Abstract
Gastrointestinal absorption remains indispensable in the systemic delivery of most drugs, even though it presents several challenges that, paradoxically, may also provide opportunities that can be exploited to achieve maximal bioavailability. Drug delivery systems made from nanoparticle carriers and especially, lipid carriers, have the potential to traverse gastrointestinal barriers and deploy in the lymphatic pathway, which aptly, is free from first pass via the liver. Several poorly soluble drugs have presented improved systemic bioavailability when couriered in lipid nanoparticle carriers. In this review, we propose an additional frontier to enhancing the bioavailability of poorly soluble drugs when encapsulated in lipid nano-carriers by imparting muco-adhesion to the particles through application of appropriate polymeric coating to the lipid carrier. The combined effect of gastrointestinal muco-adhesion followed by lymphatic absorption is a promising approach to improving systemic bioavailability of poorly soluble drugs following oral administration. Evidence to the potential of this approach is backed-up by recent studies within the review.
Keywords: nanoparticle, nanostructured lipid carrier, solid lipid nanoparticle, muco-adhesion, polymer, gastrointestinal, bioavailability
1. Introduction
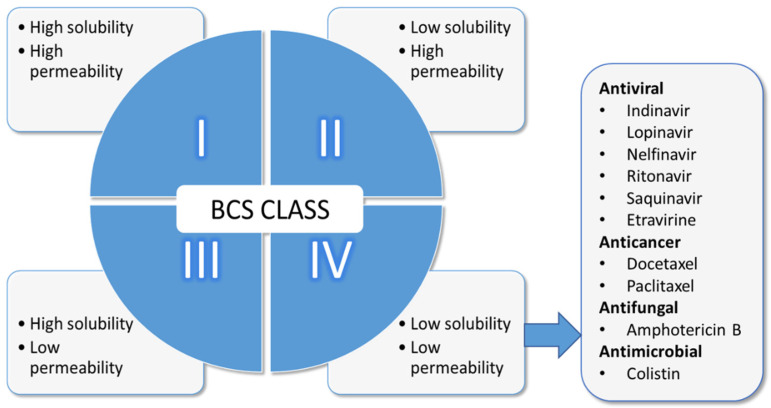
The oral route of administration remains most popular among patients because it is the most natural way to get medicines into the body. More than 60% of active pharmaceutical ingredients (API) are almost exclusively administered orally, and this represents a strong global share in the order of 90% of all dosage forms [1]. The popularity of the oral route of administration also correlates with patient compliance [2], yet, lower blood levels are observed compared to parenteral routes. This is a consequence of the epithelial barriers APIs must traverse before becoming systemically available as well as physicochemical properties of the API and physical factors related to the dosage form [3,4]. Furthermore, perturbation in systemic blood concentration occurs following first pass via the liver. The effect of food on gastrointestinal physiology, including altered gastric emptying times of dosage forms and physical interaction between the API and the food play a role in how much is presented at the target site. Apart from the effect of food on gastric emptying of dosage forms, food can alter the pharmacokinetics of APIs indirectly by interacting with gut-wall metabolizing enzymes. The best-known examples is altered pharmacokinetics of certain drugs when co-administered with grapefruit juice. This interaction is the result of inhibition of CYP3A4 metabolism as well as inhibition of the efflux transporters resident on the gut wall [5]. The consequence is an increase or decrease in bioavailability of drugs depending on whether the drug is a substrate to these enzymes. Food containing xenobiotics can also interfere with gut-wall metabolizing enzymes. Noteworthy are indole-containing vegetables such as cabbage and broccoli. Nonspecific alteration in bioavailability of APIs by certain foods may arise from physical interaction, for example dietary calcium ions (Ca2+) interacting with tetracycline or iron-containing formulations (F2+ or F3+) with tannic acid [6], both of which result in reduced bioavailability. High calorie diet can interfere with drugs absorption in a variety of ways. Poorly soluble drugs such as griseofulvin exhibits improved absorption when administered with high calorie diet, due to improved solubility in lipid milieu. On the other hand, fat receptors are believed to delay gastric emptying and hence, may impeded the rate of absorption of some drugs [6]. Besides, the Biopharmaceutics Classification System (BCS) developed by Amidon’s team [7] informs that the solubility of APIs in the GI tract and permeability through the GI membrane are both the crucial parameters that control the rate and extent of absorption (Figure 1).
Figure 1.
BCS classification system with examples of BCS Class IV drugs.
Low solubility/high permeability and low solubility/permeability drugs (Class II and IV) are of great interest to scientists because they make up 70–90% of newly discovered APIs and about 40% of currently marketed dosage forms [4,8,9]. Research on orally administered APIs has traditionally focused mainly on Class IV, APIs, for example amphotericin B, furosemide, acetazolamide, saquinavir and ritonavir [4,10,11], due to the challenges that these drugs present following administration and subsequent traversing across the GI epithelia, which aptly, results in erratic absorption and low bioavailability [4,12].
The mucous layer within the gastrointestinal tract serves as a protective barrier to the underlying epithelia. One of the key component of the mucous layer is mucin and its effect on API bioavailability is often overlooked [13]. Mucin is either secreted or cell-bound [13] and the high content of sialic acid gives it a net negative charge. Secreted mucins are linked via disulfide (S=S) bonds to form macromolecules that entangle and give rise to the typical viscoelastic gel with shear-thinning properties [14]. They are primarily responsible for the gel-like appearance but the viscoelasticity is controlled by lipid, water and ion content [15]. The protective function of mucin over the underlying epithelia means that the turnover rate of adherent mucin must be balanced by production rate. In human, this balance maintains a thickness of approximately 200 µm in the small intestine, although this is highly variable [14]. The meshwork formed from the macromolecules creates random pores, with size in the order of 300 nm, which serves as a conduit for the delivery of submicron particles. Nanoparticles can serve as drug delivery carriers for gastrointestinal absorption of poorly soluble APIs [16,17,18]. In this regard, it is desirable that the nanoparticulate delivery systems be engineered to interact with the mucous layer but not to be withheld within the meshwork. Nanoparticles with very strong positive surface charge will strongly interact with mucin and be held up within, thus preventing traversing. On the other hand, nanoparticles possessing slightly positive or close to a net neutral charge will offer the required level of electrostatic interaction with mucin to prompt muco-adhesion but not muco-retention [13]. Favorable muco-adhesive interactions with mucin will promote passive conduit toward underlying epithelia.
The Payer’s patches within the gastrointestinal epithelia can phagocytose whole particles that deploy into the lymphatic system [19]. Thus, nanoparticles with adequate surface charge can be used to cargo poorly soluble APIs across the epithelia for improved systemic bioavailability. In this regard, lipid-based carriers such as solid lipid nanoparticles (SLN) or nanostructured lipid carriers (NLC) have a prime advantage for being lipidic and hence following a lymphatic trajectory whilst avoiding the first pass via the liver [20,21].
In this review, we present an observation from the literature that points to the proposition of utilizing a combined approach of nano-formulation through lipid carrier systems and muco-adhesion as a rational approach to improving bioavailability of APIs destined for gastrointestinal absorption.
2. Lipid-Based Nanoparticulate Drug Delivery
With the advent of nanotechnology, it is now possible to formulate APIs into suitable delivery systems, which possess requisite physicochemical properties for deployment via the gastrointestinal route (GI) in a safe and/or targeted manner.
Nanotechnology may be applied to address the principal constraints akin to poorly soluble/absorbable APIs, through improvement in (i) API solubilization (ii) muco-adhesion behavior of delivery system and (iii) cellular uptake of particulate delivery systems [22,23,24,25].
Lipid-based nanoparticulate delivery systems have gained increased attention by researchers in recent years because they are biocompatible, mostly biodegradable, and have wide biomedical applications [4,26,27]. Furthermore, they have proven commercial, pharmaceutical and therapeutic benefits for compounds such as cyclosporine A, lipid soluble vitamins and protease inhibitors [4,26,28]. They have the potential for use as drug delivery systems in various routes of administrations such as pulmonary [29,30], dermal [31,32,33], ocular [34,35,36], parental [37,38] and oral [25,27,39].
As per the remit of the present review, our focus is on the delivery of API via lipid-based nanoparticles via the GI tract. The nano-sized dimension coupled with the lipidic nature of the particles offers this class of nanoparticles superiority over other nanoparticulate delivery formulations. For example, lipid nanoparticles have better tolerability in vivo as they are biocompatible, biodegradable and capable of mimicking the natural digestive process of the dietary fat [11,27]. Additionally, the use of organic solvents during formulation can potentially be avoided, in contrast to polymer-based nanoparticles and thus, show safer toxicology profiles [26,30,40,41]. Besides, the absorption of poorly soluble APIs is enhanced by virtue of stimulation of biliary and pancreatic secretions by the particles [42,43], as evidenced by improved bioavailability of lipophilic vitamins (vitamin A, D, E and K), testosterone and halofantrine co-administered with fat-rich diet [22,30,44]. The simultaneous absorption of the drug along with the lipids is also known as the “Trojan Horse effect” [26,45].
Lipids offer protection to susceptible APIs that degrade via chemical, electromagnetic, oxidation and enzymatic reactions [26,30,40,41]. Moreover, lipid-based nanoparticles can alter the pharmacokinetic profiles of APIs through the manifestation of slow release behaviour from the delivery system [46]. Therefore, abrupt exposure to high drug concentration in vulnerable organs is minimised. In this context, the pharmacokinetic profile of API is now governed by the particle size, charge, type and concentration of the lipids rather than the intrinsic physicochemical properties of the API [47,48].
Due to their lipidic nature, this class of nanoparticles permit absorption of APIs through the lymphatic pathway [26,30,40,41], avoiding liver first pass and thus improving their systemic bioavailability [4,46,49].
Typical lipid-based nanoparticles are formulated as solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) [26,45,50,51]. Generally, they comprise of various types of physiological lipids with surfactants as stabilisers (Table 1) [30,51]. The following sections reviews the salient differences between SLNs and NLCs.
Table 1.
Composition of SLNs and NLCs.
| Class | Examples | References |
|---|---|---|
| Solid lipids | Cetylpalmitate | [54,55] |
| Glyceral behenate (Compritol 888 ATO) | [56,57,58] | |
| Glyceryl monostearate | [55,58,59,60] | |
| Glyceryl tripalmitate | [61,62] | |
| Stearic acid | [63,64,65] | |
| Tristearin | [60,66] | |
| Beeswax | [18,20,67,68] | |
| Liquid oils | Coconut oil | [18,68] |
| Oleic acid | [63,69,70] | |
| Miglyol 812 | [56,58,59] | |
| Castor oil | [70,71] | |
| Surfactants | Poloxamer 188 (Pluronic® F-68) |
[57,58,59,72] |
| Polysorbate 20 (Tween-20) |
[73,74] | |
| Polysorbate 80 (Tween-80) |
[62,75] | |
| Sodium cholate | [20,69] | |
| Sodium glycocholate | [59] | |
| Sodium taurocholate | [56,64] | |
| Sodium dodecyl sulfate | [63] | |
| Soybean lecithin | [20] |
2.1. Solid Lipid Nanoparticles (SLNs)
SLNs are considered as the first-generation lipid-based nanoparticles. They are colloidal suspensions with particle size range in the order of 40–1000 nm and solid at both room and body temperatures and typically comprise of lipids as the matrix system (Table 1) [52,53].
SLNs possess all the advantages of lipid-based nanoparticles. For example, idarubicin-loaded SLNs showed a 21-fold increase in systemic bioavailability following oral administration, reflected through the area under the curve (AUC) and a 30-fold extended elimination half-life as compared to idarubicin solution [76]. In another study, cyclosporine A-loaded SLNs also showed high systemic bioavailability with no nephrotoxicity observed due to the slow release behavior from the SLNs compared to its marketed microemulsion formulation, Sandimmun® Optoral/Neoral [26,77].
Despite these advantages, SLNs suffer from low API loading capacity with significant drug expulsion during storage, due to the polymorphic transformation of the lipids [22,30,53]. The poor loading capacity of SLNs is due to the densely packed lipid crystal lattice with few imperfections and thus, offering only little room for accommodation of APIs [30,31,53]. Hence, in order to address these SLN-related constraints, a second generation of lipid nanoparticles, nanostructured lipid carriers (NLCs) has emerged [4,22,26].
2.2. Nanostructured Lipid Carriers (NLCs)
NLCs formulations were developed in 2000 and within five years, two NLCs products were approved to be marketed (Nanorepair Q10 cream and Nanorepair Q10 serum, Dr. Rimpler, Wedemark, Germany) [78,79]. NLC represent nanocarriers with the shortest time between invention and marketing and considered very promising by the scientific community.
In contrast to the SLNs, NLCs formulations comprise of a blend of solid lipid with liquid oil (Table 1). Despite the presence of liquid oil, the NLCs retains the solid matrix at room and body temperature [40,80]. The liquid oil disrupts the crystallinity of the solid lipid matrix and thus, creates imperfections that allows incorporation high loads of API [12,22,81]. So, a five-fold increase in encapsulation efficiency (EE) of retinoids was observed upon inclusion of small amount of liquid oil into glyceryl behenate SLN matrix [82]. Similarly, an increase in EE was observed for AmpB encapsulated as SLNs from 52.77 to 75.33% when encapsulated as NLCs [83].
NLCs can be categorised as imperfect, amorphous or multiple NLCs according to the structure of the lipid matrix [51,84]. In imperfect NLCs there is distortion of the perfect crystal lattice due to the presence of a small amount liquid oils whilst amorphous NLCs are solid but non-crystalline state of lipids [51,84]. The amorphous NLCs are formed through the inclusion of liquid lipids such as hydroxyl octacosanyl, hydroxyl stearate, isopropyl myristate that prevent lipid crystallisation [48,51]. The multiple type NLCs contain numerous nano-sized liquid oil domains within the solid lipid matrix, so that the release of the API is prolonged because of the tortuous paths required to be traversed before release [51,84]. The composition and structure of NLCs allows high drug payloading, increased stability of the formulation upon storage [84].
Several highly lipophilic drugs have been encapsulated within NLCs for systemic bioavailability enhancement, most belonging to the BCS Class II, such as vinpocetine [58], lovastatin [80,85], simvastatin [86,87], atorvastatin [88], miconazole [89], quercetin [39,61], resveratrol [90], testosterone [91], tamoxifen [92], fenofibrate [93] and tacrolimus [94]. BCS Class IV encapsulated in NLCs include etoposide [95], saquinavir [96], oleate-docetaxel [97] and artemether-lumefantrine [88].
3. GI Uptake of Lipid-Based Nanoparticle Formulations
Sufficient GI uptake of the lipid nanoparticle along with its API cargo is the first and crucial step toward the systemic delivery of the API. However, it is important to recognize other potential barriers within the GI tract as well. For example, the mucosal layer as mentioned earlier, which serves as a protective layer to the underlying epithelia, presents a frontier that particulate delivery system must traverse. Fortunately, lipid nanoparticles have some tendency to adhere onto the mucosal layer and therefore able to oppose dislodging imposed by propulsive forces from GI motility [22,45]. Such shear forces will normally detach particulate delivery systems from the mucosa layer, but the smaller the particle size, the lower the translated shear stress from GI motility. Hence smaller sized particles are more likely to remain muco-adhered to the epithelia longer [98].
In a cell culture study on the permeability of NLCs across Caco-2 and HT-29 MTX co-culture, significant variation was observed between type of cell, where lower NLCs uptake occurred in cells without mucus (Caco-2) compared to HT-29 MTX, which expresses the mucosal layer [12].
Furthermore, charged particles may adhere electrostatically or be repelled from the mucosal layer depending on the resident surface charge on the particles, noting that, mucin is negatively charged due to sialic acid groups [99,100]. Although positively charged particles have strong muco-adhesion to mucin, they can potentially be entrapped within the layer and thus unable to effectively traverse the underlying epithelial cells [12,99]. This entrapment could be beneficial eventually, because prolonged GI transit time, improves the chances for lateral incursions toward uptake of particles the epithelia [101,102].
Upon oral ingestion, part of the lipid nanoparticles may become degraded or digested by gastric lipase aided by shear forces (e.g., propulsion, grinding and retropulsion) in the stomach, which forms a crude emulsion [4,103]. In the small intestine, the lipids may become degraded by enzymes (e.g., lipase-colipase complex) forming surface active mono-, diglycerides and fatty acids [4,22,26,84]. The presence of exogenous lipids stimulates the secretion of biliary lipids from the gall bladder, including bile salts, phospholipids and cholesterol, which react with and disperse the surface active mono-, diglycerides and fatty acids into fine oil droplets or micelles [4,26,45,103]. The micelles are incorporated into a series of colloidal structures, including mixed-micelles, unilamellar and multi-lamellar vesicles [4,22,26,45]. Prior to the formation of these colloidal structures, the API initially encapsulated within the NLC may be taken up or solubilised into the micelles [26,27,84]. Through that, the drugs are simultaneously taken up by the epithelial cells during the absorption process of the mixed-micelles [22,26,45].
Furthermore, the colloidal structures or some intact lipid nanoparticles can either be taken up through membranous epithelial cells (M-cells) or gut enterocytes [84,104]. M-cells are specialised epithelial cells, consisting of only 10% of cells on the dome of Peyer’s patches (PP) [22,51,105]. PP are gut-associated lymphoid tissues that comprise of aggregated or isolated lymphoid follicles and they provide entry points to the lymphatic system [76,106]. They possess high transcytotic capacity and able to transport a broad range of materials including macromolecules and microbes thus, can be exploited for the delivery of drugs, vaccines and bioactive materials [101,107,108].
The uptake of lipid nanoparticles into gut enterocytes is mainly through the transcellular active transport of endocytosis [76,106,109]. Endocytosis is internalisation of macromolecules into transport vesicles, derived from the plasma membrane [110,111]. It can be further categorised as phagocytosis or pinocytosis [23,109,112]. Phagocytosis involves M-cells and immune cells such as macrophages, dendritic cells, monocytes and nucleophiles [106,109,111]. Unlike phagocytosis, pinocytosis is not restricted to specialised cells but could occur in any type of cells [23,112].
Pinocytosis is more complex and may include clathrin-mediated endocytosis (CME), caveolae-mediated endocytosis (CvME), macropinocytosis, clathrin or caveolae-independent endocytosis. CME occurs in clathrin-enriched membrane and includes receptor-ligand interactions [109,112]. CME leads to the formation of early endosome, late endosome and finally lysosome. The lysosome has an acidic (pH 4.5–5.5) and is an enzyme-rich environment thus, may promote degradation of labile therapeutic agent or nanocarriers [108,109,112].
CvME involves cholesterol-rich or sphingolipids membranes that are lined with caveolin, a dimeric protein with a flask-shape membrane invaginations characteristic [112,113]. Caveolae vesicles may protect the nanoparticles from lysosomal degradation, thus, it is a preferred pathway for delivery of enzymes and proteins in contrast to the CME pathway [108]. However, CvME occurs at a slower rate than CME pathway due to its highly regulated process that involves complex signaling mechanisms [110,112].
Similar to phagocytosis, macropinocytosis involves protrusion and fusion of the membrane, encapsulating the particles and forms vesicles of about 1 µm. However, the process of macropinocytosis is non-selective and highly dependent on the difference in the solute concentration [108,111].
Lipid nanoparticles can also traverse the paracellular route after transient disruption of tight junctions between adjacent epithelial cells by permeation enhancers [76,84,105]. However this mode of absorption is highly variable and dependent on the permeation enhancer but typically up to 2000 kDa opening is possible as evidenced by permeation of Flourescein isothiocynate (FICT) [114]. The tight junctions are modulated by proteins such as junctional adhesion molecules (JAM), occludins and claudins that regulate the passage of particles. Studies have shown that some polymers like chitosan [99,115] and thiolated polymers [116] are capable of reversibly disrupting the tight junctions, and thus, could be further exploited for the transport of macromolecules or nanoparticles. However, transcellular route remains the predominant passage route as the fraction of epithelial cells are greater than the percentage of tight junctions [109,117].
After the formed micelles are taken up by the enterocytes cells, they become converted into chylomicrons upon re-esterification via monoacyl glycerol or phosphatidic acid pathway and subsequently, stabilised by phospholipids [4,76,118]. Finally, the formed chylomicrons are transported via the intestinal lymphatic pathway [4,119].
The lymphatic system is an additional pathway for the absorption of lipid nanoparticles or other lipophilic compounds (e.g., long-chain fatty acids, cholesterol esters and fat-soluble vitamins), in contrast to most orally administered drugs, which are transported mostly via the portal blood vein before reaching the systemic circulation [51,120]. The lymphatic system consists of lymph, capable of maintaining the homeostasis through the regulation of extracellular fluid and helps in body defense system by transporting immune cells to injury sites [76,121].
Lipid nanoparticles that are absorbed by the intestinal lymphatics are transported through the mesenteric lymph duct that enter into the thoracic duct and empty in the systemic circulation via left jugular and subclavian veins [102,122]. The lymphatic system is a formidable absorption pathway for drug delivery because it (i) bypasses the first pass metabolism that increases drug bioavailability, (ii) has prolonged drug delivery due to longer duration of drug transport and (iii) offers the possibility of targeting drugs to lymph, potential application in lymphatic cancers and relevant infections such as leishmaniasis, malaria and AIDS [26,51,76,102,105].
Lymphatic absorption of lipid nanoparticles has been extensively explored as a viable means for delivering poorly soluble APIs. A commercial formulation of testosterone (Andriol®) dissolved in oleic acid has been exploited for lymphatic absorption which accounted for 91.5% of the total bioavailability [123]. In another study, methotrexate-loaded SLNs showed a 10-fold increase in bioavailability attributable to the lymphatic system [60]. Furthermore, Khan et al. (2013) reported that vincopecetine-loaded NLCs observed a two-fold increase in the Cmax compared to pure vincopecetine solution. The lymphatic pathway was hypothesised to be the main transportation route for the NLCs as opposed to the solution, which suffered significant first pass [76].
4. Importance of GI Muco-Adhesion
Conventional formulations often face challenges due to inability to withstand the strong involuntary muscular contractions and the washing effects due to GI luminal content [124]. This limitation results in the loss of crucial amounts of administered API, and thus, necessitates multiple dosage administration in order to achieve blood therapeutic levels required to elicit pharmacological responses [34,125]. Such API washing can potentially result in the failure of the therapy and result in an overall increase in treatment costs [124].
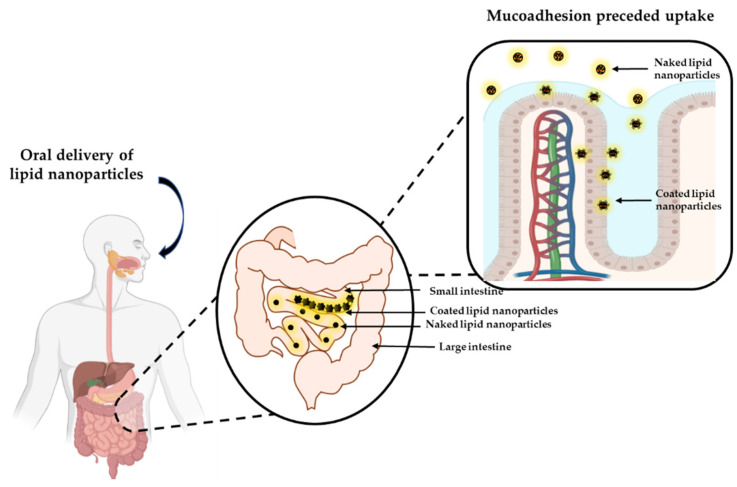
Muco-adhesive GI delivery systems can adhere to the mucous layer of the GI epithelia and hence prolong the residence time of the formulation at the absorption site (Figure 2) [100,126]. This intimate contact with the epithelium confers a higher permeation propensity which, subsequently increases the systemic bioavailability of the API [124,127]. Nanoparticulate delivery systems are inherently muco-adhesive [32,33,34,35], however, this property may be enhanced further by coating the particles with appropriate polymers. Such coated particles also protect labile drugs from the GI milieu so that unaltered form of the API traverses across the epithelia [124,128].
Figure 2.
Schematic diagram of muco-adhesion behavior of lipid nanoparticles. Created with Biorender.com and ChemDraw Professional.
GI muco-adhesion by nanoparticulate dosage may occur via a variety of mechanisms including: (i) electronic, (ii) wetting, (iii) adsorption, (iv) diffusion and (v) mechanical. The electronic phenomenon is based on the establishment of attractive forces through the transfer of electrons (e.g., between cationic polymer and negatively charged mucin), forming an electrical double layer [100,127,129]. In the wetting mechanism, a measure of the spreadability of the dosage form on the mucous layer, and hence the magnitude of its contact angle (immersional contact angle) to the mucus layer is used to assess muco-adhesivity. Lower contact angles indicate better propensity to muco-adhesion [100,127,129]. The adsorption mechanism is based on semi-permanent interactions such as hydrogen bonds, van der Waals and hydrophobic interactions as the driving forces for muco-adhesion and they require less energy to detach. Based on diffusion mechanism, adhesive forces are established through a time-dependent diffusion of the polymer chains into the glycoprotein chain network of the mucosal layer [127,130]. The mechanical theory proposes that adhesion occurs because of the diffusion of the polymer into the irregularities of the mucosal surface, which increases the interfacial area for interactions [100,126,131].
Taking advantage of the lymphatic pathway, lipid-based delivery systems, and especially submicron carriers, provide an avenue for the deployment of payloads via the lymphatic route into the systemic circulation. Whilst this is true in in vitro and animal models, GI delivery of lipid-based systems still fall short in the clinical settings and therefore warrants attention [132]. Oral delivery of AmpB has been widely investigated and includes polymeric nanoparticle carbon nanotubes, nanosuspensions, polymer lipid hybrid nanoparticles, solid lipid nanoparticles (SLN), cubosomes, emulsions and cochleates [20,67,133,134,135]. In the work by Amekyeh et al. (2015), the extent of absorption, rather than the rate from the GI tract, was the key contributor to improved bioavailability of amphotericin B [20]. This is indicative of uptake via the lymphatic route.
Within the context of the present review, attendant muco-adhesion imparted by the delivery system appear to be a rational approach for achieving both localized therapeutic action and systemic delivery through uptake by enterocytes. SLN uptake via the gastrointestinal tract can be enhanced if the transit of the particles can be extended. SLN and NLCs are inherently neutral or exhibit negative charge potential due to the lipids. However, it is possible to impart positive potential to SLN or NLC from positively charged surfactants, such as cetyltrimethylammonium bromide (CTAB). Although muco-adhesivity is achievable from such particles, toxicity concerns limit the use of positively charged surfactants [136], especially for internal applications. On the other hand, cationic lipids such as cationic lipid N, N-dioleyl-N, N-dimethylammonium chloride (DODAC) can be formulated as cationic SLN or NLC but toxicity remains a concern [137]. In addressing toxicity concerns, SLNs or NLC can be coated with muco-adhesive polymers that exhibit low or no toxicity to cells. Chitosan, a natural, non-toxic, biocompatible polycationic polysaccharide, derived from partial deacetylation of chitin is well studied and reported to possess muco-adhesive properties [132]. SLN have also been investigated for deployment in the small intestinal region for systemic absorption [73] or the colonic region of the gastrointestinal tract in the management of colon cancer, whereby doxorubicin and docetaxel were primed for local delivery [138]. In order to facilitate a controlled release of docetaxel SLN have been coated with glycolic acid [139], which is likely to impart some degree of muco-adhesion. In terms of muco-adhesion for therapeutic applications, folic acid-grafted on SLN containing irinotecan were encapsulated as microbeads of alginates and coated with Eudragit S100, which is a pH responsive enteric polymer [140,141,142]. It swells and dissolves at resident colonic pH values. This colon-targeting capability of the SLN was confirmed via a radiolabeled biodistribution study, where the microbeads showed a residence time of over 48 h in cancer tissue [142]. Thus, oral delivery of the SLN/microbeads enhanced anti-colon cancer efficacy. A high local GI concentration of SiRN was reported when the latter was delivered orally as lipoidoid nanoparticles through muco-adhesion [143]. The authors add that SiRN delivery to immune cells is possible by this approach. Furthermore, bees wax modified with phospholipids had shown muco-adhesion attributes and thus used to enhance mucosal delivery of antifungal drugs such as miconazole nitrate [144]. Lou et al. (2015) showed that the uptake of coumarin 6-loaded chitosan coated SLN by Caco2 cells was 44% in contrast to 10% from non-coated SLN [125]. Enhanced absorption was also observed from Caco2 monolayers of an insulin containing chitosan coated SLN. However, further improvement in insulin absorption was observed from HT29 cells, which are mucus producing. Oral administration of the chitosan coated SLN to diabetic rats resulted in a significant hypoglycemic effect in rats [145]. Amphotericin B containing chitosan-coated NLC demonstrated significant improvement in bioavailability after oral administration in rats compared to uncoated NLC or Amphotret® [18]. In a related study, chitosan coated NLC displayed biocompatibility with enhanced antifungal properties compared to uncoated NLC [146].
5. Muco-Adhesion Polymers
Muco-adhesion polymers can be classified as non-ionic, anionic or cationic. Non-ionic polymers (e.g., polyethylene oxides) exert their muco-adhesion through a buildup of the hydrogen bonds and entanglement of the polymer chains, independent of the surrounding pH conditions. However, they were reported to be less adhesive as compared to anionic or cationic polymers [130,147]. Anionic polymers such as sodium carboxymethylcellulose (NaCMC) and poly (-acrylic acid) (PAA) with its derivatives, exert their muco-adhesion behaviour through hydrogen bonding via their carboxyl functional groups with the hydroxyl groups of the oligosaccharide side chains of the mucus protein [100,130]. The disadvantage of anionic polymers is that they tend to precipitate in the presence of multivalent cations like Mg2+ and Ca2+ and which reduces their muco-adhesion capacity [130].
Cationic polymers exhibit muco-adhesion behavior through ionic interactions between polymers and the anionic sialic acid groups of the mucus [68,124]. Among the cationic polymers, chitosan is undoubtedly the most extensively investigated in the literature [129,130], owing to its biocompatibility and biodegradability.
6. In-Vitro/Ex Vivo Muco-Adhesion Studies
The muco-adhesion properties of conventional dosage forms or materials have been preferentially measured using methods such as tensile and shear strength tests, which directly measures the time or force needed to detach the dosage form from a model membrane [127]. These methods may involve different detachment techniques but overall they are easy and fast to perform [100,148]. However, those tests impose limitations due to their bulky nature and as such difficult to ascertain whether the detachment has occurred at muco-adhesion interface or simply due to the loss of cohesion between polymers molecules or mucin components. Additionally, the surface properties of nanosystems (e.g., morphology, curvature and polymer disposition) as well as the closer proximity of the interactions of the nanosystem with the mucosal environment, justify the development of techniques used to evaluate muco-adhesion at nanoscale [100,127].
The following sections will focus on the different experimental approaches used to assess the muco-adhesion potential of nanoformulations, which can be classified as indirect or direct methods (Table 2).
Table 2.
Methods used to evaluate the muco-adhesion properties of the formulations and comparison of their key features.
| Techniques | Principle of the Technique | Mechanism | Real Time | Relevant | Feasibility | Cost | References |
|---|---|---|---|---|---|---|---|
| Indirect | |||||||
| Mucin particle | Determined through variation in size, ζ and turbidity. | + | No | + | +++ | + | [149,158] |
| Microgravimetric | Evaluation of material adsorbed using quartz crystal microbalance based on resonance frequency. | ++ | Yes | + | ++ | ++ | [125,155] |
| AFM | Probe tip modified with polymer/mucin, measure attractive/repulsive forces exerted at molecular level. | +++ | Yes | + | + | +++ | [157,159] |
| Optical techniques | Changes in properties of incident light on surface immobilised mucin on binding with nanoparticle (ellipsometry and surface plasmon resonance). | ++ | Yes | + | ++ | +++ | [150,160] |
| Diffusion/particle tracking | Impediment to the unhindered diffusive movement of nanoparticles (multiple particle tracking). | +++ | Yes | ++ | +++ | ++ | [161] |
| Direct | |||||||
| Cytoadhesion | Adhesion of fluorescent-labelled nanoparticles in cell culture using fluorescent microscopy. | ++ | Optional | ++ | ++ | ++ | [160] |
| Ex vivo methods | Retention of labelled nanoparticles in mucosal tissue/based on weight difference of nanoparticles. | + | Optional | ++ | ++ | ++ | [162] |
| In vivo administration/ ex vivo analysis |
Administration of labelled nanoparticles to living animals, evaluation upon sacrifice. | + | No | +++ | ++ | ++ | [163,164] |
| In vivo imaging | Natural trafficking of labelled-particles (e.g., barium sulphate, technetium-99 m). | + | Yes | +++ | + | +++ | [29,165] |
Indirect methods evaluate the balance between contributing and detrimental interactions between the nanoformulation and mucins whereas direct methods evaluate muco-adhesion in vivo or using biological tissue (ex vivo).
6.1. Indirect Methods
6.1.1. Mucin Particle Method
Mucin particle method is an indirect method used to assess the muco-adhesion properties of the nanoformulation. It evaluates the degree of adsorption of nanoparticles to mucin particles through the measurement in the variation of size [149], zeta potential or electrophoretic mobility of the formed complexes [150,151].
Besides, the interaction between mucin in solution and nanoparticles could also be assessed through turbidity concept whereby the transmittance [152] or absorbance [146,153] of the dispersion after an adequate incubation time is measured. The interaction between nanoparticles and mucin will form macro aggregates, which scatters light more intensely and thus increases the turbidity of the sample [127,154]. Although mucin particle method is an easy and cheap method, additional studies are needed since higher concentrations of mucin than those observed in in vivo are often reported [127].
6.1.2. Microgravimetric Method
Microgravimetric method evaluates the amount of material adsorbed onto the mucin using a quartz crystal microbalance [155]. The muco-adhesive behavior is evaluated through the changes in the resonance frequency calculated using the Sauerby equation [156]. It is a sensitive method and allows real-time data to be acquired, providing valuable kinetic profile of muco-adhesion process. However, there is a lack of published data to prove the real value of this method.
6.1.3. Atomic Force Microscopy (AFM)
AFM measures the attractive and repulsive forces exerted at molecular level through the modification of the probe tip with polymer or mucin. Interaction with different samples (e.g., cell layers) will result in deflection the cantilever attached to the probe and thus, the interaction forces (both attractive and repulsive) can be measured [157]. Besides, AFM allows measurement to be carried out in milieu mimicking biological systems in real-time data. However, cost, complexity and reproducibility are the major disadvantages of this method [127].
6.1.4. Optical Technique
In the literature, several optical techniques employ changes in properties of incident light on surface immobilised mucin when nanoparticles adsorbs to the mucin. For example, ellipsometry measures the muco-adhesion (weight of nanoparticles adsorbed per area) based on the refractive index from the mucin layer. This is shown through the study by Svensson et al. (2008), utilising chitosan-modified particles and cubosomes on mucin coated surfaces [150].
Surface plasmon resonance optical technique utilises a resonant mirror biosensor to evaluate changes in the refractive index based on evanescent waves. The association between the nanoparticles and mucin can be obtained through the mathematical model utilising the quantitative data produced [155].
6.1.5. Diffusion/Particle Tracking
Multiple particle tracking (MPT) has been extensively used to measure the interaction between unhindered diffusive movement of nanoparticles and mucus or mucus simulating fluid. Briefly, the fluorescent nanoparticles are incubated with mucus and placed under video microscopy to obtain high resolution 2D trajectories. The muco-adhesion properties can be evaluated based on quantitative parameters such as diffusivity/immobility and transport mode of tracked nanosystems [127,161].
6.2. Direct Methods
6.2.1. Cyto-Adhesion
Generally, this type of study evaluates the adhesion of fluorescent labelled nanoparticles in epithelial cell monolayer using fluorescent microscopy. It is simple to execute but suffers from disadvantages such as lack of tissue architecture and functionality [160]. Hence, a more refined cell model was proposed, using a co-culture of intestinal epithelial cells (Caco-2) and mucus producing cells (HT-29). Study by Prego et al. (2006) demonstrated that chitosan nanocapsule showed a higher degree of association to co-culture of Caco-2/HT-29 compared to monoculture of Caco-2, revealing the muco-adhesive properties of chitosan [166].
6.2.2. Ex Vivo Muco-Adhesion Method
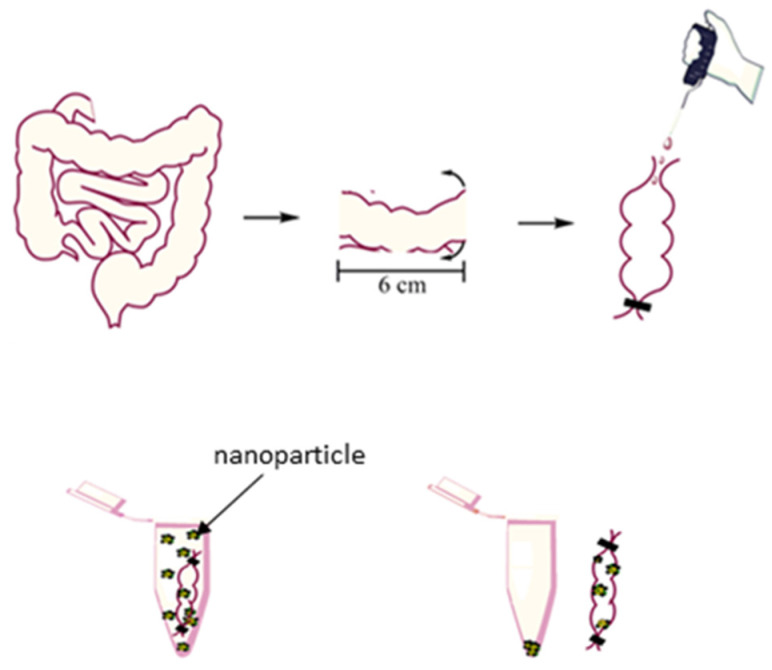
The ex vivo muco-adhesion method evaluates the degree of retention of radiolabeled or fluorescent-labelled nanoparticles in mucosal tissue explants [167]. Simpler method eliminates the need for additional radioisotopes or fluorescence in the nanosystem as such utilization requires a balance between the weight of the initially applied nanoparticles and remaining upon incubation with the mucosal explants [154,162]. The advantages of this method are that it utilizes natural mucus and is thus more physiologically relevant as well as less ethically ambiguous as it allows higher number of experiments to be performed [127]. With regard to ex vivo muco-adhesion of lipid nanoparticles, the particles can be suspended in an everted sac and the amount adsorbed estimated, Figure 3.
Figure 3.
Ex vivo estimation of muco-adhesion propensity.
6.2.3. In Vivo Administration/Ex Vivo Analysis
This method involves administration of fluorescent compounds (e.g., Cy 5.5 [168], sulforhodamine B [80] and coumarin-6 [99] or radioisotope-labelled (e.g., technetium-99 m) nanoparticles to the living animals following which the muco-adhesion is evaluated through harvesting relevant region of the GI tract in sacrificed animals over time (Table 2) [29]. The amount of the nanoparticles present at the region-of-interest is evaluated qualitatively using confocal microscopy [99] or quantitatively through fluorometric assay or gamma counter [165].
The major limitation to this method is the ethical constraints associated with the number of animals required for the experiment. Nevertheless, this technique allows good representation of the physiological washing, which is absent in the indirect and ex vivo methods as such could potentially influence the adhesion and retention of the nanoparticles [127].
7. Application and Future Perspectives of Muco-Adhesion Lipid Nanoparticle
Lipid nanoparticulate carriers provides a means for deploying APIs cargoes systemically, when delivered orally, by virtue of lymphatic uptake of the particles. In this regard, the bioavailability of several class III or IV drugs have been improved when formulated as orally administered lipid nanoformulations. Further improvement in systemic bioavailability can be achieved through delayed GI transit of the particles, when the latter is coated with muco-adhesion polymers. It is the view of the authors that more of the above classes of drugs that previously were delivered by alternative routes will now be prospective candidates for oral delivery via lipid nanoformulation cum GI muco-adhesion. Furthermore, lipid polymers that possess muco-adhesion properties should be investigated as possible carrier systems. Chemical conjugation of lipid carriers to muco-ahesive polymers will be a rational approach for use in nanoformulation. Muco-adhesion of nanoparticles as semisolid dosage form will find wide applications on mucosal surfaces for the treatment of localized diseases. In regard case, gradual release of the API from the resident formulation provides an effective therapeutic supply to concerned tissue.
8. Conclusions
Lipid nanoformulation presents an alternative means to delivering poorly soluble APIs to the systemic circulation following oral administration and GI absorption, noting that insignificant bioavailability results from administration of such pure APIs. This review captures evidence supporting the emergence of an additional dimension in the utilization of lipid nanoformulation in for enhancing the systemic bioavailability of the API. This can be achieved through use of suitable polymers adequately adsorbed to the lipid nanoparticle in order to impart muco-adhesivity, thus extending the GI transit times. This allows lateral incursions across the epithelia ultimately feeding into the lymph. The combined approach works better in enhancing the bioavailability of the API than either used alone. One area where this approach will find wide application is the treatment of local conditions within the GI tract whereby lipid nanocarriers deploy through muco-adhesion and gradual release of the API.
Author Contributions
Conceptualization, N.B. writing—original draft preparation, S.L.J.T.; writing—N.B.; S.L.J.T. review and editing, N.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sedo K., Kararli T. Global Drug Delivery & Formulation. J. Innov. Image Process. 2020;20:18–23. [Google Scholar]
- 2.De Geest S., Sabaté E. Adherence to Long-Term Therapies: Evidence for Action. Eur. J. Cardiovasc. Nurs. 2003;2:323. doi: 10.1016/S1474-5151(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 3.Savjani K.T., Gajjar A.K., Savjani J.K. Drug solubility: Importance and enhancement techniques. ISRN Pharm. 2012;2012:195727. doi: 10.5402/2012/195727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghadi R., Dand N. BCS class IV drugs: Highly notorious candidates for formulation development. J. Control. Release. 2017;248:71–95. doi: 10.1016/j.jconrel.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Disler P.B., Lynch S.R., Charlton R.W., Torrance J.D., Bothwell T.H., Walker R.B., Mayet F. The effect of tea on iron absorption. Gut. 1975;16:193–200. doi: 10.1136/gut.16.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahan A., Altman H. Food-drug interaction: Grapefruit juice augments drug bioavailability—Mechanism, extent and relevance. Eur. J. Clin. Nutr. 2004;58:1–9. doi: 10.1038/sj.ejcn.1601736. [DOI] [PubMed] [Google Scholar]
- 7.Amidon G.L., Lennernäs H., Shah V.P., Crison J.R. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995;12:413–420. doi: 10.1023/A:1016212804288. [DOI] [PubMed] [Google Scholar]
- 8.Davis M., Walker G. Recent strategies in spray drying for the enhanced bioavailability of poorly water-soluble drugs. J. Control. Release. 2018;269:110–127. doi: 10.1016/j.jconrel.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Kalepu S., Nekkanti V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B. 2015;5:442–453. doi: 10.1016/j.apsb.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindenberg M., Kopp S., Dressman J.B. Classification of orally administered drugs on the World Health Organization Model list of Essential Medicines according to the biopharmaceutics classification system. Eur. J. Pharm. Biopharm. 2004;58:265–278. doi: 10.1016/j.ejpb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Beloqui A., Solinís M., Delgado A., Évora C., del Pozo-Rodríguez A., Rodríguez-Gascón A. Biodistribution of Nanostructured Lipid Carriers (NLCs) after intravenous administration to rats: Influence of technological factors. Eur. J. Pharm. Biopharm. 2013;84:309–314. doi: 10.1016/j.ejpb.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 12.Beloqui A., Solinís M., Rodríguez-Gascón A., Almeida A., Préat V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomed. Nanotechnol. Biol. Med. 2016;12:143–161. doi: 10.1016/j.nano.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Lai S.K., Wang Y.-Y., Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Copeman M., Matuz J., Leonard A.J., Pearson J.P., Dettmar P.W., Allen A. The gastroduodenal mucus barrier and its role in protection against luminal pepsins: The effect of 16,16 dimethyl prostaglandin E2, carbopol-polyacrylate, sucralfate and bismuth subsalicylate. J. Gastroenterol. Hepatol. 1994;9:S55–S59. doi: 10.1111/j.1440-1746.1994.tb01303.x. [DOI] [PubMed] [Google Scholar]
- 15.Lai S.K., Wang Y.-Y., Wirtz D., Hanes J. Micro- and macrorheology of mucus. Adv. Drug Deliv. Rev. 2009;61:86–100. doi: 10.1016/j.addr.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee S.A., Joung H.J., Park H.J., Shin G.H. Preparation of Chitosan-Coated Nanostructured Lipid Carriers (CH-NLCs) to Control Iron Delivery and Their Potential Application to Food Beverage System. J. Food Sci. 2017;82:904–912. doi: 10.1111/1750-3841.13655. [DOI] [PubMed] [Google Scholar]
- 17.Oussoren C., Zuidema J., Crommelin D., Storm G. Lymphatic uptake and biodistribution of liposomes after subcutaneous injection.: II. Influence of liposomal size, lipid composition and lipid dose. Biochim. Biophys. Acta BBA—Biomembr. 1997;1328:261–272. doi: 10.1016/S0005-2736(97)00122-3. [DOI] [PubMed] [Google Scholar]
- 18.Tan J.S.L., Roberts C., Billa N., Ling J.T.S. Pharmacokinetics and tissue distribution of an orally administered mucoadhesive chitosan-coated amphotericin B-Loaded nanostructured lipid carrier (NLC) in rats. J. Biomater. Sci. Polym. Ed. 2019;31:141–154. doi: 10.1080/09205063.2019.1680926. [DOI] [PubMed] [Google Scholar]
- 19.Li H., Zhao X., Ma Y., Zhai G., Li L., Lou H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J. Control. Release. 2009;133:238–244. doi: 10.1016/j.jconrel.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Amekyeh H., Billa N., Yuen K.H., Chin S.L.S. A Gastrointestinal Transit Study on Amphotericin B-Loaded Solid Lipid Nanoparticles in Rats. AAPS PharmSciTech. 2015;16:871–877. doi: 10.1208/s12249-014-0279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.AlKhader E., Roberts C.J., Rosli R., Yuen K.H., Seow E.K., Lee Y.Z., Billa N. Pharmacokinetic and anti-colon cancer properties of curcumin-containing chitosan-pectinate composite nanoparticles. J. Biomater. Sci. Polym. Ed. 2018;29:2281–2298. doi: 10.1080/09205063.2018.1541500. [DOI] [PubMed] [Google Scholar]
- 22.Yoon G., Park J.W., Yoon I.-S. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs): Recent advances in drug delivery. J. Pharm. Investig. 2013;43:353–362. doi: 10.1007/s40005-013-0087-y. [DOI] [Google Scholar]
- 23.Fonte P., Araújo F., Silva C., Pereira C., Reis S., Santos H.A., Sarmento B. Polymer-based nanoparticles for oral insulin delivery: Revisited approaches. Biotechnol. Adv. 2015;33:1342–1354. doi: 10.1016/j.biotechadv.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Simsek-ege F.A., Bond G.M., Stringer J. Polyelectrolye complex formation between alginate and chitosan as a function of pH. J. Appl. Polym. Sci. 2002;88:346–351. doi: 10.1002/app.11989. [DOI] [Google Scholar]
- 25.Liu M., Chen M., Yang Z. Design of amphotericin B oral formulation for antifungal therapy. Drug Deliv. 2017;24:1–9. doi: 10.1080/10717544.2016.1225852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muchow M., Maincent P., Müller R.H. Lipid Nanoparticles with a Solid Matrix (SLN®, NLC®, LDC®) for Oral Drug Delivery. Drug Dev. Ind. Pharm. 2008;34:1394–1405. doi: 10.1080/03639040802130061. [DOI] [PubMed] [Google Scholar]
- 27.Qi J., Zhuang J., Lu Y., Dong X., Zhao W., Wu W. In vivo fate of lipid-based nanoparticles. Drug Discov. Today. 2016;22:166–172. doi: 10.1016/j.drudis.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 28.Salminen H., Gömmel C., Leuenberger B.H., Weiss J. Influence of encapsulated functional lipids on crystal structure and chemical stability in solid lipid nanoparticles: Towards bioactive-based design of delivery systems. Food Chem. 2015;190:928–937. doi: 10.1016/j.foodchem.2015.06.054. [DOI] [PubMed] [Google Scholar]
- 29.Videira M.A., Botelho M.F., Santos A.C., Gouveia L., De Lima J.P., Almeida A. Lymphatic Uptake of Pulmonary Delivered Radiolabelled Solid Lipid Nanoparticles. J. Drug Target. 2002;10:607–613. doi: 10.1080/1061186021000054933. [DOI] [PubMed] [Google Scholar]
- 30.Weber S., Zimmer A., Pardeike J. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) for pulmonary application: A review of the state of the art. Eur. J. Pharm. Biopharm. 2014;86:7–22. doi: 10.1016/j.ejpb.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 31.Pardeike J., Hommoss A., Müller R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009;366:170–184. doi: 10.1016/j.ijpharm.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Santos C.M., De Oliveira R.B., Arantes V.T., Caldeira L.R., De Oliveira M.C., Egito E.S.T., Ferreira L. Amphotericin B-Loaded Nanocarriers for Topical Treatment of Cutaneous Leishmaniasis: Development, Characterization, and In Vitro Skin Permeation Studies. J. Biomed. Nanotechnol. 2012;8:322–329. doi: 10.1166/jbn.2012.1385. [DOI] [PubMed] [Google Scholar]
- 33.Silva L.A.D., Andrade L.M., de Sá F.A.P., Marreto R., Lima E., Gratieri T., Taveira S.F. Clobetasol-loaded nanostructured lipid carriers for epidermal targeting. J. Pharm. Pharmacol. 2016;68:742–750. doi: 10.1111/jphp.12543. [DOI] [PubMed] [Google Scholar]
- 34.Luo Q., Zhao J., Zhang X., Pan W. Nanostructured lipid carrier (NLC) coated with Chitosan Oligosaccharides and its potential use in ocular drug delivery system. Int. J. Pharm. 2011;403:185–191. doi: 10.1016/j.ijpharm.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 35.Araújo J., Garcia M.L., Mallandrich M., Souto E.B., Calpena A.C. Release profile and transscleral permeation of triamcinolone acetonide loaded nanostructured lipid carriers (TA-NLC): In vitro and ex vivo studies. Nanomed. Nanotechnol. Biol. Med. 2012;8:1034–1041. doi: 10.1016/j.nano.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 36.Fu T., Yi J., Lv S., Zhang B. Ocular amphotericin B delivery by chitosan-modified nanostructured lipid carriers for fungal keratitis-targeted therapy. J. Liposome Res. 2016;27:228–233. doi: 10.1080/08982104.2016.1224899. [DOI] [PubMed] [Google Scholar]
- 37.Brime B., Frutos P., Bringas P., Nieto A., Ballesteros M.P., Frutos G. Comparative pharmacokinetics and safety of a novel lyophilized amphotericin B lecithin-based oil-water microemulsion and amphotericin B deoxycholate in animal models. J. Antimicrob. Chemother. 2003;52:103–109. doi: 10.1093/jac/dkg266. [DOI] [PubMed] [Google Scholar]
- 38.Jung S.H., Lim D.H., Jung S.H., Lee J.E., Jeong K.-S., Seong H., Shin B.C. Amphotericin B-entrapping lipid nanoparticles and their in vitro and in vivo characteristics. Eur. J. Pharm. Sci. 2009;37:313–320. doi: 10.1016/j.ejps.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 39.Aditya N., Macedo A., Doktorovova S., Souto E.B., Kim S., Chang P.-S., Ko S. Development and evaluation of lipid nanocarriers for quercetin delivery: A comparative study of solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), and lipid nanoemulsions (LNE) LWT—Food Sci. Technol. 2014;59:115–121. doi: 10.1016/j.lwt.2014.04.058. [DOI] [Google Scholar]
- 40.Das S., Ng W.K., Tan R.B. Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): Development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs? Eur. J. Pharm. Sci. 2012;47:139–151. doi: 10.1016/j.ejps.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Jain V., Gupta A., Pawar V.K., Asthana S., Jaiswal A.K., Dube A., Chourasia M.K. Chitosan-Assisted Immunotherapy for Intervention of Experimental Leishmaniasis via Amphotericin B-Loaded Solid Lipid Nanoparticles. Appl. Biochem. Biotechnol. 2014;174:1309–1330. doi: 10.1007/s12010-014-1084-y. [DOI] [PubMed] [Google Scholar]
- 42.Dahan A., Hoffman A. Rationalizing the selection of oral lipid based drug delivery systems by an in vitro dynamic lipolysis model for improved oral bioavailability of poorly water soluble drugs. J. Control. Release. 2008;129:1–10. doi: 10.1016/j.jconrel.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 43.Silva A.E., Barratt G., Chéron M., Egito E.S.T. Development of oil-in-water microemulsions for the oral delivery of amphotericin B. Int. J. Pharm. 2013;454:641–648. doi: 10.1016/j.ijpharm.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 44.Charman W.N., Porter C.J.H., Mithani S., Dressman J.B. Physicochemical and Physiological Mechanisms for the Effects of Food on Drug Absorption: The Role of Lipids and pH. J. Pharm. Sci. 1997;86:269–282. doi: 10.1021/js960085v. [DOI] [PubMed] [Google Scholar]
- 45.Shegokar R., Keck C.M. 20 Years of Lipid Nanoparticles (SLN & NLC): Present State of Development & Industrial Applications. Curr. Drug Discov. Technol. 2011;8:207–227. doi: 10.2174/157016311796799062. [DOI] [PubMed] [Google Scholar]
- 46.Neupane Y.R., Srivastava M., Ahmad N., Kumar N., Bhatnagar A., Kohli K. Lipid based nanocarrier system for the potential oral delivery of decitabine: Formulation design, characterization, ex vivo, and in vivo assessment. Int. J. Pharm. 2014;477:601–612. doi: 10.1016/j.ijpharm.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Joseph S., Bunjes H. Drug Delivery Strategies for Poorly Water Soluble Drugs. John Wiley & Sons; Somersat, NJ, USA: 2013. Solid lipid nanoparticles for drug delivery; pp. 103–1499. [Google Scholar]
- 48.Shah R., Eldridge D., Palombo E., Harding I. Springer Briefs in Pharmaceutical Science & Drug Development. Springer; Cham, Switzerland: 2015. Lipid nanoparticles: Production, characterization and stability; pp. 11–23. [Google Scholar]
- 49.Suresh G., Manjunath K., Venkateswarlu V., Satyanarayana V. Preparation, characterization, and in vitro and in vivo evaluation of lovastatin solid lipid nanoparticles. AAPS PharmSciTech. 2007;8:E162–E170. doi: 10.1208/pt0801024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang T., Xue J., Hu Q., Zhou M., Luo Y. Preparation of lipid nanoparticles with high loading capacity and exceptional gastrointestinal stability for potential oral delivery applications. J. Colloid Interface Sci. 2017;507:119–130. doi: 10.1016/j.jcis.2017.07.090. [DOI] [PubMed] [Google Scholar]
- 51.Khosa A., Reddi S., Saha R.N. Nanostructured lipid carriers for site-specific drug delivery. Biomed. Pharmacother. 2018;103:598–613. doi: 10.1016/j.biopha.2018.04.055. [DOI] [PubMed] [Google Scholar]
- 52.Geszke-Moritz M., Moritz M. Solid lipid nanoparticles as attractive drug vehicles: Composition, properties and therapeutic strategies. Mater. Sci. Eng. C. 2016;68:982–994. doi: 10.1016/j.msec.2016.05.119. [DOI] [PubMed] [Google Scholar]
- 53.Ganesan P., Narayanasamy D. Lipid nanoparticles: Different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain. Chem. Pharm. 2017;6:37–56. doi: 10.1016/j.scp.2017.07.002. [DOI] [Google Scholar]
- 54.Teeranachaideekul V., Souto E.B., Junyaprasert V.B., Müller R.H. Cetyl palmitate-based NLC for topical delivery of Coenzyme Q10-Development, physicochemical characterization and in vitro release studies. Eur. J. Pharm. Biopharm. 2007;67:141–148. doi: 10.1016/j.ejpb.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 55.Kumar V.V., Chandrasekar D., Ramakrishna S., Kishan V., Rao Y.M., Diwan P.V. Development and evaluation of nitrendipine loaded solid lipid nanoparticles: Influence of wax and glyceride lipids on plasma pharmacokinetics. Int. J. Pharm. 2007;335:167–175. doi: 10.1016/j.ijpharm.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 56.Patlolla R.R., Chougule M., Patel A.R., Jackson T., Tata P.N., Singh M. Formulation, characterization and pulmonary deposition of nebulized celecoxib encapsulated nanostructured lipid carriers. J. Control. Release. 2010;144:233–241. doi: 10.1016/j.jconrel.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu L., Jia Y., Ding W. Preparation and characterization of solid lipid nanoparticles loaded with epirubicin for pulmonary delivery. Pharmazie. 2010;65:585–587. [PubMed] [Google Scholar]
- 58.Zhuang C.-Y., Li N., Wang M., Zhang X.-N., Pan W.-S., Peng J.-J., Pan Y.-S., Tang X. Preparation and characterization of vinpocetine loaded nanostructured lipid carriers (NLC) for improved oral bioavailability. Int. J. Pharm. 2010;394:179–185. doi: 10.1016/j.ijpharm.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 59.Han F., Li S., Yin R., Liu H., Xu L. Effect of surfactants on the formation and characterization of a new type of colloidal drug delivery system: Nanostructured lipid carriers. Colloids Surf. A Physicochem. Eng. Asp. 2008;315:210–216. doi: 10.1016/j.colsurfa.2007.08.005. [DOI] [Google Scholar]
- 60.Paliwal R., Rai S., Vaidya B., Khatri K., Goyal A.K., Mishra N., Mehta A., Vyas S.P. Effect of lipid core material on characteristics of solid lipid nanoparticles designed for oral lymphatic delivery. Nanomed. Nanotechnol. Biol. Med. 2009;5:184–191. doi: 10.1016/j.nano.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 61.Sun M., Nie S., Pan X., Zhang R., Fan Z., Wang S. Quercetin-nanostructured lipid carriers: Characteristics and anti-breast cancer activities in vitro. Colloids Surf. B Biointerfaces. 2014;113:15–24. doi: 10.1016/j.colsurfb.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 62.Gainza G., Celdran D., Moreno B., Javier J., Borja F., Villullas S., Luis J., Igartua M., Maria R. The topical administration of rhEGF-loaded nanostructured lipid carriers (rhEGF-NLC) improves healing in a porcine full-thickness excisional wound model. J. Control. Release. 2015;197:41–47. doi: 10.1016/j.jconrel.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 63.Hu F.-Q., Jiang S.-P., Du Y.-Z., Yuan H., Ye Y.-Q., Zeng S. Preparation and characterization of stearic acid nanostructured lipid carriers by solvent diffusion method in an aqueous system. Colloids Surf. B Biointerfaces. 2005;45:167–173. doi: 10.1016/j.colsurfb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 64.Dharmala K., Yoo J.W., Lee C.H. Development of Chitosan–SLN Microparticles for chemotherapy: In vitro approach through efflux-transporter modulation. J. Control. Release. 2008;131:190–197. doi: 10.1016/j.jconrel.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 65.Noor N.M., Sheikh K., Somavarapu S., Taylor K.M. Preparation and characterization of dutasteride-loaded nanostructured lipid carriers coated with stearic acid-chitosan oligomer for topical delivery. Eur. J. Pharm. Biopharm. 2017;117:372–384. doi: 10.1016/j.ejpb.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 66.Manjunath K., Venkateswarlu V. Pharmacokinetics, tissue distribution and bioavailability of clozapine solid lipid nanoparticles after intravenous and intraduodenal administration. J. Control. Release. 2005;107:215–228. doi: 10.1016/j.jconrel.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 67.Tan C., Billa N., Roberts C., Scurr D. Properties of an oral nanoformulation of a molecularly dispersed amphotericin B comprising a composite matrix of theobroma oil and beeswax. Nanomaterials. 2014;4:905–916. doi: 10.3390/nano4040905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tan J.S.L., Roberts C., Billa N. Mucoadhesive chitosan-coated nanostructured lipid carriers for oral delivery of amphotericin B. Pharm. Dev. Technol. 2018;24:504–512. doi: 10.1080/10837450.2018.1515225. [DOI] [PubMed] [Google Scholar]
- 69.Tan S.W., Billa N. Lipid Effects on Expulsion Rate of Amphotericin B from Solid Lipid Nanoparticles. AAPS PharmSciTech. 2013;15:287–295. doi: 10.1208/s12249-013-0056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sütő B., Weber S., Zimmer A., Farkas G., Kelemen A., Budai-Szucs M., Berkó S., Szabó-Révész P., Csányi E. Optimization and design of an ibuprofen-loaded nanostructured lipid carrier with a 23 full factorial design. Chem. Eng. Res. Des. 2015;104:488–496. doi: 10.1016/j.cherd.2015.09.010. [DOI] [Google Scholar]
- 71.Sosa L., Clares B., Alvarado H.L., Bozal N., Domenech O., Calpena A.C. Amphotericin B releasing topical nanoemulsion for the treatment of candidiasis and aspergillosis. Nanomed. Nanotechnol. Biol. Med. 2017;13:2303–2312. doi: 10.1016/j.nano.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 72.Venkateswarlu V., Manjunath K. Preparation, characterization and in vitro release kinetics of clozapine solid lipid nanoparticles. J. Control. Release. 2004;95:627–638. doi: 10.1016/j.jconrel.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 73.Yuan H., Wang L.-L., Du Y.-Z., You J., Hu F.-Q., Zeng S. Preparation and characteristics of nanostructured lipid carriers for control-releasing progesterone by melt-emulsification. Colloids Surf. B Biointerfaces. 2007;60:174–179. doi: 10.1016/j.colsurfb.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 74.Manea A.-M., Vasile B.S., Meghea A. Antioxidant and antimicrobial activities of green tea extract loaded into nanostructured lipid carriers. Comptes Rendus Chim. 2014;17:331–341. doi: 10.1016/j.crci.2013.07.015. [DOI] [Google Scholar]
- 75.Rudolph C., Schillinger U., Ortiz A., Tabatt K., Plank C., Müller R.H., Rosenecker J. Application of novel solid lipid nanoparticle (SLN)-gene vector formulations based on a dimeric HIV-1 TAT-peptide in vitro and in vivo. Pharm. Res. 2004;21:1662–1669. doi: 10.1023/B:PHAM.0000041463.56768.ec. [DOI] [PubMed] [Google Scholar]
- 76.Darwis Y., Khan A.A., Mudassir J., Mohtar N. Advanced drug delivery to the lymphatic system: Lipid-based nanoformulations. Int. J. Nanomed. 2013;8:2733–2744. doi: 10.2147/IJN.S41521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Müller R., Runge S., Ravelli V., Mehnert W., Thünemann A., Souto E. Oral bioavailability of cyclosporine: Solid lipid nanoparticles (SLN®) versus drug nanocrystals. Int. J. Pharm. 2006;317:82–89. doi: 10.1016/j.ijpharm.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 78.Müller R., Petersen R., Hommoss A., Pardeike J. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv. Drug Deliv. Rev. 2007;59:522–530. doi: 10.1016/j.addr.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 79.Iqbal A., Shadab M., Sahni J.K., Baboota S., Dang S., Ali J. Nanostructured lipid carriers system: Recent advances in drug delivery. J. Drug Target. 2012;20:813–830. doi: 10.3109/1061186X.2012.716845. [DOI] [PubMed] [Google Scholar]
- 80.Chen C.-C., Tsai T.-H., Huang Z.-R., Fang J.-Y. Effects of lipophilic emulsifiers on the oral administration of lovastatin from nanostructured lipid carriers: Physicochemical characterization and pharmacokinetics. Eur. J. Pharm. Biopharm. 2010;74:474–482. doi: 10.1016/j.ejpb.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 81.Rabelo R.S., Oliveira I.F., da Silva V.M., Prata A.S., Hubinger M. Chitosan coated nanostructured lipid carriers (NLCs) for loading Vitamin D: A physical stability study. Int. J. Biol. Macromol. 2018;119:902–912. doi: 10.1016/j.ijbiomac.2018.07.174. [DOI] [PubMed] [Google Scholar]
- 82.Jenning V., Gohla S.H. Encapsulation of retinoids in solid lipid nanoparticles (SLN) J. Microencapsul. 2001;18:49–158. doi: 10.1080/02652040010000361. [DOI] [PubMed] [Google Scholar]
- 83.Phatsawee J., Wiwat P., Garnpimol R.C. Amphotericin B loaded solid lipid nanoparticles (SLNs) and nanostructured lipid carrier (NLCs): Part II. Effect of drug loading and biopharmaceutical characterizations. Drug Dev. Ind. Pharm. 2018;44:1693–1700. doi: 10.1080/03639045.2018.1492606. [DOI] [PubMed] [Google Scholar]
- 84.Beloqui A., del Pozo-Rodriguez A., Isla A., Rodríguez-Gascón A., Solinís M. Nanostructured lipid carriers as oral delivery systems for poorly soluble drugs. J. Drug Deliv. Sci. Technol. 2017;42:144–154. doi: 10.1016/j.jddst.2017.06.013. [DOI] [Google Scholar]
- 85.Zhou J., Zhou D. Improvement of oral bioavailability of lovastatin by using nanostructured lipid carriers. Drug Des. Dev. Ther. 2015;9:5269–5275. doi: 10.2147/DDDT.S90016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fathi H.A., Allam A., Elsabahy M., Fetih G., El-Badry M. Nanostructured lipid carriers for improved oral delivery and prolonged antihyperlipidemic effect of simvastatin. Colloids Surf. B Biointerfaces. 2018;162:236–245. doi: 10.1016/j.colsurfb.2017.11.064. [DOI] [PubMed] [Google Scholar]
- 87.Harisa G.I., Alomrani A.H., Badran M.M. Simvastatin-loaded nanostructured lipid carriers attenuate the atherogenic risk of erythrocytes in hyperlipidemic rats. Eur. J. Pharm. Sci. 2017;96:62–71. doi: 10.1016/j.ejps.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 88.Prabhu P., Suryavanshi S., Pathak S., Sharma S., Patravale V. Artemether-lumefantrine nanostructured lipid carriers for oral malaria therapy: Enhanced efficacy at reduced dose and dosing frequency. Int. J. Pharm. 2016;511:473–487. doi: 10.1016/j.ijpharm.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 89.Mendes A., Silva A., Catita J., Cerqueira F., Gabriel C., Lopes C. Miconazole-loaded nanostructured lipid carriers (NLC) for local delivery to the oral mucosa: Improving antifungal activity. Colloids Surf. B Biointerfaces. 2013;111:755–763. doi: 10.1016/j.colsurfb.2013.05.041. [DOI] [PubMed] [Google Scholar]
- 90.Reis S., Neves A.R., Lúcio M., Martins S., Lima J.L.C. Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. Int. J. Nanomed. 2013;8:177–187. doi: 10.2147/IJN.S37840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Muchow M., Maincent P., Müller R.H., Keck C.M. Testosterone undecanoate–increase of oral bioavailability by nanostructured lipid carriers (NLC) J. Pharm. Technol. Drug Res. 2013;2:4. doi: 10.7243/2050-120X-2-4. [DOI] [Google Scholar]
- 92.Shete H.K., Selkar N., Vanage G.R., Patravale V.B. Tamoxifen nanostructured lipid carriers: Enhanced in vivo antitumor efficacy with reduced adverse drug effects. Int. J. Pharm. 2014;468:1–14. doi: 10.1016/j.ijpharm.2014.03.056. [DOI] [PubMed] [Google Scholar]
- 93.Tran T.H., Ramasamy T., Truong D.H., Choi H.-G., Yong C.S., Kim J.O. Preparation and Characterization of Fenofibrate-Loaded Nanostructured Lipid Carriers for Oral Bioavailability Enhancement. AAPS PharmSciTech. 2014;15:1509–1515. doi: 10.1208/s12249-014-0175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khan S., Yar M.S., Fazil M., Baboota S., Ali J. Tacrolimus-loaded nanostructured lipid carriers for oral delivery–Optimization of production and characterization. Eur. J. Pharm. Biopharm. 2016;108:277–288. doi: 10.1016/j.ejpb.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 95.Zhang T., Chen J., Zhang Y., Shen Q., Pan W. Characterization and evaluation of nanostructured lipid carrier as a vehicle for oral delivery of etoposide. Eur. J. Pharm. Sci. 2011;43:174–179. doi: 10.1016/j.ejps.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 96.Beloqui A., Solinís M., Gascón A.R., del Pozo-Rodríguez A., Rieux A.D., Préat V. Mechanism of transport of saquinavir-loaded nanostructured lipid carriers across the intestinal barrier. J. Control. Release. 2012;166:115–123. doi: 10.1016/j.jconrel.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 97.Sun B., Luo C., Li L., Wang M., Du Y., Di D., Zhang D., Ren G., Pan X., Fu Q., et al. Core-matched encapsulation of an oleate prodrug into nanostructured lipid carriers with high drug loading capability to facilitate the oral delivery of docetaxel. Colloids Surf. B Biointerfaces. 2016;143:47–55. doi: 10.1016/j.colsurfb.2016.02.065. [DOI] [PubMed] [Google Scholar]
- 98.Patil V.R.S., Campbell C.J., Yun Y.H., Slack S.M., Goetz D.J. Particle Diameter Influences Adhesion under Flow. Biophys. J. 2001;80:1733–1743. doi: 10.1016/S0006-3495(01)76144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shi L.-L., Xie H., Lu J., Cao Y., Liu J.-Y., Zhang X.-X., Zhang H., Cui J.-H., Cao Q.-R. Positively Charged Surface-Modified Solid Lipid Nanoparticles Promote the Intestinal Transport of Docetaxel through Multifunctional Mechanisms in Rats. Mol. Pharm. 2016;13:2667–2676. doi: 10.1021/acs.molpharmaceut.6b00226. [DOI] [PubMed] [Google Scholar]
- 100.Mansuri S., Kesharwani P., Jain K., Tekade R.K., Jain N. Mucoadhesion: A promising approach in drug delivery system. React. Funct. Polym. 2016;100:151–172. doi: 10.1016/j.reactfunctpolym.2016.01.011. [DOI] [Google Scholar]
- 101.Florence A.T. Nanoparticle uptake by the oral route: Fulfilling its potential? Drug Discov. Today Technol. 2005;2:75–81. doi: 10.1016/j.ddtec.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 102.Kim H., Kim Y., Lee J. Liposomal formulations for enhanced lymphatic drug delivery. Asian J. Pharm. Sci. 2013;8:96–103. doi: 10.1016/j.ajps.2013.07.012. [DOI] [Google Scholar]
- 103.Porter C.J.H., Charman W.N. Intestinal lymphatic drug transport: An update. Adv. Drug Deliv. Rev. 2001;50:61–80. doi: 10.1016/S0169-409X(01)00151-X. [DOI] [PubMed] [Google Scholar]
- 104.Brocks D.R., Davies N.M. Lymphatic Drug Absorption via the Enterocytes: Pharmacokinetic Simulation, Modeling, and Considerations for Optimal Drug Development. J. Pharm. Pharm. Sci. 2018;21:254s–270s. doi: 10.18433/jpps30217. [DOI] [PubMed] [Google Scholar]
- 105.Harde H., Das M., Jain S. Solid lipid nanoparticles: An oral bioavailability enhancer vehicle. Expert Opin. Drug Deliv. 2011;8:1407–1424. doi: 10.1517/17425247.2011.604311. [DOI] [PubMed] [Google Scholar]
- 106.Hussain N., Jaitley V., Florence A.T. Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv. Drug Deliv. Rev. 2001;50:107–142. doi: 10.1016/S0169-409X(01)00152-1. [DOI] [PubMed] [Google Scholar]
- 107.Pukanud P., Peungvicha P., Sarisuta N. Development of mannosylated liposomes for bioadhesive oral drug delivery via M cells of Peyer’s patches. Drug Deliv. 2009;16:289–294. doi: 10.1080/10717540902989738. [DOI] [PubMed] [Google Scholar]
- 108.Gamboa J.M., Leong K.W. In vitro and in vivo models for the study of oral delivery of nanoparticles. Adv. Drug Deliv. Rev. 2013;65:800–810. doi: 10.1016/j.addr.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Beloqui A., Rieux A.D., Préat V. Mechanisms of transport of polymeric and lipidic nanoparticles across the intestinal barrier. Adv. Drug Deliv. Rev. 2016;106:242–255. doi: 10.1016/j.addr.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 110.Conner S.D., Schmid S. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 111.Duncan R., Richardson S.C.W. Endocytosis and Intracellular Trafficking as Gateways for Nanomedicine Delivery: Opportunities and Challenges. Mol. Pharm. 2012;9:2380–2402. doi: 10.1021/mp300293n. [DOI] [PubMed] [Google Scholar]
- 112.Hillaireau H., Couvreur P. Nanocarriers’ entry into the cell: Relevance to drug delivery. Cell. Mol. Life Sci. 2009;66:2873–2896. doi: 10.1007/s00018-009-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.de Garibay A.P.R., Aspiazu M.S., Gascón A.R., Ganjian H., Fuchs R. Role of endocytic uptake in transfection efficiency of solid lipid nanoparticles-based nonviral vectors. J. Gene Med. 2013;15:427–440. doi: 10.1002/jgm.2749. [DOI] [PubMed] [Google Scholar]
- 114.Yu Q., Wang Z., Li P., Yang Q. The effect of various absorption enhancers on tight junction in the human intestinal Caco-2 cell line. Drug Dev. Ind. Pharm. 2012;39:587–592. doi: 10.3109/03639045.2012.692376. [DOI] [PubMed] [Google Scholar]
- 115.Sonaje K., Lin K.-J., Tseng M.T., Wey S.-P., Su F.-Y., Chuang E.-Y., Hsu C.-W., Chen C.-T., Sung H.-W. Effects of chitosan-nanoparticle-mediated tight junction opening on the oral absorption of endotoxins. Biomaterials. 2011;32:8712–8721. doi: 10.1016/j.biomaterials.2011.07.086. [DOI] [PubMed] [Google Scholar]
- 116.Dünnhaupt S., Barthelmes J., Köllner S., Sakloetsakun D., Shahnaz G., Düregger A., Bernkop-Schnürch A. Thiolated nanocarriers for oral delivery of hydrophilic macromolecular drugs. Carbohydr. Polym. 2015;117:577–584. doi: 10.1016/j.carbpol.2014.09.078. [DOI] [PubMed] [Google Scholar]
- 117.Ghaffarian R., Muro S. Models and Methods to Evaluate Transport of Drug Delivery Systems Across Cellular Barriers. J. Vis. Exp. 2013;80:e50638. doi: 10.3791/50638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tzachev C., Svilenov H. Lipid nanoparticles at the current stage and prospects—A review article. Int. J. Pharm. Sci. Rev. Res. 2013;18:103–115. [Google Scholar]
- 119.Porter J.H., Charman W.N. Uptake of drugs into the intestinal lymphatics after oral administration. Adv. Drug Deliv. Rev. 1997;25:71–89. doi: 10.1016/S0169-409X(96)00492-9. [DOI] [Google Scholar]
- 120.Narang J.K., Khan S., Baboota S., Ali J., Khan S., Narang R.S. Nanostructured lipid carriers: An emerging platform for improving oral bioavailability of lipophilic drugs. Int. J. Pharm. Investig. 2015;5:182–191. doi: 10.4103/2230-973X.167661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Trevaskis N.L., Hu L., Caliph S.M., Han S., Porter C.J. The Mesenteric Lymph Duct Cannulated Rat Model: Application to the Assessment of Intestinal Lymphatic Drug Transport. J. Vis. Exp. 2015;97:e52389. doi: 10.3791/52389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Venishetty V.K., Chede R., Komuravelli R., Adepu L., Sistla R., Diwan P.V. Design and evaluation of polymer coated carvedilol loaded solid lipid nanoparticles to improve the oral bioavailability: A novel strategy to avoid intraduodenal administration. Colloids Surf. B Biointerfaces. 2012;95:1–9. doi: 10.1016/j.colsurfb.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 123.Shackleford D.M., Faassen W.A., Houwing N., Lass H., Edwards G.A., Porter C., Charman W.N. Contribution of Lymphatically Transported Testosterone Undecanoate to the Systemic Exposure of Testosterone after Oral Administration of Two Andriol Formulations in Conscious Lymph Duct—Cannulated Dogs. J. Pharmacol. Exp. Ther. 2003;306:925–933. doi: 10.1124/jpet.103.052522. [DOI] [PubMed] [Google Scholar]
- 124.Ways T.M.M., Lau W.M., Khutoryanskiy V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers. 2018;10:267. doi: 10.3390/polym10030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Luo Y., Teng Z., Li Y., Wang Q. Solid lipid nanoparticles for oral drug delivery: Chitosan coating improves stability, controlled delivery, mucoadhesion and cellular uptake. Carbohydr. Polym. 2015;122:221–229. doi: 10.1016/j.carbpol.2014.12.084. [DOI] [PubMed] [Google Scholar]
- 126.Roy S., Pal K., Anis A., Pramanik K., Prabhakar B. Polymers in Mucoadhesive Drug-Delivery Systems: A Brief Note. Des. Monomers Polym. 2009;12:483–495. doi: 10.1163/138577209X12478283327236. [DOI] [Google Scholar]
- 127.Das Neves J., Bahia M.F., Amiji M.M., Sarmento B. Mucoadhesive nanomedicines: Characterization and modulation of mucoadhesion at the nanoscale. Expert Opin. Drug Deliv. 2011;8:1085–1104. doi: 10.1517/17425247.2011.586334. [DOI] [PubMed] [Google Scholar]
- 128.Ying X.-Y., Cui D., Yu L., Du Y.-Z. Solid lipid nanoparticles modified with chitosan oligosaccharides for the controlled release of doxorubicin. Carbohydr. Polym. 2011;84:1357–1364. doi: 10.1016/j.carbpol.2011.01.037. [DOI] [Google Scholar]
- 129.Andrews G.P., Laverty T.P., Jones D.S. Mucoadhesive polymeric platforms for controlled drug delivery. Eur. J. Pharm. Biopharm. 2009;71:505–518. doi: 10.1016/j.ejpb.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 130.Laffleur F. Mucoadhesive polymers for buccal drug delivery. Drug Dev. Ind. Pharm. 2014;40:591–598. doi: 10.3109/03639045.2014.892959. [DOI] [PubMed] [Google Scholar]
- 131.Sosnik A., das Neves J., Sarmento B. Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes: A review. Prog. Polym. Sci. 2014;39:2030–2075. doi: 10.1016/j.progpolymsci.2014.07.010. [DOI] [Google Scholar]
- 132.Anselmo A.C., Mitragotri S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019;4:e10143. doi: 10.1002/btm2.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wasan E.K., Bartlett K., Gershkovich P., Sivak O., Banno B., Wong Z., Gagnon J., Gates B., Leon C.G., Wasan K.M. Development and characterization of oral lipid-based Amphotericin B formulations with enhanced drug solubility, stability and antifungal activity in rats infected with Aspergillus fumigatus or Candida albicans. Int. J. Pharm. 2009;372:76–84. doi: 10.1016/j.ijpharm.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 134.Jain S., Valvi P.U., Swarnakar N.K., Thanki K. Gelatin Coated Hybrid Lipid Nanoparticles for Oral Delivery of Amphotericin B. Mol. Pharm. 2012;9:2542–2553. doi: 10.1021/mp300320d. [DOI] [PubMed] [Google Scholar]
- 135.Chaudhari M.B., Desai P., Patel P.A., Patravale V.B. Solid lipid nanoparticles of amphotericin B (AmbiOnp): In vitro and in vivo assessment towards safe and effective oral treatment module. Drug Deliv. Transl. Res. 2015;6:1–11. doi: 10.1007/s13346-015-0267-6. [DOI] [PubMed] [Google Scholar]
- 136.Isomaa B., Reuter J., Djupsund B.M. The subacute and chronic toxicity of cetyltrimethylammonium bromide (CTAB), a cationic surfactant, in the rat. Arch. Toxicol. 1976;35:91–96. doi: 10.1007/BF00372762. [DOI] [PubMed] [Google Scholar]
- 137.Oliveira A.C.N., Sárria M.P., Moreira P., Fernandes J., Castro L., Lopes I., Côrte-Real M., Cavaco-Paulo A., Oliveira M.E.C.D.R., Gomes A.C. Counter ions and constituents combination affect DODAX: MO nanocarriers toxicity in vitro and in vivo. Toxicol. Res. 2016;5:1244–1255. doi: 10.1039/C6TX00074F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shen M.-Y., Liu T.-I., Yu T.-W., Kv R., Chiang W.-H., Tsai Y.-C., Chen H.-H., Lin S.-C., Chiu H.-C. Hierarchically targetable polysaccharide-coated solid lipid nanoparticles as an oral chemo/thermotherapy delivery system for local treatment of colon cancer. Biomaterials. 2019;197:86–100. doi: 10.1016/j.biomaterials.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 139.Kim K.S., Youn Y.S., Bae Y.H. Immune-triggered cancer treatment by intestinal lymphatic delivery of docetaxel-loaded nanoparticle. J. Control. Release. 2019;311–312:85–95. doi: 10.1016/j.jconrel.2019.08.027. [DOI] [PubMed] [Google Scholar]
- 140.Rajpoot K., Jain S.K. Colorectal cancer-targeted delivery of oxaliplatin via folic acid-grafted solid lipid nanoparticles: Preparation, optimization, and in vitro evaluation. Artif. Cells Nanomed. Biotechnol. 2017;46:1236–1247. doi: 10.1080/21691401.2017.1366338. [DOI] [PubMed] [Google Scholar]
- 141.Rajpoot K., Jain S.K. Irinotecan hydrochloride trihydrate loaded folic acid-tailored solid lipid nanoparticles for targeting colorectal cancer: Development, characterization, and in vitro cytotoxicity study using HT-29 cells. J. Microencapsul. 2019;36:659–676. doi: 10.1080/02652048.2019.1665723. [DOI] [PubMed] [Google Scholar]
- 142.Rajpoot K., Jain S.K. Oral delivery of pH-responsive alginate microbeads incorporating folic acid-grafted solid lipid nanoparticles exhibits enhanced targeting effect against colorectal cancer: A dual-targeted approach. Int. J. Biol. Macromol. 2020;151:830–844. doi: 10.1016/j.ijbiomac.2020.02.132. [DOI] [PubMed] [Google Scholar]
- 143.Ball R.L., Bajaj P., Whitehead K.A. Oral delivery of siRNA lipid nanoparticles: Fate in the GI tract. Sci. Rep. 2018;8:1–12. doi: 10.1038/s41598-018-20632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kenechukwu F.C., Attama A.A., Ibezim E.C., Nnamani P., Umeyor C.E., Uronnachi E.M., Momoh M.A., Akpa P.A. Tailor-made mucoadhesive lipid nanogel improves oromucosal antimycotic activity of encapsulated miconazole nitrate. Eur. J. Nanomed. 2017;9:115–126. doi: 10.1515/ejnm-2017-0010. [DOI] [Google Scholar]
- 145.Fonte P., Andrade F., Araújo F., Andrade C., das Neves J., Sarmento B. Chitosan-Coated Solid Lipid Nanoparticles for Insulin Delivery. Methods Enzymol. 2012;508:295–314. doi: 10.1016/b978-0-12-391860-4.00015-x. [DOI] [PubMed] [Google Scholar]
- 146.Ling J.T.S., Roberts C.J., Billa N. Antifungal and Mucoadhesive Properties of an Orally Administered Chitosan-Coated Amphotericin B Nanostructured Lipid Carrier (NLC) AAPS PharmSciTech. 2019;20:136. doi: 10.1208/s12249-019-1346-7. [DOI] [PubMed] [Google Scholar]
- 147.Mazzarino L., Travelet C., Ortega-Murillo S., Otsuka I., Pignot-Paintrand I., Lemos-Senna E., Borsali R. Elaboration of chitosan-coated nanoparticles loaded with curcumin for mucoadhesive applications. J. Colloid Interface Sci. 2012;370:58–66. doi: 10.1016/j.jcis.2011.12.063. [DOI] [PubMed] [Google Scholar]
- 148.Reineke J., Cho D.Y., Dingle Y.L., Chiefetz P., Laulicht B., Lavin D., Furtado S., Mathiowitz E. Can bioadhesive nanoparticles allow for more effective particle uptake from the small intestine? J. Control. Release. 2013;170:477–484. doi: 10.1016/j.jconrel.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 149.Mugabe C., Hadaschik B.A., Kainthan R.K., Brooks D.E., So A.I., Gleave M.E., Burt H.M. Paclitaxel incorporated in hydrophobically derivatized hyperbranched polyglycerols for intravesical bladder cancer therapy. BJU Int. 2009;103:978–986. doi: 10.1111/j.1464-410X.2008.08132.x. [DOI] [PubMed] [Google Scholar]
- 150.Svensson O., Thuresson K., Arnebran T. Interactions between drug delivery particles and mucin in solution and at interfaces. Langmuir. 2008;24:2573–2579. doi: 10.1021/la702680x. [DOI] [PubMed] [Google Scholar]
- 151.AlKhader E., Billa N., Roberts C. Mucoadhesive Chitosan-Pectinate Nanoparticles for the Delivery of Curcumin to the Colon. AAPS PharmSciTech. 2016;18:1009–1018. doi: 10.1208/s12249-016-0623-y. [DOI] [PubMed] [Google Scholar]
- 152.Trapani A., Lopedota A., Franco M., Cioffi N., Ieva E., Garcia-Fuentes M., Alonso M.J. A comparative study of chitosan and chitosan/cyclodextrin nanoparticles as potential carriers for the oral delivery of small peptides. Eur. J. Pharm. Biopharm. 2010;75:26–32. doi: 10.1016/j.ejpb.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 153.Rossi S., Ferrari F., Bonferoni M.C., Caramella C. Characterization of chitosan hydrochloride–mucin interaction by means of viscosimetric and turbidimetric measurements. Eur. J. Pharm. Sci. 2000;10:251–257. doi: 10.1016/S0928-0987(00)00065-8. [DOI] [PubMed] [Google Scholar]
- 154.Bonferoni M., Sandri G., Ferrari F., Rossi S., Larghi V., Zambito Y., Caramella C. Comparison of different in vitro and ex vivo methods to evaluate mucoadhesion of glycol-palmitoyl chitosan micelles. J. Drug Deliv. Sci. Technol. 2010;20:419–424. doi: 10.1016/S1773-2247(10)50073-X. [DOI] [Google Scholar]
- 155.Chayed S., Winnik F.M. In vitro evaluation of the mucoadhesive properties of polysaccharide-based nanoparticulate oral drug delivery systems. Eur. J. Pharm. Biopharm. 2007;65:363–370. doi: 10.1016/j.ejpb.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 156.Marx K.A. Quartz Crystal Microbalance: A Useful Tool for Studying Thin Polymer Films and Complex Biomolecular Systems at the Solution−Surface Interface. Biomacromolecules. 2003;4:1099–1120. doi: 10.1021/bm020116i. [DOI] [PubMed] [Google Scholar]
- 157.Patel D., Smith J.R., Smith A.W., Grist N., Barnett P., Smart J.D. An atomic force microscopy investigation of bioadhesive polymer adsorption onto human buccal cells. Int. J. Pharm. 2000;200:271–277. doi: 10.1016/S0378-5173(00)00396-3. [DOI] [PubMed] [Google Scholar]
- 158.Svensson O., Thuresson K., Arnebrant T. Interactions between chitosan-modified particles and mucin-coated surfaces. J. Colloid Interface Sci. 2008;325:346–350. doi: 10.1016/j.jcis.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 159.Iijima M., Yoshimura M., Tsuchiya T., Tsukada M., Ichikawa H., Fukumori Y., Kamiya H. Direct Measurement of Interactions between Stimulation-Responsive Drug Delivery Vehicles and Artificial Mucin Layers by Colloid Probe Atomic Force Microscopy. Langmuir. 2008;24:3987–3992. doi: 10.1021/la7038043. [DOI] [PubMed] [Google Scholar]
- 160.Lamprecht A., Koenig P., Ubrich N., Maincent P., Neumann D. Low molecular weight heparin nanoparticles: Mucoadhesion and behaviour in Caco-2 cells. Nanotechnology. 2006;17:3673–3680. doi: 10.1088/0957-4484/17/15/009. [DOI] [Google Scholar]
- 161.Suh J., Dawson M., Hanes J. Corrigendum to “Real-time multiple-particle tracking: Applications to drug and gene delivery” [Advanced Drug Delivery Reviews 57(2005)63–78] Adv. Drug Deliv. Rev. 2005;57:1551. doi: 10.1016/j.addr.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 162.Sajeesh S., Sharma C.P. Novel polyelectrolyte complexes based on poly(methacrylic acid)-bis(2-aminopropyl)poly(ethylene glycol) for oral protein delivery. J. Biomater. Sci. Polym. Ed. 2007;18:1125–1139. doi: 10.1163/156856207781554082. [DOI] [PubMed] [Google Scholar]
- 163.Arangoa M.A., Campanero M.A., Renedo M.J., Ponchel G., Irache J.M. Gliadin Nanoparticles as Carriers for the Oral Administration of Lipophilic Drugs. Relationships Between Bioadhesion and Pharmacokinetics. Pharm. Res. 2001;18:1521–1527. doi: 10.1023/A:1013018111829. [DOI] [PubMed] [Google Scholar]
- 164.Takeuchi H., Matsui Y., Sugihara H., Yamamoto H., Kawashima Y. Effectiveness of submicron-sized, chitosan-coated liposomes in oral administration of peptide drugs. Int. J. Pharm. 2005;303:160–170. doi: 10.1016/j.ijpharm.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 165.Cahouet A., Denizot B., Hindré F., Passirani C., Heurtault B., Moreau M., Le Jeune J., Benoît J. Biodistribution of dual radiolabeled lipidic nanocapsules in the rat using scintigraphy and γ counting. Int. J. Pharm. 2002;242:367–371. doi: 10.1016/S0378-5173(02)00218-1. [DOI] [PubMed] [Google Scholar]
- 166.Prego C., Fabre M., Torres D., Alonso M.J. Efficacy and Mechanism of Action of Chitosan Nanocapsules for Oral Peptide Delivery. Pharm. Res. 2006;23:549–556. doi: 10.1007/s11095-006-9570-8. [DOI] [PubMed] [Google Scholar]
- 167.Feng C., Wang Z., Jiang C., Kong M., Zhou X., Li Y., Cheng X., Chen X. Chitosan/o-carboxymethyl chitosan nanoparticles for efficient and safe oral anticancer drug delivery: In vitro and in vivo evaluation. Int. J. Pharm. 2013;457:158–167. doi: 10.1016/j.ijpharm.2013.07.079. [DOI] [PubMed] [Google Scholar]
- 168.Lim S.T., Jeong H.J., Kim D.W., Sohn M.H., Lee J.K., Jeong J., Lee C.-M. Optical imaging to trace near infrared fluorescent zinc oxide nanoparticles following oral exposure. Int. J. Nanomed. 2012;7:3203–3209. doi: 10.2147/IJN.S32828. [DOI] [PMC free article] [PubMed] [Google Scholar]