Abstract
Pretreatment with mild heat shock is known to protect cells from severe stress (acquired thermotolerance). Here we addressed the mechanism of this phenomenon by using primary human fibroblasts. Severe heat shock (45°C, 75 min) of the fibroblasts caused cell death displaying morphological characteristics of apoptosis; however, it was caspase independent. This cell death process was accompanied by strong activation of Akt, extracellular signal-regulated kinase 1 (ERK1) and ERK2, p38, and c-Jun N-terminal (JNK) kinases. Suppression of Akt or ERK1 and -2 kinases increased cell thermosensitivity. In contrast, suppression of stress kinase JNK rendered cells thermoresistant. Development of thermotolerance was not associated with Akt or ERK1 and -2 regulation, and inhibition of these kinases did not reduce acquired thermotolerance. On the other hand, acquired tolerance to severe heat shock was associated with downregulation of JNK. Using an antisense-RNA approach, we found that accumulation of the heat shock protein Hsp72 is necessary for JNK downregulation and is critical for thermotolerance. The capability of naive cells to withstand moderate heat treatment also appears to be dependent on the accumulation of Hsp72 induced by this stress. Indeed, exposure to 45°C for 45 min caused only transient JNK activation and was nonlethal, while prevention of Hsp72 accumulation prolonged JNK activation and led to massive cell death. We also found that JNK activation by UV irradiation, interleukin-1, or tumor necrosis factor was suppressed in thermotolerant cells and that Hsp72 accumulation was responsible for this effect. Hsp72-mediated suppression of JNK is therefore critical for acquired thermotolerance and may play a role in tolerance to other stresses.
Cells exposed to nonlethal elevated temperatures develop resistance to a subsequent severe heat stress, a phenomenon called acquired thermotolerance. Thermotolerant cells also become more resistant to some other stressful treatments, such as ethanol, UV irradiation, doxorubicin (Adriamycin), or tumor necrosis factor (TNF) (acquired cross-tolerance) (see references 9 and 12 for review). This protection has mainly been attributed to members of the Hsp70 family which are induced by mild heat shock. In fact, expression of recombinant human heat-inducible Hsp70 (Hsp72) in many cell lines increased their resistance to stresses (1, 8, 22, 23, 30).
On the other hand, priming of cells with mild heat shock induces the whole group of heat shock proteins (Hsps) besides Hsp72, including Hsp104, Hsp90, Hsp27, and Hsp40. Some of these proteins function in protein protection and refolding in cooperation with Hsp70 family members (e.g., Hsp40 or Hsp90), while others function independently of Hsp70 family members (e.g., small Hsps or Hsp104) (see reference 7 for review). Furthermore, in addition to induction of Hsps, mild heat shock activates phosphorylation of Hsp27, which may be important for thermotolerance (21). Therefore, protective effects of cell preheating may be potentially unrelated to induction of Hsp72. This possibility is also supported by the fact that much higher levels of recombinant Hsp72 are usually required for cell protection than the levels of endogenous Hsp72 achieved by preheating (33). The first question addressed here is whether Hsp72 is indeed critical for acquired thermotolerance.
It is well known that all stresses, including heat shock, may potentially kill cells by three distinct modes: reproductive (clonogenic) cell death, apoptosis, or necrosis. Originally the phenomenon of thermotolerance was demonstrated by assessment of colony-forming ability, but later an acquired tolerance to heat-induced apoptosis as well as to necrosis was also reported (10, 23, 26, 32). While little is known about mechanisms of heat-induced necrosis or reproductive death, in heat-induced apoptosis (or programmed cell death) initial damage does not kill cells directly but turns on specific signaling pathways that lead to the cells' suicide. Suppression of these pathways prevents the loss of cell viability despite initial stress-evoked damage. Stress resistance of cells primed with mild heat shock may be due to downregulation of the signal transduction pathway that initiates programmed cell death. Indeed, recombinant Hsp72 has been demonstrated to suppress stress-induced activation of protein kinases c-Jun N-terminal kinase (JNK) and p38 (8, 30, 43), which were implicated in apoptosis induced by various stressful treatments (4, 34, 38, 40, 45, 46). However, the relevance of this regulation to acquired thermotolerance has not been clarified.
Certain kinases, such as Akt (protein kinase B) and extracellular signal-regulated kinase (ERK) (p42 and p44 mitogen-activated protein [MAP] kinases), suppress rather than activate apoptotic signaling, since inhibition of these kinases decreases cell survival (16, 44, 45). Therefore, if Akt and ERK are involved in protection from heat-induced apoptosis, their upregulation after mild heat shock pretreatment could contribute to acquired thermotolerance. Here we investigated the roles of Akt, ERK, p38, and JNK in heat-induced programmed death and acquired thermotolerance in human fibroblasts.
MATERIALS AND METHODS
Cell culture.
IMR90 human lung fibroblasts that underwent 10 to 20 population doublings were grown in minimal essential medium with 20% fetal bovine serum and 2 mM glutamine and were used for all experiments at 50 to 70% confluence.
Adenovirus-based expression of antisense Hsp72 RNA, Hsp72, and SEK(K/R).
A recombinant adenovirus vector expressing Hsp72 antisense RNA was constructed by cloning a dicistronic transcription unit encoding this RNA and the green fluorescent protein (GFP) gene, separated by the encephalomyocarditis virus internal ribosome entry site (25, 31) into an adenovirus vector. Expression of this transcription unit is controlled by the tetracycline-regulated transactivator protein tTA (31), which was expressed from the separate recombinant adenovirus (ADCMVTTA [24]). Recombinant adenoviruses were generated by standard techniques as detailed by Jani et al. (15). Administration of 3 × 107 PFU of each virus per 35-mm dish was sufficient to infect almost 100% of the cells. This was confirmed each time by observation under a fluorescent microscope of a proportion of the cells expressing GFP. A similar adenovirus encoding sense Hsp72 RNA (ADTR5Hsp72-GFP) was used for expression of Hsp72 (25, 28). As a control, adenovirus expressing GFP under the regulation of tTA was used. Adenovirus expressing dominant-negative SEK1 (dnSEK1) (K/R), a kinase-inactive mutant of JNK-activating kinase SEK1 tagged with M2 FLAG epitope at its amino terminus was also used (5). Adenoviruses were propagated in 293 cells, and high-titer stocks were obtained and purified by CsCl2 density gradient centrifugation.
Measurement of JNK activity.
Cells were washed twice with phosphate-buffered saline on a dish, aspirated, and lysed by thoroughly scraping with a plastic scraper in 200 μl of lysis buffer per 35-mm dish (40 mM HEPES [pH 7.5], 50 mM KCl, 1% Triton X-100, 2 mM dithiothreitol, 1 mM Na3 VO4, 50 mM β-glycerophosphate, 50 mM NaF, 5 mM EDTA, 5 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, and 5 μg each of leupeptin, pepstatin A, and aprotinin/ml). The lysates were clarified by centrifugation in a microcentrifuge at 12,000 × g for 5 min. The total protein concentration was measured in the supernatants by Bio-Rad protein assay reagent, after which they were diluted with the lysis buffer to achieve equal protein concentrations in all samples. All procedures were performed at 4°C.
To measure total JNK activity in fibroblasts, 5 μl of extract was added to a reaction mixture (20-μl final volume), containing final concentrations of 40 mM HEPES (pH 7.5), 1 mM Na3 VO4, 25 mM β-glycerophosphate, 10 mM MgCl2, 20 μM ATP, 15 μCi of [γ-32P]ATP, and 40 ng of recombinant c-Jun. The reaction was allowed to proceed for 30 min at 30°C and was then stopped by adding 10 μl of loading sodium dodecyl sulfate-polyacrylamide gel electrophoresis buffer. Samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and were transferred to nitrocellulose; membranes were exposed to a Molecular Imager (Bio-Rad) for quantification. Subsequently, membranes were immunoblotted with a JNK1 antibody to verify equivalent protein loading.
Another assay for JNK activity in fibroblasts allowed us to measure separately the activity of two major isoforms, JNK1 and JNK2 (46 and 54 kDa, respectively), using an antibody specific to the activated (phosphorylated) form of JNK (Promega, Madison, Wis.).
Measurement of Akt, ERK1 and -2, and p38 kinase activities.
Activities of the Akt, ERK1 and -2 (p42 and p44), and p38 kinases were measured by immunoblotting with corresponding antibodies specific to phosphorylated (active) kinases either in total cell lysates (Akt and ERK1 and -2) or in a soluble (supernatant) fraction (p38) which was obtained as described above for measurement of JNK activity. The following antibodies (all from New England Biolabs) were used: phospho-Akt (Thr308), phospho-p42 and -p44 (Thr202 and Tyr204), and phospho-p38 (Thr180 and Tyr182). Secondary antibodies conjugated with peroxidase were visualized with enhanced-chemiluminescence substrates (Amersham, Arlington Heights, Ill.), and resulting films were quantified by densitometry.
Since phosphorylation of p38 is not suitable for measuring the effect of the inhibitor SB203580 on p38 activity (20), another assay of p38 activity was used. A substrate of p38, MAP kinase-activated protein 2 (MAPKAP-2) kinase, was immunoprecipitated with MAPKAP-2 antibodies for 2 h at 4°C and was washed three times with the kinase buffer. MAPKAP-2 kinase activity was measured using Hsp27 (StressGene) as a substrate (20).
Cell viability and apoptosis.
Cell viability was measured by fluorescent microscopy using acridine orange-ethidium bromide staining as described earlier (30). Cells which were not stained by ethidium bromide, with normal morphology and noncondensed nuclei, were considered viable. We counted 300 to 500 cells in each experiment, which was repeated at least twice. Apoptotic caspase activation was assayed by immunoblotting of total cell lysates either with antibodies to caspase 3 (Santa Cruz) or with antibodies (C2 to -10; G. Poirier) to one of the caspase substrates, poly(ADP-ribose) polymerase (PARP).
RESULTS
Acquired thermotolerance in fibroblasts depends on Hsp72 accumulation.
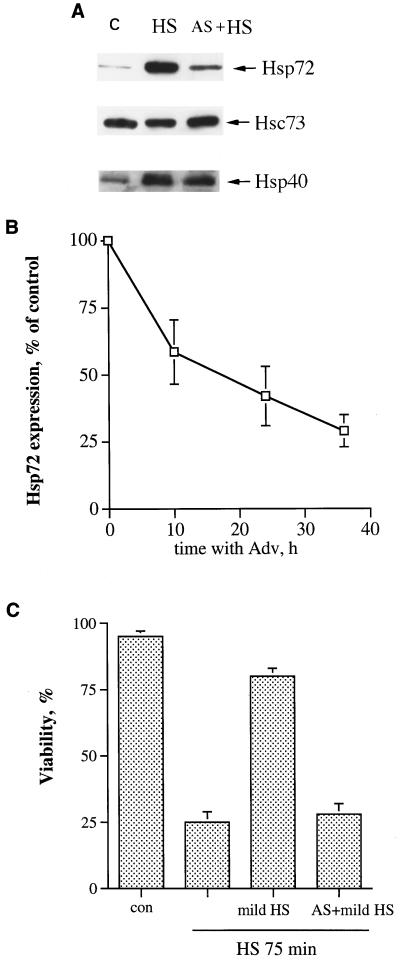
Challenging IMR90 human fibroblasts with severe heat shock (45°C, 75 min) led to a loss of viability of 80% of the cells. However, pretreatment at 45°C for 30 min followed by 16 h of recovery dramatically reduced cell death (only about 20% of the cells died) (Fig. 1C). Although the conditions used for preheating (45°C, 30 min) may be toxic for other types of cells (e.g., lymphoid cells), we consider it a mild heat shock for IMR90 fibroblasts, since it does not affect the viability of these cells but strongly induces Hsps, including Hsp72 (Fig. 1A).
FIG. 1.
Prevention of heat-induced Hsp72 accumulation abolishes acquired thermotolerance in fibroblasts. (A) Expression of Hsp72 antisense RNA prevents heat-induced accumulation of Hsp72 but not that of other Hsps. IMR90 human fibroblasts were infected with an adenovirus expressing Hsp72 antisense RNA (AS) and 36 h after infection were subjected to mild heat shock (45°C, 30 min), followed by recovery for 16 h at 37°C. The levels of Hsp72, Hsc73, and Hsp40 were then assayed by immunoblotting with corresponding antibodies. C, control cells; HS, cells incubated with tetracycline (doxycycline, 1 μg/ml) and exposed to heat shock; AS + HS, cells incubated without tetracycline and exposed to heat shock. (B) Time course of suppression of heat-induced Hsp72 accumulation by the Hsp72 antisense-RNA-expressing adenovirus. Fibroblasts were infected with the Hsp72 antisense-RNA-expressing adenovirus as explained for panel A and were incubated without tetracycline; at various times after infection, they were subjected to mild heat shock with recovery as explained for panel A, and the level of Hsp72 was measured by immunoblotting. The data shown are the means ± standard deviations of three replicates. Adv, adenovirus. (C) Prevention of Hsp72 accumulation by antisense-RNA-expressing adenovirus blocks the acquisition of thermotolerance. Fibroblasts infected with Hsp72 antisense-RNA-expressing adenovirus and pretreated with mild heat shock as explained for panel A were exposed to severe heat shock (45°C, 75 min), and cell viability was assayed 24 h later by acridine orange-ethidium bromide staining. con, control.
Pretreatment of cells with mild heat shock induces the entire set of Hsps, activates stress kinases, and may also initiate some other adaptive responses. To evaluate the role of Hsp72 in acquired thermotolerance in fibroblasts, we blocked accumulation of this protein specifically, utilizing a novel approach based on adenovirus-driven expression of the Hsp72 antisense RNA. Fibroblasts were infected with an adenovirus encoding Hsp72 antisense RNA along with GFP under the control of a tetracycline-sensitive transactivator (see Materials and Methods for details). In this system GFP serves as an indicator of Hsp72 antisense RNA expression. To induce expression of Hsp72 antisense RNA, infected cells were incubated without tetracycline, while as a control we used infected cells incubated in the presence of tetracycline. The virus titer and time of infection were chosen to provide expression of GFP (and, correspondingly, Hsp72) in 90 to 100% of cells in the absence of tetracycline. No fluorescence was observed if cells were incubated in the presence of tetracycline (data not shown).
To assess the effect of the Hsp72 antisense RNA on Hsp expression, cells were infected with the virus for 36 h and were then subjected to mild heat shock with recovery as described above. When expression of Hsp72 and other Hsps was tested by immunoblotting with the corresponding antibodies, we found that in cells expressing antisense RNA, Hsp72 induction was dramatically suppressed, while neither induction of a distinct Hsp, Hsp40, nor the level of the heat shock cognate protein Hsc73 in these cells was changed (Fig. 1A). Inhibition of the Hsp72 accumulation was dependent on the time after viral infection (Fig. 1B) and correlated well with the increase of GFP expression as judged by fluorescence microscopy (not shown). Thus, the adenoviral expression of Hsp72 antisense RNA caused strong and specific suppression of Hsp72 induction. Inhibition of Hsp72 accumulation in adenovirus-infected cells after mild heat shock pretreatment by removal of tetracycline almost completely suppressed acquisition of the tolerance to severe heat shock (Fig. 1C). In contrast, incubation of infected cells in the presence of tetracycline to prevent expression of Hsp72 antisense RNA did not significantly affect acquisition of thermotolerance (Fig. 1C). Therefore, accumulation of Hsp72 is essential for acquisition of thermotolerance in fibroblasts.
Heat-induced cell death in fibroblasts.
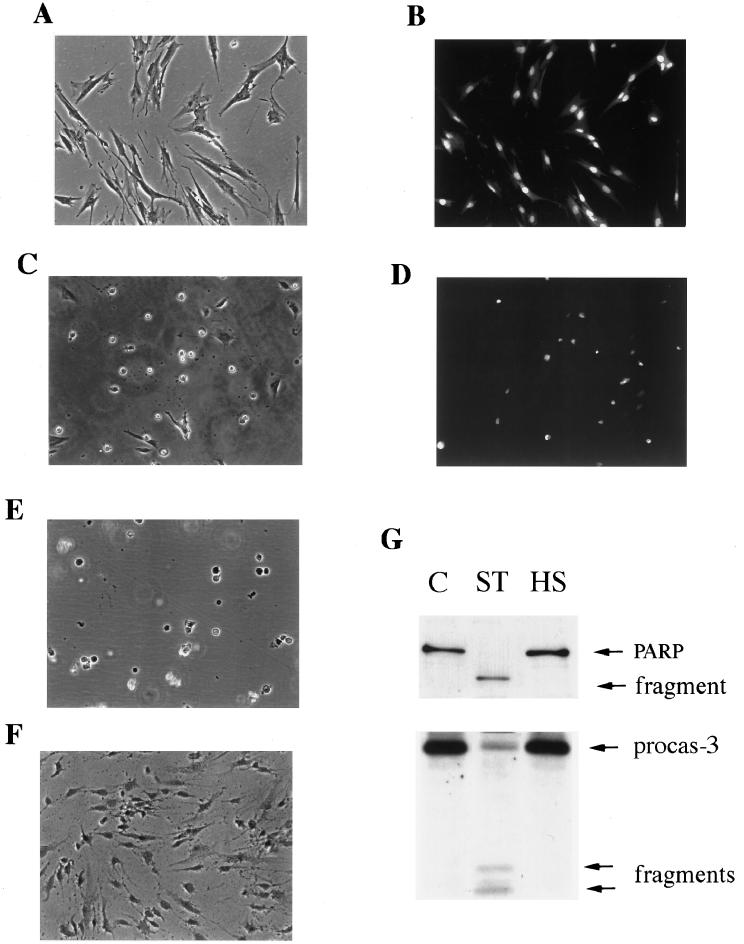
To investigate the mechanism of Hsp72 action in acquired thermotolerance, we first studied how heat shock kills human fibroblasts. It was reported that under heat shock, various cells can undergo classical apoptosis manifested by cell shrinkage, chromatin condensation, activation of caspase 3, and cleavage of substrates such as PARP, while cells remained impermeable to supravital dyes (8, 23, 30, 32). The death of heat-shocked fibroblasts resembled such apoptotic processes (43). Following heat shock, cells first shrank and then rounded and detached (Fig. 2A and C). Furthermore, they remained impermeable to trypan blue or ethidium bromide while demonstrating chromatin condensation as judged by staining with acridine orange (Fig. 2B and D) or Hoechst-33342 (not shown). The morphology of heat-shocked cells was similar to that of cells treated with the well-known apoptotic inducer staurosporine (Fig. 2E) or TNF (not shown). On the other hand, such morphology was clearly different from that of necrotic cells (e.g., cells which were heat shocked in serum-free medium): the latter remained attached to the substratum but became permeable to trypan blue (Fig. 2F). Therefore, heat shock kills IMR90 human fibroblasts by a mechanism that morphologically resembles apoptosis.
FIG. 2.
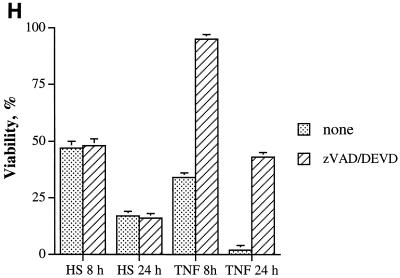
Characteristics of heat-induced cell death in fibroblasts. (A to F) Morphological characteristics of dying IMR90 fibroblasts. (A and B) Control cells. (A) Light microscopy; (B) fluorescent microscopy after staining with acridine orange. (C and D) Cells subjected to heat shock at 45°C for 75 min and observed 24 h later. (C) Light microscopy; (D) fluorescent microscopy after staining with acridine orange. (E) Cells were treated with 1 μM staurosporine for 16 h. (F) Cells were subjected to the same heat shock as explained for panels C and D but in serum-free medium and were stained with trypan blue. (G) Apoptosis-associated PARP cleavage (upper panel) and caspase 3 activation (lower panel) in control cells (C), cells treated with 1 μM staurosporine (ST), or heat-shocked cells (45°C, 75 min) (HS). PARP and procaspase 3 cleavages were assayed by immunoblotting. procas-3, procaspase 3. (H) Effect of caspase inhibitor zVAD-fmk or Ac-DEVD-CHO on heat-induced or TNF-induced cell death. Cells were exposed to heat shock (45°C, 75 min) or TNF (10 ng/ml) plus emetine (10 μg/ml) and incubated either with vehicle (1% dimethyl sulfoxide) or with zVAD-fmk (100 μM) for 8 h or Ac-DEVD-CHO (100 μM) for 24 h after the stresses. Cell viability was assayed by acridine orange-ethidium bromide staining. HS, heat shock.
Surprisingly, this heat shock-induced cell death lacked certain other important characteristics of apoptosis. In fact, neither the activation of caspase 3 nor the cleavage of PARP could be observed in heat-shocked cells at any time during chromatin condensation and massive cell detachment (Fig. 2G). In addition, no effect on caspase 3 activation or on PARP cleavage was observed when we varied the parameters of heat treatment, i.e., temperature (from 43 to 46°C), time of heating (from 15 to 90 min), and time after heat shock (from 12 to 72 h) (not shown). In contrast, staurosporine-treated (Fig. 2G) or TNF-treated (not shown) cells showed both activation of caspase 3 and PARP cleavage, indicating that caspase-dependent apoptosis can be activated in human fibroblasts. Accordingly, heat-induced cell death, unlike TNF-induced apoptosis, was not suppressed by the caspase 3 inhibitor Ac-DEVD-CHO or general caspase inhibitor zVAD-fmk (Fig. 2H). These data indicate that heat-induced cell death of fibroblasts, although it morphologically resembles apoptosis, is caspase independent.
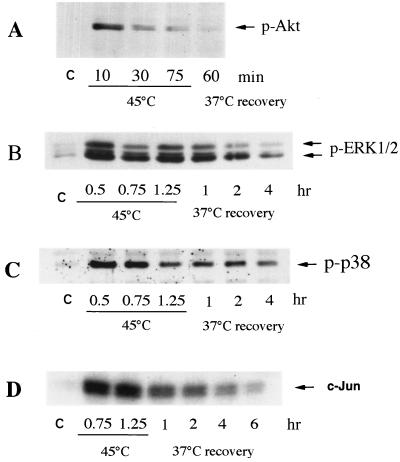
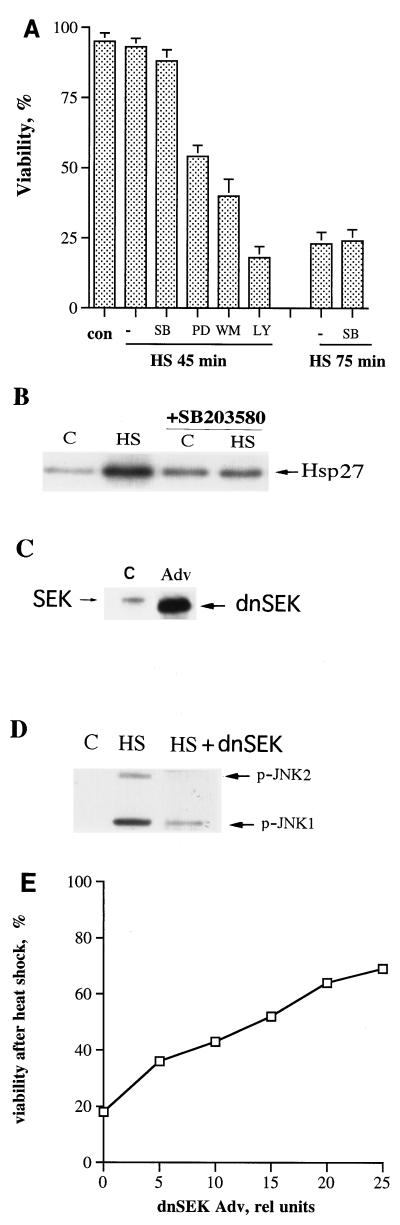
In several cell lines, heat shock was reported to activate protein kinases which are implicated in cell survival and death, including Akt kinase (35), MAP kinases ERK1 and -2 (44), and stress kinases JNK and p38 (8, 30). To address the question of whether any of these kinases plays a role in the heat-induced, caspase-independent death of human fibroblasts, we tested their activation following heat treatment. Cells were heat shocked at 45°C for different time periods, and activities of the kinases were assessed by immunoblotting with antibodies against phosphorylated (active) forms of Akt, the ERKs, p38, and JNK. All these kinases were activated by heat shock (Fig. 3A through D). We then tested whether inhibition of these kinases affects the survival of heat-shocked fibroblasts. In these experiments we employed wortmannin and LY294,002, inhibitors of the Akt signaling pathway; PD98059, an inhibitor of the ERK1 and -2 signaling pathway; and a p38 kinase inhibitor, SB203580. Cells preincubated with one of these inhibitors for 30 min were subjected to heat shock at 45°C for 45 or 75 min, and their viability was measured after 24 h of incubation at 37°C in the presence of the inhibitors. Inhibition of p38 kinase did not affect the thermosensitivity of cells, while inhibition of Akt kinase or ERKs dramatically sensitized cells to heat shock (Fig. 4A and B). Indeed, 50 to 80% of cells treated with wortmannin, LY294,002, or PD98059 died after heat shock at 45°C for 45 min, while the viability of control cells was not compromised by such heat treatment (Fig. 4A). It should be noted that any of these inhibitors alone (without heat shock) did not affect the viability of fibroblasts (not shown). Thus, it appears that both Akt kinase and ERK facilitate cell survival under heat shock conditions.
FIG. 3.
Effect of heat shock treatment on the activity of Akt (A), ERK1 and -2 (B), p38 (C), and JNK (D) kinases in fibroblasts. IMR90 fibroblasts were exposed to heat shock (45°C) for the indicated time periods and were then transferred to normal temperature (37°C, recovery). (A to C) The activity of the Akt, ERK, and p38 kinases was measured by immunoblotting with antibodies to phosphorylated (active) kinases. (D) The activity of JNK was assayed by c-Jun phosphorylation as described in Materials and Methods.
FIG. 4.
Involvement of Akt, ERK1 and -2 and JNK in heat-induced death of fibroblasts. (A) Inhibition of Akt and ERK1 and -2 kinases but not p38 kinase decreases cell viability under moderate heat shock treatment. IMR90 fibroblasts were pretreated for 30 min with p38 kinase inhibitor SB203580 (SB) (10 μM), ERK1 and -2 inhibitor PD98059 (PD) (50 μM), or IP3 kinase inhibitor wortmannin (WM) (100 nM) or LY294,002 (LY) (50 μM) and were then subjected to heat shock (HS) for 45 or 75 min. Vehicle (0.1% dimethyl sulfoxide) was added as a control to heat-shocked cells, and cell death was measured 24 h later as shown in Fig. 1C. con, control. (B) SB203580 inhibits p38 activity. Fibroblasts were pretreated with SB203580 as described above and were exposed to heat shock (45°C, 75 min). p38 activity was measured immediately after heat shock by an in vitro assay of its downstream kinase (MAPKAP-2), using Hsp27 as a substrate (see Materials and Methods for further details). (C to E) Inhibition of JNK activity suppresses heat-induced cell death. Fibroblasts were infected with an adenovirus expressing dnSEK; 72 h after infection, cells were subjected to heat shock (45°C, 75 min) and expression of dnSEK (C), JNK activity (D), and cell viability (E) were assayed. Expression of dnSEK was assayed by immunoblotting with antibodies to SEK1; JNK activity was measured with antibodies to active (phosphorylated) JNK. Cell viability was assayed as explained for Fig. 1C. The amount of added dnSEK adenovirus shown in panels C and D was 25 relative units (25 × 109 particles per 35-mm plate). This experiment was reproduced three times. Data shown in panels B to E represent typical experiments. Adv, adenovirus; rel, relative.
To test whether JNK is involved in the heat-induced death of fibroblasts, we inhibited activation of JNK by expression of dnSEK1. Cells infected with dnSEK1-expressing adenovirus for 72 h were challenged with severe heat shock (45°C, 75 min), and activation of JNK and cell viability were monitored. Expression of dnSEK1 led to a dramatic reduction of heat-induced JNK activation (Fig. 4C and D) and to dose-dependent protection from heat-induced death almost to the same degree as achieved by mild heat pretreatment (cf. Fig. 4E and 1C). As a control for dnSEK1-expressing adenovirus, we used GFP-expressing adenovirus and found that neither JNK activation nor cell death was affected by this adenovirus (data not shown). Thus, despite heat-induced damage, the inactivation of JNK in fibroblasts confers thermoresistance. This finding indicates that although cell death after heat shock is caspase independent, it is apparently a programmed event which is upregulated by activation of proapoptotic kinase JNK and is downregulated by activation of antiapoptotic kinases ERKs and Akt.
Hsp72-mediated acquired tolerance is associated with suppression of stress kinase JNK.
Previous studies indicated that Hsp72 and other members of the Hsp70 family are involved in regulation of several protein kinases that are activated by stresses, including heme-regulated kinase HRI (39), double-stranded-RNA-activated kinase PKR (27), p38, and JNK (8, 30, 43). Therefore, since Akt kinase, ERK, and JNK activities affect the sensitivity of fibroblasts to heat shock (see above), Hsp72-dependent thermotolerance may be associated with Hsp72-mediated regulation of any (or all) of these kinases.
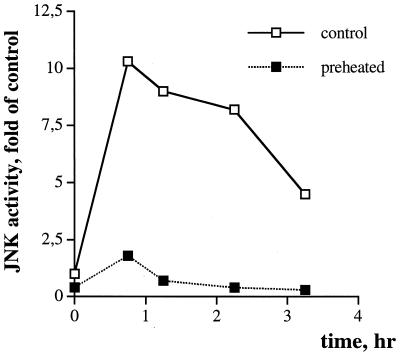
To address this problem, fibroblasts that acquired thermotolerance (see “Acquired thermotolerance in fibroblasts depends on Hsp72 accumulation” above) were treated with PD98059, wortmannin, or LY294,002 and were then exposed to severe heat shock. Treatment of cells with the inhibitors did not reduce the survival of preheated cells challenged with a second severe heat shock (not shown). Furthermore, pretreatment with mild heat shock did not enhance activation of either ERKs or Akt by the challenging heat shock (not shown). These findings indicate that acquired thermotolerance is not associated with upregulation of ERKs or Akt kinases. On the other hand, pretreatment with mild heat shock dramatically suppressed the activation of JNK by severe heat shock (Fig. 5). These data suggest that the protective effect of mild heat shock pretreatment is mediated by suppression of JNK and that when JNK is suppressed, no activities of Akt or ERKs are required.
FIG. 5.
Heat-induced JNK activity is suppressed in thermotolerant fibroblasts. IMR90 fibroblasts were pretreated with mild heat shock as explained for Fig. 1 and were then exposed to severe heat shock (45°C, 75 min). JNK activity was measured at the time points indicated. c-Jun was the substrate.
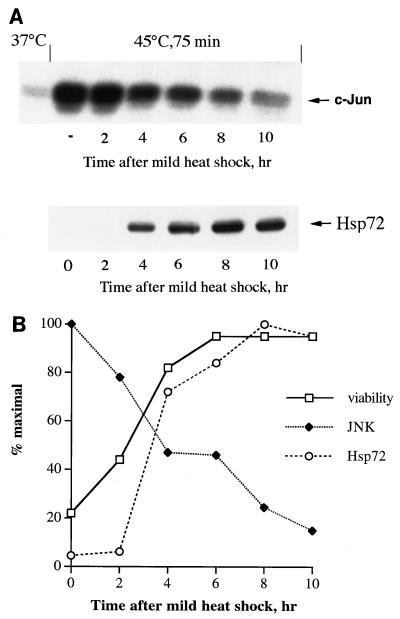
Is the effect of heat pretreatment on JNK activation due to accumulation of Hsp72? To test whether the development of thermotolerance and suppression of JNK correlate with induction of Hsp72, fibroblasts were preheated at 45°C for 30 min, were returned to normal temperature, and after various time intervals were exposed to a second severe heat shock (45°C, 75 min). JNK activity was measured immediately after the second heat shock, and cell viability was assessed 24 h later. A slight decline in both JNK activity and cell death in response to the second heat shock was already observed at 2 h after the preheating, when no increase in the level of Hsp72 was yet observed (Fig. 6). However, further inhibition of JNK and development of thermotolerance closely correlated with accumulation of Hsp72 (Fig. 6), suggesting that Hsp72-mediated suppression of JNK may be the basis for acquired thermotolerance.
FIG. 6.
Suppression of JNK activation and cell death in fibroblasts subjected to severe heat shock correlates with Hsp72 accumulation. (A) Suppression of JNK activation in response to severe heat shock correlates with Hsp72 accumulation after mild heat shock. Cells were pretreated with mild heat shock (45°C, 30 min), incubated for different time periods at 37°C, and then subjected to severe heat shock (45°C, 75 min). JNK activity (upper panel) was measured using c-Jun as the substrate. JNK activity in nonpreheated cells (−) after severe heat shock is also shown. The Hsp72 level (lower panel) was measured by immunoblotting with antibodies to inducible Hsp72 as described for Fig. 1A and B. (B) Increase in viability of cells subjected to severe heat shock correlates with JNK suppression. Cells were treated as explained for panel A, and their viability was measured 24 h later as explained for Fig. 1C. JNK activity and Hsp72 accumulation were measured as explained for panel A. This experiment was reproduced three times. Data shown here represent a typical experiment.
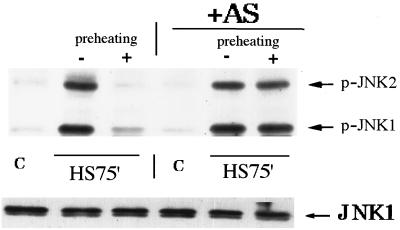
To test more directly whether suppression of JNK in acquired thermotolerance is due to accumulation of Hsp72, the antisense-RNA approach was employed. Fibroblasts infected with the adenovirus encoding Hsp72 antisense RNA, as described in “Acquired thermotolerance in fibroblasts depends on Hsp72 accumulation” above, were exposed to heat shock, and JNK activity was measured. As shown previously, in control cells, JNK activation after challenging heat shock was suppressed by preheating (Fig. 7). By contrast, expression of Hsp72 antisense RNA completely eliminated the suppression of JNK activation by the mild heat shock pretreatment (Fig. 7). Thus, Hsp72 accumulation is required for the inhibitory effect of preheating on activation of JNK in response to challenging heat shock.
FIG. 7.
Prevention of heat-induced Hsp72 accumulation eliminates suppression of JNK in fibroblasts subjected to severe heat shock. Fibroblasts were infected with adenovirus expressing Hsp72 antisense RNA, incubated either with (control) or without (+AS) tetracycline. Then the cells were pretreated with mild heat shock, and after 16 h of recovery, were exposed to severe heat shock as described for Fig. 1C. JNK activity was assayed immediately after severe heat shock by immunoblotting with antibodies to phosphorylated (active) JNK1 and -2 (upper panel) or JNK1 (lower panel). C, control.
In line with previously reported data (28), heat shock did not upregulate the JNK-activating kinase SEK1, while it inhibited JNK dephosphorylation (not shown). Accordingly, pretreatment with mild heat shock did not affect the basal level of SEK1 activity, whereas it facilitated JNK dephosphorylation after severe heat shock (not shown). As discussed previously (28), the basal SEK1 activity is critical for activation of JNK upon inhibition of a phosphatase in heat-shocked cells, which explains the inhibitory effect of dnSEK1 expression.
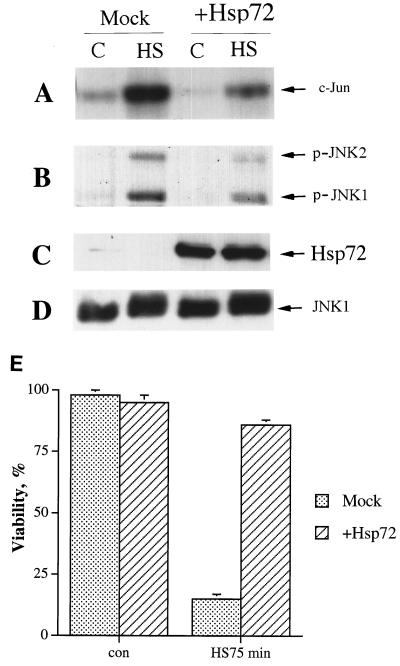
We also checked whether Hsp72 overexpression alone is sufficient to suppress heat shock-induced JNK activation and cell death. To express Hsp72 at high levels, we used an adenovirus construct encoding sense Hsp72 RNA in a dicistronic transcription unit together with GFP. Incubation of adenovirus-infected cells in the absence (but not in the presence) of tetracycline led to GFP fluorescence in 80 to 100% of cells (not shown) and caused a marked induction of Hsp72 (Fig. 8C). This treatment led to strong suppression of heat-induced JNK activation and cell death (Fig. 8A, B, and E). As stated earlier, expression of GFP alone had no effect on JNK activity and cell viability under these conditions. Therefore, Hsp72 accumulation is necessary and sufficient for the inhibitory effect of preheating on JNK activation and cell death in response to severe heat shock. It should be noted that the level of recombinant Hsp72 necessary for suppression of JNK and cell protection was higher than the level induced by mild heat shock.
FIG. 8.
Expression of Hsp72 at high levels suppresses heat-induced JNK activation and cell death. Fibroblasts were infected with recombinant adenovirus expressing Hsp72 and were incubated for 36 h either with (Mock) or without (+Hsp72) tetracycline. Then the cells were exposed to heat shock (HS) (45°C, 75 min), and JNK activity was assayed immediately afterward. Viability was assayed 24 h later. (A to D) C, control. (A) JNK activity as assayed using c-Jun as the substrate; (B) JNK activity as assayed by immunoblotting with antibodies to phosphorylated (active) JNK1 and -2; (C) Hsp72 expression as assayed by immunoblotting; (D) JNK1 expression as assayed by immunoblotting; (E) Cell viability as assayed by acridine orange-ethidium bromide staining. con, control.
Accumulation of Hsp72 is necessary for intrinsic thermoresistance and inactivation of JNK.
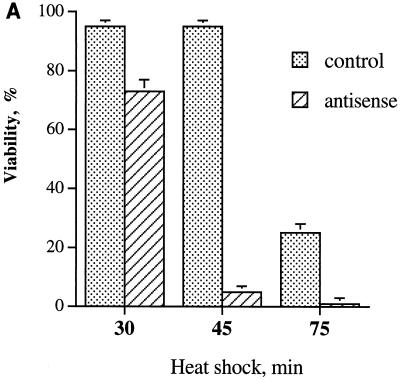
Adenovirus expressing Hsp72 antisense RNA in fibroblasts, which is nontoxic at normal temperatures, unexpectedly caused toxicity under heat shock. Indeed, heating at 45°C for only 30 min caused the death of 25% of the cells (Fig. 9A). Furthermore, exposure to 45°C for 45 min, which is nontoxic in control cells, caused loss of viability in almost 100% of the Hsp72 antisense-RNA-expressing cells; i.e., it was more toxic than heating control cells for 75 min (Fig. 9A). The thermosensitizing effect of Hsp72 antisense RNA is not due to adenoviral infection itself, since incubation of infected cells with tetracycline to block RNA expression abolished this effect (Fig. 9A). Therefore, Hsp72 not only is essential for development of thermotolerance (Fig. 1) but also is involved in intrinsic cellular thermoresistance. This finding indicates that heat stress simultaneously triggers synthesis of Hsps and activates the cell death program and that Hsp72, when accumulated, blocks this program. Consequently, failure to induce Hsp72 leads to cell death.
FIG. 9.
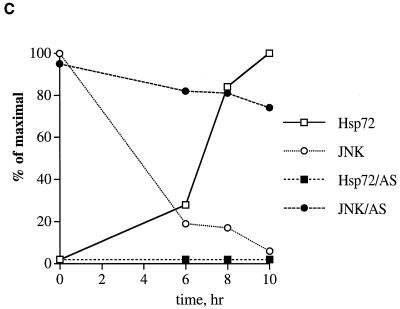
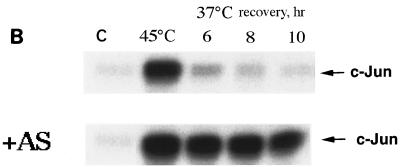
Prevention of heat shock-induced Hsp72 accumulation in fibroblasts delays JNK inactivation after moderate heat shock and decreases cell viability. (A) Expression of Hsp72 antisense RNA decreases the thermoresistance of fibroblasts. Fibroblasts infected with adenovirus expressing Hsp72 antisense RNA and incubated in the presence or absence of tetracycline as described for Fig. 1A were subjected to heat shock (45°C) for various time periods. Cell viability was assayed 24 h later as described for Fig. 1C. (B and C) Expression of Hsp72 antisense RNA delays JNK inactivation after moderate heat shock. Fibroblasts were infected with adenovirus as explained for panel A. JNK inactivation in control (with tetracycline) or in Hsp72 antisense (AS)-RNA-expressing fibroblasts (without tetracycline) after heat shock (45°C, 45 min) was measured at the indicated time points using c-Jun as the substrate. C, control. (C) Quantification of JNK deactivation and Hsp72 accumulation after the same heat shock in control (open symbols) or in Hsp72 antisense-RNA-expressing cells (filled symbols) is shown. The Hsp72 level was assayed as described for Fig. 1A and B.
How can Hsp72, which accumulates in cells only several hours after heat treatment (Fig. 6), turn off the death program initiated by this treatment? It has recently been demonstrated that heat-induced death of Rat-1 cells depends on the duration of JNK activation rather than its initial heat-induced activity (41). On the other hand, expression of recombinant Hsp72 accelerates JNK inactivation, thus rescuing cells from apoptosis (41, 42). It was suggested that Hsp72 accumulated after nontoxic heat shock is responsible for the decline of JNK activity. To test this suggestion, we compared the dynamics of JNK activity after heat shock in control and Hsp72 antisense-RNA-expressing cells. In control cells exposed to 45°C for 45 min, JNK was rapidly activated and then the activity declined, so that after 6 h of recovery, JNK activity fell almost to the background level (Fig. 9B and C). However, in Hsp72 antisense-RNA-expressing cells, JNK inactivation was drastically slowed down, so that even after 10 h the level of JNK activity was still about 80% of the maximum (Fig. 9C). In these cells, the death program was activated, and they died within 24 h (Fig. 9A). Therefore, in cells exposed to moderate heat shock, Hsp72-mediated JNK inactivation is apparently required for survival.
Hsp72 is required for suppression of JNK activation in preheated cells in response to various stresses.
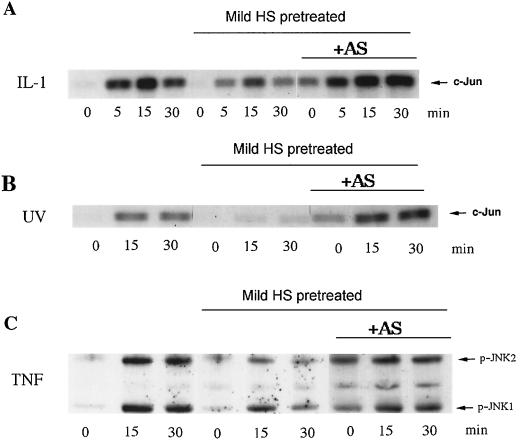
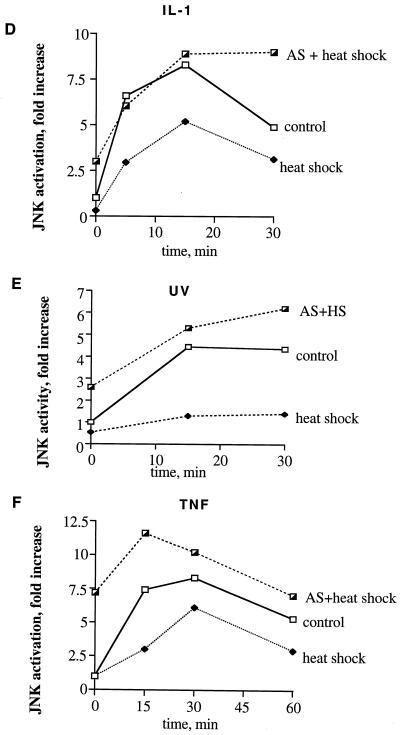
Can preheating of cells suppress JNK activation induced by various stresses besides heat shock, and what is the role of Hsp72 in this suppression? As JNK-activating stimuli, we chose interleukin-1 (IL-1), UV-C, and TNF. Preheating of fibroblasts at 45°C for 30 min, which blocked JNK activation by severe heat shock (Fig. 5), did not significantly suppress JNK activation by these stimuli (not shown). However, stronger heat shock pretreatment (45°C for 45 min), which led to a higher level of Hsp72 (not shown), caused marked suppression of JNK activation by all these stimuli (Fig. 10). To test whether suppression of JNK activation under these conditions depends on accumulation of Hsp72, cells were infected with the virus expressing Hsp72 antisense RNA. As described above, when induction of Hsp72 was inhibited, heat shock at 45°C for 45 min became toxic due to sustained JNK activation (Fig. 9A). This toxicity, however, was manifested only after 24 h of recovery, and at 12 to 16 h of recovery, most cells did not demonstrate any signs of death (i.e., chromatin condensation and detachment) (not shown). Thus, at 16 h after the heat shock treatment, control cells and cells expressing Hsp72 antisense RNA were exposed to UV-C radiation, TNF, or IL-1. Prevention of Hsp72 induction by antisense RNA increased the basal level of JNK activity due to inhibition of JNK inactivation and completely abolished the inhibitory effects of preheating on JNK activation by these stimuli (Fig. 10). Therefore, accumulation of Hsp72 in preheated cells plays a critical role in suppression of JNK activation not only by heat shock but also by other stimuli.
FIG. 10.
Heat shock (HS)-induced suppression of JNK under diverse stresses depends on Hsp72 accumulation. Fibroblasts were subjected to heat shock (45°C, 45 min) followed by recovery for 16 h and were then exposed to IL-1 (20 ng/ml) (A and D), UV-C irradiation (400 J/M2) (B and E), or TNF (10 ng/ml) plus cycloheximide (CHX) (10 μg/ml) (C and F). JNK activity was assayed either by using c-Jun as the substrate (A, B, D, and E) or by immunoblotting with antibodies to phosphorylated (active) JNK (C and F). “AS + heat shock” shows JNK activity after heat shock in cells infected with Hsp72 antisense-RNA-expressing adenovirus as described for Fig. 1A. In panels D to F, densitometric data of the experiments shown in panels A to C are presented.
DISCUSSION
Priming of cells with mild heat shock leads to thermotolerance (3, 10, 23, 32, 43); however, the role of Hsp72 in this process has not been addressed specifically. Indeed, mild heat shock can induce a variety of Hsps besides Hsp72 (e.g., Hsp40, Hsp27, and Hsp90) through heat shock factor activation, as well as some proteins whose expression depends on activation of transcriptional factors, such as AP-1 (6; see also reference 17 for review). In addition, heat pretreatment activates a number of posttranscriptional events (e.g., heat-induced Hsp27 phosphorylation [21]) which may contribute to heat tolerance. Therefore, to evaluate the role of Hsp72 in thermotolerance, it is necessary to specifically inhibit Hsp72 accumulation without affecting the expression of other proteins.
Inspired by previous publications, we first approached this goal by using 15- to 24-mer synthetic Hsp72 antisense oligonucleotides, including oligonucleotides complementary to the 5′ translated region of Hsp72. However, the effects of these oligonucleotides were quite nonspecific (i.e., similar to that of sense or random-sequence oligonucleotides) (unpublished data). Therefore, in this study we applied a novel approach: expression of the full antisense Hsp72 RNA by a recombinant adenovirus under the control of a tetracycline-regulated promoter. We found that this treatment was very effective and specific. Indeed, expression of Hsp72 antisense RNA markedly (by about 75%) reduced heat-induced accumulation of Hsp72 without any effect on the closely related Hsc73 or on Hsp40 (Fig. 1A). Thus, an adenovirus expressing Hsp72 antisense RNA appears to be a very helpful tool for elucidating the role of this protein in various heat-inducible processes. Using this approach, we demonstrated that Hsp72 accumulation is crucial for acquired thermotolerance (Fig. 1C). In other words, the whole scope of other events triggered by mild heat shock was insufficient to significantly protect cells. As a model for these experiments, we used an IMR90 primary human fibroblast culture at early passages (10 to 20), since it is more physiologically relevant than stable cultures of immortalized cells. In later passages of fibroblasts (30 to 40), antisense-RNA-expressing adenovirus was also effective but to a lesser extent (unpublished data).
An unexpected finding of this work is that heat shock induces an unusual caspase-independent type of cell death in the primary fibroblasts which is distinct from classical apoptosis. On the other hand, this death is a regulated process, since it requires activation of JNK and can be suppressed by the ERKs and Akt kinases (Fig. 3 and 4). Therefore, it appears to be a programmed cell death, which may be similar to a caspase-independent apoptosis (see reference 2 for review), and morphologically this kind of death is very different from necrosis (Fig. 2F). Accordingly, Hsp72 appears to regulate various types of cell death that are activated by JNK.
Another problem addressed in this study is the mechanism of acquired thermotolerance. Earlier works suggested that this mechanism may be based on the Hsp72-mediated suppression of stress kinase JNK (8, 30, 43). In the present study we obtained evidence in favor of this mechanism. This evidence is based on the following observations. (i) The time course of acquisition of thermotolerance correlated with that of Hsp72 accumulation and JNK suppression (Fig. 6). (ii) Prevention of heat-induced Hsp72 accumulation eliminated the suppression of JNK activity and development of thermotolerance (Fig. 1 and 7). (iii) Suppression of heat-induced JNK activity by expression of dnSEK1, an upstream component of the JNK signaling pathway, markedly reduced heat-induced cell death (Fig. 4). Therefore, Hsp72-mediated suppression of JNK appears to be an important component of acquired thermotolerance (Fig. 11). On the other hand, pretreatment of cells with mild heat shock did not upregulate either ERKs or Akt in response to the challenging heat shock. Therefore, the development of thermotolerance is unrelated to upregulation of these kinases.
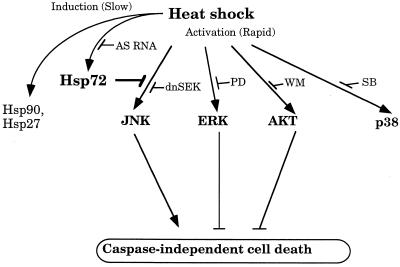
FIG. 11.
Participation of Hsp72 and heat shock-activated kinases in caspase-independent cell death of fibroblasts. Heat shock causes rapid activation of JNK, ERK, Akt, and p38 kinases and slow induction of Hsp72. JNK activation leads to cell death, while ERK and Akt activation enhances survival. Hsp72 can prevent cell death via suppression of JNK but not through activation of ERK or Akt. Prevention of Hsp72 accumulation by antisense-RNA expression (AS RNA) eliminates the inhibitory effect of mild heat shock on JNK activity and abolishes thermotolerance. PD, PD98059; WM, wortmannin; SB, SB203580.
It should be noted that acquired thermotolerance may partly depend on heat-induced (activated) factors besides Hsp72. Indeed, in fibroblasts a slight decline in both JNK activation and death in response to a challenging heat shock was observed at 2 h after the preheating, when no increase in the Hsp72 level could be detected yet (Fig. 6A and B). Therefore, this initial (though rather minor) JNK suppression and thermotolerance acquisition are independent of Hsp72 accumulation. Interestingly, this Hsp72-independent protection in fibroblasts appears to be also associated with JNK suppression (Fig. 6A and B). This protection may be related to rapid induction of a JNK phosphatase (e.g., mitogen-kinase phosphatase 1) whose expression is also activated by heat shock (18, 19), or it could also be independent of the synthesis of novel proteins (21). On the other hand, it is clear that the major and limiting factor in JNK regulation is Hsp72, since its overexpression is sufficient to suppress JNK, while specific inhibition of Hsp72 induction prevents JNK suppression (Fig. 11).
In addition to Hsp72, other heat-induced proteins may also participate in suppression of JNK. Indeed, to block JNK activation by expression of Hsp72 alone, much higher levels of Hsp72 are needed than that induced by mild heat shock (Fig. 8) (8, 43). One of the heat-induced proteins implicated in JNK suppression in cooperation with Hsp72 may be Hsp40, a well-known cochaperone which increases the binding of Hsp72 to substrates (11, 29). Other Hsp72 cofactors, like BAG-1 or Hip, as well as Hsp90, may also play a role in this regulation. In fact, BAG-1 was reported to function in suppression of apoptosis (37). It is possible that its prosurvival activity is mediated by the interaction of BAG-1 with Hsp72 and consequently with JNK.
A rather unexpected observation made in this study is that the intrinsic resistance of cells to moderate heat shock is dependent on the accumulation of Hsp72. Indeed, exposure of fibroblasts to 45°C for 45 min, which was not toxic in control cells, evoked a massive death of cells expressing Hsp72 antisense RNA (Fig. 9A). This phenomenon is probably related to the previous observation that heat-induced apoptosis requires prolonged JNK activation, while transient although strong JNK activation is insufficient to induce cell death (41). Accordingly, in control fibroblasts which were able to accumulate Hsp72, JNK activity after heat shock returned to the basal level within 6 h. In contrast, in the cells where Hsp72 accumulation was blocked, JNK activity remained elevated for a much longer time period (Fig. 9C). Therefore, accumulated Hsp72 may participate in the decline of JNK activity after heat stress, thus preventing induction of cell death.
Apparently, there is a fine balance in a cell between Hsp72 synthesis and the triggering of death after heat shock (Fig. 11). It appears that during the first hours after heat shock, cells try to synthesize Hsp72 in order to repair damaged molecules and survive. If they fail to synthesize enough Hsp72 during this initial period (e.g., in the presence of Hsp72 antisense virus), the death program prevails. Interference with the death program during this time (e.g., by suppression of JNK via dnSEK1-expressing adenovirus) leads to cell survival, although the cells' reparation capability is likely to remain unchanged (Fig. 11).
It is known that both pretreatment with mild heat shock and expression of recombinant Hsp72 can protect cells from apoptosis caused by a variety of stresses, including UV irradiation (36) and TNF (3, 13, 14). On the other hand, JNK was shown to be essential for initiation of apoptosis by these stimuli, at least in certain cell lines (4, 38, 40, 46). Therefore, we have previously proposed that mild heat shock or expression of Hsp72 suppresses activation of JNK by these agents, thus preventing apoptosis (9). In this work we tested an important prediction of this proposal and found that heat shock pretreatment did suppress JNK activation by such diverse stresses as UV irradiation and TNF and that accumulation of Hsp72 is necessary for this regulation (Fig. 10). These data suggest that Hsp72-mediated JNK suppression may play an important role not only in acquired thermotolerance but also in tolerance of various other stressful stimuli.
ACKNOWLEDGMENTS
This study was supported by an RO1 grant from the NIH to M.Y.S. and by a BBRI scholarship to J.A.Y.
We thank Sophia Ritz-Volloch for help with experiments.
REFERENCES
- 1.Angelidis C E, Lazardis I, Pagoulatos G N. Constitutive expression of heat-shock protein 70 in mammalian cells confers thermotolerance. Eur J Biochem. 1991;199:35–39. doi: 10.1111/j.1432-1033.1991.tb16088.x. [DOI] [PubMed] [Google Scholar]
- 2.Borner C, Monney L. Apoptosis without caspases: an inefficient molecular guillotine? Cell Death Differ. 1999;6:497–507. doi: 10.1038/sj.cdd.4400525. [DOI] [PubMed] [Google Scholar]
- 3.Buzzard K A, Giaccia A J, Killender M, Anderson R L. Heat shock protein 72 modulates pathways of stress-induced apoptosis. J Biol Chem. 1998;273:17147–17153. doi: 10.1074/jbc.273.27.17147. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y R, Wang X, Templeton D, Davis R J, Tan T H. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation. Duration of JNK activation may determine cell death and proliferation. J Biol Chem. 1996;271:31929–31936. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]
- 5.Choukroun G, Hajjar R, Kyriakis J M, Bonventre J V, Rosenzweig A, Force T. Role of stress-activated protein kinases in endothelin-induced cardiomyocyte hyperthrophy. J Clin Investig. 1998;102:1311–1320. doi: 10.1172/JCI3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elbirt K K, Whitmarsh A J, Davis R J, Bonkovsky H L. Mechanism of sodium arsenite-mediated induction of heme oxygenase-1 in hepatoma cells. Role of mitogen-activated protein kinases. J Biol Chem. 1998;273:8922–8931. doi: 10.1074/jbc.273.15.8922. [DOI] [PubMed] [Google Scholar]
- 7.Feige U, Morimoto R, Yahara I, Polla B. Stress-inducible cellular responses. Basel, Switzerland: Birkhauser Verlag; 1996. [Google Scholar]
- 8.Gabai V L, Meriin A B, Mosser D D, Caron A W, Rits S, Shifrin V I, Sherman M Y. Hsp70 prevents activation of stress kinases. A novel pathway of cellular thermotolerance. J Biol Chem. 1997;272:18033–18037. doi: 10.1074/jbc.272.29.18033. [DOI] [PubMed] [Google Scholar]
- 9.Gabai V L, Meriin A B, Yaglom J A, Volloch V Z, Sherman M Y. Role of Hsp70 in regulation of stress-kinase JNK: implication in apoptosis and aging. FEBS Lett. 1998;438:1–4. doi: 10.1016/s0014-5793(98)01242-3. [DOI] [PubMed] [Google Scholar]
- 10.Gabai V L, Zamulaeva I V, Mosin A F, Makarova Y M, Mosina V A, Budagova K R, Malutina Y V, Kabakov A E. Resistance of Ehrlich tumor cells to apoptosis can be due to accumulation of heat shock proteins. FEBS Lett. 1995;375:21–26. doi: 10.1016/0014-5793(95)01152-5. [DOI] [PubMed] [Google Scholar]
- 11.Greene M K, Maskos K, Landry S J. Role of the J-domain in the cooperation of Hsp40 with Hsp70. Proc Natl Acad Sci USA. 1998;95:6108–6113. doi: 10.1073/pnas.95.11.6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaattela M. Escaping cell death: survival proteins in cancer. Exp Cell Res. 1999;248:30–43. doi: 10.1006/excr.1999.4455. [DOI] [PubMed] [Google Scholar]
- 13.Jaattela M. Overexpression of major heat shock protein hsp70 inhibits tumor necrosis factor-induced activation of phospholipase A2. J Immunol. 1993;151:4286–4294. [PubMed] [Google Scholar]
- 14.Jaattela M, Wissing D, Kokholm K, Kallunki T, Egeblad M. Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J. 1998;17:6124–6134. doi: 10.1093/emboj/17.21.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jani A, Lochmuller H, Acsadi G, Simoneau M, Huard J, Garnier A, Karpati G, Massie B. Generation, validation, and large scale production of adenoviral recombinants with large size inserts such as a 6.3 kb human dystrophin cDNA. J Virol Methods. 1997;64:111–124. doi: 10.1016/s0166-0934(96)02138-6. [DOI] [PubMed] [Google Scholar]
- 16.Kandel E S, Hay N. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp Cell Res. 1999;253:210–229. doi: 10.1006/excr.1999.4690. [DOI] [PubMed] [Google Scholar]
- 17.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. Philos Trans R Soc Lond B. 1996;351:127–134. doi: 10.1098/rstb.1996.0008. [DOI] [PubMed] [Google Scholar]
- 18.Keyse S M. Protein phosphatases and the regulation of MAP kinase activity. Semin Cell Dev Biol. 1998;9:143–152. doi: 10.1006/scdb.1997.0219. [DOI] [PubMed] [Google Scholar]
- 19.Keyse S M, Emslie E A. Oxidative stress and heat shock induce a human gene encoding a protein tyrosine phosphatase. Nature. 1992;359:644–647. doi: 10.1038/359644a0. [DOI] [PubMed] [Google Scholar]
- 20.Kumar S, Jiang M S, Adams J L, Lee J C. Pyridinylimidazole compound SB 203580 inhibits the activity but not the activation of p38 mitogen-activated protein kinase. Biochem Biophys Res Commun. 1999;263:825–831. doi: 10.1006/bbrc.1999.1454. [DOI] [PubMed] [Google Scholar]
- 21.Lavoie J N, Lambert H, Hickey E, Weber L A, Landry J. Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol Cell Biol. 1995;15:505–516. doi: 10.1128/mcb.15.1.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li G C, Li L, Liu Y-K, Mak J Y, Chen L, Lee W M F. Thermal response of rat fibroblasts stably transfected with the human 70-kDa heat shock protein-encoding gene. Proc Natl Acad Sci USA. 1991;88:1681–1685. doi: 10.1073/pnas.88.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W X, Chen C H, Ling C C, Li G C. Apoptosis in heat-induced cell killing: the protective role of hsp-70 and the sensitization effect of the c-myc gene. Radiat Res. 1996;145:324–330. [PubMed] [Google Scholar]
- 24.Massie B, Couture F, Lamoureux L, Mosser D D, Guilbault C, Jolicoeur P, Belanger F, Langelier Y. Inducible overexpression of a toxic protein by an adenovirus vector with a tetracycline-regulatable expression cassette. J Virol. 1998;72:2289–2296. doi: 10.1128/jvi.72.3.2289-2296.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Massie B, Mosser D D, Koutroumanis M, Vitte-Mony I, Lamourex L, Couture F, Paquet L, Guilbault C, Dionne J, Chahla D, Jolicoeur P, Langelier Y. New adenoviral vectors for protein production and gene transfer. Cytotechnology. 1998;28:53–64. doi: 10.1023/A:1008013211222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMillan D R, Xiao X, Shao L, Graves K, Benjamin I J. Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J Biol Chem. 1998;273:7523–7528. doi: 10.1074/jbc.273.13.7523. [DOI] [PubMed] [Google Scholar]
- 27.Melville M W, Tan S L, Wambach M, Song J, Morimoto R I, Katze M G. The cellular inhibitor of the PKR protein kinase, P58(IPK), is an influenza virus-activated co-chaperone that modulates heat shock protein 70 activity. J Biol Chem. 1999;274:3797–3803. doi: 10.1074/jbc.274.6.3797. [DOI] [PubMed] [Google Scholar]
- 28.Meriin A B, Yaglom J A, Gabai V L, Mosser D D, Zon L, Sherman M Y. Protein damaging stresses activate JNK via inhibition of its phosphatase: a novel pathway controlled by Hsp72. Mol Cell Biol. 1999;19:2547–2555. doi: 10.1128/mcb.19.4.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minami Y, Hohfeld J, Ohtsuka K, Hartl F U. Regulation of the heat-shock protein 70 reaction cycle by the mammalian DnaJ homolog, Hsp40. J Biol Chem. 1996;271:19617–19624. doi: 10.1074/jbc.271.32.19617. [DOI] [PubMed] [Google Scholar]
- 30.Mosser D D, Caron A W, Bourget L, Denis-Larose C, Massie B. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol Cell Biol. 1997;17:5317–5327. doi: 10.1128/mcb.17.9.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosser D D, Caron A W, Bourget L, Jolicoeur P, Massie B. Use of a dicistronic expression cassette encoding the green fluorescent protein for the screening and selection of cells expressing inducible gene products. BioTechniques. 1997;22:150–161. doi: 10.2144/97221rr02. [DOI] [PubMed] [Google Scholar]
- 32.Mosser D D, Martin L H. Induced thermotolerance to apoptosis in a human T lymphocyte cell line. J Cell Physiol. 1992;151:561–570. doi: 10.1002/jcp.1041510316. [DOI] [PubMed] [Google Scholar]
- 33.Nollen E A A, Brunsting J F, Roelofsen H, Weber L A, Kampinga H H. In vivo chaperone activity of heat shock protein 70 and thermotolerance. Mol Cell Biol. 1999;19:2069–2079. doi: 10.1128/mcb.19.3.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seimiya H, Mashima T, Toho M, Tsuruo T. c-Jun NH2-terminal kinase-mediated activation of interleukin-1beta converting enzyme/CED-3-like protease during anticancer drug-induced apoptosis. J Biol Chem. 1997;272:4631–4636. doi: 10.1074/jbc.272.7.4631. [DOI] [PubMed] [Google Scholar]
- 35.Shaw M, Cohen P, Alessi D R. The activation of protein kinase B by H2O2 or heat shock is mediated by phosphoinositide 3-kinase and not by mitogen-activated protein kinase-activated protein kinase-2. Biochem J. 1998;336:241–246. doi: 10.1042/bj3360241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simon M M, Reikerstorfer A, Schwarz A, Krone C, Luger T A, Jaattela M, Schwarz T. Heat shock protein 70 overexpression affects the response to ultraviolet light in murine fibroblasts. Evidence for increased cell viability and suppression of cytokine release. J Clin Investig. 1995;95:926–933. doi: 10.1172/JCI117800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takayama S, Sato T, Krajewski S, Kochel K, Irie S, Millan J A, Reed J C. Cloning and functional analysis of BAG-1: a novel bcl-2-binding protein with anti-cell death activity. Cell. 1995;80:279–284. doi: 10.1016/0092-8674(95)90410-7. [DOI] [PubMed] [Google Scholar]
- 38.Tournier C, Hess P, Yang D D, Xu J, Turner T K, Nimnual A, Bar-Sagi D, Jones S N, Flavell R A, Davis R J. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- 39.Uma S, Thulasiraman V, Matts R L. Dual role for Hsc70 in the biogenesis and regulation of the heme-regulated kinase of the α subunit of eukaryotic translation initiation factor 2. Mol Cell Biol. 1999;19:5861–5871. doi: 10.1128/mcb.19.9.5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verheij M, Bose R, Lin X H, Yao B, Jarvis W D, Grant S, Birrer M J, Szabo E, Zon L I, Kyriakis J M, Haimovitz-Friedman A, Fuks Z, Kolesnick R N. Requirement for ceramide-initiated SAPK/JNK signaling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 41.Volloch V, Gabai V L, Rits S, Force T, Sherman M Y. HSP72 can protect cells from heat-induced apoptosis by accelerating the inactivation of stress kinase JNK. Cell Stress Chaperones. 2000;5:139–147. doi: 10.1379/1466-1268(2000)005<0139:hcpcfh>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Volloch V, Gabai V L, Rits S, Sherman M Y. ATPase activity of the heat shock protein Hsp72 is dispensable for its effects on dephosphorylation of stress kinase JNK and on heat-induced apoptosis. FEBS Lett. 1999;461:73–76. doi: 10.1016/s0014-5793(99)01428-3. [DOI] [PubMed] [Google Scholar]
- 43.Volloch V, Mosser D D, Massie B, Sherman M Y. Reduced thermotolerance in aged cells results from loss of an hsp72-mediated control of JNK signaling pathway. Cell Stress Chaperones. 1998;3:265–271. [PMC free article] [PubMed] [Google Scholar]
- 44.Woessmann W, Meng Y H, Mivechi N F. An essential role for mitogen-activated protein kinases, ERKs, in preventing heat-induced cell death. J Cell Biochem. 1999;74:648–662. doi: 10.1002/(sici)1097-4644(19990915)74:4<648::aid-jcb14>3.3.co;2-y. [DOI] [PubMed] [Google Scholar]
- 45.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 46.Zanke B W, Boudreau K, Rubie E, Winnett E, Tibbles L A, Zon L, Kyriakis J, Liu F F, Woodgett J R. The stress-activated protein kinase pathway mediates cell death following injury induced by cis-platinum, UV irradiation or heat. Curr Biol. 1996;6:606–613. doi: 10.1016/s0960-9822(02)00547-x. [DOI] [PubMed] [Google Scholar]