Abstract
Platelet-derived growth factor BB (PDGF BB) is a potent mitogen for fibroblasts as well as many other cell types. Interaction of PDGF BB with the PDGF β receptor (PDGF-βR) activates numerous signaling pathways and leads to a decrease in receptor expression on the cell surface. PDGF-βR downregulation is effected at two levels, the immediate internalization of ligand-receptor complexes and the reduction in pdgf-βr mRNA expression. Our studies show that pdgf-βr mRNA suppression is regulated by the c-myc proto-oncogene. Both constitutive and inducible ectopic Myc protein can suppress pdgf-βr mRNA and protein. Suppression of pdgf-βr mRNA in response to Myc is specific, since expression of the related receptor pdgf-αr is not affected. We further show that Myc suppresses pdgf-βr mRNA expression by a mechanism which is distinguishable from Myc autosuppression. Analysis of c-Myc-null fibroblasts demonstrates that Myc is required for the repression of pdgf-βr mRNA expression in quiescent fibroblasts following mitogen stimulation. In addition, it is evident that the Myc-mediated repression of pdgf-βr mRNA levels plays an important role in the regulation of basal pdgf-βr expression in proliferating cells. Thus, our studies suggest an essential role for Myc in a negative-feedback loop regulating the expression of the PDGF-βR.
The platelet-derived growth factor beta receptor (PDGF-βR) has been extensively studied in the context of wound healing and carcinogenesis, where many of the biological activities of the PDGF-βR clearly play a role (reviewed in references 6, 7, 12, and 13). The activated PDGF-βR can elicit diverse and seemingly paradoxical biological activities, including cell growth, cell survival, differentiation, and cellular transformation. Activation of the PDGF-βR occurs rapidly following PDGF ligand-receptor interaction. PDGF A and B polypeptides can form homodimers (AA and BB) or a heterodimer (AB), and they bind two distinct PDGF receptors (PDGF-αR and PDGF-βR) with differing affinities. In fibroblasts, the PDGF-αR is expressed at low abundance and binds with high affinity to all three PDGF isoforms, whereas the PDGF-βR is highly abundant and exhibits restricted binding to two isoforms: PDGF BB with high affinity and PDGF AB with lower affinity (reviewed in reference 28). Ligand binding results in receptor dimerization, which induces autophosphorylation at multiple tyrosine residues and activates the receptor (61). The phosphorylated tyrosine residues serve as binding sites for a host of SH2 domain-containing proteins, which include phospholipase C-γ1, the GTPase-activating protein of Ras, the regulatory subunit of phosphatidylinositol-3-kinase, the phosphotyrosine phosphatase Syp, Src-family members (Src, Yes and Fyn), Nck, Shc, and additional uncharacterized proteins (12). Following receptor binding, these proteins are activated through phosphorylation and subsequently stimulate a number of signal transduction cascades, which are dependent on the receptor-associated proteins recruited. These signaling pathways include the activation of mitogen-activated protein kinase and the induction of immediate-early growth response genes such as the c-myc proto-oncogene. The mechanisms used to fine-tune receptor-induced signaling and the full diversity of the signaling potential of the receptor remain unclear.
Elevated Myc protein expression following mitogen stimulation induces a number of biological activities. These include driving cell cycle progression, blocking differentiation, inducing apoptosis, and contributing to tumorigenesis (reviewed in reference 21). Deregulated Myc expression is a strong potentiator of tumorigenesis and is a common hallmark of a wide range of tumors (reviewed in reference 44). Myc is thought to function as a central regulator of such diverse cellular activities by both activating and repressing gene transcription. Interestingly, recent studies employing Myc mutants or the cMycS isoform suggest that the role of Myc as a negative regulator of gene expression is central to transformation (8, 33, 64). Despite the important role of Myc repression of gene transcription, relatively few targets have been identified to date.
Myc induces or represses gene transcription by distinct mechanisms of action. Myc functions as a transcriptional activator when heterodimerized with its partner Max (reviewed in reference 37), forming a DNA binding domain which binds directly to E-box elements to activate transcription of target genes including the carbamoyl-phosphate synthase (glutamine hydrolyzing)–aspartate carbamoyltransferase–dihydroorotase gene (cad) (42), the ornithine decarboxylase gene (odc) (5), and cdc25A (22). Identified Myc-repressed genes include growth arrest and DNA damage-inducible gene, gadd45 (38), the H-ferritin gene (63), c/ebpα (2, 34), the adenovirus 5 major late promoter gene (AdMLP) (34), and c-myc (19). Although the precise mechanism through which Myc represses gene transcription remains unclear, evidence suggests that there may be more than one mechanism for this function (reviewed in reference 21). The c-myc gene is one of the best characterized Myc repressed target genes. Myc autosuppression is a homeostatic regulatory mechanism which results in the repression of endogenous c-myc expression at the level of transcription initiation. This feedback mechanism functions in a Myc dose-dependent manner, requires the interaction of Myc with Max, and occurs through the minimal promoter region of the c-myc gene in the absence of E-box elements (19, 25, 46, 47). Analysis of further repressed targets has shown that Myc represses the transcription of certain genes, such as the H-ferritin gene AdMLP, and c/ebpα through initiator (Inr) elements in the core promoter (2, 34, 63) which can occur through competition or interference with the transcription machinery (36, 54). Other genes, such as gadd45, are repressed in an Inr-independent manner (38). In this case, an enhancer-dependent mechanism of repression has been suggested (1). Investigation of additional Myc-repressed genes will allow us to delineate the molecular mechanism(s) of repression and help us to better understand how Myc modulates proliferation in normal and transformed cells. To this end, examination of new target genes in a novel c-myc null cell line (41) will facilitate the identification of true Myc targets. These cells contain no measurable c-, N-, or L-Myc expression and allow the investigation of proposed targets in a Myc null background for the first time. Indeed, many proposed target genes have been examined to date using this system, and cad, gadd45, and c-myc have been verified as bona fide Myc-regulated genes (9).
Given the potent mitogenic stimulus initiated by the ligand-activated PDGF-βR, it is not surprising that the cell possesses negative-feedback mechanisms to regulate receptor expression. Proliferation of nontransformed cells following exposure to mitogen is controlled through the down-regulation of growth factor receptors. Receptor down-regulation involves the internalization and degradation of receptor-ligand complexes (45, 53, 57). Additional mechanisms also play a role in this process, since ligand-receptor interaction also leads to receptor mRNA suppression, as shown for the colony-stimulating factor type 1 (c-fms) receptor (23), the c-kit receptor (23), and the PDGF-βR (62), all of which are members of the PDGF-R family (65). The mechanism of receptor mRNA suppression is unclear; however, recent studies have provided clues to the constituents of this pathway. Ectopic expression of activated ras or src in fibroblasts results in pdgf-βr mRNA down-regulation (62, 66). Since activated src can target Myc (4), the latter may serve as an effector of pdgf-βr mRNA suppression. Further support for this hypothesis comes from studies of small cell lung carcinoma by Plummer et al. (50), which showed an inverse correlation between the expression of Myc and the c-kit receptor. In this paper, we show that PDGF-βR expression is down-regulated in nontransformed fibroblast cells following exposure to either serum, PDGF BB ligand, or Myc-activation. The suppression of PDGF-βR levels is specific, since Myc activation has no effect on pdgf-αr expression. Myc suppression of pdgf-βr mRNA is effected at the RNA level by a unique mechanism which is distinct from Myc autosuppression. We demonstrate that Myc is required for the repression of pdgf-βr expression following serum stimulation. Moreover, we show that Myc repression of pdgf-βr mRNA levels plays an important role in regulating basal pdgf-βr expression in proliferating cells. Our results support a role for Myc in the homeostatic regulation of pdgf-βr mRNA levels in proliferating fibroblasts.
MATERIALS AND METHODS
Cell culture and somatic cell hybridizations.
Primary rat embryo fibroblasts (REF) were prepared as described previously (32). Rat-1, Rat-1 pMV7hc-myc(wt)/ER (wtMycER), and Rat-1 pMV7hc-myc(Δ106–143)/ER (ΔMycER) were described previously (15, 18, 46). The KPREF cell line is a population of spontaneously immortalized REF (46). Rat-1 wtMycERTM, and Rat-1 ΔMycERTM cell lines were generated by infecting Rat-1 cells with replication-incompetent retroviruses, either Babepuro c-mycERTM or Babepuro Δ106–143c-mycERTM. Cells were selected in medium supplemented with puromycin, and individual drug-resistant colonies were cloned. Clones were characterized for MycERTM expression, to ascertain that expression was within physiological levels and that wtMycERTM activation would induce cells to progress from the G0/G1 to the S phase of the cell cycle. Clones of our variant NIH 3T3 cell line, NIH 3T3v3 and NIH 3T3v10, were derived from and possessed identical properties to the parental population first described by Penn et al. (46). Wild-type TGR-1 (+/+) and c-Myc null HO15.19 (−/−) cell lines were described previously (41). c-Myc null HO15.19 gfp (−/− gfp) and c-Myc null HO15.19 gfpmyc (−/− gfpmyc) cells were generated by infecting c-Myc null HO15.19 (−/−) cells with either the retrovirus BabeMNIRESgfp or BabeMNIRESgfpmyc. Rat-1 wtMycER and Rat-1 ΔMycER cells were maintained in alpha modified Eagle's medium (αMEM) without phenol red but supplemented with 10% charcoal-treated serum (HyClone). Wild-type Rat-1 clone TGR-1 (+/+), c-Myc null HO15.19 (−/−), HO15.19 gfp (−/− gfp), and HO15.19 gfpmyc (−/− gfpmyc) cells were grown in Dulbecco's modified Eagle's medium (DMEM) H21 supplemented with 10% calf serum, as described previously (51). All other fibroblast cell lines were cultured in αMEM supplemented with 10% fetal bovine serum (FBS) (Sigma). The culture medium was supplemented with 100 μg of kanamycin per ml and 2 μg of gentamicin per ml or with 100 μg of penicillin per ml and 100 μg of streptomycin sulfate per ml. Somatic cell hybridizations were conducted as previously described (20, 46). To analyze the response to PDGF BB stimulation, subconfluent cells were cultured for 3 days in 0.3% FBS–αMEM before being stimulated with 40 ng of PDGF BB (Upstate Biotechnology) per ml. To activate ectopic Myc activity, Rat-1 wtMycER cells were exposed to 100 nM β-estradiol (Sigma) in ethanol, Rat-1 wtMycERTM cells were exposed to 100 nM 4-hydroxy (Z) tamoxifen (OH-T) in ethanol (Research Biochemical International, Natick, Mass.), and controls were exposed to ethanol alone. To analyze expression in the presence of cycloheximide, Rat-1 wtMycERTM cells were incubated with cycloheximide (10 μg/ml) (Sigma), OH-T and cycloheximide, or OH-T alone. To analyze the response to serum stimulation, subconfluent wild-type TGR-1 (+/+) and c-Myc null HO15.19 (−/−) cells were maintained in 0.25% calf serum–DMEM H21 for 2 days and then stimulated with 10% calf serum–DMEM H21. Unless otherwise stated, all cells were analyzed as subconfluent proliferating cultures.
Retroviral vectors.
The pDOR/neo, pDok/v-mycneo, pBabe/hygro, pBabe/v-mychygro, pBabe/puro, and pBabe/v-mycpuro retroviral vectors were constructed as previously described (20, 43, 46, 52). The pBabepuro c-mycERTM, and pBabepuro Δ106–143c-mycERTM retroviral vectors were the kind gift of G. Evan and T. Littlewood and were described previously (35). To generate the pBabeMNIRESgfpmyc retroviral vector, human c-myc exon II and III sequences excised from pBluescript KS(+) Hc-myc were cloned into EcoRI-XhoI-digested pBabeMNIRESgfp (a kind gift of G. Nolan) (29).
Retroviral infection.
To produce infectious replication-defective ecotropic retroviral particles, recombinant retroviral constructs were transfected, using calcium phosphate precipitation (24), into either the GP+E packaging cell line (39) or the Phoenix-Eco cell line (American Type Culture Collection) and selected by using 0.5 mg of G418 sulfate (Sigma) per ml, 2 mg of puromycin (Sigma) per ml, 300 μg of hygromycin B (Sigma) per ml or green fluorescent protein (GFP) expression. After drug selection, drug-resistant clones were pooled and expanded for virus production. Primary rodent embryo fibroblasts and cell lines were infected with retrovirus and selected in either G418, hygromycin B, or puromycin. Individual drug-resistant colonies were either isolated to produce clonal populations or combined to generate pooled populations. GFP-positive c-Myc null HO15.19 gfp (−/− gfp) and HO15.19 gfpmyc (−/− gfpmyc) cells were isolated with a Coulter 753 cell sorter using an absorption wavelength of 488 nm and pooled.
RNase protection.
RNA was prepared by the guanidinium isothiocyanate method of Chirgwin et al. (11). RNase protection was conducted essentially as described previously (46). The probes were generated using T3 RNA polymerase (Stratagene) from linearized Bluescript KS and SK cloning vectors (Stratagene) containing the following DNA fragments: rat c-myc exon I (46), the gag gene derived from the avian myelocytomatosis virus MC29 (46), and the murine glyceraldehyde-3-phosphate dehydrogenase (gapdh) gene (20). The mouse pdgf-βr probe comprised nucleotides 1 to 227 of the mouse pdgf-βr cDNA. This cDNA fragment was excised from λ-N16 (a kind gift of Y. Yarden) with EcoRI and SmaI and then cloned into a Bluescript KS vector (Stratagene). The Bluescript KS mouse pdgf-βr cDNA plasmid was linearized with BamHI, and the pdgf-βr RNase protection probe was transcribed using T3 RNA polymerase, preparing a probe which is 230 bp, whereas the protected fragment after digestion is 100 bp. The protected probes were resolved by electrophoresis on 6% denaturing polyacrylamide gels and visualized using a phosphorimager or by autoradiography on X-Omat film (Kodak). Densitometry was performed by phosphorimager analysis.
Northern blot analysis.
RNA was prepared by the guanidinium isothiocyanate method of Chirgwin et al. (11). Northern blot analysis was conducted as described previously (15). The rat pdgf-αr probe consisted of a 510-bp EcoRV fragment derived from pCRIIPDGFαR (58). The probe for 36B4, a gene encoding an acidic ribosomal protein, consisted of an 800-bp PstI fragment derived from p36B4 (40). Densitometry was performed by phosphorimager analysis.
PDGF BB binding studies.
Subconfluent rodent fibroblasts were washed twice with ice-cold phosphate-buffered saline containing 1% (wt/vol) bovine serum albumin (1% BSA–PBS). To determine high-affinity saturable [125I]PDGF BB binding, cells were incubated at 4°C in 500 μl of 1% BSA–PBS with increasing concentrations of [125I]PDGF BB in the absence (total binding) or presence (nonspecific binding) of a 100-fold excess of unlabeled PDGF BB. After 2 h of incubation, the medium was aspirated and the cells were washed five times with 1 ml of ice-cold 1% BSA–PBS. The remaining cells were extracted for 1 h by adding 1 ml of lysing buffer (1% [vol/vol] Triton X-100, 10 mM glycine, 20 mM HEPES [pH 7.4]) per well, and total radioactivity was determined by gamma spectrophotometry.
Luciferase assays.
The mouse pdgf-βr promoter luciferase construct has been described previously (3). Each plate was transfected with 10 μg of the pdgf-βr promoter luciferase construct, 0.1 μg of a vector carrying the cytomegalovirus (CMV) promoter driving the β-galactosidase gene (pCMVβgal), and 10 μg of pBluescript KS(+) plasmid, using the calcium phosphate method. Luciferase and β-galactosidase assays were performed as previously described (19).
RESULTS
PDGF-βR mRNA expression is suppressed following serum or PDGF BB stimulation of Rat-1 cells.
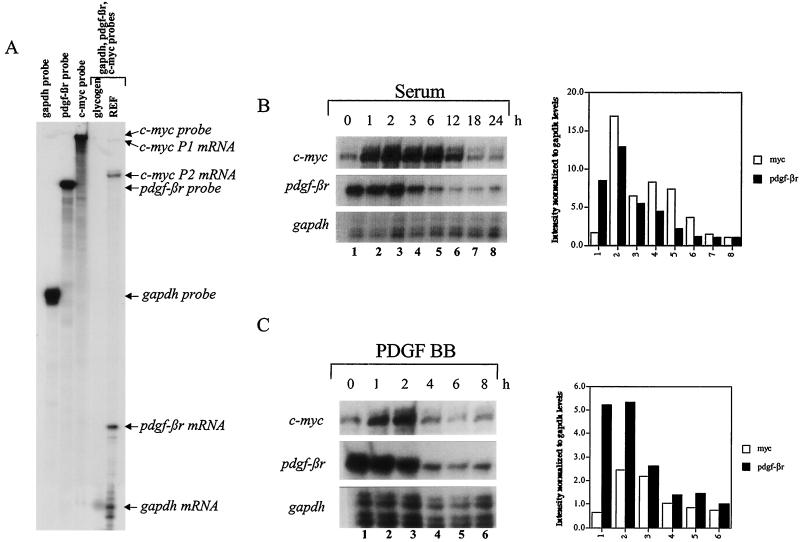
PDGF BB is one of the primary growth factors for fibroblasts, yet little is known of the pattern or regulatory mechanisms which negatively govern the mRNA expression of its key receptor, PDGF-βR, following ligand-receptor interaction in normal and transformed cells. Earlier reports have implicated src and ras as negative regulators of pdgf-βr expression (62). Since Myc has been placed downstream of src in the PDGF BB signaling pathway (4), we explored the relationship between c-myc and pdgf-βr expression following serum stimulation of quiescent cells. To investigate this relationship, we employed the RNase protection assay using probes specific for pdgf-βr and c-myc, as well as gapdh as a loading control (Fig. 1A). Hybridization to tRNA or glycogen control led to complete probe digestion (Fig. 1A), whereas hybridization to RNA derived from primary REF resulted in the resolution of all three protected fragments showing the relative expression level of these three genes in a single sample (Fig. 1A). The rat c-myc probe allowed the detection of mRNA transcribed from both the P1 and P2 promoters (Fig. 1A). Confluent Rat-1 cells were incubated in medium containing limited fetal bovine serum (0.3% FBS–αMEM) for 3 days to reduce the levels of PDGF in the medium and subsequently exposed to either fresh 10% FBS or PDGF BB growth factor. RNA was extracted at the times indicated and analyzed by the RNase protection assay, as described above, to detect endogenous c-myc, pdgf-βr, and gapdh-specific transcripts. As expected (10, 31), exposure of serum-deprived Rat-1 cells to 10% serum induced a rapid and transient increase of endogenous c-myc mRNA expression (Fig. 1B, top panel). c-myc mRNA expression peaked at approximately 2 h and then gradually decreased, reaching steady-state levels 12 to 18 h poststimulation. Exposure to PDGF BB alone also induced c-myc mRNA expression; however, compared to serum stimulation, c-myc mRNA expression was not sustained, since the levels returned to basal approximately 4 h poststimulation (Fig. 1C, top panel). Basal pdgf-βr RNA is readily detectable in cells cultured under low-serum conditions and is down-regulated approximately 3 to 4 h after exposure of cells to serum or PDGF BB alone (Fig. 1B and C, middle panel). The sequential induction of Myc expression followed by the suppression of pdgf-βr RNA suggested that Myc may play a role in initiating receptor repression following growth factor stimulation.
FIG. 1.
Expression of c-myc and pdgf-βr mRNAs in quiescent Rat-1 cells stimulated with serum or PDGF BB. (A) Single-stranded riboprobes complementary to endogenous c-myc-, pdgf-βr-, and gapdh-specific sequences were prepared and simultaneously hybridized to glycogen controls or primary REF RNA. Protected fragments were resolved on a denaturing 6% polyacrylamide gel. (B and C) 10% FBS (B) or 40 ng of PDGF BB per ml (C) was added to Rat-1 cells maintained in low serum (0.3% FBS–αMEM) for 3 days, and total RNA was extracted at the indicated intervals for analysis by the RNase protection assay using probes complementary to endogenous c-myc-, pdgf-βr-, and gapdh-specific sequences (A). The signal intensity was quantitated by densitometry using ImageQuant software and is shown in histograms, normalized to the gapdh loading control.
PDGF-βR RNA is down-regulated in Myc-activated nontransformed cells.
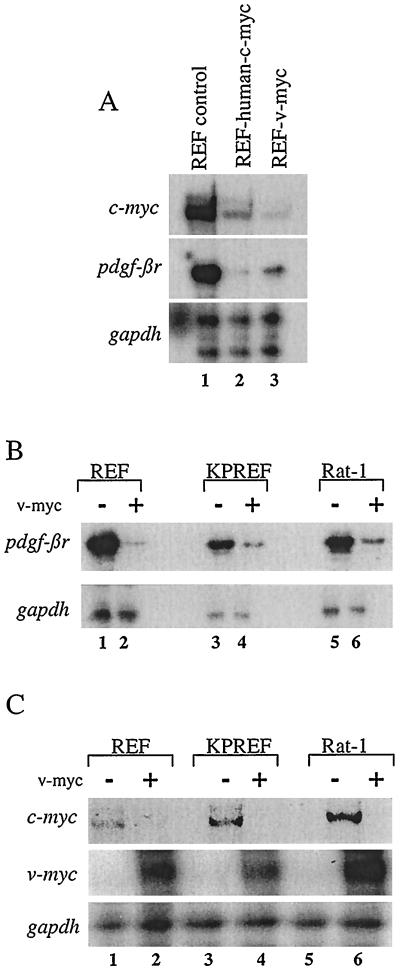
To determine whether constitutive ectopic Myc expression can regulate the expression of pdgf-βr mRNA, we infected primary REF with ecotropic retrovirus (BabeMNIRESgfp) carrying either the human c-myc cDNA or v-myc gene. RNA from control REF or REF expressing the v-Myc or c-Myc protein was harvested from subconfluent proliferating cultures and analyzed by the RNase protection assay (Fig. 2A). Previous studies have shown that the functions of these two Myc proteins are essentially interchangeable (46). Indeed, we showed that exogenous c-Myc and v-Myc were both able to down-regulate endogenous c-myc mRNA as well as pdgf-βr mRNA (Fig. 2A, compare lane 1 with lanes 2 and 3). Interestingly, repression of pdgf-βr mRNA was greater in cells expressing c-Myc than in those expressing v-Myc (Fig. 2A, middle panel, compare lanes 2 and 3), whereas down-regulation of c-myc mRNA, due to the Myc autosuppression (14, 25, 46), was achieved by v-Myc to a greater extent than by c-Myc, as previously reported (Fig. 2A, top panel, lanes 2 and 3) (46). To further confirm Myc repression of pdgf-βr mRNA, we infected primary REF and the immortal, nontransformed cell lines KPREF and Rat-1 with control replication-incompetent retrovirus (DOR/neo) carrying only the neomycin resistance gene or with retrovirus (Dok/v-mycneo) carrying the v-myc gene in addition to the neomycin resistance gene. RNA from drug-resistant subconfluent proliferating cultures was analyzed by the RNase protection assay (Fig. 2B). pdgf-βr mRNA expression was readily visible in control cells and was clearly suppressed in the cells expressing ectopic v-Myc (Fig. 2B, compare lanes 1, 3, and 5 with lanes 2, 4, and 6, respectively). As a positive control for exogenous Myc activity, ectopic v-myc mRNA expression and endogenous c-myc mRNA expression were assayed. Our results demonstrate that ectopic v-Myc protein was expressed and functionally active, since endogenous c-myc mRNA levels are suppressed in v-Myc-expressing cells, due to the Myc negative-feedback mechanism (Fig. 2C, compare lanes 1, 3, and 5 with lanes 2, 4, and 6, respectively). These results show that enforced Myc expression down-regulates pdgf-βr expression in primary and nontransformed cells.
FIG. 2.
Suppression of endogenous c-myc and pdgf-βr mRNA levels in rodent cells constitutively expressing exogenous c-Myc or v-Myc. (A) Total RNA (10 μg) from subconfluent primary control REF or REF infected with retrovirus carrying either the v-myc or human c-myc gene was analyzed by the RNase protection assay to detect endogenous rat c-myc (top panel), pdgf-βr (middle panel), and gapdh (bottom panel) mRNA levels. (B and C) RNA (10 μg) from subconfluent primary REF or rodent fibroblast cell lines (KPREF and Rat-1) infected with a control retrovirus (−) or retrovirus carrying the v-myc gene (+) was analyzed by the RNase protection assay to detect the presence of endogenous pdgf-βr and control gapdh genes (B) and endogenous rat c-myc, ectopic v-myc, and control gapdh genes (C).
The suppression of PDGF-βR mRNA levels following Myc activation occurs under subconfluent or confluent conditions and with rapid kinetics.
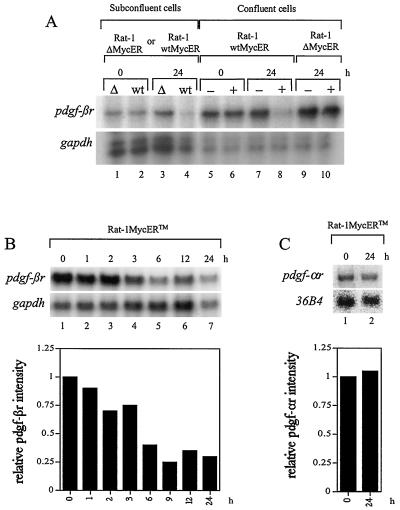
To compare pdgf-βr mRNA expression under subconfluent and confluent conditions in response to Myc induction in the same genetic background, we employed an estrogen-inducible MycER system in Rat-1 cells (Rat-1 wtMycER). These Rat-1 cells constitutively express an inactive fusion protein consisting of the human c-Myc protein fused to the estrogen binding domain of the estrogen receptor. This MycER fusion protein is rapidly activated in response to β-estradiol or the β-estradiol antagonist OH-T, allowing Myc activity to develop within minutes of ligand exposure (15, 17, 18, 55). As a control, we analyzed the Rat-1 ΔMycER cell line, which expresses a ΔMycER fusion protein containing a deletion of amino acids 106 to 143 within the Myc protein. This mutant fusion protein is unable to repress transcription, induce apoptosis or transformation, or inhibit differentiation (reviewed in reference 48). Subconfluent, proliferating Rat-1 wtMycER and Rat ΔMycER cells were exposed to β-estradiol for 24 h, and RNA was harvested for analysis by the RNase protection assay. In addition, confluent quiescent serum-deprived Rat-1 wtMycER cells were either exposed to ethanol as a control or treated with β-estradiol dissolved in ethanol, to induce Myc activity. pdgf-βr expression was elevated in confluent quiescent cells in comparison to subconfluent proliferating cells (approximately fourfold higher, as determined by densitometry [Fig. 3A]). Importantly, suppression of endogenous pdgf-βr mRNA was clearly detectable in both subconfluent (threefold) and confluent (sixfold) Rat-1 wtMycER cells in response to activated Myc expression (Fig. 3A, compare lanes 2 and 4 and lanes 6 and 8). This repression of the receptor was not evident in confluent ethanol-treated Rat-1 wtMycER cells (lanes 5 and 7), subconfluent Rat-1 ΔMycER cells in response to β-estradiol (lanes 1 and 3), or confluent Rat-1 ΔMycER cells which were or were not exposed to β-estradiol (lanes 9 and 10). Thus, our results demonstrate that pdgf-βr mRNA expression is suppressed on Myc induction in both quiescent and proliferating cells. To examine the kinetics of pdgf-βr mRNA expression, we next employed Rat-1 cells expressing the wtMycERTM construct (Rat-1 wtMycERTM) (35). Confluent quiescent Rat-1 wtMycERTM cells were treated with OH-T to induce Myc activity, and RNA was harvested at the times indicated for analysis by the RNase protection assay. Repression of pdgf-βr mRNA was first evident approximately 3 h after Myc activation and increased during the period of analysis (Fig. 3B). As a positive control for exogenous wtMycERTM activity, endogenous c-myc mRNA expression was assayed and showed the expected suppression due to the Myc negative-feedback mechanism as described previously (reference 19 and data not shown). Myc suppression of pdgf-βr mRNA levels is specific, since induction of Myc activity has no effect on the mRNA expression of the related growth factor receptor, (pdgf-αr) (Fig. 3C). The suppression of endogenous pdgf-βr mRNA levels is not the result of a Myc-induced autocrine negative-feedback pathway, since MycER activation had no effect on the expression of pdgf-B mRNA (data not shown). Therefore, the suppression of pdgf-βr mRNA by Myc represents a specific regulatory mechanism.
FIG. 3.
pdgf-βr mRNA expression is suppressed in response to Myc induction. (A) pdgf-βr mRNA expression in Rat-1 wtMycER (wt) and Rat-1 ΔMycER (Δ) cells was analyzed under both subconfluent and confluent conditions. Cell lines were grown either as asynchronous subconfluent populations and treated with β-estradiol (lanes 1 to 4) or as confluent quiescent cultures and treated with either ethanol as control (−) or β-estradiol (+) (lanes 5 to 10). At the indicated intervals after β-estradiol treatment, RNA was extracted and total RNA (10 μg) was analyzed by the RNase protection assay to detect the expression of endogenous pdgf-βr and control gapdh genes. (B) Confluent Rat-1 wtMycERTM cells were maintained in low serum (0.1% FBS–αMEM) for 2 days and then exposed to 100 nM OH-T to induce exogenous MycERTM activity. At the indicated intervals after OH-T treatment, RNA was extracted and 10 μg of total RNA was analyzed by the RNase protection assay using single-stranded probes complementary to endogenous pdgf-βr- and gapdh-specific sequences. The histogram shows quantitation of the pdgf-βr signal normalized to gapdh, as determined by densitometry. (C) Northern blot analysis of pdgf-αr and 36B4 expression in OH-T-stimulated Rat-1 wtMycERTM cells. Confluent quiescent Rat-1 wtMycERTM cells were exposed to 100 nM OH-T to induce exogenous MycERTM activity. At the indicated intervals after OH-T treatment, RNA was extracted and 10 μg of total RNA was analyzed by Northern blotting using gene-specific probes for endogenous pdgf-αr- and 36B4-specific sequences. The plotted data show the pdgf-αr signal as normalized to 36B4, as determined by densitometry.
Myc-induced repression of cell surface PDGF-βR.
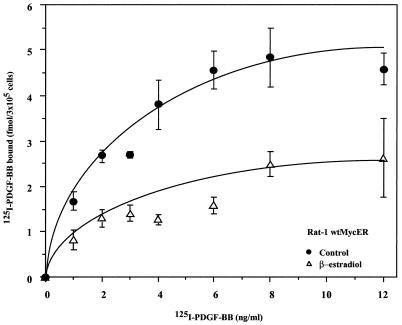
To determine whether Myc suppression of pdgf-βr mRNA affected receptor protein expression, the number of PDGF-βRs expressed at the cell surface before and after ectopic Myc induction was measured in direct ligand binding assays. Specifically, subconfluent Rat-1 wtMycER cells were exposed to ethanol as a control or to β-estradiol in ethanol for 24 h and then incubated with increasing concentrations of [125I]PDGF BB. Saturable binding was observed for both control and β-estradiol-treated cells at 12 ng of [125I]PDGF BB per ml. The binding capacity of control Rat-1 wtMycER cells was 5.0 fmol of [125I]PDGF BB per 3 × 105 cells, whereas at 24 h following MycER activation, Rat-1 wtMycER cells bound only 2.4 fmol of [125I]PDGF BB per 3 × 105 cells at saturation (Fig. 4). Scatchard analysis showed that the specific binding affinity remained unchanged (data not shown), suggesting that our results reflect a decrease in the number of receptors at the cell surface. The results obtained from the PDGF BB binding assays correlate with the decrease in pdgf-βr mRNA expression as shown by RNase protection analysis. The reduction in the number of PDGF BB binding sites in cells expressing ectopic Myc activity can be attributed solely to the down-regulation in PDGF-βR levels, since expression of the PDGF-αR, which also binds to the PDGF BB ligand, is not affected by ectopic Myc expression. Thus constitutive or induced Myc expression leads to approximately a 50% reduction in cell surface PDGF-βRs compared to control cells.
FIG. 4.
Myc suppresses PDGF-βR expression in Rat-1 cells. Binding of [125I]PDGF BB was examined in Rat-1 wtMycER cells. Subconfluent cultures were treated with ethanol as control or exposed to 100 nM β-estradiol. Both control and β-estradiol-treated cells were incubated with increasing concentrations of [125I]PDGF BB. Nonspecific binding was determined in the presence of a 100-fold excess of unlabeled PDGF BB. The plotted values represent the average specific binding to triplicate wells. Standard deviations were less than 10%.
The mechanism of PDGF-βR suppression differs from that of Myc autosuppression.
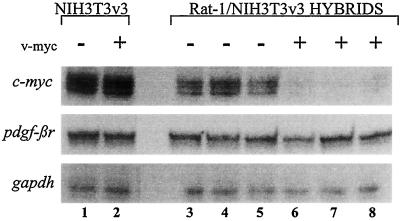
A number of similarities exist between the mechanisms employed by Myc to repress the endogenous pdgf-βr and c-myc mRNA expression. First, constitutive Myc expression resulted in the suppression of both endogenous pdgf-βr and c-myc mRNA levels (Fig. 2) (46, 47). Second, the suppression of both endogenous c-myc (19) and pdgf-βr (Fig. 3B) mRNA levels in response to Myc activation occurred with similar kinetics, given that the half-life of c-myc mRNA is approximately 20 min while the half-life of pdgf-βr mRNA is 4 to 6 h. Taken together, these results suggested that the repression of c-myc and pdgf-βr mRNA by Myc may be functioning through a common pathway. However, we have also shown that unlike c-myc mRNA, pdgf-βr mRNA is repressed to a greater degree by exogenous c-Myc protein than by v-Myc. Therefore, to further elucidate whether repression of these genes occurs by common or distinct pathways, we employed a variant mouse NIH 3T3v3 cell line. In contrast to other NIH 3T3 cells (K. Funa and L. Z. Penn, unpublished data) and Rat-1 cells (Fig. 2C), expression of ectopic Myc in this variant NIH 3T3v3 cell line did not result in the suppression of either c-myc or pdgf-βr mRNAs (Fig. 5, lanes 1 and 2). Stable intraspecies somatic cell hybrids were generated by fusion of the Rat-1 cell line to the mouse NIH 3T3v3 cell line. By using this approach, we can determine whether the trans-acting factors in Rat-1 cells can complement the dysfunction in the mouse NIH 3T3v3 cells for Myc suppression of pdgf-βr mRNA, as we have previously shown for Myc autosuppression (46).
FIG. 5.
The suppression of endogenous pdgf-βr and c-myc mRNA levels by Myc appear to be mediated by different pathways. Total RNA (10 μg) was analyzed by the RNase protection assay to detect the expression of endogenous mouse c-myc, pdgf-βr, and gapdh genes. The cells analyzed include control NIH 3T3v3 (lane 1), NIH 3T3v3 v-myc (lane 2), and three random, independent clones of the stable somatic cell hybrids, generated from the intraspecies fusion of Rat-1 × NIH 3T3v3 cells (lanes 3 to 5) and Rat-1 v-myc × NIH 3T3v3 v-myc (lanes 6 to 8).
To establish basal levels of endogenous mouse pdgf-βr and c-myc mRNAs in the control somatic cell hybrids, Rat-1 cells were fused to NIH 3T3v3 cells in the absence of ectopic Myc expression. Following drug selection, stable somatic cell hybrids were cloned and assayed by RNase protection for the expression of mouse pdgf-βr and c-myc mRNAs (Fig. 5, lanes 3 to 5). To distinguish whether the Myc suppression of endogenous c-myc and pdgf-βr mRNAs are mediated by the same or different pathways, Rat-1 cells expressing ectopic Myc were fused to mouse NIH 3T3v3 cells expressing exogenous Myc protein. The resultant stable hybrids were similarly analyzed (lanes 6 to 8). As previously shown, endogenous mouse c-myc mRNA is readily detectable in the control hybrids and is suppressed in somatic cell hybrids expressing ectopic Myc (Fig. 5, compare lanes 3 to 5 with lanes 6 to 8) (46). In contrast, expression of pdgf-βr mRNA in control and Myc-expressing somatic cell hybrids is identical (compare lanes 3 to 5 with lanes 6 to 8). Thus trans-acting components of Rat-1 cells were able to complement the Myc autosuppression but not the pdgf-βr repression mechanism in NIH 3T3v3 cells. Identical results were obtained with similar somatic cell hybrids derived from another clone, NIH 3T3v10, as well as the original parental variant NIH 3T3 cell line (reference 46 and data not shown). Therefore, the repression of mouse c-myc and pdgf-βr mRNAs in response to Myc appear to be mediated by different pathways.
Myc represses transcription of the pdgf-βr promoter.
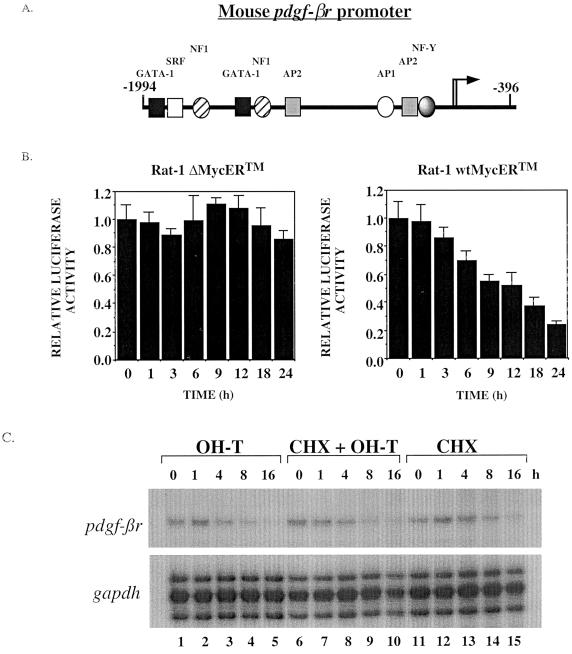
To determine whether the pdgf-βr promoter is responsive to Myc activity, we performed in vitro transient-transfection assays. Subconfluent Rat-1 wtMycERTM and control Rat-1 ΔMycERTM cells were transfected with a 1.6-kb SacI reporter plasmid containing sequences between nucleotides −1994 and −396 relative to the translation start site of the mouse pdgf-βr gene (Fig. 6A). To control for transfection efficiency, a vector carrying the β-galactosidase (lacZ) gene under the control of the CMV promoter was cotransfected with the promoter luciferase construct and luciferase activity relative to the transfection efficiency was recorded. Cells were treated with OH-T to induce ectopic Myc activity, and at the indicated time intervals, whole-cell lysates were prepared and then assayed for both luciferase and β-galactosidase activity. Induction of Myc activity in Rat-1 wtMycERTM cells resulted in a clear and reproducible suppression of luciferase activity (Fig. 6B), reflecting the suppression of transcription from the mouse pdgf-βr promoter by Myc. The loss in luciferase activity was first evident approximately 6 h following Myc activation. This suppression is due solely to the activity of Myc, since OH-T-treated Rat-1 ΔMycERTM cells exhibited no change in luciferase activity (Fig. 6B). Since the half-lives of pdgf-βr mRNA and luciferase are both approximately 4 to 6 h (60, 62), the level and kinetics of repression as measured in the pdgf-βr promoter-luciferase assay (Fig. 6B) closely mimicked the suppression of endogenous pdgf-βr mRNA in response to MycERTM activation (Fig. 3B).
FIG. 6.
The pdgf-βr promoter activity is suppressed by Myc. (A) Schematic diagram of the genomic structure of the mouse pdgf-βr promoter, showing putative transcription factor binding sites. Numbers indicate position relative to the translational start site (+1). (B) Rat-1 ΔMycERTM and Rat-1 wtMycERTM cells were transfected with a mouse pdgf-βr promoter-luciferase construct, containing pdgf-βr sequences from nucleotides −1994 to −396. To assay the transfection efficiency, cells were cotransfected with a plasmid carrying the lacZ gene under the control of the CMV promoter. Cell lines were subsequently treated with 100 nM OH-T for the times indicated to induce ectopic MycERTM activity. Whole-cell lysates were prepared and analyzed for luciferase and β-galactosidase activity. Histograms represent the measured luciferase activity. The data were normalized to β-galactosidase activity to adjust for minor differences in transfection efficiency among the samples. The data are representative of three repeated experiments. Error bars denote standard deviations within an experiment. (C) To determine whether Myc represses pdgf-βr mRNA directly or whether de novo protein synthesis is required, we investigated expression levels in Rat-1 wtMycERTM cells incubated with OH-T (lanes 1 to 5), OH-T plus cycloheximide (CHX) (lanes 6 to 10), or cycloheximide alone (lanes 11 to 15) for the periods indicated. pdgf-βr and gapdh mRNA levels were analyzed by the RNase protection assay.
In an attempt to determine whether the down-regulation of pdgf-βr mRNA is directly due to Myc, we employed the translational inhibitor cycloheximide. Subconfluent proliferating Rat-1 wtMycERTM cells were treated with OH-T, OH-T plus cycloheximide, or cycloheximide alone (Fig. 6C, lanes 1 to 5, 6 to 10, and 11 to 15, respectively) and pdgf-βr mRNA levels were analyzed by the RNase protection assay. The levels of pdgf-βr mRNA were decreased in OH-T-treated cells approximately 4 h after induction of MycERTM, as shown above (Fig. 6C, lanes 1 to 5, and Fig. 3B). The levels of pdgf-βr mRNA were also decreased when MycERTM was induced with OH-T in the presence of cycloheximide (Fig. 6C, lanes 6 to 10) and in the presence of cycloheximide alone (lanes 11 to 15). This indicates that cycloheximide itself has a negative effect on the levels of pdgf-βr mRNA in the cell, and it is difficult to determine whether Myc is directly down-regulating the expression of this gene. Thus, we determined that Myc expression results in a suppression of transcription from the pdgf-βr promoter, and this suppression activity requires the nucleotide sequence between −1994 and −396 relative to the translation start site of the mouse pdgf-βr gene.
Myc is required for the suppression in pdgf-βr mRNA levels following mitogen stimulation.
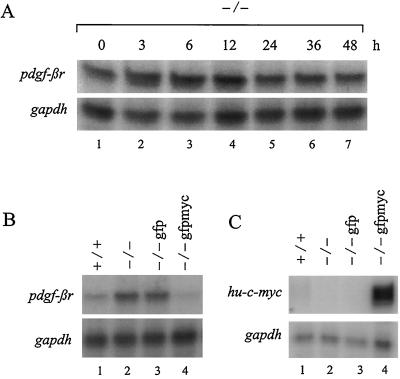
To examine the role of Myc in the repression of pdgf-βr mRNA levels following mitogen stimulation, we analyzed pdgf-βr mRNA expression in serum-deprived c-Myc null HO15.19 (−/−) rodent fibroblasts upon stimulation with 10% serum. HO15.19 (−/−) cells, derivatives of parental Rat-1 fibroblasts, lack c-Myc expression due to targeted disruption of both alleles of the c-myc gene with aminoglycoside transferase (neo) and histidine-marked targeting vectors (41). Transcription of the disrupted c-myc genes produces hybrid truncated transcripts consisting of c-myc exon I sequences fused to the coding sequences of either the neo or histidine genes. Quiescence of c-Myc null (−/−) rodent cells was achieved by culturing the cells in 0.25% calf serum plus DMEM H21 for 2 days. These cells were subsequently stimulated to enter the cell cycle with 10% serum, and RNA was extracted over one full cell cycle (45 to 60 h) (41) at the indicated time intervals for analysis of endogenous pdgf-βr and gapdh expression. Concomitant analysis by flow cytometry confirmed that both quiescence and entry into the cell cycle were achieved in response to serum withdrawal and stimulation, respectively (data not shown), as previously shown under these conditions (41). As shown in Fig. 1B, wild-type Rat-1 (+/+) fibroblasts exhibited a gradual reduction in pdgf-βr mRNA levels upon mitogen stimulation of serum-deprived cultures. In contrast, serum stimulation of c-Myc null (−/−) fibroblasts failed to elicit a reduction in endogenous pdgf-βr mRNA levels (Fig. 7A). Indeed, pdgf-βr mRNA expression was invariant despite prolonged (48-h) exposure to serum. Thus, Myc is essential for the repression of pdgf-βr mRNA levels in serum-deprived cells following serum stimulation.
FIG. 7.
Myc is required for the repression of pdgf-βr mRNA levels in both serum-stimulated quiescent cells and proliferating cultures. (A) Rat-1 cells, lacking c-Myc expression (−/−), were maintained in low serum (0.25% calf serum–DMEM H21) for 2 days and then treated with 10% calf serum–DMEM H21. At the indicated intervals, RNA was extracted and analyzed by the RNase protection assay using single-stranded probes complementary to endogenous pdgf-βr- and gapdh-specific sequences. (B and C) RNA (10 μg), extracted from subconfluent cultures of wild-type rodent fibroblasts (+/+), rodent fibroblasts lacking endogenous Myc expression (−/−), and c-Myc null fibroblasts which have been stably infected with either a control retrovirus expressing gfp (−/− gfp) or a retrovirus containing human c-myc exon II and III cDNA as well as gfp (−/− gfpmyc), was analyzed by the RNase protection assay for endogenous pdgf-βr and gapdh sequences (B) and ectopic human c-myc and endogenous gapdh-specific sequences (C). Similar results were obtained in three separate experiments.
Myc also plays a role in the regulation of basal pdgf-βr mRNA levels in subconfluent proliferating cells. Analysis of endogenous pdgf-βr mRNA levels in asynchronously proliferating cultures of wild-type (+/+) and c-Myc null (−/−) fibroblasts revealed that pdgf-βr mRNA levels in c-Myc null cells were elevated in comparison to wild-type fibroblasts (Fig. 7B, compare lane 2 to lane 1). Thus, in subconfluent proliferating cultures, Myc represses basal pdgf-βr mRNA expression. The endogenous pdgf-βr gene remained responsive to Myc, since repression of this gene could be rescued through the reconstitution of c-Myc expression in HO15.19 (−/−) cells. c-Myc null HO15.19 gfpmyc (−/− gfpmyc) cells constitutively expressing both ectopic human c-Myc and GFP as a selectable marker elicited a clear repression of pdgf-βr mRNA levels compared to the parental c-Myc null (−/−) cells as well as the control cell line HO15.19 gfp (−/− gfp), which constitutively expressed only GFP (Fig. 7B, compare lane 4 to lanes 2 and 3). Analysis of human c-myc mRNA expression clearly demonstrated that ectopic human c-myc was expressed in the appropriate cell populations (Fig. 7C, compare lane 4 to lanes 1 to 3). Thus, studies with c-Myc null fibroblasts have demonstrated that Myc is required for the suppression of pdgf-βr mRNA levels in serum-starved rodent fibroblasts following serum stimulation. Moreover, c-Myc plays a role in maintaining basal pdgf-βr mRNA levels by repressing pdgf-βr mRNA expression in asynchronous proliferating cells.
DISCUSSION
We show that the physiological down-regulation of cell surface PDGF-βRs following mitogen stimulation involves the product of the myc proto-oncogene and occurs at the RNA level. Constitutive or induced expression of Myc results in pdgf-βr mRNA down-regulation in nontransformed cells by a mechanism which appears to be distinct from that of the Myc negative-feedback mechanism. Moreover, analysis of Myc-null fibroblasts shows that pdgf-βr repression is dependent on Myc for basal expression in proliferating rodent fibroblasts, as well as for mitogen-regulated expression. In addition, a recent microarray screen performed in our laboratory independently identified pdgf-βr as a Myc down-regulated gene. Taken together, our results show that Myc is integral to the regulation of pdgf-βr expression.
The molecular mechanism of pdgf-βr RNA suppression in response to Myc was explored using the inducible MycERTM system in Rat-1 cells. We show that Myc induction specifically down-regulates pdgf-βr RNA and does not affect RNA expression of the related growth factor receptor, the pdgf-αr. This effect is evident in both growth-arrested confluent and asynchronous proliferating cells expressing an inducible Myc protein, suggesting that receptor down-regulation occurs in response to Myc per se and is not a consequence of the cellular transition from the G0/G1 to the S phase of the cell cycle. Ectopic Myc expression does not induce the expression of pdgf-B mRNA, and so it is unlikely that Myc is suppressing receptor expression via a ligand-induced autocrine feedback mechanism. Receptor suppression is detectable within 3 to 6 h of either growth factor stimulation or Myc induction. Since the half-life of pdgf-βr RNA is 4 to 6 h, this is the earliest point at which down-regulation of receptor RNA can be visualized (62).
Given the long half-life of the pdgf-βr RNA, the repression of the pdgf-βr RNA following induction of ectopic Myc activity is rapid, suggesting that Myc may exert a direct effect on the pdgf-βr promoter rather than inducing the expressing of another protein, which, in turn, causes this suppression. We have shown that Myc suppresses transcription from a fragment of the mouse pdgf-βr promoter, consisting of sequences between −1994 and −396 relative to the translational start site. Analysis of the Myc-repressed genes identified to date suggests that Myc can repress the transcription of some genes through core regulatory elements such as Inr or TATA boxes (16, 19, 34, 49, 54, 63). However, the upstream regulatory region of the mouse pdgf-βr gene is relatively simple, lacking both of these elements, as well as Myc-Max DNA binding sites (CACGTG) involved in Myc transactivation of gene transcription. Another Myc-repressed gene, gadd45, also has an Inr-minus, TATA-less promoter, and it has been suggested that repression is mediated through an enhancer-dependent mechanism (1, 38). The pdgf-βr promoter contains several putative binding sites for transcription factors such as GATA-1, SRF, AP1, NF1, AP2, and NF-Y, and it is possible that one or all of these sites are required to mediate the repression of pdgf-βr mRNA by Myc. The NF-Y site is a promising target, since mutational analysis of the mouse pdgf-βr promoter by Ishisaki et al. has demonstrated that the NF-Y binding site is singularly crucial to maintaining basal mRNA transcription (30). Moreover, Myc has recently been shown to interact with NF-Y to inhibit the expression of hsp70 by storing the activator away from the promoter (59). Indeed, more recent experiments have shown that Myc may bind specific subunits of the transcription factor NF-Y to hinder its ability to transactivate pdgf-βr gene expression through a CCAAT motif in the promoter (H. Izumi, C. Molander, L. Penn, and K. Funa, submitted for publication). Hence, this site is a likely candidate through which Myc can directly repress the transcription of the pdgf-βr gene in an enhancer-dependent manner. To address whether de novo protein synthesis is required for Myc to inhibit pdgf-βr mRNA expression, we attempted to use the translational inhibitor cycloheximide. This compound has been used successfully to elucidate the mechanism of action of Myc on other target genes, the H-ferritin gene and cdc25A (22, 63). Upon addition of cycloheximide to the cells, further protein synthesis was prevented and the regulation of mRNA could be attributed directly to activated MycERTM protein. However, exposure of control, uninduced Rat1 MycERTM cells to cycloheximide led to a reduction of pdgf-βr mRNA, showing that cycloheximide alone had a negative effect on pdgf-βr mRNA expression. These results precluded further interpretation, and we were unable to determine whether Myc can repress pdgf-βr mRNA expression in the absence of de novo protein synthesis. Thus, our analysis shows that Myc can repress the transcription of the pdgf-βr promoter, possibly through an enhancer-dependent mechanism and inhibition of activator-driven gene transcription.
Many characteristics of Myc suppression of pdgf-βr are similar to features associated with the well-characterized Myc negative autoregulation mechanism (14, 19, 25, 46, 47). Myc suppression of pdgf-βr and c-myc RNA expression are both evident in primary REF and established nontransformed cell lines. Moreover, both mechanisms are affected by Myc in a dose-dependent manner. The critical Myc box II domain of Myc which is required for negative autoregulation is mandatory for pdgf-βr suppression. Both Myc autosuppression and Myc suppression of the pdgf-βr occur at the RNA level. Interestingly, constitutive expression of Myc in a variant NIH 3T3v3 cell line does not lead to the suppression of either c-myc or pdgf-βr RNA expression. The use of a somatic cell hybridization approach with mouse NIH 3T3v3 and Rat-1 cell lines showed that rat cell factors restored suppression of the mouse c-myc gene (46); however, the mouse pdgf-βr gene was unaffected in the hybrid cellular background. In addition, stronger repression of endogenous c-myc is achieved with expression of exogenous v-Myc compared to c-Myc, and these results are reversed when pdgf-βr expression is examined. Taken together, these results show that the regulatory mechanisms through which Myc suppresses the endogenous c-myc and pdgf-βr genes are clearly distinct and further suggest that Myc repression of gene transcription can occur by multiple mechanisms.
Interestingly, introduction of transforming ras or src oncogenes can lead to reduced pdgf-βr RNA expression in rodent fibroblasts (62, 66). Indeed, the characteristics of this suppression are similar to those seen in response to Myc and suggest that Myc may lie in the same pathway, downstream of Ras and Src in the repression of PDGF-βR. Studies by Barone and Courtneidge lend support to this model, since it was demonstrated that Myc is the target of the src signaling pathway following PDGF-βR activation (4). Thus, pathways downstream of Src and possibly Ras probably signal through Myc to suppress pdgf-βr RNA and protein expression, suggesting that Myc is a component of a homeostatic regulatory mechanism controlling pdgf-βr RNA expression.
Indeed, through the use of c-Myc null rodent fibroblasts, we were able to demonstrate that Myc is an essential component of a regulatory pathway effecting the repression of pdgf-βr mRNA levels. While repression of pdgf-βr mRNA levels in wild-type Rat-1 cells was maximal 12 h after mitogen exposure, c-Myc null fibroblasts did not exhibit a reduction in pdgf-βr mRNA levels through one full cell cycle. Thus, the abrogation of the serum-induced repression of pdgf-βr mRNA expression is due to the absence of Myc expression and is not the indirect result of a reduced rate of transit through the cell cycle. This is further demonstrated by our analysis of endogenous pdgf-βr mRNA expression in proliferating c-Myc null fibroblasts. Basal expression of pdgf-βr mRNA in asynchronous subconfluent growing cells was elevated in null cells compared to wild-type Rat-1 cells. Moreover, reconstitution of the null cells with ectopic human c-Myc protein elicited a reduction in pdgf-βr mRNA levels, showing that the pdgf-βr gene is indeed responsive to Myc.
The repression of PDGF-βR levels by Myc represents a negative-feedback pathway, which is functionally analogous to the Myc-negative autoregulation mechanism in that Myc functions through both pathways to curtail a proliferative stimulus. It is interesting that Myc repression of pdgf-βr mRNA led to only a 50% reduction in the level of PDGF-βR protein on the cell surface. Harrington et al. have previously shown that addition of PDGF ligand to serum-deprived Rat1 cells expressing activated MycER leads to a decrease in observed apoptosis (27). This indicates that receptors remaining on the cell surface are at least functional in mediating survival. It is possible that the 50% reduction of PDGF-βR on the cell surface due to mitogen stimulation may allow a decrease in proliferation while maintaining responsiveness to survival factors. This mechanism would serve to temper the growth impetus, guarding against deregulated cell proliferation while maintaining responsiveness to survival factors. Indeed, experimental evidence suggests that the inhibition of this negative-feedback loop can contribute to the formation of tumors. Glioblastomas and astrocytomas constitutively express high levels of both the PDGF-βR and its ligand, which creates an autocrine feedback pathway that contributes to the deregulated growth of these tumors (26, 56). The critical nature of receptor down-regulation is supported by the many mechanisms which have evolved to ensure its successful execution: internalization, intracellular degradation, and RNA suppression. We show that Myc can suppress pdgf-βr expression at the RNA level, and we propose that Myc is a key component of the pathway responsible for receptor suppression following ligand stimulation.
ACKNOWLEDGMENTS
S.K.O. and W.W.M. contributed equally to this work.
We thank the members of the laboratory for helpful discussions and critical reading of the manuscript, and we thank Y. Yarden, G. Evan, T. Littlewood, and G. Nolan for valuable reagents. In addition, we extend our thanks to E. Fish and C. Lingwood for their assistance with the PDGF BB ligand binding assays.
Studentship support was kindly provided through the Ontario Graduate Scholarship Program (to W.W.M. and S.K.O.) and the Medical Research Council of Canada (to L.M.F. and S.K.O.). C.A. was supported by the Swedish Medical Research Council, the Karolinska-University of Toronto Exchange Program, and a grant from the Swedish Cancer Society. K.F. was supported by grants from the Swedish Medical Research Council, the Swedish Cancer Society, and the Barncancerfonden. This work was supported by a grant from the National Cancer Institute of Canada with funds from the Canadian Cancer Society (to L.Z.P.).
REFERENCES
- 1.Amundson S A, Zhan Q, Penn L Z, Fornace A J., Jr Myc suppresses induction of the growth arrest genes gadd34, gadd45, and gadd153 by DNA-damaging agents. Oncogene. 1998;17:2149–2154. doi: 10.1038/sj.onc.1202136. [DOI] [PubMed] [Google Scholar]
- 2.Antonson P, Pray M G, Jacobsson A, Xanthopoulos K G. Myc inhibits CCAAT/enhancer-binding protein alpha-gene expression in HIB-1B hibernoma cells through interactions with the core promoter region. Eur J Biochem. 1995;232:397–403. doi: 10.1111/j.1432-1033.1995.397zz.x. [DOI] [PubMed] [Google Scholar]
- 3.Ballagi A E, Ishizaki A, Nehlin J O, Funa K. Isolation and characterization of the mouse PDGF beta-receptor promoter. Biochem Biophys Res Commun. 1995;210:165–173. doi: 10.1006/bbrc.1995.1642. [DOI] [PubMed] [Google Scholar]
- 4.Barone M V, Courtneidge S A. Myc but not Fos rescue of PDGF signalling block caused by kinase-inactive Src. Nature. 1995;378:509–512. doi: 10.1038/378509a0. [DOI] [PubMed] [Google Scholar]
- 5.Bello-Fernandez C, Packham G, Cleveland J L. The ornithine decarboxylase gene is a transcription target of c-Myc. Proc Natl Acad Sci USA. 1993;90:7804–7808. doi: 10.1073/pnas.90.16.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett N T, Schultz G S. Growth factors and wound healing: biochemical properties of growth factors and their receptors. Am J Surg. 1993;165:728–737. doi: 10.1016/s0002-9610(05)80797-4. [DOI] [PubMed] [Google Scholar]
- 7.Bornfeldt K E, Raines E W, Graves L M, Skinner M P, Krebs E G, Ross R. Platelet-derived growth factor. Distinct signal transduction pathways associated with migration versus proliferation. Ann NY Acad Sci. 1995;766:416–430. doi: 10.1111/j.1749-6632.1995.tb26691.x. [DOI] [PubMed] [Google Scholar]
- 8.Brough D E, Hofmann T J, Ellwood K B, Townley R A, Cole M D. An essential domain of the c-myc protein interacts with a nuclear factor that is also required for E1A-mediated transformation. Mol Cell Biol. 1995;15:1536–1544. doi: 10.1128/mcb.15.3.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bush A, Mateyak M, Dugan K, Obaya A, Adachi S, Sedivy J, Cole M. c-Myc null cells misregulate cad and gadd45 but not other proposed c-Myc targets. Genes Dev. 1998;12:3797–3802. doi: 10.1101/gad.12.24.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campisi J, Gray H E, Pardee A B, Dean M, Sonenshein G. Cell-cycle control of c-myc but not c-ras expression is lost following chemical transformation. Cell. 1984;36:241–247. doi: 10.1016/0092-8674(84)90217-4. [DOI] [PubMed] [Google Scholar]
- 11.Chirgwin J M, Przybyla A E, MacDonald R J, Rutter W J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 12.Claesson-Welsh L. Mechanism of action of platelet-derived growth factor. Int J Biochem Cell Biol. 1996;28:373–385. doi: 10.1016/1357-2725(95)00156-5. [DOI] [PubMed] [Google Scholar]
- 13.Claesson-Welsh L. Signal transduction by the PDGF receptors. Prog Growth Factor Res. 1994;5:37–54. doi: 10.1016/0955-2235(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 14.Cleveland J L, Huleihel M, Bressler P, Siebenlist U, Akiyama L, Eisenman R N, Rapp U R. Negative regulation of c-myc transcription involves myc family proteins. Oncogene Res. 1988;3:357–375. [PubMed] [Google Scholar]
- 15.Daksis J I, Lu R Y, Facchini L M, Marhin W W, Penn L J Z. Myc induces cyclin D1 expression in the absence of de novo protein synthesis and links mitogen-stimulated signal transduction to the cell cycle. Oncogene. 1994;9:3635–3645. [PubMed] [Google Scholar]
- 16.Desbarats L, Gaubatz S, Eilers M. Discrimination between different E-box-binding proteins at an endogenous target gene of c-myc. Genes Dev. 1996;10:447–460. doi: 10.1101/gad.10.4.447. [DOI] [PubMed] [Google Scholar]
- 17.Eilers M, Picard D, Yamamoto K R, Bishop J M. Chimaeras between Myc oncoprotein and steroid receptors cause hormone-dependent transformation of cells. Nature. 1989;340:66–68. doi: 10.1038/340066a0. [DOI] [PubMed] [Google Scholar]
- 18.Evan G I, Wyllie A H, Gilbert C S, Littlewood T D, Land H, Brooks M, Waters C M, Penn L Z, Hancock D C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- 19.Facchini L, Chen S, Marhin W, Lear J, Penn L. The Myc negative autoregulation mechanism requires Myc-Max association and involves the c-myc P2 minimal promoter. Mol Cell Biol. 1997;17:100–114. doi: 10.1128/mcb.17.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Facchini L M, Chen S, Penn L J Z. Dysfunction of the Myc-induced apoptosis mechanism accompanies c-myc activation in the tumorigenic L929 cell line. Cell Growth Differ. 1994;5:637–646. [PubMed] [Google Scholar]
- 21.Facchini L M, Penn L Z. The molecular role of Myc in growth and transformation: recent discoveries lead to new insights. FASEB J. 1998;12:633–651. [PubMed] [Google Scholar]
- 22.Galaktionov K, Chen X, Beach D. Cdc25 cell-cycle phosphatase as a target of c-myc. Nature. 1996;382:511–517. doi: 10.1038/382511a0. [DOI] [PubMed] [Google Scholar]
- 23.Gliniak B C, Rohrschneider L R. Expression of the M-CSF receptor is controlled by the dominant actions of GM-CSF or multi-CSF. Cell. 1990;63:1073–1083. doi: 10.1016/0092-8674(90)90510-l. [DOI] [PubMed] [Google Scholar]
- 24.Graham F L, van der Eb A J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 25.Grignani F, Lombardi L, Giorgio I, Sternas L, Cechova K, Dalla-Favera R. Negative autoregulation of c-myc gene expression is inactivated in transformed cells. EMBO J. 1990;9:3913–3922. doi: 10.1002/j.1460-2075.1990.tb07612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guha A, Dashner K, Black P M, Wagner J A, Stiles C D. Expression of PDGF and PDGF receptors in human astrocytoma operation specimens supports the existence of an autocrine loop. Int J Cancer. 1995;60:168–173. doi: 10.1002/ijc.2910600206. [DOI] [PubMed] [Google Scholar]
- 27.Harrington E A, Bennett M R, Fanidi A, Evan G I. c-Myc-induced apoptosis in fibroblasts is inhibited by specific cytokines. EMBO J. 1994;13:3286–3295. doi: 10.1002/j.1460-2075.1994.tb06630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heldin C H, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 29.Hitoshi Y, Lorens J, Kitada S I, Fisher J, LaBarge M, Ring H Z, Francke U, Reed J C, Kinoshita S, Nolan G P. Toso, a cell surface, specific regulator of Fas-induced apoptosis in T cells. Immunity. 1998;8:461–471. doi: 10.1016/s1074-7613(00)80551-8. [DOI] [PubMed] [Google Scholar]
- 30.Ishisaki A, Murayama T, Ballagi A E, Funa K. Nuclear factor Y controls the basal transcription activity of the mouse platelet-derived-growth-factor beta-receptor gene. Eur J Biochem. 1997;246:142–146. doi: 10.1111/j.1432-1033.1997.t01-2-00142.x. [DOI] [PubMed] [Google Scholar]
- 31.Kelly K, Cochran B, Stiles C, Leder P. Cell specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983;35:603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- 32.Land H, Parada L F, Weinberg R A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983;304:596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- 33.Lee L A, Dolde C, Barrett J, Wu C S, Dang C V. A link between c-Myc-mediated transcriptional repression and neoplastic transformation. J Clin Investig. 1996;97:1687–1695. doi: 10.1172/JCI118595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li L-H, Nerlov C, Prendergast G, MacGregor D, Ziff E B. c-Myc represses transcription in vivo by a novel mechanism dependent on the initiator element and Myc box II. EMBO J. 1994;13:4070–4079. doi: 10.1002/j.1460-2075.1994.tb06724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Littlewood T D, Hancock D C, Danielian P S, Parker M G, Evan G I. A modified oestrogen receptor ligand-binding domain as an improved switch for the regulation of heterologous proteins. Nucleic Acids Res. 1995;23:1686–1690. doi: 10.1093/nar/23.10.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lucas J M, Wilkie N M, Lang J C. c-MYC repression of promoter activity through core promoter elements. Biochem Biophys Res Commun. 1993;194:1446–1452. doi: 10.1006/bbrc.1993.1987. [DOI] [PubMed] [Google Scholar]
- 37.Luscher B, Larsson L G. The basic region/helix-loop-helix/leucine zipper domain of Myc proto- oncoproteins: function and regulation. Oncogene. 1999;18:2955–2966. doi: 10.1038/sj.onc.1202750. [DOI] [PubMed] [Google Scholar]
- 38.Marhin W W, Chen S, Facchini L M, Fornace A J, Jr, Penn L Z. Myc represses the growth arrest gene gadd45. Oncogene. 1997;14:2825–2834. doi: 10.1038/sj.onc.1201138. [DOI] [PubMed] [Google Scholar]
- 39.Markowitz D, Goff S, Bank A. A safe packaging line for gene transfer: separating viral genes on two different plasmids. J Virol. 1988;62:1120–1124. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masiakowski P, Breathnach R, Bloch J, Gannon F, Krust A, Chambon P. Cloning of cDNA sequences of hormone-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res. 1982;10:7895–7903. doi: 10.1093/nar/10.24.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mateyak M K, Obaya A J, Adachi S, Sedivy J M. Phenotypes of c-Myc-deficient rat fibroblasts isolated by targeted homologous recombination. Cell Growth Differ. 1997;8:1039–1048. [PubMed] [Google Scholar]
- 42.Miltenberger R J, Sukow K A, Farnham P J. An E-box-mediated increase in cad transcription at the G1/S-phase boundary is suppressed by inhibitory c-myc mutants. Mol Cell Biol. 1995;15:2527–2535. doi: 10.1128/mcb.15.5.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morgenstern J P, Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nesbit C E, Tersak J M, Prochownik E V. MYC oncogenes and human neoplastic disease. Oncogene. 1999;18:3004–3016. doi: 10.1038/sj.onc.1202746. [DOI] [PubMed] [Google Scholar]
- 45.Nilsson J, Thyberg C H, Heldin B, Westermark A, Wasteson A. Surface binding and internalization of platelet-derived growth factor in human fibroblasts. Proc Natl Acad Sci USA. 1983;80:5592–5596. doi: 10.1073/pnas.80.18.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Penn L J Z, Brooks M W, Laufer E M, Land H. Negative autoregulation of c-myc transcription. EMBO J. 1990;9:1113–1121. doi: 10.1002/j.1460-2075.1990.tb08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Penn L J Z, Brooks M W, Laufer E M, Littlewood T D, Morgenstern J P, Evan G I, Lee W M F, Land H. Domains of human c-myc protein required for autosuppression and cooperation with ras oncogenes are overlapping. Mol Cell Biol. 1990;10:4961–4966. doi: 10.1128/mcb.10.9.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Penn L J Z, Laufer E M, Land H. C-MYC: Evidence for multiple regulatory functions. Semin Cancer Biol. 1990;1:69–80. [PubMed] [Google Scholar]
- 49.Philipp A, Schneider A, Väsrik I, Finke K, Xiong Y, Beach D, Alitalo K, Eilers M. Repression of cyclin D1: a novel function of Myc. Mol Cell Biol. 1994;14:4032–4043. doi: 10.1128/mcb.14.6.4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Plummer H, III, Catlett J, Leftwich J, Armstrong B, Carlson P, Huff T, Krystal G. c-myc expression correlates with suppression of c-kit protooncogene expression in small cell lung cancer cell lines. Cancer Res. 1993;53:4337–4342. [PubMed] [Google Scholar]
- 51.Prouty S M, Hanson K D, Boyle A L, Brown J R, Shichiri M, Follansbee M R, Kang W, Sedivy J M. A cell culture model system for genetic analyses of the cell cycle by targeted homologous recombination. Oncogene. 1993;8:899–907. [PubMed] [Google Scholar]
- 52.Ridley A J, Paterson H F, Noble M, Land H. Ras-mediated cell cycle arrest is altered by nuclear oncogenes to induce Schwann cell transformation. EMBO J. 1988;7:1635–1645. doi: 10.1002/j.1460-2075.1988.tb02990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenfeld M E, Bowen-Pope D, Ross R. Platelet-derived growth factor: morphologic and biochemical studies of binding, internalization, and degradation. J Cell Physiol. 1985;121:263–274. doi: 10.1002/jcp.1041210202. [DOI] [PubMed] [Google Scholar]
- 54.Roy A L, Carruthers C, Gutjahr T, Roeder R G. Direct role for Myc in transcription initiation mediated by interactions with TFII-I. Nature. 1993;365:359–361. doi: 10.1038/365359a0. [DOI] [PubMed] [Google Scholar]
- 55.Selvakumaran M, Liebermann D, Hoffman-Liebermann B. Myeloblastic leukemia cells conditionally blocked by myc-estrogen receptor chimeric transgenes for terminal differentiation coupled to growth arrest and apoptosis. Blood. 1993;81:2257–2262. [PubMed] [Google Scholar]
- 56.Silver B J. Platelet-derived growth factor in human malignancy. Biofactors. 1992;3:217–227. [PubMed] [Google Scholar]
- 57.Sorkin A, Westermark B, Heldin C H, Claesson-Welsh L. Effect of receptor kinase inactivation on the rate of internalization and degradation of PDGF and the PDGF beta-receptor. J Cell Biol. 1991;112:469–478. doi: 10.1083/jcb.112.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Souza P, Kuliszewski M, Wang J, Tseu I, Tanswell A K, Post M. PDGF-AA and its receptor influence early lung branching via an epithelial-mesenchymal interaction. Development. 1995;121:2559–2567. doi: 10.1242/dev.121.8.2559. [DOI] [PubMed] [Google Scholar]
- 59.Taira T, Sawai M, Ikeda M, Tamai K, Iguchi-Ariga S M, Ariga H. Cell cycle-dependent switch of up- and down-regulation of human hsp70 gene expression by interaction between c-Myc and CBF/NF-Y. J Biol Chem. 1999;274:24270–24279. doi: 10.1074/jbc.274.34.24270. [DOI] [PubMed] [Google Scholar]
- 60.Thompson J F, Hayes L S, Lloyd D B. Modulation of firefly luciferase stability and impact on studies of gene regulation. Gene. 1991;103:171–177. doi: 10.1016/0378-1119(91)90270-l. [DOI] [PubMed] [Google Scholar]
- 61.Ullrich A, Schlessenger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- 62.Vaziri C, Faller D V. Repression of platelet-drived growth factor β-receptor expression by mitogenic growth factor and transforming oncogenes in murine 3T3 fibroblasts. Mol Cell Biol. 1995;15:1244–1253. doi: 10.1128/mcb.15.3.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu K J, Polack A, Dalla-Favera R. Coordinated regulation of iron-controlling genes, H-ferritin and IRP2, by c-MYC. Science. 1999;283:676–679. doi: 10.1126/science.283.5402.676. [DOI] [PubMed] [Google Scholar]
- 64.Xiao Q, Claassen G, Shi J, Adachi S, Sedivy J, Hann S R. Transactivation-defective c-MycS retains the ability to regulate proliferation and apoptosis. Genes Dev. 1998;12:3803–3808. doi: 10.1101/gad.12.24.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yarden Y, Kuang W J, Yang-Feng T, Coussens L, Munemitsu S, Dull T J, Chen E, Schlessinger J, Francke U, Ulrich A. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987;6:3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Q X, Walker F, Burgess A W, Baldwin G S. Reduction in platelet-derived growth factor receptor mRNA in v-src-transformed fibroblasts. Biochim Biophys Acta. 1995;1266:9–15. doi: 10.1016/0167-4889(94)00232-4. [DOI] [PubMed] [Google Scholar]