Abstract
Colony-stimulating factor 1 (CSF-1) supports the proliferation, survival, and differentiation of bone marrow-derived cells of the monocytic lineage. In the myeloid progenitor 32D cell line expressing CSF-1 receptor (CSF-1R), CSF-1 activation of the extracellular signal-regulated kinase (ERK) pathway is both Ras and phosphatidylinositol 3-kinase (PI3-kinase) dependent. PI3-kinase inhibition did not influence events leading to Ras activation. Using the activity of the PI3-kinase effector, Akt, as readout, studies with dominant-negative and oncogenic Ras failed to place PI3-kinase downstream of Ras. Thus, PI3-kinase appears to act in parallel to Ras. PI3-kinase inhibitors enhanced CSF-1-stimulated A-Raf and c-Raf-1 activities, and dominant-negative A-Raf but not dominant-negative c-Raf-1 reduced CSF-1-provoked ERK activation, suggesting that A-Raf mediates a part of the stimulatory signal from Ras to MEK/ERK, acting in parallel to PI3-kinase. Unexpectedly, a CSF-1R lacking the PI3-kinase binding site (ΔKI) remained capable of activating MEK/ERK in a PI3-kinase-dependent manner. To determine if Src family kinases (SFKs) are involved, we demonstrated that CSF-1 activated Fyn and Lyn in cells expressing wild-type (WT) or ΔKI receptors. Moreover, CSF-1-induced Akt activity in cells expressing ΔKI is SFK dependent since Akt activation was prevented by pharmacological or genetic inhibition of SFK activity. The docking protein Gab2 may link SFK to PI3-kinase. CSF-1 induced Gab2 tyrosyl phosphorylation and association with PI3-kinase in cells expressing WT or ΔKI receptors. However, only in ΔKI cells are these events prevented by PP1. Thus in myeloid progenitors, CSF-1 can activate the PI3-kinase/Akt pathway by at least two mechanisms, one involving direct receptor binding and one involving SFKs.
Colony-stimulating factor-1 (CSF-1) is a homodimeric growth factor secreted by numerous cell types including fibroblasts and bone marrow stroma. It promotes the proliferation, survival, and differentiation of cells of the monocyte/macrophage lineage and their bone marrow progenitors (reviewed in reference 72). The cell surface receptor for CSF-1, the CSF-1R, is normally expressed in monocytes/macrophages, osteoclasts, and trophoblasts and abnormally in a significant number of human breast cancers and other cancers of the female reproductive system (38). The CSF-1R is a receptor tyrosine kinase (RTK) of the platelet-derived growth factor (PDGF) receptor family that also includes c-Kit and the Flt3/Flk2 receptor (reviewed in references 34, 45, and 73). The importance of CSF-1 in vivo is revealed by the functional defects of the naturally occurring osteopetrotic (op/op) mouse, which makes a truncated CSF-1 protein devoid of biological activity (93). These mice exhibit many developmental and functional abnormalities, implying potentially essential roles for CSF-1 in human diseases such as osteopetrosis (94), atherogenesis (77), and tumorigenesis (63). Such abnormalities are the consequence of the severe deficiency observed in certain macrophage populations, including reductions in hematopoietic stem cells and progenitors (94). The latter finding indicates that CSF-1 acts on early precursors as well as on the more mature monocytes and macrophages.
CSF-1 binding activates the kinase function of the CSF-1R, leading to receptor autophosphorylation and rapid stimulation of tyrosine phosphorylation on a variety of intracellular signaling molecules. Several tyrosine autophosphorylation sites have been mapped in the CSF-1R, including Tyr 697, Tyr 706, and Tyr 721 in the so-called kinase insert (KI) region that divides the catalytic domain, Tyr 807 in the activation loop of the catalytic domain, and Tyr 559 in the juxtamembrane region. Autophosphorylation converts some of these tyrosines to binding sites for proteins containing Src homology 2 (SH2) domains. Tyr 697 is the binding site for the adapter molecule Grb2 (86), which is constitutively associated with the Ras guanine nucleotide exchange factor (Sos). Tyr 721 binds the p85 regulatory subunit of phosphatidylinositol 3-kinase (PI3-kinase) (68), thus bringing PI3-kinase into proximity with its substrates in the plasma membrane. Tyr 706 is required for STAT1 activation (62), while phosphorylated Tyr 559 allows Src family kinases (SFKs) to bind (2). In addition to molecules that associate directly with the activated CSF-1R, several other proteins also become tyrosine phosphorylated upon CSF-1 binding, including the adapter Shc (55), c-Cbl, which is likely to be involved in the multiubiquitination and downregulation of CSF-1R (50), Ship, a 5′-phosphatase for phosphatidylinositol 3,4,5-trisphosphate (PI-3,4,5-P3) and inositol 1,3,4,5-tetrakisphosphate (56), Gab2, a multidomain docking/scaffolding protein (29), and the tyrosine phosphatases SHP-1 in macrophages (10) and SHP-2 in myeloid progenitors (8). The role of these molecules in CSF-1-mediated signaling has not been determined. Although it has never been directly demonstrated, Grb2 binding to autophosphorylated Tyr 697 is presumed to be the initiating event in the CSF-1-mediated activation of the Ras/ERK (extracellular signal-regulated kinase) pathway (34). However, CSF-1 also induces tyrosine-phosphorylated Shc to associate with Grb2 (55), SHP-2 can serve as an adapter linking activated PDGF and epidermal growth factor (EGF) receptors to the Grb2/Sos complex (4, 54), and overexpression of Gab2 enhances cytokine-stimulated ERK activation (29, 61, 99). There are clearly other potential mechanisms for coupling activated CSF-1R to the Ras/ERK pathway in addition to that mediated by direct docking of Grb2 to the receptor.
The well-established paradigm linking Grb2/Sos to MEK/ERK is primarily based on work in fibroblasts and COS cells. In this model, Grb2/Sos is recruited to the plasma membrane, where Sos stimulates the exchange of GTP for GDP on membrane-bound Ras, leading to sequential activation of Raf-1, MEK, and ERK. Depending on cell type and stimulus, variations on this theme include activation of MEK/ERK by Ras-independent mechanisms (7) and utilization of B-Raf instead of Raf-1 (91). Although growth factors can activate A-Raf (79), a physiological role for A-Raf in mediating ERK activation remains to be clarified. Activity of the ERK pathway is also modulated by cross-talk with other intracellular signaling pathways. Many studies have documented the involvement of PI3-kinase in regulating stimulus-induced ERK activation (13, 31, 43, 79), but reports to the contrary also abound (9, 64, 74). Such apparently contradictory findings are likely to reflect, in large part, the different cell types and stimuli used in the various studies. Moreover, the point at which PI3-kinase feeds into the Ras/mitogen-activated protein kinase (MAPK) cascade also varies with different reports, and PI3-kinase has been placed both upstream and downstream of Ras. Two studies have highlighted the importance of signal strength as a determinant of PI3-kinase involvement (21, 92). Thus, under conditions of low receptor occupancy by PDGF in fibroblasts or EGF in COS-7 cells, inhibition of PI3-kinase activity leads to almost complete inhibition of growth factor-induced Ras and ERK activation. As PI3-kinase is not activated by growth factors under these conditions, the suggestion has been made that basal, not growth factor-stimulated, PI3-kinase activity is required for Ras activation (92). At intermediate growth factor concentrations, ERK but not Ras activation is sensitive to PI3-kinase inhibition; at high growth factor concentrations, PI3-kinase activity is not required at all, implying the utilization of redundant pathways for activating Ras/MAPK. These studies have helped to resolve some of the confusion in the literature; however, the generality of these findings and precisely how PI3-kinase modulates growth factor-stimulated ERK activity are still not entirely clear. In some systems, novel (75, 83) and atypical (5, 75) protein kinase C (PKC) isoforms have been implicated as the link between PI3-kinase and the ERK pathway.
We have previously shown that CSF-1-stimulated ERK activity is required for optimal protection of CSF-1R-expressing myeloid progenitor cells from apoptosis induced by mitogen deprivation (46). Similarly, expression of a constitutively activated ERK kinase (MEK) in 3T3 fibroblasts was found to restore mitogenicity to a mitogenically defective CSF-1R mutant (11). These results implicate the ERK pathway in positive signaling by the CSF-1R. In contrast, using estrogen-dependent breast cancer cells transfected with the CSF-1R as a model system for studying CSF-1's role in human breast cancer, we found that CSF-1 treatment led to growth arrest as a consequence of ERK-dependent induction of the cyclin-dependent kinase inhibitor p21Waf1 (49). Such disparate behavior illustrates the now well-documented concept that cellular context plays an important role in determining outcome of ERK activation (reviewed in reference 53). A clue to the underlying basis for such differential biological responses might come from a detailed understanding of the pathways emanating from the activated receptor that converge on MEK/ERK in different cell systems. In this report, we have analyzed the contribution of PI3-kinase and Src signaling pathways to CSF-1-mediated ERK activation in the 32D myeloid progenitor cell line transfected with wild-type (WT) or mutant CSF-1R. This cell line lacks endogenous CSF-1R but expresses many of the signaling molecules known to play an important role in cytokine-mediated signaling in hematopoietic cells, such as c-Cbl (50), Ship (56), and Gab2 (29). PI3-kinase activity is found to be required for maximal CSF-1-induced ERK activation; unexpectedly, CSF-1 can stimulate PI3-kinase independent of direct receptor binding. The PI3-kinase requirement occurred at concentrations that optimally activate Akt/PKB, a downstream target of PI3-kinase, indicating that in myeloid cells, CSF-1R activation of PI3-kinase constitutes an integral component of the ERK pathway. We demonstrate that while Src kinase activity played a minimal role in CSF-1-dependent PI3-kinase and ERK activation in cells expressing a significant number of WT CSF-1R molecules, contribution from the Src pathway was more prominent in cells expressing low receptor numbers and was indispensable in cells expressing a mutant CSF-1R lacking the KI. These results imply that CSF-1 can recruit redundant pathways to induce PI3-kinase and ERK activity.
MATERIALS AND METHODS
Antibodies and reagents.
Cell culture reagents were from GIBCO BRL (Gaithersburg, Md.), wortmannin was from Sigma (St. Louis, Mo.), LY294002 was from Calbiochem (La Jolla, Calif.), 4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine 1 (PP1) was from BioMol (Plymouth Meeting, Pa.), and bovine phosphatidylinositol (PI) was from Avanti Polar Lipids (Alabaster, Ala.). Recombinant human CSF-1 was a gift of Genetics Institute (Cambridge, Mass.), and recombinant murine interleukin-3 (IL-3) was purchased from Becton Dickinson (Bedford, Mass.). All other reagents were from Sigma.
Rabbit antisera 421 (raised against the conserved KI region), 423 (against the C terminus), and 425 (against the cytoplasmic domain of the CSF-1R) were generated in our laboratory using glutathione S-transferase (GST) fusion proteins containing residues 675 to 749, 906 to 979, and 580 to 970, respectively, of the murine CSF-1R. Anti-CSF-1R antisera were purified over protein A-Sepharose (PAS) columns. Polyclonal antibodies against Raf-1, A-Raf, ERK2, Fyn, Lyn, Akt1, and SHP-2 were from Santa Cruz Biotechnology (Santa Cruz, Calif.); a polyclonal antibody against Shc was from Transduction Labs (Lexington, Ky.); polyclonal antibodies against Hck (06-833) and p85 (06-195) were from Upstate Biotechnology (Lake Placid, N.Y.). The following monoclonal antibodies were used: anti-Myc (9E10) from Santa Cruz; anti-hemagglutinin epitope (HA) from BAbCo (Berkeley, Calif.) and Roche Diagnostics (Indianapolis, Ind.); anti-Flag (M2) from Sigma; antibodies against MEK1, Grb2, and Shc from Transduction Labs; Ras Ab-4 and Y13-259 from Oncogene Research (Cambridge, Mass.); anti-ERK2 from Zymed (San Francisco, Calif.); and antiphosphotyrosine (anti-PY; clone 4G10) and anti-avian c-Src (clone EC10) from Upstate Biotechnology. Phosphospecific antibodies that recognize Akt phosphorylated at Thr 308 and dually phosphorylated ERK were from New England BioLabs (Beverly, Mass.). The Gab2 rabbit antiserum was raised against GST-Gab2 and was a generous gift from Toshio Hirano and Masahiko Hibi, Osaka University Medical School, Osaka, Japan.
Plasmids.
Cloning of the murine CSF-1R cDNA has been described previously (47). The ΔKI-CSF-1R mutant was constructed by replacing the internal HincII fragment spanning the KI in the WT receptor with the corresponding HincII fragment lacking residues 678 to 747 produced by the two-step PCR method described previously (48). The WT and ΔKI-CSF-1R cDNA inserts were introduced into vector pCEN/MPSV (46) or LTR-2 (18). To construct a dominant-negative PI3-kinase (p110-N), residues 171 to 1034 were deleted from the catalytic subunit of murine PI3-kinase, p110α, leaving intact the N-terminal p85 binding domain. p110-N was constructed by isolating the 5-kb AccI-HindIII fragment from pCG-p110ΔKin (provided by Anke Klippel, Chiron Corp., San Francisco, Calif.) followed by religation with an AccI-HindIII linker that encodes residues 167 to 170. This leaves intact the Myc epitope (EQKLISEEDL) fused to the C terminus of p110ΔKin. A Myc-tagged ERK2 plasmid was constructed by amplifying full-length rat ERK2 from HA-tagged ERK2 cloned into pCEP4Δ (46) with a 5′ primer encoding a KpnI restriction site, 9 bases upstream of the ATG start site (Kozak consensus sequence), and the first 24 bases of the coding region and a 3′ primer that overlapped with the last 27 bases of the coding region followed by the Myc epitope, a stop codon, and an EcoRV restriction site. The digested PCR product was subcloned into the KpnI and EcoRV sites of pCEP4Δ. All PCR-amplified segments were confirmed by DNA sequencing.
Expression construct encoding 61LRas plasmid has been described previously (46). 17NRas in expression vector pcDNA1 was kindly provided by Jeffrey Pessin (University of Iowa, Iowa City). Flag-tagged WT Akt in the simian virus 40-based expression vector pECE was generously donated by Ushio Kikkawa (Kobe University, Kobe, Japan). cDNAs encoding HA-tagged Raf-1 K375W (KD [kinase dead]-Raf-1-HA) and HA-tagged A-Raf K336W (KD-A-Raf-HA) cloned into pEF-BOS-ΔR1 downstream of the elongation factor 1α promoter have been described previously (79) and were kindly provided by Larry Karnitz (Mayo Clinic, Rochester, Minn.). The cDNA encoding Hck K269M (KD-Hck) was a gift from Dan Link (Washington University, St. Louis, Mo.) and cloned into the EcoRI site of pEF-BOSΔR1. WTc-Src and Myc-tagged Src251 consisting of the first 251 residues of chicken c-Src followed by the Myc epitope both cloned downstream of the cytomegalovirus promoter in the vector HyTCX have been described previously (39) and were kind gifts of Pam Schwartzberg (National Human Genome Research Institute, National Institutes of Health).
Recombinant proteins.
Recombinant bacterially produced His-tagged KD-MAPK and KD-MEK were expressed and purified as described elsewhere (46). GST-RBD, containing the Ras binding domain of Raf (residues 51 to 131) (RBD) fused to GST (plasmid generously provided by Johannes Bos, Utrecht University, Utrecht The Netherlands), was expressed and purified according to the procedure of de Rooij and Bos (16).
Cell culture and transfections.
The IL-3-dependent nonleukemic murine myeloid cell line 32Dcl23 and its transfectants were maintained in RPMI 1640 medium (RPMI) supplemented with 10% fetal bovine serum (FBS) and 5 to 10% WEHI conditioned medium as a source of murine IL-3. Exponentially growing cells were washed thoroughly in Hanks' buffered salt solution and deprived of serum and IL-3 for 2 to 4 h prior to treatment with inhibitors and growth factors.
Stable cell lines expressing WT CSF-1R have been described elsewhere (46). Stable cell lines expressing ΔKI-CSF-1R were similarly generated by electroporation and individual drug-resistant clones isolated by limiting dilution. Clones were screened for CSF-1R expression by saturation binding with 125I-CSF-1, and selected clones were further characterized by Scatchard analysis as previously described (47). Except where noted, two clones each of WT CSF-1R and ΔKI-CSF-1R were used in the studies shown. WT clones 1 and 2 expressed 4.5 × 104 and 1.0 × 104 receptors/cell, respectively, with an average binding affinity for CSF-1 of 1.84 × 1010/M; ΔKI clones 1 and 2 expressed 4.9 × 104 and 4.3 × 104 receptors/cell, respectively, with an average binding affinity of 2.0 × 1010/M. The protocol for transient transfection has also been previously described (46); transfected cells were allowed to express for 24 h before starvation and analysis. The amounts of DNA transfected were adjusted to yield approximately equivalent expression of the reporter plasmid (HA-ERK2, ERK2-Myc, and Flag-Akt). The total amount of transfected DNA was kept constant with vector DNA.
Immunoprecipitation and immunoblotting.
Starved cells were washed in RPMI and then resuspended in RPMI for treatment. Cells were immediately lysed in an equal volume of ice-cold 2× lysis buffer (LB; 1× LB is 20 mM Tris [pH 7.4], 2 mM EDTA, 100 mM NaCl, 50 mM NaF, 50 mM β-glycerophosphate, 1% NP-40, 10% glycerol, and 1 mM dithiothreitol) with protease inhibitors and 1 mM Na3VO4. Protein concentration was determined with the Bio-Rad assay kit. For immunoprecipitations, 0.5- to 1.0 mg of lysate was added to PAS conjugated to the appropriate antibody and allowed to rock end over end for 2 to 4 h at 4°C. Immune complexes were washed four times in lysis buffer and once in HEPES-buffered saline before boiling in Laemmli sample buffer. After separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transfer to polyvinylidene difluoride (PVDF) membranes (Millipore), blots were blocked for 1 h in 5% nonfat dry milk in Tris-buffered saline with Tween (TBST) except when probing for PY, in which case blots were blocked in 3% bovine serum albumin–TBST. Incubations with primary and horseradish peroxidase-conjugated secondary antibodies were at the recommended dilutions, and blots were developed by enhanced chemiluminescence (Amersham). For reprobing, blots were stripped by five 15-min washes in 0.1 M glycine (pH 2.5). Blot quantitation was carried out as described previously (46) by scanning multiple exposures with Adobe Photoshop software and quantitating band intensities with NIH Image 1.62 software.
Kinase assays. (i) Raf, MEK, and ERK assays.
The assays were carried out essentially as described previously (46), with the following modifications for Raf assays to decrease background counts in the gels. Immunoprecipitation was carried out with Raf-1 or A-Raf antibodies already conjugated to PAS; after separation by SDS-PAGE, kinase products were transferred to PVDF membranes. Blots were first quantitated with a Storm PhosphorImager (Molecular Dynamics) before probing with the appropriate antibodies to ensure equal loading. For transient cotransfections with HA-ERK2 or ERK2-Myc, expression of the transfected ERK2 was first quantitated by immunoblotting and the amount of lysate used for each condition was adjusted to contain equivalent levels of transfected ERK2 as described elsewhere (46). ERK kinase gels were cut at the 30-kDa marker such that the bottom part containing myelin basic protein (MBP) was fixed, stained with Coomassie blue, and subjected to quantitation with the PhosphorImager, while the top part was transferred to a PVDF membrane for probing with ERK antibodies.
(ii) Src autophosphorylation.
Cells were lysed in a buffer containing 50 mM HEPES (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 25 mM NaF, 20 mM β-glycerophosphate, 10% glycerol, and 1% Triton X-100, supplemented with protease inhibitors and Na3VO4; 900 μg of lysate was used for each immunoprecipitation and first precleared with normal rabbit immunoglobulin G (IgG) bound to PAS before incubation with 1 μg of anti-Fyn or anti-Lyn conjugated to PAS. Immune complexes were washed four times with lysis buffer, washed twice with kinase buffer (50 mM HEPES [pH 7.4], 10 mM MnCl2, 1 mM dithiothreitol), and then resuspended in kinase buffer with 15 μCi of [γ-32P]ATP and incubated for 15 min at 30°C. The reaction was terminated by the addition of Laemmli buffer and boiling.
(iii) PI3-kinase assay.
PI3-kinase activity in CSF-1R or PY immunoprecipitates was determined as described elsewhere (48).
(iv) Akt kinase assay.
Starved cells were resuspended in RPMI, stimulated with CSF-1, and subjected to lysis in 2× LB as described above; 200 μg of lysates was immunoprecipitated with 1 μg of goat anti-Akt antibodies and protein G-Sepharose. Lysates from transient transfections with Flag-Akt were immunoprecipitated with 2 μg of M2 antibody and rabbit anti-mouse secondary antibody preconjugated to PAS. Immune complexes were washed twice in 1× LB, twice in wash buffer (25 mM HEPES [pH 7.4], 1 M NaCl, 10% glycerol, 1% Triton X-100, 1% bovine serum albumin), and twice in kinase buffer (same as for ERK assays) and resuspended in 25 μl of kinase buffer containing 2 μg of histone H2B, 6 μM ATP, and 10 μCi of [γ-32P]ATP. Reactions proceeded for 25 min at 30°C before termination. After SDS-PAGE, the bottom part of the gel containing histone H2B was fixed and quantitated by PhosphorImager, while the top part containing Akt was transferred and immunoblotted with anti-Akt antibodies.
Analysis of GTP and GDP bound to Ras.
The assay was performed essentially as described previously (20). Cells were starved for 15 h in RPMI containing reduced FBS (5%) and WEHI (0.5%) and then incubated for 1 h in phosphate-free RPMI with 1% dialyzed FBS. [32P]orthophosphate was added to a final concentration of 0.25 mCi/ml, and cells were labeled for 3 h followed by stimulation and lysis. Ras was immunoprecipitated with Y13-259-conjugated agarose and extensively washed; GTP and GDP were eluted and developed by thin-layer chromatography on polyethyleneimine-cellulose plates in 1.2 M NH4COOH–0.8 N HCl. Spots corresponding to GDP and GTP were cut and Cerenkov counted.
GST-RBD Ras activation assay.
Extract equivalent to 25 μg of GST-RBD was bound to glutathione-Sepharose for 1 h at 4°C, washed six times with Ras lysis buffer (50 mM Tris [pH 7.4], 1% NP-40, 150 mM NaCl, 10% glycerol, 20 mM MgCl2), and stored on ice until use. 32D cells were starved, stimulated, and lysed in Ras lysis buffer supplemented with protease inhibitors; 500 μg of lysate was mixed with GST-RBD for 30 min to 1 h at 4°C and washed three times with Ras lysis buffer; Ras-GTP bound to GST-RBD was eluted by boiling. Ras proteins were separated by SDS-PAGE and detected by immunoblotting with a pan-Ras antibody.
RESULTS
Inhibition of PI3-kinase prevents CSF-1-induced ERK2 and MEK1 activation but does not inhibit Raf-1 activity.
IL-3-dependent 32D myeloid progenitor cells transfected with the murine WT CSF-1R proliferate indefinitely in the presence of CSF-1 (46, 47). Addition of CSF-1 induces a transient but robust increase in ERK activity which is required for maximal CSF-1-mediated protection against apoptosis induced by mitogen deprivation (46). CSF-1 also stimulates PI3-kinase activity in several cell types (48, 76, 89, 98). A number of studies have demonstrated that PI3-kinase may play a role in ERK activation (see the introduction). We therefore investigated whether CSF-1-provoked ERK activation required PI3-kinase activity.
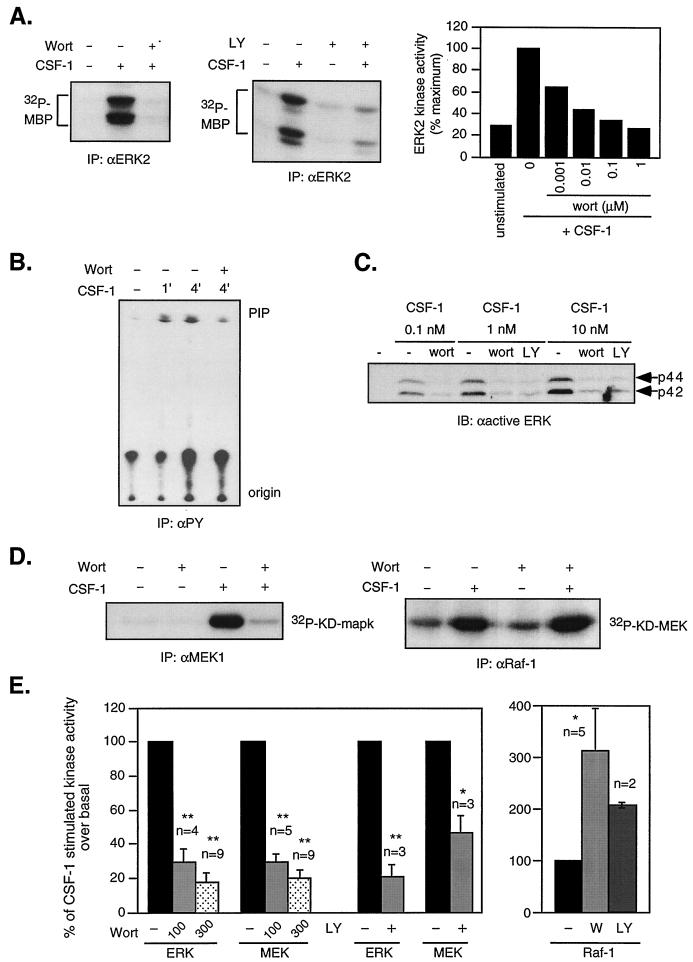
In 32D cells expressing WT CSF-1R, CSF-1 stimulated ERK transiently, with peak activity at 4 min (46). Cells were starved, pretreated with the PI3-kinase inhibitor wortmannin or LY294002, and then stimulated with 10 nM CSF-1 for 4 min. ERK activation was assessed by an immune complex in vitro kinase (IVK) assay using MBP as substrate. Pretreatment with 300 nM wortmannin or 50 μM LY294002 reduced CSF-1-stimulated ERK activity by 94 and 81%, respectively (Fig. 1A, left and middle panels). Wortmannin did not affect JNK activation by CSF-1 (data not shown), indicating that the inhibitory action on ERK was not due to nonspecific effects of the agent. Moreover, dose dependence studies with wortmannin showed that the 50% inhibitory concentration for ERK was ≈1 nM (Fig. 1A, right panel), consistent with a specific inhibition of PI3-kinase. We confirmed that wortmannin inhibited CSF-1-induced PI3-kinase activity. As shown (Fig. 1B). PI3-kinase activity assayed in anti-PY immunoprecipitates increased sevenfold after 4 min of CSF-1 stimulation and was inhibited by ≈80% when cells were pretreated with 300 nM wortmannin. In COS cells and fibroblasts (21, 92), the dependence of growth factor-stimulated ERK activity on PI3-kinase varies with signal strength. We therefore examined the sensitivity of ERK activity to PI3-kinase inhibition over a 100-fold variation in CSF-1 concentration (0.1 to 10 nM). As a reference, 0.5 nM CSF-1 is comparable to 10% WEHI conditioned medium (the amount used for cell maintenance) in supporting proliferation. ERK activity was detected by immunoblotting with an antibody that recognizes the activating phosphorylation sites on ERK. As shown (Fig. 1C), wortmannin or LY294002 dramatically suppressed ERK phosphorylation at all CSF-1 concentrations tested, indicating that in myeloid progenitors, PI3-kinase activity was an integral part of the ERK response to CSF-1.
FIG. 1.
PI3-kinase inhibitors block CSF-1-stimulated MEK/ERK but not Raf-1 activities. (A) ERK activity. 32D cells expressing the WT CSF-1R were starved and then pretreated or not with 300 nM wortmannin (Wort) or 50 μM LY294002 (LY) for 20 min before stimulation with 10 nM CSF-1 for 4 min. Lysates were immunoprecipitated (IP) with anti-ERK2 antibodies and subjected to an IVK reaction with MBP as substrate. The right panel shows the results of a dose-dependent experiment with the indicated amounts of wortmannin. (B) PI3-kinase activity. Cells were starved, not treated, or pretreated with 300 nM wortmannin before CSF-1 stimulation for the indicated times. PI3-kinase activity was assayed in anti-PY (4G10) immunoprecipitates with PI as substrate. Origin and PIP indicate the loading position and migration of a PI-4-P standard. (C) Effect of PI3-kinase inhibition on ERK phosphorylation at different CSF-1 concentrations. Cells were starved and pretreated or not with 200 nM wortmannin or 30 μM LY294002 before stimulation with the indicated CSF-1 concentrations. Total cell lysates were immunoblotted (IB) with an antibody that recognized only dually phosphorylated ERK. (D) MEK and Raf-1 activities. Cells were treated as described for panel A, and a MEK1 (left panel) or Raf-1 (right panel) immune complex kinase assay was performed with KD-MAPK or KD-MEK, respectively, as substrate. (E) Summary of results from the indicated number (n) of experiments showing the means ± standard errors. Data for ERK and MEK were averaged from experiments using 100 or 300 nM wortmannin as indicated or 50 μM LY294002; data for Raf-1 were averaged from experiments using 300 nM wortmannin or 50 μM LY294002. Data are expressed as the percentages of CSF-1-mediated increase in kinase activity over unstimulated cells, where 100% denotes the activation in the presence of CSF-1 only. Statistically significant differences between untreated and wortmannin or LY-treated samples are denoted by asterisks: ∗, P < 0.05; ∗∗, P < 0.005 (Student's two-sided t test).
To more precisely define where PI3-kinase may function in the ERK pathway, we determined the effect of wortmannin on the activity of the ERK activator, MEK1 in immune complexes with KD-MAPK as substrate. MEK2 was not investigated since CSF-1 had minimal effect on this kinase (46). Figure 1D (left panel) shows that wortmannin similarly inhibited CSF-1-induced MEK1 activity. Because Raf-1 is frequently the MEK activator, we determined the effect of PI3-kinase inhibition on CSF-1-induced Raf-1 activity. Raf-1 was immunoprecipitated, and its activity was assayed with KD-MEK as substrate. CSF-1 stimulated a fourfold increase in Raf-1 activity, which was further increased by wortmannin pretreatment (Fig. 1D, right panel). This could be consistent with PI3-kinase modulating the ERK pathway at a point between Raf-1 and MEK. The enhanced Raf-1 activity in the presence of PI3-kinase inhibitors is likely to be due to suppression of downstream feedback mechanisms mediated by MEK/ERK (1, 85). We summarize results from multiple experiments analyzing the effect of PI3-kinase inhibition on CSF-1-stimulated ERK, MEK1, and Raf-1 activities in Fig. 1E.
Effect of PI3-kinase inhibitors and cAMP-elevating agents on CSF-1 and IL-3-induced A-Raf activity.
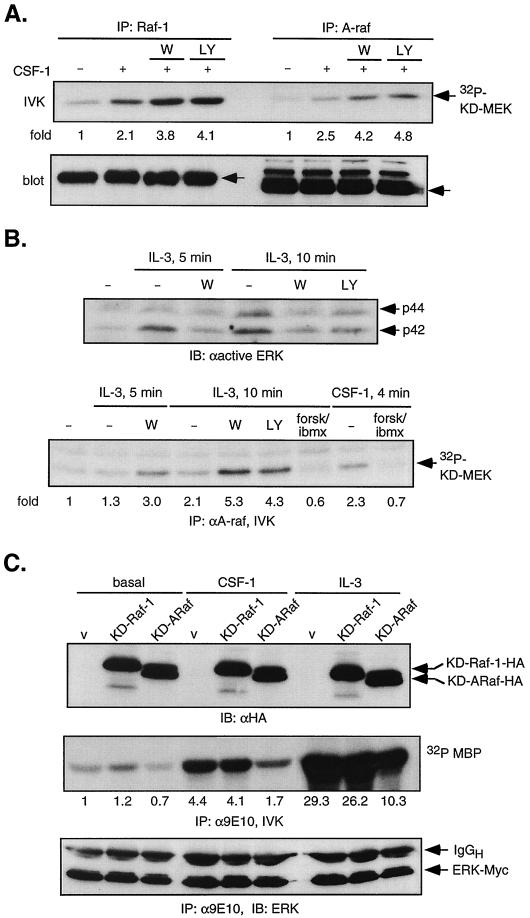
We had previously observed that cyclic AMP (cAMP) synergized with CSF-1 to greatly enhance ERK activation but to completely suppress Raf-1 activity (46), suggesting that in the presence of increased cAMP levels a MAPKKK other than Raf-1 functions as the major CSF-1-induced MEK kinase. Recently, in the FDC-P1 myeloid cell line, wortmannin was shown to inhibit IL-3-induced MEK/ERK, and A-Raf was proposed to be the IL-3-induced MEK activator since it was inhibited by wortmannin but resistant to cAMP, paralleling the effects of these inhibitors on ERK activity (79). To clarify the role of A-Raf in 32D cells, we modified the Raf assay to enhance detection of weak signals that might have been obscured by high gel background (see Materials and Methods), as we had previously not been able to detect A-Raf activity in response to CSF-1 (46). In this way, we were able to observe CSF-1-induced A-Raf activity (Fig. 2A). We cannot interpret the significantly weaker A-Raf activity induced by CSF-1, as different antibodies were used to immunoprecipitate Raf-1 and A-Raf. Similar to Raf-1, A-Raf activity was enhanced by PI3-kinase inhibition (Fig. 2A) and inhibited by cAMP elevation (Fig. 2B, bottom panel, forsk/ibmx). To examine the generality of these observations, we determined how PI3-kinase inhibition affected IL-3-induced ERK pathway. In 32D cells, cAMP also synergized with IL-3 to increase ERK activity (46). Pretreatment with 200 nM wortmannin or 50 μM LY294002 was found to significantly reduce IL-3-stimulated ERK activation (Fig. 2B, top panel). Importantly, although IL-3-induced a weak activation of A-Raf, its activity was inhibited by cAMP and increased by PI3-kinase inhibition (Fig. 2B, lower panel). Thus, the ERK pathway in 32D cells is influenced similarly by CSF-1- or IL-3-induced PI3-kinase activity. Our results show that in the presence of cAMP, neither Raf-1 nor A-Raf mediates the activating signal from CSF-1 or IL-3 to ERK; however, in the absence of cAMP, their involvement cannot be excluded.
FIG. 2.
Role of Raf-1 and A-Raf in CSF-1-mediated ERK activation. (A) CSF-1-induced Raf-1 and A-Raf activity. 32D-CSF-1R cells were pretreated or not with 200 nM wortmannin (W) or 50 μM LY294002 (LY) before stimulation with 10 nM CSF-1 for 4 min. Equal amounts of lysates were immunoprecipitated (IP) with either Raf-1 or A-Raf antibodies and subjected to an IVK assay. The gel was transferred to a PVDF membrane, and the amount of 32P incorporation into KD-MEK was quantitated by a Storm PhosphorImager. The blot was subsequently blotted with either Raf-1 or A-Raf antibodies to ensure that equal amounts of protein were immunoprecipitated. Arrows indicate the positions of p74Raf-1 and p68A-Raf. (B) IL-3-induced A-Raf and ERK activity. (Top) 32D-CSF-1R cells were pretreated as indicated followed by stimulation with recombinant IL-3 (200 U/ml) for the indicated times. Total cell lysates were analyzed for ERK phosphorylation by immunoblotting (IB) with an anti-active ERK antibody. (Bottom) Cells were pretreated or not with 50 μM forskolin and 0.5 mM 3-isobutyl-1-methylxanthine (ibmx) to activate PKA or with wortmannin (200 nM) or LY294002 (50 μM) and then stimulated with 10 nM CSF-1 or IL-3 (200 U/ml). Equal amounts of lysates were analyzed for A-Raf IVK activity. (C) Dominant-negative A-Raf and Raf-1. 32D-CSF-1R cells were transiently coelectroporated with 4 μg of ERK2-Myc and 20 μg of either vector (v; pEF-BOSΔR1), KD-Raf-1-HA, or KD-A-Raf-HA. Transfected proteins were allowed to express for 24 h before cells were starved and either left unstimulated (basal) or stimulated with 10 nM CSF-1 for 4 min or with 200 U of IL-3 per ml for 10 min. (Top) Immunoblotting of total cell lysates with anti-HA antibody. (Middle) ERK2-Myc was immunoprecipitated with anti-Myc (9E10) antibody and subjected to an IVK assay. After SDS-PAGE, the bottom part of the gel containing MBP was subjected to PhosphorImager analysis and autoradiography. Fold refers to the fold increase relative to unstimulated, vector-control cells. (Bottom) The top part of the gel was transferred and immunoblotted with an ERK monoclonal antibody, demonstrating equal amounts of immunoprecipitated ERK2-Myc.
Dominant-negative A-Raf but not dominant-negative Raf-1 partially inhibits CSF-1 and IL-3-induced ERK activation.
To further investigate the role of Raf-1 and A-Raf in mediating CSF-1 and IL-3-dependent ERK activation in the absence of cAMP, 32D-CSF-1R cells were transiently cotransfected with ERK2-Myc and either control vector, KD-Raf-1-HA or KD-A-Raf-HA. Such KD constructs have been used previously to block Raf activation (79). Immunoblotting with anti-HA antibody showed that both KD-Raf proteins were expressed at equivalent levels (Fig. 2C, top panel). ERK2-Myc was immunoprecipitated with anti-Myc antibodies, and its activity was determined (Fig. 2C, middle panel). The immunoprecipitates were also blotted with an ERK antibody to demonstrate comparable amounts of ERK2-Myc in all samples (Fig. 2C, bottom panel). Overexpression of KD-Raf-1 had no significant effect on either CSF-1- or IL-3-stimulated ERK activity, whereas KD-A-Raf inhibited by ≈60% the ERK activity induced by these cytokines. These results are in agreement with those reported by Sutor et al. (79) in their studies of IL-3-stimulated ERK activation in FDC-P1 cells. The lack of inhibition in the presence of KD-Raf-1 and the incomplete inhibition in the case of KD-A-Raf could be due to inadequate expression or because part of the activating signal is being relayed by another MAPKKK. Total cell lysates were blotted with Raf-1 or A-Raf antibodies and by densitometry, Raf in the cells transfected with KD constructs was overexpressed threefold compared with vector-transfected cells (data not shown). Given that the efficiency of transient transfection in 32D cells does not much exceed 20% (46), this implies that in cells expressing KD-Rafs, the mutant protein was present at ≈10-fold excess over the endogenous protein, making inadequate expression a less likely explanation for the observed results. Taking these data together, we suggest that in 32D cells, CSF-1 and IL-3 activate MEK/ERK in a manner that is dependent on both A-Raf, whose activity is inhibited by cAMP, and another MAPKKK that is cAMP insensitive.
CSF-1-induced ERK activation is Ras dependent, and Ras activity is not affected by inhibition of PI3-kinase.
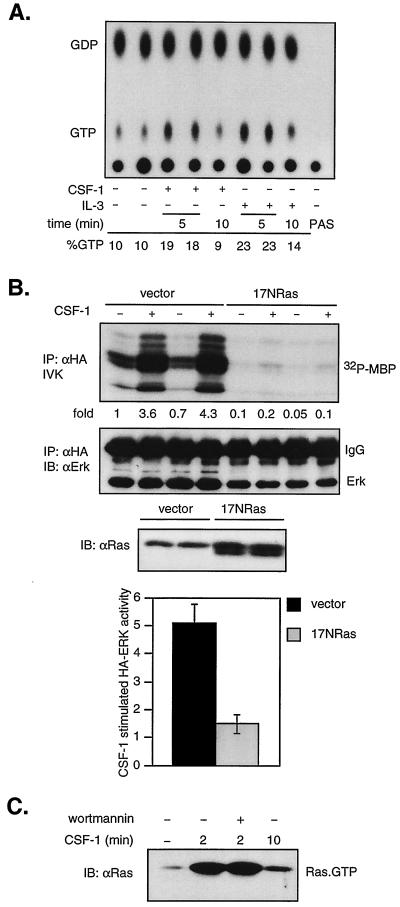
Ras is required for the activation of MEK/ERK in response to many but not all stimuli. We first confirmed that CSF-1 was able to activate Ras in vivo. Ras was immunoprecipitated from 32P-labeled 32D-CSF-1R cells that were unstimulated or stimulated with CSF-1 or IL-3 for the indicated times. Ras-bound GTP and GDP were eluted. As shown, both CSF-1 and IL-3 induced a transient twofold increase in the ratio of Ras-GTP to GDP (Fig. 3A).
FIG. 3.
CSF-1 activation of ERK is Ras dependent, and PI3-kinase inhibition does not affect Ras activation. (A) In vivo Ras activation. 32D-CSF-1R cells were starved in medium containing reduced serum and WEHI, labeled with [32P]orthophosphate, and stimulated with CSF-1 or IL-3 for the indicated times. Ras was immunoprecipitated, and bound nucleotides were eluted and analyzed by thin-layer chromatography as described in Materials and Methods. PAS refers to a sample where lysates were bound to PAS in the absence of primary antibodies. Percent GTP is calculated as 100 × cpm (GTP)/[1.5 × cpm (GDP) + cpm (GTP)]. (B) Effect of dominant-negative Ras on CSF-1 activation of ERK. 32D-CSF-1R cells were electroporated with either 7 μg of pcDNA and 2 μg of HA-ERK or 5 μg of 17NRas and 4 μg of HA-ERK. Transfections were carried out in duplicate on the same clone as indicated. The amount of HA-ERK plasmid DNA was selected to yield approximately equivalent expression levels. (Top) HA-ERK was immunoprecipitated (IP) with anti-HA antibodies and sub- jected to an IVK assay. (Middle) The amount of immunoprecipitated HA-ERK was determined by immunoblotting (IB) with an ERK monoclonal antibody. (Lower) The expression of 17NRas was assessed by immunoblotting with a pan-Ras antibody. (Bottom) Summary of results from five independent, matched transfections with either vector or 17NRas. The graph depicts the average fold increase in HA-ERK activity induced by CSF-1 over untreated cells. The difference in CSF-1-stimulated HA-ERK activity between vector- or 17NRas-transfected cells is statistically significant (P < 0.001, two-sided Student's t test). (C) Effect of wortmannin on Ras activation. Cells were starved, pretreated or not with 200 nM wortmannin, and then stimulated with CSF-1 for the indicated times. The amount of active Ras (Ras-GTP) in total cell detergent lysates was determined in a GST-RBD pull-down assay (see Materials and Methods). Active Ras was detected by immunoblotting with a pan-Ras antibody.
We next investigated if CSF-1-mediated induction of ERK activity required Ras. Cells were transiently cotransfected with HA-ERK2 and either vector or a dominant-negative Ras (17NRas). Expression of 17NRas was verified by immunoblotting with a Ras antibody (Fig. 3B, lower panel). HA-ERK2 was immunoprecipitated, and its activity was determined. As shown (Fig. 3B, top panel), 17NRas expression resulted in almost complete inhibition of both basal and CSF-1-stimulated ERK activity. The transfection was repeated multiple times, and similar degrees of inhibition were observed (Fig. 3B, bottom panel), indicating that in 32D cells, CSF-1 induction of ERK is dependent on Ras. To determine the effect of PI3-kinase inhibition on Ras activation, we made use of the sensitive assay that exploits the ability of the RBD in Raf to discriminate between Ras-GTP and Ras-GDP (16, 81). Ras-GTP was extracted from cells using a GST-RBD fusion protein, and the amount was determined by immunoblotting. Starved cells had low Ras-GTP levels which increased rapidly and markedly in response to CSF-1 stimulation (Fig. 3C). The kinetics of activation as determined by the GST-RBD pull-down assay parallels that measured by in vivo 32P labeling (Fig. 3A), but the pull-down assay is significantly more sensitive. Wortmannin had no effect, even at concentrations of up to 1 μM. These results show that PI3-kinase acts either downstream of or in parallel to Ras.
Dominant-negative Ras does not block CSF-1-activated Akt/PKB, and oncogenic Ras does not increase basal Akt activity.
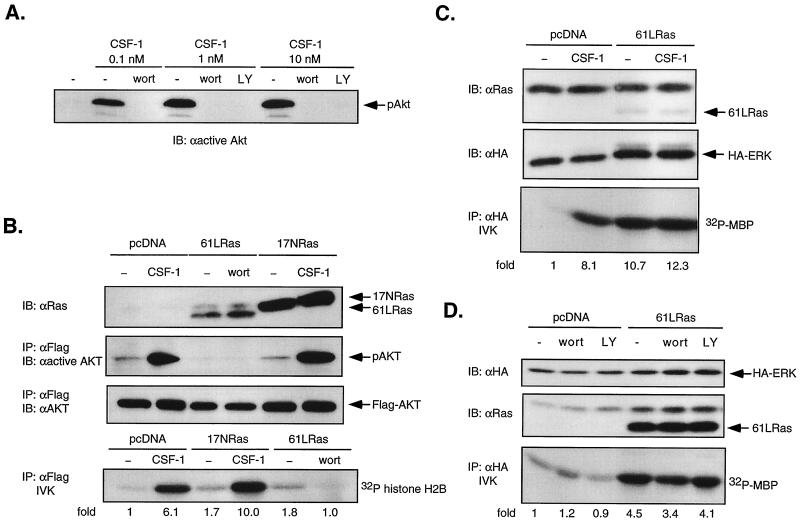
PI3-kinase can function as a Ras effector in COS and PC12 cells (71). To address the possibility that PI3-kinase functions downstream of Ras in 32D-CSF-1R cells, we used as readout the activity of the serine/threonine kinase Akt/PKB, a well-established downstream target of PI3-kinase (19) that is activated by CSF-1 in monocytes (41). In COS cells (44) and fibroblasts (40), expression of oncogenic Ras led to strong activation of Akt. We first verified that CSF-1-mediated Akt activation in 32D-CSF-1R cells is PI3-kinase dependent (Fig. 4A). Next, using a transient transfection approach, we asked if oncogenic 61LRas could increase basal activity of cotransfected WT Flag-Akt and if dominant-negative 17NRas could prevent CSF-1-induced Akt activation. Immunoblotting verified that both 61LRas and 17NRas were expressed at levels significantly above that of endogenous Ras (Fig. 4B). Akt was immunoprecipitated with anti-Flag antibodies, and its activation status was determined by analyzing Akt phosphorylation on Thr 308, one of two phosphorylation sites required for full activation (19). Figure 4B shows that CSF-1-induced Akt phosphorylation was not inhibited by 17NRas. Moreover, 61LRas did not elevate basal Akt phosphorylation. Similar results were obtained when Akt activity was monitored by an IVK assay with histone H2B as substrate (Fig. 4B). We confirmed the functionality of 61LRas in a parallel experiment in which it was cotransfected with HA-ERK. An MBP kinase assay showed that 61LRas increased basal HA-ERK activity 10-fold, comparable to that induced by CSF-1 (Fig. 4C). There was also minimal effect of PI3-kinase inhibition on ERK activity induced by oncogenic Ras (Fig. 4D), in agreement with previous observations in COS cells (31). Thus, no evidence can be found for PI3-kinase functioning in a linear pathway downstream of Ras leading to ERK activation in 32D cells. Also, we were unable to detect any interaction between Ras and the p110 subunit of PI3-kinase by coimmunoprecipitation (data not shown).
FIG. 4.
Dominant-negative Ras does not block CSF-1-activated Akt/PKB, and oncogenic Ras does not stimulate Akt activity in 32D-CSF-1R cells. (A) Effect of PI3-kinase inhibition on Akt phosphorylation. 32D-CSF-1R cells were pretreated or not with either wortmannin (wort; 200 nM) or LY294002 (LY; 30 μM) for 20 min before stimulation with 0.1, 1, or 10 nM CSF-1. Total cell lysates were analyzed for Akt phosphorylation by immunoblotting (IB) with anti-active Akt antibodies. (B) Akt activity in cells transfected with oncogenic or dominant-negative Ras. Cells were transfected with 5 μg of WT Flag-Akt and 10 μg of either vector, 61LRas, or 17NRas. Transfected Ras was verified by immunoblotting with anti-Ras antibodies. Lysates were immunoprecipitated (IP) with anti-Flag antibodies and either immunoblotted with an antibody that recognizes phosphorylated Akt (anti-active Akt) or subjected to an IVK assay with histone H2B as substrate. The amount of immunoprecipitated Akt was determined by immunoblotting with anti-Akt antibodies. (C) Oncogenic Ras activation of ERK. Cells were transfected with either 7 μg of pcDNA and 2 μg of HA-ERK or 6 μg of 61LRas, 2 μg of pcDNA, and 1 μg of HA-ERK. Ras and HA-ERK expression was detected by immunoblotting with anti-Ras and anti-HA antibodies, respectively. Lysates were analyzed for MBP kinase activity in anti-HA immunoprecipitates. (D) Effect of PI3-kinase inhibition on ERK activity stimulated by oncogenic Ras. Cells were transfected with either 4 μg of pcDNA and 4 μg of HA-ERK or 7 μg of 61LRas and 1 μg HA-ERK, starved, and left untreated or else treated with either 200 nM wortmannin or 50 μM LY294002 prior to lysis. MBP kinase activity was determined in anti-HA immunoprecipitates.
CSF-1R lacking the Grb2 and PI3-kinase binding sites can still activate ERK in a PI3-kinase-dependent manner.
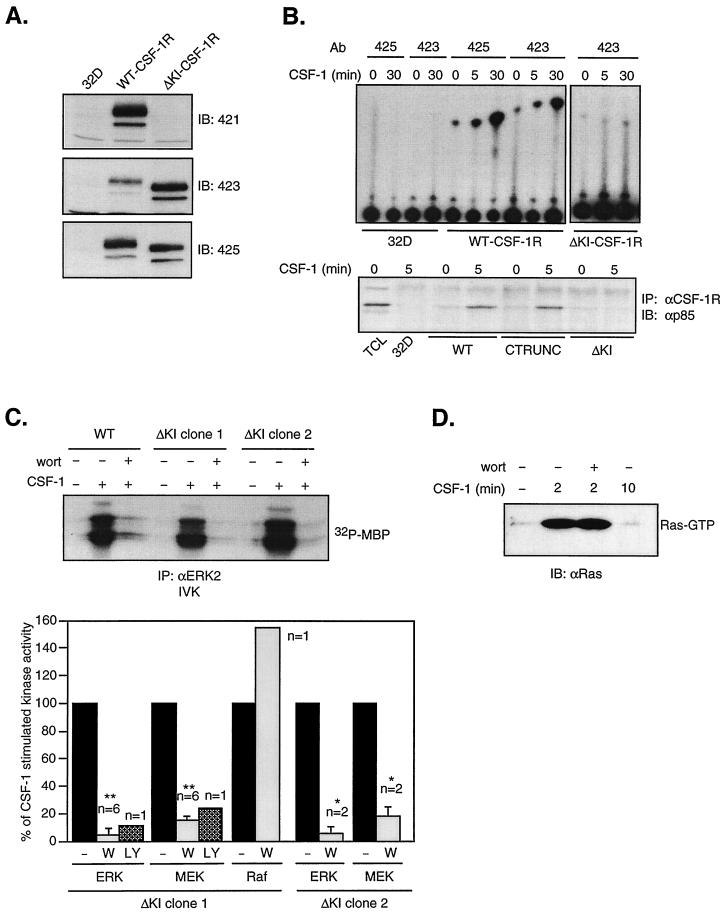
To determine if the involvement of PI3-kinase in ERK activation required recruitment of PI3-kinase to the p85 docking site on the receptor, we stably expressed in 32D cells a mutant CSF-1R which lacks the KI region, thus deleting both the p85 and Grb2 binding sites. Immunoblotting with CSF-1R antibodies directed against the KI, the C terminus, or the majority of the cytoplasmic domain showed that ΔKI-CSF-1R expressed in 32D cells to be of the expected size and to lack immunoreactivity corresponding to the KI (Fig. 5A). To confirm that the KI deletion eliminated PI3-kinase binding to the receptor in response to CSF-1, CSF-1R-associated PI3-kinase activity was measured in an IVK assay (Fig. 5B, top panel). In cells expressing WT CSF-1R, CSF-1 stimulated an increase in receptor-associated PI3-kinase activity after 5 min at 37°C. When stimulation was performed at 4°C for 30 min to reduce receptor internalization and degradation, the increase was significantly greater. Under the same conditions, CSF-1 did not increase receptor-associated PI3-kinase activity in cells expressing ΔKI-CSF-1R. We also examined the co precipitation of CSF-1R with the p85 subunit of PI3-kinase and found that p85 associated with WT CSF-1R but not ΔKI-CSF-1R after CSF-1 stimulation (Fig. 5B, bottom panel). Also shown is the association of p85 with a CSF-1R mutant lacking the C terminus (CTRUNC). This region contains several tyrosines conserved among CSF-1R from different species but whose functions are not known. Since their removal has no effect on p85–CSF-1R association, they are not involved in direct binding of PI3-kinase to the receptor.
FIG. 5.
A mutant CSF-1R lacking the PI3-kinase and Grb2 binding sites can still activate MEK/ERK in a PI3-kinase sensitive manner. (A) Expression of WT CSF-1R and ΔKI-CSF-1R. Equal amounts of lysates from parental 32D cells or cells expressing WT CSF-1R or ΔKI-CSF-1R were analyzed by immunoblotting (IB) with anti-CSF-1R antibodies (Ab): 421 directed against the KI, 423 against the C terminus, and 425 against the cytoplasmic domain. (B) (Top) CSF-1R-associated PI3-kinase activity. Parental 32D cells or cells expressing WT CSF-1R or ΔKI-CSF-1R were starved and treated or not with CSF-1 for 5 min at 37°C or for 30 min at 4°C before lysis. Equal amounts of cell lysates were immunoprecipitated (IP) with the indicated anti-CSF-1R antibodies, and an in vitro PI3-kinase assay was performed. Compared to the assay shown in Fig. 1B, the chromatograph was developed for a shorter period. (Bottom) Lysates were also immunoprecipitated with a mixture of anti-CSF-1R antibodies, and the immunoprecipitates were analyzed by immunoblotting with an anti-p85 antibody. TCL, total cell lysate. (C) ERK, MEK, and Raf-1 activity. (Top) An MBP kinase assay was carried out on the indicated cell lines as described for Fig. 1. (Bottom) Plot summarizes the results from multiple experiments where ΔKI cells (clone 1 or clone 2) were pretreated with either 200 nM wortmannin (W) or 50 μM LY294002 (LY). CSF-1-stimulated kinase activity over unstimulated cells in the absence of inhibitors is set to 100%. The statistical significance is as described for Fig. 1. (D) Ras activation. 32D-ΔKI-CSF-1R (clone 1) cells were pretreated or not with 200 nM wortmannin (wort) prior to CSF-1 stimulation for the indicated times. Ras activation was determined using a GST-RBD pull-down assay as described for Fig. 3.
We next examined CSF-1-induced ERK activation in 32D-ΔKI-CSF-1R cells. Surprisingly, wortmannin markedly inhibited CSF-1 induction of ERK activity, findings confirmed by a second clone and by the use of LY294002 (Fig. 5C). Figure 5C (bottom panel) summarizes the results from multiple experiments demonstrating that in ΔKI-CSF-1R, PI3-kinase activity was required upstream of MEK/ERK, analogous to that observed for the WT receptor. Consistent with the ability of ΔKI-CSF-1R to activate ERK, Ras activation was also retained and was insensitive to wortmannin pretreatment (Fig. 5D). As the Grb2 binding site is also missing in ΔKI-CSF-1R, the observation that this receptor remained capable of activating Ras/ERK in a manner comparable to the WT receptor indicates that direct Grb2 binding to CSF-1R is not required for ERK activation.
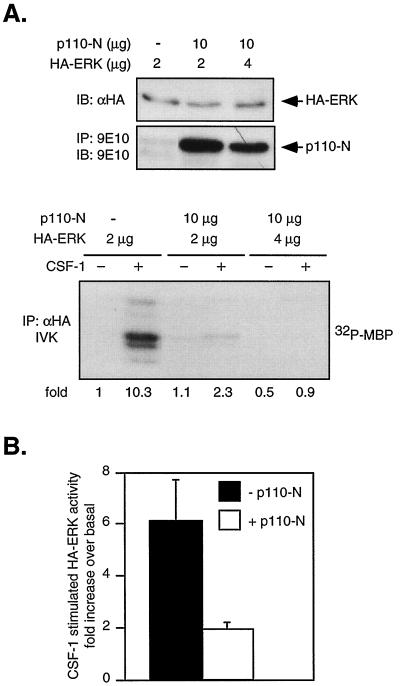
The results of Fig. 5 imply that deletion of the PI3-kinase binding site had no apparent effect on CSF-1R's ability to activate PI3-kinase. As this conclusion is rather unexpected, we sought to confirm our findings by blocking PI3-kinase function with expression of a dominant-negative PI3-kinase. A Myc-tagged truncation mutant of p110α containing only the N-terminal p85 binding domain (p110-N) was transiently cotransfected with HA-ERK2 into 32D-ΔKI-CSF-1R cells. p110-N competes with endogenous p110 for the regulatory p85 subunit and prevents functional complex formation. A similar mutant was shown to have dominant-negative properties in erythropoietin-stimulated cells (43). Figure 6A shows that p110-N substantially inhibited CSF-1-induced ERK activation, in agreement with the inhibitor studies. Figure 6B summarizes results from four independent transfections, showing an average of 81% inhibition by p110-N (P < 0.05). Since there is residual ERK activity not blocked by PI3-kinase inhibitors or dominant-negative PI3-kinase, a PI3-kinase-independent route cannot be entirely excluded. Taken together, our data indicate that PI3-kinase played a major role in the activation of ERK by ΔKI-CSF-1R and that activation of PI3-kinase by ΔKI-CSF-1R is not mediated by direct binding of PI3-kinase to the receptor.
FIG. 6.
Dominant-negative PI3-kinase inhibits CSF-1-stimulated ERK activity in ΔKI cells. (A) Effect of p110-N expression on CSF-1 activation of ERK. ΔKI cells were transfected with the indicated amounts of Myc-tagged p110-N and HA-ERK plasmids. Expression of transfected HA-ERK was analyzed by immunoblotting (IB) of total cell lysates with anti-HA antibodies. Myc-p110-N was immunoprecipitated (IP) with anti-Myc (9E10) antibody and immunoblotted with the same antibody. Lysates containing the same amount of HA-ERK were immunoprecipitated with anti-HA antibodies and subjected to an IVK assay. (B) Summary of results from four independent transfections. In the absence of p110-N, the average CSF-1-stimulated HA-ERK activity is 6.1 ± 2.8-fold above basal level and in its presence, the average fold activation is 1.95 ± 0.45.
Src family kinases couple ΔKI-CSF-1R to the PI3-kinase/Akt pathway.
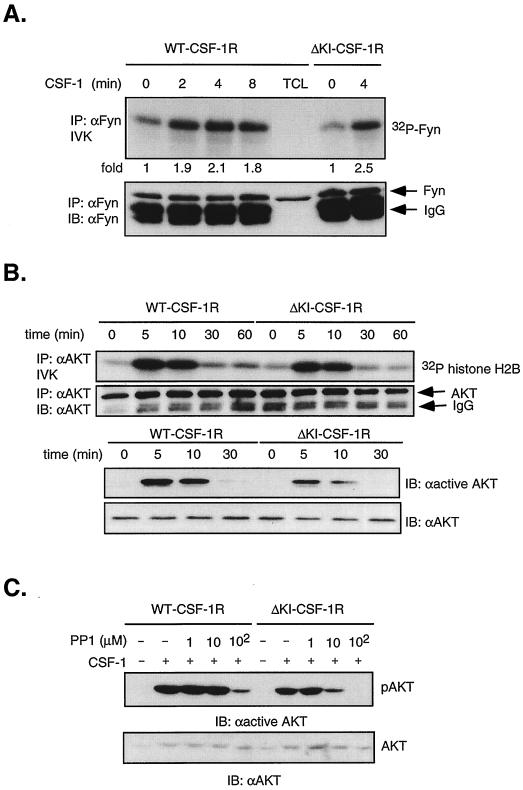
The CSF-1R contains a Src binding site at Tyr 559 which is retained in ΔKI-CSF-1R. Thus, it is possible that the ΔKI receptor utilizes SFKs to signal to PI3-kinase. 32D cells express Fyn and Lyn. Hck expression in 32D cells has been reported by some (3) but not others (23); however, we have not been able to detect Hck either by immunoblotting of total cell lysates with a polyclonal anti-Hck antibody (see Fig. 8B) or by anti-PY or anti-Hck blotting of Hck immunoprecipitates. We first confirmed that CSF-1 can activate SFKs in 32D cells expressing WT or ΔKI receptors. Fyn was immunoprecipitated and subjected to in vitro autophosphorylation (Fig. 7A). Src activity as measured by autophosphorylation has been shown to track activity assayed by phosphorylation of exogenous substrates (12). After 4 min of stimulation, CSF-1 induced a maximal 2- to 2.5-fold increase in Fyn autophosphorylation in both WT and ΔKI cells. The fold increase in Src activity is in agreement with what has been reported for CSF-1 (12). Similar results were obtained with Lyn (data not shown).
FIG. 8.
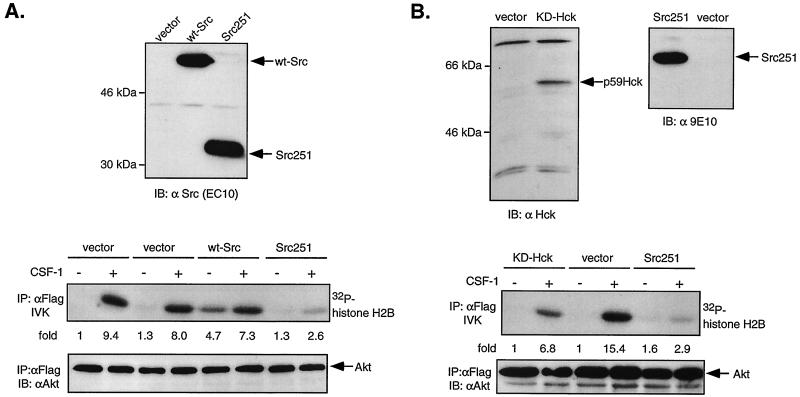
Dominant-negative SFKs inhibit CSF-1-stimulated Akt activity in ΔKI-CSF-1R cells. (A) ΔKI-CSF-1R cells were transiently transfected with either 21 μg of pEF-BOSΔR1 vector and 1 μg of Flag-Akt, 20 μg of pcDNA vector and 2 μg of Flag-Akt, 20 μg of WT Src and 2 μg of Flag-Akt, or 20 μg of Src251 and 2 μg of Flag-Akt. Cells were starved and stimulated with 10 nM CSF-1 for 2 min. (Top) Total cell lysates were immunoblotted (IB) with an anti-Src antibody. (Middle) Lysates were immunoprecipitated (IP) with anti-Flag antibodies and analyzed for histone H2B kinase activity. (Bottom) The amount of immunoprecipitated Flag-Akt was detected with anti-Akt antibodies. (B) ΔKI-CSF-1R cells were transiently transfected with 2 μg of Flag-Akt and 20 μg of either vector (pEF-BOSΔR1), KD-Hck, or Src251. (Top) Total cell lysates were analyzed for Hck expression with an anti-Hck antibody and for Src251 expression with anti-Myc antibody. (Middle and bottom) Cells were processed as described for panel A.
FIG. 7.
Activation of PI3-kinase/Akt by the ΔKI receptor is sensitive to PP1, a Src kinase inhibitor. (A) Fyn autophosphorylation. Starved 32D-CSF-1R and 32D-ΔKI-CSF-1R cells were stimulated with 10 nM CSF-1 for the indicated times, and equal amounts of lysates were immunoprecipitated (IP) with anti-Fyn antibodies and subjected to an IVK reaction. Products were separated by SDS-PAGE and transferred to a membrane, and 32P incorporation into Fyn was quantitated by PhosphorImager analysis. Subsequently, the blot was probed with anti-Fyn antibodies. IB, immunoblot. (B) Akt activity and phosphorylation. Cells were starved and stimulated with 10 nM CSF-1 for the indicated times. Lysates were immunoprecipitated with anti-Akt antibodies, and activity was assayed with histone H2B as substrate. The amount of Akt immunoprecipitated was analyzed by immunoblotting with anti-Akt antibodies. Total cell lysates from the same experiment were also immunoblotted with an antibody that recognizes phosphorylated Thr 308 on Akt (anti-active Akt). The blot was subsequently stripped and reprobed with anti-Akt antibodies. (C) PP1 dependence of Akt phosphorylation. Starved cells were pretreated with the indicated PP1 concentrations for 20 min before stimulation with 10 nM CSF-1 for 2 min and Western analysis with anti-active Akt antibodies. The blot was stripped and reprobed with anti-Akt antibodies.
To test the hypothesis that SFKs may mediate PI3-kinase activation by the ΔKI receptor, we turned to an extensively used, specific chemical inhibitor of Src kinases, PP1, reported to inhibit T-cell tyrosine phosphorylation in the micromolar range (30). Activation of the PI3-kinase pathway was monitored by assaying Akt activity in an immune complex kinase assay. We first demonstrated that both WT CSF-1R and ΔKI-CSF-1R can activate Akt (Fig. 7B), thus supporting our earlier conclusion that CSF-1 activates the PI3-kinase pathway in ΔKI cells. Akt activation was transient, with maximal activity at 5 to 10 min after CSF-1 addition. Akt phosphorylation at Thr 308 was found to follow the same trend as Akt kinase activity (Fig. 7B). Next, the role of Src in Akt activation was assessed. Dose-dependent studies demonstrated that 10 μM PP1 inhibited CSF-1-induced Akt phosphorylation in ΔKI cells by 80% but that an equivalent inhibition in WT cells required a 10-fold-higher concentration of PP1 (Fig. 7C). These findings suggest that the ΔKI receptor activates PI3-kinase by Src-dependent mechanisms but that the WT receptor utilizes predominantly Src-independent means to activate PI3-kinase, most likely through direct binding of PI3-kinase to the KI. We sought to confirm the PP1 results by examining the effect of dominant-negative SFK expression on CSF-1-dependent Akt activity in ΔKI-CSF-1R cells. Src251 is a c-Src truncation mutant lacking the catalytic domain and C-terminal tail (39) and functions as a dominant-negative for multiple SFKs (6, 22). Cells were cotransfected with Flag-Akt and either vector (pEF-BOSΔR1 or pcDNA), WT Src, or Src251. CSF-1 stimulated comparable increases in Flag-Akt activity for either vector (Fig. 8A). WT Src increased basal Akt activity 4.7-fold, indicating that when overexpressed, WT Src is constitutively active. In contrast, Src251 inhibited CSF-1-stimulated Akt activity by ≈70%. We also tested a second dominant-negative Src construct, KD-Hck. Figure 8B shows that KD-Hck inhibited CSF-1-induced Akt activity by ≈55%, compared to an inhibition of ≈80% by Src251. KD-Hck was a less effective inhibitor than Src251, probably because of lower expression level and perhaps because the SH2 domain in KD-Hck is more constrained due to interactions with the C-terminal phosphorylated tyrosine (97) which is absent in Src251. These results, together with those utilizing PP1, strongly support a role for SFKs in the activation of PI3-kinase by the ΔKI-CSF-1R, although we cannot exclude a minor contribution from SFK-independent mechanisms.
Src family kinases may also contribute to ERK activation by the ΔKI-CSF-1R.
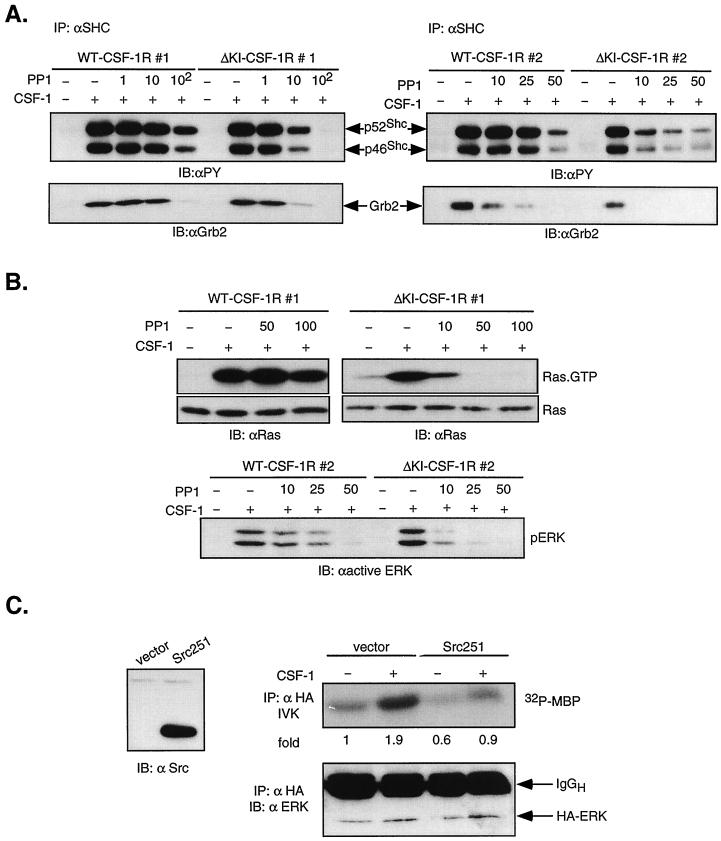
The observation that ΔKI-CSF-1R can activate ERK independent of Grb2 binding to the receptor prompted us to examine if ERK activation is initiated by Shc tyrosine phosphorylation and subsequent association with Grb2. Shc was immunoprecipitated and examined for tyrosine phosphorylation content and association with Grb2. In both WT and ΔKI cells, CSF-1 stimulated robust tyrosine phosphorylation of the p46 and p52 isoforms of Shc and their binding to Grb2 (Fig. 9A, left panel). Moreover, when cells were treated with PP1 prior to CSF-1 stimulation, ΔKI cells showed a dose-dependent inhibition of Shc tyrosine phosphorylation and Grb2 binding, similar to that observed for Akt phosphorylation. WT cells showed inhibition only at 100 μM PP1. To confirm these findings, the same experiment was performed with a second pair of clones (Fig. 9A, right panel). The two clones of ΔKI expressed approximately the same number of receptors per cell, but WT clone 2 expressed only 25% as many receptors as clone 1 (see Materials and Methods). While the two clones of ΔKI behaved similarly, PP1 was a more potent inhibitor of WT clone 2 than of clone 1, suggesting that the Src pathway makes a greater contribution when fewer receptors are engaged. In data not shown, pretreatment with wortmannin had no effect on Shc tyrosine phosphorylation and Grb2 association.
FIG. 9.
Role of SFKs in activating the Ras/ERK pathway in ΔKI-CSF-1R cells. (A) Shc tyrosine phosphorylation and association with Grb2. WT clones 1 and 2 and ΔKI clones 1 and 2 were pretreated with PP1 at the concentration (micromolar) as indicated and stimulated with 10 nM CSF-1 for 2 min. Lysates were immunoprecipitated (IP) with anti-Shc antibodies, separated by SDS-PAGE, and transferred to a membrane. The top part was immunoblotted (IB) with anti-PY antibodies, and the bottom part was probed with anti-Grb2 antibodies. (B) Ras and ERK activation. (Top) WT clone 1 and ΔKI clone 1 cells were pretreated with PP1 at the concentration (micromolar) indicated and stimulated with 10 nM CSF-1 for 2 min. Ras activation was assayed by binding 500 μg of lysates to GST-RBD. Total Ras was determined in 50 μg of total cell lysates. (Bottom) WT clone 2 and ΔKI clone 2 cells were treated as indicated, and total cell lysates were analyzed with anti-active ERK antibodies. (C) Effect of dominant-negative Src expression. ΔKI-CSF-1R cells were transiently transfected with 5 μg HA-ERK2 and 20 μg of either vector or Src251. Lysates were analyzed for MBP kinase activity (IVK) and blotted for ERK levels in anti-HA immunoprecipitates. This experiment has been repeated twice with similar results.
We examined if Src kinases could mediate Ras/ERK activation in ΔKI cells. Figure 9B shows that in ΔKI clone 1 cells, PP1 markedly inhibited CSF-1-induced Ras activation measured by the GST-RBD pulldown assay and ERK activation assessed by Western blotting with an anti-active ERK antibody. Similar results were obtained with ΔKI clone 2 (not shown). Thus, there is a strong correlation between diminished Shc-Grb2 association and reduction in activity of the Ras/ERK pathway. Analogous to its effect on SHC and Grb2 association, PP1 exerted a modest inhibitory effect on ERK activation in WT clone 2. Transient transfection of ΔKI-CSF-1R cells with the plasmid encoding Src251 inhibited both basal and CSF-1-induced ERK activation, supporting a role for SFKs (Fig. 9C). Together, these results are consistent with the model described for ΔKI cells, in which CSF-1-mediated recruitment of SFKs may be the primary mechanism by which the PI3-kinase/Akt and Ras/ERK pathways are activated, the latter possibly proceeding through phosphorylation of Shc and association with Grb2. On the other hand, in WT cells, SFKs appear to play a less important role, particularly when a larger number of receptors are engaged.
The contribution of Src family kinases to ERK and Akt activation induced by the WT CSF-1R is dependent on receptor expression levels.
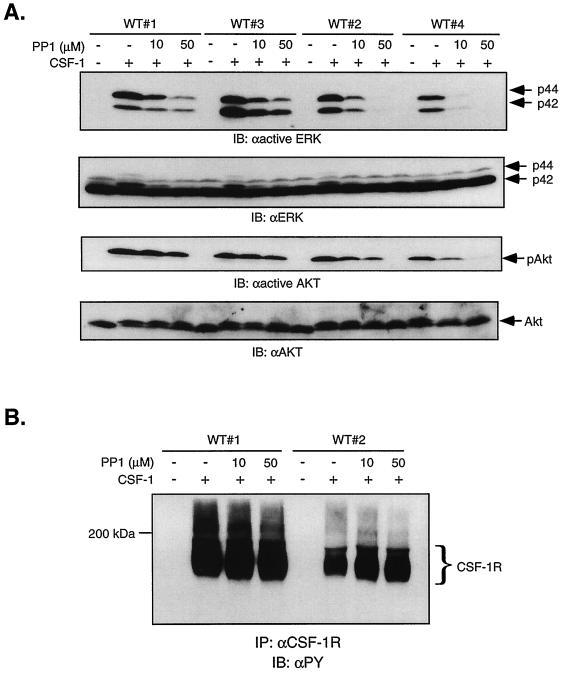
The results in Fig. 9 suggest that the contribution of SFKs to CSF-1-stimulated ERK and Akt activities may depend on WT receptor expression level. This possibility has physiological relevance since CSF-1R expression increases with maturation: whereas early myeloid precursors express 2,000 receptors/cell, mature macrophages have 50,000 receptors/cell (72). To further investigate the role of SFKs in mediating signaling by WT CSF-1R, we used WT clones that our laboratory has established with CSF-1R expression levels spanning the physiological range (clone 1, 45,000 receptors/cell; clone 3, 25,000 receptors/cell; clone 2, 10,000 receptors/cell; clone 4, 3,900 receptors/cell). PP1 was used to assess the role of SFKs in mediating CSF-1-induced ERK and Akt activation. The effectiveness of PP1 inhibition was clearly dependent on CSF-1R expression levels: CSF-1-mediated ERK phosphorylation in clone 4 was almost completely abolished by 10 μM PP1; the same dose was much less effective in clones 1 and 3, although an inhibitory effect was still evident (Fig. 10A). The decrease in ERK phosphorylation in the presence of PP1 and the dependence on receptor expression was not due to differences in ERK protein levels, as the same blot when stripped and reprobed with anti-ERK antibodies showed equivalent levels in all samples. Also, PP1 did not directly affect CSF-1R autophosphorylation (Fig. 10B). We note for clone 1 that whereas CSF-1-induced Ras activation was unaffected by 100 μM PP1 (Fig. 9B), ERK activation was partially inhibited even at 10 μM PP1 (Fig. 10A). This suggests that in addition to acting upstream of Shc/Grb2 recruitment, Src may also act downstream of Ras. Although the mechanism of activation of A-Raf is not well understood, maximal activation of Raf-1 requires tyrosine phosphorylation possibly mediated by SFKs (24, 37, 57). PP1 inhibition of CSF-1-induced Akt phosphorylation showed a similar dependence on CSF-1R levels, although the extent of inhibition is less than that observed for ERK phosphorylation. These results show that the contribution of SFKs to CSF-1-dependent signaling may depend on receptor expression levels.
FIG. 10.
WT receptor expression levels may determine the involvement of the SFKs. (A) 32D cells expressing 45,000 (clone 1), 25,000 (clone 3), 10,000 (clone 2), and 3,900 (clone 4) WT receptors/cell were starved and pretreated with the indicated doses of PP1 before stimulation with 10 nM CSF-1. Equal amounts of total cell lysates were immunoblotted (IB) for anti-active ERK or anti-active Akt. The blots were stripped and reprobed with anti-ERK or anti-Akt antibodies. (B) Lysates shown in panel A were immunoprecipitated (IP) with anti-CSF-1R antibodies, and phosphotyrosine content was analyzed by immunoblotting with 4G10.
Gab2 may be an intermediate linking Src family kinases to PI3-kinase during CSF-1-mediated signaling in cells expressing ΔKI-CSF-1R.
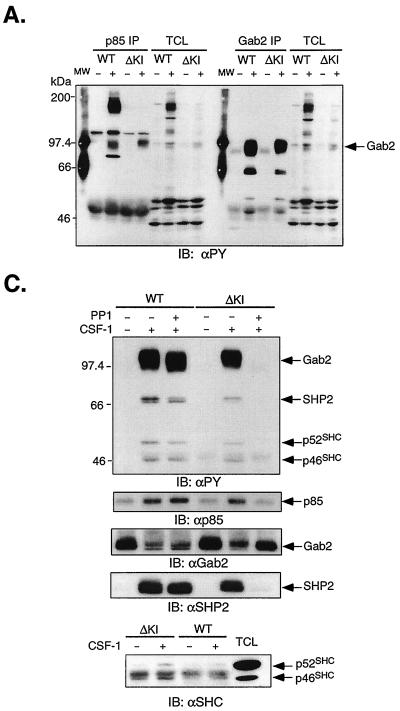
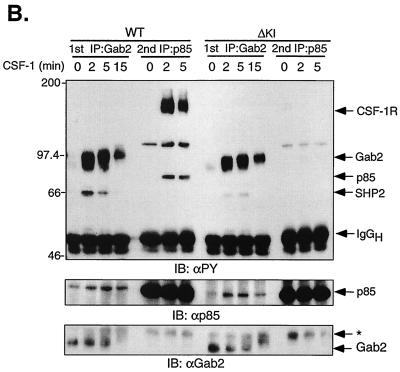
Recently, a multisite docking protein was identified that responds to a variety of cytokines by undergoing tyrosine phosphorylation and creating binding sites for signaling molecules including the p85 subunit of PI3-kinase (29, 61, 99). This is Gab2, which shares ≈35% identity with Gab1, another docking protein demonstrated to act downstream of RTKs and cytokine receptors (32, 33, 70, 80). To determine if Gab2 might be involved in linking ΔKI-CSF-1R to PI3-kinase, we compared the spectra of tyrosine-phosphorylated proteins that coimmunoprecipitated with p85 or Gab2 in 32D-WT-CSF-1R and 32D-ΔKI-CSF-1R cells. In WT cells, CSF-1 stimulated the tyrosine phosphorylation of p85 and induced its coimmunoprecipitation with a broad, tyrosine-phosphorylated band at 160 kDa and a second broad, tyrosine-phosphorylated band at 97 kDa (p97) (Fig. 11A). In contrast, p85 from ΔKI cells was not tyrosine phosphorylated and coprecipitated predominantly with p97. The 160-kDa band is most likely CSF-1R, consistent with its absence in ΔKI cells, as the ΔKI receptor lacks the p85 binding site. A third coprecipitating protein at 110 to 120 kDa was not pursued since CSF-1 did not consistently induce its tyrosine phosphorylation in ΔKI cells. p97 appears to comigrate with tyrosine-phosphorylated Gab2 detected in Gab2 immunoprecipitates (Fig. 11A) which was strongly stimulated by CSF-1 in both WT and ΔKI cells. Gab2 coprecipitated a tyrosine-phosphorylated protein at ≈66 kDa which is SHP-2 (Fig. 11C). To further confirm that p97 detected in p85 immunoprecipitates is Gab2, we took an immunodepletion approach. Lysates were first immunoprecipitated with anti-Gab2; the depleted lysates were then reimmunoprecipitated with anti-p85. The two sets of immunoprecipitates were analyzed side by side (Fig. 11B). The results showed that immunodepletion with anti-Gab2 effectively removed p97 from p85 immunoprecipitates from both WT and ΔKI cells. Note that the blot was overexposed so as to detect any residual p97. The blot was stripped and reprobed with anti-p85, confirming its presence in Gab2 immunoprecipitates. The association of p85 with Gab2 was transient and had decreased after 15 min of CSF-1 treatment (Fig. 11B).
FIG. 11.
Role of Gab2 in linking SFKs to PI3-kinase. (A) 32D-CSF-1R (clone 1) and 32D-ΔKI-CSF-1R (clone 1) cells were starved and stimulated (+) or not (−) with 10 nM CSF-1. Total cell lysates were immunoprecipitated (IP) with p85 or Gab2 antiserum and immunoblotted (IB) with anti-PY. TCL, 35 μg of total cell lysates from the same experiment that were also loaded; MW, molecular weight markers. (B) Cells were starved and stimulated with CSF-1 for the indicated times. Total cell lysates were first immunoprecipitated with Gab2 antiserum (1st IP); the depleted supernatant was subsequently reimmunoprecipitated with p85 antiserum (2nd IP). The immunoprecipitates were first blotted with anti-PY, stripped, and sequentially blotted with anti-p85 and anti-Gab2. The asterisk refers to a nonspecific band detected by the Gab2 antiserum. (C) Cells were starved and pretreated with 10 μM PP1 prior to CSF-1 stimulation for 2 min. Total cell lysates were immunoprecipitated with Gab2 antiserum and sequentially blotted with anti-PY, anti-p85, anti-Gab2, and anti-SHP-2. A parallel Gab2 immunoprecipitation was performed and blotted with a monoclonal antiShc antibody.
We next asked if Gab2 could be a link between SFKs and PI3-kinase during CSF-1-mediated signaling in ΔKI cells. 32D-WT-CSF-1R (clone 1) and 32D-ΔKI-CSF-1R (clone 1) cells were pretreated or not with 10 μM PP1 prior to CSF-1 stimulation (Fig. 11C). PP1 completely eliminated CSF-1-stimulated Gab2 tyrosine phosphorylation in ΔKI but not WT cells, an effect accompanied by the loss of CSF-1-inducible association of Gab2 with p85 and SHP-2 (Fig. 11C). The anti-PY blots in Fig. 11A and C also showed two bands migrating at 46 and 52 kDa upon CSF-1 treatment, which could correspond to p46Shc and p52Shc. Gab2 immunoprecipitates probed with a monoclonal Shc antibody to avoid obscuration by the IgG heavy chain showed the faint presence of p46Shc and p52Shc associated with Gab2 (Fig. 11C), similar to what has been reported elsewhere (29). Taken together, our data demonstrate that CSF-1 stimulated the tyrosine phosphorylation of Gab2 and its association with PI3-kinase in both WT and ΔKI cells, but only in the latter case does Src kinase activity appear to be required for these events to occur.
DISCUSSION
The primary aim of this work was to develop a detailed understanding of how CSF-1 activates the ERK/MAPK pathway in hematopoietic cells. IL-3-dependent myeloid progenitor cell lines expressing transduced CSF-1R have been used extensively to investigate CSF-1 signaling since they provide the appropriate cellular context and permit analysis of CSF-1R mutants (8, 46–48, 55, 65). The major conclusions of the present study are that CSF-1-mediated ERK activation in the 32D myeloid cell line depends on Ras and requires CSF-1-induced PI3-kinase activity which feeds into the ERK pathway at a point upstream of MEK/ERK, probably in parallel to Ras. Activation of PI3-kinase by the CSF-1R can occur by direct recruitment of PI3-kinase to the receptor or indirectly, by a Src-dependent mechanism. The Src pathway is mainly utilized at low CSF-1 receptor occupancy, such as one might find in early myeloid precursors, or when the direct pathway is not available. To the best of our knowledge, this is the first study demonstrating that an RTK such as the CSF-1R which binds PI3-kinase directly (68, 76) can also activate PI3-kinase by a separate mechanism independent of direct PI3-kinase–receptor interactions. Other RTKs such as the insulin/IGF-1 receptor (reviewed in reference 90), EGF receptor (70), and TrkA (33) also activate PI3-kinase via recruitment of docking proteins, but they either do not bind or weakly bind PI3-kinase.
We had previously demonstrated that costimulation of the CSF-1/CSF-1R and cAMP/PKA pathways, while producing a large synergistic enhancement of ERK activity, concomitantly suppressed the activity of Raf-1 (46). In addition, IL-3 also exhibited the same type of cross-talk between the ERK and cAMP/PKA pathways. Since then, Sutor et al. (79) have reported very similar findings for IL-3 acting on the FDC-P1 myeloid progenitor cell line. Here, we showed that CSF-1-stimulated-A-Raf activity, in addition to Raf-1, was suppressed at a time when ERK activity was significantly increased by cAMP-elevating agents and upregulated at a time when ERK activity was inhibited by PI3-kinase inhibitors (Fig. 1, Fig. 2, and reference 46). Since it is well established that PKA phosphorylates Raf-1 on sites that prevent interaction with Ras (96) and inhibit catalytic activity (59), the latter site being conserved in all three Rafs, our findings in the presence of cAMP are most consistent with the explanation that neither Raf-1 or A-Raf is responsible for relaying the activating signal to MEK/ERK.
In the absence of cAMP, dominant-negative A-Raf but not dominant-negative Raf-1 partially blocked ERK activity stimulated by CSF-1 or IL-3 (Fig. 2C). A concern with dominant-negative Rafs is that they may exert their inhibitory function by sequestering a shared upstream activator (Ras) away from the authentic MAPKKK, so that the block may not necessarily suggest a role for the Rafs themselves. This concern does not appear to be an issue for dominant-negative Raf-1 since its presence had no significant effect. As we do not have a way of determining if dominant-negative A-Raf competes with the authentic MAPKKK, the most straightforward explanation for our observations is that CSF-1-stimulated MEK/ERK activity is mediated by A-Raf-dependent and -independent mechanisms. We did not specifically investigate the role of B-Raf in this study, as B-Raf was previously found to have high constitutive activity which was not further stimulated by CSF-1 (46). Our findings regarding Raf-1 and B-Raf are in agreement with those of Sutor et al. (79). In contrast, they reported that IL-3-induced A-Raf activity in FDC-P1 cells is suppressed by PI3-kinase inhibition and resistant to cAMP elevation. For B-Raf the susceptibility to PKA inhibition may be isoform dependent, with the 68-kDa but not 95-kDa form of B-Raf being inhibited by PKA (91). Since 32D and FDC-P1 cells have somewhat different lineages (17, 28), it is conceivable that they contain different A-Raf variants with distinct PKA inhibition profiles, although we are not aware of any reports to this effect.
The results presented in this study strongly support a major role for PI3-kinase in the regulation of the MEK/ERK pathway by CSF-1. Evidence is obtained with two unrelated PI3-kinase inhibitors, wortmannin and LY294002, in a dose range generally accepted to be specific for PI3-kinase (26) and with a dominant-negative PI3-kinase, p110-N. Although the dependence on PI3-kinase appears to vary with signal strength in COS cells and fibroblasts (21, 92), in 32D-CSF-1R cells, PI3-kinase activity is required over a 100-fold variation in CSF-1 concentration (0.1 to 10 nM). Akt phosphorylation, used as a convenient readout for PI3-kinase activation, was induced at all doses of CSF-1 tested, indicating that CSF-1-dependent rather than basal PI3-kinase activity contributes to MEK/ERK activation. PI3-kinase activity was not necessary for Ras activation or activation of the events leading to Ras-GTP accumulation, namely, Shc tyrosine phosphorylation and subsequent association with Grb2. In some cell types, PI3-kinase functions as a downstream effector of Ras (71). However, we obtained no evidence to indicate that PI3-kinase is on a linear pathway between Ras and MEK, although we cannot exclude the possibility that PI3-kinase is activated by multiple mechanisms which might obscure blockade by 17NRas. Our results are in agreement with those of Genot et al. (27), who also used Akt activity as a readout for PI3-kinase activation in Jurkat T cells. They also showed that PI3-kinase did not activate Rac/PAK, a potential link to MEK (25, 42). Although we did not specifically investigate the role of Rac/PAK in CSF-1-mediated ERK activation, the available data do not strongly support such an involvement. While EGF-mediated ERK activity is potentiated by constitutively active PAK, it is not blocked by dominant-negative Rac or PAK, implying that EGF does not utilize Rac/PAK to activate ERK. Rather, Raf/MEK may represent a regulatory point for divergent signals (25, 92). The precise mechanism by which PI3-kinase regulates MEK/ERK activity in 32D-CSF-1R cells is unknown. We have some preliminary evidence suggesting that PI3-kinase may feed into the MEK/ERK pathway via atypical PKCs (A. W.-M. Lee and D. J. States, unpublished data). Another possibility is that a lipid product of PI3-kinase or a PI3-kinase protein effector acts at the level of MEK, e.g., by affecting the stability of a MEK/ERK complex.
An intriguing finding is our observation that the CSF-1R can activate PI3-kinase by more than one mechanism, revealed when the mutant CSF-1R lacking the KI was found to be still capable of activating Akt and regulating MEK/ERK in a PI3-kinase-sensitive manner. We used a combination of pharmacological and genetic approaches to conclude that the ΔKI receptor stimulated an SFK-dependent mechanism to activate both the Ras/ERK and PI3-kinase/Akt pathways. Although the SFK inhibitor PP1 can antagonize the EGF receptor kinase activity when added directly to in vitro assays (30), it is unlikely that our findings are due to inactivation of the CSF-1R kinase function because in vivo CSF-1R autophosphorylation was unaffected at PP1 concentrations up to 50 μM (Fig. 10B), consistent with studies showing that PP1 had no effect on EGF receptor autophosphorylation when added to cells (95). In addition, the inhibitory action of PP1 on ERK and Akt activation in cells expressing WT CSF-1R depended on receptor expression levels (Fig. 10A), an observation not easily explained by inhibition of CSF-1R kinase activity. In support of the PP1 results, CSF-1-induced ERK and Akt activities were also inhibited in ΔKI cells overexpressing dominant-negative SFKs. Presumably the SH2 and SH3 domains in these mutant SFKs compete with endogenous SFKs to bind to cellular proteins with Src binding sites, including the CSF-1R. Hence our findings with dominant-negative SFKs cannot be taken to imply that the SFK pathway leading to ERK and PI3-kinase activation depends on SFK binding to the CSF-1R. CSF-1 activates both Fyn and Lyn, but we have not examined expression of all SFKs. The question of which SFK member is utilized by the CSF-1R in 32D cells to initiate signaling is an important one, as SFKs play nonredundant roles in vascular endothelial growth factor-mediated angiogenesis (22) and T-cell receptor signaling (15).
How do SFKs couple the CSF-1R to PI3-kinase? There are a number of possibilities. Upon activation by binding to the CSF-1R, SFKs can directly recruit PI3-kinase, a mechanism that may be utilized by the T- and B-cell receptors (66, 67). Alternately, activated SFKs can phosphorylate docking/scaffolding proteins which contain binding sites for the SH2 domain of the p85 subunit of PI3-kinase. Examples of docking proteins implicated in RTK signaling are the IRS family members, which include Gab1/2 and the proto-oncogene product c-Cbl. 32D cells have no endogenous IRS-1, IRS-2 and IRS-4 (84) and anti-PY blots of p85 immunoprecipitates from ΔKI cells did not consistently reveal CSF-1-induced phosphorylation of proteins in the 110-kDa range (Fig. 11A and B), which would have suggested the presence of Gab1. Both Gab2 and c-Cbl are tyrosine phosphorylated in response to CSF-1 in WT and ΔKI cells (Fig. 11; A. W.-M. Lee, unpublished data). c-Cbl has been shown to function as a negative regulator of CSF-1 signaling by promoting multiubiquitination and hence degradation of the CSF-1R (50) similar to that observed for the PDGF and EGF receptors (52, 60). Such a role in negative regulation of signaling has not been reconciled with the observations that tyrosine-phosphorylated c-Cbl also recruits signaling molecules (e.g., PI3-kinase). It is not known if these signaling molecules bound to c-Cbl are sequestered and destined for degradation or are normally presented to their substrates and downstream effectors. In this report we have focused on Gab2 as a possible intermediary between SFKs and PI3-kinase. Data supporting a role for Gab2 are our observations that CSF-1 induced the association of Gab2 with p85/PI3-kinase in WT and ΔKI cells and in ΔKI cells, PP1 prevented CSF-1-stimulated Gab2 tyrosine phosphorylation and binding to the p85 subunit of PI3-kinase, suggesting possibly that Gab2 is a substrate for SFKs. In WT cells, PP1 had no effect on CSF-1-induced Gab2 tyrosine phosphorylation; Gab2 may be phosphorylated by the CSF-1R itself or by non-RTKs other than SFKs. In addition to being a downstream effector of Gab1, PI3-kinase can also function as an upstream activator since the PI3-kinase product PI-3,4,5-P3 binds to the pleckstrin homology (PH) domain of Gab1 and recruits it to the plasma membrane (58, 70). PI-3,4,5-P3 will likely also bind to the PH domain of Gab2, as the PH domains of Gab1 and Gab2 are 73% identical (29). Therefore, in WT cells, there is a potential for PI-3,4,5-P3 generated upon direct binding of PI3-kinase to Tyr 721 in the activated CSF-1R to recruit Gab2, a mechanism that is not available to the ΔKI-CSF-1R.
With regard to activation of the Ras/ERK pathway by the CSF-1R, likely mechanisms include binding of Grb2/Sos to Tyr 697 on the activated CSF-1R and the recruitment and tyrosine phosphorylation of Shc and subsequent association with Grb2/Sos. We have not observed coprecipitation of Shc with the CSF-1R, implying that Shc tyrosine phosphorylation does not require stable complex formation with the receptor. In ΔKI cells, the activating signal to Ras is presumably sent via Shc, as the route involving direct Grb2 binding is eliminated. In these cells, Src kinase activity was shown to play a significant role in mediating the tyrosine phosphorylation of Shc and association with Grb2 (Fig. 9A). SFKs can directly phosphorylate Shc (87), but the relationship may also be an indirect one since Shc is bound to Gab2 upon CSF-1 stimulation (Fig. 11C), similar to that previously reported for IL-3 (29), and Gab2 is phosphorylated in an SFK-dependent manner in ΔKI cells. Whether Gab2-bound Shc is involved in mediating Ras/ERK activation remains to be elucidated. When overexpressed, Gab1 and Gab2 potentiate cytokine-mediated ERK activation (29, 61, 80); moreover, embryonic fibroblasts from Gab1−/− mice show much diminished ERK activation in response to IL-6, EGF, PDGF, and hepatocyte growth factor (36). The tyrosine phosphatase SHP-2 binds to Gab1/2 (29, 32, 33, 61, 80) and is required for activation of Ras/ERK in response to a variety of signals (reviewed in reference 35). The published data concerning whether Gab1/2 and SHP-2 act in concert to influence Ras/ERK activation are confusing since mutation of the SHP-2 binding sites in Gab1/2 either had no effect (29) or blocked (14) growth factor-induced ERK activity. In 32D-CSF-1R cells, CSF-1 induced significant tyrosine phosphorylation of SHP-2, and SHP-2 coprecipitated Grb2 (data not shown) and Gab2 (Fig. 11C). Although tyrosine-phosphorylated SHP-2 has binding sites for Grb2/Sos (4, 54), Gab2 can bind Grb2 constitutively (29); hence we cannot exclude a three-way complex (SHP-2–Gab2–Grb2). Additional experiments utilizing Gab2 and SHP-2 mutants will be required to determine the contribution of Gab2 and SHP-2 to CSF-1-mediated ERK activation.
Src's contribution to CSF-1-mediated ERK and PI3K/Akt activation in 32D clones expressing WT CSF-1R appears to depend on receptor levels. This suggests that under conditions when fewer receptors are engaged such as what occurs in clone 4 (3,900 receptors/cell) or early myeloid precursors (2,000 receptors/cell) or when ligand concentration is low, fewer redundant pathways are activated and the Src-dependent mechanism plays a more prominent role. That different signaling pathways may require different signal strengths for activation has been noted previously. Soler et al. (78) found that whereas phospholipase C-γ1 and RasGAP were poorly phosphorylated by EGF in cells expressing <104 receptors/cell, Shc remained efficiently phosphorylated; all three proteins were strongly phosphorylated when >4 × 104 receptors/cell were occupied. Signal strength, determined by receptor levels (21) or ligand concentration (92), has also been shown to be a primary factor in the PI3-kinase contribution to ERK activation (see the introduction). Factors that might explain why the Src-dependent pathway was more important at low signal strength include differences in the stoichiometry of phosphorylation of the Src versus p85 and Grb2 binding sites, in the binding affinity of Src, p85, and Grb2 to their respective sites, and in the efficiency with which the different pathways couple to their downstream targets. Recently, it was reported that a Kit/SCF receptor mutant lacking the Src binding site when expressed in endothelial cells exhibited much reduced ligand-induced ERK activation (51), while the same mutant expressed in mast cells had an intact ERK response (82). Our findings suggest two possible explanations: in mast cells the mutant might have been able to activate redundant mechanisms not available in endothelial cells. Alternately, expression levels of Kit might have altered the signaling pathways used.
The experiments described in this report extend our current model for how the CSF-1R mediates activation of the Ras/ERK and PI3-kinase/Akt pathways in myeloid cells. Importantly, our findings point to the utilization of redundant mechanisms by the CSF-1R to activate downstream pathways that affect the cell's ability to proliferate and survive. A corollary of our observations is that PI3-kinase exerts its downstream effects in myeloid progenitors in part via the ERK pathway. Inhibitors of MEK/ERK and of PI3-kinase independently induced apoptosis and reduced proliferation in 32D-CSF-1R cells in the presence of CSF-1 (H.-L. Lin and A. W.-M. Lee, unpublished data). Further work will be needed to distinguish the ERK from the Akt branch in mediating PI3-kinase's survival and proliferative functions in these cells. The role of PI3-kinase in mediating CSF-1-induced DNA synthesis has been examined previously. Utilizing fibroblasts transfected with the CSF-1R, Roche et al. (69) showed that microinjected anti-p110α antibodies had no effect on CSF-1-induced DNA synthesis. In marked contrast, Ridley's group found that microinjected anti-p110α antibodies completely abrogated CSF-1-dependent DNA synthesis in BAC1 macrophages (88). Possibly, cell type difference played a role in determining the outcome of the two studies. Clearly there is a need to fully identify and characterize the signaling pathways activated by the CSF-1R in both myeloid precursors and mature macrophages and the cellular context that permits redundant regulation of growth and survival.
ACKNOWLEDGMENTS
This work was supported by National Science Foundation Research Planning grant MCB-9306519 (to A.W.-M.L.) and by National Institutes of Health grant RO1-DK48929 (to A.W.-M.L.).
We acknowledge the contribution of Raymond Yeh, who performed the Akt kinase assay shown in Fig. 7B, the advice of Johan de Rooij regarding the GST-RBD pulldown assay, and discussions with Masahiko Hibi regarding Gab2 immunoprecipitations. We also appreciate the continuing generosity of the Genetics Institute for recombinant human CSF-1 and Johannes Bos, Melanie Cobb, P. Di Fiore, Toshio Hirano, Gary Johnson, Ushio Kikkawa, Anke Klippel, Dan Link, Jeffrey Pessin, and Pam Schwartzberg for reagents.
REFERENCES
- 1.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;280:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 2.Alonso G, Koegl M, Mazurenko N, Courtneidge S. Sequence requirements for binding of Src family tyrosine kinases to activated growth factor receptors. J Biol Chem. 1995;270:9840–9848. doi: 10.1074/jbc.270.17.9840. [DOI] [PubMed] [Google Scholar]
- 3.Anderson S M, Jorgensen B. Activation of src-related tyrosine kinases by IL-3. J Immunol. 1995;155:1660–1670. [PubMed] [Google Scholar]
- 4.Bennett A M, Tang T L, Sugimoto S, Walsh C T, Neel B G. Protein-tyrosine-phosphatase SHPTP2 couples platelet-derived growth factor receptor beta to Ras. Proc Natl Acad Sci USA. 1994;91:7335–7339. doi: 10.1073/pnas.91.15.7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berra E, Diaz-Meco M T, Lozano J, Frutos S, Municio M M, Sanchez P, Sanz L, Moscat J. Evidence for a role of MEK and MAPK during signal transduction by protein kinase Cζ. EMBO J. 1995;14:6157–6163. doi: 10.1002/j.1460-2075.1995.tb00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broome M, Hunter T. Requirement for c-Src catalytic activity and the SH3 domain in platelet-derived growth factor BB and epidermal growth factor mitogenic signaling. J Biol Chem. 1996;271:16798–16806. doi: 10.1074/jbc.271.28.16798. [DOI] [PubMed] [Google Scholar]
- 7.Burgering B M, de Vries-Smits A M, Medema R H, van Weeren P C, Tertoolen L G, Bos J L. Epidermal growth factor induces phosphorylation of extracellular signal-regulated kinase 2 via multiple pathways. Mol Cell Biol. 1993;13:7248–7256. doi: 10.1128/mcb.13.12.7248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlberg K, Rohrschneider L R. Characterization of a novel tyrosine phosphorylated 100-kDa protein that binds to SHP-2 and phosphatidylinositol 3′-kinase in myeloid cells. J Biol Chem. 1997;272:15943–15950. doi: 10.1074/jbc.272.25.15943. [DOI] [PubMed] [Google Scholar]
- 9.Cheatham B, Vlahos C J, Cheatham L, Wang L, Blenis J, Kahn C R. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol Cell Biol. 1994;14:4902–4911. doi: 10.1128/mcb.14.7.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H E, Chang S, Trub T, Neel B G. Regulation of colony-stimulating factor 1 receptor signaling by the SH2 domain-containing tyrosine phosphatase SHPTP1. Mol Cell Biol. 1996;16:3685–3697. doi: 10.1128/mcb.16.7.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng M, Wang D, Roussel M. Expression of c-myc in response to colony-stimulating factor-1 requires mitogen-activated protein kinase kinase-1. J Biol Chem. 1999;274:6553–6558. doi: 10.1074/jbc.274.10.6553. [DOI] [PubMed] [Google Scholar]
- 12.Courtneidge S, Dhand R, Pilat D, Twamley G, Waterfield M, Roussel M. Activation of Src family kinases by colony stimulating factor-1, and their association with its receptor. EMBO J. 1993;12:943–950. doi: 10.1002/j.1460-2075.1993.tb05735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cross D, Alessi D, Vandenheede J, McDowell H, Hundal H, Cohen P. The inhibition of glycogen synthase kinase-3 by insulin or insulin-like growth factor 1 in the rat skeletal muscle cell line L6 is blocked by wortmannin but not by rapamycin: evidence that wortmannin blocks activation of the mitogen-activated protein kinase pathway in L6 cells between Ras and Raf. Biochem J. 1994;303:21–26. doi: 10.1042/bj3030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunnick J, Dorsey J, Munoz-Antonia T, Mei L, Wu J. Requirement of SHP2 binding to Grb2-associated binder-1 for mitogen-activated protein kinase activation in response to lysophosphatidic acid and epidermal growth factor. J Biol Chem. 2000;275:13842–13848. doi: 10.1074/jbc.275.18.13842. [DOI] [PubMed] [Google Scholar]
- 15.Denny M, Patai B, Straus D. Differential T-cell antigen receptor signaling mediated by the Src family kinases Lck and Fyn. Mol Cell Biol. 2000;20:1426–1435. doi: 10.1128/mcb.20.4.1426-1435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Rooij J, Bos J L. Minimal Ras-binding domain of Raf1 can be used as an activation-specific probe for Ras. Oncogene. 1997;14:623–625. doi: 10.1038/sj.onc.1201005. [DOI] [PubMed] [Google Scholar]
- 17.Dexter T M, Garland J, Scott D, Scolnick E, Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980;152:1036–1047. doi: 10.1084/jem.152.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Fiore P, Pierce J, Kraus M, Segatto O, King C, Aaronson S A. ErbB-2 is a potent oncogene when overexpressed in NIH 3T3 cells. Science. 1987;237:178–182. doi: 10.1126/science.2885917. [DOI] [PubMed] [Google Scholar]
- 19.Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- 20.Downward J, Graves J, Warne P, Rayter S, Cantrell D. Stimulation of p21ras upon T-cell activation. Nature. 1990;346:719–723. doi: 10.1038/346719a0. [DOI] [PubMed] [Google Scholar]
- 21.Duckworth B, Cantley L. Conditional inhibition of the mitogen-activated protein kinase cascade by wortmannin. J Biol Chem. 1997;272:27665–27670. doi: 10.1074/jbc.272.44.27665. [DOI] [PubMed] [Google Scholar]
- 22.Eliceiri B, Paul R, Schwartzberg P, Hood J, Leng J, Cheresh D. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4:915–924. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- 23.English B. Expression of the activated (Y501-F501) hck tyrosine kinase in 32Dcl3 myeloid cells prolongs survival in the absence of IL-3 and blocks granulocytic differentiation in response to G-CSF. J Leukoc Biol. 1996;60:667–673. doi: 10.1002/jlb.60.5.667. [DOI] [PubMed] [Google Scholar]
- 24.Fabian J, Daar I, Morrison D K. Critical tyrosine residues regulate the enzymatic and biological activity of Raf-1 kinase. Mol Cell Biol. 1993;13:7170–7179. doi: 10.1128/mcb.13.11.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frost J A, Steen H, Shapiro P, Lewis T, Ahn N, Shaw P, Cobb M H. Cross-cascade activation of ERKs and ternary complex factors by Rho family proteins. EMBO J. 1997;16:6426–6438. doi: 10.1093/emboj/16.21.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fruman D, Meyers R, Cantley L. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 27.Genot E, Reif K, Beach S, Kramer I, Cantrell D. p21ras initiates Rac-1 but not phosphatidylinositol 3-kinase/PKB mediated signaling pathways in T lymphocytes. Oncogene. 1998;17:1731–1738. doi: 10.1038/sj.onc.1202101. [DOI] [PubMed] [Google Scholar]
- 28.Greenberger J S, Sakakeeny M, Humphries R, Eaves C, Eckner R. Demonstration of permanent factor-dependent multipotential (erythroid/neutrophil/basophil) hematopoietic progenitor cell lines. Proc Natl Acad Sci USA. 1983;80:2931–2935. doi: 10.1073/pnas.80.10.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu H, Pratt J, Burakoff S, Neel B. Cloning of p97/Gab2, the major SHP2-binding protein in hematopoietic cells, reveals a novel pathway for cytokine-induced gene activation. Mol Cell. 1998;2:729–740. doi: 10.1016/s1097-2765(00)80288-9. [DOI] [PubMed] [Google Scholar]
- 30.Hanke J, Gardner J, Dow R, Changelian P, Brissette W, Weringer E, Pollok B, Connelly P. Discovery of a novel, potent and src family-selective tyrosine kinase inhibitor. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 31.Hawes B, Luttrell L, van Biesen T, Lefkowitz R J. Phosphatidylinositol 3-kinase is an early intermediate in the Gβγ-mediated mitogen-activated protein kinase signaling pathway. J Biol Chem. 1996;271:12133–12136. doi: 10.1074/jbc.271.21.12133. [DOI] [PubMed] [Google Scholar]
- 32.Holgado-Madruga M, Emlet D, Moscatello D, Godwin A, Wong A J. A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature. 1996;379:560–564. doi: 10.1038/379560a0. [DOI] [PubMed] [Google Scholar]
- 33.Holgado-Madruga M, Koscatello D, Emlet D, Dietrich R, Wong A. Grb2-associated binder-1 mediates phosphatidylinositol 3-kinase activation and the promotion of cell survival by nerve growth factor. Proc Natl Acad Sci USA. 1997;94:12419–12424. doi: 10.1073/pnas.94.23.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunter T. The Croonian Lecture 1997. The phosphorylation of proteins on tyrosine: its role in cell growth and disease. Philos Trans R Soc Lond B. 1998;353:583–605. doi: 10.1098/rstb.1998.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huyer G, Alexander D. Immune signaling: SHP-2 docks at multiple ports. Curr Biol. 1999;9:R129–R132. doi: 10.1016/s0960-9822(99)80080-3. [DOI] [PubMed] [Google Scholar]
- 36.Itoh M, Yoshida Y, Nishida K, Narimatsu M, Hibi M, Hirano T. Role of Gab1 in heart, placenta, and skin development and growth factor- and cytokine-induced extracellular signal-regulated kinase mitogen-activated protein kinase activation. Mol Cell Biol. 2000;20:3695–3704. doi: 10.1128/mcb.20.10.3695-3704.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jelinek T, Dent P, Sturgill T W, Weber M J. Ras-induced activation of Raf-1 is dependent on tyrosine phosphorylation. Mol Cell Biol. 1996;16:1027–1034. doi: 10.1128/mcb.16.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kacinski B M. CSF-1 and its receptor in breast carcinomas and neoplasms of the female reproductive tract. Mol Reprod Dev. 1997;46:71–74. doi: 10.1002/(SICI)1098-2795(199701)46:1<71::AID-MRD11>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 39.Kaplan K, Bibbins K, Swerdlow J, Arnaud M, Morgan D, Varmus H. Association of the amino-terminal half of c-Src with focal adhesions alters their properties and is regulated by phosphorylation of tyrosine 527. EMBO J. 1994;13:4745–4756. doi: 10.1002/j.1460-2075.1994.tb06800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature. 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- 41.Kelley T, Graham M, Doseff A, Pomerantz R, Lau S, Ostrowski M, Franke T, Marsh C. Macrophage colony-stimulating factor promotes cell survival through Akt/protein kinase B. J Biol Chem. 1999;274:26393–26398. doi: 10.1074/jbc.274.37.26393. [DOI] [PubMed] [Google Scholar]
- 42.King A, Sun H, Diaz B, Barnard D, Miao W, Bagrodia S, Marshall M S. The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of Serine 338. Nature. 1998;396:180–183. doi: 10.1038/24184. [DOI] [PubMed] [Google Scholar]
- 43.Klingmuller U, Wu H, Gsiao J, Toker A, Duckworth B, Cantley L, Lodish H F. Identification of a novel pathway important for proliferation and differentiation of primary erythroid progenitors. Proc Natl Acad Sci USA. 1997;94:3016–3021. doi: 10.1073/pnas.94.7.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klippel A, Reinhard C, Kavanaugh W, Apell G, Escobedo M, Williams L T. Membrane localization of phosphatidylinositol 3-kinase is sufficient to activate multiple signal-transducing kinase pathways. Mol Cell Biol. 1996;16:4117–4127. doi: 10.1128/mcb.16.8.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee A W-M. Signal transduction by the colony stimulating factor-1 receptor; comparison to other receptor tyrosine kinases. Curr Top Cell Regul. 1992;32:73–181. doi: 10.1016/b978-0-12-152832-4.50005-7. [DOI] [PubMed] [Google Scholar]
- 46.Lee A W-M. Synergistic activation of mitogen-activated protein kinase by cyclic AMP and myeloid growth factors opposes cyclic AMP's growth-inhibitory effects. Blood. 1999;93:537–553. [PubMed] [Google Scholar]
- 47.Lee A W-M, Nienhuis A W. Mechanism of kinase activation in the receptor for colony-stimulating factor 1. Proc Natl Acad Sci USA. 1990;87:7270–7274. doi: 10.1073/pnas.87.18.7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee A W-M, Nienhuis A W. Functional dissection of structural domains in the receptor for colony-stimulating factor-1. J Biol Chem. 1992;267:16472–16483. [PubMed] [Google Scholar]
- 49.Lee A W-M, Nambirajan S, Moffat J G. CSF-1-activates MAPK-dependent and p53-independent pathways to induce growth arrest of hormone-dependent human breast cancer cells. Oncogene. 1999;18:7475–7492. doi: 10.1038/sj.onc.1203123. [DOI] [PubMed] [Google Scholar]
- 50.Lee P S, Wang Y, Dominguez M, Yeung Y, Murphy M, Bowtell D, Stanley E R. The Cbl protooncoprotein stimulates CSF-1 receptor multiubiquitination and endocytosis, and attenuates macrophage proliferation. EMBO J. 1999;18:3616–3628. doi: 10.1093/emboj/18.13.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lennartsson J, Blume-Jensen P, Hermanson M, Ponten E, Carlberg M, Ronnstrand L. Phosphorylation of Shc by Src family kinases is necessary for stem cell factor/c-kit mediated activation of the Ras/MAP kinase pathway and c-fos induction. Oncogene. 1999;18:5546–5553. doi: 10.1038/sj.onc.1202929. [DOI] [PubMed] [Google Scholar]
- 52.Levkowitz G, Waterman H, Ettenberg S, Katz M, Tsygankov A, Alroy I, Lavi S, Iwai K, Reiss Y, Ciechanover A, Lipkowitz S, Yarden Y. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol Cell. 1999;4:1029–1040. doi: 10.1016/s1097-2765(00)80231-2. [DOI] [PubMed] [Google Scholar]
- 53.Lewis T, Shapiro P, Ahn N G. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- 54.Li W, Nishimura R, Kashishian A, Batzer A G, Kim W J, Cooper J A, Schlessinger J. A new function for a phosphotyrosine phosphatase: linking GRB2-Sos to a receptor tyrosine kinase. Mol Cell Biol. 1994;14:509–517. doi: 10.1128/mcb.14.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lioubin M, Myles G, Carlberg K, Bowtell D, Rohrschneider L. Shc, Grb2, Sos1, and a 150-kilodalton tyrosine-phosphorylated protein form complexes with Fms in hematopoietic cells. Mol Cell Biol. 1994;14:5682–5691. doi: 10.1128/mcb.14.9.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lioubin M, Algate P, Tsai S, Carlberg K, Aebersold A, Rohrschneider L R. p150Ship, a signal transduction molecule with inositol polyphosphate-5-phosphatase activity. Genes Dev. 1996;10:1084–1095. doi: 10.1101/gad.10.9.1084. [DOI] [PubMed] [Google Scholar]
- 57.Marais R, Light Y, Paterson H F, Marshall C J. Ras recruits Raf-1 to the plasma membrane for activation by tyrosine phosphorylation. EMBO J. 1995;14:3136–3145. doi: 10.1002/j.1460-2075.1995.tb07316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maroun C, Holgado-Madruga M, Royal I, Naujokas M, Fournier T, Wong A, Park M. The Gab1 PH domain is required for localization of Gab1 at sites of cell-cell contact and epithelial morphogenesis downstream from the Met receptor tyrosine kinase. Mol Cell Biol. 1999;19:1784–1799. doi: 10.1128/mcb.19.3.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mischak H, Seitz T, Janosch P, Eulitz M, Steen H, Schellerer M, Philipp A, Kolch W. Negative regulation of Raf-1 by phosphorylation of serine 621. Mol Cell Biol. 1996;16:5409–5418. doi: 10.1128/mcb.16.10.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miyake S, Lupher M, Druker B, Band H. The tyrosine kinase regulator Cbl enhances the ubiquitination and degradation of the platelet-derived growth factor receptor alpha. Proc Natl Acad Sci USA. 1998;95:7927–7932. doi: 10.1073/pnas.95.14.7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nishida K, Yoshida Y, Itoh M, Fukada T, Ohtani T, Shirogane T, Atsumi T, Takahashi-Tezuka M, Ishihara K, Hibi M, Hirano T. Gab-family adapter proteins act downstream of cytokine and growth factor receptors and T- and B-cell antigen receptors. Blood. 1999;93:1809–1816. [PubMed] [Google Scholar]
- 62.Novak U, Nice E, Hamilton J, Paradiso J. Requirement for Y706 of the murine (or Y708 of the human) CSF-1 receptor for STAT1 activation in response to CSF-1. Oncogene. 1996;13:2607–2613. [PubMed] [Google Scholar]
- 63.Nowicki A, Szenajch J, Ostrowska G, Wojtowicz A, Wojtowicz K, Kruszewski A, Maruszynski M, Aukerman S, Wiktor-Jedrzejczak W. Impaired tumor growth in colony-stimulating factor 1 (CSF-1)-deficient, macrophage-deficient op/op mouse: evidence for a role of CSF-1-dependent macrophages in formation of tumor stroma. Int J Cancer. 1996;65:112–119. doi: 10.1002/(SICI)1097-0215(19960103)65:1<112::AID-IJC19>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 64.Petritsch C, Woscholski R, Edelmann H, Parker P, Ballou L. Selective inhibition of p70s6 kinase activation by phosphatidylinositol 3-kinase inhibitors. Eur J Biochem. 1995;230:431–438. doi: 10.1111/j.1432-1033.1995.0431h.x. [DOI] [PubMed] [Google Scholar]
- 65.Pierce J, Marco E, Cox G, Lombardi D, Ruggiero M, Varesio L, Wong L, Choudhury G, Sakaguchi A, DiFiore P, Aaronson S. Macrophage-colony-stimulating factor (CSF-1) induces proliferation, chemotaxis, and reversible monocytic differentiation in myeloid progenitor cells transfected with the human c-fms/CSF-1 receptor cDNA. Proc Natl Acad Sci USA. 1990;87:5613–5617. doi: 10.1073/pnas.87.15.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pleiman C, Hertz W, Cambier J C. Activation of phosphatidylinositol 3-kinase by Src-family kinase SH3 binding to the p85 subunit. Science. 1994;263:1609–1612. doi: 10.1126/science.8128248. [DOI] [PubMed] [Google Scholar]
- 67.Prasad K, Janssen O, Kapeller R, Raab M, Cantley L, Rudd C. Src-homology 3 domain of protein kinase p59fyn mediates binding to phosphatidylinositol 3-kinase in T cells. Proc Natl Acad Sci USA. 1993;90:7366–7370. doi: 10.1073/pnas.90.15.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reedijk M, Liu X, van der Geer P, Letwin K, Waterfield M, Hunter T, Pawson T. Tyr721 regulates specific binding of the CSF-1 receptor kinase insert to PI 3′-kinase SH2 domains: a model for SH2-mediated receptor-target interactions. EMBO J. 1992;11:1365–1372. doi: 10.1002/j.1460-2075.1992.tb05181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roche S, Koegl M, Courtneidge S A. The phosphatidylinositol 3-kinase alpha is required for DNA synthesis induced by some, but not all, growth factors. Proc Natl Acad Sci USA. 1994;91:9185–9189. doi: 10.1073/pnas.91.19.9185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rodrigues G, Falasca M, Zhang Z, Ong S, Schlessinger J. A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Mol Cell Biol. 2000;20:1448–1459. doi: 10.1128/mcb.20.4.1448-1459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rodriguez-Viciana P, Warne P H, Dhand R, Vanhaesebroeck B, Gout I, Fry M J, Waterfield M D, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 72.Roth P, Stanley E R. The biology of CSF-1 and its receptor. Curr Top Microbiol Immunol. 1992;181:141–167. doi: 10.1007/978-3-642-77377-8_5. [DOI] [PubMed] [Google Scholar]
- 73.Roussel M F. Signal transduction by the macrophage-colony-stimulating factor receptor (CSF-1R) J Cell Sci Suppl. 1994;18:105–108. doi: 10.1242/jcs.1994.supplement_18.15. [DOI] [PubMed] [Google Scholar]
- 74.Scheid M P, Duronio V. Phosphatidylinositol 3-OH kinase activity is not required for activation of mitogen-activated protein kinase by cytokines. J Biol Chem. 1996;271:18134–18139. doi: 10.1074/jbc.271.30.18134. [DOI] [PubMed] [Google Scholar]
- 75.Schonwasser D, Marais R, Marshall C J, Parker P J. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol. 1998;18:790–798. doi: 10.1128/mcb.18.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shurtleff S, Downing J, Rock C, Hawkins S, Roussel M, Sherr C. Structural features of the colony-stimulating factor 1 receptor that affect its association with phosphatidylinositol 3-kinase. EMBO J. 1990;9:2415–2421. doi: 10.1002/j.1460-2075.1990.tb07417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smith J D, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci USA. 1995;92:8264–8268. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Soler C, Alvarez C, Beguinot L, Carpenter G. Potent SHC tyrosine phosphorylation by epidermal growth factor at low receptor density or in the absence of receptor autophosphorylation sites. Oncogene. 1994;9:2207–2215. [PubMed] [Google Scholar]
- 79.Sutor S, Vroman B, Armstrong E, Abraham R, Karnitz L M. A phosphatidylinositol 3-kinase-dependent pathway that differentially regulates c-raf and A-raf. J Biol Chem. 1999;274:7002–7010. doi: 10.1074/jbc.274.11.7002. [DOI] [PubMed] [Google Scholar]
- 80.Takahashi-Tezuka M, Yoshida Y, Fukada T, Ohtani T, Yamanaka Y, Nishida K, Nakajima K, Sibi M, Hirano T. Gab1 acts as an adapter molecule linking the cytokine receptor gp130 to ERK mitogen-activated protein kinase. Mol Cell Biol. 1998;18:4109–4117. doi: 10.1128/mcb.18.7.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taylor S J, Shalloway D. Cell cycle dependent activation of Ras. Curr Biol. 1996;6:1621–1627. doi: 10.1016/s0960-9822(02)70785-9. [DOI] [PubMed] [Google Scholar]
- 82.Timokhina I, Kissel H, Stella G, Besmer P. Kit signaling through PI3-kinase and src kinase pathways: an essential role for Rac1 and JNK activation in mast cell proliferation. EMBO J. 1998;17:6250–6262. doi: 10.1093/emboj/17.21.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Toker A, Meyers M, Reddy K K, Falck J, Aneja R, Aneja S, Parra A, Burns D, Ballas L, Cantley L C. Activation of protein kinase C family members by the novel polyphosphoinositides PtdIns-3,4-P2 and PtdIns-3,4,5-P3. J Biol Chem. 1994;269:32358–32367. [PubMed] [Google Scholar]
- 84.Uchida T, Myers M, White M. IRS-4 mediates protein kinase B signaling during insulin stimulation without promoting antiapoptosis. Mol Cell Biol. 2000;20:126–138. doi: 10.1128/mcb.20.1.126-138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ueki K, Matsud S, Tobe K, Gotoh Y, Tamemoto H, Yachi M, Akanuma Y, Yazaki Y, Nishida E, Kadowaki T. Feedback regulation of mitogen-activated protein kinase kinase kinase activity of c-Raf-1 by insulin and phorbol ester stimulation. J Biol Chem. 1994;269:15756–15761. [PubMed] [Google Scholar]
- 86.van der Geer P, Hunter T. Mutation of Tyr697, a Grb2-binding site, and Tyr721, a PI 3-kinase binding site, abrogates signal transduction by the murine CSF-1 receptor expressed in Rat-2 fibroblasts. EMBO J. 1993;12:5161–5172. doi: 10.1002/j.1460-2075.1993.tb06211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van der Geer P, Wiley S, Gish G, Pawson T. The Shc adaptor protein is highly phosphorylated at conserved, twin tyrosine residues (Y239/240) that mediate protein-protein interactions. Curr Biol. 1996;6:1435–1444. doi: 10.1016/s0960-9822(96)00748-8. [DOI] [PubMed] [Google Scholar]
- 88.Vanhaesebroeck B, Jones G, Allen W, Zicha D, Hooshmand-Rad R, Sawyer C, Wells C, Waterfield M, Ridley A. Distinct PI(3)Ks mediate mitogenic signalling and cell migration in macrophages. Nat Cell Biol. 1999;1:69–71. doi: 10.1038/9045. [DOI] [PubMed] [Google Scholar]
- 89.Varticovski L, Druker B, Morrison D, Cantley L, Roberts T. The colony stimulating factor-1 receptor associates with and activates phosphatidylinositol-3 kinase. Nature. 1989;342:699–702. doi: 10.1038/342699a0. [DOI] [PubMed] [Google Scholar]
- 90.Virkamaki A, Ueki K, Kahn C R. Protein-protein interaction in insulin signaling and the molecular mechanisms of insulin resistance. J Clin Investig. 1999;103:931–943. doi: 10.1172/JCI6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vossler M R, Yao H, York R D, Pan M G, Rim C S, Stork P J. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell. 1997;89:73–82. doi: 10.1016/s0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- 92.Wennstrom S, Downward J. Role of phosphoinositide 3-kinase in activation of Ras and mitogen-activated protein kinase by epidermal growth factor. Mol Cell Biol. 1999;19:4279–4288. doi: 10.1128/mcb.19.6.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wiktor-Jedrzejczak W, Bartocci A, Ferrante A, Ahmed-Ansari A, Sell K, Pollard J, Stanley E R. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci USA. 1990;87:4828–4832. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wiktor-Jedrzejczak W, Ratajczak M, Ptasznik A, Sell K, Ahmed-Ansari A, Ostertag W. CSF-1 deficiency in the op/op mouse has differential effects on macrophage populations and differentiation stages. Exp Hematol. 1992;20:1004–1010. [PubMed] [Google Scholar]
- 95.Wilde A, Beattie E, Lem L, Riethof D, Liu S, Mobley W, Soriano P, Brodsky F. EGF receptor signaling stimulates Src kinase phosphorylation of clathrin, influencing clathrin redistribution and EGF uptake. Cell. 1999;96:677–687. doi: 10.1016/s0092-8674(00)80578-4. [DOI] [PubMed] [Google Scholar]
- 96.Wu J, Dent P, Jelinek T, Wolfman A, Weber M, Sturgill T. Inhibition of the EGF-activated MAP kinase signaling pathway by adenosine 3′5′-monophosphate. Science. 1993;262:1065–1069. doi: 10.1126/science.7694366. [DOI] [PubMed] [Google Scholar]
- 97.Xu W, Doshi A, Lei M, Eck M, Harrison S C. Crystal structures of c-Src reveal features of its autoinhibitory mechanism. Mol Cell. 1999;3:629–638. doi: 10.1016/s1097-2765(00)80356-1. [DOI] [PubMed] [Google Scholar]
- 98.Yusoff P, Hamilton J, Nolan R, Phillips W. Haematopoietic colony stimulating factors CSF-1 and GM-CSF increase phosphatidylinositol 3-kinase activity in murine bone marrow-derived macrophages. Growth Factors. 1994;10:181–192. doi: 10.3109/08977199409000236. [DOI] [PubMed] [Google Scholar]
- 99.Zhao C, Yu D, Shen R, Feng G-S. Gab2, a new pleckstrin homology domain-containing adapter protein, acts to uncouple signaling from ERK kinase to Elk-1. J Biol Chem. 1999;274:19649–19654. doi: 10.1074/jbc.274.28.19649. [DOI] [PubMed] [Google Scholar]