Summary
Lipids play crucial roles in signal transduction, contribute to the structural integrity of cellular membranes, and regulate energy metabolism. Questions remain as to which lipid species maintain metabolic homeostasis and which disrupt essential cellular functions leading to metabolic disorders. Here we discuss recent advances in understanding lipid metabolism with a focus on catabolism, synthesis, and signaling. Technical advances including functional genomics, metabolomics, lipidomics, lipid-protein interaction maps, and advances in mass spectrometry have uncovered new ways to prioritize molecular mechanisms mediating lipid function. By reviewing what is known about the distinct effects of specific lipid species in physiological pathways, we provide a framework for understanding newly identified targets regulating lipid homeostasis with implications for ameliorating metabolic diseases.
Keywords: Lipids, lipotoxicity, lipid metabolism, obesity, free fatty acids (FFAs), cellular metabolism, triacylglycerol accumulation, lipidomics, cancer
eTOC Blurb
Yoon et al., discuss recent advances in understanding lipid metabolism with a focus on catabolism, synthesis, and signaling. Through the lens of recent technical advances including functional genomics, metabolomics, lipidomics, lipid-protein interaction maps, and advances in mass spectrometry, the authors discuss new ways to prioritize molecular mechanisms mediating lipid function.
INTRODUCTION
Cellular lipids contain a diverse collection of individual molecular components that give rise to many tens of thousands of lipid species, the compendium of the cell, collectively called the lipidome (Yang et al., 2009). Lipid metabolism affects many cellular processes critical for homeostasis including membrane synthesis and the use of lipids (i.e. triglycerides) as an energy store. Fatty acids (FAs) are essential lipids that constitute the major structural components of membrane lipids (i.e., glycerophospholipids and sphingolipids) while also serving as an important energy source through mitochondria-mediated beta-oxidation and tricarboxylic acid (TCA) cycle catabolism.
Excessive levels of circulating lipids have been linked to metabolic diseases (Musunuru and Kathiresan, 2016, 2019) and cancer (Beloribi-Djefaflia et al., 2016). The harmful effects of prolonged exposure to excess lipids is referred to as “lipotoxicity” (Lytrivi et al., 2020; Sharma and Alonso, 2014) -- a term first coined by Roger Unger and colleagues to explain the inhibition of pancreatic β-cell function and the development of type 2 diabetes in the pancreatic islets of rats overloaded with lipids (Lee et al., 1994). The molecular mechanisms underlying lipotoxicity include endoplasmic reticulum (ER) stress, oxidative stress, mitochondrial dysfunction, impaired autophagy, and inflammation (Lytrivi et al., 2020). Specifically, in metabolic disorders where there is an imbalance between the uptake or synthesis and consumption of fatty acids (FAs), lipid intermediates accumulate intracellularly resulting in cellular dysfunction and death in diverse tissues including the kidney, brain, skeletal muscle, and heart (Goldberg et al., 2012). Effectively channeling free FAs to structural lipids, lipid droplets, or to the mitochondria for beta-oxidation has the potential to mitigate harmful effects of lipid accumulation, leading to new questions: (i) How do imbalances in the uptake or synthesis of lipids and their consumption or destruction affect downstream signaling pathways? (ii) How does the intracellular accumulation of lipid intermediates directly contribute to cellular dysfunction?
In this review, we highlight key roles for lipids across diverse cell types in order to provide a framework for understanding the mechanisms that link excess lipids and lipotoxicity to dysfunction in metabolic diseases including chronic kidney disease, fatty liver, heart failure, obesity, neurodegeneration and cancer. Understanding the mechanisms regulating the fate of lipids within cells will provide clues into tightly regulated mechanisms of homeostasis. We discuss fatty acid synthesis, uptake, degradation, and signaling in the context of homeostasis as well as in disease states (Figure 1). Finally, we highlight emerging technologies including functional genomics, lipid-protein interaction maps, and advances in mass spectrometry as tools to identify therapeutic targets for metabolic diseases.
Figure 1. Overview of Lipid Metabolism.
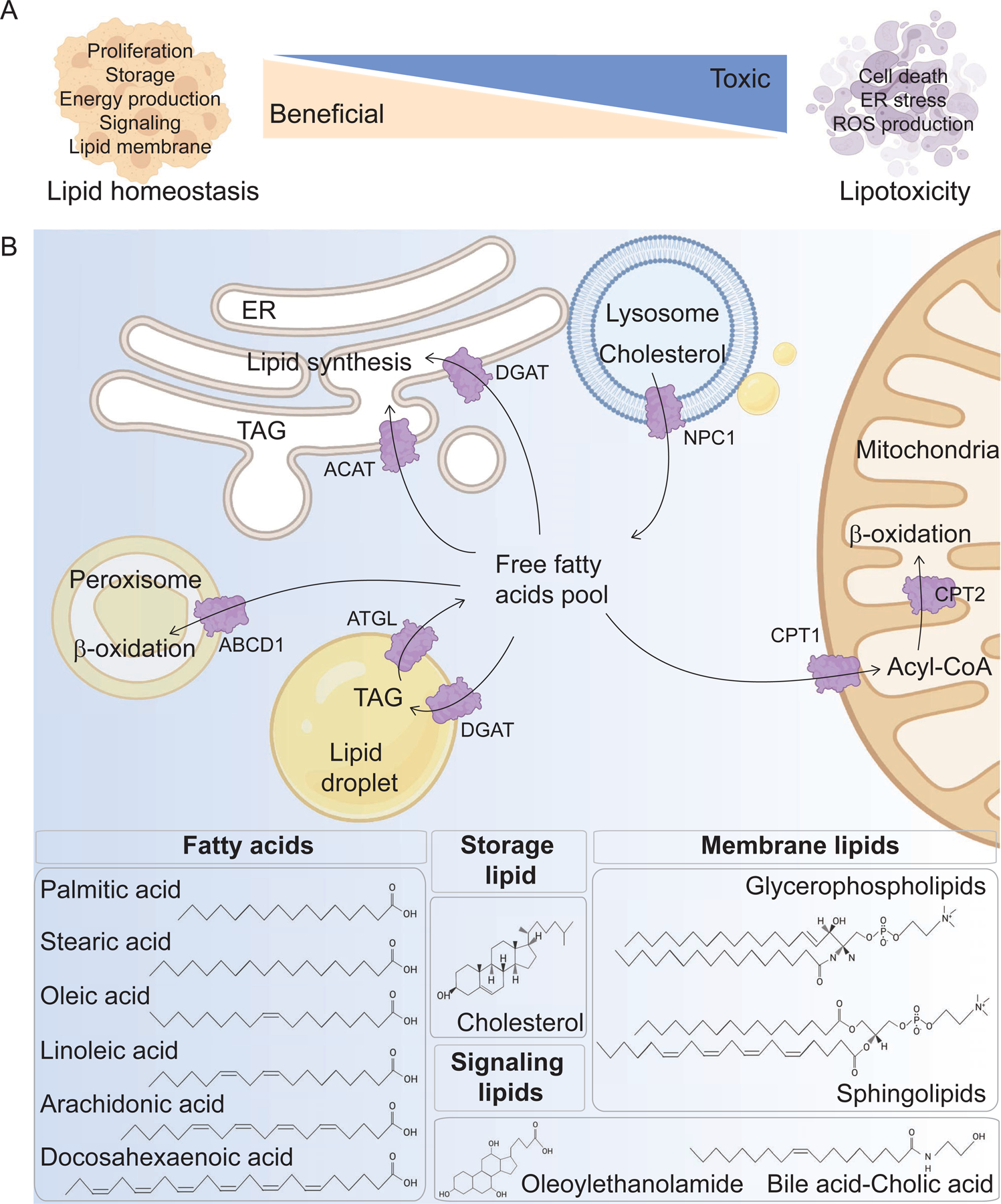
(A) A systematic approach is necessary to categorize lipids as beneficial or lipotoxic. Bioactive lipid species have different roles in cellular responses, including beneficial roles in lipid homeostasis through the regulation of proliferation, storage, energy production, cell signaling, and lipid membrane composition. Lipids play a lipotoxic role by influencing cell death, ER stress, and ROS production. It is important to understand which lipid species maintain metabolic homeostasis and which disrupt essential cellular functions leading to metabolic disorders. (B) The role and structure of lipids are determined by uptake, synthesis, storage and consumption across different cellular organelles. In the anabolic pathway, lipids are taken to the ER and cytosol for lipid synthesis. For catabolism, lipids are transmitted to the mitochondria and peroxisomes. These pathways generate fatty acids, storage lipids, such as cholesterol, and triglycerides, signaling lipids containing N-acetylethanolamines (oleoylethanolamide) and cholesterol-derived bile acids. Membrane lipids include glycerophospholipids and sphingolipids. Fatty acids are building blocks of all lipids, and palmitic acid, stearic acid, oleic acid, linoleic acid, arachidonic acid, docosahexaneoic acid are illustrated as examples for saturated, monounsaturated, and polyunsaturated FAs. ABCD1, ATP Binding Cassette Subfamily D Member 1; ACAT, acyl-CoA:cholesterol acyltransferase; ATGL, adipose triglyceride lipase; CPT, carnitine palmitoyl-transferase; DGAT1, diacylglycerol O-acyltransferase 1; NPC1, Niemann Pick type-C 1; TAG, triacylglycerol.
LIPID BIOLOGY
Over 40,000 lipids have been identified across the kingdoms of life (http://www.lipidmaps.org), yet we still have an incomplete understanding of the roles most of these lipids play in cell biology and physiology. By definition, lipids are complex molecules generated from simpler constituents through enzymatic reactions. Typically, each lipid consists of a head group with a unique chemical composition that is esterified to hydrophobic tails made up of fatty acyl chains or sphingoid bases (Raghu, 2020). The biological functions of different lipid classes are defined by the lipid head group. Fatty acids have diverse biological roles and serve as building blocks in cells, important biochemical intermediates, major determinants of membrane properties, modulators of cellular signaling pathways, and as a fuel source (Figure 2). For a deeper analysis of the chemical diversity that regulates lipid function, we direct readers to an excellent comprehensive review on this topic (Harayama and Riezman, 2018).
Figure 2. Major Processes of Lipid Metabolism: uptake, synthesis, and consumption.
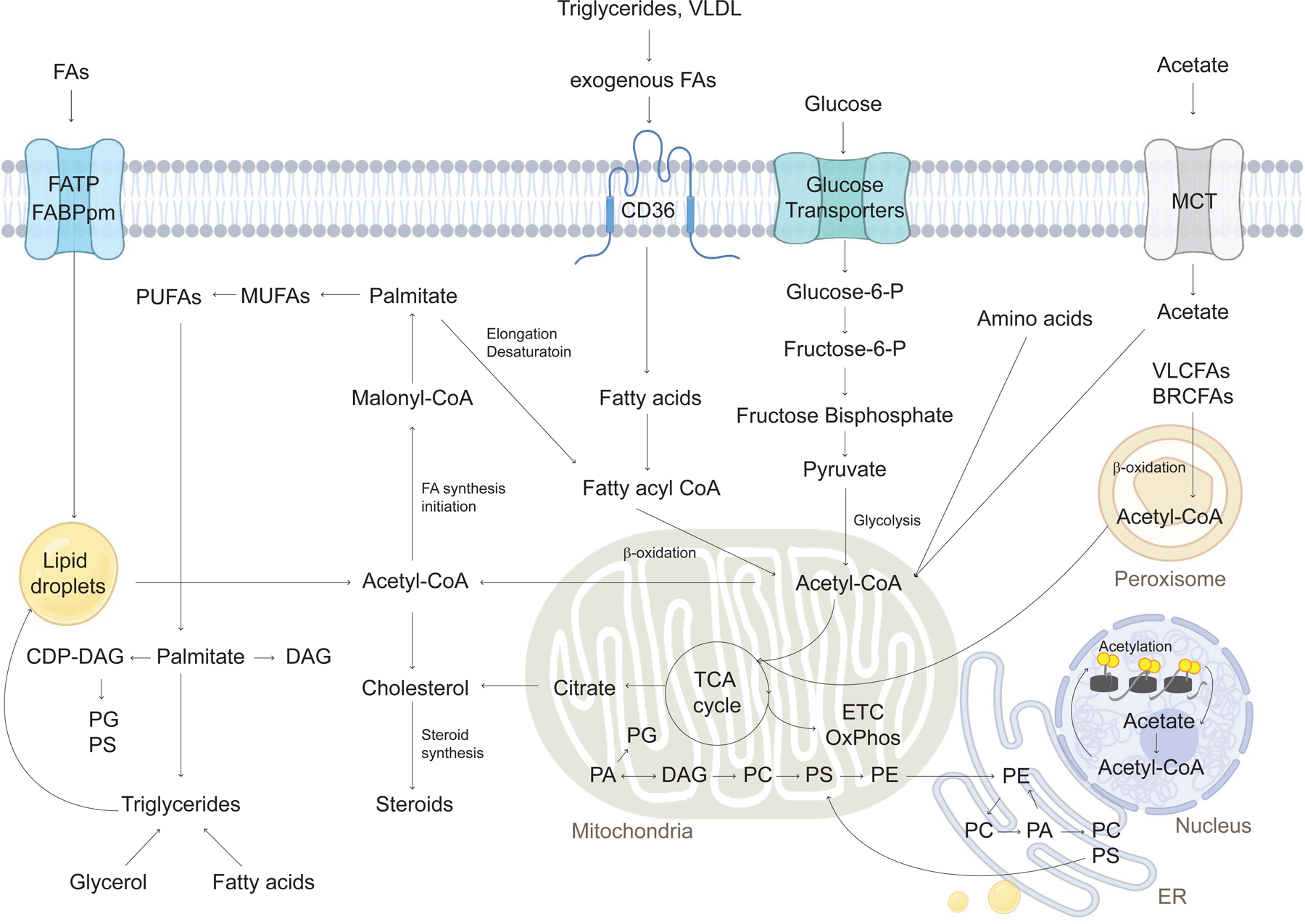
Lipid metabolism includes catabolic processes that generate energy, and anabolic processes that create diverse lipid species. Lipids are transmitted into cells using FA transport or translocase proteins, including FATPs and CD36. Once cells take up lipids, fatty acids are transported into the mitochondria using membrane proteins. In the mitochondria, lipids are oxidized to produce acetyl-CoA, which is further used to make ATP. Glucose uptake through glucose transporters contributes to pyruvate and acetyl-CoA to support the TCA cycle in mitochondria. Acetate uptake using MCT is another source of acetyl-CoA in the cytosol. Acetyl-CoA can affect histone and protein acetylation for epigenetic alteration in the nucleus. In addition to fatty acid oxidation in mitochondria, VLCFAs and BRCFAs are oxidized in peroxisomes contributing to TCA metabolism. Citrate is synthesized during TCA cycling and exported from the mitochondria for de novo lipogenesis (FA and cholesterol synthesis). Fatty acids can be synthesized from malonyl-CoA by fatty acid synthase. Acetyl CoA and malonylCoA are used to produce palmitate and further elongate to MUFAs and PUFAs. Long-chain fatty acids are combined into triglyceride species and stored in lipid droplets. Palmitate is converted to CDP-DAG, DAG, and triglycerides. DAG is also used in the synthesis of phospholipids for membranes, with predominant species including PC, PE, PI, and PS in ER membrane, mitochondria, and cytosol. Lipid metabolism pathways intersect to coordinate cellular metabolic state. BRCFA, branched-chain fatty acid; CDP-DAG, cytidine diphosphate diacylglycerol; DAG, diacylglycerol; ETC, electron transport chain; FA, fatty acid; FATP, fatty acid transport protein; MUFAs, monounsaturated fatty acids; PA phosphatidic acid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PS, phosphatidylserine; PUFA, polyunsaturated fatty acid; TCA cycle, tricarboxylic acid cycle; VLCFA, very-long-chain fatty acid.
While all lipids are insoluble in water, broad categories help classify lipids as fatty acids (FA), phospholipids, or neutral lipids (triglycerides and cholesteryl esters) (Mutlu et al., 2021). FAs, the building blocks of all lipids, serve as a primer for the synthesis of other lipids including glycerolipids, glycerophospholipids, sphingolipids, sterols, and saccharolipids (de Carvalho and Caramujo, 2018). Imbalances between FA uptake and oxidation lead to the accumulation of long-chain FAs that are incorporated into triglycerides (TG) and phospholipids as well as into other lipid species (Goldberg et al., 2012). Ceramides, diacylglycerols, and acylcarnitines, all regulators of intracellular signaling cascades and metabolism (Itani et al., 2002; Koves et al., 2008), are largely considered to be toxic signaling lipid species (Goldberg et al., 2012). Defective mitochondrial FA oxidation increases medium-chain acyl carnitines, another toxic species (Wajner and Amaral, 2015). Studying the diverse roles played by lipids, especially in the context of metabolic disease and cancer, offers an entry point for understanding lipid-mediated toxicity.
FATTY ACID METABOLISM
FA Uptake
Cellular uptake of fatty acids is a key component of metabolic regulation. While FAs can diffuse across phospholipid bilayers, much of fatty acid uptake in mammalian cells is facilitated by integral or membrane associated proteins. Several transporters, across multiple classes, mediate cellular fatty acid uptake including the scavenger receptor CD36 (fatty acid translocase, FAT), plasma membrane fatty acid-binding protein (FABPpm), and six fatty acid transport proteins (FATPs, solute carrier family SLC27A1–6) (Kazantzis and Stahl, 2012; Schwenk et al., 2010; Stahl et al., 2001; Su and Abumrad, 2009). Once at the inner side of the membrane, fatty acids are bound by cytoplasmic FABP (FABPc) before entering metabolic or signaling pathways. Interestingly, a series of studies have shown that FABPpm and mitochondrial aspartate aminotransferase (mAspAt) are identical proteins involved in amino acid metabolism (Birsoy et al., 2015; Cechetto et al., 2002). Additionally, fatty acids are activated by a set of acyl coenzyme A (CoA) synthetase (ACS) enzymes, which catalyze the activation of free fatty acids (FAs) to CoA esters (Roche et al., 2013). CoA conjugation contributes to the maintenance of the concentration gradient by directly pulling fatty acids into the cell. Furthermore, the FATPs are a group of membrane proteins that facilitate the import of long-chain fatty acids (LCFAs), and use ACS activity to regulate intracellular polyunsaturated fatty acids (Coe et al., 1999). Among the six FATP/SLC27A family members, overexpression of FATP1 in 3T3-L1 cells, a mouse embryonic fibroblast cell line that can differentiate into adipocyte-like cells, results in the internalization of palmitic acid (PA), oleic acid (OA) and arachidonic acid (AA) without any selective preference for these fatty acids (Schaffer and Lodish, 1994). Subcellular fractionation indicates that FATP is localized to the plasma membrane and transports LCFAs into the cell for use as an energy substrate.
FA Synthesis
FA synthesis is an anabolic process that creates diverse lipid species. The multifunctional enzyme fatty acid synthase (FASN) directly converts dietary carbohydrates into long-chain saturated fatty acids, predominately the 16-carbon palmitate, by using acetyl-CoA as a primer (Figure 2). FASN is used to supply additional lipids, to support membrane structure, and for cytosolic signaling. Several metabolic enzymes are involved in the conversion of carbons from citrate in the citric acid cycle (TCA) to bioactive fatty acids. ATP citrate lyase (ACLY) generates acetyl-CoA, a precursor for FA synthesis, from mitochondrial TCA-generated citrate in the cytosol (Zaidi et al., 2012). Additional molecular components include acetyl-CoA carboxylases (ACCs) which generate malonyl-CoA. Malonyl-CoA decarboxylase (MCD) converts malonyl-CoA to acetyl-CoA, reversing the reaction catalyzed by ACC (Zhou et al., 2009). The serial condensation of seven malonyl-CoA molecules and one priming acetyl-CoA by FASN generates palmitate, the initial product of FA synthesis. This 16‑ carbon saturated FA (16:0) is then activated by fatty acid-CoA ligase (ACS), elongated by fatty acid protein 5 (ELOVL5), and desaturated by stearoyl-CoA desaturase (SCD) and fatty acid desaturase 2 (FADS2) to produce molecules of various lengths and degrees of saturation (Bogie et al., 2020; Jakobsson et al., 2006). Synthesized fat is stored as triglycerides in cells. Diglyceride acyltransferase, DGAT, involves the TG synthesis pathway to convert diacylglycerol (DAG) to triacylglycerols (TAG). DGAT enzymes catalyze the final step in the known pathways of triglyceride synthesis. Although the 2 enzymes are dissimilar in protein sequences, both enzymes use fatty acyl CoA substrates (Stone et al., 2006). TGs synthesized by DGAT enzymes are then either stored in cytosolic lipid droplets or in other organs such as the liver and small intestine where they are secreted as components of lipoproteins. Both DGAT enzymes are universally expressed in tissues, and highly expressed in organs associated with TG metabolism including adipose tissue and the liver (Cases et al., 1998).
FA synthesis enzymes are regulated at the transcriptional level by sterol regulatory element-binding protein 1 (SREBP-1) transcription factors (Dihingia et al., 2018). Recently, a genome-wide CRISPR screen systematically mapped genetic interactions (GIs) in human HAP1 cells (a near-haploid human cell line derived from chronic myelogenous leukemia (CML) to investigate how cells adapt to the loss of de novo fatty acid synthesis (Aregger et al., 2020). Cells carrying a loss-of-function mutation in FASN, whose product catalyses the formation of long-chain fatty acids, show a strong dependence on lipid uptake that is reflected in negative GIs with genes involved in the low-density lipoprotein receptor signaling pathway (Aregger et al., 2020). A previously unrecognized role emerged for C12orf49 in the regulation of exogenous lipid uptake through a sterol regulatory element binding protein, SREBF2. This study demonstrates how pooled genome-wide CRISPR screens can nominate new metabolic targets in human cells.
Whereas most normal cells preferentially use extracellular lipids for the synthesis of new structural lipids, cancer cells elevate de novo FA synthesis to sustain proliferation in a lipid-poor microenvironment without extracellular lipids (Röhrig and Schulze, 2016). SREBP increases phospholipid, TAG, and cholesterol synthesis to promote cancer cell survival and tumor growth (Griffiths et al., 2013; Lewis et al., 2015). Cancer progression is accelerated through SREBP-1 signaling where the RNA-binding protein LIN-28 accelerates de novo fatty acid synthesis and promotes the conversion from saturated to unsaturated fatty acids (Zhang et al., 2019). Together with essential FAs including linolenic acid taken up through the diet, they form a complex collection of substrates to synthesize FA-containing lipids (Figure 2). Working in concert with membrane receptor tyrosine kinase (RTKs) and serine/threonine kinase mTOR, FASN regulates survival signaling by providing second messenger signaling lipids (Röhrig and Schulze, 2016). As a consequence, de novo fatty acid synthesis generates diverse lipids involved in regulating cellular signaling and lipid homeostasis. For a comprehensive review of fatty acid dysregulation in cancer cells, we direct readers to a recent review on the subject (Broadfield et al., 2021).
FA Regulation
Fatty acids interact with diverse metabolic enzymes to become incorporated into complex lipid species, including DAGs and TAGs or to be converted into phosphoglycerides, such as phosphatidic acid (PA), phosphatidylethanolamine (PE), and phosphatidylserine (PS) (Fagone and Jackowski, 2009; Koundouros and Poulogiannis, 2020)(Figure 2). Acyl groups of fatty acids - predominantly stearoyl groups in mammalian cells - can determine the diversity of phosphatidylcholine (PtdCho) and phosphatidylinositol (PtdIns) (Anaokar et al., 2019; de Carvalho and Caramujo, 2018; Vance, 2014). PtdIns are among the best-characterized secondary messengers in signal transduction pathways (Cantley, 2002). PtdIns can be converted to several phosphoinositide species by phosphorylation, containing phosphatidylinositol 4,5-bisphosphate and phosphatidylinositol (3,4,5)-trisphosphate (PIP2/3) (Cantley, 2002). PIP3 activates AKT to induce pro-tumorigenic signaling, through phosphoinositide-dependent kinase 1 (PDK1), tuberous sclerosis complex (TSC) 1/2, and mTORC2 (Koundouros and Poulogiannis, 2020). Moreover, fatty acids can be used for ceramide de novo synthesis in the endoplasmic reticulum (ER). The initial step is the condensation of the activated C16 fatty acid palmitoyl-CoA and the amino acid L-serine, which is catalyzed by pyridoxal 5’-phosphate (PLP)-dependent serine palmitoyltransferase (SPT). This produces 3-ketosphinganine (3KS), which can be rapidly reduced to sphinganine (dihydrosphingosine, d18:0 Sph) by 3-ketosphinganine reductase (KDSR) in a NADPH dependent manner (Wigger et al., 2019).
Fatty acid synthesis is activated by hypoxia-inducible factor (HIF) signaling (Wagner et al., 2017). Carnitine palmitoyltransferase 1 (CPT1) is repressed by HIF, reducing fatty acid transport into the mitochondria, and directing fatty acids to lipid droplets for storage (Du et al., 2017). Both HIF-1α and HIF-2α are upregulated upon ER stress (Pereira et al., 2014), leading to the formation of lipid droplets in an attempt to decrease cytotoxic ER stress responses (Qiu et al., 2015). An increase in lipid droplets in cells is commonly associated with lipotoxicity and altered metabolism that contributes to cellular dysfunction. Lipid droplet composition and catabolism is a key regulatory node that integrates physiological inputs, such as dietary lipids and lipolytic stimuli, to coordinate cellular signaling and metabolism. Lipid droplets maintain lipid homoeostasis, prevent lipotoxicity, and generate ATP by breaking down lipids stored in droplets during conditions of metabolic stress (Olzmann and Carvalho, 2019). Moreover, repression of SREBP or limitation of FASN can also trigger the HIF-1α signaling pathway and the UPR (Griffiths et al., 2013). In the context of energy-deficiency mediated stress, HIF signaling pathways coordinate with AMP-activated protein kinase (AMPK) and mTOR to compensate for the limitation of FASN and activate lipid metabolism to rescue lipid-mediated ER stress.
FASN antagonists are increasingly being investigated as a therapeutic approach to treat cancer. The use of techniques like MALDI-MS/MS, liquid chromatography-matrix assisted laser desorption/ionization mass spectrometry, enable greater proteome analysis (Mueller et al., 2007). Applications of mass spectrometry indicate that FASN-inhibitors such as C75 and G28UCM increase polyunsaturated fatty acids and decrease signaling lipids like DAG and PIP3 in ovarian cancer cell lines (Wagner et al., 2017). FASN inhibition influences multiple downstream targets suggesting that a greater understanding of pathway cross-talk will enhance drug target efficacy by influencing activity across multiple pathways. Specifically, FASN inhibition affects ERBB-PI3K-mTORC1 activity by blocking phosphorylation of EGF-receptor/ERBB/HER, inhibiting GRB2-EGF-receptor recruitment, and suppressing PI3K-AKT signaling (Giró-Perafita et al., 2016; Kumar-Sinha et al., 2003; Menendez et al., 2004; Wagner et al., 2017). Moreover, fatty acid synthesis is elevated in metastatic breast cancer, especially in the brain (Ferraro et al., 2021). This phenotype is an adaptation to decreased lipid availability in the brain compared to other tissues, resulting in site-specific dependency on fatty acid synthesis for breast tumors growing in the brain. Inhibition of fatty acid synthase reduces human EGF receptor 2-positive breast tumor growth in the brain, pointing to the emergence of new cancer targets based on differential nutrient availability across metastatic sites (Ferraro et al., 2021). FABP5 is an intracellular chaperone that delivers cytosolic fatty acids to nuclear receptors to enhance metastasis. FASN and monoacylglycerol lipase (MAGL) promote nuclear receptor activation and PCa metastasis are critically dependent upon co-expression of FABP5 (Carbonetti et al., 2019). Moreover, the expression level of FASN affects the PI3/AKT signaling through phosphatase and tensin homolog deleted on chromosome 10 (PTEN) (Van de Sande et al., 2002). A greater understanding of how these pathways intersect will enhance the ability to design effective FASN inhibitors that regulate multiple, interconnected targets.
In addition to HIF-dependent pathways, fatty acid synthesis is regulated by other metabolic enzymes. Lipid biosynthesis and oxidation is regulated by a master regulator of FA metabolism, acetyl-CoA carboxylase (ACC), which converts acetyl-CoA to malonyl-CoA. This conversion serves as a precursor for fat synthesis and inhibits fatty acid oxidation. ACC1 is localized in the cytosol and promotes the production of FAs, while ACC2 is localized to the mitochondrial outer membrane and generates malonyl-CoA to inhibit the fatty acid transport protein CPT1 (German et al., 2016). Cellular stress directly affects enzymatic actions related to ACC and regulates FA metabolism. In energy stress conditions, AMPK activates fat synthesis and catabolism by inhibiting both ACCs (Park et al., 2002). In contrast, under conditions of nutrient abundance, AMPK is downregulated and no longer represses ACC1 and ACC2. Moreover, ACC2 is regulated by post-translational modifications. For example, prolyl-hydroxylase 3 protein (PHD3) presents a metabolic barrier to fatty acid utilization by hydroxylating and activating ACC2 (German et al., 2016; Yoon et al., 2020). ACC is therefore a signaling node that senses nutrient abundance and adjusts anabolic versus catabolic FA metabolism accordingly. In the setting of acute myeloid leukemia, PHD3 levels are decreased, fueling a dependence on fats that can be targeted with fatty acid oxidation (FAO) inhibitors. In addition to AML, many cancer cells use fat synthesis to induce proliferation. Questions remain regarding the extent to which modulating PHD3 in metabolic disorders can confer a therapeutic benefit.
LIPID METABOLISM IN MITOCHONDRIA AND PEROXISOMES
Fatty acid metabolism includes catabolic processes that generate energy, and anabolic processes that create diverse lipid species (DeBerardinis et al., 2007; Yoon et al., 2020). Mitochondria play an important role in the regulation of FA metabolism, including anabolic and catabolic pathways. Fatty acids provide twice as much ATP as carbohydrates and six times more when comparing stored fatty acids to stored glycogen (Carracedo et al., 2013). FAO occurs through a series of reactions that result in the shortening of fatty acids by two carbons per cycle. Each round generates NADH, FADH2, and acetyl CoA until the last cycle when two acetyl-CoA molecules are generated (Figure 2). The NADH and FADH2 that are generated by FAO enter the electron transport chain (ETC) in order to generate ATP. Fatty acid availability is a key signal for adaptations in mitochondria-rich muscle cells and their specific enzymes involved in lipid metabolism. Numerous studies underscore that an efficient capacity to oxidize fatty acids, and the ability to adapt fatty acid utilization to fatty acid availability, is of great importance for both lipid and glucose homeostasis and insulin action (Matoba et al., 2017; Santoro et al., 2021; Zhou et al., 2019).
β-oxidation of stored lipids leads to the production of acetyl-CoA through oxidative degradation of FAs (Figure 2). The acetyl-CoA produced from each round of β-oxidation can subsequently enter the TCA cycle to generate NADH and FADH2 for the electron transport chain (Fritz and McEWEN, 1959). Once cells take up lipids, FAs are transported into the mitochondria and oxidized in a multi-step pathway known as beta-oxidation (McGarry and Foster, 1980). As long-chain fatty acids are prepared for the multi-step process of mitochondrial import, they are regulated by CPT1, which is a transferase that converts acyl-CoAs into acyl-carnitines for transport. These FAs are processed by acyl-CoA dehydrogenase to form long-chain acyl-CoA, enoyl-CoA hydratase to acyl-chain forming hydroxy-acyl-CoA, by hydroxy-acyl-CoA dehydrogenase to the substrate forming a second keto-group, and by thiolase to acetyl-CoA and a free CoA with the new substrate. Using these processes, FAs are oxidized into acetyl-CoA, which is subsequently used to make ATP (Huynh et al., 2014). Cells containing increased FAO through metabolic alteration use acyl-CoA for producing ATP through the TCA cycle. To compensate for the acyl-CoA, levels of acyl-carnitine are increased (Yoon et al., 2020). Cancer cells exhibit a decrease in ATP and NADPH, due to decreased flux through the pentose phosphate pathway (PPP), which inhibits glycolysis and leads to elevated levels of reactive oxygen species (ROS) that repress FAO activity (Schafer et al., 2009). In conclusion, this crosstalk between FAO and metabolic signaling pathways includes redox systems and directly impacts cell survival, but questions remain as to which lipid species are specifically participating in FAO and the detailed mechanism of how FAO induces cell survival.
Peroxisomes regulate shortening of long-chain and very-long-chain fatty acyl-CoAs, dicarboxylic fatty acids, 2-methyl-branched fatty acids, eicosanoid inflammatory mediators, prostaglandins, and bile acid intermediates. Moreover, fatty acid oxidation of very long chain fatty acids or branched fatty acids occurs in peroxisomes. This process generates hydrogen peroxide. Peroxisomal β-oxidation does not degrade fatty acids completely as it is not coupled to oxidative phosphorylation for ATP synthesis. Peroxisome proliferator–activated receptors (PPAR) are the most important transcriptional regulators of peroxisomal β-oxidation (Reddy and Hashimoto, 2001; Vanhove et al., 1993). Recently, acyl-CoA oxidase 1 (Acox1) has been reported to activate peroxisome-derived acetyl-CoA to increase peroxisomal β-oxidation. The induction of cytosolic acetyl-CoA levels activates mTORC1, inhibits autophagy, and induces hepatic triglycerides (He et al., 2020). To date, the physiological significance of peroxisomal beta-oxidation is still an open question and the subject of active investigation.
LIPID-MEDIATED MODULATION OF CHROMATIN STATE
Lipids modulate chromatin states through histone and protein acetylation by generating acetyl-CoAs, which are generated from mitochondria and peroxisomes (Galdieri et al., 2013; McDonnell et al., 2016). Lipids can provide up to 90% of acetyl-carbon for histone acetylation using lipid-derived acetyl-CoA (Ac-CoA). We hypothesize that metabolites like Ac-CoA directly affect histone modification by regulating gene expression specific to lipid homeostasis and control of lipotoxicity. Understanding how Ac-CoA regulates gene expression specific to lipid homeostasis will provide important insights into the genes important for homeostatic or lipotoxic programs. The Ac-CoA pool regulates glucose which in turn drives a gene expression program characterized by activating genes involved in its metabolism, in part by increasing glucose-derived histone acetylation. Lipid-derived acetyl-CoA is a major source of carbon for histone acetylation (Galdieri et al., 2013; McDonnell et al., 2016). Using 13C-carbon tracing combined with acetyl-proteomics, up to 90% of acetylation on certain histone lysines can be derived from fatty acid carbon, even in the presence of excess glucose (Galdieri et al., 2013; McDonnell et al., 2016). This suggests a new mechanism for how Acetyl-CoA fluxes could regulate genes important for homeostatic/lipotoxic programs.
Free acetate is converted to Ac-CoA by acetyl-CoA synthetase (ACSS) and promotes lipid synthesis under hypoxic conditions through epigenetic reprogramming (Gao et al., 2016). ATP citrate lyase (ACLY) is the primary enzyme responsible for the synthesis of cytosolic acetyl-CoA in many tissues, and is critical for histone acetylation (Wellen et al., 2009). ACLY generates nucleus and cytosolic Ac-CoA by cleaving citrate derived from TCA cycle intermediates released from mitochondria (Figure 2). Although acetyl-CoA provided by ACLY activity regulates histone acetylation in adipocytes, other metabolites shunted from the TCA cycle also regulate the epigenome (Felix et al., 2021). For histone modification, α-KG is used as cofactor for 2-oxoglutarate dependent dioxygenases, such as Jumonji C domain-containing lysine histone demethylases. During this modification process, succinate is converted from α-KG. The ways in which energy balance impacts lipid synthesis is an ongoing area of investigation This in combination with the immune effects of acetate, succinate, and α-KG, suggest that nutrient metabolism must also match demand across a diverse mixture of adipocytes and stromal cells in WAT (Felix et al., 2021). ACLY is phosphorylated by AKT to induce histone acetylation, and pAkt(Ser473) levels correlate significantly with histone acetylation markers in human gliomas and prostate tumors (Lee et al., 2014). Moreover, histone methylation modifiers influence mono-unsaturated fatty acids (MUFA) metabolism (Han et al., 2017). The MUFA oleic acid plays a key role in the longevity of H3K4me3 methyltransferase-deficient worms. The role of oleic acid in lifespan regulation in the context of histone modification suggests the importance of MUFAs and their downstream polyunsaturated fatty acids (PUFAs) in the regulation of lifespan under physiological conditions (Han et al., 2017). In sum, lipids take part in a complex, interconnected regulatory network that includes signaling, epigenetics, aging, and metabolism.
LIPOTOXICITY ACROSS DIVERSE ORGAN SYSTEMS
Impairments in fatty acid metabolism have significant consequences for a range of human diseases. The application of CRISPR-based genetic screens and unbiased lipidomics has identified a new approach to studying the enzymes responsible for regulating how fatty acids incorporate into membrane and storage glycerolipids. Lipid accumulation in tissues is increasingly recognized as a contributor to cellular dysfunction. Many cells across organ systems are not equipped to handle large lipid loads, and the mechanism by which excess lipids cause cellular injury, or lipotoxicity, is an area of investigation across the kidney, liver, heart, skeletal muscle, bone, pancreas, and brain (Figure 3). Saturated fatty acids are thought to be particularly harmful to cells invoking a diverse array of harmful cellular responses: apoptosis, inflammation, ceramides, reactive oxygen species (ROS), small nucleolar RNAs (Michel et al., 2011), and ER stress. An ongoing priority in the field is nominating bioactive lipid species that modulate the lipotoxic cellular response.
Figure 3. Lipid homeostasis and disease.
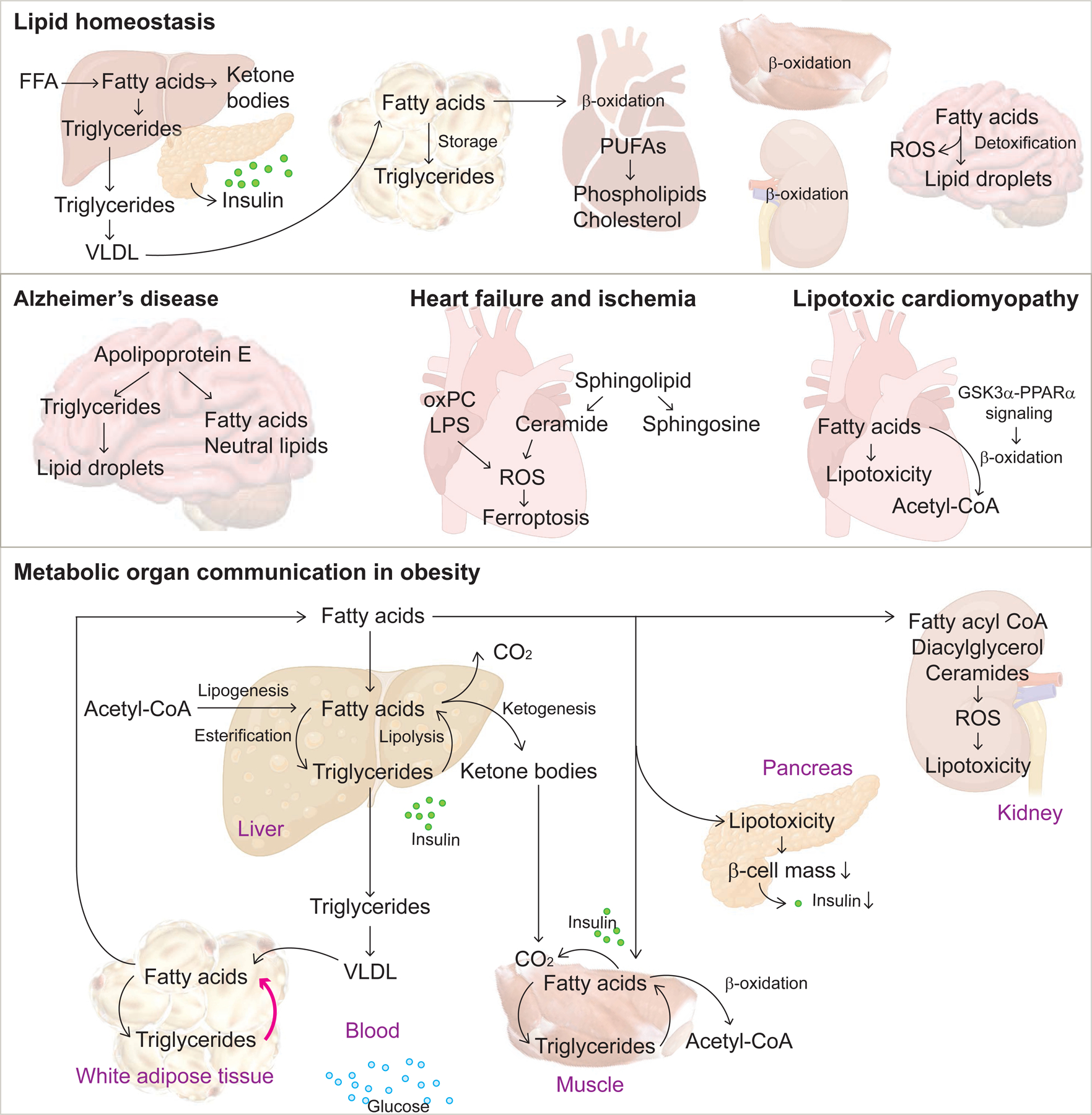
Under conditions of excess nutrients, there is less lipolysis, conversion of triglycerides to fatty acids, in favor of increased lipogenesis or triglyceride storage in adipose tissue. In muscle cells, glucose oxidation is increased. In fasting conditions, lipolysis is increased in adipose tissue and fatty acid oxidation increases in muscle cells to provide energy. Changes in metabolism occur in the brain, heart, liver, pancreas, kidney, and adipose tissue.
Lipid Accumulation in Adipose Tissue
Adipose tissue is a major regulator of energy homeostasis, and its dysregulation results in an imbalance in energy homeostasis due to inappropriate loads in peripheral tissues. Adipocytes act as a reservoir for energy storage, but also sense energy demands and secrete paracrine factors to regulate other metabolic tissues. Mammals have two types of adipose tissue: white adipose tissue (WAT) which stores excess energy, and brown adipose tissue (BAT) which releases excess energy as heat. A major function of WAT is the release of nonesterified fatty acids (NEFAs) into the bloodstream during periods of energy-demand. Recent studies have investigated which byproducts of lipid metabolism affect the function in adipocytes. Specifically, there is an emerging role for short-chain fatty acids (SCFAs) and TCA cycle metabolites that connect lipogenesis to WAT energy balance (Felix et al., 2021). SCFAs including acetate, butyrate, and propionate inhibit lipolysis and promote adipogenesis in WAT, and provide substrates for glucose and lipid synthesis. SCFAs act on G protein-coupled receptors (GPR41 and GPR43) to inhibit lipolysis and decrease plasma levels of FFAs (Felix et al., 2021). Applying highly-sensitive, mass-spectrometry-based proteomics to human adipocytes identified 471 secreted proteins including hormones, growth factors, extracellular matrix proteins that are differentially regulated between brown and white adipose tissue (Deshmukh et al., 2019). Interestingly, brown and white adipocytes have distinct secretory profiles and metabolic functions. Mammalian ependymin-related protein 1 (EPDR1) is selectively secreted from brown adipocytes where it plays a vital role in promoting the development into functional thermogenic adipocytes by activating UCP1 expression. Thus, this recent profiling of the secretome of human white adipocytes and energy-burning brown adipocytes identified important regulators of human metabolism. Since this secretome analysis was done in mature adipocytes, questions remain about the dynamic regulation of lipid metabolism and secretome throughout the differentiation process. Studies conducted at different differentiation stages in fat cells would provide additional insights into the mechanism responsible for preserved metabolic health in people with obesity, severe insulin resistance, and type 2 diabetes.
Lipid Accumulation in Kidney
Extensive work in animal models has demonstrated a link between kidney dysfunction and lipid accumulation in models of metabolic disease including obesity, metabolic syndrome, diabetes mellitus, chronic kidney disease, and acute kidney injury (Jiang et al., 2005; Kume et al., 2007; Wang et al., 2005). Lipid accumulation and kidney dysfunction have been widely documented in human clinical studies including focal segmental glomerulosclerosis (FSGS), minimal change disease, Fabry’s disease, and lipoprotein glomerulopathy (Bobulescu, 2010). The kidney can use multiple substrates as fuel, depending on availability (Elhamri et al., 1993; Guder et al., 1986; Klein et al., 1981). Substrate use varies across regions on the basis of energy demand (Bobulescu, 2010). The proximal tubules have a high energy demand, second only to cardiac myocytes. Therefore, they have relatively little glycolytic capacity and rely instead on mitochondrial β-oxidation of FFAs to maximize ATP production (Balaban and Mandel, 1988; Gullans et al., 1984; Uchida and Endou, 1988). Recent evidence indicates that during diabetic kidney disease, the proximal tubule expresses kidney injury molecule (KIM)-1. KIM-1 mediates uptake of palmitic acid leading to enhanced tubule injury characterized by DNA damage, interstitial inflammation, and fibrosis (Mori et al., 2021). A small molecule inhibitor of KIM-1, TW-37, ameliorates kidney inflammation and fibrosis. These studies highlight that small molecule targets upstream of FAO can be a novel therapeutic strategy in kidney injury.
The kidney responds to lipid toxicity through the upregulation of regulators involved in lipid peroxidation and the accumulation of toxic metabolites including fatty acyl CoA, diacylglycerol, and ceramides. Lipotoxic cellular dysfunction results in the generation of reactive oxygen species, organelle damage, disruption of intracellular signaling pathways, release of proinflammatory and pro-fibrotic factors, and lipid-induced apoptosis. Lipid peroxidation occurs when oxidants such as free radicals attack lipids containing carbon-carbon double bonds, especially PUFAs. Even though many studies have shown that PUFAs reduce kidney disease by decreasing triglycerides and inflammation, it is hypothesized that PUFAs are converted to oxidized lipid by lipoxygenases (LOX), cyclooxygenases (COX), and cytochrome P450 (CYP) (Hajeyah et al., 2020). The kidney, in particular, is susceptible to changes in gene expression in sterol regulatory element-binding proteins (SREBPs) in response to diabetes, and results in TG accumulation, mesangial expansion, and glomerulosclerosis (Sun et al., 2002). This suggests that activation of renal SREBP-1 results in alterations in renal lipid metabolism and renal lipid accumulation plays an important role in the pathogenesis of diabetic nephropathy.
Lipid accumulation is a major contributor to diabetic kidney disease, the most rapidly growing cause of kidney failure worldwide (Alicic et al., 2017). As essential components of the kidney filter, podocytes are post-mitotic, highly-differentiated epithelial cells that are particularly vulnerable to lipid accumulation and toxicity (D’Agati et al., 2016). Coenzyme Q10 (CoQ), a ubiquitous lipid present in all cellular membranes, protects against polyunsaturated fatty acid-mediated (PUFA-mediated) lipid peroxidation (Sidhom et al., 2021). With mitochondrial dysfunction, the absence of protection from lipid peroxidation sensitizes cells to death (To et al., 2019). Elevation of lipid peroxidation, shown by upregulated glutathione peroxidase 4 (GPX4), has been reported in podocytes of CoQ deficient mice (Sidhom et al., 2021). Moreover, loss of GPX4 triggers ferroptosis death in the kidney resulting in renal degeneration (Angeli et al., 2014). Recent efforts to understand the connection between kidney disease, PUFAs, and dysregulated pathways in podocytes used single-nucleus RNA-Seq (sNuc-Seq) and integrated metabolomics and transcriptomics to identify a therapeutically relevant Braf/MAPK pathway (Sidhom et al., 2021). In addition, JAML (junctional adhesion molecule-like protein) is expressed in podocytes and induced under diabetic conditions (Fu et al., 2020). Podocyte-specific deletion of Jaml ameliorates podocyte injury and proteinuria in two different models of diabetic mice (Fu et al., 2020). Junctional adhesion molecules, members of an immunoglobulin subfamily, play an emerging role in lipid metabolism. Specifically, Jam-A knockout mice fed a high-saturated fat, fructose, and cholesterol diet (HFCD) develop severe non-alcoholic steatohepatitis (Rahman et al., 2016). Deploying LC/MS-based lipidomics analysis revealed that JAML deletion in podocytes reduces levels of lipids including free fatty acids, cholesteryl esters, and phosphatidylcholines (Fu et al., 2020). JAML regulates podocyte lipid metabolism through SIRT1-mediated SREBP1 signaling, and is higher in the glomeruli of patients with kidney disease. In the future, clinical studies aimed at preventing lipid accumulation and preserving glomerular function may be an attractive therapeutic target for diabetic kidney disease and other types of proteinuric kidney diseases.
Roles of Liver, Bone, and Skeletal Muscle in Lipotoxicity
Fatty acids are delivered to the liver through the blood following lipolysis of triglycerides in adipose tissue. Fatty acids in the liver bind to FABP-1 and are metabolized by mitochondrial β-oxidation. Obesity and type 2 diabetes are frequently complicated by excess fat accumulation in the liver, which is known as nonalcoholic fatty liver disease (NAFLD). The major genetic determinants of NAFLD are PNPLA3, HSD17B13, and TM6SF2, and liver steatosis develops due to the dysregulation of pathways controlling de novo lipogenesis and fat catabolism. Recent evidence suggests that reduction in the activity of lysosomal acid lipase (LAL), which is a key enzyme for intracellular fat disposal, is of clinical relevance for patients with NAFLD (Baratta et al., 2019). With the advance of high-throughput sequencing technology, liver transcriptome sequencing results have identified potential gene candidates affecting fat deposition. FABP1 is a liver-specific FABP that plays important roles in intracellular lipid metabolism in the liver. Knockdown of FABP1 blocks lipid accumulation in hepatocytes (Mukai et al., 2017). FABP1 affects the regulation of fat deposition through PPAR signaling and biosynthesis of fatty acids (Wang et al., 2019). Palmitic acid hydroxystearic acids (PAHSAs) are endogenous lipids with anti-diabetic and anti-inflammatory effects. Chronic PAHSA treatment augments insulin-stimulated glucose uptake in glycolytic muscle and heart in high-fat diet-fed mice by enhancing hepatic insulin sensitivity and inhibiting lipolysis in adipose tissue (Zhou et al., 2019). Moreover, PAHSAs mediate GPR40 receptors to regulate improvements in glucose tolerance and insulin sensitivity (Syed et al., 2018).
Beyond the systems associated with metabolism, bone has an important role in the clearance of circulating lipoproteins and non-esterified fatty acids (Kushwaha et al., 2018). Interestingly, long-chain fatty acid oxidation affects postnatal bone development by altering fatty acid utilization. Eicosapentaenoic acid (EPA, long-chain polyunsaturated n-3 fatty acids) affects substrate cycling in human skeletal muscle cells by altering lipolysis rate of intracellular triacylglycerol and re-esterification of fatty acids by increasing fatty acid turnover (Løvsletten et al., 2018). In the future, further studies of how ER stress and the UPR pathways underlie lipotoxicity in peripheral tissues may provide an important point of therapeutic intervention for tissue damage.
Lipids and Cardiovascular Function
The heart has both the greatest caloric need and the most extensive oxidation of fatty acids (Goldberg et al., 2012) and adeptly acquires lipids from circulating, non-esterified fatty acids and esterified FAs bound to lipoproteins. Lipid energy metabolism is an important factor for heart disease including heart failure and ischemia. Specifically, extensive clinical evidence links lipid oxidation and the inflammatory response to cardiovascular diseases. Polyunsaturated fatty acids (PUFAs) affect the levels of phospholipids and cholesterol esters in lipoproteins during the development of atherosclerosis (Berliner et al., 2009). Free radical lipids and modified lipoproteins generated from oxidized lipid peroxidation play a key role in modulating inflammatory responses (Binder et al., 2016; Hansson and Hermansson, 2011). Higher lipid availability promotes ischemia-induced cardiac dysfunction and decreases myocardial mitochondrial efficiency. Myocardial fatty acid-linked respiration and oxidative stress are increased, whereas mitochondrial efficiency is decreased. Increased lipid availability favors susceptibility to ischemia-induced cardiac dysfunction (Jelenik et al., 2018).
Cell death occurs through different mechanisms, and ferroptosis, a programmed iron-dependent cell death, is driven by damage to the lipid membrane in ischemia/reperfusion-induced cardiomyopathy as well as peroxidation of lipids. Recent studies have suggested that free iron accumulates in mitochondria to cause oxidative stress and ferroptosis-induced heart damage (Fang et al., 2019). The glutathione metabolic pathway and reactive oxygen species (ROS) pathway are significantly downregulated during myocardial infarction (Park et al., 2019). Interestingly, GPX4, which protects cells from ferroptosis, is downregulated in myocardial infarction. Moreover, oxidized phospholipids promote inflammation in global myocardial ischemia/reperfusion injury. Cytokine IL-10 plays an anti-inflammatory role and modulates the production of oxidized phosphatidylcholines in cardiomyocytes thereby mitigating inflammation and cell death (Bagchi et al., 2020).
Lipid levels and composition in patient blood during myocardial infarction have been shown to predict the risk of complications (Meeusen et al., 2017). Specifically, sphingolipids serve as a biomarker for both recurrence and mortality after myocardial infarction (MI) (Hadas Yoav et al., 2020). Ceramides are simple membrane sphingolipids that form the backbone of all complex sphingolipids and can trigger programmed cell death upon reaching high cellular levels (Arana et al., 2010). Studies have shown that ceramide levels are high in the heart tissues of humans during acute MI (Hadas Yoav et al., 2020). 24 hours post-MI, 30% of sphingolipid metabolism genes are significantly upregulated and the levels of C16-ceramide, C20-ceramide, C20:1-ceramide, and C24-ceramide are significantly higher (Hadas Yoav et al., 2020). In hypoxic conditions that mimic myocardial infarction, several inhibitors limit ceramide degradation including the pan-ceramidase inhibitor B13 and the acid ceramidase (AC) specific inhibitor ARN14974. Additionally, a pan-sphingosine kinase inhibitor SK1-II significantly increases cardiomyocyte cell death levels (Hadas Yoav et al., 2020). Alterations in sphingolipid metabolism by ceramidase, which hydrolyzes proapoptotic ceramide and generates sphingosine, is necessary for regulating ceramide levels and cell survival in ischemic heart disease (Hadas Yoav et al., 2020). Transcriptomic and protein analyses reveal that altering ceramide metabolism through chemical inhibitor modulation of sphingolipid metabolism can induce cardioprotection after MI. Furthermore, the expression of microsomal triglyceride transport protein (MTTP) is associated with structural and perfusion abnormalities in patients with ischemic heart disease, suggesting that triglycerides play an important role in cardiac function as it relates to ischemic events (Klevstig et al., 2019).
Decreases in the level of FA oxidation are commonly associated with heart failure (Neubauer, 2007). Genetic overexpression of PPARα in the heart recapitulates the phenotype of lipotoxic cardiomyopathy (Finck et al., 2002) whereas knockdown attenuates the phenotype (Finck et al., 2003). Questions related to PPARα activity are emerging including (1) how is endogenous PPARα regulated in the context of diabetes and obesity, and (2) how does this modification contribute to lipotoxicity? PPARα is a nuclear receptor transcription factor known to play a role in controlling the expression of genes involved in FA metabolism, specifically, uptake, storage, and oxidation. Since saturated FAs including palmitic acid, monounsaturated FAs (such as oleic acid), and polyunsaturated FAs (such as linoleic acid) have distinct effects on metabolic diseases (Roberts et al., 2014), the effect of different FAs on the GSK-3α-PPARα signaling pathways is a current area of interest. Specifically, palmitic acid increases PPARα activity; however, knockdown of GSK-3α abolishes the palmitic-acid induced increase (Nakamura et al., 2019). The functional role of PPARα phosphorylation is an effect on energy metabolism. Specifically, PPARα-S280D increased expression of genes related to FA uptake and pyruvate dehydrogenase PDH kinase 4 (PDK4) which inactivates the PDH complex and inhibits glucose oxidation. Overall, this study suggests that, in the heart, FA exposure increases GSK-3α activity as part of a feedforward axis with PPARα that induces lipotoxic cardiomyopathy in obesity. Importantly, constitutively active GSK-3α and GSK-3β exert opposite effects on genes involved in FA uptake and transport. As a result, the therapeutic potential for GSK-3α inhibitors will depend on an isoform specific small-molecule inhibitor as the beneficial effects of GSK-3α inhibition could be negated by unintended GSK-3β inhibition.
Aberrant Lipid Metabolism in Neurodegenerative Diseases
The central nervous system regulates systemic metabolism and lipid balance. Highly coordinated interactions between the brain and metabolic organs maintain energy and glucose homeostasis. The energy state of the body is assessed by sensing regions of the brain including key nuclei within the hypothalamus, including the ventromedial nucleus (VMH), arcuate nucleus (ARC), dorsomedial hypothalamic nucleus (DMH), and the paraventricular nucleus. Alterations in plasma levels of key nutrients, including glucose, fatty acids, and amino acids, provide information about nutrient availability. Increasing malonyl CoA content in hypothalamic neurons acts as a fuel gauge: adding the fatty acid synthase inhibitor, C75, to hypothalamic neurons, decreases food intake. Additionally, increasing long-chain fatty acyl-CoA (LCFA-CoA) content in hypothalamic neurons decreases food intake through hypothalamic inhibition of carnitine palmitoyltransferase-1 (Gao et al., 2013; Obici et al., 2003).
Neurons do not typically make lipid droplets and have a low capacity for fatty acid consumption in mitochondria for energy production (Schönfeld and Reiser, 2013). When neurons undergo periods of sustained activity, high levels of reactive oxygen species induce peroxidation of FAs (Reynolds and Hastings, 1995). Highly active neurons are particularly susceptible to peroxidated FAs as they do not have the ability to divert them into lipid droplets. In contrast, lipid droplet accumulation in glia has been demonstrated to protect neurons (Bailey et al., 2015). In the brain, hyperactivity in neurons results in the production of toxic fatty acids. The transfer of these toxic fatty acids via lipid particles to astrocytes provides a mechanism of detoxification, especially during periods of enhanced activity (Ioannou et al., 2019). Astrocytes endocytose neuron-derived lipid particles to deliver the fatty acid to lipid droplets. Thus, activity-dependent stimulation of lipid metabolism in astrocytes represents a dynamic process that regulates prevention of FA toxicity in neurons.
In contrast to neurons, astrocytes make LDs and produce antioxidants. Oxidative stress in neurons induces lipid droplet formation in neighboring astrocytes. Glial and neuronal monocarboxylate transporters (MCTs), fatty acid transport proteins (FATPs), and apolipoproteins are important for this type of lipid droplet formation (Liu et al., 2017a). MCTs enable glia to secrete lactate which is converted to pyruvate and acetyl-CoA in neurons. Lactate metabolites provide a substrate for fatty acid synthesis. In the presence of elevated levels of ROS, inhibiting lactate transfer or lowering FATP or apolipoprotein levels has been shown to decrease glial lipid droplet accumulation (Liu et al., 2017a).
Dysregulation of lipids has recently emerged as a key factor in neurodegenerative diseases including Alzheimer’s disease (AD). Many lipid species have been used as markers for early diagnosis of AD and identified as playing a role in neurotoxicity. The most validated genetic risk factor for late-onset Alzheimer’s disease is the 4 allele of the APOE gene (APOE4). The presence of APOE4 lowers the age of AD onset. Apolipoprotein E (APOE) is a component of many lipoprotein particles and acts as a ligand for membrane receptors that mediate lipoprotein uptake. Recent efforts to shed light on how APOE4 alters the composition of lipids have demonstrated that human iPSC-derived APOE4 astrocytes accumulate unsaturated triacylglycerols stored in lipid droplets to a greater extent than isogenic APOE3 counterparts. Using liquid chromatography-mass spectrometry (LC-MS), the authors compared the lipid composition of APOE3- and APOE4-expressing human iPSC-derived astrocytes and observed that the degree of unsaturation of the fatty acids attached to triacylglycerides in APOE4-expressing astrocytes was higher (Sienski et al., 2021). To determine if the iPSC-derived human astrocyte cultures reflected APOE4-related dysfunction in human brains, the authors examined transcriptomic data from postmortem human brain samples using the Genotype-Tissue Expression (GTEx) project. Genes that emerged as significantly differentially expressed and of relevance to lipid metabolism included up-regulated genes involved in the metabolism of neutral lipids (FA2H, ACSL1, SQLE, HMCGR, and MVK) and downregulated genes involved in the metabolism of fatty acids and neutral lipids (OLAH, CNEP1R1, and GPAM). It remains to be seen whether a clinical intervention that takes advantage of genotype-specific dietary supplementation might mitigate disease progression.
LIPID SIGNALING AND THE IMMUNE SYSTEM
Lipid-Immune Interactions
Different fatty acids and lipids differentially influence immune cell subsets. Omega-3 PUFAs possess potent immunomodulatory activities and play a role in regulating inflammatory and autoimmune diseases including arthritis, Crohn’s disease, ulcerative colitis and lupus erythematosus (Simopoulos, 2002). PUFAs suppress the production of interleukin 1 (IL-1) and the expression of Cyclooxygenase (COX) 2 mRNA that is induced by IL-1. Moreover, α-linolenic acid (ALA), the precursor to the omega-3 family compounds, increases pro-inflammatory cytokine secretion (IL-1, IL-2, and tumor necrosis factor α). In addition to these mediators with pro-inflammatory effects, ALA has been known to suppress prostaglandins and leukotrienes and induce anti-inflammatory and resolvin functions (Marton et al., 2019; Simopoulos, 2002). PUFAs and related FAs represent a potential target for diseases of inflammation.
The immune system plays a critical role in removing cancerous cells. The cross-talk between immune cells and cancer is demonstrated by the ways in which tumor-infiltrating T lymphocytes (TILs) adapt to the metabolic constraints within the tumor microenvironment (TME) introducing a route to combat tumor progression. T cells can exhibit some degree of metabolic flexibility. Since CD36, in the plasma membrane, is essential for facilitating exogenous FA uptake, the upregulation of CD36 in intratumoral Treg cells has an important role in tumor progression and T cell function (Wang et al., 2020). In TMEs with low levels of glucose, CD8+ T cells enhance PPAR-alpha signaling and fatty acid catabolism under hypoglycemic and hypoxic conditions to partially preserve effector functions. Importantly, metabolic reprogramming of T cells using a PPAR-alpha agonist inhibits tumor growth, an effect that is enhanced in combination with PD-1 inhibition (Zhang et al., 2017). As FAO is important for the differentiation of Tregs, FAO inhibition could prevent the accumulation of this immunosuppressive T cell population (Bader et al., 2020). These studies suggest that lipid metabolism provides intriguing opportunities to modulate the TME and specific immune cell populations.
Beyond T cells, metabolic rewiring has been linked to a protumoural phenotype in tumor associated macrophages. Alternative (M2) activation of macrophages is dependent on fat oxidation. The triacylglycerol substrates are taken up through the scavenger receptor CD36, and their lipolysis by lysosomal acid lipase (LAL) leads to prolonged survival and M2 activation in ovarian cancer, suggesting that CD36 inhibition is an important strategy for combating cancer (Huang et al., 2014). Moreover, the production of a-ketoglutarate from glutaminolysis promotes fatty acid oxidation and epigenetic activation in alternative (M2) activation of macrophages (Liu et al., 2017b). This study suggests that macrophage responses are fine-tuned through FA metabolism and epigenetic reprogramming. Metabolic adaptations in tumor and immune cells within the tumor microenvironment likely occur in response to changes in local nutrient levels, and we will discuss how this lipid metabolism affects cancer biology.
Fat Metabolism in Cancer
Different lipid species have opposing effects on cancer proliferation and death requiring careful mechanistic interrogation. Cellular proliferation is a common feature of all cancers which require fatty acids in order to synthesize membranes and signaling molecules. Tumor cells acquire abundant lipids for rapid cell growth, but despite this seeming overload of FAs, tumors avoid toxicity. Indeed, highly proliferative cancer cells have been found to upregulate enzymes involved in lipid and cholesterol biosynthesis leading to increased aggressiveness in certain cancers (Bader et al., 2020). How cancers metabolize fat is an emerging area of investigation. Numerous tumors forgo the use of glucose and glutamine in favor of fatty acid oxidation. In many tissues, fatty acids are not the predominant fuel choice, rather, fatty acid metabolism is reserved for conditions of stress or nutrient depletion as a means to restore metabolic homeostasis. Metabolic rewiring in fatty acid metabolism occurs in mouse hepatocellular carcinomas, primary human liver, and in lung carcinomas. Cancer cells desaturate palmitate to the unusual fatty acid sapienate to support membrane biosynthesis (Vriens et al., 2019). Moreover, plasma membrane remodeling is a necessary component of oncogenic signaling. Lipids are the main component of cellular membranes and play a crucial role in creating a barrier between subcellular compartments. Subtle changes in the structure, composition, and interactions of lipids in cellular membranes can dramatically alter biological functions. Advances in lipidomics have identified the lipid species that make up mammalian membranes. The major membrane lipids are glycerophospholipids (GPL), sphingolipids, and sterols (Harayama and Riezman, 2018). Membrane lipid composition, levels of saturation and cellular distribution of lipids are underexplored aspects that are crucial for organelle homeostasis, cell signaling, and the management of nutrient and oxidative stress (Röhrig and Schulze, 2016; Rysman et al 2010; Young et al. 2013). Lysophosphatidylcholine acyltransferase (LPCAT1) enhances saturated phosphatidylycholine content in the composition of the plasma membrane and also drives tumor growth by activating oncogenic signals (Bi et al., 2019).
Interestingly, high levels of MUFAs suppress ferroptosis by competing with PUFAs for insertion into the membrane (Magtanong et al, 2019). This membrane lipid composition is related to suppression of ROS at the plasma membrane and decreased levels of phospholipids containing oxidizable polyunsaturated fatty acids. This effect requires MUFA activation by acyl-coenzyme A synthetase long-chain family member (ACSL) 3 (Magtanong et al, 2019). Like ACSL3, lysophosphatidylcholine acyltransferase 3 (LPCAT3) has an important role in PUFA incorporation into phospholipids. Lack of LPCAT3 leads to modulation of membrane phospholipid composition by drastic reductions of AA, which is a polyunsaturated fatty acid present in phospholipids (Hashidate-Yoshida et al., 2015). ACSL4 is also implicated in the localized release of AA in the mitochondria by catalyzing the conversion of long-chain fatty acids to their active form, acyl-CoA, for synthesis of cellular lipids (Kuwata and Hara, 2019). ACSL4 limits the cytotoxicity associated with elevated cellular pools of unesterified AA by producing arachidonoyl-CoA, thereby increasing the apoptotic threshold and survival of castration-resistant prostate cancer (Kuwata and Hara, 2019). This suggests that the localized accumulation of PUFAs in the mitochondria can contribute to membrane depolarization and electron transport chain uncoupling, leading to increased ROS production. Therefore, specific fatty acids trigger ROS generation, ER stress and ferroptosis, suggesting the need for studies that define the function of lipid properties in cancer (Figure 4).
Figure 4. Lipid signaling in cancer.
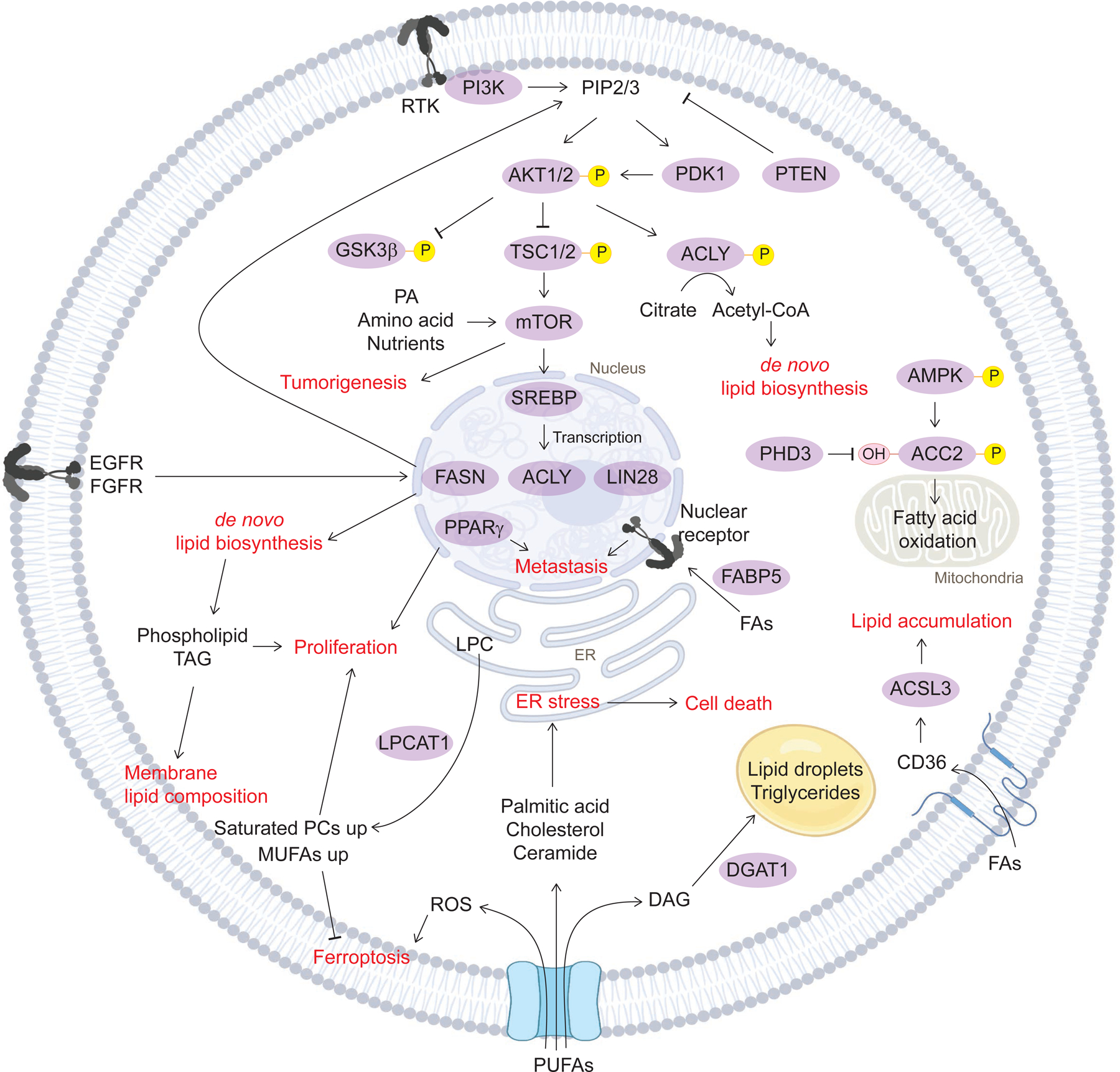
Lipid metabolism directly affects cancer signaling. AKT phosphorylation, the major cancer signaling pathway, activates de novo lipid biosynthesis through ACLY. This phosphorylation cascade coordinates with PA, amino acids, mTOR signaling to boost tumorigenesis, and increase SREBP transcription, resulting in fatty acid synthesis. Phospholipids and TAGs from de novo lipid biosynthesis activate proliferation and rewire membrane lipid composition by increasing saturated phospholipids. Fatty acid uptake into the nucleus directly activates cancer metastasis by nuclear receptor and the transcription factor PPAR. PUFAs induce ROS-dependent ferroptosis. Different lipid signaling employs different adaptive responses in cancer -- either survival or death. ACC2, acetyl-CoA carboxylase 2; ACLY, ATP-citrate lyase; ACSL3, acyl-CoA synthetase long-chain family member 3/4; AKT, RAC-alpha serine/threonine-protein kinase; AMPK, AMP-activated protein kinase; DAG, Diacylglycerol; DGAT1, diacylglycerol O-acyltransferase 1; FAs fatty acids; FABP5, fatty-acid binding proteins 5; FASN, fatty acid synthase; LPC, Lysophosphatidylcholine; RTK, Receptor tyrosine kinase.
Lipid Toxicity in Cancer and Immune Cells
Tumors hijack pathways that protect normal cells from lipotoxicity as a mechanism to circumvent the harsh tumor microenvironment. Cancer cells prevent the accumulation of toxic cellular lipids and waste products using autophagy, which targets intracellular products and organelles to the lysosomal compartment for degradation (Poillet-Perez and White, 2019). Palmitate and other saturated FAs induce apoptosis in breast cancer, while unsaturated FAs, such as oleate, are non-toxic for cancer cells (Hardy et al. 2003; Yang et al. 2018). Cancer cells also protect against lipid toxicity by converting potentially toxic lipids, including fatty acids, DAG, cholesterol and ceramide to triglycerides, cholesterol esters and acylceramides that can be stored in lipid droplets (Senkal et al., 2017). Moreover, DGAT1 converts excess fatty acids into triglycerides and lipid droplets to protect glioblastoma cells from oxidative damage (Cheng et al. 2020). Therefore, inhibiting DGAT1 results in an excess of fatty acids moving into the mitochondria for oxidation, leading to the generation of high levels of ROS, mitochondrial damage, cytochrome c release, and apoptosis. Moreover, blocking DGAT1 can also channel fatty acids into phospholipids and increase ferroptosis (Dierge et al., 2021).
Ferroptosis (Stockwell et al. 2017), also operative in cancer cells, is the result of lipid peroxidation of PUFAs present in phospholipids to generate various lipid hydroperoxides (Kuhn et al. 2015). The expression of ELOVL5 and fatty acid desaturase 1 (FADS1) is upregulated in gastric cancer cells, leading to ferroptosis sensitization. AA supplementation restores sensitivity to ferroptosis in gastric cancer cells (Lee et al. 2020). The enzyme GPX4 is a central regulator of ferroptosis, and protects cells by neutralizing lipid peroxides. Ferroptosis suppression by ferroptosis suppressor protein 1 (FSP1) reduces CoQ, which acts as a lipophilic radical-trapping antioxidant that inhibits the propagation of lipid peroxides in several cancer cells (Bersuker et al., 2019; Doll et al., 2019). A recent study showed that immunotherapy-activated CD8+ T cells enhance ferroptosis-specific lipid peroxidation in tumour cells, and that increased ferroptosis contributes to the anti-tumour efficacy of immunotherapy (Wang et al. 2019). Therefore, modulation of biosynthetic and peroxisomal oxidation pathways may offer new opportunities for ferroptosis-mediated cancer therapy.
LIPID METABOLISM AND THE MICROBIOME
Interactions between the gut microbiome and host lipid homeostasis are highly relevant to metabolic disease susceptibility. Microbiota generate monounsaturated fatty acids by stearoyl-CoA desaturase 1 and polyunsaturated fatty acids via elongation by fatty acid elongase 5, leading to significant alterations in glycerophospholipid acyl-chain profiles. Interestingly, gut microbiota also generate acetate from dietary fiber, which serves as a precursor for hepatic long-chain fatty acids and their related glycerophospholipid species (Kindt et al., 2018). Also, Streptococcus pneumoniae, Gram-positive spherical bacteria, respond to exogenous fatty acids by suppressing de novo biosynthetic pathways and exclusively utilizing extracellular fatty acids for membrane phospholipid synthesis. This suggests that Streptococcus pneumoniae permits the utilization of the entire spectrum of mammalian fatty acid structures to construct its membrane (Gullett et al., 2019). For a more detailed discussion of lipids and the microbiome, we refer the reader to the following comprehensive review on the subject (Schoeler and Caesar, 2019).
TARGETING LIPID METABOLISM: DRUG DISCOVERY
Fatty acid biology may illuminate new targets for the treatment of metabolic diseases. While there are currently no direct fatty acid targets, there are drugs that inhibit long-chain acyl carnitine import into mitochondria, including fatty acid oxidation inhibitors such as Etomoxir (German et al., 2016; Pike et al., 2011), Ranolazine (Samudio et al., 2010), Soraphen-A (Beckers et al., 2007), TOFA (5-(tetradecyloxy)-2-furoic acid) (Guo et al., 2009; Pizer et al., 2000), and A-769662 (Göransson et al., 2007). Ranolazine is FDA approved as an anticancer drug, based on its targeting of FAO. While the inhibition of de novo fatty acid synthesis may cause a multitude of side effects, TVB-2640, a FASN-antagonist, is currently studied in Phase II trials as another putative cancer therapy (Brenner et al., 2015). Metformin (Pollak, 2012) and AICAR are AMPK activators that increase both FAO and FAS with anti-diabetic properties (Jose et al., 2011; Swinnen et al., 2005). Drugs that target the FAS pathway include SB-204990 (Hatzivassiliou et al., 2005; Ros et al., 2012) and LY294002 (Migita et al., 2008) modulators of the PI3K signaling pathway. A more comprehensive list of FAS inhibitors is included in Table 1.
Table 1. Fatty acid targeting drug development.
Summary of drugs developed to target fatty acid oxidation, fatty acid synthesis, and fatty acid uptake.
| Mechanism of Action | Target | Inhibitor | Selected Reference | Target Disease or Condition | Clinical trials (https://clinicaltrials.gov) |
|---|---|---|---|---|---|
| Fatty acid oxidation | |||||
| Inhibition of fatty acid β-oxidation and activation of pyruvate dehydrogenase | ACC | Ranolazine | Zacharowski et al., 2001; Samudio et al., 2010 | Chronic angina (FDA approval); acute myocardial infarction; leukemia | FDA approved (NDA #021526) |
| ACC (acetyl-CoA carboxylase) inhibitor, a PPAR-α agonist | ACC, PPARα | TOFA (5-(tetradecyloxy)-2-furoic acid) | Ottemann Abbamonte et al., 2021; Pizer et al., 2000; Guo et al., 2009 | Cutaneous lupus; systemic lupus erythematosus, glioblastoma; breast cancer | Phase 2 clinical trials (NCT03288324) |
| FAO activator | AMPK | A-769662 | Kemmerer et al., 2015; Goransson et al., 2007 | Type 2 diabetes (T2D); macrophages | NA |
| Inhibition of long-chain fatty acid import into mitochondria | CPT1 | Etomoxir | German et al., 2016; Pike et al., 2011 | Leukemia; glioblastoma | NA |
| Blocking FAO | Long-chain 3-ketoacyl-CoA thiolase (LCTH) | Trimetazidine | Gatta et al., 2017 | Precapillary pulmonary hypertension; muscle wasting (cachexia) | Phase 2 clinical trials (NCT03273387) |
| Fatty acid synthesis | |||||
| Fatty acid elongation | ACC | Soraphen-A | Beckers et al., 2007 | Prostate cancer; high fat diet-induced insulin resistance; hepatic steatosis | NA |
| Block lipigenesis | ACC | Firsocostat (GS-0976, NDI-010976, ND-630 | Alkhouri et al., 2020 | Non-alcoholic Steatohepatitis (NASH) | Phase 1 clinical trials (NCT02891408) |
| Inhibit TAG accumulation into lipid droplets | Acyl-CoA synthetases (ACS) | Triacscin C | Mashima et al., 2005 | Lung cancer; colon cancer; stomach cancer; brain cancer; and breast cancer | NA |
| FAS inhibitor | Acylglycerolphosphate acyltransferase (AGPAT) | CT-32501 | Takeuchi and Reue, 2009 | Prostate cancer | NA |
| ACLY inhibitor and AMPK activator in liver | ATP-citrate lyase (ACLY) and AMPK | ETC-1002 | Chen et al., 2020 | Hyperlipidemia | Phase 2 clinical trials (NCT02659397) |
| FAS activator | AMPK | Metformin | Bhansali et al., 2020 | Type 2 diabetes; solid cancer | FDA approved, Phase 4 clinical trials |
| FAS activator | AMPK | Aminoimidazole Carboxamide Riboside (AICAR) | Jose et al., 2011; Swinnen et al., 2005 | Lesch-Nyhan Syndrome; cancer | Phase 2 clinical trials (NCT00004314) |
| Blocking generation of acetyl-CoA for cholesterol and de novo fatty acid synthesis | ATP citrate lyase (ACLY) | SB-204990 | Hatzivassili ou et al., 2005; Ros et al., 2012 | Non-small cell lung cancer; solid cancer | NA |
| Blocking phosphatidylcholine (PC) biosynthesis | Choline kinase alpha (CKα) | TCD-717 | Glunde et al., 2011 | Solid cancer | Phase 1 clinical trials (NCT01215864) |
| Blocking fatty acid synthase and glutamate dehydrogenase activity | DNA methyltransferase, EGF receptors, HER-2 receptors, FASN | Epigallocatechin-3-gallate (EGCG) | Humbert et al., 2021 | Lung neoplasms; esophagitis | Phase 2 clinical trials (NCT02577393) |
| Blocking triglyceride (TG) synthesis | Diacylglycerol acyltransferase 1 (DGAT1) | A922500 | Zhao et al., 2008 | Obesity; dyslipidemia | NA |
| Suppressing triacylglyceride (TAG) plasma excursion and adipose tissue TAG synthesis | DGAT | AZD3988 | McCoull et al., 2012 | Type 2 diabetes (T2D); obesity | NA |
| Blocking TG synthesis | DGAT | AZD7687 | Morentin et al., 2019 | Obesity; overweight | Phase 1 clinical trials (NCT01119352) |
| Blocking TG synthesis | DGAT | JNJ DGAT2-A | Irshad et al., 2016 | Type 2 diabetes (T2D); solid cancer | NA |
| Blocking hydrolysis of triglycerides and the absorption of FFA | FASN | Orlistat | Schcolnik-Cabrera et al, 2018; Zhi et al., 1995 | Obesity; overweight | FDA approved |
| FASN antagonistic drug | FASN | TVB-2640 | Lolkema et al., 2015 | Non-alcoholic fatty liver disease | Phase 2 clinical trials (NCT049064210) |
| FAS inhibitor | Fatty acid synthase (FASN) and HMG-CoA reductase (HMGR) | Cerulenin | Currie et al., 2013; Lupu and Menendez, 2006; Ros et al., 2012 | Hepatic steatosis; solid cancer | NA |
| FAS inhibitor | Mitochondrial citrate transporter (CIC) | Benzene-tricarboxylate analog (BTA) | Catalina-Rodriguez et al., 2012 | Solid cancer | NA |
| Fatty acid amide hydrolase inhibitor | Monoacylglycerol lipase (MAGL) | JZL184 | Walenna et al., 2020; Taïb et al., 2019 | Type 2 diabetes (T2D); glioblastoma | NA |
| Blocking biosynthesis of monounsaturated fatty acids | Stearoyl-coA desaturase (SCD) | A939572 | von Roemeling et al., 2013 | Renal cell carcinoma | NA |
| Alters membrane fatty acid composition | SCD | BZ36 | Fritz et al., 2010 | Prostate cancer | NA |
| Blocking the conversion of saturated, long-chain fatty acyl-CoAs to monounsaturated | SCD | CAY10566 | Liu et al., 2007 | Breast cancer; lung cancer; colorectal cancer | NA |
| FAS inhibitor in liver | SCD | MK-8245 | Oballa et al., 2011 | Type 2 diabetes (T2D) | Phase 1 clinical trials (NCT00790556) |
| Inhibits the ER-Golgi translocation of SREBPs | SREBP1/2 | Fatostatin | Williams et al., 2013 | Prostate cancer | NA |
| Blocks biosynthesis and accumulation of fat | SREBP1/2 | FGH10019 | Williams et al., 2013, Kamisuki et al., 2011 | Prostate cancer | NA |
| Blocking SREBP activity | PI3Kα/β/δ | LY294002 | Migita et al., 2008 | Neuroblastoma; solid cancer | Phase 1 clinical trials |
| PPAR agonists drug for T2DM | PPARδ | Thiazolidinediones (TZDs) | Kim et al., 2001 | Type 2 diabetes (T2D) | FDA approved |
| Fat uptake | |||||
| Peptide mimetics of thrombospondin-1 (TSP-1) | CD36 | ABT-510 | Markovic et al., 2007 | Melanoma, renal cell carcinoma, lymphoma | Phase 2 clinical trials (NCT00602199) |
Emerging evidence suggests that lipid transporters may represent a new therapeutic target in cancer and metabolic disorders. CD36 is one such transporter that plays an important role in facilitating intracellular FFA uptake and trafficking (Coburn et al., 2001). Importantly, CD36 membrane levels and turnover rates are disrupted in diabetes leading to dysfunctional FA utilization, and variants in the CD36 gene influence susceptibility for metabolic syndrome (Love-Gregory et al., 2008). In the context of cancer, neutralizing antibodies used to block CD36 cause complete inhibition of metastasis in mouse models of human oral cancers and encouragingly impair metastasis in human melanoma- and breast cancer derived-tumors. Palmitic acid or a high-fat diet specifically enhance the metastatic potential of CD36+ metastasis-initiating cells suggesting that metastasis-initiating cells respond to dietary lipids (Pascual et al., 2017). The therapeutic relevance of CD36 is not restricted to oral cancers since CD36 is upregulated in intratumoral Treg cells where it acts as a central metabolic modulator. Furthermore, genetic ablation of CD36 in Treg cells suppresses tumor growth and enhances antitumor reactivity in tumor-infiltrating lymphocytes while preserving immune homeostasis (Wang et al., 2020). Selectively disrupting intratumoral Treg cells is a sought-after approach for cancer immunotherapy.
TECHNICAL ADVANCES
Historically, cellular lipids were detected based on their biochemical and morphologic features using BODIPY and oil red O staining (French et al., 1993; Mehlem et al., 2013). Moreover, certain FFAs have been used as markers of diabetes (Boden, 2008; Reaven et al., 1988). With advances in technology, our ability to identify lipid species with greater granularity grows, directly contributing to a greater understanding of lipid homeostasis in physiology as well as in metabolic diseases. Analytical chemistry methods applied to understanding the entire lipid content of a cell have illuminated the lipidome and have revealed exciting new roles for lipids in cell biology and physiology (Han, 2016). Advances in mass spectrometry have expanded analytical sensitivity and specificity in lipidomic analyses, including (1) the characterization of lipids in relevant cellular compartments and structures by cellular fractionation, (2) the measurement of the physical properties of lipids, and (3) the description of the phenotypic and functional consequences of specific lipid perturbations. Chemical imaging, including coherent Raman scattering (CRS) microscopy, enables label-free visualization of lipid molecules in live cells allowing unprecedented visualization of the distribution and heterogeneity of lipids (Chen et al., 2020b). Addressing the challenge of tracing metabolic reactions within the complex network of cellular lipid metabolism, click-chemistry mass spectrometry reporter strategies now enable parallel quantitative monitoring of as many as 120 distinct, labeled lipid species to trace alkyne-labeled lipids (Thiele et al., 2019). Advances in metabolic tracing continue to enable a deeper investigation into how fatty acids are incorporated into membrane lipids. Applying this method to follow de novo fatty acid synthesis or degradation by beta oxidation will be an interesting future application.
A substantial number of drug targets are lipid-binding proteins and a map of lipid-protein interactions could uncover new modes of signaling of relevance to pharmacological perturbation (Niphakis et al., 2015). Lipid-based chemical proteomic probes identify the proteins that participate in lipid pathways in cells (Niphakis et al., 2015). Ligand-receptor, substrate-enzyme, and client-carrier relationships are just some of the interactions regulated by lipid-protein interactions. To identify proteins that interact with fatty acid-derived lipids, a set of probes containing binding groups that resembled common fatty acids, including arachidonic (C20:4), oleic (C18:1), palmitic (C16:0), and stearic (C18:0) was used to demonstrate that arachidonoyl lipids preferentially interact with proteins. In situ drug profiling with arachidonoyl lipid probes revealed that the lipid-interaction proteome is enriched in known drug targets. Combining a lipid probe approach with high-throughput drug screening identified NUCB1 as a previously unknown protein involved in facilitating the intracellular transfer of FA-derived lipid messengers, fatty acyl ethanolamides (NAEs)/NATs, for delivery to metabolic enzymes, such as FAAH and PTGS2 (Niphakis et al., 2015). Applying this approach to structurally distinct drugs has the potential to uncover additional ligand-protein interactions. The current gold standard for visualizing lipids in complex with membrane proteins is to use cryo-electron microscopy and X-ray crystallography. In the past, extraction-analysis coupled with LC-MS-based quantitative lipidomics was used to compare the lipid profile that co-purifies with a protein of interest with that of the native membrane to provide indirect evidence of interaction. The limitation of this approach is that it reports on lipids that are co-purified with a particular protein complex without differentiating regulatory lipids that are linked to a specific biological function. A recently published approach combines high-energy native mass spectrometry (HE-nMS) and solution-phase lipid profiling to determine the identity of lipids that directly interact with a protein of interest (Gupta et al., 2018). This approach offers a way to identify lipids that interact with a membrane protein and has been applied to understand how specific lipids maintain oligomeric states.
Studies combining CRISPR-based genetic screens, unbiased lipidomics, and transcriptomics have the potential to identify novel regulators of lipotoxicity (Piccolis et al., 2019; Zhu et al., 2019). Recent investigations using the known toxic saturated fatty acid, palmitate (C16:0), identified genetic modifiers of lipotoxicity in a cellular model using human leukemia cells. The authors nominated two novel targets for follow-up studies: a putative E3 ligase and ER-localized glycerol-3-phosphate acyltransferase (GPAT) enzymes (Piccolis et al., 2019). In the context of this study, one lipid class emerged as central to lipotoxicity: di-saturated glycerolipids (Piccolis et al., 2019). Palmitic acid exposure increases the relative amounts and saturation levels of glycerolipids including TG, DAG, phosphatidic acid, and lysophospholipids. A parallel study used a combination of a CRISPR-based genetic screen using palmitate and unbiased lipidomics to identify calcineurin B homologous protein 1 (CHP1) as a major regulator of ER glycerolipid synthesis (Zhu et al., 2019). CHP1 binds and activates GPAT4, which catalyzes the initial rate-limiting step in glycerolipid synthesis. Knockout of CHP1 in Jurkat cells, a human leukemic T-cell line, surprisingly resulted in cell proliferation due to partial compensation for this CHP1-knockout-mediated loss of ER lipid synthesis by GNPAT, a peroxisomal enzyme (Zhu et al., 2019). This compensation indicates a degree of plasticity and dynamic regulation of glycerolipid metabolism and lipid synthesis of proliferating cells. Furthermore, ongoing efforts to use systematic and unbiased approaches to identify alterations in lipid droplets include the Lipid Droplet Knowledge Portal (LD-Portal, lipiddroplet.org), a platform that integrates transcriptional profiles of lipid storage, organelle proteomics, genome-wide screens, and human genetics to characterize determinants of lipid storage (Mejhert et al.). Systematic studies of lipid biology utilizing new technologies will help identify targets for therapeutic intervention for diseases related to disrupted lipid metabolism.
A compelling future direction for the field of lipid biology is the use of spatial information to investigate changes in metabolites using advanced imaging techniques. Mass spectrometry imaging (MSI) is a technique used to simultaneously visualize the spatial distribution of molecules in a biological sample with implications for pharmacological target screening. For example, in the aftermath of drug treatment, lipid disturbance analysis can be performed using liquid chromatography-mass spectrometry (LC-MS) based lipidomics combined with matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI-MSI) to map the location of biomolecules within tissue (Djambazova et al., 2020). Fully characterizing the structural diversity of lipids is an ongoing challenge, but recent efforts showed separation and imaging of lipid isomers with distinct spatial distributions from a whole-body mouse pup using MALDI trapped ion mobility separation (TIMS) MS (Djambazova et al., 2020). Spatial information can have profound implications for understanding biological function. In the mouse liver, investigators analyzed lipid composition in the nucleus and mitochondria as well as the temporal changes in lipid composition throughout the day and in response to time restricted feeding. Applying shotgun lipidomics (Han et al., 2012) revealed a total of 222 individual lipid species of which 147 were present in both organelles, 5 were exclusive to the nucleus, and 70 were unique to the mitochondria (Aviram et al., 2016). Of interest, restricting food availability and intake to nighttime induced dramatic changes in the lipid composition of different organelles.
Conclusions and Future Perspectives
In this review, we investigate the role of lipids as mediators of the progression of metabolic disorders. Increasing evidence suggests that the fatty acid composition of fats and the individual concentrations of FAs (i.e. monounsaturated vs. polyunsaturated, ratio of saturated to unsaturated, odd or even chain saturated FAs, omega position, etc.) have distinct effects on metabolism at the cellular level. Currently, high-throughput methods that take advantage of transcriptomics and functional genomics can nominate genes that are most relevant for metabolic disorders; however, in the future, there is a need to develop comprehensive platforms that can probe the biological effects of a diversity of lipid species in an effort to tease out the mechanisms by which structurally distinct lipids contribute to disease progression. Future work to understand lipid metabolism will rely on advances in chemical imaging, functional genomics, and biochemical characterization of the spatiotemporal dynamics of lipids. Emerging efforts to understand lipid-protein interactions and spatial transcriptomics in metabolic disorders and cancer will identify drug targets with the potential to promote beneficial lipid regulatory mechanisms. Advances in lipidomics have enabled the identification and characterization of lipid species; however, in comparison to gene expression or protein interaction networks, the subcellular distribution of lipids is an ongoing challenge. Spatiotemporal analyses provide an opportunity to move beyond snapshots of lipids within the whole cell or tissue at a single time point.
Acknowledgments
We regret that we were unable to cite all the primary literature relevant to this topic due to space limitations. This work was made possible by support from NIH grants DK095045 and DK099465, the Cure Alzheimer’s Fund, and the Slim Initiative for Genomic Medicine in the Americas (SIGMA), a collaboration of the Broad Institute with the Carlos Slim Foundation (A.G.). M.C.H. is supported by the Ludwig Center at Harvard Medical School, the Paul F. Glenn Foundation for Medical Research, and NIH grants RO1CA213062 and U54CA224088.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
A.G. has a financial interest in Goldfinch Biopharma, which was reviewed and is managed by Brigham and Women’s Hospital, Mass General Brigham (MGB) and the Broad Institute of MIT and Harvard in accordance with their conflict of interest policies.
References
- Alicic RZ, Rooney MT, and Tuttle KR (2017). Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol 12, 2032–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhouri N, Lawitz E, Noureddin M, DeFronzo R, and Shulman GI (2020). GS-0976 (Firsocostat): an investigational liver-directed acetyl-CoA carboxylase (ACC) inhibitor for the treatment of non-alcoholic steatohepatitis (NASH). Expert Opin. Investig. Drugs 29, 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anaokar S, Kodali R, Jonik B, Renne MF, Brouwers JFHM, Lager I, de Kroon AIPM, and Patton-Vogt J (2019). The glycerophosphocholine acyltransferase Gpc1 is part of a phosphatidylcholine (PC)-remodeling pathway that alters PC species in yeast. J. Biol. Chem 294, 1189–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeli JPF, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, et al. (2014). Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nature Cell Biology 16, 1180–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arana L, Gangoiti P, Ouro A, Trueba M, and Gómez-Muñoz A (2010). Ceramide and ceramide 1-phosphate in health and disease. Lipids Health Dis 9, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aregger M, Lawson KA, Billmann M, Costanzo M, Tong AHY, Chan K, Rahman M, Brown KR, Ross C, Usaj M, et al. (2020). Systematic mapping of genetic interactions for de novo fatty acid synthesis identifies C12orf49 as a regulator of lipid metabolism. Nature Metabolism 2, 499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviram R, Manella G, Kopelman N, Neufeld-Cohen A, Zwighaft Z, Elimelech M, Adamovich Y, Golik M, Wang C, Han X, et al. (2016). Lipidomics Analyses Reveal Temporal and Spatial Lipid Organization and Uncover Daily Oscillations in Intracellular Organelles. Mol. Cell 62, 636–648. [DOI] [PubMed] [Google Scholar]
- Bader JE, Voss K, and Rathmell JC (2020). Targeting Metabolism to Improve the Tumor Microenvironment for Cancer Immunotherapy. Mol. Cell 78, 1019–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi AK, Surendran A, Malik A, Jassal DS, Ravandi A, and Singal PK (2020). IL-10 attenuates OxPCs-mediated lipid metabolic responses in ischemia reperfusion injury. Sci. Rep 10, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey AP, Koster G, Guillermier C, and Hirst EMA (2015). Antioxidant role for lipid droplets in a stem cell niche of Drosophila. Cell [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban RS, and Mandel LJ (1988). Metabolic substrate utilization by rabbit proximal tubule. An NADH fluorescence study. Am. J. Physiol 254, F407–F416. [DOI] [PubMed] [Google Scholar]
- Baratta F, Pastori D, Ferro D, Carluccio G, Tozzi G, Angelico F, Violi F, and Del Ben M (2019). Reduced lysosomal acid lipase activity: A new marker of liver disease severity across the clinical continuum of non-alcoholic fatty liver disease? World J. Gastroenterol 25, 4172–4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers A, Organe S, Timmermans L, Scheys K, Peeters A, Brusselmans K, Verhoeven G, and Swinnen JV (2007). Chemical inhibition of acetyl-CoA carboxylase induces growth arrest and cytotoxicity selectively in cancer cells. Cancer Res 67, 8180–8187. [DOI] [PubMed] [Google Scholar]
- Beloribi-Djefaflia S, Vasseur S, and Guillaumond F (2016). Lipid metabolic reprogramming in cancer cells. Oncogenesis 5, e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berliner JA, Leitinger N, and Tsimikas S (2009). The role of oxidized phospholipids in atherosclerosis. J. Lipid Res 50 Suppl, S207–S212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al. (2019). The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575, 688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhansali S, Bhansali A, and Dhawan V (2020). Metformin promotes mitophagy in mononuclear cells: a potential in vitro model for unraveling metformin’s mechanism of action. Ann. N. Y. Acad. Sci 1463, 23–36. [DOI] [PubMed] [Google Scholar]
- Bi J, Ichu TA, Zanca C, Yang H, Zhang W, Gu Y, Chowdhry S, Reed A, Ikegami S, Turner KM, Zhang W, Villa GR, Wu S, Quehenberger O, Yong WH, Kornblum HI, Rich JN, Cloughesy TF, Cavenee WK, Furnari FB, Cravatt BF, Mischel PS (2019). Oncogene Amplification in Growth Factor Signaling Pathways Renders Cancers Dependent on Membrane Lipid Remodeling. Cell Metab 30, 525–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder CJ, Papac-Milicevic N, and Witztum JL (2016). Innate sensing of oxidation-specific epitopes in health and disease. Nat. Rev. Immunol 16, 485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birsoy K, Wang T, Chen WW, Freinkman E, Abu-Remaileh M, and Sabatini DM (2015). An Essential Role of the Mitochondrial Electron Transport Chain in Cell Proliferation Is to Enable Aspartate Synthesis. Cell 162, 540–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobulescu IA (2010). Renal lipid metabolism and lipotoxicity. Curr. Opin. Nephrol. Hypertens 19, 393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G (2008). Obesity and free fatty acids. Endocrinol. Metab. Clin. North Am 37, 635–646, viii – ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogie JFJ, Grajchen E, Wouters E, Corrales AG, Dierckx T, Vanherle S, Mailleux J, Gervois P, Wolfs E, Dehairs J, et al. (2020). Stearoyl-CoA desaturase-1 impairs the reparative properties of macrophages and microglia in the brain. J. Exp. Med 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner AJ, Von Hoff DD, Infante JR, Patel MR, Jones SF, Burris HA, Rubino C, McCulloch W, Zhukova-Harrill V, and Kemble G (2015). First-in-human investigation of the oral first-in-class fatty acid synthase (FASN) inhibitor, TVB-2640. J. Clin. Orthod 33, TPS2615–TPS2615. [Google Scholar]
- Broadfield LA, Pane AA, Talebi A, Swinnen JV, and Fendt S-M (2021). Lipid metabolism in cancer: New perspectives and emerging mechanisms. Dev. Cell 56, 1363–1393. [DOI] [PubMed] [Google Scholar]
- Cantley LC (2002). The phosphoinositide 3-kinase pathway. Science. 296, 1655–1657. [DOI] [PubMed] [Google Scholar]
- Carbonetti G, Wilpshaar T, Kroonen J, Studholme K, Converso C, d’Oelsnitz S, and Kaczocha M (2019). FABP5 coordinates lipid signaling that promotes prostate cancer metastasis. Sci. Rep 9, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carracedo A, Cantley LC, and Pandolfi PP (2013). Cancer metabolism: fatty acid oxidation in the limelight. Nat. Rev. Cancer 13, 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho CCCR, and Caramujo MJ (2018). The Various Roles of Fatty Acids. Molecules 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cases S, Smith SJ, Zheng YW, Myers HM, Lear SR, Sande E, Novak S, Collins C, Welch CB, Lusis AJ, et al. (1998). Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc. Natl. Acad. Sci. U. S. A 95, 13018–13023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalina-Rodriguez O, Kolukula VK, Tomita Y, Preet A, Palmieri F, Wellstein A, Byers S, Giaccia AJ, Glasgow E, Albanese C, et al. (2012). The mitochondrial citrate transporter, CIC, is essential for mitochondrial homeostasis. Oncotarget 3, 1220–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechetto JD, Sadacharan SK, Berk PD, and Gupta RS (2002). Immunogold localization of mitochondrial aspartate aminotransferase in mitochondria and on the cell surface in normal rat tissues. Histol. Histopathol 17, 353–364. [DOI] [PubMed] [Google Scholar]
- Chen F, Wu X, Niculite C, Gilca M, Petrusca D, Rogozea A, Rice S, Guo B, Griffin S, Calin GA, et al. (2020a). Classic and targeted anti-leukaemic agents interfere with the cholesterol biogenesis metagene in acute myeloid leukaemia: Therapeutic implications. J. Cell. Mol. Med 24, 7378–7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W-W, Lemieux GA, Camp CH, Chang T-C, Ashrafi K, and Cicerone MT (2020b). Spectroscopic coherent Raman imaging of Caenorhabditis elegans reveals lipid particle diversity. Nat. Chem. Biol 16, 1087–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Palanisamy AP, Evans ZP, Sutter AG, Jin L, Singh I, May H, Schmidt MG, and Chavin KD (2013). Cerulenin blockade of fatty acid synthase reverses hepatic steatosis in ob/ob mice. PLoS One 8, e75980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn CT, Hajri T, Ibrahimi A, and Abumrad NA (2001). Role of CD36 in membrane transport and utilization of long-chain fatty acids by different tissues. J. Mol. Neurosci 16, 117–121. [DOI] [PubMed] [Google Scholar]
- Coe NR, Simpson MA, and Bernlohr DA (1999). Targeted disruption of the adipocyte lipid-binding protein (aP2 protein) gene impairs fat cell lipolysis and increases cellular fatty acid levels. J. Lipid Res 40, 967–972. [PubMed] [Google Scholar]
- Currie E, Schulze A, Zechner R, Walther TC, and Farese RV Jr (2013). Cellular fatty acid metabolism and cancer. Cell Metab 18, 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agati VD, Chagnac A, de Vries APJ, Levi M, Porrini E, Herman-Edelstein M, and Praga M (2016). Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat. Rev. Nephrol 12, 453–471. [DOI] [PubMed] [Google Scholar]
- DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, and Thompson CB (2007). Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proceedings of the National Academy of Sciences 104, 19345–19350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh AS, Peijs L, Beaudry JL, Jespersen NZ, Nielsen CH, Ma T, Brunner AD, Larsen TJ, Bayarri-Olmos R, Prabhakar BS, et al. (2019). Proteomics-Based Comparative Mapping of the Secretomes of Human Brown and White Adipocytes Reveals EPDR1 as a Novel Batokine. Cell Metab 30, 963–975.e7. [DOI] [PubMed] [Google Scholar]
- Dierge E, Debock E, Guilbaud C, Corbet C, Mignolet E, Mignard L, Bastien E, Dessy C, Larondelle Y, and Feron O (2021). Peroxidation of n-3 and n-6 polyunsaturated fatty acids in the acidic tumor environment leads to ferroptosis-mediated anticancer effects. Cell Metab [DOI] [PubMed] [Google Scholar]
- Dihingia A, Bordoloi J, Dutta P, Kalita J, and Manna P (2018). Hexane-Isopropanolic Extract of Tungrymbai, a North-East Indian fermented soybean food prevents hepatic steatosis via regulating AMPK-mediated SREBP/FAS/ACC/HMGCR and PPARα/CPT1A/UCP2 pathways. Sci. Rep 8, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djambazova KV, Klein DR, Migas LG, Neumann EK, Rivera ES, Van de Plas R, Caprioli RM, and Spraggins JM (2020). Resolving the Complexity of Spatial Lipidomics Using MALDI TIMS Imaging Mass Spectrometry. Anal. Chem 92, 13290–13297. [DOI] [PubMed] [Google Scholar]
- Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, Goya Grocin A, Xavier da Silva TN, Panzilius E, Scheel CH, et al. (2019). FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575, 693–698. [DOI] [PubMed] [Google Scholar]
- Du W, Zhang L, Brett-Morris A, Aguila B, Kerner J, Hoppel CL, Puchowicz M, Serra D, Herrero L, Rini BI, et al. (2017). HIF drives lipid deposition and cancer in ccRCC via repression of fatty acid metabolism. Nat. Commun 8, 1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhamri M, Martin M, Ferrier B, and Baverel G (1993). Substrate uptake and utilization by the kidney of fed and starved rats in vivo. Ren. Physiol. Biochem 16, 311–324. [DOI] [PubMed] [Google Scholar]
- Fagone P, and Jackowski S (2009). Membrane phospholipid synthesis and endoplasmic reticulum function. J. Lipid Res 50 Suppl, S311–S316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Wang H, Han D, Xie E, Yang X, Wei J, Gu S, Gao F, Zhu N, Yin X, et al. (2019). Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. U. S. A 116, 2672–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix JB, Cox AR, and Hartig SM (2021). Acetyl-CoA and Metabolite Fluxes Regulate White Adipose Tissue Expansion. Trends Endocrinol. Metab 32, 320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro GB, Ali A, Luengo A, Kodack DP, Deik A, Abbott KL, Bezwada D, Blanc L, Prideaux B, Jin X, et al. (2021). Fatty acid synthesis is required for breast cancer brain metastasis. Nature Cancer 2, 414–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, Han X, Gross RW, Kozak R, Lopaschuk GD, et al. (2002). The cardiac phenotype induced by PPARα overexpression mimics that caused by diabetes mellitus. J. Clin. Invest 109, 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck BN, Han X, Courtois M, Aimond F, Nerbonne JM, Kovacs A, Gross RW, and Kelly DP (2003). A critical role for PPAR -mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: Modulation by dietary fat content. Proceedings of the National Academy of Sciences 100, 1226–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French AN, Wilson SR, Welch MJ, and Katzenellenbogen JA (1993). A synthesis of 7 alpha-substituted estradiols: synthesis and biological evaluation of a 7 alpha-pentyl-substituted BODIPY fluorescent conjugate and a fluorine-18-labeled 7 alpha-pentylestradiol analog. Steroids 58, 157–169. [DOI] [PubMed] [Google Scholar]
- Fritz IB, and McEWEN B (1959). Effects of carnitine on fatty-acid oxidation by muscle. Science 129, 334–335. [DOI] [PubMed] [Google Scholar]
- Fritz V, Benfodda Z, Rodier G, Henriquet C, Iborra F, Avancès C, Allory Y, de la Taille A, Culine S, Blancou H, et al. (2010). Abrogation of de novo lipogenesis by stearoyl-CoA desaturase 1 inhibition interferes with oncogenic signaling and blocks prostate cancer progression in mice. Mol. Cancer Ther 9, 1740–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Sun Y, Wang M, Hou Y, Huang W, Zhou D, Wang Z, Yang S, Tang W, Zhen J, et al. (2020). Elevation of JAML Promotes Diabetic Kidney Disease by Modulating Podocyte Lipid Metabolism. Cell Metab 32, 1052–1062.e8. [DOI] [PubMed] [Google Scholar]
- Galdieri L, Chang J, Mehrotra S, and Vancura A (2013). Yeast phospholipase C is required for normal acetyl-CoA homeostasis and global histone acetylation. J. Biol. Chem 288, 27986–27998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Serra D, Keung W, Hegardt FG, and Lopaschuk GD (2013). Important role of ventromedial hypothalamic carnitine palmitoyltransferase-1a in the control of food intake. American Journal of Physiology-Endocrinology and Metabolism 305, E336–E347. [DOI] [PubMed] [Google Scholar]
- Gao X, Lin SH, Ren F, Li JT, Chen JJ, Yao CB, Yang HB, Jiang SX, Yan GQ, Wang D, et al. (2016). Acetate functions as an epigenetic metabolite to promote lipid synthesis under hypoxia. Nat Commun 7: 11960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatta L, Vitiello L, Gorini S, Chiandotto S, Costelli P, Giammarioli AM, Malorni W, Rosano G, and Ferraro E (2017). Modulating the metabolism by trimetazidine enhances myoblast differentiation and promotes myogenesis in cachectic tumor-bearing c26 mice. Oncotarget 8, 113938–113956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German NJ, Yoon H, Yusuf RZ, Murphy JP, Finley LWS, Laurent G, Haas W, Satterstrom FK, Guarnerio J, Zaganjor E, et al. (2016). PHD3 Loss in Cancer Enables Metabolic Reliance on Fatty Acid Oxidation via Deactivation of ACC2. Mol. Cell 63, 1006–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giró-Perafita A, Palomeras S, Lum DH, Blancafort A, Viñas G, Oliveras G, Pérez-Bueno F, Sarrats A, Welm AL, and Puig T (2016). Preclinical Evaluation of Fatty Acid Synthase and EGFR Inhibition in Triple-Negative Breast Cancer. Clin. Cancer Res 22, 4687–4697. [DOI] [PubMed] [Google Scholar]
- Glunde K, Bhujwalla ZM, and Ronen SM (2011). Choline metabolism in malignant transformation. Nat. Rev. Cancer 11, 835–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg IJ, Trent CM, and Schulze PC (2012). Lipid metabolism and toxicity in the heart. Cell Metab. 15, 805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göransson O, McBride A, Hawley SA, Ross FA, Shpiro N, Foretz M, Viollet B, Hardie DG, and Sakamoto K (2007). Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. J. Biol. Chem 282, 32549–32560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths B, Lewis CA, Bensaad K, Ros S, Zhang Q, Ferber EC, Konisti S, Peck B, Miess H, East P, et al. (2013). Sterol regulatory element binding protein-dependent regulation of lipid synthesis supports cell survival and tumor growth. Cancer Metab 1, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guder WG, Wagner S, and Wirthensohn G (1986). Metabolic fuels along the nephron: pathways and intracellular mechanisms of interaction. Kidney Int 29, 41–45. [DOI] [PubMed] [Google Scholar]
- Gullans SR, Brazy PC, Mandel LJ, and Dennis VW (1984). Stimulation of phosphate transport in the proximal tubule by metabolic substrates. Am. J. Physiol. 247, F582–F587. [DOI] [PubMed] [Google Scholar]
- Gullett JM, Cuypers MG, Frank MW, White SW, and Rock CO (2019). A fatty acid-binding protein of Streptococcus pneumoniae facilitates the acquisition of host polyunsaturated fatty acids. J. Biol. Chem 294, 16416–16428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Hildebrandt IJ, Prins RM, Soto H, Mazzotta MM, Dang J, Czernin J, Shyy JY-J, Watson AD, Phelps M, et al. (2009). The AMPK Agonist AICAR Inhibits the Growth of EGFRvlll-Expressing Glioblastomas by Inhibiting Lipogenesis. Proc. Natl. Acad. Sci. U. S. A 106, 12932–12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K, Li J, Liko I, Gault J, Bechara C, Wu D, Hopper JTS, Giles K, Benesch JLP, and Robinson CV (2018). Identifying key membrane protein lipid interactions using mass spectrometry. Nat. Protoc 13, 1106–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadas Yoav, Vincek Adam S., Youssef Elias, Żak Magdalena M., Chepurko Elena, Sultana Nishat, Sharkar Mohammad Tofael Kabir, Guo Ningning, Komargodski Rinat, Kurian Ann Anu, et al. (2020). Altering Sphingolipid Metabolism Attenuates Cell Death and Inflammatory Response After Myocardial Infarction. Circulation 141, 916–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajeyah AA, Griffiths WJ, Wang Y, Finch AJ, and O’Donnell VB (2020). The Biosynthesis of Enzymatically Oxidized Lipids. Front. Endocrinol 11, 591819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X (2016). Lipidomics for studying metabolism. Nat. Rev. Endocrinol 12, 668–679. [DOI] [PubMed] [Google Scholar]
- Han S, Schroeder EA, Silva-García CG, Hebestreit K, Mair WB, and Brunet A (2017). Mono-unsaturated fatty acids link H3K4me3 modifiers to C. elegans lifespan. Nature 544, 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Yang K, and Gross RW (2012). Multi-dimensional mass spectrometry-based shotgun lipidomics and novel strategies for lipidomic analyses. Mass Spectrometry Reviews 31, 134–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson GK, and Hermansson A (2011). The immune system in atherosclerosis. Nat. Immunol 12, 204–212. [DOI] [PubMed] [Google Scholar]
- Harayama T, and Riezman H (2018). Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol 19, 281–296. [DOI] [PubMed] [Google Scholar]
- Hashidate-Yoshida T, Harayama T, Hishikawa D, Morimoto R, Hamano F, Tokuoka SM, Eto M, Tamura-Nakano M, Yanobu-Takanashi R, Mukumoto Y, Kiyonari H, Okamura T, Kita Y, Shindou H, and Shimizu T (2015). Fatty acid remodeling by LPCAT3 enriches arachidonate in phospholipid membranes and regulates triglyceride transport. Elife. 4, e06328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D, Hingorani SR, Tuveson DA, and Thompson CB (2005). ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell 8, 311–321. [DOI] [PubMed] [Google Scholar]
- He A, Chen X, Tan M, Chen Y, Lu D, Zhang X, Dean JM, Razani B, and Lodhi IJ (2020). Acetyl-CoA Derived from Hepatic Peroxisomal β-Oxidation Inhibits Autophagy and Promotes Steatosis via mTORC1 Activation. Mol. Cell 79, 30–42.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SC-C, Everts B, Ivanova Y, O’Sullivan D, Nascimento M, Smith AM, Beatty W, Love-Gregory L, Lam WY, O’Neill CM, et al. (2014). Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat. Immunol 15, 846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert M, Seiler K, Mosimann S, Rentsch V, Sharma K, Pandey AV, McKenna SL, and Tschan MP (2021). Reducing FASN expression sensitizes acute myeloid leukemia cells to differentiation therapy. Cell Death Differ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh FK, Green MF, Koves TR, and Hirschey MD (2014). Measurement of fatty acid oxidation rates in animal tissues and cell lines. Methods Enzymol 542, 391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou MS, Jackson J, Sheu S-H, Chang C-L, Weigel AV, Liu H, Pasolli HA, Xu CS, Pang S, Matthies D, et al. (2019). Neuron-Astrocyte Metabolic Coupling Protects against Activity-Induced Fatty Acid Toxicity. Cell 177, 1522–1535.e14. [DOI] [PubMed] [Google Scholar]
- Irshad Z, Dimitri F, Christian M, and Zammit VA (2017). Diacylglycerol acyltransferase 2 links glucose utilization to fatty acid oxidation in the brown adipocytes. J. Lipid Res 58, 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itani SI, Ruderman NB, Schmieder F, and Boden G (2002). Lipid-Induced Insulin Resistance in Human Muscle Is Associated With Changes in Diacylglycerol, Protein Kinase C, and IκB-α. Diabetes 51, 2005–2011. [DOI] [PubMed] [Google Scholar]
- Jakobsson A, Westerberg R, and Jacobsson A (2006). Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog. Lipid Res 45, 237–249. [DOI] [PubMed] [Google Scholar]
- Jelenik T, Flögel U, Álvarez-Hernández E, Scheiber D, Zweck E, Ding Z, Rothe M, Mastrototaro L, Kohlhaas V, Kotzka J, et al. (2018). Insulin Resistance and Vulnerability to Cardiac Ischemia. Diabetes 67, 2695–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Wang Z, Proctor G, Moskowitz S, Liebman SE, Rogers T, Lucia MS, Li J, and Levi M (2005). Diet-induced Obesity in C57BL/6J Mice Causes Increased Renal Lipid Accumulation and Glomerulosclerosis via a Sterol Regulatory Element-binding Protein-1c-dependent Pathway*. J. Biol. Chem 280, 32317–32325. [DOI] [PubMed] [Google Scholar]
- Jose C, Hébert-Chatelain E, Bellance N, Larendra A, Su M, Nouette-Gaulain K, and Rossignol R (2011). AICAR inhibits cancer cell growth and triggers cell-type distinct effects on OXPHOS biogenesis, oxidative stress and Akt activation. Biochim. Biophys. Acta 1807, 707–718. [DOI] [PubMed] [Google Scholar]
- Kamisuki S, Shirakawa T, Kugimiya A, Abu-Elheiga L, Choo H-YP, Yamada K, Shimogawa H, Wakil SJ, and Uesugi M (2011). Synthesis and evaluation of diarylthiazole derivatives that inhibit activation of sterol regulatory element-binding proteins. J. Med. Chem 54, 4923–4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazantzis M, and Stahl A (2012). Fatty acid transport proteins, implications in physiology and disease. Biochim. Biophys. Acta 1821, 852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmerer M, Finkernagel F, Cavalcante MF, Abdalla DSP, Müller R, Brüne B, and Namgaladze D (2015). AMP-Activated Protein Kinase Interacts with the Peroxisome Proliferator-Activated Receptor Delta to Induce Genes Affecting Fatty Acid Oxidation in Human Macrophages. PLoS One 10, e0130893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lewin TM, and Coleman RA (2001). Expression and characterization of recombinant rat Acyl-CoA synthetases 1, 4, and 5. Selective inhibition by triacsin C and thiazolidinediones. J. Biol. Chem 276, 24667–24673. [DOI] [PubMed] [Google Scholar]
- Kindt A, Liebisch G, Clavel T, Haller D, Hörmannsperger G, Yoon H, Kolmeder D, Sigruener A, Krautbauer S, Seeliger C, et al. (2018). The gut microbiota promotes hepatic fatty acid desaturation and elongation in mice. Nat. Commun 9, 3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein KL, Wang MS, Torikai S, Davidson WD, and Kurokawa K (1981). Substrate oxidation by isolated single nephron segments of the rat. Kidney Int 20, 29–35. [DOI] [PubMed] [Google Scholar]
- Klevstig M, Arif M, Mannila M, Svedlund S, Mardani I, Ståhlman M, Andersson L, Lindbom M, Miljanovic A, Franco-Cereceda A, et al. (2019). Cardiac expression of the microsomal triglyceride transport protein protects the heart function during ischemia. J. Mol. Cell. Cardiol 137, 1–8. [DOI] [PubMed] [Google Scholar]
- Koundouros N, and Poulogiannis G (2020). Reprogramming of fatty acid metabolism in cancer. Br. J. Cancer 122, 4–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JRB, Newgard CB, et al. (2008). Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7, 45–56. [DOI] [PubMed] [Google Scholar]
- Kumar-Sinha C, Ignatoski KW, Lippman ME, Ethier SP, and Chinnaiyan AM (2003). Transcriptome analysis of HER2 reveals a molecular connection to fatty acid synthesis. Cancer Res 63, 132–139. [PubMed] [Google Scholar]
- Kume S, Uzu T, Araki S-I, Sugimoto T, Isshiki K, Chin-Kanasaki M, Sakaguchi M, Kubota N, Terauchi Y, Kadowaki T, et al. (2007). Role of altered renal lipid metabolism in the development of renal injury induced by a high-fat diet. J. Am. Soc. Nephrol 18, 2715–2723. [DOI] [PubMed] [Google Scholar]
- Kushwaha P, Wolfgang MJ, and Riddle RC (2018). Fatty acid metabolism by the osteoblast. Bone 115, 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebovitz HE (2019). Thiazolidinediones: the Forgotten Diabetes Medications. Curr. Diab. Rep 19, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JV, Carrer A, Shah S, Snyder NW, Wei S, Venneti S, Worth AJ, Yuan ZF, Lim HW, Liu S, Jackson E, Aiello NM, Haas NB, Rebbeck TR, Judkins A, Won KJ, Chodosh LA, Garcia BA, Stanger BZ, Feldman MD, Blair IA, Wellen KE (2014). Akt-dependent metabolic reprogramming regulates tumor cell histone acetylation. Cell Metab 20, 306–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Hirose H, Ohneda M, Johnson JH, McGarry JD, and Unger RH (1994). Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proc. Natl. Acad. Sci. U. S. A 91, 10878–10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CA, Brault C, Peck B, Bensaad K, Griffiths B, Mitter R, Chakravarty P, East P, Dankworth B, Alibhai D, et al. (2015). SREBP maintains lipid biosynthesis and viability of cancer cells under lipid- and oxygen-deprived conditions and defines a gene signature associated with poor survival in glioblastoma multiforme. Oncogene 34, 5128–5140. [DOI] [PubMed] [Google Scholar]
- Liu G, Lynch JK, Freeman J, Liu B, Xin Z, Zhao H, Serby MD, Kym PR, Suhar TS, Smith HT, et al. (2007). Discovery of potent, selective, orally bioavailable stearoyl-CoA desaturase 1 inhibitors. J. Med. Chem 50, 3086–3100. [DOI] [PubMed] [Google Scholar]
- Liu L, MacKenzie KR, Putluri N, Maletić-Savatić M, and Bellen HJ (2017a). The Glia-Neuron Lactate Shuttle and Elevated ROS Promote Lipid Synthesis in Neurons and Lipid Droplet Accumulation in Glia via APOE/D. Cell Metab 26, 719–737.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P-S, Wang H, Li X, Chao T, Teav T, Christen S, Di Conza G, Cheng W-C, Chou C-H, Vavakova M, et al. (2017b). α-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat. Immunol 18, 985–994. [DOI] [PubMed] [Google Scholar]
- Lolkema MP, Bohets HH, Arkenau H-T, Lampo A, Barale E, de Jonge MJA, van Doorn L, Hellemans P, de Bono JS, and Eskens FALM (2015). The c-Met Tyrosine Kinase Inhibitor JNJ-38877605 Causes Renal Toxicity through Species-Specific Insoluble Metabolite Formation. Clin. Cancer Res 21, 2297–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love-Gregory L, Sherva R, Sun L, Wasson J, Schappe T, Doria A, Rao DC, Hunt SC, Klein S, Neuman RJ, et al. (2008). Variants in the CD36 gene associate with the metabolic syndrome and high-density lipoprotein cholesterol. Hum. Mol. Genet 17, 1695–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Løvsletten NG, Bakke SS, Kase ET, Ouwens DM, Thoresen GH, and Rustan AC (2018). Increased triacylglycerol - Fatty acid substrate cycling in human skeletal muscle cells exposed to eicosapentaenoic acid. PLoS One 13, e0208048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytrivi M, Castell A-L, Poitout V, and Cnop M (2020). Recent Insights Into Mechanisms of β-Cell Lipo- and Glucolipotoxicity in Type 2 Diabetes. J. Mol. Biol 432, 1514–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magtanong L, Ko PJ, To M, Cao JY, Forcina GC, Tarangelo A, Ward CC, Cho K, Patti GJ, Nomura DK, Olzmann JA, Dixon SJ (2019). Exogenous Monounsaturated Fatty Acids Promote a Ferroptosis-Resistant Cell State. Cell Chem Biol 26, 420–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovic SN, Suman VJ, Rao RA, Ingle JN, Kaur JS, Erickson LA, Pitot HC, Croghan GA, McWilliams RR, Merchan J, et al. (2007). A phase II study of ABT-510 (thrombospondin-1 analog) for the treatment of metastatic melanoma. Am. J. Clin. Oncol 30, 303–309. [DOI] [PubMed] [Google Scholar]
- Marton LT, Goulart R. de A., Carvalho A.C.A. de, and Barbalho SM (2019). Omega Fatty Acids and Inflammatory Bowel Diseases: An Overview. Int. J. Mol. Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashima T, Oh-hara T, Sato S, Mochizuki M, Sugimoto Y, Yamazaki K, Hamada J-I, Tada M, Moriuchi T, Ishikawa Y, et al. (2005). p53-defective tumors with a functional apoptosome-mediated pathway: a new therapeutic target. J. Natl. Cancer Inst 97, 765–777. [DOI] [PubMed] [Google Scholar]
- Matoba K, Lu Y, Zhang R, Chen ER, Sangwung P, Wang B, Prosdocimo DA, and Jain MK (2017). Adipose KLF15 Controls Lipid Handling to Adapt to Nutrient Availability. Cell Rep 21, 3129–3140. [DOI] [PubMed] [Google Scholar]
- McCoull W, Addie MS, Birch AM, Birtles S, Buckett LK, Butlin RJ, Bowker SS, Boyd S, Chapman S, Davies RDM, et al. (2012). Identification, optimisation and in vivo evaluation of oxadiazole DGAT-1 inhibitors for the treatment of obesity and diabetes. Bioorg. Med. Chem. Lett 22, 3873–3878. [DOI] [PubMed] [Google Scholar]
- McDonnell E, Crown SB, Fox DB, Kitir B, Ilkayeva OR, Olsen CA, Grimsrud PA, and Hirschey MD (2016). Lipids Reprogram Metabolism to Become a Major Carbon Source for Histone Acetylation. Cell Rep 17, 1463–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry JD, and Foster DW (1980). Regulation of hepatic fatty acid oxidation and ketone body production. Annu. Rev. Biochem 49, 395–420. [DOI] [PubMed] [Google Scholar]
- Meeusen JW, Donato LJ, and Jaffe AS (2017). Lipid Biomarkers for Risk Assessment in Acute Coronary Syndromes. Curr. Cardiol. Rep 19, 48. [DOI] [PubMed] [Google Scholar]
- Mehlem A, Hagberg CE, Muhl L, Eriksson U, and Falkevall A (2013). Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat. Protoc 8, 1149–1154. [DOI] [PubMed] [Google Scholar]
- Mejhert N, Gabriel KR, Krahmer N, Kuruvilla L, Chitraju C, Boland S, Jang D-K, von Grotthuss M, Costanzo MC, Flannick J, et al. The Lipid Droplet Knowledge Portal: A resource for systematic analyses of lipid droplet biology [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez JA, and Lupu R (2007). Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer 7, 763–777. [DOI] [PubMed] [Google Scholar]
- Menendez JA, Vellon L, Mehmi I, Oza BP, Ropero S, Colomer R, and Lupu R (2004). Inhibition of fatty acid synthase (FAS) suppresses HER2/neu (erbB-2) oncogene overexpression in cancer cells. Proc. Natl. Acad. Sci. U. S. A 101, 10715–10720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel CI, Holley CL, Scruggs BS, Sidhu R, Brookheart RT, Listenberger LL, Behlke MA, Ory DS, and Schaffer JE (2011). Small nucleolar RNAs U32a, U33, and U35a are critical mediators of metabolic stress. Cell Metab 14, 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migita T, Narita T, Nomura K, Miyagi E, Inazuka F, Matsuura M, Ushijima M, Mashima T, Seimiya H, Satoh Y, et al. (2008). ATP Citrate Lyase: Activation and Therapeutic Implications in Non–Small Cell Lung Cancer. Cancer Res 68, 8547–8554. [DOI] [PubMed] [Google Scholar]
- Morentin Gutierrez P, Yates J, Nilsson C, and Birtles S (2019). Evolving data analysis of an Oral Lipid Tolerance Test toward the standard for the Oral Glucose Tolerance Test: Cross species modeling effects of AZD7687 on plasma triacylglycerol. Pharmacol Res Perspect 7, e00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori Y, Ajay AK, Chang J-H, Mou S, Zhao H, Kishi S, Li J, Brooks CR, Xiao S, Woo H-M, et al. (2021). KIM-1 mediates fatty acid uptake by renal tubular cells to promote progressive diabetic kidney disease. Cell Metab 33, 1042–1061.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller DR, Voshol H, Waldt A, Wiedmann B, and Van Oostrum J (2007). LC-MALDI MS and MS/MS—an efficient tool in proteome analysis. Subcellular Proteomics 355–380. [DOI] [PubMed] [Google Scholar]
- Mukai T, Egawa M, Takeuchi T, Yamashita H, and Kusudo T (2017). Silencing of FABP1 ameliorates hepatic steatosis, inflammation, and oxidative stress in mice with nonalcoholic fatty liver disease. FEBS Open Bio 7, 1009–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musunuru K, and Kathiresan S (2016). Surprises From Genetic Analyses of Lipid Risk Factors for Atherosclerosis. Circ. Res 118, 579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musunuru K, and Kathiresan S (2019). Genetics of Common, Complex Coronary Artery Disease. Cell 177, 132–145. [DOI] [PubMed] [Google Scholar]
- Mutlu AS, Duffy J, and Wang MC (2021). Lipid metabolism and lipid signals in aging and longevity. Dev. Cell 56, 1394–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Liu T, Husain S, Zhai P, Warren JS, Hsu C-P, Matsuda T, Phiel CJ, Cox JE, Tian B, et al. (2019). Glycogen Synthase Kinase-3α Promotes Fatty Acid Uptake and Lipotoxic Cardiomyopathy. Cell Metab. 29, 1119–1134.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer S (2007). The Failing Heart — An Engine Out of Fuel. New England Journal of Medicine 356, 1140–1151. [DOI] [PubMed] [Google Scholar]
- Niphakis MJ, Lum KM, Cognetta AB 3rd, Correia BE, Ichu T-A, Olucha J, Brown SJ, Kundu S, Piscitelli F, Rosen H, et al. (2015). A Global Map of Lipid-Binding Proteins and Their Ligandability in Cells. Cell 161, 1668–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oballa RM, Belair L, Black WC, Bleasby K, Chan CC, Desroches C, Du X, Gordon R, Guay J, Guiral S, et al. (2011). Development of a liver-targeted stearoyl-CoA desaturase (SCD) inhibitor (MK-8245) to establish a therapeutic window for the treatment of diabetes and dyslipidemia. J. Med. Chem 54, 5082–5096. [DOI] [PubMed] [Google Scholar]
- Obici S, Feng Z, Arduini A, Conti R, and Rossetti L (2003). Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production. Nat. Med 9, 756–761. [DOI] [PubMed] [Google Scholar]
- Olzmann JA, and Carvalho P (2019). Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol 20, 137–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottemann Abbamonte CJ, Overton TR, Beaulieu AD, and Drackley JK (2021). Effects of in vivo phlorizin treatment and in vitro addition of carnitine, propionate, acetate, and 5-tetradecyloxy-2-furoic acid on palmitate metabolism in ovine hepatocytes. J. Dairy Sci 104, 7749–7760. [DOI] [PubMed] [Google Scholar]
- Park SH, Gammon SR, Knippers JD, Paulsen SR, Rubink DS, and Winder WW (2002). Phosphorylation-activity relationships of AMPK and acetyl-CoA carboxylase in muscle. Journal of Applied Physiology 92, 2475–2482. [DOI] [PubMed] [Google Scholar]
- Park T-J, Park JH, Lee GS, Lee J-Y, Shin JH, Kim MW, Kim YS, Kim J-Y, Oh K-J, Han B-S, et al. (2019). Quantitative proteomic analyses reveal that GPX4 downregulation during myocardial infarction contributes to ferroptosis in cardiomyocytes. Cell Death Dis 10, 835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual G, Avgustinova A, Mejetta S, Martín M, Castellanos A, Attolini CS-O, Berenguer A, Prats N, Toll A, Hueto JA, et al. (2017). Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 541, 41–45. [DOI] [PubMed] [Google Scholar]
- Pereira ER, Frudd K, Awad W, and Hendershot LM (2014). Endoplasmic Reticulum (ER) Stress and Hypoxia Response Pathways Interact to Potentiate Hypoxia-inducible Factor 1 (HIF-1) Transcriptional Activity on Targets Like Vascular Endothelial Growth Factor (VEGF). Journal of Biological Chemistry 289, 3352–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolis M, Bond LM, Kampmann M, Pulimeno P, Chitraju C, Jayson CBK, Vaites LP, Boland S, Lai ZW, Gabriel KR, et al. (2019). Probing the Global Cellular Responses to Lipotoxicity Caused by Saturated Fatty Acids. Mol. Cell 74, 32–44.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike LS, Smift AL, Croteau NJ, Ferrick DA, and Wu M (2011). Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochim. Biophys. Acta 1807, 726–734. [DOI] [PubMed] [Google Scholar]
- Pizer ES, Thupari J, Han WF, Pinn ML, Chrest FJ, Frehywot GL, Townsend CA, and Kuhajda FP (2000). Malonyl-coenzyme-A is a potential mediator of cytotoxicity induced by fatty-acid synthase inhibition in human breast cancer cells and xenografts. Cancer Res. 60, 213–218. [PubMed] [Google Scholar]
- Poillet-Perez L, and White E (2019). Role of tumor and host autophagy in cancer metabolism. Genes Dev 33, 610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak MN (2012). Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discov 2, 778–790. [DOI] [PubMed] [Google Scholar]
- Qiu B, Ackerman D, Sanchez DJ, Li B, Ochocki JD, Grazioli A, Bobrovnikova-Marjon E, Alan Diehl J, Keith B, and Celeste Simon M (2015). HIF2α-Dependent Lipid Storage Promotes Endoplasmic Reticulum Homeostasis in Clear-Cell Renal Cell Carcinoma. Cancer Discovery 5, 652–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu P (2020). Functional diversity in a lipidome. Proc. Natl. Acad. Sci. U. S. A 117, 11191–11193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman K, Desai C, Iyer SS, Thorn NE, Kumar P, Liu Y, Smith T, Neish AS, Li H, Tan S, et al. (2016). Loss of Junctional Adhesion Molecule A Promotes Severe Steatohepatitis in Mice on a Diet High in Saturated Fat, Fructose, and Cholesterol. Gastroenterology 151, 733–746.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven GM, Hollenbeck C, Jeng CY, Wu MS, and Chen YD (1988). Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes 37, 1020–1024. [DOI] [PubMed] [Google Scholar]
- Reddy JK, and Hashimoto T (2001). Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor alpha: an adaptive metabolic system. Annu. Rev. Nutr 21, 193–230. [DOI] [PubMed] [Google Scholar]
- Reynolds IJ, and Hastings TG (1995). Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J. Neurosci 15, 3318–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LD, Koulman A, and Griffin JL (2014). Towards metabolic biomarkers of insulin resistance and type 2 diabetes: progress from the metabolome. Lancet Diabetes Endocrinol 2, 65–75. [DOI] [PubMed] [Google Scholar]
- Roche CM, Blanch HW, Clark DS, and Glass NL (2013). Physiological role of Acyl coenzyme A synthetase homologs in lipid metabolism in Neurospora crassa. Eukaryot. Cell 12, 1244–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemeling C.A. von, von Roemeling CA, Marlow LA, Wei JJ, Cooper SJ, Caulfield TR, Wu K, Tan WW, Tun HW, and Copland JA (2013). Stearoyl-CoA Desaturase 1 Is a Novel Molecular Therapeutic Target for Clear Cell Renal Cell Carcinoma. Clinical Cancer Research 19, 2368–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhrig F, and Schulze A (2016). The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer 16, 732–749. [DOI] [PubMed] [Google Scholar]
- Ros S, Santos CR, Moco S, Baenke F, Kelly G, Howell M, Zamboni N, and Schulze A (2012). Functional Metabolic Screen Identifies 6-Phosphofructo-2-Kinase/Fructose-2,6-Biphosphatase 4 as an Important Regulator of Prostate Cancer Cell Survival. Cancer Discov. 2, 328–343. [DOI] [PubMed] [Google Scholar]
- Samudio I, Harmancey R, Fiegl M, Kantarjian H, Konopleva M, Korchin B, Kaluarachchi K, Bornmann W, Duvvuri S, Taegtmeyer H, et al. (2010). Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. J. Clin. Invest 120, 142–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro A, McGraw TE, and Kahn BB (2021). Insulin action in adipocytes, adipose remodeling, and systemic effects. Cell Metab 33, 748–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer ZT, Grassian AR, Song L, Jiang Z, Gerhart-Hines Z, Irie HY, Gao S, Puigserver P, and Brugge JS (2009). Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature 461, 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer JE, and Lodish HF (1994). Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell 79, 427–436. [DOI] [PubMed] [Google Scholar]
- Schcolnik-Cabrera A, Chávez-Blanco A, Domínguez-Gómez G, Taja-Chayeb L, Morales-Barcenas R, Trejo-Becerril C, Perez-Cardenas E, Gonzalez-Fierro A, and Dueñas-González A (2018). Orlistat as a FASN inhibitor and multitargeted agent for cancer therapy. Expert Opin. Investig. Drugs 27, 475–489. [DOI] [PubMed] [Google Scholar]
- Schoeler M, and Caesar R (2019). Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord 20, 461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönfeld P, and Reiser G (2013). Why does brain metabolism not favor burning of fatty acids to provide energy?-Reflections on disadvantages of the use of free fatty acids as fuel for brain. J. Cereb. Blood Flow Metab [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenk RW, Holloway GP, Luiken JJFP, Bonen A, and Glatz JFC (2010). Fatty acid transport across the cell membrane: regulation by fatty acid transporters. Prostaglandins Leukot. Essent. Fatty Acids 82, 149–154. [DOI] [PubMed] [Google Scholar]
- Senkal CE, Salama MF, Snider AJ, Allopenna JJ, Rana NA, Koller A, Hannun YA, and Obeid LM (2017). Ceramide Is Metabolized to Acylceramide and Stored in Lipid Droplets. Cell Metab 25, 686–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma RB, and Alonso LC (2014). Lipotoxicity in the pancreatic beta cell: not just survival and function, but proliferation as well? Curr. Diab. Rep 14, 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhom E-H, Kim C, Kost-Alimova M, Ting MT, Keller K, Avila-Pacheco J, Watts AJ, Vernon KA, Marshall JL, Reyes-Bricio E, et al. (2021). Targeting a Braf/Mapk pathway rescues podocyte lipid peroxidation in CoQ-deficiency kidney disease. J. Clin. Invest 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sienski G, Narayan P, Bonner JM, Kory N, Boland S, Arczewska AA, Ralvenius WT, Akay L, Lockshin E, He L, et al. (2021). APOE4 disrupts intracellular lipid homeostasis in human iPSC-derived glia. Sci. Transl. Med 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simopoulos AP (2002). Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr 21, 495–505. [DOI] [PubMed] [Google Scholar]
- Stahl A, Gimeno RE, Tartaglia LA, and Lodish HF (2001). Fatty acid transport proteins: a current view of a growing family. Trends Endocrinol. Metab 12, 266–273. [DOI] [PubMed] [Google Scholar]
- Stone SJ, Levin MC, and Farese RV Jr (2006). Membrane topology and identification of key functional amino acid residues of murine acyl-CoA:diacylglycerol acyltransferase-2. J. Biol. Chem 281, 40273–40282. [DOI] [PubMed] [Google Scholar]
- Su X, and Abumrad NA (2009). Cellular fatty acid uptake: a pathway under construction. Trends Endocrinol. Metab 20, 72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulciner ML, Serhan CN, Gilligan MM, Mudge DK, Chang J, Gartung A, Lehner KA, Bielenberg DR, Schmidt B, Dalli J, et al. (2018). Resolvins suppress tumor growth and enhance cancer therapy. J. Exp. Med 215, 115–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Halaihel N, Zhang W, Rogers T, and Levi M (2002). Role of sterol regulatory element-binding protein 1 in regulation of renal lipid metabolism and glomerulosclerosis in diabetes mellitus. J. Biol. Chem 277, 18919–18927. [DOI] [PubMed] [Google Scholar]
- Swinnen JV, Beckers A, Brusselmans K, Organe S, Segers J, Timmermans L, Vanderhoydonc F, Deboel L, Derua R, Waelkens E, et al. (2005). Mimicry of a cellular low energy status blocks tumor cell anabolism and suppresses the malignant phenotype. Cancer Res 65, 2441–2448. [DOI] [PubMed] [Google Scholar]
- Syed I, Lee J, Moraes-Vieira PM, Donaldson CJ, Sontheimer A, Aryal P, Wellenstein K, Kolar MJ, Nelson AT, Siegel D, et al. (2018). Palmitic Acid Hydroxystearic Acids Activate GPR40, Which Is Involved in Their Beneficial Effects on Glucose Homeostasis. Cell Metab 27, 419–427.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taïb B, Aboussalah AM, Moniruzzaman M, Chen S, Haughey NJ, Kim SF, and Ahima RS (2019). Lipid accumulation and oxidation in glioblastoma multiforme. Sci. Rep 9, 19593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K, and Reue K (2009). Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am. J. Physiol. Endocrinol. Metab 296, E1195–E1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele C, Wunderling K, and Leyendecker P (2019). Multiplexed and single cell tracing of lipid metabolism. Nat. Methods 16, 1123–1130. [DOI] [PubMed] [Google Scholar]
- To T-L, Cuadros AM, Shah H, Hung WHW, Li Y, Kim SH, Rubin DHF, Boe RH, Rath S, Eaton JK, et al. (2019). A Compendium of Genetic Modifiers of Mitochondrial Dysfunction Reveals Intra-organelle Buffering. Cell 179, 1222–1238.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S, and Endou H (1988). Substrate specificity to maintain cellular ATP along the mouse nephron. American Journal of Physiology-Renal Physiology 255, F977–F983. [DOI] [PubMed] [Google Scholar]
- Vance JE (2014). MAM (mitochondria-associated membranes) in mammalian cells: lipids and beyond. Biochim. Biophys. Acta 1841, 595–609. [DOI] [PubMed] [Google Scholar]
- Van de Sande T, De Schrijver E, Heyns W, Verhoeven G, and Swinnen JV (2002). Role of the phosphatidylinositol 3’-kinase/PTEN/Akt kinase pathway in the overexpression of fatty acid synthase in LNCaP prostate cancer cells. Cancer Res. 62, 642–646. [PubMed] [Google Scholar]
- Vanhove GF, Van Veldhoven PP, Fransen M, Denis S, Eyssen HJ, Wanders RJ, and Mannaerts GP (1993). The CoA esters of 2-methyl-branched chain fatty acids and of the bile acid intermediates di- and trihydroxycoprostanic acids are oxidized by one single peroxisomal branched chain acyl-CoA oxidase in human liver and kidney. Journal of Biological Chemistry 268, 10335–10344. [PubMed] [Google Scholar]
- Vriens K, Christen S, Parik S, Broekaert D, Yoshinaga K, Talebi A, Dehairs J, Escalona-Noguero C, Schmieder R, Cornfield T, et al. (2019). Evidence for an alternative fatty acid desaturation pathway increasing cancer plasticity. Nature 566, 403–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R, Stübiger G, Veigel D, Wuczkowski M, Lanzerstorfer P, Weghuber J, Karteris E, Nowikovsky K, Wilfinger-Lutz N, Singer CF, et al. (2017). Multi-level suppression of receptor-PI3K-mTORC1 by fatty acid synthase inhibitors is crucial for their efficacy against ovarian cancer cells. Oncotarget 8, 11600–11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajner M, and Amaral AU (2015). Mitochondrial dysfunction in fatty acid oxidation disorders: insights from human and animal studies. Biosci. Rep 36, e00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walenna NF, Kurihara Y, Chou B, Ishii K, Soejima T, and Hiromatsu K (2020). Chlamydia pneumoniae infection-induced endoplasmic reticulum stress causes fatty acid-binding protein 4 secretion in murine adipocytes. J. Biol. Chem 295, 2713–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Franco F, Tsui Y-C, Xie X, Trefny MP, Zappasodi R, Mohmood SR, Fernández-García J, Tsai C-H, Schulze I, et al. (2020). CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nat. Immunol 21, 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Tang K, Zhang W, Guo W, Wang Y, Zan L, and Yang W (2019). Fatty acid-binding protein 1 increases steer fat deposition by facilitating the synthesis and secretion of triacylglycerol in liver. PLoS One 14, e0214144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Jiang T, Li J, Proctor G, McManaman JL, Lucia S, Chua S, and Levi M (2005). Regulation of renal lipid metabolism, lipid accumulation, and glomerulosclerosis in FVBdb/db mice with type 2 diabetes. Diabetes 54, 2328–2335. [DOI] [PubMed] [Google Scholar]
- Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, and Thompson CB (2009). ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324, 1076–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigger D, Gulbins E, Kleuser B, and Schumacher F (2019). Monitoring the Sphingolipid de novo Synthesis by Stable-Isotope Labeling and Liquid Chromatography-Mass Spectrometry. Front Cell Dev Biol 7, 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KJ, Argus JP, Zhu Y, Wilks MQ, Marbois BN, York AG, Kidani Y, Pourzia AL, Akhavan D, Lisiero DN, et al. (2013). An essential requirement for the SCAP/SREBP signaling axis to protect cancer cells from lipotoxicity. Cancer Res 73, 2850–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Cheng H, Gross RW, and Han X (2009). Automated lipid identification and quantification by multidimensional mass spectrometry-based shotgun lipidomics. Anal. Chem 81, 4356–4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H, Spinelli JB, Zaganjor E, Wong SJ, German NJ, Randall EC, Dean A, Clermont A, Paulo JA, Garcia D, et al. (2020). PHD3 Loss Promotes Exercise Capacity and Fat Oxidation in Skeletal Muscle. Cell Metab 32, 215–228.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi N, Swinnen JV, and Smans K (2012). ATP-citrate lyase: a key player in cancer metabolism. Cancer Res 72, 3709–3714. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kurupati R, Liu L, Zhou XY, Zhang G, Hudaihed A, Filisio F, Giles-Davis W, Xu X, Karakousis GC, et al. (2017). Enhancing CD8+ T Cell Fatty Acid Catabolism within a Metabolically Challenging Tumor Microenvironment Increases the Efficacy of Melanoma Immunotherapy. Cancer Cell 32, 377–391.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li C, Hu C, Wu Q, Cai Y, Xing S, Lu H, Wang L, Huang D, Sun L, et al. (2019). Lin28 enhances de novo fatty acid synthesis to promote cancer progression via SREBP-1. EMBO Rep 20, e48115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Souers AJ, Voorbach M, Falls HD, Droz B, Brodjian S, Lau YY, Iyengar RR, Gao J, Judd AS, et al. (2008). Validation of diacyl glycerolacyltransferase I as a novel target for the treatment of obesity and dyslipidemia using a potent and selective small molecule inhibitor. J. Med. Chem 51, 380–383. [DOI] [PubMed] [Google Scholar]
- Zhi J, Melia AT, Eggers H, Joly R, and Patel IH (1995). Review of limited systemic absorption of orlistat, a lipase inhibitor, in healthy human volunteers. J. Clin. Pharmacol 35, 1103–1108. [DOI] [PubMed] [Google Scholar]
- Zhou P, Santoro A, Peroni OD, Nelson AT, Saghatelian A, Siegel D, and Kahn BB (2019). PAHSAs enhance hepatic and systemic insulin sensitivity through direct and indirect mechanisms. J. Clin. Invest 129, 4138–4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Tu Y, Simpson PJ, and Kuhajda FP (2009). Malonyl-CoA decarboxylase inhibition is selectively cytotoxic to human breast cancer cells. Oncogene 28, 2979–2987. [DOI] [PubMed] [Google Scholar]
- Zhu XG, Nicholson Puthenveedu S, Shen Y, La K, Ozlu C, Wang T, Klompstra D, Gultekin Y, Chi J, Fidelin J, et al. (2019). CHP1 Regulates Compartmentalized Glycerolipid Synthesis by Activating GPAT4. Mol. Cell 74, 45–58.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Watters A, Cheng N, Perry CE, Xu K, Alicea GM, Parris JLD, Baraban E, Ray P, Nayak A, et al. (2019). Polyunsaturated Fatty Acids from Astrocytes Activate PPARγ Signaling in Cancer Cells to Promote Brain Metastasis. Cancer Discov 9, 1720–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]


