Abstract
Background: Studies reporting the long-term humoral response after receiving the BNT162b2 COVID-19 vaccine are important to drive future vaccination strategies. Yet, available literature is scarce. Covidiagnostix is a multicenter study designed to assess the antibody response in >1000 healthcare professionals (HCPs) who received the BNT162b2 vaccine. Methods: Serum was tested at time-0 (T0), before the first dose, T1, T2, and T3, respectively, 21, 42, and 180 days after T0. Antibodies against the SARS-CoV-2 nucleocapsid-protein were measured to assess SARS-CoV-2 infections, whereas antibodies against the receptor-binding domain of the spike protein were measured to assess the vaccine response. Neutralization activity against the D614G, B.1.1.7, and B.1.351 variants were also analyzed. Results: Six months post-vaccination HCPs showed an antibody titer decrease of approximately 70%, yet, the titer was still one order of magnitude higher than that of seropositive individuals before vaccination. We identified 12 post-vaccination infected HCPs. None showed severe symptoms. Interestingly, most of them showed titers at T2 above the neutralization thresholds obtained from the neutralization activity experiments. Conclusion: Vaccination induces a humoral response which is well detectable even six months post-vaccination. Vaccination prevents severe COVID-19 cases, yet post-vaccination infection is possible even in the presence of a high anti-S serum antibody titer.
Keywords: COVID-19, serological test, mRNA vaccine, Roche Anti-SARS-CoV-2-S, immune response, vaccination
1. Introduction
As of 6 October 2021, the COVID-19 pandemic, caused by Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), is responsible for more than 230 million infected people and nearly five million deaths [1]. The BNT162b2 mRNA COVID-19 vaccine (Comirnaty®, Pfizer BioNTech) was one of the first vaccine formulations to be developed and approved under an Emergency Use Authorization (EUA) on 11 December 2020 by the FDA, and on 21 December by the European Union; on 23 August it received full approval by the FDA [2]. Its administration protocol has been based on a double dose approach which proved to be effective in preventing 95% of COVID-19 cases [3,4]. However, Pfizer’s CEO recently announced the need of a third vaccine dose, likely within 12 months of the first dose [5], which has been recently authorized in several countries for some frail population [6]. The Comirnaty vaccine exploits mRNA, encoding the SARS-CoV-2 full-length spike protein (S-protein). In order to promote the production of receptor binding domain (RBD) neutralizing antibodies, the mRNA nucleotide sequence has been modified by two proline mutations to lock the S-protein in the prefusion conformation [7,8]. Anti-S antibodies can be detected with quantitative serological tests, but also with neutralization tests, which measure the ability of the subject’s antibodies to protect cells from infection [9,10,11,12,13]. Several studies have monitored the antibody response after the first dose of the Comirnaty vaccine observing a higher anti-S antibody response in persons previously infected by SARS-CoV-2 compared to subjects never infected by SARS-CoV-2 [9,10,11,12,14,15]. A few studies also evaluated the response after the second vaccine administration, however, the follow-up of enrolled subjects was up to 3 months only [16,17,18] and the number of participants was limited (≤400). To the best of our knowledge only one study followed the serological response after a 6 month follow-up period yet the cohort was limited (n = 33) and was injected with a different vaccine (Moderna) [19]. A longer follow-up period and large cohorts of subjects are thus sought to better assess the antibody kinetics and the neutralizing activity of sera against the emerging virus variants of concern, as well as to better inquire into the need of a third vaccine dose.
We took advantage of our ongoing multicenter longitudinal study (Covidiagnostix), funded by the Italian Ministry of Health, to investigate the antibody responses in a six month period of over 1000 healthcare professionals (HCPs) injected with the Comirnaty vaccine [20]. The objective of the study was the evaluation of the antibody response induced by the current Comirnaty vaccine administration protocol in different sex/age groups, as well as in subjects seropositive/seronegative for anti-SARS-CoV-2 antibodies before vaccine administration. In addition, as serum antibody neutralizing activity represents, so far, the best surrogate of protection for COVID-19, we evaluated the sera’s neutralizing activity stratified according to their anti-RBD-S-protein antibody titer, at different time points. We speculate that inferring anti-RBD-S-protein antibody titer thresholds, possibly related to serum neutralizing activity, could bring new important information for the implementation of new vaccine administration protocols [5,6].
2. Materials and Methods
2.1. Covidiagnostix Study
The Covidiagnostix is an ongoing multicenter study, approved by the San Raffaele Hospital, Milan, Italy, Institutional Ethical Review Boards (CE:199/INT/2020), which aims to monitor the antibody response of a population of HCPs who were offered the BNT162b2 mRNA COVID-19 (Comirnaty) vaccine.
2.2. Inclusion Criteria and Methodology
This study included 1052 HCPs from the San Raffaele Hospital, Milan, Italy. All of the HCPs received two doses of the BNT162b2 vaccine (21 ± 1 day’s interval between the two doses) during January and February 2021; no exclusion criteria were applied. Blood samples were withdrawn for serological evaluation, as previously described [21] at: time 0 (T0), 1–2 min before receiving the first vaccination dose. Time 1 (T1), 21 ± 2 days after T0, before (1–2 min) the injection of the second dose. Time 2 (T2), 42 ± 4 days after T0. Time 3 (T3), 180 ± 10 days after T0. At T0, samples were tested for the presence of SARS-CoV-2 antibodies using the Roche Anti-SARS-CoV-2, an electrochemiluminescence immunoassay (ECLIA) (Sensitivity: 100%; Specificity: 99.8%, by adopting the manufacturer’s suggested cutoff of 1 U/mL), on a COBAS 601 platform (Roche, Basel, Switzerland), targeted on total Immunoglobulins (IgTot: IgA, IgG and IgM) against the viral nucleocapsid protein (N-protein) [22,23]. Tests results between 0.165 and 1 U/mL were considered dubious [24], thus, when available, previous diagnostic tests (RT-PCR tests) were used to discriminate between SARS-CoV-2 previously infected and non-previously infected individuals. Thanks to an instrument query, upon dubious/positive results (>0.165 U/mL), samples were further tested on the same platform with the Roche anti-SARS-CoV-2-S test (Roche, Basel, Switzerland): an electrochemiluminescence immunoassay (ECLIA) detecting IgTot against the receptor binding domain (RBD) of the viral S-protein. The quantification range is between 0.4 and 250.0 U/mL, which is further extended to 2500.0 U/mL by a 1:10 dilution of the sample automatically performed by the instrument. Specificity and sensitivity (≥14 days after diagnosis) are 99.98% and 98.8%, respectively, by adopting the manufacturer’s suggested cutoff of 0.8 U/mL. At T1, T2, and T3 the samples were tested for the presence of anti-Spike protein antibodies with the Roche anti-SARS-CoV-2-S test. At T3, when needed, samples were also tested for the presence of antibodies against the N-protein (Roche Anti-SARS-CoV-2). It must be noted that for the anti-RBD-S titers the manufacturer claims a Unit per milliliter (U/mL) to Binding Antibody Units per milliliter (BAU/mL, proposed by the WHO to standardize any device to the WHO International Standard) conversion factor equal to 1. Therefore, anti-RBD-S titers expressed in U/mL throughout the paper correspond to BAU/mL [25].
2.3. COVID-19 Diagnostic Data
From the beginning of the COVID-19 pandemic, as part of a follow-up institutional program, real-time PCR swab tests were performed both routinely and whenever a HCP showed symptoms consistent with COVID-19. Nasopharyngeal swabs were analyzed using the Tib-Molbiol 2019-nCoV real-time reverse-transcription PCR Kit (cat# 61011896) on a Roche Cobas Z480 thermocycler (Roche Diagnostic, Basel, Switzerland). RNA purification was performed using the Roche Magna pure system (cat# A42352) [26].
2.4. Viruses and Cells
Vero E6 (Vero C1008, clone E6-CRL-1586; ATCC) cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with non-essential amino acids (NEAA), penicillin/streptomycin (P/S), HEPES buffer, and 10% (v/v) fetal bovine serum (FBS). Three clinical isolates of SARS-CoV-2 were obtained and propagated in Vero E6 cells as previously described [27]: D614G (hCoV-19/Italy/UniSR1/2020; GISAID Accession ID: EPI_ISL_413489), B.1.1.7 (Alpha) (19/Italy/LOM-UniSR7/2021; GSAID Accession ID: EPI_ISL_1924880), B.1.351 (Beta) (hCoV-19/Italy/LOM-UniSR6/2021, GISAID Accession ID: EPI_ISL_1599180).
2.5. Micro-Neutralization Experiments
Samples from 48 different HCPs underwent a neutralization activity test at T2 and T3. The 48 HCPs’ samples were randomly chosen, at T1, to cover a broad antibody titer range (Roche Anti-SARS-CoV-2-S) from below the detection level (<0.4 U/mL) to above the high instrument limit (>2500 U/mL). Samples showing antibody titers exceeding the upper instrument limits were further diluted with a pool of pre-pandemic sera to obtain test results within the quantification range.
Vero E6 cells (4 × 105 cells/mL) were seeded into 96-well plates 24 h before the experiment was performed at 95% cell confluency for each well. Serum samples were decomplemented by incubation at 56 °C for 30 min, diluted to 1:80 and 1:160 and incubated with SARS-CoV-2 strains at 0.01 multiplicity of infection (MOI) at 1:80 and 1:160 dilution for 1 h at 37 °C [28]. Virus-serum mixtures, as well as positive infection control, were applied to Vero E6 monolayers after washing cells with PBS 1X, and virus adsorption was carried out at 37 °C for 1 h. Cells were then washed with PBS 1X to remove cell-free virus particles and virus-containing mixtures, while controls were replaced with complete DMEM supplemented with 2% FBS. The plates were incubated at 37 °C in the presence of CO2 for 72 h. The experiments were performed in triplicate. Neutralization activity was evaluated by comparing the percentage of cytopathic effect (CPE) detected in the virus-serum mixtures with the positive infection control. Neutralization activity was ranked as follow: 100%, 66.7%, 33.3%, and 0% if all, two, one, and none of the triplicate experiments showed neutralization, respectively.
Live images were acquired (Olympus CKX41 inverted phase-contrast microscopy), CPE was assessed using a scoring system (0 = uninfected; 0.5 to 2.5 = increasing number/area of plaques; 3 = all cells infected) to evaluate all of the tested conditions. Infection control (score 3) was set as 0% infection inhibition and uninfected cells (score 0) as 100% infection inhibition. The whole surface of the wells was considered for the analysis (5x magnification). All conditions were tested in triplicate.
2.6. Statistical Analysis
Observed categorical measures were summarized by means of frequencies or percentages. Numeric observed measures were summarized with mean and standard deviations (SD), or median and Inter Quartile Range (IQR) when in the presence of an excess of censored data.
Differences in antibody titer between genders were assessed by the Mann–Whitney U statistic test. The relationship between antibody titer and age classes was analyzed by means of the exact Jonckheere trend test [29]. To investigate the antibody titer variation between T2 and T3 as function of the antibody titer at T2, a robust linear model based on MM estimates with high breakdown point was fitted to keep into account possible outlier values [30]. The kinetic of the mean antibody titer over time was fitted by Generalized Additive Mixed Effect Models with spline functions as smooth term and random effect for subject.
For each of the three variants, Receiver Operating Characteristic (ROC) curves and the associated Area Under the Curve (AUC) for the neutralizing activity was computed assuming a linear mixed model with fixed effect for test and crossed random effects for subject and time. More details about this approach can be found in [30]. For graphical purposes, ROC curves were fitted by Kernel density estimates. One sample was defined as “neutralizing” if at least one of the triplicates showed neutralizing activity. Best threshold was defining according to the “top-left” rule, that is the threshold minimizing (1 − sensitivity)2 + (1 − specificity)2.
Exact p-values were computed by means of permutation methods to avoid any distributional approximation. All analyses were performed in R environment (ver. 4.1.1).
3. Results
3.1. Serological Evaluation at T0
Of the 1052 HCPs tested, 81 showed the presence of anti-N antibody (>1 U/mL), consistent with previous SARS-CoV-2 infection, whereas 18 showed dubious results (0.165–1 U/mL). Diagnostic information collected as part of the institutional follow-up program showed that only 1 HCP of the above mentioned 18 had previously experienced COVID-19, bringing the number of seropositive subjects to 82 (7.8%). Seropositive HCPs were further tested for the presence of anti-S antibodies. Their anti-RBD-S antibody titers median value was 58.3 (IQR: 135.9) U/mL with females showing slightly higher antibody titers (Table 1).
Table 1.
Serological evaluation at 0 (T0), 21 (T1), 42 (T2) and 180 (T3) days post first vaccination dose. Test results are expressed as median (IQR). Results above the 2500 U/mL instrument limit were rounded to 2500 U/mL.
| Subjects (Year ± SD) | N° a | Test Results U/mL | |||
|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | ||
| COV+ (44.3 ± 12.6) | 82 | 58.3 (135.9) | 2500 b | 2500 c | 2500 d |
| M (44.2 ± 14.6) | 30 | 42.9 (79.4) | 2500 b | 2500 c | 2500 d |
| F (44.4 ± 11.5) | 52 | 67.2 (151) | 2500 b | 2500 c | 2500 d |
| COV− (49.4 ± 11.1) | 958 | / | 31.9 (73.0) | 1622 (1613) | 591 (612.8) |
| M (51.1 ± 11.7) | 319 | / | 27 (61.3) | 1427 (1541) | 499 (497) |
| M 20–30 | 16 | / | 95.9 (141.3) | 1408 (1831) | 975 (1376) |
| M 31–40 | 49 | / | 42.6 (104.7) | 1829 (1249) | 621 (518) |
| M 41–50 | 61 | / | 28.3 (63.9) | 1157 (1440) | 568 (491) |
| M 51–60 | 103 | / | 20 (57.3) | 1554 (1432) | 500 (436) |
| M 61–70 | 83 | / | 16.5 (41.1) | 1329 (1466) | 404 (403) |
| M 71–80 | 7 | / | 9.6 (99.8) | 573 (949) | 265 (201) |
| F (48.5 ± 10.7) | 639 | / | 35.8 (75.3) | 1755 (1569) | 645 (658) |
| F 20–30 | 39 | / | 69.4 (147.8) | 2500 e | 1229 (741) |
| F 31–40 | 98 | / | 45.5 (58.6) | 1947 (1199) | 660 (483) |
| F 41–50 | 172 | / | 38.2 (78.2) | 1916 (1496) | 614 (628) |
| F 51–60 | 235 | / | 29.6 (79.9) | 1595.5 (1646) | 630 (668) |
| F 61–70 | 90 | / | 26.7 (54.9) | 1464 (1757) | 568 (734) |
| F 71–80 | 5 | / | 3.4 (4.2) | 287 (136) | 370 (164) |
a The 12 HCPs who got post-vaccine infection were omitted from this table. b Median was above the 2500 U/mL higher instrument limit. Of the 82 test results 11 (2 males and 9 females) were below the limit. c Median was above the 2500 U/mL higher instrument limit. Of the 82 test results 4 (all females) were below the limit. d Median was above the 2500 U/mL higher instrument limit. Of the 82 test results 36 (10 males and 26 females) were below the limit. e Median was above the 2500 U/mL higher instrument limit. Of the 46 test results 22 were below the limit.
3.2. Serological Evaluation at T1
3.2.1. Seropositive Group
As observed in previous studies [11,12,14,16,31] the 82 HCPs identified at T0 as seropositive showed an exceptional antibody response that was above the 2500 U/mL instrument limit in 71 (86.6%) subjects (Table 1). The remaining 11 HCPs showed a median of antibody titers equal to 658 U/mL (IQR 1433 U/mL).
3.2.2. Seronegative Group
Of the 970 HCPs seronegative at T0, 12 were retrospectively excluded from this group because they became infected by SARS-CoV-2 after or during the vaccination protocol and will be discussed in Section 3.5. The remaining 958 HCPs, except 34 (3.5%), all showed a post-vaccination humoral response at T1. Their anti-RBD-S antibody titers were of the same order of magnitude as those observed in the seropositive group at T0 (Table 1) and decreased significantly with age (p < 0.00001) (Figure 1). In contrast, no significant differences were observed when comparing males and females. Only one subject showed an antibody titer >2500 U/mL. The 17 HCPs showing dubious anti-N antibodies results, listed as seronegative in Section 3.1, did not show the exceptionally high response (>2500 U/mL) observed in seropositive subjects.
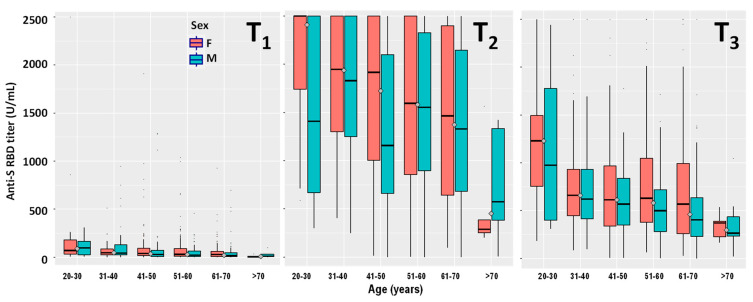
Figure 1.
Stratification by age and gender of the serological responses at 21 days (T1), 42 days (T2), and 180 days (T3) after the first dose of the Comirnaty mRNA vaccine in HCPs never infected by SARS-CoV-2 (n = 958).
3.3. Serological Evaluation at T2
3.3.1. Seropositive Group
Compared to T1 the number of seropositive HCPs showing antibody titers >2500 U/mL increased from 71 (86.6%) to 78 (95.1%) (Table 1). The remaining four HCPs showed antibody titers at T2 higher than their corresponding T1 titers (median value 1045 U/mL, IQR 1247.3 U/mL).
3.3.2. Seronegative Group
The antibody titers of the seronegative group, measured 21 days after receiving the second vaccine dose, increased approximately two-orders of magnitude when compared to T1 (Table 1, Figure 1). The number of HCPs that did not even respond to the second dose was three (0.3%). In contrast, 283 (29.5%) HCPs showed antibody titers >2500 U/mL. As observed for T1, the antibody titers decreased significantly with age for both males (p = 0.00857) and females (p < 0.00001) (Figure 1), whereas no significant differences were observed when comparing the two genders.
3.4. Serological Evaluation at T3
3.4.1. Seropositive Group
Six months after the first vaccination dose the median values for both males and females were still above the 2500 U/mL instrument limit (Table 1). However, the number of HCPs showing antibody titers within the assay reading range increased from 4 (4.9%) at T2 to 36 (43.9%) at T3 (median value 1262.5 U/mL, IQR 977.5 U/mL), 10 of which were males and 26 females (Table 1).
3.4.2. Seronegative Group
At T3 the number of HCPs showing antibody titers >2500 U/mL dropped from 283 (29.5%) at T2 to 17 (1.8%), showing a general remarkable decrease in antibody titers with time (Figure 1). The median values for the different age groups showed a large anti-RBD-S titer drop at T3 when compared with T2 (Table 1). The antibody titers measured at T3 decreased significantly with age for both males (p < 0.00001) and females (p < 0.00001) (Figure 1), whereas no significant differences were observed when comparing the two genders. Out of the three HCPs that didn’t even respond to the second vaccination dose (T2), two of them showed detectable, albeit low, antibody titers at T3.
3.5. Post-Vaccination Infections
In contrast to the general downward antibody titers’ trend, 67 (6.4%) HCPs (42 females and 25 males; 52.6 ± 11.2 years, all seronegative at T0) showed increased antibody titers at T3. Among them, seven individuals (four females and three males) showed antibody titers at T3 above the 2500 U/mL instrument limit, whereas the remaining 60 showed a median T2 to T3 titer increase equal to 167 U/mL (IQR 375.5 U/mL). The T3 sera of the 67 HCPs were tested for the presence of anti-N antibody: five were above the 1 U/mL cutoff level (Table S1: subjects 1, 4, 5, 9, 10), thus consistent with post-vaccination infection, whereas two showed dubious results (0.165–1 U/mL) (Table S1: subjects 3, 8). Post-vaccination infection was confirmed for the latter two HCPs by the presence of positive RT-PCR swab tests performed in the post-vaccination period. Positive swab tests were also present for three of the five HCPs showing anti-N antibody titer >1 U/mL.
Out of the 1052 HCPs involved in the study, 65 (6.2%) HCPs (41 females and 24 males, 45.3 ± 13.3 years old) showed anti-RBD titers above >2500 U/mL, both at T2 and T3, and were listed as “indefinite”. Forty-six of them were seropositive at T0, the remaining 19 subjects, seronegative at T0, were tested for the presence of anti-N antibodies at T3. Out of 19 HCPs, two were above the 1 U/mL cutoff limit (Table S1: subjects 6, 7) (their post-vaccination infection was also confirmed by previous diagnostic tests), whereas two subjects had dubious anti-N antibody titers. One of the latter had a previous positive swab tests in the post-vaccination period (Table S1: subject 2), whereas the second one had no history of COVID-19, showed dubious anti-N antibody titers also at T0, yet did not exhibit the distinguished high antibody titer response at T1 observed in seropositive subjects [11,12,14,16,32].
Two more HCPs, (seronegative at T0) were infected after receiving the second dose (Table S1: subjects 11, 12). They did not show T3 minus T2 Ab titers <0 and were identified thanks to post-vaccination nasopharyngeal swab-RT-PCR test results available as part of the institutional follow-up program. Both females were infected shortly after receiving the second dose (7 and 14 days, respectively). As expected, their T3 serum showed the presence of anti-N antibodies. As a control group, 300 HCPs (seronegative at T0) showing a T2 to T3 decreased antibody titer were also tested for the presence of anti-N antibody. All of them showed both negative results and no presence of post-vaccination positive swab tests.
Altogether, 12 (1.2%) HCPs were infected after receiving the Comirnaty vaccine. According to the available diagnostic information, one subject was infected between the first and the second dose, two were infected between 7 and 14 days after the second dose, seven were infected between 57 and 100 days after the second dose, whereas two HCPs were oblivious of having been infected. Half of the subjects were asymptomatic, whereas the other six claimed mild anosmia and ageusia, accompanied in two cases by cold and in one case by a generalized pain. Eight post-vaccination infected HCPs reported the presence of a SARS-CoV-2 positive family member (not vaccinated) at the time of their infection (Table S1). Interestingly, 10 out of 12 subjects had antibody titers at T2 above 1000 U/mL, and seven of them were above 2000 U/mL (Table S1). Only two had a rather low titer (<400 U/mL). The 12 HCPs performed, within the hospital, different tasks and belonged to different medical departments (Table S1).
3.6. Antibody Titer Decrease between T2 and T3
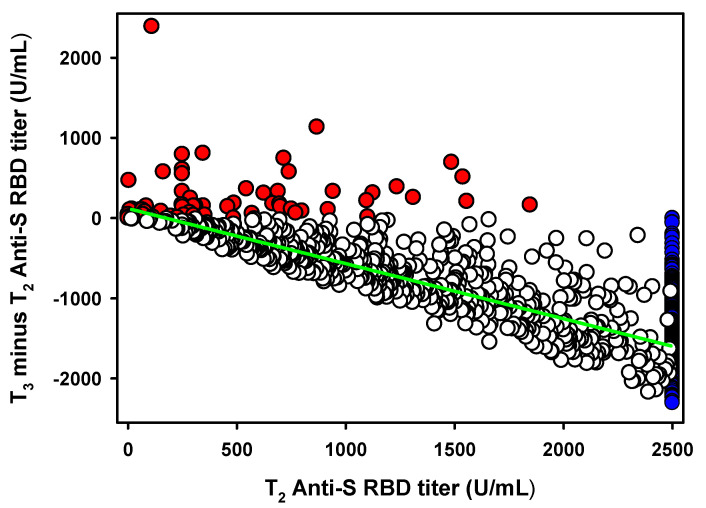
To quantify the antibody titer decrease occurring after the anti-RBD-S antibody titer peaks which, according to Favresse et al. [16] is represented by T2, we plotted the T2 values of the 958 seronegative groups’, versus their subsequent antibody titer variations (T3-T2) (Figure 2). We obtained a statistically significant (p < 0.0001) linear decrease with a slope equal to −0.62 (CI95%: −0.59 to −0.66). However, the value was biased by the several antibody titers above the 2500 U/mL instrument limit (n = 213). Excluding these values from the linear regression analysis we obtained a slope equal to −0.73 (CI95%: −0.69 to −0.77). By further removing the small subset of HCPs (n = 62) which, in contrast to the general trend, increased their antibody titer from T2 to T3 and were treated as an exception, we obtained a final slope value equal to −0.69 (CI95%: −0.65 to −0.72) (Figure 1). Thus, the average antibody titer decrease from its highest peak (T2) six months post-vaccination is approximately 70%.
Figure 2.
Association between antibody titer peak at T2 versus T3 minus T2 variation in the seronegative groups (n = 958). Red dots (n = 62) represent the small HCPs with an antibody titer increase between T2 and T3. Blue dots (n = 213) represent the HCPs showing an antibody titer >2500 U/mL at T2 or T3. Empty dots (683) represent HCPs with an antibody titer decrease between T2 and T3. The green line represents the linear regression analysis.
After stratifying for age (Table 2), the youngest showed the smallest decrease with slopes equal to −0.54 (CI95%: −0.35 to −0.73), whereas HCPs aged 31 to 70 exhibited very similar linear regression slopes ranging from −0.66 to −0.74 (Table 2). The small group of HCPs older than 70 (n = 5) showed a larger, yet significant, antibody titer decrease equal to −0.88 associated to a rather large CI95% (−0.40 to −1.36) (Table 2). Stratifying for gender showed slopes equal to −0.66 (CI95%: −0.61 to −0.70) and −0.73 (CI95%: −0.68 to −0.79) for females and males, respectively (Table 2).
Table 2.
Linear regression parameters calculated by analyzing the antibody peak at T2 versus the subsequent antibody titers decrease (T3-T2 serological evaluation).
| Subjects’ Age Interval | N a | Linear Regression Parameters | ||
|---|---|---|---|---|
| Slope (95%CI) | p Value | R2 | ||
| 20–30 | 31 | −0.54 (−0.35 to −0.73) | <0.0001 | 0.533 |
| 31–40 | 95 | −0.74 (−0.63 to −0.85) | <0.0001 | 0.660 |
| 41–50 | 151 | −0.72 (−0.66 to −0.79) | <0.0001 | 0.769 |
| 51–60 | 230 | −0.69 (−0.64 to −0.75) | <0.0001 | 0.723 |
| 61–70 | 101 | −0.66 (−0.59 to −0.73) | <0.0001 | 0.778 |
| 71–80 | 5 | −0.88 (−0.40 to −1.36) | <0.05 | 0.912 |
| Males | 227 | −0.73 (−0.68 to −0.79) | <0.0001 | 0.770 |
| Females | 386 | −0.66 (−0.61 to −0.70) | <0.0001 | 0.692 |
| Total | 613 | −0.69 (−0.65 to −0.72) | <0.0001 | 0.720 |
a Analysis was limited to the 615 seronegative HCPs showing antibody titers within the instrument readout range and decreasing at T3.
The 213 samples with titers above the 2500 U/mL instrument limit seemed to undergo the same 70% decrease as shown by the analysis of the 48 samples described later in Section 3.7 (Figure S1). Such samples, when needed, were diluted with pre-pandemic serum to obtain a titer within the instrument readout range. By applying the same criteria (i.e., excluding the HCPs showing a T2 to T3 antibody titer increase) we obtained an anti-RBD-S antibody titer decrease from T2 to T3 for the considered seronegative individuals equal to −0.64 ± 0.21 (Table S2, Figure S1). It must be noted that 9 of the 48 HCPs were seropositive at T0 (Table S2) and they showed an averaged T2 to T3 decrease equal to -0.85 (−0.81 to −0.89).
3.7. Neutralization Assays and Diluted Samples
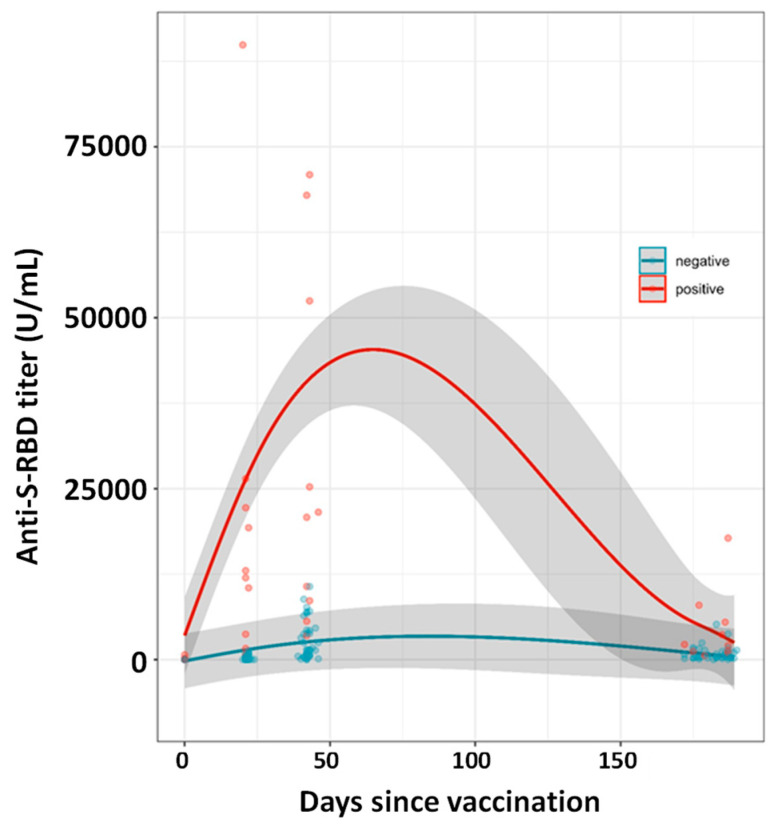
Forty-eight HCPs samples, nine of which were seropositive at T0 (Table S2), were chosen at T1 to cover the anti-RBD titers instrumental range used in this study: 4 were below the 0.4 U/mL detection limit, 36 were within the instrument readout range and 8 were above the 2500 U/mL instrument limit (Table S2). The 48 HCPs were also followed at T2 and T3. Each sample exceeding the 2500 U/mL instrument limit was diluted to 1:50 with a pool of pre-pandemic sera to obtain results within the quantification range. At T1, the 9 seropositive HCPs showed the well described exceptional antibody anti-RBD titers’ increase (Figure 3) (median 12,480 U/mL, IQR: 17,225 U/mL) whereas the 39 seronegative HCPs showed a median value approximately 500-folds lower (22.5 U/mL, IQR 266.8 U/mL). At T2, the seropositive HCPs showed an anti-RBD titers median value equal to 21,175 U/mL (IQR: 50,525 U/mL) approximately 16-fold higher than the seronegative one (1392 U/mL, IQR: 3737 U/mL), whereas at T3 the seropositive/seronegative median anti-RBD titers ratio drops to approximately 5.0 (2130 (IQR: 5448) and 427 (IQR: 986) U/mL for the seropositive and seronegative HCPs, respectively).
Figure 3.
Kinetics of SARS-CoV-2 spike antibodies (U/mL) in seronegative (blue) and seropositive (red) individuals based on 48 HCPs (9 seropositives) described in Section 3.7. Samples above the 2500 U/mL instrument limit were diluted with pre-pandemic serum to bring the signal within the instrument readout range. The kinetics models are based on Generalized Additive Mixed effect Models with spline function as nonlinear term. Shadow area represents standard deviation.
We also evaluated the neutralizing activity at T2 and T3 of the 48 collected sera (diluted to 1:80 and 1:160) against three SARS-CoV-2 strains isolated in our laboratory and belonging to the variants D614G, B.1.1.7, and B.1.351 (hCoV-19/Italy/LOM-UniSR-1/2020; hCoV-19/Italy/LOM-UniSR7/2021, and hCoV-19/Italy/LOM-UniSR6/2021 all available on GISAID sequence database—https://www.gisaid.org/ (accessed on 3 November 2021)). Results reported in Figure S2 show that all tested isolates have a sensitivity profile to serum neutralization proportional to their corresponding anti-RBD titers.
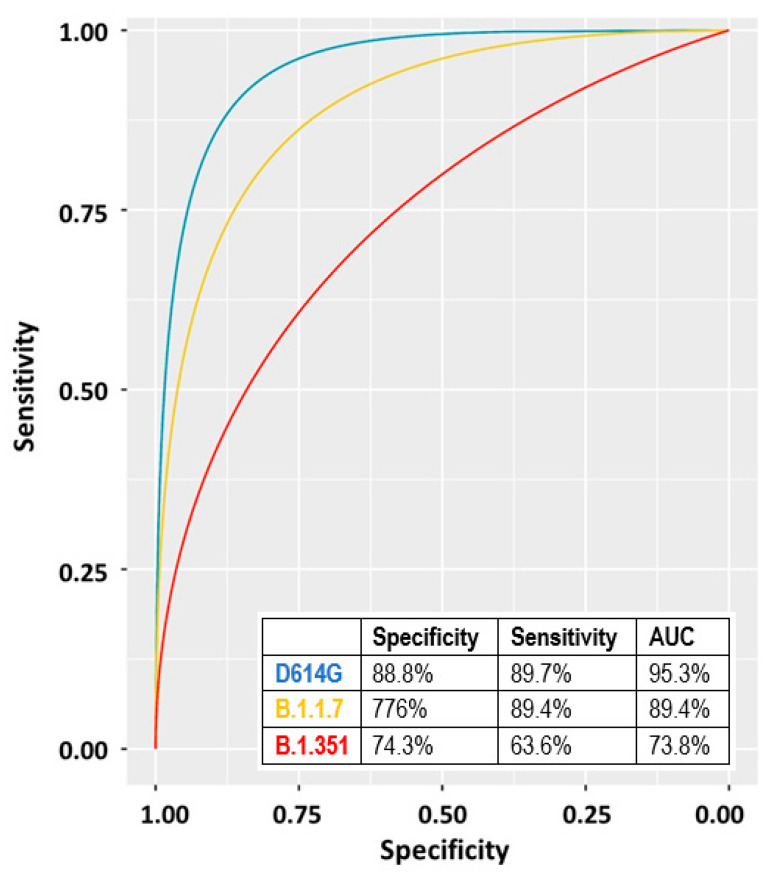
We calculated the ROC curve for the three variants by using the 1:80 data of both T2 and T3 (Figure 4). The highest AUC was observed for the D614G variant followed by the B.1.1.7 and the B.1.351 (Figure 4).
Figure 4.
ROC curves and corresponding parameters obtained by considering the antibody titers of the 48 individual samples described in Section 3.7. Blue line: D614G, yellow line: B.1.1.7, red line: B.1.351.
4. Discussion
Our study reports the antibody kinetics 6 months post-vaccination in both seronegative and seropositive individuals who received two doses of the BNT162b2 vaccine. Seropositive HCPs showed, as already described, exceptional increase in antibody titer upon receiving the first vaccine dose [9,10,11,12,14]. In contrast, seronegative subjects showed the production of limited amounts of anti-RBD-S at T1, which was boosted by the second dose (T2), as described in Figure 1. Upon receiving the second dose (T2), the anti-S-RBD antibody titer also increased in individuals previously infected by SARS-CoV-2, reaching values approximately 10-fold higher than seronegative individuals. Six months after receiving the first dose, the average antibody titer decrease (from the T2 peak) was approximately 70% (within individuals aged 30 to 70). Such a decrease is less pronounced in young individuals in their twenties (~55% decrease). Although females showed a less pronounced antibody titer decrease than males, no statistically significant differences were observed between genders. Individuals who experienced COVID-19 before vaccination also showed a T2 to T3 decrease which, on average, is higher than seronegative individuals (~85%). We might speculate that the reason for this large decrease is due to the exceptional vaccine response, observed in this HCPs’ category, which cannot be sustained for a long time by the organism.
Despite the 70% decrease (Figure 2), the mean antibody titer at T3 of the HCPs was still one order of magnitude higher than that of seropositive individuals before vaccination (T0).
In contrast to the general decreasing trend, a small subset of HCPs showed an increased antibody titer from T2 to T3. Such behavior, except for a few cases with a diagnosed post-vaccination infection, was observed mainly in older individuals with a low vaccination response. Further information is needed to inquire whether this behavior is related to a physiological slow vaccination response occurring in elderly individuals [33,34], or rather to an ongoing pathology/medical treatment. Notably, 12 HCPs were infected by SARS-CoV-2 post vaccination (Table S1). Eleven out of twelve received two vaccination doses, whereas one was infected between the two doses. None of them showed severe symptoms, thus confirming the vaccines’ efficacy previously described in clinical trials [3]. Closer investigation showed that 8 out of 12 post-vaccination infected HCPs had been in close contact with a non-vaccinated SARS-CoV-2 positive family member. This observation further stresses that post-vaccination infection can be possible, even in the presence of a high anti-S serum antibody titer. Furthermore, the possibility of an in-hospital outbreak was ruled out since the 12 HCPs perform, within the hospital, different tasks.
Neutralizing activity experiments showed that the vaccine elicited antibodies are also effective against the B.1.1.7 and B.1.351 variants, yet, to a slightly lesser extent. A previous work [25] showed that in vitro, anti-RBD-S titers around 1400 U/mL provide protection from infection, yet HCPs with even higher anti-RBD-S titers at T2 were SARS-CoV-2 infected also shortly after the second dose. These data highlight both the difficulty to find a reliable correlate of protection by assessing the serum neutralizing antibody titers only, and, that other immune mechanisms, such as long-lived memory B and T cells play major protective roles against SARS-CoV-2 infection [35].
Our study, performed on well-stratified cohorts of individuals receiving COVID-19 vaccinations, highlights the importance of vaccination and stresses the need for further studies investigating the contribution of memory immunity after vaccination on protection from severe COVID-19.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/vaccines9111357/s1. Figure S1: Demographic characteristics, serological results and COVID-19 related information of the 12 HCPs post-vaccination infected by SARS-CoV-2, Figure S2: Antibody titers at T0, T1, T2 and T3 of the 48 HCPs selected for neutralization activity tests, Table S1: Association between antibody titer peak at T2 versus T3 minus T2 variation in the 48 samples described in Section 3.7, Table S2. Neutralization assay. Neutralizing activity was assessed using two sera dilutions (1:80 and 1:160) against 0.01 MOI of three SARS-CoV-2 variants: D614G, B.1.1.7, B.1.351. Mean values + SD are reported, each condition was tested in triplicate. Sera are ordered according to the anti-RBD titer at T1: dark blue, >2500 U/mL; blue, 2500–500 U/mL; light blue, 500–50 U/mL; dark grey 50–10 U/mL; light gray 10–1 U/mL, white <1 U/mL.
Author Contributions
Conceptualization, D.F., N.C. and M.L.; methodology, E.C. and F.C.; software, A.A.; validation, M.P., G.B. and N.M.; formal analysis, D.F.; investigation, D.F.; resources, M.L.; data curation, N.C. and N.M.; writing—original draft preparation, D.F.; writing—review and editing, D.F. and A.A.; visualization, D.F. and A.A.; supervision, C.D.R., R.T., N.M., G.B. and M.P.; project administration, C.D.R.; funding acquisition, G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by Ministry of Health of Italy, “Bando Ricerca COVID-19”; project number: COVID-2020-12371619; project title: COVIDIAGNOSTIX—Health Technology Assessment in Covid serological diagnostics.
Institutional Review Board Statement
The Covidiagnostix is an ongoing multicenter study, approved by the San Raffaele Hospital, Milan, Italy, Institutional Ethical Review Boards (CE:199/INT/2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data available on request due to restrictions eg privacy or ethical.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Worldometers COVID-19 Statistics. [(accessed on 6 October 2021)]. Available online: https://www.worldometers.info/coronavirus/
- 2.FDA FDA Approves First COVID-19 Vaccine. [(accessed on 3 September 2021)];2021 Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine.
- 3.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Marc G.P., Moreira E.D., Zerbini C., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulligan M.J., Lyke K.E., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Raabe V., Bailey R., Swanson K.A., et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 5.Berkeley L.J. Pfizer CEO Says Third Covid Vaccine Dose Likely Needed within 12 Months. CNBC. 2021. [(accessed on 6 October 2021)]. Available online: https://www.cnbc.com/2021/04/15/pfizer-ceo-says-third-covid-vaccine-dose-likely-needed-within-12-months.html.
- 6.Howard J. A Third Dose of COVID-19 Vaccine Is Now Authorized for Some. Here’s What You Need to Know about Boosters for All. CNN. 2021. [(accessed on 6 October 2021)]. Available online: https://edition.cnn.com/2021/08/14/health/covid-19-vaccine-boosters-explainer-fda-wellness/index.html.
- 7.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B., Mclellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pallesen J., Wang N., Corbett K.S., Wrapp D., Kirchdoerfer R.N., Turner H.L., Cottrell C.A., Becker M.M., Wang L., Shi W., et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc. Natl. Acad. Sci. USA. 2017;114:E7348–E7357. doi: 10.1073/pnas.1707304114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saadat S., Rikhtegaran Z.T., James L., Michelle N., Matthew B.F., Harris A.D., Sajadi M.M. Binding and Neutralization Antibody Titers After a Single Vaccine Dose in Health CareWorkers Previously InfectedWith SARS-CoV-2. JAMA J. Am. Med. Assoc. 2021;325:1467–1469. doi: 10.1001/jama.2021.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krammer F., Srivastava K., Alshammary H., Amoako A.A., Awawda M.H., Beach K.F., Bermúdez-González M.C., Bielak D.A., Carreño J.M., Chernet R.L., et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N. Engl. J. Med. 2021;384:1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prendecki M., Clarke C., Brown J., Cox A., Gleeson S., Guckian M., Randell P., Pria A.D., Lightstone L., Xu X.-N., et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. [(accessed on 6 October 2021)];Lancet. 2021 397:1178–1181. doi: 10.1016/S0140-6736(21)00502-X. Available online: http://www.ncbi.nlm.nih.gov/pubmed/33640037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manisty C., Otter A.D., Treibel T.A., McKnight I., Altmann D.M., Brooks T., Noursadeghi M., Boyton R.J., Semper A., Moon J.C. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397:1057–1058. doi: 10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y., Liu J., Xia H., Zhang X., Fontes-Garfias C.R., Swanson K.A., Cai H., Sarkar R., Chen W., Cutler M., et al. Neutralizing Activity of BNT162b2-Elicited Serum. N. Engl. J. Med. 2021;384:1466–1468. doi: 10.1056/NEJMc2102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrari D., Di Resta C., Sabetta E.T.R., Pontillo M., Motta A., Locatelli M. Long-term antibody persistence and exceptional vaccination response on previously SARS-CoV-2 infected subjects. Vaccine. 2021;39:4256–4260. doi: 10.1016/j.vaccine.2021.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrari D., Mangia A., Spanò M.S., Zaffarano L., Viganò M., Di Resta C., Locatelli M., Ciceri F., De Vecchi E. Quantitative serological evaluation as a valuable tool in the COVID-19 vaccination campaign. Clin. Chem. Lab. Med. 2021 doi: 10.1515/cclm-2021-0364. in press. [DOI] [PubMed] [Google Scholar]
- 16.Favresse J., Bayart J.-L., Mullier F., Elsen M., Eucher C., Van Eeckhoudt S., Roy T., Wieers G., Laurent C., Dogné J.-M., et al. Antibody titres decline 3-month post-vaccination with BNT162b2. Emerg. Microbes Infect. 2021;10:1495–1498. doi: 10.1080/22221751.2021.1953403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Padoan A., Dall’Olmo L., Rocca F.D., Barbaro F., Cosma C., Basso D., Cattelan A., Cianci V., Plebani M. Antibody response to first and second dose of BNT162b2 in a cohort of characterized healthcare workers. Clin. Chim. Acta. 2021;519:60–63. doi: 10.1016/j.cca.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cocomazzi G., Piazzolla V., Squillante M.M., Antinucci S., Giambra V., Giuliani F., Maiorana A., Serra N., Mangia A. Early Serological Response to BNT162b2 mRNA Vaccine in Healthcare Workers. Vaccines. 2021;9:913. doi: 10.3390/vaccines9080913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doria-Rose N., Suthar M.S., Makowski M., O’Connell S., McDermott A.B., Flach B., Ledgerwood J.E., Mascola J.R., Graham B.S., Lin B.C., et al. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for Covid-19. N. Engl. J. Med. 2021;384:2259–2261. doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomaiuolo R., Derrico P., Ritrovato M., Locatelli M., Milella F., Restelli U., Lago P., Giuliani F., Banfi G. COVIDIAGNOSTIX: Health technology assessment of serological tests for SARS-CoV-2 infection. Int. J. Technol. Assess. Health Care. 2021;37:1–5. doi: 10.1017/S0266462321000441. [DOI] [PubMed] [Google Scholar]
- 21.Ferrari D., Manca M., Premaschi S., Banfi G., Locatelli M. Toxicological investigation in blood samples from suspected impaired driving cases in the Milan area: Possible loss of evidence due to late blood sampling. Forensic Sci. Int. 2018;288:211–217. doi: 10.1016/j.forsciint.2018.04.038. [DOI] [PubMed] [Google Scholar]
- 22.Perkmann T., Perkmann-Nagele N., Breyer M.K., Breyer-Kohansal R., Burghuber O.C., Hartl S., Aletaha D., Sieghart D., Quehenberger P., Marculescu R., et al. Side-by-Side Comparison of Three Fully Automated SARS-CoV-2 Antibody Assays with a Focus on Specificity. Clin. Chem. 2020;66:1405–1413. doi: 10.1093/clinchem/hvaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kittel M., Muth M.C., Zahn I., Roth H.J., Thiaucourt M., Gerhards C., Haselmann V., Neumaier M., Findeisen P. Clinical evaluation of commercial automated SARS-CoV-2 immunoassays. Int. J. Infect. Dis. 2021;103:590–596. doi: 10.1016/j.ijid.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Favresse J., Eucher C., Elsen M., Tré-Hardy M., Dogné J.M., Douxfils J. Clinical Performance of the Elecsys Electrochemiluminescent Immunoassay for the Detection of SARS-CoV-2 Total Antibodies. Clin. Chem. 2020;66:1104–1106. doi: 10.1093/clinchem/hvaa131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrari D., Clementi N., Spanò S.M., Albitar-Nehmee S., Ranno S., Colombini A., Criscuolo E., Di Resta C., Tomaiuolo R., Viganó M., et al. Harmonization of six quantitative SARS-CoV-2 serological assays using sera of vaccinated subjects. Clin. Chim. Acta. 2021;522:144–151. doi: 10.1016/j.cca.2021.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrari D., Motta A., Strollo M., Banfi G., Locatelli M. Routine blood tests as a potential diagnostic tool for COVID-19. [(accessed on 6 October 2021)];Clin. Chem. Lab. Med. 2020 58:1095–1099. doi: 10.1515/cclm-2020-0398. Available online: http://www.ncbi.nlm.nih.gov/pubmed/32301746. [DOI] [PubMed] [Google Scholar]
- 27.Castelli M., Baj A., Criscuolo E., Ferrarese R., Diotti R., Sampaolo M., Novazzi F., Gasperina D.D., Focosi D., Ferrari D., et al. Characterization of a Lineage C.36 SARS-CoV-2 Isolate with Reduced Susceptibility to Neutralization Circulating in Lombardy, Italy. Viruses. 2021;13:1514. doi: 10.3390/v13081514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Criscuolo E., Diotti R.A., Strollo M., Rolla S., Ambrosi A., Locatelli M., Burioni R., Mancini N., Clementi M., Clementi N. Weak correlation between antibody titers and neutralizing activity in sera from SARS-CoV-2 infected subjects. J. Med. Virol. 2021;93:2160–2167. doi: 10.1002/jmv.26605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jonckheere A.R. A Distribution-Free k-Sample Test Against Ordered Alternatives. Biometrika. 1954;41:133. doi: 10.1093/biomet/41.1-2.133. [DOI] [Google Scholar]
- 30.Yohai V.J. High Breakdown-Point and High Efficiency Robust Estimates for Regression. Ann. Stat. 1987;15:642–656. doi: 10.1214/aos/1176350366. [DOI] [Google Scholar]
- 31.Liu W., Pantoja-Galicia N., Zhang B., Kotz R.M., Pennello G., Zhang H., Jacob J., Zhang Z. Generalized linear mixed models for multi-reader multi-case studies of diagnostic tests. Stat. Methods Med. Res. 2017;26:1373–1388. doi: 10.1177/0962280215579476. [DOI] [PubMed] [Google Scholar]
- 32.Di Resta C., Ferrari D., Viganò M., Moro M., Sabetta E., Minerva M., Ambrosio A., Locatelli M., Tomaiuolo R. The Gender Impact Assessment among Healthcare Workers in the SARS-CoV-2 Vaccination-An Analysis of Serological Response and Side Effects. [(accessed on 6 October 2021)];Vaccines. 2021 9:522. doi: 10.3390/vaccines9050522. Available online: https://www.mdpi.com/2076-393X/9/5/522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiedermann U., Garner-Spitzer E., Wagner A. Primary vaccine failure to routine vaccines: Why and what to do? Hum. Vaccines Immunother. 2016;12:239–243. doi: 10.1080/21645515.2015.1093263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ciabattini A., Nardini C., Santoro F., Garagnani P., Franceschi C., Medaglini D. Vaccination in the elderly: The challenge of immune changes with aging. Semin. Immunol. 2018;40:83–94. doi: 10.1016/j.smim.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 35.Dan J.M., Mateus J., Kato Y., Hastie K.M., Yu E.D., Faliti C.E., Grifoni A., Ramirez S.I., Haupt S., Crotty S., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371:1–13. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available on request due to restrictions eg privacy or ethical.