Abstract
We report on the rapid and specific detection of bacteria commonly isolated from clinical specimens from cystic fibrosis (CF) patients by fluorescent in situ hybridization (FISH). On the basis of comparative sequence analysis, we designed oligonucleotide probes complementary to species-specific 16S rRNA regions of these microorganisms and demonstrated the specificities of the probes by hybridization of different remotely related as well as closely related reference strains. Furthermore, in a pilot project we investigated 75 sputum samples and 10 throat swab specimens from CF patients by FISH and detected Pseudomonas aeruginosa, Burkholderia cepacia, Stenotrophomonas maltophilia, Haemophilus influenzae, and Staphylococcus aureus within these specimens. The specificity of FISH was 100% in comparison to the results of conventional microbial culture. In contrast, the sensitivity of standard laboratory cultivation was moderately higher, since the limit for microscopic detection of bacteria within sputum samples by FISH was approximately 4 × 105 CFU/ml of sputum (resulting in a 90% sensitivity for FISH). Moreover, we demonstrated that FISH will be useful for the rapid detection of bacteria that cause acute pulmonary exacerbations in CF patients, as demonstrated in patients with H. influenzae, S. aureus, and P. aeruginosa exacerbations. Therefore, FISH is a valuable additional method for the rapid and specific detection of bacteria in clinical samples from CF patients, in particular, patients with pulmonary exacerbations.
Cystic fibrosis (CF) is the most frequent inherited lethal disorder of Caucasian populations and arises from mutations of the CF transmembrane conductance regulator (18). CF transmembrane conductance regulator dysfunction promotes the secretion of a sticky dehydrated mucus in various exocrine glands. The major clinical manifestation of CF occurs in the respiratory tract. The impaired mucociliary clearance leads to persistent microbial colonization and chronic bacterial infections of the lungs of CF patients, with periods of relative well-being complicated by episodes of debilitating infections. With each exacerbation, lung function declines, resulting in progressive pulmonary failure and eventually death (11, 22, 23).
Bacterial lung infections in CF patients are usually associated with a limited number of microbial species. Early in life CF patients become infected with Staphylococcus aureus and Haemophilus influenzae. Afterward, Pseudomonas aeruginosa is the most common and most important pathogen that infects patients with CF, being isolated from more than 80% of CF patients aged 26 or older, and once established, it is seldom eradicated (7, 23). During the last decade Stenotrophomonas maltophilia and Burkholderia cepacia have increasingly been isolated from sputum specimens of CF patients. Whereas the clinical significance of S. maltophilia is still under debate, the occurrence of B. cepacia has already been correlated with a poorer prognosis for CF patients (4, 5).
Deterioration of pulmonary function is the major problem of CF patients nowadays. Therefore, microbiological monitoring of the respiratory tract flora, including quantification of the isolated microorganisms, is a landmark in the clinical management of CF patients for the laboratory diagnosis of pulmonary exacerbations and the initiation of an appropriate antimicrobial treatment. Unfortunately, conventional microbiological methods require at least 1 to 3 days for definite detection of a microbe. Moreover, administration of antibiotics before clinical sampling may impede cultivation of bacteria. Furthermore, H. influenzae, an important pathogen in CF patients, may be difficult to detect in cultures of respiratory secretions of CF patients because of overgrowth with P. aeruginosa or S. aureus.
It is well established that fluorescent in situ hybridization (FISH) is a highly valuable tool for the specific and rapid detection of pathogenic bacteria in clinical samples without cultivation (2, 3, 17; K. Trebesius, L. Leitritz, K. Adler, S. Schubert, I. B. Authenrieth, and J. Heesemann, submitted for publication). Therefore, the objective of this pilot project was to evaluate the practicability, specificity, and sensitivity of FISH as a rapid method for the identification of microorganisms from clinical specimens of CF patients.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
All bacterial strains used in this study are listed in Table 1. For in situ hybridization they were grown aerobically in Luria-Bertani (LB) broth at 37°C, harvested while in the exponential growth phase, and fixed as described previously (1, 12).
TABLE 1.
Oligonucleotide probes used for FISH
| Probe | Target species | Probe sequence (5′-3′) | Target site | Reference |
|---|---|---|---|---|
| EUB338 | Bacteria | GCTGCCTCCCGTAGGAGT | 16S rRNA | 1 |
| PseaerA | P. aeruginosa | GGTAACCGTCCCCCTTGC | 16S rRNA | Trebesius et al., submitted |
| PseaerB | P. aeruginosa | TCTCGGCCTTGAAACCCC | 23S rRNA | Trebesius et al., submitted |
| Stemal | S. maltophilia | GTCGTCCAGTATCCACTGC | 16S rRNA | Present study |
| Burcep | B. cepacia | CTGTGCGCCGGTTCTCTT | 16S rRNA | Present study |
| Burkho | Burkholderia spp. | ACCCTCTGTTCCGACCAT | 16S rRNA | Present study |
| Haeinf | H. influenzae | CCGCACTTTCATCTTCCG | 16S rRNA | Present study |
| Staaur | S. aureus | GAAGCAAGCTTCTCGTCCG | 16S rRNA | Trebesius et al., submitted |
| Caal | C. albicans | GCCAAGGCTTATACTCGCT | 18S rRNA | 9 |
| Strpyo | Streptococcus pyogenes | CTAACATGCGTTAGTCTCTC | 16S rRNA | Trebesius et al., submitted |
| BET42a | Beta subclass of Proteobacteria | GCCTTCCCACTTCGTTT | 16S rRNAa | 10 |
Unlabeled probe BET42a was added to hybridization buffer to reduce nonspecific binding of labeled oligonucleotide probes.
Conventional microbiological laboratory diagnosis.
Sputum samples and throat swab specimens were collected from patients in the University Hospital of Munich and were submitted to the diagnostic laboratory of the Max von Pettenkofer-Institut. The specimens were prepared for conventional microbial culture as follows. Each clinical sputum specimen was diluted 1:2 with freshly prepared dithiothreitol (DTT; Sigma, Deisenhofen, Germany) solution (1 mg/ml) for liquefaction. Sputum samples were then plated on tryptone soy agar and MacConkey agar in dilutions of 10−1, 10−4, and 10−5 for quantitative determination (numbers of CFU) of cultivable bacteria and were then incubated aerobically at 32°C. An aliquot of each sample was plated on sheep blood agar and chocolate blood agar and incubated anaerobically at 32°C to prevent overgrowth of clinically important bacteria (H. influenzae, group A streptococci) by P. aeruginosa. For cultivation of B. cepacia, sputum samples were plated on tryptone soy agar supplemented with polymyxin B (16 μg/ml). Sabouraud agar was used for detection of Aspergillus spp. and Candida spp. Throat swabs were cultured qualitatively on the same set of media described above. All clinical samples were incubated for at least 72 h and were then examined for bacterial growth. Further identification of the cultivated microorganisms was done by standard microbiological techniques.
16S rRNA-directed FISH.
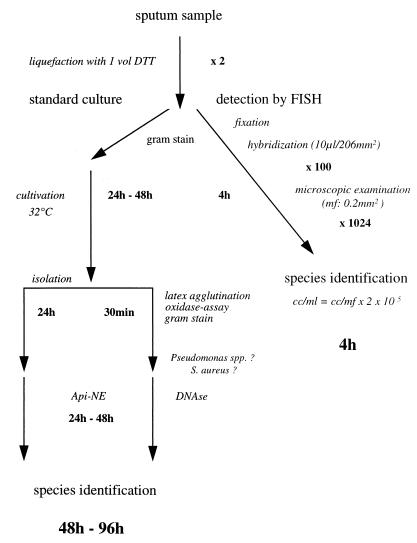
Oligonucleotide probes that hybridize to the 16S rRNAs of microorganisms typically associated with CF were synthesized and 5′ labeled with the cyanine dye Cy3 or with fluorescein (FLUOS) (Metabion, Munich, Germany), which represent red (Cy3) and green (FLUOS) fluorescence, respectively. In situ hybridization was performed on six-field glass slides (Marienfeld, Bad Mergentheim, Germany) with fixed bacterial strains or with 10 μl of DTT-treated and fixed sputum samples per field (206 mm2) for 1.5 h at 46°C in an isotonically equilibrated humid chamber (1). For hybridization of throat swab specimens, the specimens were smeared onto glass slides and heat fixed. Before hybridization the samples were air dried and dehydrated in an ascending ethanol series (50, 80, and 96%; 3 min each). Samples investigated for S. aureus were pretreated for 15 min with lysozyme (1 mg/ml in 10 mM Tris-HCl [pH 8.0]) and for 5 min with lysostaphin (10 μg/ml in 10 mM Tris-HCl [pH 8.0]). The species- and group-specific probes were applied simultaneously with probe EUB338, whose sequence is complementary to the sequence of a region of 16S rRNA that is unique for all bacteria. Clinical samples were preincubated for 30 min with 10 μl of hybridization buffer (0.9 M NaCl, 20 mM Tris-HCl [pH 7.6], 0.01% sodium dodecyl sulfate, 30% formamide) containing 50 ng of unlabeled oligonucleotide probe BET42a to reduce nonspecific binding of labeled oligonucleotide probes. Fixed samples were hybridized by application of 10 μl of hybridization buffer containing 5 ng of each specific oligonucleotide probe and 50 ng of probe BET42a. Stringent washing was performed by incubating the slide in washing buffer (20 mM Tris-HCl [pH 7.6], 0.01% sodium dodecyl sulfate, 112 mM NaCl) at 48°C for 15 min. Finally, the slides were rinsed with phosphate-buffered saline, air dried, and mounted in Citifluor (Citifluor Ltd., London, United Kingdom). The hybridized slides were further examined with a Leica fluorescent microscope equipped with a standard filter set (Leica Microsystems, Wetzlar, Germany) and the SPOT digital camera system (Diagnostic Instruments, Sterling Heights, Mich.) (Fig. 1A to D). For the semiquantitative microscopic determination of P. aeruginosa within sputum samples of CF patients, the visible-stained rRNA of bacteria (by FISH) in at least 30 randomly chosen microscopic fields (1 microscopic field = 0.2 mm2) were examined (bacterial cell count per milliliter of liquefied sputum = bacterial cell count per microscopic field × 2 × 100 × 1,024; see Fig. 2). Figure 1E and F were acquired with a Leica (Heerbrugg, Switzerland) TCS NT scanning confocal microscope. The standard software delivered by the manufacturer was used to further process the digitized images.
FIG. 1.
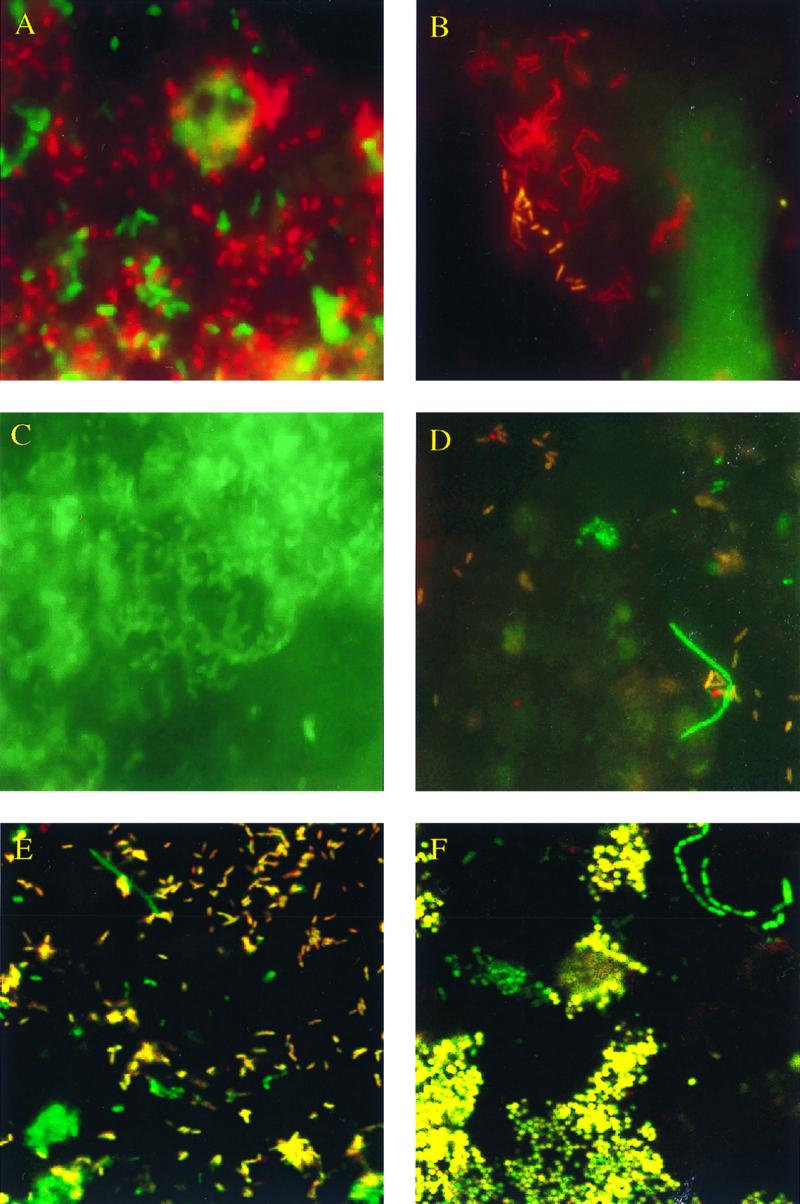
Specific detection of different bacteria in sputum samples by in situ hybridization with labeled oligonucleotide probes within 4 h. (A) P. aeruginosa (PseaerA-FLUOS–PseaerB-FLUOS) and H. influenzae (Haeinf-Cy3). (B) S. maltophilia (Stemal-FLUOS) and eubacterial probe (EUB338-Cy3). (C) P. aeruginosa (PseaerA-FLUOS–PseaerB-FLUOS). (D) B. cepacia (Burcep-Cy3) and (EUB338-FLUOS). (E) P. aeruginosa (PseaerA-Cy3–PseaerB-Cy3) and eubacterial probe (EUB338-FLUOS). (F) S. aureus (Staaur-Cy3) and eubacterial probe (EUB338-FLUOS). The images in panels E and F were acquired with a Leica TCS NT scanning confocal microscope. The dual combination of the red (Cy3) and the green (FLUOS) colors results in yellow.
FIG. 2.
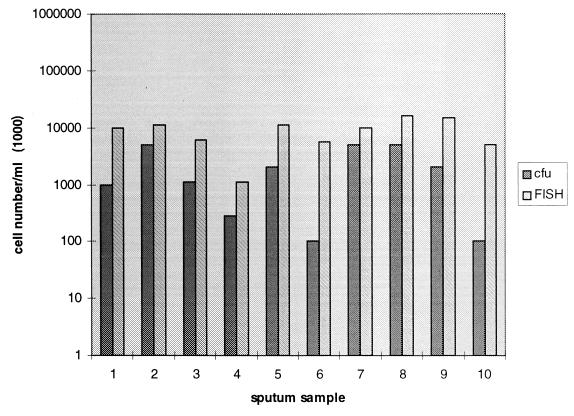
P. aeruginosa cell numbers within 10 different sputum samples determined by conventional culture (numbers of CFU) and FISH.
Probe design and evaluation of probe specificity.
For FISH of sputum samples of CF patients, species-specific oligonucleotide probes complementary to 16S rRNA regions of S. maltophilia, B. cepacia, and H. influenzae as well as a Burkholderia group-specific probe (Table 1) were developed. Oligonucleotide probes specific for S. aureus, Candida albicans, and P. aeruginosa were previously published (9; Trebesius et al., submitted), including a Burkholderia group-specific probe with approximately the same specificity and 16S rRNA position as probe Burkho (13). The specificities of the oligonucleotide probes (probes Burcep, Burkho, PseaerA, PseaerB, Stemal, and Haeinf) that were developed were tested by FISH of reference strains and of clinical isolates of the respective target organisms as well as of closely related strains according to sequence analysis (O. Strunk and W. Ludwig, ftp://ftp.mikro.biologie.tu-muenchen.de/pub/ARB; ARB software).
The optimal hybridization conditions for the different probes were determined by gradually increasing the formamide concentration in the hybridization buffer (from 0 to 30% in steps of 10%). Specificity testing of the oligonucleotide probes demonstrated that the probes were highly specific in the presence of 30% formamide and hybridized exclusively to their respective target organisms but not to any of the negative controls (Table 2). With probes specific for P. aeruginosa (probes PseaerA and PseaerB), nonmucoid and mucoid strains in control samples and clinical samples of CF patients could also be hybridized. Moreover, the simultaneous hybridization of probes PseaerA and Pseaer complementary to 16S rRNA (PseaerA) and 23S rRNA (PseaerB) improved the fluorescent signal and facilitated examination of clinical samples. The microscopic examination of 75 hybridized sputum samples revealed that in the presence of 30% formamide probe PseaerA but not probe probe PseaerB hybridized nonspecifically with Alcaligenes xylosoxidans (cultivated from four sputum samples which were culture negative for P. aeruginosa but positive with probe PseaerA). However, an increase in the formamide concentration in the hybridization buffer to 40% prevented the nonspecific binding of probe PseaerA to these species, and the remaining oligonucleotide probes listed in Table 1 also hybridized to the target organisms when 40% formamide was used.
TABLE 2.
Target organisms and reference strains used for FISH
| Organisms and strains | Source | Hybridization signal with the following probes (30% formamide):
|
||||||
|---|---|---|---|---|---|---|---|---|
| Eub338 | PseaerA | PseaerB | Stemal | Burcep | Burkho | Haeinf | ||
| Pseudomonas | ||||||||
| P. aeruginosa | DSM 50071 | + | + | + | − | − | − | − |
| P. aeruginosa | ATCC 25853 | + | + | + | − | − | − | − |
| P. aeruginosa | CF (10×) | + | + | + | − | − | − | − |
| P. putida | DSM 291 | + | − | − | − | − | − | − |
| P. stutzeri | DSM 5190 | + | − | − | − | − | − | − |
| P. chlororaphis | DSM 50083 | + | − | − | − | − | − | − |
| P. fluorescens | DSM 50090 | + | − | − | − | − | − | − |
| P. alcaligenes | DSM 50342 | + | − | − | − | − | − | − |
| Stenotrophomonas | ||||||||
| S. maltophilia | DSM 50170 | + | − | − | + | − | − | − |
| S. maltophilia | CF (10×) | + | − | − | + | − | − | − |
| Burkholderia | ||||||||
| B. cepacia | ATCC 25416 | + | − | − | − | + | + | − |
| B. cepacia | CF (10×) | + | − | − | − | + | + | − |
| B. caryophilli | DSM 50341 | + | − | − | − | − | + | − |
| B. andropogonis | DSM 9511 | + | − | − | − | − | + | − |
| B. gladioli | DSM 4285 | + | − | − | − | − | + | − |
| B. vietnamiensis | LMG 10929 | + | − | − | − | − | + | − |
| Alcaligenes | ||||||||
| A. faecalis | DSM 30030 | + | − | − | − | − | − | − |
| A. xylosoxidans | CF (4×) | + | +a | − | − | − | − | − |
| Haemophilus | ||||||||
| H. influenzae | ATCC 3391 | + | − | − | − | − | − | + |
| H. influenzae | CF (5×) | + | − | − | − | − | − | + |
| H. parainfluenzae | DSM 8978 | + | − | − | − | − | − | − |
| Bordetella pertussis | DSM 5571 | + | − | − | − | − | − | − |
| Streptococcus | ||||||||
| S. pneumonia | DSM 20566 | + | − | − | − | − | − | − |
| S. pyogenes | DSM 2071 | + | − | − | − | − | − | − |
| Escherichia coli | ATCC 25922 | + | − | − | − | − | − | − |
Negative in the presence of 40% formamide.
RESULTS
In situ detection of bacteria in specimens of CF patients by FISH in comparison with conventional microbiological culture.
To evaluate the practicability of FISH for detection of microorganisms from clinical specimens of CF patients, 75 sputum samples and 10 throat swab specimens of CF patients were sampled and investigated by convential culture techniques and by FISH (Table 3). FISH was performed with a set of oligonucleotide probes specific for bacteria commonly isolated from clinical samples of CF patients (probes PseaerA-PseaerB, Staaur, Haeinf, Burcep, Burkho, and Stemal) and allowed the detection of these microorganisms in sputum samples (Fig. 1A to F) and in throat swab specimens.
TABLE 3.
Examination of 75 sputum samples with various oligonucleotide probes by FISH and by conventional microbial culture
| Procedure and result | No. of samples containing the following species (probesa):
|
|||||
|---|---|---|---|---|---|---|
| P. aeruginosa (PsaerA-PsaerB) | S. aureus (Staaur) | H. influenzae (Haeinf) | B. cepacia (Burcep) | Burkholderia spp. (Burkho) | S. maltophilia (Stemal) | |
| Culture | ||||||
| Positive | 59 | 14 | 3 | 6 | 6 | 6 |
| Negative | 16 | 61 | 72 | 54 | 54 | 54 |
| Culture positive | ||||||
| FISH positive | 55 | 10 | 3 | 6 | 6 | 5 |
| FISH negative | 4 | 4 | 1 | |||
| Culture negative | ||||||
| FISH positive | 4 | 1 | ||||
| FISH negative | 12 | 25b | 24b | 25b | 25b | 25b |
Oligonucleotide probes used for FISH.
Only 25 culture-negative sputum samples were examined by FISH with the respective FISH probes.
By conventional culture 59 of 75 tested sputum samples were culture positive and 16 samples were culture negative for P. aeruginosa. In contrast P. aeruginosa could be detected by FISH in 55 of 75 sputum samples. All FISH-positive sputum samples were culture positive for P. aeruginosa (100% specificity). The numbers of CFU of P. aeruginosa determined within these 55 sputum samples were in the range of 4 × 105 to 4 × 109 CFU/ml, while the bacterial cell numbers in the remaining four culture-positive but FISH-negative sputum samples were less than 1 × 105 CFU/ml (sputum sample S1, 1 × 105 CFU/ml; sputum sample S2, 2 × 104 CFU/ml; sputum sample S3, 5 × 103 CFU/ml; sputum sample S4, 4 × 102 CFU/ml; [sensitivity of FISH, 93%]).
Moreover, 4 of 16 culture-negative sputum samples were FISH positive for P. aeruginosa by simultaneous hybridization with probes PseaerA and PseaerB in the presence of 30% formamide. A. xylosoxidans was isolated from these four sputum samples. Probe PseaerA nonspecifically hybridized to this species. Reexamination of these sputum samples with hybridization buffer containing 40% formamide prevented this nonspecific binding of probe PseaerA. Furthermore, 12 sputum specimens that were culture negative for P. aeruginosa were FISH negative for P. aeruginosa as well, including 3 samples containing other Pseudomonas spp. (P. putida, P. stutzeri, and P. fluorescens).
Furthermore, from S. aureus was cultivable 14 of 75 investigated sputum specimens. S. aureus was detectable by FISH from 10 culture-positive sputum samples, whereas 25 of 25 culture-negative sputum samples tested negative by FISH (100% specificity).
The numbers of S. aureus cells in these four culture-positive but FISH-negative samples were 5 × 104 cfu/ml or less (sputum sample S1, 5 × 104 CFU/ml; sputum sample S2, 1 × 104 CFU/ml; sputum sample S3, 2 × 103 CFU/ml; serum sample S4, 1 × 103 CFU/ml [sensitivity of FISH, 71%]).
Moreover, the cultivation result for B. cepacia in 6 culture-positive (>107 CFU/ml each) and 25 culture-negative sputum samples could be confirmed by FISH with Burkholderia species-specific probe Burcep and Burkholderia group-specific probe Burkho. The intensity of the fluorescent signal of the hybridized B. cepacia bacteria in these samples was remarkably low compared with those of the other bacteria simultaneously hybridized with eubacterial probe EUB338. This indicates that the B. cepacia organisms detected in the sputum samples of the CF patients investigated in the present study carry a smaller number of ribosomes than other bacteria. Nevertheless, despite this small number of ribosomes, B. cepacia was successfully detected by FISH (Fig. 1D).
Furthermore, of 75 investigated sputum samples, 3 tested positive for H. influenzae by conventional culture and by FISH as well, whereas only 5 of 6 culture-positive samples were positive for S. maltophilia by FISH. Similar to other culture-positive but FISH-negative samples, the count of S. maltophilia cells in the culture-positive but FISH-negative sample was beyond the microscopic detection limit (105 CFU/ml). The examination of 25 H. influenzae culture-negative sputum specimens with probe Haeinf found that 24 samples were FISH negative and 1 was FISH positive. Twenty-five sputum samples that were culture negative for S. maltophilia hybridized with the corresponding probe Stemal and were found to be FISH negative. Moreover, 25 sputum samples culture positive for C. albicans were investigated with FISH probe Caal and were FISH negative, but the concentration of C. albicans in all these samples was less than 104 CFU/ml.
These data demonstrate that the specificity of the FISH probes for detection of bacteria in clinical specimens of CF patients with respect to the results of conventional microbiological culture (the “gold standard”) was 100%. However, in situ hybridization of sputum samples turned out to be less sensitive than conventional culture techniques. The overall sensitivity for all specific oligonucleotide probes tested in this study was about 90%. The microscopic detection limit of FISH turned out to be approximately 4 × 105 CFU/ml of liquefied sputum. This limit was confirmed by an in vitro experiment in which a serial dilution of P. aeruginosa grown in LB broth was plated for CFU determination and was simultaneously examined by FISH. Fluorescently labeled bacteria could be detected microscopically up to a bacterial cell count of 2 × 105 CFU/ml of LB broth (an aliquot of 10 μl was hybridized).
Furthermore, 10 nonselected throat swab specimens from CF patients were investigated by conventional culture and by FISH: 3 throat swab specimens were culture positive and FISH positive for P. aeruginosa and 5 were culture positive and FISH positive for S. aureus, whereas S. maltophilia, B. cepacia, and H. influenzae were not detectable by conventional culture or FISH.
Quantitative determination by FISH of P. aeruginosa in sputum samples of CF patients.
Quantitative cultures of sputum samples of CF patients were commonly performed to monitor changes in bacterial cell number, especially for P. aeruginosa and S. aureus, with the aim of predicting the beginning of pulmonary exacerbations or controlling the efficacy of antimicrobial therapy.
We determined by FISH the numbers of P. aeruginosa cells in 10 sputum samples from CF patients who had not received intravenous or oral therapy but who had received inhalative (tobramycin or colistin) antipseudomonal therapy to compare the numbers of visible bacterial cells with the numbers of CFU of cultivable bacteria. The influence of antibiotic inhalation therapy might have led to reduced numbers of CFU in sputum samples. Sputum samples with less than 107 CFU of P. aeruginosa per ml were examined quantitatively by FISH because higher bacterial concentrations resulted in abundant background fluorescence, preventing the differentiation of single cells. This investigation demonstrated that the in situ cell number for P. aeruginosa visualized by FISH was about 10 times higher then the corresponding number of CFU (Fig. 2). Therefore, determination of the numbers of CFU in sputum samples of CF patients may not reflect the true bacterial concentration because of the insufficient disintegration of bacterial clumps embedded in mucus and impairment of microorganism growth due to antipseudomonal inhalation therapy.
Rapid identification by FISH of P. aeruginosa, H. influenzae, and S. aureus as etiologic agents of acute pulmonary exacerbations.
Herein we present three case reports of the rapid and specific detection by FISH of microorganisms that cause pulmonary exacerbations in CF patients. FISH was performed with a standard set of oligonucleotide probes (probes PseaerA-PseaerB, Burcep, Haeinf, Stemal, and Staaur) specific for bacteria commonly found in CF patients, including a probe specific for group A streptococci (Strpyo).
A 20-year-old female CF patient presented with clinical symptoms of pulmonary exacerbation 2 months after her last follow-up examination. The patient was known to be chronically infected with nonmucoid P. aeruginosa, with 4 × 107 CFU/ml found within the last investigated sputum sample. Immediately, intravenous empiric antipseudomonal therapy with ceftazidime and tobramycin was started. Microscopic examination of sputum samples revealed negative results with FISH probes Burcep, Stemal, Staaur, and Haeinf and positive signals with FISH probes PseaerA and PseaerB within 4 h. The counts for the P. aeruginosa cells visible in this sample were approximately >109 cells/ml (Fig. 1C and E), with a mass of bacterial cells embedded in sputum particles (Fig. 1C). Because of the exclusive identification of high counts for P. aeruginosa cells with probes PseaerA and PseaerB, we concluded that the clinical symptoms were induced by P. aeruginosa. In fact, by conventional culture 5 × 108 CFU of nonmucoid P. aeruginosa per ml and 5 × 108 CFU of mucoid P. aeruginosa per ml were detected (the organisms were susceptible to the combination of tobramycin and ceftazidime).
Second, a 16-year-old CF patient who had been chronically colonized with P. aeruginosa (nonmucoid) for 3 years (P. aeruginosa counts in the range of 1 × 107 to 2 × 107 CFU/ml for the last three sputum samples investigated at intervals of 3 months) presented with clinical symptoms of acute respiratory tract infection. H. influenzae, S. aureus, and other clinically important bacteria were not cultured, but P. aeruginosa was cultured from these sputum samples. Ciprofloxacin was directly administered per os. The actual sputum sample was FISH positive for P. aeruginosa and H. influenzae (1 × 107 CFU/ml for P. aeruginosa and 3 × 107 CFU/ml for H. influenzae; Fig. 1A). Hybridization with probes Staaur, Burcep, Burkho, Stemal, and Strpyo revealed negative results. Eventually, conventional culture revealed 106 CFU/ml for P. aeruginosa and additional large numbers (semiquantitative determination) of H. influenzae cells (H. influenzae was susceptible to ciprofloxacin).
Third, a 4-year-old female CF patient presented with fever, dyspnea, and cough 3 weeks after her last examination. The normal flora of the upper respiratory tract was cultivated from the last two throat swabs taken (every 3 months) from the patient, whereas before this low concentrations of S. aureus had been grown and P. fluorescens had been grown once. Ciprofloxacin was administered as empiric antibiotic prophylaxis. The FISH techniques revealed S. aureus, with an estimated visible cell count of about 108/ml within the current sputum sample (Fig. 1F), within 4 h. Moreover, FISH was negative with probes for P. aeruginosa, H. influenzae, group A streptococci, B. cepacia, and S. maltophilia. Finally, by conventional culture 4 × 107 CFU of S. aureus (ciprofloxacin sensitive) per ml was detectable.
The results for these three patients demonstrate that FISH rapidly detects P. aeruginosa, H. influenzae, and S. aureus within sputum samples of CF patients with pulmonary exacerbation. The results of FISH were confirmed 24 to 72 h later by conventional culture. Microscopic examination by FISH included identification to the species level, semiquantitative determination of microorganism numbers, and the rapid exclusion (within the detection limit) of other pathogens (e.g., B. cepacia and S. maltophilia).
DISCUSSION
CF is a common genetic disorder characterized by chronic bacterial colonization and recurrent bacterial infections of the upper and lower respiratory tracts. The outcome for CF patients is mainly lung disease. A rapid microbiological diagnosis and appropriate antibiotic therapy for pulmonary infections can prevent rapid deterioration because of lung disease. Therefore, once CF is diagnosed, all patients with CF are periodically assessed for airway colonization or pulmonary infection. Conventional laboratory methods require at least 24 to 72 h for definite laboratory diagnosis, and therefore, in severe cases of infection antimicrobial therapy frequently starts without knowledge of the causative microorganism.
FISH is a suitable method for the rapid and specific detection of pathogenic bacteria in clinical samples without time-consuming cultivation, delivers additional information concerning cell count and cell morphology, and is an in situ means of differentiation of mixed infections. Therefore, we evaluated the practicability of FISH for rapid identification of microorganisms in specimens of patients with CF. For this purpose, we developed 16S rRNA-targeted oligonucleotide probes directed to the most prevalent bacteria found in clinical samples of CF patients. Specificity testing of the oligonucleotide probes demonstrated that with 30% formamide all the FISH probes except probe PseaerA were highly specific and hybridized only with target organisms. By increasing the formamide concentration to 40%, nonspecific binding of probe PseaerB to A. xylosoxidans was prevented.
Furthermore, the practicability of FISH was demonstrated by the detection of CF-relevant bacterial species, including P. aeruginosa, S. aureus, B. cepacia, S. maltophilia, and H. influenzae, in sputum samples of CF patients. The specificity of the FISH technique when the results were compared with those of conventional microbiological culture was 100%, whereas the sensitivity of FISH was lower than that of microbiological culture. The detection limit of FISH was approximately 4 × 105 CFU/ml of sputum because of high background fluorescence. Therefore, the sensitivity of FISH for detection of bacteria from sputum samples may range between 4 × 105 and 1 × 106 CFU/ml of visible rRNA-stained bacteria. Eighty-five of 94 culture-positive sputum samples were found to be FISH positive as well (90% sensitivity), whereas the bacterial cell count in 9 sputum samples was beyond the microscopic detection limit.
Moreover, we demonstrated in patients with exacerbations due to H. influenzae, S. aureus, and P. aeruginosa that FISH is useful for the rapid detection of microorganisms that cause acute pulmonary infections. Pulmonary exacerbations in CF patients may be initiated by newly established bacterial infections or by reactivation of chronic lung infections. By using the FISH technique with the described set of oligonucleotide probes, we were able to detect pathogens commonly responsible for pulmonary exacerbations in CF patients. FISH may help to identify or at least to exclude an overload (>105 CFU/ml) of potential pathogens and, for example, may direct further laboratory investigations for detection of atypical bacteria or organisms of nonbacterial origin. Therefore, FISH will support the rapid diagnosis of pulmonary exacerbations and an adequate pathogen-directed antibiotic therapy for CF patients with exacerbations and will probably improve the clinical outcomes for CF patients. In fact, because of the multidrug-resistant nature of P. aeruginosa, it is important to know whether P. aeruginosa is responsible for pulmonary exacerbations in CF patients, especially if airway colonization with P. aeruginosa has not yet been established or if B. cepacia emerges as an additional pathogen, so that an appropriate antimicrobial therapy can be initiated.
Furthermore, H. influenzae is an important pathogen in patients with CF and commonly colonizes the upper respiratory tract of humans. Therefore, caution concerning the interpretation of isolation of this organism from sputum samples or throat swab specimens from CF patients with lower respiratory tract infections must be used. Moreover, H. influenzae may be difficult to detect in respiratory secretions of CF patients because of overgrowth with P. aeruginosa and S. aureus. FISH will be a rapid and specific method for detection of H. influenzae, including differentiation from Haemophilus parainfluenzae with quantitative determination of bacterial cell number. Therefore, rapid results from in situ investigations of clinical specimens by FISH may improve the diagnosis of pulmonary exacerbations caused by H. influenzae.
B. cepacia, a phytopathogen first described in the 1950s (5), is resistant to many of the traditional antipseudomonal antibiotics. When recovered from the sputum of CF patients, B. cepacia is associated with a poor clinical prognosis, because fatal pulmonary infections occur frequently (5, 6, 14). However, in some CF patients long-term colonization occurred without adversely affecting lung function, whereas in others, chronic infections associated with slowly declining lung function were seen. Finally, in another group of patients acute fulminant lung infection that led to death in weeks to months was observed (8, 15, 16). B. cepacia strains associated with acute clinical decline usually belong to genomovar III (20), but the factors that determine its pathogenetic potential are still unclear. During this study we observed that in all B. cepacia-positive sputum samples of chronically infected patients investigated, these species showed only a low fluorescent signal. A low fluorescent signal correlates with a low ribosome content and probably a low rate of in situ metabolic activity during chronic infection.
This study further demonstrated that the in situ cell numbers determined by FISH were about 10 times higher than the numbers of CFU (Fig. 2) in some instances. In general, exact determination of the bacterial cell count present in sputum samples is not reliable because of the viscous nature of the clinical material and the density of bacterial cell clusters found in sputum samples. FISH applied to sputum samples reveals properly that liquefaction of sputum by pretreatment with DTT does not dissociate all bacterial clumps and thus results in smaller numbers of CFU after plating. In fact, many bacteria are embedded together in tiny sputum particles and prevent microscopic counting of all single bacterial cells. Therefore, FISH is reliable only for semiquantitative determination of the bacterial cell count within sputum samples of CF patients.
Although antibiotic activity within sputum samples may impede bacterial cultivation, there was no significant difference in the bacterial cell concentration determined by FISH in comparison to that determined by culture between sputum of patients without antibiotic treatment (Fig. 2) or with antibiotic treatment (intravenous therapy; data not shown). Nontreated patients, if they were chronically infected, at least received intermittent antipseudomonal inhalation therapy that could also impair the growth of microorganisms. P. aeruginosa was still cultivable from the investigated sputum samples, despite antibiotic therapy. Nevertheless, negative culture results for sputum samples of CF patients can occur during antibiotic treatment, and use of these samples may be more suitable for underscoring the advantage of the FISH technique for the detection of microorganisms from antibiotic-treated patients.
Furthermore, bacterial species identification by conventional microbiological laboratory methods is time-consuming and not always definite. In fact, the first diagnosis of colonization with P. aeruginosa or B. cepacia, which is usually correlated with a decline in pulmonary function and a poorer prognosis for CF patients, must be absolutely reliable. Moreover, some CF centers recommend aggressive treatment after the first isolation of P. aeruginosa in an attempt to eradicate it (19, 21). Therefore, the identification of these microbial species must be substantiated. The use of specific rRNA-targeted oligonucleotide probes may help to overcome this problem because FISH is a rapid and inexpensive method for bacterial identification to the species level, is appropriate for daily routine application, and is as reliable as 16S rRNA sequencing.
In summary, the potential to detect and identify pathogenic bacteria in clinical samples of CF patients within a few hours without time-consuming cultivation and identification by standard bacterial techniques may prove that FISH offers alternatives to traditional diagnostic methods. FISH is an inexpensive, rapid (Fig. 3), and highly valuable method for the specific identification of P. aeruginosa, S. maltophilia, B. cepacia, H. influenzae, and S. aureus, the most prevalent bacteria associated with pulmonary infections in patients with CF. Moreover, FISH will be useful for diagnosis of respiratory exacerbations of microbial infections and for diagnosis of infections when clinical sampling occurs while the patient is receiving antimicrobial therapy. However, it should be emphasized that FISH will only partially replace cultivation of pathogens because of the limited sensitivity of FISH and the need for antibiotic susceptibility testing.
FIG. 3.
Time course and management of sputum samples for rapid bacterial detection by FISH and by standard culture. mf, microscopic field; cc, cell count.
The possibility of the rapid and simultaneous detection of pathogenic bacteria in clinical samples of CF patients will help to improve diagnosis of a microbiological infection and clinical management of these patients. Furthermore, FISH will provide additional important information about total bacterial cell count, bacterial activity, and the morphology, localization, and distribution of bacteria within clinical samples. However, further studies are necessary to determine if these data provide more information about the in situ situation and therefore may help to provide a better understanding of the pathogenicity of respiratory tract infections in CF patients.
ACKNOWLEDGMENT
We thank Kristin Adler for expert technical assistance.
REFERENCES
- 1.Amann R I, Krumholz L, Stahl D. Fluorescent oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J Bacteriol. 1990;172:762–770. doi: 10.1128/jb.172.2.762-770.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann R I, Ludwig W, Schleifer K-H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59:225–229. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeLong E F, Wickham G S, Pace N R. Phylogenetic strains: ribosomal RNA-based probes for the identification of single cells. Science. 1989;243:1360–1363. doi: 10.1126/science.2466341. [DOI] [PubMed] [Google Scholar]
- 4.Denton M, Todd N J, Kerr K G, Hawkey P M, Littlewood J M. Molecular epidemiology of Stenotrophomonas maltophilia isolated from clinical specimens from patients with cystic fibrosis and associated environmental samples. J Clin Microbiol. 1998;36:1953–1958. doi: 10.1128/jcm.36.7.1953-1958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Govan J R, Hugues J E, Vandamme P. Burkholderia cepacia: medical, taxonomic and ecological issues. J Med Microbiol. 1996;45:395–407. doi: 10.1099/00222615-45-6-395. [DOI] [PubMed] [Google Scholar]
- 6.Govan J R, Deretic V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev. 1996;60:539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoiby N. Microbiology of lung infections in cystic fibrosis patients. Acta Paediatr Scand Suppl. 1982;301:33–54. [PubMed] [Google Scholar]
- 8.Isles A, Maclusky I, Corey M, Gold R, Prober C, Fleming P, Levison H. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J Pediatr. 1984;104:206–210. doi: 10.1016/s0022-3476(84)80993-2. [DOI] [PubMed] [Google Scholar]
- 9.Kempf, V. A. J., K. Trebesius, and I. B. Autenrieth. Rapid identification of microbial pathogens in blood cultures by fluorescently labelled rRNA-targeted oligonucleotides. Ann. Intern. Med., in press.
- 10.Manz W, Amann R, Ludwig W, Wagner M, Schleifer K-H. Phylogenetic oligodeoxynucleotide probes for the major subclasses of proteobacteria: problems and solutions. Syst Appl Microbiol. 1992;5:593–600. [Google Scholar]
- 11.Pedersen S S, Hoiby N, Espersen F, Koch C. Role of alginate in infection with mucoid Pseudomonas aeruginosa in cystic fibrosis. Thorax. 1992;47:6–13. doi: 10.1136/thx.47.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roller C, Wagner M, Amann R, Ludwig W, Schleifer K-H. In situ probing of gram-positive bacteria with high DNA G+C content using 23S rRNA-targeted oligonucleotides. Microbiology. 1994;140:2849–2858. doi: 10.1099/00221287-140-10-2849. [DOI] [PubMed] [Google Scholar]
- 13.Stoffels M, Amann R, Ludwig W, Hekmat D, Schleifer K-H. Bacterial community dynamics during start-up of a trickle-bed bioreactor degrading aromatic compounds. Appl Environ Microbiol. 1998;64:930–939. doi: 10.1128/aem.64.3.930-939.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tablan O C, Chorba T L, Schidlow D V, White J W, Hardy K A, Gilligan P H, Morgan W M, Carson L A, Martone W J, Jason J M, Jarvis W R. Pseudomonas cepacia colonization in patients with cystic fibrosis: risk factors and clinical outcome. J Pediatr. 1985;107:382–387. doi: 10.1016/s0022-3476(85)80511-4. [DOI] [PubMed] [Google Scholar]
- 15.Thomassen M J, Demko C A, Doershuk C F, Stern R C, Klinger J D. Pseudomonas cepacia: decrease in colonization in patients with cystic fibrosis. Am Rev Respir Dis. 1986;134:669–671. doi: 10.1164/arrd.1986.134.4.669. [DOI] [PubMed] [Google Scholar]
- 16.Thomassen M J, Demko C A, Klinger J D, Stern R C. Pseudomonas cepacia colonization among patients with cystic fibrosis: a new opportunist. Am Rev Respir Dis. 1995;131:791–796. doi: 10.1164/arrd.1985.131.5.791. [DOI] [PubMed] [Google Scholar]
- 17.Trebesius K, Harmsen D, Rakin A, Schmelz J, Heesemann J. Developement of rRNA-targeted PCR and in situ hybridization with fluorescently labelled oligonucleotides for detection of Yersinia species. J Clin Microbiol. 1998;36:2557–2564. doi: 10.1128/jcm.36.9.2557-2564.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsui L C, Buchwald M, Barker D, Braman J C, Knowlton R, Schumm J W, Eiberg H, Mohr J, Kennedy D, Plavsic N, et al. Cystic fibrosis locus defined by a genetically linked polymorphic DNA marker. Science. 1985;230:1054–1057. doi: 10.1126/science.2997931. [DOI] [PubMed] [Google Scholar]
- 19.Valerius N H, Koch C, Hoiby N. Prevention of chronic Pseudomonas aeruginosa colonisation in cystic fibrosis by early treatment. Lancet. 1991;338:725–726. doi: 10.1016/0140-6736(91)91446-2. [DOI] [PubMed] [Google Scholar]
- 20.Vandamme P, Holmes B, Vancanneyt M, Coenye T, Hoste B, Coopman R, Revets H, Lauwers S, Gillis M, Kersters M, Govan J R. Occurrence of multiple genomovars of Burkholderia cepacia in cystic fibrosis patients and proposal of Burkholderia multivorans sp. nov. Int J Syst Bacteriol. 1997;47:1188–1200. doi: 10.1099/00207713-47-4-1188. [DOI] [PubMed] [Google Scholar]
- 21.Vazquez C, Municio M, Corera M, Gaztelurrutia L, Sojo A, Vitoria J C. Early treatment of Pseudomonas aeruginosa colonization in cystic fibrosis. Acta Paediatr. 1993;82:308–309. doi: 10.1111/j.1651-2227.1993.tb12668.x. [DOI] [PubMed] [Google Scholar]
- 22.Wood R E, Boat T F, Doershuk C F. State of the art: cystic fibrosis. Am Rev Respir Dis. 1976;113:833–878. doi: 10.1164/arrd.1976.113.6.833. [DOI] [PubMed] [Google Scholar]
- 23.Zach M S. Lung disease in cystic fibrosis—an updated concept. Pediatr Pulmonol. 1990;8:188–202. doi: 10.1002/ppul.1950080311. [DOI] [PubMed] [Google Scholar]