for arterial stiffness independent of traditional cardiovascular risk factors.
Methods
Patients
The ARIC study is a prospective, population cohort of adults who were middle-aged (aged 45–64 years at visit 1) when recruited from 4 U.S. communities between 1987–1989. Patients were followed during multiple subsequent study visits. The study protocol was approved by the institutional review boards of all participating centers, and all participants provided written informed consent. For the present analysis, of the 6,538 individuals who participated in ARIC visit 5 (2011–2013), we excluded those with missing gal-3 measurements (N=130); those missing cfPWV or with evidence of biased PWV waveforms (N=1033), those of race other than white or black (N=18); black race at the Minneapolis and Washington County centers due to small numbers (N=24); history of aortic aneurysms (N=99); history of aortic or peripheral revascularization or graft (N=46); moderate or greater aortic stenosis (peak velocity across the aortic valve of >3m/s) (N=27); moderate or greater aortic insufficiency (determined by visual estimation on echocardiography) (N=25); body mass index (BMI) ≥40 (N=233); prevalent HF at visit 5 (N=376); and missing co-variates (N=222) (19). After exclusions (Figure 1), there were 4275 participants included for analyses. Prevalent HF was defined as signs and symptoms of HF by the Gothenberg criteria at visit 1 or adjudicated HF hospitalization between ARIC visit 1 and visit 5 as determined by ICD-9 code 428 (20). Medical history, demographic data, anthropometric data, blood pressure measurements, and lipid measurements were obtained at visit 5. Hypertension was defined by systolic blood pressure (SBP) ≥140 mm Hg, diastolic blood pressure (DBP) ≥90 mm Hg, or reported use of antihypertensive medications. Mean arterial pressure was calculated as 1/3 SBP + 2/3 DBP. Diabetes mellitus was defined as either self-reported diabetes diagnosed by a physician, use of hypoglycemic medications, non-fasting serum glucose levels ≥200 mg/dL, or fasting serum glucose level ≥126 mg/dL (21). Estimated glomerular filtration rate (eGFR) was based on the creatinine-based Chronic Kidney Disease Epidemiology Collaboration Equation. Prevalent peripheral artery disease (PAD) at visit 5 was defined as having prevalent PAD at visit 4, incident PAD between visit 4 and visit 5, or ankle-brachial index (ABI) <0.9 at visit 5, where ABI was determined as the ratio of the ankle SBP to brachial SBP using the higher value of the right or left brachial SBP as the denominator (22).
Figure 1.
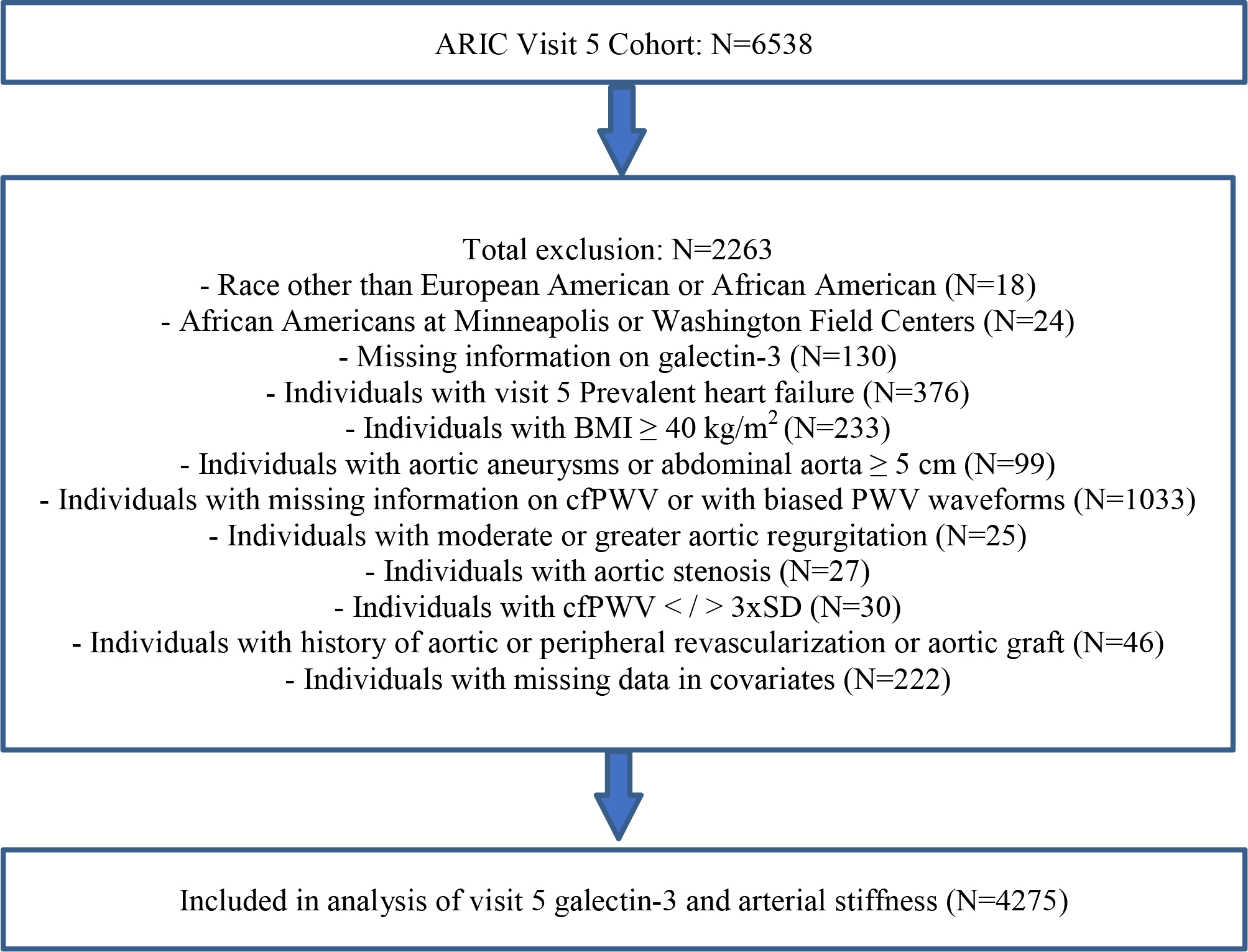
Inclusion and exclusion criteria.
Galectin-3 and Other Biomarker Measurements
Gal-3 was measured using a chemiluminescent immunoassay on an Architect i 2000sr platform (Abbott, Abbott Park, IL) from EDTA-plasma samples. Samples were collected at ARIC visit 5 and were stored at −70°C before measurement (March 2017 – December 2017). The limit of detection for the assay was 1.1 ng/mL and limit of quantitation was 4.0 ng/mL. Interassay coefficients of variation were 5.2%, 3.3%, and 2.3% at mean galectin-3 levels of 8.8 ng/mL, 19.2 ng/mL, and 72.0 ng/ml, respectively. The reliability coefficient was r=0.92. The coefficient of variation was 7.5% based on 402 blinded quality-control samples.
High-sensitivity C-reactive protein (hsCRP) was measured by using an immunonephelometric assay on a BNII autoanalyzer (Siemens Healthcare Diagnostics, Deerfield, Illinois) with a reliability coefficient of 0.99 (23).
Pulse Wave Velocity Measurements
Detailed descriptions of measurement and quality assurance of cfPWV in ARIC has been described previously (24). Briefly, carotid-femoral PWV was measured following a standardized protocol with the automated waveform analyzer VP-1000 Plus (Omron, Kyoto, Japan). A minimum of 2 measurements were taken per participant and the last 2 measurements were averaged.
Statistical Analysis
Baseline characteristics for the study population at visit 5 were tabulated by quartiles of gal-3 concentration. P-values for linear trend across gal-3 quartiles were calculated using test of trend. Gal-3 was evaluated as a continuous variable (natural log transformed due to non-normality) and as a categorical variable grouped by quartiles (quartile 1 as the reference group). Linear regression analyses were performed to assess the cross-sectional association between visit 5 gal-3 and cfPWV treated as a continuous variable. The beta-coefficient from the linear coefficient is expressed as cm/s change in cfPWV per log unit change in gal-3. Logistic regression analyses were also performed to assess the association between gal-3 and cfPWV treated as a categorical variable (≥75 percentile vs <75 percentile as the reference). Step-wise adjustment models were constructed with co-variates selected a-priori, first accounting for likely confounders (models 1 and 2) and then including covariates that may be potential modifiers (model 3). Model 1 adjusted for age, sex, race-center while model 2 adjusted for model 1 plus heart rate, SBP, anti-hypertensive medication use, current smoking status and finally model 3 adjusted for model 2 plus diabetes status, low-density lipoprotein cholesterol (LDL-C), eGFR, hsCRP, and body mass index (BMI). We then performed stratified analyses with respect to sex, race, diabetes status, hypertension status, inflammation status (based on hsCRP < 2mg/L vs ≥ 2mg/L), and prevalent PAD to evaluate whether these factors may be effect modifiers of the association between gal-3 and cfPWV.
Results
Baseline characteristics of participants at visit 5 gal-3 quartiles are shown in Table 1. The mean age at visit 5 was 75.2±5.0 years and 59.5% were women. The median (interquartile range) gal-3 level at visits 5 was 16.5 (13.8, 19.8) ng/mL respectively while the mean cfPWV at visit 5 was 1163±303 cm/s. Overall, individuals with higher gal-3 at visit 5 were more likely to be older, female, black, have higher pulse pressure, heart rate, BMI; were more likely to be users of anti-hypertensive and cholesterol lowering medication, and were more likely to have hypertension, diabetes and prevalent coronary heart disease. Higher gal-3 was also associated with lower DBP, mean arterial pressure, LDL-C and eGFR. Finally, higher gal-3 categories were associated with higher frequencies of prevalent PAD.
Table 1.
Baseline characteristics across galectin-3 quartiles at visit 5 of the ARIC Study.
| Visit 5 galectin-3 quartiles (ng/mL) | P trend | ||||
|---|---|---|---|---|---|
| Q1 (4.2–13.8) | Q2 (13.9–16.5) | Q3 (16.6–19.8) | Q4 (19.9–94.3) | ||
| cfPWV (cm/s) | 1128±293.2 | 1155±294.8 | 1177±301.0 | 1188±319.3 | <0.001 |
| Age (year) | 74.3±4.9 | 74.6±4.6 | 75.5±5.0 | 76.5±5.3 | <0.001 |
| Female % | 46.8 | 57.2 | 63.5 | 70.7 | <0.001 |
| Black % | 16.0 | 19.9 | 23.6 | 27.9 | <0.001 |
| SBP (mmHg) | 129.5±17.1 | 130.2±16.9 | 130.5±17.3 | 130.0±18.3 | 0.297 |
| DBP (mmHg) | 67.1±10.3 | 66.8±10.1 | 66.6±10.5 | 64.6±10.4 | <0.001 |
| Pulse pressure (mmHg) | 62.4±13.7 | 63.4±13.7 | 63.9±14.1 | 65.4±15.1 | <0.001 |
| Mean arterial pressure (mmHg) | 87.9±11.3 | 87.9±11.1 | 87.9±11.4 | 86.4±11.5 | 0.003 |
| Heart beat (beat/min) | 64.1±10.8 | 65.1±10.7 | 65.6±10.9 | 65.6±11.1 | 0.001 |
| Hypertensive medication % | 59.7 | 69.7 | 72.9 | 86.4 | <0.001 |
| Hypertension % | 62.6 | 70.4 | 72.8 | 83.7 | <0.001 |
| BMI (kg/m2) | 27.2±4.3 | 27.6±4.4 | 27.8±4.5 | 28.6±4.6 | <0.001 |
| Diabetes (%) | 26.1 | 23.1 | 30.5 | 36.3 | <0.001 |
| LDL-C (mg/dL) | 108.3±32.9 | 107.1±33.9 | 106.8±35.7 | 102.0±34.8 | <0.001 |
| Cholesterol lower medication (%) | 48.4 | 53.8 | 54.9 | 62.6 | <0.001 |
| Current smoking (%) | 6.0 | 5.0 | 6.1 | 5.6 | 0.982 |
| eGFR (mL/min/1.73m2) | 77.7±13.1 | 73.9±14.2 | 70.9±15.0 | 61.2±18.3 | <0.001 |
| Hs-CRP (mg/L) | 1.5 (0.8, 3.1) | 1.8 (0.9, 3.6) | 1.9 (0.9, 3.9) | 2.3 (1.1, 4.7) | <0.001 |
| Prevalent CHD (%) | 9.7 | 12.0 | 11.6 | 14.6 | 0.001 |
| Prevalent PAD (%) | 4.3 | 6.8 | 8.8 | 14.1 | <0.001 |
Data presented as mean±sd, median (25th, 75th percentile), or percentage; P trend were calculated by test of trend across ordered groups. Abbreviations: cfPWV = carotid-femoral pulse wave velocity, SBP = systolic blood pressure, DBP = diastolic blood pressure, BMI = body mass index, LDL-C = low-density lipoprotein cholesterol, eGFR = estimated glomerular filtration rate, CHD = coronary heart disease, hs-CRP = high-sensitivity C-reactive protein, PAD = peripheral artery disease
When analyzed as a continuous variable, higher gal-3 was significantly associated with greater central arterial stiffness as measured by cfPWV after adjustments for model 1 (β 53.9 cm/s change in cfPWV per log unit change in gal-3, 95% CI 22.8, 85.0, p=0.001) and model 2 covariates (β 36.4, 95% CI 7.2, 65.5, p=0.015). However, the association was attenuated and became statistically non-significant after additional adjustments with model 3 covariates (β 17.3, 95% CI −14.4, 49.0).
In an analysis stratified by diabetes status, there was a significant association between gal-3 and arterial stiffness observed in adults without diabetes (β 35.1 95% CI 0.8, 69.3, p=0.045) and without hypertension (β 73.9, 95% CI 19.5, 128.2, p=0.008) subgroups up to model 2 adjustment but not in their respective subgroups with disease. The association was attenuated after model 3 adjustment (β 24.9, 95% CI −12.1, 61.9, p=0.186 for individuals without diabetes; β 52.2, 95% CI −3.72, 108.1, p=0.067 for individuals without hypertension). In the subgroup of participants without both diabetes and hypertension, there was a significant association between gal-3 and arterial stiffness even after adjustment for model 3 (β 67.2 95% CI 9.2, 125.3, p=0.023). However, the p-interaction was not significant. There were no significant differences in association noted between white and black race or for any other subgroups (all p-interaction >0.05). Furthermore, there were no significant associations in other subgroups when adjusting for model 2 or model 3 covariates (Table 2).
Table 2.
Linear regression showing the association of visit 5 galectin-3 as a continuous variable (natural log transformed) and arterial stiffness as measured by visit 5 carotid-femoral pulse wave velocity in the overall included participants as well as by subgroups.
| Β coefficient | 95% CI | P value | ||
|---|---|---|---|---|
| Overall (N=4275) | Model 1 | 53.9 | 22.8, 85.0 | 0.001 |
| Model 2 | 36.4 | 7.2, 65.5 | 0.015 | |
| Model 3 | 17.3 | −14.4, 49.0 | 0.285 | |
| Men (N=1731) | Model 1 | 81.6 | 31.8, 131.5 | 0.001 |
| Model 2 | 46.1 | −1.1, 93.3 | 0.055 | |
| Model 3 | 26.5 | −25.1, 78.1 | 0.314 | |
| Women (N=2544) | Model 1 | 34.4 | −5.3, 74.2 | 0.090 |
| Model 2 | 28.5 | −8.6, 65.7 | 0.132 | |
| Model 3 | 11.9 | −28.4, 52.2 | 0.563 | |
| Whites (N=3342) | Model 1 | 51.8 | 17.9, 85.8 | 0.003 |
| Model 2 | 27.4 | −4.5, 59.4 | 0.093 | |
| Model 3 | 5.6 | −28.7, 39.8 | 0.751 | |
| Blacks (N=933) | Model 1 | 60.8 | −13.3, 134.9 | 0.108 |
| Model 2 | 68.9 | −0.2, 138.1 | 0.051 | |
| Model 3 | 62.0 | −16.8, 140.9 | 0.123 | |
| Non-diabetics (N=3037) | Model 1 | 52.0 | 15.4, 88.5 | 0.005 |
| Model 2 | 35.1 | 0.8, 69.3 | 0.045 | |
| Model 3 | 24.9 | −12.1, 61.9 | 0.186 | |
| Diabetes (N=1238) | Model 1 | 14.9 | −43.2, 72.9 | 0.615 |
| Model 2 | 24.2 | −30.7, 79.2 | 0.387 | |
| Model 3 | 3.2 | −58.2, 64.6 | 0.918 | |
| Non-hypertension (N=1176) | Model 1 | 109.2 | 51.7, 166.8 | <0.001 |
| Model 2 | 73.9 | 19.5, 128.2 | 0.008 | |
| Model 3 | 52.2 | −3.7, 108.1 | 0.067 | |
| Hypertension (N=3073) | Model 1 | 12.5 | −24.5, 49.6 | 0.507 |
| Model 2 | 25.0 | −9.7, 59.6 | 0.158 | |
| Model 3 | 7.9 | −30.4, 46.2 | 0.685 | |
| Non-hypertension & non-diabetes (N=975) | Model 1 | 103.4 | 44.2, 162.6 | 0.001 |
| Model 2 | 72.8 | 16.7, 128.9 | 0.011 | |
| Model 3 | 67.2 | 9.2, 125.3 | 0.023 | |
| Hypertension or diabetes (N=3281) | Model 1 | 18.0 | −18.0, 54.0 | 0.327 |
| Model 2 | 26.9 | −7.0, 60.9 | 0.120 | |
| Model 3 | 4.6 | −32.7, 42.0 | 0.808 | |
| hs-CRP<2 mg/L (N=2289) | Model 1 | 59.9 | 18.4, 101.4 | 0.005 |
| Model 2 | 32.0 | −7.0, 71.0 | 0.108 | |
| Model 3 | 22.0 | −19.9, 63.9 | 0.304 | |
| hs-CRP≥2 mg/L (N=1986) | Model 1 | 29.7 | −17.2, 76.6 | 0.214 |
| Model 2 | 16.2 | −8.0, 80.4 | 0.108 | |
| Model 3 | 9.8 | −38.6, 58.3 | 0.691 | |
| Non-prevalent PAD (N=3905) | Model 1 | 45.3 | 13.0, 77.6 | 0.006 |
| Model 2 | 32.2 | 1.9, 62.5 | 0.037 | |
| Model 3 | 11.7 | −20.9, 44.3 | 0.483 | |
| Prevalent PAD (N=363) | Model 1 | 71.7 | −46.0, 189.3 | 0.232 |
| Model 2 | 29.7 | −82.4, 141.8 | 0.603 | |
| Model 3 | 53.3 | −79.2, 185.8 | 0.429 |
Model 1: Adjusted by v5 age, gender, and race-center; model 2: model 1 plus v5 heart rate, anti-hypertensive medication user, current smoking, and SBP; model 3: model 2 plus LDL-C, eGFR, BMI, diabetes status and hs-CRP
When treating gal-3 as a categorical variable, there was not a consistent association between higher categories of gal-3 with cfPVW. For instance, there was a statistically significant association between higher levels of gal-3 with cfPWV after model 1 adjustment when comparing Quartile 3 vs Quartile1 (β 30.9, 95% CI 6.0, 55.7), but Quartile 4 vs Quartile 1 was not significant (β 23.9, 95% CI −1.6, 49.4). There was also no significant association between quartiles of gal-3 with cfPWV after adjustments with model 2 and model 3 covariates (data not shown).
In logistic regression analysis, there was a significant association between higher gal-3 with increased odds of ≥75 percentile vs <75 percentile cfPWV after model 2 adjustment (OR 1.42, 95% CI 1.07, 1.88, p=0.014). The association was attenuated after additional adjustments with model 3 covariates (OR 1.21, 95% CI 0.89, 1.65) (Table 3). There was a significant odds for elevated cfPWV when comparing quartile 4 with quartile 1 gal-3 after model 1 adjustment (OR 1.25, 95% CI 1.01, 1.55). But the association between quartiles of gal-3 with cfPWV was attenuated after adjustments with model 2 and model 3 covariates (data not shown).
Table 3.
Logistic regression with visit 5 gal-3 as a continuous as the independent variable and visit 5 cfPWV as dependent variable treated as dichotomous variable (≥75 percentile of cfPWV vs <75 percentile as reference).
| Odds ratio | 95% CI | P value | |
|---|---|---|---|
| Model 1 | 1.52 | 1.17–1.96 | 0.001 |
| Model 2 | 1.42 | 1.07–1.88 | 0.014 |
| Model 3 | 1.21 | 0.89–1.65 | 0.218 |
Model 1: Adjusted by v5 age, gender, and race-center; model 2: model 1 plus v5 heart rate, anti-hypertensive medication user, current smoking, and SBP; model 3: model 2 plus LDL-C, eGFR, BMI, diabetes status and hs-CRP
Discussion
In this analysis of the association between circulating gal-3 levels with central arterial stiffness in older adults, we found that gal-3 was significantly associated with cfPWV after adjustment for age, sex, race-center, heart rate, SBP, anti-hypertensive medication use, and current smoking status. However, the association was attenuated and became non-significant after further adjustment for additional cardiovascular risk factors including diabetes, LDL-C, eGFR, hsCRP, and BMI. The association of gal-3 with arterial stiffness appeared to be stronger in subgroups of participants without hypertension and diabetes. The results suggest that gal-3 demonstrate significant association with arterial stiffness in older adults but this relationship likely shares common pathways, and thus are not independent of other cardiovascular risk factors such as diabetes and hypertension.
Our results differ from data from previous studies that showed significant association between circulating gal-3 levels and arterial stiffness even after adjustment of multiple cardiovascular risk factors (16–18). The differences may be due to a difference in the study population. Notably, individuals included in our study were older, of white and black race who did not have prevalent HF, which differs from those that were examined in prior studies: younger patients of black race, patients with HF and end-stage renal disease on dialysis. Our results therefore build upon findings of previous studies and suggest that while gal-3 demonstrate significant association with arterial stiffness in selected patient populations, this relationship is likely not independent of traditional cardiovascular risk factors in older adults.
Gal-3 levels were (positively) correlated with risk factors such as hypertension and diabetes, suggesting that gal-3 may be a potential marker, or mediator between these risk factors and arterial stiffness. Though higher gal-3 levels were correlated with decreased DBP, this is likely reflective of the widening pulse pressure observed, another marker of arterial stiffness. A correlation with lower MAP is likely related to lower DBP as well as the increased use of anti-hypertensive medication in these patients. Likewise, the lower LDL-C noted in the higher gal-3 categories likely reflect an increase in cholesterol lowering medication use among these patients who have higher risk for CVD. Risk factors such as hypertension and diabetes contribute to endothelial dysfunction, inflammation and vascular fibrosis (25, 26). These risk factors may induce increased expression of gal-3, a known modulator of inflammation and fibrosis, in activated macrophages, endothelial and vascular smooth muscle cells ultimately leading to pathological vascular remodeling (27). Meanwhile, in the absence of hypertension and diabetes, gal-3 may represent a marker for unexplained vascular stiffness. More detailed mechanism by which these co-morbidities relate to gal-3 and vascular health deserves further investigation.
Age may also be an important modifier in the relationship between gal-3 and arterial stiffness. Both gal-3 levels and arterial stiffness measures are lower at younger age. For instance, in the study by Libhaber et al (mean age ~43 years), the mean gal-3 level was 8.88 (SD 4.02) and the mean cfPWV was 6.43 (SD 2.69), thus much lower than those observed in our study (16). Elevated gal-3 may reflect ongoing vascular remodeling. In younger age, the ability of gal-3 to identify increased arterial stiffness may be relatively larger as risk factors for vascular health such as hypertension and diabetes are less prevalent and exposure time to these risk factors are shorter. For instance, we found that gal-3 was more significantly associated with arterial stiffness in individuals without hypertension and diabetes (but less so among those with these conditions). As gal-3 appears to be significantly correlated with traditional cardiovascular risk factors, prolonged exposure to these risk factors with aging likely attenuates the effects of gal-3 as an independent biomarker of arterial stiffness. Furthermore, gal-3 may reflect vascular pathogenesis, a process that may not be as robust in older age when subclinical or clinical vascular disease is more likely to have already established.
A study of gal-3 from an earlier visit from the ARIC study found that gal-3 was significantly associated with incident PAD independent of traditional cardiovascular risk factors (28). In our current analysis, gal-3 was also associated with an increased frequency of prevalent PAD. Interestingly, the median levels of gal-3 of included patients in our analysis (median gal-3 of 16.5 ng/mL) was numerically similar to those individuals with incident PAD (median gal-3 16.9 ng/mL) in the previous ARIC analysis. However, the participants were more than a decade older in our current analysis. Taken together, gal-3 appears to contribute to development of arterial pathology as reflected by increased risk of PAD at earlier age as well as central arterial stiffening in older adults.
There are some limitations to our study. While we measured galectin-3 in blood, it remains unclear how circulating biomarkers reflect tissue specific expression and future studies to clarify this relationship are needed. Also, as this was an observational study, there is possibility of residual confounding and selection bias.
Conclusion
In this study, circulating blood levels of gal-3 was significantly associated with central arterial stiffness in older adults. However, our results suggest that this association is not independent of traditional cardiovascular risk factors and that gal-3 likely shares common pathways with risk factors such as hypertension and diabetes in the development of arterial stiffness in older age.
Acknowledgement
The authors thank the staff and participants of the ARIC study for their important contributions.
Funding
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I).
Footnotes
Conflict of interest
V.N.: site PI study sponsored by Merck. S.S.V.: honorarium: American College of Cardiology (Associate editor for Innovations acc.org); Steering Committee member: PALM registry at Duke Clinical Research Institute (no financial remuneration). E.S.: honoraria from Novo Nordisk. R.H.: grant support and consulting fees from Denka Seiken outside the submitted work. C.M.B.: grants/research support (significant; paid to institution, not individual): NIH R01 HL134320 Abbott Diagnostic, Roche Diagnostic; consultant (modest): Abbott Diagnostic, Denka Seiken, Roche Diagnostic.
X.J., C.S., H.T., M.A.R., D.A., C.N., G.H.: none.
References
- 1.Ho JE, Liu C, Lyass A, Courchesne P, Pencina MJ, Vasan RS, et al. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. 2012;60(14):1249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fashanu OE, Norby FL, Aguilar D, Ballantyne CM, Hoogeveen RC, Chen LY, et al. Galectin-3 and incidence of atrial fibrillation: The Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2017;192:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madrigal-Matute J, Lindholt JS, Fernandez-Garcia CE, Benito-Martin A, Burillo E, Zalba G, et al. Galectin-3, a biomarker linking oxidative stress and inflammation with the clinical outcomes of patients with atherothrombosis. J Am Heart Assoc. 2014;3(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barman SA, Chen F, Li X, Haigh S, Stepp DW, Kondrikov D, et al. Galectin-3 Promotes Vascular Remodeling and Contributes to Pulmonary Hypertension. Am J Respir Crit Care Med. 2018;197(11):1488–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Vark LC, Lesman-Leegte I, Baart SJ, Postmus D, Pinto YM, de Boer RA, et al. Prognostic Value of Serial Galectin-3 Measurements in Patients With Acute Heart Failure. J Am Heart Assoc. 2017;6(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aguilar D, Sun C, Hoogeveen RC, Nambi V, Selvin E, Matsushita K, et al. Levels and Change in Galectin-3 and Association With Cardiovascular Events: The ARIC Study. J Am Heart Assoc. 2020;9(13):e015405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McEvoy JW, Chen Y, Halushka MK, Christenson E, Ballantyne CM, Blumenthal RS, et al. Galectin-3 and Risk of Heart Failure and Death in Blacks and Whites. J Am Heart Assoc. 2016;5(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez B, Gonzalez A, Querejeta R, Zubillaga E, Larman M, Diez J. Galectin-3 and histological, molecular and biochemical aspects of myocardial fibrosis in heart failure of hypertensive origin. Eur J Heart Fail. 2015;17(4):385–92. [DOI] [PubMed] [Google Scholar]
- 9.Calvier L, Miana M, Reboul P, Cachofeiro V, Martinez-Martinez E, de Boer RA, et al. Galectin-3 mediates aldosterone-induced vascular fibrosis. Arterioscler Thromb Vasc Biol. 2013;33(1):67–75. [DOI] [PubMed] [Google Scholar]
- 10.Meijers WC, Januzzi JL, deFilippi C, Adourian AS, Shah SJ, van Veldhuisen DJ, et al. Elevated plasma galectin-3 is associated with near-term rehospitalization in heart failure: a pooled analysis of 3 clinical trials. Am Heart J. 2014;167(6):853–60 e4. [DOI] [PubMed] [Google Scholar]
- 11.Weisbrod RM, Shiang T, Al Sayah L, Fry JL, Bajpai S, Reinhart-King CA, et al. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension. 2013;62(6):1105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galis ZS, Thrasher T, Reid DM, Stanley DV, Oh YS. Investing in high blood pressure research: a national institutes of health perspective. Hypertension. 2013;61(4):757–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63(7):636–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(13):1318–27. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, et al. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study. Circulation. 2010;122(14):1379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Libhaber E, Woodiwiss AJ, Raymond A, Gomes M, Maseko MJ, Sareli P, et al. Independent associations of circulating galectin-3 concentrations with aortic pulse wave velocity and wave reflection in a community sample. Hypertension. 2015;65(6):1356–64. [DOI] [PubMed] [Google Scholar]
- 17.Oikonomou E, Karlis D, Tsalamadris S, Siasos G, Chrysohoou C, Vogiatzi G, et al. Galectin-3 and Arterial Stiffness in Patients with Heart Failure: A Pilot Study. Curr Vasc Pharmacol. 2019;17(4):396–400. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Q, Yin K, Zhu M, Lin X, Fang Y, Lu J, et al. Galectin-3 is associated with arterial stiffness among hemodialysis patients. Biomark Med. 2019;13(6):437–43. [DOI] [PubMed] [Google Scholar]
- 19.Shah AM, Claggett B, Loehr LR, Chang PP, Matsushita K, Kitzman D, et al. Heart Failure Stages Among Older Adults in the Community: The Atherosclerosis Risk in Communities Study. Circulation. 2017;135(3):224–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol. 2008;101(7):1016–22. [DOI] [PubMed] [Google Scholar]
- 21.Kucharska-Newton AM, Couper DJ, Pankow JS, Prineas RJ, Rea TD, Sotoodehnia N, et al. Diabetes and the risk of sudden cardiac death, the Atherosclerosis Risk in Communities study. Acta Diabetol. 2010;47 Suppl 1:161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsushita K, Ballew SH, Sang Y, Kalbaugh C, Loehr LR, Hirsch AT, et al. Ankle-brachial index and physical function in older individuals: The Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis. 2017;257:208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Heiss G, et al. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2004;109(7):837–42. [DOI] [PubMed] [Google Scholar]
- 24.Meyer ML, Tanaka H, Palta P, Cheng S, Gouskova N, Aguilar D, et al. Correlates of Segmental Pulse Wave Velocity in Older Adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Hypertens. 2016;29(1):114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spinetti G, Kraenkel N, Emanueli C, Madeddu P. Diabetes and vessel wall remodelling: from mechanistic insights to regenerative therapies. Cardiovasc Res. 2008;78(2):265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrie JR, Guzik TJ, Touyz RM. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can J Cardiol. 2018;34(5):575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pugliese G, Iacobini C, Pesce CM, Menini S. Galectin-3: an emerging all-out player in metabolic disorders and their complications. Glycobiology. 2015;25(2):136–50. [DOI] [PubMed] [Google Scholar]
- 28.Ding N, Yang C, Ballew SH, Kalbaugh CA, McEvoy JW, Salameh M, et al. Fibrosis and Inflammatory Markers and Long-Term Risk of Peripheral Artery Disease: The ARIC Study. Arterioscler Thromb Vasc Biol. 2020;40(9):2322–31. [DOI] [PMC free article] [PubMed] [Google Scholar]


