Abstract
Sarcopenic obesity is closely associated with knee osteoarthritis (KOA) and has high risk of total knee replacement (TKR). In addition, poor nutrition status may lead to sarcopenia and physical frailty in KOA and is negatively associated with surgery outcome after TKR. This study investigated the effects of sarcopenic obesity and its confounding factors on recovery in range of motion (ROM) after total knee replacement (TKR) in older adults with KOA. A total of 587 older adults, aged ≥60 years, who had a diagnosis of KOA and underwent TKR, were enrolled in this retrospective cohort study. Sarcopenia and obesity were defined based on cutoff values of appendicular mass index and body mass index for Asian people. Based on the sarcopenia and obesity definitions, patients were classified into three body-composition groups before TKR: sarcopenic-obese, obese, and non-obese. All patients were asked to attend postoperative outpatient follow-up admissions. Knee flexion ROM was measured before and after surgery. A ROM cutoff of 125 degrees was used to identify poor recovery post-surgery. Kaplan-Meier curve analysis was performed to measure the probability of poor ROM recovery among study groups. Cox multivariate regression models were established to calculate the hazard ratios (HRs) of postoperative poor ROM recovery, using potential confounding factors including age, sex, comorbidity, risk of malnutrition, preoperative ROM, and outpatient follow-up duration as covariates. Analyses results showed that patients in the obese and sarcopenic-obese groups had a higher probability of poor ROM recovery compared to the non-obese group (all p < 0.001). Among all body-composition groups, the sarcopenic-obese group yielded the highest risk of postoperative physical difficulty (adjusted HR = 1.63, p = 0.03), independent to the potential confounding factors. Sarcopenic obesity is likely at the high risk of poor ROM outcome following TKR in older individuals with KOA.
Keywords: sarcopenic obesity, malnutrition, osteoarthritis, total knee replacement, range of motion
1. Introduction
Obesity has become epidemic worldwide in populations with knee osteoarthritis (KOA) and has growing impacts for those who are undergoing total knee replacement (TKR) [1]. Due to this, overweight or obesity is strongly associated with disease progression of KOA [2,3], obesity has an increased risk of TKR [4,5], and the prevalence of obesity in patients undergoing TKR surgery is expected to increase [6]. In addition, obesity is a predominant risk factor for post-surgery complications and may affect short-term or long-term outcomes after TKR [7,8], among which the recovery in knee flexion range of motion (ROM) is of particular interest [9,10,11,12,13]. However, the effects of obesity on surgery outcomes, including ROM, regain after TKR remains conflict [1]. A number of previous studies have indicated that obesity negatively affects ROM recovery following TKR [7,14], whereas others had inconsistent conclusions [15]. Due to the fact that the rate of TKR grows rapidly in obese older individuals with KOA [16,17] and that the postoperative ROM gains are significantly associated with function recovery [12,18,19,20,21], there is an urgent need to further identify the effect of obesity on ROM recovery following TKR.
In addition to obesity, low muscle mass (i.e., sarcopenia) has been given as a risk factor for KOA and its disease progression [22,23,24]. Notably, the obese older individuals with KOA who are classified as having sarcopenia (i.e., sarcopenic obesity) may suffer potential risks of physical decline and disability [25,26,27]. Sarcopenic obesity is a condition referring to the coexistence of sarcopenia and obesity and threatens physical health in elderly populations [28], with an overall global prevalence of 11% in older adults [29]. Sarcopenic obesity has been identified to be closely associated with chronic diseases such as knee osteoarthritis (KOA) [30,31]. Because low muscle mass is associated with greater functional impairments [32] and preoperative functional status predicts the functional recovery pattern [33,34,35], potential sarcopenia in such obese older people with KOA who are undergoing TKR may have additive impacts on postoperative outcomes. In addition, post-surgery muscle attenuation commonly occurs by decreased muscle mass [36] and muscle volume [37,38,39,40,41] from early weeks up to two years after total joint replacement in legs. Moreover, obesity is associated with muscle attenuation. Therefore, obese older adults may suffer potential risks of sarcopenia, especially those who are undergoing TKR. Sarcopenia may occur in end-stage KOA and persist even after TKR, especially for those who are obese, since the prevalence of sarcopenic obesity ranges from 3% to 35% in KOA population, dependent on the diagnostic criteria for classification of sarcopenia and obesity [27,42]. However, whether sarcopenic obesity interferes with surgical outcomes remains unclear. Identifying the role of sarcopenic obesity in recovery after TKR might facilitate the optimization of treatment and identify patients with a poor outcome risk.
Malnutrition is a high risk factor for physical frailty for elder individuals who have KOA [43]. Especially, preoperative nutritional status is significantly associated with surgery complications after TKR [44,45,46]. Therefore, preoperative undernutrition may affect recovery from TKR in elder people with KOA. Whether preoperative undernutrition has any influence on the associations between sarcopenic obesity and ROM recovery needs to be further investigated.
The purpose of this study was to identify the effects of sarcopenic obesity on the knee flexion ROM outcome after TKR in older adults with KOA. We also identified potential confounding factors which may affect the associations between sarcopenic obesity and ROM restoration after TKR. The study hypothesis was that obese patients with or without low muscle mass exhibited higher risks in poor surgical outcomes, identified based on knee flexion ROM after TKR, compared to non-obese patients.
2. Materials and Methods
2.1. Study Design
This study was conducted based on a retrospective cohort design, used to evaluate the effects of high body mass index (BMI) on post-TKR outcomes [47]. This design adheres to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines [48]. The Institutional Review Board of Taipei Medical University approved our analysis of the patient data contained in the database (Trial number: N202107102). All data used in our study were collected from a rehabilitation center database. Two research assistants, who were trained for standardization of study procedure, collected and extracted the data from the database; in addition, all of the extracted data were further confirmed by one author (C-DL). Patient characteristics and demographics data including age, BMI, comorbidity, nutrition status, side of TKR surgical leg, Kellgren and Lawrence (K-L) grade of the surgical leg, surgical time, length of inpatient stay (LOS) after surgery (in day), and outpatient follow-up time period (in week) were extracted and analyzed. The patients’ global comorbidities were evaluated using Cumulative Illness Rating Scale, which assesses the comorbidity status of older individuals [49].
2.2. Participants
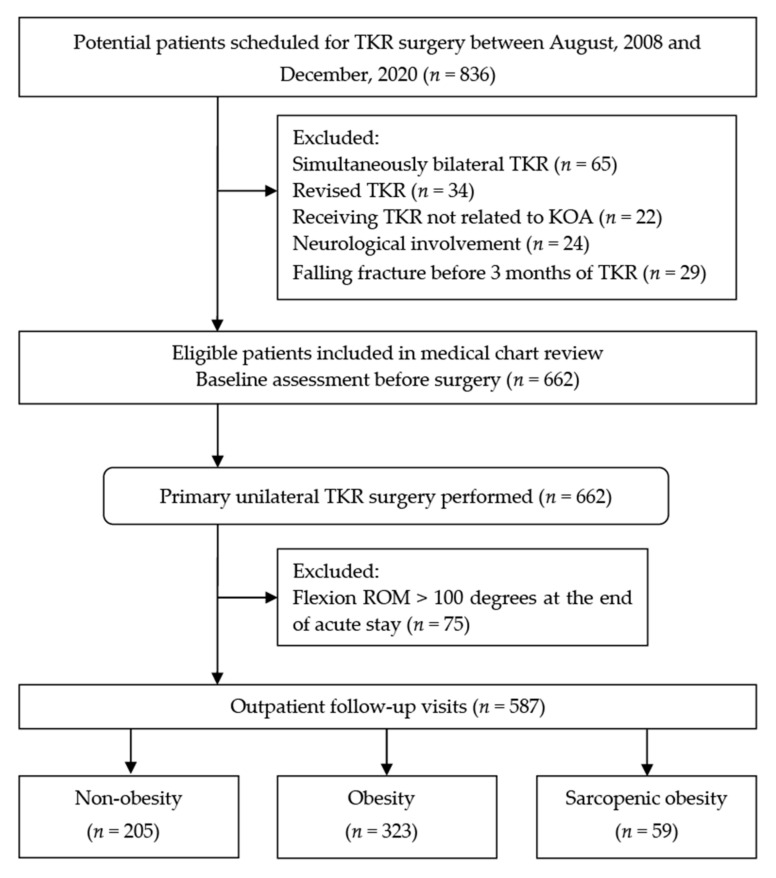
The records of patients who were aged 60 years or older, were diagnosed with end-stage KOA (K-L grade ≥3), and had undergone primary unilateral TKR between August 2008 and December 2020, were reviewed. Enrollment criteria were designed to exclude subjects from entry who (1) had comorbidity which was not regularly controlled by medicine including insulin-dependent diabetes, hypertension, and hepatic disease. Such uncontrolled comorbidities may have impacts on an individual’s physiologic reserve [50], especially those who are undergoing an acute event (e.q. TKR surgery) [51]; (2) a history of internal cancer requiring surgery, neurological diseases, or falling fracture in legs before six months of surgery; (3) underwent simultaneously bilateral or a revision TKR; (4) patients who had a knee flexion ROM < 125 degrees at inpatient discharge; the details were described in the following (Section 2.6). A flowchart depicting the patient selection process and study group assignment is illustrated in Figure 1.
Figure 1.
Flow of patient enrollment and allocation throughout the present study. TKR, total knee replacement; KOA, knee osteoarthritis; ROM, range of motion.
2.3. Assessment of Nutritional Status and Risk of Undernutrition
Preoperative nutritional status was assessed using Mini Nutritional Assessment Short Form scale (MNA-SF) [52,53]. The MNA-SF significantly predicts survival outcome for Asian hospitalized geriatric patients [54] and has good validity for screening malnutrition in elder populations [55,56]. Two senior research assistants, who had been trained by a nutritionist with a two-week nutritional course, collected the data on the inpatient admission (before surgery) and performed the assessment of nutritional status for each patient.
The total sum scores for the MNA-SF range from 0 to 14 points, with 14 points indicating the best nutritional state possible [52]. An MNA-SF score of ≤7, 8–11, and ≥12 points indicates malnutrition, possible malnutrition, and well-nourished, respectively. In the present study, the patients who obtained a total score of MNA-SF < 12 points were identified as having potential risk of undernutrition [53].
2.4. Identification of Sarcopenic Obesity
A BMI cutoff of value 25.0 kg/m2, identified by the World Health Organization for Asian population, was used to define obesity in this study cohort [57]. Low muscle mass was identified based on appendicular lean mass (ALM) measurements (in kg), normalized for height to provide AMI [= ALM/height2 (in kg/m2)]. We followed Wen’s method using an established equation model to estimate ALM [58], which has been validated and used to assess muscle mass for older Chinese population [59,60]. The ALM equation model was established based on anthropometric measurements as follows:
| ALM = 0.193 × body weight (kg) + 0.107 × height (m) − 4.157 × gender (male = 1, female = 2) − 0.037 × age (year) − 2.631 |
Using dual-energy X-ray absorptiometry as the standard reference, the adjusted R2 of the ALM equation model was 0.90 with a standard error of 1.63 kg [58]. A cutoff value of ALM to define low muscle mass in Asians were based on the diagnostic criteria established by the Asian Working Group for Sarcopenia [61]. Patients were classified as having low muscle mass if the ALM <7.0 kg/m2 in men and <5.4 kg/m2 in women.
2.5. Measurement and Follow up of Knee Flexion Range of Motion
Active knee flexion ROM was measured by two orthopedic doctors before surgery (baseline), at the end of inpatient stay, and at outpatient follow-up admissions. After inpatient discharge, each patient was asked to admit outpatient follow-up clinic one to two times per month until a full recovery of knee flexion ROM (i.e., 130 to 135 degrees [62,63]) was achieved. In addition, the outpatient follow up for each patient was continued for a time period as long as nine months to ensure the maximum gains in knee ROM after TKR, despite recovery of knee flexion ROM reaching a peak at 3−6 months after TKR [13,62].
Active knee flexion ROM was measured using a long-arm goniometer. Patients were asked to lie in supine position and actively bend the involved knee to the maximum extent. Reproducibility of goniometric measurement of active knee flexion is acceptable with an intraclass correlation coefficient of 0.89 for patients receiving TKR [64].
2.6. Identification of Poor Surgical Outcome
Poor surgical outcome was identified based on knee flexion ROM, which is an indicator for clinicians to determine the success of TKR surgery [9,10,11,12,13]. In the present study, a cut-off value of 125 degrees of knee flexion was used for poor outcome following TKR based on the following reasons: (1) a knee flexion of 125 degrees permits older individuals to perform the majority of activities in daily life [65,66,67]; (2) a knee flexion ROM < 125 degrees leads to function limitations after TKR [21]; and (3) those who have a knee flexion ROM < 125 degrees tend to experience lower walking capability after TKR [68]. At the end of nine-month outpatient follow-up period, which is defined above, the patients who exhibited a knee flexion ROM < 125 degrees were classified as suffering poor outcome after TKR surgery. The present analysis was limited to patients who had a knee flexion ROM < 125 degrees at inpatient discharge, indicating that all patients were functioning at a low level of ROM at the beginning of outpatient follow-up.
In addition, for each patient, the time period (in week) required to yield a knee flexion ROM ≥ 125 degrees from inpatient discharge was estimated on the basis of the outpatient follow-up data. As mentioned above, all data were collected, assessed, and calculated by the two trained research assistants and the author (C-DL) confirmed all of the extracted data.
2.7. Statistical Analysis
One-way ANOVA was used to analyze continuous variables and chi-squared analysis was employed for categorical variables. Post-hoc analysis was performed using the Bonferroni method where the equal variance was assumed. If the homogeneity of group variance was not assumed, the Games-Howell test was performed.
Kaplan-Meier curve analysis was performed to measure the probability of poor postoperative outcome using time period for yielding a knee flexion ROM ≥ 125 degrees as the underlying time scale. In the Kaplan-Meier curve analysis, patients were divided into subgroups by body composition. The log-rank test was used to compare the probabilities of poor surgical outcome among body composition subgroups.
For estimating the associations between different obese groups and ROM restoration after TKR, Cox regression models were established to calculate the hazard ratios (HRs) and the corresponding 95% confidence intervals (CIs) of postoperative poor outcome, with reference to nonobese group and using potential confounders as covariates. The confounding factors which may affect post-TKR outcomes included patients’ age [69,70,71,72], sex [70,72,73,74], comorbidity [75,76], preoperative ROM of knee flexion [69,72,74,77,78], LOS [79], preoperative nutritional status [80,81,82], and outpatient follow-up duration.
A statistician who was blinded to the group allocation was consulted regarding the statistical approach in this study. All differences with p < 0.05 were defined as statistically significant. SPSS Statistics (version 22.0; IBM, Armonk, NY, USA) was used for all analyses.
3. Results
3.1. Patient Demographics and Clinical Characteristics
After 174 patients who did not match the inclusion criteria were excluded, 662 eligible patients who underwent a primary unilateral TKR were included in the medical chart review. Finally, a total of 587 patients (144 men, 443 women) were included for follow up and analyses whereas 69 patients who exhibited a knee flexion < 125 degrees at inpatient discharge were excluded from further investigations. Based on the BMI and AMI cutoff values for obesity and low muscle mass in Asian individuals, respectively, all included patients were stratified into to three body-composition groups: non-obesity (n = 205), obesity without low muscle mass (n = 323), and obese with low muscle mass (n = 59).
Demographic characteristics of the included patients are listed in Table 1. The sarcopenic-obese patients aged nearly 4 years older than those in the non-obese group (p < 0.001), whereas obese patients were younger compared with the non-obese peers (mean = 67.8 versus 70.9 years). Between the sarcopenic obesity and non-obesity groups, the mean values of comorbidity score (10.9 versus 8.4 points), number of patients who were at risk of undernutrition (27/59 versus 76/205), preoperative (101 versus 118 degrees) and inpatient-discharge (94 versus 102 degrees) knee flexion ROMs, LOS (6.2 versus 5.3 days), and outpatient follow-up duration (19.5 versus 13.7 weeks) differed significantly (all p < 0.01); similar results were observed between the obesity and non-obesity groups. No significant intergroup difference in proportion rates of women, surgical time, side of surgery leg, or K-L grade of the involved leg was observed (all p > 0.01).
Table 1.
Demographic characteristics of participants.
| Items | Non-Obese | Obese | Obese-Sarcopenic | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| mean | ± | SD | mean | ± | SD | mean | ± | SD | ||
| n | 205 | 323 | 59 | |||||||
| Women, n (%) | 160 | (78.0) | 249 | (77.1) | 39 | (66.1) | 0.15 d | |||
| Age (years) | 70.9 | ± | 6.9 | 67.8 | ± | 6.3a | 75.1 | ± | 6.1 a | <0.001 b |
| Age category, n (%) | <0.001 d | |||||||||
| <65 years | 44 | (21.5) | 156 | (48.3) | 10 | (16.9) | ||||
| 70−74.9 years | 101 | (17.1) | 105 | (32.5) | 21 | (35.6) | ||||
| ≥75 years | 60 | (29.2) | 62 | (19.2) | 28 | (47.5) | ||||
| BMI (kg/m2) | 22.9 | ± | 1.8 | 30.6 | ± | 3.9 a | 27.0 | ± | 2.1 a | <0.001 c |
| ALM (kg) | 16.0 | ± | 3.2 | 17.9 | ± | 3.6 a | 13.8 | ± | 2.8 a | <0.001 c |
| AMI (kg/m2) | 6.64 | ± | 0.91 | 7.56 | ± | 0.98 a | 5.96 | ± | 0.75 a | <0.001 c |
| CIRS score | 8.4 | ± | 5.7 | 10.1 | ± | 5.5 a | 10.9 | ± | 6.2 a | 0.001 c |
| Surgical time (min) | 149 | ± | 35 | 152 | ± | 39 | 155 | ± | 33 | 0.35 b |
| LOS (day) | 5.3 | ± | 1.6 | 6.5 | ± | 2.4 a | 6.2 | ± | 1.9 a | <0.001 c |
| Follow-up time (week) | 13.7 | ± | 7.3 | 16.4 | ± | 7.7 a | 19.5 | ± | 8.9 a | <0.001 c |
| TKR leg, Right, n (%) | 123 | (60.0) | 181 | (56.0) | 31 | (52.5) | 0.51 d | |||
| KL grade, TKR leg, n (%) | 0.33 d | |||||||||
| 3 | 103 | (50.2) | 165 | (51.1) | 24 | (40.7) | ||||
| 4 | 102 | (49.8) | 158 | (48.9) | 35 | (59.3) | ||||
| MNA-SF <12 points | 76 | (37.1) | 92 | (28.5) | 27 | (45.8) | 0.01 d | |||
| Number of comorbidities, n (%) | ||||||||||
| Hypertension | 129 | (62.9) | 228 | (70.6) | 40 | (67.8) | 0.19 d | |||
| DM | 37 | (18.0) | 139 | (43.0) | 22 | (37.3) | <0.001 d | |||
| Cardiopulmonary disease | 94 | (45.9) | 174 | (53.9) | 29 | (49.2) | 0.19 d | |||
| Leg fracture | 12 | (5.9) | 15 | (4.6) | 3 | (5.1) | 0.83 d | |||
| Knee flexion ROM (degree) | ||||||||||
| Presurgery | 118 | ± | 14 | 108 | ± | 13 a | 101 | ± | 15 a | <0.001 b |
| Inpatient discharge |
102 | ± | 10 | 95 | ± | 10 a | 94 | ± | 10 a | <0.001 b |
a A significant difference compared with the reference group, p < 0.05. b One-way analysis of variance. Post-hoc analysis was performed using the Bonferroni test. c One-way analysis of variance. Post-hoc analysis was performed using the Games-Howell test. d Chi-Square Test. AMI, appendicular mass index; BMI, body mass index; CIRS, Cumulative Illness Rating Scale; DM, diabetes mellitus; ROM, gait speed; K-L grade, Kellgren-Lawrence grade; MNA-SF, Mini Nutritional Assessment Short Form scale; SD, standard deviation; TKR, total knee replacement.
3.2. Survival Time for ROM Recovery after TKR
A total of 378 (64.4%) patients in the study cohort recovered a knee flexion ROM > 125 degrees during an overall follow-up duration of 15.8 weeks (range 1–36 weeks), among which 159 (77.6%) in the non-obesity, 194 (60.1%) in the obesity, and 25 (42.4%) in the sarcopenic-obesity group.
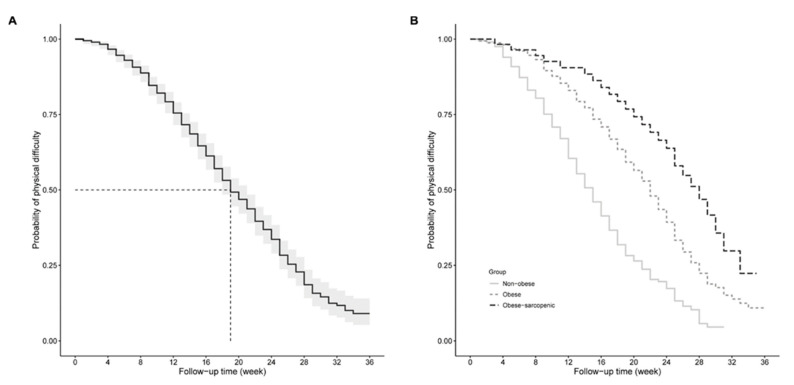
Kaplan-Meier analysis showed an overall median time of 19 weeks for all patients in the study to yield successful recovery in knee flexion ROM after TKR (Figure 2A). Patients in the obesity and sarcopenic obesity groups had a higher probability of poor surgery outcome (i.e., ROM < 125 degrees) over the postoperative follow-up time period compared to their non-obese peers (all p < 0.001; Figure 2B). In addition, those who were classified as having sarcopenic obesity experienced a higher probability of poor surgery outcome compared with purely obese patients (p < 0.01). The median survival time of poor surgery outcome for non-obese patients was 15 weeks whereas the obesity group (median survival time = 22 weeks) as well as the sarcopenic obesity group (median survival time = 28 weeks) took relatively longer time periods to recover a knee flexion ROM ≥ 125 degrees (Figure 2B).
Figure 2.
Kaplan-Meier survival curve for time to yield poor surgery outcome by (A) overall study cohort and (B) body composition type. Time scale on X-axis represents the time point exceeding a knee range of motion of 125 degrees after inpatient discharge.
3.3. Associations of Sarcopenic Obesity with ROM Restoration following TKR
Table 2 shows the results of Cox regression analyses determining the effect of different body-composition types on poor ROM recovery. The crude HR of 3.63 (95% CI, 2.37−5.56) indicates a risk of poor ROM recovery that was 3.6 times higher in sarcopenic-obese patients compared with the referent peers who were neither sarcopenic nor obese preoperatively. Controlling for age, sex, comorbidity score, risk of undernutrition, preoperative ROM, LOS, and outpatient follow-up duration, the HR of poor ROM recovery in sarcopenic obesity group reduced to 1.68 (95% CI, 1.06−2.66) with reference to non-obese group; similar results were observed in the obesity group (adjusted HR = 1.35, p < 0.05).
Table 2.
Hazard ratios for proportional hazards models evaluating the associations of body-composition types with poor recovery in knee flexion.
| Body-Composition Group | Crude Model | Adjusted Model a | ||||
|---|---|---|---|---|---|---|
| HR | (95% CI) | p | HR | (95% CI) | p | |
| Non-obesity | Reference | Reference | ||||
| Obesity | 2.10 | (1.68, 2.62) | <0.001 | 1.35 | (1.04, 1.75) | 0.02 |
| Sarcopenic obesity | 3.63 | (2.37, 5.56) | <0.001 | 1.68 | (1.06, 2.66) | 0.03 |
a Model was adjusted using age, sex (coded as men = 1, sex = 2), comorbidity score, risk of undernutrition (coded as yes = 1, no = 0), preoperative range of motion, length of inpatient stay, and outpatient follow-up time as covariates. HR, hazard ratio; 95% CI, 95% confidence interval.
4. Discussion
4.1. Summary of the Main Findings
This study determined the effects of sarcopenic obesity and its confounding factors on surgery outcome in terms of ROM restoration for individuals with KOA. The results indicated that patients with sarcopenic obesity, as well as those with pure obesity, suffered a higher probability of poor ROM recovery over a follow-up time period of 34 weeks after TKR, compared with the non-obese peers. In addition, the sarcopenic obesity group was at greater risk of suffering poor ROM recovery with an HR 1.6 times greater than the reference group, adjusted for age, sex, comorbidity, risk of undernutrition, LOS, preoperative ROM, and outpatient follow-up duration.
4.2. Demographics and Characteristics in the Study Cohort
Demographic results in this study showed that patients were older in the sarcopenic obesity group (mean age = 75.1 years) compared with the non-obese group (mean age = 70.9 years). In addition, the proportions of patients who were aged ≥ 70 years within the sarcopenic obesity group (49/59, 83%) were higher than that within the non-obesity group (161/205, 46%). Our results are supported by previous studies reporting dramatic muscle mass loss occurring at ≥70 years of age [83,84]. In addition, obese patients in this study tended to be significantly younger than the non-obese patients. Similar results concerning effects of obesity on surgery outcome following TKR have been reported in prior literatures [7,8,85].
Another finding of particular interest was that patients in both obesity and sarcopenic obesity groups experienced an inpatient stay approximately one day longer than the non-obese peers did. Our results were consistent with previous studies demonstrating that obese patients (BMI ≥ 30 kg/m2) expended a significantly longer time period of hospital stay than those with BMIs < 30 kg/m2 did (p < 0.001) after total knee or hip arthroplasty [7,85,86,87]. Additionally, a higher comorbidity score along with a longer period of inpatient stay was noted in our obese patients compared with the referent peers and such findings were consistent with prior results [7,85]. Such obese patients who have higher BMIs along with higher comorbidity scores are expected to experience a longer period of hospital stay since BMI and comorbidities predict length of hospital stay after TKR [87,88].
4.3. Prevalence of Sarcopenic Obesity and Nutritional Status in End-Stage Knee Osteoarthritis
Sarcopenic obesity is closely associated with radiographic KOA [31], and has become a phenotype in the elder population with lower-limb osteoarthritis [23,89]. Low muscle mass as well as high body fat become evident with disease progression of KOA [24,90], sarcopenic obesity becomes prevalent at late stage of KOA. The overall sarcopenic obesity prevalence at end-stage KOA has been estimated to be 2.7−17.2% for the elder population in Asia [26,31,91] and 2.6−9.4% in other areas worldwide [27,32], regardless of sex, age, and identification criteria of sarcopenic obesity. In this study cohort, 59 out of 587 (10.1%) patients with moderate or severe KOA were classified as having sarcopenic obesity, which agrees with previous results.
In the present study, 195 out of 587 patients (33.2%) had an MNA-SF score < 12, indicating high risk of undernutrition. Our findings were in agreement with prior results reporting 32.4% of older individuals with KOA are at high risk for malnutrition assessed by MNA-SF [43]. We further identified that the proportion of patients at high risk of undernutrition was greater in the sarcopenic obesity (45.8%) compared with non-obese control (37.1%). Our findings are supported by previous results indicating that the prevalence of patients at high risk of malnutrition (47%) is greater among frail individuals with KOA compared with the non-frail peers (19%) [43]. Based on our results, sarcopenic obesity was more likely to experience undernutrition than non-obese peers did in KOA population.
4.4. Obesity and Range of Motion in Knee Osteoarthritis
Preoperative ROM has been identified as the principal predictor of eventual knee flexion after TKR [69,72,92]. In the present study, significant differences among the body-composition groups were noted in knee flexion ROM before surgery. After adjustment for such potential confounding variables, our patients in the obesity and sarcopenic obesity groups exhibited higher probabilities of poor ROM recovery compared with their non-obese peers. Our results may indicate that obesity exerted negative effects on restoration of ROM after TKR. The underlying mechanism through which obesity affects knee flexion ROM in older individuals with KOA remains unclear. The following conclusions of previous studies may support our findings: first, our results were in agreement with prior literatures indicating that obesity is associated with poorer postoperative knee flexion ROM [93,94,95,96]; secondly, given the fact that earlier calf-thigh impingement of the thigh and lower leg results from increased posterior soft tissue girth behind the knee [95,97], it is rational that obese patients experience reduced postoperative ROM.
4.5. Sarcopenic Obesity and Its Risk of Poor Recovery in Range of Motion after Total Knee Replacement
Recovery in knee ROM following TKR is likely to be negatively impacted by either obesity [93,96] or sarcopenia [98]. In agreement with prior findings, survival analysis results in the current study demonstrated that the obesity group took a time period of 7 weeks longer than the non-obese control did to recover a knee flexion over 125 degrees. We further identified that the sarcopenic obesity group took a longer time period to achieve knee flexion ≥ 125 degrees after TKR than the obesity and non-obesity group did. Accordingly, sarcopenic-obese patients were determined to have the highest probability of poor surgery outcome (i.e., flexion ROM < 125 degrees) among the body-composition groups. Our results may indicate that obesity alone exerted negative impacts on surgery outcome after TKR whereas sarcopenic obesity had greater negative effects on post-TKR function recovery than obesity alone did, particularly the restoration of knee flexion ROM.
Cox regression analyses results in this study showed that sarcopenic-obese patients as well as purely obese patients suffered high risks of poor ROM recovery post-surgery, with reference to the non-obese peers. In addition, sarcopenic-obese patients were likely to yield a relatively higher risk of post-TKR poor ROM recovery compared with the obese peers without sarcopenia. Our findings in obesity group were supported by previous studies concerning associations between high body fat (or BMI) and ROM restoration after TKR [7,93,94,95,96]. However, few of the prior authors discussed the associations between sarcopenic obesity and surgery outcome following TKR. According to our results, sarcopenic obesity appears to exert impacts on function recovery, particularly the restoration of knee flexion ROM, independent to age, sex, comorbidity, malnutrition risk, LOS, preoperative flexion ROM, and the outpatient follow-up period. Some reasons may explain our findings as follows: first, knee ROM at inpatient discharge has been identified as a relevant indicator for knee flexion outcome after TKR [74]; in addition, insufficient gains in knee flexion ROM at early period after TKR predict its poor recovery at later follow up [9]. Therefore, our sarcopenic-obese patients who exhibited a minor ROM may take a longer period to regain ROM and obtain minor knee flexion at a long-term follow up, compared to the non-obese patients.
4.6. Study Limitations
A number of certain limitations to this study need to be highlighted. First, our study included only older individuals with moderate to severe KOA; therefore, the results cannot be generalized to those who have mild KOA (K-L grade < 3). In addition, our results might not be generalizable to all TKR types. Further investigation is necessary regarding whether sarcopenic obesity affects function outcomes post-surgery in patients with other preoperative diagnoses, such as rheumatoid arthritis, are similar to those for KOA. Secondly, the needs or prescriptions for outpatient rehabilitation were not assessed. Because of this, the rehabilitation exercise program following TKR such as stretch or ROM exercises may have positive effects on recovery rate of knee flexion ROM, the time duration required to exceed a knee ROM ≥ 125 degrees may be underestimated in our analyses. Further studies are needed to assess whether outpatient rehabilitation, including exercise training types and durations, has any effect on the associations between sarcopenic obesity and ROM restorations following TKR. Thirdly, medication prescribed for pain control also represents a potential confounding factor. We did not consider drug use for pain in our analysis of the results of the ROM measure. Future studies on whether pain medications have a significant intragroup contribution to the recovery rate of knee ROM after TKR are warranted. Fourth, the assessment of nutritional status using MNA-SF may underdiagnose malnutrition for the patients enrolled in this study [99,100]. Fifth, we did not conduct a blinding process performed either at the data collection or data interpretation stage despite all of the collected and extracted data being further confirmed by one study author. Results in the current study should be cautiously interpreted due to potential detection bias. Finally, potential confounders, such as disease duration [101,102], may have made contributions when measuring acute outcomes after TKR. However, disease duration was not assessed or included as covariates for analysis in this study; therefore, differences in risk of poor ROM outcome among the body-composition groups may had been overestimated.
5. Conclusions
This study demonstrated that obesity, as well as sarcopenic obesity, independently had negative effects on surgery outcome following TKR in terms of yielding high risks of poor recovery in knee flexion ROM. Sarcopenic obesity appears to have greater risk of poor ROM recovery after TKR compared with obesity alone for older individuals who had moderate to severe KOA. The results of the current study have implications for clinicians setting rehabilitation strategies to optimize ROM restorations after TKR, especially for those who are identified as sarcopenic obesity pre-surgery.
Acknowledgments
This manuscript was edited by the professional editing. We would like to acknowledge all the research assistances who helped us in identifying eligible patients and collecting the data.
Author Contributions
The authors’ responsibilities are outlined as follows: C.-D.L., S.-W.H. and C.-L.L. conceptualized and designed the experiments; C.-D.L. acquired, collected, and assembled the data; C.-D.L. and S.-W.H. analyzed and interpreted the data; C.-D.L. and C.-L.L. drafted and prepared the manuscript; S.-W.H., Y.-Y.H. and C.-L.L. reviewed the paper and verified the accuracy of its content; and C.-D.L., S.-W.H. and C.-L.L. supervised and visualized the study and take responsibility for the integrity of the work as a whole. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by grants from Taipei Medical University-Shuang Ho Hospital, Ministry of Health and Welfare, Taiwan (grant no. 110TMU-SHH-04). The funding sources played no role in the design, implementation, data analysis, interpretation, or reporting of the study. The contents of this publication are solely the responsibility of the authors.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Joint Institutional Review Board of Taipei Medical University (protocol code N202107102 and date of final approval on 17 August 2021).
Informed Consent Statement
Due to a retrospective study design, the informed consent was not necessary from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. Public data sharing is not applicable to this article due to ethical considerations and privacy restrictions.
Conflicts of Interest
The authors declare that they have no conflicts of interest regarding the publication of this article.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jester R., Rodney A. The relationship between obesity and primary Total Knee Replacement: A scoping review of the literature. Int. J. Orthop. Trauma Nurs. 2021;42:100850. doi: 10.1016/j.ijotn.2021.100850. [DOI] [PubMed] [Google Scholar]
- 2.Pishgar F., Guermazi A., Ashraf-Ganjouei A., Haj-Mirzaian A., Roemer F.W., Zikria B., Sereni C., Hakky M., Demehri S. Association between Patellofemoral and medial Tibiofemoral compartment osteoarthritis progression: Exploring the effect of body weight using longitudinal data from osteoarthritis initiative (OAI) Skelet. Radiol. 2021:1–10. doi: 10.1007/s00256-021-03749-0. [DOI] [PubMed] [Google Scholar]
- 3.Haase C.L., Eriksen K.T., Lopes S., Satylganova A., Schnecke V., McEwan P. Body mass index and risk of obesity-related conditions in a cohort of 2.9 million people: Evidence from a UK primary care database. Obes Sci Pract. 2021;7:137–147. doi: 10.1002/osp4.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munugoda I., Wills K., Cicuttini F., Graves S., Lorimer M., Jones G., Callisaya M., Aitken D. The association between ambulatory activity, body composition and hip or knee joint replacement due to osteoarthritis: A prospective cohort study. Osteoarthr. Cartil. 2018;26:671–679. doi: 10.1016/j.joca.2018.02.895. [DOI] [PubMed] [Google Scholar]
- 5.Lim Y.Z., Wang Y., Cicuttini F.M., Giles G.G., Graves S., Wluka A.E., Hussain S.M. Obesity defined by body mass index and waist circumference and risk of total knee arthroplasty for osteoarthritis: A prospective cohort study. PLoS ONE. 2021;16:e0245002. doi: 10.1371/journal.pone.0245002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasarhelyi E.M., Macdonald S.J. Obesity and Total Joint Arthroplast. Semin. Arthroplast. 2012;23:10–12. doi: 10.1053/j.sart.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Liao C.-D., Huang Y.-C., Chiu Y.-S., Liou T.-H. Effect of body mass index on knee function outcomes following continuous passive motion in patients with osteoarthritis after total knee replacement: A retrospective study. Physiotherapy. 2017;103:266–275. doi: 10.1016/j.physio.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Xu S., Chen J.Y., Lo N.N., Chia S.L., Tay D.K.J., Pang H.N., Hao Y., Yeo S.J. The influence of obesity on functional outcome and quality of life after total knee arthroplasty: A ten-year follow-up study. Bone Joint, J. 2018;100:579–583. doi: 10.1302/0301-620X.100B5.BJJ-2017-1263.R1. [DOI] [PubMed] [Google Scholar]
- 9.Kornuijt A., De Kort G.J.L., Das D., Lenssen A.F., Van Der Weegen W. Recovery of knee range of motion after total knee arthroplasty in the first postoperative weeks: Poor recovery can be detected early. Musculoskelet. Surg. 2019;103:289–297. doi: 10.1007/s12306-019-00588-0. [DOI] [PubMed] [Google Scholar]
- 10.Wimmer M.A., Nechtow W., Schwenke T., Moisio K.C. Knee Flexion and Daily Activities in Patients following Total Knee Replacement: A Comparison with ISO Standard 14243. BioMed Res. Int. 2015;2015:1–7. doi: 10.1155/2015/157541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito H., Ichihara K., Tamari K., Amano T., Tanaka S., Uchida S., Morikawa S. Determination of reference intervals for knee motor functions specific to patients undergoing knee Arthroplast. PLoS ONE. 2021;16:e0249564. doi: 10.1371/journal.pone.0249564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miner A.L., Lingard E.A., Wright E.A., Sledge C.B., Katz J.N. Knee range of motion after total knee arthroplasty: How important is this as an outcome measure? J. Arthroplast. 2003;18:286–294. doi: 10.1054/arth.2003.50046. [DOI] [PubMed] [Google Scholar]
- 13.Stratford P., Kennedy D.M., Robarts S.F. Modelling Knee Range of Motion Post Arthroplasty: Clinical Applications. Physiother. Can. 2010;62:378–387. doi: 10.3138/physio.62.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Razak H.R.B.A., Chong H.C., Tan A.H.C. Obesity Does Not Imply Poor Outcomes in Asians after Total Knee Arthroplast. Clin. Orthop. Relat. Res. 2013;471:1957–1963. doi: 10.1007/s11999-012-2721-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonnefoy-Mazure A., Martz P., Armand S., Sagawa Y., Suva D., Turcot K., Miozzari H.H., Lübbeke A. Influence of Body Mass Index on Sagittal Knee Range of Motion and Gait Speed Recovery 1-Year After Total Knee Arthroplast. J. Arthroplast. 2017;32:2404–2410. doi: 10.1016/j.arth.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Vasarhelyi E.M., Macdonald S.J., Munro J.T., Garbuz D.S., Masri B.A., Duncan C.P. The influence of obesity on total joint Arthroplast. J. Bone Jt. Surg. Br. Vol. 2012;94:100–102. doi: 10.1302/0301-620X.94B11.30619. [DOI] [PubMed] [Google Scholar]
- 17.Gillespie G., Porteous A. Obesity and knee Arthroplast. Knee. 2007;14:81–86. doi: 10.1016/j.knee.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Zeni J.A., Snyder-Mackler L. Early Postoperative Measures Predict 1- and 2-Year Outcomes After Unilateral Total Knee Arthroplasty: Importance of Contralateral Limb Strength. Phys. Ther. 2010;90:43–54. doi: 10.2522/ptj.20090089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park K.K., Chang C.B., Kang Y.G., Seong S.C., Kim T.K. Correlation of maximum flexion with clinical outcome after total knee replacement in Asian patients. J. Bone Jt. Surg. Br. Vol. 2007;89:604–608. doi: 10.1302/0301-620x.89b5.18117. [DOI] [PubMed] [Google Scholar]
- 20.Meneghini R.M., Pierson J.L., Bagsby D., Ziemba-Davis M., Berend M.E., Ritter M.A. Is there a functional benefit to obtaining high flexion after total knee arthroplasty? J Arthroplast. 2007;22:43–46. doi: 10.1016/j.arth.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Devers B.N., Conditt M.A., Jamieson M.L., Driscoll M.D., Noble P.C., Parsley B.S. Does greater knee flexion increase patient function and satisfaction after total knee arthroplasty? J Arthroplast. 2011;26:178–186. doi: 10.1016/j.arth.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Papalia R., Zampogna B., Torre G., Lanotte A., Vasta S., Albo E., Tecame A., Denaro V. Sarcopenia and its relationship with osteoarthritis: Risk factor or direct consequence? Musculoskelet Surg. 2014;98:9–14. doi: 10.1007/s12306-014-0311-6. [DOI] [PubMed] [Google Scholar]
- 23.Karlsson M., Magnusson H., Cöster M., Karlsson C., Rosengren B. Patients With Knee Osteoarthritis Have a Phenotype With Higher Bone Mass, Higher Fat Mass, and Lower Lean Body Mass. Clin. Orthop. Relat. Res. 2015;473:258–264. doi: 10.1007/s11999-014-3973-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kemnitz J., Wirth W., Eckstein F., Culvenor A. The role of thigh muscle and adipose tissue in knee osteoarthritis progression in women: Data from the Osteoarthritis Initiative. Osteoarthr. Cartil. 2018;26:1190–1195. doi: 10.1016/j.joca.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 25.Jin W.S., Choi E.J., Lee S.Y., Bae E.J., Lee T.H., Park J. Relationships among Obesity, Sarcopenia, and Osteoarthritis in the Elderly. J. Obes. Metab. Syndr. 2017;26:36–44. doi: 10.7570/jomes.2017.26.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim B.S., Lee S.Y., Kim B.R., Choi J.H., Kim S.R., Lee H.J., Lee S.J. Associations Between Obesity with Low Muscle Mass and Physical Function in Patients with End-Stage Knee Osteoarthritis. Geriatr. Orthop. Surg. Rehabil. 2021;12:21514593211020700. doi: 10.1177/21514593211020700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godziuk K., Prado C., Woodhouse L., Forhan M. Prevalence of sarcopenic obesity in adults with end-stage knee osteoarthritis. Osteoarthr. Cartil. 2019;27:1735–1745. doi: 10.1016/j.joca.2019.05.026. [DOI] [PubMed] [Google Scholar]
- 28.Rossi A.P., Urbani S., Fantin F., Nori N., Brandimarte P., Martini A., Zoico E., Mazzali G., Babbanini A., Muollo V., et al. Worsening Disability and Hospitalization Risk in Sarcopenic Obese and Dynapenic Abdominal Obese: A 5.5 Years Follow-Up Study in Elderly Men and Women. Front. Endocrinol. 2020;11:314. doi: 10.3389/fendo.2020.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao Q., Mei F., Shang Y., Hu K., Chen F., Zhao L., Ma B. Global prevalence of sarcopenic obesity in older adults: A systematic review and meta-analysis. Clin. Nutr. 2021;40:4633–4641. doi: 10.1016/j.clnu.2021.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Misra D., Fielding R.A., Felson D.T., Niu J., Brown C., Nevitt M., Lewis C.E., Torner J., Neogi T., the MOST study Risk of Knee Osteoarthritis with Obesity, Sarcopenic Obesity, and Sarcopenia. Arthritis Rheumatol. 2019;71:232–237. doi: 10.1002/art.40692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee S., Kim T.N., Kim S.-H. Sarcopenic obesity is more closely associated with knee osteoarthritis than is nonsarcopenic obesity: A cross-sectional study. Arthritis Rheum. 2012;64:3947–3954. doi: 10.1002/art.37696. [DOI] [PubMed] [Google Scholar]
- 32.Jeanmaire C., Mazières B., Verrouil E., Bernard L., Guillemin F., Rat A.-C. Body composition and clinical symptoms in patients with hip or knee osteoarthritis: Results from the KHOALA cohort. Semin. Arthritis Rheum. 2018;47:797–804. doi: 10.1016/j.semarthrit.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy D.M., Hanna S.E., Stratford P., Wessel J., Gollish J.D. Preoperative Function and Gender Predict Pattern of Functional Recovery After Hip and Knee Arthroplast. J. Arthroplast. 2006;21:559–566. doi: 10.1016/j.arth.2005.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fortin P.R., Clarke A.E., Joseph L., Liang M.H., Tanzer M., Ferland D., Phillips C., Partridge A.J., Fossel A.H., Mahomed N., et al. Outcomes of total hip and knee replacement: Preoperative functional status predicts outcomes at six months after surgery. Arthritis Rheum. 1999;42:1722–1728. doi: 10.1002/1529-0131(199908)42:8<1722::AID-ANR22>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 35.Parent É., Moffet H. Preoperative predictors of locomotor ability two months after total knee arthroplasty for severe osteoarthritis. Arthritis Rheum. 2003;49:36–50. doi: 10.1002/art.10906. [DOI] [PubMed] [Google Scholar]
- 36.Hopkins S.J., Toms A.D., Brown M., Welsman J.R., Ukoumunne O.C., Knapp K.M. A study investigating short- and medium-term effects on function, bone mineral density and lean tissue mass post-total knee replacement in a Caucasian female post-menopausal population: Implications for hip fracture risk. Osteoporos. Int. 2016;27:2567–2576. doi: 10.1007/s00198-016-3546-2. [DOI] [PubMed] [Google Scholar]
- 37.Kitsuda Y., Tanimura C., Inoue K., Park D., Osaki M., Hagino H. Effectiveness of ultrasonographic skeletal muscle assessment in patients after total knee arthroplast. Osteoporos. Sarcopenia. 2019;5:94–101. doi: 10.1016/j.afos.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mizner R.L., Petterson S.C., Stevens J.E., Vandenborne K., Snyder-Mackler L. Early quadriceps strength loss after total knee Arthroplast. The contributions of muscle atrophy and failure of voluntary muscle activation. J. Bone Jt. Surg. Am. 2005;87:1047–1053. doi: 10.2106/JBJS.D.01992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petterson S.C., Barrance P., Marmon A.R., Handling T., Buchanan T.S., Snyder-Mackler L. Time Course of Quad Strength, Area, and Activation after Knee Arthroplasty and Strength Training. Med. Sci. Sports Exerc. 2011;43:225–231. doi: 10.1249/MSS.0b013e3181eb639a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rasch A., Byström A.H., Dalén N., Martinez-Carranza N., Berg H.E. Persisting muscle atrophy two years after replacement of the hip. J. Bone Jt. Surgy. Br. Vol. 2009;91:583–588. doi: 10.1302/0301-620X.91B5.21477. [DOI] [PubMed] [Google Scholar]
- 41.Reardon K., Galea M., Dennett X., Choong P., Byrne E. Quadriceps muscle wasting persists 5 months after total hip arthroplasty for osteoarthritis of the hip: A pilot study. Intern. Med. J. 2001;31:7–14. doi: 10.1046/j.1445-5994.2001.00007.x. [DOI] [PubMed] [Google Scholar]
- 42.Godziuk K., Prado C.M., Woodhouse L.J., Forhan M. The impact of sarcopenic obesity on knee and hip osteoarthritis: A scoping review. BMC Musculoskelet Disord. 2018;19:271. doi: 10.1186/s12891-018-2175-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wanaratna K., Muangpaisan W., Kuptniratsaikul V., Chalermsri C., Nuttamonwarakul A. Prevalence and Factors Associated with Frailty and Cognitive Frailty among Community-Dwelling Elderly with Knee Osteoarthritis. J. Community Health. 2019;44:587–595. doi: 10.1007/s10900-018-00614-5. [DOI] [PubMed] [Google Scholar]
- 44.Courtney P.M., Boniello A.J., Berger R.A. Complications Following Outpatient Total Joint Arthroplasty: An Analysis of a National Database. J. Arthroplast. 2017;32:1426–1430. doi: 10.1016/j.arth.2016.11.055. [DOI] [PubMed] [Google Scholar]
- 45.Gu A., Malahias M.-A., Strigelli V., Nocon A.A., Sculco T.P., Sculco P.K. Preoperative Malnutrition Negatively Correlates With Postoperative Wound Complications and Infection after Total Joint Arthroplasty: A Systematic Review and Meta-Analysis. J. Arthroplast. 2019;34:1013–1024. doi: 10.1016/j.arth.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Hanada M., Hotta K., Matsuyama Y. Prognostic nutritional index as a risk factor for aseptic wound complications after total knee arthroplast. J. Orthop. Sci. 2020;26:827–830. doi: 10.1016/j.jos.2020.07.019. [DOI] [PubMed] [Google Scholar]
- 47.Liao C.-D., Huang Y.-C., Lin L.-F., Huang S.-W., Liou T.-H. Body Mass Index and Functional Mobility Outcome Following Early Rehabilitation after a Total Knee Replacement: A Retrospective Study in Taiwan. Arthritis Rheum. 2015;67:799–808. doi: 10.1002/acr.22474. [DOI] [PubMed] [Google Scholar]
- 48.Von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 49.Abizanda Soler P., Paterna Mellinas G., Martinez Sanchez E., Lopez Jimenez E. Comorbidity in the elderly: Utility and validity of assessment tools. Rev. Espanola. Geriatr. Gerontol. 2010;45:219–228. doi: 10.1016/j.regg.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 50.Valderas J.M., Starfield B., Sibbald B., Salisbury C., Roland M. Defining Comorbidity: Implications for Understanding Health and Health Services. Ann. Fam. Med. 2009;7:357–363. doi: 10.1370/afm.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sinclair S.T., Emara A.K., Orr M.N., McConaghy K.M., Klika A.K., Piuzzi N.S. Comorbidity indices in orthopaedic surgery: A narrative review focused on hip and knee Arthroplast. EFORT Open Rev. 2021;6:629–640. doi: 10.1302/2058-5241.6.200124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaiser M.J., MNA-International Group. Bauer J.M., Ramsch C., Uter W., Guigoz Y., Cederholm T., Thomas D.R., Anthony P., Charlton K., et al. Validation of the Mini Nutritional Assessment short-form (MNA®-SF): A practical tool for identification of nutritional status. J. Nutr. Health Aging. 2009;13:782–788. doi: 10.1007/s12603-009-0214-7. [DOI] [PubMed] [Google Scholar]
- 53.Rubenstein L.Z., Harker J.O., Salvà A., Guigoz Y., Vellas B. Screening for Undernutrition in Geriatric Practice: Developing the Short-Form Mini-Nutritional Assessment (MNA-SF) J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2001;56:M366–M372. doi: 10.1093/gerona/56.6.M366. [DOI] [PubMed] [Google Scholar]
- 54.Zhang X., Zhang X., Zhu Y., Tao J., Zhang Z., Zhang Y., Wang Y., Ke Y., Ren C., Xu J. Predictive Value of Nutritional Risk Screening 2002 and Mini Nutritional Assessment Short Form in Mortality in Chinese Hospitalized Geriatric Patients. Clin. Interv. Aging. 2020;15:441–449. doi: 10.2147/CIA.S244910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lilamand M., The Toulouse Frailty Platform Team. Kelaiditi E., Cesari M., Raynaud-Simon A., Ghisolfi A., Guyonnet S., Vellas B., Van Kan G.A. Validation of the Mini Nutritional Assessment-Short Form in a population of frail elders without disability. Analysis of the Toulouse Frailty Platform population in 2013. J. Nutr. Health Aging. 2015;19:570–574. doi: 10.1007/s12603-015-0457-4. [DOI] [PubMed] [Google Scholar]
- 56.Soysal P., Veronese N., Arik F., Kalan U., Smith L., Isik A.T. Mini Nutritional Assessment Scale-Short Form can be useful for frailty screening in older adults. Clin. Interv. Aging. 2019;14:693–699. doi: 10.2147/CIA.S196770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weir C.B., Jan A. BMI Classification Percentile and Cut off Points. StatPearls Publishing LLC.; Treasure Island, FL, USA: 2021. [PubMed] [Google Scholar]
- 58.Wen X., Wang M., Jiang C.-M., Zhang Y.-M. Anthropometric equation for estimation of appendicular skeletal muscle mass in Chinese adults. Asia Pac. J. Clin. Nutr. 2011;20:551–556. [PubMed] [Google Scholar]
- 59.Wu X., Li X., Xu M., Zhang Z., He L., Li Y. Sarcopenia prevalence and associated factors among older Chinese population: Findings from the China Health and Retirement Longitudinal Study. PLoS ONE. 2021;16:e0247617. doi: 10.1371/journal.pone.0247617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang M., Hu X., Wang H., Zhang L., Hao Q., Dong B. Sarcopenia predicts readmission and mortality in elderly patients in acute care wards: A prospective study. J. Cachex-Sarcopenia Muscle. 2016;8:251–258. doi: 10.1002/jcsm.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen L.-K., Woo J., Assantachai P., Auyeung T.-W., Chou M.-Y., Iijima K., Jang H.C., Kang L., Kim M., Kim S., et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med Dir. Assoc. 2020;21:300–307.e2. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 62.Mehta S., Rigney A., Webb K., Wesney J., Stratford P.W., Shuler F.D., Oliashirazi A. Characterizing the recovery trajectories of knee range of motion for one year after total knee replacement. Physiother. Theory Pr. 2018;36:176–185. doi: 10.1080/09593985.2018.1482980. [DOI] [PubMed] [Google Scholar]
- 63.McClure G., Frisch N.B. Range of Motion (ROM) after Knee Replacement Surgery: The Basics. [(accessed on 5 July 2017)]. Available online: https://peerwell.co/blog/range-of-motion-after-knee-replacement/
- 64.Lenssen A.F., Van Dam E.M., Crijns Y.H., Verhey M., Geesink R.J., Brandt P.A.V.D., De Bie R.A. Reproducibility of goniometric measurement of the knee in the in-hospital phase following total knee Arthroplast. BMC Musculoskelet Disord. 2007;8:83. doi: 10.1186/1471-2474-8-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hyodo K., Masuda T., Aizawa J., Jinno T., Morita S. Hip, knee, and ankle kinematics during activities of daily living: A cross-sectional study. Braz. J. Phys. Ther. 2017;21:159–166. doi: 10.1016/j.bjpt.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pfeiffer J.L., Zhang S., Milner C.E. Knee biomechanics during popular recreational and daily activities in older men. Knee. 2014;21:683–687. doi: 10.1016/j.knee.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 67.Rowe P.J., Myles C.M., Walker C., Nutton R. Knee joint kinematics in gait and other functional activities measured using flexible electrogoniometry: How much knee motion is sufficient for normal daily life? Gait Posture. 2000;12:143–155. doi: 10.1016/S0966-6362(00)00060-6. [DOI] [PubMed] [Google Scholar]
- 68.Hoogeboom T.J., Van Meeteren N.L.U., Kim R.H., Stevens-Lapsley J.E. Linear and Curvilinear Relationship between Knee Range of Motion and Physical Functioning in People with Knee Osteoarthritis: A Cross-Sectional Study. PLoS ONE. 2013;8:e76173. doi: 10.1371/journal.pone.0076173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Farahini H., Moghtadaei M., Bagheri A., Akbarian E. Factors Influencing Range of Motion after Total Knee Arthroplast. Iran. Red Crescent Med. J. 2012;14:417–421. [PMC free article] [PubMed] [Google Scholar]
- 70.Hewlett-Smith N.A., Pope R.P., Hing W.A., Simas V.P., Furness J.W. Patient and surgical prognostic factors for inpatient functional recovery following THA and TKA: A prospective cohort study. J. Orthop. Surg. Res. 2020;15:1–19. doi: 10.1186/s13018-020-01854-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alzahrani K., Gandhi R., Debeer J., Petruccelli D., Mahomed N. Prevalence of Clinically Significant Improvement Following Total Knee Replacement. J. Rheumatol. 2011;38:753–759. doi: 10.3899/jrheum.100233. [DOI] [PubMed] [Google Scholar]
- 72.Ritter M.A., Harty L.D., Davis K.E., Meding J.B., Berend M.E. Predicting range of motion after total knee arthroplast. JBJS. 2003;85:1278–1285. doi: 10.2106/00004623-200307000-00014. [DOI] [PubMed] [Google Scholar]
- 73.Parsley B.S., Bertolusso R., Harrington M., Brekke A., Noble P.C. Influence of Gender on Age of Treatment with TKA and Functional Outcome. Clin. Orthop. Relat. Res. 2010;468:1759–1764. doi: 10.1007/s11999-010-1348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Naylor J.M., Ko V., Rougellis S., Green N., Mittal R., Heard R., Yeo A.E.T., Barnett A., Hackett D., Saliba C., et al. Is discharge knee range of motion a useful and relevant clinical indicator after total knee replacement? Part 2. J. Eval. Clin. Pr. 2011;18:652–658. doi: 10.1111/j.1365-2753.2011.01656.x. [DOI] [PubMed] [Google Scholar]
- 75.Elmallah R.D.K., Cherian J.J., Robinson K., Harwin S.F., Mont M.A. The Effect of Comorbidities on Outcomes following Total Knee Arthroplast. J. Knee Surg. 2015;28:411–416. doi: 10.1055/s-0035-1549023. [DOI] [PubMed] [Google Scholar]
- 76.Podmore B., Hutchings A., Van Der Meulen J., Aggarwal A., Konan S. Impact of comorbid conditions on outcomes of hip and knee replacement surgery: A systematic review and meta-analysis. BMJ Open. 2018;8:e021784. doi: 10.1136/bmjopen-2018-021784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harvey I.A., Barry K., Kirby S.P., Johnson R., Elloy M.A. Factors affecting the range of movement of total knee Arthroplast. J. Bone Jt. Surg. 1993;75:950–955. doi: 10.1302/0301-620X.75B6.8245090. [DOI] [PubMed] [Google Scholar]
- 78.Kawamura H., Bourne R.B. Factors affecting range of flexion after total knee Arthroplast. J. Orthop. Sci. 2001;6:248–252. doi: 10.1007/s007760100043. [DOI] [PubMed] [Google Scholar]
- 79.Fatima M., Scholes C., Tutty A., Ebrahimi M., Genon M., Martin S.J. Outcomes following short hospital stay after total knee replacement in a regional setting: A prospective analysis of an observational cohort in a public hospital treated 2018–2019. medRxiv. 2020 medRxiv: 2020.2003.2008.20031989. [Google Scholar]
- 80.Font-Vizcarra L., Lozano L., Ríos J., Forga M.T., Soriano A. Preoperative Nutritional Status and Post-Operative Infection in Total Knee Replacements: A Prospective Study of 213 Patients. Int. J. Artif. Organs. 2011;34:876–881. doi: 10.5301/ijao.5000025. [DOI] [PubMed] [Google Scholar]
- 81.Alfargieny R., Bodalal Z., Bendardaf R., El-Fadli M., Langhi S. Nutritional status as a predictive marker for surgical site infection in total joint Arthroplast. Avicenna J. Med. 2015;5:117–122. doi: 10.4103/2231-0770.165122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lavernia C.J., Sierra R.J., Baerga L. Nutritional parameters and short term outcome in Arthroplast. J. Am. Coll. Nutr. 1999;18:274–278. doi: 10.1080/07315724.1999.10718863. [DOI] [PubMed] [Google Scholar]
- 83.Cheng Q., Zhu X., Zhang X., Li H., Du Y., Hong W., Xue S., Zhu H. A cross-sectional study of loss of muscle mass corresponding to sarcopenia in healthy Chinese men and women: Reference values, prevalence, and association with bone mass. J. Bone Miner. Metab. 2013;32:78–88. doi: 10.1007/s00774-013-0468-3. [DOI] [PubMed] [Google Scholar]
- 84.Liu L.-K., Lee W.-J., Liu C.-L., Chen L.-Y., Lin M.-H., Peng L.-N. Age-related skeletal muscle mass loss and physical performance in Taiwan: Implications to diagnostic strategy of sarcopenia in Asia. Geriatr. Gerontol. Int. 2013;13:964–971. doi: 10.1111/ggi.12040. [DOI] [PubMed] [Google Scholar]
- 85.Singh V., Yeroushalmi D., Lygrisse K.A., Simcox T., Long W.J., Schwarzkopf R. The influence of obesity on achievement of a ‘forgotten joint’ following total knee Arthroplast. Arch. Orthop. Trauma Surg. 2021:1–9. doi: 10.1007/s00402-021-03840-0. [DOI] [PubMed] [Google Scholar]
- 86.Singh V., Yeroushalmi D., Lygrisse K.A., Schwarzkopf R., Davidovitch R.I. Impact of Obesity on the Forgotten Joint Score Following Primary Total Hip Arthroplast. J. Arthroplast. 2020;36:1342–1347. doi: 10.1016/j.arth.2020.10.027. [DOI] [PubMed] [Google Scholar]
- 87.Husted H., Jørgensen C.C., Gromov K., Kehlet H. Does BMI influence hospital stay and morbidity after fast-track hip and knee arthroplasty? Acta Orthop. 2016;87:466–472. doi: 10.1080/17453674.2016.1203477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Raut S., Mertes S.C., Muniz-Terrera G., Khanduja V. Factors associated with prolonged length of stay following a total knee replacement in patients aged over 75. Int. Orthop. 2012;36:1601–1608. doi: 10.1007/s00264-012-1538-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kemmler W., Teschler M., Goisser S., Bebenek M., Von Stengel S., Bollheimer L.C., Sieber C.C., Freiberger E. Prevalence of sarcopenia in Germany and the corresponding effect of osteoarthritis in females 70 years and older living in the community: Results of the FORMoSA study. Clin. Interv. Aging. 2015;10:1565–1573. doi: 10.2147/CIA.S89585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Erturk C., Altay M.A., Sert C., Levent A., Yaptı M., Yüce K. The body composition of patients with knee osteoarthritis: Relationship with clinical parameters and radiographic severity. Aging Clin. Exp. Res. 2015;27:673–679. doi: 10.1007/s40520-015-0325-4. [DOI] [PubMed] [Google Scholar]
- 91.Ho K.K.-W., Lau L.C.-M., Chau W.-W., Poon Q., Chung K.-Y., Wong R.M.-Y. End-stage knee osteoarthritis with and without sarcopenia and the effect of knee arthroplasty—A prospective cohort study. BMC Geriatr. 2021;21:1–11. doi: 10.1186/s12877-020-01929-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shyam A.K., Joshi R., Patil K., Jain A., Sancheti K.H., Sancheti P.K. Factors affecting range of motion in total knee arthroplasty using high flexion prosthesis: A prospective study. Indian J. Orthop. 2013;47:50–56. doi: 10.4103/0019-5413.106901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Järvenpää J., Kettunen J., Kröger H., Miettinen H. Obesity May Impair the Early Outcome of Total Knee Arthroplasty a Prospective Study of 100 Patients. Scand. J. Surg. 2010;99:45–49. doi: 10.1177/145749691009900110. [DOI] [PubMed] [Google Scholar]
- 94.Gadinsky N.E., Ehrhardt J.K., Urband C., Westrich G.H. Effect of Body Mass Index on Range of Motion and Manipulation After Total Knee Arthroplast. J. Arthroplast. 2011;26:1194–1197. doi: 10.1016/j.arth.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 95.Shoji H., Solomonow M., Yoshino S., D’Ambrosia R., Dabezies E. Factors affecting postoperative flexion in total knee arthroplast. Orthopedics. 1990;13:643–649. doi: 10.3928/0147-7447-19900601-08. [DOI] [PubMed] [Google Scholar]
- 96.Järvenpää J., Kettunen J., Soininvaara T., Miettinen H., Kröger H. Obesity Has a Negative Impact on Clinical Outcome after Total Knee Arthroplast. Scand. J. Surg. 2012;101:198–203. doi: 10.1177/145749691210100310. [DOI] [PubMed] [Google Scholar]
- 97.Lizaur A., Marco L., Cebrian R., Lizaur-Utrilla A. Preoperative factors influencing the range of movement after total knee arthroplasty for severe osteoarthritis. J. Bone Jt. Surg. Br. Vol. 1997;79:626–629. doi: 10.1302/0301-620X.79B4.0790626. [DOI] [PubMed] [Google Scholar]
- 98.Liao C.-D., Chen H.-C., Huang S.-W., Liou T.-H. Impact of sarcopenia on rehabilitation outcomes after total knee replacement in older adults with knee osteoarthritis. Ther. Adv. Musculoskelet Dis. 2021;13:1759720X21998508. doi: 10.1177/1759720X21998508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bell J., Bauer J., Capra S., Pulle R.C. Concurrent and predictive evaluation of malnutrition diagnostic measures in hip fracture inpatients: A diagnostic accuracy study. Eur. J. Clin. Nutr. 2014;68:358–362. doi: 10.1038/ejcn.2013.276. [DOI] [PubMed] [Google Scholar]
- 100.Bell J.J., Bauer J., Capra S., Pulle R.C. Quick and Easy Is Not without Cost: Implications of Poorly Performing Nutrition Screening Tools in Hip Fracture. J. Am. Geriatr. Soc. 2014;62:237–243. doi: 10.1111/jgs.12648. [DOI] [PubMed] [Google Scholar]
- 101.Aghdam A.R.M., Kolahi S., Hasankhani H., Behshid M., Varmaziar Z. The relationship between pain and physical function in adults with Knee Osteoarthritis. Int. Res. J. Appl. Basic Sci. 2013;4:1102–1106. [Google Scholar]
- 102.Pisters M., Veenhof C., van Dijk G., Heymans M., Twisk J., Dekker J. The course of limitations in activities over 5 years in patients with knee and hip osteoarthritis with moderate functional limitations: Risk factors for future functional decline. Osteoarthr. Cartil. 2012;20:503–510. doi: 10.1016/j.joca.2012.02.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. Public data sharing is not applicable to this article due to ethical considerations and privacy restrictions.