Abstract
The polypeptides encoded by the chloroplast ndh genes and some nuclear genes form the thylakoid NADH dehydrogenase (Ndh) complex, homologous to the mitochondrial complex I. Except for Charophyceae (algae related to higher plants) and a few Prasinophyceae, all eukaryotic algae lack ndh genes. Among vascular plants, the ndh genes are absent in epiphytic and in some species scattered among different genera, families, and orders. The recent identification of many plants lacking plastid ndh genes allows comparison on phylogenetic trees and functional investigations of the ndh genes. The ndh genes protect Angiosperms under various terrestrial stresses, maintaining efficient photosynthesis. On the edge of dispensability, ndh genes provide a test for the natural selection of photosynthesis-related genes in evolution. Variable evolutionary environments place Angiosperms without ndh genes at risk of extinction and, probably, most extant ones may have lost ndh genes recently. Therefore, they are evolutionary endpoints in phylogenetic trees. The low number of sequenced plastid DNA and the long lifespan of some Gymnosperms lacking ndh genes challenge models about the role of ndh genes protecting against stress and promoting leaf senescence. Additional DNA sequencing in Gymnosperms and investigations into the molecular mechanisms of their response to stress will provide a unified model of the evolutionary and functional consequences of the lack of ndh genes.
Keywords: Ndh complex, photosynthesis, plant evolution, plastid DNA, stress protection
1. Introduction
The ndh genes are homologous to those encoding components of mitochondrial and bacterial respiratory complex I (NADH dehydrogenase, EC 1.6.5.3). Their identification in chloroplast DNA, by the 1980s [1,2], was a surprise because the respiratory electron transport chain and the photosynthetic electron transport chain are characteristic of mitochondria and chloroplast respectively, and there was no evidence for the presence of any complex I-like or respiratory-like process in chloroplasts. Therefore, the role, if any, of the ndh genes in the chloroplast became an active field of research that continues today.
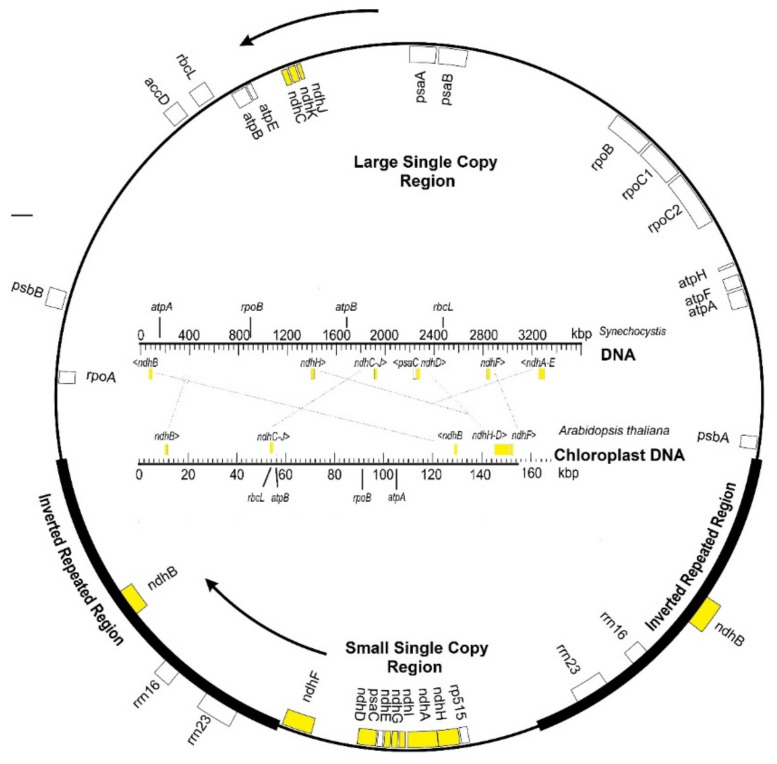
Chloroplasts evolutionarily derive from primitive endosymbiont cyanobacteria in host cells [3,4]. Many genes from cyanobacteria were progressively transferred to the nucleus of host cells, and the engulfed cyanobacteria evolved into chloroplasts that are only partially autonomous. Most chloroplast proteins are encoded in nuclear DNA, and only a few chloroplast proteins (about a hundred) are encoded in genes retained in plastid DNA; among them, the ndh genes. These genes, although lost in most algae lineages, are conserved in the plastid DNAs in the phylum Streptophyta and in the derived land plants. This suggests that ndh genes provide some advantages for the adaptation from aquatic to terrestrial environments. Figure 1 compares the chloroplast DNAs of Arabidopsis thaliana (154.5 kbp), as a model Angiosperm, and Synechocystis (3600 kbp), as a model cyanobacterium. Transpositions of the ndh genes (yellow) accompanied the reduction of about 95% of the cyanobacterial genome size to become the chloroplast DNA of angiosperms. The ndh genes are present in the plastid DNA of bryophytes, ferns, and photosynthetic higher plants, except for a few species that will be considered here. The conservation and the expression of ndh genes in chloroplasts vary among photosynthetic eukaryotes, revealing how environmental factors determine the conservation or the loss of genes through natural selection.
Figure 1.
Map of ndh genes (yellow) in the circular plastid DNA typical of higher plants. Some other representative genes are also indicated. Those inside the circle are transcribed clockwise (inner arrow) and those on the complementary strand counterclockwise (outer arrow) and are depicted outside. The thick lines in the circle correspond to the inverted repeated regions. Inside the circle, the ndh gene map in Arabidopsis is compared with the homologous gene map of Synechocystis as a model cyanobacterium.
Indeed, sequencing of chloroplast DNA from many plants, and immunological and proteomic identification of chloroplast proteins and protein complexes, have demonstrated the presence of ndh genes and a complex I-like (the Ndh complex) in the chloroplast of most land plants. Parasitic angiosperms, such as Epifagus virginiana and Cuscuta reflexa, which have low or no photosynthetic activity, were soon found to lack ndh genes [5,6,7], which suggested that the protein products of the ndh genes play a role in photosynthesis. However, as we will see below, some fully photosynthetic plants have been found to lack the plastid ndh genes and the Ndh complex, making their removal as intriguing as their functional role. The low relative amount of Ndh protein (about 0.2% of the thylakoid protein) [8,9] and the absence of chloroplast ndh genes in several plants [10] suggest that the functional role of the Ndh complex in the chloroplast might be dispensable in some environments and in some plant lines.
Early evidence [11,12] still suggested that ndh genes are involved in the response to different stresses, which could be related to the evolutionary loss of ndh genes. However, other functions of the Ndh complex in photosynthesis and leaf senescence could explain the loss of ndh genes. Plastid DNA from many plants have recently been sequenced and provide extensive taxonomic and phylogenetic information on the loss of plastid ndh genes. This review will seek correlations of biochemical, functional, and protective properties attributable to the Ndh complex with the estimated time and mode of evolutionary loss of ndh genes.
2. Chloroplast ndh Genes
Table 1 shows the usual name of the 11 chloroplast ndh genes (ndhA to ndhK), their encoded polypeptides, and their homologous subunits in respiratory complex I of mitochondria and respiratory bacteria. In most plants, the ndh H, A, I, G, E, and D genes with the psaC gene (encoding the photosystem I protein PsaC and located between ndhE and ndhD genes) are organized into a transcriptional unit (operon) that maps into the small single-copy region of plastid DNA. The ndhC, K, and J genes constitute another operon located in the large single-copy region of plastid DNA (Figure 1). The ndhF (mapping into the small single-copy region) and two identical copies of the ndhB gene (one in each of the two inverted repeated regions) are transcribed independently as monocistronic mRNA. Transpositions, inversions, and deletions within the DNA strand account for the deviations from the canonical gene organization, prevalent in Angiosperms (Figure 1). Each of the ndhA and ndhB genes has an intron of approximately 700 and 1000 nucleotides, respectively.
Table 1.
Chloroplast ndh genes, encoded polypeptides, and homologous subunits in the respiratory complex I.
|
Ndh Gene |
Encoded Polypeptide |
Homologous Polypeptides in Respiratory Complex I (References) |
|---|---|---|
| ndhA | NDH-A | ND1/NuoH/FpoH, EchB, NQ08 [11,13] |
| ndhB | NDH-B | ND2/NuoN/FpoO, NQO14 [13,14,15] |
| ndhC | NDH-C | ND3/NuoA/FpoA, NQ07 [14,15,16] |
| ndhD | NDH-D | ND4/NuoM/FpoM, NQ013 [13] |
| ndhE | NDH-E | ND4L/NuoK/FpoK, NQ011 [13,15,16] |
| ndhF | NDH-F | ND5/NuoL/FpoL, NQ012 [13,17] |
| ndhG | NDH-G | ND6/NuoJ/FpoJ, NQ010 [13,18] |
| ndhH | NDH-H | 49(IP)/NuoD/FpoD, EchE, NQ05 [13,19,20] |
| ndhI | NDH-I | TYKY/NuoI/FpoI, EchF, NQ09 [13,20] |
| ndhJ | NDH-J | 30(IP)/NuoC/FpoC, EchD, NQ04 [13,20] |
| ndhK | NDH-K | PSTT/NuoB/FpoB, EchC, NQ06 [13,20] |
Consistent with a functional role of the proteins encoded by the ndh genes in photosynthesis, several of them are absent or pseudogenized in heterotrophic species of the genera Orobanche and the family Orchidaceae [21,22,23]. However, ndh genes were not found in some fully photosynthetic competent plants, such as the Gymnosperm Pinus thunbergii [24]. Except for Gnetales and several conifers, plastid DNA of Gymnosperms have ndh genes [25]. Thus, the plastid DNA of Cycas taitungensis [26] and of the Conifers Cryptomeria japonica (a Cupressaceae) [27] and Thuja plicata [28] contain the ndh genes. Frequently, ndh pseudogenes with large nucleotide deletions are found in the plastid DNA of plants lacking ndh genes, and comparison among phylogenetically close genera suggests that the functional genes were recently lost.
Among fully photosynthetic Angiosperms, species of several genera and families’ (e.g., Erodium, Ericaceae, Najas) ndh genes rarely correlate with taxonomic or evolutionary relationships [23] and, at least in the Orchids family, occurred after evolution into subfamilies [29,30,31,32,33]. Similarly, only two out of thirteen Erodium species (E. texanum and E. carvifolium) retain all eleven ndh genes intact in the chloroplast [29]. The ndh genes were reported to be absent in species of Gentiana sect. Kudoa [34], but they are present in species of Gentiana sect. Cruciata [35]. Extensive sequencing of plastid DNA within families and genera over the past few years frequently reports the absence of ndh genes in a few plants. As a recent example, of 25 complete DNA sequences from the genera Aragoa, Littorella, and Plantago of Plantaginaceae, only those of the aquatic genus Littorella lack ndh genes [36].
Independent losses of ndh genes have been found in families of the order Alismatales, where 10 of 94 plants tested lack ndh genes [37], in the family Tofieldiaceae, in the aquatic species Najas flexilis of the family Hydrocharitaceae [31], and specifically, in Capparis spinosa var. herbacea of the genus Capparis [38]. In contrast, through chloroplast DNA re-ordering, some Ericales duplicate six ndh genes (in the inverted repeat regions), while they lose one copy of the usually duplicated ndhB gene, and the ndhF remains alone in the small single-copy region [39,40] (compare with Figure 1). Not surprisingly, the loss of ndh genes and the inverted repeat region are found in some Cactaceae [41] and may be associated with crassulacean acid metabolism.
Representative phylogenetic trees for Ericaceae (Figure 3 of Reference [30]), Erodium (Figure 1 of Reference [29]), and Alismatales (Figure 3 of Reference [37]) show that losses of chloroplast DNA ndh genes from some Angiosperms appear to be recent (less than 50 Ma) and independent evolutionary events after most species’ diversification occurred. Possibly, most of the older losses of ndh genes produced plants unable to evolve and diversify. Plant species that have lost the ndh genes could be endpoints of the evolutionary tree and will become extinct. Arguably, many Angiosperms lost the ndh genes in mild environments, where the Ndh complex was dispensable [10,23,32,42,43,44,45]. They and their offspring were unable to survive and diversify in variable stress environments. Probably, the same considerations are also valid for Gymnosperms, where ongoing intensive sequencing programs for Pinus species, which probably diverge only less than 10 Mya [46,47], should clarify what species and how many contain ndh genes [48].
3. Functional Role of the Thylakoid Ndh Complex
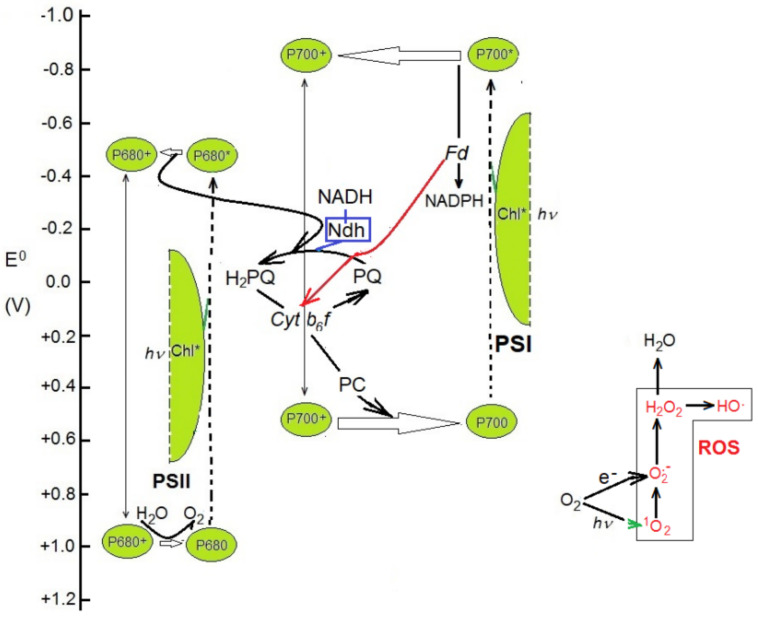
Understanding the fate of the ndh genes during land plant evolution must be based on the reactions catalyzed by the Ndh complex in chloroplasts. These appear to be related to the cyclic photosynthetic electron transport and photophosphorylation. The Ndh complex is found in the stromal thylakoids [9,49,50] and catalyzes an oxidoreduction reaction whose electron acceptor is oxidized plastoquinone (PQ). There are some discrepancies about the identity of the electron donor. The similarity to respiratory complex I, as well as in vitro and zymogram assays, suggests that NADH is the electron donor [8,9,17,28,51,52,53], in accordance with the reaction:
| NADH + H+ + PQ → NAD+ + H2PQ |
NADPH has no or negligible donor activity in assays with most plant preparations. Therefore, the Ndh complex would provide a pathway for PQ reduction not dependent on photosynthetic electron transport, that would initiate (Figure 2) a chlororespiratory electron transport chain [9,50,53]. Through dynamic oxidoreduction of plastoquinone, chlororespiration adjusts (poises) the redox levels of intermediaries [9,54,55,56] to optimize [57] cyclic electron transport (CET) following photoinhibition of photosystem II, under fluctuating light intensities or when temperature or humidity strongly affect CO2 availability or the rate of its reduction. CET complements linear electron transport (LET) (especially under different stress conditions and high CO2 concentrations) to polarize the thylakoid membrane, which is required to synthetize ATP and dissipate the excess of energy from excited chlorophylls (non-photosynthetic quenching of chlorophyll fluorescence, NPQ) via zeaxanthin (xanthophyll cycle).
Figure 2.
Connection of the Ndh complex with the photosynthetic electron transport. Electron transporters are displayed on the reference scale of the redox potential (E0). Arrows are marked in red for cyclic-specific electron transport (CET), in blue for electrons through the Ndh complex, and in green for electron excitation transfer. Box on the bottom right schematizes the main transformations of reactive oxygen species.
At a sudden decrease in light intensity, the reductive CO2 cycle drains more electrons than PSII can supply, and the transporters would be over-oxidized if the Ndh complex does not supply extra electrons from the NADH formed, for example, by malate dehydrogenase. Conversely, at high light intensity and a low rate of CO2 fixation (due to low temperature or stomate closure under dry conditions), the electron transporters are reduced in excess, and electrons are drained by the Mehler reaction, producing anion superoxide (O2•−). The additional electrons are drained by the subsequent action of superoxide dismutase and plastoquinol peroxidase, which scavenge superoxide and hydrogen peroxide (H2O2) respectively, keeping the level of reactive oxygen species (ROS) under control.
Terminal oxidase [50,55] could be an additional electron-draining process of cyclic over-reduced electron transporters. The combined actions of the Ndh complex and oxidative reactions constitute the chlororespiratory electron transport chain that rapidly buffers the redox shifts of electron transporters while maintaining active CET. The balanced ratio of Ndh and oxidative reactions prevents the burst of ROS levels that can led to cell death [58,59,60,61,62,63,64,65].
Based on assays with Arabidopsis membrane preparations, Yamamoto et al. [66] reported evidence for reduced ferredoxin as an electron donor and postulated that the Ndh complex transports electrons from photosystem I to PQ, providing a route of CET additional to that of the commonly accepted model in which ferredoxin donates electrons to the intermediary electron pool, PQ/cyt.b6f [67]. Be that as it may, the involvement of the Ndh complex optimizing CET and the associated photophosphorylation is widely accepted.
4. Dispensing with the Role of the ndh Genes
The involvement of the Ndh complex optimizing photosynthesis would explain the absence of the plastid ndh genes in parasitic plants and some carnivorous plants [68,69] that rely on low or no photosynthetic activity. Logic suggests that unused genes would accumulate mutations and, eventually, be eliminated to economize plant chemical and energy consumption [10,23,32,42,43,44,45]. Among the mutations, T-to-C mutations are frequently corrected by C-to-U editing at the RNA level of the transcript [42] and, less frequently, by C-to-T reversion in DNA [41]. However, the pseudogenization and deletion stages of ndh genes should be affected by the effects of environmental changes on the evolution of other functional traits of plants. Thus, detailed analysis of gene loss in Orchid and Ericaceae species [21,30] revealed that ndh genes were among the first pseudogenized genes in the chloroplast during the evolutionary transition from phototrophic or mycoheterotrophic to wholly heterotrophic metabolism, raising the question of whether species without ndh genes would survive for a long time without the development of heterotrophic structural and functional adaptations.
Transgenic plants defective in ndh genes point to some clues based on the role of the Ndh complex in protection against different stresses. Increased expression of ndh genes under different environments provides additional lines of evidence. Thus, transgenic tobacco plants whose ndh genes have been inactivated show impaired photosynthetic activity [17,70], especially under fluctuating light intensities and high atmospheric CO2 concentrations [56].
Plants under stress demand extra ATP consumption. Consequently, to satisfy it, heat stress was reported to increase CET and photophosphorylation in grape leaves [71]. The plastid Ndh complex is an efficient proton pump that increases CET and associated phosphorylation [72]. More specifically, chlororespiration has been found to increase under stress [55,73,74,75,76] and protects against photo-damage of oxygen-evolving complex and PSII [77,78].
Many biochemical and functional assays using transgenic plants indicate that the Ndh complex improves photosynthesis efficiency, decreases entropy production [44,56], and protects the leaves against a variety of stresses. However, extensive research using plant species lacking ndh genes is required to confirm the selective advantages provided by the Ndh complex. In this line of research, Sun et al. [79] reported intense variability and loss of ndh genes in the critically endangered Kingdonia uniflora. Similarly, Folk et al. [80] found intense pseudogenization and loss of ndh genes in the semi-aquatic plant Saniculiphyllum guangxiense, which contrasts with the strong conservatism of the plastid genes in other Saxifragales. Plausibly, the precariousness of Kingdonia uniflora and Saniculiphyllum guangxiense could be related to the poor photooxidative protection due to the absence of ndh genes. Plants lacking ndh genes have more difficulty adapting to changing environments. Thus, while the non-invasive weed Mikania cordata lacks the ndhF gene, the invasive Mikania micrantha retains it [81] and could invade new environments.
Current explanations assume that ndh genes could be dispensed with in mild environments. However, their loss only slowly drove plants to the heterotrophic alternative or, eventually, to extinction when abiotic stress episodes affected terrestrial habitats [82]. Be that as it may, gene dispensation implies that plants without ndh have some evolutionary advantages over plants with ndh in mild environments. Metabolism economy may be one advantage, but not necessarily the only one.
Being involved in stress protection, comparative analyses of plastid genes in different plants frequently report positive selection of the ndh genes [83,84,85]. However, the subtle function of ndh genes makes it difficult to functionally and ecologically compare species that differ only by the presence of ndh genes. Moreover, in stress protection, several activities interact with that of the Ndh complex in different ways, which involve ROS signaling and transcriptional factors, and may result in either a protective response [86] or cell death [63,64,87]. The Ndh complex and ndh gene transcripts increase early during leaf senescence [9,59]. Accordingly, transgenic tobacco plants defective in the ndhF gene show delayed leaf senescence [62] (Figure 3), and chloroplast-related ROS activities are required for senescence and cell death in different plant systems [28,88,89,90,91,92].
Figure 3.
Delayed leaf senescence in T181A and T181S tobacco as compared with wt (Petit Havana), showing basal leaf senescence even before blooming. Tobacco T181A and T181S are point mutants obtained from wt tobacco in which the phosphorylable threonine at position 181 of the NDH-F subunit of the Ndh complex is changed to non-phosphorylable alanine and serine, respectively [17].
Due to their involvement in ROS metabolism, ndh genes participate in complex crossroads regulating defense response, aging, and programmed cell death [63,64,65], three related cellular possibilities critical for plant survival and evolution.
ndh gene losses may have occurred frequently during the evolution of land plants, but the co-existence of ndh-less and ndh-containing plants of the same or newly diversified genera suggests: (1) that plants that lost ndh genes a long time ago (e.g., 10 or more Mya) became extinct, and (2) that the ancestors of extant ndh-less plants lost ndh genes recently. Therefore, ndh gene pseudogenizations are recent events in plant evolutionary trees that only include data from extant plants. An obvious conclusion would be that plant species lacking ndh genes are in danger of extinction.
5. Plant Death or Species Extinction, a ndh Dilemma?
Extinction of species lacking ndh genes requires recurrent periods of stress in most lands and over many generations. Their survival requires poorly investigated mechanisms of protection alternative to ndh genes, or a drastic decrease of ROS-generating metabolism, such as photosynthesis, as occurs in the heterotrophic metabolism of epiphytic and carnivorous plants.
Gnetales and some Pinus, which could have lost the ndh genes early in evolution, require further functional investigations and sequencing of the plastid DNA from many closely related species and subspecies.
Gnetales and some Pinus that lack ndh genes are surprisingly long-lived. The Gnetal Welwitschia mirabilis is a well-documented case of a plant lacking ndh genes [93]. It is the only species in the only genera of the Welwitschiaceae family of Gnetales, and estimates put its lifespan at up to 1000 years. Several Pinus, such as some individuals of Pinus longaeva, probably live for longer periods, and their needles remain alive and photosynthetically active for up to 30 years. As long-lived plants, they have surely suffered many periods of stress, but have survived without ndh genes. On the other hand, their longevity could be due in part to the absence of ndh genes, as found in transgenic tobaccos. To answer this question and, in general, the relationships among plant longevity, species survival, extinction, and diversification, requires detailed investigations on the distribution of ndh genes and pseudogenes in Gymnosperms and on the molecular mechanisms that protect long-lived species against stress.
In Angiosperms’ leaves, ROS not only destroy cell components, but are also transduction signals within the complex networks of molecules that modulate the response to various stresses and cell survival and death [94]. The destructive and signaling actions of ROS co-exist in responses to stress, aging, and senescence, and it is often difficult to distinguish between the two actions and among the three responses. Three major ROS, singlet oxygen (1O2), superoxide anion radical (O2•−), and hydrogen peroxide (H2O2), are effective wreckers of cellular components and are also cellular signals that affect gene expression and enzymatic activities. In general, 1O2 and O2•− appear to promote cellular aging and programmed cell death (senescence), and H2O2 promotes the stress defense response [64,65,95,96]. In chloroplasts, 1O2 is generated by transfer of an electronic excitation from chlorophyll to O2. O2•− is generated by transfer of an electron from reduced intermediaries to O2 or to 1O2. The Ndh complex, in concerted action with electron-draining reactions (chlororespiration, including superoxide dismutase, SOD), keeps the thylakoid membrane polarized, which allows dissipation as heat of excess energy from excited chlorophylls and decreases the formation of 1O2. SOD delays senescence by removing O2•− and forming H2O2. Therefore, low levels of SOD are associated with senescence in plant [58,59,61,97,98,99,100] and animal [101] tissues. In contrast, mRNA translatable expression of ndh genes increases early in leaf senescence, and the Ndh complex increases during tissue senescence and fruit ripening [9,11,28,59,91,102,103]. Increased Ndh with lower SOD levels in senescence and aging, far from balancing the redox level of transporters, increases their reduced forms, thus hindering thylakoid polarization and dissipation of excess light energy, and worse, increasing 1O2 and O2•− formation, as observed during barley flag leaf senescence [104]. Therefore, SOD and Ndh complex responses, as photosynthetic tissues age, emerge as key determinants of the leaf fate towards death (increased Ndh and decreased SOD) or survival.
Although the presence of ndh genes in nuclear DNA and their encoded polypeptides in chloroplasts cannot be excluded, Gnetales and some Pinus probably lack the Ndh complex. In addition, investigations on SOD and other activities that may be involved in protection of photosynthetic machinery in Gymnosperms are needed to understand their survival and longevity, often in extreme environments.
The molecular mechanisms of senescence have been investigated mainly in leaves of monocarpic Angiosperms and show great similarity with the programmed cell death (PCD) investigated in animals and plants [62]. When the tissue reaches an advanced, but poorly defined, stage of development, the death program triggers the expression of senescence-related genes involved in the ordered macromolecular breakdown, leading to cell death. In animal and non-photosynthetic plant tissues, mitochondria are critical in triggering PCD through cellular signals, including ROS, that re-program gene expression from live metabolic processes to cell death [105,106,107]. Often, the ROS damage makes it difficult to distinguish between ROS-mediated PCD and ROS-mediated cellular aging, in which the cell dies by accumulation of hazardous damages of cellular components. In photosynthetic tissues, chloroplasts replace mitochondria as the signal factory (including ROS) that triggers PCD and/or cellular aging [62]. Most responses to biotic and abiotic stresses are also mediated by ROS produced by mitochondria [108] and chloroplasts [12], adding further complexity to the time-course of death and defense responses in plants that are permanently exposed to variable environments. However, the molecular mechanisms of death and defense responses are reasonably well-known in Angiosperms, but are scarcely known in most Gymnosperms, where the absence of ndh genes in some of them is a factor to be considered.
The absence or low expression of ndh genes could explain why needles and the whole plant in several Gymnosperms do not appear to show PCD but show aging by accumulation of hazardous damages. Intriguingly, evidence links heritable deficiencies of complex I (the mitochondrial homologous of Ndh complex) to human longevity [109], in clear correspondence with the longevity observed in Gymnosperms lacking ndh genes. However, this does not explain why, without ndh genes, Gymnosperms can survive the stresses to which Angiosperms are supposedly protected by ndh genes. Further molecular and physiological investigations should shed light on the uncertainties surrounding the life extension of some plants, the dispensation of the ndh genes, and the extinction of most ndh-less plants.
6. Concluding Remarks and Future Perspectives
Recent analyses of the absence of plastid ndh genes in a number of plants and comparison with phylogenetic trees, as well as with functional investigations of the role of the ndh genes, allowed us to advance some conclusions and propose open questions about the functional and evolutionary consequences of the presence of the ndh genes.
The ndh genes allow Angiosperm species to survive in many stressful terrestrial environments and to maintain efficient photosynthesis. They provide little or no advantages in mild environments, where they consequently accumulate mutations and, eventually, become pseudogenes and are deleted from plastid DNA. Angiosperms that have lost plastid ndh genes survive in mild and moderate stress environments or adopt a heterotrophic or carnivorous metabolism that compensates for their low or absent photosynthetic efficiency. Variable environments along the scale time of biological evolution place Angiosperms without ndh at permanent risk of extinction, as may be occurring with the endangered Kingdonia uniflora [79]. Consequently, phylogenetic analyses indicate that Angiosperm species lacking plastid ndh genes lost them recently (typically less than 10 Mya). Most hypothetical species that lacked ndh genes more than 10 Mya became extinct, and the extant Angiosperms without ndh are probably evolutionary endpoints on phylogenetic trees.
In Gymnosperms, the still small number of available sequences makes a comprehensive phylogenetic analysis of ndh gene loss difficult. Although a low relevance of PCD is assumed for leaves and the whole plant in Gymnosperms, the long lifespan of many of them poses formidable problems of understanding from our knowledge of ndh genes and stress responses in Angiosperms. Therefore, in addition to advances in plastid and nuclear DNA sequencing, future research should reveal the mechanisms of the stress response in Gymnosperms, from the molecular and cellular levels, including ROS generation and scavenging, to membrane-protecting mechanisms. A satisfactory understanding of the dispensability of the ndh genes may only be achieved with a broad view that includes the peculiarities of Angiosperms and Gymnosperms in terms of photosynthetic metabolism, stress response, longevity, reproduction, diversification, and vulnerability to extinction, that could be affected by the loss of ndh genes.
Acknowledgments
The author wishes to thank the stimulating criticism and suggestions of Carmen Díaz-Sala and Mercedes Martín (University of Alcalá, Spain).
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ohyama K., Fukuzawa H., Kochi T., Shira H., Sano T., Umesono K., Shiki Y., Takeuchi M., Chang Z., Aota S., et al. Chloroplast gene organization deduced from complete sequence of liverwort Marchantia polymorpha chloroplast DNA. Nature. 1986;322:572–574. doi: 10.1038/322572a0. [DOI] [Google Scholar]
- 2.Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K., et al. The complete nucleotide sequence of the tobacco chloroplast genome. Its gene organization and expression. EMBO J. 1986;5:2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin W., Herrmann R.G. Gene transfer from organelles to nucleus: How much, what happens, and why? Plant Physiol. 1998;118:9–17. doi: 10.1104/pp.118.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin W., Rujan T., Richly E., Hansen A., Cornelsen S., Lins T., Leister D., Stoebe B., Hasegawa M., Penny D. Evolutionary analysis of Arabidopsis, cyanobacterial, and chloroplast genomes reveals plastid phylogeny and thousands of cyanobacterial genes in the nucleus. Proc. Natl. Acad. Sci. USA. 2002;99:12246–12251. doi: 10.1073/pnas.182432999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolfe K.H., Morden C.W., Palmer J.D. Function and evolution of a minimal plastid genome from a non-photosynthetic parasitic plant. Proc. Natl. Acad. Sci. USA. 1992;89:10648–10652. doi: 10.1073/pnas.89.22.10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haberhausen G., Zetsche K. Functional loss of all ndh genes in an otherwise relatively unaltered plastid genome of the holoparasitic flowering plant Cuscuta reflexa. Plant Mol. Biol. 1994;24:217–222. doi: 10.1007/BF00040588. [DOI] [PubMed] [Google Scholar]
- 7.Funk H.T., Berg S., Krupinska K., Maier U.G., Krause K. Complete DNA sequences of the plastid genomes of two parasitic flowering plant species, Cuscuta reflexa and Cuscuta gronovii. BMC Plant Biol. 2007;7:45. doi: 10.1186/1471-2229-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sazanov L., Burrows P.A., Nixon P.J. The plastid ndh genes code for an NADH-specific dehydrogenase: Purification and characterization of a mitochondrial-like complex I from pea thylakoid membranes. Proc. Natl. Acad. Sci. USA. 1998;95:1319–1324. doi: 10.1073/pnas.95.3.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casano L.M., Zapata J.M., Martín M., Sabater B. Chlororespiration and poising of cyclic electron transport: Plastoquinone as electron transporter between thylakoid NADH dehydrogenase and peroxidase. J. Biol. Chem. 2000;275:942–948. doi: 10.1074/jbc.275.2.942. [DOI] [PubMed] [Google Scholar]
- 10.Martín M., Sabater B. Plastid ndh genes in plant evolution. Plant Physiol. Biochem. 2010;48:636–645. doi: 10.1016/j.plaphy.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Martín M., Casano L.M., Sabater B. Identification of the product of ndhA gene as a thylakoid protein synthesized in response to photooxidative treatment. Plant Cell Physiol. 1996;37:293–298. doi: 10.1093/oxfordjournals.pcp.a028945. [DOI] [PubMed] [Google Scholar]
- 12.Martín M., Casano L.M., Zapata J.M., Guéra A., del Campo E.M., Schmitz-Linneweber C., Maier R.M., Sabater B. Role of thylakoid Ndh complex and peroxidase in the protection against photo-oxidative stress: Fluorescence and enzyme activities in wild-type and ndhF-deficient tobacco. Physiol. Plant. 2004;122:443–452. doi: 10.1111/j.1399-3054.2004.00417.x. [DOI] [Google Scholar]
- 13.Friedrich T., Scheide D. The respiratory complex I of bacteria, archaea and eukarya and its module common with membrane-bound multisubunit hydrogenases. FEBS Lett. 2000;479:1–5. doi: 10.1016/S0014-5793(00)01867-6. [DOI] [PubMed] [Google Scholar]
- 14.Freyer R., López C., Maier R.M., Martín M., Sabater B., Kossel H. Editing of chloroplast ndhB encoded transcript shows divergence between closely related members of the grass family (poaceae) Plant Mol. Biol. 1995;29:679–684. doi: 10.1007/BF00041158. [DOI] [PubMed] [Google Scholar]
- 15.Maier R.M., Neckermann K., Igloi G.L., Kossel H. Complete sequence of the maize chloroplast genome: Gene content, hotspots of divergence and fine tuning of genetic information by transcript editing. J. Mol. Biol. 1995;251:614–628. doi: 10.1006/jmbi.1995.0460. [DOI] [PubMed] [Google Scholar]
- 16.Hiratsuka J., Shimada H., Whittier R., Ishibashi T., Sakamoto M., Mori M., Kondo C., Honji Y., Sun C.-R., Meng B.-Y., et al. The complete sequence of the rice (Oryza sativa) chloroplast genome: Intermolecular recombination between distinct tRNA genes accounts for a major plastid DNA inversion during the evolution of the cereals. Mol. Gen. Genet. 1989;217:185–194. doi: 10.1007/BF02464880. [DOI] [PubMed] [Google Scholar]
- 17.Martín M., Funk H.T., Serrot P.H., Poltnigg P., Sabater B. Functional characterization of the thylakoid Ndh complex phosphorylation by site-directed mutations in the ndhF gene. Biochim. Biophys. Acta BBA Bioenerg. 2009;1787:920–928. doi: 10.1016/j.bbabio.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Martínez P., López C., Roldán M., Sabater B., Martín M. Plastid DNA of five ecotypes of Arabidopsis thaliana: Sequence of ndhG gene and maternal inheritance. Plant Sci. 1997;123:113–122. doi: 10.1016/S0168-9452(96)04563-3. [DOI] [Google Scholar]
- 19.Berger S., Ellersiek U., Westhoff P., Steinmuller K. Studies on the expression of NDH-H, a subunit of the NAD(P)H-plastoquinone-oxidoreductase of higher plant chloroplasts. Planta. 1993;190:25–31. doi: 10.1007/BF00195671. [DOI] [Google Scholar]
- 20.Burrows P.A., Sazanov L.A., Svab Z., Maliga P., Nixon P.J. Identification of a functional respiratory complex in chloroplasts through analysis of tobacco mutants containing disrupted plastid ndh genes. EMBO J. 1998;17:868–871. doi: 10.1093/emboj/17.4.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barrett C.F., Freudenstein J.V., Li J., Mayfield-Jones D.R., Perez L., Santos C. Investigating the path of plastid genome degradation in an early-transitional clade of heterotrophic orchids, and implications for heterotrophic angiosperms. Mol. Biol. Evol. 2014;31:3095–3112. doi: 10.1093/molbev/msu252. [DOI] [PubMed] [Google Scholar]
- 22.Luo J., Hou B.-W., Niu Z.-T., Liu W., Xue Q.-Y., Ding X.-Y. Comparative chloroplast genomes of photosynthetic orchids: Insights into evolution of the Orchidaceae and development of molecular markers for phylogenetic applications. PLoS ONE. 2014;9:e99016. doi: 10.1371/journal.pone.0099016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin C.S., Chen J.J., Huang Y.T., Chan M.T., Daniell H., Chang W.J., Hsu C.T., Liao D.C., Wu F.H., Lin S.Y., et al. The location and translocation of ndh genes of chloroplast origin in the Orchidaceae family. Sci. Rep. 2015;5:9040. doi: 10.1038/srep09040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wakasugi T., Tsudzuki J., Ito S., Nakashima K., Tsudzuki T., Sugiura M. Loss of all ndh genes as determined by sequencing the entire chloroplast genome of the black pine Pinus thunbergii. Proc. Natl. Acad. Sci. USA. 1994;91:9794–9798. doi: 10.1073/pnas.91.21.9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braukmann T.W.A., Kuzmina M., Stefanovíc S. Loss of all ndh genes in Gnetales and conifers: Extent and evolutionary significance for seed plant phylogeny. Curr. Genet. 2009;55:323–337. doi: 10.1007/s00294-009-0249-7. [DOI] [PubMed] [Google Scholar]
- 26.Wu C.S., Wang Y.N., Liu S.M., Chaw S.M. Chloroplast genome (cpDNA) of Cycas taitungensis and 56 Cp Protein-coding genes of Gnetum parvifolium: Insights into CpDNA evolution and phylogeny of extant seed plants. Mol. Biol. Evol. 2007;24:1366–1379. doi: 10.1093/molbev/msm059. [DOI] [PubMed] [Google Scholar]
- 27.Hirao T., Watanabe A., Kurita M., Kondo T., Takata K. Complete nucleotide sequence of the Cryptomeria japonica D. Don. chloroplast genome and comparative chloroplast genomics: Diversified genomic structure of coniferous species. BMC Plant Biol. 2008;8:70. doi: 10.1186/1471-2229-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serrot P.H., Sabater B., Martín M. Activity, polypeptide and gene identification of thylakoid Ndh complex in trees: Potential physiological relevance of fluorescence assays. Physiol. Plant. 2012;146:110–120. doi: 10.1111/j.1399-3054.2012.01598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blazier J.C., Guisinger M.M., Jansen R.K. Recent loss of plastid-encoded ndh genes within Erodium (Geraniaceae) Plant Mol. Biol. 2011;76:263–272. doi: 10.1007/s11103-011-9753-5. [DOI] [PubMed] [Google Scholar]
- 30.Braukmann T.W.A., Stefanovíc S. Plastid genome evolution in mycoheterotrophic Ericaceae. Plant Mol. Biol. 2012;79:5–20. doi: 10.1007/s11103-012-9884-3. [DOI] [PubMed] [Google Scholar]
- 31.Peredo E.L., King U.M., Les D.H. The plastid genome of Najas flexilis: Adaptation to submersed environments is accompanied by the complete loss of the NDH Complex in an Aquatic Angiosperm. PLoS ONE. 2013;8:e68591. doi: 10.1371/journal.pone.0068591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruhlman T.A., Chang W.J., Chen J.J., Huang Y.T., Chan M.T., Zhang J., Liao D.C., Blazier J.C., Jin X., Shih M.C., et al. NDH expression marks major transitions in plant evolution and reveals coordinate intracellular gene loss. BMC Plant Biol. 2015;15:100. doi: 10.1186/s12870-015-0484-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong W.-L., Wang R.-N., Zhang N.-Y., Fan W.-B., Fang M.-F., Li Z.-H. Molecular evolution of chloroplast genomes of orchid species: Insights into phylogenetic relationship and adaptive evolution. Int. J. Mol. Sci. 2018;19:716. doi: 10.3390/ijms19030716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun S.-S., Fu P.-C., Zhou X.-J., Cheng Y.-W., Zhang F.-Q., Chen S.-L., Gao Q.-B. The complete plastome sequences of seven species in Gentiana sect. Kudoa (Gentianaceae): Insights into plastid gene loss and molecular evolution. Front. Plant Sci. 2018;9:493. doi: 10.3389/fpls.2018.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou T., Wang J., Jia Y., Li W., Fusheng Xu F., Wang X. Comparative chloroplast genome analyses of species in Gentiana section Cruciata (Gentianaceae) and the development of authentication markers. Int. J. Mol. Sci. 2018;19:1962. doi: 10.3390/ijms19071962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mower J.P., Guo W., Partha R., Fan W., Levsen N., Wolff K., Jacqueline M., Nugent J.M., Pabón-Mora N., González F. Plastomes from tribe Plantagineae (Plantaginaceae) reveal infrageneric structural synapormorphies and localized hypermutation for Plantago and functional loss of ndh genes from Littorella. Mol. Phylogenet. Evol. 2021;162:107217. doi: 10.1016/j.ympev.2021.107217. [DOI] [PubMed] [Google Scholar]
- 37.Ross T.G., Barrett C.F., Gomez M.S., Lam V.K.Y., Henriquez C.L., Les D.H., Davis J.I., Cuenca A., Petersen G., Seberg O., et al. Plastid phylogenomics and molecular evolution of Alismatales. Cladistics. 2016;32:160–178. doi: 10.1111/cla.12133. [DOI] [PubMed] [Google Scholar]
- 38.Maurya S., Darshetkar A.M., Datar M.N., Tamhankar S., Li P., Choudhary R.K. Plastome data provide insights into intra and interspecific diversity and ndh gene loss in Capparis (Capparaceae) Phytotaxa. 2020;432:206–220. doi: 10.11646/phytotaxa.432.2.10. [DOI] [Google Scholar]
- 39.Fajardo D., Senalik D., Ames M., Zhu H., Steffan S.A., Harbut R., Polashock J., Vorsa N., Gillespie E., Kron K., et al. Complete Plastid Genome Sequence of Vaccinium macrocarpon: Structure, Gene Content, and Rearrangements Revealed by Next Generation Sequencing. Tree Gen. Genom. 2013;9:489–498. doi: 10.1007/s11295-012-0573-9. [DOI] [Google Scholar]
- 40.Szczecinska M., Gomolinska A., Szkudlarz P., Sawick J. Plastid and nuclear genomic resources of a relict and endangered plant species: Chamaedaphne calyculata (L.) Moench (Ericaceae) Turk. J. Bot. 2014;38:1229–1238. doi: 10.3906/bot-1405-80. [DOI] [Google Scholar]
- 41.Sanderson M.J., Copetti D., Búrquez A., Bustamante E., Charboneau J.L.M., Luis E., Eguiarte L.E., Kumar S., Lee H.O., Lee J., et al. Exceptional reduction of the plastid genome of saguaro cactus (Carnegiea gigantea): Loss of the ndh gene suite and inverted repeat. Am. J. Bot. 2015;102:1115–1127. doi: 10.3732/ajb.1500184. [DOI] [PubMed] [Google Scholar]
- 42.Sabater B., Martín M., Schmitz-Linneweber C., Maier R.M. Is clustering of plastid RNA editing sites a consequence of transitory loss of gene function?—Implications for past environmental and evolutionary events in plants. Perspect. Plant Ecol. Evol. Syst. 2002;5:81–90. doi: 10.1078/1433-8319-00024. [DOI] [Google Scholar]
- 43.Marín D., Martín M., Serrot P., Sabater B. Thermodynamic balance of photosynthesis and transpiration at increasing CO2 concentrations and rapid light fluctuations. BioSystems. 2014;116:21–26. doi: 10.1016/j.biosystems.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 44.Martín M., Marín D., Serrot P.H., Sabater B. Evolutionary reversion of editing sites of ndh genes suggests their origin in the Permian-Triassic before the increase of atmospheric CO2. Front. Ecol. Evol. 2015;3:81. doi: 10.3389/fevo.2015.00081. [DOI] [Google Scholar]
- 45.Zhe M., Zhang L., Liu F., Huang Y., Fan W., Yang J., Zhu A. Plastid RNA editing reduction accompanied with genetic variations in Cymbidium, a genus with diverse lifestyle modes. Plant Divers. 2021 doi: 10.1016/j.pld.2021.07.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ni Z., Ye Y., Bai T., Xu M., Xu L.A. Complete chloroplast genome of Pinus massoniana (Pinaceae): Gene rearrangements, loss of ndh genes, and short inverted repeats contraction, expansion. Molecules. 2017;22:1528. doi: 10.3390/molecules22091528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeb U., Dong W.-L., Zhang T.-T., Wang R.-N., Shahzad K., Ma X.-F., Li Z.-H. Comparative plastid genomics of Pinus species: Insights into sequence variations and phylogenetic relationships. J. Syst. Evol. 2020;58:118–132. doi: 10.1111/jse.12492. [DOI] [Google Scholar]
- 48.Sokołowska J., Fuchs H., Celínski K. New insight into taxonomy of European mountain pines, Pinus mugo complex, based on complete chloroplast genomes sequencing. Plants. 2021;10:1331. doi: 10.3390/plants10071331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Casano L.M., Lascano H.R., Martín M., Sabater B. Topology of the plastid Ndh complex and its NDH-F subunit in thylakoid membranes. Biochem. J. 2004;382:145–155. doi: 10.1042/BJ20031828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lennon A.M., Prommeenate P., Nixon P.J. Location, expression, and orientation of the putative chlororespiratory enzymes, Ndh, and IMMUTANS, in higher plant plastids. Planta. 2003;218:254–260. doi: 10.1007/s00425-003-1111-7. [DOI] [PubMed] [Google Scholar]
- 51.Cuello J., Quiles M.J., Albacete M.E., Sabater B. Properties of a large complex of NADH dehydrogenase from barley leaves. Plant Cell Physiol. 1995;36:265–271. doi: 10.1093/oxfordjournals.pcp.a078758. [DOI] [Google Scholar]
- 52.Corneille S., Courmac L., Guedeney G., Havaux M., Peltier G. Reduction of the plastoquinone pool by exogenous NADH and NADPH in higher plant chloroplasts. Characterization of a NAD(P)H-plastoquinone oxidoreductase activity. Biochim. Biophys. Acta BBA Bioenerg. 1998;1363:59–69. doi: 10.1016/S0005-2728(97)00074-1. [DOI] [PubMed] [Google Scholar]
- 53.Díaz M., de Haro V., Muñoz R., Quiles M.J. Chlororespiration is involved in the adaptation of Brassica plants to heat and high light intensity. Plant Cell Environ. 2007;30:1578–1585. doi: 10.1111/j.1365-3040.2007.01735.x. [DOI] [PubMed] [Google Scholar]
- 54.Joët T., Cournac L., Peltier G., Havaux M. Cyclic electron flow around Photosystem I in C3 plants. In vivo control by the redox state of chloroplast and involvement of the NADH-dehydrogenase complex. Plant Physiol. 2002;128:760–769. doi: 10.1104/pp.010775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rumeau D., Peltier G., Cournac L. Chlororespiration and cyclic electron flow around PSI during photosynthesis and plant stress response. Plant Cell Environ. 2007;30:1041–1051. doi: 10.1111/j.1365-3040.2007.01675.x. [DOI] [PubMed] [Google Scholar]
- 56.Martín M., Marín D., Serrot P.H., Sabater B. The rise of the photosynthetic rate when light intensity increases is delayed in ndh gene-defective tobacco at high but not at low CO2 concentrations. Front. Plant Sci. 2015;6:34. doi: 10.3389/fpls.2015.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heber U., Walker D. Concerning a dual function of coupled cyclic electron transport in leaves. Plant Physiol. 1992;100:1621–1626. doi: 10.1104/pp.100.4.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Casano L.M., Martín M., Sabater B. Sensitivity of superoxide dismutase transcript levels and activities to oxidative stress is lower in mature-senescent than in young barley leaves. Plant Physiol. 1994;106:1033–1039. doi: 10.1104/pp.106.3.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Casano L.M., Martín M., Zapata J.M., Sabater B. Leaf age- and paraquat concentration-dependent effects on the levels of enzymes protecting against photooxidative stress. Plant Sci. 1999;149:13–22. doi: 10.1016/S0168-9452(99)00138-7. [DOI] [Google Scholar]
- 60.Abarca D., Martín M., Sabater B. Differential leaf stress responses in young and senescent plants. Physiol. Plant. 2001;113:409–415. doi: 10.1034/j.1399-3054.2001.1130315.x. [DOI] [PubMed] [Google Scholar]
- 61.Abarca D., Roldán M., Martín M., Sabater B. Arabidopsis thaliana ecotype Cvi shows an increased tolerance to photo-oxidative stress and contains a new chloroplastic copper/zinc superoxide dismutase isoenzyme. J. Exp. Bot. 2001;52:1417–1425. doi: 10.1093/jexbot/52.360.1417. [DOI] [PubMed] [Google Scholar]
- 62.Zapata J.M., Guera A., Esteban-Carrasco A., Martín M., Sabater B. Chloroplasts regulate leaf senescence: Delayed senescence in transgenic ndhF-defective tobacco. Cell Death Differ. 2005;12:1277–1284. doi: 10.1038/sj.cdd.4401657. [DOI] [PubMed] [Google Scholar]
- 63.Sabater B., Martín M. Chloroplast control of leaf senescence. In: Biswal B., Krupinska K., Biswal U., editors. Plastid Development in Leaves during Growth and Senescence. Advances in Photosynthesis and Respiration. Volume 36. Springer Science+Business Media; Dordrecht, The Netherlands: 2013. pp. 529–550. [DOI] [Google Scholar]
- 64.Sabater B., Martín M. Hypothesis: Increase of the ratio singlet oxygen plus superoxide radical to hydrogen peroxide changes stress defense response to programmed leaf death. Front. Plant Sci. 2013;4:479. doi: 10.3389/fpls.2013.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sabater B., Martín M. Control of leaf cell death by reactive oxygen species. In: Rice J., editor. Programmed Cell Death in Plants and Animals. Nova Science Publishers, Inc.; New York, NY, USA: 2016. pp. 75–93. Chapter 3. [Google Scholar]
- 66.Yamamoto H., Peng L., Fukao Y., Shikanai T. A Src homology 3 domain-like fold protein forms a ferredoxin binding site for the chloroplast NADH dehydrogenase-like complex of Arabidopsis. Plant Cell. 2011;23:1480–1493. doi: 10.1105/tpc.110.080291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kurisu G., Zhang H., Smith J.L., Cramer W.A. Structure of the cytochrome b6f complex of oxygenic photosynthesis: Tuning the cavity. Science. 2003;302:1009–1014. doi: 10.1126/science.1090165. [DOI] [PubMed] [Google Scholar]
- 68.Silva S.R., Diaz Y.C., Penha H.A., Pinheiro D.G., Fernandes C.C., Miranda V.F., Michael T.P., Varani A.M. The Chloroplast Genome of Utricularia reniformis Sheds Light on the Evolution of the ndh Gene Complex of Terrestrial Carnivorous Plants from the Lentibulariaceae Family. PLoS ONE. 2016;11:e0165176. doi: 10.1371/journal.pone.0165176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gruzdev E.V., Kadnikov V.V., Beletsky A.V., Kochieva E.Z., Mardanov A.V., Skryabin K.G., Ravin N.V. Plastid genomes of carnivorous plants Drosera rotundifolia and Nepenthes × ventrata reveal evolutionary patterns resembling those observed in parasitic plants. Int. J. Mol. Sci. 2019;20:4107. doi: 10.3390/ijms20174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Horváth E.M., Peter S.O., Joët T., Rumeau D., Cournac L., Horváth G.V., Kavanagh T.A., Schäfer C., Peltier G., Medgyesy P. Targeted inactivation of the plastid ndhB gene in tobacco results in an enhanced sensitivity of photosynthesis to moderate stomatal closure. Plant Physiol. 2000;123:1337–1349. doi: 10.1104/pp.123.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun Y., Geng Q., Du Y., Yang X., Zhai H. Induction of cyclic electron flow around photosystem I during heat stress in grape leaves. Plant Sci. 2017;256:65–71. doi: 10.1016/j.plantsci.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 72.Strand D.D., Fisher N., Kramer D.M. The higher plant plastid NAD(P)H dehydrogenase-like complex (NDH) is a high efficiency proton pump that increases ATP production by cyclic electron flow. J. Biol. Chem. 2017;292:11850–11860. doi: 10.1074/jbc.M116.770792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Paredes M., Quiles M.J. Stimulation of chlororespiration by drought under heat and high illumination in Rosa meillandina. J. Plant Physiol. 2013;170:165–171. doi: 10.1016/j.jplph.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 74.Segura M.V., Quiles M.J. Involvement of chlororespiration in chilling stress in the tropical species Spathiphyllum wallisü. Plant Cell Environ. 2015;38:525–533. doi: 10.1111/pce.12406. [DOI] [PubMed] [Google Scholar]
- 75.Li Q., Yao Z.J., Mi H. Alleviation of photoinhibition by co-ordination of chlororespiration and cyclic electron flow mediated by NDH under heat stressed condition in tobacco. Front. Plant Sci. 2016;7:285. doi: 10.3389/fpls.2016.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alafari H.A., Abd-Elgawadab M.E. Differential expression gene/protein contribute to heat stress-responsive in Tetraena propinqua in Saudi Arabia. Saudi J. Biol. Sci. 2021;28:5017–5027. doi: 10.1016/j.sjbs.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tan Y., Zhang Q.S., Zhao W., Liu Z., Ma M.Y., Zhong M.Y., Wang M.X., Xu B. Chlororespiration serves as photoprotection for the photo-inactivated oxygen-evolving complex in Zostera marina, a marine angiosperm. Plant Cell Physiol. 2020;61:1517–1529. doi: 10.1093/pcp/pcaa075. [DOI] [PubMed] [Google Scholar]
- 78.Zhao J., Yu W., Zhang L., Liu J. Chlororespiration protects the photosynthetic apparatus against photoinhibition by alleviating inhibition of photodamaged-PSII repair in Haematococcus pluvialis at the green motile stage. Algal Res. 2021;54:102140. doi: 10.1016/j.algal.2020.102140. [DOI] [Google Scholar]
- 79.Sun Y., Deng T., Zhang A., Moore M.J., Landis J.B., Lin N., Zhang H., Zhang X., Huang J., Zhang X., et al. Genome sequencing of the endangered Kingdonia uniflora (Circaeasteraceae, Ranunculales) reveals potential mechanisms of evolutionary specialization. iScience. 2020;23:101124. doi: 10.1016/j.isci.2020.101124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Folk R.A., Sewnath N., Xiang C.-L., Sinn B.T., Guralnick R.P. Degradation of key photosynthetic genes in the critically endangered semi-aquatic flowering plant Saniculiphyllum guangxiense (Saxifragaceae) BMC Plant Biol. 2020;20:324. doi: 10.1186/s12870-020-02533-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Su Y., Huang L., Wang Z., Wang T. Comparative chloroplast genomics between the invasive weed Mikania micrantha and its indigenous congener Mikania cordata: Structure variation, identification of highly divergent regions, divergence time estimation, and phylogenetic analysis. Mol. Phylogenet. Evol. 2018;126:181–195. doi: 10.1016/j.ympev.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 82.Scobeyeva V.A., Artyushin I.V., Krinitsina A.A., Nikitin P.A., Antipin M.I., Kuptsov S.V., Belenikin M.S., Omelchenko D.O., Logacheva M.D., Konorov E.A., et al. Gene loss, pseudogenization in plastomes of genus Allium (Amaryllidaceae), and putative selection for adaptation to environmental conditions. Front. Genet. 2021;12:674783. doi: 10.3389/fgene.2021.674783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ivanova Z., Sablok G., Daskalova E., Zahmanova G., Apostolova E., Yahubyan G., Baev V. Chloroplast genome analysis of resurrection tertiary relict Haberlea rhodopensis highlights genes important for desiccation stress response. Front. Plant Sci. 2017;8:204. doi: 10.3389/fpls.2017.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xie D.-F., Yu Y., Deng Y.-Q., Li J., Liu H.-Y., Dong Zhou S.-D., He X.-J. Comparative analysis of the chloroplast genomes of the Chinese endemic genus Urophysa and their contribution to chloroplast phylogeny and adaptive evolution. Int. J. Mol. Sci. 2018;19:1847. doi: 10.3390/ijms19071847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu M.-L., Fan W.-B., Wang N., Dong P.-B., Zhang T.-T., Yue M., Li Z.-H. Evolutionary analysis of plastid genomes of seven Lonicera L. species: Implications for sequence divergence and phylogenetic relationships. Int. J. Mol. Sci. 2018;19:4039. doi: 10.3390/ijms19124039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hu Y., Zhong S., Zhang M., Liang Y., Gong G., Chang X., Tan F., Yang H., Qiu X., Luo L., et al. Potential role of photosynthesis in the regulation of reactive oxygen species and defence responses to Blumeria graminis f. sp. tritici in wheat. Int. J. Mol. Sci. 2020;21:5767. doi: 10.3390/ijms21165767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu Y., Ren D., Pike S., Pallardy S., Gassmann W., Zhang S. Chloroplast-generated reactive oxygen species are involved in hypersensitive response-like cell death mediated by a mitogen-activated protein kinase cascade. Plant J. 2007;51:941–954. doi: 10.1111/j.1365-313X.2007.03191.x. [DOI] [PubMed] [Google Scholar]
- 88.Reape T.J., Molony E.M., McCabe P.F. Programmed cell death in plants: Distinguishing between different modes. J. Exp. Bot. 2008;59:435–444. doi: 10.1093/jxb/erm258. [DOI] [PubMed] [Google Scholar]
- 89.Doyle S.M., Diamond M., McCabe P.F. Chloroplast and reactive oxygen species involvement in apoptotic-like programmed cell death in Arabidopsis suspension cultures. J. Exp. Bot. 2010;61:473–482. doi: 10.1093/jxb/erp320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang M.S., Braun D.M. Genetic analyses of cell death in maize (Zea mays, poaceae) leaves reveal a distinct pathway operating in the camouflage1 mutant. Am. J. Bot. 2010;97:357–364. doi: 10.3732/ajb.0900233. [DOI] [PubMed] [Google Scholar]
- 91.Nilo R., Saffie C., Lilley K., Baeza-Yates R., Cambiazo V., Campos-Vargas R., González M., Meisel L.A., Retamales J., Silva H., et al. Proteomic analysis of peach fruit mesocarp softening and chilling injury using difference gel electrophoresis (DIGE) BMC Genom. 2010;11:43. doi: 10.1186/1471-2164-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mayta M.L., Hajirezaei M.-R., Carrillo N., Lodeyro A.F. Leaf senescence: The chloroplast connection comes of age. Plants. 2019;8:495. doi: 10.3390/plants8110495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McCoy S.R., Kuehl J.V., Boore J.L., Raubeson L.A. The complete plastid genome sequence of Welwitschia mirabilis: An unusually compact plastome with accelerated divergence rates. BMC Evol. Biol. 2008;8:130. doi: 10.1186/1471-2148-8-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gechev T.S., Van Breusegem F., Stone J.M., Denev J., Laloi C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssays. 2006;28:1091–1101. doi: 10.1002/bies.20493. [DOI] [PubMed] [Google Scholar]
- 95.Mubarakshina M.M., Ivanov B.N., Naydov I.A., Hillier W., Badger M.R., Krieger-Liszkay A. Production and diffusion of chloroplastic H2O2 and its implication to signalling. J. Exp. Bot. 2010;61:3577–3587. doi: 10.1093/jxb/erq171. [DOI] [PubMed] [Google Scholar]
- 96.Wiciarz M., Gubernator B., Kruk J., Niewiadomska E. Enhanced chloroplastic generation of H2O2 in stress resistant Thellungiella salsuginea in comparison to Arabidopsis thaliana. Physiol. Plant. 2015;153:467–476. doi: 10.1111/ppl.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bowler C., Van Montagu M., Inzé D. Superoxide dismutase and stress tolerance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992;43:83–116. doi: 10.1146/annurev.pp.43.060192.000503. [DOI] [Google Scholar]
- 98.Kurepa J., Hérouart D., Van Montagu M., Inzé D. Differential expression of CuZn- and Fe-superoxide dismutase genes of tobacco during development, oxidative stress and hormonal treatments. Plant Cell Physiol. 1997;38:463–470. doi: 10.1093/oxfordjournals.pcp.a029190. [DOI] [PubMed] [Google Scholar]
- 99.Prochazkova D., Sairam R.K., Srivastava G.C., Singh D.V. Oxidative stress and antioxidant activity as the basis of senescence in maize. Plant Sci. 2001;161:765–771. doi: 10.1016/S0168-9452(01)00462-9. [DOI] [Google Scholar]
- 100.Ohe M., Rapolu M., Mieda T., Miyagawa Y., Yabuta Y., Yoshimura K., Shigeoka S. Decline of leaf photooxidative stress tolerance with age in tobacco. Plant Sci. 2005;168:1487–1493. doi: 10.1016/j.plantsci.2005.01.020. [DOI] [Google Scholar]
- 101.Orr W.C., Sohal R.S. Extension of lifespan by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994;263:1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- 102.Lascano H.R., Casano L.M., Martín M., Sabater B. The activity of the chloroplastic Ndh complex is regulated by phosphorylation of the NDH-F subunit. Plant Physiol. 2003;132:256–262. doi: 10.1104/pp.103.020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nashilevitz S., Melamed-Bessudo C., Izkovich Y., Rogachev I., Osorio S., Itkin M., Adato A., Pankratov J., Hirschberg J., Femie A.R., et al. An orange ripening mutant links plastid NAD(P)H dehydrogenase complex activity to central and specialized metabolism during tomato fruit maturation. Plant. Cell. 2010;22:1977–1997. doi: 10.1105/tpc.110.074716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Krieger-Liszkay A., Trosch M., Krupinska K. Generation of reactive oxygen species in thylakoids from senescing flag leaves of the barley varieties Lomerit and Carina. Planta. 2015;241:1497–1508. doi: 10.1007/s00425-015-2274-8. [DOI] [PubMed] [Google Scholar]
- 105.Kane D.J., Sarafian T.A., Anton R., Hahn H., Gralla E.B., Valentine J.S., Örd T., Bredesen D.E. Bcl-2 inhibition of neural death: Decreased generation of reactive oxygen species. Science. 1993;262:1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- 106.Green D.R., Reed J.C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 107.Yu Z.-H., Perdue T.D., Heimer Y.M., Jones A.M. Mitochondrial involvement in tracheary element programmed cell death. Cell Death Differ. 2002;9:189–198. doi: 10.1038/sj.cdd.4400940. [DOI] [PubMed] [Google Scholar]
- 108.Lam E., Kato N., Lawton M. Programmed cell death, mitochondria and the plant hypersensitive response. Nature. 2001;411:848–853. doi: 10.1038/35081184. [DOI] [PubMed] [Google Scholar]
- 109.Ruiz-Pesini E., Mishmar D., Brandon M., Procaccio V., Wallace D.C. Effects of purifying and adaptive selection on regional variation in human mtDNA. Science. 2004;303:223–226. doi: 10.1126/science.1088434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.