Abstract
Chronic kidney disease (CKD) is characterized by the accumulation of uremic toxins which exert deleterious effects on various organ systems. Several of these uremic toxins originate from the bacterial metabolization of aromatic amino acids in the colon. This study assessed whether the gut microbial composition varies among patients in different stages of CKD. Uremic metabolites were quantified by UPLC/fluorescence detection and microbial profiling by 16S rRNA amplicon sequencing. Gut microbial profiles of CKD patients were compared among stages 1–2, stage 3 and stages 4–5. Although a substantial inter-individual difference in abundance of the top 15 genera was observed, no significant difference was observed between groups. Bristol stool scale (BSS) correlated negatively with p-cresyl sulfate and hippuric acid levels, irrespective of the intake of laxatives. Butyricicoccus, a genus with butyrate-generating properties, was decreased in abundance in advanced stages of CKD compared to the earlier stages (p = 0.043). In conclusion, in this cross-sectional study no gradual differences in the gut microbial profile over the different stages of CKD were observed. However, the decrease in the abundance of Butyricicoccus genus with loss of kidney function stresses the need for more in-depth functional exploration of the gut microbiome in CKD patients not on dialysis.
Keywords: chronic kidney disease, uremic toxins, gut microbial composition, p-cresyl sulfate, p-cresyl glucuronide
1. Introduction
In chronic kidney disease (CKD), uremic toxins accumulate in the blood circulation [1,2,3], exerting deleterious effects on various organ systems of the human body [4] and contributing to cardiovascular morbidity and mortality [5,6,7,8,9]. The gut microbiota is responsible for the generation of the precursor metabolites of the protein-bound uremic toxins (PBUTs) such as p-cresyl sulfate (pCS), p-cresyl glucuronide (pCG), indoxyl sulfate (IxS) and indole-3-acetic acid (IAA). Aromatic amino acids (i.e., tyrosine, phenylalanine and tryptophan) are predominantly metabolized in the distal colon by gut bacteria into p-cresol, indole and IAA [10,11,12,13,14]. Subsequently, p-cresol and indole are detoxified through sulfation and glucuronidation by the colon mucosa and liver into pCS, pCG and IxS [15,16], whereas IAA enters the blood circulation unmodified [17,18]. In the circulation, these PBUTs reversibly bind to albumin [19]. Since only the free fraction can be removed by dialysis therapy [20,21], albumin-binding hampers their removal at end-stage kidney disease (ESKD).
As shown in previous studies [11,22,23,24,25,26,27,28], the abundance of specific gut bacteria in patients with CKD is altered. A previous study by our group using 16S rRNA amplicon sequencing [29] revealed that hemodialysis (HD) patients do not have a uniform altered gut microbial composition, when compared to healthy volunteers with similar genetic and environmental backgrounds from the Flemish Gut Flora Project (FGFP) [30]. However, when dividing the CKD patient group according to their serum uremic toxin levels into a high pCS and low IxS group and a high IxS and low pCS group, the bacterial genera Enterococcus, Dialister, Akkermansia and Ruminococcus were comparatively overrepresented in the high pCS/low IxS group, whereas members of Bacteroides and Blautia were comparatively overrepresented in the high IxS/low pCS group [29]. Nevertheless, because only patients who had reached ESKD were included in this study, it cannot be ruled out that dialysis-related conditions co–influenced microbiome readouts.
In the present study it was investigated whether the gut microbial profile changes over the different stage of CKD and whether gut microbial composition correlates to plasma levels of colon derived uremic toxins. The aim was to point out potential gut microbial targets to prevent the accumulation of uremic toxins from the early stages of CKD on, which could in its turn decrease morbidity and mortality of patients with CKD and/or slow down disease progression.
2. Materials and Methods
2.1. Study Population and Sample Collection
A total of 111 patients with CKD [CKD 1 (n = 13); CKD 2 (n = 23); CKD 3 (n = 44); CKD 4 (n = 22); CKD 5 (n = 9)] were included. The ‘Chronic kidney disease epidemiology collaboration (CKD-EPI)’-creatinine equation was used to determine the estimated glomerular filtration rate (eGFR) of each patient. Based on their eGFR, the total group of patients with CKD was divided into three groups: (i) eGFR above 60 mL/min/1.73 m2 corresponding to CKD stages 1 and 2 (n = 36), (ii) eGFR between 30 and 60 mL/min/1.73 m2 corresponding to CKD stage 3 (n = 44), and (iii) eGFR below 30 mL/min/1.73 m2 corresponding to CKD stages 4 and 5 (n = 31). From each patient of the CKD population, a single blood and fecal sample was collected in parallel. Bristol Stool Scale (BSS), a visual scale of the aspect of stool, from hard (1) to liquid (7) [31] was indicated by the lab technician immediately after obtaining the fresh stool sample from the patients. Patient characteristics and clinical parameters have been described previously [32].
Exclusion criteria were age < 18 years, active infection (C-reactive protein > 20 mg/L), active malignancy, cardiovascular event in the past three months, immunosuppressive therapy, inflammatory bowel disease, obesity (Body Mass Index > 35 kg/m2), pregnancy, transplantation, and/or use of non-steroidal anti-inflammatory drugs within the past month. Patients were asked to provide information on general diet and health status in a questionnaire including the occurrence of stomach, gut, liver and bile diseases, gastro-intestinal infection and antibiotic, pre- and probiotic and laxative intake and different types of surgery. All patients gave written informed consent before inclusion and the study was conducted following the Declaration of Helsinki, and approved by the Medical Ethics Committee of the Ghent University Hospital (Ref 2010/033, B67020107926).
2.2. Determination of Uremic Metabolites in Blood
Plasma urea [60 Dalton (Da)], creatinine (133 Da), phosphorus (31 Da) and total protein content were measured with standard laboratory methods in the routine laboratory of the Ghent University Hospital, Belgium. In plasma, total concentrations of the PBUTs pCS (187 Da), pCG (284 Da), IxS (213 Da), IAA (175 Da), 3-carboxy-4-methyl-5-propyl-2-furanpropionic acid (CMPF, 240 Da) and hippuric acid (HA, 179 Da) were measured by ultra-performance liquid chromatography (UPLC) and fluorescence detection as previously described [32,33].
2.3. Illumina-Based Microbial Profiling
Fecal DNA extraction using the RNeasy PowerMicrobiome Kit® (Qiagen, Hilden, Germany) and Illumina-based microbial profiling were performed as described previously [29]. The V4 region of the 16S rRNA gene was amplified using the 515F/806R primer set. Sequencing data were analyzed using the DADA2 pipeline, filtering and trimming forward and reverse reads truncated after 130 and 200 bases. Thirty bases were removed from the start of forward and reverse reads. Minimum quality score of each read was >11 and reads with more than 2 expected errors (EE) were discarded. Identified chimeras were removed using removeBimeraDenovo. For taxonomic classification the 16S rRNA reference (RDP) training set, version 16, formatted for DADA2, revealed 4069 amplicon sequence variants (ASVs). Reads were rarefied to 21,046 reads per sample and ASVs that had no reads left after rarefaction were removed, resulting in 3574 ASVs (this number can vary slightly upon re-rarefaction). Metadata parameters for which more than half of the values were missing were discarded. Metadata, sequencing data and bacterial cell count data were obtained from a total of 111 subjects, for whom the metadata and bacterial cell count data were summarized in a previous paper including the same CKD cohort [32].
2.4. Statistical Analysis
Statistical analyses were performed with R. To identify, in an unsupervised manner, the main correlates of variation of the microbial composition in this cohort, two different variants of the same approach were applied: (i) a Principal Coordinates Analysis (PCoA) on rarefied data using Bray Curtis dissimilarities, and (ii) centered log-ratio (CLR) transformation in combination with Euclidean distances. PCoA was carried out on a sub-set of 102 samples without missing metadata values. Envfit was used to assess the co-variation of principal components with metadata in the triplot [34]. Differences between the gut microbial composition on genus levels between earlier CKD stages (CKD 1–2) and later stages of CKD (CKD 4–5) were assessed with ALDEx2 [35], with Wilcoxon rank sum test, p-values were Benjamini-Hochberg corrected.
3. Results
3.1. Gut Microbiome Profiles in Different Stages of CKD
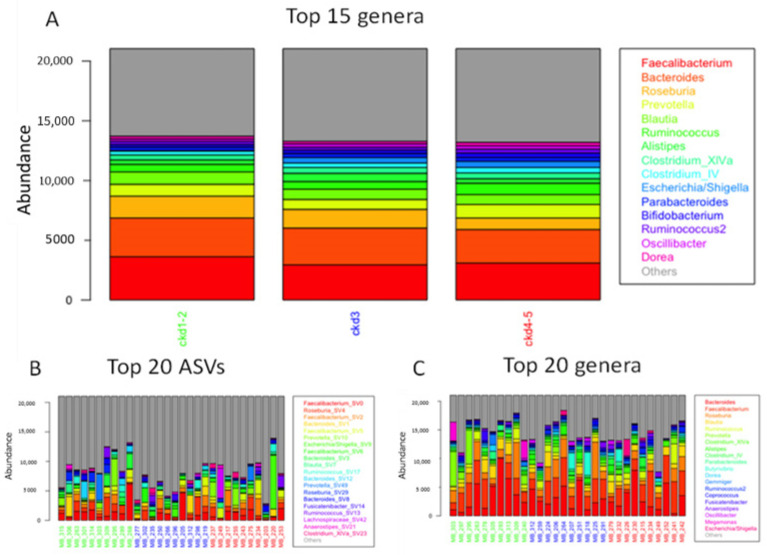
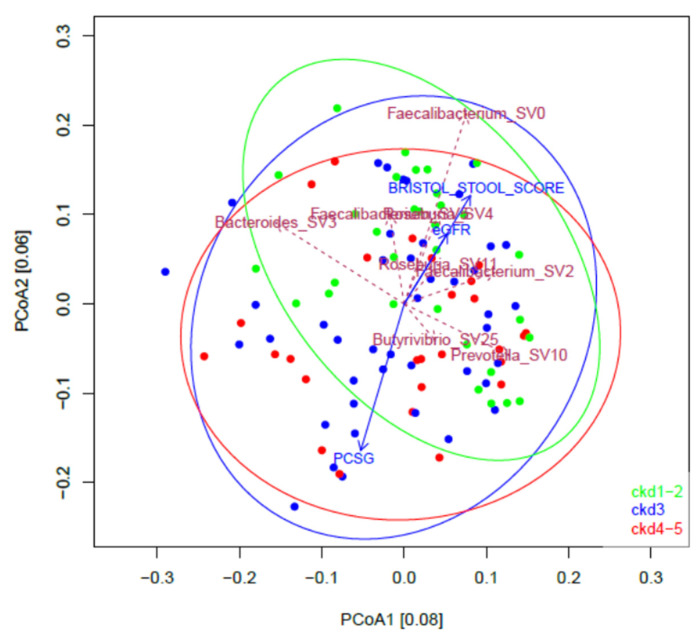
Figure 1A illustrates the mean abundance of the top 15 genera in different groups of progressive CKD stages (CKD1–2, CKD3 and CKD4–5), computed across all CKD stage-specific samples and ranked across all samples. Across all groups, the three most abundant genera were Faecalibacterium, Bacteroides and Roseburia. No significant difference in genus abundances between the different CKD groups was observed. Nevertheless, inter-individual variation was apparent across abundance profiles of the top 20 ASVs (species level) and genera (sum of ASVs) of 30 randomly selected samples (10 per CKD group) (Figure 1B,C). Also based on PCoA (Figure 2), CKD clusters largely overlapped in ordination.
Figure 1.
Mean abundance of the top genera or ASVs computed across all CKD stage-specific samples. (A) mean abundance of the top 15 genera in the different CKD stage groups; (B) mean abundance of the top 20 ASVs in 10 randomly selected samples per CKD stage group; (C) mean abundance of the top 20 genera in 10 randomly selected samples per CKD stage group. CKD: chronic kidney disease; ASV: 16S rRNA amplicon sequence variant.
Figure 2.
Principal coordinate analysis of the microbial composition of the fecal samples of patients with CKD. Colors and ellipses are coding for CKD stages; SV: (amplicon) sequencing variant; PCSG: p-cresyl sulfate + p-cresyl glucuronide; eGFR: estimated glomerular filtration rate.
3.2. Correlates of Intestinal Microbiota Composition in CKD
A total of 3574 ASVs were found in the cohort. The missing-value-free metadata contained 62 parameters (Table S1), including toxin concentrations and confounders for microbiota research [29]. The (Bray-Curtis-based) variation of the microbial composition in this cohort was correlated to BSS, eGFR and pCSG, of which the latter is the sum of plasma pCS and pCG (Figure 2). The length of scaled arrows reflects the correlation with overall community composition. BSS and eGFR point in the same direction, and in the opposite direction as pCSG (Figure 2). This finding was confirmed by using Centred Log-Ratio (clr) transformation and Euclidean distances.
The correlation between plasma levels of intestinally generated uremic toxins and transit time was assessed by analyzing their correlation with BSS [1 (severe constipation) to 7 (severe diarrhea)]. A significant negative correlation was found between BSS and plasma HA in the total CKD cohort and in stages 1–2; and between BSS and pCS in the total CKD cohort and stages 4–5 (Table 1).
Table 1.
Correlation between intestinally generated uremic toxins and transit time of patients with CKD.
| Correlation to BSS | All CKD Stages (n = 111) |
CKD Stage 1–2 (n = 37) |
CKD Stage 3 (n = 44) |
CKD Stage 4–5 (n = 33) |
||||
|---|---|---|---|---|---|---|---|---|
| Uremic Toxin | rs | p-Value | rs | p-Value | rs | p-Value | rs | p-Value |
| Indoxyl sulfate | −0.173 | ns | −0.130 | ns | −0.054 | ns | −0.223 | ns |
| Indole-3-acetic acid | −0.150 | ns | −0.035 | ns | −0.041 | ns | −0.206 | ns |
| Hippuric acid | −0.343 | <0.001 | −0.366 | 0.036 | −0.153 | ns | −0.318 | ns |
| p-Cresyl sulfate | −0.287 | 0.003 | −0.335 | ns | −0.134 | ns | −0.443 | 0.012 |
| p-Cresyl glucuronide | −0.175 | ns | −0.105 | ns | −0.116 | ns | −0.340 | ns |
BSS: Bristol stool scale which is used to assess transit time (slow transit = low BSS); CKD: chronic kidney disease; rs: Spearman’s correlation coefficient; bold: significant; ns: not significant.
On the triplot in Figure 2, it is also apparent that pCSG points in the opposite direction as BSS. Because part of the samples were taken from participants on laxatives and to avoid distortion due to laxative usage, analyses were also repeated excluding the samples of patients on laxatives (n = 101). Similar significant correlations between BSS and pCS and HA were found (Table S2).
3.3. Variation in Gut Microbiota Profiles in Different Stages of CKD
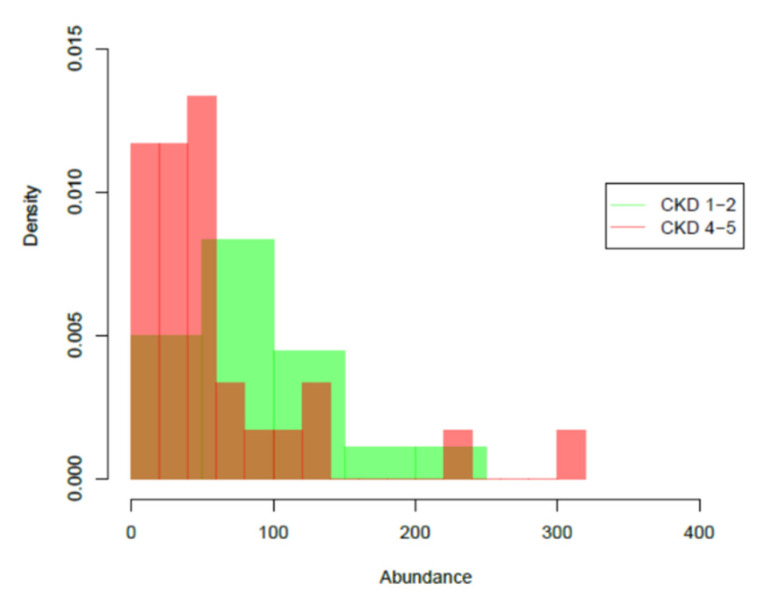
Potential microbial differences between the early stage and the most advanced stage CKD patients were assessed. No differences survived multiple testing correction using all 3574 ASVs nor using the 100 top-abundant sequencing variants. However, at genus level, a significantly lower abundance of Butyricicoccus was found in latest stages (CKD 4–5) of the disease compared to the earliest stages (CKD 1–2) (p = 0.043; Figure 3).
Figure 3.
Significant differences of the abundance of Butyricicoccus, a genus with butyrate-generating properties, between earlier (CKD stages 1–2; green) and later (CKD stages 4–5; pink) stages of CKD. Brown: the intersection of both graphs. p = 0.043.
4. Discussion
In this study, the gut microbial composition was assessed among patients at different stages of CKD not on dialysis, i.e., CKD stages 1 to 5, taking their plasma levels of PBUTs into account. No specific microbial profile was linked to different degrees of kidney function decline, as was also observed in ESKD patients compared to the control group in our previous study [29]. However, at the genus level, a significantly lower abundance of Butyricicoccus was found in advanced stages of CKD compared to the earliest stages. This is in line with our previously reported quantitative polymerase chain reaction (qPCR) data showing that the abundance of Butyricicoccus spp. but also of other gut bacteria such as Roseburia spp., Faecalibacterium prausnitzii, and Bifidobacterium spp. declined with advancing stages of CKD [11] and confirm earlier qPCR findings in CKD by Jiang et al. for Roseburia spp. and Faecalibacterium prausnitzii [36]. In addition, a recent metagenome study showed that butyrate-producing species such as Roseburia inulinivorans, Ruminococcus torques and Ruminococcus lactaris were already less abundant in early CKD compared to the control group and this in the absence of clear gut microbiome changes [37]. Moreover, other studies in patients with ESKD also showed that butyrate-generating bacteria are reduced compared to controls [28,36,38,39] and that a decreased abundance of Faecalibacterium prausnitzii is also associated with inflammatory bowel disease (IBD) and irritable bowel syndrome [40,41,42]. These results justify further exploration of butyrate-producing gut bacteria for their modulatory potential in intestinal disorders [43].
The variation of the microbial composition in this cohort correlated to BSS, eGFR and the sum of pCS and pCG (Figure 2), which is in line with earlier findings in healthy cohorts (BSS, eGFR) [30] and also with our earlier findings for HD patients (BSS, p-cresyl conjugates) [29]. Similar to the HD cohort, BSS points in the opposite direction as the p-cresyl conjugates (pCS and pCG) (Figure 2). Interestingly, in the present CKD cohort, IxS was not identified as a main correlate of the intestinal microbial composition, although in the HD cohort, this was a main co-variate pointing in the same direction as BSS [29]. In addition, the overall negative correlation between BSS, as a marker of transit time, and plasma levels of HA and pCS, especially in a more advanced stage of CKD, suggests that it should be explored whether preservation/modulation of transit time could affect circulating levels of the pCS. Similar observations were made in patients on automatized peritoneal dialysis [44] and in in patients with non-dialysis-dependent CKD [45].
The lack of overt difference in gut microbiota composition between the different stages of CKD observed in the present study and in a previous study on HD patients by our group points to potential limitations of 16S rDNA-based approaches, and calls for more in-depth studies focusing on functional characteristics of gut microbiota. This is exemplified by the recent finding that dietary intake of sulfide donors can tune microbiota function via post-translational modification without altering microbial community composition [46]. Shotgun metagenomic approaches revealing relative abundances of gene pathways and metatranscriptomic studies in CKD cohorts are still in their infancy, but should be further explored to build a more comprehensive systems biology framework for the gut-kidney axis.
The major strength of this study is that it covers the whole range of pre-dialysis CKD, while most studies focus on patients with ESKD. To follow up this cross-sectional approach, however, longitudinal sampling is preferred to also allow other covariates to be included in data analysis. In addition, given the high inter-individual variation observed, expansion of the cohort’s sample size could provide more power to reveal potential microbiota differences between subsequent CKD stages.
5. Conclusions
In this cross-sectional study we observed no gradual differences in the gut microbial composition in patients at different stages of CKD. However, a non-supervised comparison of CKD stage 1–2 with CKD 4–5 revealed a decrease in the abundance of the butyrate-producing genus Butyricicoccus with loss of kidney function. Additional in-depth studies taking into account the functional capacity of the gut microbiome will be required to identify potential targets to tackle chronic inflammation and to decrease levels of intestinally generated uremic toxins and their precursors.
Acknowledgments
The authors thank the attending nephrologists (especially J. Vanmassenhove, S. Van Laecke and A. Van Der Tol) and the nurses of the Nephrology Unit of the Ghent University Hospital for their effort in recruiting patients and for the collection of the samples, respectively. The authors thank the patients for their participation in the study. The authors thank Raul Yhossef Tito Tadeo for the DADA2 script.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jpm11111118/s1, Table S1: Metadata (n = 62) taken into account for correlation to overall taxon composition.; Table S2: Correlation between intestinally generated uremic toxins and transit time of patients with CKD not on laxatives.
Author Contributions
G.G., M.J. and T.G. conceived the original idea and designed the study. T.G., W.V.B., F.V., M.S. and G.G. collected the data for the study which were analyzed by K.F. and M.J. The data interpretation and manuscript drafting were performed by T.G., M.J., K.F., M.V., G.R.B.H., J.R. and G.G. T.G., K.F., W.V.B., G.R.B.H., F.V., M.S., J.R., M.V., M.J. and G.G. reviewed and edited the manuscript and gave the final approval for submission. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Foundation-Flanders (FWO Vlaanderen; No. G017815N).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Medical Ethics Committee of the Ghent University Hospital (Ref 2010/033, B67020107926).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data are fully available without restriction, in an anonymized format, upon request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Duranton F., Cohen G., De Smet R., Rodriguez M., Jankowski J., Vanholder R., Argiles A. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 2012;23:1258–1270. doi: 10.1681/ASN.2011121175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vanholder R., De Smet R., Glorieux G., Argilés A., Baurmeister U., Brunet P., Clark W., Cohen G., De Deyn P.P., Deppisch R., et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003;63:1934–1943. doi: 10.1046/j.1523-1755.2003.00924.x. [DOI] [PubMed] [Google Scholar]
- 3.Vanholder R., Glorieux G., De Smet R., Lameire N. New insights in uremic toxins. Kidney Int. Suppl. 2003;63((Suppl. S84)):S6–S10. doi: 10.1046/j.1523-1755.63.s84.43.x. [DOI] [PubMed] [Google Scholar]
- 4.Vanholder R., Pletinck A., Schepers E., Glorieux G. Biochemical and Clinical Impact of Organic Uremic Retention Solutes: A Comprehensive Update. Toxins. 2018;10:33. doi: 10.3390/toxins10010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bammens B., Evenepoel P., Keuleers H., Verbeke K., Vanrenterghem Y. Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int. 2006;69:1081–1087. doi: 10.1038/sj.ki.5000115. [DOI] [PubMed] [Google Scholar]
- 6.Barreto F.C., Barreto D.V., Liabeuf S., Meert N., Glorieux G., Temmar M., Choukroun G., Vanholder R., Massy Z.A. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2009;4:1551–1558. doi: 10.2215/CJN.03980609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dou L., Sallée M., Cerini C., Poitevin S., Gondouin B., Jourde-Chiche N., Fallague K., Brunet P., Calaf R., Dussol B., et al. The cardiovascular effect of the uremic solute indole-3 acetic acid. J. Am. Soc. Nephrol. 2015;26:876–887. doi: 10.1681/ASN.2013121283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liabeuf S., Glorieux G., Lenglet A., Diouf M., Schepers E., Desjardins L., Choukroun G., Vanholder R., Massy Z.A. Does p-cresylglucuronide have the same impact on mortality as other protein-bound uremic toxins? PLoS ONE. 2013;8:e67168. doi: 10.1371/journal.pone.0067168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin C.J., Chuang C.K., Jayakumar T., Liu H.L., Pan C.F., Wang T.J., Chen H.H., Wu C.J. Serum p-cresyl sulfate predicts cardiovascular disease and mortality in elderly hemodialysis patients. Arch. Med. Sci. 2013;9:662–668. doi: 10.5114/aoms.2013.36901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evenepoel P., Meijers B.K., Bammens B.R., Verbeke K. Uremic toxins originating from colonic microbial metabolism. Kidney Int. Suppl. 2009;76((Suppl. S114)):S12–S19. doi: 10.1038/ki.2009.402. [DOI] [PubMed] [Google Scholar]
- 11.Gryp T., Huys G.R.B., Joossens M., Van Biesen W., Glorieux G., Vaneechoutte M. Isolation and Quantification of Uremic Toxin Precursor-Generating Gut Bacteria in Chronic Kidney Disease Patients. Int. J. Mol. Sci. 2020;21:1986. doi: 10.3390/ijms21061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gryp T., Vanholder R., Vaneechoutte M., Glorieux G. p-Cresyl Sulfate. Toxins. 2017;9:52. doi: 10.3390/toxins9020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mair R.D., Sirich T.L., Plummer N.S., Meyer T.W. Characteristics of Colon-Derived Uremic Solutes. Clin. J. Am. Soc. Nephrol. 2018;13:1398–1404. doi: 10.2215/CJN.03150318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishima E., Fukuda S., Mukawa C., Yuri A., Kanemitsu Y., Matsumoto Y., Akiyama Y., Fukuda N.N., Tsukamoto H., Asaji K., et al. Evaluation of the impact of gut microbiota on uremic solute accumulation by a CE-TOFMS-based metabolomics approach. Kidney Int. 2017;92:634–645. doi: 10.1016/j.kint.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 15.de Loor H., Bammens B., Evenepoel P., De Preter V., Verbeke K. Gas chromatographic-mass spectrometric analysis for measurement of p-cresol and its conjugated metabolites in uremic and normal serum. Clin. Chem. 2005;51:1535–1538. doi: 10.1373/clinchem.2005.050781. [DOI] [PubMed] [Google Scholar]
- 16.Poesen R., Windey K., Neven E., Kuypers D., De Preter V., Augustijns P., D’Haese P., Evenepoel P., Verbeke K., Meijers B. The Influence of CKD on Colonic Microbial Metabolism. J. Am. Soc. Nephrol. 2016;27:1389–1399. doi: 10.1681/ASN.2015030279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taleb S. Tryptophan Dietary Impacts Gut Barrier and Metabolic Diseases. Front. Immunol. 2019;10:2113. doi: 10.3389/fimmu.2019.02113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zelante T., Iannitti R.G., Cunha C., De Luca A., Giovannini G., Pieraccini G., Zecchi R., D’Angelo C., Massi-Benedetti C., Fallarino F., et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Deltombe O., Van Biesen W., Glorieux G., Massy Z., Dhondt A., Eloot S. Exploring Protein Binding of Uremic Toxins in Patients with Different Stages of Chronic Kidney Disease and during Hemodialysis. Toxins. 2015;7:3933–3946. doi: 10.3390/toxins7103933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee C.T., Kuo C.C., Chen Y.M., Hsu C.Y., Lee W.C., Tsai Y.C., Ng H.Y., Kuo L.C., Chiou T.T., Yang Y.K., et al. Factors associated with blood concentrations of indoxyl sulfate and p-cresol in patients undergoing peritoneal dialysis. Perit. Dial. Int. 2010;30:456–463. doi: 10.3747/pdi.2009.00092. [DOI] [PubMed] [Google Scholar]
- 21.Martinez A.W., Recht N.S., Hostetter T.H., Meyer T.W. Removal of P-cresol sulfate by hemodialysis. J. Am. Soc. Nephrol. 2005;16:3430–3436. doi: 10.1681/ASN.2005030310. [DOI] [PubMed] [Google Scholar]
- 22.Fukuuchi F.H.M., Aiba Y., Koga Y., Endoh M., Kurokawa K., Sakai H. Intestinal bacteria-derived putrefactants in chronic renal failure. Clin. Exp. Nephrol. 2002;6:6. doi: 10.1007/s101570200016. [DOI] [Google Scholar]
- 23.Hida M., Aiba Y., Sawamura S., Suzuki N., Satoh T., Koga Y. Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron. 1996;74:349–355. doi: 10.1159/000189334. [DOI] [PubMed] [Google Scholar]
- 24.Lun H., Yang W., Zhao S., Jiang M., Xu M., Liu F., Wang Y. Altered gut microbiota and microbial biomarkers associated with chronic kidney disease. Microbiologyopen. 2019;8:e00678. doi: 10.1002/mbo3.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi K., Wang F., Jiang H., Liu H., Wei M., Wang Z., Xie L. Gut bacterial translocation may aggravate microinflammation in hemodialysis patients. Dig. Dis. Sci. 2014;59:2109–2117. doi: 10.1007/s10620-014-3202-7. [DOI] [PubMed] [Google Scholar]
- 26.Vaziri N.D., Wong J., Pahl M., Piceno Y.M., Yuan J., DeSantis T.Z., Ni Z., Nguyen T.H., Andersen G.L. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83:308–315. doi: 10.1038/ki.2012.345. [DOI] [PubMed] [Google Scholar]
- 27.Wong J., Piceno Y.M., DeSantis T.Z., Pahl M., Andersen G.L., Vaziri N.D. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am. J. Nephrol. 2014;39:230–237. doi: 10.1159/000360010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu K.Y., Xia G.H., Lu J.Q., Chen M.X., Zhen X., Wang S., You C., Nie J., Zhou H.W., Yin J. Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients. Sci. Rep. 2017;7:1445. doi: 10.1038/s41598-017-01387-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joossens M., Faust K., Gryp T., Nguyen A.T.L., Wang J., Eloot S., Schepers E., Dhondt A., Pletinck A., Vieira-Silva S., et al. Gut microbiota dynamics and uraemic toxins: One size does not fit all. Gut. 2019;68:2257–2260. doi: 10.1136/gutjnl-2018-317561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Falony G., Joossens M., Vieira-Silva S., Wang J., Darzi Y., Faust K., Kurilshikov A., Bonder M.J., Valles-Colomer M., Vandeputte D., et al. Population-level analysis of gut microbiome variation. Science. 2016;352:560–564. doi: 10.1126/science.aad3503. [DOI] [PubMed] [Google Scholar]
- 31.Lewis S.J., Heaton K.W. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol. 1997;32:920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 32.Gryp T., De Paepe K., Vanholder R., Kerckhof F.M., Van Biesen W., Van de Wiele T., Verbeke F., Speeckaert M., Joossens M., Couttenye M.M., et al. Gut microbiota generation of protein-bound uremic toxins and related metabolites is not altered at different stages of chronic kidney disease. Kidney Int. 2020;97:1230–1242. doi: 10.1016/j.kint.2020.01.028. [DOI] [PubMed] [Google Scholar]
- 33.Snauwaert E., Holvoet E., Van Biesen W., Raes A., Glorieux G., Vande Walle J., Roels S., Vanholder R., Askiti V., Azukaitis K., et al. Uremic Toxin Concentrations are Related to Residual Kidney Function in the Pediatric Hemodialysis Population. Toxins. 2019;11:235. doi: 10.3390/toxins11040235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oksanen J.G.F., Friendly B., Friendly M., Kindt R., Legendre P., McGlinn D., Minchin P.R., O’Hara R.B., Simpson G.L., Solymos P., et al. Vegan: Community Ecology Package. 2020. [(accessed on 27 October 2021)]. R Package Version 2.5-7. Available online: https://CRAN.R-project.org/package=vegan.
- 35.Fernandes A.D., Reid J.N., Macklaim J.M., McMurrough T.A., Edgell D.R., Gloor G.B. Unifying the analysis of high-throughput sequencing datasets: Characterizing RNA-seq, 16S rRNA gene sequencing and selective growth experiments by compositional data analysis. Microbiome. 2014;2:15. doi: 10.1186/2049-2618-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang S., Xie S., Lv D., Zhang Y., Deng J., Zeng L., Chen Y. A reduction in the butyrate producing species Roseburia spp. and Faecalibacterium prausnitzii is associated with chronic kidney disease progression. Antonie Leeuwenhoek. 2016;109:1389–1396. doi: 10.1007/s10482-016-0737-y. [DOI] [PubMed] [Google Scholar]
- 37.Sato N., Kakuta M., Hasegawa T., Yamaguchi R., Uchino E., Murashita K., Nakaji S., Imoto S., Yanagita M., Okuno Y. Metagenomic profiling of gut microbiome in early chronic kidney disease. Nephrol. Dial. Transpl. 2020;36:1675–1684. doi: 10.1093/ndt/gfaa122. [DOI] [PubMed] [Google Scholar]
- 38.Jiang S., Xie S., Lv D., Wang P., He H., Zhang T., Zhou Y., Lin Q., Zhou H., Jiang J., et al. Alteration of the gut microbiota in Chinese population with chronic kidney disease. Sci. Rep. 2017;7:2870. doi: 10.1038/s41598-017-02989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stadlbauer V., Horvath A., Ribitsch W., Schmerböck B., Schilcher G., Lemesch S., Stiegler P., Rosenkranz A.R., Fickert P., Leber B. Structural and functional differences in gut microbiome composition in patients undergoing haemodialysis or peritoneal dialysis. Sci. Rep. 2017;7:15601. doi: 10.1038/s41598-017-15650-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang S., Denman S.E., Morrison M., Yu Z., Dore J., Leclerc M., McSweeney C.S. Dysbiosis of fecal microbiota in Crohn’s disease patients as revealed by a custom phylogenetic microarray. Inflamm. Bowel Dis. 2010;16:2034–2042. doi: 10.1002/ibd.21319. [DOI] [PubMed] [Google Scholar]
- 41.Rajilić-Stojanović M., Biagi E., Heilig H.G., Kajander K., Kekkonen R.A., Tims S., de Vos W.M. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141:1792–1801. doi: 10.1053/j.gastro.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 42.Sokol H., Seksik P., Furet J.P., Firmesse O., Nion-Larmurier I., Beaugerie L., Cosnes J., Corthier G., Marteau P., Doré J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm. Bowel Dis. 2009;15:1183–1189. doi: 10.1002/ibd.20903. [DOI] [PubMed] [Google Scholar]
- 43.Van Immerseel F., Ducatelle R., De Vos M., Boon N., Van De Wiele T., Verbeke K., Rutgeerts P., Sas B., Louis P., Flint H.J. Butyric acid-producing anaerobic bacteria as a novel probiotic treatment approach for inflammatory bowel disease. J. Med. Microbiol. 2010;59:141–143. doi: 10.1099/jmm.0.017541-0. [DOI] [PubMed] [Google Scholar]
- 44.Pereira N.B.F., Ramos C.I., de Andrade L.S., Teixeira R.R., Nakao L.S., Rodrigues S.D., de Lima J.D., Dalboni M.A., Cuppari L. Influence of bowel habits on gut-derived toxins in peritoneal dialysis patients. J. Nephrol. 2020;33:1049–1057. doi: 10.1007/s40620-020-00819-9. [DOI] [PubMed] [Google Scholar]
- 45.Ramos C.I., Armani R.G., Canziani M.E., Ribeiro Dolenga C.J., Nakao L.S., Campbell K.L., Cuppari L. Bowel Habits and the Association with Uremic Toxins in Non-Dialysis-Dependent Chronic Kidney Disease Patients. J. Ren. Nutr. 2020;30:31–35. doi: 10.1053/j.jrn.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Lobel L., Cao Y.G., Fenn K., Glickman J.N., Garrett W.S. Diet posttranslationally modifies the mouse gut microbial proteome to modulate renal function. Science. 2020;369:1518–1524. doi: 10.1126/science.abb3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are fully available without restriction, in an anonymized format, upon request.