Abstract
Noninvasive imaging plays an important role in acute stroke towards diagnosis and ongoing management of patients. Systemic thrombolysis and endovascular thrombectomy (EVT) are proven treatments currently used in standards of care in acute stroke settings. The role of computed tomography angiography (CTA) in selecting patients with large vessel occlusion for EVT is well established. However, the value of CT perfusion (CTP) imaging in predicting outcomes after stroke remains ambiguous. This article critically evaluates the value of multimodal CT imaging in early diagnosis and prognosis of acute ischemic stroke with a focus on the role of CTP in delineating tissue characteristics, patient selection, and outcomes after reperfusion therapy. Insights on various technical and clinical considerations relevant to CTP applications in acute ischemic stroke, recommendations for existing workflow, and future areas of research are discussed.
Neuroimaging is currently the mainstay of acute stroke workflow as it is used to rule out hemorrhage and select patients eligible for systemic thrombolysis and endovascular thrombectomy (EVT) (1). Advanced imaging has shown promise in acute stroke; however, the prognostic utility of computed tomography perfusion (CTP) imaging has drawn considerable debate. Given that stroke is associated with long-term morbidity and mortality, efforts to develop diagnostic imaging biomarkers have been pursued. However, the clinical translation of certain imaging modalities has been limited, especially CTP imaging. This could be attributed to the limited availability of CTP in under-resourced settings, variations in the diagnostic accuracy of CTP maps, and differences in the thresholds used in creating perfusion maps. In this paper, we will present a comprehensive overview of various imaging modalities in acute stroke with a particular focus on the clinically relevant utility of CTP and provide roadmap for future research.
Current recommendations in stroke workflow
The 2018 (updated 2019) American Heart Association/American Stroke Association (AHA/ASA) recommendations provide a comprehensive guide to the management of patients presenting with acute ischemic stroke (AIS), through prehospital settings to 2 weeks post-stroke (1). Brain imaging is essential for the diagnosis of stroke and each imaging modality contributes to clinical decision making and prognostication. The current stroke recommendations are presented in Table 1. Briefly, noncontrast computed tomography (NCCT) is used to exclude stroke mimics, such as brain tumors, as well as intracerebral hemorrhage, which is a contraindication to intravenous thrombolysis. Computed tomographic angiography (CTA) is useful in confirming a large vessel occlusion that is amenable to EVT. Computed tomography perfusion (CTP) imaging and perfusion magnetic resonance imaging (MRP) provides tissue characteristics, including infarct and penumbral volumes (highlighting tissue at risk of infarction), hence reinforcing clinical decisions for thrombectomy in patients who present outside of the conventional time-window. A case study with the application of multimodal CT imaging in acute stroke is shown in Figs. 1 and 2. In the next section, we will discuss the various imaging techniques and look at current and emerging evidence regarding the use of these imaging modalities.
Table 1.
Current recommendations by the AHA/ASA
| Recommendation | Class | Evidence |
|---|---|---|
| Initial imaging | ||
| All patients with suspected stroke should receive emergency brain imaging upon first arrival to the hospital before initiating specific therapy for AIS. | I | A |
| Systems should be established to ensure that brain imaging studies can be performed as quickly as possible in patients who may be candidates for IV fibrinolytics, mechanical thrombectomy or both. | I | B-NR |
|
| ||
| IV alteplase eligibility (within 4.5 hours of symptom onset) | ||
| Both NCCT and MRI are effective to exclude ICH before IV alteplase administration. | I | CT: A |
| MRI: B-NR | ||
| Administration of intravenous alteplase in eligible patients without first obtaining MRI to exclude CMBs is recommended. | I | B-NR |
| Since the benefit of alteplase therapy is time-dependent, treatment should be initiated as quickly as possible for eligible patients, and not delayed by additional multimodal neuroimaging, such as CTP/MRP. | I | B-NR |
| The extent and severity of acute hypoattenuation, early ischemic changes or the hyperdense MCA sign should not be used as a criterion to withhold IV alteplase therapy for patients who otherwise qualify. | III | B-R |
| Use of imaging criteria to select patients who awoke with stroke or have an unclear time of symptom onset for IV alteplase therapy is not recommended outside of a clinical trial. | III | B-NR |
| In patients with AIS who awake with stroke symptoms or have unclear time of onset > 4.5 hours from last known well or at baseline state, MRI to identify diffusion-positive FLAIR-negative lesions can be useful for selecting those who can benefit from IV alteplase administration within 4.5 hours of stroke symptom recognition. | IIA | B-R |
|
| ||
| Mechanical thrombectomy eligibility | ||
| Noninvasive intracranial vascular study (using CTA/MRA) is recommended for patients deemed to be eligible for endovascular treatment. For patients with suspected LVO who have not had noninvasive vessel imaging as part of their initial imaging assessment for stroke, noninvasive vessel imaging should then be obtained as quickly as possible (e.g., during alteplase infusion if feasible). | I | A |
| For patients with suspected intracranial LVO who meet the criteria for endovascular treatment, it is reasonable to proceed with CTA before obtaining a serum creatinine concentration in patients without a history of renal impairment. | IIA | B-NR |
| In patients who are potential candidates for EVT, imaging of the extracranial carotid and vertebral arteries, in addition to the intracranial circulation, is reasonable to provide useful information regarding patient eligibility and endovascular procedural planning. | IIB | C-EO |
| It may be reasonable to incorporate collateral flow status into clinical decision making in some candidates to determine eligibility for mechanical thrombectomy. | IIB | C-LD |
| When evaluating patients with AIS within 6 hours of last known normal with LVO and ASPECTS of ≥6, selection for mechanical thrombectomy based on CT/CTA or MRI/MRA is recommended in preference to performance of additional imaging such as perfusion studies. | I | B-NR |
| In patients who have a large vessel occlusion in the anterior circulation from 6–24 hours of last known well, CTP/MRP/DWI-MRI is recommended to aid in patient selection for mechanical thrombectomy, but only when imaging and other eligibility criteria from RCTs are strictly applied. | I | A |
|
| ||
| Class of recommendation: | ||
| I = strong recommendation (benefit >>>risk) | ||
| IIA = moderate recommendation (benefit >>risk) | ||
| IIB = weak recommendation (benefit ≥ risk) | ||
| III stratified into moderate recommendation (benefit = risk) and strong recommendation (risk > harm) | ||
|
| ||
| Level of evidence: | ||
| Level A: high quality evidence from more than 1 RCT, meta-analyses of high quality RCTs | ||
| Level B-R: moderate quality evidence from 1 or more RCTs, or meta-analysis of moderate quality RCTs | ||
| Level B-NR: moderate quality evidence from well-designed observational studies, meta-analyses of such studies | ||
| Level C-LD (limited data): moderate quality evidence from observational studies with flawed design | ||
| Level C-EO: expert opinions | ||
Based on the American Heart Association/American Stroke Association (AHA/ASA) guidelines (1).
AIS, acute ischemic stroke; IV, intravenous; NCCT, noncontrast computed tomography; MRI, magnetic resonance imaging; ICH, intracerebral hemorrhage; CMB, cerebral microbleeds; CTP, computed tomography perfusion; MRP, perfusion magnetic resonance; MCA, middle cerebral artery; FLAIR, fluid-attenuated inversion recovery; CTA, computed tomographic angiography; MRA, magnetic resonance angiography; LVO, large vessel occlusion; EVT, endovascular therapy; ASPECTS, Alberta Stroke Program Early CT Score; DWI, diffusion-weighted imaging; RCT, randomized controlled trial.
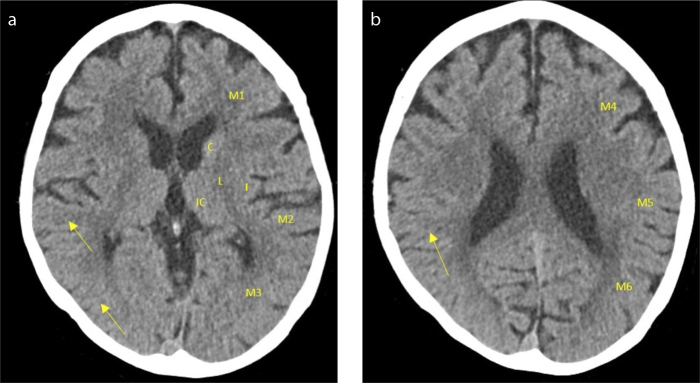
Figure 1. a, b.
Noncontrast computed tomography (NCCT) and ASPECTS score in acute ischemic stroke. Axial NCCT image at the basal ganglia (a), and the supra-ganglionic (b) levels, respectively, show loss of grey-white matter differentiation in the M2, M3, and M5 territory of the right hemisphere, confirming a right middle cerebral artery (MCA) ischemic stroke (arrows) with an ASPECTS score of 7/10. The patient, in the 80–90 years age group, presented with left-sided hemiparesis and hemianesthesia. Following triage, the patient underwent NCCT, which indicated a right MCA stroke. IC, internal capsule; C, caudate nucleus; L, lentiform nucleus; I, insular ribbon; M1, anterior MCA cortex; M2, MCA cortex lateral to the insular ribbon; M3, posterior MCA cortex; M4, anterior MCA superior territory; M5, lateral MCA superior territory; M6, posterior MCA superior territory.
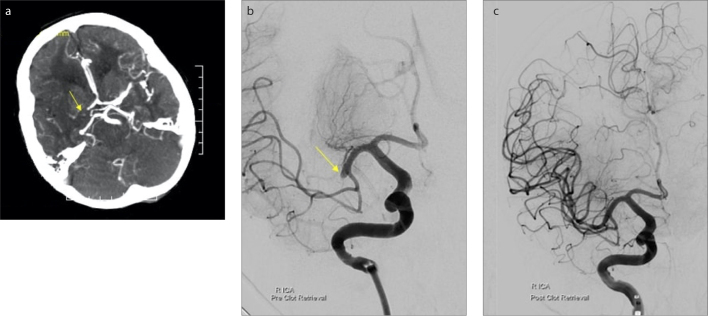
Figure 2. a–c.
Computed tomographic angiography (CTA) and digital subtraction angiography (DSA) in acute ischemic stroke. Axial CTA (a) at the level of the basal ganglia show a mid-M1-MCA occlusion. Axial pre-intervention DSA (b) confirms the CTA findings (yellow arrow). Post-endovascular thrombectomy (EVT) DSA (c) reveal restoration of the blood flow to the right cerebral hemisphere. The patient presented with left-sided hemiparesis and hemianesthesia. Following triage, the patient underwent NCCT, which indicated a right MCA stroke. The patient subsequently underwent EVT for the clot retrieval.
Role of noncontrast CT in initial stroke evaluation
The NCCT is the main imaging modality for the initial evaluation of patients with suspected stroke, allowing clinicians to identify hemorrhage, which produces a good contrast between the “bright”, high attenuating clot, and the “dark” low attenuating cerebrospinal fluid (2). This is critical in selecting patients for intravenous thrombolysis (IVT) via tissue plasminogen activator (t-PA) within 4.5 hours since hemorrhage is an absolute contraindication and must be excluded (3). Moreover, the presence of various early ischemic changes (EIC), including parenchymal hypoattenuation, cortical sulcal effacement (4), and loss of grey-white matter differentiation may be visualized up to 6 hours from symptom onset and inform treatment decisions. In a multicenter trial, von Kummer et al. (5) showed that the presence of parenchymal hypoattenuation in over one-third of the middle cerebral artery (MCA) territory correlated with larger infarct size and worse long-term functional outcomes in patients despite reperfusion with t-PA. As an alternative to the qualitative one-third rule of von Kummer et al., the Alberta Stroke Program Early Computed Tomography Score (ASPECTS) was developed and validated to systematically detect and quantify EICs (6). The ASPECTS score is graded by deducting points for every region of the MCA territory demonstrating hypoattenuation, from a total of 10 (Fig. 1). ASPECTS has been used as an indirect measure of ischemic core size and has shown utility in assessing the duration and degree of tissue hypoperfusion (7). A baseline ASPECTS score ≤7 has been associated with worse 90-day functional outcomes and increased risk of symptomatic intracerebral hemorrhage (sICH) following t-PA (6, 8), supporting its use as a prognostic marker in clinical settings. However, the most significant limitation of ASPECTS is the subtle nature of EICs, which, despite adjustments to the CT window level in providing enhanced contrast of these lesions (9), still results in variability even amongst experienced clinicians (10); this is especially problematic given the fact that ASPECTS has been invoked in the inclusion criteria of multiple clinical trials (11).
Furthermore, a “hyperdense vessel” sign may be visualized on NCCT, providing evidence of a possible intraluminal thrombus indicative of a large-vessel occlusion (LVO). However, this hyperdense sign has limited clinical use because its diagnostic capability varies by location, often with high specificity but poor to moderate sensitivity (12). Although NCCT alone is useful in predicting patients most likely to be harmed by IVT, it is insufficient to guide the selection of patients in whom the benefits from reperfusion therapy outweigh the potential risks of sICH. The pooled meta-analysis by the Third International Stroke Trial (IST-3) Collaboration group demonstrated no statistically significant subgroup difference (p = 0.94) for IV alteplase effect on the functional outcome for ASPECTS subgroups (0–7 versus 8–10), or patients with hypo-attenuated arteries (p = 0.517) (13). This is consistent with the AHA/ASA guidelines whereby the use of ASPECTS and hyperdense MCA sign is not indicated for withholding t-PA treatment (1).
CT angiography in identifying vessel status and patency
Computed tomographic angiography (CTA) of the head and neck enables clinicians to delineate the vasculature from the aortic arch to distal divisions of the intracranial vessels, allowing confirmation of LVOs, evaluation of the patency of blood vessels, and the assessment of collateral blood flow (14, 15). Studies have also demonstrated that in comparison to NCCT, CTA-source images (CTA-SI) provide greater visual demarcation between normal and abnormal tissue, enhancing the diagnostic capacity and providing greater concordance between clinicians regardless of their level of experience (14, 16). Compared to gold-standard digital subtraction angiography (DSA), CTA-SI has a sensitivity and specificity of 98%–100% for detecting large vessel occlusion (17, 18). The prognostic capacity of CTA has shown promise through the clot-burden score (CBS), a 10-point system analogous to the ASPECTS of NCCT, which quantifies the extent of intracranial thrombosis. Despite heterogeneity in diagnostic protocols and workflow across studies, lower CBS values have been validated to independently predict lower recanalization rates with t-PA, larger infarcts, poor functional outcomes, and greater risk of hemorrhagic transformation (19–21).
Given the transition of stroke care from IVT alone to additional EVT, CTA is critical in establishing a lesion amenable to neuro-intervention, typically an occlusion within the terminal internal carotid artery or MCA-M1 segment. Other MCA-segment occlusions require careful patient selection, for example, based upon the presence of leptomeningeal collateral supply, a key determinant in the progression of the salvageable tissue (known as the penumbra) to irreversible infarction (22, 23). Various collateral scoring systems exist; however, there is no standardization regarding the best scoring method. Nevertheless, the consensus is that good collateral supply correlates with smaller final infarct volumes (FIV) and good functional outcomes (24–28). However, key issues highlighted in these studies were marked variability in collateral supply between patients and limited accuracy in the evaluation of collateral supply in branches of the anterior and posterior cerebral arteries, often due to small vessel size and complex routes, which may impact infarct progression.
Fig. 2 compares CTA with DSA performed during the EVT procedure, highlighting the restoration of blood supply in the brain. Despite having utility in prognostication and guiding treatment decisions, the inability to visualize the penumbral tissue using CTA limits its ability in guiding the selection of patients who are more likely to have a poor outcome despite EVT. Patient selection based on collateral supply is classified under moderate recommendations as per the AHA/ASA guidelines (1) and requires further validation in clinical trials to be incorporated in standards of care in acute stroke.
Role of CT perfusion imaging in defining tissue characteristics
Computed tomography perfusion (CTP) maps demonstrate various parameters within the brain parenchyma, for example, cerebral blood flow within a region suspected of infarction. These perfusion maps are derived by measuring the attenuation of injected intravenous iodinated contrast through large arteries and the brain parenchyma over time, with post-processing conducted by software to generate CTP parameter maps (29). CTP parameters are explained in Table 2.
Table 2.
Parameters of CT perfusion imaging
| Parameter | Units | Definition | Hypoperfused state |
|---|---|---|---|
| Cerebral blood volume (CBV) | mL/100 g | Total volume of blood present within a region of the brain. Relative CBV (rCBV) is often calculated by dividing the average CBV values in the affected area with those of the contralateral hemisphere | Normal, elevated or decreased* |
| Mean transit time (MTT) | s | The average time difference between the arterial inflow and venous outflow in a region of brain | Increased |
| Cerebral blood flow (CBF) | mL/100 g/s | The flow rate of blood within a region of the brain. Calculated as CBV/MTT | Decreased |
| Time to peak (TTP) | s | The time from the beginning of contrast injection to the maximum concentration of contrast material within a region of interest | Increased |
| Time to maximum (Tmax) | s | The time to the maximum of the residue function, reflecting the delay of bolus between the site of the arterial input and the brain tissue | Increased |
| Delay time (DT) | s | Tmax with mathematical correction for delay and dispersion of bolus | Increased |
Diffusion-weighted imaging on magnetic resonance imaging (MRI) is typically considered the gold standard in detecting the ischemic core; however, it is less readily available compared to CTP and excludes patients with pacemakers and other metallic implants (12). In their comprehensive meta-analysis, Biesbroek et al. (30) established that CTP has a high sensitivity of 80% (95% confidence interval [CI]: 72%–86%) and very high specificity of 95% (95% CI: 86%–98%) in stroke detection, with false negatives mainly arising through a limited capability to detect smaller lacunar infarcts and infra-tentorial lesions. Despite this, the concordance between MRI- and CTP-identified mismatch has been demonstrated to be 90%, thus facilitating the use of either modality in acute stroke settings (31). However, there is still great contention about the extent of infarction using CTP. Previously, manual interpretation of CTP data required time and specialized skill, which was undesirable given that delays would result in greater neuronal loss. Moreover, visual assessment of CTP maps to identify penumbral tissue reveals moderate inter-rater reliability with inexperienced clinicians most likely to misinterpret these maps (32). Although this hurdle was overcome using automated software in calculating core and penumbral volumes, Bivard et al. (33) identified marked variability in computed volumes amongst different deconvolution techniques employed by different software packages. A key finding was that cerebral blood flow (CBF) best approximated the infarct core amongst all these algorithms, supporting results by Campbell et al. (34) and shifting away from the use of cerebral blood volume (CBV) in earlier studies (35); delay time (DT) may also be substituted with time to the maximum of the residue function (Tmax), as demonstrated in a recent validation study (36). Heterogeneity in CTP parameter thresholds and imaging processing techniques amongst different commercial software result in significant variability in computed volumes with poor reproducibility (37), highlighting the need for standardization between stroke centers. Recent clinical trials have employed Rapid processing of perfusion and diffusion (RAPID) (iSchemaView©) software utilizing the thresholds Tmax>6 seconds for total hypoperfused tissue and CBF<30% for the infarcted core; the difference in the two volumes is the penumbra (8–41). RAPID has been validated to have 100% sensitivity and 91% specificity in a review by Straka et al. (42), whilst more accurately predicting FIV in comparison with Philips and Siemens (43). RAPID’s extensive validation in the aforementioned trials has lent support to its widespread use for patient evaluation; however, its low availability necessitates the need for alternative platforms, techniques, or open-access software that can create perfusion maps that reliably assess perfusion parameters. Fig. 3 demonstrates the application of CTP maps in delineating the core and penumbral volumes.
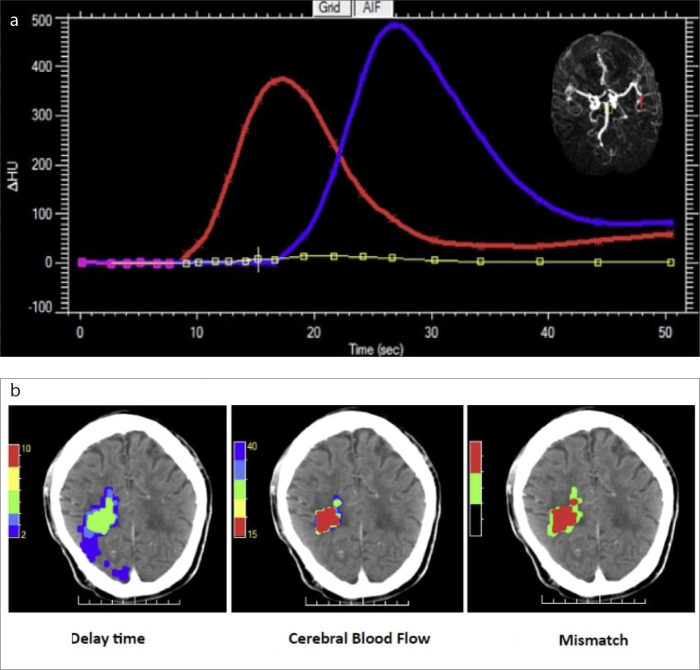
Figure 3. a, b.
CTP perfusion maps in delineating the infarcted core and penumbra. The arterial (red) and venous (blue) input curves (a), measured from the right MCA and the venous sinus, are shown. A MIP is shown in the top right (a), showing an occlusion of the MCA. These maps were processed by MiStar™ to create perfusion maps (b), namely the delay time (DT) map, cerebral blood flow (CBF) map, and mismatch map (left to right). The axial DT map shows volumes of critically hypoperfused tissue, including both the infarcted core and the penumbral tissue at risk of infarction, based on various thresholds: DT >6.0 s, 0 mL; DT >4.0 s, 4 mL; DT >3.0 s, 12 mL; DT >2.0 s, 95 mL. A threshold DT >3.0 s indicated a volume of 12 mL. The axial CBF map shows the infarcted core volumes, based on various thresholds: CBF <40%, 7 mL; CBF <35%, 6 mL; CBF <30%, 5 mL; CBF <20%, 3 mL; CBF <15%, 1 mL. A threshold of CBF <30% indicated a volume of 5 mL (42% of the total lesion). The axial mismatch map shows the infarcted core and penumbra superimposed on the NCCT image to better identify the mismatch volume (7 mL, equivalent to 58% of the total lesion). The penumbral to the core volume ratio of 2.4. Hence, there is a benefit associated with endovascular thrombectomy within 24 hours of stroke symptom onset and in the absence of other clinical contraindications.
Role of CT perfusion imaging in patient selection
The implementation of CTP has largely been marred due to its limited availability, increased radiation and contrast doses, and greater susceptibility to motion artifacts compared to NCCT (30, 44). Moreover, the additional benefit conferred by CTP imaging in guiding the selection of patients presenting more than 4.5 hours of the onset of stroke, initially demonstrated mixed results. In their meta-analysis, Mishra et al. (45) failed to demonstrate a statistically significant improvement in functional outcomes when t-PA was administered to patients with CTP-derived mismatch between penumbral and core volumes beyond 4.5 hours, reflecting the time-threshold in current AHA/ASA guidelines (1). Moreover, updated AHA/ASA guidelines for patients presenting within 6 hours of last known well and ASPECTS ≥6, advanced perfusion imaging such as CTP is not required for patient selection, because numerous multi-center randomized controlled trials (Randomized Assessment of Rapid Endovascular Treatment of Ischemic Stroke (ESCAPE) (98), SWIFT PRIME (99), REVASCAT (100)) demonstrated the efficacy of EVT in patients selected using CTA/magnetic resonance angiography (MRA).
Previously, the Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy (MR RESCUE) trial (46) investigating CTP mismatch-based patient selection for EVT using first-generation clot-retrievers had failed to demonstrate clinical significance; however, it was limited by a poor workflow that resulted in delays in receiving EVT and changes in clinical protocols during the 8-year trial. In contrast, the DEFUSE 2 trial (47) provided support for the use of magnetic resonance perfusion (MRP) in mismatch-based patient selection, despite being limited by its nonrandomized approach. Of prime importance was the trial’s inability to establish a statistically significant difference between patients who were treated ≤6 hours and >6 hours in terms of a favorable response to EVT. This result has since been supported by recent trials, thus dispensing with the widely accepted belief that time from symptom onset was the principal determinant of success in EVT (22, 48, 49). This has provided support in recognizing the importance of collateral circulation in preserving the penumbral tissue for longer periods of time (50).
With the introduction of new stent-retrievers that have superior recanalization rates compared to first-generation clot-retrievers in 2012 and the abandoning of time-sensitive treatment modalities (12, 51), the recent multicenter, randomized controlled trials such as Extending the Time for Thrombolysis in Emergency Neurological Deficits — Intra-Arterial Trial (EXTEND-IA) (38), Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke Trial (DEFUSE-3) (39) and Diffusion-weighted imaging or computed tomography perfusion Assessment with clinical mismatch in the triage of Wake-up and late presenting strokes undergoing Neurointervention with Trevo Trial (DAWN) (41) were conducted to investigate the efficacy of EVT in an extended time window. In particular, the DEFUSE-3 trial (39) demonstrated that in patients with target mismatch (TMM), where the ratio of penumbral to core volume >1.8 who presented 6–16 hours after the onset of stroke, undergoing EVT provided greater rates of 90-day functional independence (modified Rankin Score, mRS: 0–2) compared to those in the control group receiving conservative medical management (45% versus 17%, p < 0.001), with no significant difference in rates of sICH (7% versus 4%, p = 0.75). This has been supported by a sub-analysis of the Solitaire with the Intention for Thrombectomy as Primary Endovascular Treatment Trial (SWIFT-PRIME) trial (52), whereby patients with TMM profile at baseline had a highly favorable response to EVT with higher rates of reperfusion, smaller FIV, and better functional outcomes. The DAWN trial (41) highlighted the benefits of EVT by innovatively selecting patients with a mismatch between clinical indexes with perfusion imaging-derived baseline core volume, up to 24 hours from onset of stroke. This has been monumental towards extending the time window for EVT in the recent AHA/ASA guidelines (1) and lends support for further clinical trials in investigating the utility of combining imaging data and clinical scores in patient selection for reperfusion therapy.
Current challenges in CTP based patient selection
The randomized controlled trials EXTEND-IA (38), DEFUSE-3 (39), and DAWN (41) each excluded patients with core volumes >70 mL, a parameter with great debate regarding its utility. Whilst previous studies have demonstrated that core volumes above this threshold have been associated with worse functional outcomes and higher mortality (53, 54), Sim et al. (55) highlighted that EVT may lead to a favorable shift in 90-day mRS scores compared to standard treatment. This has been supported by numerous studies (56–59); the recent Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials (HERMES)-collaboration meta-analysis (59) incorporating 7 high-quality randomized controlled trials highlighted improved functional outcomes from treatment with EVT over a wide range of infarct core volumes, particularly in younger patients. A proposed explanation is that reperfusion of the infarct core may still enhance recovery by inhibiting edema formation, rather than saving tissue at risk (60). There is also limited evidence on the efficacy of EVT in patients with unfavorable mismatch volumes, where there is scarce penumbral tissue despite having small core volumes (61) (Fig. 4). This highlights the need for large prospective trials that explore the utility of higher baseline core volume thresholds and different penumbral patterns in patient selection for reperfusion therapy. Analysis of these randomized controlled trials also reveals the heterogeneity in the inclusion and exclusion criteria used for patient selection (Table 3). Coupled with inconsistencies in imaging modalities as MRP and CTP were used interchangeably at some centers, technical errors such as over-estimation of baseline lesions are likely. This highlights the need for standardizing criteria for patient selection in each neuroimaging modality (CTP and MRP) with consistency in the modality of follow-up imaging to streamline workflow across stroke centers.
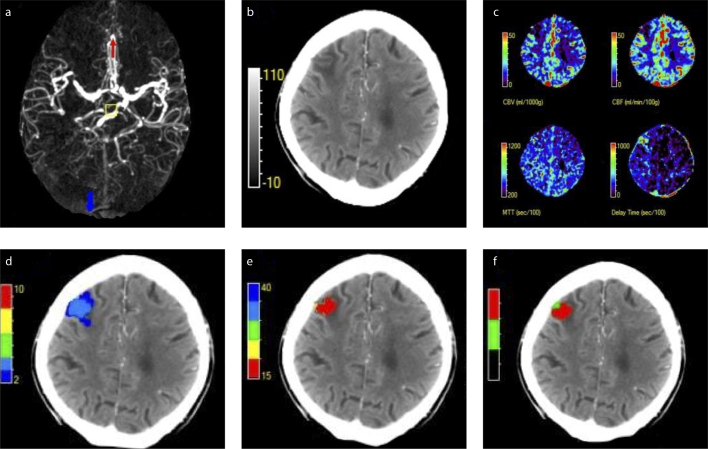
Figure 4. a–f.
Penumbral pattern and its role in patient selection by CTP. A male patient in the 70–80 years age group presented with left-sided hemiparesis and hemianesthesia. Subsequently, he underwent multimodal CT imaging. Axial MIP image (a) shows a distal MCA stroke. Axial NCCT (b) shows acute ischemic changes in the M1-MCA territory. Axial CTP maps (c) show cerebral blood volume (CBV), cerebral flow (CBF), mean transit time (MTT), and delay time (DT) from left to right. Axial DT band map (d) shows the total hypoperfused volume. The volumes at various thresholds: DT>2.0s+, 10ml; DT>3.0s+, 5 ml; DT>4.0s+, 0 ml; DT>6.0s+, 0 ml. Axial CBF band map (e) shows the infarct core volume. The volumes at various thresholds: CBF<40%, 4 mL; CBF<35%, 4 mL; CBF<30%, 4 mL; CBF<25%, 3 mL; CBF<20%, 2 mL; CBF<15%, 1 mL. CTP-derived post-processed map using MiStar™ (f) demonstrates an infarct core volume of 4 mL (80%) and penumbral volume of 1 mL (20%), which constitutes to a mismatch ratio of 1.3. Scarce penumbral tissue and anticipated risks of treatment may outweigh the benefit of neurointervention. The DEFUSE 3 (39) used a threshold core to penumbral volume >1.8 as an inclusion criterion, hence this patient would not be considered for EVT in that context. Further clinical trials are required to verify appropriate penumbral patterns that are amenable to neurointervention and those that are not. DEFUSE 3, Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke Trial 3.
Table 3.
Inclusion and exclusion criteria in the DAWN, DEFUSE 3 and EXTEND-IA trials
| Study | Year | Patients (n) | Inclusion time (hours) | Inclusion criteria | Neuroimaging exclusion criteria | |
|---|---|---|---|---|---|---|
| Clinical | Neuroimaging | |||||
| DAWN (38) | 2018 | 206 | 6–24 |
|
|
|
| DEFUSE 3 (39) | 2018 | 182 | 6–16 |
|
|
|
| EXTEND-IA (41) | 2015 | 70 | 0–6 |
|
|
|
t-PA, tissue plasminogen activator; NIHSS, National Health Institute Stroke Severity Score; MCA, middle cerebral artery, with M1 and M2 segments; NCCT, noncontrast enhanced computed tomography; CTP, computed tomography perfusion imaging; MRP, magnetic resonance perfusion imaging; ICA, internal carotid artery; CTA, computed tomographic angiography; MRA, magnetic resonance angiography; ASPECTS, Alberta Stroke Program Early CT Score; MRI, magnetic resonance imaging.
Value of CTP imaging in prognosis and tissue fate
Baseline core volume
Pre-intervention CTP parameters may have value in predicting FIV and long-term functional outcomes, independently of receiving reperfusion therapy. An earlier retrospective cohort study by Zhu et al. (62) argued that imaging the penumbra would predict the FIV and functional outcome only if recanalization would be achieved. Indeed, Rao et al. (29) demonstrated in patients achieving >90% recanalization, the initial baseline core infarct correlated with the 24-hour FIV (r=0.83; p < 0.0001). However, since the recanalization status cannot be known when administering treatment, pre-intervention CTP may not be effective in predicting the FIV. Chen et al. (63) showed that the reperfused penumbral volume was a strong predictor of good functional outcome (area under the curve [AUC]=0.946, p < 0.0001). Moreover, the HERMES-collaboration meta-analysis (59) involving 1764 patients demonstrated that each 10 mL increase in CTP-derived baseline ischemic core volume was independently associated with reduced likelihood of 90-day functional independence by 20%–30% (odds ratio=0.77, 95% CI: 0.69–0.86, per 10 mL). However, the primary objective of the randomized controlled trials included in the HERMES meta-analysis was to investigate the efficacy of second-generation stent-retrievers; there is currently limited evidence on prospective trials that specifically analyze the linear relationship between baseline core volume, functional outcomes, and tissue outcomes in meaningful ways that may be implemented across stroke centers. Thus, the HERMES meta-analysis has paved the road for future trials in the assessment of patient prognosis using advanced neuroimaging modalities.
CTP ASPECTS
As an alternative to automated software, earlier studies adapted the semi-quantitative ASPECTS technique to CTP-source images and parameters. Parsons et al. (65) first established the additional benefit of CTP-SI and CBV-ASPECTS in more accurately predicting infarcted tissue and 90-day functional outcomes compared to NCCT or CTA-SI alone. Aviv et al. (66) further added information to this field by demonstrating that using a CBV-ASPECTS threshold of 8 discriminated patients with good long-term functional outcomes (mRS 0–2), providing moderate sensitivity of 60% and specificity of 100%. Numerous subsequent studies have demonstrated the utility of CBV-ASPECTS in predicting functional outcomes and FIV; however, these studies have demonstrated heterogeneity in the optimal thresholds (range, 6–9) used to discriminate between patients with good and poor functional outcomes (67–69). Moreover, using CBV-ASPECTS may be problematic given that CBV-ASPECTS can be affected by the presence of spontaneous recanalization in some patients, resulting in the reversibility of the CBV lesion (that defines the infarcted core) and an overestimation of the FIV (68). Taking this another step forward, Tang et al. (70) demonstrated in a retrospective analysis of 144 patients that the combination of CTA-SI-ASPECTS, the Regional leptomeningeal collateral score (rLMC) collateral scoring system in multimodal CT assessment, and CBV-ASPECTS provided a greater area under the ROC curve (AUC=0.87) for the detection of poor functional outcomes following a stroke, significantly greater than for any single parameter used alone. This warrants further investigation in a larger prospective cohort of patients, in comparison with CTP-derived quantitative thresholds.
Collateral status
Collateral status has also been validated in an extensive meta-analysis of 29 studies in independently predicting functional outcomes, FIV, sICH, and mortality. Of notable importance, however, is that whilst 22 of these studies utilized CTA, only 3 studies utilized CTP imaging (71). CTP imaging is underutilized in determining collateral status because the function is fulfilled by CTA; both imaging modalities reflect similar pathophysiological aspects of ischemic stroke. Arenillas et al. (72) demonstrated a positive association between CTP-derived relative CBV (rCBV) and collateral circulation grade, which in turn predicted infarct growth in subgroups of patients in patients receiving EVT (p = 0.038). The use of rCBV as a prognostic marker has been supported by another study (73); however, limitations in sample size reduce the strength of their evidence. While Menon et al. (44) argues that multiphase CTA may be sufficient as a prognostic tool, there is limited evidence in the additional benefit of using CTP imaging over conventional CTA for collateral assessment. This offers opportunities to investigate a combination of baseline core volume and collateral status using CTP in predicting clinical and tissue-based outcomes.
Hemorrhagic transformation
Hemorrhagic transformation (HT) often occurs following reperfusion therapy, hypothesized to occur as a consequence of the disruption of blood-brain barrier integrity. Lin et al. (74) first highlighted the utility of permeability surface area product (PS) values, computed by measuring the extravasation of iodinated contrast into the brain, with elevated values in regions of infarction in patients with HT. Aviv et al. (75) further investigated this, highlighting that a PS threshold of 0.23 mL/min/100 g as demonstrating 77% sensitivity and 94% specificity for detection of HT. The utility of PS has been supported by various other retrospective studies, providing support for the role of PS in early identification of patients at risk of HT (76–79); however, these studies have been limited by small sample sizes. When validated in a larger cohort of 545 patients, Horsch et al. (80) concluded that although PS values predicted HT, it did not improve prediction compared to clinical parameters such as age above 60 years and admission stroke severity (determined using the National Institutes of Health Stroke Scale [NIHSS]). Key issues identified were the high number of patent exclusions (19%) due to technical processing difficulties and the substitution of follow-up CTP with MRI in some patients. As an alternative, Yassi et al. (81) utilized baseline CTP-parameters, showing that regions on CTP where Tmax >14 s exceeding a total of 5 mL enabled prediction of HT with a sensitivity of 79%, the specificity of 68%, and a negative likelihood ratio of 3.16. Overall, the disputed results of these trials highlight the need for larger prospective trials exploring the efficacy of CTP-based parameters in detecting HT and evaluating its utility in clinical settings in excluding patients for reperfusion therapy if required.
Limitations of CTP, technical and clinical considerations
Although various pre-intervention CTP parameters are useful in the assessment of patient prognosis, the current AHA/ASA guidelines mainly incorporate multimodal CT imaging as a diagnostic tool. Large multicenter randomized controlled trials are required to assess the potential for CTP as a prognostic tool and investigate its utility in individualizing stroke rehabilitation programs in clinical settings. Table 4 highlights areas where further research is warranted in improving guidelines for stroke intervention in the future. Further points of discussion are listed below:
Table 4.
Various imaging modalities in acute stroke
| Imaging techniques | Role in terms of current AHA/ASA recommendations | Further research avenues |
|---|---|---|
| Multi-modal computed tomography imaging | ||
| NCCT |
|
The utility of combining: |
| CTA |
|
The utility of combining: in randomized trials in excluding patients for whom potential risks outweigh the benefits |
| CTP |
|
|
|
| ||
| Multi-modal magnetic resonance imaging | ||
| MRI |
|
|
| MRA | Same as CTA:
|
|
| MRP | Same as CTP:
|
|
| MRI - ASL | Nil. |
|
|
| ||
| Other modalities | ||
| PET | Nil. Access to radiopharmaceuticals, long radioisotope uptake times, and lack of standardized protocols impede use in acute stroke situations. |
|
| TCD | Nil. In acute stroke situations, diagnosis is confirmed through NCCT/MRI to exclude hemorrhage and extracranial carotid arteries are visualized through CTA/MRA to rule out dissection. TCD results are more operator dependent. |
|
NCCT, noncontrast computed tomography; t-PA, tissue plasminogen activator; MCA, middle cerebral artery; ASPECTS, Alberta Stroke Program Early CT Score; CTA, computed tomographic angiography; LVO, large vessel occlusion; EVT, endovascular thrombectomy; CTP, computed tomography perfusion; FIV, final infarct volume; HT, hemorrhagic transformation; MRI, magnetic resonance imaging; AIS, acute ischemic stroke; FLAIR, fluid-attenuated inversion recovery; IV, intravenous; sICH, symptomatic intracranial hemorrhage; HDMCA, hyperdense middle cerebral artery sign; MRA, magnetic resonance angiography; MRP, perfusion magnetic resonance; RCT, randomized controlled trial; ASL, arterial spin labelling; CBF, cerebral blood flow; PET, positron emission tomography; TCD, transcranial Doppler ultrasound.
As highlighted in Table 2, autoregulatory vasodilation may ensure normal or elevated CBV in hypoperfused tissue to preserve oxygenation in an otherwise hypoxic state (82). Therefore, reduced CBV is not consistent with the ischemic penumbra unlike other CTP parameters (CBF or Tmax), and should not be used for penumbral evaluation.
Perfusion maps can be inaccurate in patients with poor cardiac output, atrial fibrillation, and severe proximal arterial stenosis (83). Specifically, perfusion maps tend to overestimate MTT and underestimate CBF in these conditions. This can lead to an erroneous diagnosis of extensive hypoperfusion or ischemia. These limitations also apply due to poor placement of arterial and venous density regions of interest (84).
With the advent of improved efficacy of EVT and efficient stroke workflow in the future, ultra-fast reperfusion (≈60 min) may be achieved, hence alternative time-stratified CTP thresholds may need to be validated to accurately depict ischemic lesions (85, 86).
Standardized CTP protocols exclude a large volume of the brain, including the superior cerebral hemisphere and the posterior fossa as the scans are focused on the basal ganglia and supra-ganglionic level. This can cause erroneous diagnosis to patients with posterior circulation stroke. However, some studies have demonstrated that whole-brain CTP may provide additional diagnostic accuracy over conventional CTA/NCCT in detecting acute posterior circulation stroke (101, 102).
CTP maps are not good to evaluate lacunar infarcts due to their lower resolution and may result in false-negative results (30).
Patients presenting with seizures may cause a diagnostic dilemma in that post-ictal paralysis mimics stroke symptoms and CTP imaging may demonstrate hyperperfusion in the ictal region (87), which can lead to misinterpretation of hypoperfusion in the contralateral hemisphere.
Recommendation for future work
Various key issues have been highlighted in this article including heterogeneity in CTP-parameter thresholds and imaging processing techniques amongst different commercial software; the need for alternative platforms, techniques, or open-access software that can reliably create perfusion maps; the need to develop standardized inclusion criteria with advanced neuroimaging modalities (MRP or CTP) in selecting patients for reperfusion therapy; and further research regarding the utility of pre-intervention CTP features (baseline core volume, collateral status, permeability surface area product maps) in predicting tissue-based and clinical outcomes in patients with acute ischemic stroke. Future randomized controlled trials in exploring these key issues are warranted given that robust patient selection criteria and assessment of prognosis would enable a more streamlined and consistent workflow across stroke centers. Given the significant burden of disease posed by stroke globally, clinicians need to be adequately trained worldwide in using neuroimaging effectively so that patients can receive evidence-based treatment and have improved outcomes.
Conclusion
CTP perfusion imaging has a value in stratifying subgroups of acute ischemic stroke patients who are more likely to respond favorably to appropriate reperfusion therapy intervention. In the milieu of EVT, the prognostic value of CTP in determining the threshold of infarct core to accurately predict tissue status and clinical outcome is still not clear. Futile recanalization, which does not translate to clinical benefit, is an ongoing challenge. Lack of standardized CTP methods, reporting variations, ambiguity in infarct thresholds, and differences in follow-up outcome measures are factors that need to be considered while designing future acute stroke trials. For CTP imaging to be incorporated into routine clinical practice and appropriate guideline, further research leveraging randomized controlled trials are warranted.
Main points.
CT perfusion (CTP) imaging has a value in stratifying subgroups of acute ischemic stroke patients who are more likely to respond favorably to appropriate reperfusion therapy intervention.
In the milieu of endovascular thrombectomy, the prognostic value of CTP in determining the threshold of infarct core to accurately predict tissue status and clinical outcome is still not clear.
Lack of standardized CTP methods, reporting variations, ambiguity in infarct thresholds, and differences in follow-up outcome measures are factors that need to be considered while designing future acute stroke trials.
Acknowledgments
We would like to acknowledge the support from the administrative staff and NSW wide partnering clinicians and investigators.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
Financial disclosure
Seed funding from the UNSW Medicine Mindgardens CAG funding 2017 for the Thrombolysis and Endovascular FLow Network (TEFLON) trial (Chief Investigator: Dr. Sonu Bhaskar) and funding for the NSW Brain Clot Bank (Chief Investigator: Dr. Sonu Bhaskar) from the NSW Ministry of Health (2019–2022) is acknowledged.
References
- 1.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 2.Gabrielli A, Layon AJ, Yu M. Civetta, Taylor, & Kirby’s Manual of Critical Care. Lippincott Williams & Wilkins; Philadelphia: 2012. [Google Scholar]
- 3.Demaerschalk BM, Kleindorfer DO, Adeoye OM, et al. Scientific rationale for the inclusion and exclusion criteria for intravenous alteplase in acute ischemic stroke. Stroke. 2016;47:581–641. doi: 10.1161/STR.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 4.Na DG, Kim EY, Ryoo JW, et al. CT sign of brain swelling without concomitant parenchymal hypoattenuation: comparison with diffusion- and perfusion-weighted MR imaging. Radiology. 2005;235:992–948. doi: 10.1148/radiol.2353040571. [DOI] [PubMed] [Google Scholar]
- 5.von Kummer R, Allen KL, Holle R, et al. Acute stroke: usefulness of early CT findings before thrombolytic therapy. Radiology. 1997;205:327–333. doi: 10.1148/radiology.205.2.9356611. [DOI] [PubMed] [Google Scholar]
- 6.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Lancet. 2000;355:1670–1674. doi: 10.1016/S0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 7.Hirano T, Yonehara T, Inatomi Y, Hashimoto Y, Uchino M. Presence of early ischemic changes on computed tomography depends on severity and the duration of hypoperfusion. Stroke. 2005;36:2601–2608. doi: 10.1161/01.STR.0000189990.31225.82. [DOI] [PubMed] [Google Scholar]
- 8.Dzialowski I, Hill MD, Coutts SB, et al. Extent of early ischemic changes on computed tomography (CT) before thrombolysis: prognostic value of the Alberta Stroke Program Early CT Score in ECASS II. Stroke. 2006;37:973–978. doi: 10.1161/01.STR.0000206215.62441.56. [DOI] [PubMed] [Google Scholar]
- 9.Lev MH, Farkas J, Gemmete JJ, et al. Acute Stroke: Improved nonenhanced CT detection—benefits of soft-copy interpretation by using variable window width and center level settings. Radiology. 1999;213:150–155. doi: 10.1148/radiology.213.1.r99oc10150. [DOI] [PubMed] [Google Scholar]
- 10.McTaggart RA, Jovin TG, Lansberg MG, et al. Alberta stroke program early computed tomographic scoring performance in a series of patients undergoing computed tomography and MRI. Stroke. 2015;46:407–412. doi: 10.1161/STROKEAHA.114.006564. [DOI] [PubMed] [Google Scholar]
- 11.Suh CH, Jung SC, Kim B, et al. Neuroimaging in randomized, multi-center clinical trials of endovascular treatment for acute ischemic stroke: a systematic review. Korean J Radiol. 2020;21:42–57. doi: 10.3348/kjr.2019.0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catanese L, Tarsia J, Fisher M. Acute ischemic stroke therapy overview. Circulation Res. 2017;120:541–558. doi: 10.1161/CIRCRESAHA.116.309278. [DOI] [PubMed] [Google Scholar]
- 13.IST-3 Collaborative Group. Association between brain imaging signs, early and late outcomes, and response to intravenous alteplase after acute ischemic stroke in the third International Stroke Trial (IST-3): secondary analysis of a randomized controlled trial. Lancet Neurol. 2015;14:485–496. doi: 10.1016/S1474-4422(15)00012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ezzeddine MA, Lev MH, McDonald CT, et al. CT angiography with whole brain perfused blood volume imaging: added clinical value in the assessment of acute stroke. Stroke. 2002;33:959–966. doi: 10.1161/hs0402.105388. [DOI] [PubMed] [Google Scholar]
- 15.Schramm P, Schellinger PD, Fiebach JB, et al. Comparison of CT and CT angiography source images with diffusion-weighted imaging in patients with acute stroke within 6 hours after onset. Stroke. 2002;33:2426–2432. doi: 10.1161/01.STR.0000032244.03134.37. [DOI] [PubMed] [Google Scholar]
- 16.Aviv RI, Malam SS, Chakraborty S, et al. Early stroke detection and extent: impact of experience and the role of computed tomography angiography source images. Clin Radiol. 2007;62:447–452. doi: 10.1016/j.crad.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen-Huynh MN, Wintermark M, English J, et al. How accurate is CT angiography in evaluating intracranial atherosclerotic disease? Stroke. 2008;39:1184–1188. doi: 10.1161/STROKEAHA.107.502906. [DOI] [PubMed] [Google Scholar]
- 18.Lev MH, Farkas J, Rodriguez VR, et al. CT angiography in the rapid triage of patients with hyperacute stroke to intraarterial thrombolysis: accuracy in the detection of large vessel thrombus. J Comput Assist Tomogr. 2001;25:520–528. doi: 10.1097/00004728-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Tan IYL, Demchuk AM, Hopyan J, et al. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol. 2009;30:525. doi: 10.3174/ajnr.A1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fanou EM, Knight J, Aviv RI, et al. Effect of collaterals on clinical presentation, baseline imaging, complications, and outcome in acute stroke. AJNR Am J Neuroradiol. 2015;36:2285. doi: 10.3174/ajnr.A4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puetz V, Dizialowski I, Hill MD, et al. Intracranial thrombus extent predicts clinical outcome, final infarct size and hemorrhagic transformation in ischemic stroke: the clot burden score. Int J Stroke. 2008;3:230–236. doi: 10.1111/j.1747-4949.2008.00221.x. [DOI] [PubMed] [Google Scholar]
- 22.Lansberg MG, Cereda CW, Mlynash M, et al. Response to endovascular reperfusion is not time-dependent in patients with salvageable tissue. Neurology. 2015;85:708–714. doi: 10.1212/WNL.0000000000001853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Baumgarten L, Thierfelder KM, Beyer SE, et al. Early CT perfusion mismatch in acute stroke is not time-dependent but relies on collateralization grade. Neuroradiology. 2016;58:357–365. doi: 10.1007/s00234-016-1643-8. [DOI] [PubMed] [Google Scholar]
- 24.Kim JJ, Fischbein NJ, Lu Y, Pham D, Dillon WP. Regional angiographic grading system for collateral flow. Stroke. 2004;35:1340–1344. doi: 10.1161/01.STR.0000126043.83777.3a. [DOI] [PubMed] [Google Scholar]
- 25.Kucinski T, Koch C, Eckert B, et al. Collateral circulation is an independent radiological predictor of outcome after thrombolysis in acute ischeemic stroke. Neuroradiology. 2003;45:11–18. doi: 10.1007/s00234-002-0881-0. [DOI] [PubMed] [Google Scholar]
- 26.Hendrikse J, Hartkamp MJ, Hillen B, Mali WP, van der Grond J. Collateral ability of the circle of Willis in patients with unilateral internal carotid artery occlusion. Stroke. 2001;32:2768–2773. doi: 10.1161/hs1201.099892. [DOI] [PubMed] [Google Scholar]
- 27.Campbell BCV, Christensen S, Tress BM, et al. Failure of collateral blood flow is associated with infarct growth in ischemic stroke. J Cereb Blood Flow Metab. 2013;33:1168–1172. doi: 10.1038/jcbfm.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menon BK, Smith EE, Modi J, et al. Regional leptomeningeal score on CT angiography predicts clinical and imaging outcomes in patients with acute anterior circulation occlusions. AJNR Am J Neuroradiol. 2011;32:1640. doi: 10.3174/ajnr.A2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konstas AA, Goldmakher GV, Lee TY, Lev MH. Theoretic basis and technical implementations of CT perfusion in acute ischemic stroke, part 1: Theoretic basis. AJNR Am J Neuroradiol. 2009;30:662. doi: 10.3174/ajnr.A1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biesbroek JM, Niesten JM, Dankbaar JM, et al. Diagnostic accuracy of CT perfusion imaging for detecting acute ischemic stroke: a systematic review and meta-analysis. Cerebrovasc Dis. 2013;35:493–501. doi: 10.1159/000350200. [DOI] [PubMed] [Google Scholar]
- 31.Campbell BCV, Christensen S, Levi CR, et al. Comparison of computed tomography perfusion and magnetic resonance imaging perfusion-diffusion mismatch in ischemic stroke. Stroke. 2012;43:2648–2653. doi: 10.1161/STROKEAHA.112.660548. [DOI] [PubMed] [Google Scholar]
- 32.Campbell BCV, Christensen S, Foster SJ, et al. Visual assessment of perfusion-diffusion mismatch is inadequate to select patients for thrombolysis. Cerebrovasc Dis. 2010;29:592–596. doi: 10.1159/000311080. [DOI] [PubMed] [Google Scholar]
- 33.Bivard A, Levi C, Spratt N, Parsons M. Perfusion CT in acute stroke: a comprehensive analysis of infarct and penumbra. Radiology. 2013;267:543–550. doi: 10.1148/radiol.12120971. [DOI] [PubMed] [Google Scholar]
- 34.Campbell BCV, Christensen S, Levi CR, et al. Cerebral blood flow is the optimal CT perfusion parameter for assessing infarct core. Stroke. 2011;42:3435–3440. doi: 10.1161/STROKEAHA.111.618355. [DOI] [PubMed] [Google Scholar]
- 35.Wintermark M, Flanders AE, Velthuis B, et al. Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke. 2006;37:979–985. doi: 10.1161/01.STR.0000209238.61459.39. [DOI] [PubMed] [Google Scholar]
- 36.Olivot JM, Mlynash M, Thijs VN, et al. Optimal Tmax threshold for predicting penumbral tissue in acute stroke. Stroke. 2009;40:469–475. doi: 10.1161/STROKEAHA.108.526954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fiorella D, Heiserman J, Prenger E, Partovi S. Assessment of the reproducibility of postprocessing dynamic CT perfusion data. AJNR Am J Neuroradiol. 2004;25:97–107. [PMC free article] [PubMed] [Google Scholar]
- 38.Campbell BCV, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. New Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 39.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. New Engl J Med. 2018;378:708–718. doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saver JL, Goyal M, Bonafe M, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. New Engl J Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 41.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. New Engl J Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 42.Straka M, Albers GW, Bammer R. Real-time diffusion-perfusion mismatch analysis in acute stroke. J Magn Reson Imaging. 2010;32:1024–1037. doi: 10.1002/jmri.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Austein F, Riedel C, Kerby T, et al. Comparison of perfusion ct software to predict the final infarct volume after thrombectomy. Stroke. 2016;47:2311–2317. doi: 10.1161/STROKEAHA.116.013147. [DOI] [PubMed] [Google Scholar]
- 44.Menon BK, d’Esterre CD, Qazi M, et al. Multiphase CT angiography: a new tool for the imaging triage of patients with acute ischemic stroke. Radiology. 2015;275:510–520. doi: 10.1148/radiol.15142256. [DOI] [PubMed] [Google Scholar]
- 45.Mishra NK, Albers GW, Davis SM, et al. Mismatch-based delayed thrombolysis. Stroke. 2010;41:e25–e33. doi: 10.1161/STROKEAHA.109.566869. [DOI] [PubMed] [Google Scholar]
- 46.Kidwell CS, Jahan R, Gorbein J, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. New Engl J Med. 2013;368:914–923. doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lansberg MG, Straka M, Kemp S, et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol. 2012;11:860–867. doi: 10.1016/S1474-4422(12)70203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lansberg MG, Christensen S, Kemp S, et al. Computed tomographic perfusion to predict response to recanalization in ischemic stroke. Ann Neurol. 2017;81:849–856. doi: 10.1002/ana.24953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turk AS, Magarick JA, Frei D, et al. CT perfusion-guided patient selection for endovascular recanalization in acute ischemic stroke: a multicenter study. J Neurointerv Surg. 2013;5:523–527. doi: 10.1136/neurintsurg-2012-010491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manning NW, Campbell BCV, Oxley TJ, Chapot R. Acute ischemic stroke. Stroke. 2014;45:640–644. doi: 10.1161/STROKEAHA.113.003798. [DOI] [PubMed] [Google Scholar]
- 51.Fanous AA, Siddiqui AH. Mechanical thrombectomy: Stent retrievers vs. aspiration catheters. Cor Vasa. 2016;58:e193–e203. doi: 10.1016/j.crvasa.2016.01.004. [DOI] [Google Scholar]
- 52.Albers GW, Goyal M, Jahan R, et al. Relationships between imaging assessments and outcomes in solitaire with the intention for thrombectomy as primary endovascular treatment for acute ischemic stroke. Stroke. 2015;46:2786–2794. doi: 10.1161/STROKEAHA.115.010710. [DOI] [PubMed] [Google Scholar]
- 53.Bivard A, Levi C, Krishnamurty V, et al. Perfusion computed tomography to assist decision making for stroke thrombolysis. Brain. 2015;138:1919–1931. doi: 10.1093/brain/awv071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanak D, Nosal V, Horak D, et al. Impact of diffusion-weighted MRI-measured initial cerebral infarction volume on clinical outcome in acute stroke patients with middle cerebral artery occlusion treated by thrombolysis. Neuroradiology. 2006;48:632–639. doi: 10.1007/s00234-006-0105-0. [DOI] [PubMed] [Google Scholar]
- 55.Sim K, Yan B, Dowling R, Bush S, Mitchell P. Endovascular clot retrieval in acute stroke with large ischemic core is not always associated with poor outcomes. Intern Med J. 2019;49:490–494. doi: 10.1111/imj.14116. [DOI] [PubMed] [Google Scholar]
- 56.Chen Z, Zhang R, Zhou Y, et al. Patients with ischemic core ≥70 ml within 6 h of symptom onset may still benefit from endovascular treatment. Front Neurol. 2018;9:933. doi: 10.3389/fneur.2018.00933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Borst J, Berkhemer OA, Roos YB, et al. Value of computed tomographic perfusion-based patient selection for intra-arterial acute ischemic stroke treatment. Stroke. 2015;46:3375–3382. doi: 10.1161/STROKEAHA.115.010564. [DOI] [PubMed] [Google Scholar]
- 58.Rebello LC, Bouslama M, Haussen DC, et al. Endovascular treatment for patients with acute stroke who have a large ischemic core and large mismatch imaging profile. JAMA Neurol. 2017;74:34–40. doi: 10.1001/jamaneurol.2016.3954. [DOI] [PubMed] [Google Scholar]
- 59.Campbell BCV, Majoie CBLM, Albers GW, et al. Penumbral imaging and functional outcome in patients with anterior circulation ischemic stroke treated with endovascular thrombectomy versus medical therapy: a meta-analysis of individual patient-level data. Lancet Neurol. 2019;18:46–55. doi: 10.1016/S1474-4422(18)30314-4. [DOI] [PubMed] [Google Scholar]
- 60.Broocks G, Hanning U, Flottman F, et al. Clinical benefit of thrombectomy in stroke patients with low ASPECTS is mediated by edema reduction. Brain. 2019;142:1399–1407. doi: 10.1093/brain/awz057. [DOI] [PubMed] [Google Scholar]
- 61.Lin L, Bivard A, Parsons MW. Perfusion patterns of ischemic stroke on computed tomography perfusion. J Stroke. 2013;15:164–173. doi: 10.5853/jos.2013.15.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu G, Michel P, Aghaebrahim A, et al. Prediction of recanalization trumps prediction of tissue fate. Stroke. 2013;44:1014–1019. doi: 10.1161/STROKEAHA.111.000229. [DOI] [PubMed] [Google Scholar]
- 63.Chen C, Parsons MW, Clapham M, et al. Influence of penumbral reperfusion on clinical outcome depends on baseline ischemic core volume. Stroke. 2017;48:2739–2745. doi: 10.1161/STROKEAHA.117.018587. [DOI] [PubMed] [Google Scholar]
- 64.Rao V, Chirstensen S, Yennu A, et al. Ischemic core and hypoperfusion volumes correlate with infarct size 24 hours after randomization in DEFUSE 3. Stroke. 2019;50:626–631. doi: 10.1161/STROKEAHA.118.023177. [DOI] [PubMed] [Google Scholar]
- 65.Parsons MW, Pepper M, Chen V, et al. Perfusion computed tomography: prediction of final infarct extent and stroke outcome. Ann Neurol. 2005;58:672–679. doi: 10.1002/ana.20638. [DOI] [PubMed] [Google Scholar]
- 66.Aviv RI, Maldelcorn J, Chakraborty S, et al. Alberta Stroke Program Early CT Scoring of CT perfusion in early stroke visualization and assessment. AJNR Am J Neuroradiol. 2007;28:1975–1980. doi: 10.3174/ajnr.A0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Psychogios MN, Schramm P, Frölich AM, et al. Alberta Stroke Program Early CT Scale evaluation of multimodal computed tomography in predicting clinical outcomes of stroke patients treated with aspiration thrombectomy. Stroke. 2013;44:2188–2193. doi: 10.1161/STROKEAHA.113.001068. [DOI] [PubMed] [Google Scholar]
- 68.Padroni M, Bernardoni A, Tamborino C, et al. Cerebral blood volume ASPECTS is the best predictor of clinical outcome in acute ischemic stroke: a retrospective, combined semi-quantitative and quantitative assessment. PloS one. 2016;11:e0147910–e0147910. doi: 10.1371/journal.pone.0147910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sillanpaa N, Saarinan JT, Rusanen H, et al. CT perfusion ASPECTS in the evaluation of acute ischemic stroke: thrombolytic therapy perspective. Cerebrovasc Dis Extra. 2011. Jan, pp. 6–16. [DOI] [PMC free article] [PubMed]
- 70.Tang B, Zeng J, Liu L, et al. Evaluating the prognosis of ischemic stroke using low-dose multimodal computed tomography parameters in hyperacute phase. J Comput Assist Tomogr. 2019;43:22–28. doi: 10.1097/RCT.0000000000000783. [DOI] [PubMed] [Google Scholar]
- 71.Wufuer A, Wubuli A, Mijiti P, et al. Impact of collateral circulation status on favorable outcomes in thrombolysis treatment: A systematic review and meta-analysis. Exp Ther Med. 2018;15:707–718. doi: 10.3892/etm.2017.5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arenillas JF, Cortijo E, Garcia-Bermejo P, et al. Relative cerebral blood volume is associated with collateral status and infarct growth in stroke patients in SWIFT PRIME. J Cereb Blood Flow Metab. 2018;38:1839–1847. doi: 10.1177/0271678X17740293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cortijo E, Calleja AI, Garcia-Bermejo P, et al. Relative cerebral blood volume as a marker of durable tissue-at-risk viability in hyperacute ischemic stroke. Stroke. 2014;45:113–118. doi: 10.1161/STROKEAHA.113.003340. [DOI] [PubMed] [Google Scholar]
- 74.Lin K, Kazmi KS, Law M, et al. Measuring elevated microvascular permeability and predicting hemorrhagic transformation in acute ischemic stroke using first-pass dynamic perfusion CT imaging. AJNR Am J Neuroradiol. 2007;28:1292. doi: 10.3174/ajnr.A0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aviv RI, d’Esterre CD, Murphy BD, et al. Hemorrhagic transformation of ischemic stroke: prediction with CT perfusion. Radiology. 2009;250:867–877. doi: 10.1148/radiol.2503080257. [DOI] [PubMed] [Google Scholar]
- 76.Ozkul-Wermester O, Guegan-Massardier E, Triquenot A, et al. Increased blood-brain barrier permeability on perfusion computed tomography predicts hemorrhagic transformation in acute ischemic stroke. Eur Neurol. 2014;72:45–53. doi: 10.1159/000358297. [DOI] [PubMed] [Google Scholar]
- 77.Puig J, Blasco G, Daunis-I-Estadella P, van Eendendburg C, et al. High-permeability region size on perfusion CT predicts hemorrhagic transformation after intravenous thrombolysis in stroke. PLoS One. 2017;12:e0188238. doi: 10.1371/journal.pone.0188238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hom J, Dankbaar JW, Soares BP, et al. Blood-brain barrier permeability assessed by perfusion CT predicts symptomatic hemorrhagic transformation and malignant edema in acute ischemic stroke. AJNR Am J Neuroradiol. 2011;32:41–48. doi: 10.3174/ajnr.A2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yen P, Cobb A, Shankar JJS. Does computed tomography permeability predict hemorrhagic transformation after ischemic stroke? World J Radiol. 2016;8:594–599. doi: 10.4329/wjr.v8.i6.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Horsch AD, Bennink E, van Seeters T, et al. Computed tomography perfusion derived blood-brain barrier permeability does not yet improve prediction of hemorrhagic transformation. Cerebrovasc Dis. 2018;45:26–32. doi: 10.1159/000485043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yassi N, Parsons MW, Christensen S, et al. Prediction of poststroke hemorrhagic transformation using computed tomography perfusion. Stroke. 2013;44:3039–3043. doi: 10.1161/STROKEAHA.113.002396. [DOI] [PubMed] [Google Scholar]
- 82.Murphy BD, Fox AJ, Lee DH, et al. Identification of penumbra and infarct in acute ischemic stroke using computed tomography perfusion–derived blood flow and blood volume measurements. Stroke. 2006;37:1771–1777. doi: 10.1161/01.STR.0000227243.96808.53. [DOI] [PubMed] [Google Scholar]
- 83.Heit JJ, Wintermark M. Perfusion computed tomography for the evaluation of acute ischemic stroke. Stroke. 2016;47:1153–1158. doi: 10.1161/STROKEAHA.116.011873. [DOI] [PubMed] [Google Scholar]
- 84.Lui YW, Tang ER, Allmendinger AM, Spektor V. Evaluation of CT perfusion in the setting of cerebral ischemia: patterns and pitfalls. AJNR Am J Neuroradiol. 2010;31:1552. doi: 10.3174/ajnr.A2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bivard A, Kleinig T, Miteff F, et al. Ischemic core thresholds change with time to reperfusion: A case control study. Ann Neurol. 2017;82:995–1003. doi: 10.1002/ana.25109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Najm M, Al-Ajlan FS, Boesen ME, et al. Defining CT perfusion thresholds for infarction in the golden hour and with ultra-early reperfusion. Can J Neurol Sci. 2018;45:339–342. doi: 10.1017/cjn.2017.287. [DOI] [PubMed] [Google Scholar]
- 87.Masterson K, Vargas MI, Delavelle J. Postictal deficit mimicking stroke: Role of perfusion CT. J Neuroradiol. 2009;36:48–51. doi: 10.1016/j.neurad.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 88.Shang WJ, Chen HB, Shu LM, et al. The association between FLAIR vascular hyperintensity and stroke outcome varies with time from onset. AJNR Am J Neuroradiol. 2019;40:1317. doi: 10.3174/ajnr.A6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Walberer M, Backes H, Rueger MA, et al. Potential of early [(18)F]-2-fluoro-2-deoxy-D-glucose positron emission tomography for identifying hypoperfusion and predicting fate of tissue in a rat embolic stroke model. Stroke. 2012;43:193–198. doi: 10.1161/STROKEAHA.111.624551. [DOI] [PubMed] [Google Scholar]
- 90.Sobrado M, Delgado M, Fernandez-Valle E, et al. Longitudinal studies of ischemic penumbra by using 18F-FDG PET and MRI techniques in permanent and transient focal cerebral ischemia in rats. Neuroimage. 2011;57:45–54. doi: 10.1016/j.neuroimage.2011.04.045. [DOI] [PubMed] [Google Scholar]
- 91.Zazulia AR, Videen TO, Powers WJ. Transient focal increase in perihematomal glucose metabolism after acute human intracerebral hemorrhage. Stroke. 2009;40:1638–1643. doi: 10.1161/STROKEAHA.108.536037. [DOI] [PubMed] [Google Scholar]
- 92.Lin W, Powers WJ. Oxygen metabolism in acute ischemic stroke. J Cereb Blood Flow Metab. 2018;38:1481–1499. doi: 10.1177/0271678X17722095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dundar A, Bold MS, Agac B, Kendi AT, Friedman SN. Stroke detection with 3 different PET tracers. Radiol Case Rep. 2019;14:1447–1451. doi: 10.1016/j.radcr.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Molyneux AJ, Kerr RSC, Yu LM, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366:809–817. doi: 10.1016/S0140-6736(05)67214-5. [DOI] [PubMed] [Google Scholar]
- 95.Sarkar S, Ghosh S, Ghosh SK, Collier A. Role of transcranial Doppler ultrasonography in stroke. Postgrad Med J. 2007;83:683–689. doi: 10.1136/pgmj.2007.058602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Toni D, Fiorelli M, Zanette EM, et al. Early spontaneous improvement and deterioration of ischemic stroke patients. A serial study with transcranial Doppler ultrasonography. Stroke. 1998;29:1144–1148. doi: 10.1161/01.STR.29.6.1144. [DOI] [PubMed] [Google Scholar]
- 97.Alexandrov AV, Black SE, Ehrlich LE, Caldwell CB, Norris JW. Predictors of hemorrhagic transformation occurring spontaneously and on anticoagulants in patients with acute ischemic stroke. Stroke. 1997;28:1198–1202. doi: 10.1161/01.STR.28.6.1198. [DOI] [PubMed] [Google Scholar]
- 98.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. New Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 99.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. New Engl J Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 100.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. New Engl J Med. 2015;372:2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 101.van der Hoeven EJ, Dankbaar JW, Algra A, et al. Additional diagnostic value of computed tomography perfusion for detection of acute ischemic stroke in the posterior circulation. Stroke. 2015;46:1113–1115. doi: 10.1161/STROKEAHA.115.008718. [DOI] [PubMed] [Google Scholar]
- 102.Sporns P, Schmidt R, Minnerup J, et al. Computed tomography perfusion improves diagnostic accuracy in acute posterior circulation stroke. Cerebrovasc Dis. 2016;41:242–247. doi: 10.1159/000443618. [DOI] [PubMed] [Google Scholar]