Abstract
Background:
With significant improvements in the diagnosis and treatment of HIV, the number of people with HIV in the United States steadily increases. Monitoring trends in HIV-related care outcomes is needed to inform programs aimed at reducing new HIV infections in the United States.
Setting:
The setting is 33 United States jurisdictions that had mandatory and complete reporting of all levels of CD4 and viral load test results for each year during 2014–2018.
Methods:
Estimated annual percentage change and 95% confidence intervals were calculated to assess trends in stage of disease at time of diagnosis, linkage to HIV medical care within 1 month of HIV diagnosis, and viral suppression within 6 months after HIV diagnosis. Differences in percentages were analyzed by sex, age, race/ethnicity, and transmission category for persons with HIV diagnosed from 2014 to 2018.
Results:
Among 133,477 persons with HIV diagnosed during 2014–2018, the percentage of persons who received a diagnosis classified as stage 0 increased 13.7%, stages 1–2 (early infections) increased 2.9%, stage 3 (AIDS) declined 1.5%, linkage to HIV medical care within 1 month of HIV diagnosis increased 2.3%, and viral suppression within 6 months after HIV diagnosis increased 6.5% per year, on average. Subpopulations and areas that showed the least progress were persons aged 45–54 years, American Indian/Alaska Native persons, Asian persons, Native Hawaiian/other Pacific Islander persons, and rural areas with substantial HIV prevalence, respectively.
Conclusions:
New infections will continue to occur unless improvements are made in implementing the Ending the HIV Epidemic: A Plan for America strategies of diagnosing, treating, and preventing HIV infection.
Keywords: HIV, ending the HIV epidemic, EHE, care continuum, stage of disease, linkage to care, viral suppression, rural
INTRODUCTION
As the diagnosis and treatment of HIV continues to improve, the number of people with HIV in the United States steadily increases and with that comes the potential for increased transmission and increased need for treatment and care services.1–3 In 2018, an estimated 1.2 million people in the United States were living with HIV and an estimated 36,400 new HIV infections occurred4; consequently, HIV continues to pose a substantial threat to the health and well-being of Americans. In 2019, the federal initiative Ending the HIV Epidemic: A Plan for America (EHE) was announced, with the goal to reduce new HIV infections by 75% in 5 years and by at least 90% in 10 years.5 The EHE initiative aims to reduce new HIV infections through improving care outcomes and targeting existing gaps among priority populations and geographic areas. The EHE initiative goal focuses on 4 key strategies that together can end the HIV epidemic in the United States: diagnose all persons with HIV as early as possible after infection; treat persons with HIV rapidly and effectively to reach sustained viral suppression; prevent new HIV transmissions by using proven interventions; and respond quickly to potential HIV outbreaks to get needed prevention and treatment services to persons who need them. Phase I of the EHE initiative focuses on 48 counties, Washington, D.C., San Juan, Puerto Rico, and 7 states (Alabama, Arkansas, Kentucky, Mississippi, Missouri, Oklahoma, and South Carolina) with a substantial rural HIV burden that made up more than 50 percent of the new HIV diagnoses in 2016 and 2017.
Monitoring population outcomes along the HIV care continuum has been a useful tool for measuring progress toward EHE goals in the United States.6 The continuum allows for the evaluation and comparison of trends in diagnosed HIV infection, prompt linkage to HIV care, and viral suppression as measures of success in HIV care and treatment in the United States. Trends in diagnosed HIV infection are assessed by stage of disease at diagnosis to monitor progress in the EHE goal of all persons with HIV being diagnosed as early as possible after infection; diagnosis and initiation of treatment at an earlier stage of disease improves outcomes and reduces HIV-related mortality.7 As a result of multiple health inequities that may be associated with gender, age, race/ethnicity, HIV transmission risk, and area of residence; significant differences in some care continuum outcomes have been identified between subpopulations of persons with HIV infection in the United States—for example, heterosexual men have a higher percentage of stage 3 (AIDS) diagnoses compared with persons in other transmission categories, Black/African American persons are promptly linked to care less often, and American Indian/ Alaska Native persons have a lower percentage of viral suppression compared with other racial and ethnic populations.8–11
Monitoring trends in HIV-related care outcomes is important to achieve the EHE initiative goal and help focus resources and prevention efforts among subpopulations and in geographic areas with substantial HIV prevalence in the United States. The objective of this analysis is to assess trends in the following HIV care outcomes: stage of disease at time of diagnosis, linkage to HIV medical care within 1 month gender HIV diagnosis, and viral suppression within 6 months after HIV diagnosis, by selected characteristics (gender, age, race/ethnicity, and transmission category) and area of residence at diagnosis.
METHODS
Data on HIV cases diagnosed during 2014–2018 and reported to the Centers for Disease Control and Prevention’s (CDC) National HIV Surveillance System (NHSS) by December 2019 were used to assess annual changes in percentage distributions in stage of disease at the time of diagnosis, linkage to HIV medical care within 1 month of HIV diagnosis, and viral suppression within 6 months after HIV diagnosis among persons aged 13 years and older (ie, adolescents and adults) in 33 United States jurisdictions. The analysis was limited to the 33 United States jurisdictions that as of December 2019 had complete laboratory reporting—mandatory reporting of all levels of CD4 and viral load (i.e., detectable and undetectable) results—for each year during 2014–2018 (Fig. 1).
FIGURE 1.
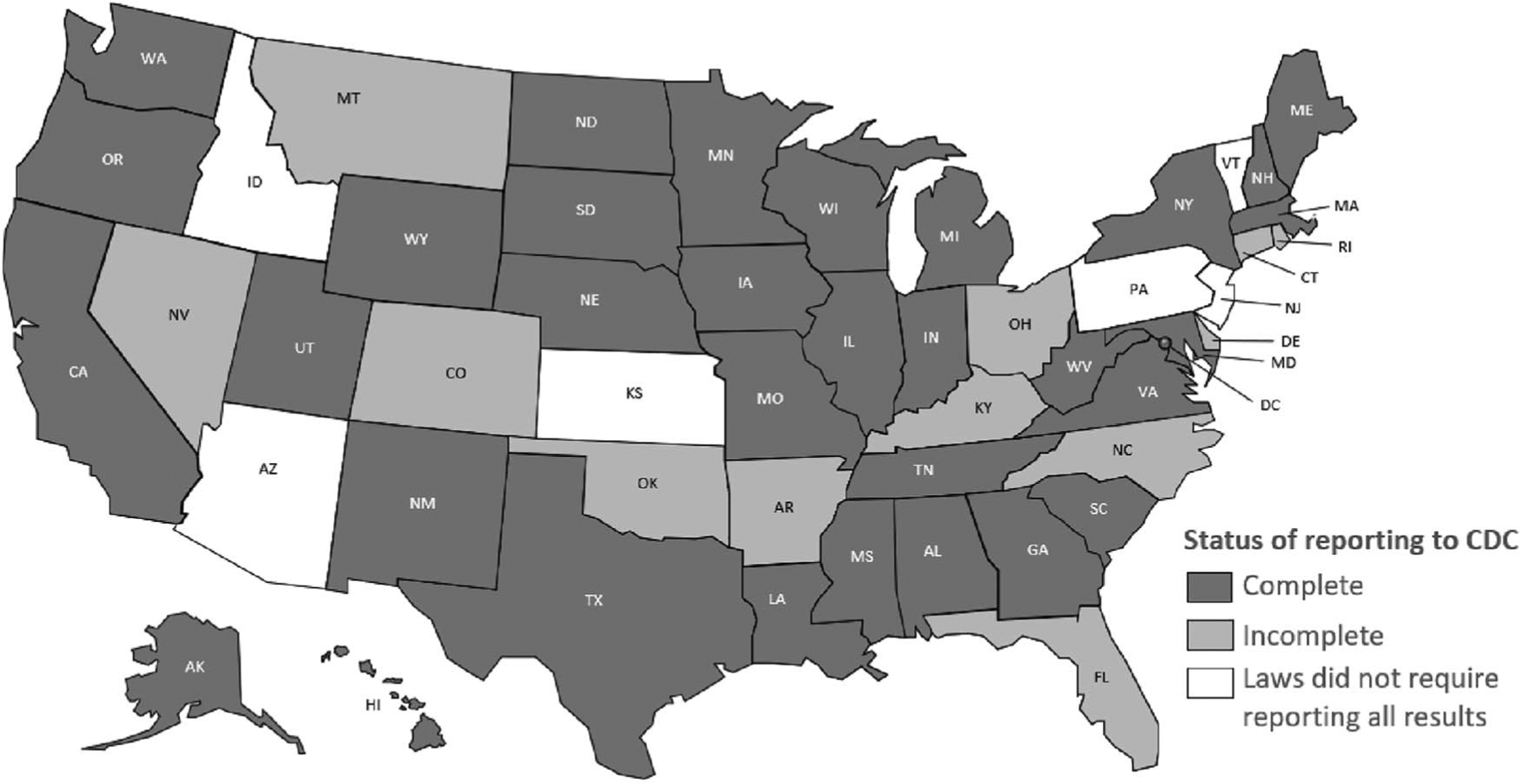
HIV surveillance reporting areas with complete reporting of CD4 and viral load test results to CDC, 2014–2018. Complete reporting was defined as follows: The jurisdiction’s laws/regulations required the reporting of all CD4 and viral load results to the state or local health department. Laboratories that perform HIV-related testing for the jurisdiction had reported a minimum of 95% of HIV-related test results to the state or local health department. The jurisdiction had reported (to CDC) at least 95% of all CD4 and viral load test results received each year from 2014 to 2018. As of December 2019, 33 jurisdictions had complete laboratory reporting for specimens through September 2019.
Stage of disease at diagnosis of HIV infection was measured using the first CD4 test performed or documentation of an AIDS-defining condition ≤3 months after a diagnosis of HIV infection: stage 0—first positive test result within 6 months after a negative test result; stages 1–2 (early infection)—CD4 lymphocyte count of ≥200/μL and a CD4 percentage of total lymphocytes of ≥14; stage 3 (AIDS) (late infection)—CD4 lymphocyte count of <200/μL or a CD4 percentage of total lymphocytes of <14 or documentation of an AIDS-defining condition. Linkage to HIV medical care was measured by documentation of ≥1 CD4 or viral load (VL) tests ≤1 month after HIV diagnosis. Viral suppression within 6 months of diagnosis of HIV infection was measured by documentation of a VL test result of <200 copies per milliliter at any time within 6 months of diagnosis of HIV infection.
Estimated annual percentage change (EAPC) and 95% confidence intervals (CIs) were calculated to assess trends for each care outcome. Differences in percentages were analyzed by gender (male, female, transgender male-to-female, and transgender female-to-male), age (13–24, 25–34, 35–44, 45–54, and ≥55 years), race/ethnicity (American Indian/Alaska Native, Asian, Black/African American, Hispanic/Latino, Native Hawaiian/other Pacific Islander, White, and multiracial persons), transmission category (male-to-male sexual contact, injection drug use, male-to-male sexual contact and injection drug use, and heterosexual contact), and jurisdiction of residence for persons with HIV diagnosed from 2014 to 2018. We analyzed the data using SAS version 9.4 (SAS Institute, Cary NC) and considered trends statistically significant if the EAPC CIs excluded 0. Comparisons of trends by gender, age group, and transmission category should be made cautiously because subpopulations vary in size and some have small numbers. Groups with small case counts (≤12) in at least 4 of 5 years (persons of additional gender identity, examples include “bigender,” “gender queer,” and “two-spirit”; and persons residing in Wyoming at time of diagnosed HIV infection) were not analyzed. Approximately 20% of persons with HIV diagnosed during 2014–2018 were reported to the CDC without an identified risk factor. Missing transmission category values were multiply imputed.12
RESULTS
Stage of Disease
Among 133,477 persons with HIV diagnosed during 2014–2018, the percentage distribution of diagnoses by stage of disease was as follows: 6% (8,320) classified as stage 0; 54% (71,830) as stages 1–2, early infection; and 21% (28,042) as stage 3 (AIDS), late infection. Approximately 19% had an unknown stage of disease at time of HIV diagnosis.
Stage of Disease: Stage 0 at Time of HIV Diagnosis
From 2014 through 2018, the percentage of persons who received a diagnosis classified as stage 0 increased 13.7% per year (95%CI: 12.0 to 15.4), on average, from 4.3% in 2014 to 7.5% in 2018 (Fig. 2; see Table 1, Supplemental Digital Content 1, http://links.lww.com/QAI/B703 for annual counts of care outcomes and EAPCs). By subpopulation, increases ranged from 10.6% among persons aged 45–54 years (CI: 5.5 to 15.9) to 26.8% among men with infection attributed to injection drug use (CI: 11.3 to 44.6) per year, on average. By jurisdiction of residence, 16 of the 33 jurisdictions had increases in the percentage of persons with HIV classified at stage 0 and accounted for 89% of stage 0 classifications. Percentages of stage 0 classifications did not significantly change among the populations of trans-gender male-to-female, American Indian/Alaska Native and Asian persons, and men with HIV attributed to male-to-male sexual contact and injection drug use. By jurisdiction of residence, 7 of the 33 jurisdictions had no significant change (District of Columbia, Hawaii, Indiana, Minnesota, Missouri, South Carolina, and Washington) and accounted for 10% of stage 0 classifications. Overall, no declines in the percentage of stage 0 classifications were observed by subpopulation or jurisdiction of residence.
FIGURE 2.

Percentage distribution by stage of disease at time of HIV diagnosis*, 2014–2018, 33 jurisdictions**. *Stage of disease at diagnosis of HIV infection was measured using the first CD4 test performed or documentation of an AIDS-defining condition ≤3 months after a diagnosis of HIV infection [stage 0: first positive test result within 6 months after a negative test result; stages 1–2: Early infection; stage 3 (AIDS): late infection]. **The 33 jurisdictions included Alabama, Alaska, California, the District of Columbia, Georgia, Hawaii, Illinois, Indiana, Iowa, Louisiana, Maine, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Nebraska, New Hampshire, New Mexico, New York, North Dakota, Oregon, South Carolina, South Dakota, Tennessee, Texas, Utah, Virginia, Washington, West Virginia, Wisconsin, and Wyoming. §EAPCs indicate the annual percentage change in the proportion of persons diagnosed with HIV infection at a specific stage per-year.
Stage of Disease: Stages 1–2 (Early Infection) at Time of HIV Diagnosis
From 2014 through 2018, the percentage of persons with diagnosed HIV classified as stages 1–2 (early infections) increased 2.9% per year (CI: 2.6 to 3.2), on average, from 51.9% in 2014 to 55.7% in 2018 (Fig. 2; see Table 1, Supplemental Digital Content 1, http://links.lww.com/QAI/B703 for annual counts of care outcomes and EAPCs). By subpopulation, increases ranged from 1.8% among persons aged 45–54 years (CI: 0.8 to 2.8) to 8.9% among the subpopulation of transgender female-to-male (CI: 1.9 to 16.2) per year, on average. By jurisdiction of residence, 18 of the 33 jurisdictions had increases in the percentage of persons with early infection and accounted for 86% of stage 1–2 classifications. Percentages of early infections did not significantly change among American Indian/Alaska Native, Asian, and Native Hawaiian/other Pacific Islander persons and women with infection attributed to injection drug use. By jurisdiction of residence, 12 of the 33 jurisdictions had no significant change (Alaska, Hawaii, Indiana, Maine, Massachusetts, Minnesota, Nebraska, New Mexico, South Carolina, South Dakota, West Virginia, and Wisconsin) in the percentage of persons with early infection and accounted for 11% of stage 1–2 classifications. Overall, no declines in the percentage of persons with early infection were observed by subpopulation, and 2 of the 33 jurisdictions had a decline (Mississippi: EAPC −10.1; CI −12.8 to −7.2. Missouri: EAPC −2.9; CI −5.4 to −0.2).
Stage of Disease: Stage 3 (AIDS) (Late Infection) at Time of HIV Diagnosis
From 2014 through 2018, the percentage of persons who received an HIV diagnosis classified as stage 3 (AIDS) declined 1.5% per year (CI −2.2 to −0.8), on average, from 22.0% in 2014 to 20.6% in 2018 (Fig. 2; see Table 1, Supplemental Digital Content 1, http://links.lww.com/QAI/B703 for annual counts of care outcomes and EAPCs). By subpopulation, declines ranged from −1.2% among men (CI −2.1 to −0.4) to −7.4% among men with HIV attributed to injection drug use (CI −11.0 to −3.6) per year, on average. By jurisdiction of residence, 4 of the 33 jurisdictions had declines in the percentage of persons with late-stage disease and accounted for 18% of stage 3 (AIDS) classifications. Percentages of late-stage classifications did not significantly change among the subpopulations of transgender male-to-female; persons aged 13–24, 25–34, and 45–54 years; all race/ethnicities (except Black/African American and White persons); and all transmission categories (except men with infection attributed to injection drug use and women with infection attributed to heterosexual contact). By jurisdiction of residence, 23 of the 33 jurisdictions had no significant change (all jurisdictions except Alaska, Hawaii, Iowa, Maine, New Hampshire, North Dakota, Oregon, South Dakota, Texas, and Wyoming) in the percentage of persons with late-stage classification and accounted for 81% of all late-stage classifications. Overall, no increases in the percentage of persons with late-stage HIV classification were observed by subpopulation or jurisdiction of residence.
Linkage to HIV Medical Care
From 2014 through 2018, 75.9% (101,356 of 133,477) of persons who received an HIV diagnosis were linked to HIV medical care within 1 month of diagnosis. Overall, the percentage of persons linked to HIV medical care within 1 month increased 2.3% per year (CI: 2.0 to 2.5), on average, from 73.2% in 2014 to 79.7% in 2018 (Fig. 3; see Table 2, Supplemental Digital Content 1, http://links.lww.com/QAI/B703 for annual counts of care outcomes and EAPCs). By subpopulation, increases ranged from 1.0% among persons aged 45–54 years (CI: 0.5 to 1.6) and 55 years and older (CI: 0.3 to 1.6) to 5.4% among the transgender male-to-female population (CI: 3.5 to 7.5) per year, on average. By jurisdiction of residence, 15 of the 33 jurisdictions had increases in the percentage and accounted for 86% of persons linked to care. Percentages of persons linked to care did not significantly change among the subpopulations of transgender female-to-male, American Indian/Alaska Native and Native Hawaiians/other Pacific Islander persons, and women with HIV attributed to injection drug use. By jurisdiction of residence, 16 of 33 jurisdictions had no significant change (Alaska, Hawaii, Indiana, Iowa, Maine, Massachusetts, Minnesota, Missouri, Nebraska, New Hampshire, New Mexico, North Dakota, Oregon, South Dakota, West Virginia, and Wisconsin) and accounted for 12% of persons linked to care. No decline in the percentage of persons linked to care was observed by subpopulation, and one of the 33 jurisdictions had a decline (Mississippi: EAPC −4.8, CI −6.3 to −3.3).
FIGURE 3.
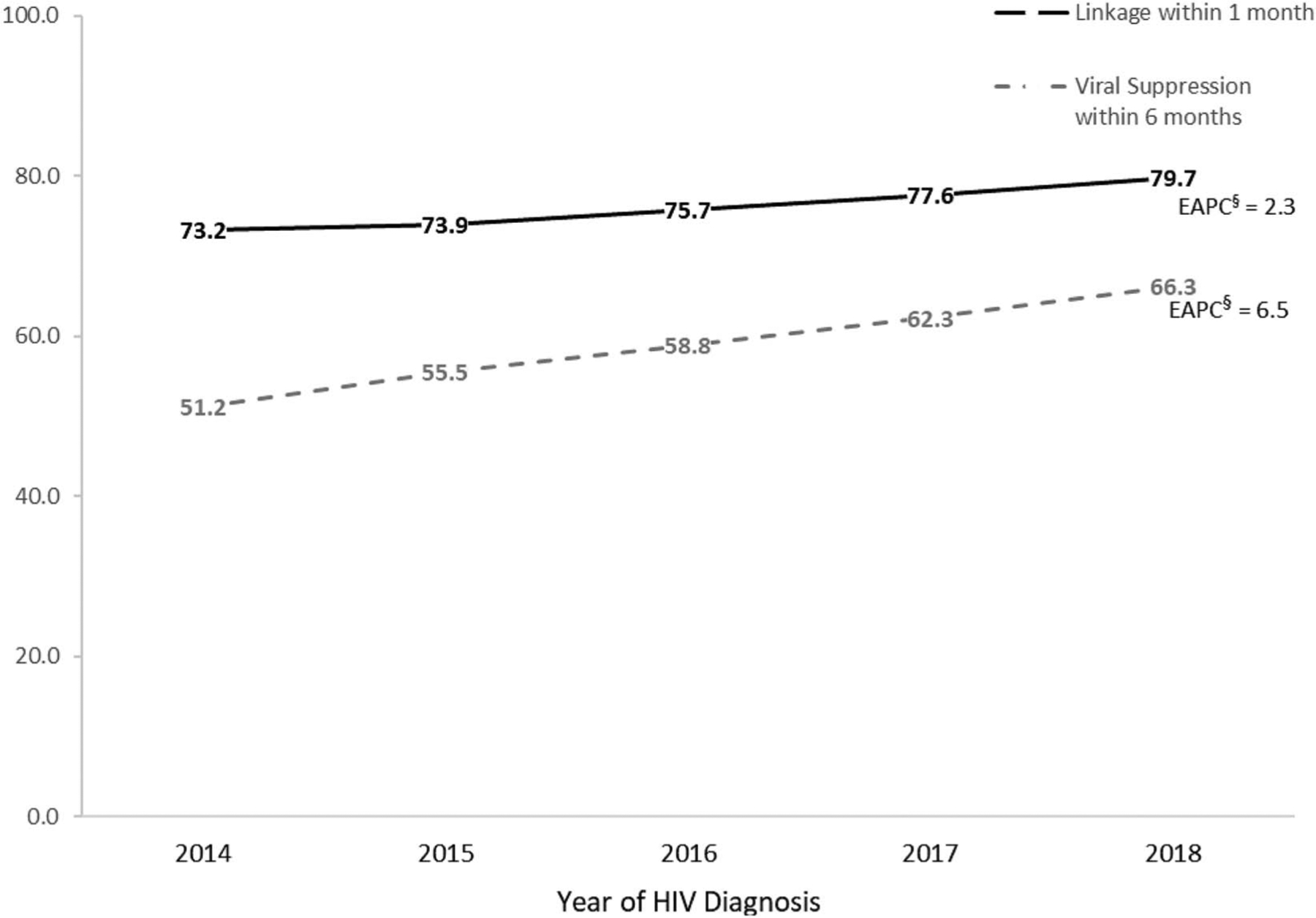
Percentage linkage to HIV medical care within 1 month after HIV diagnosis and HIV viral suppression within 6 Months of HIV diagnosis*, 2014–2018, 33 jurisdictions**. *Linkage to HIV medical care was measured by documentation of ≥1 CD4 or VL tests ≤1 month of HIV diagnosis. A VL test result of <200 copies/mL indicates HIV viral suppression. VL test results are within 6 months of diagnosis of HIV infection during the specified year. **The 33 jurisdictions included Alabama, Alaska, California, the District of Columbia, Georgia, Hawaii, Illinois, Indiana, Iowa, Louisiana, Maine, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Nebraska, New Hampshire, New Mexico, New York, North Dakota, Oregon, South Carolina, South Dakota, Tennessee, Texas, Utah, Virginia, Washington, West Virginia, Wisconsin, and Wyoming. §EAPCs indicate the per-year change, on average, in the percentage linked to HIV medical care or virally suppressed.
Viral Suppression
From 2014 through 2018, 58.7% (78,301 of 133,477) of persons who received an HIV diagnosis had viral suppression within 6 months after diagnosis. Overall, the percentage of persons with viral suppression increased 6.5% per year (CI: 6.1 to 6.8), on average, from 51.2% in 2014 to 66.3% in 2018 (Fig. 3; see Table 3, Supplemental Digital Content 1, http://links.lww.com/QAI/B703 for annual counts of care outcomes and EAPCs). By subpopulation, increases ranged from 3.9% among Asian persons (CI: 2.3 to 5.5) to 9.7% among men with HIV attributed to male-to-male sexual contact and injection drug use (CI: 7.2 to 12.2) per year, on average. By jurisdiction of residence, 24 of 33 jurisdictions had increases in the percentage. Percentage of persons with viral suppression did not significantly change among sub-populations of American Indian/Alaska Native and Native Hawaiian/other Pacific Islander persons. By jurisdiction, 8 of 33 jurisdictions had no significant change (Hawaii, Maine, Nebraska, New Hampshire, North Dakota, Oregon, South Dakota, and West Virginia). No declines in the percentage of persons with viral suppression were observed by subpopulation or jurisdiction.
DISCUSSION
From 2014 through 2018 in the 33 jurisdictions, trends in HIV-related care outcomes among adults and adolescents showed overall progress—with improvements in the timeliness of receiving diagnoses at earlier stages (stage 0, 1, or 2) and a decline in late-diagnosed infections [stage 3 (AIDS)]. Improvements were also observed as linkage to care within 1 month of HIV diagnosis and viral suppression within 6 months after HIV diagnosis increased from 2014 through 2018. These findings are promising because there are clear health advantages to diagnosing HIV early and starting treatment as soon as possible to reach viral suppression.13 Increased linkage to care promotes quick access to treatment and earlier viral suppression, which effectively prevents HIV transmission.14,15 Although overall progress was made in the care outcomes, progress in outcomes varied by subpopulation and jurisdiction of residence.
Subpopulations that showed the least progress and had no significant change in percentages from 2014 through 2018 were persons aged 45–54 years [for stage 3 (AIDS)], American Indian/Alaska Native persons (for all stages of diagnoses, linkage to care, and viral suppression), Asian persons (for all stages of diagnoses), and Native Hawaiian/other Pacific Islander persons (for stages 1–2 of diagnoses, linkage to care, and viral suppression). For persons aged 45–54 years, studies have found that health care providers may not routinely ask persons of this age group about risk for HIV, and persons aged 45–54 years may not consider themselves to be at risk for HIV or may mistake HIV symptoms for those of normal aging.16 Lack of access to HIV-related services and HIV-related stigma may explain the lack of progress among American Indian/Alaska Native persons.17 A higher percentage of undiagnosed HIV among Asian persons compared with other race/ethnicities and cultural factors (eg, stigma, immigration issues, or fear of bringing shame to their families) may explain lack of progress in the population.18 Socioeconomic issues such as poverty, inadequate or no health care coverage, language barriers, and lower education attainment may make it difficult for some Native Hawaiian/other Pacific Islander persons to access HIV testing and care.19
American Indian, Asian, and Native Hawaiian/other Pacific Islander persons living with HIV are stigmatized and vulnerable groups who are frequently unable to access medical care and social services. In addition, each of these racial and ethnic groups is diverse, and many medical and social service agencies are unable to provide culturally and linguistically competent services to such a broad spectrum. As a result, many persons in these racial and ethnic groups living with HIV are unable to access needed care and treatment.20,21 Although race and ethnicity are not risk factors but are instead markers for many underlying problems of greater relevance to health, including socioeconomic status and cultural behavior characteristics, which are social and not biological.22,23 Racial and ethnic differences in health are more likely to reflect profound differences in people’s experience based on the relatively advantaged or disadvantaged position in society into which they are born.23,24 Social determinants of health factors, shaped by income, education, wealth, and socioeconomic conditions, vary systematically by race and ethnicity and are important in explaining differences in health outcomes.24
Jurisdictions of residence that showed the least progress and had no significant change or declines in trend percentages from 2014 through 2018 were mostly in states with a substantial HIV prevalence in rural areas,5 had reported an HIV outbreak among persons who inject drugs,25,26 or were low morbidity (<10 diagnoses per 100,000 persons) jurisdictions with diagnoses in less urbanized areas.27 In addition, phase I of the EHE initiative includes 7 states that have a disproportionate occurrence of HIV in rural areas, 3 of which are among the jurisdictions in our study that show the least progress (Mississippi, Missouri, and South Carolina).5 Factors associated with trends in HIV-related care outcomes in these states might be explained by the fact that many of these rural communities have experienced increases in nonprescription use of opioids and heroin that have led to increases in injection drug use and potential for localized HIV outbreaks.26 The risk of accelerated HIV transmission associated with opioid use in rural communities is well documented.28–31 In addition, residents of and clinicians in less urbanized areas may have less awareness than their more urban counterparts of individual HIV risk and the benefits of prevention strategies such as routine HIV testing and use of preexposure prophylaxis.8 Rural areas also are more likely to lack the services and support required for HIV care and treatment and to have greater HIV stigma in the community.8 Moreover, this analysis found no significant change in stage 1–2 classifications and linkage to HIV medical care among women with HIV attributed to injection drug use and no significant change among identification of stage 0 infection in men with HIV attributed to male-to-male sexual contact and injection drug use. Outcomes among these subpopulations may affect trends in HIV-related care outcomes in jurisdictions with a substantial rural burden or with diagnoses in less urbanized areas. Variations in trends by subpopulation and jurisdiction of residence in HIV-related care outcomes were expected and may partially be due to differences in testing behaviors, targeted HIV testing initiatives, or changes in the numbers of new HIV infections (incidence) in some subpopulations.8
LIMITATIONS
This analysis was subject to several limitations. First, data were available from 33 United States jurisdictions with complete reporting of HIV-related laboratory data to the CDC during all 5 years analyzed; these jurisdictions account for 68% of persons living with diagnosed HIV infections in the United States and may not be representative of all people with diagnosed HIV infection in the United States during the study period. Second, this analysis included persons with HIV in jurisdictions based on their residence at diagnosis. Laboratory reports may have been missed if a person moved to an area outside the 33 jurisdictions. Third, because CD4 and VL test results reported to HIV surveillance programs were relied on to monitor the outcomes, not having these tests performed or reported limits the inclusion criteria for all the outcomes. Data on CD4 and VL test results during the follow-up period may be missing for people who moved to a jurisdiction (after HIV diagnosis) that did not report complete test results to CDC.
CONCLUSIONS
Although overall trends in stage of disease at diagnosis, linkage to HIV medical care within 1 month, and viral suppression within 6 months are promising, some subpopulations and jurisdictions have made little to no significant progress. In addition, some subpopulations or jurisdictions are not making progress toward meeting the EHE initiative targets of at least 95% of persons with HIV infection having received a diagnosis, 95% of persons with diagnosed HIV infection linked to HIV medical care, and 95% of persons with diagnosed HIV infection having a suppressed viral load27—and more specifically, some areas (including those with substantial rural HIV burden) are declining in progress. These findings confirm substantial gaps in diagnosing, treating, and preventing HIV infection and underscore the need for expanded efforts not only among groups making little to no progress but continued progress among subpopulations that are disproportionately affected with diagnosed HIV infection (eg, Black/African American and Hispanic/Latino persons and men with HIV attributed to male-to-male sexual contact).32 Expanded efforts include full implementation of EHE initiative strategies; for example, that all persons are tested for HIV at least once and persons with HIV as early as possible after infection, HIV treatment is initiated promptly after diagnosis, there are no gaps in care, and persons with HIV are promptly linked to and receive treatment with the goal of viral suppression. New infections will continue to occur in the United States unless substantial improvements are made in implementing the EHE strategies of diagnosing, treating, and preventing HIV infection.
Supplementary Material
Footnotes
The authors have no funding or conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.jaids.com).
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Chen M, Rhodes PH, Hall IH, et al. Prevalence of undiagnosed HIV infection among persons aged ≥13 years—National HIV Surveillance System, United States, 2005–2008. MMWR Morb Mortal Wkly Rep. 2012;61:57–64. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Monitoring Selected National HIV Prevention and Care Objectives by Using HIV Surveillance Data. 2016. Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed December 14, 2020.
- 3.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. 2019. Available at: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultandAdolescentGL.pdf. Accessed December 2, 2020.
- 4.Centers for Disease Control and Prevention. Estimated HIV Incidence and Prevalence in the United States, 2014–2018. 2020. Available at: https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hivsurveillance-supplemental-report-vol-25-1.pdf. Accessed December 2, 2020.
- 5.Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844–845. [DOI] [PubMed] [Google Scholar]
- 6.Gardner EM, McLees MP, Steiner JF, et al. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375:830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson JA, Kinder A, Johnson AS, et al. Differences in selected HIV care continuum outcomes among people residing in rural, urban, and metropolitan areas—28 US jurisdictions. J Rural Health. 2018;34:63–70. [DOI] [PubMed] [Google Scholar]
- 9.Gordon D, Bian F, Anderson BJ, et al. Timing of entry to care by newly diagnosed HIV cases before and after the 2010 New York State HIV testing law. J Acquir Immune Defic Syndr. 2015;68:S54–S58. [DOI] [PubMed] [Google Scholar]
- 10.Maulsby C, Charles V, Kinsky S, et al. Positive charge: filling the gaps in the U.S. HIV continuum of care. AIDS Behav. 2015;19:2097–2107. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg ES, Millett GA, Sullivan PS, et al. Understanding the HIV disparities between black and white men who have sex with men in the USA using the HIV care continuum: a modelling study. Lancet HIV. 2014;1:e112–e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrison KM, Kajese T, Hall HI, et al. Risk factor redistribution of the national HIV/AIDS surveillance data: an alternative approach. Public Health Rep. 2008;123:618–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lundgren JD, Babiker AG, Gordin F, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373: 795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall HI, Holtgrave DR, Maulsby C. HIV transmission rates from persons living with HIV who are aware and unaware of their infection. AIDS. 2012;26:893–896. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. HIV and Older Americans. 2020. Available at: https://www.cdc.gov/hiv/group/age/olderamericans/index.html. Accessed October 16, 2020.
- 17.Negin J, Aspin C, Gadsden T, et al. HIV among indigenous peoples: a review of the literature on HIV-related behaviour since the beginning of the epidemic. AIDS Behav. 2015;19:1720–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russ LW, Meyer AC, Takahashi LM, et al. Examining barriers to care: provider and client perspectives on the stigmatization of HIV-positive Asian-Americans with or without viral hepatitis co-infection. AIDS Care. 2012;24:1302–1307. [DOI] [PubMed] [Google Scholar]
- 19.Adih WK, Campsmith M, Williams CL, et al. Epidemiology of HIV among asians and pacific islanders in the United States, 2001–2008. J Int Assoc Physicians AIDS Care. 2011;10:150–159. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. HIV and American Indian/ Alaska Native People. 2021. Available at: https://www.cdc.gov/hiv/group/racialethnic/aian/index.html. Accessed June 15, 2021.
- 21.Chin JJ, Kang E, Kim JH, et al. Serving asians and pacific islanders with HIV/AIDS: challenges and lessons learned. J Health Care Poor Underserved. 2006;17:910–927. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Use of race and ethnicity in public health surveillance summary of the CDC/ATSDR workshop. MMWR Morb Mortal Wkly Rep. 1993;42:1–28.8418395 [Google Scholar]
- 23.Doubeni CA, Simon M, Krist AH. Addressing systemic racism through clinical preventive service recommendations from the US Preventive Services Task Force. JAMA. 2021;325:627–628. [DOI] [PubMed] [Google Scholar]
- 24.Braveman PA, Egerter SA, Mockenhaupt RE. Broadening the focus: the need to address the social determinants of health. Am J Prev Med. 2011; 40:S4–S18. [DOI] [PubMed] [Google Scholar]
- 25.Minnesota Department of Health. Health Advisory: HIV Outbreak in Persons Who Inject Drugs (PWID). 2020. Available at: https://www.health.state.mn.us/communities/ep/han/2020/feb3hiv.pdf. Accessed February 6, 2020.
- 26.Peters PJ, Pontones P, Hoover KW, et al. HIV infection linked to injection use of oxymorphone in Indiana, 2014–2015. New Engl J Med. 2016;375:229–239. [DOI] [PubMed] [Google Scholar]
- 27.US Department of Health and Human Services. What Is “Ending the HIV Epidemic: A Plan for America?”. 2019. Available at: https://www.hiv.gov/federal-response/ending-the-hiv-epidemic/overview. Accessed June 14, 2021.
- 28.Runfola JK, House J, Miller L, et al. Community outbreak of HIV infection linked to injection drug use of Oxymorphone—Indiana. MMWR Morb Mortal Wkly Rep. 2015;64:443–444. [PMC free article] [PubMed] [Google Scholar]
- 29.Lerner AM, Fauci AS. Opioid injection in rural areas of the United States: a potential obstacle to ending the HIV epidemic. JAMA. 2019; 322:1041–1042. [DOI] [PubMed] [Google Scholar]
- 30.Evans ME, Labuda SM, Hogan V, et al. Notes from the field: HIV infection investigation in a rural area—West Virginia, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:257–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bradley H, Hogan V, Agnew-Brune C, et al. Increased HIV diagnoses in West Virginia counties highly vulnerable to rapid HIV dissemination through injection drug use: a cautionary tale. Ann Epidemiol. 2019;34:12–17. [DOI] [PubMed] [Google Scholar]
- 32.McCree DH, Chesson H, Bradley EL, et al. Exploring changes in racial/ethnic disparities of HIV diagnosis rates under the “Ending the HIV epidemic: a plan for America” initiative. Public Health Rep. 2020;135: 685–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


