Abstract
Laboratory confirmation of pertussis by culture, PCR, or detection of antibody increase in paired sera is hampered by low sensitivity in the later stages of the disease. Therefore, we investigated whether, and at which level, concentrations of immunoglobulin G (IgG) antibodies against pertussis toxin (PT), IgG-PT, in a single serum sample are indicative of active or recent pertussis. IgG-PT, measured by enzyme-linked immunosorbent assay in units per milliliter, was analyzed in 7,756 sera collected in a population-based study in The Netherlands, in the sera of 3,491 patients with at least a fourfold increase of IgG-PT, in paired sera of 89 patients with positive cultures and/or PCR results, and in the sera of 57 patients with clinically documented pertussis with a median follow-up of 1.4 years. We conclude that, independently of age, IgG-PT levels of at least 100 U/ml are diagnostic of recent or active infection with Bordetella pertussis. Such levels are present in less than 1% of the population and are reached in most pertussis patients within 4 weeks after disease onset and persist only temporarily.
Whooping cough is a highly contagious bacterial infection of the respiratory tract, caused by Bordetella pertussis. It is most severe in unvaccinated infants. Evidence is increasing that B. pertussis infections occur more frequently in older children and adults in vaccinated populations than has been commonly recognized (1, 4, 6, 8, 17, 22, 26). These individuals may play an important role in the transmission to infants too young to be vaccinated (4, 7, 19, 25). Adequate laboratory diagnosis is important for the control and prevention of pertussis.
In The Netherlands, the case definition for notification of pertussis includes defined typical clinical symptoms and laboratory confirmation. Laboratory confirmation is defined as either a positive culture or a positive PCR for B. pertussis or B. parapertussis, or positive two-point serology, i.e., a significant increase (at least fourfold) of antibodies against (antigens of) B. pertussis. This case definition for notification is highly specific, but it results in low sensitivity, especially when laboratory diagnosis is initiated at a late stage of the disease. Other countries also report that pertussis diagnosis is hampered by low sensitivity (16, 20, 27, 30, 31). Culture of B. pertussis is laborious and insensitive; the ability to isolate B. pertussis by culture decreases progressively during the disease (12, 13). The sensitivity of PCR is superior to that of culture; however, this sensitivity, like that of culture, rapidly decreases by the time the paroxysmal phase has developed and with increasing age (15, 34). In The Netherlands, confirmation of suspected pertussis is attempted often by serology. However, in our serodiagnostic practice, for more than 50% of the suspected cases, only one serum sample is submitted or else high titers are found in paired sera without a significant increase. Similar problems have also been reported by others (9, 23, 28).
Because pertussis toxin (PT) is expressed only by B. pertussis and cross-reacting antigens have not been described (14, 23) and because immunoglobulin G (IgG) responses occur in most patients with B. pertussis infection, we investigated whether, and at which level, titers of IgG antibodies against PT (IgG-PT) in a single serum sample are indicative for active or recent pertussis.
We analyzed IgG-PT in the sera of a large cross-section of the population (n = 7,756), in the paired sera of patients of all ages in whom clinical suspicion of pertussis was confirmed by at least a fourfold increase of IgG-PT (n = 3,491), and in the paired sera of patients in whom pertussis had been confirmed by culture of B. pertussis or by positive pertussis PCR (n = 89). The course of IgG-PT after natural infection, i.e., the duration of high levels, was assessed in long-term follow-up sera of 57 patients after pertussis had been clinically documented.
MATERIALS AND METHODS
Collection of sera and data from the general population and patients. (i) Cross-section of the general population (n = 7,756).
The study design and data collection have been published elsewhere (21). Briefly, eight municipalities with probabilities proportional to their population sizes were sampled within each of five geographical Dutch regions with similar population sizes. An age-stratified sample (classes 0, 1 to 4, 5 to 9, … , 75 to 79 years) of 380 individuals was randomly selected from each municipality. These individuals were requested to give a blood sample and to fill out a questionnaire in which the participants were asked whether they had had a period with coughing attacks that had lasted for more than 2 weeks. They were also asked whether a physician had diagnosed pertussis, either during the past year or for more than 1 year previously. No information was available as to whether the physician had diagnosed pertussis by symptomatology, serology, culture, or PCR. The participation rate was 55%. Sufficient serum for pertussis serology was available from 7,756 of the 8,359 participants. Sera were collected in 1995 and 1996 and stored at −70°C until use.
(ii) Patients with serologically confirmed pertussis.
Until 1997, the National Institute of Public Health and the Environment was the only laboratory in The Netherlands that performed pertussis serology examinations for patients with a suspected pertussis infection. Routinely, the submitted sera were assayed for both IgG-PT and IgA against B. pertussis. In all cases, if the date of onset of symptoms and/or date of sampling of serum was missing upon submission of serum, a standard questionnaire was sent to collect the data. From January 1989 onwards, all data and results were registered in an electronic database.
For the purpose of this study, patient data and serologic results were obtained in the period from 1989 to 1996 from 3,491 patients in whom the clinical suspicion of pertussis had been confirmed by the detection, in paired sera, of a ≥4-fold increase of IgG-PT to ≥20 U/ml.
Likewise, data were analyzed for 15,319 patients whose first submitted serum sample contained ≥100 U of IgG-PT per ml without (in the case of paired sera) a fourfold IgG-PT increase.
(iii) Patients with typically symptomatic infection with B. pertussis and their longitudinal sera.
During the period from 1989 to 1998, we obtained follow-up serum samples from 57 patients with a clinical diagnosis of typical pertussis (paroxysmal cough lasting more than 2 weeks) so that we could study the longitudinal course of IgG-PT after infection. Twenty-three patients showed at least a fourfold increase in IgG-PT in paired sera, and 34 patients had an IgG-PT level in a first serum sample of at least 75 U/ml. The IgG-PT level was at least 100 U/ml for 31 of these 34 patients. The follow-up period varied from 6.5 months to 6.7 years after the acute phase of infection (mean, 1.8 years; median, 1.4 years). The number of serum samples collected in the follow-up periods varied from two to seven (mean and median of three). All patients were from a single pediatric practice. The patients were treated with macrolides for their pertussis. Only those patients participated who continued to be treated by the pediatrician after the episode of pertussis because of other medical conditions (mostly allergic conditions and/or asthma) or who consulted the pediatrician again at a later stage because of new medical problems. In addition, sera from parents with clinical pertussis were selected. The median age of the patients in which the longitudinal course of IgG-PT was studied was 3.5 years (range, 0 to 35 years). A total of 10 patients were less than 6 months old; 7 patients were 6 to 11 months old; 19 patients were between 1 and 4 years old, 16 patients were between 5 and 9 years old; 2 patients were 11 or 12 years old; and 3 patients were 30 to 35 years old. Thirty-nine of the patients were vaccinated, six patients were unvaccinated, and for twelve patients the vaccination status was unknown. In all cases the follow-up sera used for the study were “left over” from samples obtained for some other diagnostic procedure. Informed consent for the study was obtained from the patients or their parents.
(iv) Patients with PCR and/or culture-proven pertussis.
In the period from 1993 to 1998, the diagnosis of pertussis for 89 patients had been confirmed by culture of B. pertussis and/or a positive pertussis-specific PCR, while paired sera from these patients had also been submitted for serology. Of the 89 patients, 58 had participated in a clinical study to assess the sensitivity of the pertussis or parapertussis PCR in comparison with culture and serology (34). For each of the remaining patients, a pertussis PCR test was performed in the regional public health laboratory in Tilburg, The Netherlands. In all cases, the first serum of the pair was obtained on the same day that material for culture and/or PCR had been obtained: the second sample was obtained 2 to 4 weeks later. Of the 89 patients, 37.1% were 0 to 5 months old, 7.9% were 6 to 11 months old, 21.3% were 1 to 4 years old, 25.2% were 5 to 9 years old, 2.2% were 10 to 14 years old, and 5.6% were ≥15 years old.
In-house IgG-PT ELISA.
The patient sera had been submitted immediately after sampling and were assayed in the routine setting of the serology laboratory of our institute within 4 days after receipt. The population sera, which had been collected in 1995 and 1996, were assayed in 1997 and 1998 in the same routine setting at a rate of approximately 200 sera per week. The IgG-PT was measured by enzyme-linked immunosorbent assay (ELISA) as previously described (34). In short, the procedure was as follows. Purified PT (National Institute of Public Health and the Environment) was used to coat 96-well ELISA plates after precoating with fetuin (50 mg/liter in phosphate-buffered saline). Peroxidase-labeled rabbit anti-human IgG was used as a conjugate, and 3,3′,5,5′-tetramethylbenzidine (TMB) was used as the substrate. Negative, low-positive, and medium-positive control sera with defined IgG-PT contents were run on each plate. The sera were tested in duplicate in 1:100 and 1:400 dilutions against serial dilutions of a positive reference serum with a range of 1.6 to 100 “local” U/ml. The optical density (OD) of the 1:100 dilution was used to calculate the IgG-PT concentration. When the OD of the 1:100 dilution of a serum was above the range that constituted the steep part of the dose-response curve, the OD of the 1:400 dilution was used.
Due to the use of only two dilutions for the sera, the IgG-PT assay has an upper limit (500 U/ml) above which the values are not further differentiated. The lower detection limit of the assay is 5 U/ml.
Results are expressed in “local” units per milliliter. The reference serum was also calibrated against the FDA preparation lot 3 (Food and Drug Administration Laboratory of Pertussis, Rockville, Md.). The formula for conversion of local to FDA units per milliliter appeared to be as follows: local U/ml × 0.8 = FDA U/ml within this assay.
Data analysis.
For the cross-sectional population-based study, frequencies of IgG-PT levels from <5 U/ml (lower detection limit) to ≥500 U/ml (upper differentiation limit) within each municipality were weighted by the proportion of the age group in the population. To produce national estimates for percentiles 1, 2.5, 10, 25, 50, 75, 97.5, and 99, these weighted and age-specific frequencies were averaged over the 40 municipalities (5). The following age groups were separately analyzed: 0 to 5 months, 6 to 11 months, 12 to 17 months, 18 to 23 months, 2 years, 3 years, 4 years, 5 to 9 years, 10 to 14 years, and ≥15 years.
The proportions with IgG-PT levels of <5U/ml, 5 to 9 U/ml, 10 to 49 U/ml, 50 to 99 U/ml, 100 to 499 U/ml, and ≥500 U/ml were also assessed. The proportion of these groups that reported a pertussis diagnosis or a period with coughing attacks during the past year was calculated. The proportion of those who had a pertussis diagnosis and/or coughing attacks more than one year ago and the proportion without any pertussis diagnosis or coughing attacks were also calculated.
Likewise, for the patients with serologically proven pertussis (i.e., a ≥4-fold increase of IgG-PT in paired sera), the total and (similar) age-specific percentiles 1, 2.5, 10, 25, 50, 75, 97.5, and 99 for IgG-PT levels were calculated for the second sera of the serum pairs. The median duration of disease at the time of initiation of laboratory diagnosis, i.e., the time of sampling of first sera, was assessed for these patients in the following age groups: 0 to 5 months, 6 to 11 months, 1 year, 2 years, 3 years, 4 years, 5 to 9 years, 10 to 14 years, and ≥15 years.
The median duration of the disease was also assessed for the same age groups for the selection of patients from the serological database with ≥100 U/ml at the time of the first serum sampling without a fourfold IgG-PT increase.
The Wilcoxon signed ranked test was used to test differences between age groups in the IgG-PT distributions in the patients with positive cultures and/or PCR with a P value of 0.05, which was considered statistically significant.
To study the longitudinal course of IgG-PT levels after pertussis infection for patients in the follow-up study, the association between the IgG-PT level and time in 2log days after the first day of illness (and the effect of age) was analyzed with a mixed linear model (PROC MIXED in SAS version 6.12) (29). A 2log transformation of both the IgG-PT level in units per milliliter and of time in days since the first day of illness was performed. This way, linear regression yielded the best fit.
For those patients with follow-up data at 0 to 5 months, 6 to 11 months, 12 to 23 months, 2 to 3 years, and 4 to 7 years after onset of the disease, the proportions with IgG-PT levels of <20, 20 to 49, 50 to 99, and ≥100 U/ml were calculated. If multiple serum samples were available for one patient within one follow-up period, the serum sample with the highest IgG-PT level was used.
RESULTS
The IgG-PT distribution in the population compared to the IgG-PT distribution in second sera of serum pairs of patients with serologically confirmed pertussis: choice of cut-offs to be validated.
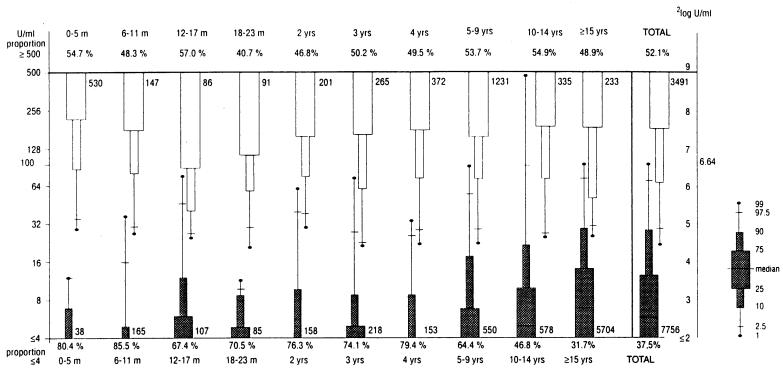
The IgG-PT levels in individuals of the population-based study were low in comparison to those in second sera (i.e., reconvalescence sera) of patients with serologically confirmed pertussis; these distributions showed little overlap (Fig. 1). In the population-based study, the median IgG-PT was 6 U/ml, and for percentiles 97.5 and 99 the values were 69 and 97 U/ml, respectively. In the second sera of patients with serologically confirmed pertussis, the median IgG-PT was ≥500 U/ml; the 10th percentile was 66 U/ml (Fig. 1).
FIG. 1.
IgG-PT distribution in the population-based study and in pertussis patients (second sera) with at least a fourfold increase in IgG-PT level in paired sera. Results for the population-based study are given in shaded bars, and results for second sera of pertussis patients are given in open bars; the total numbers of individuals/patients are given beside the bars.
In the population-based study, the median IgG-PT level was <5 U/ml in the age group <10 years old, rose to 5 U/ml for 10- to 14-year-olds, and was 7 U/ml for patients 15 years old or older (Fig. 1). For those 0 to 5 months old, 6 to 11 months old and 18 to 23 months old, the percentile 97.5 value was less than 20 U/ml, while in the other age groups the values given by percentile 97.5 ranged from 26 U/ml (4-year-olds) to 98 U/ml (10- to 14-year-olds). Further differentiation in 5-year age classes from 15 to 19 years to 75 to 79 years showed a stable IgG-PT distribution for those who were ≥15 years old (data not shown separately). With the exception of the 10- to 14-year-olds, the age-specific percentile 99 value was below 100 U/ml.
The percentages with undetectable IgG-PT (i.e., <5 U/ml) decreased from 85.5% for infants aged 6 to 11 months to 67.4% for infants 12 to 17 months and increased again to 79.4% at the age of 4 years (Fig. 1). The proportion with undetectable IgG-PT decreased to 31.7% in those ≥15 years of age. From this age onwards, this percentage remained stable (data not shown separately).
The median and the 10th percentile of the distributions of IgG-PT in the second sera of patients with serologically proven pertussis showed little variation in the different age groups; the median IgG-PT values ranged from 316 to ≥500 U/ml, and the 10th percentile values ranged from 40 to 87 U/ml (Fig. 1). The percentage of patients with an IgG-PT level of ≥500 U/ml ranged from 40.7 to 57.0% within the various age groups (Fig. 1). Thus, the IgG-PT distributions in the population differed for the various age categories, but the IgG-PT immune responses in pertussis patients did not. Therefore, for further assessment of specificity and sensitivity of certain IgG-PT levels for recent and/or active infection with B. pertussis, we chose cutoffs of IgG-PT levels which were age independent, i.e., which were based on the comparison of the IgG-PT distribution in the total population and the IgG-PT distribution in second sera of the total group of serologically confirmed pertussis patients. Cutoffs of 50 and 100 U/ml were chosen because IgG-PT levels of ≥50 and ≥100 U/ml were detected in, respectively, 92.7 and 81.0% of patients (second sera) with serologically confirmed pertussis. Such levels were rare, i.e., respectively, 3.6 and 0.8% in the general population. That is to say, they gave specificities of such values as 96.4 and 99.2%, respectively. The specificity of the proposed cutoff of ≥100 U/ml was maximally 100% for the ages of 0 to 5 months, 12 to 17 months, 18 to 23 months, and 2 years; minimally 97.9% for 10- to 14-year-olds; and for the cutoff value, ≥50 U/ml, maximally 100% for the ages of 0 to 5 months and 18 to 23 months and minimally 96.2% for 10- to 14-year-olds and ≥15-year-olds.
Validation of the proposed IgG-PT cutoffs as markers for recent or active infection with B. pertussis. (i) Relationship between high IgG-PT levels in the population and recall of pertussis diagnosis and/or paroxysmal coughing.
In the population-based study, while 9.6% of those with an IgG-PT level of <5 U/ml reported coughing attacks or pertussis during the last year, this percentage was statistically significantly greater: 19.9% for those with an IgG-PT level between 50 and 99 U/ml and 25.5% for those with an IgG-PT level of ≥100 U/ml (Table 1).
TABLE 1.
Percentages of individuals with a history of pertussis or coughing attacks during the last year, of individuals with pertussis or coughing attacks more than 1 year ago, and of individuals without pertussis or coughing attacks according to IgG antibody levels in the population-based studya
| Occurrence pertussis or coughing attack status | n | % Individuals with IgG concn of:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| <5 U/ml
|
5–9 U/ml
|
10–49 U/ml
|
50–99 U/ml
|
≥100 U/ml
|
|||||||
| % | 95% CIa | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | ||
| Yes, during last yearb | 886 | 9.6 | 8.0–11.3 | 11.6 | 9.4–13.8 | 11.3 | 9.7–12.9 | 19.9 | 13.0–26.9 | 25.5 | 12.2–38.8 |
| Yes, more than 1 year agoc | 463 | 5.5 | 4.3–6.7 | 6.1 | 4.6–7.6 | 6.7 | 5.2–8.5 | 7.3 | 3.5–11.0 | 14.1 | 2.7–25.5 |
| Nod | 6,407 | 84.9 | 82.8–87.0 | 82.3 | 79.9–84.6 | 81.8 | 80.1–83.5 | 72.8 | 65.2–80.5 | 60.4 | 45.3–75.5 |
| Total | 7,756 | 100 | 100 | 100 | 100 | 100 | |||||
CI, confidence interval.
Participants who reported that a physician diagnosed pertussis during the last year or participants who reported coughing attacks that had lasted for more than 2 weeks during the last year. Among the 886 individuals, 15 individuals reported pertussis, and 12 of these 15 individuals reported coughing attacks.
Participants who reported that a physician diagnosed pertussis more than 1 year ago or participants who reported coughing attacks that had lasted for more than 2 weeks more than 1 year ago. No pertussis was diagnosed and no coughing attacks were reported during the last year. Among the 473 individuals, 126 individuals reported pertussis, and 45 of these individuals reported coughing attacks.
Participants who did not report pertussis or coughing attacks during the last year or more than one year ago.
The percentage of individuals who reported that pertussis was diagnosed or who reported to have had coughing attacks more than 1 year ago showed a smaller increase with increasing IgG-PT. The differences in the percentage were not statistically significant (Table 1).
(ii) Longitudinal course of IgG-PT levels after infection.
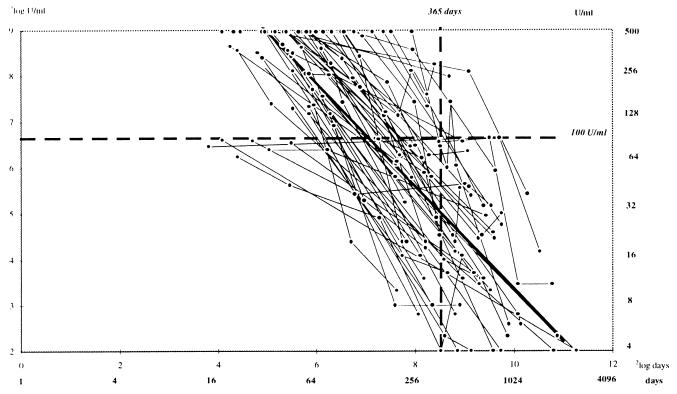
In each of the 57 patients with clinical pertussis for whom there were follow-up serum samples, the IgG-PT decreased with time after the onset of the disease (Fig. 2). In the mixed linear model with the IgG-PT in 2log U/ml and time from the onset of the disease in 2log days, the intercept amounted to 14.61 and the slope was −1.128. Thus, this model predicts that the mean time of persistence of an IgG-PT level of ≥100 U/ml amounts to 134 days (4.4 months) and that after 365 days a level of 32 U/ml is reached. Although IgG-PT levels of ≥500 U/ml were not further differentiated, the slopes did not change statistically significantly assuming that the levels of ≥500 U/ml were 1,000 U/ml or restricting the analysis to those samples with IgG-PT levels of <500 U/ml. No effect of age or vaccination status was shown in the mixed linear model. The data points for each individual patient were connected linearly. The resulting lines show that the IgG-PT level was below 100 U/ml within 1 year for 47 of the 57 patients. For 7 of the remaining 10 patients, the IgG-PT levels decreased below 100 U/ml during the subsequent follow-up period, which ranged from 1.4 to 4 years. For two of the remaining three patients, the follow-up period was less than 1 year. For these two patients, the IgG-PT levels amounted to 160 and 304 U/ml after 0.84 and 0.92 year of follow-up, respectively. The remaining patient had an IgG-PT level of 252 U/ml at 1.1 years after the first day of illness.
FIG. 2.
IgG-PT levels (in 2log U/ml) versus time elapsed (in 2log days) since date of onset for 57 patients with clinical pertussis.
As shown in Table 2, the highest IgG-PT level detected between 0 and 5 months after onset of the disease was ≥100 U/ml in 90% of the patients, while the highest IgG-PT level detected in the remaining patients was between 50 and 100 U/ml. At 6 to 11 months after the onset of the disease, the level had declined to <50 U/ml in 40% of the patients. A decrease to <50 U/ml had occurred in 72% after 12 to 23 months, in 86% after 2 to 3 years, and in 100% after 4 to 7 years (Table 2).
TABLE 2.
IgG-PT levels in patients with clinical pertussis versus time elapsed since disease onset
| IgG-PT level (U/ml) | % of patients with indicated IgG-PT level at time of serum collection after onset of diseasea
|
||||
|---|---|---|---|---|---|
| 0–5 mo (n = 57) | 6–11 mo (n = 30) | 12–23 mo (n = 25) | 2–3 yr (n = 14) | 4–7 yr (n = 8) | |
| ≥100 | 90 | 20 | 16 | 7 | 0 |
| 50–99 | 11 | 40 | 12 | 7 | 0 |
| 20–49 | 0 | 13 | 28 | 36 | 13 |
| <20 | 0 | 27 | 44 | 50 | 88 |
n, number of patients.
(iii) Time between onset of the disease and development of high IgG-PT levels.
Sera from patients for all age groups with an IgG-PT level of ≥100 U/ml in the first serum sample without a fourfold increase in IgG-PT level had been sampled at a later stage of the disease (median 30 days) than the first sera of patients showing a fourfold increase in IgG-PT level (median 17 days) (Table 3). The first samples from patients younger than 3 years of age were collected a few days earlier than the samples from older age groups, particularly for patients with at least a fourfold increase in IgG-PT.
TABLE 3.
Time between onset of the disease and first blood sampling for patients with at least a fourfold IgG-PT increase and for patients with at least 100 U/ml in the first serum sample without a fourfold IgG-PT increase
| Age | Patients with at least a fourfold IgG-PT increase
|
Patients with at least 100 U/ml in first serum sample without a fourfold IgG-PT increase
|
||||||
|---|---|---|---|---|---|---|---|---|
| No. of patients | Median (days) | Percentiles 2.5–97.5 | Mean (days) | No. of patients | Median (days) | Percentiles 2.5–97.5 | Mean (days) | |
| 0–5 mo | 517 | 14 | 2–53 | 17.0 | 497 | 30 | 4–108 | 36.8 |
| 6–11 mo | 144 | 14 | 2–61 | 16.8 | 305 | 29 | 2–105 | 35.4 |
| 1 yr | 174 | 15 | 2–62 | 19.2 | 426 | 28 | 4–122 | 36.3 |
| 2 yr | 195 | 15 | 4–88 | 19.6 | 763 | 30 | 6–109 | 38.1 |
| 3 yr | 262 | 18 | 3–60 | 19.7 | 966 | 30 | 7–119 | 38.5 |
| 4 yr | 362 | 21 | 5–55 | 21.9 | 1,499 | 31 | 7–112 | 39.0 |
| 5–9 yr | 1,191 | 18 | 3–58 | 20.6 | 5,655 | 31 | 7–103 | 37.6 |
| 10–14 yr | 327 | 18 | 4–61 | 21.3 | 2,180 | 31 | 7–111 | 40.0 |
| ≥15 yr | 222 | 16 | 3–67 | 19.3 | 3,028 | 30 | 6–108 | 37.2 |
| Total | 3,394a | 17 | 3–59 | 19.8 | 15,319 | 30 | 6–109 | 38.0 |
n < 3,491 due to missing values for time of disease onset.
The results given in Table 3 also show the number of patients with an IgG-PT level of ≥100 U/ml in a first serum that in our serodiagnostic database of 1989 to 1996 is considerably larger (4.5-fold) than the number of patients with at least a 4-fold increase in IgG-PT. This discrepancy increases with age from 1-fold for the 0- to 5-month-old ages to 13.6-fold for those of ≥15 years.
(iv) Sensitivity of the proposed cutoffs for IgG-PT in PCR and/or culture-confirmed pertussis cases.
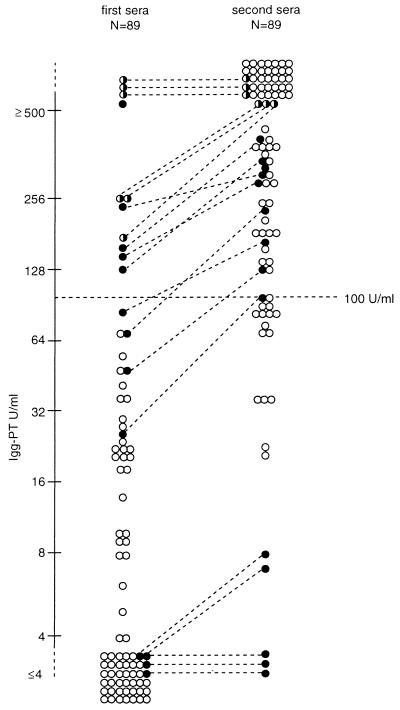
Among the 89 patients for whom pertussis was confirmed by a positive culture and/or a PCR both in the IgG-PT distributions in the first and second serum samples, no statistically significant differences were found between age groups. The distributions of IgG-PT in the first and second sera of the serum pairs of these patients are shown in Fig. 3. In 3 of the 89 patients, IgG-PT was undetectable (<5 U/ml) in both the first and the second serum samples. In two other patients the IgG-PT rose from <5 to 7 and 8 U/ml. In 69 patients a ≥4-fold increase of IgG-PT was detected, in 11 patients the IgG-PT in the first serum sample was ≥100 U/ml and no fourfold rise was detected, and in 4 patients the rise in IgG-PT was <4-fold and the IgG-PT level in the first serum sample was <100 U/ml. Thus, if detection of a ≥4-fold increase of IgG-PT in paired sera and detection of ≥100 U of IgG-PT per ml in a single serum sample are used as the criteria for the serodiagnosis of active or recent infection with B. pertussis, the sensitivity of IgG-PT serology in this “gold standard” group of patients is enhanced from 77.5% (69 of 89) to 89.9% (80 of 89). Overall, the sensitivity of IgG-PT levels of ≥50 and ≥100 U/ml amounted to 88.8 and 76.4%, respectively; i.e., 79 and 68 of the 89 patients had IgG-PT levels of ≥50 and ≥100 U/ml in the first and/or second serum samples, respectively.
FIG. 3.
IgG-PT distribution in the first and second serum samples of patients with a positive culture and/or PCR for B. pertussis. White circles indicate sera with at least a fourfold increase in IgG-PT to a level of at least 20 U/ml. Black circles indicate sera without a fourfold increase in IgG-PT; pairs of sera are connected with a dotted line. Half-black, half-white circles indicate sera in which the degree of increase of IgG-PT could not be determined due to the upper limit of differentiation of the IgG-PT assay of ≥500 U/ml; pairs of sera are connected with a dotted line.
DISCUSSION
Our results show that an IgG-PT level of at least 100 U/ml is a specific tool in laboratory confirmation of patients with a suspected pertussis infection in The Netherlands. The levels of IgG-PT in the Dutch population are lower than, and overlap only slightly, IgG-PT levels that are reached in patients with clinical symptoms of pertussis and a significant immune response to B. pertussis. IgG-PT levels of at least 100 U/ml were observed in <1% of the overall general population, varying between maximally 2.5% for 10- to 14-year-olds and <0.5% for those 2 years of age or younger. Furthermore, such levels were present in the second serum sample of 80% of those patients who had at least a fourfold increase in IgG-PT.
Our longitudinal study of pertussis patients shows that the levels decreased within less than 1 year to a level below 100 U/ml after natural infection with B. pertussis for almost all patients who had had high IgG-PT levels. The regression model predicts that a level of 100 U/ml is reached in 4.5 months and a level of <40 U/ml is reached within 1 year after onset of the disease. For seven of eight patients with a longer follow-up time, the IgG-PT levels were below 20 U/ml by 4 to 7 years after the disease onset. Thus, IgG-PT levels of at least 100 U/ml for patients with suspected whooping cough are indicative of recent or active infection with B. pertussis.
In our population-based study, the percentage of individuals who reported pertussis or coughing attacks in the last year increased from 10% for those with IgG-PT levels of <5 U/ml to 26% for those with levels of at least 100 U/ml. This finding offers further support for our conclusion that a high IgG-PT level is indicative of recent or active infection with B. pertussis.
Ten percent of the individuals in the population study with an IgG-PT level of <5 U/ml reported pertussis or coughing attacks in the last year. On the one hand, this may be explained by other respiratory tract infections that cause “pertussis-like” symptoms (35), rapid decline of a previously high IgG-PT level, or absence of an IgG-PT response after B. pertussis infection. It is also possible that not all subjects answered the question properly. On the other hand, the large percentage (60%) of individuals with high antibody titers who did not report pertussis or long-lasting coughing attacks might be due to very mild, atypical, or even asymptomatic infection with B. pertussis. In a household exposure study, a B. pertussis infection was shown to exist in 46% of the exposed subjects who remained well (7). In another study, only 26% of the adults with laboratory evidence of a B. pertussis infection reported recent symptoms compatible with pertussis (30).
Although IgG-PT is induced after whole-cell vaccination only in children aged 12 to 17 months and thus after the fourth dose given at the age of 11 months, a temporary and small increase was observed. These results are consistent with observations in a vaccine trial showing very low levels of IgG-PT after the first to third vaccinations and a small increase just after the fourth vaccination, as Nagel and others have observed (18, 24). Thus, it is very unlikely that high levels of IgG-PT are induced by previous vaccination with Dutch whole-cell vaccine. However, other vaccines might induce higher IgG-PT levels since the response to PT varies between different whole-cell vaccines and acellular vaccines (2, 11, 32, 33). However, even when a level of at least 100 U/ml is reached, it is likely to decline shortly afterwards (11, 32, 33). Giuliano et al. (11) reported that mean titers were close to the limit of detection 15 months after the primary immunization with acellular vaccine. High IgG-PT levels must be interpreted more cautiously in children recently vaccinated with a vaccine known to induce relatively high levels of such antibodies.
In addition to specificity, both sensitivity and positive predictive value are important as diagnostic tools. Using paired sera of patients with positive PCRs and/or positive cultures (gold standard group), a sensitivity of IgG-PT of at least 100 U/ml was 76%. At least a fourfold increase was found in most of the remaining patients in whose paired sera the IgG-PT level remained lower than 100 U/ml. One might speculate that a level of at least 100 U/ml may have been reached at a later point. Only 6% of all patients had very low IgG-PT levels in both sera without significant dynamics. With the exception of those aged 10 to 14 years, a level of IgG-PT of at least 100 U/ml exceeded the 99th percentile in the general population. Furthermore, as described above, it was likely that individuals in the general population with IgG-PT levels above this value had had a recent or active B. pertussis infection. Based on this 99th percentile, the positive predictive values will still amount to 90 and 80%, assuming the proportions of true pertussis patients to be 9 and 4%, respectively, among those who submitted serum samples [i.e., 9/(9 + 1) and 4/(4 + 1)]. However, depending on the clinical presentation and the epidemiological situation, the a priori chance of true positivity in most cases will be higher. Using a more conservative estimate, i.e., percentile 97.5 in the general population, the positive predictive value will not be below 80%, assuming a percentage of true positives of 10%.
Even an IgG-PT level of at least 50 U/ml has some predictive value, since it amounts to 70%, assuming a percentage of true positives of 10%. This suggests that the diagnosis of pertussis is likely among patients with clinical symptoms of pertussis with such IgG-PT levels. However, we interpret such a result to be indicative of, but not definite proof of, a recent B. pertussis infection, and we advise submission of a second serum sample. If no further change of IgG-PT level has occurred as evidenced by a second serum sample, we conclude that “recent or active infection with B. pertussis is possible.”
Serological data obtained at our laboratory show that using our cutoff value for the IgG-PT level of at least 100 U/ml would increase the number of patients with serologically proven pertussis by more than fourfold. The increase is smallest for infants and greatest for adults, which is probably related to a longer delay in consulting a physician and/or initiating laboratory testing in older children and adults. This is supported by the similar median time (28 to 30 days) between the first blood sampling and the onset of symptoms for the various age groups for those with an IgG-PT level of at least 100 U/ml. For patients with at least a fourfold increase in IgG-PT the median time (17 days) between the first blood sampling and the first symptoms is about 2 weeks shorter than that for those without a fourfold increase and IgG-PT levels above 100 U/ml. The most useful method for pertussis diagnosis depends on the time of initiation. PCRs and cultures are most useful early in the disease. However, if they are negative, the diagnosis is indeterminate, and serology tests should be initiated. Late in the disease, PCRs and cultures are fairly insensitive (with an exception for infants less than 1 year old) and serology is then the method of choice (15, 20, 34).
To diagnose pertussis, other investigators have also used single serum samples from a control group for defining a cutoff, but most studies were limited to a specific study setting and were not meant for routine diagnosis (1, 6, 19, 20, 22, 26, 27, 31). Our control group consisted of a large number of participants from a population-based study so the representativeness is probably better guaranteed.
Cattaneo et al. point out that it is unlikely that a single serology value can be used to define infected persons in a broad age range because age, geographic area, prevalence of infection, and history of vaccination all have to be taken into account (3). Yet an IgG-PT level of at least 100 U/ml in a single serum sample might be a specific diagnostic tool for pertussis in other countries too. After all, it is likely that such high IgG-PT levels will not or will be reached only temporarily no matter which vaccine is used. After the initial increase, the IgG-PT level decreases again after B. pertussis infection, high predictive values are calculated under different assumptions about prevalence of infection and, finally, a large proportion of individuals with a B. pertussis infection show high IgG-PT levels later in the course of the disease. Thus, we believe that high IgG-PT levels could provide a useful laboratory tool for the diagnosis of pertussis in both the individual patient and in epidemiological studies (20, 28, 31). It might be worthwhile to validate our results in other countries.
ACKNOWLEDGMENTS
We acknowledge the Public Health Services, the Pienter Project Team, H. G. L. Boshuis, I. Belmouden, and P. van der Kraak for their very useful contributions.
REFERENCES
- 1.Addiss D G, Davis J P, Meade B D, Burstyn D G, Meissner M, Zastrow J A, Berg J L, Drinka P, Philips R. A pertussis outbreak in a Wisconsin nursing home. J Infect Dis. 1991;164:704–710. doi: 10.1093/infdis/164.4.704. [DOI] [PubMed] [Google Scholar]
- 2.Baker J D, Halperin S A, Edwards K, Miller B, Decker M, Stephens D. Antibody response to Bordetella pertussis antigens after immunization with American and Canadian whole-cell vaccines. J Pediatr. 1992;121:523–527. doi: 10.1016/s0022-3476(05)81138-2. [DOI] [PubMed] [Google Scholar]
- 3.Cattaneo L A, Reed G W, Haase D H, Wills M J, Edwards K M. The seroepidemiology of Bordetella pertussis infections: a study of persons aged 1–65 years. J Infect Dis. 1996;173:1256–1259. doi: 10.1093/infdis/173.5.1256. [DOI] [PubMed] [Google Scholar]
- 4.Cherry J D. The role of Bordetella pertussis infections in adults in the epidemiology of pertussis. Dev Biol Stand. 1997;89:181–186. [PubMed] [Google Scholar]
- 5.Cochran W G. Sampling techniques. 3rd ed. New York, N.Y: John Wiley & Sons; 1997. [Google Scholar]
- 6.Cromer B A, Goydos J, Hackell J, Mezzatesta J, Dekker C, Mortimer E A. Unrecognized pertussis infection in adolescents. Am J Dis Child. 1993;147:575–577. doi: 10.1001/archpedi.1993.02160290081031. [DOI] [PubMed] [Google Scholar]
- 7.Deen J L, Mink C A M, Cherry J D, Cherry J D, Christenson P D, Pineda E F, Lewis K, Blumberg D A, Ross L A. Household contact study of Bordetella pertussis infections. Clin Infect Dis. 1995;21:1211–1219. doi: 10.1093/clinids/21.5.1211. [DOI] [PubMed] [Google Scholar]
- 8.Deville J G, Cherry J D, Christenson P D, Pineda E, Leach C T, Kuhls T L, Viker S. Frequency of unrecognized Bordetella pertussis infections in adults. Clin Infect Dis. 1995;21:639–642. doi: 10.1093/clinids/21.3.639. [DOI] [PubMed] [Google Scholar]
- 9.Giammanco A, Taormina S, Genovese M, Mangiaracina G, Giammanco G, Chiarini A. Serological responses to infection with B. pertussis. Dev Biol Stand. 1997;89:213–220. [PubMed] [Google Scholar]
- 10.Giammanco A, Chiarini A, Stroffolini T, Chiaramonte M, Moschen M E, Mura I, Rigo G, Taormina S, Sarzana A, Mazza G, Scarpa B. Seroepidemiology of pertussis in Italy. Rev Infect Dis. 1991;13:1216–1220. doi: 10.1093/clinids/13.6.1216. [DOI] [PubMed] [Google Scholar]
- 11.Giuliano M, Mastrantonio P, Giammanco A, Piscitelli A, Salmaso S, Wassilak S G F. Antibody responses and persistence in the two years after immunization with two acellular vaccines and one whole-cell vaccine against pertussis. J Pediatr. 1998;132:983–988. doi: 10.1016/s0022-3476(98)70395-6. [DOI] [PubMed] [Google Scholar]
- 12.Granström G, Wretlind B, Granström M. Diagnostic value of clinical and bacteriological findings in pertussis. J Infect. 1991;22:17–26. doi: 10.1016/0163-4453(91)90842-g. [DOI] [PubMed] [Google Scholar]
- 13.Halperin S A, Bortolussi R, Wort A J. Evaluation of culture, immunofluorescence, and serology for diagnosis of pertussis. J Clin Microbiol. 1989;27:752–757. doi: 10.1128/jcm.27.4.752-757.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halperin S A, Bortolussi R, Kasina A, Wort A J. Use of a Chinese hamster ovary cell cytotoxicity assay for the rapid diagnosis of pertussis. J Clin Microbiol. 1990;28:32–38. doi: 10.1128/jcm.28.1.32-38.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He Q, Mertsola J, Soini H, Skurnik M, Ruuskanen O, Viljanen M K. Comparison of polymerase chain reaction with culture and enzyme immunoassay for diagnosis of pertussis. J Clin Microbiol. 1993;31:642–645. doi: 10.1128/jcm.31.3.642-645.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isacson J, Trollfors B, Hedvall G, Taranger J, Zackrisson G. Response and decline of serum IgG antibodies to pertussis toxin, filamentous hemagglutinin and pertactin in children with pertussis. Scand J Infect Dis. 1995;27:273–277. doi: 10.3109/00365549509019021. [DOI] [PubMed] [Google Scholar]
- 17.Jansen D L, Gray G C, Putnam S D, Lynn F, Meade B D. Evaluation of pertussis in U.S. Marine Corps trainees. Clin Infect Dis. 1997;25:1099–1107. doi: 10.1086/516099. [DOI] [PubMed] [Google Scholar]
- 18.Labadie J, Sundermann L C, Rümke H C the DTP-IPV–Hib Vaccine Study Group. Multicenter study on the simultaneous administration of DTP-IPV and Hib PRP-T vaccines. Part 1. Immunogenicity. Report 124001003. Bilthoven, The Netherlands: National Institute of Public Health and the Environment; 1996. [Google Scholar]
- 19.Long S S, Welkon C J, Clark J L. Widespread silent transmission of pertussis in families: antibody correlates of infection and symptomatology. J Infect Dis. 1990;161:480–486. doi: 10.1093/infdis/161.3.480. [DOI] [PubMed] [Google Scholar]
- 20.Marchant C D, Loughin A M, Lett S M, Todd C W, Wetterlow L H, Bicchieri R, Higham S, Etkind P, Silva E, Siber G R. Pertussis in Massachusetts, 1981–1991: incidence, serologic diagnosis, and vaccine effectiveness. J Infect Dis. 1994;169:1297–1305. doi: 10.1093/infdis/169.6.1297. [DOI] [PubMed] [Google Scholar]
- 21.Melker H E, Conyn-van Spaendonck M A E. Immunosurveillance and the evaluation of national immunisation programmes: a population-based approach. Epidemiol Infect. 1998;121:637–643. doi: 10.1017/s0950268898001587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mink C M, Cherry J D, Christenson P, Lewis K, Pineda E, Shlian D, Dawson J A, Blumberg D A. A search for Bordetella pertussis infection in university students. Clin Infect Dis. 1992;14:464–471. doi: 10.1093/clinids/14.2.464. [DOI] [PubMed] [Google Scholar]
- 23.Müller F M, Hoppe J E, Wirsing-von König C H. Laboratory diagnosis of pertussis: state of the art in 1997. J Clin Microbiol. 1997;35:2435–2443. doi: 10.1128/jcm.35.10.2435-2443.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagel J, de Graaf S, Schijf-Evers D. Improved serodiagnosis of whooping cough caused by Bordetella pertussis by determination of IgG anti-LFP antibody levels. Dev Biol Stand. 1985;61:325–330. [PubMed] [Google Scholar]
- 25.Nelson J D. The changing epidemiology of pertussis in young infants: the role of adults as a reservoir of infection. Am J Dis Child. 1978;132:371–373. doi: 10.1001/archpedi.1978.02120290043006. [DOI] [PubMed] [Google Scholar]
- 26.Nennig H E, Shinefield H R, Edwards K M, Black S B, Fireman B H. Prevalence and incidence of adult pertussis in an urban population. JAMA. 1996;275:1672–1674. [PubMed] [Google Scholar]
- 27.Patriarca P A, Biellik R J, Sanden G, Burstyn D G, Mitchell P D, Silverman P R, Davis J P, Manclark C R. Sensitivity and specificity of clinical case definitions for pertussis. Am J Public Health. 1988;78:833–836. doi: 10.2105/ajph.78.7.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenthal S, Strebel P, Cassiday P, Sanden G, Brusuelas K, Wharton M. Pertussis infection among adults during the 1993 outbreak in Chicago. J Infect Dis. 1995;171:1650–1652. doi: 10.1093/infdis/171.6.1650. [DOI] [PubMed] [Google Scholar]
- 29.SAS Institute, Inc. STAT software: changes and enhancement through release 6.12. Cary, N.C: SAS Institute, Inc.; 1997. [Google Scholar]
- 30.Schmitt-Grohé S, Cherry J D, Heininger U, Überall M A, Pineda E, Stehr K. Pertussis in German adults. Clin Infect Dis. 1995;21:860–866. doi: 10.1093/clinids/21.4.860. [DOI] [PubMed] [Google Scholar]
- 31.Steketee R W, Burstyn D G, Wassilak S G F, Adkins W N, Polyak M B, Davis J P, Manclark C R. A comparison of laboratory and clinical methods for diagnosis of pertussis in an outbreak in a facility for the developmentally disabled. J Infect Dis. 1988;157:441–449. doi: 10.1093/infdis/157.3.441. [DOI] [PubMed] [Google Scholar]
- 32.Storsaeter J, Hallander H O, Gustafsson L, Olin P. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine. 1998;16:1907–1916. doi: 10.1016/s0264-410x(98)00227-8. [DOI] [PubMed] [Google Scholar]
- 33.Tomoda T, Ogura H, Kurashige T. Immune responses to Bordetella pertussis infection and vaccination. J Infect Dis. 1991;163:559–563. doi: 10.1093/infdis/163.3.559. [DOI] [PubMed] [Google Scholar]
- 34.van der Zee A, Agterberg C, Peeters M, Mooi F, Schellekens J. A clinical validation of Bordetella pertussis and Bordetella parapertussis polymerase chain reaction: comparison with culture and serology using samples of patients with suspected whooping cough from a highly immunized population. J Infect Dis. 1996;174:89–96. doi: 10.1093/infdis/174.1.89. [DOI] [PubMed] [Google Scholar]
- 35.Wirsing van König C H, Rott H, Bogaerts H, Schmitt H J. A serologic study of organisms possibly associated with pertussis-like coughing. Pediatr Infect Dis J. 1998;17:645–649. doi: 10.1097/00006454-199807000-00013. [DOI] [PubMed] [Google Scholar]