To the editor,
Primary mediastinal large B cell lymphoma (PMLBCL) is a distinct subtype of diffuse large B cell lymphoma (DLBCL) of putative thymic-B cell origin. RDA-EPOCH (rituximab, etoposide, doxorubicin, cyclophosphamide, vincristine and prednisone) regimen is commonly used to treat PMLBCL. Rituximab acts by antibody-dependent cell-mediated cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC) and induces apoptosis. Brentuximab vedotin is an antibody drug conjugate that consists of anti-CD30 chimeric monoclonal antibody linked to the antimitotic agent monomethyl auristatin E (MMAE). Brentuximab induces responses in relapsed/ refractory diffuse large B cell lymphomas and T cell lymphomas but it has a lower response rate in PMBCL [1]. Anti-CD19 chimeric antigen receptors (CAR) consist of a FMC63 anti-CD19 single chain variable fragment linked to CD28 costimulatory and TCRζ chain signaling domains [2]. CD19-directed CARs (CART-19) induces sustained remissions in chronic lymphocytic leukemia and B lymphoblastic leukemia (B-ALL). CART-19 has proven effective in adult DLBCL and has shown limited responses in adult PMLBCL, inducing remission in one out of three treated patients [3]. Here we report on a case of pediatric PMLBCL treated who relapsed with antigen negative disease after targeted immune therapy.
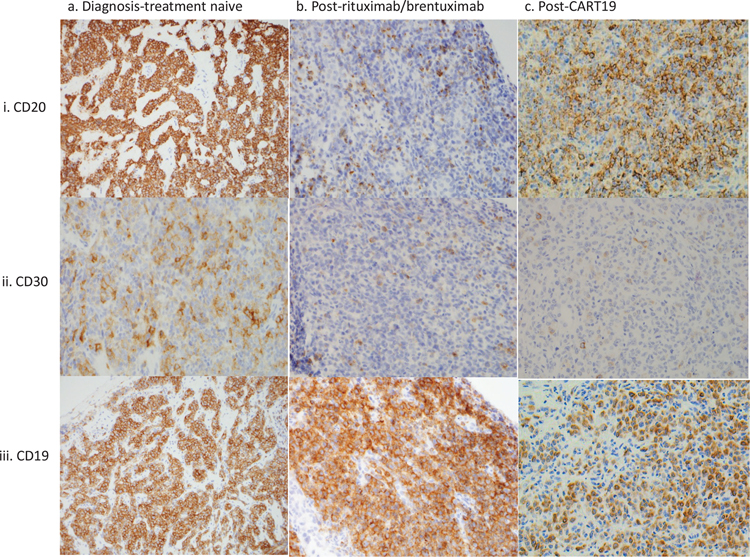
A 12 year old female with no significant past medical history presented with cough, tachycardia, weight loss and a solitary 10 cm anterior mediastinal mass. Biopsy showed diffuse infiltration by large mature B cells (CD20+, CD19+, CD30subset+) with abundant cytoplasm and delicate fibrosis consistent with involvement by PMLBCL (Figure 1; column a). The patient was treated with RDA-EPOCH. Upon completion of 6 cycles of chemotherapy, PET imaging showed a 7.4 × 8.5 × 7.1 cm mass that was confirmed by biopsy to be active PMLBCL. Salvage was attempted with rituximab and high dose methotrexate followed by high-dose cytarabine, dexamethasone, platinol and rituximab (R-DHAP) as well as proton radiation. Follow up imaging revealed new renal and pancreatic lesions that were confirmed by biopsy to be additional involvement by PMLBCL which was now negative for CD20 (Figure 1; column b, row i). Treatment with brentuximab and local radiation to the pancreatic and renal lesion was unsuccessful in halting the progression of disease. A subsequent biopsy showed predominantly CD30-negative PMLBCL (Figure 1; column b, row ii). She was started on CART-19 therapy and showed adequate expansion of CAR T cells and expected B cell aplasia. However, two months after CART-19 infusion, she developed renal insufficiency and a renal biopsy revealed persistent involvement by PMLBCL that was remarkable for absence of surface CD19 by flow cytometry but positive for cytoplasmic expression by immunohistochemistry (Figure 2). Interestingly, CD20 and CD30 were positive in a subset of these tumor cells (Figure 1 column c row ii). Flow cytometry and immunohistochemistry studies on the biopsy showed increased CD3+, CD4 and CD8 double positive cells that are consistent with infiltration of the tumor by activated CART cells. Due to progression of disease despite CART-19 therapy, the patient transitioned into hospice and passed away a few days later.
Figure 1. Target antigen expression at various treatment time points.
CD20, CD30 and CD19 expression at diagnosis, post-rituximab or post-brentuximab/pre-CART19 and Post-CART19 infusion are shown. CD20 and CD30 are lost after rituximab and brentuximab respectively while CD19 expression changes after CART-19 therapy. The cytology and morphology of tumor was similar across the three time points.
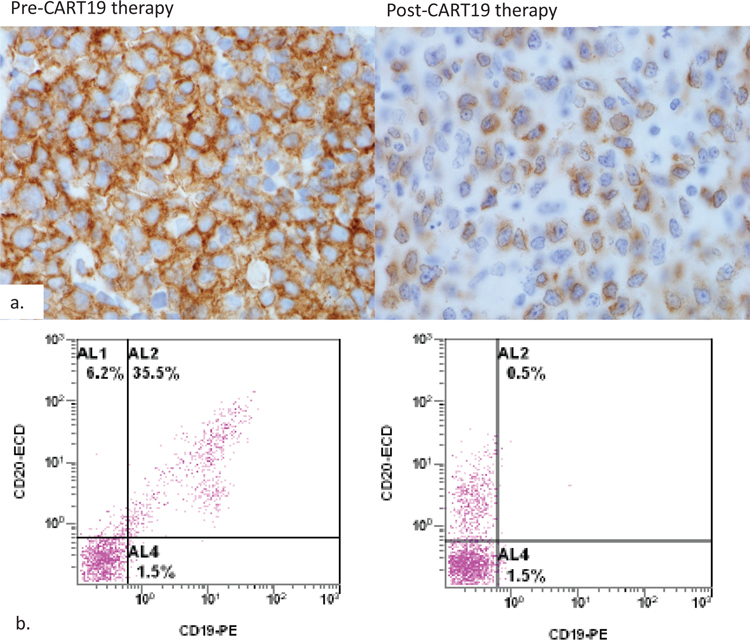
Figure 2. Loss of membrane expression of CD19 post CART19 therapy.
CD19 immunostain and flow cytometric data from pre and post-CART therapy biopsies are shown. CD19 expression changes from strong membranous pattern to dim cytoplasm pattern (panel a). Flow cytometric evaluation of surface CD19 reveals loss of CD19 but intact CD20 after CART therapy (panel b).
Sequencing of the CD19 gene in the tumor identified a novel heterozygous missense mutation (G210D) in exon 4 that substitutes glycine, a highly conserved amino acid localized in the extracellular domain of CD19, with a negatively charged aspartic acid (supplemental figure 1A and 1B). Mutations replacing this residue are predicted to be extremely damaging by polyphen-2 and SIFT computational protein prediction models (Suppl. Fig. 1C and 1D). This mutation was present in the pre and post-CART samples suggesting it was a pre-existing clone that was likely selected under CART-19 treatment pressure. In order to understand how this patient’s tumor was able to evade multiple treatment regimens, we performed genome-wide SNP array on relapse sample. PMLBCL usually show gains in chromosome 9p24, (75%), 2p15(50%), XP11.4–21 (33%) and Xq2–26(33%). In the present case, we detected abundant chromosomal alterations, including loss of parts of 2p, 6p, 20q, 22q and gain of an X. The 2p region lost contains MSH2 and MSH6 loci that, along with MLH1, MLH3, MSH3, PMS1 and PMS2 genes, work coordinately in sequential steps to initiate repair of DNA mismatch. Immunostaining for mismatch repair proteins confirmed the loss of MSH2 and MSH6 expression with intact MLH1 and PMS2 expression (supplemental figure 2). These findings raise the possibility that the extreme mutability of this patient’s tumor is related to deficiency of DNA repair genes. There is no suspicion of Lynch syndrome here since the mutations are somatic and there is no patient or family history of colorectal/endometrial cancers. DNA repair genes are known to be mutated in diffuse large B cell lymphomas [4] and in lymphomas non-responsive to chemotherapy suggesting that mutations in DNA repair genes could be the cause of their refractoriness to therapy.
This patient’s tumor was remarkably refractory to treatment and showed antigen escape under immunotherapy. Despite the different mechanisms of action of the immunotherapy agents, target antigen loss is a common mechanism of immune escape. CD20 downregulation after rituximab occurs by multiple mechanisms including mutations in CD20, changes in binding region and shaving of antigen [5]. Dynamics of CD30 expression has not been evaluated extensively in relapsed DLBCL patients after brentuximab. Failure of treatment after CART-19 therapy is either due to failure of CART cells to expand/function or due to loss of CD19 expression on the surface of the tumor cells. T molecular mechanisms underlying loss of CD19 in B-ALL is multifactorial and includes mutations in CD19 and alternative splicing [6]. In many of those cases, mRNA of alternatively spliced CD19 can be detected in relapse samples and, while CD19 protein cannot be detected by flow cytometry, alternative versions of the protein are evidenced by western blot. None of the previously described alternative isoforms of CD19 were found in the patient’s tumor. CAR treatment pressure can differentiation of CLL to a CD19-negative plasmablastic lymphoma [7] or B-ALL to CD19-negative leukemia with myeloid phenotype [8]. Our case is the first report of CD19 membrane negative (but dim cytoplasmic expression) relapse after CART-19 therapy in PMLBCL. The findings suggest that both cytoplasmic and membranous expression should be evaluated by flow cytometry and immunohistochemistry. Our findings also suggest that loss of DNA repair proteins could be a cause of extreme mutability of lymphomas. A combination of immunotherapeutic agents might needed to eradicate the tumor as with chemotherapy regimens.
Supplementary Material
References
- 1.Jacobsen ED, Sharman JP, Oki Y, et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood 2015;125:1394–1402. [DOI] [PubMed] [Google Scholar]
- 2.Maus MV, Grupp SA, Porter DL, et al. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood 2014;123:2625–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 2015;33:540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Miranda NF, Peng R, Georgiou K, et al. DNA repair genes are selectively mutated in diffuse large B cell lymphomas. J Exp Med 2013;210:1729–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez-Callejo D, Gonzalez-Rincon J, Sanchez A, et al. Action and resistance of monoclonal CD20 antibodies therapy in B-cell Non-Hodgkin Lymphomas. Cancer Treat Rev 2015;41:680–689. [DOI] [PubMed] [Google Scholar]
- 6.Sotillo E, Barrett DM, Black KL, et al. Convergence of Acquired Mutations and Alternative Splicing of CD19 Enables Resistance to CART-19 Immunotherapy. Cancer Discov 2015;5:1282–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans AG, Rothberg PG, Burack WR, et al. Evolution to plasmablastic lymphoma evades CD19-directed chimeric antigen receptor T cells. Br J Haematol 2015;171:205–209. [DOI] [PubMed] [Google Scholar]
- 8.Jacoby E, Nguyen SM, Fountaine TJ, et al. CD19 CAR immune pressure induces B-precursor acute lymphoblastic leukaemia lineage switch exposing inherent leukaemic plasticity. Nat Commun 2016;7:12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.