Abstract
Although screening for Trypanosoma cruzi antibodies is mandatory in most South American countries, current tests are insensitive and have poor specificity. A recently optimized line immunoassay (the INNO-LIA Chagas assay) for the serological confirmation of Chagas' disease was evaluated at a large blood bank in São Paulo, Brazil. Sera from blood donors who reacted in at least one of three serological screening assays (n = 1,604) and who returned for a follow-up were retested, and the donors were interviewed to assess their epidemiological risk. The results obtained by the confirmatory assay evaluated in this study were compared to those obtained by the three different screening assays. Upon consideration of the consensus results obtained by the three different screening assays as a “gold standard,” the INNO-LIA Chagas assay showed a sensitivity of 99.4% (95% confidence interval [CI], 98.3 to 99.9) and a specificity of 98.1% (95% CI, 96.6 to 99.0) for positive (n = 503) and negative (n = 577) sera. The INNO-LIA Chagas assay confirmed the results for significantly larger numbers of positive samples of at-risk individuals independent of the number of positive screening tests (P = 0.017, Mantel-Haenszel test). In conclusion, the INNO-LIA Chagas assay reliably confirmed the presence of antibodies to T. cruzi and can be implemented as a confirmatory assay for Chagas' disease serology.
In Brazilian blood banks, the screening for antibodies directed against Trypanosoma cruzi is mandatory. Screening assays include the indirect immunofluorescence assay (IFA), the indirect hemagglutination assay (IHA), and the enzyme-linked immunosorbent assay. The use of at least two assays based either on different methodologies or on different antigen preparations is currently recommended. At Fundação Pro-Sangue Hemocentro de São Paulo (FPS/HSP), a state blood bank in the city of São Paulo, Brazil, all donated blood is tested by three different assays for Chagas' disease: IFA, IHA, and enzyme immunoassay (EIA). If a sample is repeatedly reactive by one or more assays, the blood unit is discarded and the corresponding donor is asked to return for follow-up testing. However, only some of the donors return, and 70% of the discarded blood donations show discrepant results between initial and follow-up screening. On the basis of epidemiological data, we have previously suggested that some of the discrepant results may represent a true infection (11).
Most of the tests that are commercially available in Brazil today use crude parasite extracts or subcellular fractions as antigen preparations. In recent years, various investigators have characterized T. cruzi-specific immunoreactive antigens, and several studies have evaluated the diagnostic potential of these antigens either in the form of recombinant proteins or as synthetic peptides (1, 2, 5, 10, 14). Recombinant antigens are more specific than parasite extracts that cross-react with sera from patients with other diseases such as leishmaniasis (12), Trypanosoma rangeli infection (3), syphilis, or rheumatic fever. The sensitivities reported for the different recombinant antigens vary with the clinical status of the patient and the manifestation of the disease; the combined use of different recombinant antigens in the same test improves diagnostic sensitivity (12). However, some recombinant antigens or synthetic peptides may exhibit amino acid stretches that resemble those of other organisms, thereby resulting in cross-reactivity by means of molecular mimicry. These reactivities must be carefully interpreted. Moreover, use of a multiparametric assay that measures independently the reactivities to several antigens dramatically reduces the combined probability of occurrence of cross-reactions. In this study, we evaluated the use of a new multiparameter confirmatory assay that combines relevant, immunodominant recombinant and synthetic antigens derived from T. cruzi proteins as a confirmatory diagnostic test for Chagas' disease.
MATERIALS AND METHODS
Study populations.
During 1995 and 1996, about 400,000 blood units were screened at FPS/HSP; about 1% of the units were discarded due to an initial reactivity by at least one of three screening assays. The 1,604 serum samples used in this retrospective study were obtained from Brazilian blood donors who returned and participated in an epidemiological survey during this period (11). Medical counseling was offered for those who returned to obtain the results of the testing, at which time they were interviewed about their risk of exposure to Chagas' disease. Risk factors were evaluated on the basis of “yes” or “no” answers to a series of questions concerning housing style, place of birth, places of residence, the presence of the triatomine vector at home or in the neighborhood, and, finally, the occurrence of Chagas' disease in the family. For purpose of simple comparison, we combined the five different answers into a single binary risk-factor variable (i.e., the risk was absent or present) that refers to the likelihood of contact with the parasite. If at least one of the answers indicated a risk for contact with the bug vector, the donor was considered at risk (n = 815). Donors who replied to all five questions negatively were considered at low risk (n = 147). Data for the donors who failed to reply to all of the questions (for whom one or more answers were missing) were discarded from the risk-factor analysis (n = 642) unless they indicated the presence of a risk factor in their partially answered questionnaire.
Screening assays.
All sera were serologically characterized by a set of three different techniques: IHA and IFA (both from Biolab, Jacarepaguá, Brazil) and an EIA (Embrabio, São Paulo, Brazil). These techniques were applied according to the corresponding manufacturer's instructions. Each sample was given a Chagas' disease screening score (CSS) that ranged from 0 to 3, reflecting the number of screening tests in which it showed reactivity.
INNO-LIA Chagas assay.
The INNO-LIA Chagas antibody assay consists of seven recombinant and synthetic T. cruzi antigens coated as discrete lines onto a nylon membrane with plastic backing. In addition, the strips contain control lines for sera with strong, moderate, and weak (cutoff) reactivities and a streptavidin background control. The antigens used in this assay were either Escherichia coli-expressed protein Tc24 (9) or N-terminally biotinylated synthetic peptides derived from the following proteins: Ag 39, TcD, SAPA, MAP, CRA, and FRA (1, 5, 6, 10, 13). The strips were incubated with the sera at a 1/100 dilution for 18 h at 25°C, and after washing, the immune complexes were detected by incubation with an anti-human immunoglobulin G conjugate and subsequent color development. The results were determined by visually comparing the intensities of the antigen lines with those of the controls. The intensities were scored as follows: 0 (−), no line or intensity less than that of the cutoff line; 0.5 (±), intensity equal to that of the cutoff line; 1 (+), intensity greater than that of the cutoff line or equal to that of the 1+ control line; 2 (++), intensity between those of the 1+ control line and the 3+ control line; 3 (+++), intensity equal to that of the 3+ control line; 4 (++++), intensity greater than that of the 3+ control line.
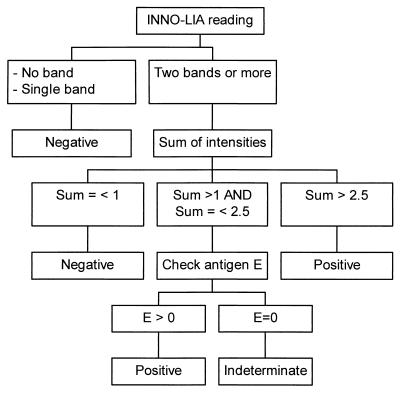
The interpretation criteria shown in Fig. 1 have been validated in a previous study in terms of sensitivity and specificity (8). Briefly, a sample was considered negative if either no band or only a single band appeared or if two or more bands appeared with a total score of less than or equal to 1. A sample was considered positive when at least two bands appeared and the sum of their intensities was greater than 2.5. If two or more bands with a sum of intensities greater than 1 but less than or equal to 2.5 appeared, the result for the sample was considered indeterminate if the score for the E-antigen line was 0 and positive if the score for the E-antigen line was higher than 0.
FIG. 1.
Algorithm for interpretation of INNO-LIA Chagas assay results.
Statistical methods.
StatMate software (version 1.01; GraphPad, San Diego, Calif.) was used for the calculation of 95% confidence intervals for proportions. Samples from low- and high-risk subjects were compared for their INNO-LIA Chagas assay reactivities (positive versus nonpositive) stratified for CSS (CSS of 0, 1, 2, or 3). Mantel-Haenszel test inference based on two-by-two tables and a test for the difference between two binomial proportions were performed with StatXact, version 3, software (Cytel Software Corporation, Cambridge, Mass.).
RESULTS
Confirmation of results by INNO-LIA Chagas assay.
A total of 1,604 screened serum samples were analyzed by the INNO-LIA Chagas confirmatory assay. The comparative results are summarized in Table 1. A total of 577 serum samples (36.0%) were negative by all three screening assays. Of these, 566 (98.1%) were also negative by the INNO-LIA Chagas assay, and the remaining samples were either indeterminate (n = 5) or positive (n = 6). Of the 471 serum samples that were reactive by only one screening assay, 438 (93.0%) were negative, 16 were indeterminate, and 17 were positive upon confirmation of the results by the INNO-LIA Chagas assay. Of 53 samples that reacted in two screening assays, 35 (66.0%) were confirmed to be positive, 17 (32.1%) were confirmed to be negative, and 1 (1.9%) was indeterminate by the INNO-LIA Chagas assay. Finally, the INNO-LIA Chagas assay confirmed the results for 500 of 503 serum samples that reacted in the three screening assays (99.4%); the remaining 3 samples were confirmed to be negative.
TABLE 1.
INNO-LIA Chagas confirmatory assay results compared to CSS
| CSS | No. (%) of samples with the following result by INNO-LIA Chagas assay:
|
|||
|---|---|---|---|---|
| Negative | Indeterminate | Positive | Total | |
| 0 | 566 (98.1) | 5 (0.9) | 6 (1.0) | 577 |
| 1 | 438 (93.0) | 16 (3.4) | 17 (3.6) | 471 |
| 2 | 17 (32.1) | 1 (1.9) | 35 (66.0) | 53 |
| 3 | 3 (0.6) | 0 (0.0) | 500 (99.4) | 503 |
| Total | 1,024 (63.8) | 22 (1.4) | 558 (34.8) | 1,604 |
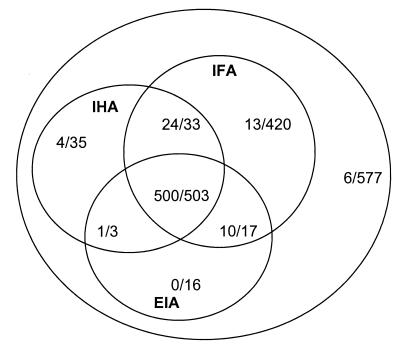
Figure 2 depicts the results found by each of the screening techniques and the proportion of samples with positive results whose results were confirmed by the INNO-LIA Chagas assay. Figure 2 and Table 1 show that most of the samples with a CSS of 1 were positive by IFA (420 of 471). Of these, the INNO-LIA Chagas assay confirmed the results for 13 samples. Samples with a CSS of 2 were predominantly positive by IFA and IHA techniques (33 of 53), and the INNO-LIA Chagas assay confirmed that 24 were positive. The results for none of the 16 samples reactive only by EIA were confirmed by the INNO-LIA Chagas assay.
FIG. 2.
Comparison of screening and confirmation assay results. The outer circle includes 1,604 screened serum samples. Each inner circle represents one of the screening techniques (IFA, IHA, and EIA). Ratios indicate the number of INNO-LIA Chagas assay-positive samples/the number of samples reactive by the technique being considered.
Evaluation of discrepant results with epidemiological information.
Only 962 of 1,604 serum samples for which complete epidemiological data were available could be classified into two relative risk groups depending on the epidemiological information. The epidemiological data were used to classify the sera into a low-risk (n = 147) or a high-risk (n = 815) population (Table 2). For instance, as shown in Table 2, among the 244 serum samples that did not react by any screening assay (CSS = 0), 3 were found to be positive by the INNO-LIA Chagas assay; all 3 serum samples belonged to the group at high risk. Conversely, among the subset of samples with a CSS of 3 (n = 418), two serum samples from at-risk individuals were found to be negative by the INNO-LIA Chagas assay. Figure 2 shows the frequency of positive samples as confirmed by the INNO-LIA Chagas assay compared to the number of samples that were reactive by the screening test (CSS) for each of the risk groups. A higher proportion of INNO-LIA Chagas assay positivity was obtained among subjects at high risk for Chagas' disease, and this result was statistically significant and independent of the number of samples reactive by screening assays (Mantel-Haenszel test, stratifying for CSS, P = 0.017).
TABLE 2.
Evaluation of discrepant samples by serology with risk-factor groups
| CSS (no. of serum samples) | Result by INNO-LIA Chagas assay | No. of samples with epidemiological risk factors:
|
||
|---|---|---|---|---|
| Absent | Present | Total | ||
| 0 (244) | Negative | 71 | 165 | 236 |
| Indeterminate | 0 | 5 | 5 | |
| Positive | 0 | 3 | 3 | |
| 1 (257) | Negative | 56 | 177 | 233 |
| Indeterminate | 0 | 11 | 11 | |
| Positive | 0 | 13 | 13 | |
| 2 (43) | Negative | 1 | 9 | 10 |
| Indeterminate | 0 | 1 | 1 | |
| Positive | 1 | 31 | 32 | |
| 3 (418) | Negative | 0 | 2 | 2 |
| Indeterminate | 0 | 0 | 0 | |
| Positive | 18 | 398 | 416 | |
| Total | 147 | 815 | 962 | |
DISCUSSION
The confirmation of human infections with T. cruzi cannot be based solely on clinical manifestations due to a lack of overt symptoms in most infected persons. Xenodiagnosis and hemoculture are useful techniques for demonstration of the presence of the parasite in the blood. Nevertheless, although very specific, these techniques are insensitive due to variable parasitemia levels (4). On the other hand, conventional serological assays lack specificity for the confirmation of T. cruzi antibodies due to uncontrollable cross-reactivities.
In this study, we evaluated the reliability of the INNO-LIA Chagas assay to confirm the presence of antibodies to T. cruzi in human serum samples with evidence of Chagas' disease. Samples that reacted in three different screening assays (CSS = 3) were considered positive, while those samples with a CSS equal to zero were thought to be negative for T. cruzi antibodies. The results for all samples with an intermediate CSS of 1 or 2 were considered doubtful. The results obtained by the confirmatory assay, the INNO-LIA Chagas assay, were first evaluated in comparison to the CSS. As shown in Table 1, INNO-LIA Chagas assay results tend to be in very good agreement for both negative samples (566 of 577) and positive samples (500 of 503), showing a specificity of 98.1% and a sensitivity of 99.4%.
In Table 1, as epidemiological data are not shown, the reasons for the discrepancies for samples with CSSs of 0 and indeterminate (n = 5) or positive (n = 6) patterns by the INNO-LIA Chagas assay cannot be elucidated. Therefore, screening and confirmatory assay results were also looked at in the context of epidemiological information, when it was available (Table 2). When analyzed for their relative risk factors, the doubtful samples by screening (CSS = 1 and CSS = 2) were resolved into those with negative or positive results by the INNO-LIA Chagas assay. More negative samples were found in the subset with a CSS of 1 (233 of 257; 90.6%) than in the subset with a CSS of 2 (10 of 43; 23.2%). This suggests that the results for most of the samples with a CSS of 1 could be linked to a false reactivity by one screening assay (the reacting assay), while the results for most of the samples with a CSS of 2 are probably due to a lack of sensitivity of the one screening assay (the nonreacting assay). On the basis of the previously demonstrated performance of the INNO-LIA Chagas assay (8), we provide additional evidence for an increased sensitivity of the confirmatory assay over those of the screening assays used. This can be illustrated by the fact that three samples were positive by the INNO-LIA Chagas assay but had a CSS of 0. All three samples were from individuals who belong to a risk group. Interestingly, these samples were from individuals with cumulative geography- and vector-related risks (data not shown). On the other hand, the two samples that had a CSS of 3 and that were confirmed to be negative by the INNO-LIA Chagas assay may exemplify the higher specificity of the INNO-LIA Chagas assay versus those of the screening assays. This specificity was demonstrated with samples potentially infected with Leishmania (the area of endemicity for which overlaps that for Chagas' disease) (8). The observations are in line with the recommendation for the use of two screening assays for all blood donations. On the other hand, the use of only two screening assays without any reliable confirmation indicates a yet underperforming approach.
Finally, of 503 samples, only 3 (0.6%) with a CSS of 3 were confirmed to be negative by the INNO-LIA Chagas assay. Table 2 shows that two of these samples were from individuals in a risk group; complete epidemiological information was not available for the third sample. Table 2 also shows that the proportion of positive samples as determined by the INNO-LIA Chagas assay increases with both the CSS and the number of relative risk factors. Nevertheless, among the 418 samples that had a CSS of 3 and that were confirmed to be positive by the INNO-LIA Chagas assay, 18 (4.3%) belonged to individuals in a low-risk group. This can be explained by the relatively weak accuracy of questionnaire-based estimations compared to those of laboratory measurements. Since implementation of the epidemiological survey is laborious, especially in a routine setting such as blood banks, the risk-factor determination can be advantageously replaced by a reliable confirmatory assay. The need for such an assay has been clearly highlighted for blood bank supply safety as well as for the clinical diagnosis of Chagas' disease. In conclusion, on the basis of the results of the present study as well as those of the independent previous investigation (8), the INNO-LIA Chagas assay is a reliable assay for the serological confirmation of Chagas' disease.
ACKNOWLEDGMENTS
We thank Fred Shapiro for critically reviewing and editing the manuscript.
REFERENCES
- 1.Affranchino J L, Ibanez C F, Luquetti A O, Rassi A, Reyes M B, Macina R A, Aslund L, Pettersson U, Frasch A C. Identification of a Trypanosoma cruzi antigen that is shed during the acute phase of Chagas' disease. Mol Biochem Parasitol. 1989;34:221–228. doi: 10.1016/0166-6851(89)90050-9. [DOI] [PubMed] [Google Scholar]
- 2.Carvalho M R, Krieger M A, Almeida E, Oelemann W, Shikanai-Yassuda M A, Ferreira A W, Pereira J B, Saez-Alquezar A, Dorlhiac-Llacer P E, Chamone D F. Chagas' disease diagnosis: evaluation of several tests in blood bank screening. Transfusion. 1993;33:830–834. doi: 10.1046/j.1537-2995.1993.331094054620.x. [DOI] [PubMed] [Google Scholar]
- 3.Coura J R, Fernandes O, Arboleda M, Barrett T V, Carrara N, Degrave W, Campbell D A. Human infection by Trypanosoma rangeli in the Brazilian Amazon. Trans R Soc Trop Med Hyg. 1996;90:278–279. doi: 10.1016/s0035-9203(96)90247-3. [DOI] [PubMed] [Google Scholar]
- 4.Gomes M L, Galvao L M, Macedo A M, Pena S D, Chiari E. Chagas' disease diagnosis: comparative analysis of parasitologic, molecular, and serologic methods. Am J Trop Med Hyg. 1999;60:205–210. doi: 10.4269/ajtmh.1999.60.205. [DOI] [PubMed] [Google Scholar]
- 5.Ibanez C F, Affranchino J L, Macina R A, Reyes M B, Leguizamon S, Camargo M E, Aslund L, Pettersson U, Frasch A C. Multiple Trypanosoma cruzi antigens containing tandemly repeated amino acid sequence motifs. Mol Biochem Parasitol. 1988;30:27–38. doi: 10.1016/0166-6851(88)90129-6. [DOI] [PubMed] [Google Scholar]
- 6.Kerner N, Liegeard P, Levin M J, Hontebeyrie-Joskowicz M. Trypanosoma cruzi: antibodies to a MAP-like protein in chronic Chagas' disease cross-react with mammalian cytoskeleton. Exp Parasitol. 1991;73:451–459. doi: 10.1016/0014-4894(91)90069-9. [DOI] [PubMed] [Google Scholar]
- 7.Oelemann W M, Teixeira M G, Verissimo Da Costa G C, Borges-Pereira J, De-Castro J A, Coura J R, Peralta J M. Evaluation of three commercial enzyme-linked immunosorbent assays for diagnosis of Chagas' disease. J Clin Microbiol. 1998;36:2423–2427. doi: 10.1128/jcm.36.9.2423-2427.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oelemann W M, Vanderborght B O M, Verissimo-Da-Costa G C, Teixeira M D, Borges-Pereira J, De-Castro J A, Coura J R, Stoops E, Hulstaert F, Zrein M, Peralta J M. A recombinant peptide antigen line immunoassay optimized for the confirmation of Chagas' disease. Transfusion. 1999;39:711–717. doi: 10.1046/j.1537-2995.1999.39070711.x. [DOI] [PubMed] [Google Scholar]
- 9.Ouaissi A, Aguirre T, Plumas-Marty B, Piras M, Schoneck R, Gras-Masse H, Taibi A, Loyens M, Tartar A, Capron A. Cloning and sequencing of a 24-kDa Trypanosoma cruzi specific antigen released in association with membrane vesicles and defined by a monoclonal antibody. Biol Cell. 1992;75:11–17. doi: 10.1016/0248-4900(92)90119-l. [DOI] [PubMed] [Google Scholar]
- 10.Pastini A C, Iglesias S R, Carricarte V C, Guerin M E, Sanchez D O, Frasch A C. Immunoassay with recombinant Trypanosoma cruzi antigens potentially useful for screening donated blood and diagnosing Chagas' disease. Clin Chem. 1994;40:1893–1894. [PubMed] [Google Scholar]
- 11.Salles N A, Sabino E C, Cliquet M G, Eluf-Neto J, Mayer A, Almeida-Neto C, Mendonca M C, Dorliach-Llacer P, Chamone D F, Saez-Alquezar A. A risk exposure for Chagas' disease among seroreactive Brazilian blood donors. Transfusion. 1996;36:969–973. doi: 10.1046/j.1537-2995.1996.36111297091740.x. [DOI] [PubMed] [Google Scholar]
- 12.Umezawa E S, Bastos S F, Camargo M E, Yamauchi L M, Santos M R, Gonzalez A, Zingales B, Levin M J, Sousa O, Rangel Aldao R, da Silveira J F. Evaluation of recombinant antigens for serodiagnosis of Chagas' disease in South and Central America. J Clin Microbiol. 1999;37:1554–1560. doi: 10.1128/jcm.37.5.1554-1560.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vergara U, Lorca M, Veloso C, Gonzalez A, Engstrom A, Aslund L, Pettersson U, Frasch A C C. Assay for detection of Trypanosoma cruzi antibodies in human sera based on reaction with synthetic peptides. J Clin Microbiol. 1991;29:2034–2037. doi: 10.1128/jcm.29.9.2034-2037.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zingales B, Gruber A, Ramalho C B, Umezawa E S, Colli W. Use of two recombinant proteins of Trypanosoma cruzi in the serological diagnosis of Chagas' disease. Mem Inst Oswaldo Cruz. 1990;85:519–522. doi: 10.1590/s0074-02761990000400024. [DOI] [PubMed] [Google Scholar]