Abstract
PURPOSE
Our purpose is to clarify the optimal timing of surgery after transarterial embolization (TAE) for renal cell carcinoma (RCC) bone metastases.
METHODS
This retrospective study included 41 patients with RCC bone metastases embolized between 2013 and 2019. Different-sized particulate and/or liquid embolic agents were used for TAE. Embolizations were categorized into groups 1–3 according to the interval between TAE and surgery (group 1: <1 day, group 2: 1–3 days, group 3: >3 days). Degree of embolization after TAE was graded visually based on angiographic images (<50%, 50%–75%, 75%–90%, >90%). The relationship between the TAE–surgery interval and intraoperative blood loss (IBL) and the correlation between IBL and embolization grade were examined. Lesion sizes and the relationships among lesion localizations and contrast media usage, intervention time, and IBL were also analyzed.
RESULTS
Forty-six pre-operative TAEs (single lesion at each session) were performed in this study (26 in group 1, 13 in group 2, 7 in group 3). Lesion sizes and distributions were similar between groups (p = 0.897); >75% devascularization was achieved in 40 (TAEs 86.96%), but the IBL showed no correlation with the embolization rate (r=0.032, p = 0.831). The TAE–surgery interval was 1–7 days. The median IBL in group 1 (750 mL; range, 150–3000 mL) was significantly lower than those in the other groups (p = 0.002). Contrast media usage (p = 0.482) and intervention times (p = 0.261) were similar for metastases at different localizations. IBL values after TAE were lower for extremity metastases (p = 0.003).
CONCLUSION
Bone metastases of RCC are well-vascularized, and to achieve lowest IBL values, surgery should preferably be performed <1 day after TAE.
Renal cell carcinomas (RCCs) are one of the leading causes of cancer-related death worldwide (1). About one-third of RCCs are metastatic at initial diagnosis, and skeletal metastases are the second most frequent type of RCC metastases following lung metastases (43%) (2, 3). Surgical intervention is an option for the treatment of skeletal metastases of RCCs. Although local ablative therapies like thermal ablation may be preferred for tumors <3 cm, systemic chemotherapy and radiotherapy are other options for suitable patients (4). However, RCCs are usually chemo/radio-resistant (50%), and these treatment options are usually favored for palliative intent (3–5).
The 5-year overall survival of patients with RCC bone metastases increases when surgical metastasectomy is performed (4). However, since RCC metastases in the skeletal system are usually hypervascular, the operative blood loss could be as high as 18500 mL, which could threaten patients’ lives (6). Transarterial embolization (TAE) of bone tumors was first described in 1975 (7). The operative blood loss can be reduced by adequate devascularization after TAE of the bone metastases (8–11), and a blood loss of less than 3000 mL was defined as clinical success for spinal tumor surgeries (12). Selective TAE of bone metastases can be performed pre-operatively in a single session. Successful embolization can clarify the tumor margins from the surrounding tissue planes, simplifying surgical manipulation of the tumors. Thus, recurrence rates may be lower in patients undergoing this treatment combination (13, 14). Different types of permanent and temporary embolic agents can be selected for TAE of bone metastases. The rationale behind TAE is occlusion of the capillary bed of the tumors; therefore, proximal occlusion should not be preferred due to the presence of numerous collateral capillary vessels (5, 8, 15).
This study aimed to clarify the optimal interval between TAE and surgery for RCC bone metastasis to minimize blood loss at the time of surgery.
Methods
This retrospective study was approved by our university’s local ethics committee (İ6-383-20) and informed consent was obtained from all patients. We included patients with single bone metastases who underwent embolization before the surgical operation and had proven RCC bone metastases diagnosed histopathologically after the surgical operation. The exclusion criteria were the presence of secondary tumors and insufficient visualization of pathologic vascularity in angiographic images. The study was conducted with 41 patients (26 males, 15 females). The patients’ mean age was 62.24±9.36 years (range, 43–91 years). Between 2013 and 2019, a total of 46 TAEs were performed at our institution at different intervals (single lesion at each session) before 46 surgical procedures. All patients in this cohort had single metastases before surgical procedures and 46 TAEs were performed to the patients at different sessions because some of the patients had recurrent metastases in their follow-up thus they needed another surgical operation and another pre-operative TAEs. Therefore there is a number discrepancy between patient numbers and TAE. Indications for the surgical procedures were palliation to prevent fractures, repair of pathologic fractures, prevention of neurological deficit, and enhancement of patient survival. Embolization procedures were performed with coaxial catheters to achieve superselective embolization. Patients were embolized with different-sized trisacryl gelatin microspheres, N-butyl cyanoacrylate (NBCA), and/or Onyx. Patients in this cohort were categorized and analyzed according to the interval between TAE and the surgical operation and the embolization grades of the metastases.
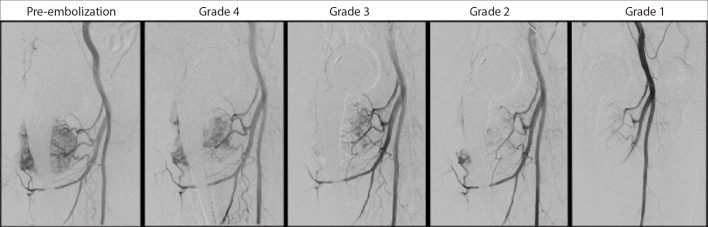
Patients in this cohort were operated between 1 and 7 days after TAE and were divided into three groups according to the interval between TAE and the surgical operation: group 1 consisted of patients operated <1 day after TAE; group 2 included patients operated between 1 and 3 days after TAE; and group 3 included patients operated >3 days after TAE. Since embolization process and grading of the embolization rate after TAE is a very subjective process, we have tried to propose an objective grading system for TAE procedures. Lesion devascularization after TAEs was graded and classified by the operators based on the estimation of reduction in tumoral blush visualized in angiographic images (Fig. 1). Embolization rates were classified as grade 1 >90% devascularization, grade 2 75%–90% devascularization, grade 3 50%–75% devascularization, and grade 4 <50% devascularization, with visual estimations as described in the previous literature (16–18). Data on this cohort were also evaluated according to metastasis sizes and localizations. Since pelvic and spinal (PS) metastases more often needed complex surgical approaches and have higher bleeding potential than upper and lower extremity (UL) metastases, PS and UL metastases were examined and analyzed separately (17, 19).
Figure 1.
Demonstrative images of the devascularization after transarterial embolization (TAE); reduction of tumor blush was visually estimated and graded by the operator according to the reduction of tumoral blush visualized in angiographic images. Grade 1, >90% devascularization; Grade 2, 75%–90% devascularization; Grade 3, 50%–75% devascularization; Grade 4, <50% devascularization.
The intraoperative blood loss volume (IBL), contrast media volume used during angiography, TAE procedure time, and complications related to the TAE were also recorded and analyzed. IBL was calculated on the basis of operative reports including transfusion requirements and peri-operative laboratory findings, as described previously (11, 18). An IBL lower than 1500 mL was considered to be satisfactory for the entire cohort in our institutional protocol.
Angiography and pre-operative TAE technique
All embolization procedures were performed with Siemens Artis Zee system (Siemens Healthcare) under local anesthesia. Right femoral artery access with a 5 F or 6 F sheath was the preferred mode of access in most cases. A manual pulse-palpation technique was used for femoral artery catheterization for most of the cohort; thus, ultrasound guidance was needed in some cases because of an insufficient arterial pulse, antegrade catheterization, or scar tissue in the femoral region. Long sheaths were used whenever needed and in cases with an unfavorable anatomy during contralateral access for pelvic region embolizations or extremity embolizations. TAE procedures were performed with a coaxial technique for all patients with combinations of different microcatheters (2.4 F, 2.7 F) and microguidewires (0.014-inch, 0.016-inch, 0.018-inch, and 0.021-inch). A non-ionic contrast media was used (350 mg/mL) for all procedures, and the total contrast media doses were recorded in milliliters. Trisacryl gelatin microspheres (300–500 μm to 700–900 μm) were preferred as particulate embolic agents in this study. Onyx 18 (EV3) was the preferred viscosity and used for the spinal metastases. N-butyl cyanoacrylate (NBCA) was prepared in a 3:1 lipiodol-to-NBCA ratio and sandwiched with dextrose 5% solution. Nonselective angiography was performed with a flush catheter to determine the vascular pattern of metastases and potential feeders whenever needed. After nonselective imaging, selective angiography was performed from the potential feeders according to the initial angiographic or other imaging findings.
A lidocaine provocative test was performed for some patients with spinal metastases whenever needed or preferred by the operator. The time required to complete TAE was calculated by determining the interval between the time at which the first image was recorded after femoral sheath insertion and the time at which the last angiographic image was recorded after TAE. Unless antegrade femoral access was preferred, the puncture site was closed with a vascular closure device in most of the patients. Manual compression of the access artery was preferred for hemostasis, unless a vascular closure device was used. Complications related to the angiographic procedure were recorded and categorized according to the Society of Interventional Radiology quality improvement guidelines for transcatheter embolization and adverse event classification (20, 21).
Statistical analysis
IBM-SPSS 20.0 (IBM Corp) was used to conduct statistical analysis. Continuous data were presented as mean ± standard deviation or median and range, while categorical data were presented as numbers and percentages. Independent t-test was used to compare the mean ages of different groups categorized by sex. The relationships between categorical variables were analyzed using Pearson chi-squared test. The compliance of the data to normal distribution was tested with Shapiro Wilk test for comparison of continuous data in patients undergoing TAE. Kruskal–Wallis test was used for comparisons involving more than two groups. Spearman correlation coefficient was used for the correlation analysis. Statistical significance was considered at p < 0.05.
Results
The study population consisted of 26 male (63.41%) and 15 female (36.59%) patients. A total of 46 RCC bone metastases were embolized preoperatively in these 41 patients. In each embolization session, TAE was performed for a single lesion. Forty metastases (86.96%) were embolized with particulate embolic agents and six (13.04%) were embolized with liquid embolic agents. Nineteen (41.30%) of the metastases were localized at the pelvis or spine, while 27 (58.70%) were localized at the extremities (Table 1). The mean maximum diameter of the metastases was 6.22±2.13 cm, and the size distribution of the metastases was similar for all groups (p = 0.897).
Table 1.
Localizations of bony metastases
| Localizations of the RCC bone metastases | (n) |
|---|---|
| Extremity metastases | |
| Femur | 13 |
| Humerus | 12 |
| Scapula | 2 |
|
| |
| Pelvic metastases | |
| Iliac crest | 5 |
| Ischium | 2 |
| Acetabulum | 5 |
|
| |
| Spine metastases | |
| Thoracic spine | 1 |
| Lumbar spine | 5 |
| Cervical spine | 1 |
|
| |
| Total metastases | 46 |
A total of 26 metastases (56.52%) were operated <1 day after TAE (group 1); 13 metastases (28.26%) were operated between 1 and 3 days (group 2) after TAE, and seven metastases (15.22%) were operated >3 days after TAE (group 3). There was one (2.17%) grade 4 (<50%) devascularization, five (10.87%) grade 3 (50%–75%) devascularization, 20 (43.48%) grade 2 (75%–90%) devascularization, and 20 (43.48%) grade 1 (>90%) devascularization. There was no correlation between the IBL and embolization grades (r=0.032, p = 0.831) (Table 2).
Table 2.
Patient distributions, operation timing, embolization grades and relation with intraoperative blood loss (r=0.032, p = 0.831)
| n (%) | Median IBL (mL) (min–max) | p | |
|---|---|---|---|
| Embolization grade | |||
| Grade 4 (<50%) | 1 (2.17) | 1200 | NA |
| Grade 3 (50%–75%) | 5 (10.87) | 1000 (250–3000) | 0.746 |
| Grade 2 (75%–90%) | 20 (43.48) | 900 (150–2500) | 0.746 |
| Grade 1 (>90%) | 20 (43.48) | 800 (400–1650) | 0.746 |
|
| |||
| TAE to operation time | |||
| Group 1 (<1 day) | 26 (56.52) | 750 (150–3000) | 0.002 |
| Group 2 (1–3 day) | 13 (28.26) | 1500 (550–2500) | 0.002 |
| Group 3 (>3 day) | 7 (15.22) | 2000 (600–2500) | 0.002 |
IBL, intraoperative blood loss; NA, non-applicable.
Group 1- Group 2 (median IBL): p = 0.021; Group1- Group 3 (median IBL): p = 0.008; Group 2- Group 3 (median IBL): p = 1.000.
p < 0.05 is considered significant.
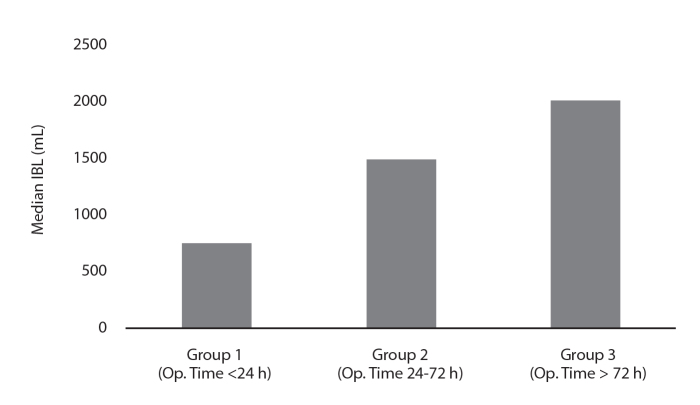
The relationship between operative timing and IBL values was analyzed. The median IBL was 750 mL (150–3000 mL) in group 1, 1500 mL (550–2500 mL) in group 2, and 2000 mL (600–2500 mL) in group 3. The differences between groups 1 and 2 (p = 0.021) and groups 1 and 3 (p = 0.008 ) reached significance. Although the IBL values in group 2 were less than those in group 3, the difference did not reach statistical significance (p = 1.000) (Fig. 2). The median IBL was 1431.58±709.50 mL for PS metastases and 959.26±642.27 mL for UL metastases. These differences were statistically significant (p = 0.014).
Figure 2.
Median intraoperative blood loss (IBL) values of 46 TAEs based on three different operative time groups (p = 0.002).
A subgroup analysis was also performed for the PS and UL lesions according to the operation timing after TAE. The median IBL values of patients with PS metastases were as follows: group 1: 975 mL (250–3000 mL), group 2: 1575 mL (550–1750 mL), and group 3: 2000 mL (2000–2500 mL). In contrast, the mean IBL values of patients with UL metastases were as follows: group 1: 575 mL (150–1200 mL), group 2: 1500 mL (550–2500 mL), group 3: 1375 mL (600–2500 mL). Although the differences did not reach statistical significance, the mean IBL values were slightly lower for patients with PS metastases when they were operated <1 day after TAE (p = 0.074). On the other hand, the IBL could be reduced significantly for patients with UL metastases if they were operated <1 day (group 1) after TAE (p = 0.003) (Table 3).
Table 3.
Mean intraoperative blood loss values for metastases at different localizations
| Group 1 (<1 day) | Group 2 (1–3day) | Group 3 (>3 day) | ||
|---|---|---|---|---|
| Localization | Median IBL (mL) (min–max) | Median IBL (mL) (min–max) | Median IBL (mL) (min–max) | p |
| PS | 975 (250–3000) | 1575 (550–1750) | 2000 (2000–2500) | 0.074 |
|
| ||||
| UL | 575 (150–1200) | 1500 (550–2500) | 1375 (600–2500) | 0.003 |
IBL, intraoperative blood loss; PS, pelvic and spinal; UL, upper or lower extremity.
p < 0.05 is considered significant.
Grade 1 devascularization rate was 36.84% (7 metastases) and 48.15% (13 metastases) for PS and UL metastases, respectively. This difference did not reach statistical significance (p = 0.446). Contrast media usage during TAE tended to be higher for PS metastases, although this difference did not reach statistical significance (96.32±38.11 mL vs. 89.07±31.04 mL; p = 0.482). The procedure times were similar for TAEs at different locations (p = 0.261).
No major complications were recorded after TAE procedures. Ten patients presented with postembolization syndrome and 4 patients had a mild groin hematoma (<3 cm). One patient presented with a pseudoaneurysm in the common femoral artery.
Discussion
Most RCCs are metastatic to the bone on initial diagnosis. Surgical metastatectomy is one of the best treatment options, particularly for oligometastatic RCCs; aggressive surgery can prolong patient survival (22). Despite some conflicting data in the literature, most previous reports (17, 18, 23–27) demonstrated that TAE has a positive influence in terms of lower IBL, operative time, and recurrence rates and improved resectability and tumor margin recognition (13, 14, 26).
In a recent review, IBL was demonstrated to be independent from the embolization grade (17, 18). The IBL values of the patients in this study after preoperative TAE were similar to those reported in literature and showed no correlation with the devascularization grade. However, IBL was related to the interval between TAE and surgery. Group 1 had the least mean and median IBL, followed by groups 2 and 3. The results of this study demonstrate that a longer interval between TAE and surgery increases the IBL. These differences reached significance in the comparisons between groups 1 and 2 and between groups 1 and 3. If a median IBL lower than 1500 mL is considered to be sufficient, then patients in groups 1 and 2 have met this goal. Moreover, we showed that the least IBL could be achieved with a shorter waiting period between TAE and surgery (Group 1). These results are similar to the findings reported in the literature (10).
Tumor size is another important factor related to IBL and is moderately correlated with IBL (18). However, since the mean maximal diameters of the metastases in our cohort were similar across all groups, the relationship between IBL and metastasis size was not assessed in this study.
Although the results did not reach statistical significance, PS metastases had slightly lower IBL when operated <1 day after TAE. In contrast, the IBL for UL metastases was significantly lower if they were operated <1 day after TAE (Table 3). These results may guide surgeons and interventional radiologists to further understand the potential benefits of early operation after TAE (operation time < 1 day), particularly for tumors located at the extremities. Complications related to TAE of spinal metastases have been reported in the literature, and surgical operations shortly after TAE have been questioned (23). Our results suggest that a waiting period of 6–12 h within a 24 h window after TAE is a prudent option for planning the surgical operation. This option could exclude neurologic and/or other complications that may be related to TAE and ensure the best operative results regarding the IBL.
The mean IBL for UL metastases was lower than that for PS metastases. This difference could be attributed to the complexity of the anatomy, operative techniques, and the rich capillary vascularity of PS metastases in contrast to UL metastases. However, the median procedure times for TAE of PS and UL metastases were similar, despite slight nonsignificant differences related to devascularization grades and contrast media usage. Grade 1 devascularization rates were lower for the PS metastases than for UL metastases (36.84% vs. 48.15%). We hypothesize that these differences in embolization grades were due to the complex vascularity and the risk of potential complications related to tumor localizations. The contrast media volumes used for PS metastases (96.32±38.11 mL) were also higher than those used for UL metastases (89.07±31.04 mL), but these differences were not significant. This slight difference may be due to the usage of flush catheters, especially for tumors located in the spine.
Complications related to pre-operative TAE have been reported in the literature (28, 29). We used the Society of Interventional Radiology guidelines in this study to understand the importance of complications related to the angiography procedure (20, 21). Our cohort showed no complications greater than category B. The patient who showed a pseudoaneurysm underwent surveillance with weekly Doppler ultrasound examination, and the pseudoaneurysm was thrombosed spontaneously three weeks after the intervention. Postembolization syndrome is a common adverse effect of TAE procedures, and systemic antiinflammatory drugs were used for these patients. Four hematomas related to the angiographic procedures also dissolved spontaneously on surveillance after a couple of weeks. Vascular closure devices were used in most cases in our cohort, except in cases that required an antegrade approach. Moreover, for spinal tumors above the diaphragmatic level, we used a provocative testing method whenever needed to avoid any unexpected complications. These measures may have enhanced patient safety in our cohort.
The limitations of this study include its retrospective nature and the low number of patients in different groups. No correlation was demonstrated between embolization grade and IBL during the current study, though, IBL could be affected by different embolic agents, particle sizes and embolization techniques such as using a microcatheter or not. Most TAEs achieved an embolization grade >75% in current study. Although no difference in IBL could be found between grade 1 (>90%) and grade 2 (75%–90%) devascularizations, one could speculate that an embolization grade >75% could be the critical TAE threshold for RCC metastases. More studies will be needed to have an absolute conclusion. Also particulate agent sizes used during TAEs in current study ranged from 300–500 μm to 700–900 μm, which could be a limitation. As another limitation; IBL can be affected by the surgical technique and surgeon performing surgery. In our institution, the oncologic orthopedic surgery team consists of 2 senior and 1 junior surgeons. All major surgeries, including all cases presented here, were performed by this team. Therefore surgeon-dependent errors can be neglected in this study. However, uniformity of surgical techniques could not be achieved. Wide or radical resection was performed in some cases, while intralesional intervention had to be performed in others. Correction for this factor can be achieved in future studies with higher patient numbers. These factors precluded analysis of IBL values according to different surgical techniques. Nevertheless, our results were consistent with the findings of previous reports in the literature, and the findings suggested that pre-operative embolization of RCC metastases is a valuable option for hypervascular tumors, particularly when the operation is performed <1 day after TAE.
In conclusion, no correlation was found between devascularization grade after TAE and IBL in patients with RCC bone metastases. However, TAE still had a positive influence in reducing the IBL, if the surgical operation was scheduled <1 day after TAE.
Main points.
Renal cell carcinoma (RCC) bone metastases present as hypervascular lesions at angiographic images.
Pre-operative embolization could be used to lower intraoperative blood loss at RCC bone metastases.
Surgery should be scheduled <1 day after transarterial embolization.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Capitanio U, Bensalah K, Bex A, et al. Epidemiology of renal cell carcinoma. Eur Urol. 2019;75:74–84. doi: 10.1016/j.eururo.2018.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chatziioannou AN, Johnson ME, Pneumaticos SG, et al. Preoperative embolization of bone metastases from renal cell carcinoma. Eur Radiol. 2000;10:593–596. doi: 10.1007/s003300050969. [DOI] [PubMed] [Google Scholar]
- 3.Fottner A, Szalantzy M, Wirthmann L, et al. Bone metastases from renal cell carcinoma: patient survival after surgical treatment. BMC Musculoskelet Disord. 2010;11:145. doi: 10.1186/1471-2474-11-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grünwald V, Eberhardt B, Bex A, et al. An interdisciplinary consensus on the management of bone metastases from renal cell carcinoma. Nature Rev Urol. 2018;15:511–521. doi: 10.1038/s41585-018-0034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun S. Bone metastases from renal cell carcinoma: preoperative embolization. In: Golzarian J, Sun S, Sharafuddin MJ, editors. Vascular Embolotherapy Medical Radiology (Diagnostic Imaging) Heidelberg: Springer; 2006. pp. 189–199. [DOI] [Google Scholar]
- 6.Barton PP, Waneck ER, Karnel JF, et al. Embolization of bone metastases. J Vasc Interv Radiol. 1996;7:81–88. doi: 10.1016/S1051-0443(96)70738-8. [DOI] [PubMed] [Google Scholar]
- 7.Feldman F, Casarella WJ, Dick HM, et al. Selective intraarterial embolization of bone tumors. A useful adjunct in the management of selected lesions. Am J Roentgenol Radium Ther Nucl Med. 1975;123:130–139. doi: 10.2214/ajr.123.1.130. [DOI] [PubMed] [Google Scholar]
- 8.Geraets SEW, Bos PK, Van der Stok J. Preoperative embolization in surgical treatment of long bone metastasis: a systematic literature review. EFORT Open Reviews. 2020;5:17–25. doi: 10.1302/2058-5241.5.190013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aggarwal V, Bhardwaj R, Thakur RK. Is pre-operative angio-embolisation an effective modality to control intraoperative blood loss in extremity bone tumors? JMSCR. 2017;5:27578–27586. doi: 10.18535/jmscr/v5i9.36. [DOI] [Google Scholar]
- 10.Kato S, Hozumi T, Takaki Y, et al. Optimal schedule of preoperative embolization for spinal metastasis surgery. Spine. 2013;38:1964–1969. doi: 10.1097/BRS.0b013e3182a46576. [DOI] [PubMed] [Google Scholar]
- 11.Kumar N, Tan B, Zaw AS, et al. The role of preoperative vascular embolization in surgery for metastatic spinal tumors. Eur Spine J. 2016;25:3962–3970. doi: 10.1007/s00586-016-4494-4. [DOI] [PubMed] [Google Scholar]
- 12.Gellad FE, Sadato N, Numaguchi Y, et al. Vascular metastatic lesions of the spine: preoperative embolization. Radiology. 1990;176:683–686. doi: 10.1148/radiology.176.3.2389026. [DOI] [PubMed] [Google Scholar]
- 13.Mavrogenis AF, Rossi G, Rimondi E, et al. Embolization of bone tumors. Orthopedics. 2011;34:303–310. doi: 10.3928/01477447-20110228-20. [DOI] [PubMed] [Google Scholar]
- 14.Chu JP, Chen W, Li JP, et al. Clinicopathological features and results of transcatheter arterial chemoembolization for osteosarcoma. Cardiovasc Intervent Radiol. 2007;30:201–206. doi: 10.1007/s00270-005-0302-y. [DOI] [PubMed] [Google Scholar]
- 15.Ghobriala GM, Chalouhia N, Harropa J, et al. Preoperative spinal tumor embolization: An institutional experience with Onyx. Clin Neurol Neurosurg. 2013;115:2457–2463. doi: 10.1016/j.clineuro.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 16.Kwon JH, Shin JH, Kim JH, et al. Preoperative transcatheter arterial embolization of hypervascular metastatic tumors of long bones. Acta Radiol. 2010;51:396–401. doi: 10.3109/02841851003660081. [DOI] [PubMed] [Google Scholar]
- 17.Reitz M, Mende KC, Cramer C, et al. Surgical treatment of spinal metastases from renal cell carcinoma-effects of preoperative embolization on intraoperative blood loss. Neurosurgical Review. 2018;41:861–867. doi: 10.1007/s10143-017-0935-8. [DOI] [PubMed] [Google Scholar]
- 18.Ma J, Tullius T, Van Ha TG. Update on preoperative embolization of bone metastases. Semin Intervent Radiol. 2019;36:241–248. doi: 10.1055/s-0039-1693120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang X, Guo W, Yang R, Tang S, Ji T. Evaluation of blood loss during limb salvage surgery for pelvic tumours. Int Orthop. 2009;33:751–756. doi: 10.1007/s00264-008-0695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khalilzadeh O, Baerlocher MO, Shyn PB, et al. Proposal of a new adverse event classification by the Society of Interventional Radiology-Standards of Practice Committee. J Vasc Interv Radiol. 2017;28:1432–1437e3. doi: 10.1016/j.jvir.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 21.Angle JF, Siddiqi NH, Wallace MJ, et al. Society of Interventional Radiology Standards of Practice Committee. Quality improvement guidelines for percutaneous transcatheter embolization: Society of Interventional Radiology Standards of Practice Committee. J Vasc Interv Radiol. 2010;21:1479–1486. doi: 10.1016/j.jvir.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Ouzaid I, Capitanio U, Staehler M, et al. Surgical metastasectomy in renal cell carcinoma: a systematic review. Eur Urol Oncol. 2019;2:141–149. doi: 10.1016/j.euo.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 23.Wilson MA, Cooke DL, Ghodke B, et al. Retrospective analysis of preoperative embolization of spinal tumors. AJNR Am J Neuroradiol. 2010;31:656–660. doi: 10.3174/ajnr.A1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Börüban S, Sancak T, Yıldız Y, et al. Embolization of benign and malignant bone and soft tissue tumors of the extremities. Diagn Interv Radiol. 2007;13:164–171. [PubMed] [Google Scholar]
- 25.Radeleff B, Eiers M, Lopez-Benitez R, et al. Transarterial embolization of primary and secondary tumors of the skeletal system. Eur J Radiol. 2006;58:68–75. doi: 10.1016/j.ejrad.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Awad AW, Almefty KK, Ducruet AF, et al. The efficacy and risks of preoperative embolization of spinal tumors. J NeuroIntervent Surg. 2016;8:859–864. doi: 10.1136/neurintsurg-2015-011833. [DOI] [PubMed] [Google Scholar]
- 27.Ratasvuori M, Sillanpaa N, Rikard W, et al. Surgery of non-spinal skeletal metastases in renal cell carcinoma: No effect of preoperative embolization? Acta Orthopaedica. 2016;87:183–188. doi: 10.3109/17453674.2015.1127726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manke C, Bretschneider T, Lenhart M, et al. Spinal metastases from renal cell carcinoma: effect of preoperative particle embolization on intraoperative blood loss. AJNR Am J Neuroradiol. 2001;22:997–1003. [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Hadithy N, Gikas P, Perera J, et al. Pre-operative embolization of primary and secondary bone tumours is safe and effective: a retrospective study. World J Oncol. 2011;2:319–322. doi: 10.4021/wjon389w. [DOI] [PMC free article] [PubMed] [Google Scholar]