Abstract
Repetitive behaviors (e.g., stereotypic movements, compulsions, rituals) are common features of a number of neurodevelopmental disorders. Clinical and animal model studies point to the importance of cortical-basal ganglia circuitry in the mediation of repetitive behaviors. In the current study, we tested whether a drug cocktail (dopamine D2 receptor antagonist + adenosine A2A receptor agonist + glutamate mGlu5 positive allosteric modulator) designed to activate the indirect basal ganglia pathway would reduce repetitive behavior in C58 mice after both acute and sub-chronic administration. In addition, we hypothesized that sub-chronic administration (i.e. 7 days of twice-daily injections) would increase the functional activation of the subthalamic nucleus (STN), a key node of the indirect pathway. Functional activation of STN was indexed by dendritic spine density, analysis of GABA, glutamate, and synaptic plasticity genes, and cytochrome oxidase activity. The drug cocktail used significantly reduced repetitive motor behavior in C58 mice after one night as well as seven nights of twice-nightly injections. These effects did not reflect generalized motor behavior suppression as non-repetitive motor behaviors such as grooming, digging and eating were not reduced relative to vehicle. Sub-chronic drug treatment targeting striatopallidal neurons resulted in significant changes in the STN, including a four-fold increase in brain-derived neurotrophic factor (BDNF) mRNA expression as well as a significant increase in dendritic spine density. The present findings are consistent with, and extend, our prior work linking decreased functioning of the indirect basal ganglia pathway to expression of repetitive motor behavior in C58 mice and suggest novel therapeutic targets.
Keywords: Neurodevelopmental disorders, Repetitive behaviour, Obsessive compulsive disorder, Autism spectrum disorder, Subthalamic nucleus, Indirect pathway, Basal ganglia
1. Introduction
Repetitive behaviors, including stereotypic movements, compulsions, rituals, and circumscribed interests, are common features of a number of neurodevelopmental, psychiatric, and neurological disorders. The presentation of repetitive behaviors can be variable across individuals and clinical disorders, but they are generally described as apparently purposeless, inappropriate to the context, and exhibiting little variation in form. With regard to neurodevelopmental disorders, repetitive behavior is diagnostic for autism spectrum disorder (ASD) and a common behavioral phenotype of genetic or syndromic (e.g. Prader-Willi, Rett, Lesch-Nyhan syndromes) and non-syndromic intellectual disabilities [1]. Compulsions in obsessive-compulsive disorder and tics in Tourette syndrome also meet the definition of repetitive behaviors [2,3]. Unfortunately, for most children and adolescents within these clinical populations, pharmacological treatment is generally not effective [4–8].
A better understanding of the neurobiological mechanisms that mediate repetitive behaviors will undoubtedly improve our ability to develop novel, efficacious drug treatments [9]. Behavioral and genetic heterogeneity within and across the clinical disorders associated with repetitive behavior complicates these neurobiological investigations. Cortico-basal ganglia circuitry has received the most attention in the study of repetitive behavior and involves widespread cortical inputs to striatal projection neurons that make up the two complementary basal ganglia tracts, the direct and indirect pathways [1,10].
Animal models of repetitive behavior have also indicated a role for cortico-basal ganglia circuitry [10]. Genetic disruption of the cortico-striatal synapse [11–13], inactivation of basal ganglia regions [14,15], and exacerbating dopaminergic modulation of the direct [16] and/or indirect pathways [17–19] can all induce repetitive behaviors. In addition, utilization of mouse models that exhibit repetitive behaviors in the context of impoverished laboratory housing have also elucidated a key role for basal ganglia circuitry.
Deer mice (Peromyscus maniculatus) and C58 mice (Mus musculus) exhibit spontaneous repetitive behaviors that include hindlimb jumping and backward somersaulting [20,21]. These repetitive behaviors are seen in very young mice of both species and are expressed at asymptotic levels by five weeks post-weaning [22–25]. In addition, five weeks of environmental enrichment (EE) starting at weaning significantly reduced repetitive motor behavior in both models [20,23].
Our previous work using both mouse models has also provided evidence for an important role for reduced activity of the indirect basal ganglia pathway in the expression of repetitive motor behavior. For example, we have found lower neuronal activation, as indexed by cytochrome oxidase (CO) staining, in the subthalamic nucleus (STN) in high versus low repetitive behavior deer mice [26]. Similarly, lower CO staining in STN was found in C58 mice when compared to control C57BL/6 (B6) mice. We have also shown higher levels of CO staining in STN in F2 mice (from a C58 X B6 cross) that exhibited low levels of repetitive behavior versus F2 mice displaying high levels of repetitive behavior [27]. Moreover, EE which significantly reduced repetitive behavior in deer mice, was associated with higher levels of CO staining in STN as well as globus pallidus [28], although EE did not induce higher levels of CO staining in STN in C58 mice [27]. We have also reported lower dendritic spine density in STN of C58 mice compared to B6 controls. In addition, EE was associated with increased dendritic spine density in the STN of both deer mice and C58 mice [27,28]. We have also shown that a drug cocktail designed to activate striatopallidal neurons, significantly and selectively attenuated repetitive motor behavior in both deer mice and C58 mice [26,29]. In these studies, we identified the A2A receptor as a primary druggable target based on its enriched expression in striatum and selective expression on striatopallidal neurons. We found, however, an A2A agonist alone had no effect on repetitive behavior but addition of an A1 agonist resulted in significant reductions.
Reduced function of the STN in models of repetitive behavior is consistent with our current understanding of the role the STN in behavioral inhibition [30–32]. Activation of the STN is important for stopping on-going behavior [33].
We recently demonstrated that a drug cocktail made up of a dopamine D2 receptor antagonist, an adenosine A2A receptor agonist, and a glutamate mGlu5 positive allosteric modulator (PAM) significantly and selectively reduced repetitive behavior in deer mice [34]. These neurotransmitter receptors were targeted based on evidence that they form a heteromeric receptor complex on indirect pathway neurons of the striatum [35,36]. We found low doses of each drug (doses that did not reduce repetitive behavior alone or in double drug combinations) selectively reduced repetitive behavior when the three drugs were administered together. This drug cocktail was formulated to increase activation of the striatal indirect pathway neurons.
In the current study, we sought to systematically replicate our previous work by testing the generalizability of this drug cocktail to reduce repetitive behavior in C58 mice after both acute and sub-chronic administration. In addition, we hypothesized that sub-chronic administration (i.e. 7 days of twice-daily injections) would increase the functional activation of the STN. We measured functional activation using measurements of dendritic spine density, analysis of GABA, glutamate, and synaptic plasticity genes, and cytochrome oxidase activity.
2. Methods and materials
2.1. Animals
Fifty-three male C58 mice (Mus musculus) were acquired from our established breeding colony, weaned at 21 days of age, and group housed 3–6 mice per cage. Standard housing cages for C58 mice measured 29 × 18 × 13 cm and Sani-chips were used for bedding. Food and water were available ad libitum and two Nestlet squares were provided for nest construction. Housing cages were changed every two weeks and new Nestlet squares were added. The housing room was maintained at 70−75 °F and humidity ranged from 50 to 70%. The housing room was kept on a 12 h:12 -h light:dark cycle, with lights off at 8:00 PM. All procedures were performed in accordance with the guidelines set forth in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Florida.
2.2. Drugs
To target the indirect pathway neurons of the striatum pharmacologically, we formulated a drug cocktail consisting of a dopamine D2 receptor antagonist, an adenosine A2A receptor agonist, and a glutamate mGlu5 receptor positive allosteric modulator (PAM). The dopamine D2 receptor antagonist, L-741,626 (4-(4-Chlorophenyl)-1-(1H-indol-3-ylmethyl)-4-piperidinol), was purchased from Tocris Bioscience (Bio-Techne Corporation, Minneapolis, MN). The adenosine A2A receptor agonist, CGS21680 (2-p-(2-Carboxyethyl)phenethylamino-5′-Nethylcarboxamidoadenosine hydrochloride), and the glutamate mGlu5 receptor PAM, CDPPB (3-Cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide), were acquired from the National Institute of Mental Health Chemical Synthesis and Drug Supply Program. Based on our previous dose response analyses in deer mice [34], we suspended L-741,626 at 0.5 mg/mL, CGS21680 at 0.03 mg/mL, and CDPPB at 1.5 mg/mL in peanut oil and injected the drug cocktail at 5 mg/kg for L-741,626, 0.3 mg/kg for CGS21680, and 15 mg/kg for CDPPB. Drugs were injected at 1 mL per 100 g body weight and ranged from 0.18−0.28 mL (i.e. mouse body weights ranged from 18.3 to 28.2 g). The drug cocktail was administered subcutaneously in a single injection and injections were alternated between the left and right flanks. Each of these drugs has been tested at these doses in single and double drug combinations, in male deer mice, and they had no significant effects on repetitive behavior [34]. In addition, the three drug cocktail did not affect body weight over seven days of administration and did not have significant effects on non-repetitive behaviors (e.g. locomotion [34] in deer mice.
2.3. Sub-chronic drug treatment
In adult male deer mice, this drug cocktail formulation reduced repetitive behavior for five hours following injection when it was administered at the start of their 8 -h dark cycle [34]. A preliminary study in C58 mice showed drug effects on repetitive behavior lasted only 6 h. Since we were interested in showing long lasting brain plasticity changes and given their 12 h dark cycle, we injected each mouse with either peanut oil vehicle (n = 26) or the drug cocktail (n = 27) twice per dark cycle (at 8:00 PM and 2:00 AM) each day for seven days. Beginning at five weeks post-weaning (post-natal day 56), each mouse was randomly assigned to either the vehicle or drug cocktail group and all mice continued to be socially housed throughout the seven days of injections. On days 1 and 7, the mice were tested for repetitive behavior (see following section). On days 2–6, mice were weighed (at 8:00 PM), injected (at 8:00 PM and 2:00 AM), and then returned to their home cage in the housing room.
2.4. Assessment of behavior
On days 1 and 7 of vehicle or drug cocktail administration, each mouse was removed from its cage, weighed, and placed singly in a clear plastic testing cage (22 × 15 × 28 cm) at least one hour before lights off (i.e. 7:00 PM). These testing cages had Sani-chip bedding, food pellets, and a water bottle. Following habituation, each animal was assessed for the entire 12 -h dark cycle (i.e. 8:00 PM to 8:00 AM). Vertical hindlimb jumping and backward somersaulting, the primary repetitive motor behaviors in C58 mice, were quantified using photobeam arrays (Columbus Instruments, Columbus, OH). These vertical behaviors resulted in photobeam interruptions that were recorded as “repetitive behavior counts” with accompanying time stamps [23]. The apparatus was set so rearing or other non-stereotyped vertical activity did not result in photobeam interruptions. All test sessions were video recorded to identify the topography of repetitive behavior and verify the accuracy of the automated counters. The number of repetitive behavior counts per 15-minute period was calculated for each mouse for Day 1 and Day 7 for graphical purposes and summed for each of those testing days for use in statistical analysis.
Assessment of non-repetitive motor behaviors was performed using the video recordings from randomly selected vehicle- (n = 8) and drug cocktail-treated mice (n = 8) at 3:00 am, 3:30 am, 4:00 am, 4:30 am, 5:00 am, 5:30 am, 6:00 am, and 6:30 am on day 7 of drug administration. Five-minute (300 s) videos were scored at each of these 8 times, providing 2400 total seconds of video. These times were selected because the repetitive behavior counts at these times on day 7 were the lowest values documented throughout the experiment (Fig. 1). Duration of grooming, digging, eating, drinking, inactivity, rearing, and locomotion was scored by a human observer, who was blind to treatment condition, using Behavioral Observation Research Interactive Software (BORIS), which is open-source software for video coding of behavioral events [37]. Grooming was defined as the licking of the paws or body and paw strokes over the head and face. Digging was scored when the mouse moved the bedding with its paws or its face. Eating was scored when the mouse held a food pellet with the paws and was actively gnawing at it. Drinking was scored when the mouse approached and kept in contact with the spout of the water bottle. Inactivity was scored when the mouse was still for longer than three seconds. Rearing was scored when the mouse placed its forepaws on the cage wall or was free-standing. Locomotion was scored when the mouse was actively walking around the cage. This metric for locomotion (duration) differs from the more conventional metric of distance travelled that is measured automatically in an open field arena.
Fig. 1.
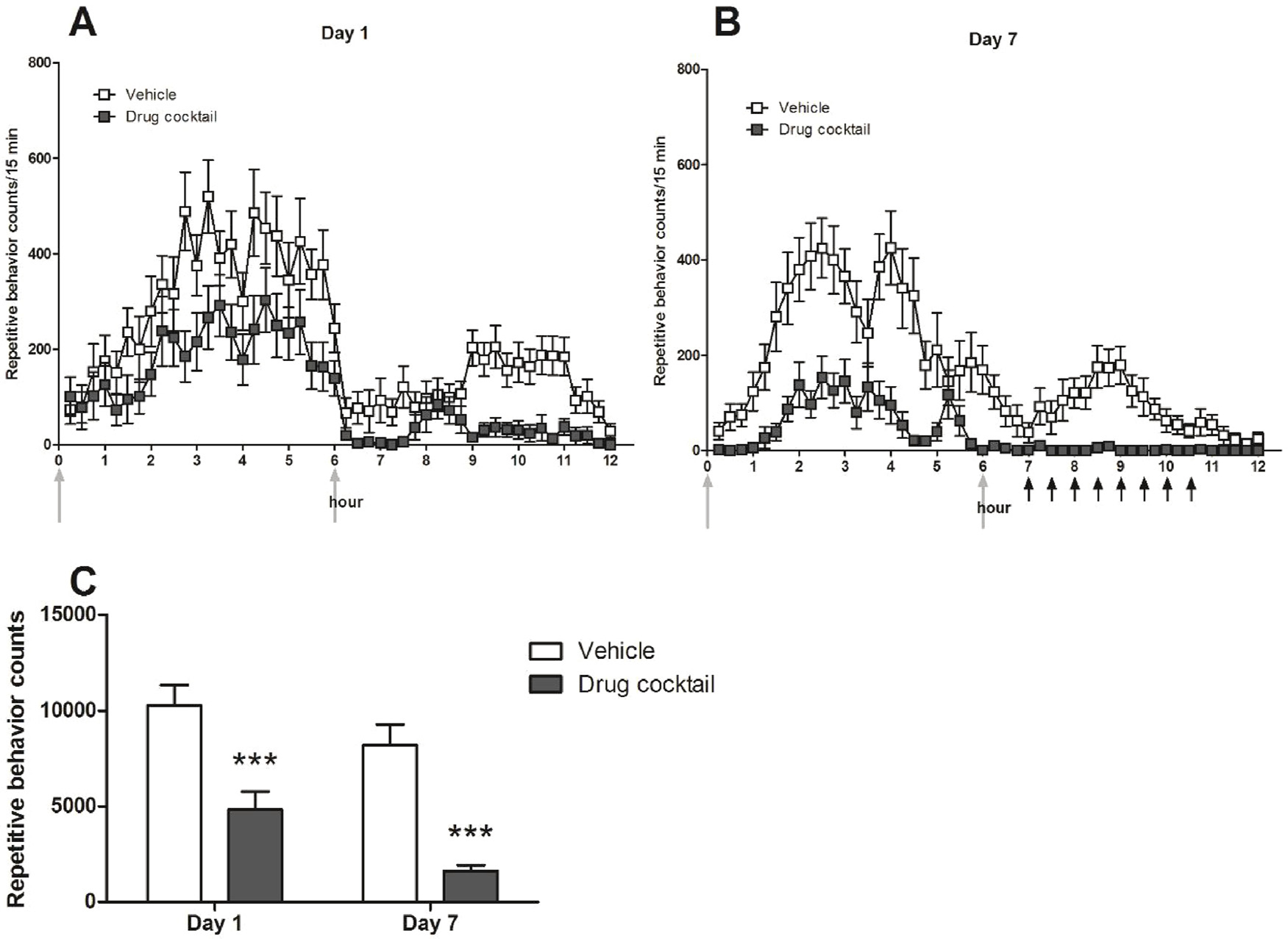
Repetitive behavior counts per 15 min period (mean ± standard error of the mean (SEM)) across the 12 -h dark cycle for Day 1 (1A) and Day 7 (1B), respectively. Upward light-colored arrows indicate time of drug or vehicle administration. Upward black arrows indicate times that non-repetitive behaviors were sampled. Fig. 1C: Total repetitive behavior counts over the 12 -h dark cycle for days 1 and 7. Drug cocktail-treated mice exhibited significantly less repetitive behavior on days 1 and 7 during the seven days of repeated administration (mean ± SEM; *** p < 0.001 for Bonferroni post-tests).
2.5. Gene expression assays
Ten male C58 mice were randomly selected for the gene expression analyses from the larger cohort of drug- and vehicle-treated mice. Thirteen hours (9:00 PM) after the final day of injections and repetitive behavior testing (that concluded at 8:00 AM), mice used for STN gene expression analyses (vehicle: n = 5; drug cocktail: n = 5) were subjected to cervical dislocation followed by rapid decapitation. As described previously [27], brains were quickly removed from the skull and frozen in cold 2-methylbutane. Whole brains were stored at −80 °C until they were transferred to a cryostat held at −10 °C and sliced at 300 μm. A 0.5 mm tissue puncher was used to dissect STN from the coronal slices. RNA was isolated using the RNeasy Micro Kit and cDNA was synthesized using RT2 First Strand Kit. The cDNA from each STN sample was added to RT2 Sybr Green mastermix and loaded onto both the RT2 Profiler PCR GABA and glutamate array (cat. no. PAMM-126Z, Qiagen, Germantown, Maryland) and the synaptic plasticity array (cat. no. PAMM-152Z, Qiagen). These PCR arrays were run on an ABI 7500 H T thermocycler. Each of these arrays contain primer sets for 84 genes and 5 housekeeping genes.
Data were analyzed using the GeneGlobe Data Analysis Center software (Qiagen, available from qiagen.com) which used the ΔΔCT method. Two housekeeping genes (Hprt and Actb) were used for normalization of the data. An unadjusted significance level of 0.05 was used for data reporting. We also employed the Benjamini-Hochberg procedure to control for multiple comparisons. This involved rank ordering the gene hits by p-value then dividing by the total number of comparisons and adjusting for a false discovery rate (FDR) of 20 %. The FDR was selected based on the exploratory nature of the study.
2.6. Golgi-Cox staining
Six male C58 mice were randomly selected for the dendritic spine analysis from the larger cohort of drug- and vehicle-treated mice. Golgi-Cox staining was used to assess dendritic morphology in the STN in the vehicle- and drug cocktail-treated groups. We had previously shown significant spine density differences in the same brain region in C58 versus B6 mice with only a small number of animals per group. In addition, environmental enrichment, which also markedly attenuated repetitive behavior in C58 mice, also increased dendritic spine density in STN. Thirteen hours after behavioral testing (i.e. 9:00 PM), mice (vehicle: n = 3; drug cocktail: n = 3) were anesthetized with 5% isoflurane and sacrificed by decapitation. Golgi-Cox staining was completed according to FD Rapid GolgiStain Kit guidelines (FD NeuroTechnologies, Inc., Colombia, MD) as we have previously described [27]. Dendritic spine density was quantified using ImagePro (Media Cybernetics, Rockville, MD) and a Zeiss microscope with Leica DFC camera with a 40x air objective, a 2.5x camera tube objective, 1.5x on the magnification changer, and a built-in 1.25x magnifier in the scope (40 × 4.6875 total) by an observer blind to treatment group. Within the STN, spines along unobstructed dendritic segments > 15 μm were marked on graphic overlays on live digital video images during continuous manual focus adjustment. Any protrusion from the main cylindrical column of the dendrite was counted as a spine. After labeling all the spines along a sample, the approximate dendritic cylinder centerline was traced for return of calibrated length by the software. Dendrites were measured starting beyond the first bifurcation point and at least 50 μm from the soma. For each mouse, 11 dendrites within the STN were measured ensuring samples represented both hemispheres and multiple tissue sections. Spine densities were calculated as the total number of spines divided by the total length of dendrite segments, such that each mouse had one density measurement.
2.7. Cytochrome oxidase histochemistry
Sixteen male C58 mice were randomly selected for the cytochrome oxidase analysis from the larger cohort of drug- and vehicle-treated mice. Cytochrome oxidase staining, using histochemistry, reflects the oxidative metabolic capacity of neurons to generate ATP [38]. Cytochrome oxidase activity is a relative measure of long-term (days to weeks) neuronal metabolic activity [39], and has previously been used to detect differences in activation of basal ganglia nuclei in mouse models of repetitive behavior [27,28,40,41]. Thirteen hours after behavioral testing (i.e. 9:00 PM), mice (vehicle: n = 8; drug cocktail: n = 8) were killed by cervical dislocation followed by decapitation. The cytochrome oxidase staining protocol [42] was performed on brains that were first frozen in chilled 2-methylbutane and stored in a −80 °C freezer, as previously described [27]. Sagittal sections (20 μm) sliced on a cryostat (−20 °C) were collected from both hemispheres at the level of the STN and mounted onto microscope slides (Superfrost Plus, FisherBrand, Fisher Scientific, Hampton, NH).
The optical density dependent variable is a metric for the amount of light that passes through the tissue stained for cytochrome oxidase. Thus, darker staining will have a higher optical density value indicating more cytochrome oxidase activity in the days to weeks prior to decapitation [43]. Optical density measurements were taken (ImagePro software, Media Cybernetics) from the basal ganglia regions of interest, dorsal striatum, the external segment of the globus pallidus (GPe), STN, and substantia nigra pars reticulata (SNr), as well as the primary motor cortex. These brain regions were selected as the drug cocktail was designed to activate indirect pathway neurons of the striatum. The GPe and STN are key downstream nodes of the indirect pathway and so were also selected to assess potential drug effects. The SNr is the major output nucleus of the basal ganglia and motor cortex sends afferents to the STN. As the SNr is innervated by both the direct and indirect pathways and motor cortex is the origin of the hyperdirect pathway, these regions were not expected to reflect neuronal activation changes induced by the drug cocktail. For each brain region, traces were drawn around each individual region across multiple adjacent sections. The optical density values were averaged for each brain region for each mouse.
2.8. Data analysis
Repeated measures ANOVAs with Geisser-Greenhouse correction for lack of sphericity were used to determine the main effects of time and drug and their interaction on body weights as well as repetitive behavior frequency. Bonferroni post-tests were performed for further comparisons within the two test days. One mouse in the vehicle group was an outlier on the repetitive behavior frequency measure but removal of these data did not change the results. The effects of drug versus vehicle on the duration of non-repetitive behaviors were analyzed using unpaired, two-tailed t-tests. Eating and drinking measures were non-normal and the variances of the locomotion and rearing measures were non-homogeneous. Independent, two-tailed t-tests, with normality and homogeneity of variance confirmed, were also used to compare the effects of drug versus vehicle on dendritic spine density as well as optical density of cytochrome oxidase histochemical stains in each brain region.
3. Results
3.1. Behavior
Drug cocktail-treated mice had fewer repetitive behavior counts at each 15-minute interval on day 1 (Fig. 1A) and day 7 (Fig. 1B) than vehicle treated mice. When aggregating total repetitive behavior counts for the 12 -h period, we found significantly lower total repetitive behavior counts in C58 mice that received the drug cocktail compared to vehicle-treated mice (Fig. 1C). In addition, mice exhibited significantly fewer repetitive behaviors on day 7, compared to day 1. A two-way RM-ANOVA showed a significant main effect of drug (F(1,51) = 37.54, p <0.0001), as well as a significant main effect of time (F(1,51) = 19.29, p< 0.0001). There was no significant drug × time interaction (F(1,51) = 0.68, p = 0.41).
After seven days of drug administration, no differences were found between drug cocktail and vehicle treated mice on measures of grooming, digging, and eating (p >0.05). Vehicle-treated C58 mice exhibited significantly longer durations of drinking (t(14) = 3.69; p <0.01), however. As expected, inactivity was significantly greater in drug-treated mice (t(14) = 10.57; p <0.0001) due to reductions in repetitive behavior. As depicted in Fig. 2, vehicle treated C58 mice were inactive for an average of 540 s across the 2400 s scored. Drug cocktail treated C58 mice were inactive an average of 1649 s. This is comparable to the 1290 s average inactivity time of untreated male C57BL/6 mice (n = 4) that were being assessed for repetitive behavior as controls for another study during the period in which this study was being conducted. C57BL/6 mice exhibit very low levels of repetitive behavior and have served as a control strain for a number of our studies. We coded inactivity as a reference for the inactivity data from drug treated C58 mice. The comparison indicates that inactivity under drug conditions is similar to drug-free control mice that do not exhibit any appreciable repetitive behavior rather than being indicative of drug-induced general motor suppression. Conversely, locomotion and rearing were significantly higher in vehicle mice (p < 0.001) reflecting the involvement of these behaviors in the pattern of backward somersaulting and hindlimb jumping. Finally, no main effect of drug was found for body weight (p >0.05), nor was there any difference in body weight found at either day 1 or day 7 when comparing drug treated mice to vehicle treated mice.
Fig. 2.
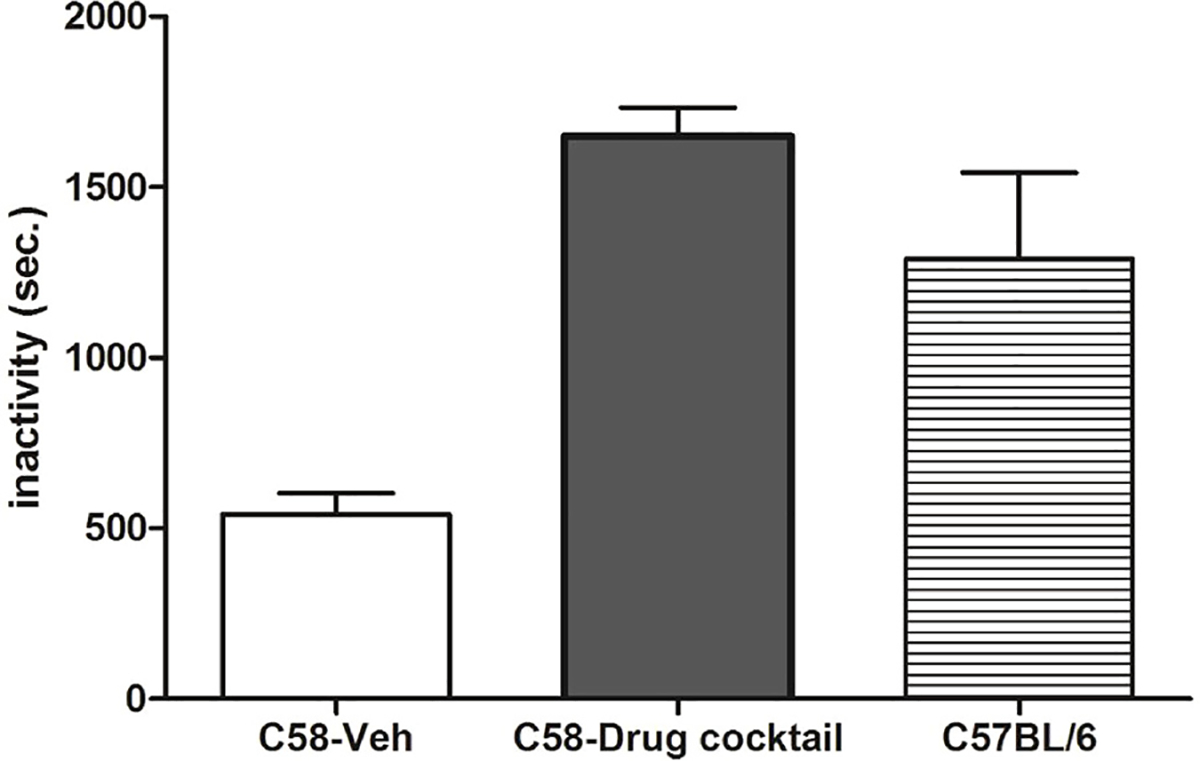
Duration of inactivity (in secs) for vehicle-treated C58 mice compared to drug cocktail-treated C58 mice (mean ± SEM; p < 0.0001) and contrasted with untreated male C57BL/6 mice (n = 4) scored at the same times, in the same cages for another study.
3.2. Gene expression assays
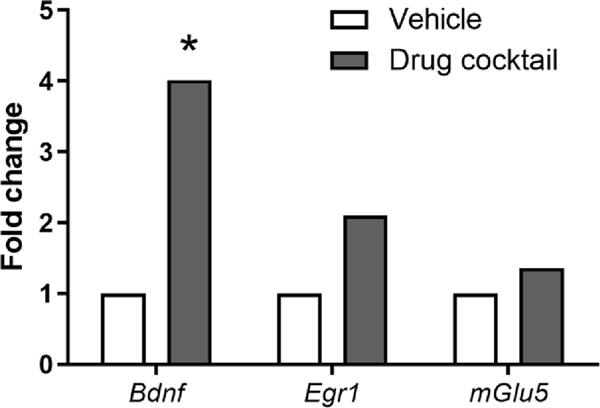
Although the drug cocktail was formulated to selectively activate the indirect pathway neurons in the striatum, we were primarily interested in testing whether sub-chronic administration would affect the STN, a region we have previously shown to have differential gene expression relative to the C57BL/6 strain, which does not exhibit repetitive motor behaviors [27]. Our previous analysis of these gene expression differences suggested reduced excitatory drive in the STN of C58 mice. Following seven days of twice-daily administration of the drug cocktail, we found increases in the expression (fold change) of three genes, Bdnf, Egr1, and Grm5 (mGlu5; Table 1; Fig. 3). Only the Bdnf expression remained statistically significant after applying the Benjamini-Hochberg procedure with a 20 % FDR. For the analysis, vehicle-treated mice were treated as the control so positive-fold regulation changes indicate higher gene expression in the drug cocktail-treated mice.
Table 1.
Gene expression differences in the STN between vehicle- and drug cocktail-treated mice. Only differential Bdnf expression remained significant after the Benjamini-Hochberg procedure using a 20 % FDR.
| Gene | RefSeq number | Fold regulation | Unadjusted p-value |
|---|---|---|---|
| Bdnf | NM_007540 | 4.0 | 0.001 |
| Egr1 | NM_007913 | 2.1 | 0.023 |
| Grm5 (mGlu5) | NM_001081414 | 1.36 | 0.031 |
Fig. 3.
Increased expression (fold change) of three genes, Bdnf, Egr1, and Grm5 following seven days of twice-daily administration of the drug cocktail. Only the Bdnf expression remained statistically significant after applying the Benjamini-Hochberg procedure with a 20 % FDR.
3.3. Golgi-Cox staining
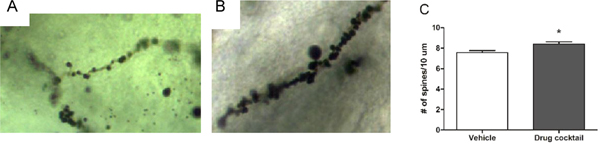
We have previously demonstrated that dendritic spine density in the STN is lower in C58 mice compared to C57BL/6 mice and increased by environmental enrichment, which significantly reduces repetitive behavior in C58 mice [27]. Considering this, we assessed dendritic spine density in the STN of vehicle- (Fig. 4A) and drug cocktail-treated (Fig. 4B) C58 mice. Using a two-tailed unpaired t-test, we found that the sub-chronic administration of the drug cocktail significantly increased dendritic spine density (t(4) = 2.87, p< 0.05; Fig. 4C) in the STN.
Fig. 4.
Comparison of STN dendritic spine density. A) A single segment of STN dendrite from a vehicle-treated mouse. B) A single segment of STN dendrite from a drug cocktail-treated mouse. C) Mice treated with the drug cocktail have significantly higher dendritic spine density in the STN compared to vehicle-treated mice (mean ± SEM; *p < 0.05).
3.4. Cytochrome oxidase histochemistry
Neuronal activation within discrete brain regions of drug cocktail- and vehicle treated mice was analyzed by cytochrome oxidase histochemistry. We have previously shown that this measure of neuronal activation is reduced in the STN of C58 mice, relative to C57BL/6 mice [27]. No other brain regions were significantly different between these two groups. Using two-tailed unpaired t-tests, we found no significant differences in cytochrome oxidase staining in any of the five brain regions assessed in the vehicle- and drug cocktail-treated C58 mice.
4. Discussion
Our previous findings in deer mice revealed that a drug cocktail targeting dopamine D2, adenosine A2A, and glutamate mGlu5 heteromeric receptor complexes, expressed on indirect pathway neurons of the striatum, significantly and selectively reduced repetitive behavior [34]. The present findings provide an important systematic replication of this drug manipulation with generalization to a different mouse model representing a different genus. This drug cocktail significantly reduced repetitive behavior in C58 mice after one night of twice-nightly injections and continued to be effective when this treatment regimen was continued for another six days.
These effects did not reflect generalized motor behavior suppression as non-repetitive motor behaviors such as grooming, digging and eating were not reduced relative to vehicle, although a significant reduction in drinking was found. We would expect a higher level of inactivity associated with a drug-induced decrease in repetitive behavior. The concern, of course, is that inactivity may simply reflect general motor suppression induced by the drug cocktail. However, inactivity levels of drug-treated mice were comparable to those measured in drug-naïve C57BL/6 mice that show only very low levels of repetitive motor behavior. Thus, assessment of other non-repetitive motor behaviors plus the comparable levels of inactivity between drug treated C58 mice and drug naïve B6 mice clearly indicate that the drug cocktail was selectively affecting repetitive behavior. In addition, sub-chronic administration of the drug cocktail did not affect body weight.
One limitation of this study, however, is the potential role that extra-striatal D2, A2A, and mGlu5 receptors may have had in the behavioral outcomes obtained. Targeting of striatopallidal neurons cannot be selective using systemic administration. Although A2A receptors are highly enriched in striatum and selectively expressed on indirect pathway neurons, D2 and mGlu5 receptors have a broader range of expression outside of striatum (e.g., cortex) and activation of these receptors may have contributed to the results obtained. Subsequent studies will need to establish the importance of striatal indirect pathway neurons using site-specific intra-cranial injections. An additional limitation of the present study is that it was limited to male mice. This was to provide a more direct comparison with our previous findings using deer mice [34]. Although we consistently fail to find a sex difference in the expression of repetitive behavior in our animals, this is a limitation of the present study. Demonstrating generalizability of the pharmacological effects of the drug cocktail to female mice will be important in subsequent studies.
This drug treatment used targeted striatopallidal neurons (i.e. striatal indirect pathway neurons) and its effects corresponded with significant changes in the STN. Unfortunately, no selective druggable targets have been identified in the STN. We hypothesized, however, that driving the indirect pathway could change STN function and reduce repetitive behavior. We found a four-fold increase in Bdnf mRNA expression in STN following drug cocktail administration. BDNF expression is linked to synaptic plasticity and is released following depolarization. The significantly increased BDNF expression is consistent with a drug cocktail induced increase in dendritic spine density. It was surprising that no other genes survived FDR correction. PCR arrays are designed to be focused panels for specific pathways. Future studies, including strain, environment, and drug treatment, should use next generation sequencing (NGS) for gene expression profiling and, perhaps, laser capture microdissection for subregion analyses of STN.
The drug cocktail also resulted in a significant increase in STN dendritic spine density. The increase in dendritic spine density also indicates that glutamatergic excitation of the STN is increased following a drug cocktail administration targeting striatopallidal neurons. We hypothesize that this increase is due to a drug cocktail-induced reduction of GABAergic inhibition on the STN. Sub-chronic administration of the drug cocktail did not change neuronal metabolic activity in a manner that could be measured by changes in cytochrome oxidase staining, however. This was somewhat surprising given the drug-induced increases in dendritic spine density and Bdnf mRNA expression. It is consistent, however, with our previous finding in C58 mice that another intervention, environmental enrichment, that markedly reduced repetitive behavior also did not significantly increase cytochrome oxidase staining [27].
The present findings are consistent with, and extend, our prior work linking decreased functioning of the indirect basal ganglia pathway to expression of repetitive motor behavior in C58 mice. For example, Lewis et al. [27] showed that C58 mice, versus C57BL/6 showed less CO staining as well as fewer dendritic spines in STN. Moreover, in F2 generation mice (products of a C58 × C57BL/6 intercross), the mice that exhibited high rates of repetitive behavior exhibited significantly reduced cytochrome oxidase staining selectively in STN, relative to mice that exhibited low rates of repetitive behavior [27]. In addition, reductions in repetitive behavior induced by environmental enrichment in C58 mice were associated with increased STN dendritic spine density. Using magnetic resonance imaging (MRI), we also showed that C58 mice exhibited reduced volumes of STN, when compared to C57BL/6 mice, and STN volumetric measures positively correlated with repetitive motor behavior [44]. Evidence for indirect basal ganglia pathway mediation of repetitive behavior in C58 mice is highly consistent with our previous work in deer mice [26,28,41,45].
Clinical and other animal studies have also found a link between repetitive behavior and the STN implicating the indirect basal ganglia pathway. Clinically, deep brain stimulation (DBS) of the STN reduced symptom severity in previously treatment refractory OCD patients [46]. In animal models, Amodeo et al. [47] found that the A2A agonist CGS21680 significantly reduced repetitive grooming behavior in BTBR mice, presumably through activation of the indirect basal ganglia pathway. Excessive grooming behavior expressed by the Shank3B mutant mouse was rescued by selective activation of indirect basal ganglia pathway neurons in the striatum using chemogenetic techniques [12]. High frequency stimulation of the STN reduced excessive self-grooming in two mouse models relevant to autism spectrum disorder [48]. Pharmacological induction of stereotyped motor behavior in non-human primates was attenuated by DBS of the STN [14,49]. High frequency stimulation of STN also reduced compulsive checking in rats [50].
The findings reported here provide additional, important evidence for the role of the indirect basal ganglia pathway in mediating repetitive behavior. In addition, these findings point to potential therapeutic targets that could spur the development of novel therapeutic agents for treatment of repetitive behavior in neurodevelopmental disorders.
Acknowledgments
We would like to acknowledge the support of the National Institute of Mental Health Chemical Synthesis and Drug Supply Program for providing the adenosine A2A receptor agonist, CGS21680 and the glutamate mGlu5 receptor PAM, CDPPB. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under University of Florida Clinical and Translational Science Awards KL2 TR000065 and UL1 TR000064.
Footnotes
Declarations of Competing Interest
None.
References
- [1].Muehlmann AM, Lewis MH, Abnormal repetitive behaviours: shared phenomenology and pathophysiology, J. Intellect. Disabil. Res 56 (5) (2012) 427–440, 10.1111/j.1365-2788.2011.01519.x. [DOI] [PubMed] [Google Scholar]
- [2].Jiujias M, Kelley E, Hall L, Restricted, repetitive behaviors in autism spectrum disorder and obsessive-compulsive disorder: a comparative review, Child Psychiatry Hum. Dev 48 (6) (2017) 944–959, 10.1007/s10578-017-0717-0. [DOI] [PubMed] [Google Scholar]
- [3].Singer HS, Motor control, habits, complex motor stereotypies, and Tourette syndrome, Ann. N. Y. Acad. Sci 1304 (2013) 22–31, 10.1111/nyas.12281. [DOI] [PubMed] [Google Scholar]
- [4].Carrasco M, Volkmar FR, Bloch MH, Pharmacologic treatment of repetitive behaviors in autism spectrum disorders: evidence of publication bias, Pediatrics 129 (5) (2012) e1301–1310, 10.1542/peds.2011-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Skapinakis P, Caldwell D, Hollingworth W, Bryden P, Fineberg N, Salkovskis P, Welton N, Baxter H, Kessler D, Churchill R, Lewis G, A systematic review of the clinical effectiveness and cost-effectiveness of pharmacological and psychological interventions for the management of obsessive-compulsive disorder in children/adolescents and adults, Health Technol. Assess 20 (43) (2016) 1–392, 10.3310/hta20430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ji NY, Findling RL, Pharmacotherapy for mental health problems in people with intellectual disability, Curr. Opin. Psychiatry 29 (2) (2016) 103–125, 10.1097/YCO.0000000000000233. [DOI] [PubMed] [Google Scholar]
- [7].Bloch MH, et al. , Commentary: Are alpha-2 agonist really effective in children with tics with comorbid ADHD? A commentary on Whittington (2016), J. Child Psychol. Psychiatry 57 (9) (2016) 1005–1007, 10.1111/jcpp.12592. [DOI] [PubMed] [Google Scholar]
- [8].Whittington C, Pennant M, Kendall T, Glazebrook C, Trayner P, Groom M, Hedderly T, Heyman I, Jackson G, Jackson S, Murphy T, Rickards H, Robertson M, Stern J, Hollis C, Practitioner review: treatments for Tourette syndrome in children and young people - a systematic review, J. Child Psychol. Psychiatry 57 (9) (2016) 988–1004, 10.1111/jcpp.12556. [DOI] [PubMed] [Google Scholar]
- [9].Lacivita E, Perrone R, Margari L, Leopoldo M, Targets for drug therapy for autism Spectrum disorder: challenges and future directions, J. Med. Chem. 60 (22) (2017) 9114–9141, 10.1021/acs.jmedchem.7b00965. [DOI] [PubMed] [Google Scholar]
- [10].Wilkes BJ, Lewis MH, The neural circuitry of restricted repetitive behavior: magnetic resonance imaging in neurodevelopmental disorders and animal models, Neurosci. Biobehav. Rev 92 (2018) 152–171, 10.1016/j.neubiorev.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, Feliciano C, Chen M, Adams JP, Luo J, Dudek SM, Weinberg RJ, Calakos N, Wetsel WC, Feng G, Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice, Nature 448 (7156) (2007) 894–900, 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang W, Li C, Chen Q, van der Goes MS, Hawrot J, Yao AY, Gao X, Lu C, Zang Y, Zhang Q, Lyman K, Wang D, Guo B, Wu S, Gerfen CR, Fu Z, Feng G, Striatopallidal dysfunction underlies repetitive behavior in Shank3-deficient model of autism, J. Clin. Invest 127 (5) (2017) 1978–1990, 10.1172/JCI87997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nagarajan N, Jones BW, West PJ, Marc RE, Capecchi MR, Corticostriatal circuit defects in Hoxb8 mutant mice, Mol. Psychiatry 23 (9) (2018) 1–10, 10.1038/mp.2017.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Grabli D, McCairn K, Hirsch EC, Agid Y, Feger J, Francois C, Tremblay L, Behavioural disorders induced by external globus pallidus dysfunction in primates: I. Behavioural study, Brain 127 (Pt 9) (2004) 2039–2054, 10.1093/brain/awh220. [DOI] [PubMed] [Google Scholar]
- [15].Baumeister AA, Frye GD, Self-injurious behavior in rats produced by intranigral microinjection of GABA agonists, Pharmacol. Biochem. Behav 21 (1) (1984) 89–95, 10.1016/0091-3057(84)90136-9. [DOI] [PubMed] [Google Scholar]
- [16].Lee Y, Kim H, Kim JE, Park JY, Choi J, Lee JE, Lee EH, Han PL, Excessive D1 dopamine receptor activation in the dorsal striatum promotes autistic-like behaviors, Mol. Neurobiol 55 (7) (2018) 5658–5671, 10.1007/s12035-017-0770-5. [DOI] [PubMed] [Google Scholar]
- [17].Djodari-Irani A, Klein J, Banzhaf J, Joel D, Heinz A, Harnack D, Lagemann T, Juckel G, Kupsch A, Morgenstern R, Winter C, Activity modulation of the globus pallidus and the nucleus entopeduncularis affects compulsive checking in rats, Behav. Brain Res 219 (1) (2011) 149–158, 10.1016/j.bbr.2010.12.036. [DOI] [PubMed] [Google Scholar]
- [18].Kitanaka N, Kitanaka J, Hall FS, Kayama M, Sugimori H, Uhl GR, Takemura M, Pretreatment or posttreatment with aripiprazole attenuates methamphetamine-induced stereotyped behavior in mice, J. Exp. Neurosci 9 (Suppl 1) (2015) 1–10, 10.4137/JEN.S27733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Capper-Loup C, Canales JJ, Kadaba N, Graybiel AM, Concurrent activation of dopamine D1 and D2 receptors is required to evoke neural and behavioral phenotypes of cocaine sensitization, J. Neurosci 22 (14) (2002) 6218–6227, 10.1523/JNEUROSCI.22-14-06218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Powell SB, Newman HA, Pendergast JF, Lewis MH, A rodent model of spontaneous stereotypy: initial characterization of developmental, environmental, and neurobiological factors, Physiol. Behav 66 (2) (1999) 355–363, 10.1016/s0031-9384(98)00303-5. [DOI] [PubMed] [Google Scholar]
- [21].Moy SS, Nadler JJ, Young NB, Nonneman RJ, Segall SK, Andrade GM, Crawley JN, Magnuson TR, Social approach and repetitive behavior in eleven inbred mouse strains, Behav. Brain Res 191 (1) (2008) 118–129, 10.1016/j.bbr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ryan BC, Young NB, Crawley JN, Bodfish JW, Moy SS, Social deficits, stereotypy and early emergence of repetitive behavior in the C58/J inbred mouse strain, Behav. Brain Res 208 (1) (2010) 178–188, 10.1016/j.bbr.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Muehlmann AM, Edington G, Mihalik AC, Buchwald Z, Koppuzha D, Korah M, Lewis MH, Further characterization of repetitive behavior in C58 mice: developmental trajectory and effects of environmental enrichment, Behav. Brain Res 235 (2) (2012) 143–149, 10.1016/j.bbr.2012.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Muehlmann AM, Bliznyuk N, Duerr I, Lewis MH, Repetitive motor behavior: further characterization of development and temporal dynamics, Dev. Psychobiol 57 (2) (2015) 201–211, 10.1002/dev.21279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tanimura Y, Yang MC, Ottens AK, Lewis MH, Development and temporal organization of repetitive behavior in an animal model, Dev. Psychobiol 52 (8) (2010) 813–824, 10.1002/dev.20477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tanimura Y, Vaziri S, Lewis MH, Indirect basal ganglia pathway mediation of repetitive behavior: attenuation by adenosine receptor agonists, Behav. Brain Res 210 (1) (2010) 116–122, 10.1016/j.bbr.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lewis MH, Lindenmaier Z, Boswell K, Edington G, King MA, Muehlmann AM, Subthalamic nucleus pathology contributes to repetitive behavior expression and is reversed by environmental enrichment, Genes Brain Behav. (2018) e12468, 10.1111/gbb.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bechard AR, Cacodcar N, King MA, Lewis MH, How does environmental enrichment reduce repetitive motor behaviors? Neuronal activation and dendritic morphology in the indirect basal ganglia pathway of a mouse model, Behav. Brain Res 299 (2016) 122–131, 10.1016/j.bbr.2015.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lewis MH, Rajpal H, Muehlmann AM, Reduction of repetitive behavior by coadministration of adenosine receptor agonists in C58 mice, Pharmacol. Biochem. Behav 181 (2019) 110–116, 10.1016/j.pbb.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Baker PM, Ragozzino ME, The prelimbic cortex and subthalamic nucleus contribute to cue-guided behavioral switching, Neurobiol. Learn. Mem 107 (2014) 65–78, 10.1016/j.nlm.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Eagle DM, Baunez C, Hutcheson DM, Lehmann O, Shah AP, Robbins TW, Stop-signal reaction-time task performance: role of prefrontal cortex and subthalamic nucleus, Cereb. Cortex 18 (1) (2008) 178–188, 10.1093/cercor/bhm044. [DOI] [PubMed] [Google Scholar]
- [32].Yoon JH, Cui E, Minzenberg MJ, Carter CS, Subthalamic nucleus activation occurs early during stopping and is associated with trait impulsivity, J. Cogn. Neurosci (2019) 1–12, 10.1162/jocn_a_01370. [DOI] [PubMed] [Google Scholar]
- [33].Fife KH, Gutierrez-Reed NA, Zell V, Bailly J, Lewis CM, Aron AR, Hnasko TS, Causal role for the subthalamic nucleus in interrupting behavior, Elife 6 (2017), 10.7554/eLife.27689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lewis MH, Primiani CT, Muehlmann AM, D.2 Targeting Dopamine, Adenosine A2A, and glutamate mGlu5 receptors to reduce repetitive behaviors in deer mice, J. Pharmacol. Exp. Ther 369 (1) (2019) 88–97, 10.1124/jpet.118.256081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fuxe K, Agnati LF, Jacobsen K, Hillion J, Canals M, Torvinen M, Tinner-Staines B, Staines W, Rosin D, Terasmaa A, Popoli P, Leo G, Vergoni V, Lluis C, Ciruela F, Franco R, Ferre S, Receptor heteromerization in adenosine A2A receptor signaling: relevance for striatal function and Parkinson’s disease, Neurology 61 (11 Suppl 6) (2003) S19–23, 10.1212/01.wnl.0000095206.44418.5c. [DOI] [PubMed] [Google Scholar]
- [36].Cabello N, Gandia J, Bertarelli DC, Watanabe M, Lluis C, Franco R, Ferre S, Lujan R, Ciruela F, Metabotropic glutamate type 5, dopamine D2 and adenosine A2a receptors form higher-order oligomers in living cells, J. Neurochem 109 (5) (2009) 1497–1507, 10.1111/j.1471-4159.2009.06078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Friard O, Gamba M, BORIS: A Free, Versatile Open-source Event-logging Software for video/audio Coding and Live Observations, (2016).
- [38].Wong-Riley MTT, Nie F, Hevner RF, Liu S, Brain cytochrome oxidase: functional significance and biogenomic regulation in the CNS, in: Gonzalez-Lima F (Ed.), Cytochrome Oxidase in Neuronal Metabolism and Alzheimer’s Disease, Plenum Press, New York, NY, 1998, pp. 1–53. [Google Scholar]
- [39].Sakata JT, Crews D, Gonzalez-Lima F, Behavioral correlates of differences in neural metabolic capacity, Brain Res. Brain Res. Rev 48 (1) (2005) 1–15, 10.1016/j.brainresrev.2004.07.017. [DOI] [PubMed] [Google Scholar]
- [40].Tanimura Y, King MA, Williams DK, Lewis MH, Development of repetitive behavior in a mouse model: roles of indirect and striosomal basal ganglia pathways, Int. J. Dev. Neurosci 29 (4) (2011) 461–467, 10.1016/j.ijdevneu.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Turner CA, Yang MC, Lewis MH, Environmental enrichment: effects on stereotyped behavior and regional neuronal metabolic activity, Brain Res. 938 (1–2) (2002) 15–21, 10.1016/s0006-8993(02)02472-1. [DOI] [PubMed] [Google Scholar]
- [42].Gonzalez-Lima F, Cada A, Quantitative histochemistry of cytochrome oxiase activity: theory, methods, and regional brain vulnerability, in: Gonzalez-Lima F (Ed.), Cytochrome Oxidase in Neuronal Metabolism and Alzheimer’s Disease, Plenum Press, New York, NY, 1998, pp. 55–90. [Google Scholar]
- [43].Wong-Riley MT, Cytochrome oxidase: an endogenous metabolic marker for neuronal activity, Trends Neurosci. 12 (3) (1989) 94–101, 10.1016/0166-2236(89)90165-3. [DOI] [PubMed] [Google Scholar]
- [44].Wilkes BJ, Bass C, Korah H, Febo M, Lewis MH, Lewis MH, Magnetic resonance and diffusion tensor imaging of inbred C58/J mice: neural correlates of repetitive behavior, Brain Imaging Behav. (2019) (2019), 10.1007/s11682-019-00158-9. [DOI] [PubMed] [Google Scholar]
- [45].Presti MF, Lewis MH, Striatal opioid peptide content in an animal model of spontaneous stereotypic behavior, Behav. Brain Res 157 (2) (2005) 363–368, 10.1016/j.bbr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- [46].Burdick A, Goodman WK, Foote KD, Deep brain stimulation for refractory obsessive-compulsive disorder, Front Biosci (Landmark Ed) 14 (2009) 1880–1890, 10.2741/3348. [DOI] [PubMed] [Google Scholar]
- [47].Amodeo DA, Cuevas L, Dunn JT, Sweeney JA, Ragozzino ME, The adenosine A2A receptor agonist, CGS 21680, attenuates a probabilistic reversal learning deficit and elevated grooming behavior in BTBR mice, Autism Res. 11 (2) (2018) 223–233, 10.1002/aur.1901. [DOI] [PubMed] [Google Scholar]
- [48].Chang AD, Berges VA, Chung SJ, Fridman GY, Baraban JM, Reti IM, High-frequency stimulation at the subthalamic nucleus suppresses excessive self-grooming in autism-like mouse models, Neuropsychopharmacology 41 (7) (2016) 1813–1821, 10.1038/npp.2015.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Baup N, Grabli D, Karachi C, Mounayar S, Francois C, Yelnik J, Feger J, Tremblay L, High-frequency stimulation of the anterior subthalamic nucleus reduces stereotyped behaviors in primates, J. Neurosci 28 (35) (2008) 8785–8788, 10.1523/JNEUROSCI.2384-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Winter C, Mundt A, Jalali R, Joel D, Harnack D, Morgenstern R, Juckel G, Kupsch A, High frequency stimulation and temporary inactivation of the subthalamic nucleus reduce quinpirole-induced compulsive checking behavior in rats, Exp. Neurol 210 (1) (2008) 217–228, 10.1016/j.expneurol.2007.10.020. [DOI] [PubMed] [Google Scholar]