Abstract
Cytotoxic necrotizing factor 1 (CNF1) is a bacterial virulence factor, the target of which is represented by Rho GTPases, small proteins involved in a huge number of crucial cellular processes. CNF1, due to its ability to modulate the activity of Rho GTPases, represents a widely used tool to unravel the role played by these regulatory proteins in different biological processes. In this review, we summarized the data available in the scientific literature concerning the observed in vitro effects induced by CNF1. An article search was performed on electronic bibliographic resources. Screenings were performed of titles, abstracts, and full-texts according to PRISMA guidelines, whereas eligibility criteria were defined for in vitro studies. We identified a total of 299 records by electronic article search and included 76 original peer-reviewed scientific articles reporting morphological or biochemical modifications induced in vitro by soluble CNF1, either recombinant or from pathogenic Escherichia coli extracts highly purified with chromatographic methods. Most of the described CNF1-induced effects on cultured cells are ascribable to the modulating activity of the toxin on Rho GTPases and the consequent effects on actin cytoskeleton organization. All in all, the present review could be a prospectus about the CNF1-induced effects on cultured cells reported so far.
Keywords: Rho GTP-binding proteins, cytotoxic necrotizing factor type 1, actin cytoskeleton, mitochondria, apoptosis, primary cell culture, transformed cell line, cancer cell line
1. Introduction
Cytotoxic necrotizing factor 1 (CNF1) is a bacterial virulence factor associated with some pathogenic Escherichia coli strains causing urinary tract infection and meningitis [1]. It belongs to the cytotoxic necrotizing factors family that includes proteins from E. coli (CNF1, CNF2, and CNF3) and Yersinia pseudotuberculosis (CNFY). CNF1 is an AB-type toxin, composed of a cell-binding domain and the C-terminal catalytic domain, bearing deamidase activity. The cell-binding domain encompasses two interaction sites in CNF1: an N-terminus domain, which interacts with the 37-kDa laminin receptor precursor (LRP), and a domain directly adjacent to the catalytic domain, which is a high affinity interaction site for the Lutheran (Lu) adhesion glycoprotein/basal cell adhesion molecule (BCAM) Lu/BCAM [2,3]. Following endocytosis, the catalytic domain of CNF1 is cleaved off and released into the cytosol [4]. The CNF1 target is represented by small GTPases belonging to the Rho family, Rho, Rac, and Cdc42. CNF1 deamidates a specific glutamine residue, located in the switch 2 domain and involved in GTP hydrolysis (glutamine 63 in RhoA [3,5,6] or 61 in Cdc42 and Rac1 [7]) and this modification results in the constitutive association of the Rho GTPase with GTP, namely, its constitutive activation.
Nonetheless, some of the Rho-regulated signaling pathways have been found to be only transiently activated. That is because, once constitutively activated by CNF1, Rho proteins are rapidly conveyed to the ubiquitin-mediated proteasomal degradation pathway [8,9]. Interestingly, degradation seems to be cell type-specific. For example, while in HUVECs, macrophages, keratinocytes, fibroblasts, and 804G cells, the three CNF1-activated GTPases undergo efficient ubiquitin-mediated proteasomal degradation, in HEp-2, Vero, and HEK293 cells the ubiquitination level of specific Rho proteins is quite low. In these cell lines, a specific absence of cellular depletion of Rho (Vero), Cdc42 (HEK293), and Rac (HEp-2) is shown, indicating the existence of three independent and differently expressed ubiquitination pathways for the three GTPases [8,10,11].
Rho GTPases are signaling nodes, regulated by diverse upstream extracellular stimuli, and interacting with a wide range of downstream effectors that initiate a number of cellular signaling cascades. Rho GTPases are mainly known for their role in the modulation of cytoskeletal dynamics and, as a consequence, of cell adhesion, migration, and endocytosis [12,13]. Rho controls actin stress fiber formation and contractile actomyosin bundles found in many cultured non-muscle cells and plays a central role in cell adhesion and morphogenesis. Rac regulates the formation of membrane ruffles, while Cdc42 is involved in the formation of filopodial extensions at the leading edge, both characteristic features of many actively migrating cells.
Beyond the involvement in direct regulation of the actin cytoskeleton, Rho GTPases play a key role in a huge number of crucial cellular processes, such as the regulation of transcription, cell polarity, cell cycle progression and inflammation [10]. They are also involved in physiological processes, including embryonic development [14], neuronal differentiation and neurite formation [15,16], maintenance of stem cells [17,18], and both innate and adaptive immune cell processes [19].
Hence, due to its ability to modulate the activity of Rho GTPases, CNF1 represents a widely used tool to unravel the role played by these regulatory proteins in several biological processes [20].
From a bacterial point of view, Rho GTPases, by activating a panel of effectors, confer to pathogens the ability to alter the architecture of host cells and tissues, thereby promoting their ability to evade host defenses and spread within and among hosts [21]. In this context, CNF1 has been investigated as a potential risk factor for cancer onset and/or progression, especially in those anatomical areas that naturally host E. coli pathogenic strains (colon, uroepithelial tract) [22,23].
The aim of this systematic review is to summarize the data available in the scientific literature concerning the observed in vitro effects induced by CNF1 in different cell lines of finite/primary, transformed/immortalized, and tumoral origin.
2. Materials and Methods
2.1. Literature Search Strategy
The search was oriented on original peer-reviewed scientific articles in which any CNF1-induced effect observed in in vitro studies was reported.
According to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [24], a systematic review of the published literature from inception to 30 June 2021 was performed in electronic bibliographic databases (US National Library of Medicine MEDLINE/PubMed, EMBASE, Web of Science, and Scopus). An internet-based search was also performed. The electronic search was conducted by three authors independently (F.C., Z.M., and A.F.) using a combination of keywords (“CNF1”, “primary cells”, “transformed cells”), according to the different search instructions and techniques adopted in each database (Figure S1).
Selected publications were compiled into a single database and duplicates removed.
Based on the inclusion/exclusion criteria, two authors independently (F.C. and S.T.) screened all the publication records identified and reviewed the abstracts to identify articles requiring an additional full-text review. The final decision was reached through consensus, solving discrepancies by discussion with a third author (A.F.).
Full-texts of the selected publications were obtained. The reference lists of the selected articles were also checked, in order to identify further works eligible for this study.
2.2. Inclusion Criteria
For this review, only original peer-reviewed scientific articles reporting morphological or biochemical modifications induced in vitro by soluble CNF1 (recombinant or from pathogenic E. coli extracts highly purified with chromatographic methods) were selected. Results related to both human and animal cells were included. Only English-written studies were included.
2.3. Exclusion Criteria
Irrelevant articles, letters to the editor, editorials, case reports, reviews, short communications, bioinformatic meta-analyses, and articles written in languages other than English were excluded. Papers focused on in vivo studies, on receptor studies or those in which pathogenic E. coli strains were used for infection, were not included, as well as CNF1-induced effects reported as “data not shown”.
2.4. Data Extraction
Two authors (F.C. and S.T., independently) screened the abstracts and the full-text versions of the selected articles to double-check their eligibility and achieve data extraction. The selected articles were further verified by two authors (C.F. and A.F.). Of the 239 articles examined, 76 met the eligibility criteria fixed in the present review.
The following information was obtained from each paper: cell line name, cell line, tissue origin, bibliographic references, summary of the observed effects ascribed to the toxin, and the type of CNF1 preparation used.
All eligible studies were grouped according to the cell line in which CNF1 effects have been examined: cancer, immortalized/transformed, and finite/primary.
3. Results
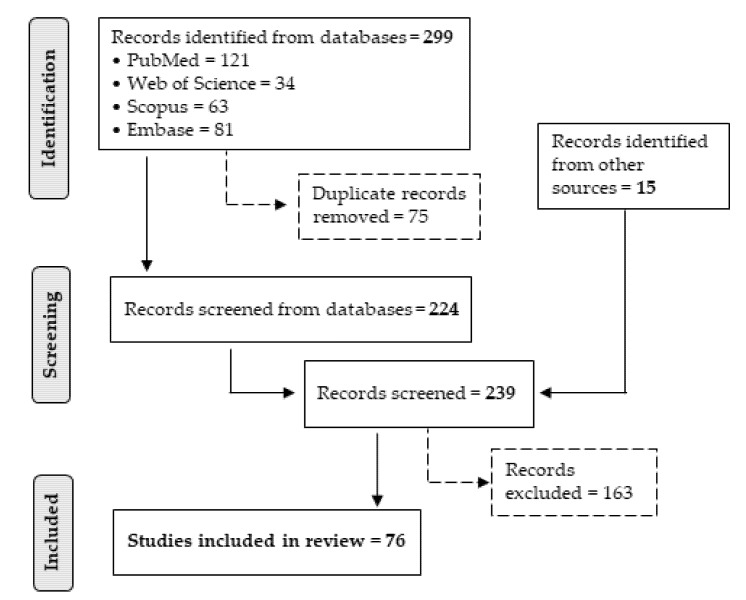
Using combinations of the keywords “CNF1”, “primary cells”, and “transformed cells” (Figure S1), a total of 299 studies were identified through literature research in the search engine, of which 75 were removed as duplicates. Fifteen records were identified from other sources. After screening the abstracts and full-text, 163 studies were excluded. The remaining 76 papers were finally assessed for eligibility.
The flow diagram in Figure 1 summarizes the selection process, showing the number of records passing through each step.
Figure 1.
PRISMA flow diagram. Flowchart of the selection process for the inclusion of studies.
The search identified 76 experimental studies focused on 74 cell lines.
In Table 1, Table 2 and Table 3, the observed effects induced by CNF1 toxin and the related references have been grouped together for each cell type origin i.e., cancer, immortalized/transformed, and finite/primary.
Table 1.
Cancer cell lines.
| Cell Line | Tissue Origin and Morphology | References | CNF1 Described Effects | CNF1 Preparation |
|---|---|---|---|---|
| T24 | hu bladder, carcinoma, epithelial | [11,25,26] * [27,28] § |
|
recombinant, * purified by chromatography § His-tagged protein |
| UMUC3 | hu bladder, carcinoma, epithelial | [28] | MMP-9 activity increase | recombinant, His-tagged protein |
| 5637 | hu bladder, carcinoma, epithelial | [26] * [27,28] § |
|
recombinant, * purified by chromatography § His-tagged protein |
| J82 | hu bladder, carcinoma, epithelial | [27] |
|
recombinant, His-tagged protein |
| SH-SY5Y | hu brain, neuroblastoma, epithelial | [29] |
|
recombinant, purified by chromatography |
| SK-N-SH | hu brain, neuroblastoma, epithelial | [30] |
|
recombinant, purified by chromatography |
| U87 | hu brain, glioblastoma like, epithelial | [31] |
|
recombinant, purified by chromatography |
| GBM (Glioblastoma) |
hu brain, glioblastoma multiforme | [31,32] |
|
recombinant, purified by chromatography |
| MCF7 | hu breast, ductal carcinoma, epithelial | [11] | Rho, Rac, and Cdc42 efficient depletion | recombinant, purified by chromatography |
| HeLa | hu cervix, adenocarcinoma, epithelial | [7,9,33,34,35,36,37,38] |
|
recombinant, GST fusion protein |
| Caco-2 | hu colon, adenocarcinoma, epithelial | [33,39,40] |
|
recombinant, GST fusion protein |
| SW480 | hu colon, adenocarcinoma, epithelial | [22] | enhanced migration and invasion | recombinant, purified by chromatography |
| SW620 | hu colon, adenocarcinoma, epithelial | [11] | Rho, Rac, and Cdc42 efficient depletion | recombinant, purified by chromatography |
| HT-29 | hu colon, adenocarcinoma, epithelial | [22] * [37] ‡ |
|
recombinant: * purified by chromatography ‡ GST fusion protein |
| T84 | hu colon, adenocarcinoma, epithelial | [41,42,43,44] |
|
recombinant, purified by chromatography |
| HCT-116 | hu colon carcinoma, epithelial | [45] |
|
recombinant, His-tagged protein |
| ACHN | hu kidney, adenocarcinoma, epithelial | [27] |
|
recombinant, His-tagged protein |
| A-498 | hu kidney, carcinoma, epithelial | [27,28] |
|
recombinant, His-tagged protein |
| HEp-2 (Human Epidermoid carcinoma #2) |
hu larynx, carcinoma, epithelial | [2,7,10,21,26,45,46,47,48,49,50,51,52,53,54,55,56,57,58] |
|
recombinant, purified by chromatography |
| IGR-Heu | hu non-small-cell, lung carcinoma, epithelial | [59] |
|
recombinant, GST fusion protein |
| IGR-Heu R8 | hu non-small-cell, lung carcinoma, epithelial | [59] |
|
recombinant, GST fusion protein |
| THP-1 | hu peripheral blood, acute monoblastic/monocytic leukemia, monocyte | [60] * [61] § |
|
recombinant, *purified by chromatography § His-tagged protein |
| JURKAT | hu peripheral blood, acute T cell leukemia, lymphoblast | [44] |
|
recombinant, purified by chromatography |
| PC3 | hu prostate, adenocarcinoma, epithelial | [62] * [63] ‡ |
|
recombinant, *purified by chromatography ‡ GST fusion protein |
| LNCaP (Lymph Node Carcinoma of the Prostate) |
hu prostate, carcinoma, epithelial | [62] * [63] ‡ |
|
recombinant, *purified by chromatography ‡ GST fusion protein |
| 22Rv1 | hu prostate, carcinoma, epithelial | [62] |
|
recombinant, purified by chromatography |
| VCaP (Vertebral Cancer of the Prostate) |
hu prostate, carcinoma, epithelial | [62] | enhanced migration and invasion | recombinant, purified by chromatography |
| Me-665 | hu skin, melanoma, epithelial | [52] | formation of stress fibers, ruffles and filopodia | recombinant, purified by chromatography |
| RAW264.7 | mouse abelson murine leukemia virus-induced tumor, monocyte/macrophage | [61] |
|
recombinant, His-tagged protein |
| Y-1 | mouse adrenal cortical carcinoma, epithelial | [27] |
|
recombinant, His-tagged protein |
| GL261 (Glioma 261) |
mouse brain, glioblastoma, fibroblastoid | [31,32] |
|
recombinant, purified by chromatography |
| BL6-10 | mouse skin, melanoma, epithelial | [64,65] |
|
recombinant, GST-fusion protein |
| B16-F10 | mouse skin, melanoma, mixture of spindle-shaped and epithelial-like | [66] |
|
not specified |
| 804G | rat bladder, carcinoma, epithelial | [8,11] |
|
recombinant, purified by chromatography |
* purified by chromatography; § His-tagged protein; ‡ GST fusion protein
Table 2.
Immortalized/transformed cell lines
| Cell Line | Tissue Origin and Morphology | References | CNF1 Described Effects | CNF1 Preparation |
|---|---|---|---|---|
| HMEC-1 (Human Microvascular Endothelial Cell line-1) |
hu dermal endothelium, immortalized (SV40 T-antigen), endothelial-like | [67] |
|
recombinant, GST fusion protein |
| HEK 293 (Human Embryonic Kidney 293) |
hu embryonic kidney, transformed, tumorigenic, epithelial | [11] * [9] ‡ |
|
recombinant, * purified by chromatography ‡ GST fusion protein |
| HEK 293T (Human Embryonic Kidney 293T) |
hu embryonic kidney, transformed, HEK 293 derivative expressing SV40 T-antigen, epithelial | [68] |
|
recombinant, His-tagged protein |
| HBMEC-60 (Human Bone Marrow Endothelial Cell line-60) |
hu bone marrow, immortalized (HPV16 E6/E7), endothelial | [69] |
|
recombinant, purified by chromatography |
| HBEC-5i (Human Brain Endothelial Cell line-5i) |
hu brain cerebral cortex, immortalized (SV40 T-antigen), endothelial | [22,69] |
|
recombinant, purified by chromatography |
| SV-HUC-1 | hu ureter, immortalized (SV40 T-antigen), epithelial | [27] | multinucleation | recombinant, His-tagged protein |
| MesEnd (Mesenteric Endothelial) |
mouse mesenteric microvascular, immortalized (SV40 T-antigen), | [70] |
|
recombinant, GST fusion protein |
| MyEnd (Myocardial Endothelial) |
mouse microvascular myocardial, immortalized (SV40 T-antigen) | [70,71,72] |
|
recombinant, GST fusion protein |
| HaCaT | hu skin, spontaneously immortalized, keratinocyte | [73] |
|
not specified |
| NIH 3T3 | mouse embryonic, spontaneously immortalized, fibroblasts | [6,33,37,74] |
|
recombinant, GST fusion protein |
| 3T3-Swiss albino | mouse embryo, spontaneously immortalized, fibroblasts | [53] |
|
recombinant, His-tagged protein |
| 3T3-L1 | mouse embryo, substrain of 3T3-Swiss albino, preadipocytes fibroblasts |
[75] |
|
recombinant, His-tagged protein |
| C2C12 | mouse muscle, spontaneously immortalized, myoblast | [76] |
|
recombinant, purified by chromatography |
| Vero | monkey kidney, spontaneously immortalized, epithelial | [5,11,56] |
|
recombinant, purified by chromatography |
| MDCK (Madin-Darby Canine Kidney) |
dog kidney, spontaneously immortalized, epithelial | [77] |
|
recombinant, GST fusion protein |
| PAE (p23 clone) (Porcine Aorta-derived Endothelial) |
pig aorta | [78] |
|
recombinant, GST fusion protein |
* purified by chromatography; ‡ GST fusion protein
Table 3.
Finite/primary cell lines
| Cell line | Tissue Origin and Morphology | References | CNF1 Described Effects | CNF1 Preparation |
|---|---|---|---|---|
| HPECC (HPCEC) | hu colon, finite cell line, epithelial | [22] | cell motility decrease | recombinant, purified by chromatography |
| HDMEC (Human Dermal Microvascular Endothelial Cells) |
hu dermal, finite cell line, microvascular endothelial cells | [70,79] |
|
recombinant, GST fusion protein |
| HUVEC (Human Umbilical Vein Endothelial Cell) |
hu umbilical, finite cell line, endothelial | [8,10,11,28,80] |
|
recombinant, purified by chromatography, His-tagged protein |
| IEC-6 (Intestinal Epithelioid Cell line #6) |
rat small intestine, finite cell line, epithelial | [22,81] |
|
recombinant, purified by chromatography |
| T-lymphocytes | hu blood, primary, lymphocyte | [44] |
|
recombinant, purified by chromatography |
| NK | hu blood, primary, large granular lymphocyte | [82] |
|
recombinant, purified by chromatography |
| monocytes | hu blood, primary, monocyte | [60] |
|
recombinant, purified by chromatography |
| macrophages | hu blood, primary, macrophage | [11] | Rho, Rac, and Cdc42 depletion | recombinant, purified by chromatography |
| DC (dendritic cell) monocytes | hu blood, primary, monocyte | [83] |
|
recombinant, purified by chromatography |
| HBMEC (Human Brain Microvascular Endothelial Cells) |
hu brain, primary, microvascular endothelium | [3] |
|
recombinant, GST fusion protein |
| keratinocytes | hu neonatal foreskin, primary, keratinocyte | [11] | RhoA, Rac1, and Cdc42 depletion | recombinant, purified by chromatography |
| MERRF (Myoclonic Epilepsy with Ragged-Red Fibers) fibroblasts | hu skin, primary, from myoclonic epilepsy with ragged-red fibers, fibroblast | [84] |
|
recombinant, purified by chromatography |
| fibroblasts | hu skin/neonatal foreskin, primary, fibroblast | [11,84] |
|
recombinant, purified by chromatography |
| BMDM (Bone-Marrow-Derived Macrophages) |
mouse bone marrow derived macrophages | [85] * [61] § |
|
recombinant, * purified by chromatography § His-tagged protein |
| MEFs (Mouse Embryonic Fibroblasts) |
mouse embryo, primary, fibroblast | [11] |
|
recombinant, purified by chromatography |
| mouse peritoneal macrophages | mouse peritoneal lavage, primary, macrophage | [61] |
|
recombinant, His-tagged protein |
| rat mesangial primary cells | rat kidney, primary | [86] | increase of Cox2 mRNA levels | recombinant, His-tagged protein |
| rat embryonic primary astrocytes | rat embryo cortex, primary, astrocytes | [87] |
|
recombinant, purified by chromatography |
| rat embryonic primary neurons | rat embryonic hippocampus | [87] |
|
recombinant, purified by chromatography |
| rat embryonic primary neurons | rat newborn hippocampus | [88] | dendrite and axon retraction | recombinant, GST-fusion |
| rat embryonic primary neurons | rat embryonic substantia nigra | [89] |
|
recombinant, His-SUMO tag protein |
| OPC (Oligodendrocyte Precursor Cells) |
rat/mouse newborn cortex, primary | [90] |
|
recombinant, GST fusion protein |
| PAEC (Porcine Aorta Endothelial Cells) |
pig pulmonary artery, primary, endothelial | [70] |
|
recombinant, GST fusion protein |
| dog thyroid epithelial cells | dog thyroid, primary, epithelial-like | [91] |
|
recombinant, GST fusion protein |
* purified by chromatography; § His-tagged protein
3.1. Effects on Rho GTPases and on Actin Cytoskeleton
Not all selected papers report the description of the CNF1 effects on its direct targets, Rho, Rac, and Cdc42 (i.e., analyzed by pulldown, band shift, or glutamine 63 deamidation experiments), probably depending on the specific purpose of the single experimental work.
Overall, the specific activity of CNF1 is to permanently activate Rho, Rac, and Cdc42 GTPases in all the cell systems where its effects have been studied [2,4,5,7,8,9,10,25,26,27,32,33,34,35,36,37,38,39,41,42,43,47,52,55,56,57,58,59,60,61,62,63,64,68,69,75,77,78,79,80,81,84,89,90,91,92]. However, the timing and level of activation of the distinct members of the Rho GTPase family may turn out to be variable between different cell types and lines. It is known that Rho proteins and/or their regulators (GDIs, GAPs and GEFs) can be differentially expressed, due to the specific phenotype and/or physiological conditions of a specific cell line [93]. Moreover, of great importance is the efficiency of the ubiquitination/proteasomal degradation system of the host cell. Indeed, CNF1-activated Rho GTPases undergo polyubiquitination, a modification targeting Rho proteins to the degradative proteasome machinery, a crucial mechanism in the control of both Rho small GTPases and their modulators. The observed Rho GTPase depletion after CNF1 treatment is strictly dependent on its sustained activation [8,9,11,33,37,59,94,95].
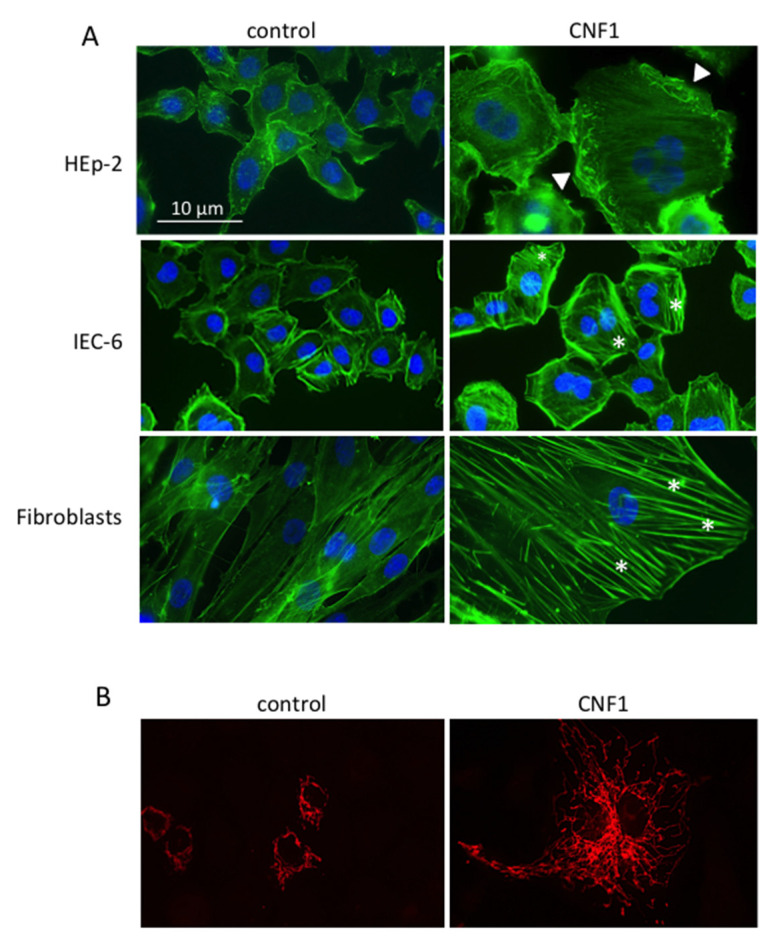
In most studies, the induction of at least some of the morphological effects characteristic of Rho GTPase activation is described, that is, changes in the actin cytoskeleton organization, demonstrated by actin stress fibers or the formation of actin cables, membrane ruffles, filopodia and lamellipodia assembly (Figure 2A) [6,7,8,10,25,26,32,34,35,36,38,40,41,44,45,46,47,50,51,52,56,94,95].
Figure 2.
Example of morphological effects of CNF1 on actin and mitochondria. (A) F-actin and nuclei staining of different cell lines untreated or treated with CNF1. Asterisks: stress fibers; arrow-heads: ruffles. (B) Mitochondrial staining of control and CNF1-treated IEC-6 cells. Note the enrichment of the mitochondrial network in treated cells.
3.1.1. Actin Cytoskeleton-Dependent Phenomena (Motility, Focal Adhesion, Permeability, Phago-Pinocytosis)
Regardless of the method used to identify the impact of CNF1-induced cytoskeletal modifications on cell movement ability (migration—invasion test, scratch wound healing assay), CNF1 treatment stimulated an increase of cell motility in 13 cell lines (T24, 5637, HUVEC [28]; HT-29, SW480, HEp-2 [22]; PC3, LNCaP, 22Rv1, VCaP [62]; BL6-10 [64]; 804G, HUVEC [8]; T-lymphocytes [44]). Among these, in PC3 [62] and BL6-10 [64] cells, this effect was accompanied by an in vivo increase in metastatic ability.
By contrast, in five cell lines, the toxin showed an inhibitory effect on cell motility (T84 [43]; HeLa [36,38]; GL216 [32]; HPECC, HBEC-5i [22]).
Of interest, in T-lymphocytes and IEC-6 cells, CNF1 was able to raise cell motility, but only in the presence of SDF-1α [44] or inflammation mediators [22].
In four CNF1-treated cell lines, an increase in focal adhesion formation was observed, irrespective of whether cell motility was increased or inhibited (BL6-10 [64,65]; Caco-2 [39]; HeLa [36]; IGR-Heu-R8 [59]).
Effects of CNF1 treatment on phagocytic activity has been observed. In particular, two publications [60,95] report that, in different monocyte/macrophagic cell lines, of both primary and cancer origin (BMDM, mouse peritoneal macrophages, human monocytes, THP-1, Raw264.7), CNF1 reduced the phagocytosis of nonopsonized beads and of nonopsonized bacteria. Conversely, in HEp-2 and in 804G epithelial cancer cell lines, CNF1 confers the ability to ingest latex beads as well as bacteria [8,46,47,54].
These results suggest that CNF1 might contribute to bacterial infection by favoring epithelium colonization and/or affecting the host innate immune defense, thus reducing the pathogenic E. coli clearance ability of macrophages (by decreasing scavenger receptor CD36 expression).
Along with cytoskeletal modifications, alterations in the distribution or in the amount of intercellular junction proteins following CNF1 treatment are described in eight cells lines (HT-29, IEC-6 [22]; T84 [42]; MyEnd [71,72]; HMEC1 [67]; MDCK [77]; HDMEC, PAEC [70,79]). For example, E-cadherin, β-catenin, zonula occludens-1 (ZO-1), caveolin-1, as well as junction adhesion molecule-1 were reorganized away from the TJ membrane (MyEnd [71]; MDCK [77]; HT-29 [22]). In some cases, cytoskeletal and tight junction alterations were accompanied by modifications in cell monolayer permeability, as in two colon carcinoma cell lines in which CNF1 caused a transient rise in cell monolayer paracellular permeability (Caco-2 [39,40]; T84 [41,42,54]) and the transepithelial migration of polymorphonuclear neutrophils (PMN) (T84 [41,42,54]).
On cell lines of endothelial origin, activation of Rho GTPases by CNF1 seems to have different effects on the regulation of cell permeability depending on the background of the endothelial cell lines. Baumer and co-workers [70] show, in fact, that CNF1-induced activation of Rho GTPases reduces permeability in microvascular endothelial cell types; whereas, in macrovascular endothelial cells CNF1 stabilizes barrier functions.
3.1.2. Multinucleation, Cell Cycle, Cell Death, Apoptosis, and Senescence
It is well known that Rho signaling pathways are involved in cell proliferation and cell cycle regulation, also through actin cytoskeleton regulation.
Multinucleation after treatment with CNF1 is reported for 19 cell lines (HEp-2 [27,49,50,53,57,96]; HeLa [33,34,36]; Caco-2 [39]; HT-29 [22]; T24 [26,27]; 5637 [26,27]; Y-1 [27]; A-498 [27]; J82 [27]; SV-HUC-1 [27]; ACHN [27]; human GMB [32]; GL261 [32]; JURKAT [44]; PAE [78]; NIH 3T3 [6,37]; 3T3-Swiss Albino [53]; 3T3-L1 [75]; HCT-116 [45]) (Figure 2A). These morphological changes are probably a consequence of CNF1-induced mitosis/cytokinesis failure [1,49,97]. In fact, it is well known that Rho GTPases are involved in several stages of mitosis, such as spindle formation and attachment to the kinetochore, as well as in the cytokinesis process [98]. Moreover, CNF1 is classified as a cyclomodulin, thus being able to perturb the host cell cycle [99]. Actually, along with multinucleation in some of the reported works, CNF1 treatment is shown to induce a block or a partial inhibition of cell proliferation (GL261 [32]; 3T3-L1 [75]; BL6-10 [64]; Hs 738 [100]) and/or G2/M arrest (T24, 5637 [26]; HEp-2 [50]; HeLa [34]), accompanied by a downregulation of cyclin B1 expression and its cytoplasmatic sequestration (T24 [26]; HEp-2 [50]).
In four cell lines, the inhibition of cell cycle progression leads cells to a senescence state (U87 GL261, human GBM [31]; HCT-116 [45]). Of interest, Zhang and co-workers [45] showed that in HCT-116 human colon cancer cells, CNF1 elicited endoreplication and polyploidization driving cells into a reversible senescence state, which provided a survival route to the cells via depolyploidization. Indeed, authors showed that when CNF1-induced polyploid cells were cultured in fresh medium, in the absence of the toxin, a population of depolyploidized cells able to re-enter the mitotic cycle was selected [45]. Importantly, progeny derived from the CNF1 treatment exhibited genomic instability exemplified by an increased aneuploidy.
In three cell lines, after prolonged treatment, the block of proliferation resulted in cell death (5637 [27]; 3T3-L1 [75]; GL261 [32]), which in one case occurred by an apoptotic mechanism (5637 [27]). In other cell lines, CNF1 treatment seems to protect cells from apoptosis induced by exposure to UV [57,96] or in simulated microgravity conditions [64,65]. Although the molecular mechanisms are still unknown, it seems reasonable, that the fate (senescence, cell death, or survival) of CNF1-treated cells largely depends on the cell type and on the transformation degree of the cells exposed.
3.2. Mitochondria and Mitochondria-Related Phenomena
The effect of CNF1 on mitochondrial activity has been analyzed in seven cell lines (BL6-10 [64]; HEp-2 [47,51,58,96]; T24 [25]; SH-SYS5 [29]; IEC-6 [81]; human fibroblasts MERRF [84]). Overall, CNF1 seems to affect mitochondrial metabolism by stimulating an increase in ATP synthesis (IEC-6 [81]; human fibroblasts MERRF [84]) and counteracting the negative effects produced on these organelles under particular experimental or pathological conditions [29,64,84]. In four of the above-mentioned cell lines (SH-SY5Y, HEp-2, IEC-6, human fibroblasts MERRF), metabolic stimulation was accompanied by a prominent modification in the mitochondrial morphology, consisting of the formation of a complex network of elongated organelles (Figure 2B). This was probably due to phosphorylation/inactivation of dynamin-related protein 1 (Drp1), one of the large GTPases that control the mitochondrial fission process.
Indeed, in IEC-6 and SH-SY5Y cells a significant increase in the phospho-Drp1 protein was observed (IEC-6 [81]; SH-SY5Y [29]).
3.3. CNF1 on Immune Cells
Few articles investigated CNF1-induced effects in cells of the immune system.
In both finite/primary (human monocytes, macrophages, DC monocytes, mouse peritoneal macrophages) and cancer monocytic/macrophagic cell lines (THP-1, RAW264.7), CNF1 is able to modulate CR3 activation and its colocalization with the actin cytoskeleton (THP-1 e monocytes [60]) and downregulate CD36 transcription/expression [96], leading to a reduced phagocytic ability of nonopsonized beads and/or E. coli bacteria (THP-1, RAW264.7, mouse peritoneal macrophages [96]; THP-1, human monocytes [60]).
On the other hand, one paper shows that CNF1 triggers the activation and phenotypic maturation of cultured monocyte-derived DCs, with an increased level of IL-6 and TNF-α secretion and the proliferation of allogenic naïve CD4+ T cells (DC monocytes [83]). In bone-derived macrophages, CNF1 toxin activates the NLRP3 inflammasome via a signaling cascade that involves PAK1/Rac2, thus inducing caspase-1 activation and IL1-β secretion [85].
In cells of lymphoid origin, both primary (T-lymphocytes, BMDM) and leukemic (Jurkat), CNF1 treatment enhances cell migration ability across acellular filters and their adherence to colonic epithelial cell monolayers. In particular, treated T-lymphocytes are able to adhere more tightly to monolayers of human intestinal epithelial cell lines resulting in cytotoxicty for the epithelial cells. In these cells, CNF1 also stimulates the production of high levels of TGF-β1, TGF-β2, TGF-β3, and TNF-α proteins (Jurkat, T-lymphocytes [44]).
In NK cells, CNF1 causes a strong increase in the binding efficiency and killing capacity of effector cells. An augmented expression of cell adhesion and activation-associated molecules, as well as reshaping of the actin and microtubule networks, are also described and probably represent the basis of the enhanced binding ability and cytotoxicity of NK-treated cells [82].
Overall, the in vitro described effects of CNF1 toxin on cells of immune origin suggest its ability to affect innate immune defenses, facilitating bacterial infection and increasing the virulence of E. coli pathogenic strains (in the intestinal epithelia). On the other hand, CNF1 also seems to elicit a protective immune mechanism, which is consistent with in vivo studies indicating CNF1 as promoter of antibacterial immunity [101,102]. This apparent discrepancy between pro- and antibacterial activity induced by CNF1 probably depends on the experimental settings and on the specific purpose of the study.
3.4. CNF1 Effects on Different Cellular Pathways
Rho proteins have over 60 known downstream effectors, which determine the outcome of activation for a given Rho GTPase protein. The activation of CNF1-induced Rho GTPases affects different cellular pathways that, in turn, drive different new cell states. Actually, in the reviewed papers, CNF1 cell intoxication resulted in a number of proteins being modulated. The described effects on actin organization and cytoskeletal rearrangement (see Section 3.1.1.) are often accompanied by modifications in the distribution and/or expression of proteins involved in specific signal transduction pathways regulating cytoskeleton organization, as well as cell adhesion, motility, and migration.
Both Rho GTPases and cytoskeletal rearrangements are also known to influence gene transcription [93]. Actually, after CNF1 intoxication, in 17 cell lines (HeLa [38]; BL6-10 [65]; HEp-2 [48,52]; GL-261 [31]; C2C12 [76]; HUVEC [10]; 3T3-L1 [75]; mouse peritoneal macrophages, Raw264.1, BMDM, THP-1 [96]; 5637, T24 [28]; HT-29, IEC-6 [22]; 293T [68]) a number of transcription factors (TFs) also result in being modulated. For example, in HEp-2 epithelial cells, CNF1 activates NF-κB through the Rac1/PI3K/Akt/IKK prosurvival pathway, with the ensuing modulation of the antiapoptotic proteins Bcl-2 and Bcl-XL [51,95].
Moreover, CNF1 promotes transcription and release of proinflammatory cytokines, such as IL-6, IL-8 and TNF-α, in cells of different origin (T24 [25]; HEK 293T [68]; HUVEC [10]; human T-lymphocytes [44]) and DC monocytes [83]. Furthermore, CNF1 upregulates the transcription of cyclooxygenase-2 [86], as well as the cell adhesion molecule ICAM-1 (human NK [82]), and the cell cycle related genes p21 and p16 (U87, GL261, human GBM [31,45]). Interestingly, the genes coding for all the above-mentioned proteins are under NF-κB TF control [103,104].
One more example shows how, in both 5637 and T24 uroepithelial cell lines, under hypoxic conditions, CNF1 indirectly promotes VEGF secretion and angiogenesis through RhoC-dependent activation of the HSF1-HSP90α-HIF1α axis. In particular, activated RhoC induces HIF1α stabilization and VEGF production by increasing HSP90α expression and the interaction between HIF1α and HSP90 [28]. Beyond the mentioned examples, other TFs, as well as proteins, are described in the literature as modulated after CNF1 cell treatment (IκB-α, c-Jun [10]; MyoD [76]; C/EBP-α, PPAR-γ [75]; AP-1 [38]; FoxG1 [31]; C/EBP-α, LXR-β [96]; Snail1, ZEB1 [22]).
It is evident that the heterogeneity of the reported results reflects the great variability of the experimental models, cell lines, experimental conditions, and authors’ purpose between the different reviewed articles.
4. Discussion and Conclusions
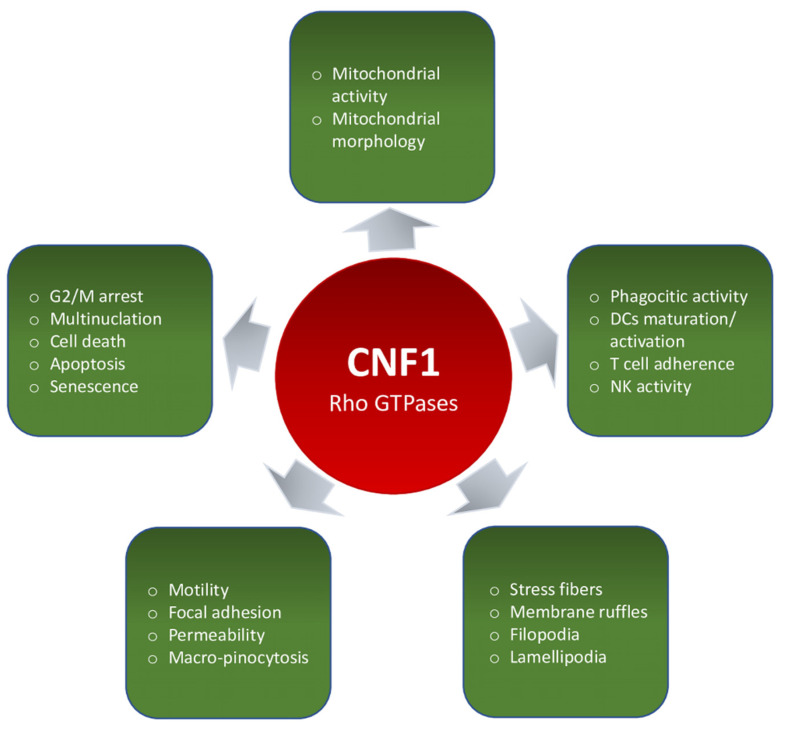
CNF1 is a bacterial protein toxin mainly produced by E. coli, associated with extra-intestinal disease, but occasionally detected in intestinal infections [105]. For this reason, many studies have been carried out on epithelial cells that represent the actual target of the toxin, in an attempt to analyze its role in E. coli pathogenesis. However, due to its specific activity on Rho GTPases, CNF1 has also been used as a tool for studies aimed at deciphering the involvement of Rho GTPases in certain pathways. In the present review, we aimed at giving a comprehensive examination of the CNF1 effects described in different human and animal cell lines, in an attempt to provide an easy-to-use guide of the results obtained so far. A schematic summary of the overall CNF1-induced effects reported in the literature is shown in Figure 3.
Figure 3.
Graphical summary of the overall CNF1-induced effects reported in the literature.
From a general analysis of the published studies, a different activity of CNF1 does not emerge between primary, transformed and cancer cells. All in all, it is evident that the heterogeneity of the reported results reflects the great variability between the different reviewed articles in terms of experimental models, cell line tissue and type origin, experimental conditions and authors’ purpose.
Almost all the in vitro studies report the direct CNF1 enzymatic activation of Rho proteins and/or its effects on cytoskeletal organization, irrespective of the cell type and transformation status of a cell. It is also interesting to note that the depletion of Rho GTPases, ensuing CNF1 exposure, does not seem to be related to the transformation status or cell type, but rather to a specific alteration of the ubiquitin pathway in certain cells (see introduction). Rho GTPases are important transducers in signaling pathways crucial for the maintenance of normal tissue. It is well known that the same signaling pathway regulated by a specific Rho can elicit distinct responses in different cell types, depending on the biological context, such as the extracellular stimuli and signaling pathways involved in that particular cell type [93]. This is also evident from reviewing the effects of CNF1, since various TFs are activated and different proteins are regulated in many of the experimental models analyzed.
Finally, we would like to point out that none of the published papers seem to take into account the possible further consequence elicited by CNF1 interaction with its receptors, the 37 kDa LRP (37LRP) and the Lutheran adhesion glycoprotein/basal cell adhesion molecule (Lu/BCAM) [2,3]. Actually, these two distinct laminin receptors are known to be involved in a number of cellular functions, resembling those regulated by CNF1. In particular, 37LRP, acting as a mediator of cell adhesion, cell proliferation and differentiation, hampers apoptosis, plays a major role as a cell surface receptor in prion disorders and could possibly be involved in the cell biology of neurodegenerative diseases, such as Alzheimer’s disease (AD) [106,107]. In this context, it is interesting to underline that CNF1 is able to rescue cognitive deficits in a murine model of AD by increasing brain energy levels and counteracting neuroinflammatory markers [108]. Furthermore, the overexpression of 37LRP is evident in several cancer types and has been demonstrated to enhance the invasiveness of cancer cells [109].
Another aspect that could be taken into account in future studies is the possible effect due to the alteration of the balance between the F- and G-actin cellular pools, as a consequence of the CNF1-induced actin polymerization state. It has now been established that, in addition to its function in the cytoplasm, actin is actively imported into the nucleus, where it directly regulates transcription and participates in chromatin organization, mRNA transport, translation, post-translational modifications, chromosome positioning, DNA rearrangements and repair. All these functions are tightly linked to the balance between nuclear actin monomers and polymers in the nucleus and indirectly, to actin polymerization/depolymerization in the cytoplasm, which affects nuclear import/export [110].
Not least, the carcinogenic capacity of CNF1, in line with other toxins, is an emerging feature. Several modifications induced by CNF1 are, in fact, reminiscent of a procarcinogenic potential [111,112]. In recent years, studies have been carried out to corroborate this hypothesis [21,113], but the subject of study is still in its infancy.
In conclusion, although several aspects have already been addressed in studies dealing with CNF1, there are still completely new fields of investigation concerning the cellular activity of the toxin that deserve careful investigation.
Acknowledgments
The authors thank Rossella Di Nallo for her invaluable technical contribution.
Abbreviations
| AKT | RAC- serine/threonine-protein kinase |
| AMPK | 5′-AMP-activated protein kinase catalytic subunit alpha-2 |
| AP-1 | activator protein 1 |
| Bcl-2 | apoptosis regulator Bcl-2 (B-cell lymphoma 2) |
| Bcl-XL | apoptosis regulator Bcl-X (B-cell lymphoma-extra-large) |
| C/EBPα | CCAAT/enhancer-binding protein alpha |
| cAMP | cyclic adenosine monophosphate |
| CD11a | integrin alpha-L |
| CD11b | integrin alpha-M |
| CD18 | integrin beta-2 |
| CD29 | integrin beta-1 |
| CD32 | low affinity immunoglobulin gamma Fc region receptor II-b |
| CD36 | platelet glycoprotein 4 |
| CD49d | integrin alpha-4 |
| CD83 | cluster of differentiation 83 antigen |
| CD86 | T-lymphocyte activation antigen CD86 |
| CD96 | T-cell surface protein tactile |
| c-Myc | proto-oncogene protein Myc |
| c-Jun | AP-1 transcription factor subunit |
| CR3 | complement receptor 3 C |
| DLK1 | protein delta homolog 1 |
| Drp1 | dynamin-1-like protein |
| Dsg3 | desmoglein-3 |
| EEA-1 | early endosome antigen 1 |
| EGFR | epidermal growth factor receptor |
| EIF4E | eukaryotic translation initiation factor 4E |
| EMT | epithelial–mesenchymal transition |
| ERK1/2, (p42-44, MAPK) | mitogen-activated protein kinases 3 and 1 |
| FAK | focal adhesion kinase |
| FoxG1 | forkhead box protein G1 |
| GFAP | glial fibrillary acidic protein |
| HIF-1α | hypoxia-inducible factor 1-alpha |
| HLA-DR | HLA class II histocompatibility antigen gamma chain |
| HMGA-2 | high mobility group protein HMGI-C |
| HSF1 | heat shock factor protein 1 |
| HSP90α | heat shock protein HSP 90-alpha |
| ICAM-1 | intercellular adhesion molecule 1 |
| IFN-γ | interferon gamma |
| IL-1β | interleukin 1 beta |
| IL-2R | interleukin 2 receptor |
| IL-6 | interleukin 6 |
| IL-8 | interleukin 8 |
| IκB-α, IKK | NF-κB inhibitor alpha |
| JAM-1 | junctional adhesion molecule A |
| JNK | mitogen-activated protein kinase 8 |
| LAMP1 | lysosome-associated membrane glycoprotein 1 |
| LC3-II | microtubule-associated proteins 1A/1B light chain 3B |
| LXRβ | liver X receptor beta |
| MAL | myocardin-related transcription factor A |
| MCP-1 | monocyte chemoattractant protein 1 |
| Met72 | melanoma cell-surface 72 Kd-glycoprotein |
| MHC | major histocompatibility complex |
| MIP-3α | macrophage inflammatory protein-3 alpha |
| MMP-2 | matrix metalloproteinase-2 |
| MMP-9 | matrix metalloproteinase-9 |
| moDC | monocyte-derived dendritic cells |
| MORs | mu-opioid receptors |
| mTOR | mammalian target of rapamycin |
| mTORC1 | mTOR complex 1 |
| MyoD | myoblast determination protein 1 |
| NADH | nicotinamide adenine dinucleotide, reduced form |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NIS | sodium/iodide cotransporter |
| Notch1 | neurogenic locus notch homolog protein 1 |
| p16 | cyclin-dependent kinase inhibitor 2A |
| p21 | cyclin-dependent kinase inhibitor 1 |
| p38 | mitogen-activated protein kinase 11 |
| p53 | cellular tumor antigen p53 |
| PAK1 | serine/threonine-protein kinase PAK1 |
| pAKT | RAC- serine/threonine-protein kinase, phosphorylate form |
| PDGFR | platelet-derived growth factor receptor |
| PdtIns-4-P 5-kinase | phosphatidylinositol 4-phosphate 5-kinase |
| PKA | cAMP-dependent protein kinase |
| PMN | polymorphonuclear neutrophils |
| PPARγ | peroxisome proliferator-activated receptor gamma |
| pRb | retinoblastoma-associated protein |
| pRBC | Plasmodium falciparum-parasitized erythrocytes |
| Pref1 | preadipocyte factor |
| pS6K | ribosomal protein S6 kinase, phosphorylate form |
| pULK1 | unc-51 like autophagy activating kinase 1, phosphorylate form |
| PV-IgG | pemphigus vulgaris autoantibodies |
| Rab11 | Ras-related protein Rab-11 |
| RagC | Ras-related GTP-binding protein C |
| rpS6 | 40S ribosomal protein S6 |
| Rip1, Rip2 | receptor-interacting serine/threonine-protein kinase 1 and 2 |
| ROS | reactive oxygen species |
| SA-β-gal | senescence-associated beta-galactosidase |
| SDF-1α | stromal cell-derived factor 1 |
| SMG | simulated microgravity |
| Snail1 | snail family transcriptional repressor 1 |
| TF | transcription factor |
| Tg | thyroglobulin |
| ThOX | thyroid oxidase |
| TGF-β | transforming growth factor beta |
| TJ/AJ | tight junction/adherent junction |
| TNF-α | tumor necrosis factor alpha |
| Tom20 | mitochondrial import receptor subunit |
| TRAF1 | TNF receptor-associated factor 1 |
| UPP1 | uridine-phosphorylase 1 |
| VEGF | vascular endothelial growth factor |
| VASP | vasodilator-stimulated phosphoprotein |
| ZEB1 | zinc finger E-box-binding homeobox 1 |
| ZO-1 | Zonula occudens-1 (tight junction protein 1) |
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms222212610/s1.
Author Contributions
Conceptualization: F.C. and A.F.; investigation: F.C., Z.M., S.T. and A.F.; data curation, F.C., Z.M., S.T. and A.F.; validation: A.F.; visualization: F.C. and Z.M.; writing-original draft preparation: F.C., Z.M., S.T. and A.F.; writing-review and editing: F.C., Z.M., C.F., S.T. and A.F.; supervision: A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Boquet P. The cytotoxic necrotizing factor 1 (CNF1) from Escherichia coli. Toxicon. 2001;39:1673–1680. doi: 10.1016/S0041-0101(01)00154-4. [DOI] [PubMed] [Google Scholar]
- 2.Piteau M., Papatheodorou P., Schwan C., Schlosser A., Aktories K., Schmidt G. Lu/BCAM Adhesion Glycoprotein Is a Receptor for Escherichia coli Cytotoxic Necrotizing Factor 1 (CNF1) PLoS Pathog. 2014;10:3884. doi: 10.1371/annotation/6eec6403-e090-4283-aa34-34cc58ca0bbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung J.W., Hong S.J., Kim K.J., Goti D., Stins M.F., Shin S., Dawson V.L., Dawson T.M., Kim K.S. 37-kDa laminin receptor precursor modulates cytotoxic necrotizing factor 1-mediated RhoA activation and bacterial uptake. J. Biol. Chem. 2003;278:16857–16862. doi: 10.1074/jbc.M301028200. [DOI] [PubMed] [Google Scholar]
- 4.Contamin S., Galmiche A., Doye A., Flatau G., Benmerah A., Boquet P. The p21 Rho-activating toxin cytotoxic necrotizing factor 1 is endocytosed by a clathrin-independent mechanism and enters the cytosol by an acidic-dependent membrane translocation step. Mol. Biol. Cell. 2000;11:1775–1787. doi: 10.1091/mbc.11.5.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flatau G., Lemichez E., Gauthier M., Chardin P., Paris S., Fiorentini C., Boquet P. Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. Nature. 1997;387:729–733. doi: 10.1038/42743. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt G., Sehr P., Wilm M., Selzer J., Mann M., Aktories K. Gln 63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-1. Nature. 1997;387:725–729. doi: 10.1038/42735. [DOI] [PubMed] [Google Scholar]
- 7.Lerm M., Selzer J., Hoffmeyer A., Rapp U.R., Aktories K., Schmidt G. Deamidation of Cdc42 and Rac by Escherichia coli cytotoxic necrotizing factor 1: Activation of c-Jun N-terminal kinase in HeLa cells. Infect. Immun. 1999;67:496–503. doi: 10.1128/IAI.67.2.496-503.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doye A., Mettouchi A., Bossis G., Clément R., Buisson-Touati C., Flatau G., Gagnoux L., Piechaczyk M., Boquet P., Lemichez E. CNF1 exploits the ubiquitin-proteasome machinery to restrict Rho GTPase activation for bacterial host cell invasion. Cell. 2002;111:553–564. doi: 10.1016/S0092-8674(02)01132-7. [DOI] [PubMed] [Google Scholar]
- 9.Lerm M., Pop M., Fritz G., Aktories K., Schmidt G. Proteasomal degradation of cytotoxic necrotizing factor 1-activated rac. Infect. Immun. 2002;70:4053–4058. doi: 10.1128/IAI.70.8.4053-4058.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munro P., Flatau G., Doye A., Boyer L., Oregioni O., Mege J.-L., Landraud L., Lemichez E. Activation and Proteasomal Degradation of Rho GTPases by Cytotoxic Necrotizing Factor-1 Elicit a Controlled Inflammatory Response. J. Biol. Chem. 2004;279:35849–35857. doi: 10.1074/jbc.M401580200. [DOI] [PubMed] [Google Scholar]
- 11.Boyer L., Turchi L., Desnues B., Doye A., Ponzio G., Mege J.L., Yamashita M., Zhang Y.E., Bertoglio J., Flatau G., et al. CNF1-induced ubiquitylation and proteasome destruction of activated RhoA is impaired in Smurf1−/− cells. Mol. Biol. Cell. 2006;17:2489–2497. doi: 10.1091/mbc.e05-09-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodge R.G., Ridley A.J. Regulating Rho GTPases and their regulators. Nat. Rev. Mol. Cell Biol. 2016;17:496–510. doi: 10.1038/nrm.2016.67. [DOI] [PubMed] [Google Scholar]
- 13.Ridley A. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–529. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Duquette P., Lamarche-Vane N. Rho GTPases in embryonic development. Small GTPases. 2014;5:e972857. doi: 10.4161/sgtp.29716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang G., Sun Z., Li H., Feng D. Rho GTPase-activating proteins: Regulators of Rho GTPase activity in neuronal development and CNS diseases. Mol. Cell. Neurosci. 2017;80:18–31. doi: 10.1016/j.mcn.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Niftullayev S., Lamarche-Vane N. Regulators of rho GTPases in the nervous system: Molecular implication in axon guidance and neurological disorders. Int. J. Mol. Sci. 2019;20:1497. doi: 10.3390/ijms20061497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chai L., Cao C., Bi S., Dai X., Gan L., Guo R., Li R. Small Rho GTPase Rac1 determines human epidermal stem cell fate in vitro. Int. J. Mol. Med. 2010;25:723–727. doi: 10.3892/IJMM_00000397. [DOI] [PubMed] [Google Scholar]
- 18.Yang L., Wang L., Geiger H., Cancelas J., Mo J., Zheng Y. Rho GTPase Cdc42 coordinates hematopoietic stem cell quiescence and niche interaction in the bone marrow. Proc. Natl. Acad. Sci. USA. 2007;104:5091–5096. doi: 10.1073/pnas.0610819104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bros M., Haas K., Moll L., Grabbe S. RhoA as a Key Regulator of Innate and Adaptive Immunity. Cells. 2019;8:733. doi: 10.3390/cells8070733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Etienne-Manneville S., Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 21.Popoff M.R. Bacterial factors exploit eukaryotic Rho GTPase signaling cascades to promote invasion and proliferation within their host. Small GTPases. 2014;5:e983863. doi: 10.4161/sgtp.28209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fabbri A., Travaglione S., Rosadi F., Ballan G., Maroccia Z., Giambenedetti M., Guidotti M., Ødum N., Krejsgaard T., Fiorentini C. The Escherichia coli protein toxin cytotoxic necrotizing factor 1 induces epithelial mesenchymal transition. Cell Microbiol. 2019;22:e13138. doi: 10.1111/cmi.13138. [DOI] [PubMed] [Google Scholar]
- 23.Buc E., Dubois D., Sauvanet P., Raisch J., Delmas J., Darfeuille-Michaud A., Pezet D., Bonnet R. High Prevalence of Mucosa-Associated, E. coli Producing Cyclomodulin and Genotoxin in Colon Cancer. PLoS ONE. 2013;8:e56964. doi: 10.1371/journal.pone.0056964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement David Moher and colleagues introduce PRISMA, an update of the QUOROM guidelines for reporting systematic reviews and meta-analyses. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falzano L., Quaranta M.G., Travaglione S., Filippini P., Fabbri A., Viora M., Donelli G., Fiorentini C. Cytotoxic necrotizing factor 1 enhances reactive oxygen species-dependent transcription and secretion of proinflammatory cytokines in human uroepithelial cells. Infect. Immun. 2003;71:4178–4181. doi: 10.1128/IAI.71.7.4178-4181.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falzano L., Filippini P., Travaglione S., Miraglia A.G., Fabbri A., Fiorentini C. Escherichia coli cytotoxic necrotizing factor 1 blocks cell cycle G2/M transition in uroepithelial cells. Infect. Immun. 2006;74:3765–3772. doi: 10.1128/IAI.01413-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mills M., Meysick K.C., O’Brien A.D. Cytotoxic necrotizing factor type 1 of uropathogenic Escherichia coli kills cultured human uroepithelial 5637 cells by an apoptotic mechanism. Infect. Immun. 2000;68:5869–5880. doi: 10.1128/IAI.68.10.5869-5880.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo Y., Wang J., Zhou K., Lv J., Wang L., Gao S., Keller E.T., Zhang Z.S., Wang Q., Yao Z. Cytotoxic necrotizing factor 1 promotes bladder cancer angiogenesis through activating RhoC. FASEB J. 2020;34:7927–7940. doi: 10.1096/fj.201903266RR. [DOI] [PubMed] [Google Scholar]
- 29.Travaglione S., Loizzo S., Vona R., Ballan G., Rivabene R., Giordani D., Guidotti M., Dupuis M.L., Maroccia Z., Baiula M., et al. The Bacterial Toxin CNF1 Protects Human Neuroblastoma SH-SY5Y Cells against 6-Hydroxydopamine-Induced Cell Damage: The Hypothesis of CNF1-Promoted Autophagy as an Antioxidant Strategy. Int. J. Mol. Sci. 2020;21:3390. doi: 10.3390/ijms21093390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pavone F., Luvisetto S., Marinelli S., Straface E., Fabbri A., Falzano L., Fiorentini C., Malorni W. The Rac GTPase-activating bacterial protein toxin CNF1 induces analgesia up-regulating mu-opioid receptors. Pain. 2009;145:219–229. doi: 10.1016/j.pain.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 31.Vannini E., Olimpico F., Middei S., Ammassari-Teule M., de Graaf E.L., McDonnell L., Schmidt G., Fabbri A., Fiorentini C., Baroncelli L., et al. Electrophysiology of glioma: A Rho GTPase-activating protein reduces tumor growth and spares neuron structure and function. Neuro. Oncol. 2016;18:1634–1643. doi: 10.1093/neuonc/now114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vannini E., Panighini A., Cerri C., Fabbri A., Lisi S., Pracucci E., Benedetto N., Vannozzi R., Fiorentini C., Caleo M., et al. The bacterial protein toxin, cytotoxic necrotizing factor 1 (CNF1) provides long-term survival in a murine glioma model. BMC Cancer. 2014;14:449. doi: 10.1186/1471-2407-14-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stoll T., Markwirth G., Reipschläger S., Schmidt G. A new member of a growing toxin family--Escherichia coli cytotoxic necrotizing factor 3 (CNF3) Toxicon. 2009;54:745–753. doi: 10.1016/j.toxicon.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 34.Huelsenbeck S., May M., Schmidt G., Genth H. Inhibition of cytokinesis by Clostridium difficile toxin B and cytotoxic necrotizing factors--reinforcing the critical role of RhoA in cytokinesis. Cell Motil. Cytoskeleton. 2009;66:967–975. doi: 10.1002/cm.20390. [DOI] [PubMed] [Google Scholar]
- 35.Dong N., Liu L., Shao F. A bacterial effector targets host DH-PH domain RhoGEFs and antagonizes macrophage phagocytosis. EMBO J. 2010;29:1363–1376. doi: 10.1038/emboj.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.May M., Kolbe T., Wang T., Schmidt G., Genth H. Increased Cell-Matrix Adhesion upon Constitutive Activation of Rho Proteins by Cytotoxic Necrotizing Factors from E. Coli and Y. Pseudotuberculosis. J. Signal Transduct. 2012;2012:1–10. doi: 10.1155/2012/570183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huelsenbeck S.C., Roggenkamp D., May M., Huelsenbeck J., Brakebusch C., Rottner K., Ladwein M., Just I., Fritz G., Schmidt G., et al. Expression and cytoprotective activity of the small GTPase RhoB induced by the Escherichia coli cytotoxic necrotizing factor 1. Int. J. Biochem. Cell Biol. 2013;45:1767–1775. doi: 10.1016/j.biocel.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 38.Pfaumann V., Lang A.E., Schwan C., Schmidt G., Aktories K. The actin and Rho-modifying toxins PTC3 and PTC5 of Photorhabdus luminescens: Enzyme characterization and induction of MAL/SRF-dependent transcription. Cell. Microbiol. 2015;17:579–594. doi: 10.1111/cmi.12386. [DOI] [PubMed] [Google Scholar]
- 39.Gerhard R., Schmidt G., Hofmann F., Aktories K. Activation of Rho GTPases by Escherichia coli cytotoxic necrotizing factor 1 increases intestinal permeability in Caco-2 cells. Infect. Immun. 1998;66:5125–5131. doi: 10.1128/IAI.66.11.5125-5131.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schlegel N., Meir M., Spindler V., Germer J., Wascher J. Differential role of Rho GTPases in intestinal epithelial barrier regulation in vitro. J. Cell. Physiol. 2011;226:1196–1203. doi: 10.1002/jcp.22446. [DOI] [PubMed] [Google Scholar]
- 41.Hofman P., Flatau G., Selva E., Gauthier M., Le Negrate G., Fiorentini C., Rossi B., Boquet P. Escherichia coli cytotoxic necrotizing factor 1 effaces microvilli and decreases transmigration of polymorphonuclear leukocytes in intestinal T84 epithelial cell monolayers. Infect. Immun. 1998;66:2494–2500. doi: 10.1128/IAI.66.6.2494-2500.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hopkins A., Walsh S., Verkade P., Boquet P., Nusrat A. Constitutive activation of Rho proteins by CNF-1 influences tight junction structure and epithelial barrier function. J. Cell Sci. 2003;116:725–742. doi: 10.1242/jcs.00300. [DOI] [PubMed] [Google Scholar]
- 43.Brest P., Turchi L., Le’Negrate G., Berto F., Moreilhon C., Mari B., Ponzio G., Hofman P. Escherichia coli cytotoxic necrotizing factor 1 inhibits intestinal epithelial wound healing in vitro after mechanical injury. Infect. Immun. 2004;72:5733–5740. doi: 10.1128/IAI.72.10.5733-5740.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brest P., Mograbi B., Hofman V., Loubat A., Rossi B., Auberger P., Hofman P. Rho GTPase is activated by cytotoxic necrotizing factor 1 in peripheral blood T lymphocytes: Potential cytotoxicity for intestinal epithelial cells. Infect. Immun. 2003;71:1161–1169. doi: 10.1128/IAI.71.3.1161-1169.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Z., Aung K.M., Uhlin B.E., Wai S.N. Reversible senescence of human colon cancer cells after blockage of mitosis/cytokinesis caused by the CNF1 cyclomodulin from Escherichia coli. Sci. Rep. 2018;8:1–11. doi: 10.1038/s41598-018-36036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Falzano L., Fiorentini C., Donelli G., Michel E., Kocks C., Cossart P., Cabanié L., Oswald E., Boquet P. Induction of phagocytic behaviour in human epithelial cells by Escherichia coli cytotoxic necrotizing factor type 1. Mol. Microbiol. 1993;9:1247–1254. doi: 10.1111/j.1365-2958.1993.tb01254.x. [DOI] [PubMed] [Google Scholar]
- 47.Fiorentini C., Donelli G., Matarrese P., Fabbri A., Paradisi S., Boquet P. Escherichia coli cytotoxic necrotizing factor 1: Evidence for induction of actin assembly by constitutive activation of the p21 Rho GTPase. Infect. Immun. 1995;63:3936–3944. doi: 10.1128/iai.63.10.3936-3944.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fiorentini C., Fabbri A., Flatau G., Donelli G., Matarrese P., Lemichez E., Falzano L., Boquet P. Escherichia coli cytotoxic necrotizing factor 1 (CNF1), a toxin that activates the Rho GTPase. J. Biol. Chem. 1997;272:19532–19537. doi: 10.1074/jbc.272.31.19532. [DOI] [PubMed] [Google Scholar]
- 49.Fiorentini C., Matarrese P., Straface E., Falzano L., Donelli G., Boquet P., Malorni W. Rho-dependent cell spreading activated by E.coli cytotoxic necrotizing factor 1 hinders apoptosis in epithelial cells. Cell Death Differ. 1998;5:921–929. doi: 10.1038/sj.cdd.4400422. [DOI] [PubMed] [Google Scholar]
- 50.Falzano L., Rivabene R., Santini M.T., Fabbri A., Fiorentini C. An Escherichia coli cytotoxin increases superoxide anion generation via Rac in epithelial cells. Biochem. Biophys. Res. Commun. 2001;283:1026–1030. doi: 10.1006/bbrc.2001.4894. [DOI] [PubMed] [Google Scholar]
- 51.Fiorentini C., Falzano L., Fabbri A., Stringaro A., Logozzi M., Travaglione S., Contamin S., Arancia G., Malorni W., Fais S. Activation of Rho GTPases by Cytotoxic Necrotizing Factor 1 Induces Macropinocytosis and Scavenging Activity in Epithelial Cells. Mol. Biol. Cell. 2001;12:2061–2073. doi: 10.1091/mbc.12.7.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Falzano L., Rivabene R., Fabbri A., Fiorentini C. Epithelial cells challenged with a Rac-activating E. coli cytotoxin acquire features of professional phagocytes. Toxicol. Vitr. 2002;16:421–425. doi: 10.1016/S0887-2333(02)00027-9. [DOI] [PubMed] [Google Scholar]
- 53.Boyer L., Travaglione S., Falzano L., Gauthier N.C., Popoff M.R., Lemichez E., Fiorentini C., Fabbri A. Rac GTPase Instructs Nuclear Factor-κB Activation by Conveying the SCF Complex and IkBα to the Ruffling Membranes. Mol. Biol. Cell. 2004;15:1124–1133. doi: 10.1091/mbc.e03-05-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malorni W., Fiorentini C. Is the Rac GTPase-activating toxin CNF1 a smart hijacker of host cell fate? FASEB J. 2006;20:606–609. doi: 10.1096/fj.05-4706hyp. [DOI] [PubMed] [Google Scholar]
- 55.Giamboi-Miraglia A., Travaglione S., Filippini P., Fabbri A., Fiorentini C., Falzano L. A multinucleating Escherichia coli cytotoxin perturbs cell cycle in cultured epithelial cells. Toxicol. Vitro. 2007;21:235–239. doi: 10.1016/j.tiv.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 56.Miraglia A.G., Travaglione S., Meschini S., Falzano L., Matarrese P., Quaranta M.G., Viora M., Fiorentini C., Fabbri A. Cytotoxic necrotizing factor 1 prevents apoptosis via the Akt/IkappaB kinase pathway: Role of nuclear factor-kappaB and Bcl-2. Mol. Biol. Cell. 2007;18:2735–2744. doi: 10.1091/mbc.e06-10-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fabbri A., Cori S., Zanetti C., Guidotti M., Sargiacomo M., Loizzo S., Fiorentini C. Cell-to-cell propagation of the bacterial toxin CNF1 via extracellular vesicles: Potential impact on the therapeutic use of the toxin. Toxins. 2015;7:4610–4621. doi: 10.3390/toxins7114610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McNichol B.A., Rasmussen S.B., Meysick K.C., O’Brien A.D. A single amino acid substitution in the enzymatic domain of cytotoxic necrotizing factor type 1 of Escherichia coli alters the tissue culture phenotype to that of the dermonecrotic toxin of Bordetella spp. Mol. Microbiol. 2006;60:939–950. doi: 10.1111/j.1365-2958.2006.05157.x. [DOI] [PubMed] [Google Scholar]
- 59.Abouzahr-Rifai S., Hasmim M., Boukerche H., Hamelin J., Janji B., Jalil A., Kieda C., Mami-Chouaib F., Bertoglio J., Chouaib S. Resistance of tumor cells to cytolytic T lymphocytes involves Rho-GTPases and focal adhesion kinase activation. J. Biol. Chem. 2008;283:31665–31672. doi: 10.1074/jbc.M800078200. [DOI] [PubMed] [Google Scholar]
- 60.Capo C., Meconi S., Sanguedolce M.V., Bardin N., Flatau G., Boquet P., Mege J.L. Effect of cytotoxic necrotizing factor-1 on actin cytoskeleton in human monocytes: Role in the regulation of integrin-dependent phagocytosis. J. Immunol. 1998;161:4301–4308. [PubMed] [Google Scholar]
- 61.Yang H., Li Q., Wang C., Wang J., Lv J., Wang L., Zhang Z.S., Yao Z., Wang Q. Cytotoxic Necrotizing Factor 1 Downregulates CD36 Transcription in Macrophages to Induce Inflammation During Acute Urinary Tract Infections. Front. Immunol. 2018;9:1987. doi: 10.3389/fimmu.2018.01987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo Y., Zhang Z., Wei H., Wang J., Lv J., Zhang K., Keller E.T., Yao Z., Wang Q. Cytotoxic necrotizing factor 1 promotes prostate cancer progression through activating the Cdc42-PAK1 axis. J. Pathol. 2017;243:208–219. doi: 10.1002/path.4940. [DOI] [PubMed] [Google Scholar]
- 63.Augspach A., List J., Wolf P., Bielek H., Schwan C., Elsässer-Beile U., Aktories K., Schmidt G. Activation of RhoA,B,C by Yersinia Cytotoxic Necrotizing Factor (CNFy) induces apoptosis in LNCaP prostate cancer cells. Toxins. 2013;5:2241–2257. doi: 10.3390/toxins5112241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tan X., Xu A., Zhao T., Zhao Q., Zhang J., Fan C., Deng Y., Freywald A., Genth H., Xiang J. Simulated microgravity inhibits cell focal adhesions leading to reduced melanoma cell proliferation and metastasis via FAK/RhoA-regulated mTORC1 and AMPK pathways. Sci. Rep. 2018;8:1–12. doi: 10.1038/s41598-018-20459-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao T., Li R., Tan X., Zhang J., Fan C., Zhao Q., Deng Y., Xu A., Lukong K.E., Genth H., et al. Simulated microgravity reduces focal adhesions and alters cytoskeleton and nuclear positioning leading to enhanced apoptosis via suppressing FAK/Rhoa-mediated mTORC1/NF-κB and ERK1/2 pathways. Int. J. Mol. Sci. 2018;19:1994. doi: 10.3390/ijms19071994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buscà R., Bertolotto C., Abbe P., Englaro W., Ishizaki T., Narumiya S., Boquet P., Ortonne J.P., Ballotti R. Inhibition of Rho is required for cAMP-induced melanoma cell differentiation. Mol. Biol. Cell. 1998;9:1367–1378. doi: 10.1091/mbc.9.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stray A., Janning A., Barth H., Gerke V. Endothelial Rho signaling is required for monocyte transendothelial migration. FEBS Lett. 2002;517:261–266. doi: 10.1016/S0014-5793(02)02643-1. [DOI] [PubMed] [Google Scholar]
- 68.Boyer L., Magoc L., Dejardin S., Cappillino M., Paquette N., Hinault C., Charriere G., Ip W., Fracchia S., Hennessy E., et al. Pathogen-derived effectors trigger protective immunity via activation of the Rac2 enzyme and the IMD or Rip kinase signaling pathway. Immunity. 2011;35:536–549. doi: 10.1016/j.immuni.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Messina V., Loizzo S., Travaglione S., Bertuccini L., Condello M., Superti F., Guidotti M., Alano P., Silvestrini F., Fiorentini C. The bacterial protein CNF1 as a new strategy against Plasmodium falciparum cytoadherence. PLoS ONE. 2019;14:e0213529. doi: 10.1371/journal.pone.0213529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baumer Y., Burger S., Curry F.E., Golenhofen N., Drenckhahn D., Waschke J. Differential role of Rho GTPases in endothelial barrier regulation dependent on endothelial cell origin. Histochem. Cell Biol. 2008;129:179–191. doi: 10.1007/s00418-007-0358-7. [DOI] [PubMed] [Google Scholar]
- 71.Schlegel N., Burger S., Golenhofen N., Walter U., Drenckhahn D., Waschke J. The role of VASP in regulation of cAMP- and Rac 1-mediated endothelial barrier stabilization. Am. J. Physiol. Cell Physiol. 2008;294:C178–C188. doi: 10.1152/ajpcell.00273.2007. [DOI] [PubMed] [Google Scholar]
- 72.Waschke J., Burger S., Curry F.R.E., Drenckhahn D., Adamson R.H. Activation of Rac-1 and Cdc42 stabilizes the microvascular endothelial barrier. Histochem. Cell Biol. 2006;125:397–406. doi: 10.1007/s00418-005-0080-2. [DOI] [PubMed] [Google Scholar]
- 73.Gliem M., Heupel W.M., Spindler V., Harms G.S., Waschke J. Actin reorganization contributes to loss of cell adhesion in pemphigus vulgaris. Am. J. Physiol. Cell Physiol. 2010;299:C606–C613. doi: 10.1152/ajpcell.00075.2010. [DOI] [PubMed] [Google Scholar]
- 74.Chiariello M., Marinissen M.J., Gutkind J.S. Regulation of c-myc expression by PDGF through Rho GTPases. Nat. Cell Biol. 2001;3:580–586. doi: 10.1038/35078555. [DOI] [PubMed] [Google Scholar]
- 75.Bannai Y., Aminova L.R., Faulkner M.J., Ho M., Wilson B.A. Rho/ROCK-dependent inhibition of 3T3-L1 adipogenesis by G-protein-deamidating dermonecrotic toxins: Differential regulation of Notch1, Pref1/Dlk1, and β-catenin signaling. Front. Cell. Infect. Microbiol. 2012;2:80. doi: 10.3389/fcimb.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Travaglione S., Messina G., Fabbri A., Falzano L., Giammarioli A.M., Grossi M., Rufini S., Fiorentini C. Cytotoxic necrotizing factor 1 hinders skeletal muscle differentiation in vitro by perturbing the activation/deactivation balance of Rho GTPases. Cell Death Differ. 2005;12:78–86. doi: 10.1038/sj.cdd.4401522. [DOI] [PubMed] [Google Scholar]
- 77.Kazmierczak B.I., Jou T.S., Mostov K., Engel J.N. Rho GTPase activity modulates Pseudomonas aeruginosa internalization by epithelial cells. Cell. Microbiol. 2001;3:85–98. doi: 10.1046/j.1462-5822.2001.00091.x. [DOI] [PubMed] [Google Scholar]
- 78.Moreau V., Tatin F., Varon C., Génot E. Actin Can Reorganize into Podosomes in Aortic Endothelial Cells, a Process Controlled by Cdc42 and RhoA. Mol. Cell. Biol. 2003;23:6809–6822. doi: 10.1128/MCB.23.19.6809-6822.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baumer Y., Drenckhahn D., Waschke J. cAMP induced Rac 1-mediated cytoskeletal reorganization in microvascular endothelium. Histochem. Cell Biol. 2008;129:765–778. doi: 10.1007/s00418-008-0422-y. [DOI] [PubMed] [Google Scholar]
- 80.Vouret-Craviari V., Grall D., Flatau G., Pouysségur J., Boquet P., Van Obberghen-Schilling E. Effects of cytotoxic necrotizing factor 1 and lethal toxin on actin cytoskeleton and VE-cadherin localization in human endothelial cell monolayers. Infect. Immun. 1999;67:3002–3008. doi: 10.1128/IAI.67.6.3002-3008.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Travaglione S., Loizzo S., Rizza T., Del Brocco A., Ballan G., Guidotti M., Vona R., Di Nottia M., Torraco A., Carrozzo R., et al. Enhancement of mitochondrial ATP production by the Escherichia coli cytotoxic necrotizing factor 1. FEBS J. 2014;281:3473–3488. doi: 10.1111/febs.12874. [DOI] [PubMed] [Google Scholar]
- 82.Malorni W., Quaranta M.G., Straface E., Falzano L., Fabbri A., Viora M., Fiorentini C. The Rac-activating toxin cytotoxic necrotizing factor 1 oversees NK cell-mediated activity by regulating the actin/microtubule interplay. J. Immunol. 2003;171:4195–4202. doi: 10.4049/jimmunol.171.8.4195. [DOI] [PubMed] [Google Scholar]
- 83.Gall-Mas L., Fabbri A., Namini M.R.J., Givskov M., Fiorentini C., Krejsgaard T. The bacterial toxin CNF1 induces activation and maturation of human monocyte-derived dendritic cells. Int. J. Mol. Sci. 2018;19:1408. doi: 10.3390/ijms19051408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fabbri A., Travaglione S., Maroccia Z., Guidotti M., Pierri C.L., Primiano G., Servidei S., Loizzo S., Fiorentini C. The bacterial protein CNF1 as a potential therapeutic strategy against mitochondrial diseases: A pilot study. Int. J. Mol. Sci. 2018;19:1825. doi: 10.3390/ijms19071825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dufies O., Doye A., Courjon J., Torre C., Michel G., Loubatier C., Jacquel A., Chaintreuil P., Majoor A., Guinamard R.R., et al. Escherichia coli Rho GTPase-activating toxin CNF1 mediates NLRP3 inflammasome activation via p21-activated kinases-1/2 during bacteraemia in mice. Nat. Microbiol. 2021;6:401–412. doi: 10.1038/s41564-020-00832-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hahn A., Barth H., Kress M., Mertens P.R., Goppelt-Struebe M. Role of Rac and Cdc42 in lysophosphatidic acid-mediated cyclo-oxygenase-2 gene expression. Biochem. J. 2002;362:33–40. doi: 10.1042/bj3620033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Malchiodi-Albedi F., Paradisi S., Di Nottia M., Simone D., Travaglione S., Falzano L., Guidotti M., Frank C., Cutarelli A., Fabbri A., et al. CNF1 improves astrocytic ability to support neuronal growth and differentiation in vitro. PLoS ONE. 2012;7:e34115. doi: 10.1371/journal.pone.0034115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boutillier S., Rapp J., Staeb T., Olenik C., Schmidt G., Meyer D.K., Leemhuis J. Cytotoxic necrotizing factor-2 of Escherichia coli alters the morphology of cultured hippocampal neurons. Naunyn. Schmiedebergs. Arch. Pharmacol. 2003;368:513–519. doi: 10.1007/s00210-003-0830-4. [DOI] [PubMed] [Google Scholar]
- 89.Musilli M., Ciotti M.T., Pieri M., Martino A., Borrelli S., Dinallo V., Diana G. Therapeutic effects of the Rho GTPase modulator CNF1 in a model of Parkinson’s disease. Neuropharmacology. 2016;109:357–365. doi: 10.1016/j.neuropharm.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 90.Czopka T., Von Holst A., Schmidt G., Ffrench-Constant C., Faissner A. Tenascin C and tenascin R similarly prevent the formation of myelin membranes in a RhoA-dependent manner, but antagonistically regulate the expression of myelin basic protein via a separate pathway. Glia. 2009;57:1790–1801. doi: 10.1002/glia.20891. [DOI] [PubMed] [Google Scholar]
- 91.Fortemaison N., Blancquaert S., Dumont J.E., Maenhaut C., Aktories K., Roger P.P., Dremier S. Differential involvement of the actin cytoskeleton in differentiation and mitogenesis of thyroid cells: Inactivation of Rho proteins contributes to cyclic adenosine monophosphate-dependent gene expression but prevents mitogenesis. Endocrinology. 2005;146:5485–5495. doi: 10.1210/en.2005-0329. [DOI] [PubMed] [Google Scholar]
- 92.Doye A., Boyer L., Mettouchi A., Lemichez E. Ubiquitin-mediated proteasomal degradation of Rho proteins by the CNF1 toxin. Methods Enzymol. 2006;406:447–456. doi: 10.1016/S0076-6879(06)06033-2. [DOI] [PubMed] [Google Scholar]
- 93.Haga R.B., Ridley A.J. Rho GTPases: Regulation and roles in cancer cell biology. Small GTPases. 2016;7:207–221. doi: 10.1080/21541248.2016.1232583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ding F., Yin Z., Wang H.-R. Ubiquitination in Rho Signaling. Curr. Top. Med. Chem. 2011;11:2879–2887. doi: 10.2174/156802611798281357. [DOI] [PubMed] [Google Scholar]
- 95.Yang S., Rosenwald A. Small GTPase proteins in macroautophagy. Small GTPases. 2018;9:409–414. doi: 10.1080/21541248.2016.1246280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fiorentini C., Matarrese P., Straface E., Falzano L., Fabbri A., Donelli G., Cossarizza A., Boquet P., Malorni W. Toxin-Induced Activation of Rho GTP-Binding Protein Increases Bcl-2 Expression and Influences Mitochondrial Homeostasis. Exp. Cell Res. 1998;242:341–350. doi: 10.1006/excr.1998.4057. [DOI] [PubMed] [Google Scholar]
- 97.Horiguchi Y. Escherichia coli cytotoxic necrotizing factors and Bordetella dermonecrotic toxin: The dermonecrosis-inducing toxins activating Rho small GTPases. Toxicon. 2001;39:1619–1627. doi: 10.1016/S0041-0101(01)00149-0. [DOI] [PubMed] [Google Scholar]
- 98.Chircop M. Rho GTPases as regulators of mitosis and cytokinesis in mammalian cells. Small GTPases. 2014;5:e29770. doi: 10.4161/sgtp.29770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.El-Aouar Filho R.A., Nicolas A., De Paula Castro T.L., Deplanche M., De Carvalho Azevedo V.A., Goossens P.L., Taieb F., Lina G., Le Loir Y., Berkova N. Heterogeneous family of cyclomodulins: Smart weapons that allow bacteria to hijack the eukaryotic cell cycle and promote infections. Front. Cell. Infect. Microbiol. 2017;7:208. doi: 10.3389/fcimb.2017.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Island M.D., Cui X., Warren J.W. Effect of Escherichia coli cytotoxic necrotizing factor 1 on repair of human bladder cell monolayers in vitro. Infect. Immun. 1999;67:3657–3661. doi: 10.1128/IAI.67.7.3657-3661.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Munro P., Flatau G., Lemichez E. Intranasal immunization with tetanus toxoid and CNF1 as a new mucosal adjuvant protects BALB/c mice against lethal challenge. Vaccine. 2007;25:8702–8706. doi: 10.1016/j.vaccine.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 102.Diabate M., Munro P., Garcia E., Jacquel A., Michel G., Obba S., Goncalves D., Luci C., Marchetti S., Demon D., et al. Escherichia coli α-Hemolysin Counteracts the Anti-Virulence Innate Immune Response Triggered by the Rho GTPase Activating Toxin CNF1 during Bacteremia. PLoS Pathog. 2015;11:1–20. doi: 10.1371/journal.ppat.1004732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ahmed A.U., Williams B.R.G., Hannigan G.E. Transcriptional activation of inflammatory genes: Mechanistic insight into selectivity and diversity. Biomolecules. 2015;5:3087–3111. doi: 10.3390/biom5043087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Collins P.W., Noble K.E., Reittie J.R., Hoffbrand A.V., Pasi K.J., Yong K.L. Induction of tissue factor expression in human monocyte/endothelium cocultures. Br. J. Haematol. 1995;91:963–970. doi: 10.1111/j.1365-2141.1995.tb05420.x. [DOI] [PubMed] [Google Scholar]
- 105.Fabbri A., Travaglione S., Fiorentini C. Escherichia coli cytotoxic necrotizing factor 1 (CNF1): Toxin biology, in vivo applications and therapeutic potential. Toxins. 2010;2:283–296. doi: 10.3390/toxins2020282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Omar A., Jovanovic K., Da Costa Dias B., Gonsalves D., Moodley K., Caveney R., Mbazima V., Weiss S.F. Patented biological approaches for the therapeutic modulation of the 37 kDa/67 kDa laminin receptor. Expert Opin. Ther. Pat. 2011;21:35–53. doi: 10.1517/13543776.2011.539203. [DOI] [PubMed] [Google Scholar]
- 107.Gopalakrishna R., Bhat N.R., Zhou S., Mack W.J. Cell signaling associated with internalization of 67 kDa laminin receptor (67LR) by soluble laminin and its implication for protection against neurodegenerative diseases. Neural Regen. Res. 2019;14:1513–1514. doi: 10.4103/1673-5374.255965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Loizzo S., Rimondini R., Travaglione S., Fabbri A., Guidotti M., Ferri A., Campana G., Fiorentini C. CNF1 Increases Brain Energy Level, Counteracts Neuroinflammatory Markers and Rescues Cognitive Deficits in a Murine Model of Alzheimer’s Disease. PLoS ONE. 2013;8:e65898. doi: 10.1371/annotation/8da0f878-fcab-4f65-bad0-c5bdda8181ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cloutier J.M., Charville G.W. Diagnostic classification of soft tissue malignancies: A review and update from a surgical pathology perspective. Curr. Probl. Cancer. 2019;43:250–272. doi: 10.1016/j.currproblcancer.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 110.Kashina A.S. Regulation of actin isoforms in cellular and developmental processes. Semin. Cell Dev. Biol. 2020;102:113–121. doi: 10.1016/j.semcdb.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Travaglione S., Fabbri A., Fiorentini C. The Rho-activating CNF1 toxin from pathogenic E. coli: A risk factor for human cancer development? Infect. Agent. Cancer. 2008;3:4. doi: 10.1186/1750-9378-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fabbri A., Travaglione S., Ballan G., Loizzo S., Fiorentini C., Fabbri A., Travaglione S., Ballan G., Loizzo S., Fiorentini C. The Cytotoxic Necrotizing Factor 1 from E. Coli: A Janus Toxin Playing with Cancer Regulators. Toxins. 2013;5:1462–1474. doi: 10.3390/toxins5081462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Piciocchi A., Germinario E.A.P., Garcia Etxebarria K., Rossi S., Sanchez-Mete L., Porowska B., Stigliano V., Trentino P., Oddi A., Accarpio F., et al. Association of polygenic risk score and bacterial toxins at screening colonoscopy with colorectal cancer progression: A multicenter case-control study. Toxins. 2021;13:569. doi: 10.3390/toxins13080569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.