Abstract
G protein-coupled receptors (GPCRs) have been shown to stimulate extracellular regulated kinases (ERKs) through a number of linear pathways that are initiated by Gq/11 or Gi proteins. We studied signaling to the ERK cascade by receptors that simultaneously activate both G protein subfamilies. In HEK293T cells, bradykinin B2 receptor (B2R)-induced stimulation of ERK2 and transcriptional activity of Elk1 are dependent on Gαq-mediated protein kinase C (PKC) and on Gαi-induced Ras activation, while they are independent of Gβγ subunits, phosphatidylinositol 3-kinase, and tyrosine kinases. Similar results were obtained with m1 and m3 muscarinic receptors in HEK293T cells and with the B2R in human and mouse fibroblasts, indicating a general mechanism in signaling toward the ERK cascade. Furthermore, the bradykinin-induced activation of ERK is strongly reduced in Gαq/11-deficient fibroblasts. In addition, we found that constitutively active mutants of Gαq/11 or Gαi proteins alone poorly stimulate ERK2, whereas a combination of both led to synergistic effects. We conclude that dually coupled GPCRs require a cooperation of Gαi- and Gq/11-mediated pathways for efficient stimulation of the ERK cascade. Cooperative signaling by multiple G proteins thus might represent a novel concept implicated in the regulation of cellular responses by GPCRs.
The family of G-protein-coupled receptors (GPCRs) is the largest and most complex group of integral membrane proteins involved in signal transduction. These receptors can be activated by a diverse array of external stimuli, including growth factors, vasoactive peptides, chemoattractants, neurotransmitters, hormones, phospholipids, photons, odorants, and taste ligands. Following ligand binding they promote the GDP-GTP exchange of heterotrimeric G proteins. In turn, GTP-bound α subunits and released βγ complexes initiate a broad range of intracellular signaling events, including the activation of classical effectors such as adenylyl cyclases, phosphodiesterases, and phospholipases and the regulation of the activity of ion channels, ion transporters, and several kinases (22, 23, 41, 59). Recently, it has become increasingly apparent that, like receptor tyrosine kinases, GPCRs and G proteins are also involved in the regulation of cell growth and differentiation. A number of human proliferative diseases have been linked to mutations of GPCRs or G proteins (5, 15, 16). Furthermore, overexpression of constitutively active GPCRs or G proteins, as well as prolonged agonist stimulation of GPCRs, can induce cellular transformation in cultured fibroblasts (2, 15, 25).
The question of how GPCRs control signals that regulate gene expression in the nucleus, even though intensively studied during the last years, is not yet fully answered. It has been shown that GPCRs can activate mitogen-activated kinase (MAPK) pathways, which is sufficient and necessary for the control of proliferation in different cellular systems (26, 39). Mechanisms by which GPCRs activate MAPK cascades appear to be different. Gαi-coupled receptors preferentially utilize a Gβγ-dependent route via phosphatidylinositol (PI) 3-kinase γ, Src, and Ras (12, 37). In contrast, Gαq/11-coupled receptors employ protein kinase C (PKC) to directly target Raf-1 (33, 50) or calcium to activate the MAPK module via Pyk2, Src, and Ras (17, 34). Furthermore, in certain cells transactivation of epidermal growth factor (EGF) or platelet-derived growth factor receptors has been shown to be essential for extracellular-regulated-kinase (ERK) activation by Gαi- as well as by Gαq/11-coupled receptors (13, 30). The vast majority of the currently described pathways leading to MAPK stimulation have been considered as linear, initiated either by Gαq/11 or Gβγ protein subunits (26, 57). However, most GPCRs can couple to several G proteins within a single cell (22, 23). For example m1 and m3 muscarinic receptors, α2-adrenergic receptors, and receptors for thrombin and lysophosphatidic acid have been shown to stimulate Gi and Gq/11 proteins even though efficacies could differ among cell types (22, 23, 59). It is unknown at present whether one pathway initiated by a distinct G protein subfamily dominates over the other(s) or whether these receptors signal via parallel routes that might converge at a certain point. Coupling to multiple G proteins has rarely been considered with respect to MAPK activation, even though it should have an important impact on specific cellular responses elicited by GPCRs.
In order to address the question whether multiple G proteins are involved in ERK activation, we have analyzed signaling pathways linking the bradykinin B2 receptor (B2R) to the ERK/MAPK cascade. Even though often described as a prototypical Gαq/11-coupled receptor, the B2R can also catalyze the GDP-GTP exchange of Gαi/o, Gαs, and Gα12/13 proteins (20, 27, 28, 35, 36). Activation of the ERK/MAPK cascade by bradykinin has been reported to occur via PKC and/or calcium-dependent pathways, involving the protein tyrosine kinases Pyk2 and Src or the EGF receptor (1, 17, 58, 62). In this study we demonstrate that a cooperative action of Gαq/11 and Gαi is required for efficient signal transmission to the ERK/MAPK cascade by the B2R and other dually coupled GPCRs.
MATERIALS AND METHODS
Antibodies and reagents.
An antiserum against the C terminus of βARK1/2 was provided by R. J. Lefkowitz (Durham, N.C.), and the antiserum against Csk was a gift from S. Courtneidge (Sugen, Inc.). A polyclonal antiserum against ERK2 and the hemagglutinin (HA) tag were provided by L. Rönnstrand (Uppsala, Sweden), and the antiserum against Raf-1 was from U. Rapp (Würzburg, Germany). Polyclonal antibodies against ERK2 (C-14), Gαi proteins (C-10), Gαq/11 proteins (C-19), and Src (N-16) were from Santa Cruz. The monoclonal pan-Ras antibody (R02120) was purchased from Transduction, and the phospho-specific antibodies against MEK (9121/9122) were from New England BioLabs. [3H]Palmitate and Rainbow protein marker were from Amersham, bradykinin was from Bachem; aprotinin (Trasylol©) was from Bayer, AG-X8 anion-exchanger resin was from Bio-Rad; leupeptin was from Boehringer Mannheim; AG1478, GF109203X, Genistein, Gö6976, Gö6983, LY294002, PD98059, and PP1 were from Calbiochem; Pefabloc was from Fluka; Lipofectamine and protein ladder markers (10 to 200 kDa) were from Life Technologies; the luciferase assay system kit was from Promega; ATP, carbachol, glutathione agarose, myelin basic protein (MBP), and all tissue culture reagents were from Sigma; thin-layer chromatography (TLC) plates LK5 D were from Whatman; and protein A agarose was from Zymed.
Plasmids.
The following constructs were used: βARK-ct in pRK5 (from R. J. Lefkowitz); CD8-βARK1 in pcDNA3 (from S. Gutkind); human B2R in pcDNA3 (from A. Pizard); Csk in pSV and kinase-inactive Src in pSGT (from S. Courtneidge); pFA-Elk1 and pFR-Luc reporter plasmids of the PathDetect Reporting System (from Stratagene); HA-ERK2 in pRK5 (from C. J. Marshall); Gαi1Q204C, Gαi2Q205L, and Gαi3Q204L in pCDNA1 from (M. Faure); GαqQ209L and Gα11Q209L in pCIS (from M. Simon); human m1 muscarinic receptor in pCD-PS (from C. van Koppen); human m3 muscarinic receptor in pcDNA3 (from A. Tobin); kinase-inactive Raf-1 and Raf1-RBD in pCMV5 (from W. Kolch); GST–Raf-1(1-149)–RBD in pGEX2 (from S. Taylor); pTKCIII and β-galactosidase in pCMV5 (from J. Ericsson); and dominant-negative Ras (N17-Ras) in pZipNeo (from M. Karin).
Cell culture and transfections.
Human embryonic kidney cells HEK293T were grown to about 50 to 70% confluence in Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS). Transfections were done in serum-free medium with the indicated cDNAs using 0.2 to 0.4 μg/well of a 24-well plate, 0.4 to 1 μg/well of a 12-well plate, 1 to 2 μg/well of a 6-well plate, or 10 μg/10-cm dish by the Lipofectamine method according to the supplier's manual. About 24 h after transfection cells were serum starved in DMEM containing 0.1% (wt/vol) bovine serum albumin (BSA) for another 24 h and then used for experiments. HF-15 human foreskin fibroblasts (provided by W. Müller-Esterl) were grown in DMEM containing 10% FBS. Mouse embryonic fibroblasts were kept in the same medium supplemented with 1 mM sodium pyruvate and nonessential amino acids. These cells were transfected using a modified calcium phosphate method according to the supplier's manual (MBS; Stratagene) with 1.2 to 1.6 μg cDNA/well of a 12-well plate.
PLC and PLD assays.
Phospholipase C (PLC) activity was measured by analyzing cellular inositol phosphate accumulation (11). Cells grown on 24-well plates were labeled with 1 μCi of myo-[3H]inositol per ml for 24 h in inositol-free Ham's F-12 including 0.1% (wt/vol) BSA, treated for 10 min with 10 mM LiCl, and then challenged with 1 μM bradykinin for 10 min. Reactions were stopped by addition of 1 ml of ice-cold 10 mM formic acid. Water-soluble inositols were extracted for 2 to 12 h at 4°C and separated by anion-exchange chromatography using AG-X8 as a resin. Finally, isolated inositol phosphates were quantified by liquid scintillation counting.
PLD activity was measured as described elsewhere (10). Briefly, cells grown on 12-well plates were labeled with 5 μCi of [3H]palmitate per ml for 24 h in DMEM–1% FBS. Cells were preincubated with 30 mM butanol for 5 min prior to stimulation with 1 μM bradykinin for 10 min. Reactions were terminated, and lipids were extracted and separated by TLC. The [3H]phosphatidylbutanol band was located by iodine staining, scraped and quantified by liquid scintillation counting.
ERK in vitro kinase reactions.
ERK activity was analyzed as previously described (4). Briefly, serum-starved cells on six-well plates were stimulated with 1 μM bradykinin at 37°C, followed by lysis in 0.5 ml of ice-cold buffer containing 50 mM HEPES (pH 7.2), 150 mM NaCl, 1 mM EDTA, 20 mM NaF, 2 mM sodium orthovanadate, 1% (wt/vol) Triton X-100, 10% (wt/vol) glycerol, and protease inhibitors (1 mM Pefabloc, 10 μg of leupeptin per ml, and 1% Trasylol). Equal amounts of soluble fractions were subjected to immunoprecipitation with 5 μl of a polyclonal antiserum against the HA tag or ERK2 and 35 μl of protein A-agarose slurry for 3 h at 4°C. Resultant precipitates were washed three times with lysis buffer, followed by two washes with kinase buffer (10 mM Tris, pH 7.5; 10 mM MgCl2). Kinase reactions were started by the addition of 30 μl of kinase buffer including 200 μM ATP, 1 μCi of [γ-32P]ATP, and 10 μg of MBP. After 20 min at room temperature, reactions were stopped by the addition of 20 μl of sodium dodecyl sulfate (SDS) sample buffer and boiling for 2 min. Proteins were separated by SDS–12% polyacrylamide gel electrophoresis (PAGE), and gels were cut at the 30-kDa marker band. Upper parts were transferred onto nitrocellulose membranes and probed with 0.5 μg of anti-ERK2 antibodies per ml in 5% BSA in TBS (50 mM Tris, pH 7.6; 150 mM NaCl) to check for equal immunoprecipitation of HA-ERK2. Lower parts were stained with Coomassie brilliant blue R250 to monitor the amounts of MBP. Substrate phosphorylation was analysed using a phosphorimager (Fuji BAS2000). When dominant-negative constructs were used, Western blotting of total cell lysates with respective antibodies was performed to prove their expression as well as the equal HA-ERK2 levels. For statistic analysis, Student t tests for independent samples were performed using the QuickTTest program from S. Ashcroft.
Ras pulldown.
The activation status of Ras was assayed using the glutathione S-transferase (GST) fusion protein of the Ras-binding domain (RBD) of Raf-1 (positions 1 to 149) which has a high affinity for GTP-loaded, active Ras (53). Prior to the experiment, baits were prepared by incubating GST–Raf-1–RBD-containing bacterial lysates with glutathione agarose for 1 h at 4°C. Beads were washed three times with lysis buffer (20 mM Tris, pH 7.5; 100 mM NaCl; 10 mM NaF; 10 mM MgCl2; 1 mM sodium orthovanadate; 1% [wt/vol] Triton X-100; 10% [wt/vol] glycerol; and protease inhibitors [see above]) and stored on ice. Starved and bradykinin-treated cells were lysed in 500 μl of lysis buffer, and soluble fractions (1 to 2 mg of protein) were incubated with RBD baits for 1 h at 4°C. After three washing steps (lysis buffer with 0.5% Triton X-100), 30 μl of SDS sample buffer was added and samples were boiled for 2 min. Precipitated Ras was analyzed by SDS–15% PAGE, followed by Western blotting using 1 μg of a monoclonal pan-Ras antibody per ml.
Elk1 reporter gene assay.
Luciferase assays were done using pFA-Elk1 and pFR-Luc reporter plasmids of the PathDetect Reporting System (Stratagene) and the Luciferase Assay System kit (Promega). HEK293T cells grown on 24-well plates to 50 to 70% confluence were transfected using the Lipofectamine method, with the following amounts of cDNA per well: 100 ng of B2R, 50 ng of β-galactosidase (pCMV5-βGal), 25 ng of luciferase (pFR-Luc), and 25 ng Elk1 (pFA-Elk1). Mouse embryonic fibroblasts grown on 12-well plates were transfected using a modified calcium phosphate method (MBS; Stratagene) with the following amounts of cDNA per well: 250 ng of B2R, 500 ng of pCMV5-βGal, 125 ng of pFR-Luc, 60 ng of pFA-Elk1, and 565 ng of pTKCIII. After 4 to 6 h of incubation, transfection medium was replaced with regular culture medium, and cells were allowed to regenerate for 12 h. Thereafter, cells were serum starved for 24 h, stimulated with 1 μM bradykinin for 12 h, and lysed in 100 μl of 1% Triton X-100–10% glycerol–25 mM Tris phosphate (pH 7.8)–2 mM dithiothreitol–2 mM CDTA per well. Luciferase activity in cleared lysates was measured using the Luciferase Assay System from Promega and a Wallac 1420 multilabel counter. To determine transfection efficiency, parts of the lysates were analyzed for β-galactosidase activity.
RESULTS
The B2R-mediated ERK2 and Elk1 activation in HEK293T cells involves pertussis toxin (Ptx)-sensitive G proteins and PKC.
For biochemical studies on the mechanisms of GPCR-mediated ERK activation, we transiently coexpressed the B2R with HA-tagged ERK2 and dominant-interfering constructs in HEK293T cells. B2R expression was confirmed by [3H]bradykinin binding assays, demonstrating a single population of binding sites with an apparent Kd of 2 nM, a value which is similar to values obtained with cells with endogenous receptors (28, 48). Functional coupling to PLC was observed with a 50% effective concentration value for bradykinin of about 5 nM, which is close to the affinity of the B2R for this agonist (data not shown). Furthermore, we have demonstrated in former studies that the B2R is phosphorylated and sequestrated upon bradykinin treatment in HEK293T cells and that it is linked to the ERK/MAPK cascade (4, 47). Thus, the transiently expressed B2R in HEK293T cells is functionally coupled to major signaling pathways and is pharmacologically similar to endogenous receptors on native cells.
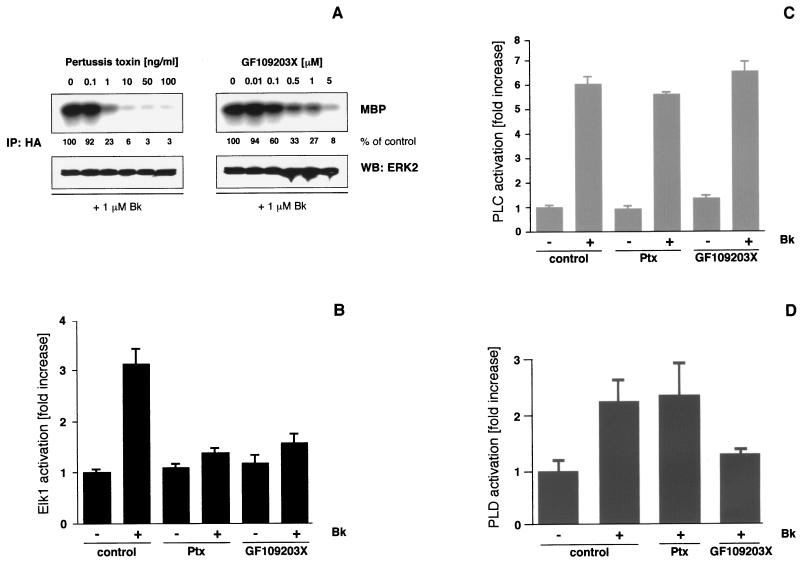
The B2R has been reported to couple to multiple G proteins such as Gαq/11, Gαi/o, Gαs, and Gα12/13 (20, 27, 28, 35, 36). With respect to ERK/MAPK signaling, Gαq/11 and Gi activation appear to be most relevant. To analyze the contribution of these particular G proteins, we applied different concentrations of Ptx to inactivate Gi/o or the specific PKC inhibitor GF109203X to block the major downstream effector of Gαq/11. Both treatments interfered in a dose-dependent manner with ERK2 activation in HEK293T cells that had been cotransfected with the B2R and HA-ERK2 and challenged with 1 μM bradykinin for 5 min (Fig. 1A). Whereas the inhibition by Ptx was almost complete, displaying a 50% inhibitory concentration of approximately 0.5 ng/ml, GF109203X led to a 75 to 95% reduction of ERK2 activity with a half-maximal effect observed with a <0.1 μM concentration of the inhibitor. A similar inhibition of bradykinin-induced ERK2 activity was observed after downregulation of PKC by prolonged phorbol ester treatment of cells (cf. Fig. 3C). Identical manipulations did not affect the corresponding EGF-induced ERK2 activation, thus demonstrating the absence of any nonspecific effects associated with the inhibitors (not shown).
FIG. 1.
Effects of Ptx and PKC inhibition on the B2R-mediated signal transduction in HEK293T cells. (A) HEK293T cells grown on six-well plates were transiently transfected with B2R (1 μg/well) and HA-ERK2 (0.5 μg/well) using the Lipofectamine method (6 μl/well). After 24 h of serum starvation and 16 h of Ptx or 30 min of GF109203X pretreatment with the indicated concentrations, 1 μM bradykinin (Bk) was applied for 5 min. Cells were lysed, and ERK2 activity was determined after immunoprecipitation (IP) with a polyclonal anti-HA antibody using MBP as a substrate. Western blots (WB) with polyclonal ERK2 antibodies (0.5 μg/ml) were performed to confirm that equal amounts of ERK2 were present in the precipitates, and phosphorylated MBP was detected by autoradiography and quantified by phosphorimager analysis (Fuji BAS2000). MBP phosphorylation is expressed as a percentage of the signal obtained in the particular experiment in the absence of any inhibitor. In general, the background of basal ERK activity was about 2 to 10% of the signal seen with agonist stimulation. Representative autoradiograms and blots from three experiments with identical results are shown. (B) To analyze Elk1 activation, a reporter gene assay was used. Luciferase activities in cells stimulated with 1 μM bradykinin for 12 h were measured and compared with those obtained after pretreatment with Ptx (50 ng/ml, 16 h) or GF109203X (5 μM, 30 min) prior to agonist challenge. Means ± the standard deviation (SD) of three independent experiments performed in triplicates are shown. (C and D) In B2R-transfected HEK293T cells grown on 12-well plates, the increase of total inositol phosphates (PLC activation) and the production of phosphatidic acid, trapped as [3H]phosphatidylbutanol (PLD activation), were measured without or with 10 min of 1 μM bradykinin stimulation. Results were compared with those obtained under identical conditions but with inhibitor pretreatment prior to bradykinin challenge. Means ± the SD of triplicates from typical experiments are shown.
FIG. 3.
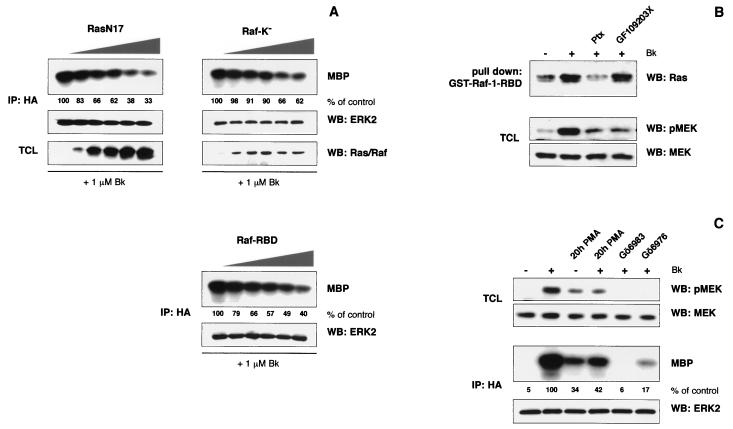
The bradykinin-induced ERK2 activation is Ras and Raf dependent. (A) HEK293T cells grown on six-well plates were cotransfected with B2R (0.25 μg/well), HA-tagged ERK2 (0.1 μg/well), and increasing amounts of dominant-interfering Ras and Raf constructs (0, 0.25, 0.5, 0.75, 1, and 1.25 μg of RasN17, Raf-K−, or Raf-RBD per well). The total amount of cDNA was adjusted to 1.6 μg/well with respective empty expression vectors. After serum starvation, cells were challenged with 1 μM bradykinin for 5 min and ERK2 activity was analyzed by in vitro kinase reactions after immunoprecipitation. Levels of HA-ERK2 and expression of dominant-interfering constructs were controlled by Western blotting, with the respective antibodies diluted 1:400 to 1:2,000 (0.2 to 1 μg/ml) in 5% BSA-TBS. We could not prove Raf-RBD expression since the protein does not cross-react with our Raf antibodies. (B) B2R-transfected HEK293T cells grown on 10-cm plates were starved for 24 h and stimulated for 1 min with 1 μM bradykinin. After lysis, Ras activation was analyzed by GST–Raf-1–RBD pulldown experiments using 1 to 2 mg of cell lysate and ca. 5 μg of GST–Raf-1–RBD bound to glutathione agarose. Bound Ras was eluted and analyzed by SDS–15% PAGE and Western blotting using a monoclonal Ras antibody (1 μg/ml). After antibody detection, the use of equal amounts of bait was checked by staining membranes with amido black. To indirectly monitor Raf activity, MEK phosphorylation was analyzed in total cell lysates using a phospho-specific antibody (pMEK; 0.2 μg/ml). To prove equal MEK levels, the membranes were stripped and blots were reprobed with a MEK antibody (MEK; 0.2 μg/ml). Experiments were repeated at least three times, and representative autoradiograms and blots are shown. (C) MEK phosphorylation and ERK2 in vitro kinase activity were measured after pretreatment of B2R- or B2R-HA-ERK2-expressing cells with 0.2 μM PMA for 20 h and 5 μM Gö6983 or 1 μM Gö6976 for 15 min. A representative experiment, including the control of MEK and HA-ERK2 levels, is shown.
It has been previously shown that MAPK activation leads to phosphorylation of transcription factors of the Elk family and to an increase in their transcriptional activity (54). We have studied long-term effects of bradykinin-induced ERK activation by monitoring Elk1 transcriptional activation using a luciferase reporter assay (4). Bradykinin stimulation of B2R-expressing HEK293T cells for 12 h led to a 3.1 ± 0.3-fold increase in Elk1-driven luciferase activity (Fig. 1B). Similar to ERK2, the activation of Elk1 transcriptional activity was largely abolished by Ptx and by GF109203X pretreatment. These data suggest that Gαi/o and PKC signal downstream of the B2R and that both are necessary to link the receptor to ERK2 and Elk1 activation.
Full activation of PKC requires, depending on the respective isoform, either diacylglycerol and Ca2+ (conventional PKCs), diacylglycerol alone (novel PKCs), or other, not yet completely identified factors (atypical PKCs) (42, 43). Cellular diacylglycerol and Ca2+ are elevated as a consequence of Gαq/11- or Gβγ-mediated PLCβ activation with Gαi-type G proteins regarded as a major source of βγ subunits (7, 43). To distinguish whether the bradykinin-induced PLC stimulation in transiently transfected HEK293T cells involves Gαq/11 and/or Gβγ of Gi, we measured inositol phosphate production in untreated and Ptx-pretreated cells. Incubation of B2R-expressing HEK293T cells with bradykinin led to a 6.1 ± 0.3-fold increase in cellular inositol phosphate levels (Fig. 1C). Pretreatment of cells with 100 ng of Ptx per ml for 16 h slightly decreased B2R-mediated inositol phosphate production by about 10%, indicating a minor contribution of Gi protein βγ subunits to PLC stimulation. The inhibition of PKC by GF109203X application failed to affect PLC activity (Fig. 1C). Bradykinin stimulation also led to a substantial PLD activation in HEK293T cells that was completely insensitive to Ptx, while considerably reduced by the PKC inhibitor GF109203X (Fig. 1D). We observed identical signaling profiles with bradykinin concentrations ranging from 1 nM to 10 μM and B2R expression levels of 50 to 2,000 fmol of B2R/mg of protein, indicating that dual Gi-Gq/11 coupling is independent of receptor expression and agonist concentration (not shown). Altogether, these results show that PKC is not a relevant downstream effector of Gi proteins in HEK293T cells. We therefore conclude that Ptx and the PKC inhibitor GF109203X target two independent pathways, which are simultaneously activated through the B2R and are both necessary for efficient bradykinin-induced ERK activation in HEK293T cells.
The B2R-mediated ERK2 activation in HEK293T cells is independent of tyrosine kinases and Gβγ signaling.
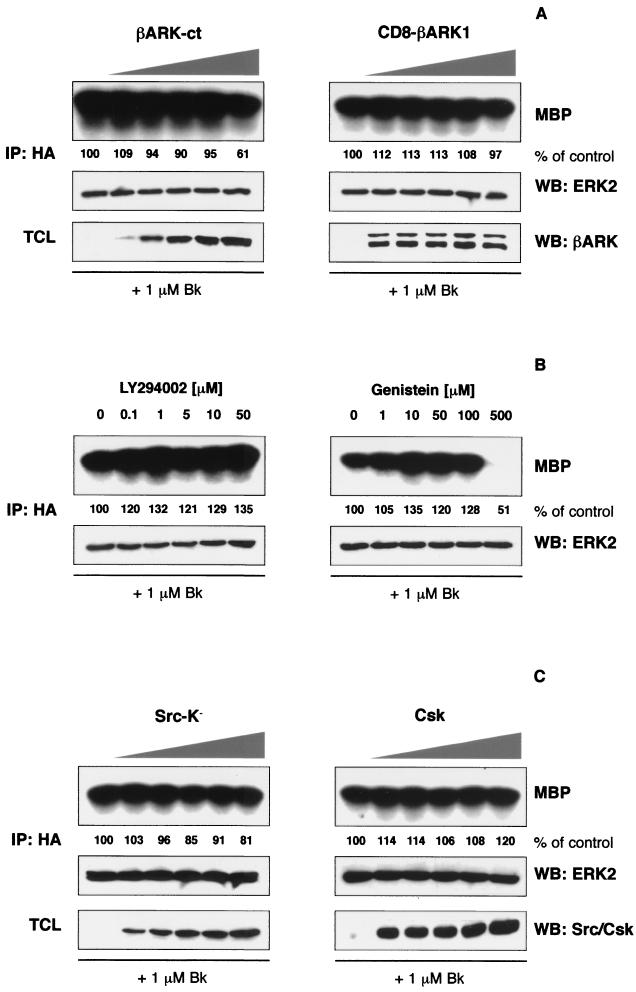
The inhibitory effect of Ptx on the B2R-mediated ERK2 and Elk1 activation points to an involvement of Gi proteins. It is well established that mitogenic signaling of Gi is primarily mediated by βγ subunits, which can associate with and activate PI 3-kinase γ (12, 37). Subsequently, Src-family tyrosine kinases become involved, phosphorylating the adapter protein Shc that recruits Grb2-Sos complexes to the membrane, enhancing the GDP-GTP exchange of Ras, and finally linking to the ERK/MAPK cascade (18, 26). To elucidate the role of Gβγ subunits in B2R signaling, we coexpressed increasing amounts of two βγ sequestrating constructs, a minigene coding for the C terminus of β-adrenergic receptor kinase 1 (βARK1), together with constant amounts of B2R and HA-ERK2. In vitro kinase reactions after bradykinin challenge revealed a very small inhibitory effect of these constructs on ERK2, which was only observed with the highest expression levels (Fig. 2A). Cellular levels of the dominant-interfering proteins and HA-tagged ERK2 were monitored by Western blotting with respective antibodies. Since PI 3-kinase β is involved in B2R signaling in human colon carcinoma cells (21) and to further exclude an involvement of Gβγ pathways, we blocked PI 3-kinase activity that is downstream of Gβγ subunits (37). Application of a concentration of up to 50 μM of the potent and specific PI 3-kinase inhibitor LY294002 did not decrease bradykinin-induced ERK2 activation (Fig. 2B), thus excluding a role of PI 3-kinase in bradykinin signaling to ERK/MAPK in HEK293T cells. Therefore, we considered it unlikely that Gβγ proteins or PI 3-kinase play a major role in the B2R-mediated ERK activation, as was convincingly demonstrated for other GPCRs such as the LPA and the m2 muscarinic receptor (26, 37).
FIG. 2.
The B2R-mediated ERK2 activation in HEK293T cells is independent of Gβγ, PI 3-kinase and tyrosine kinases. HEK293T cells grown on six-well plates were cotransfected with B2R (0.25 μg/well), HA-tagged ERK2 (0.1 μg/well), and increasing amounts of dominant-interfering constructs (0, 0.25, 0.5, 0.75, 1, and 1.25 μg of βARK-ct, CD8-βARK1, Src-K−, or Csk per well). The total amount of cDNA was adjusted to 1.6 μg/well with respective empty expression vectors. After 24 h of serum starvation cells were stimulated with 1 μM bradykinin for 5 min and the ERK2 activity was analyzed by in vitro kinase reactions using MBP as a substrate. Levels of precipitated HA-ERK2 and expression of dominant-interfering constructs in total cell lysates (TCL) were controlled by Western blotting, with the respective antibodies diluted 1:400 to 1:2,000 (0.2 to 1 μg/ml) in 5% BSA-TBS. For inhibitor experiments, cells were pretreated with the indicated concentrations of LY294002 and genistein 30 min before bradykinin stimulation. Assays were repeated at least three times, and representative autoradiograms and blots are shown.
Tyrosine kinases have a central function in MAPK activation by several GPCRs. In particular, signaling by Ptx-sensitive G proteins is dependent on the activation of tyrosine kinases (18, 26). Therefore, we initially analyzed bradykinin-mediated ERK2 activation after pretreatment of cells with increasing concentrations of the broad-spectrum tyrosine kinase inhibitor genistein. As shown in Fig. 2B, doses of genistein that should inhibit tyrosine kinases (10 to 100 μM) did not affect the B2R-induced ERK activation. The reduction in ERK2 activity observed with a 500 μM concentration of genistein is most likely due to toxic effects or inhibition of other kinases, such as PKC. In addition, the specific EGF receptor tyrosine kinase inhibitor AG1478 applied at concentrations of up to 10 μM was completely ineffective, even though abolishing EGF receptor autophosphorylation and ERK activation induced by EGF (not shown). We also investigated the function of Src-like kinases, which are necessary intermediates between GPCRs and the MAPK cascade in several cellular systems (18, 26). Coexpression of increasing amounts of a kinase-inactive Src mutant (Src-K−), as well as Csk, which decreases the activity of Src-family kinases by an inhibitory phosphorylation, did not affect bradykinin-induced ERK2 activation (Fig. 2C). Both proteins are expressed, as shown by Western blotting with respective antibodies, and both constructs have been proven to interfere with Src functions in HEK293T cells (5, 17). In agreement with these findings, the Src-specific inhibitor PP1 applied in concentrations of up to 10 μM had not any effect on the bradykinin-induced ERK2 activation (not shown). We conclude that signal transduction from the B2R to the ERK/MAPK cascade involves Ptx-sensitive G proteins but is independent of Gβγ subunits, PI 3-kinase activity and tyrosine kinase functions.
Ras and Raf are intermediates in the B2R-induced ERK2 activation in HEK293T cells.
The small G protein Ras is a common intermediate in GPCR-initiated mitogenic signaling. In particular, the link between Ptx-sensitive G proteins and ERK/MAPK was shown to be Ras dependent in several cell types. Gαq/11-PKC can target the ERK/MAPK cascade via Ras as well, but also a direct activation of Raf-1 and MEK has been observed (17, 26, 33, 38, 50). To investigate the role of Ras and Raf in B2R signaling, we coexpressed increasing amounts of dominant-negative RasN17 and two inhibitory Raf-1 constructs, a kinase-inactive mutant (Raf-K−) and the Ras-binding domain of Raf (Raf-RBD), together with the B2R and HA-tagged ERK2. All three constructs significantly decreased the bradykinin-mediated ERK2 activation in correlation with their expression levels (Fig. 3A). Thus, the B2R utilizes Ras and its effectors, such as Raf, to transmit signals to the ERK/MAPK cascade in HEK293T cells.
Collectively, our data obtained with chemical inhibitors and coexpression studies using dominant-interfering constructs indicate that coupling of the B2R to both Gαq/11 and Gαi proteins is necessary to transmit signals to the ERK/MAPK module. To define at which point signals from both G proteins converge, we analyzed the contribution of Gαq/11 and Gαi to the Ras and Raf activation. The activity of Ras was measured using a recently developed assay that is based on the high affinity of the isolated Ras-binding domain of Raf-1 for GTP-bound Ras (53). In B2R-expressing HEK293T cells, we consistently observed a weak Ras activation upon bradykinin treatment (Fig. 3B). Ptx application clearly decreased the amount of activated Ras, whereas PKC inhibition by GF109203X was ineffective. Furthermore, in contrast to our findings in PC12 cells, activation of PKC by phorbol esters failed to induce GTP-binding to Ras in HEK293T cells (not shown), supporting a role for Gαi rather than Gαq in Ras activation.
Next, we analyzed the activity of Raf indirectly by monitoring MEK phosphorylation using phospho-specific antibodies. Bradykinin stimulation of B2R-expressing HEK293T cells substantially increased MEK phosphorylation (Fig. 3B). In contrast to the activation of Ras, the phosphorylation of MEK was almost completely blocked by either Ptx or GF109203X treatment. To further corroborate a role of PKC in the activation of MEK and ERK, we monitored MEK phosphorylation and ERK2 kinase activity after downregulation of PKC by prolonged phorbol ester treatment. Incubation of B2R-expressing HEK293T cells with a 0.2 μM concentration of the phorbol ester phorbol myristate acetate (PMA) for 20 h resulted in a considerable elevation of the basal MEK phosphorylation and ERK activity, which only slightly further increased upon bradykinin stimulation (Fig. 3C). In addition, two other different PKC inhibitors (Gö6983 at 5 μM and Gö6976 at 1 μM) largely abolished the B2R-mediated MEK phosphorylation and ERK activation. In summary, our data suggest that the B2R activates PKC via Gαq/11, which in turn directly acts on Raf or on the Ras-Raf interface. However, this does not seem to be sufficient to trigger the ERK/MAPK cascade, and an additional input from Gαi subunits is necessary for a substantial Ras and Raf stimulation.
Cooperation of G proteins leads to ERK2 activation in HEK293T cells.
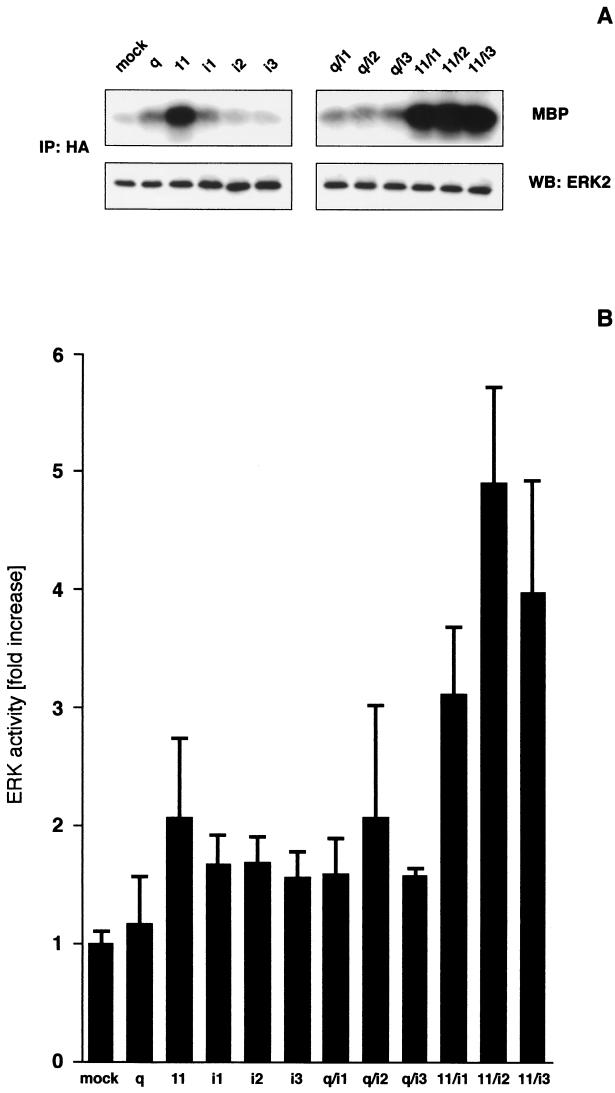
Our data obtained with the B2R in HEK293T cells suggest a cooperation of signals derived from Gαq/11 and Gαi proteins in ERK/MAPK activation. To verify this hypothesis we cotransfected HEK293T cells with constitutive active mutants of heterotrimeric G proteins Gαq, Gα11, Gαi1, Gαi2, and Gαi3, either alone or in combination with HA-ERK2. In every experiment HA-ERK2 and G protein expression were confirmed by Western blotting with the respective antibodies (Fig. 4A and data not shown). In Fig. 4A a representative experiment is shown, and Figure 4B displays a quantitative analysis of a range of independent experiments. Whereas a transfection with activated Gαq alone was ineffective, mutant Gα11 increased ERK2 activity 2.1 ± 0.6-fold, a result which is slightly above the levels of the Gαi variants (Fig. 4). Coexpression of Gαq with Gαi2 and Gα11 with Gαi1, Gαi2, or Gαi3 amplified the signal seen with either of the G proteins alone. In most experiments the Gα11-Gαi2 combination was more potent than all others, resulting in a 4.9 ± 0.8-fold increase in ERK2 activity. These receptor-independent experiments indicate that a cooperation of Gαq/11- and Gαi-derived signals could lead to a more efficient activation of ERK/MAPK in HEK293T cells.
FIG. 4.
Cooperation of activated G proteins leads to a stimulation of ERK2 in HEK293T cells. To study G protein cooperation HEK293T cells grown on six-well plates were transfected with 1.4 μg of constitutively activated Gα mutants and 0.2 μg of HA-ERK2 per well. After 24 h of serum starvation, ERK2 activity was measured by in vitro kinase reactions and compared with cells transfected with empty expression vector (mock). (A) A representative experiment, including the control of HA-ERK2 levels in the kinase reactions, is shown. (B) Quantitative evaluation of data (means ± the SD) from up to 17 independent experiments. The increased activation of ERK2 by Gα11, Gαi1, Gαi2, and Gαi3 and all G protein combinations was statistically significant as judged by Student t tests for independent samples (P < 0.001 to P < 0.05). The P values of all Gα11-Gαi combinations were significantly above those of the individual G proteins (P < 0.001 to P < 0.05), Gαq-Gαi1 and Gαq-Gαi3 (P < 0.001 to P < 0.01).
Gαq/11-Gαi cooperation is observed in different cell types with other GPCRs.
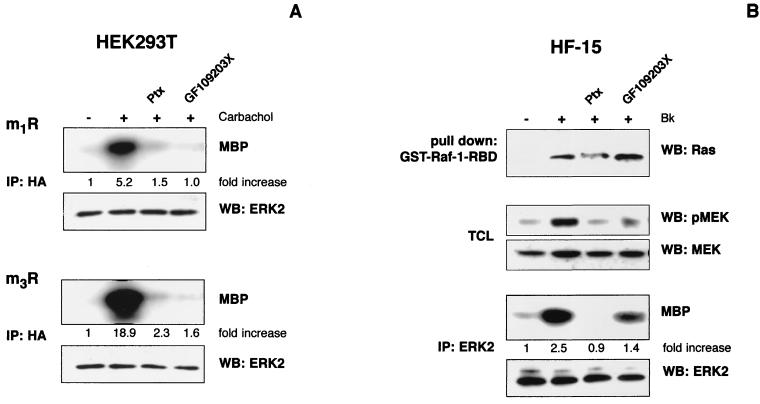
The preceding experiments with constitutively active G protein mutants suggest that the G protein cooperation observed for the bradykinin-induced ERK activation might have more general implications. Therefore, we tested additional Gi-Gq-coupled GPCRs, such as m1 and m3 muscarinic receptors, for their ability to activate ERK in HEK293T cells. We coexpressed these receptors with HA-ERK2 and challenged them with 1 mM carbachol after pretreatment with Ptx and GF109203X, respectively. Both inhibitors largely abolished the carbachol-induced ERK2 activation (Fig. 5A), suggesting a cooperative mechanism similar to that observed with the B2R (cf. Fig. 1 to 3).
FIG. 5.
Cooperative G protein signaling by muscarinic receptors in HEK293T cells and the endogenous B2R in human fibroblasts. (A) HEK293T cells were cotransfected with m1 or m3 muscarinic receptors (m1R and m3R) and HA-ERK2. After serum starvation, control and inhibitor-treated cells (16 h, 50 ng of Ptx per ml, and 30 min, 5 μM GF109203X) were stimulated with 1 mM carbachol, and ERK2 activity was analyzed by in vitro kinase reactions. HA-ERK2 levels were checked by Western blots, and kinase activities expressed as the fold increase of unstimulated cells of a representative experiment are shown. (B) Serum-starved HF-15 cells grown on 10-cm plates were pretreated with Ptx (16 h, 50 ng/ml) or GF109203X (30 min, 5 μM). B2R-induced Ras activation was analyzed by GST–Raf-1–RBD pulldown after challenge with 1 μM bradykinin for 1 min. Results were compared with those obtained without inhibitor application. In the same lysates, Raf activation was monitored by analyzing MEK phosphorylation with phospho-specific antibodies. In addition, ERK activity after 5 min of 1 μM bradykinin stimulation was measured by in vitro kinase reactions. A representative result from three independent experiments, including quantitative analysis of the increase of kinase activity compared to unstimulated cells, is shown.
We next analyzed GPCRs in physiologically more relevant cellular environments. Human foreskin fibroblasts (HF-15) with endogenously expressed B2R have been used as a model to study bradykinin-induced signaling (3). Stimulation with 1 μM bradykinin for 5 min led to a 2.5-fold increase in ERK activation, as measured by in vitro kinase reactions after immunoprecipitation with specific antibodies against ERK2 (Fig. 5B). Furthermore, we observed a bradykinin-induced Ras-GTP loading and MEK phosphorylation. Both Ptx and GF109203X substantially decreased the B2R-mediated ERK activity and MEK phosphorylation, while Ras activation was only affected by Ptx application (Fig. 5B). Thus, the endogenous B2R in HF-15 cells utilizes pathways to activate the Ras/MAPK cascade that are very similar to those of the exogenous receptor in HEK293T cells (cf. Fig. 1A and Fig. 3B). Collectively, these data confirm that dual coupling and cooperation of Gi and Gq/11 proteins might represent a general mechanism in signal transduction by the B2R and possibly other GPCRs that are also able to activate both G protein families, such as m1 and m3 muscarinic receptors.
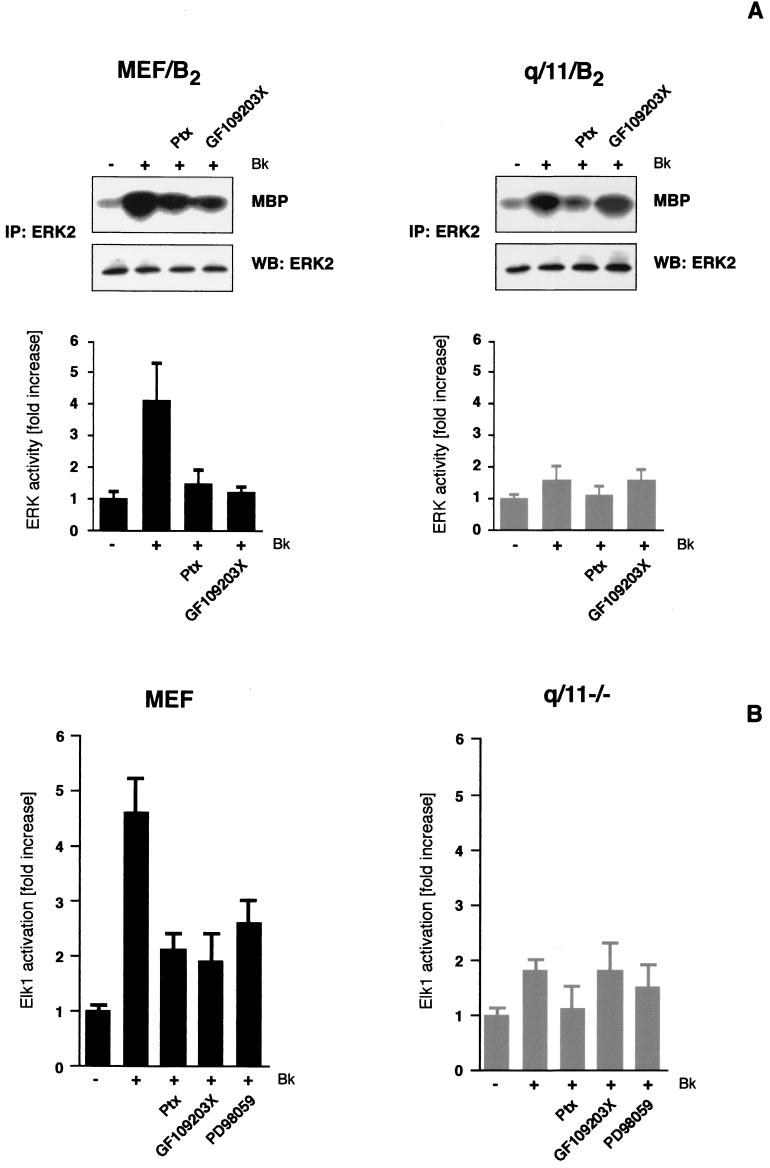
The availability of fibroblasts from Gαq/11-deficient mice enabled us to study the issue of G protein cooperation in a Gαq/11-free background (45). Since mouse embryonic fibroblasts, like many other cells, lose B2R expression during culturing conditions, we established several B2R-expressing clones of wild-type (MEF/B2) and Gαq/11-deficient (q/11/B2) mouse fibroblasts. Similar levels of B2R expression (200 to 600 fmol of B2R/mg of protein) in wild-type and Gαq/11-deficient fibroblasts were confirmed by [3H]bradykinin binding assays, and functional coupling was analyzed by inositol phosphate measurements. As expected, bradykinin only induced a detectable inositol phosphate accumulation in MEF/B2 but not in q/11/B2 cells, confirming that βγ subunits of Gi proteins do not significantly contribute to PLC stimulation (not shown and reference 20). Analyzing ERK activity by in vitro kinase reactions, we observed a 4.5 ± 1.5-fold increase upon bradykinin treatment in three different MEF/B2 clones (Fig. 6A). Ptx and GF109203X significantly decreased ERK activation induced by B2R. When we analyzed B2R signaling in three different clones of Gαq/11-deficient cells, we observed only a faint ERK activity (1.6 ± 0.4-fold increase) that was completely blocked by Ptx but not affected by GF109203X. Similar results were obtained with endogenous receptors for bombesin and bradykinin in early-passage parental cells, whereas the thrombin-induced ERK activation was only blocked by Ptx (not shown). We also monitored the long-term effects of B2R stimulation by measuring Elk1 transcriptional activation in parental mouse embryonic fibroblasts and Gq/11-deficient cells (q/11−/−) cotransfected with the B2R and respective reporter constructs. As shown in Fig. 6B, bradykinin stimulation led to a 4.6 ± 0.6-fold increase in Elk1-driven luciferase activity in MEF cells. Both Ptx and GF109203X decreased the Elk1 activation to the same level as had the specific MEK inhibitor PD98059, indicating that for the MEK/ERK-dependent Elk1 transcriptional activity both Gαi and Gαq/11-PKC are necessary. The B2R-mediated Elk1 activation was much weaker in Gαq/11-deficient cells (1.8 ± 0.2-fold increase) than in mouse embryonic fibroblasts. Ptx pretreatment decreased the Elk1 activation slightly below the level of the MEK inhibition, whereas GF109203X was completely ineffective. Collectively, these results confirm that a cooperation of Gαi and Gαq/11 is necessary for a full ERK and Elk1 activation by dually coupled GPCRs.
FIG. 6.
B2R signaling in Gαq/11-deficient cells. (A) Stable B2R-expressing clones of wild-type mouse embryonic fibroblasts (MEF/B2) and Gαq/11-deficient (q/11/B2) cells (200 to 600 fmol of B2R/mg of protein) were analyzed for the Gi and PKC dependence of bradykinin-induced ERK activation by performing in vitro kinase reactions. Means ± the SD from 6 to 10 independent experiments are presented. Furthermore, a typical set of in vitro kinase reactions, including blots of precipitated ERK is shown. (B) To analyze Elk1 transcriptional activation in parental (MEF) and Gαq/11-deficient cells (q/11−/−), a reporter gene assay was used. Cells grown on 12-well plates were cotransfected with B2R and respective reporter constructs. Luciferase activities in cells stimulated with 1 μM bradykinin for 12 h were measured and compared with those obtained after pretreatment with Ptx (16 h, 50 ng/ml) or GF109203X (30 min, 5 μM) prior to agonist challenge. Means ± the SD of three independent experiments performed in triplicates are presented.
DISCUSSION
Many GPCRs can simultaneously couple to members of various G protein families. Furthermore, several cells express more than one subtype of a particular receptor, each potentially able to stimulate different G proteins (22, 59). Therefore, the activation of several G protein subtypes within a single cell at the same time reflects a physiological relevant situation. Mutual interactions between the signal transduction pathways initiated by different G protein subtypes are well established (22). One such example is the m3 muscarinic receptor for which a Gi-mediated potentiation of the Gq-dependent PLC activation has been described (49). The aim of our study was to investigate how dually Gi-Gq/11-coupled receptors activate the ERK/MAPK cascade, one of the major pathways that mediates mitogenic effects of GPCRs. The majority of the mechanisms of GPCR-induced ERK/MAPK activation has been considered as linear, initiated either by βγ subunits of Ptx-sensitive G proteins or by Gαq (26). By coexpression studies in HEK293T cells and using inhibitory compounds for key elements of signal transduction pathways, we have obtained evidence for a cooperation of Gαi and Gαq/11 proteins in ERK/MAPK activation by the B2R. Both, Ptx-sensitive G proteins and PKC were identified as being indispensable for a significant stimulation of ERK2 and transcriptional activity of Elk1. A similar situation has been reported for the α2-adrenergic receptor expressed in CHO cells, where PKC was found to be downstream of Ptx-sensitive Gαo proteins (56). However, we failed to detect Gαo expression in HEK293T cells, thus excluding such a pathway as an explanation for our findings (data not shown and reference 58). Della Rocca et al. have suggested PLCβ as a common intermediate in the signal transduction of Gq-coupled α1B-adrenergic and Gi-coupled α2A-adrenergic receptors toward ERK/MAPK in HEK293 cells (14). We observed only a marginal effect of Ptx on the B2R-induced inositol phosphate accumulation, indicating only a minor contribution of Gi to PLC activity which is unlikely to account for the strong inhibition of ERK by Ptx. In addition, PLD stimulation was not modified by Gαi inhibition, whereas it was efficiently blocked by GF109203X. Therefore, we consider Gi-mediated signals to be independent from those carried by the Gαq/PLC/PKC cascade induced through the B2R. However, both pathways are necessary for efficient activation of the ERK/MAPK cascade.
Dual regulation of the ERK/MAPK module has only been observed for a limited number of GPCRs. Chen et al. have revealed that Gi and Gq-PKC signals mediate ERK1/2 activation by the calcitonin receptor expressed in HEK293 cells. They found that both pathways contribute equally to ERK activation and suggest a convergence at the level of the adapter protein Shc (8). Coexpression of the human anaphylatoxin C5a receptor together with the Gq family member Gα16 has been shown to result in a PKC-dependent amplification of the otherwise-moderate and solely Gi-mediated ERK activation in HEK293 cells (6). Recently, the B2R exogenously expressed in COS-7 cells has been reported to activate ERK1 via parallel signals from PKC and transactivated EGF receptors (1). A common feature of these findings is the generation of two independent signals, which contribute in an additive manner to ERK/MAPK activation. In contrast, we have observed that inhibition of one signaling pathway activated by the B2R is sufficient to completely block ERK and Elk1 activation, suggesting a cooperative interaction. Similar to Adomeit et al., we propose the Ras-Raf complex as a point of convergence for the two signaling branches (1). Bradykinin caused a weak activation of Ras in HEK293T and HF-15 cells, which was blocked by Ptx but only slightly affected by PKC inhibitors. In contrast, MEK phosphorylation that is presumably catalyzed by Raf decreased after both treatments. It has previously been shown that in some cells Ras is involved in the PKC-mediated stimulation of Raf (38, 44). Marais et al. found that phorbol ester treatment of COS cells results in a Ras-dependent Raf activation (38). Surprisingly, RasN17, which is thought to inhibit guanine nucleotide exchange factors, did not affect Raf-1 functions under these conditions. Furthermore, Marais et al. showed that PKC inhibitors block the m1 muscarinic receptor-mediated Ras and Raf-1 stimulation. We failed to detect any Ras-GTP loading after phorbol ester treatment in HEK293T cells. Furthermore, Ptx but not GF109203X abolished the B2R-mediated Ras activation in HEK293T and HF-15 cells. Therefore, the Ras activation we measured most likely results from Gαi signaling and is independent of PKC. In addition, we have observed an inhibitory effect of RasN17 and of the Ras-binding domain of Raf-1, indicating a different mechanism in B2R-expressing HEK293T cells. We propose that two signals are required for efficient ERK activation by the B2R: a Gαi-mediated Ras stimulation and the action of PKC on the Ras-Raf complex. This is similar to the model recently suggested by Chiloeches et al., who analyzed several GPCRs in rat ventricular myocytes and proposed a Gi-mediated priming of Ras-Raf for activation by PKC (9). The exact mechanism of the signal integration at the Ras-Raf levels remains to be further investigated.
It is well established that βγ subunits of Ptx-sensitive G proteins play a major role in mediating signal transmission to Ras and MAPK cascades (12, 19, 55). We found that Gβγ-sequestrating constructs were largely ineffective in inhibiting ERK activation by the B2R in HEK293T cells, suggesting instead an important function of Gαi subunits. Furthermore, the Ptx-sensitive branch of B2R signaling to the MAPK cascade involves neither protein tyrosine kinases nor PI 3-kinase, which is in contrast to results found for the majority of typical Gi-coupled receptors (26, 37). Recently, the δ-opioid receptor expressed in Jurkat T cells has also been shown to utilize a Ptx-sensitive but Gβγ- and PI 3-kinase-independent pathway to activate ERK and mobilize AP-1 transcription factors (29). Currently, it is unclear which intermediates connect Gαi subunits with Ras/MAPK signaling, but the search for novel G protein interactions partners might give an answer. Recently, GTPase-activating proteins for Rap1 have been identified in yeast two-hybrid screenings as potential links between Gαi/o and the regulation of MAPK cascades (32, 40). Therefore, GTPase-activating proteins or guanine nucleotide exchange factors might be potential candidates that could establish a junction between heterotrimeric G proteins and small GTPases such as Ras and Rap.
By coexpressing mutationally activated Gα subunits and HA-ERK2, we studied G protein cross-talk in a receptor-independent approach. Contradicting data have been published as to whether or not G protein α subunits can increase ERK activity (12, 19, 24, 31). The specific cell type and experimental conditions seem to have an important impact on the results obtained. We found in HEK293T cells that activated Gαi subunits caused a rather weak ERK stimulation, whereas Gαq was almost ineffective. Surprisingly, Gα11, even though expressed at levels similar to those of other G proteins, was most potent in stimulating the ERK cascade. Considering the high degree of redundancy of Gq and G11 in signal transduction (23, 46), further studies are needed to judge whether the apparently stronger ERK activation by G11 reflects a property that distinguishes it from Gq in vivo. However, we cannot entirely exclude that slight differences in the expression levels of G proteins or an increased apoptosis induced by some constitutive active mutants (15) might also have an impact on their ability to activate ERK in these assays. In contrast to the rather weak effect of the different G protein subtypes when expressed alone, several combinations of Gαq and Gα11 with Gαi proteins led to a considerable overadditive ERK activation. In agreement with a former study performed in COS cells (12), coexpression of Gαq and Gαi2 had a rather moderate effect compared to the Gα11-Gαi2 combination, which caused a four- to five-fold increase in ERK activity. Perhaps the most important observation is that the synergistic effects, at least for the Gα11-Gαi2 combination, were more than additive, indicating cooperative rather than parallel mechanisms in signaling. The fact that ERK activation measured with this approach was much lower than that obtained with stimulation of dually coupled GPCRs might reflect cellular desensitization and downregulation in response to chronic stimulation by constitutively activated G protein α subunits.
Using fibroblasts from Gαq/11-deficient mice, we confirmed a requirement for dual G protein coupling in signaling by the B2R. In different stable B2R-expressing clones of control cells, bradykinin-induced ERK and Elk1 activation was sensitive to Ptx and GF109203X. In Gαq/11-deficient cells, signaling of the B2R to ERK and Elk1 was in general reduced and not affected by PKC inhibition, though still sensitive to Ptx treatment. Though Elk1 transcriptional activity was not quantitatively blocked by Ptx and GF109203X, the application of these substances led to a reduction to the same level that seen with the specific MEK inhibitor PD98059. These findings suggest a contribution of additional MAPK, such as the G12/13-activated c-Jun N-terminal kinase to Elk1 regulation in mouse fibroblasts. The fact that there was still a considerable B2R-mediated ERK stimulation detectable in Gαq/11 knockout cells might reflect the redundancy of intracellular signaling and adaptation processes occurring during the embryonic development of Gαq/11-deficient mice.
We have also provided evidence that dual Gαq/11-Gαi signaling is a more general mechanism implicated in GPCR signaling to ERK/MAPK cascades. ERK activities induced by m1 and m3 muscarinic receptors expressed in HEK293T and bombesin receptors in mouse embryonic fibroblasts were also sensitive to Ptx and GF109203X treatment. Identical observations have previously been reported for m1 and m3 receptors expressed in CHO cells (56, 60). Furthermore, we have observed a dual B2R signaling in human fibroblasts (HF-15) and in H-69 small cell lung cancer cells (unpublished results), for which bradykinin acts as a potent mitogen (51). Given the fact that several G protein family members are commonly expressed in the same cell, their cooperation might contribute to the careful control of strength and specificity in signal transduction. We provide here a detailed molecular characterization of the pathways involved in the quantitative regulation of the ERK/MAPK pathway through cooperation of Gαq/11 and Gαi signals. In a recent study, Stam et al. suggested a requirement for signals from small G proteins of the Rho family, which are presumably activated by Gα12/13 and Gαq/11 PLC pathways in regulating the invasion of T-lymphoma cells into monolayers of rat embryo fibroblasts (52). Therefore, cooperative signaling by multiple G proteins might represent a novel concept implicated in the regulation of different cellular responses induced by GPCRs.
ACKNOWLEDGMENTS
We thank all scientists who generously provided reagents used in this study. We acknowledge A. Pizard and R. M. Rajerison (INSERM U367, Paris, France) for the initial characterization of B2R expression in HEK293T cells.
A. Barac was supported by a scholarship from the Swedish Institute, M. J. Cross is supported by a Marie Curie TMR fellowship, and A. Blaukat is a recipient of a postdoctoral fellowship from the Deutsche Forschungsgemeinschaft. I. Dikic is a research fellow of the Boehringer Ingelheim Fonds.
REFERENCES
- 1.Adomeit A, Graness A, Gross S, Seedorf K, Wetzker R, Liebmann C. Bradykinin B2 receptor-mediated mitogen-activated protein kinase activation in COS-7 cells requires dual signaling via both protein kinase C pathway and epidermal growth factor receptor transactivation. Mol Cell Biol. 1999;19:5289–5297. doi: 10.1128/mcb.19.8.5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen L F, Lefkowitz R J, Caron M G, Cotecchia S. G protein-coupled receptor genes as proto-oncogenes: constitutively activating mutation of the α1B-adrenergic receptor enhances mitogenesis and tumorigenicity. Proc Natl Acad Sci USA. 1991;88:11354–11358. doi: 10.1073/pnas.88.24.11354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaukat A, AbdAlla S, Lohse M J, Müller-Esterl W. Ligand-induced phosphorylation/dephosphorylation of the endogenous bradykinin B2 receptor from human fibroblasts. J Biol Chem. 1996;271:32366–32374. doi: 10.1074/jbc.271.50.32366. [DOI] [PubMed] [Google Scholar]
- 4.Blaukat A, Pizard A, Rajerisson R M, Alhenc-Gelas F, Müller-Esterl W, Dikic I. Activation of mitogen-activated protein kinase by the bradykinin B2 receptor is independent of receptor phosphorylation and phosphorylation-triggered internalization. FEBS Lett. 1999;451:337–341. doi: 10.1016/s0014-5793(99)00613-4. [DOI] [PubMed] [Google Scholar]
- 5.Blaukat A, Ivankovic-Dikic I, Grönroos E, Dolfi F, Tokiwa G, Vuori C, Dikic I. Adaptor proteins Grb2 and Crk couple Pyk2 with activation of specific mitogen-activated protein kinase cascades. J Biol Chem. 1999;274:14893–14901. doi: 10.1074/jbc.274.21.14893. [DOI] [PubMed] [Google Scholar]
- 6.Buhl A M, Osawa S, Johnson G L. Mitogen-activated protein kinase activation requires two signal inputs from the human anaphylatoxin C5a receptor. J Biol Chem. 1995;270:19828–19832. doi: 10.1074/jbc.270.34.19828. [DOI] [PubMed] [Google Scholar]
- 7.Camps M, Carozzi A, Schnabel P, Scheer A, Parker P J, Gierschik P. Isozyme-selective stimulation of phospholipase Cβ 2 by G protein βγ-subunits. Nature. 1992;360:684–686. doi: 10.1038/360684a0. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Shyu J-F, Santhanagopal A, Inoue D, David J-P, Dixon S J, Horne W C, Baron R. The calcitonin receptor stimulates Shc tyrosine phosphorylation and Erk1/2 activation. J Biol Chem. 1998;273:19809–19816. doi: 10.1074/jbc.273.31.19809. [DOI] [PubMed] [Google Scholar]
- 9.Chiloeches A, Paterson H F, Marais R, Clerk A, Marshall C J, Sugden P H. Regulation of Ras-GTP loading and Ras-Raf association in neonatal rat ventricular myocytes by G protein-coupled receptor agonists and phorbol ester. J Biol Chem. 1999;274:19762–19770. doi: 10.1074/jbc.274.28.19762. [DOI] [PubMed] [Google Scholar]
- 10.Cook S J, Briscoe C P, Wakelam M J O. The regulation of phospholipase D activity and its role in sn-1,2-diacylglycerol formation in bombesin- and phorbol 12-myristate 13-acetate-stimulated Swiss 3T3 cells. Biochem J. 1991;280:431–438. doi: 10.1042/bj2800431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cramer H, Müller-Esterl W, Schroeder C. Subtype-specific desensitization of human endothelin ETA and ETB receptors reflects differential receptor phosphorylation. Biochemistry. 1997;36:13325–13332. doi: 10.1021/bi9708848. [DOI] [PubMed] [Google Scholar]
- 12.Crespo P, Xu N, Simons W F, Gutkind S. Ras-dependent activation of MAP kinase pathway mediated by G-protein βγ subunits. Nature. 1994;369:418–420. doi: 10.1038/369418a0. [DOI] [PubMed] [Google Scholar]
- 13.Daub H, Weiss F U, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G protein-coupled receptors. Nature. 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 14.Della Rocca G J, van Biesen T, Daaka Y, Luttrell D K, Luttrell L M, Lefkowitz R J. Ras-dependent mitogen-activated protein kinase activation by G protein-coupled receptors. J Biol Chem. 1997;272:19125–19132. doi: 10.1074/jbc.272.31.19125. [DOI] [PubMed] [Google Scholar]
- 15.Dhanasekaran N, Tsim S T, Dermott J M, Onesime D. Regulation of cell proliferation by G proteins. Oncogene. 1998;17:1383–1394. doi: 10.1038/sj.onc.1202242. [DOI] [PubMed] [Google Scholar]
- 16.Dhanasekaran N, Heasley L E, Johnson G L. G protein-coupled receptor systems involved in cell growth and oncogenesis. Endocr Rev. 1995;16:259–270. doi: 10.1210/edrv-16-3-259. [DOI] [PubMed] [Google Scholar]
- 17.Dikic I, Tokiwa G, Lev S, Courtneidge S A, Schlessinger J. A role for Pyk2 and Src in linking G protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 18.Dikic I, Blaukat A. Protein tyrosine kinase-mediated pathways in G protein-coupled receptor signaling. Cell Biochem Biophys. 1999;30:369–387. doi: 10.1007/BF02738120. [DOI] [PubMed] [Google Scholar]
- 19.Faure M, Voyno-Yasenetskaya T A, Bourne H R. cAMP and βγ subunits of heterotrimeric G proteins stimulate the mitogen-activated protein kinase pathway in COS-7 cells. J Biol Chem. 1994;269:7851–7854. [PubMed] [Google Scholar]
- 20.Gohla A, Offermanns S, Wilkie T M, Schultz G. Differential involvement of Gα12 and Gα13 in receptor-mediated stress fiber formation. J Biol Chem. 1999;274:17901–17907. doi: 10.1074/jbc.274.25.17901. [DOI] [PubMed] [Google Scholar]
- 21.Graness A, Adomeit A, Heinze R, Wetzker R, Liebmann C. A novel mitogenic signaling pathway of bradykinin in the human colon carcinoma cell line SW-480 involves sequential activation of a Gq/11 protein, phosphatidylinositol 3-kinase β, and protein kinase Cɛ. J Biol Chem. 1998;273:32016–32022. doi: 10.1074/jbc.273.48.32016. [DOI] [PubMed] [Google Scholar]
- 22.Gudermann T, Kalkbrenner F, Schultz G. Diversity and selectivity of receptor-G-protein interaction. Annu Rev Pharmacol Toxicol. 1996;36:429–459. doi: 10.1146/annurev.pa.36.040196.002241. [DOI] [PubMed] [Google Scholar]
- 23.Gudermann T, Schöneberg T, Schultz G. Functional and structural complexity of signal transduction via G-protein-coupled receptors. Annu Rev Neurosci. 1997;20:399–427. doi: 10.1146/annurev.neuro.20.1.399. [DOI] [PubMed] [Google Scholar]
- 24.Gupta S K, Gallego C, Johnson G L, Heasley L E. MAP kinase is constitutively activated in gip2 and src transformed rat 1a fibroblasts. J Biol Chem. 1992;267:7987–7990. [PubMed] [Google Scholar]
- 25.Gutkind J S, Novotny E A, Brann M R, Robbins K C. Muscarinic acetylcholine receptor subtypes as agonist-dependent oncogenes. Proc Natl Acad Sci USA. 1991;88:4703–4707. doi: 10.1073/pnas.88.11.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gutkind J S. The pathways connecting G protein-coupled receptors to the nucleus through divergent mitogen-activated protein kinase cascades. J Biol Chem. 1998;273:1839–1842. doi: 10.1074/jbc.273.4.1839. [DOI] [PubMed] [Google Scholar]
- 27.Gutowski S, Smrcka A, Nowak L, Wu D G, Simon M, Sternweis P C. Antibodies to the αq subfamily of guanine nucleotide-binding regulatory protein α subunits attenuate activation of phosphatidylinositol 4,5-bisphosphate hydrolysis by hormones. J Biol Chem. 1991;266:20519–20524. [PubMed] [Google Scholar]
- 28.Hall J M. Bradykinin receptors: pharamcological responses and biological roles. Pharmacol Ther. 1993;56:131–190. doi: 10.1016/0163-7258(92)90016-s. [DOI] [PubMed] [Google Scholar]
- 29.Hedin K E, Bell M P, Huntoon C J, Karnitz L M, McKean D J. Gi proteins use a novel βγ- and Ras-independent pathway to activate extracellular signal-regulated kinase and mobilize AP-1 transcription factors in Jurkat T lymphocytes. J Biol Chem. 1999;274:19992–2001. doi: 10.1074/jbc.274.28.19992. [DOI] [PubMed] [Google Scholar]
- 30.Herrlich A, Daub A, Knebel A, Ullrich A, Schultz G, Guderman T. Ligand-independent activation of platelet-derived growth factor receptor is a necessary intermediate in lysophosphatidic-acid stimulated mitogenic activity in L cells. Proc Natl Acad Sci USA. 1998;95:8985–8990. doi: 10.1073/pnas.95.15.8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ito A, Satoh T, Kaziro Y, Itoh H. G protein βγ subunits activate Ras, Raf, and MAP kinase in HEK293 cells. FEBS Lett. 1995;368:183–187. doi: 10.1016/0014-5793(95)00643-n. [DOI] [PubMed] [Google Scholar]
- 32.Jordan J D, Carey K D, Storck P J S, Iyengar R. Modulation of Rap activity by direct interaction of Gα0 with Rap1 GTPase-activating protein. J Biol Chem. 1999;274:21507–21510. doi: 10.1074/jbc.274.31.21507. [DOI] [PubMed] [Google Scholar]
- 33.Kolch W, Heidecker G, Kochs G, Hummel R, Vahadi H, Mischak H, Finkenzeller G, Marme D, Rapp U R. Protein kinase Cα activates Raf-1 by direct phosphorylation. Nature. 1993;364:249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- 34.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio J M, Plowman G D, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca2+-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 35.Liebmann C, Offermanns S, Spicher K, Hinsch K D, Schnittler M, Morgat J L, Reissmann S, Schultz G, Rosenthal W. A high-affinity bradykinin receptor in membranes from rat myometrium is coupled to Ptx-sensitive G-proteins of the Gi family. Biochem Biophys Res Commun. 1990;167:910–917. doi: 10.1016/0006-291x(90)90610-y. [DOI] [PubMed] [Google Scholar]
- 36.Liebmann C, Graness A, Ludwig B, Adomeit A, Boehmer A, Boehmer F D, Nürnberg B, Wetzker R. Dual bradykinin B2 receptor signalling in A431 human epidermoid carcinoma cells: activation of protein kinase C is counteracted by a GS-mediated stimulation of the cyclic AMP pathway. Biochem J. 1996;313:109–118. doi: 10.1042/bj3130109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez-Ilasaca M, Crespo P, Pellici P G, Gutkind J S, Wetzker R. Linkage of G protein-coupled receptors to the MAPK signaling pathway through PI 3-kinase γ. Science. 1997;275:394–397. doi: 10.1126/science.275.5298.394. [DOI] [PubMed] [Google Scholar]
- 38.Marais R, Light Y, Mason C, Paterson H, Olson M F, Marshall C J. Requirement of Ras-GTP-Raf complexes for activation of Raf-1 by protein kinase C. Science. 1998;280:109–112. doi: 10.1126/science.280.5360.109. [DOI] [PubMed] [Google Scholar]
- 39.Marshall C J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 40.Mochizuki N, Ohba Y, Kiyokawa E, Kurata T, Murakami T, Ozaki T, Kitabatake A, Nagashima K, Matsuda M. Activation of the ERK/MAPK pathway by an isoform of rap1GAP associated with Gα. Nature. 1999;400:891–894. doi: 10.1038/23738. [DOI] [PubMed] [Google Scholar]
- 41.Neer E J. Heterotrimeric G proteins: organizers of transmembrane signals. Cell. 1995;80:249–257. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- 42.Newton A C. Protein kinase C: structure, function, and regulation. J Biol Chem. 1995;270:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- 43.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 44.Nori M, L'Allemain G, Weber M J. Regulation of tetradecanoyl phorbol acetate-induced responses in NIH 3T3 cells by GAP, the GTPase-activating protein associated with p21c-ras. Mol Cell Biol. 1992;12:936–945. doi: 10.1128/mcb.12.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Offermanns S, Zhao L P, Sarosi I, Simon M I, Wilkie T M. Embryonic cardiomyocyte hypoplasia and craniofacial defects in Gαq/Gα11-mutant mice. EMBO J. 1998;17:4304–4312. doi: 10.1093/emboj/17.15.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Offermanns S, Simon M I. Genetic analysis of mammalian G-protein signaling. Oncogene. 1998;17:1375–1381. doi: 10.1038/sj.onc.1202173. [DOI] [PubMed] [Google Scholar]
- 47.Pizard A, Blaukat A, Müller-Esterl W, Alhenc-Gelas F, Rajerison R M. Bradykinin-induced internalization of the human B2 receptor requires phosphorylation of three serine and two threonine residues at its carboxyl tail. J Biol Chem. 1999;274:12738–12747. doi: 10.1074/jbc.274.18.12738. [DOI] [PubMed] [Google Scholar]
- 48.Regoli D, Jukic D, Gobeil F, Rhaleb N E. Receptors for bradykinin and related kinins: a critical analysis. Can J Physiol Pharmacol. 1993;71:556–567. doi: 10.1139/y93-079. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt M, Lohman B, Hammer K, Haupenthal S, Voss M, Nehls C, Jakobs K H. Gi- and protein kinase C-mediated heterologous potentiation of phospholipase C signaling by G protein-coupled receptors. Mol Pharmacol. 1998;53:1139–1148. [PubMed] [Google Scholar]
- 50.Schönwasser D C, Marais R M, Marshall C J, Parker P J. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol. 1998;18:790–798. doi: 10.1128/mcb.18.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sethi T, Rozengurt E. Multiple neuropeptides stimulate clonal growth of small cell lung cancer: effects of bradykinin, vasopressin, cholecystokinin, galanin, and neurotensin. Cancer Res. 1991;51:3621–3623. [PubMed] [Google Scholar]
- 52.Stam J C, Michiels F, van der Kammen R A, Moolenaar W H, Collard J G. Invasion of T-lymphoma cells: cooperation between Rho family GTPases and lysophosphatidic receptor signaling. EMBO J. 1998;17:4066–4074. doi: 10.1093/emboj/17.14.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taylor S J, Shalloway D. Cell cycle-dependent activation of Ras. Curr Biol. 1996;6:1621–1627. doi: 10.1016/s0960-9822(02)70785-9. [DOI] [PubMed] [Google Scholar]
- 54.Treisman R. Regulation of transcription by MAP kinase cascades. Curr Opin Cell Biol. 1996;8:205–215. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- 55.van Biesen T, Hawes B E, Luttrell D K, Krueger K M, Touhara K, Porfiri E, Sakaue M, Luttrell L M, Lefkowitz R J. Receptor-tyrosine-kinase- and Gβγ-mediated MAP kinase activation by a common signalling pathway. Nature. 1995;376:781–784. doi: 10.1038/376781a0. [DOI] [PubMed] [Google Scholar]
- 56.van Biesen T, Hawes B E, Raymond J R, Luttrell L M, Koch W J, Lefkowitz R J. G0-protein α-subunits activate mitogen-activated protein kinase via a novel protein kinase C-dependent mechanism. J Biol Chem. 1996;271:1266–1269. doi: 10.1074/jbc.271.3.1266. [DOI] [PubMed] [Google Scholar]
- 57.van Biesen T, Luttrell L M, Hawes B E, Lefkowitz R J. Mitogenic signaling via G protein-coupled receptors. Endocr Rev. 1996;17:698–714. doi: 10.1210/edrv-17-6-698. [DOI] [PubMed] [Google Scholar]
- 58.Velarde V, Ullian M E, Morinelli T A, Mayfield R K, Jaffa A A. Mechanisms of MAPK activation by bradykinin in vascular smooth muscle cells. Am J Physiol. 1999;277:C253–C261. doi: 10.1152/ajpcell.1999.277.2.C253. [DOI] [PubMed] [Google Scholar]
- 59.Wess J. G-protein-coupled receptors: molecular mechanisms involved in receptor activation and selectivity of G-protein recognition. FASEB J. 1997;11:346–354. [PubMed] [Google Scholar]
- 60.Wylie P G, Challiss J, Blank J L. Regulation of extracellular-signal regulated kinase and c-Jun N-terminal kinase by G-protein-linked muscarinic acetylcholine receptors. Biochem J. 1999;338:619–628. [PMC free article] [PubMed] [Google Scholar]
- 61.Yamauchi J, Kaziro Y, Itoh H. Different regulation of mitogen-activated protein kinase kinase 4 (MKK4) by signaling from G protein βγ subunits in human embryonal kidney 293 cells. J Biol Chem. 1999;274:1957–1965. doi: 10.1074/jbc.274.4.1957. [DOI] [PubMed] [Google Scholar]
- 62.Zwick E, Daub H, Aoki N, Yamaguchi-Aoki Y, Tinhofer I, Maly K, Ullrich A. Critical role of calcium-dependent epidermal growth factor receptor transactivation in PC12 cell membrane depolarization and bradykinin signaling. J Biol Chem. 1997;272:24767–24770. doi: 10.1074/jbc.272.40.24767. [DOI] [PubMed] [Google Scholar]