Abstract
In addition to the small intestine’s well-known function of nutrient absorption, the small intestine also plays a major role in nutrient sensing. Similar to taste sensors seen on the tongue, GPCR-coupled nutrient sensors are expressed throughout the intestinal epithelium and respond to nutrients found in the lumen. These taste receptors respond to specific ligands, such as digested carbohydrates, fats, and proteins. The activation of nutrient sensors in the intestine allows for the induction of signaling pathways needed for the digestive system to process an influx of nutrients. Such processes include those related to glucose homeostasis and satiety. Defects in intestinal nutrient sensing have been linked to a variety of metabolic disorders, such as type 2 diabetes and obesity. Here, we review recent updates in the mechanisms related to intestinal nutrient sensors, particularly in enteroendocrine cells, and their pathological roles in disease. Additionally, we highlight the emerging nutrient sensing role of tuft cells and recent work using enteroids as a sensory organ model.
Keywords: small intestine, GPCR, enteroendocrine cell, tuft cell, enteroid
1. Introduction
The small intestine is a major site of nutrient absorption. Enterocytes, the largest population of small intestinal epithelium, show dynamic changes in nutrient transporter expression in response to luminal nutrient composition, suggesting that nutrient absorption mechanisms have the flexibility to adapt to nutrient availability. Chemosensory molecules in the intestinal mucosa recognize luminal nutrients and activate different signaling pathways to regulate enterocyte function, digestive functions (bile and exocrine pancreas secretion), systemic glucose homeostasis (insulin secretion), and satiety [1,2]. A variety of G protein-coupled receptors (GPCRs) are localized in different types of intestinal epithelial cells, in particular, sensory enteroendocrine and tuft cells. Enteroendocrine cells (EECs) scattered through the intestinal epithelium produce a variety of gut hormones that play important roles in the signal transduction of those physiological responses. The first section of this review summarizes numerous studies on different types of nutrient receptors and their functions, with a focus on EECs.
Another rare population of sensory cell is the tuft cell, which possesses similar sensing machinery as taste receptor cells seen in the tongue. Intestinal tuft cell had been considered as a subpopulation of EECs, however, recently identified tuft cell markers have revealed that tuft cell is a different cell lineage than other types of epithelial cells. Although several chemoreceptors are identified in tuft cells, their function as nutrient sensors has not been fully understood. Latter section of this review summarizes recent findings on intestinal tuft cells and discuss possible strategies to study sensory cells using enteroid models. The nomenclatures of receptors are following the current NCBI Gene database.
2. Nutrient Sensors in Enteroendocrine Cells (EECs)
2.1. Sugar-Sensing Receptors
In the mouth and intestine, complex carbohydrates (i.e., polysaccharides) are broken down into oligosaccharides and/or simple sugars (glucose, galactose, and fructose) that are then detected by carbohydrate-sensing molecules in the small intestinal mucosa. There are several well-characterized glucose-sensing proteins, including the sweet taste receptor (TAS1R2+TAS1R3, also known as T1R2+T1R3) and sodium-coupled glucose cotransporter-1 (SGLT1, a gene product of SLC5A1). TAS1R2 and TAS1R3 mRNA expression was first detected in the mouse small intestine by Dyer et. al, with expression of both receptor subunits being highest in the jejunum [3]. The authors also found a similar pattern of protein expression in intestinal epithelial cell membranes from the proximal, mid, and distal intestine. They also showed that the sequence of intestinal TAS1R1–3 is homologous to the mouse sequences of TAS1R1–3 found in lingual taste buds. Later, T1R1–3 expressions were confirmed throughout the human intestine [4]. TAS1R2+TAS1R3 is a mammalian heterodimeric taste receptor that is expressed in EEC cell lines, such as GLUTag and STC-1 [5,6]. Like naturally occurring sugars, non-nutritive sweeteners have high affinities for TAS1R2+TAS1R3 [6,7].
The activation of TAS1R2+TAS1R3 promotes the secretion of a variety of EEC-derived hormones, such as peptide 1 (GLP-1) and gastric inhibitory peptide (GIP). Inhibition of T1R3 in human L cells and T1R3 KO mouse intestines demonstrate a reduction in sugar-stimulated GLP-1 expression [8,9]. Similarly, T1R2 KO mice have reduced plasma GLP-1 expression following a intragastric glucose administration compared to wild-type [10]. In GLUTag cells, a mouse L cell line, the inhibition of sweet taste receptor activity by gurmarin blocked sucralose-mediated GLP-1 and GIP secretion [6]. Studies in healthy human subjects suggest similar findings, as the administration of lactisole, a sweet taste receptor antagonist, reduced intragastric and intraduodenal glucose-mediated secretion of GLP-1 and PYY [11,12]. Interestingly, the role of TAS1R2+TAS1R3 in CCK secretion is less clear. Nasogastric administration of artificial sweeteners led to an increase in plasma CCK, however, in another study, lactisole administration did not affect glucose-induced CCK secretion in human subjects [12,13]. Neither of these studies assessed TAS1R2 or 3 expression and there is a lack of literature studying the effects of TAS1R2 and 3 activities on CCK secretion by EECs.
To promote the release of these peptides, TAS1R2+TAS1R3 acts with a heterotrimeric G protein, gustducin (Figure 1). The association of T1R2+3 with gustducin was first found in lingual taste cells, and their colocalization was later identified in the human duodenum via immunofluorescence [6,14]. Gustducin is made up of three components: α-gustducin (Gαgust, a gene product of GNAT3), Gβ3, and Gγ13. Intestinal Gαgust expression was first evaluated by PCR and immunostaining of the rat duodenum [15]. Later, PCR and Western blotting revealed that Gαgust is expressed throughout the small intestine (highest in the jejunum) and in STC-1 cells [3]. As a Gi/o-coupled GPCR, the Gαgust inhibits adenylyl cyclase (AC) activity, leading to a decrease in cAMP concentration [16]. Meanwhile, the βγ subunits activate phospholipase Cβ2 (PLCβ2), which catalyzes the formation of inositol triphosphate (IP3) to promote an increase in intracellular calcium concentrations [17]. This increase in calcium thereby activates TRPM5 to allow cation (i.e., Na+) entry into the cell [18,19]. Gustducin mediates GLP-1 release by the gut, as siRNA for Gαgust prevents sugar-induced GLP-1 secretion in human L cells [8]. The release of peptide hormones, such as GLP-1, by EECs target nearby enterocytes and afferent nerves to carry out signaling activity. However, the direct connection between the TAS1R2+TAS1R3 heterodimer and incretin expression is controversial, as the intraluminal administration of non-nutritive sweeteners (TAS1R2+TAS1R3 agonists) did not promote expression of either hormone in isolated rat intestine [20]. On the other hand, mice lacking TAS1R3 have deficiencies in GLP-1 expression and insulin regulation, and sweet taste receptor antagonist administration reduces glucose-induced GLP-1 and PYY secretion [9,11]. Mice lacking TAS1R2 have deficiencies in GLP-1 and -2 expression as well as glucose absorption, due to a lack of GLP-2-induced GLUT2 translocation [10].
Figure 1.
A schematic diagram of nutrient sensor signaling in an enteroendocrine cell. AA, amino acid; AC; adenylyl cyclase; cAMP, cyclic adenosine monophosphate; CaSR, calcium sensing receptor; DAG, diacylglycerol; FFAR, free fatty acid receptor; GPR119, G-protein receptor 119; IP3, inositol trisphosphate; LCFA, long chain fatty acid; PKA, protein kinase A; PKC protein kinase C; PLC, phospholipase C; PLCβ2, phospholipase C β2; SCFA, short chain fatty acid; SGLT1, sodium/glucose cotransporter 1; TAS1R, taste 1 receptor; TPRM5, transient receptor potential cation channel subfamily M member 5.
In addition to its traditional transporter niche, SLGT1 has an implied role in glucose sensing in the small intestine due to its expression pattern in the intestine and mediation of glucose-induced incretin section [21,22,23,24]. Dietary and artificial sugar responsive SGLT1 upregulation is dependent on TAS1R3 and Gαgust, as demonstrated in knockout mouse models [6]. In mice lacking SGLT1, secretion of glucose-responsive GIP and GLP-1 was diminished [25]. On the other hand, studies in diabetic rats chronically treated with selective SGLT1 inhibitors and SGLT1 knockout mice demonstrated the opposing effect of GLP-1, as inhibition of SGLT1 promoted GLP-1 secretion [26,27]. Interestingly, GLP-2 upregulates SGLT1 expression and GLP-2 promotes the relocation of glucose transporter GLUT2 (a gene product of SLC2A2) to the apical membrane in enterocytes to enhance sugar absorption [28,29]. The role of GLUT2 as a glucose sensor has been previously investigated. Studies in favor of GLUT2 as a sugar sensor emphasized the transporter’s importance in sugar-induced GLP-1 secretion using isolated rat intestine [30] and GLUT2 knockout mice [31,32]. However, a recent study using GLUT2 knockout mice demonstrated that sugar-induced GLP-1 expression did not differ between knockout and wild-type mice [25]. As mentioned by the authors, the discrepancy between the two results could be due to differences in fasting length as well as dose and route of administration of glucose.
The role of sugar sensors and their associated signaling has been explored in a variety of health conditions, including diabetes, obesity, and pathogen-induced diarrhea. As Type 2 diabetes (T2D) is a condition concerning insulin malfunction and GLP-1 secretion is involved in insulin release, the role of TAS1R2+TAS1R3 in the disorder has been explored. Although there was no difference seen in the copy number of sweet taste receptor transcripts between duodenal biopsies from T2D patients and healthy controls, the transcript number of sweet taste receptor molecules (TAS1R2, TAS1R3, Gαgust and TRPM5) was inversely correlated with blood glucose levels in T2D patients [33]. Sugar sensors have also been investigated in mouse models of obesity and obese patients. Much of the research has focused on GLP-1 expression, where obese patients have reduced postprandial GLP-1 levels [34]. However, a recent study in rats have found that, despite having significantly different plasma GLP-1 concentrations, high-fat diet (HFD)-fed rats did not have a significant difference in sweet taste receptors in the intestine compared to standard diet-fed rats [35]. Interestingly, rabbits with Escherichia coli (E. coli)-induced diarrhea had a decrease in mortality and improvement of symptoms after consuming stevia leaf extract, likely mediated through TAS1R1+TAS1R3-induced upregulation of intestinal SGLT1 [36]. TAS1R2+TAS1R3 regulation in other diarrheal disorders could be of interest in future research.
2.2. Protein-Sensing Receptors
As proteins are broken down in the stomach by peptidases and HCl, protein sensing in the intestine involves receptors responsive to amino acids and short peptides. There are multiple amino acid sensing receptors seen in the intestine, including TAS1R1+TAS1R3 dimer [3,4], extracellular Calcium sensing receptor (CaSR) [37,38,39], GPCR class C group 6 member A (GPRC6A) [40,41], metabotropic glutamate receptors (mGluRs) [42,43], and lysophosphatidic acid receptor 5 (LPAR5, previously GPR93) [44,45]. These receptors differ in amino acid/peptide ligand preference. mGluRs specifically respond to L-glutamate and their signaling promotes food digestion [46,47]. LPAR5/GPR93 is a peptone receptor found in the EECs that induces CCK secretion in STC-1 cells [48,49]. GPRC6A preferentially binds to basic L-amino acids and is primarily expressed in intestinal epithelial cells and EECs [50,51]. GPRC6A induces GLP-1 expression in GLUTag cells when treated with L-ornithine [52]. A canonical calcium sensor, CaSR, is expressed throughout the intestinal epithelium (primarily in EECs) and colon, and has demonstrated peptide-sensing ability in the intestine [38]. Beyond calcium, CaSR primarily responds to basic amino acids and oligopeptides.
TAS1R1+TAS1R3, like the sweet taste receptor TAS1R2+TAS1R3, functions as a heterodimeric GPCR that binds to a broad spectrum of amino acids [7,53] and acts through coupling with α-gustducin and α-transducin [54] (Figure 1). TAS1R3 shows similar transcription patterns to that of TAS1R2, with the highest expression in the proximal small intestine, while TAS1R1 is seen in similar levels throughout the intestine [3,4]. When activated by L-Phe, L-Leu, or L-Glu, TAS1R1+TAS1R3 promotes CCK secretion by STC-1 cells [55]. Conversely, a recent study has demonstrated that activation of GPR35, GPR93, GPR142, and the TAS1R1+TAS1R3 do not stimulate GLP-1 release in perfused rat proximal small intestine [56]. These authors only found that CaSR promotes GLP-1 secretion. However, as indicated by these authors, the use of specific inhibitors is needed to strengthen these results. CaSR promotes oligopeptide-inducible GLP-1 secretion by primary mouse L cells in an oligopeptide transporter (PEPT)-dependent manner [57]. CaSR also functions as an aromatic amino acid sensor, where the receptor promotes CCK and GLP-1 expression in the presence of L-Phe in isolated I cells and in rats [58,59]. CaSR is predominantly Gαq coupled, however it is known to couple with other G proteins [60,61]. Gαq activates phospholipase C to promote the synthesis of diacylglycerol (DAG) and IP3. Together, DAG and IP3 induce an increase in intracellular calcium for signaling events.
In addition to the different receptors, the signaling outcome of amino acid signaling is dependent on the specific amino acid ligand and its location (luminal vs. vascular side). For example, L-Val induces the greatest GLP-1 release on the luminal side of perfused rat intestine, while L-Arg and aromatic amino acids do so on the vascular side [56].
Contrary to carbohydrates, intestinal protein sensing response remains unchanged in obesity. This finding is observed as obese individuals have similar CCK and GLP-1 levels in response to intraduodenal protein infusion to what is seen in lean individuals [62]. Beyond this, protein intake reduces postprandial glucose response in T2D individuals and improvement in body composition in obese subjects [63,64]. Similar to sweet taste receptors [36], CaSR also has implicated therapeutic potential in alleviating diarrhea in isolated rat intestines, as the receptor is a modulator of intestinal fluid secretion and absorption [65,66]. However, these findings have only been reported in calcium-activated CaSR, and protein activation of CaSR has yet to be explored in these aspects.
2.3. Fatty Acid-Sensing Receptors
Dietary fats, typically triglycerides, are primarily broken down by lipases in the intestinal lumen into free medium- or long-chain fatty acids (LCFAs) prior to nutrient detection. SCFAs are ligands for free fatty acid receptor (FFAR) 2 (previously GPR43) and FFAR3 (GPR41), while LCFAs are detected by FFAR1 (GPR40), GPR119, and FFAR4 (GPR120) (Figure 1). Similar to protein sensors, the different FA receptors have specific ligand preferences [67]. For example, FFAR2 has equal potency with acetate and propionate, while FFAR3 has increased potency with propionate compared to acetate [68]. FFAR1 preferentially binds to pentadecanoic and palmitic acids, while FFAR4 demonstrates greatest potency with saturated FAs between 14–18 carbons in length and unsaturated free FAs between 16–22 carbons in length [69,70]. All four FFARs are expressed in L cells [71,72]. FFAR4 is primarily expressed in EECs of ileum [69].
Despite their similar length of FA ligands, FFAR2 and FFAR3 are coupled with different G proteins, with FFAR2 coupled to Gi/o proteins and FFAR3 having dual coupling with Gi/o and Gq proteins [73]. Both receptors primarily promote their signaling activity though IP3-induced intracellular calcium release. FFAR2 mediates metabolic homeostasis through promoting leptin secretion, a lipogenesis inhibitor [74]. In contrast, FFAR3 inhibits lipolysis and its signaling lessens plasma FA levels in vivo [75]. FFAR1 and FFAR4 are coupled to Gq and are involved in insulin synthesis via incretin secretion signaling as demonstrated in STC-1 cells and mice [69,70,76,77].
GPR119 is another fatty acid sensor, which primarily interacts with phospholipid and fatty acid amide ligands [78,79]. GPR119 is selectively coupled with Gs, as the receptor acts through increasing cAMP concentrations by promoting adenylyl cyclase (AC) activity [80].
The cluster of differentiation 36 (CD36) is a fatty acid transporter with FA-sensing abilities. As a scavenger receptor, CD36 interacts with a variety of ligands, including LCFAs [81]. CD36 is expressed on the brush border of enterocytes and EECs primarily in the proximal intestine [82,83]. CD36 activity in enterocytes has been linked to prolonging satiety though mediating the conversion of FA into oleoylethanolamide, as demonstrated in knockout mice [84]. In EECs, CD36 activation promotes the CCK secretion via cAMP activation and secretin secretion via cAMP-PKA signaling, as demonstrated in knockout mice [85]. CD36 is also involved in GLP-1 and GIP secretion, as obese African American women carrying a CD36-diminishing mutation release fewer incretins after ingesting a high-fat meal compared to controls [86]. Interestingly, the stimulation of anorectic signals by dietary fat is dependent of chylomicron formation from FAs in rats, indicating that fat sensing also occurs on the basolateral side of the intestine following FA absorption [87]. To this end, CD36 could support the hypothesis of basolateral fat sensing through its FA transport activity and promotion of chylomicron synthesis [88].
Similar to amino acid- and sugar-sensing, the role of fat-sensing in obesity and diabetes has been studied. Interestingly, duodenal CD36 and FFAR4 transcript levels are directly correlated with BMI in lean and obese subjects [89]. However, small intestinal Cd36 expression is significantly downregulated in high sucrose diet (HSD)-fed mice, but high-fat + high-sucrose (HFHSD)-fed mice have elevated CD36 transcript levels [90,91]. These studies indicate that body weight alone is not completely indicative of CD36 expression, and diet composition must be considered. The other FFARs, particularly FFAR1, have been studied as therapeutic targets for metabolic disorders. Using a combination treatment of FFAR1 agonists and DPP-IV (incretin degradation enzyme) inhibitors, insulin secretion and glucose metabolism were improved in ob/ob mice [92].
A variety of sugar-, amino acid-, and fatty acid-sensing molecules are expressed in specific cells of the intestinal epithelium that initiate physiological responses to luminal nutrients.
3. Sensory Tuft Cell in the Small Intestine
3.1. Similarity of Intestinal Tuft Cell and Lingual Taste Receptor Cell
Over the past decade, the intestinal tuft cell has been acknowledged as a different cell lineage from other types of epithelial cell, including EEC. Through electron microscopy, the distinct ultrastructure of long, stiff microvilli and the well-developed tubulovesicular system were identified in the 1950s in tuft cells, also known as brush, caveolated, multivesicular, or fibrillovesicular cells [93,94]. However, intestinal tuft cells had not been characterized well until doublecortin-like kinase 1 (DCLK1) was identified as a tuft cell-specific marker in mice [95]. In later histochemical studies, the jelly fish-like apical tuft structure has been described with the actin-associating phalloidin, gamma-actin, and advillin, but not villin unlike other types of intestinal epithelial cells [95,96,97] (Figure 2). In addition to morphological similarity, intestinal tuft cells possess chemosensory and signal transduction machineries that are seen in lingual taste receptor cells.
Figure 2.
Tuft cell morphology in mouse small intestine. Widely used tuft cell markers, F-actin (phalloidin), DCLK1, and acetylated tubulin demonstrate the microvillus structure, whole-cell shape, and dense microtubules, respectively.
Sweet, umami, or bitter compounds in foods are detected by different taste receptor cells that express distinct GPCRs, namely TAS1R2+TAS1R3, TAS1R1+TAS1R3, or TAS2R(s), respectively. A master transcription factor POU2F3 (POU Class 2 Homeobox 3) generates these taste receptor cells in the taste buds [98]. Pou2f3-deficient mice specifically lack tuft cells in the intestine, whereas other cell lineages are equivalent to that in wild-type mice [99]. Taste transduction molecules are commonly expressed in tuft and taste receptor cells alike in mice, including GNAT3, TRPM5, PLCβ2, and TAS1R members [4,15]. Single cell RNA-sequencing studies have revealed that mouse intestinal tuft cells express a variety of GPCRs, including Sucnr1 (Gpr91), Ffar3, Gprc5c, and some orphan GPCRs [100]. These transcription signatures suggest that tuft cells have the sensing ability for succinate (SUCNR1), short-chain fatty acids (FFAR3), acid/base balance (GPRC5C), and other unknown ligands in the lumen. Unlike the GPCRs for endogenous hormones that show high affinity (<100 nM), the GPCRs expressed on taste buds and intestinal tuft cells require 0.1–100 mM range of ligands and respond to broad spectrum of chemicals with similar structure [101]. This range is suitable to nutrient concentrations in the intestinal lumen. Individual tuft cell may express a limited number of GPCRs and respond to distinct ligands similar to that of the lingual taste receptor cells [98]. In contrast to the direct communication of taste receptor cells with gustatory nerves, intestinal tuft cells likely release IL-25, acetylcholine (ACh), and/or eicosanoids (prostaglandins and leukotrienes) in paracrine fashion to neighboring cells or nerves.
3.2. Intestinal Tuft Cell Markers
Most POU2F3-expressing (POU2F3+) tuft cells express signal transmitter enzymes that indicate their signal transduction function. Immunostaining for these enzymes is used as intestinal tuft cell markers in mouse and human tissues, including choline acetyl transferase (ChAT) (Figure 3), prostaglandin synthase 1 (PTGS1, also known as COX1), PTGS2 (COX2), hematopoietic prostaglandin D synthase (HPGDS), and arachidonate 5-lypoxygenase (ALOX5) [95,102]. Tuft cell-specific tyrosine phosphorylation was identified in the epidermal growth factor receptor (EGFR)(Y1068) [103] and an actin-binding protein, CCDC88A (GIRDIN)(Y1798) [104], suggesting a distinct kinase pathway in the tuft cell. Although the majority of intestinal tuft cells possess ChAT protein, other cholinergic neuron machineries, such as vesicle ACh transporter or high-affinity choline transporter (SLC5A7), have not been identified in the tuft cell [105]. Whether ACh is actually secreted into extracellular space or is acting via intracellular signaling remains unknown. DCLK1 is also widely utilized as a tuft cell marker and Cre/loxP target in genetically engineered mice, however, the DCLK1 expression in human tuft cells has not been confirmed. Esmaeilniakooshkghazi et al. recently reported that advillin protein selectively accumulates in intestinal tuft cells of mice [96]. Since the predominate expression of advillin was identified in peripheral sensory neurons, Advillin-Cre mice have been utilized to target the sensory neurons [106]. To distinguish intestinal tuft cell-specific function, this mouse strain can be used in enteroid studies.
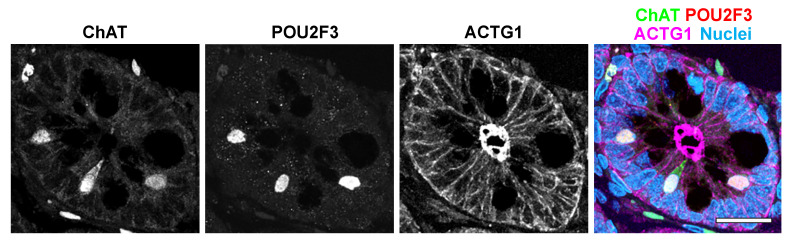
Figure 3.
Immunostaining for human intestinal tuft cells. Both ChAT and POU2F3 are detected in tuft cells in jejunum paraffin sections. Scale bar: 20 µm.
3.3. Tuft Cell Function in Mucosal Homeostasis
DCLK1+ tuft cell depletion reduces epithelial cell proliferation and survival rate of mice after mucosal injury [107,108]. These studies suggest that tuft cells may support stem cell activity and promote mucosal restitution. Indeed, our quantitative immunofluorescence studies demonstrated that tuft cell frequency was significantly decreased in celiac disease patients who showed malabsorption [109]. Several clinical trials have been reported, demonstrating that experimental helminth infections suppress inflammatory cytokines and allergic reactions in patients with autoimmune disorders, including celiac disease and inflammatory bowel disease (IBD) [110,111,112]. In mouse experimental models, helminth inoculation induces tuft cell hyperplasia and activates type 2 immune response through IL-25 secretion from tuft cells [99,113]. Tuft cell contribution to the type 2 immune response is reviewed elsewhere [114,115]. Although there is no report about the change in human tuft cell population under the parasite infections, tuft cell-mediated mucosal restitution might also be important for epithelial homeostasis in humans. Furthermore, a significant decrease in tuft cell number is correlated with the inflammation severity in duodenal ulceration [109] and ileal inflammation in Crohn’s disease [116]. Loss of tuft cell population can be an indicator of impaired mucosal integrity and activating tuft cells might be a therapeutic pathway for enteropathy. As expected by Sucnr1 transcriptional signature in intestinal tuft cells, 100–150 mM succinate in drinking water, but not short-chain fatty acids that activate FFAR3, leads tuft cell hyperplasia and type 2 immune responses via IL-25 release [117,118]. Several studies using knockout mouse strains confirmed that the succinate-induced tuft cell activation is mediated by SUCNR1, GNAT3, PLCß2, TRPM5, and TAS1R3, suggesting the same cascade as that in lingual taste receptor cells [117,118,119,120]. SUCNR1-deficient mice lack the response to protozoa-derived succinate but have intact immune response to helminth infection [119]. The sensory mechanism for helminth by the tuft cell is still unknown. Unlike mouse tissues, IL-25 production or any chemosensing receptor expression in human tuft cell has not yet been confirmed. Recently, predominant expression of Fc fragments was identified in a subset of human intestinal tuft cells, suggesting the involvement in immune response [121]. Identification of tuft cell activation pathways in human intestine might support the development of treatments for autoimmune disorders.
3.4. Enteroid/Intestinal Organoid as a Sensory Organ Model
Sato and Clevers established 3-dimentional enteroid culture technique in 2009, demonstrating that Lgr5+ stem cells can differentiate into all types of intestinal epithelial cells [122]. The term enteroid indicates primary cultures of isolated stem cell-derived epithelium, while inducible pluripotent stem (iPS) cell-derived “mini gut” cultures are termed intestinal organoids (reviewed in [123]). Enteroids recapitulate the functional characteristics of original tissues, including intestinal segmental differences and disease phenotypes, suggesting that this is a closer model to epithelial physiology than immortalized cell lines [124].
Studying luminal the chemosensing mechanism in 3-dimensional enteroid cultures has had difficulties, since the luminal volume of enteroids cannot be estimated to administer precise concentrations of compounds. The Donowitz and Zachos groups have established 2-dimensional cultures of human and mouse enteroids that allow for the measurement of epithelial ion transport and cytokine release into the luminal and basolateral spaces [125,126]. The combination of activators and/or inhibitors of Wnt and Notch signaling pathways can manipulate the cell lineage populations in enteroid systems [127]. Treatment with Notch inhibitors induces tuft cell hyperplasia in vivo and in enteroids [113,128], suggesting that tuft cell development likely requires Wnt signaling activation. We recently reported that tuft cell differentiation was specifically inhibited by myosin Vb (MYO5B) loss, a model of a congenital diarrheal disorder, microvillus inclusion disease [97]. MYO5B-deficient epithelial cells showed reduced Wnt ligands, while Notch ligands remained unchanged, thereby inducing an imbalance of Notch/Wnt signaling. A Wnt signaling inhibitor, Wnt-C59, significantly reduced tuft cell differentiation in wild-type mouse enteroids, but had no effect on MYO5B-deficient enteroids [97]. Similarly, the combination of high Wnt and a Notch inhibitor, DBZ, enhanced taste receptor cell development in gustatory organoids [129,130]. These observations suggest that a low Notch and high Wnt environment initiate chemosensory cell differentiation in the enteroid/organoid cultures. IL-4 and IL-13 supplementation increase tuft cell differentiation in mouse enteroids, at least partly mediated by Notch signaling modulation [96,99,113]. Tuft cell-enriched enteroids as monolayer on transwell inserts serves as a useful tool to identify chemoreceptors of tuft cells and to determine nutrient handling of neighboring enterocytes in response to tuft cell activation. Furthermore, immune cells or neurons can be co-cultured in the transwell system to investigate cell-cell interaction downstream of chemosensing.
The enteroid technique is a promising tool for studying tuft cell functions in healthy and disease conditions. Of note, abnormal glucose absorption and gluconeogenesis in obese patients are recapitulated in enteroids generated from these patients [131]. Enteroids could also potentially be used to model congenital diarrhea disorders. Congenital diarrheal patients with diacylglycerol acyltransferase (DGAT)1 mutation are known to be rescued by a very low-fat diet [132]. However, long-term avoidance of dietary fat induces essential fatty acid depletion that affects the development of humoral and nervous systems. Since DGAT1 knockout mice do not recapitulate the human patient phenotype, the mechanistic study for lipid-induced diarrhea in DGAT1 mutant patients is hampered. Future studies with patient enteroids may provide important clues of lipid sensing mechanism and therapeutic strategies for those patients as well as for other unclassified congenital diarrheal diseases.
4. Conclusions
Recent studies have revealed the correlation between intestinal nutrient sensors and metabolic, immune, and diarrheal disorders. Nutrient sensing pathways and tuft cell activation can be therapeutic targets to normalize nutrient absorption and mucosal restitution.
Author Contributions
A.B. and I.K. contributed to writing the original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by NIH National Institute of Diabetes and Digestive and Kidney Diseases R01DK128190 to IK and the Histochemical Society Cornerstone Award to AB.
Institutional Review Board Statement
Animal studies are approved by the Institutional Animal Care and Use Committee of Vanderbilt University Medical Center (M2000104). Histological analysis of human tissues is approved by IRB committee for Vanderbilt University Medical Center (#190693).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Moran A.W., Daly K., Al-Rammahi M.A., Shirazi-Beechey S.P. Nutrient sensing of gut luminal environment. Proc. Nutr. Soc. 2020;80:29–36. doi: 10.1017/S0029665120007120. [DOI] [PubMed] [Google Scholar]
- 2.Lu V.B., Gribble F.M., Reimann F. Nutrient-Induced Cellular Mechanisms of Gut Hormone Secretion. Nutrients. 2021;13:883. doi: 10.3390/nu13030883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dyer J., Salmon K., Zibrik L., Shirazi-Beechey S. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem. Soc. Trans. 2005;33:302–305. doi: 10.1042/BST0330302. [DOI] [PubMed] [Google Scholar]
- 4.Bezençon C., le Coutre J., Damak S. Taste-Signaling Proteins Are Coexpressed in Solitary Intestinal Epithelial Cells. Chem. Senses. 2006;32:41–49. doi: 10.1093/chemse/bjl034. [DOI] [PubMed] [Google Scholar]
- 5.Shackley M., Ma Y., Tate E.W., Brown A.J.H., Frost G., Hanyaloglu A.C. Short Chain Fatty Acids Enhance Expression and Activity of the Umami Taste Receptor in Enteroendocrine Cells via a Gα(i/o) Pathway. Front Nutr. 2020;7:568991. doi: 10.3389/fnut.2020.568991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Margolskee R.F., Dyer J., Kokrashvili Z., Salmon K.S.H., Ilegems E., Daly K., Maillet E., Ninomiya Y., Mosinger B., Shirazi-Beechey S.P. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc. Nat. Acad. Sci. USA. 2007;104:15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X., Staszewski L., Xu H., Durick K., Zoller M., Adler E. Human receptors for sweet and umami taste. Proc. Nat. Acad. Sci. USA. 2002;99:4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jang H.-J., Kokrashvili Z., Theodorakis M.J., Carlson O.D., Kim B.-J., Zhou J., Kim H.H., Xu X., Chan S.L., Juhaszova M., et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc. Natl. Acad. Sci. USA. 2007;104:15069–15074. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kokrashvili Z., Mosinger B., Margolskee R.F. T1r3 and α-Gustducin in Gut Regulate Secretion of Glucagon-like Peptide-1. Ann. N. Y. Acad. Sci. 2009;1170:91–94. doi: 10.1111/j.1749-6632.2009.04485.x. [DOI] [PubMed] [Google Scholar]
- 10.Smith K., Azari E.K., LaMoia T.E., Hussain T., Vargova V., Karolyi K., Veldhuis P.P., Arnoletti J.P., de la Fuente S.G., Pratley R.E., et al. T1R2 receptor-mediated glucose sensing in the upper intestine potentiates glucose absorption through activation of local regulatory pathways. Mol. Metab. 2018;17:98–111. doi: 10.1016/j.molmet.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinert R.E., Gerspach A.C., Gutmann H., Asarian L., Drewe J., Beglinger C. The functional involvement of gut-expressed sweet taste receptors in glucose-stimulated secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) Clin. Nutr. 2011;30:524–532. doi: 10.1016/j.clnu.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Gerspach A.C., Steinert R.E., Schönenberger L., Graber-Maier A., Beglinger C. The role of the gut sweet taste receptor in regulating GLP-1, PYY, and CCK release in humans. Am. J. Physiol. Metab. 2011;301:E317–E325. doi: 10.1152/ajpendo.00077.2011. [DOI] [PubMed] [Google Scholar]
- 13.Woelnerhanssen B.K., Cajacob L., Keller N., Doody A., Rehfeld J.F., Drewe J., Peterli R., Beglinger C., Meyer-Gerspach A.C. Gut hormone secretion, gastric emptying, and glycemic responses to erythritol and xylitol in lean and obese subjects. Am. J. Physiol. Metab. 2016;310:E1053–E1061. doi: 10.1152/ajpendo.00037.2016. [DOI] [PubMed] [Google Scholar]
- 14.McLaughlin S.K., McKinnon P.J., Margolskee R. Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature. 1992;357:563–569. doi: 10.1038/357563a0. [DOI] [PubMed] [Google Scholar]
- 15.Hofer D., Puschel B., Drenckhahn D. Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. Proc. Natl. Acad. Sci. USA. 1996;93:6631–6634. doi: 10.1073/pnas.93.13.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan W., Sunavala G., Rosenzweig S., Dasso M., Brand J.G., Spielman A.I. Bitter taste transduced by PLC-β2-dependent rise in IP3 and α-gustducin-dependent fall in cyclic nucleotides. Am. J. Physiol. Physiol. 2001;280:C742–C751. doi: 10.1152/ajpcell.2001.280.4.C742. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y., Hoon M.A., Chandrashekar J., Mueller K.L., Cook B., Wu D., Zuker C.S., Ryba N.J. Coding of Sweet, Bitter, and Umami Tastes: Different Receptor Cells Sharing Similar Signaling Pathways. Cell. 2003;112:293–301. doi: 10.1016/S0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 18.Prawitt D., Monteilh-Zoller M.K., Brixel L., Spangenberg C., Zabel B., Fleig A., Penner R. TRPM5 is a transient Ca2+-activated cation channel responding to rapid changes in [Ca2+]i. Proc. Natl. Acad. Sci. USA. 2003;100:15166–15171. doi: 10.1073/pnas.2334624100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu D., Liman E.R. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc. Natl. Acad. Sci. USA. 2003;100:15160–15165. doi: 10.1073/pnas.2334159100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saltiel M.Y., Kuhre R.E., Christiansen C.B., Eliasen R., Conde-Frieboes K.W., Rosenkilde M.M., Holst J.J. Sweet Taste Receptor Ac-tivation in the Gut Is of Limited Importance for Glucose-Stimulated GLP-1 and GIP Secretion. Nutrients. 2017;9:418. doi: 10.3390/nu9040418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freeman S.L., Bohan D., Darcel N., Raybould H.E. Luminal glucose sensing in the rat intestine has characteristics of a so-dium-glucose cotransporter. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;291:G439–G445. doi: 10.1152/ajpgi.00079.2006. [DOI] [PubMed] [Google Scholar]
- 22.Moriya R., Shirakura T., Ito J., Mashiko S., Seo T. Activation of sodium-glucose cotransporter 1 ameliorates hyperglycemia by mediating incretin secretion in mice. Am. J. Physiol. Metab. 2009;297:E1358–E1365. doi: 10.1152/ajpendo.00412.2009. [DOI] [PubMed] [Google Scholar]
- 23.Soták M., Casselbrant A., Rath E., Zietek T., Strömstedt M., Adingupu D.D., Karlsson D., Fredin M.F., Ergang P., Pácha J., et al. Intestinal sodium/glucose cotransporter 3 expression is epithelial and downregulated in obesity. Life Sci. 2020;267:118974. doi: 10.1016/j.lfs.2020.118974. [DOI] [PubMed] [Google Scholar]
- 24.Gorboulev V., Schurmann A., Vallon V., Kipp H., Jaschke A., Klessen D., Friedrich A., Scherneck S., Rieg T., Cunard R., et al. Na+-d-glucose Cotransporter SGLT1 is Pivotal for Intestinal Glucose Absorption and Glucose-Dependent Incretin Secretion. Diabetes. 2011;61:187–196. doi: 10.2337/db11-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Röder P.V., Geillinger K.E., Zietek T.S., Thorens B., Koepsell H., Daniel H. The Role of SGLT1 and GLUT2 in Intestinal Glucose Transport and Sensing. PLoS ONE. 2014;9:e89977. doi: 10.1371/journal.pone.0089977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibazaki T., Tomae M., Ishikawa-Takemura Y., Fushimi N., Itoh F., Yamada M., Isaji M. KGA-2727, a Novel Selective Inhibitor of a High-Affinity Sodium Glucose Cotransporter (SGLT1), Exhibits Antidiabetic Efficacy in Rodent Models. J. Pharmacol. Exp. Ther. 2012;342:288–296. doi: 10.1124/jpet.112.193045. [DOI] [PubMed] [Google Scholar]
- 27.Powell D.R., Smith M., Greer J., Harris A., Zhao S., Dacosta C., Mseeh F., Shadoan M.K., Sands A., Zambrowicz B., et al. LX4211 Increases Serum Glucagon-Like Peptide 1 and Peptide YY Levels by Reducing Sodium/Glucose Cotransporter 1 (SGLT1)–Mediated Absorption of Intestinal Glucose. J. Pharmacol. Exp. Ther. 2013;345:250–259. doi: 10.1124/jpet.113.203364. [DOI] [PubMed] [Google Scholar]
- 28.Cheeseman C.I. Upregulation of SGLT-1 transport activity in rat jejunum induced by GLP-2 infusion in vivo. Am. J. Physiol. Integr. Comp. Physiol. 1997;273:R1965–R1971. doi: 10.1152/ajpregu.1997.273.6.R1965. [DOI] [PubMed] [Google Scholar]
- 29.Au A., Gupta A., Schembri P., Cheeseman C.I. Rapid insertion of GLUT2 into the rat jejunal brush-border membrane pro-moted by glucagon-like peptide 2. Biochem. J. 2002;367:247–254. doi: 10.1042/bj20020393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mace O.J., Schindler M., Patel S. The regulation of K- and L-cell activity by GLUT2 and the calcium-sensing receptor CasR in rat small intestine. J. Physiol. 2012;590:2917–2936. doi: 10.1113/jphysiol.2011.223800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cani P.D., Holst J.J., Drucker D.J., Delzenne N.M., Thorens B., Burcelin R., Knauf C. GLUT2 and the incretin receptors are involved in glucose-induced incretin secretion. Mol. Cell. Endocrinol. 2007;276:18–23. doi: 10.1016/j.mce.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Mace O.J., Lister N., Morgan E., Shepherd E., Affleck J., Helliwell P., Bronk J.R., Kellett G.L., Meredith D., Boyd R., et al. An energy supply network of nutrient absorption coordinated by calcium and T1R taste receptors in rat small intestine. J. Physiol. 2009;587:195–210. doi: 10.1113/jphysiol.2008.159616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young R.L., Sutherland K., Pezos N., Brierley S.M., Horowitz M., Rayner C.K., Blackshaw L.A. Expression of taste molecules in the upper gastrointestinal tract in humans with and without type 2 diabetes. Gut. 2008;58:337–346. doi: 10.1136/gut.2008.148932. [DOI] [PubMed] [Google Scholar]
- 34.Naslund E., Hellström M. Glucagon-like peptide-1 in the pathogenesis of obesity. Drug News Perspect. 1998;11:92–97. doi: 10.1358/dnp.1998.11.2.659947. [DOI] [PubMed] [Google Scholar]
- 35.Feng R.L., Qian C., Liu L.Y., Liu Q.J., Jin Y.Q., Li S.X., Liu W., Rayner C.K., Ma J. Secretion of Gut Hormones and Expression of Sweet Taste Receptors and Glucose Transporters in a Rat Model of Obesity. Obes. Facts. 2019;12:190–198. doi: 10.1159/000497122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moran A.W., Al-Rammahi M.A., Daly K., Grand E., Ionescu C., Bravo D.M., Wall E.H., Shirazi-Beechey S.P. Consumption of a Natural High-Intensity Sweetener Enhances Activity and Expression of Rabbit Intestinal Na+/Glucose Cotransporter 1 (SGLT1) and Improves Colibacillosis-Induced Enteric Disorders. J. Agric. Food Chem. 2019;68:441–450. doi: 10.1021/acs.jafc.9b04995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gama L., Baxendale-Cox L.M., Breitwieser G. Ca2+-sensing receptors in intestinal epithelium. Am. J. Physiol. Content. 1997;273:C1168–C1175. doi: 10.1152/ajpcell.1997.273.4.C1168. [DOI] [PubMed] [Google Scholar]
- 38.Chattopadhyay N., Cheng I., Rogers K., Riccardi D., Hall A., Diaz R., Hebert S.C., Soybel D.I., Brown E.M. Identification and localization of extracellular Ca2+-sensing receptor in rat intestine. Am. J. Physiol. Content. 1998;274:G122–G130. doi: 10.1152/ajpgi.1998.274.1.G122. [DOI] [PubMed] [Google Scholar]
- 39.Sheinin Y., Kallay E., Wrba F., Kriwanek S., Peterlik M., Cross H.S. Immunocytochemical Localization of the Extracellular Calcium-Sensing Receptor in Normal and Malignant Human Large Intestinal Mucosa. J. Histochem. Cytochem. 2000;48:595–601. doi: 10.1177/002215540004800503. [DOI] [PubMed] [Google Scholar]
- 40.Kinsey-Jones J.S., Alamshah A., McGavigan A.K., Spreckley E., Banks K., Monteoliva N.C., Norton M., Bewick G.A., Murphy K.G. GPRC6a is not required for the effects of a high-protein diet on body weight in mice. Obesity. 2015;23:1194–1200. doi: 10.1002/oby.21083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baumard L., Weerts Z., Masclee A., Keszthelyi D., Michael-Titus A., Peiris M. Effect of Obesity on the Expression of Nutrient Receptors and Satiety Hormones in the Human Colon. Nutrients. 2021;13:1271. doi: 10.3390/nu13041271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu H.-Z., Ren J., Liu S., Gao C., Xia Y., Wood J.D. Functional group I metabotropic glutamate receptors in submucous plexus of guinea-pig ileum. Br. J. Pharmacol. 1999;128:1631–1635. doi: 10.1038/sj.bjp.0702980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tong Q., Kirchgessner A.L. Localization and function of metabotropic glutamate receptor 8 in the enteric nervous system. Am. J. Physiol. Liver Physiol. 2003;285:G992–G1003. doi: 10.1152/ajpgi.00118.2003. [DOI] [PubMed] [Google Scholar]
- 44.Lin S., Yeruva S., He P., Singh A.K., Zhang H., Chen M., Lamprecht G., de Jonge H.R., Tse M., Donowitz M., et al. Lysophosphatidic Acid Stimulates the Intestinal Brush Border Na+/H+ Exchanger 3 and Fluid Absorption via LPA5 and NHERF2. Gastroenterology. 2010;138:649–658. doi: 10.1053/j.gastro.2009.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Symonds E.L., Peiris M., Page A.J., Chia B., Dogra H., Masding A., Galanakis V., Atiba M., Bulmer D., Young R.L., et al. Mechanisms of activation of mouse and human en-teroendocrine cells by nutrients. Gut. 2015;64:618–626. doi: 10.1136/gutjnl-2014-306834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaudhari N., Landin A.M., Roper S.D. A metabotropic glutamate receptor variant functions as a taste receptor. Nat. Neurosci. 2000;3:113–119. doi: 10.1038/72053. [DOI] [PubMed] [Google Scholar]
- 47.Akiba Y., Watanabe C., Mizumori M., Kaunitz J.D. Luminal l-glutamate enhances duodenal mucosal defense mechanisms via multiple glutamate receptors in rats. Am. J. Physiol. Liver Physiol. 2009;297:G781–G791. doi: 10.1152/ajpgi.90605.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi S., Lee M., Shiu A.L., Yo S.J., Aponte G.W. Identification of a protein hydrolysate responsive G protein-coupled receptor in enterocytes. Am. J. Physiol. Liver Physiol. 2007;292:G98–G112. doi: 10.1152/ajpgi.00295.2006. [DOI] [PubMed] [Google Scholar]
- 49.Choi S., Lee M., Shiu A.L., Yo S.J., Halldén G., Aponte G.W. GPR93 activation by protein hydrolysate induces CCK transcription and secretion in STC-1 cells. Am. J. Physiol. Liver Physiol. 2007;292:G1366–G1375. doi: 10.1152/ajpgi.00516.2006. [DOI] [PubMed] [Google Scholar]
- 50.Christiansen B., Hansen K.B., Wellendorph P., Brauner-Osborne H. Pharmacological characterization of mouse GPRC6A, an L-alpha-amino-acid receptor modulated by divalent cations. Br. J. Pharmacol. 2007;150:798–807. doi: 10.1038/sj.bjp.0707121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mizokami A., Yasutake Y., Gao J., Matsuda M., Takahashi I., Takeuchi H., Hirata M. Osteocalcin Induces Release of Glucagon-Like Peptide-1 and Thereby Stimulates Insulin Secretion in Mice. PLoS ONE. 2013;8:e57375. doi: 10.1371/journal.pone.0057375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oya M., Kitaguchi T., Pais R., Reimann F., Gribble F., Tsuboi T. The G Protein-coupled Receptor Family C Group 6 Subtype A (GPRC6A) Receptor Is Involved in Amino Acid-induced Glucagon-like Peptide-1 Secretion from GLUTag Cells. J. Biol. Chem. 2013;288:4513–4521. doi: 10.1074/jbc.M112.402677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nelson G., Chandrashekar J., Hoon M.A., Feng L., Zhao G., Ryba N.J.P., Zuker C.S. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- 54.He W., Yasumatsu K., Varadarajan V., Yamada A., Lem J., Ninomiya Y., Margolskee R.F., Damak S. Umami taste responses are mediated by al-pha-transducin and alpha-gustducin. J. Neurosci. 2004;24:7674–7680. doi: 10.1523/JNEUROSCI.2441-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Daly K., Al-Rammahi M., Moran A., Marcello M., Ninomiya Y., Shirazi-Beechey S. Sensing of amino acids by the gut-expressed taste receptor T1R1-T1R3 stimulates CCK secretion. Am. J. Physiol. Liver Physiol. 2013;304:G271–G282. doi: 10.1152/ajpgi.00074.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Modvig I.M., Kuhre R.E., Jepsen S.L., Xu S.F.S., Engelstoft M.S., Egerod K.L., Schwartz T.W., Ørskov C., Rosenkilde M.M., Holst J.J. Amino acids differ in their capacity to stimulate GLP-1 release from the perfused rat small intestine and stimulate secretion by different sensing mechanisms. Am. J. Physiol. Metab. 2021;320:E874–E885. doi: 10.1152/ajpendo.00026.2021. [DOI] [PubMed] [Google Scholar]
- 57.Diakogiannaki E., Pais R., Tolhurst G., Parker H.E., Horscroft J., Rauscher B., Zietek T., Daniel H., Gribble F., Reimann F. Oligopeptides stimulate glucagon-like peptide-1 secretion in mice through proton-coupled uptake and the calcium-sensing receptor. Diabetologia. 2013;56:2688–2696. doi: 10.1007/s00125-013-3037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liou A.P., Sei Y., Zhao X., Feng J., Lu X., Thomas C., Pechhold S., Raybould H.E., Wank S.A. The extracellular calcium-sensing receptor is required for chole-cystokinin secretion in response to L-phenylalanine in acutely isolated intestinal I cells. Am. J. Gastrointest. Liver Physiol. 2011;300:G538–G546. doi: 10.1152/ajpgi.00342.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alamshah A., Spreckley E., Norton M., Kinsey-Jones J.S., Amin A., Ramgulam A., Cao Y., Johnson R., Saleh K., Akalestou E., et al. l-phenylalanine modulates gut hormone release and glucose tolerance, and suppresses food intake through the calcium-sensing receptor in rodents. Int. J. Obes. 2017;41:1693–1701. doi: 10.1038/ijo.2017.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Conigrave A.D., Brown E.M. Taste receptors in the gastrointestinal tract. II. L-amino acid sensing by calcium-sensing re-ceptors: Implications for GI physiology. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;291:G753–G761. doi: 10.1152/ajpgi.00189.2006. [DOI] [PubMed] [Google Scholar]
- 61.Saidak Z., Brazier M., Kamel S., Mentaverri R. Agonists and Allosteric Modulators of the Calcium-Sensing Receptor and Their Therapeutic Applications. Mol. Pharmacol. 2009;76:1131–1144. doi: 10.1124/mol.109.058784. [DOI] [PubMed] [Google Scholar]
- 62.Hutchison A.T., Feinle-Bisset C., Fitzgerald P.C., Standfield S., Horowitz M., Clifton P.M., Luscombe-Marsh N.D. Comparative effects of intra-duodenal whey protein hydrolysate on antropyloroduodenal motility, gut hormones, glycemia, appetite, and energy intake in lean and obese men. Am. J. Clin. Nutr. 2015;102:1323–1331. doi: 10.3945/ajcn.115.114538. [DOI] [PubMed] [Google Scholar]
- 63.Arciero P.J., Gentile C.L., Pressman R., Everett M., Ormsbee M.J., Martin J., Santamore J., Gorman L., Fehling P.C., Vukovich M., et al. Moderate protein intake improves total and regional body composition and insulin sensitivity in overweight adults. Metabolism. 2008;57:757–765. doi: 10.1016/j.metabol.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 64.Manders R.J., Wagenmakers A.J., Koopman R., Zorenc A.H., Menheere P.P., Schaper N.C., Saris W.H.M., van Loon L.J.C. Co-ingestion of a protein hy-drolysate and amino acid mixture with carbohydrate improves plasma glucose disposal in patients with type 2 diabetes. Am. J. Clin. Nutr. 2005;82:76–83. doi: 10.1093/ajcn/82.1.76. [DOI] [PubMed] [Google Scholar]
- 65.Lysyy T., Lalani A.S., Olek E.A., Diala I., Geibel J.P. The calcium-sensing receptor: A novel target for treatment and prophylaxis of neratinib-induced diarrhea. Pharmacol. Res. Perspect. 2019;7:e00521. doi: 10.1002/prp2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barahona M.J., Maina R.M., Lysyy T., Finotti M., Caturegli G., Baratta V., D’Amico F., Mulligan D., Geibel J.P. Activation of the Calcium Sensing Receptor Decreases Secretagogue-Induced Fluid Secretion in the Rat Small Intestine. Front. Physiol. 2019;10:439. doi: 10.3389/fphys.2019.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Priyadarshini M., Kotlo K.U., Dudeja P.K., Layden B.T. Role of Short Chain Fatty Acid Receptors in Intestinal Physiology and Pathophysiology. Compr. Physiol. 2011;8:1091–1115. doi: 10.1002/cphy.c170050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brown A.J., Goldsworthy S.M., Barnes A.A., Eilert M.M., Tcheang L., Daniels D., Muir A.I., Wigglesworth M.J., Kinghorn I., Fraser N.J., et al. The Orphan G Protein-coupled Receptors GPR41 and GPR43 Are Activated by Propionate and Other Short Chain Carboxylic Acids. J. Biol. Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 69.Hirasawa A., Tsumaya K., Awaji T., Katsuma S., Adachi T., Yamada M., Sugimoto Y., Miyazaki S., Tsujimoto G. Free fatty acids regulate gut incretin gluca-gon-like peptide-1 secretion through GPR120. Nat. Med. 2005;11:90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 70.Briscoe C.P., Tadayyon M., Andrews J.L., Benson W.G., Chambers J.K., Eilert M.M., Ellis C., Elshourbagy N.A., Goetz A.S., Minnick D.T., et al. The Orphan G Protein-coupled Receptor GPR40 Is Activated by Medium and Long Chain Fatty Acids. J. Biol. Chem. 2003;278:11303–11311. doi: 10.1074/jbc.M211495200. [DOI] [PubMed] [Google Scholar]
- 71.Reimann F., Habib A.M., Tolhurst G., Parker H.E., Rogers G.J., Gribble F. Glucose Sensing in L Cells: A Primary Cell Study. Cell Metab. 2008;8:532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oh D.Y., Talukdar S., Bae E.J., Imamura T., Morinaga H., Fan W., Li P., Lu W.J., Watkins S.M., Olefsky J.M. GPR120 Is an Omega-3 Fatty Acid Receptor Mediating Potent Anti-inflammatory and Insulin-Sensitizing Effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Le Poul E., Loison C., Struyf S., Springael J.Y., Lannoy V., Decobecq M.E., Brezillon S., Dupriez V., Vassart G., Van Damme J., et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003;278:25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 74.Xiong Y., Miyamoto N., Shibata K., Valasek M., Motoike T., Kedzierski R.M., Yanagisawa M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc. Natl. Acad. Sci. USA. 2004;101:1045–1050. doi: 10.1073/pnas.2637002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ge H., Li X., Weiszmann J., Wang P., Baribault H., Chen J.-L., Tian H., Li Y. Activation of G Protein-Coupled Receptor 43 in Adipocytes Leads to Inhibition of Lipolysis and Suppression of Plasma Free Fatty Acids. Endocrinology. 2008;149:4519–4526. doi: 10.1210/en.2008-0059. [DOI] [PubMed] [Google Scholar]
- 76.Edfalk S., Steneberg P., Edlund H. Gpr40 Is Expressed in Enteroendocrine Cells and Mediates Free Fatty Acid Stimulation of Incretin Secretion. Diabetes. 2008;57:2280–2287. doi: 10.2337/db08-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sankoda A., Harada N., Kato T., Ikeguchi E., Iwasaki K., Yamane S., Murata Y., Hirasawa A., Inagaki N. Free fatty acid receptors, G protein-coupled receptor 120 and G protein-coupled receptor 40, are essential for oil-induced gastric inhibitory polypeptide secretion. J. Diabetes Investig. 2019;10:1430–1437. doi: 10.1111/jdi.13059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Overton H.A., Babbs A.J., Doel S.M., Fyfe M.C., Gardner L.S., Griffin G., Jackson H.C., Procter M.J., Rasamison C.M., Tang-Christensen M., et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006;3:167–175. doi: 10.1016/j.cmet.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 79.Soga T., Ohishi T., Matsui T., Saito T., Matsumoto M., Takasaki J., Matsumoto S.-I., Kamohara M., Hiyama H., Yoshida S., et al. Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem. Biophys. Res. Commun. 2005;326:744–751. doi: 10.1016/j.bbrc.2004.11.120. [DOI] [PubMed] [Google Scholar]
- 80.Chu Z.-L., Jones R.M., He H., Carroll C., Gutierrez V., Lucman A., Moloney M., Gao H., Mondala H., Bagnol D., et al. A Role for β-Cell-Expressed G Protein-Coupled Re-ceptor 119 in Glycemic Control by Enhancing Glucose-Dependent Insulin Release. Endocrinology. 2007;148:2601–2609. doi: 10.1210/en.2006-1608. [DOI] [PubMed] [Google Scholar]
- 81.Abumrad N.A., El-Maghrabi M.R., Amri E.Z., Lopez E., Grimaldi P.A. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J. Biol. Chem. 1993;268:17665–17668. doi: 10.1016/S0021-9258(17)46753-6. [DOI] [PubMed] [Google Scholar]
- 82.Masson C.J., Plat J., Mensink R.P., Namiot A., Kisielewski W., Namiot Z., Fullekrug J., Ehehalt R., Glatz J.F.C., Pelsers M.M.A.L. Fatty acid- and cholesterol transporter protein expression along the human intestinal tract. PLoS ONE. 2010;5:e10380. doi: 10.1371/journal.pone.0010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nassir F., Wilson B., Han X., Gross R.W., Abumrad N.A. CD36 Is Important for Fatty Acid and Cholesterol Uptake by the Proximal but Not Distal Intestine. J. Biol. Chem. 2007;282:19493–19501. doi: 10.1074/jbc.M703330200. [DOI] [PubMed] [Google Scholar]
- 84.Schwartz G.J., Fu J., Astarita G., Li X., Gaetani S., Campolongo P., Cuomo V., Piomelli D. The Lipid Messenger OEA Links Dietary Fat Intake to Satiety. Cell Metab. 2008;8:281–288. doi: 10.1016/j.cmet.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sundaresan S., Shahid R., Riehl T.E., Chandra R., Nassir F., Stenson W.F., Liddle R.A., Abumrad N.A. CD36-dependent signaling mediates fatty ac-id-induced gut release of secretin and cholecystokinin. FASEB J. 2013;27:1191–1202. doi: 10.1096/fj.12-217703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shibao C.A., Celedonio J.E., Tamboli R., Sidani R., Love-Gregory L., Pietka T., Xiong Y., Wei Y., Abumrad N.N., Abumrad N.A., et al. CD36 Modulates Fasting and Preabsorptive Hormone and Bile Acid Levels. J. Clin. Endocrinol. Metab. 2018;103:1856–1866. doi: 10.1210/jc.2017-01982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sakata Y., Fujimoto K., Ogata S., Koyama T., Fukagawa K., Sakai T., Tso P. Postabsorptive factors are important for satiation in rats after a lipid meal. Am. J. Physiol. 1996;271:G438–G442. doi: 10.1152/ajpgi.1996.271.3.G438. [DOI] [PubMed] [Google Scholar]
- 88.Tran T.T.T., Poirier H., Clément L., Nassir F., Pelsers M.M., Petit V., Degrace P., Monnot M.-C., Glatz J.F., Abumrad N.A., et al. Luminal Lipid Regulates CD36 Levels and Downstream Signaling to Stimulate Chylomicron Synthesis. J. Biol. Chem. 2011;286:25201–25210. doi: 10.1074/jbc.M111.233551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Little T.J., Isaacs N.J., Young R.L., Ott R., Nguyen N.Q., Rayner C.K., Horowitz M., Feinle-Bisset C. Characterization of duodenal expression and local-ization of fatty acid-sensing receptors in humans: Relationships with body mass index. Am. J. Physiol. Gastrointest. Liver Physiol. 2014;307:G958–G967. doi: 10.1152/ajpgi.00134.2014. [DOI] [PubMed] [Google Scholar]
- 90.O’Brien P., Han G., Ganpathy P., Pitre S., Zhang Y., Ryan J., Sim P.Y., Harding S.V., Gray R., Preedy V.R., et al. Chronic Effects of a High Sucrose Diet on Murine Gastro-intestinal Nutrient Sensor Gene and Protein Expression Levels and Lipid Metabolism. Int. J. Mol. Sci. 2020;22:137. doi: 10.3390/ijms22010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Okamura T., Hashimoto Y., Majima S., Senmaru T., Ushigome E., Nakanishi N., Asano M., Yamazaki M., Takakuwa H., Hamagaguchi M., et al. Trans Fatty Acid Intake Induces In-testinal Inflammation and Impaired Glucose Tolerance. Front. Immunol. 2021;12:669672. doi: 10.3389/fimmu.2021.669672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tanaka H., Yoshida S., Minoura H., Negoro K., Shimaya A., Shimokawa T., Shibasaki M. Novel GPR40 agonist AS2575959 exhibits glucose metabolism improvement and synergistic effect with sitagliptin on insulin and incretin secretion. Life Sci. 2014;94:115–121. doi: 10.1016/j.lfs.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 93.Jarvi O., Keyrilainen O. On the cellular structures of the epithelial invasions in the glandular stomach of mice caused by intramural application of 20-methylcholantren. Acta Pathol. Microbiol. Scand. Suppl. 1956;39:72–73. doi: 10.1111/j.1600-0463.1956.tb06739.x. [DOI] [PubMed] [Google Scholar]
- 94.Rhodin J., Dalhamn T. Electron microscopy of the tracheal ciliated mucosa in rat. Z. Zellforsch. Mikrosk. Anat. 1956;44:345–412. doi: 10.1007/BF00345847. [DOI] [PubMed] [Google Scholar]
- 95.Gerbe F., Van Es J.H., Makrini L., Brulin B., Mellitzer G., Robine S., Romagnolo B., Shroyer N., Bourgaux J.-F., Pignodel C., et al. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J. Cell Biol. 2011;192:767–780. doi: 10.1083/jcb.201010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Esmaeilniakooshkghazi A., George S.P., Biswas R., Khurana S. Mouse intestinal tuft cells express advillin but not villin. Sci. Rep. 2020;10:8877. doi: 10.1038/s41598-020-65469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kaji I., Roland J.T., Rathan-Kumar S., Engevik A.C., Burman A., Goldstein A.E., Watanabe M., Goldenring J.R. Cell differentiation is disrupted by MYO5B loss through Wnt/Notch imbalance. JCI Insight. 2021;6:e150416. doi: 10.1172/jci.insight.150416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Matsumoto I., Ohmoto M., Narukawa M., Yoshihara Y., Abe K. Skn-1a (Pou2f3) specifies taste receptor cell lineage. Nat. Neurosci. 2011;14:685–687. doi: 10.1038/nn.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gerbe F., Sidot E., Smyth D., Ohmoto M., Matsumoto I., Dardalhon V., Cesses P., Garnier L., Pouzolles M., Brulin B., et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature. 2016;529:226–230. doi: 10.1038/nature16527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Haber A.L., Biton M., Rogel N., Herbst R.H., Shekhar K., Smillie C., Burgin G., Delorey T., Howitt M.R., Katz Y., et al. A single-cell survey of the small intestinal epithelium. Nature. 2017;551:333–339. doi: 10.1038/nature24489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ahmad R., Dalziel J.E. G Protein-Coupled Receptors in Taste Physiology and Pharmacology. Front. Pharmacol. 2020;11:587664. doi: 10.3389/fphar.2020.587664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schütz B., Ruppert A.-L., Strobel O., Lazarus M., Urade Y., Büchler M.W., Weihe E. Distribution pattern and molecular signature of cholinergic tuft cells in human gastro-intestinal and pancreatic-biliary tract. Sci. Rep. 2019;9:17466. doi: 10.1038/s41598-019-53997-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McKinley E.T., Sui Y., Al-Kofahi Y., Millis B.A., Tyska M.J., Roland J.T., Santamaria-Pang A., Ohland C.L., Jobin C., Franklin J.L., et al. Optimized multiplex immunofluorescence single-cell analysis reveals tuft cell heterogeneity. JCI Insight. 2017;2:e93487. doi: 10.1172/jci.insight.93487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kuga D., Ushida K., Mii S., Enomoto A., Asai N., Nagino M., Takahashi M., Asai M. Tyrosine Phosphorylation of an Actin-Binding Protein Girdin Specifically Marks Tuft Cells in Human and Mouse Gut. J. Histochem. Cytochem. 2017;65:347–366. doi: 10.1369/0022155417702586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schütz B., Jurastow I., Bader S., Ringer C., von Engelhardt J., Chubanov V., Gudermann T., Diener M., Kummer W., Krasteva-Christ T., et al. Chemical coding and chemosensory prop-erties of cholinergic brush cells in the mouse gastrointestinal and biliary tract. Front Physiol. 2015;6:87. doi: 10.3389/fphys.2015.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zappia K.J., O’Hara C.L., Moehring F., Kwan K.Y., Stucky C.L. Sensory Neuron-Specific Deletion of TRPA1 Results in Me-chanical Cutaneous Sensory Deficits. eNeuro. 2017;4 doi: 10.1523/ENEURO.0069-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.May R., Qu D., Weygant N., Chandrakesan P., Ali N., Lightfoot S.A., Li L., Sureban S.M., Houchen C.W. Brief Report: Dclk1 Deletion in Tuft Cells Results in Impaired Epithelial Repair After Radiation Injury. Stem Cells. 2013;32:822–827. doi: 10.1002/stem.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Westphalen C.B., Asfaha S., Hayakawa Y., Takemoto Y., Lukin D.J., Nuber A.H., Brandtner A., Setlik W., Remotti H., Muley A., et al. Long-lived intestinal tuft cells serve as colon cancer–initiating cells. J. Clin. Investig. 2014;124:1283–1295. doi: 10.1172/JCI73434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huh W.J., Roland J.T., Asai M., Kaji I. Distribution of duodenal tuft cells is altered in pediatric patients with acute and chronic enteropathy. Biomed. Res. 2020;41:113–118. doi: 10.2220/biomedres.41.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McSorley H.J., Gaze S., Daveson J., Jones D., Anderson R.P., Clouston A., Ruyssers N.E., Speare R., McCarthy J.S., Engwerda C.R., et al. Suppression of Inflammatory Immune Responses in Celiac Disease by Experimental Hookworm Infection. PLoS ONE. 2011;6:e24092. doi: 10.1371/journal.pone.0024092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fleming J.O., Weinstock J.V. Clinical trials of helminth therapy in autoimmune diseases: Rationale and findings. Parasite Immunol. 2015;37:277–292. doi: 10.1111/pim.12175. [DOI] [PubMed] [Google Scholar]
- 112.Ryan S.M., Eichenberger R.M., Ruscher R., Giacomin P.R., Loukas A. Harnessing helminth-driven immunoregulation in the search for novel therapeutic modalities. PLoS Pathog. 2020;16:e1008508. doi: 10.1371/journal.ppat.1008508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.von Moltke J., Ji M., Liang H.E., Locksley R.M. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. 2016;529:221–225. doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.O’Leary C.E., Schneider C., Locksley R.M. Tuft Cells—Systemically Dispersed Sensory Epithelia Integrating Immune and Neural Circuitry. Annu. Rev. Immunol. 2019;37:47–72. doi: 10.1146/annurev-immunol-042718-041505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Billipp T.E., Nadjsombati M.S., von Moltke J. Tuning tuft cells: New ligands and effector functions reveal tissue-specific function. Curr. Opin. Immunol. 2020;68:98–106. doi: 10.1016/j.coi.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Banerjee A., McKinley E.T., Von Moltke J., Coffey R.J., Lau K.S. Interpreting heterogeneity in intestinal tuft cell structure and function. J. Clin. Investig. 2018;128:1711–1719. doi: 10.1172/JCI120330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nadjsombati M.S., McGinty J.W., Lyons-Cohen M.R., Jaffe J.B., DiPeso L., Schneider C., Miller C.N., Pollack J.L., Gowda G.N., Fontana M.F., et al. Detection of Succinate by Intestinal Tuft Cells Triggers a Type 2 Innate Immune Circuit. Immunity. 2018;49:33–41.e7. doi: 10.1016/j.immuni.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schneider C., O’Leary C., von Moltke J., Liang H.-E., Ang Q.Y., Turnbaugh P.J., Radhakrishnan S., Pellizzon M., Ma A., Locksley R.M. A Metabolite-Triggered Tuft Cell-ILC2 Circuit Drives Small Intestinal Remodeling. Cell. 2018;174:271–284.e14. doi: 10.1016/j.cell.2018.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lei W., Ren W., Ohmoto M., Urban J., Matsumoto I., Margolskee R.F., Jiang P. Activation of intestinal tuft cell-expressed Sucnr1 triggers type 2 immunity in the mouse small intestine. Proc. Natl. Acad. Sci. USA. 2018;115:5552–5557. doi: 10.1073/pnas.1720758115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Howitt M.R., Cao Y.G., Gologorsky M.B., Li J.A., Haber A.L., Biton M., Lang J., Michaud M., Regev A., Garrett W.S. The Taste Receptor TAS1R3 Regulates Small Intes-tinal Tuft Cell Homeostasis. ImmunoHorizons. 2020;4:23–32. doi: 10.4049/immunohorizons.1900099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Elmentaite R., Kumasaka N., Roberts K., Fleming A., Dann E., King H.W., Kleshchevnikov V., Dabrowsnka M., Pritchard S., Bolt L., et al. Cells of the human intestinal tract mapped across space and time. Nature. 2021;597:250–255. doi: 10.1038/s41586-021-03852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E., van Es J.H., Abo A., Kujala P., Peters P.J., et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 123.Zachos N.C., Kovbasnjuk O., Foulke-Abel J., In J., Blutt S.E., de Jonge H.R., Estes M.K., Donowitz M. Human Enteroids/Colonoids and Intestinal Organoids Functionally Recapitulate Normal Intestinal Physiology and Pathophysiology. J. Biol. Chem. 2016;291:3759–3766. doi: 10.1074/jbc.R114.635995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sato T., Stange D., Ferrante M., Vries R.G., van Es J.H., Brink S.V.D., van Houdt W., Pronk A., van Gorp J., Siersema P.D., et al. Long-term Expansion of Epithelial Organoids From Human Colon, Adenoma, Adenocarcinoma, and Barrett’s Epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 125.Yin J., Tse C.-M., Avula L.R., Singh V., Foulke-Abel J., de Jonge H.R., Donowitz M. Molecular Basis and Differentiation-Associated Al-terations of Anion Secretion in Human Duodenal Enteroid Monolayers. Cell. Mol. Gastroenterol. Hepatol. 2018;5:591–609. doi: 10.1016/j.jcmgh.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Noel G., Baetz N.W., Staab J.F., Donowitz M., Kovbasnjuk O., Pasetti M.F., Zachos N.C. A primary human macrophage-enteroid co-culture model to investigate mucosal gut physiology and host-pathogen interactions. Sci. Rep. 2017;7:45270. doi: 10.1038/srep45270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yin X., Farin H.F., Van Es J.H., Clevers H., Langer R.D., Karp J.M. Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat. Methods. 2013;11:106–112. doi: 10.1038/nmeth.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.VanDussen K., Carulli A.J., Keeley T.M., Patel S.R., Puthoff B.J., Magness S.T., Tran I.T., Maillard I., Siebel C., Kolterud A., et al. Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development. 2012;139:488–497. doi: 10.1242/dev.070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Aihara E., Mahe M., Schumacher M.A., Matthis A.L., Feng R., Ren W., Noah T.K., Matsu-Ura T., Moore S., Hong C.I., et al. Characterization of stem/progenitor cell cycle using murine circumvallate papilla taste bud organoid. Sci. Rep. 2015;5:1–15. doi: 10.1038/srep17185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ren W., Aihara E., Lei W., Gheewala N., Uchiyama H., Margolskee R.F., Iwatsuki K., Jiang P. Transcriptome analyses of taste organoids reveal multiple pathways involved in taste cell generation. Sci. Rep. 2017;7:4004. doi: 10.1038/s41598-017-04099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hasan N.M., Johnson K.F., Yin J., Baetz N.W., Fayad L., Sherman V., Blutt S.E., Estes M.K., Kumbhari V., Zachos N.C., et al. Intestinal stem cell-derived enteroids from morbidly obese patients preserve obesity-related phenotypes: Elevated glucose absorption and gluconeogenesis. Mol. Metab. 2020;44:101129. doi: 10.1016/j.molmet.2020.101129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schlegel C., Weis V., Knowles B., Lapierre L.A., Martin M.G., Dickman P., Goldenring J.R., Shub M.D. Apical Membrane Alterations in Non-intestinal Organs in Microvillus Inclusion Disease. Dig. Dis. Sci. 2017;63:356–365. doi: 10.1007/s10620-017-4867-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.